Abstract
Background
Neutropenic fever (NF) is associated with significant morbidity and mortality for patients receiving cancer treatment in sub-Saharan Africa (sSA). However, the antibiotic management of NF in sub-Saharan Africa has not been well described. We evaluated the timing and selection of antibiotics for patients with NF at the Uganda Cancer Institute (UCI).
Methods
We conducted a retrospective chart review of adults with acute leukemia admitted to UCI from 1 January 2016 to 31 May 2017, who developed NF. For each NF event, we evaluated the association of clinical presentation and demographics with antibiotic selection as well as time to both initial and guideline-recommended antibiotics. We also evaluated the association between ordered antibiotics and the in-hospital case fatality ratio (CFR).
Results
Forty-nine NF events occurred among 39 patients. The time to initial antibiotic order was <1 day. Guideline-recommended antibiotics were ordered for 37 (75%) NF events. The median time to guideline-recommended antibiotics was 3 days. Fever at admission, a documented physical examination, and abdominal abnormalities were associated with a shorter time to initial and guideline-recommended antibiotics. The in-hospital CFR was 43%. There was no difference in in-hospital mortality when guideline-recommended antibiotics were ordered as compared to when non-guideline or no antibiotics were ordered (hazard ratio, 0.51 [95% confidence interval {CI}, .10–2.64] and 0.78 [95% CI, .20–2.96], respectively).
Conclusions
Patients with acute leukemia and NF had delayed initiation of guideline-recommended antibiotics and a high CFR. Prospective studies are needed to determine optimal NF management in sub-Saharan Africa, including choice of antibiotics and timing of antibiotic initiation.
Keywords: antimicrobial stewardship, guideline adherence, hematologic malignancy, neutropenic fever, sub-Saharan Africa
We examined adherence to guideline-recommended antibiotics in 49 neutropenic fever episodes among adults with acute leukemia at the Uganda Cancer Institute. Guideline-recommended antibiotics were ordered in 37 (75%) events, but median time to guideline-recommended antibiotic order was 3 days.
Cancer is a growing cause of morbidity and mortality in sub-Saharan Africa (sSA). By 2030 more than 1.28 million new cancer cases and 970 000 cancer-related deaths are expected in sSA annually [1]. As cancer detection and treatment has improved, treatment-related infections are a growing concern [2–7]. Chemotherapy-associated neutropenic fever (NF) is an oncologic emergency requiring rapid initiation of empiric broad-spectrum antibiotics to avoid early death [8, 9]. Consensus guidelines for NF management have been developed and validated in high-income countries (HICs) [8, 9]. According to these guidelines, patients should receive empiric broad-spectrum antibiotics that have antipseudomonal activity [8, 9]. The time from NF onset to guideline-recommended antibiotic administration is an important predictor of outcomes [10, 11]. Accordingly, antibiotic administration within 1 hour of NF onset is a key clinical care metric [12]. In HICs where NF guidelines are well-established, case fatality ratios (CFRs) range from <5% in patients with solid tumors to 15%–20% in patients with hematologic malignancies [13].
Several small retrospective studies from sSA suggest that NF is associated with high CFRs [14–20]. We recently showed that up to 46% of Ugandan patients with hematologic malignancies died within 30 days of NF onset [3]. The reasons for these high CFRs are not clear. Providers may have incomplete knowledge of the NF guidelines, there may be inconsistent access to guideline-recommended antibiotics, and international guidelines may not adequately account for the antimicrobial resistance patterns found in sSA [20, 21]. Identifying the unique factors that influence timely initiation of guideline-recommended antibiotics is key to improving antibiotic delivery and decreasing CFRs among patients with NF in sSA. While several studies from sSA describe the antibiotics prescribed to treat NF, few have evaluated provider adherence to guideline recommendations [20–22].
Our primary objective was to evaluate baseline adherence to guideline-recommended antibiotics for patients with NF at a national cancer referral center in sSA before the initiation of antimicrobial stewardship interventions. Accordingly, we completed a retrospective chart review of adult patients with acute leukemia and NF at the Uganda Cancer Institute (UCI) in Kampala, Uganda, to evaluate provider adherence to guideline-recommended antibiotics and to identify factors associated with timely antibiotic orders. Our secondary objective was to explore associations between clinical outcomes and the time from NF onset to the order for guideline-recommended antibiotics.
