Abstract
Objective
Given the variability in crisis standards of care (CSC) guidelines during the COVID‐19 pandemic, we investigated the racial and ethnic differences in prioritization between 3 different CSC triage policies (New York, Massachusetts, USA), as well as a first come, first served (FCFS) approach, using a single patient population.
Methods
We performed a retrospective cohort study of patients with intensive care unit (ICU) needs at a tertiary hospital on its peak COVID‐19 ICU census day. We used medical record data to calculate a CSC score under 3 criteria: New York, Massachusetts with full comorbidity list (Massachusetts1), and MA with a modified comorbidity list (Massachusetts2). The CSC scores, as well as FCFS, determined which patients were eligible to receive critical care under 2 scarcity scenarios: 50 versus 100 ICU bed capacity. We assessed the association between race/ethnicity and eligibility for critical care with logistic regression.
Results
Of 211 patients, 139 (66%) were male, 95 (45%) were Hispanic, 23 (11%) were non‐Hispanic Black, and 69 (33%) were non‐Hispanic White. Hispanic patients had the fewest comorbidities. Assuming a 50 ICU bed capacity, Hispanic patients had significantly higher odds of receiving critical care services across all CSC guidelines, except FCFS. However, assuming a 100 ICU bed capacity, Hispanic patients had greater odds of receiving critical care services under only the Massachusetts2 guidelines (odds ratio, 2.05; 95% CI, 1.09 to 3.85).
Conclusion
Varying CSC guidelines differentially affect racial and ethnic minority groups with regard to risk stratification. The equity implications of CSC guidelines require thorough investigation before CSC guidelines are implemented.
Keywords: COVID‐19, crisis standards of care, emergency department, health disparities
1. INTRODUCTION
1.1. Background
As of February 2021, there were over 27.3 million cases of COVID‐19 in the United States, with >148,000 cases around Boston alone. 1 In preparation for a surge in critically ill COVID‐19 patients, states developed policies known as crisis standards of care (CSC). These triage policies help distribute scarce resources while “maximizing the care delivered to the population…under austere circumstances.” 2 Simply stated, CSC guide the allocation of critical care resources, such as ventilators and ICU beds, when the demand exceeds the supply.
Varied ethical principals have guided the development of CSC. The principle of utilitarianism asserts that resources should be allocated to save the greatest number of life‐years. This often forms the essence of triage policies, 3 including the Massachusetts CSC guidelines. 2 In contrast, egalitarianism mandates that all patients should have an equal chance at receiving scarce resources. A survey of the general public found that most agree with resource allocation motivated by the utilitarian principle of maximizing benefit, however, the public favored more egalitarian principles if that meant mitigating disadvantage for people of color. 3
Because CSC frameworks are motivated by multiple allocation principles, there is wide variability in guidelines across states. 4 For example, New York incorporates explicit exclusion criteria that prohibit access to critical care resources (eg., cardiac arrest). Most states also include the Sequential Organ Failure Assessment (SOFA) score, used to assess the likelihood of survival from acute illness. 5 In Massachusetts, the initial CSC framework included both the SOFA score (for prognosis of hospital survival) and comorbidities to assess long‐term prognosis. 2 The inclusion of comorbidities into the initial Massachusetts CSC guidelines raised concern among health equity experts because many of the conditions considered for inclusion in the Massachusetts CSC, such as diabetes and renal disease, are characterized by a broad spectrum of severity and are not equitably distributed in the population, 6 , 7 with higher prevalence among Black and Hispanic patients. 8 , 9 Among the many reasons for the higher prevalence of comorbidities in Black and Hispanic patients are structural racism, unequal access to good‐quality health care, and socioeconomic factors. 10 Furthermore, these same factors may contribute to the disproportionate impact of COVID‐19 on Black and Hispanic communities 11 , 12 —as members of these communities are more likely to live in crowded housing conditions or work essential jobs that necessitate in‐person interaction. 13 , 14 These criteria may also systematically disadvantage patients with limited English proficiency or those without insurance, who may have less access to primary care. Less access to primary care leads to worsened chronic disease and thus, higher risk for deprioritization in those CSC guidelines that include comorbidities. Therefore, after advocacy efforts by health equity experts and community leaders, the Massachusetts guidelines were modified to emphasize short‐term survival, with less emphasis placed on comorbidities. A comparison of these guidelines can be found in Table 1. 15 , 16
TABLE 1.
Comparison of New York and Massachusetts crisis standard of care guidelines
| New York | Massachusetts1 c | Massachusetts2 c | |
|---|---|---|---|
| Exclusion | Cardiac arrest | ||
| Irreversible age‐specific hypotension unresponsive to fluid resuscitation or vasopressor therapy | |||
| TBI with no motor response to painful stimulus | |||
| Severe burns with predicted survival <10% | |||
| Any other conditions resulting in immediate or near‐immediate mortality even with aggressive therapy | |||
| Evaluation of current illness | SOFA | SOFA | SOFA |
| Comorbidities | |||
| Major condition a | Expected survival <5 years | ||
| CHF or NYHA class 3 CHF | |||
| Cirrhosis, ascites, encephalopathy, portal hypertension, variceal bleeding | |||
| COPD (O2 dependent) | |||
| ILD | |||
| Cystic fibrosis | |||
| Primary pulmonary hypertension | |||
| ESRD/dialysis or Cr >3 | |||
| Progressive neuromuscular disease | |||
| Stroke with chronic aspiration not responsive to speech language therapy | |||
| Dementia with FAST stage 4–6 | |||
| AIDS | |||
| Diabetes with end‐organ damage | |||
| Severe pressure ulcer not amenable to surgical intervention | |||
| Severe inoperable multivessel CAD | |||
| Severe condition b | Expected survival <1 year | Expected survival <1 year | |
| Advanced neuromuscular disease dependent in ADLs and/or requiring chronic ventilator support | Advanced neuromuscular disease dependent in ADLs and/or requiring chronic ventilator support |
Abbreviations: ADLs, activities of daily living; AIDS, acquired immunodeficiency syndrome; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Cr, creatinine; ESRD, end‐stage renal disease; FAST, functional assessment staging test; ILD, interstitial lung disease; NYHA, New York Heart Association; O2, oxygen; SOFA, Sequential Organ Failure Assessment.
aANY condition = +2 points (not cumulative).
bANY condition = +4 points (not cumulative); if both major and severe condition present, patient received +4 points for severe.
cFor Massachusetts guidelines, any documented pregnancy was worth ‐2 points, regardless of gestational age.
2. IMPORTANCE
As many hospitals, including those in Massachusetts, have not yet implemented CSC, we do not have data to understand how these standards would differentially affect the population, specifically by race/ethnicity. Notably, if CSC are implemented, measuring their impact post hoc will render it impossible to correct any structural inequities inherent in the guidelines.
