Abstract
Natural products remain a crucial source of drug discovery for accessible and affordable solutions for healthy aging. Centella asiatica (L.) Urb. (CA) is an important medicinal plant with a wide range of ethnomedicinal uses. Past in vivo and in vitro studies have shown that the plant extract and its key components, such as asiatic acid, asiaticoside, madecassic acid and madecassoside, exhibit a range of anti-inflammatory, neuroprotective, and cognitive benefits mechanistically linked to mitoprotective and antioxidant properties of the plant. Mitochondrial dysfunction and oxidative stress are key drivers of aging and neurodegenerative disease, including Alzheimer’s disease and Parkinson’s disease. Here we appraise the growing body of evidence that the mitoprotective and antioxidative effects of CA may potentially be harnessed for the treatment of brain aging and neurodegenerative disease.
Keywords: medicinal plants, neuroprotection, mitochondria, neurodegeneration, centella asiatica (L.) Urb, antioxidative, mitoprotective
Introduction
Centella asiatica (L.) Urb. (CA) is a medicinal plant commonly consumed in salads or juices in several countries, including Malaysia, India, Sri Lanka, Indonesia and China (Hashim, 2011; Maulidiani et al., 2012; Bachok et al., 2014; Singh et al., 2014). CA has a wide range of ethnomedical applications, including treatment of gastrointestinal disorders, skin diseases, fever, and cognitive and memory problems (Gohil et al., 2010; Jahan et al., 2012; Sabaragamuwa et al., 2018). Studies of the plant extract and its bioactive compounds have revealed a broad range of pharmacological and therapeutic effects, including anti-ulcer (Zheng et al., 2016), anti-microbial (Idris and Nadzir, 2017), cytoprotective (Choi et al., 2016; Tewari et al., 2016), anti-inflammatory (Choi et al., 2016; Park et al., 2017; Ho et al., 2018), anti-oxidant (Zhao et al., 2014; Dewi and Maryani, 2015; Intararuchikul et al., 2019) and mitoprotective (Gray et al., 2017; Zhang et al., 2017; Gray et al., 2018c) properties. The bioactive components of CA readily cross the blood brain barrier and exert beneficial neuroactive effects in a range of models of aging (Zweig et al., 2021) and neurodegenerative disease including Alzheimer’s disease (AD) (Gray et al., 2018c; Matthews et al., 2019) and Parkinson’s disease (PD) (Gopi and Arambakkam Janardhanam, 2017; Teerapattarakan et al., 2018). Recent studies have associated these neuroprotective and anti-inflammatory effects with increased expression of proteins essential for mitochondrial bioenergetics and antioxidant genes (Gray et al., 2018c; Lu et al., 2021; Zweig et al., 2021). Mitochondria play a pivotal role in aging and neurodegeneration, regulating energy metabolism, immune responses and cell death pathways (Moreira et al., 2010; Rizzuto et al., 2012; Mills and O'Neill, 2016; Sun et al., 2016; Shah et al., 2019). Hence, this review focuses on the potential therapeutic application of CA for the treatment of brain-aging and neurodegenerative disease through restoration of mitochondrial function and inhibition of oxidative damage.
Centella asiatica (L.) Urb. (CA): The Medicinal Plant
Botany and Geographical Distribution of Centella asiatica (L.) Urb.
CA is commonly known by several names, including gotu kola in Sinhala, pegaga in Malay, ‘léi gōng gēn’ in Chinese and Asian or Indian Pennywort in English (Jahan et al., 2012; Orhan, 2012; Singh et al., 2014; Gajbhiye et al., 2016). CA belongs to the Apiaceae family, which is native to Asian countries and parts of China as well as several other parts of the world, such as northern Australia and the Western Pacific. The plant grows horizontally, with long, slender and tender prostrate stolons that can extend up to 2 m and are characterized by long internodes and nodes. Each node of the stem bears one to three leaves that are about 2–6 cm in length and 1.5–5 cm in width with a slightly cupped circular-reniform shape and palmately netted veins. CA is odorless and flowers from April to June with fascicled umbels that consist of three to four sessile flowers. These flowers bear 4-mm-long fruits that range in shape from oval to globular. Found up to 1800 m above sea level, CA grows in a wide range of habitats, such as open sunny areas, swamps, paddy fields as well as along the banks of lakes and ponds and on stone walls and rocks (Roy et al., 2013; Singh et al., 2014; Sirichoat et al., 2015; Gajbhiye et al., 2016).
Centella asiatica (L.) Urb. and its Major Phytochemical Constituents
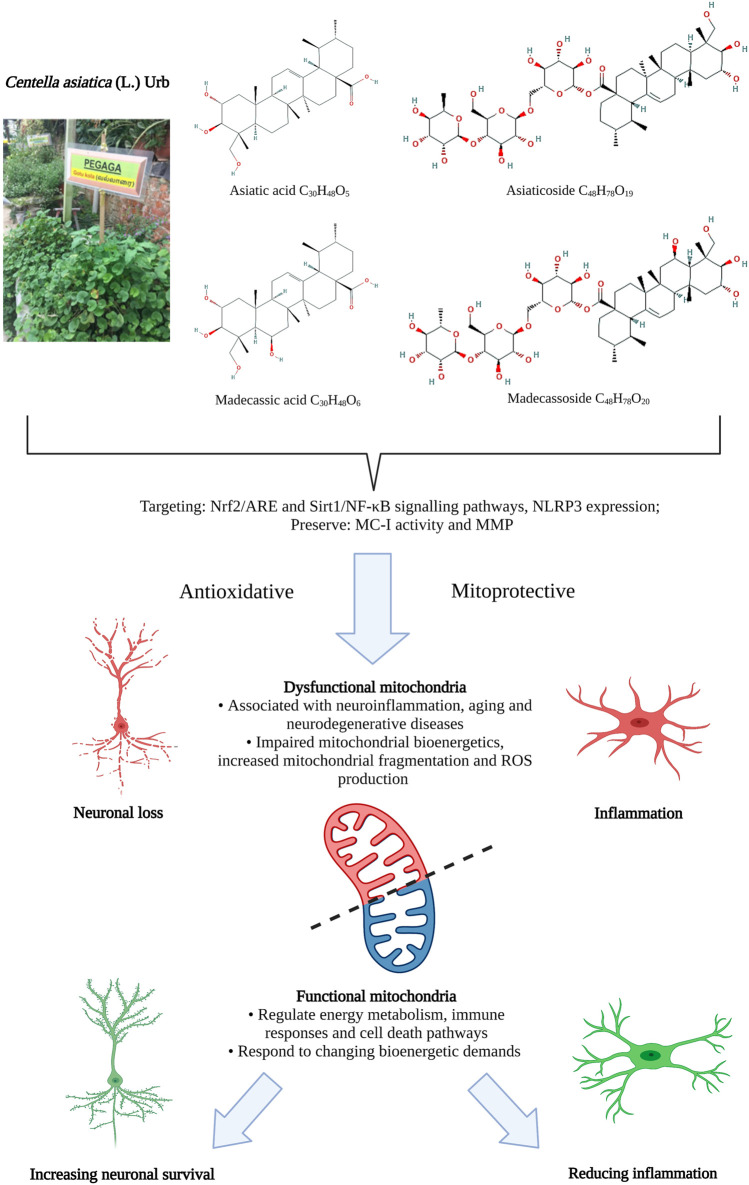
CA contains amino acids, alkaloids, carbohydrates, vitamins, minerals, terpenes of various categories (such as monoterpenes, sesquiterpenes, diterpenes, triterpenes and tetraterpene) and phenolic compounds (such as the flavonoids, tannins and other constituents). The phytochemistry of CA has previously been comprehensively reviewed by Brinkhaus et al. (2000), Gray et al. (2018a) and Torbati et al. (2021) therefore will only be summarized briefly here. Terpenes are the dominant group of chemical constituents of CA, with triterpenes being the major and most important component of CA, serving as a marker constituent for quality control analyses (Rafi et al., 2018). The triterpenes (Figure 1) found in CA are mostly pentacyclic triterpenic acids (sapogenins), such as the asiatic acid (PubChem CID: 119034, National Center for Biotechnology Information, 2021a) and madecassic acid (PubChem CID: 73412, National Center for Biotechnology Information, 2021b), and their respective triterpenoid glycosides (saponins, with a trisaccharide moiety linked to the aglycones), such as asiaticoside (PubChem CID: 52912190, National Center for Biotechnology Information, 2021c) and madecassoside (PubChem CID: 131801373, National Center for Biotechnology Information, 2021d) (Azerad, 2016; Rafi et al., 2018).