METHODS
Study Design
We conducted a single-center retrospective cohort study of adults with acute leukemia admitted to the UCI from 1 January 2016 to 31 May 2017, and developed NF during their hospital admission. The study period occurred after the release of the updated 2016 UCI neutropenic fever guidelines and before the launch of our prospective cohort study regarding the microbiology of NF in patients with acute leukemia at UCI [3].
Study Setting and Population
The UCI is a national cancer referral hospital located in Kampala, Uganda, and is the African Development Bank–designated East African Center of Excellence in Oncology. More than 5000 patients are treated at the UCI annually. Adults with acute leukemia receive treatment at the inpatient Liquid Tumor Centre (LTC). Daily patient care is conducted by medical officers. They are supervised by fellowship-trained oncologists who oversee the cancer treatment plan. All medical documentation occurs in paper charts that are stored in the UCI Medical Records Department when they are not in clinical use.
We used the LTC admission logbook to identify patients >18 years of age with acute leukemia who were hospitalized during the study period. We obtained the medical charts from the UCI Medical Records Department and reviewed each chart for inclusion criteria. We included only patients with histopathologically confirmed leukemia who experienced at least 1 NF event during a hospital admission.
The Uganda Cancer Institute Neutropenic Fever Guidelines
The “UCI Guidelines for NF Management” were established in 2014 and updated just before our study period (Table 1). The UCI NF guidelines include recommendations for the clinical identification, microbiologic evaluation, and choice of empiric antibiotics for patients with NF (Table 1). To create these guidelines, a group of UCI clinicians and pharmacists collaborated with infectious diseases specialists from the Fred Hutchinson Cancer Research Center (Seattle, Washington). The group adapted the Infectious Diseases Society of America clinical practice guidelines for the management of fever in neutropenia [8] to account for locally available microbiologic tests and antibiotics.
Table 1.
Summary of Microbiologic Evaluation and Antibiotic Recommendations for Adult Inpatients With Neutropenic Fever per the 2016 Uganda Cancer Institute Neutropenic Fever Guidelines
| Guideline | Recommendation |
|---|---|
| Microbiologic evaluation of neutropenic fever | Blood cultures obtained within 30 minutes of fever detection
Blood cultures obtained prior to antibiotic initiation |
| Empiric antibiotic regimens for neutropenic fever | First-line: If no suspicion for MDRO - Piperacillin-tazobactam - If evidence of sepsis/shock: add gentamicin |
| First-line: If high suspicion for MDRO - Chloramphenicol, meropenem, or imipenem |
|
| Second-line: if first-line regimens unavailablea - Ceftriaxone ± ciprofloxacin ± gentamicin - If suspected abdominal source: add metronidazole |
Abbreviation: MDRO, multidrug-resistant organism.
aCeftriaxone only recommended in cases where no first-line antibiotics are available due to drug shortages; fluoroquinolones and gentamicin are not considered guideline-recommended unless ordered in conjunction with ceftriaxone.
Neutropenic Fever
We defined neutropenia as an absolute neutrophil count (ANC) of <500 cells/µL. When a temperature was recorded, we defined fever as a single axillary temperature of >37.5°C. When a temperature was not recorded, we defined fever as any use of the word “fever” or “febrile” to describe the patient in the medical record. We considered a febrile patient to have NF if they had documented neutropenia within 2 days before fever onset or within 2 days after NF occurred [8]. We defined an NF event as the first neutropenic fever that occurred during that hospital admission. For patients who had >1 hospital admission during the study period, each admission was evaluated independently. Thus, each patient could have up to 1 NF event per hospital admission (Supplementary Table 1).
Antibiotic Treatment
For each NF event, we evaluated the time from NF onset to the first antibiotic ordered (time to initial antibiotics) regardless of whether the antibiotic was guideline-recommended. We also evaluated the time from NF onset to the first order for guideline-recommended antibiotics (time to guideline-recommended antibiotics). We considered an antibiotic to be guideline-recommended if it was a first- or second-line antibiotic in the UCI NF guidelines (Table 1).
Data Collection, Management, and Quality Assurance
We provided standardized training for all data collectors. We used EpiInfo (Centers for Disease Control and Prevention, Atlanta, Georgia) to abstract data into case report forms. Abstracted data included patient demographics, clinical presentation, and laboratory findings. To evaluate NF management, we abstracted all blood cultures and antibiotics ordered throughout the hospitalization. We used a checklist to record physical examination abnormalities documented within 2 days of NF onset. Finally, we recorded the date of hospital discharge or in-hospital death. We analyzed the data using R (R Foundation for Statistical Computing, Vienna, Austria) and Stata 16 (StataCorp, College Station, Texas) software.
