Abstract
Background
Mutations in the AmpC-AmpR region are associated with treatment-emergent ceftolozane-tazobactam (TOL-TAZ) and ceftazidime-avibactam (CAZ-AVI) resistance. We sought to determine if these mutations impact susceptibility to the novel cephalosporin-siderophore compound cefiderocol.
Methods
Thirty-two paired isolates from 16 patients with index P. aeruginosa isolates susceptible to TOL-TAZ and subsequent P. aeruginosa isolates available after TOL-TAZ exposure from January 2019 to December 2020 were included. TOL-TAZ, CAZ-AVI, imipenem-relebactam (IMI-REL), and cefiderocol minimum inhibitory concentrations (MICs) were determined using broth microdilution. Whole-genome sequencing of paired isolates was used to identify mechanisms of resistance to cefiderocol that emerged, focusing on putative mechanisms of resistance to cefiderocol or earlier siderophore-antibiotic conjugates based on the previously published literature.
Results
Analyzing the 16 pairs of P. aeruginosa isolates, ≥4-fold increases in cefiderocol MICs occurred in 4 of 16 isolates. Cefiderocol nonsusceptibility criteria were met for only 1 of the 4 isolates, using Clinical and Laboratory Standards Institute criteria. Specific mechanisms identified included the following: AmpC E247K (2 isolates), MexR A66V and L57D (1 isolate each), and AmpD G116D (1 isolate) substitutions. For both isolates with AmpC E247K mutations, ≥4-fold MIC increases occurred for both TOL-TAZ and CAZ-AVI, while a ≥4-fold reduction in IMI-REL MICs was observed.
Conclusions
Our findings suggest that alterations in the target binding sites of P. aeruginosa–derived AmpC β-lactamases have the potential to reduce the activity of 3 of 4 novel β-lactams (ie, ceftolozane-tazobactam, ceftazidime-avibactam, and cefiderocol) and potentially increase susceptibility to imipenem-relebactam. These findings are in need of validation in a larger cohort.
Keywords: AmpC, antimicrobial resistance, ceftazidime-avibactam, omega loop
Pseudomonas aeruginosa with difficult-to-treat resistance (DTR; ie, P. aeruginosa resistant to all traditional β-lactams and fluoroquinolones) poses significant clinical challenges [1]. Several novel β-lactam agents have become Food and Drug Administration (FDA) approved with activity against DTR P. aeruginosa, including ceftolozane-tazobactam (TOL-TAZ), ceftazidime-avibactam (CAZ-AVI), imipenem-cilastatin-relebactam (IMI-REL), and cefiderocol. Unreliable baseline susceptibility of DTR P. aeruginosa to the novel agents, as well as reports of resistance emerging during therapy, has tempered enthusiasm for several of these agents [2].
TOL-TAZ remains a preferred agent for the treatment of DTR P. aeruginosa infections [1]. We previously reported that in a cohort of 28 patients infected with DTR P. aeruginosa and paired clinical isolates before and after receipt of TOL-TAZ, half of patients had isolates that developed ≥4-fold increases in TOL-TAZ minimum inhibitory concentrations (MICs) after exposure to this agent [3].
Before the clinical use of cefiderocol, there was widespread belief that resistance would primarily result from mutations in TonB-dependent receptors (TBDRs), a series of bacterial outer membrane proteins that mediate siderophore–iron complex transport [4-6]. While such mutations have been identified [7, 8], there have also been isolated reports of changes in the ampC region contributing to cefiderocol resistance among the Enterobacterales [9, 10]. This may occur after exposure to oxyminocephalosporins, such as CAZ-AVI or cefepime, in the absence of exposure to cefiderocol. It is unknown what role exposure to TOL-TAZ, also an oxyminocephalosporin, has in contributing to cefiderocol inactivity against P. aeruginosa. A P. aeruginosa isolate infecting a 30-year-old liver transplant recipient developed a cefiderocol MIC increase from 2 to 8 mcg/mL after treatment with TOL-TAZ, in the absence of exposure to cefiderocol [11]. Mutations in the TBDR genes piuD and pirR were identified, in addition to a leucine-to-phenylalanine substitution at amino acid position 147 in the AmpC enzyme [11]. The relative role of mutations in the iron transport pathway and the role of the ampC gene in contributing to cefiderocol MIC increases in this case are unclear. Building on existing investigations, we sought to determine the frequency and putative mechanisms of cefiderocol resistance in a cohort of patients infected with DTR P. aeruginosa after TOL-TAZ exposure.