FIGURE 1.
Antioxidative and mitoprotective activities of Centella asiatica and its main components. Mitochondrial dysfunction in regulating energy metabolism in response to changing bioenergy demands is closely associated with neuroinflammation in aging and neurodegenerative diseases. The antioxidative and mitoprotective activities of CA targeting mitochondrial and oxidative functions may confer neuroprotective benefits that could potentially be harnessed to treat aging and neurodegenerative diseases and improve functional behavioral outcomes. ARE, antioxidant response element genes; MC-I, mitochondrial complex I; MMP, mitochondrial membrane potential; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing three; Nrf2, NF-E2-p45-related factor 2; Sirt1, Sirtuin 1. Figure created with BioRender.com.
CA extract has been widely studied in the form of ethanolic (Sari et al., 2014; Sari and Rochmah, 2015; Binti Mohd Yusuf Yeo et al., 2018; Suri et al., 2018; Wong et al., 2019; Wong et al., 2020), methanolic (Veerendra Kumar and Gupta, 2003; Arora et al., 2018) and aqueous (Mitha et al., 2016; Gray et al., 2018c; Chintapanti et al., 2018) extract as well as leaf juice (Rao et al., 2007; Thirawarapan et al., 2019). Of these different preparations of CA, it was found that the ethanolic extract retained the highest amount of the triterpenes asiatic acid and asiaticoside compared to other solvents (Puttarak and Panichayupakaranant, 2013; Gajbhiye et al., 2016).
Wide chemotypic variations in triterpenoids were found in CA planted in different growing regions, altitudes and localities (Long et al., 2012; Singh et al., 2014; Srivastava et al., 2014). Genotypic and phenotypic variability have been associated with differences in phytochemicals content of CA including macronutrients, micronutrients, phenolics, flavonoids, tannin, anthocyanin, carotenoids and ascorbic acid (Thomas et al., 2010; Singh et al., 2014; Lal et al., 2017; Chandrasekara et al., 2020). Other than geographical and genotypical influences, the phytochemical compositions of CA also vary due to seasonal variations associated with the cultivation and harvesting procedures, light conditions, as well as the drying conditions post-harvesting (Maulidiani et al., 2012; Rahajanirina et al., 2012; Alqahtani et al., 2015; Plengmuankhae and Tantitadapitak, 2015). This underlines the potential challenges involved in the study of CA plant extract, as differences in specific phytochemical composition may influence the efficacy of the extract.
Neuroactive Effects of Centella asiatica (L.) Urb.: Crossing the Blood Brain Barrier
Several pharmacokinetic studies have confirmed that bioactive components of CA can cross the blood brain barrier (BBB) when administered peripherally, although the transport mechanisms of these phytochemicals remain largely unknown. For example, asiatic acid, asiaticoside and madecassoside were found to accumulate in the brains of animals administered with CA extract or the respective single components (Yin et al., 2012; Anukunwithaya et al., 2017a; Anukunwithaya et al., 2017b). A recent study using primary porcine brain endothelial cells as in vitro BBB model also reported that asiatic acid, asiaticoside and madecassoside exhibit high permeability across the BBB (Hanapi et al., 2021). The bioavailability of these phytochemicals in brain tissue after peripheral administration (Yin et al., 2012; Anukunwithaya et al., 2017a; Anukunwithaya et al., 2017b) indicates they cross the BBB at adequate concentrations to exert neuroactive effects supporting the potential use of these compounds as neurotherapeutics.
Neuroactive Effects of Centella asiatica (L.) Urb.: Cognition
Cognitive-enhancing effects of CA extract have been described in numerous studies, in both normal animals and models of aging and neurodegenerative disease (Doknark et al., 2014; Sari et al., 2014; Sirichoat et al., 2015; Yolanda et al., 2015; Wong et al., 2019; Sbrini et al., 2020). In early studies, CA extract was found to improve memory and ameliorate biochemical and mitochondrial dysfunction in a mouse model of aging (Kumar et al., 2011). In other studies CA was found to confer protection against hippocampal dysfunction, a region of the brain that plays a critical role in learning and memory and is severely affected in AD (Veerendra Kumar and Gupta, 2003; Giribabu et al., 2014). Further, key bioactive components of CA have also been shown to affect learning and memory in models of aging and neurodegenerative disease. For example, asiaticoside has been found to enhance cognitive performance in aged animals (Lin et al., 2013) and a rat model of AD (Zhang et al., 2017). The cognitive effects of CA extract have been linked to changes in synaptic plasticity (Lin et al., 2013) and excitatory neurotransmission (Wanasuntronwong et al., 2018; Wong et al., 2020) as well as improved neuronal health and survival in models of aging and disease (Gray et al., 2018b; Gray et al., 2018c). Here we will examine the evidence that CA and its phytochemicals provide cognitive benefits in aging and neurodegenerative disease via mitoprotective and antioxidant mechanisms (Soumyanath et al., 2012; Chen et al., 2016; Gray et al., 2016; Gray et al., 2017; Matthews et al., 2019).
Targeting Mitochondria in Aging and Neurodegenerative Disease: Role for Centella asiatica (L.) Urb.
Mitochondrial dysfunction is closely associated with aging (López-Otín et al., 2013; Sun et al., 2016), AD (Moreira et al., 2010; Yoo et al., 2020) and PD (Yang et al., 2020). Mitochondria regulate energy metabolism, immune responses and cell-death pathways through their highly flexible and dynamic network. The mitochondrial network responds to changing bioenergetic demands by adjusting the rate of mitochondrial fission and fusion—a function that was found to be affected in most age-associated neurodegenerative conditions (Shah et al., 2019). Studies have shown that age-related toxic protein aggregates, such as Alzheimer’s beta amyloid (Aβ), induce mitochondrial dysregulation by binding to mitochondrial proteins. For example, Aβ has been found to bind to the mitochondrial fission protein (Drp1), and the mitochondrial voltage-dependent anion channel (VDAC) (Manczak et al., 2011; Manczak et al., 2018). These abnormal protein interactions affect mitochondrial biogenesis, increase mitochondrial fragmentation and induce free radical production (John and Reddy, 2020).
Mitochondria are the primary source of free radicals, otherwise known as reactive oxygen species (ROS), and ROS overproduction leads to oxidative damage. Oxidative damage further affects the mitochondrial respiratory chain function in generating energy in the form of adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) (Elfawy and Das, 2019). Perturbations in the electron transport chain function and/or reduction in the mitochondrial membrane potential lead to a vicious cycle of mitochondrial stress, which results in decreased ATP production and increased ROS production (Szalardy et al., 2015; Zorova et al., 2018). The brain is highly susceptible to both bioenergetic dysfunction and oxidative damage due to the high energy demands associated with neurotransmission and a high lipid content, respectively. The use of antioxidant strategies has been reported to provide a protective benefit against aging and neurodegenerative diseases. Further, enhancing mitochondrial biogenesis and quality control may be an efficient strategy for preventing mitochondrial disorders (Smith et al., 2012; Suliman and Piantadosi, 2016; Murphy and Hartley, 2018) and providing neuroprotection in AD and PD mouse models (Johri and Beal, 2012). Several therapeutic approaches that aim to protect against neurodegeneration and inflammation by improving brain bioenergetics, rescuing mitochondrial dysfunction and reducing oxidative damage are being developed (Cunnane et al., 2020; Fairley et al., 2021). In this section, we will focus on the mitoprotective and antioxidative effects of CA and its key phytochemicals as potential therapeutic agents that can 1) promote neuronal health and survival, and 2) reduce neuroinflammation.
