Abstract
Critical insights into the etiology of type 1 diabetes (T1D) came from genome-wide association studies that unequivocally connected genetic susceptibility to immune cell function. At the top of the susceptibility are genes involved in regulatory T-cell (Treg) function and development. The advances in epigenetic and transcriptional analyses have provided increasing evidence for Treg dysfunction in T1D. These are well supported by functional studies in mouse models and analysis of peripheral blood during T1D. For these reasons, Treg-based therapies are at the forefront of research and development and have a tangible probability to deliver a long-sought-after successful immune-targeted treatment for T1D. The current challenge in the field is whether we can directly assess Treg function at the tissue site or make informative interpretations based on peripheral data. Future studies focused on Treg function in pancreatic lymph nodes and pancreas could provide key insight into the ultimate mechanisms underlying Treg failure in T1D. In this Perspective we will provide an overview of current literature regarding Treg development and function in T1D and how this knowledge has been applied to Treg therapies.
Introduction
The discovery of Foxp3+ T cells as key regulators of the immune response provided an additional level of complexity to our understanding of self-tolerance. This added layer of protection against autoimmunity is crucial; however, to what degree loss of regulatory T-cell (Treg) function is a contributing factor in type 1 diabetes (T1D) has not been fully resolved. Although we know that deletion of Tregs in mice and mutations in FOXP3 in humans lead to pancreatic autoimmunity, we still possess limited understanding of how polygenetic susceptibilities underlie Treg dysfunction in T1D. Initial studies, using CD25 as a marker of Tregs, suggested that the numbers of Tregs measured in the peripheral blood might be deficient in T1D (1). However, as more precise methods of discrimination between Tregs and conventional T cells were used, the improved methods of Treg quantification showed no change in Treg numbers (2,3). The apparent normal size of the Treg compartment in T1D patients led to the alternative hypothesis that Treg function is somehow altered in T1D. Loss of Treg function is consistent with genetic associations affecting pathways that are known to be key for optimal Treg suppressive function, including the IL-2 and T-cell receptor (TCR) pathways (Fig. 1) (4). While the downstream effects on transcription, translation, and function of the susceptible allelic variants have been investigated in mouse models and human patients, the conclusions are complicated by the fact that the pathways could affect both Tregs and effector T cells. Nevertheless, recent studies have made exceptional effort in separating Treg-specific phenotypes in T1D. In this review, we will discuss the breakthroughs that led to improved understanding of Treg development and function in the context of autoimmune susceptibility and how this knowledge has been applied to study the etiology of human T1D.
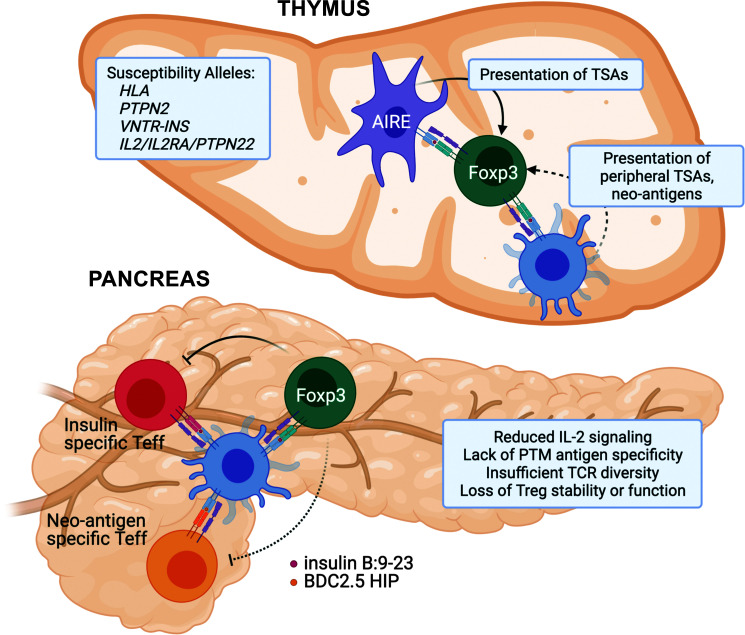
Figure 1.