The Bottom Line
In preparation for critical patient surges from COVID‐19, states developed triage policies called crisis standards of care (CSC). While using ethical principles and clinical parameters, the CSC did not consider implications for equity. In a retrospective study of 211 patients in Boston during COVID‐19, CSC guidelines differentially affected racial and ethnic minority groups with regard to risk stratification. The authors recommend consideration of the equity of CSCs before their implementation.
To address this urgent need, we investigated how 3 different CSC criteria—the New York, original Massachusetts, and modified Massachusetts guidelines—would affect the racial and ethnic composition of the patient population prioritized for critical care resources during the initial COVID‐19 surge. We compared these criteria to the more egalitarian approach of first come, first served (FCFS), which depicts what might be expected to happen in the absence of CSC guidance. Given the nuanced nature of decisions regarding withdrawal of critical care, 17 we intentionally refrained from assessing which of the three CSC guidelines maximize survival and minimize long‐term morbidity.
2.1. Goals of this investigation
Our objective was to investigate how the application of 3 different CSC criteria to the same sample population affected the racial/ethnic distribution of patients who would be prioritized for critical care resources and to compare these triage policies to FCFS.
3. METHODS
3.1. Study design
We performed a retrospective cohort study using the electronic medical record (EMR) data of patients receiving care in the ICU at a large tertiary care hospital. We modeled the impact of 3 different CSC criteria, with specific regard to the racial/ethnic distribution of treated patients. Our study was conducted in several stages (Figure 1), which are detailed next. In brief, participants were assigned CSC scores under three CSC criteria: the New York guidelines, the original Massachusetts guidelines, which included a full list of comorbidities (Massachusetts1), and the modified Massachusetts guidelines, which contained fewer comorbidities (Massachusetts2). The scores were used to determine which patients would qualify for critical care resources, defined as intubation or ICU admission, assuming 2 different resource scarcity scenarios: a hospital with 50 ICU beds versus a hospital with 100 ICU beds. These results were compared with the FCFS system. Sensitivity analyses were then performed to examine the potential effects of missing data on the triage of patients. This study was reviewed and approved by the Mass General Brigham institutional research board.
FIGURE 1.
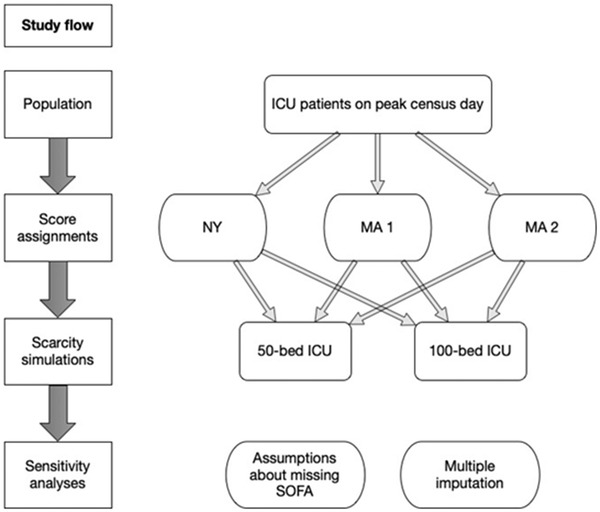
Study design
Abbreviations: MA, Massachusetts; NY, New York; SOFA, Sequential Organ Failure Assessment
3.2. Selection of participants and laboratory values
All patients receiving critical care resources at our institution on the peak ICU census day during the initial surge of COVID‐19 (April 18, 2020) underwent EMR review. Demographic and clinical data were manually abstracted to calculate each patient's CSC score under each guideline, as well as their Charlson Comorbidity Index (CCI). The CCI is a composite score that has been used to estimate 10‐year survival. 18
With regard to SOFA scoring, several assumptions were made. First, if the arterial oxygen saturation (PaO2) was missing, the peripheral oxygen saturation (SpO2) was used instead. The fraction of inspired oxygen (FiO2) was either recorded or estimated based on the type of supplemental oxygen. If the patient was intubated before arrival, an FiO2 of 100% was assumed. If the patient was using a nasal cannula, the FiO2 was assumed to be 44% (a flow rate of ≈ 6 liters per minute). If the patient was using a non‐rebreather mask, an FiO2 of 90% was assumed (≈ 15 liters per minute). Finally, for those patients who were on multiple pressors not listed in the SOFA scoring calculator (ie, vasopressin), the vasopressors were converted to equivalent doses of norepinephrine, and the total cumulative dosage was used. 19
When multiple values for a given clinical variable were available, the value most proximal to the decision to provide critical care (defined as either intubation or admission to ICU) was selected. To avoid biased comparisons between patients with acute critical illness and those who had already been resuscitated in the ICU, priority was given to those values recorded immediately before (as opposed to after) the provision of critical care services. Each patient chart was reviewed by 2 trained research assistants. The data were reviewed for inconsistencies, which were reconciled by an experienced third reviewer, with either an MD or MPH degree. This process was repeated, until all charts were consistent. Data extraction guidelines are available in Appendix A.
3.3. Outcomes
The primary outcome of the study was the odds of receiving critical care resources as determined by the CSC score or FCFS triage under 2 hypothetical scenarios simulating resource scarcity: a hospital with 50 ICU bed capacity versus a hospital with 100 ICU bed capacity.
3.4. Analysis
3.4.1. Assignment of triage priority
We triaged patients using (1) the New York system, (2) the Massachusetts system with a full comorbidity list (Massachusetts1), and (3) the modified Massachusetts system with a shorter comorbidity list (Massachusetts2) (Table 1). We then examined how the triage of patients differed under each system and compared this to FCFS.
Each patient was assigned a CSC score, as detailed in each CSC protocol. The New York protocol excluded patients with certain conditions indicating near immediate mortality and then divided patients into 3 priority groups based on SOFA score (Table 1). For the Massachusetts1 and Massachusetts2 guidelines, patients would receive 1, 2, 3, or 4 points if their SOFA score was <6, 6–9, 10–12, and >12, respectively. Two points were subtracted if they were pregnant beyond 24 weeks gestational age. Under the Massachusetts1 guidelines, patients would receive 2 points for major comorbid conditions with substantial impact on long‐term survival and 4 points for severe comorbid conditions (Table 1). However, under the Massachusetts2 guidelines, only those with major underlying conditions making death likely within 1 year would receive 4 points. The totaled points were divided into 3 categories: highest priority (red, 1–2 points), intermediate priority (orange, 3–5 points), and lowest priority (yellow, 6–8 points). Within each priority group, patients were arranged by CSC score and age. Those with the lowest CSC score (corresponding to the most severe illness) and youngest age received highest priority.