METHODS
Study Population
Sixteen unique patients from The Johns Hopkins Hospital with DTR P. aeruginosa isolates available both before and after at least 72 hours of TOL-TAZ (and up to 30 days after TOL-TAZ completion) between January 2018 and December 2019 had paired isolates available for additional testing. All initial DTR P. aeruginosa isolates were susceptible to TOL-TAZ. Patients contributing isolates were a median (range) of 55 (16–77) years, 44% had severe immunocompromise, and the most common sources of infection were pneumonia (69%) and bacteremia (31%) (Table 1). Patients received an average (range) of 12 (6–22) days of TOL-TAZ between the index and subsequent clinical P. aeruginosa isolate.
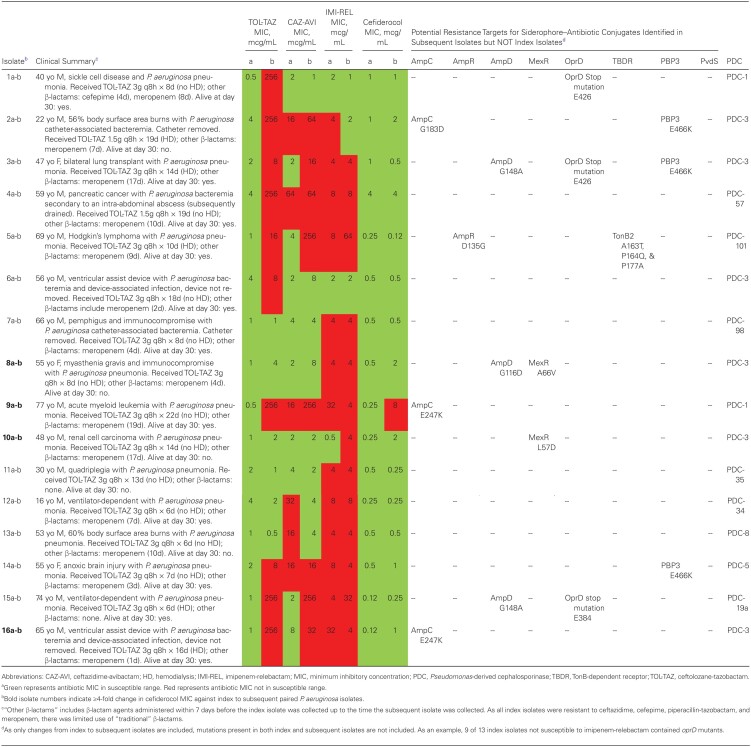
Table 1.
Antibiotic MICs of TOL-TAZ, CAZ-AVI, IMI-REL, and Cefiderocol and Whole-Genome Sequencing Results of 32 Clinical Index and Subsequent Pseudomonas aeruginosa Isolates With a Difficult-to-Treat Resistance Profile Before and After Exposure to TOL-TAZa
Microbiological Testing
Antimicrobial susceptibility testing (AST) for 32 DTR P. aeruginosa isolates from the 16 patients was determined using MDRGN2F lyophilized sensititer broth microdilution (BMD) panels (Thermo Fisher Scientific, Waltham, MA, USA) [12]. Panels contain cefiderocol concentrations ranging from 0.03 to 64 mg/L and a proprietary chelator in the wells, removing the requirement for iron-depleted cation-adjusted Mueller Hinton broth. Isolates were tested in triplicate by BMD; modal MICs were used for analysis. Clinical Laboratory and Standards Institute (CLSI) interpretive criteria were applied to all agents to determine P. aeruginosa susceptibility [13]. Quality control organisms were performed each day of testing, including E. coli ATCC 25922 and P. aeruginosa ATCC 27853. The CLSI defines P. aeruginosa isolates with cefiderocol MICs ≤ 4 mcg/mL as susceptible to cefiderocol [13].