Statistical Analysis
We used descriptive statistics to summarize categorical variables as frequencies and percentages and continuous variables as medians with interquartile ranges (IQRs). We conducted Kaplan-Meier analyses of the time to initial and guideline-recommended antibiotic orders. We measured time in days and classified orders written on the date of NF onset as 0.1 day to ensure inclusion in the survival analyses. We excluded the NF episodes in which patients already had guideline-recommended antibiotics ordered at the time of NF onset. For the time to initial antibiotics analyses, we followed patients from NF onset to the first new antibiotic order or until they were censored due to death or hospital discharge. For the time to guideline-recommended antibiotics analyses, we followed patients from NF onset to the first guideline-recommended antibiotic order or until they were censored due to death or hospital discharge. We then estimated the percentage with 95% confidence intervals (CIs) of NF episodes for which any antibiotics and guideline-recommended antibiotics were ordered within 1 day of NF onset. We used Cox proportional hazards regression to estimate hazard ratios (HRs) with 95% CIs to describe associations between the time to initial and guideline-recommended antibiotics with patient characteristics including age, sex, reason for hospital admission, time from admission to NF onset, and physical examination findings at the time of NF. We used clustered standard error estimation to account for correlation between repeated NF episodes in the same patient.
For the mortality analyses, we included the first hospitalization with an NF event. We followed patients from NF onset to death or until they were censored due to hospital discharge. We treated orders for antibiotics as a time-varying categorical exposure with 3 possible values: 0 from NF onset to any antibiotic order; 1 following the order of non-guideline-recommended antibiotics and prior to the order of guideline-recommended antibiotics; or 2 following the order of guideline-recommended antibiotics. We analyzed participant demographics and clinical characteristics as potential confounders of the association between antibiotics with in-hospital mortality. We considered P < .05 as statistically significant for all analyses.
Ethical Considerations
The University of Virginia Institutional Review Board (IRB), the Fred Hutchinson Cancer Research Center IRB, the Uganda Cancer Institute Research and Ethics Committee, and the Uganda National Council on Science and Technology approved the study with a waiver of consent.
RESULTS
Study Population and Demographics
Of the 95 patients with acute leukemia, we located 66 (69%) charts for review (Supplementary Figure 1). Of these, 39 (59%) patients had at least 1 NF event. Among these 39 unique patients, there were 49 hospitalizations with an NF event. Nine of 49 (18%) NF events occurred at admission or within 1 day of hospitalization. For the remaining 40 NF events, the median time from hospital admission to documented fever onset was 15 (IQR, 9–18) days. In 44 of 49 (90%) NF events, the measured temperature was documented at NF onset.
Among the 39 patients, the median age at the time of first hospitalization was 31 years, and approximately half were female (Table 2). The most frequently encountered cancers were acute lymphocytic leukemia (n = 22 [56%]) and acute myelogenous leukemia (n = 14 [36%]). Most patients had newly diagnosed cancer at the time of first hospitalization (n = 32 [82%]). There were 9 (23%) patients who had documented comorbidities, which included hypertension, diabetes, and renal disease. One patient was living with human immunodeficiency virus.
Table 2.
Characteristics of Adult Inpatients With Acute Leukemia and Neutropenic Fever at Time of First Hospital Admission to the Uganda Cancer Institute, 1 January 2016 to 31 May 2017
| Characteristics | Total (N = 39) |
|---|---|
| Demographics | |
| Age, y, median (IQR) | 31 (25–49) |
| Male sex | 20 (51) |
| Cancer history | |
| Cancer type | |
| ALL | 22 (56) |
| AML | 14 (36) |
| Othera | 3 (8) |
| Stage of malignancy | |
| Initial diagnosis | 32 (82) |
| Relapsed disease | 4 (10) |
| Other | 3 (8) |
| HIV | |
| Positive | 1 (3) |
| Negative | 25 (64) |
| Unknown | 13 (33) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphomatous leukemia; AML, acute myelogenous leukemia; HIV, human immunodeficiency virus; IQR, interquartile range.
aTwo with undifferentiated acute leukemia and 1 with a history of myelodysplastic syndrome with blast crisis, which was managed like AML.