With regard to FCFS, the first 50 or 100 patients—depending on the scarcity scenario—requiring ICU‐level of care and/or intubation were given highest priority. The first date of ICU admission or the ICU admission note (if date of admission was not recorded) was used to determine priority order.
3.5. Primary data analysis
We used descriptive statistics to summarize participants’ demographic characteristics and CSC scores. We used unadjusted logistic regression models to assess the association between CSC scores and race/ethnicity for patients without missing data. Patients with missing data were addressed in the sensitivity and imputation analysis (Stata IC, Version 15.1; StataCorp, College Station, Texas, USA). We compared SOFA scores across categories of race/ethnicity and the 3 CSC scores across key demographic and clinical characteristics (eg., age group, body mass index).
We used logistic regression models to examine the association between the receipt of critical care services and key patient characteristics. In order to understand how triage rankings and FCFS would affect patients’ receipt of critical care resources, we created 2 hypothetical scenarios to mimic real‐life resource scarcity. The first scenario assumed 50 available ICU beds, and the second 100 available ICU beds. After arranging patients in order of priority, the first 50 or 100 patients—depending on the scenario—were selected to receive critical care services. If ties between patients occurred (ie., same CSC score and same age), selection was random in accordance with the tiebreaker recommendations of the three CSC guidelines. 2 , 20 For FCFS, if multiple patients had the same admission date, the first 50 or 100 patients were selected randomly.
In the logistic regression analyses, we purposefully did not control for comorbidities because we suspected that comorbidities were a mediator variable, rather than a confounding variable, in the association between race/ethnicity and the receipt of critical care services. Thus, if we were to control for comorbidities, the resulting association would represent only part of the association (ie, the “direct” association) between race/ethnicity and receipt of critical care services—potentially eliminating that which was mediated by comorbidities. Using the Baron and Kenny procedure, 21 we assessed for significant associations between (1) race/ethnicity and receipt of critical care services and (2) race/ethnicity and comorbidities. We then included comorbidities in the logistic regression model to determine if the association between race/ethnicity and receipt of critical care services disappeared (full mediation) or was attenuated (partial mediation).
3.6. Sensitivity analysis
With regard to missing components of the SOFA score: a score of 4 points was assumed for the central nervous system component if Glasgow Coma Scale (GCS) score was missing and the patient had evidence of altered mental status or inability to protect their airway. Otherwise, a score of 0 points (ie, GCS 15) was assumed. For all other missing components, a score of 0 points was assumed. The analysis detailed p was then repeated.
3.7. Multiple imputation
We performed multiple imputation using chained equations to impute missing data assuming that data were missing at random. We used predictive mean matching owing to the distribution of the data, and we used 25 imputations owing to the amount of missing data. We imputed actual values, rather than derived variables, where possible. The imputed variables were bilirubin, GCS, mean arterial pressure, and the respiratory component of the SOFA score—as these were most frequently missing. The imputed variables then were used to calculate the SOFA score component, except for the imputed respiratory component, and the total SOFA score. Auxiliary variables included those that were potentially correlated with missing values or thought to be related to the missing data mechanism. 22 In particular, we hypothesized that missingness was related to patient race/ethnicity and language preference (ie, GCS was less commonly recorded for non‐English speaking patients). The auxiliary variables included demographics, clinical status (eg, CCI, inability to protect airway, SOFA components for coagulation and renal function), and past medical history. Trace plots were used to determine convergence of the imputation model. 22 The imputed data were pooled for analysis using Rubin's rules. 23 The previously outlined analysis then was repeated using the imputed data.
4. RESULTS
4.1. Characteristics of study subjects
Of the 211 patients, 139 (66%) were male; 95 (45%) were Hispanic, 23 (11%) were non‐Hispanic Black, 69 (33%) were non‐Hispanic White, and 24 (11%) were non‐Hispanic other/unknown. Eighty‐five (40%) patients spoke Spanish, 104 (49%) spoke English, and 22 (10%) spoke other languages. With regard to COVID‐19, 164 (78%) patients tested positive. Of these, 89 (54%) were Hispanic, 21 (13%) were non‐Hispanic Black, 36 (22%) were non‐Hispanic White, and 18 (11%) were non‐Hispanic other/unknown. Most patients (63%) had state/public health insurance, 42% had private health insurance, and 1% were self‐pay. With regard to comorbidities, 37% of patients had none, 30% had 1, and 33% had 2 or more. Sixty percent of patients were obese with a body mass index (BMI) of 30.0 or higher, 17% of patients were normal or underweight, and 23% of patients were overweight (BMI 25.0–30.0) (Table 2). Most patients (55%) had complete data required for CSC score calculation.
TABLE 2.
Patient demographics (n = 211)
| Characteristic | N | % a |
|---|---|---|
| Language | ||
| English | 104 | 49 |
| Spanish | 85 | 40 |
| Portuguese | 5 | 2 |
| Haitian‐Creole | 5 | 2 |
| Arabic | 2 | 1 |
| Other | 10 | 5 |
| Age | ||
| Mean (SD) | 58 | 16 |
| Median (IQR) | 60 | 48‐70 |
| Range | 19–89 | |
| Age group | ||
| 18‐49 years | 58 | 47 |
| 50‐74 years | 119 | 56 |
| ≥75 years | 34 | 16 |
| BMI (kg/m2) | ||
| Mean (SD) | 32 | 8 |
| Median (IQR) | 30 | 26‐36 |
| Range | 14–68 | |
| BMI category | ||
| Normal or underweight (<25.0) | 35 | 17 |
| Overweight (25.0 ‐ <30.0) | 49 | 23 |
| Obese (30.0 or higher) | 127 | 60 |
| Class 1 (30.0 ‐ <35.0) | 70 | 55 |
| Class 2 (35.0 ‐ <40.0) | 24 | 19 |
| Class 3 (40.0 or higher) | 33 | 26 |
| Race/ethnicity | ||
| Non‐Hispanic Black | 23 | 11 |
| Non‐Hispanic Asian | 11 | 5 |
| Non‐Hispanic White | 69 | 33 |
| Non‐Hispanic Other | 3 | 1 |
| Non‐Hispanic Unknown | 10 | 5 |
| Hispanic | 95 | 45 |
| Sex | ||
| Male | 139 | 66 |
| Female | 72 | 34 |
| Gender | ||
| Man | 17 | 8 |
| Woman | 31 | 15 |
| Unspecified | 163 | 77 |
| Health insurance (>1 possible) | ||
| State/public (eg., Medicare, Medicaid) | 133 | 63 |
| Private | 89 | 42 |
| Self‐pay | 3 | 1 |
| Undomiciled | 3 | 1 |
| Place of residence | ||
| Boston | 45 | 21 |
| Chelsea | 39 | 18 |
| Everett | 14 | 7 |
| Lynn | 15 | 7 |
| Malden | 11 | 5 |
| Medford | 9 | 4 |
| Revere | 13 | 6 |
| Somewhere else | 65 | 31 |
| Healthcare worker | 3 | 1 |
| Nurse | 0 | |
| Physician | 1 | 33 |
| Nurse practitioner or physician assistant | 0 | |
| Other | 2 | 67 |
| History | ||
| Alcohol abuse (past or current) | 17 | 8 |
| Drug abuse (past or current) | 7 | 3 |
| Smoking (past or current) | 80 | 39 |
| Missing | 5 | |
| Current tobacco smoker | 15 | 7 |
| ICU admission for trauma | 7 | 3 |
| Number of comorbidities | ||
| 0 | 78 | 37 |
| 1 | 64 | 30 |
| 2 | 35 | 17 |
| ≥3 | 34 | 16 |
Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation.
aPercentages may not total to 100% because of rounding.