Whole-Genome Sequencing
Genomic DNA was extracted from the 32 isolates using the DNeasy Blood & Tissue Kit (QIAGEN, Inc., Valencia, CA, USA). Whole-genome sequencing (WGS) was conducted using Illumina MiSeq short-read sequencing (Illumina, San Diego, CA, USA). Sequenced isolates were evaluated using FASTQC, version 0.11.6, and MultiQC, version 1.6. Trimmomatic, version 0.39, removed adapters and trimmed low-quality paired-end reads. Trimmed and de-duplicated reads (FastUniq, version 1.1) were de novo assembled with SPAdes, version 3.12.0, and annotated with Prokka, version 1.13. Quast, version 4.6.3, confirmed assembly quality. Genomic distances for cluster analysis were calculated with SourMash 2.0.0a. MUMmer3, version 3.23, was used for pairwise differential genome analysis. Gene annotations were determined with nucleotide BLAST, version 2.9.0+, against the reference genome of P. aeruginosa PA01. Resistance genes were identified using ARESdb [14]. Intergenic and synonymous variants were removed. Isolate variant analysis was carried out with Snippy 4.6.0 against the reference genome for each species using default parameters.
More specifically, isolates with cefiderocol MICs >4 mcg/mL or those that developed a ≥4-fold increase in MICs when comparing index and subsequent isolates were compared with the PA01 reference genome and compared with their paired isolate using multiple sequence alignment to identify missense mutations resulting in changes to amino acid composition. Efforts focused on examining P. aeruginosa resistance targets described for earlier siderophore-antibiotic conjugates and/or cefiderocol. These include insertions, deletions, and frameshift mutations in piuA, piuC, piuD, pirA, pirR, exbD3, tonB; or mutations in the promotor region of pvdS or fecI [4–7]—all components of the bacterial iron transport system (Table 2). Proteins associated with increased permeability were also assessed (OprD, mex-operon encoded proteins). Furthermore, based on reports of deletions, insertions, and amino acid substitutions in or proximal to the omega loop of AmpC contributing to cefiderocol resistance [9–11], this region was carefully examined. Bioinformatics analyses were conducted by Ares Genetics.
Table 2.
Previously Identified Mutations Potentially Contributing to Elevated Minimum Inhibitory Concentrations to Cefiderocol and Other Siderophore-Conjugated Antibiotic Candidates Against Pseudomonas aeruginosa and Acinetobacter baumannii Complex
| Target | Organism(s) | Function | Description of Findings |
|---|---|---|---|
| piuA | P. aeruginosa, A. baumannii | Encodes TonB-dependent receptor | Overexpression of piuA increased susceptibility to siderophore-conjugated antibiotics BAL30072 and MC-1 by 4- to 32-fold for P. aeruginosa [5]; transposon insertion in the iron transport receptor piuA increased cefiderocol MICs to P. aeruginosa but did not lead to frank resistance [6]; deletion of piuA in A. baumannii resulted in a 4- to 8-fold decrease in susceptibility to siderophore-conjugated antibiotics BAL30072 and MC-1 [5]; insertions, deletions, and frameshift mutations in the piuA gene in P. aeruginosa isolates led to increased MICs for the siderophore-conjugated antibiotic SMC-3176 [21]; piuA deleted mutants had a 8- to 32-fold reduction in cefiderocol MICs [4] |