Neuroprotective Effects of Centella asiatica (L.) Urb. and its Major Constituents: Antioxidative and Mitoprotective Effects
Neuroprotective effects of CA have been described in several models of neurodegenerative disease and injury, linked to effects on mitochondrial energy production, oxidative stress and mitochondrial-induced apoptosis. For example, the CA extract, asiatic acid has been shown to prevent mitochondrial morphology abnormalities in a rat model of kainic acid-induced seizure, which protected synaptic function and alleviated cognitive deficits (Lu et al., 2021). In a separate study, the CA phytochemical asiaticoside was found to inhibit Aβ-induced neuronal apoptosis by restoring and maintaining mitochondrial membrane potential (Song et al., 2018). Several potential molecular mechanisms mediating the mitoprotective effects of CA have been proposed, including increased conductance and stabilization of VDAC (Tewari et al., 2016). VDAC plays a critical role in cell survival, transport of substrates for energy production and maintenance of mitochondrial membrane potential (Camara et al., 2017), making it a target of interest in regulating mitochondrial function.
Meanwhile, other studies have implicated CA and its bioactive components in the regulation of important antioxidant response signaling pathways. In mouse models of AD, CA extract has been found to promote antioxidative responses, countering Aβ pathology-driven oxidative stress, mitigating neuronal loss around the plaques and improving memory function (Gray et al., 2017; Gray et al., 2018c). CA extract has also been found to protect rotenone-induced parkinsonism rats against lipid peroxidation, dopaminergic neuronal death and locomotor deficit. These protective effects were associated with increased antioxidant enzyme expression and preservation of mitochondrial complex I activity, which is responsible for the rate-limiting step in OXPHOS (Teerapattarakan et al., 2018). Madecassoside was also found to be effective at ameliorating the deficits observed in PD rat models via its antioxidative activities, maintaining the redox balance (Xu et al., 2013). Similarly, asiaticoside has been found to reduce oxidative stress induced by rotenone (Gopi and Arambakkam Janardhanam, 2017; Subaraja and Vanisree, 2019). Likewise, asiatic acid provided antioxidative benefits in a drosophila PD model, protecting mitochondria against rotenone-induced oxidative stress and apoptosis. The antioxidative properties of asiatic acid are also thought to mediate neuroprotection and improve spatial memory function in animals treated with valproic acid (Xu et al., 2012; Umka Welbat et al., 2016). Outside of the brain, antioxidative effects of CA are also observed in other organs and systems. For example, CA extract was found to inhibit lipid peroxidation in rotenone-treated rats (Intararuchikul et al., 2019) and regulate lipid metabolism via antioxidant effect (Zhao et al., 2014). These findings support the notion that the neuroprotective effects of CA and its bioactive components are at least in part mediated through enhanced antioxidative responses.
CA-induced antioxidative responses have been linked to the higher expression of antioxidant response element genes (AREs) activated via Nrf2 (NF-E2-p45-related factor two, encoded by the NFE2L2 gene) (Matthews et al., 2019). The Nrf2/ARE signaling cascade regulates a plethora of cellular activities, including metabolic reprogramming, mitochondrial physiology and biogenesis, antioxidant stress response, drug detoxification, inflammation, autophagy and unfolded protein response and proteostasis (Dinkova-Kostova and Abramov, 2015; He et al., 2020). Altered expression of Nrf2-targeted genes is associated with AD, and previous studies have demonstrated that the activation of Nrf2 ameliorates Aβ pathology and cognitive deficits in AD mouse models (Bahn et al., 2019). Consequently, activation of Nrf2 pathway represents a promising therapeutic direction for enhancing mitochondrial quality control and biogenesis in aging and neurodegenerative diseases (Kerr et al., 2017; Gureev et al., 2019; Gureev and Popov, 2019; Brandes and Gray, 2020; Bento-Pereira and Dinkova-Kostova, 2021). Subsequent studies found that Nrf2 is a crucial component of the mitoprotective effects of CA, whereby long-term CA treatment improved the cognitive performance of wild type but not Nrf2 deficient mice (Nrf2 knockout) (Zweig et al., 2021). Further, these studies associated hippocampal mitochondrial dysfunction with cognitive performance.
In addition to the general ability to induce antioxidant responses, disease-specific mitoprotective effects of CA have also been identified in models of PD. For example, CA components have been shown to block the translocation of α-synuclein to the mitochondria, therefore maintaining mitochondrial membrane integrity and ATP production (Ding et al., 2018). Further, pre-treatment with asiatic acid significantly decreased mitochondrial ROS production in a 1-methyl-4-phenyl-pyridine (MPP+)-induced neuroblastoma model of PD and protected the cells form the loss of mitochondrial membrane potential (Chen et al., 2019). Additionally, CA and its triterpenoids may also reduce ROS production (Gray et al., 2017; Nataraj et al., 2017), thus potentially restoring mitochondrial function in the central nervous system (Onyango et al., 2017). For example, madecassic acid inhibited ROS production in human retinal microvascular endothelial cells (hMRECs) following hypoxia-induced oxidative stress (Yang et al., 2016). The molecular targets mediating these effects are yet to be elucidated and whether they are generalized to other disease models remains to be determined.
Anti-Inflammatory Effects of Centella asiatica (L.) Urb. and its Major Constituents
The mitochondrial and metabolic fitness of the brain’s innate immune system plays an important role in the neuroinflammatory responses involved in neurodegenerative diseases (Paolicelli and Angiari, 2019)—a concept known as “immunometabolism” (O'Neill et al., 2016). Mitochondrial-dependent OXPHOS and fatty acid oxidation (FAO) are associated with anti-inflammatory responses (Mills and O'Neill, 2016) while, on the other hand, inflammatory responses are associated with a shift toward non-mitochondrial erobic glycolysis (Rodríguez-Prados et al., 2010; Galván-Peña and O’Neill, 2014). This switch toward erobic glycolysis causes several functional changes: 1) rapid supply of ATP, 2) proinflammatory cytokine production, 3) rearrangement of the tricarboxylic acid (TCA) cycle and accumulation of intermediate metabolites, such as succinate and citrate, and 4) repurposing of the electron transport chain (ETC) to produce ROS (Lampropoulou et al., 2016; Millet et al., 2016; Mills et al., 2016). Furthermore, microglial activation releases neurotoxic factors, such as mitochondrial-generated ROS, that exacerbate the neuroinflammation, thus resulting in neuronal death and neurodegeneration (González et al., 2014; Simpson and Oliver, 2020). Microglia are metabolically plastic and, hence, are potential therapeutic targets for the treatment of AD using metabolic reprogramming strategies (Fairley et al., 2021).
CA and its derivatives have also been shown to affect inflammatory responses through the regulation of mitochondrial and oxidative functions. Asiatic acid, asiaticoside and madecassoside have been found to demonstrate anti-inflammatory effects through a reduction of cytokine levels and the activation of microglia in stroke models (Krishnamurthy et al., 2009; Chen et al., 2014; Luo et al., 2014). Sirtuin 1 (Sirt 1) protein is an important epigenetic regulator for many physiological processes, modulating downstream pathways by targeting proteins such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and plays a role in alleviating oxidative stress (Elibol and Kilic, 2018). In an immortalized microglial cell line, asiatic acid was found to prevent LPS-induced neuroinflammation by enhancing Sirt1 expression while suppressing NF-κB activation, attenuated the production of nitric oxide and the expression of inducible nitric oxide synthase (iNOS) and reduced the expression and release of inflammatory cytokines in response to LPS-induced inflammation (Qian et al., 2018). Asiatic acid was shown to protect BV2 cells from LPS-induced damage by suppressing NLRP3 (NLR family pyrin domain containing three) expression and decreasing mitochondrial ROS, effectively ameliorating mitochondrial dysfunction (Chen et al., 2019).