5. MAIN RESULTS
5.1. Comorbidities and severity of illness
Table 3 presents the distribution of comorbidities and SOFA score across race/ethnicity. The mean CCI composite score was 3.3 (95% confidence interval [CI], 2.0 to 4.7) for non‐Hispanic Black, 1.1 (95% CI, 0.8 to 1.4) for Hispanic, and 2.0 (95% CI, 1.5 to 2.6) for non‐Hispanic White patients. Of the complete cases (no missing data), non‐Hispanic Black patients had a mean SOFA score of 5.2 (95% CI, 3.3 to 7.1), compared to 4.2 (95% CI, 3.5 to 4.9) for Hispanic, 5.2 (95% CI, 4.1 to 6.3) for non‐Hispanic White, and 3.0 (95% CI, 1.6 to 4.4) for non‐Hispanic other/unknown patients.
TABLE 3.
Comorbidities and SOFA scores by race/ethnicity
| Race/ethnicity | ||||
|---|---|---|---|---|
| Non‐Hispanic black (n = 23) | Non‐Hispanic white (n = 69) | Hispanic (n = 95) | Non‐Hispanic other or unknown (n = 24) | |
| Charlson Comorbidity Index (composite score) | ||||
| Mean (95% CI) | 3.3 (2.0–4.7) | 2.0 (1.5–2.6) | 1.1 (0.8–1.4) | 0.6 (0.2–1.0) |
| Median (IQR) | 2 (1–5) | 1 (0–3) | 1 (0–2) | 0 (0–1) |
| Number of comorbidities, n (%; 95% CI) a | ||||
| 0 | 1 (4; 1–25) | 20 (29; 19–41) | 42 (44; 35–54) | 15 (63; 42–79) |
| 1 | 11 (48; 29–68) | 18 (26; 17–38) | 29 (31; 22–41) | 6 (25; 12–46) |
| 2 | 3 (13; 4–34) | 16 (23; 15–25) | 14 (15; 9–23) | 2 (8; 2–28) |
| ≥3 | 8 (35; 18–56) | 15 (22; 14–33) | 10 (11; 6–19) | 1 (4; 1–25) |
| SOFA score (complete case, n = 116) | ||||
| Mean (95% CI) | 5.2 (3.3–7.1) | 5.2 (4.1–6.3) | 4.2 (3.5–4.9) | 3.0 (1.6–4.4) |
| Median (IQR) | 4 (3–7) | 4 (3–7) | 3 (3–5) | 3 (2–4) |
| Missing (n = 95), n (%) | 10 (43) | 32 (46) | 39 (41) | 14 (58) |
| SOFA score (sensitivity analysis) b | ||||
| Mean (95% CI) | 5.7 (4.2‐7.2) | 4.6 (3.8‐5.4) | 3.9 (3.4‐4.5) | 4.0 (3.1‐5.0) |
| Median (IQR) | 5 (3‐8) | 4 (2‐6) | 3 (3‐5) | 4 (3‐5) |
Abbreviations: CI, confidence interval; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
aPercentages may not total to 100% because of rounding.
bIf Glasgow Coma Score (GCS) was missing and there was evidence of altered mental status or inability to protect airway, then GCS was assumed to be <6 and given 4 points. Otherwise, GCS was assumed to 15 and given 0 points. Other missing components were assumed to be normal and given 0 points.
For SOFA scores, the following assumptions were made:
1. Peripheral oxygen saturation (SpO2) was used in place of arterial oxygen saturation (PaO2) if missing. Fraction of inspired oxygen (FiO2) was either recorded (n = 18) or estimated based on the type of supplemental oxygen. If the patient was intubated prior to arrival, assumed FiO2 was 100%. If the patient was using a nasal cannula, assumed FiO2 was 44% (6 lpm). If the patient was using a non‐rebreather mask, assumed FiO2 was 90% (15 lpm).
2. Maximum dose of vasopressors before intubation was used. All were converted to equivalent doses of norepinephrine.
3. Number of missing: overall = 95; respiratory = 16; central nervous system component = 88; liver component = 4. Some patients were missing more than 1 component.
5.2. Assignment of triage categories
Table 4 presents the CSC classification under the New York, Massachusetts1, and Massachusetts2 scoring systems using complete cases. Of the 116 patients with complete data, 101 (87%) under New York, 70 (60%) under Massachusetts1, and 102 (88%) under Massachusetts2 guidelines had highest priority (red). In the intermediate priority (yellow/orange) group there were 9 (8%) under NewYork, 37 (32%) under Massachusetts1, and 7 (6%) under Massachusetts2 guidelines. With regard to race/ethnicity under the NY guidelines, 77% of non‐Hispanic Black, 93% of Hispanic, and 78% of non‐Hispanic White patients had highest priority. However, under the Massachusetts1 guidelines, 38% of non‐Hispanic Black patients had highest priority, compared with 70% of Hispanic and 46% of non‐Hispanic White patients. These figures increased to 85% non‐Hispanic Black, 96% Hispanic, and 76% non‐Hispanic White in highest priority under the Massachusetts2 guidelines.
TABLE 4.