| piuC | P. aeruginosa | Encodes iron-dependent oxygenase and located adjacent to piuA | Frameshift mutation in piuC led to premature termination of translation of the PiuC protein and impacted the adjacent gene piuA, causing a reduction in expression of PiuA [22]; downregulation of the piuC gene increased MICs for siderophore-conjugated antibiotic BAL30072 8- to 16-fold [23]; insertions, deletions, and frameshift mutations in the piuC gene led to increased MICs for the siderophore-conjugated antibiotic SMC-3176 in P. aeruginosa [21] |
| piuD | P. aeruginosa | Encodes TonB-dependent receptor | Deletion of piuD increased cefiderocol MICs by 32-fold [4]; clinical isolate with no prior exposure to cefiderocol demonstrated resistance potentially associated with mutation in piuD (deletion of an A nucleotide with premature stop codon at amino acid 89) [11] |
| pirA | P. aeruginosa; A. baumannii | Encodes TonB-dependent receptor | Overexpression of piuA increased susceptibility to siderophore-conjugated antibiotics BAL30072 and MC-1 by 4- to 32-fold [5]; deletion of pirA in A. baumannii resulted in 4- to 8-fold decreased susceptibility to siderophore-conjugated antibiotics BAL30072 and MC-1 [5]; deletion of pirA led to a 2-fold increase in cefiderocol MICs [4]; pirA mutants had a 2-fold reduction in siderophore-conjugated antibiotics BAL30072 and MC-1 [4]; reduced expression of the siderophone receptor gene pirA, possibly in combination with piuA, was associated with cefiderocol resistance in A. baumannii isolates [31] |
| pirR | P. aeruginosa | Encodes the response regulator of a 2-component regulatory system predicted to activate expression of piuA | Frameshift mutations in pirR increase MICs to SMC-3176, a siderophore-conjugated antibiotic [21]; clinical isolate with no prior exposure to cefiderocol demonstrated resistance potentially associated with mutation in pirR (insertion of a G nucleotide with premature stop codon at amino acid 201) [11] |
| pvdS | P. aeruginosa | Required for pyoveridine production; mutations in pvdS lead to derepression of pyoveridine synthesis, which enhances production of the pyoveridine siderophore receptor FpvA | Mutation in promotor region of pvdS increased MICs for cefiderocol and the siderophore-conjugated antibiotic SMC-3176 [7, 8, 21] |
| fecI | P. aeruginosa | Regulator of the synthesis of the iron transporter FecA, contributing to the transport of iron citrate | Single nucleotide change in fecI promotor increased MICs to siderophore-conjugated antibiotic BAL30072 8- to 16-fold [23];. Point mutations in the fecI promoter reduced activity of the siderophore-conjugated antibiotic SMC-3176 against P. aeruginosa [21]; mutations in the promotor region of fecI increased cefiderocol [7] |
| exbD3 | A. baumannii | Component of inner membrane protein complex providing energy to TonB-dependent transporters | Frameshift mutations in exbD3 increased the MICs of siderophore-conjugated antibiotics BAL30072 and MC-1 [5] |
| tonB | A. baumannii | Component of inner membrane protein complex providing energy to TonB-dependent transporters | Frameshift mutations in tonB3 increased the MICs of siderophore-conjugated antibiotics BAL30072 and MC-1 [5] |
| ampC | P. aeruginosa | Chromosomal β-lactamase gene | Substitution of leucine for phenylalanine at Ambler amino acid position 147 in the AmpC β-lactamase enzyme, potentially increased cefiderocol MICs [11] |
| PBP3 | A. baumannii | Target site of activity for cefiderocol | A Isoleucine-to-asparagine substitution at position 236 and a histamine-to-tyrosine substitution at position 370 identified in a cefiderocol-resistant isolate. [31] |
Abbreviation: MIC, minimum inhibitory concentration.