Clinical Presentation
A review of systems was documented within 2 days of fever onset in 36 of 49 (73%) NF events (Supplementary Table 1). Among these 36 NF events, the most frequent complaints were subjective fever (n = 14 [39%]), nosebleed (n = 6 [17%]), headache (n = 6 [17%]), diarrhea (n = 6 [17%]), and abdominal pain (n = 5 [13%]). A physical examination was documented within 2 days of fever onset in 34 (69%) NF events. Among these 34 NF events, the most frequently documented abnormalities were abdominal tenderness (n = 12 [35%]), hepatosplenomegaly (n = 7 [20%]), and skin rashes or lesions (n = 7 [20%]). All patients had at least 1 ANC obtained during their hospitalization. The median ANC at the time of NF onset was 50 (IQR, 10–120) cells/µL.
Microbiologic Evaluation
Blood cultures were ordered in 9 of 49 (18%) NF events. In 1 NF event, cultures were ordered twice for a total of 10 blood cultures. The median days from fever onset to first culture order was 9 (IQR, 2–21) days. Only 4 of 10 (40%) cultures had results documented in the chart: 3 had no growth and 1 had growth of gram-negative cocci, which were susceptible to meropenem.
Antimicrobial Management
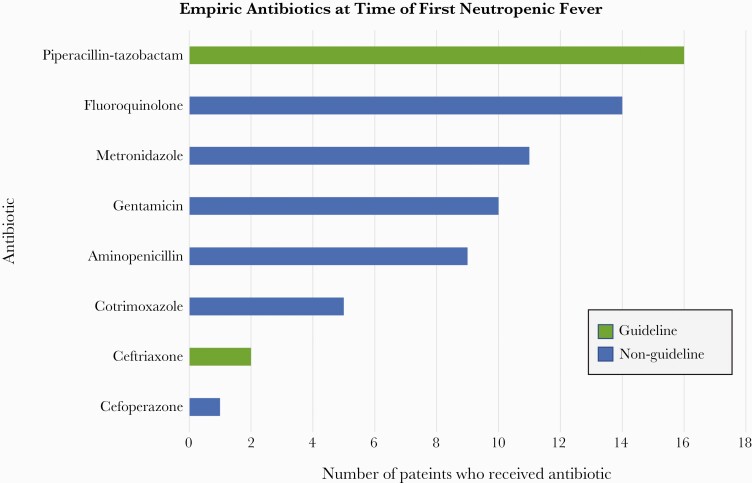
In 8 of 49 (16%) NF events, patients already had guideline-recommended antibiotics ordered at the time of fever onset (Figure 1). In the remaining 41 NF events, antibiotics were ordered in 39 (95%). In these 39 NF events, guideline-recommended antibiotics were ordered for initial treatment in 18 (46%), and non-guideline-recommended antibiotics were ordered for initial treatment in 21 (54%). In 11 (52%) of these 21 NF events, guideline-recommended antibiotics were subsequently ordered. Thus, guideline-recommended antibiotics were ordered in 37 of 49 (75%) NF events. Among the 2 NF events in which no antibiotics were ordered, 1 patient died in the hospital and 1 was discharged alive. The antibiotics ordered for initial NF treatment are shown in Figure 2.
Figure 1.
Antibiotic orders for neutropenic fever events among adult inpatients with acute leukemia at the Uganda Cancer Institute, 1 January 2016 to 31 May 2017.
Figure 2.
First antibiotic ordered for after fever onset for adult inpatients with acute leukemia and neutropenic fever at the Uganda Cancer Institute, 1 January 2016 to 31 May 2017 (N = 39). Among the 39 neutropenic fever events, 16 (41%) had 1 antibiotic ordered, 17 (44%) had 2 antibiotics ordered, and 6 (15%) had 3 antibiotics ordered.
Time to Initial Antibiotic Therapy
Among 32 of 41 NF events, antibiotics were ordered within 1 day of fever onset (78% [95% CI, 65%–89%]) (Figure 3A); the median time from NF onset to the first antibiotic order was <1 day (95% CI, 0–3 days). We found no association between sex or type of malignancy and time to initial antibiotics. For all 9 events in which NF was documented at admission, antibiotics were ordered within 1 day, compared to 23 of 32 (72% [95% CI, 56%–86%]) when NF was not documented at admission (HR, 1.50 [95% CI, 1.12–2.00]). When a physical examination was documented at NF onset, antibiotics were ordered within 1 day in 25 of 30 NF events (83%; [95% CI, 68%–94%]) compared with 7 of 11 (64% [95% CI, 37%–89%]) when no physical examination was documented (HR, 1.66 [95% CI, .98–2.81]). In the 30 NF events in which a physical examination was documented, antibiotics were ordered within 1 day for all 7 events in which there was an abnormal abdominal examination finding, compared with 18 of 23 NF events (78% [95% CI, 60%–92%]) with no documented abdominal findings (HR, 1.53 [95% CI, 1.15–2.03]).