CSC categories by patient characteristics, complete cases
| New York score (overall n = 116), row % (95% CI) | Massachusetts1 score (overall n = 116), row % (95% CI) | Massachusetts2 score (overall n = 116), row % (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Red | Yellow | Blue | Red | Orange | Yellow | Red | Orange | Yellow | |
| Overall, n (row %) | 101 (87) | 9 (8) | 6 (5) | 70 (60) | 37 (32) | 9 (8) | 102 (88) | 7 (6) | 7 (6) |
| Age group | |||||||||
| 18‐49 years | 97 (79–100) | 0 | 3 (0–21) | 83 (65–93) | 17 (7–35) | 0 | 100 | 0 | 0 |
| 50‐74 years | 83 (72–91) | 11 (5–21) | 6 (2–15) | 64 (51–74) | 27 (18–39) | 9 (4–19) | 86 (76–93) | 8 (3–17) | 6 (2–15) |
| ≥75 years | 85 (62–95) | 10 (2–33) | 5 (1–29) | 15 (5–38) | 70 (47–86) | 15 (5–38) | 75 (52–89) | 10 (2–33) | 15 (5–38) |
| Obesity | |||||||||
| BMI <35.0 | 87 (78–93) | 6 (2–13) | 7 (3–15) | 58 (47–68) | 33 (24–44) | 9 (5–18) | 87 (78–93) | 6 (2–13) | 7 (3–15) |
| BMI ≥35.0 | 87 (70–95) | 13 (5–30) | 0 | 68 (50–82) | 29 (16–47) | 3 (0–20) | 90 (74–97) | 6 (2–23) | 3 (0–20) |
| Race/ethnicity | |||||||||
| Non‐Hispanic black | 77 (48–92) | 15 (4–45) | 8 (1–40) | 38 (17–66) | 54 (28–78) | 8 (1–40) | 85 (55–96) | 15 (4–45) | 0 (0–0) |
| Non‐Hispanic white | 78 (62–89) | 16 (7–32) | 5 (1–19) | 46 (31–62) | 38 (24–54) | 16 (7–32) | 76 (59–87) | 11 (4–26) | 14 (6–29) |
| Hispanic | 93 (82–97) | 2 (0–12) | 5 (2–16) | 70 (56–80) | 27 (17–40) | 4 (1–13) | 96 (87–99) | 0 | 4 (1–13) |
| Non‐Hispanic other or unknown | 100 | 0 | 0 | 90 (53–99) | 10 (1–47) | 0 | 90 (53–99) | 10 (1–47) | 0 |
| Language preference | |||||||||
| English | 81 (68–89) | 12 (6–24) | 7 (3–17) | 49 (36–62) | 37 (25–50) | 14 (7–26) | 82 (70–90) | 7 (3–17) | 11 (5–22) |
| Spanish | 94 (82–98) | 2 (0–14) | 4 (1–15) | 71 (56–82) | 27 (16–41) | 2 (0–14) | 98 (86–100) | 0 | 2 (0–14) |
| Other | 91 (56–99) | 9 (1–44) | 0 | 73 (41–91) | 27 (9–59) | 0 | 73 (41–91) | 27 (9–59) | 0 |
| Sex a | |||||||||
| Male | 87 (78–93) | 8 (4–17) | 5 (2–12) | 60 (49–70) | 33 (23–43) | 7 (3–15) | 87 (78–93) | 8 (4–17) | 5 (2–12) |
| Female | 88 (72–95) | 6 (2–21) | 6 (2–21) | 61 (43–76) | 30 (17–48) | 9 (3–25) | 91 (75–97) | 0 | 9 (3–25) |
| Health insurance | |||||||||
| State/public (eg., Medicare, Medicaid) | 84 (73–91) | 9 (4–19) | 7 (3–17) | 60 (47–71) | 30 (20–42) | 10 (5–20) | 85 (74–92) | 7 (3–17) | 7 (3–17) |
| Private | 93 (80–98) | 5 (1–17) | 2 (0–15) | 64 (49–77) | 31 (19–46) | 5 (1–17) | 93 (80–98) | 2 (0–15) | 5 (1–17) |
| Self‐pay | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 |
| Public & private | 83 (36–98) | 17 (2–64) | 0 | 33 (8–74) | 67 (26–92) | 0 | 83 (36–98) | 17 (2–64) | 0 |
| ICU admission time | |||||||||
| Previously admitted | 87 (79–92) | 8 (4–15) | 5 (2–11) | 62 (52–71) | 31 (22–40) | 7 (3–14) | 89 (81–94) | 5 (2–11) | 6 (3–13) |
| Newly admitted (on 4/18) | 87 (59–97) | 7 (1–36) | 7 (1–36) | 47 (24–71) | 40 (19–65) | 13 (3–41) | 80 (53–93) | 13 (3–41) | 7 (1–36) |
Abbreviations: BMI, body mass index; CI, confidence interval; CSC, crisis standards of care; SOFA, Sequential Organ Failure Assessment.
aBiological sex because of missing data for gender. For patients with a gender documented, it concurred with listed sex.
Notes for CSC scores:
1. New York—Excluded conditions included near immediate mortality; severe irreversible neurological exam; hypotension unresponsive to vasopressors; traumatic brain injury with no motor response to pain; severe burn with predicted survival <10%; current history of cardiac arrest. Categories included red (highest priority; SOFA score 0–7); yellow (SOFA score 8–11); blue (lowest priority; SOFA score ≥12 or excluded condition); green was not included.
2. Massachusetts1 (based on guidance from state dated April 20, 2020; https://www.mass.gov/doc/statewide‐advisory‐committee‐recommendations‐for‐standards‐of‐care/download)–Major comorbid conditions (+2 points) included all listed in Appendix A of analysis plan (included history or current problem). Severe comorbid conditions (+4 points) included death likely within 1 year and advanced neuromuscular disease. Two points were subtracted for pregnancy. Categories included red (highest priority; 1–2 points), orange (3‐5 points), and yellow (lowest priority; 6–8 points). Because of the subtraction for pregnancy, point values of ‐1 or 0 were included in the red group.
3. Massachusetts2 (based on guidance from state dated October 6, 2020; https://www.mass.gov/doc/crisis‐standards‐of‐care‐draft‐planning‐guidance‐for‐public‐comment‐october‐6‐2020/download)–Major comorbid conditions are not included in this version. Severe comorbid conditions (same as above) are given 4 points. Two points were subtracted for pregnancy. Categories included red (highest priority; 1–2 points), orange (3‐5 points), and yellow (lowest priority; 6–8 points). Because of the subtraction for pregnancy, point values of ‐1 or 0 were included in the red group.
5.3. Scarcity scenarios
Table 5 presents the logistic regression analysis for receiving critical care services, assuming a 50 ICU bed capacity. Under this scenario, Hispanic patients had greater odds of receiving critical care services under all guidelines: New York (odds ratio, 3.00; 95% CI, 1.35 to 6.63), Massachusetts1 (OR, 2.55; 95% CI, 1.18 to 5.54), and Massachusetts2 (OR, 2.81; 95% CI, 1.30 to 6.06). For FCFS; however, there was no significant difference across race/ethnicity. Assuming a 100 ICU bed capacity (Appendix B), the odds of receiving critical care services under the New York and Massachusetts1 guidelines, as well as FCFS, were not significantly different across race/ethnicity. However, under the Massachusetts2 guidelines, Hispanic patients again had higher odds of receiving critical care services (OR, 2.05; 95% CI, 1.09 to 3.85). Non‐Hispanic Black and non‐Hispanic other/unknown patients did not have significantly different odds compared to non‐Hispanic White patients under the Massachusetts2 guidelines.