RESULTS
Phylogenetic trees were constructed to confirm relatedness between index and subsequent isolates, and sequence types were determined, also to ensure relatedness between isolates. Paired isolates for each patient met criteria for relatedness. Table 1 includes a brief description of the 16 patients, antibiotic exposures, antibiotic MIC data, and WGS results. For the 16 index isolates (ie, before TOL-TAZ exposure), susceptibility was as follows: TOL-TAZ 100%, CAZ-AVI 63%, IMI-REL 19%, and cefiderocol 100%. For the 16 subsequent isolates, susceptibility was as follows: TOL-TAZ 38%, CAZ-AVI 50%, IMI-REL 19%, and cefiderocol 94%. One pair of DTR P. aeruginosa isolates (isolates 9a-b) had cefiderocol MICs of 0.25 mcg/mL and 8 mcg/mL, respectively, transitioning from the susceptible category to the intermediate category. Isolates 8a-b, isolates 10a-b, and isolates 16a-b developed ≥4-fold increases in cefiderocol MICs following TOL-TAZ exposure, with cefiderocol MICs increasing from 0.5 to 2 mcg/mL, 0.25 to 2 mcg/mL, and 0.12 to 1 mcg/mL, respectively, prompting further examination.
For isolates 8a-8b, a substitution in MexR A66V was identified. Additionally, a glycine-to-aspartic acid substitution at position 116 on AmpD was identified. ampD mutations have the potential to lead to AmpC overproduction, increasing β-lactam MICs in organisms with a chromosomal ampC, such as P. aeruginosa [15]. Similarly, for isolates 10a-b, a leucine-to-aspartic acid substitution in position 57 was noted in MexR. Mutations in mexR result in derepression of the mexAB-oprM multidrug efflux operon [16]. Despite these observations, cefiderocol MICs remained in the susceptible range for 8a-b and 10a-b.
For isolates 9a-9b and 16a-16b, a glutamic acid-to-lysine substitution at position 247 in ampC was identified. This substitution has been previously identified as producing AmpC mutants exhibiting high-level resistance to TOL-TAZ and CAZ-AVI through reduced structural stability of the AmpC enzyme [17]. For isolates 9a-9b, TOL-TAZ MICs increased from 0.5 to 256 mcg/mL and CAZ-AVI MICs increased from 16 to 64 mcg/mL after 22 days of TOL-TAZ. Similarly, for isolates 16a-16b, TOL-TAZ MICs increased from 1 to 256 mcg/mL and CAZ-AVI MICs increased from 8 to 32 mcg/mL after 16 days of TOL-TAZ exposure. Interestingly, for both of these patients, IMI-REL MICs decreased from 32 to 4 mcg/mL, comparing index and subsequent isolates. Although remaining nonsusceptible to IMI-REL, this nonetheless represents a >4-fold decrease in IMI-REL MICs. All subsequent isolates remained resistant to all “traditional” β-lactams and fluoroquinolones.
Discussion
In a cohort of 32 paired DTR P. aeruginosa isolates from 16 patients exposed to TOL-TAZ, 4 P. aeruginosa isolates developed ≥4-fold increases in cefiderocol MICs, although MICs remained in the susceptible range for 3 of the 4 isolates. The clinical significance of increased cefiderocol MICs in the absence of frank resistance is unknown. Additionally, as none of the included isolates were exposed to cefiderocol therapy, it is unknown if a furthering of MIC elevation would be anticipated after cefiderocol therapy. Antimicrobial resistance markers potentially contributing to cefiderocol MIC increases included mutations in mexR (2 isolates), ampD (1 isolate), and ampC (2 isolates). Of these, the E247K mutations identified in AmpC enzymes for 2 of the 4 isolates have been the most frequently described mechanism of resistance to TOL-TAZ and other cephalosporins [2, 17-19].