Figure 3.
Kaplan-Meier estimates of the time from onset of neutropenic fever to initial antibiotic order (A) and first guideline-recommended antibiotic order (B) for adult inpatients with acute leukemia at the Uganda Cancer Institute, 1 January 2016 to 31 May 2017. The y-axis indicates percentage of neutropenic episodes.
Time to Guideline-Recommended Antibiotic Therapy
Among 15 of 41 NF events, guideline-recommended antibiotics were ordered within 1 day of fever onset (37% [95% CI, 24%–53%]). The median time from NF onset to the first order for guideline-recommended antibiotics was 3 days (95% CI, 1–11 days) (Figure 3B). We found no association between sex or type of malignancy and time to guideline-recommended antibiotics. When NF was documented at admission, guideline-recommended antibiotics were ordered within 1 day in 6 of 9 NF events (67% [95% CI, 38%–92%]), compared to 9 of 32 (28% [95% CI, 16%–47%]) when NF was not present on admission (HR, 2.42 [95% CI, 1.12–5.22]). When a physical examination was documented at NF onset, guideline-recommended antibiotics were ordered within 1 day for 14 of 30 NF events (47% [95% CI, 31%–66%]), compared to 1 of 11 (9% [95% CI, 1%–49%]) in which no physical examination was documented (HR, 1.96 [95% CI, .94–4.09]). Among the 30 NF events in which a physical examination was documented, antibiotics were ordered within 1 day for 5 of 7 NF events (71% [95% CI, 39%–96%]) in which there was an abnormal abdominal examination finding, compared with 3 of 23 (10% [95% CI, 23%–62%]) with no documented abdominal findings (HR, 1.84 [95% CI, .87–3.90]).
Mortality
Of the 39 patients with NF included in the study, 20 (51%) were known to have died during the study period. Seventeen patients died during their initial hospitalization for a CFR of 43%. An order for non-guideline-recommended antibiotics or guideline-recommended antibiotics was associated with a nonsignificant lower hazard of in-hospital mortality (HR, 0.51 [95% CI, .10–2.64] and 0.78 [95% CI, .20–2.96], respectively). This relationship did not change with adjustment for participant characteristics.
Discussion
In this study, we retrospectively evaluated the antibiotic management of adult inpatients with acute leukemia and NF at a single national cancer center in sSA. In most NF events, antibiotics were ordered on the day of NF onset. However, only about one-third had guideline-recommended antibiotics ordered on the day that NF occurred, and it took a median of 3 days for guideline-recommended antibiotics to be ordered. The in-hospital CFR of 43% was high, and we did not find a significant association between mortality and an order for guideline-recommended antibiotics.
Since most NF events had at least 1 antibiotic ordered on the day of NF onset, there seemed to be general recognition that NF requires rapid antibiotic initiation. However, most patients had non-guideline-recommended antibiotics ordered for initial NF treatment. Understanding why guideline-recommended antibiotics were not routinely ordered is key to improving NF management at UCI and in similar cancer centers throughout sSA. Lack of guideline knowledge is frequently identified as a barrier to guideline adherence [23, 24]. Since our goal was to assess baseline adherence to guideline recommendations, our study started shortly after the UCI NF guidelines were updated and before the launch of our ongoing prospective cohort study investigating the microbiology of NF at UCI. Thus, lack of knowledge may have contributed to low guideline implementation. Since this was a retrospective study, we were unable to assess provider knowledge as a contributing factor. We are currently evaluating the knowledge and attitudes regarding the NF guidelines among UCI clinicians. We will use these findings to determine whether targeted educational interventions (eg, lectures, online modules) could improve knowledge and increase guideline adherence [21, 25].