TABLE 5.
Unadjusted odds of receiving critical care services for each variable with 50 ICU beds, complete case analysis
| 50 ICU bed capacity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New York | Massachusetts1 | Massachusetts2 | FCFS | |||||||||
| Yes, n (%) | No, n (%) | OR a (95% CI) | Yes, n (%) | No, n (%) | OR a (95% CI) | Yes, n (%) | No, n (%) | OR a (95% CI) | Yes, n (%) | No, n (%) | OR b (95% CI) | |
| Age group | ||||||||||||
| 18‐49 years | 20 (34) | 38 (66) | Referent | 22 (38) | 36 (62) | Referent | 24 (41) | 34 (59) | Referent | 12 (40) | 18 (60) | Referent |
| 50‐74 years | 29 (24) | 90 (76) | 0.61 (0.31–1.21) | 28 (24) | 91 (76) | 0.50 (0.26–0.99) | 26 (22) | 93 (78) | 0.40 (0.20–0.78) | 30 (45) | 36 (55) | 1.25 (0.52–3.00) |
| ≥75 years | 1 (3) | 22 (97) | 0.06 (0.01–0.45) | 0 (0) | 34 (100) | NC | 0 (0) | 34 (100) | NC | 8 (40) | 12 (60) | 1.00 (0.32–3.17) |
| Obesity | ||||||||||||
| BMI <35.0 | 37 (24) | 117 (76) | Referent | 36 (23) | 118 (77) | Referent | 35 (23) | 119 (77) | Referent | 35 (41) | 50 (59) | Referent |
| BMI ≥35.0 | 13 (23) | 44 (77) | 0.93 (0.45–1.92) | 14 (25) | 43 (75) | 1.07 (0.53–2.17) | 15 (26) | 42 (74) | 1.21 (0.60–2.44) | 15 (48) | 16 (52) | 1.34 (0.59–3.06) |
| Race/ethnicity | ||||||||||||
| Non‐Hispanic White | 10 (14) | 59 (86) | Referent | 11 (16) | 58 (84) | Referent | 11 (16) | 58 (84) | Referent | 13 (35) | 24 (65) | Referent |
| Non‐Hispanic Black | 3 (13) | 20 (87) | 0.89 (0.22–3.54) | 3 (13) | 20 (87) | 0.79 (0.20–3.13) | 2 (9) | 21 (91) | 0.50 (0.10–2.46) | 7 (54) | 6 (46) | 2.15 0.60–7.77) |
| Hispanic | 32 (34) | 63 (66) | 3.00 (1.35–6.63) | 31 (33) | 64 (67) | 2.55 (1.18–5.54) | 33 (35) | 62 (65) | 2.81 (1.30–6.06) | 28 (50) | 28 (50) | 1.85 (0.79–4.34) |
| Non‐Hispanic other or unknown | 5 (21) | 19 (79) | 1.55 (0.47–5.11) | 5 (21) | 19 (79) | 1.39 (0.43–4.50) | 4 (17) | 20 (83) | 1.05 (0.30–3.69) | 2 (20) | 8 (80) | 0.46 (0.09–2.50) |
| Language preference | ||||||||||||
| English | 18 (17) | 86 (83) | Referent | 18 (17) | 86 (83) | Referent | 19 (18) | 85 (82) | Referent | 22 (39) | 35 (61) | Referent |
| Spanish | 27 (32) | 58 (68) | 2.22 (1.12–4.40) | 28 (33) | 57 (67) | 2.35 (1.19–4.63) | 28 (33) | 57 (67) | 2.20 (1.12–4.30) | 24 (50) | 24 (50) | 1.59 (0.73–3.46) |
| Other | 5 (23) | 17 (77) | 1.41 (0.46–4.30) | 4 (18) | 18 (82) | 1.06 (0.32–3.51) | 3 (14) | 19 (86) | 0.71 (0.19–2.63) | 4 (36) | 7 (64) | 0.91 (0.24–3.47) |
| Sex | ||||||||||||
| Male | 35 (25) | 104 (75) | Referent | 36 (26) | 103 (74) | Referent | 35 (25) | 104 (75) | Referent | 36 (43) | 47 (57) | Referent |
| Female | 15 (21) | 57 (79) | 0.78 (0.39–1.55) | 14 (19) | 58 (81) | 0.69 (0.34–1.39) | 15 (21) | 57 (79) | 0.78 (0.39–1.55) | 14 (42) | 19 (58) | 0.96 (0.43–2.17) |
| Health insurance | ||||||||||||
| Any private insurance | 20 (22) | 69 (78) | Referent | 22 (25) | 67 (75) | Referent | 22 (25) | 67 (75) | Referent | 22 (46) | 26 (54) | Referent |
| Public or self‐pay | 30 (25) | 92 (75) | 1.13 (0.59–2.15) | 28 (23) | 94 (77) | 0.91 (0.48–1.72) | 28 (23) | 94 (77) | 0.91 (0.48–1.72) | 28 (41) | 40 (59) | 0.83 (0.39–1.74) |
Abbreviations: BMI, body mass index; CI, confidence interval; FCFS, first come, first served; NC, not calculated; OR, odds ratio; SOFA, Sequential Organ Failure Assessment.
For the New York and Massachusetts scores, allocation was done by sorting patients by priority group, priority score, and age. If there was a tie in ages, a random lottery was used to select which patient received services. For the first come, first served strategy, allocation was done by sorting patients by admission date, with earliest admissions being highest priority. If there was a tie in admission date, a random lottery was used to select which patient received services. Patients were restricted to the same sample as was used for the New York and Massachusetts scores (ie, not missing SOFA score).
aUnadjusted odds ratios.
bRow percentages presented.
5.4. Sensitivity and multiple imputation analyses
In the sensitivity analyses, the mean SOFA scores were similar across racial/ethnic groups: non‐Hispanic Black 5.7 (95% CI, 4.2 to 7.2), Hispanic 3.9 (95% CI, 3.4 to 4.5), non‐Hispanic White 4.6 (95% CI, 3.8 to 5.4), and non‐Hispanic other/unknown 4.0 (95% CI, 3.1 to 5.0) (Table 3). With regard to triage scoring, the distribution of patients by race/ethnicity was similar to that of the complete case data (Appendix C). Most patients regardless of race/ethnicity had highest priority under the New York guidelines. The highest priority (red) group under the Massachusetts1 guidelines contained 39% of non‐Hispanic Black patients, compared to 71% of Hispanic and 58% non‐Hispanic White patients. These figures increased to 78% non‐Hispanic Black, 97% Hispanic, and 78% non‐Hispanic White patients in highest priority under the Massachusetts2 guidelines.