We did not identify mutations in TBDRs in the paired isolates contributing to cefiderocol nonsusceptibility in our cohort. However, identification of such mutations is likely more common in patients with previous exposure to cefiderocol. TBDRs are bacterial outer membrane proteins that enable uptake of specific siderophore–iron complexes across the bacterial membrane. They are dependent on 3 inner membrane proteins, TonB-ExbB-ExbD, for energy transduction [20]. TBDR expression is regulated by 2-component regulatory systems [21]. Mutations decreasing the function of components of this pathway may cause dramatic MIC increases for siderophore–antibiotic compounds. The deletion of the TBDRs PiuA and PirA in Acinetobacter baumannii decreased susceptibility to BAL30072 and MC-1, earlier siderophore-conjugated antibiotic prototypes, by 4--fold [5], while overexpression increased P. aeruginosa susceptibility to these agents by 4- to 32-fold [5, 22]. Frameshift mutations in exbD3 or tonB3 genes led to significant increases in BAL30072 and MC-1 MICs [5]. Elevations in cefiderocol MICs may also be associated with mutations in the upstream regions of pvdS (a regulator of pyoveridine synthesis) or the FecIRA operon (a regulator of iron transporter protein synthesis). Overexpression of these proteins can lead to ≥4-fold increases in cefiderocol MICs [7, 8, 21, 23].
Shields and colleagues demonstrated a 2–amino acid deletion in the R2 loop of the AmpC β-lactamase (ie, alanine and leucine at positions 292 and 293) in 2 Enterobacter hormaechei isolates from distinct patients after exposure to cefepime [9]. These deletions appear to broadly impact cephalosporin antibiotics in that they confer resistance to cefepime, CAZ-AVI, and cefiderocol—in the absence of preceding exposure to CAZ-AVI or cefiderocol. The same group described a third patient with an E. cloacae clinical isolate with a cefiderocol MIC of >16 mcg/mL with an alanine–proline deletion at positions 294 and 295 and a leucine-to-valine substitution at position 296 in AmpC [10]. Conformation changes in the R2 loop of AmpC β-lactamases expand its substrate binding site, enabling entrapment of cephalosporins with bulkier R2 side chains, increasing their hydrolysis [24]. The omega loop borders the R1 and R2 regions of AmpC, and the R1 region contains position 247, where a substitution was identified in isolates 9b and 16b in our cohort, resulting in elevated cefiderocol, TOL-TAZ, and CAZ-AVI MICs.
Although our focus was on the emergence of resistance, we found that 69% of index isolates not susceptible to IMI-REL contained oprD mutants. Previous work has found that IMI-REL can remain effective against oprD mutants even when the pseudomonal AmpC is overexpressed, because of the potent activity of relebactam against AmpC enzymes [25, 26]. However, others have found that reduced expression of oprD can be sufficient to result in IMI-REL resistance, or at a minimum an increase in IMI-REL MICs compared with isolates with oprD mutants [27-29]. Our understanding of P. aeruginosa resistance to IMI-REL remains incomplete and will likely become clearer as it is used more frequently in clinical practice. An interesting observation in our cohort was the >4-fold reduction in IMI-REL MICs in both isolates 9b and 16b, which both contained E247K AmpC mutations. A similar finding was observed by Rubio and colleagues, who found that 81% of TOL-TAZ-resistant P. aeruginosa isolates with ampC mutations were susceptible to IMI-REL, including several isolates that developed reduced IMI-REL MICs in conjunction with an elevation in TOL-TAZ MICs [29]. It is hypothesized that mutations resulting in AmpC structural modifications can enable carbapenems such as imipenem to rotate their bulky 6α-hydroxyethyl side chain to prevent hydrolysis [30].
We identified 2 different mutations in MexR, the negative regulator of the MexAB-OprM efflux pump, in isolates 8b and 10b with a ≥4-fold in cefiderocol MICs. The role of MexAM-OprM overexpression in reducing cefiderocol activity warrants further exploration as cefiderocol appears to be a substrate of this efflux pump, although other investigators did not find an association with MexAB-OprM overproduction and reduced cefiderocol activity [6]. Similarly, the role of the AmpD G116 substitution (isolate 8b) remains unclear, as this mutation did not appear to impact TOL-TAZ or CAZ-AVI MICs, leading us to suspect that there was likely incomplete disruption of this gene. Mutations in AmpD G116D, MexR A66V, and MexR L57D were associated with modest increases in cefiderocol MICs to 2 mcg/mL, with cefiderocol MICs remaining in the susceptible range.