Putative mechanisms underlying Foxp3+ Treg dysfunction in T1D. Thymus: Genetic susceptibility in T1D points to several pathways that are important for regulatory T-cell development, including TCR activation (HLA/PTPN22) and IL-2 signaling pathways (IL2/IL2RA). Variable number of tandem repeat polymorphisms upstream of the insulin promoter (VNTR-INS) may impact thymic transcript levels of insulin, while peripheral TSAs are presented to developing thymocytes by mTECs and DCs. Although DCs can migrate from periphery and have the potential to bring novel posttranslationally modified (PTM) self-antigens (neo-antigens), the process is likely inefficient, leading to escape of neo-antigen reactive autoimmune T cells and lack of development of neo-antigen reactive Tregs. Pancreas: Although we are lacking data regarding human Treg function in the pancreas during T1D development, studies in NOD mice have defined several potential issues that lead to Treg dysfunction in autoimmune diabetes. At the pancreatic tissue site, Tregs suffer from insufficient activation of the IL-2 signaling pathway, reduced TCR repertoire and diversity, and progressive loss of function as a result of chronic exposure to inflammatory mediators. Teff, effector T cell.
Tregs have been divided into three major subpopulations: thymically derived Foxp3+ sometimes referred to as “natural” Tregs (tTreg), peripherally induced Foxp3+ Tregs (pTreg), and IL-10–producing Tregs (Tr1). Evidence suggests that all three populations could participate in curbing islet autoimmunity (5). However, the absence of definitive markers that can unambiguously differentiate thymically derived and peripherally induced Foxp3+ Tregs precludes us from determining the relative contributions of these regulatory T-cell populations. TCR repertoire comparisons between Foxp3+ Tregs and effector T cells in the islets of NOD mice show little sequence overlap, suggesting that there is minimal contribution of induced Tregs to protection against T1D (6). However, deletion of the conserved noncoding DNA sequence element 1 (CNS1), a region in Foxp3 promoter critical for the development of peripheral but not thymic Tregs, led to a partial acceleration of diabetes (7), suggesting a role for peripherally induced Tregs. Two other attempts at addressing the role of induced Tregs by either deleting the SMAD response element or removing a shorter portion of CNS1 did not observe a significant effect on disease development (8,9). While functionally there exists a large degree of overlap between thymically derived and peripherally induced Foxp3+ Tregs, there are important distinctions in the pathways that guide their development (10,11). Therefore, knowing the source of the responding Treg population will hint at the allelic susceptibilities and upstream immunological deficiencies that underlie Treg dysfunction in T1D.
While this Perspective is primarily focused on the role of Foxp3-expressing Tregs, other regulatory cell populations likely have an important contribution to the suppression of pancreatic autoimmunity. For instance, Foxp3-negative, IL-10–producing Tr1 cells specific for pancreatic antigens have been identified in periphery of T1D patients (12). Importantly, higher frequencies of Tr1 cells are associated with better glycemic control post-diagnosis (13). In mouse models of autoimmunity, Tr1 cells were shown to be protective and could be induced with peptide-MHC–coated nanoparticles in an antigen-specific manner (14). These studies suggest that efforts focused on in vivo induction of antigen-specific Tregs might be feasible for Tr1 cells in the context of T1D.
Defects in Thymic Selection Lead to a Suboptimal Treg Repertoire
Studies focused on early events in the development of autoimmunity suggest that self-antigen expression during thymic selection is a critical factor necessary for deletion of autoimmune T cells and parallel development of Foxp3+ Tregs. Thymic development of Tregs is driven by interactions between developing thymocytes and professional antigen-presenting cells, notably dendritic cells (DCs) and medullary thymic epithelial cells (mTECs). While mTECs present to thymocytes a random assortment of tissue-restricted antigens (TSAs) driven by autoimmune regulator (AIRE) transcription factor, migratory DCs are unique in their capacity to present antigens acquired in the periphery as well as antigens recycled from mTECs (Fig. 1) (15,16). Mutations within AIRE in humans result in autoimmune polyendocrinopathy candidiasis ectodermal dystrophy and include severe multiorgan autoimmunity, including autoimmune targeting of pancreatic β-cells. The underlying mechanism is tied to the reduced or absent expression of AIRE-driven TSAs in the thymus, such as insulin. Given that healthy individuals exhibit self-reactive naive T cells in their periphery (17,18), it is still unclear whether deletion of self-reactive conventional T cells or the development of self-reactive Tregs is a more critical role for thymic presentation of self-antigen. Insulin-reactive T cells that develop in the NOD mouse with a mutated insulin epitope exhibit higher TCR affinity, respond with increased sensitivity to the insulin epitope, and cause accelerated diabetes (19). At the same time, there is a defect in insulin-reactive Treg development, suggesting that both pathways could be playing a role in the loss of tolerance. Conversely, ectopic overexpression of insulin B:9-23 peptide during thymic development allows for an increase of insulin-specific Tregs and complete protection from developing T1D in insulin-reactive TCR retrogenic mice (20).