The unique management of patients with neutropenia could also have affected antibiotic selection. The Uganda Ministry of Health has created the Uganda clinical guidelines for management of common conditions to provide standardized, evidence-based recommendations for managing priority health conditions. These guidelines recommend using relatively narrow-spectrum antibiotics (eg, cloxacillin) to treat febrile patients if they do not appear critically ill [26]. Since patients with neutropenia have attenuated neutrophil-mediated inflammation, they may lack the traditional signs and symptoms of severe infection [27]. In our study, patients with a documented physical examination abnormality had a shorter time to guideline-recommended antibiotic order. A previous study from Uganda showed that increased frequency of vital sign documentation was associated with higher illness acuity and increased mortality in patients with sepsis [28]. Similarly, a documented physical examination abnormality may be a surrogate marker for illness severity, indicating that those who appeared clinically unwell were more likely to receive the broad-spectrum antibiotics (eg, piperacillin-tazobactam, ceftriaxone) recommended by the UCI NF guidelines [29]. We also found that patients who were febrile at the time of hospital admission were more likely to have guideline-recommended antibiotics ordered on the day of NF onset than those who developed fever later during hospitalization. Since it is standard UCI practice to document a full history and physical examination when a patient is admitted to the hospital, those with severe illness may be more quickly recognized and guideline-recommended antibiotics more rapidly ordered. These findings suggest that educating clinicians about the need to initiate broad-spectrum antibiotics (eg, piperacillin-tazobactam) regardless of illness severity could improve guideline adherence among patients with NF.
Antibiotic stockouts (lack of product in the pharmacy) are another key barrier to guideline implementation in low-resource settings [30]. Stockouts are associated with the use of alternative antibiotics that are less effective, have increased rates of adverse events, and can drive antimicrobial resistance [25]. While we do not have records of the available antibiotics during the study period, stockouts due to local or national shortages occur periodically at the UCI. During these times, clinicians must prescribe non-guideline antibiotics or ask patients to purchase the preferred antibiotic at a local pharmacy, which may not be feasible. This reliance on antibiotic self-procurement from local pharmacies is associated with the use of substandard or falsified medications, which can result in increased mortality and drive antimicrobial resistance [25]. The extent to which stockouts resulted in the prescription of non-guideline antibiotics could not be determined in our study but should be prospectively evaluated.
We found an in-hospital CFR of 43% for the first hospitalization of patients with NF. This is consistent with findings from our ongoing prospective cohort study among patients with NF at UCI [3]. However, it is significantly higher than the 7%–22% CFR among patients who are hospitalized for sepsis in Uganda [31–33] and the 5%–20% CFR for patients with NF who live in HICs [13]. NF studies from HICs show that decreasing the time to guideline-recommended antibiotics reduces mortality [10, 11]. In our study, we found no difference in CFRs among those who received an order for guideline-recommended antibiotics compared to those who received an order for non-guideline-recommended or no antibiotics. This may reflect our relatively small sample size. It is also possible that the antibiotics that were ordered were not administered. Alternatively, it may reflect the presence of antibiotic resistance, which resulted in treatment failure even among those who received guideline-recommended antibiotics. Given the increasing prevalence of multidrug-resistant bacteria in sSA [34, 35], it is possible that the UCI NF guidelines provide evidence-based recommendations in accordance with international guidelines but do not adequately address local antimicrobial resistance patterns. Prospective studies are currently underway to investigate the presence of multidrug-resistant bacteria at UCI and to evaluate patient outcomes in relation to antibiotic administration.
Our study had other limitations, including the retrospective chart review study design. Although we identified 95 patients hospitalized with acute leukemia during our study period, only 69% of patient charts were available for review. However, there is no reason to believe that patients with missing charts experienced different antibiotic prescription patterns than those whose charts were available. Consequently, we believe that our findings can be extrapolated to other patients at the UCI. The use of paper medical records and incomplete medical documentation also posed a challenge since all microbiology results were handwritten and antibiotic administration was inconsistently documented. As noted, we did not have a list of antibiotics available at the UCI pharmacy during the time of our study. Thus, it is possible that some patients did not receive the prescribed antibiotics.
In conclusion, most patients at the UCI who had acute leukemia and developed NF had antibiotics ordered on the day that fever occurred, but experienced delayed prescription of guideline-recommended antibiotics. These findings emphasize the need for prospective studies to understand why providers do not always adhere to the NF guidelines. Given the high CFRs among this patient population, it is also vital to understand the microbiologic causes of NF in sSA in order to determine whether international guidelines adequately address local antimicrobial resistance patterns. Meanwhile, clinicians in sSA should continue to treat neutropenic fever as an emergency that requires rapid procurement of blood cultures and empiric administration of broad-spectrum guideline-recommended antibiotics when they are available. Further studies are needed to develop implementation strategies to facilitate rapid initiation of guideline-recommended antibiotics among patients with cancer and NF in sSA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Flow diagram for adult patients with acute leukemia admitted to the Uganda Cancer Institute from 1 January 2016 to 31 May 2017. Patients could have 1 neutropenic fever event per hospital admission during the study period.