Our findings suggest that substitutions in the region of the AmpC omega loop contribute to increased cefiderocol MICs to P. aeruginosa. This is particularly concerning as a single amino acid substitution has the potential to inactive 3 of the 4 novel antipseudomonal β-lactams (ie, TOL-TAZ, CAZ-AVI, and cefiderocol) while potentially increasing activity of the fourth (ie, IMI-REL). Our findings also bolster the hypothesis that resistance markers leading to P. aeruginosa nonsusceptibility to cefiderocol are diverse [32]. Our study is small and exploratory. Cloning and transformation studies are needed to confirm the significance of the mutations we identified as contributing to cefiderocol resistance.
Acknowledgments
Financial support. This work was supported by an R21-AI153580 from the National Institutes of Health and an American Lung Association Research Grant, both awarded to P.D.T.
Potential conflicts of interest. S.B. and A.E.P. are employees of Ares Genetics. Ares Genetics performed the bioinformatics analysis for this work. S.E.C. receives consulting fees from Novartis, Theravance, and Balisea outside the submitted work. P.J.S. has received grants and personal fees from Accelerate Diagnostics, OpGen Inc, and BD Diagnostics; grants from bioMerieux, Inc., Affinity Biosensors, and Hardy Diagnostics; and personal fees from Roche Diagnostics, GeneCapture, and Shionogi Inc. P.D.T. and Y.B. report no disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This work was approved by the Johns Hopkins University Institutional Review Board with a waiver of informed consent.
References
- 1. Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa) Clin Infect Dis 2021; 72:e169–e183. [DOI] [PubMed] [Google Scholar]
- 2. Papp-Wallace KM, Mack AR, Taracila MA, Bonomo RA. Resistance to novel β-lactam-β-lactamase inhibitor combinations: the “price of progress.” Infect Dis Clin North Am 2020; 34:773–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamma PD, Beisken S, Bergman Y, et al. Modifiable risk factors for the emergence of ceftolozane-tazobactam resistance. Clin Infect Dis 2020; ciaa1306. [DOI] [PubMed] [Google Scholar]
- 4. Luscher A, Moynie L, Auguste PS, et al. TonB-dependent receptor repertoire of Pseudomonas aeruginosa for uptake of siderophore-drug conjugates. Antimicrob Agents Chemother 2018; 62:e00097–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moynie L, Luscher A, Rolo D, et al. Structure and function of the PiuA and PirA siderophore-drug receptors from Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother 2017; 61:e02531–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother 2017; 62:e01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito A, Nishikawa T, Ishii R, et al. Mechanism of cefiderocol high MIC mutants obtained in non-clinical FoR studies. Poster presented at: ID Week 2018; October 3–7, 2018; San Francisco, CA. Poster 696.