Anti-inflammatory effects have also been reported in models of AD. In a study that used the intracerebroventricular infusion of toxic forms of Alzheimer’s Aβ, the neuroprotective effects of asiaticoside in Aβ-infused rats were suggested as being associated with the anti-inflammatory properties of asiaticoside, hence mitigating mitochondrial injuries and regulating the expression of apoptosis markers (Zhang et al., 2017). The mitoprotective effects of asiatic acid have been demonstrated in earlier studies that targeted the regulation of the mitochondrial membrane potential and ROS production (Xiong et al., 2009; Xu et al., 2012). Taken together, these findings demonstrate that CA and its major phytochemicals inhibit ROS production and ameliorate mitochondrial dysfunction, reducing detrimental inflammatory responses.
Conclusion
Plants produce chemically, structurally and molecularly diverse phytochemicals that determine their evolutionary success. These compounds represent biological functions and continue to provide crucial novel pharmacological leads for the treatment of human diseases. CA and its phytochemicals have wide ethnopharmacological applications in various cultures, and its biological effects have been substantiated in numerous studies. These findings suggest that CA confers pleiotropic neuroprotective and anti-inflammatory benefits through its mitoprotective and antioxidative effects, which could potentially be harnessed for the treatment of aging and neurodegenerative diseases. Further research is still needed to determine the synergistic effects, safety, efficacy, bioavailability and metabolism of these components.
Author Contributions
JW, AB, and JA wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
AB acknowledges funding support from the Nanyang Assistant Professorship from Nanyang Technological University Singapore. JW and JA acknowledge funding support from the NKEA Research Grant Scheme (NRGS Grant, NH1014D049) for the research presented in this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alqahtani A., Tongkao-on W., Li K. M., Razmovski-Naumovski V., Chan K., Li G. Q. (2015). Seasonal Variation of Triterpenes and Phenolic Compounds in australian Centella asiatica (L.) Urb. Phytochem. Anal. 26 (6), 436–443. 10.1002/pca.2578 [DOI] [PubMed] [Google Scholar]
- Anukunwithaya T., Tantisira M., Shimada T., Sai Y., Khemawoot P. (2017a). Multiple Oral Dosing Pharmacokinetics of Standardized Extract of Centella asiatica ECa 233 and its Inductive Effect on Efflux Transporters in Rats. PMIO 4 (02), e66–e73. 10.1055/S-0043-114669 [DOI] [Google Scholar]
- Anukunwithaya T., Tantisira M., Tantisira B., Khemawoot P. (2017b). Pharmacokinetics of a Standardized Extract of Centella asiatica ECa 233 in Rats. Planta Med. 83 (08), 710–717. 10.1055/s-0042-122344 [DOI] [PubMed] [Google Scholar]
- Arora R., Kumar R., Agarwal A., Reeta K. H., Gupta Y. K. (2018). Comparison of Three Different Extracts of Centella asiatica for Anti-amnesic, Antioxidant and Anticholinergic Activities: In Vitro and In Vivo Study. Biomed. Pharmacother. 105, 1344–1352. 10.1016/j.biopha.2018.05.156 [DOI] [PubMed] [Google Scholar]
- Azerad R. (2016). Chemical Structures, Production and Enzymatic Transformations of Sapogenins and Saponins from Centella asiatica (L.) Urban. Fitoterapia 114, 168–187. 10.1016/j.fitote.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Bachok M. F., Mohd Yusof B.-N., Ismail A., Hamid A. A. (2014). Effectiveness of Traditional Malaysian Vegetables ('ulam') in Modulating Blood Glucose Levels. Asia Pac. J. Clin. Nutr. 23 (3), 369–376. 10.6133/apjcn.2014.23.3.01 [DOI] [PubMed] [Google Scholar]
- Bahn G., Park J.-S., Yun U. J., Lee Y. J., Choi Y., Park J. S., et al. (2019). NRF2/ARE Pathway Negatively Regulates BACE1 Expression and Ameliorates Cognitive Deficits in Mouse Alzheimer's Models. Proc. Natl. Acad. Sci. USA 116 (25), 12516–12523. 10.1073/pnas.1819541116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento-Pereira C., Dinkova-Kostova A. T. (2021). Activation of Transcription Factor Nrf2 to Counteract Mitochondrial Dysfunction in Parkinson's Disease. Med. Res. Rev. 41 (2), 785–802. 10.1002/med.21714 [DOI] [PubMed] [Google Scholar]
- Binti Mohd Yusuf Yeo N. A., Muthuraju S., Wong J. H., Mohammed F. R., Senik M. H., Zhang J., et al. (2018). Hippocampal Amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic Acid GluA1 (AMPA GluA1) Receptor Subunit Involves in Learning and Memory Improvement Following Treatment with Centella asiatica Extract in Adolescent Rats. Brain Behav. 8 (9), e01093. 10.1002/brb3.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M. S., Gray N. E. (2020). NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 12, 175909141989978. 10.1177/1759091419899782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhaus B., Lindner M., Schuppan D., Hahn E. G. (2000). Chemical, Pharmacological and Clinical Profile of the East Asian Medical Plant Centella Aslatica. Phytomedicine 7 (5), 427–448. 10.1016/s0944-7113(00)80065-3 [DOI] [PubMed] [Google Scholar]
- Camara A. K. S., Zhou Y., Wen P.-C., Tajkhorshid E., Kwok W.-M. (2017). Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 8, 460. 10.3389/fphys.2017.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekara C. H. W. M. R. B., Sumanarathne R. A. P. I., Bandaranayake P. C. G. (2020). Centellaasiatica Morphotypes Differ Genetically as Well as Macronutrients Content, Total Phenolic Content and Chemical Fingerprints of Leaves. J. Agric. Sci. 15, 75. 10.4038/jas.v15i1.8673 [DOI] [Google Scholar]
- Chen S., Yin Z.-J., Jiang C., Ma Z.-Q., Fu Q., Qu R., et al. (2014). Asiaticoside Attenuates Memory Impairment Induced by Transient Cerebral Ischemia-Reperfusion in Mice through Anti-inflammatory Mechanism. Pharmacol. Biochem. Behav. 122, 7–15. 10.1016/j.pbb.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Chen C.-L., Tsai W.-H., Chen C.-J., Pan T.-M. (2016). Centella asiatica Extract Protects against Amyloid β1-40-induced Neurotoxicity in Neuronal Cells by Activating the Antioxidative Defence System. J. Traditional Complement. Med. 6 (4), 362–369. 10.1016/j.jtcme.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Zhang X.-Y., Sun J., Cong Q.-J., Chen W.-X., Ahsan H. M., et al. (2019). Asiatic Acid Protects Dopaminergic Neurons from Neuroinflammation by Suppressing Mitochondrial ROS Production. Biomolecules Ther. 27 (5), 442–449. 10.4062/biomolther.2018.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapanti S., Pratap Reddy K., Sreenivasula Reddy P. (2018). Behavioral and Neurochemical Consequences of Perinatal Exposure to lead in Adult Male Wistar Rats: Protective Effect by Centella asiatica . Environ. Sci. Pollut. Res. 25 (13), 13173–13185. 10.1007/s11356-018-1500-x [DOI] [PubMed] [Google Scholar]