The development of potentially autoreactive T cells in susceptible individuals is likely due in part to allelic variants in the human leukocyte antigen (HLA), the highest susceptibility allele identified in T1D that confers ˜50% of the risk (4). The mechanism behind the role of HLA in T1D may be found in the unique structures of susceptible HLAs and the stability of the peptide/HLA complex. How the HLA structure can influence the immune response is best characterized in the case of the HLA-DQ8. HLA-DQ8 has a relatively shallow peptide binding groove, and the positive charge near the ninth binding pocket results in preferred binding of peptides with negative charge at the C-terminus (21). The groove is particularly unfavorable to binding of the insulin B:9-23 peptide, which has a positive charge at the C-terminus (22). Important to our understanding of the autoimmune T-cell development, the second highest susceptibility allele is a variant of the variable number of tandem repeats of the insulin gene promoter (VNTR-INS), which controls the amount of thymic insulin transcript (23). The combination of a suboptimal HLA/MHC for peptide presentation and a reduction in the amount of insulin peptide present during thymic selection squarely sets the TCR/peptide/MHC trimolecular complex at the center of autoimmune T-cell development. Importantly, not all pancreatic antigens are efficiently presented in the thymus. In particular, posttranslationally modified antigens generated in the pancreatic β-cells are less likely to be presented to thymocytes. Lack of peripheral neo-antigen expression in the thymus results in a “hole” in the Treg TCR repertoire (24,25). These observations suggest that thymically derived Treg populations are at a disadvantage in curtailing autoimmunity that targets posttranslationally modified antigens (Fig. 1).
Challenges in Identifying Treg Deficiencies in T1D
Recent research effort has seen significant advances in our understanding of Treg function and context-dependent specialization of the suppressive mechanisms employed by Tregs. We now understand that Tregs comprise a heterogenous population made up of subsets that exhibit unique transcriptional repertoires regulated by tissue- or context-specific transcription factors. For example, Tregs that reside in the adipose tissue express transcriptional factor PPARγ, while Tregs important in regulating Th1-type responses upregulate Tbet (26–28). The challenge we face now is how to apply this knowledge to identify biomarkers of Treg function in a clinical setting. The wide gamut of Treg functions makes it difficult to pick the appropriate marker or an in vitro functional assay to provide information relevant to the disease in question. Treg studies in humans are additionally compromised because of constraints in isolating Tregs from tissues other than blood. It is especially challenging to assess the role and function of Tregs in T1D, as access to pancreatic samples can only be obtained postmortem.
Treg function is largely dependent on their ability to localize with appropriate target cells in the lymph node or traffic to the correct nonlymphoid tissue. TCR specificity for tissue antigen or tissue-specific chemokine expression can effectively target Tregs to a particular anatomical location. For example, CD62L and CCR5 expression are important for optimal Treg function in mouse models of graft-versus-host disease (GVHD), as they target Tregs to lymph nodes and GVHD-affected organs (29,30). In T1D, islet antigen-specific Tregs are significantly more protective compared with peripheral polyclonal Tregs (31,32). It is now well appreciated that Treg functional specialization, as well as Treg trafficking, can be regulated by coexpression of helper T-cell lineage-specifying transcription factors. Tbet+ Tregs are the predominant population that infiltrates the islets of NOD mice, and deletion of Tbet+ Tregs leads to accelerated diabetes, suggesting their critical function in controlling anti-islet autoimmunity (28). Tbet expression in Tregs also drives the expression of CXCR3 chemokine receptor that recruits Tregs to the same site as Th1 effector cells (26,28,33). However, it is unclear whether Tbet is important in instructing additional functions in Tregs. Moreover, expression of Tbet and IFNγ can lead to loss of Treg function (34); therefore, Tbet expression is not always a marker of optimally functional Tregs.