Notes
Acknowledgments. We gratefully acknowledge the clinicians and patients of the Uganda Cancer Institute (UCI). The authors thank Dr Rhoda Morrow for critical examination of the manuscript.
Author contributions. E. A. G., J. K., A. O., and C. C. M. contributed to conceptualization. E. A. G., B. C., R. H., J. K., A. O., and C. C. M. contributed to the methodology. E. A. G., B. C., L. F., and J. S. abstracted the data. S. A. and E. A. G. performed the data analysis. E. A. G. and S. A. drafted the manuscript, which was edited by all authors. All authors have read and agreed with the published version of the manuscript. E. A. G. takes responsibility for the integrity of the work as a whole.
Data availability. Data are available upon request.
Financial support. E. A. G. received funding from the University of Virginia Department of Infectious Diseases Internal Seed Grant the University of Virginia T32 Infectious Diseases Training Program (T32AI007046-43) and the Fred Hutchinson Cancer Research Center National Institutes of Health T32 Training Program in Infectious Diseases in the Immunocompromised Host (5T32AI118690-02). B. C. received funding through the American Society of Tropical Medicine and Hygiene Benjamin K. Kean Travel Fellowship, the Infectious Diseases Society of America Medical Scholars Program, and the University of Virginia School of Medicine. B. C., R. H., and L. F. received funding through the University of Virginia Center for Global Health.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–917. [DOI] [PubMed] [Google Scholar]
- 2. Arega B, Woldeamanuel Y, Adane K, et al. Microbial spectrum and drug-resistance profile of isolates causing bloodstream infections in febrile cancer patients at a referral hospital in Addis Ababa, Ethiopia. Infect Drug Resist 2018; 11:1511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lubwama M, Adams S, Muwonge C, et al. Multidrug-resistant bacteria are common cause of neutropenic fever and increase mortality among patients with hematologic malignancies in Uganda. Open Forum Infect Dis 2019; 6:S108–S9. [Google Scholar]
- 4. Lubwama M, Phipps W, Najjuka CF, et al. Bacteremia in febrile cancer patients in Uganda. BMC Res Notes 2019; 12:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mohammed HB, Yismaw MB, Fentie AM, Tadesse TA. Febrile neutropenia management in pediatric cancer patients at Ethiopian tertiary care teaching hospital. BMC Res Notes 2019; 12:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mvalo T, Eley B, Bamford C, et al. Bloodstream infections in oncology patients at Red Cross War Memorial Children’s Hospital, Cape Town, from 2012 to 2014. Int J Infect Dis 2018; 77:40–7. [DOI] [PubMed] [Google Scholar]
- 7. von Knorring N, Nana T, Chibabhai V. Cumulative antimicrobial susceptibility data for a tertiary-level paediatric oncology unit in Johannesburg, South Africa. South Afr J Oncol 2019; 3:8. [Google Scholar]
- 8. Freifeld AG, Bow EJ, Sepkowitz KA, et al. ; Infectious Diseases Society of America . Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 9. Averbuch D, Orasch C, Cordonnier C, et al. ; ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID, and ELN . European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013; 98:1826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butts AR, Bachmeier CC, Dressler EV, et al. Association of time to antibiotics and clinical outcomes in adult hematologic malignancy patients with febrile neutropenia. J Oncol Pharm Pract 2017; 23:278–83. [DOI] [PubMed] [Google Scholar]
- 11. Faye Anderson D, Lisa Rioux B. Implementation of an evidence-based order set to impact initial antibiotic time intervals in adult febrile neutropenia. Oncol Nurs Forum 2011; 38:661–8. [DOI] [PubMed] [Google Scholar]
- 12. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013; 31:794–810. [DOI] [PubMed] [Google Scholar]
- 13. Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006; 106:2258–66. [DOI] [PubMed] [Google Scholar]
- 14. Buys H. Klebsiella pneumoniae Bloodstream Infections in Hospitalised Children at RED Cross War Memorial Children’s Hospital: 2006–2011 [Master of Science]. Cape Town: University of Cape Town; 2015. [Google Scholar]
- 15. Malande OO, Nuttall J, Pillay V, et al. A ten-year review of ESBL and non-ESBL Escherichia coli bloodstream infections among children at a tertiary referral hospital in South Africa. PLoS One 2019; 14:e0222675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naidu G. Infectious Complications in the South African Black Child With Cancer [PhD]. Johannesburg: University of the Witwatersrand; 2017. [Google Scholar]