- Choi M.-J., Zheng H.-M., Kim J. M., Lee K. W., Park Y. H., Lee D. H. (2016). Protective Effects of Centella asiatica Leaf Extract on Dimethylnitrosamine-Induced Liver Injury in Rats. Mol. Med. Rep. 14 (5), 4521–4528. 10.3892/mmr.2016.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane S. C., Trushina E., Morland C., Prigione A., Casadesus G., Andrews Z. B., et al. (2020). Brain Energy rescue: an Emerging Therapeutic Concept for Neurodegenerative Disorders of Ageing. Nat. Rev. Drug Discov. 19 (9), 609–633. 10.1038/s41573-020-0072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewi R. T., Maryani F. (2015). Antioxidant and α-Glucosidase Inhibitory Compounds of Centella Asiatica. Proced. Chem. 17, 147–152. 10.1016/j.proche.2015.12.130 [DOI] [Google Scholar]
- Ding H., Xiong Y., Sun J., Chen C., Gao J., Xu H. (2018). Asiatic Acid Prevents Oxidative Stress and Apoptosis by Inhibiting the Translocation of α-Synuclein into Mitochondria. Front. Neurosci. 12, 431. 10.3389/fnins.2018.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Abramov A. Y. (2015). The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 88 (Pt B), 179–188. 10.1016/j.freeradbiomed.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doknark S., Mingmalairak S., Vattanajun A., Tantisira B., Tantisira M. H. (2014). Study of Ameliorating Effects of Ethanolic Extract of Centella asiatica on Learning and Memory Deficit in Animal Models. J. Med. Assoc. Thai. 97 Suppl 2 (Suppl. 2), S68–S76. [PubMed] [Google Scholar]
- Elfawy H. A., Das B. (2019). Crosstalk between Mitochondrial Dysfunction, Oxidative Stress, and Age Related Neurodegenerative Disease: Etiologies and Therapeutic Strategies. Life Sci. 218, 165–184. 10.1016/j.lfs.2018.12.029 [DOI] [PubMed] [Google Scholar]
- Elibol B., Kilic U. (2018). High Levels of SIRT1 Expression as a Protective Mechanism against Disease-Related Conditions. Front. Endocrinol. 9, 614. 10.3389/fendo.2018.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley L. H., Wong J. H., Barron A. M. (2021). Mitochondrial Regulation of Microglial Immunometabolism in Alzheimer's Disease. Front. Immunol. 12, 257. 10.3389/fimmu.2021.624538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajbhiye N. A., Makasana J., Saha A., Patel I., Jat R. S. (2016). LC-ESI-MS/MS Method for Simultaneous Determination of Triterpenoid Glycosides and Aglycones in Centella asiatica L. Chromatographia 79 (11), 727–739. 10.1007/s10337-016-3089-x [DOI] [Google Scholar]
- Galván-Peña S., O’Neill L. A. (2014). Metabolic Reprograming in Macrophage Polarization. Front. Immunol. 5, 420. 10.3389/fimmu.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribabu N., Srinivasarao N., Swapna Rekha S., Muniandy S., Salleh N. (2014). Centella asiatica Attenuates Diabetes Induced Hippocampal Changes in Experimental Diabetic Rats. Evidence-Based Complement. Altern. Med. 2014, 1–10. 10.1155/2014/592062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil K., Patel J., Gajjar A. (2010). Pharmacological Review on Centella asiatica: A Potential Herbal Cure-All. Indian J. Pharm. Sci. 72 (5), 546–556. 10.4103/0250-474X.78519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González H., Elgueta D., Montoya A., Pacheco R. (2014). Neuroimmune Regulation of Microglial Activity Involved in Neuroinflammation and Neurodegenerative Diseases. J. Neuroimmunol. 274 (1-2), 1–13. 10.1016/j.jneuroim.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Gopi M., Arambakkam Janardhanam V. (2017). Asiaticoside: Attenuation of Rotenone Induced Oxidative burden in a Rat Model of Hemiparkinsonism by Maintaining the Phosphoinositide-Mediated Synaptic Integrity. Pharmacol. Biochem. Behav. 155, 1–15. 10.1016/j.pbb.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Gray N. E., Harris C. J., Quinn J. F., Soumyanath A. (2016). Centella asiatica Modulates Antioxidant and Mitochondrial Pathways and Improves Cognitive Function in Mice. J. Ethnopharmacology 180, 78–86. 10.1016/j.jep.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. E., Zweig J. A., Matthews D. G., Caruso M., Quinn J. F., Soumyanath A. (2017). Centella asiatica Attenuates Mitochondrial Dysfunction and Oxidative Stress in Aβ-Exposed Hippocampal Neurons. Oxidative Med. Cell Longevity 2017, 1–8. 10.1155/2017/70230912017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. E., Alcazar Magana A., Lak P., Wright K. M., Quinn J., Stevens J. F., et al. (2018a). Centella asiatica: Phytochemistry and Mechanisms of Neuroprotection and Cognitive Enhancement. Phytochem. Rev. 17 (1), 161–194. 10.1007/s11101-017-9528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. E., Zweig J. A., Caruso M., Martin M. D., Zhu J. Y., Quinn J. F., et al. (2018b). Centella asiatica Increases Hippocampal Synaptic Density and Improves Memory and Executive Function in Aged Mice. Brain Behav. 8, e01024. 10.1002/brb3.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. E., Zweig J. A., Caruso M., Zhu J. Y., Wright K. M., Quinn J. F., et al. (2018c). Centella asiatica Attenuates Hippocampal Mitochondrial Dysfunction and Improves Memory and Executive Function in β-amyloid Overexpressing Mice. Mol. Cell Neurosci. 93, 1–9. 10.1016/j.mcn.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureev A. P., Popov V. N. (2019). Nrf2/ARE Pathway as a Therapeutic Target for the Treatment of Parkinson Diseases. Neurochem. Res. 44 (10), 2273–2279. 10.1007/s11064-018-02711-2 [DOI] [PubMed] [Google Scholar]
- Gureev A. P., Shaforostova E. A., Popov V. N. (2019). Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 10, 435. 10.3389/fgene.2019.00435435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanapi N. A., Mohamad Arshad A. S., Abdullah J. M., Tengku Muhammad T. S., Yusof S. R. (2021). Blood-brain Barrier Permeability of Asiaticoside, Madecassoside and Asiatic Acid in Porcine Brain Endothelial Cell Model. J. Pharm. Sci. 110 (2), 698–706. 10.1016/j.xphs.2020.09.015 [DOI] [PubMed] [Google Scholar]
- Hashim P. (2011). Centella asiatica in Food and Beverage Applications and its Potential Antioxidant and Neuroprotective Effect. Int. Food Res. J. 18 (4), 1215. [Google Scholar]
- He F., Ru X., Wen T. (2020). NRF2, a Transcription Factor for Stress Response and beyond. Ijms 21 (13), 4777. 10.3390/ijms21134777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. J., Sung J. J., Cheon K. K., Tae H. J. (2018). Anti-inflammatory Effect of Centella asiatica Phytosome in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis. Phytomedicine 43, 110–119. 10.1016/j.phymed.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Idris F. N., Nadzir M. M. (2017). Antimicrobial Activity of Centella asiatica on Aspergillus niger and Bacillus Subtilis. Chem. Eng. Trans. 56, 1381–1386. 10.3303/CET1756231 [DOI] [Google Scholar]
- Intararuchikul T., Teerapattarakan N., Rodsiri R., Tantisira M., Wohlgemuth G., Fiehn O., et al. (2019). Effects of Centella Asiatica extract on Antioxidant Status and Liver Metabolome of Rotenone-Treated Rats Using GC-MS. Biomed. Chromatogr. 33 (2), e4395. 10.1002/bmc.4395 [DOI] [PubMed] [Google Scholar]
- Jahan R., Hossain S., Seraj S., Nasrin D., Khatun Z., Das P. R., et al. (2012). Centella asiatica (L.) Urb.: Ethnomedicinal Uses and Their Scientific Validations. Am. -Eurasian J. Sustain. Agric. 6 (4), 261–270. [Google Scholar]