- 8. Kohira N, Ito A, Ota M, et al. Frequency of resistance acquisition and resistance mechanisms to cefiderocol. Poster presented at: American Society of Microbiology Annual Meeting; June 6–11, 2018; Atlanta, GA. Poster 619. [Google Scholar]
- 9. Shields RK, Iovleva A, Kline EG, et al. Clinical evolution of AmpC-mediated ceftazidime-avibactam and cefiderocol resistance in Enterobacter cloacae complex following exposure to cefepime. Clin Infect Dis 2020; 71:2713–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawai A, McElheny CL, Iovleva A, et al. Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother 2020; 64:e00198–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Streling AP, Al Obaidi MM, Lainhart WD, et al. Evolution of cefiderocol non-susceptibility in Pseudomonas aeruginosa in a patient without previous exposure to the antibiotic. Clin Infect Dis 2021; ciaa1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris CP, Bergman Y, Tekle T, et al. Cefiderocol antimicrobial susceptibility testing against multidrug-resistant gram-negative bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol. 2020. Dec 17;59(1):e01649–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira I, Beisken S, Lueftinger L, et al. Species identification and antibiotic resistance prediction by analysis of whole-genome sequence data by use of ARESdb: an analysis of isolates from the unyvero lower respiratory tract infection trial. J Clin Microbiol 2020; 58:e00273–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidtke AJ, Hanson ND. Model system to evaluate the effect of ampD mutations on AmpC-mediated beta-lactam resistance. Antimicrob Agents Chemother 2006; 50:2030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adewoye L, Sutherland A, Srikumar R, Poole K. The mexR repressor of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutations compromising activity. J Bacteriol 2002; 184:4308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slater CL, Winogrodzki J, Fraile-Ribot PA, et al. Adding insult to injury: mechanistic basis for how AmpC mutations allow Pseudomonas aeruginosa to accelerate cephalosporin hydrolysis and evade avibactam. Antimicrob Agents Chemother 2020; 64:e00894–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacVane SH, Pandey R, Steed LL, et al. Emergence of ceftolozane-tazobactam-resistant Pseudomonas aeruginosa during treatment is mediated by a single AmpC structural mutation. Antimicrob Agents Chemother 2017; 61:e01183-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schalk IJ, Mislin GL, Brillet K. Structure, function and binding selectivity and stereoselectivity of siderophore-iron outer membrane transporters. Curr Top Membr 2012; 69:37–66. [DOI] [PubMed] [Google Scholar]
- 21. Kim A, Kutschke A, Ehmann DE, et al. Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa: assessing the risk for resistance and attenuated efficacy. Antimicrob Agents Chemother 2015; 59:7743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McPherson CJ, Aschenbrenner LM, Lacey BM, et al. Clinically relevant gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob Agents Chemother 2012; 56:6334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Delden C, Page MG, Köhler T. Involvement of Fe uptake systems and AmpC β-lactamase in susceptibility to the siderophore monosulfactam BAL30072 in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:2095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berrazeg M, Jeannot K, Ntsogo Enguéné VY, et al. Mutations in β-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother 2015; 59:6248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraile-Ribot PA, Zamorano L, Orellana R, et al. Activity of imipenem-relebactam against a large collection of Pseudomonas aeruginosa clinical isolates and isogenic beta-lactam-resistant mutants. Antimicrob Agents Chemother 2020; 64:e02165–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnes MD, Bethel CR, Alsop J, et al. Inactivation of the Pseudomonas-derived cephalosporinase-3 (PDC-3) by relebactam. Antimicrob Agents Chemother 2018; 62:e02406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68:2286–90. [DOI] [PubMed] [Google Scholar]
- 28. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of imipenem with relebactam against gram-negative pathogens from New York City. Antimicrob Agents Chemother 2015; 59:5029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubio AM, Kline EG, Jones CE, et al. In vitro susceptibility of multidrug-resistant Pseudomonas aeruginosa following treatment-emergent resistance to ceftolozane-tazobactam. Antimicrob Agents Chemother 2021; 65:e00084-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lahiri SD, Walkup GK, Whiteaker JD, et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother 2015; 70:1650–8. [DOI] [PubMed] [Google Scholar]
- 31. Malik S, Kaminski M, Landman D, Quale J. Cefiderocol resistance in Acinetobacter baumannii: roles of beta-lactamases, siderophore receptors, and penicillin binding protein 3. Antimicrob Agents Chemother 2020; 64:e01221–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCreary EK, Heil EL,. Tamma PD. New perspectives on antimicrobial agents: cefiderocol. Antimicrob Agents Chemother 2021; AAC.02171-20. [DOI] [PMC free article] [PubMed] [Google Scholar]