Appendix D1 demonstrates the results of sensitivity analysis, assuming a 50 ICU bed capacity. Interestingly, although there remained no significant difference in odds of receiving critical care services among race/ethnicity under the New York guidelines and FCFS, under the Massachusetts1 and Massachusetts2 guidelines, no non‐Hispanic Black patients qualified to receive critical care services, whereas Hispanic patients had higher odds of receiving critical care services under both Massachusetts1 (OR, 4.25; 95% CI, 1.82 to 9.92) and Massachusetts2 (OR, 4.45; 95% CI, 1.91 to 10.37). Non‐Hispanic other or unknown race/ethnicity also had higher odds of receiving critical care services when compared to non‐Hispanic White patients under both Massachusetts1 (OR, 3.81; 95% CI, 1.24 to 11.73) and Massachusetts2 (OR, 3.14; 95% CI, 1.00 to 9.90). Assuming a 100 ICU bed capacity (Appendix D2), Hispanic patients had higher odds of receiving critical care resources under New York (OR, 2.16; 95% CI, 1.66 to 6.02), Massachusetts1 (OR, 2.69; 95% CI, 1.42 to 5.12) and Massachusetts2 (OR, 3.36; 95% CI, 1.76 to 6.44), as well as FCFS (OR, 2.01; 95% CI, 1.07 to 3.78), with no significant difference in odds among other races/ethnicities.
In the multiple imputation analyses assuming a 50 ICU bed capacity (Appendix E), Hispanic patients had significantly higher odds of receiving critical care services under Massachusetts1 and Massachusetts2, with OR 4.72 (95% CI, 1.84 to 12.14) and OR 6.10 (95% CI, 2.23 to 16.69), respectively, and zero non‐Hispanic Black patients qualified to receive critical care services under both MA guidelines. Assuming a 100 ICU bed capacity, Hispanic patients had higher odds of receiving critical care services across all guidelines and FCFS: New York OR 2.88 (95% CI, 1.38 to 6.01), Massachusetts1 OR 2.82 (95% CI, 1.44 to 5.53), MassachusettsOR 3.31 (95% CI ,1.68 to 6.54), and FCFS OR 2.01 (95% CI, 1.07 to 3.78).
In our assessment of comorbidities as a mediator variable, we found that comorbidities were a partial mediator in the association between race/ethnicity and receipt of critical care services. When adjusting for comorbidities in the logistic regression models, associations were either attenuated or no longer significant (Appendix F).
6. LIMITATIONS
Approximately 45% of patients were missing GCS data, making the SOFA calculation impossible for this subset. This was largely because of an absence of GCS documentation before requiring critical care for patients who underwent transfer to our facility or a lack of GCS documentation altogether. We investigated this issue by performing sensitivity analyses, in which missing SOFA data were inferred, and multiple imputation, in which the missing GCS data were assumed to be missing at random. The persistent significantly higher odds for Hispanic patients to receive critical care services compared to non‐Hispanic White patients under the Massachusetts guidelines in both sensitivity and multiple imputation analyses suggest that the missing data is unlikely to have materially influenced these results.
In real‐world attempts at maximizing the availability of critical care resources, not only will the application of CSC rules be needed to determine eligibility for critical care, but there will also be considerations about withdrawal of critical care from those with poor predicted clinical outcomes. Decisions regarding withdrawal of critical care resources are nuanced, and preferences for early withdrawal of care may vary by race and cultural values. 17 These factors make care withdrawal decisions and survival challenging to model. Because of this, we did not assess which of the 3 CSC guidelines most accurately prioritized patients to maximize survival and minimize long‐term morbidity. This question is beyond the scope of this work and requires further study. Rather, our study demonstrates the differences in racial and ethnic compositions of patients prioritized for critical care based on the application of the varying CSC rules.
Although our sample size included 211 patients, we had relatively small numbers of patients within each race/ethnicity category, which led to wide CIs in our logistic regression analysis. We attempted to address this with sensitivity and multiple imputation analyses to ensure that we could use all available data and minimize bias via potential patterns in data missingness. Further research should include multiple institutions to enroll greater numbers of patients to ensure a diverse sample population.
7. DISCUSSION
In our study, we applied three different utilitarian CSC guidelines as well as an egalitarian FCFS approach 3 to all patients receiving critical care on our hospital's peak COVID‐19 ICU census day to evaluate the odds of receiving critical care resources across racial/ethnic groups under 2 hypothetical scarce resource scenarios: 50 versus 100 ICU bed capacity. Most of the patients in our cohort were Hispanic (45%), with 33% non‐Hispanic White patients, and 11% non‐Hispanic Black patients. Hispanic patients, on average, had fewer comorbidities (CCI mean score 1.1), compared to non‐Hispanic Black (CCI 3.3) and non‐Hispanic White patients (CCI 2.0). In the logistic regression complete case analysis under the 50 ICU bed scenario, Hispanic patients had greater odds of receiving critical care services compared to non‐Hispanic White patients under all CSC guidelines, but not FCFS. When the scenario was modified to 100 available ICU beds, Hispanic patients had greater odds of receiving care only under the Massachusetts2 guidelines. The results were similar in the sensitivity and multiple imputation analyses.
The results of this study are best understood when placed in the context of the COVID‐19 pandemic. In the fiscal year 2019, the racial/ethnic composition of our hospital was 0.9% Hispanic, 5.2% Black, 74.7% White, and 19.2% other/unknown24—although experts estimate the true percentage of Hispanic patients is approximately 9%, as many are misclassified as other/unknown (Joseph Betancourt, MD, email communication, February 2021). However, in our cohort, Hispanic patients formed the largest racial/ethnic group (45%) and the majority (54%) of COVID‐19 positive patients yet had the fewest comorbidities—likely reflecting systematic disparities in exposure to COVID‐19. Thus, most Hispanic patients were classified as red (highest priority) under the Massachusetts scoring, which emphasized comorbidities. In contrast, non‐Hispanic Black patients in our cohort had the highest comorbidities, which may explain why fewer non‐Hispanic Black patients qualified for critical care services under the Massachusetts guidelines in the sensitivity analysis. This highlights the concerns regarding the inclusion of comorbidities under CSC and their potential disproportionate impact on minority populations with higher incidence of comorbidities. Although there were no differences seen across race/ethnicity in our study modeling a FCFS approach, known disparities in access to care suggest that basing triage decisions on arrival time may exacerbate existing inequities in care.