- John A., Reddy P. H. (2021). Synaptic Basis of Alzheimer's Disease: Focus on Synaptic Amyloid Beta, P-Tau and Mitochondria. Ageing Res. Rev. 65, 101208. 10.1016/j.arr.2020.101208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A., Beal M. F. (2012). Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 342 (3), 619–630. 10.1124/jpet.112.192138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr F., Sofola-Adesakin O., Ivanov D. K., Gatliff J., Gomez Perez-Nievas B., Bertrand H. C., et al. (2017). Direct Keap1-Nrf2 Disruption as a Potential Therapeutic Target for Alzheimer's Disease. Plos Genet. 13 (3), e1006593. 10.1371/journal.pgen.1006593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy R. G., Senut M.-C., Zemke D., Min J., Frenkel M. B., Greenberg E. J., et al. (2009). Asiatic Acid, a Pentacyclic Triterpene from Centella Asiatica, Is Neuroprotective in a Mouse Model of Focal Cerebral Ischemia. J. Neurosci. Res. 87 (11), 2541–2550. 10.1002/jnr.22071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Prakash A., Dogra S. (2011). Centella asiatica Attenuates D-Galactose-Induced Cognitive Impairment, Oxidative and Mitochondrial Dysfunction in Mice. Int. J. Alzheimer's Dis. 2011, 347569. 10.4061/2011/347569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal R. K., Gupta P., Dubey B. K. (2017). Genetic Variability and Associations in the Accessions of Manduk Parni {Centella asiatica (L)}. Ind. Crops Prod. 96, 173–177. 10.1016/j.indcrop.2016.11.056 [DOI] [Google Scholar]
- Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E. E., Loginicheva E., et al. (2016). Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cel Metab. 24 (1), 158–166. 10.1016/j.cmet.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Huang R., Zhang S., Wei L., Zhuo L., Wu X., et al. (2013). Beneficial Effects of Asiaticoside on Cognitive Deficits in Senescence-Accelerated Mice. Fitoterapia 87, 69–77. 10.1016/j.fitote.2013.03.023 [DOI] [PubMed] [Google Scholar]
- López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2013). The Hallmarks of Aging. Cell 153 (6), 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H. S., Stander M. A., Van Wyk B.-E. (2012). Notes on the Occurrence and Significance of Triterpenoids (Asiaticoside and Related Compounds) and Caffeoylquinic Acids in Centella Species. South Afr. J. Bot. 82, 53–59. 10.1016/j.sajb.2012.07.017 [DOI] [Google Scholar]
- Lu C.-W., Lin T.-Y., Pan T.-L., Wang P.-W., Chiu K.-M., Lee M.-Y., et al. (2021). Asiatic Acid Prevents Cognitive Deficits by Inhibiting Calpain Activation and Preserving Synaptic and Mitochondrial Function in Rats with Kainic Acid-Induced Seizure. Biomedicines 9, 284. 10.3390/biomedicines9030284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Yang Y.-P., Liu J., Li W.-H., Yang J., Sui X., et al. (2014). Neuroprotective Effects of Madecassoside against Focal Cerebral Ischemia Reperfusion Injury in Rats. Brain Res. 1565, 37–47. 10.1016/j.brainres.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Manczak M., Calkins M. J., Reddy P. H. (2011). Impaired Mitochondrial Dynamics and Abnormal Interaction of Amyloid Beta with Mitochondrial Protein Drp1 in Neurons from Patients with Alzheimer's Disease: Implications for Neuronal Damage. Hum. Mol. Genet. 20 (13), 2495–2509. 10.1093/hmg/ddr139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M., Kandimalla R., Yin X., Reddy P. H. (2018). Hippocampal Mutant APP and Amyloid Beta-Induced Cognitive Decline, Dendritic Spine Loss, Defective Autophagy, Mitophagy and Mitochondrial Abnormalities in a Mouse Model of Alzheimer's Disease. Hum. Mol. Genet. 27 (8), 1332–1342. 10.1093/hmg/ddy042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. G., Caruso M., Murchison C. F., Zhu J. Y., Wright K. M., Harris C. J., et al. (2019). Centella asiatica Improves Memory and Promotes Antioxidative Signaling in 5XFAD Mice. Antioxidants 8 (12), 630. 10.3390/antiox812063012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulidiani H., Khatib A., Shaari K., Abas F., Shitan M., Kneer R., et al. (2012). Discrimination of Three Pegaga (Centella) Varieties and Determination of Growth-Lighting Effects on Metabolites Content Based on the Chemometry of 1H Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food Chem. 60 (1), 410–417. 10.1021/jf200270y [DOI] [PubMed] [Google Scholar]
- Millet P., Vachharajani V., McPhail L., Yoza B., McCall C. E. (2016). GAPDH Binding to TNF-α mRNA Contributes to Posttranscriptional Repression in Monocytes: A Novel Mechanism of Communication between Inflammation and Metabolism. J.I. 196 (6), 2541–2551. 10.4049/jimmunol.1501345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. L., O'Neill L. A. (2016). Reprogramming Mitochondrial Metabolism in Macrophages as an Anti-inflammatory Signal. Eur. J. Immunol. 46 (1), 13–21. 10.1002/eji.201445427 [DOI] [PubMed] [Google Scholar]
- Mills E. L., Kelly B., Logan A., Costa A. S. H., Varma M., Bryant C. E., et al. (2016). Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 167 (2), 457–470.e13. 10.1016/j.cell.2016.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitha K. V., Yadav S., Ganaraja B. (2016). Improvement in Cognitive Parameters Among Offsprings Born to Alcohol Fed Female Wistar Rats Following Long Term Treatment with Centella Asiatica. Indian J. Physiol. Pharmacol. 60 (2), 167–173. [PubMed] [Google Scholar]
- Moreira P. I., Carvalho C., Zhu X., Smith M. A., Perry G. (2010). Mitochondrial Dysfunction Is a Trigger of Alzheimer's Disease Pathophysiology. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1802, 2–10. 10.1016/j.bbadis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Murphy M. P., Hartley R. C. (2018). Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 17 (12), 865–886. 10.1038/nrd.2018.174 [DOI] [PubMed] [Google Scholar]
- Nataraj J., Manivasagam T., Justin Thenmozhi A., Essa M. M. (2017). Neuroprotective Effect of Asiatic Acid on Rotenone-Induced Mitochondrial Dysfunction and Oxidative Stress-Mediated Apoptosis in Differentiated SH-SYS5Y Cells. Nutr. Neurosci. 20 (6), 351–359. 10.1080/1028415X.2015.1135559 [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information (2021a). PubChem Compound Summary for CID 119034, Asiatic acid. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/Asiatic-acid (Retrieved June 8, 2021)
- National Center for Biotechnology Information (2021b). PubChem Compound Summary for CID 73412, Madecassic acid. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/Madecassic-acid (Retrieved June 8, 2021)
- National Center for Biotechnology Information (2021c). PubChem Compound Summary for CID 52912190. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/52912190 (Retrieved June 8, 2021)
- National Center for Biotechnology Information (2021d). PubChem Compound Summary for CID 131801373. Retrieved at https://pubchem.ncbi.nlm.nih.gov/compound/131801373 (Retrieved June 8, 2021)
- O'Neill L. A. J., Kishton R. J., Rathmell J. (2016). A Guide to Immunometabolism for Immunologists. Nat. Rev. Immunol. 16 (9), 553–565. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango I. G., Khan S. M., Bennett J. P., Jr (2017). Mitochondria in the Pathophysiology of Alzheimer S and Parkinson S Diseases. Front. Biosci. 22, 854–872. 10.2741/4521 [DOI] [PubMed] [Google Scholar]