- 17. Steinhaus N, Al-Talib M, Ive P, et al. The management and outcomes of Staphylococcus aureus bacteraemia at a South African referral hospital: a prospective observational study. Int J Infect Dis 2018; 73:78–84. [DOI] [PubMed] [Google Scholar]
- 18. Fentie A, Wondimeneh Y, Balcha A, et al. Bacterial profile, antibiotic resistance pattern and associated factors among cancer patients at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist 2018; 11:2169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mvalo T, Eley B, Bamford C, et al. Bloodstream infections in oncology patients at Red Cross War Memorial Children’s Hospital, Cape Town, from 2012 to 2014. Int J Infect Dis 2018; 77:40–7. [DOI] [PubMed] [Google Scholar]
- 20. Mohammed HB, Yismaw MB, Fentie AM, Tadesse TA. Febrile neutropenia management in pediatric cancer patients at Ethiopian tertiary care teaching hospital. BMC Res Notes 2019; 12:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muchela MA. Patterns of Antibiotic Prescription During Febrile Episodes in Pediatric Patients With Cancer at the Kenyatta National Hospital [Master of Medicine]. Nairobi, Kenya: University of Nairobi; 2020. [Google Scholar]
- 22. Vanderpuye V, Yarney J, Beecham K. Management of febrile neutropenia in patients receiving chemotherapy for solid tumors: a retrospective study of twenty cases from the radiotherapy centre, Accra, Ghana. West Afr J Med 2010; 29:303–8. [DOI] [PubMed] [Google Scholar]
- 23. Hooft AM, Ripp K, Ndenga B, et al. Principles, practices and knowledge of clinicians when assessing febrile children: a qualitative study in Kenya. Malar J 2017; 16:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mula CT, Middleton L, Human N, Varga C. Assessment of factors that influence timely administration of initial antibiotic dose using collaborative process mapping at a referral hospital in Malawi: a case study of pneumonia patients. BMC Infect Dis 2018; 18:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knowles R, Sharland M, Hsia Y, et al. Measuring antibiotic availability and use in 20 low- and middle-income countries. Bull World Health Organ 2020; 98:177–187C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ministry of Health and the Uganda National Drug Authority; Uganda Clinical Guidelines 2016: Guidelines on Management of Common Disease Conditions. Kisubi, Uganda: Marianum Press Ltd; 2016. [Google Scholar]
- 27. Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med 1975; 135:715–9. [PubMed] [Google Scholar]
- 28. Asiimwe SB, Okello S, Moore CC. Frequency of vital signs monitoring and its association with mortality among adults with severe sepsis admitted to a general medical ward in Uganda. PLoS One 2014; 9:e89879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ndabarora E, Chipps JA, Uys L. Systematic review of health data quality management and best practices at community and district levels in LMIC. Inf Dev 2014; 30:103–20. [Google Scholar]
- 30. Tickell KD, Mangale DI, Tornberg-Belanger SN, et al. ; Childhood Acute Illness and Nutrition Network . A mixed method multi-country assessment of barriers to implementing pediatric inpatient care guidelines. PLoS One 2019; 14:e0212395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis JM, Abouyannis M, Katha G, et al. population incidence and mortality of sepsis in an urban African setting, 2013-2016. Clin Infect Dis 2020; 71:2547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacob ST, Moore CC, Banura P, et al. ; Promoting Resource-Limited Interventions for Sepsis Management in Uganda (PRISM-U) Study Group . Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One 2009; 4:e7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalyesubula R, Mutyaba I, Rabin T, et al. Trends of admissions and case fatality rates among medical in-patients at a tertiary hospital in Uganda; a four-year retrospective study. PLoS One 2019; 14:e0216060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madney Y, Elkady W, Elmahalawy H, Ebeid E. Infection related complications during maintainnce phase treatment for children with acute lymphoblastic leukemia in developing countries: single center experience, Egypt. Pediatr Blood Cancer 2018; 65:S136–S7. [Google Scholar]
- 35. Saravanan M, Ramachandran B, Barabadi H. The prevalence and drug resistance pattern of extended spectrum β-lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb Pathog 2018; 114:180–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.