We also investigated the impact of incorporating comorbidities into CSC guidelines by comparing the New York guidelines, which do not include comorbidities, with the original Massachusetts guidelines (heavy emphasis on comorbidities) and the modified Massachusetts guidelines (less emphasis on comorbidities). Of CSC guidelines identified from 29 states across the United States, 15 (71.4%) consider comorbid conditions, 4 which makes examining this issue especially important. In contrast to the Massachusetts guidelines, the New York guidelines were less likely to show significant differences in odds of receiving critical care across race/ethnicities—suggesting the elimination of comorbidities from these criteria may help ensure equitable access to critical care. However, the SOFA score, used in the New York guidelines and the majority of others, indirectly incorporates comorbidities. For example, creatinine is included, when it is known that Black Americans suffer disproportionately from chronic kidney disease. 8 Additionally, under the New York guidelines, most patients (>77%) were classified as highest priority. With less risk stratification, tie‐breaking rules become critically important and will themselves need to be investigated for bias.
Finally, the dearth of GCS documentation has severe implications for SOFA's inclusion into CSC guidelines. Of the CSC guidelines identified in 29 states, all incorporated SOFA. 4 Inherent in SOFA scoring is an assessment of neurologic status via GCS. In our cohort, GCS was not recorded in ≈ 45% of patients just before requiring critical care. This was because of a lack of GCS documentation at outside hospitals before transfer to our hospital, a lack of GCS documentation during emergent situations, and/or limitation in GCS assessment secondary to language barriers or inherent inability to follow commands. Thus, a reliable way of information sharing between institutions will be needed to ensure the appropriate application of CSC guidelines, as well as strategies for consistently modifying SOFA for patients with baseline disability.
The equity implications of the CSC guidelines require further examination before implementation. Institutions should examine their own CSC guidelines closely for inherent biases and consider engagement with community stakeholders. 25 Ongoing monitoring should be performed to ensure that CSC application is equitable.
8.
With the persistence of the COVID‐19 pandemic, and the disproportionate impact of COVID‐19 on communities of color, the development of equitable CSC criteria remains critically important. The results of our study suggest that various CSC guidelines differentially affect the racial/ethnic distribution of patients prioritized for critical care. They further demonstrate the importance of examining the equity implications of CSC guidelines before use.
AUTHOR CONTRIBUTIONS
MSK, WMK, and CAC conceived and designed the study. MSK, MFM, JCT, and GC supervised the conduct of the study and data collection. JP, KM, JCT,MFM,GR, NCO,OB, and DV collected the data. MFM, GC, JCT, and GR managed the data, including quality control. REC provided statistical advice on study design and analyzed the data. MSK and MFM assisted with the analysis. MFM drafted the manuscript, and all authors contributed substantially to its revision. MSK takes responsibility for the paper as a whole.
CONFLICT OF INTEREST
None.
Supporting information
Supporting material
ACKNOWLEDGMENTS
This work was supported by the Center for Social Justice and Health Equity at Massachusetts General Hospital.
Biography
Melanie F. Molina, MD, is an Emergency Medicine Physician at Massachusetts General Hospital in Boston, MA.

Molina MF, Cash RE, Carreras‐Tartak J, et al. Applying crisis standards of care to critically ill patients during the COVID‐19 pandemic: Does race/ethnicity affect triage scoring? JACEP Open. 2021;2:e12502. 10.1002/emp2.12502
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Chadd Kraus, DO, DrPH.
REFERENCES
- 1. Covid in the U.S. . Latest Map and Case Count. The New York Times. NY: Covid in the U.S; 2020. [Google Scholar]
- 2. The Commonwealth of Massachusetts . Crisis Standards of Care Planning Guidance for the COVID‐19 Pandemic. Massachusetts: The Commonwealth of Massachusetts; 2020. [Google Scholar]
- 3. Buckwalter W, Peterson A. Public attitudes toward allocating scarce resources in the COVID‐19 pandemic. PLOS ONE. 2020;15(11):e0240651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cleveland Manchanda EC, Sanky C, Appel JM. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID‐19. J Racial Ethn Health Disparities. 2020: 1‐13. 10.1007/s40615-020-00840-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zucker HA, Adler KP, Berens DP. Ventilator Allocation Guidelines. 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published online. Accessed February 11, 2021.
- 6. Nicholas SB, Kalantar‐Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):6‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Addressing Health Disparities in Diabetes | Diabetes. Atlanta, GA: CDC; 2020. [Google Scholar]
- 8. Norris KC, Agodoa LY. Race and kidney disease: the scope of the problem. J Natl Med Assoc. 2002;94(8 Suppl):1S‐6S. [PMC free article] [PubMed] [Google Scholar]
- 9. National Diabetes Statistics Report, 2020. Atlanta, GA: CDC; 2020. [Google Scholar]
- 10. Geruso M. Black‐white disparities in life expectancy: how much can the standard SES variables explain?. Demography. 2012;49(2):553‐574. [DOI] [PubMed] [Google Scholar]
- 11. Kendi IX. Stop Blaming Black People for Dying of the Coronavirus. Atlantic City: The Atlantic; 2020. [Google Scholar]
- 12. Gold JAW. Ethnicity, and age trends in persons who died from COVID‐19—United States. MMWR Morb Mortal Wkly Rep. 2020;69:1517‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Employed Persons by Detailed Industry, Sex, Race, and Hispanic or Latino Ethnicity. Washington, D.C: U.S. Bureau of Labor Statistics. 2021. [Google Scholar]
- 14. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2020;72(4):703‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bebinger M. After Uproar, Mass. Revises Guidelines On Who Gets An ICU Bed Or Ventilator Amid COVID‐19 Surge. 2020. https://www.wbur.org/commonhealth/2020/04/20/mass‐guidelines‐ventilator‐covid‐coronavirus. Accessed February 8, 2021.
- 16. CPR and Coalition Partners Secure Important Changes in Massachusetts’ Crisis Standards of Care. Madison, WI: Center for Public Representation; 2021. [Google Scholar]
- 17. Elmer J, Torres C, Aufderheide TP. Association of early withdrawal of life‐sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 19. Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score—Development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. New York City Department of Health and Mental Hygiene . COVID‐19 Pandemic Patient Surge: Preparing for Crisis Care. Long Island City, NY: New York City Department of Health and Mental Hygiene; 2020. [Google Scholar]
- 21. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173‐1182. [DOI] [PubMed] [Google Scholar]
- 22. Little R, Rubin D. Statistical Analysis with Missing Data. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 23. Rubin D. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 24. Partners Healthcare System, Inc . Substantial Capital Expenditure Don‐Required Equipment Massachusetts General Hospital. MA: Partners Healthcare System, Inc; 2019. [Google Scholar]
- 25. Society for Academic Emergency Medicine . Equity in Crisis Standards of Care Statement. https://www.saem.org/docs/default‐source/saem‐documents/equity‐in‐crisis‐standards‐of‐care‐872020.pdf?sfvrsn=c0bd03fd_2. Accessed February 9, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material