- Orhan I. E. (2012). Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evidence-Based Complement. Altern. Med. 2012, 1–8. 10.1155/2012/946259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R. C., Angiari S. (2019). Microglia Immunometabolism: From Metabolic Disorders to Single Cell Metabolism. Semin. Cel Dev. Biol. 94, 129–137. 10.1016/j.semcdb.2019.03.012 [DOI] [PubMed] [Google Scholar]
- Park J., Choi J., Son D., Park E., Song M., Hellström M., et al. (2017). Anti-inflammatory Effect of Titrated Extract of Centella asiatica in Phthalic Anhydride-Induced Allergic Dermatitis Animal Model. Ijms 18, 738. 10.3390/ijms180407384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plengmuankhae W., Tantitadapitak C. (2015). Low Temperature and Water Dehydration Increase the Levels of Asiaticoside and Madecassoside in Centella asiatica (L.) Urban. South Afr. J. Bot. 97, 196–203. 10.1016/j.sajb.2015.01.013 [DOI] [Google Scholar]
- Puttarak P., Panichayupakaranant P. (2013). A New Method for Preparing Pentacyclic Triterpene Rich Centella asiatica Extracts. Nat. Product. Res. 27 (7), 684–686. 10.1080/14786419.2012.686912 [DOI] [PubMed] [Google Scholar]
- Qian Y., Xin Z., Lv Y., Wang Z., Zuo L., Huang X., et al. (2018). Asiatic Acid Suppresses Neuroinflammation in BV2 Microgliaviamodulation of the Sirt1/NF-κB Signaling Pathway. Food Funct. 9 (2), 1048–1057. 10.1039/c7fo01442b [DOI] [PubMed] [Google Scholar]
- Rafi M., Handayani F., Darusman L. K., Rohaeti E., Wahyu Y., Sulistiyani S., et al. (2018). A Combination of Simultaneous Quantification of Four Triterpenes and Fingerprint Analysis Using HPLC for Rapid Identification of Centella asiatica from its Related Plants and Classification Based on Cultivation Ages. Ind. Crops Prod. 122, 93–97. 10.1016/j.indcrop.2018.05.062 [DOI] [Google Scholar]
- Rahajanirina V., Rakotondralambo Raoseta S. n. O., Roger E., Razafindrazaka H., Pirotais S., Boucher M., et al. (2012). The Influence of Certain Taxonomic and Environmental Parameters on Biomass Production and Triterpenoid Content in the Leaves of Centella asiatica (L.) Urb. From Madagascar. Chem. Biodiversity 9 (2), 298–308. 10.1002/cbdv.201100073 [DOI] [PubMed] [Google Scholar]
- Rao M. K., Rao M. S., Rao G. S. (2007). Treatment with Centalla Asiatica (Linn) Fresh Leaf Extract Enhances Learning Ability and Memory Retention Power in Rats. Neurosciences (Riyadh) 12 (3), 236–241. [PubMed] [Google Scholar]
- Rizzuto R., De Stefani D., Raffaello A., Mammucari C. (2012). Mitochondria as Sensors and Regulators of Calcium Signalling. Nat. Rev. Mol. Cel Biol. 13 (9), 566–578. 10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Prados J.-C., Través P. G., Cuenca J., Rico D., Aragonés J., Martín-Sanz P., et al. (2010). Substrate Fate in Activated Macrophages: a Comparison between Innate, Classic, and Alternative Activation. J.I. 185 (1), 605–614. 10.4049/jimmunol.0901698 [DOI] [PubMed] [Google Scholar]
- Roy D. C., Barman S. K., Shaik M. M. (2013). Current Updates on Centella asiatica: Phytochemistry, Pharmacology and Traditional Uses. Med. Plant Res. 3. 10.5376/mpr.2013.03.0004 [DOI] [Google Scholar]
- Sabaragamuwa R., Perera C. O., Fedrizzi B. (2018). Centella asiatica (Gotu Kola) as a Neuroprotectant and its Potential Role in Healthy Ageing. Trends Food Sci. Technol. 79, 88–97. 10.1016/j.tifs.2018.07.024 [DOI] [Google Scholar]
- Sari D. C. R., Rochmah M. A. (2015). The Effects of Ethanol Extracts of Centella asiatica Leaf on Serial Serum Brain Derived Neurotrophin Factor (BDNF) Concentration of Rats (Sprague Dawley) Following Chronic Stress. Kls 2, 159–167. 10.18502/kls.v2i1.136 [DOI] [Google Scholar]
- Sari D. C. R., Aswin S., Susilowati R., Ar-Rochmah M., Prakosa D., Romi M., et al. (2014). Ethanol Extracts of Centella asiatica Leaf Improves Memory Performance in Rats after Chronic Stress via Reducing Nitric Oxide and Increasing Brain-Derived Neurotrophic Factor (BDNF) Concentration. GSTF J. Psych 1, 9. 10.7603/s40790-014-0009-01 [DOI] [Google Scholar]
- Sbrini G., Brivio P., Fumagalli M., Giavarini F., Caruso D., Racagni G., et al. (2020). Centella asiatica L. Phytosome Improves Cognitive Performance by Promoting BDNF Expression in Rat Prefrontal Cortex. Nutrients 12 (2), 355. 10.3390/nu12020355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. I., Paine J. G., Perez C., Ullah G. (2019). Mitochondrial Fragmentation and Network Architecture in Degenerative Diseases. PLoS One 14 (9), e0223014. 10.1371/journal.pone.0223014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. S. A., Oliver P. L. (2020). ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 9 (8), 743. 10.3390/antiox9080743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Singh D. R., Banu V. S., N A. (2014). Functional Constituents (Micronutrients and Phytochemicals) and Antioxidant Activity of Centella asiatica (L.) Urban Leaves. Ind. Crops Prod. 61, 115–119. 10.1016/j.indcrop.2014.06.045 [DOI] [Google Scholar]
- Sirichoat A., Chaijaroonkhanarak W., Prachaney P., Pannangrong W., Leksomboon R., Chaichun A., et al. (2015). Effects of Asiatic Acid on Spatial Working Memory and Cell Proliferation in the Adult Rat hippocampus. Nutrients 7 (10), 8413–8423. 10.3390/nu7105401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A. J., Hartley R. C., Cochemé H. M., Murphy M. P. (2012). Mitochondrial Pharmacology. Trends Pharmacol. Sci. 33 (6), 341–352. 10.1016/j.tips.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Song D., Jiang X., Liu Y., Sun Y., Cao S., Zhang Z. (2018). Asiaticoside Attenuates Cell Growth Inhibition and Apoptosis Induced by Aβ1-42 via Inhibiting the TLR4/NF-κB Signaling Pathway in Human Brain Microvascular Endothelial Cells. Front. Pharmacol. 9, 28. 10.3389/fphar.2018.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumyanath A., Zhong Y.-P., Henson E., Wadsworth T., Bishop J., Gold B. G., et al. (2012). Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer's Disease: Investigation of a Possible Mechanism of Action. Int. J. Alzheimer's Dis. 2012, 1–9. 10.1155/2012/381974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Verma S., Gupta A., Rajan S., Rawat A. (2014). Studies on Chemotypic Variation in Centella asiatica (L.) Urban from Nilgiri Range of India. J. Planar Chromatogr. - Mod. TLC 27 (6), 454–459. 10.1556/jpc.27.2014.6.9 [DOI] [Google Scholar]
- Subaraja M., Vanisree A. J. (2019). The Novel Phytocomponent Asiaticoside-D Isolated from Centella asiatica Exhibits Monoamine Oxidase-B Inhibiting Potential in the Rotenone Degenerated Cerebral Ganglions of Lumbricus Terrestris. Phytomedicine 58, 152833. 10.1016/j.phymed.2019.152833 [DOI] [PubMed] [Google Scholar]
- Suliman H. B., Piantadosi C. A. (2016). Mitochondrial Quality Control as a Therapeutic Target. Pharmacol. Rev. 68 (1), 20–48. 10.1124/pr.115.011502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Youle R. J., Finkel T. (2016). The Mitochondrial Basis of Aging. Mol. Cel 61 (5), 654–666. 10.1016/j.molcel.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri A. A., Handayani A., Ferhad A., Farida S., Redjeki S. (2018). Effect of Centella asiatica Ethanol Extract in Spatial Working Memory on Adult Male Rats. Adv. Sci. Lett. 24 (8), 6109–6111. 10.1166/asl.2018.12641 [DOI] [Google Scholar]
- Szalárdy L., Zádori D., Klivényi P., Toldi J., Vécsei L. (2015). Electron Transport Disturbances and Neurodegeneration: From Albert Szent-Györgyi's Concept (Szeged) till Novel Approaches to Boost Mitochondrial Bioenergetics. Oxidative Med. Cell Longevity 2015, 1–19. 10.1155/2015/498401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerapattarakan N., Benya-Aphikul H., Tansawat R., Wanakhachornkrai O., Tantisira M. H., Rodsiri R. (2018). Neuroprotective Effect of a Standardized Extract of Centella asiatica ECa233 in Rotenone-Induced Parkinsonism Rats. Phytomedicine 44, 65–73. 10.1016/j.phymed.2018.04.028 [DOI] [PubMed] [Google Scholar]
- Tewari D., Mukhopadhyay M., Nekkanti M. S., Vallabhaneni S., Sahu G., Jetti S. K., et al. (2016). Cytoprotective Effect of Centella asiatica Is Mediated through the Modulation of Mitochondrial Voltage-dependent Anion Channel (VDAC) and Scavenging of Free Radicals. J. Funct. Foods 21, 301–311. 10.1016/j.jff.2015.11.047 [DOI] [Google Scholar]
- Thirawarapan S. S., Jariyapongsakul A., Suvitayavat W., Muangnongwa S., Sribusarakum A. (2019). Anti-hypertensive and Cerebral Blood Flow Improving Actions of Centella asiatica (L.) Urban Leaves Juice in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Pharm. Sci. Asia 46 (3), 184–192. 10.29090/psa.2019.03.018.0002 [DOI] [Google Scholar]
- Thomas M. T., Kurup R., Johnson A. J., Chandrika S. P., Mathew P. J., Dan M., et al. (2010). Elite Genotypes/chemotypes, with High Contents of Madecassoside and Asiaticoside, from Sixty Accessions of Centella asiatica of South India and the Andaman Islands: For Cultivation and Utility in Cosmetic and Herbal Drug Applications. Ind. Crops Prod. 32 (3), 545–550. 10.1016/j.indcrop.2010.07.003 [DOI] [Google Scholar]
- Torbati F. A., Ramezani M., Dehghan R., Amiri M. S., Moghadam A. T., Shakour N., et al. (2021). Ethnobotany, Phytochemistry and Pharmacological Features of Centella asiatica: A Comprehensive Review. Adv. Exp. Med. Biol. 1308, 451–499. 10.1007/978-3-030-64872-5_25 [DOI] [PubMed] [Google Scholar]
- Umka Welbat J., Sirichoat A., Chaijaroonkhanarak W., Prachaney P., Pannangrong W., Pakdeechote P., et al. (2016). Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients 8 (5), 303. 10.3390/nu8050303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerendra Kumar M., Gupta Y. (2003). Effect of Centella asiatica on Cognition and Oxidative Stress in an Intracerebroventricular Streptozotocin Model of Alzheimer's Disease in Rats. Clin. Exp. Pharmacol. Physiol. 30 (5-6), 336–342. 10.1046/j.1440-1681.2003.03842.x [DOI] [PubMed] [Google Scholar]
- Wanasuntronwong A., Wanakhachornkrai O., Phongphanphanee P., Isa T., Tantisira B., Tantisira M. H. (2018). Modulation of Neuronal Activity on Intercalated Neurons of Amygdala Might Underlie Anxiolytic Activity of a Standardized Extract of Centella asiatica ECa233. Evidence-Based Complement. Altern. Med. 2018, 1–8. 10.1155/2018/3853147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. H., Muthuraju S., Reza F., Senik M. H., Zhang J., Mohd Yusuf Yeo N. A. B., et al. (2019). Differential Expression of Entorhinal Cortex and Hippocampal Subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) and N-Methyl-D-Aspartate (NMDA) Receptors Enhanced Learning and Memory of Rats Following Administration of Centella asiatica . Biomed. Pharmacother. 110, 168–180. 10.1016/j.biopha.2018.11.044 [DOI] [PubMed] [Google Scholar]
- Wong J. H., Reza F., Muthuraju S., Chuang H. G., Zhang J., Senik M. H., et al. (2020). Acute Application of Centella asiatica Extract Enhanced AMPAR-Mediated Postsynaptic Currents in Rat Entorhinal Cortex. J. Integr. Neurosci. 19 (2), 217–227. 10.31083/j.jin.2020.02.50 [DOI] [PubMed] [Google Scholar]
- Xiong Y., Ding H., Xu M., Gao J. (2009). Protective Effects of Asiatic Acid on Rotenone- or H2O2-Induced Injury in SH-SY5Y Cells. Neurochem. Res. 34 (4), 746–754. 10.1007/s11064-008-9844-0 [DOI] [PubMed] [Google Scholar]
- Xu M.-f., Xiong Y.-y., Liu J.-k., Qian J.-j., Zhu L., Gao J. (2012). Asiatic Acid, a Pentacyclic Triterpene in Centella asiatica, Attenuates Glutamate-Induced Cognitive Deficits in Mice and Apoptosis in SH-SY5Y Cells. Acta Pharmacol. Sin. 33 (5), 578–587. 10.1038/aps.2012.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.-L., Qu R., Zhang J., Li L.-F., Ma S.-P. (2013). Neuroprotective Effects of Madecassoside in Early Stage of Parkinson's Disease Induced by MPTP in Rats. Fitoterapia 90, 112–118. 10.1016/j.fitote.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Yang B., Xu Y., Hu Y., Luo Y., Lu X., Tsui C. K., et al. (2016). Madecassic Acid Protects against Hypoxia-Induced Oxidative Stress in Retinal Microvascular Endothelial Cells via ROS-Mediated Endoplasmic Reticulum Stress. Biomed. Pharmacother. 84, 845–852. 10.1016/j.biopha.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Yang L., Mao K., Yu H., Chen J. (2020). Neuroinflammatory Responses and Parkinson' Disease: Pathogenic Mechanisms and Therapeutic Targets. J. Neuroimmune Pharmacol. 15 (4), 830–837. 10.1007/s11481-020-09926-7 [DOI] [PubMed] [Google Scholar]
- Yin M.-C., Lin M.-C., Mong M.-C., Lin C.-Y. (2012). Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food Chem. 60 (31), 7697–7701. 10.1021/jf302529x [DOI] [PubMed] [Google Scholar]
- Yolanda D. A., Sari D. C. R., Rochmah M. A., Suharmi S. (2015). The Dose Variations Effect of Centella asiatica Ethanol Extract o Escape Latency's Distance Morris Water Maze After Chronic Electrical Stress. Kls 2, 146–153. 10.18502/kls.v2i1.134 [DOI] [Google Scholar]
- Yoo S.-M., Park J., Kim S.-H., Jung Y.-K. (2020). Emerging Perspectives on Mitochondrial Dysfunction and Inflammation in Alzheimer's Disease. BMB Rep. 53 (1), 35–46. 10.5483/BMBRep.2020.53.1.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Li X., Li D., Luo M., Li Y., Song L., et al. (2017). Asiaticoside Ameliorates β-amyloid-induced Learning and Memory Deficits in Rats by Inhibiting Mitochondrial Apoptosis and Reducing Inflammatory Factors. Exp. Ther. Med. 13 (2), 413–420. 10.3892/etm.2016.4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Shu P., Zhang Y., Lin L., Zhou H., Xu Z., et al. (2014). Effect of Centella Asiaticaon Oxidative Stress and Lipid Metabolism in Hyperlipidemic Animal Models. Oxidative Med. Cell Longevity 2014, 1–7. 10.1155/2014/154295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.-M., Choi M.-J., Kim J. M., Cha K. H., Lee K. W., Park Y. H., et al. (2016). Centella asiatica Leaf Extract Protects against Indomethacin-Induced Gastric Mucosal Injury in Rats. J. Med. Food 19 (1), 38–46. 10.1089/jmf.2015.3464 [DOI] [PubMed] [Google Scholar]
- Zorova L. D., Popkov V. A., Plotnikov E. Y., Silachev D. N., Pevzner I. B., Jankauskas S. S., et al. (2018). Mitochondrial Membrane Potential. Anal. Biochem. 552, 50–59. 10.1016/j.ab.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig J. A., Brandes M. S., Brumbach B. H., Caruso M., Wright K. M., Quinn J. F., et al. (2021). Loss of NRF2 Accelerates Cognitive Decline, Exacerbates Mitochondrial Dysfunction, and Is Required for the Cognitive Enhancing Effects of Centella asiatica during Aging. Neurobiol. Aging 100, 48–58. 10.1016/j.neurobiolaging.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]