Tregs finalize their differentiation and acquire tissue-specific transcriptional program as they migrate from peripheral lymphoid organs to the tissue (35–38). Therefore, blood or peripheral lymphoid Tregs are quite distinct from tissue-infiltrating cells, and they might not provide a robust readout of tissue-specific Treg function. Nevertheless, several attempts have been made to use a combination of markers applied to the analysis of circulating Tregs to acquire clinically relevant information. A transcriptional signature focused on ˜30 genes was used to discriminate Treg function in T1D patients compared with healthy control subjects (39,40). The changes in the selected genes, several of which are known to be important for Treg function (e.g., FOXP3, CTLA4), could predict the accelerated rate of disease progression. The development of Treg-focused biomarkers has the potential to stratify patients based on the level of Treg deficiency, with a potential to identify subgroups that are more likely to benefit from Treg-based therapies.
Tregs use multiple cell contact-dependent and -independent mechanisms to regulate immune responses. Although some of these mechanisms, such as IL-2 consumption and expression of CTLA4, CD73, and CD39, are broadly used by the majority of Treg populations, it appears that other functions such as expression of IL-10, TGFβ, IL-35, and indolamine-2,3-dioxygenase might be selectively induced depending on tissue site or extracellular signals (41–43). Importantly, many of the suppressive mechanisms depend on antigen-specific interactions between a Treg and an APC. Continuous and strong contacts between a Treg and an APC lead to physical sequestering and stripping of peptide/MHC complexes and CD80/86 costimulatory molecules from the surface of APCs, which interferes with DC interactions and activation of cognate effector T cells (44,45). Tregs can also secrete apoptotic mediators, such as granzyme and perforin, and mediate deletion of APCs as well as conventional T cells and natural killer cells (46–48). Additionally, TCR activation is important for driving the majority of Treg functions (49), supporting the critical role for antigen specificity in optimal Treg-mediated suppression. Moreover, peptide/MHC trogocytosis was shown to require shared antigenic specificity between the Treg and the suppressed effector T cell (45). This is especially important to consider in the case of T1D, where effector T cells specific for neo-antigenic epitopes often do not have an antigen-specific Treg counterpart (12).
Evidence for Impaired Treg Function in T1D
There is no question that genetic susceptibility plays a fundamental role in T1D development, with the majority of the susceptible single nucleotide polymorphisms (SNPs) being proximal to immune genes and indicating immune dysregulation (50). The robust association is noted with genes important for Treg function, most notably IL2RA, IL2, PTPN2, CTLA4, and IL10 (51). However, few studies have been able to connect SNPs to functional outcomes. Moreover, because many of these key genes are important for both effector T-cell and Treg function, it is hard to predict the relative impact of the allelic variants on regulatory versus effector T cells. Nevertheless, several studies have shown SNP-associated defects in Treg function, most prominently IL-2 signaling (52–54). These findings and parallel observations in NOD mice showing a defect in IL-2 signaling in Tregs have spurred the efforts to exploit the pathway for therapeutic interventions. An unfortunate setback for low-dose IL-2 therapy that successfully expanded Tregs was a concomitant increase in eosinophils and natural killer cells and a reduction in C-peptide (55). However, recent efforts to develop Treg-specific IL-2 delivery should overcome off-target effects (56,57). Alternative approaches to modify pharmacokinetics of IL-2 therapy should further improve the efficacy of the therapy. One such study used a low-dose administration of IL-2/CD25 fusion protein that prevented the onset of diabetes and the control of overt diabetes in the NOD mouse model of T1D. The increased half-life of this IL-2 analog was thought to allow longer engagement with the CD25-expressing Tregs, leading to greater IL-10 production and migration to the pancreas (58).
A more recent study applied T-cell population-specific epigenetic analysis to pinpoint the location of susceptible SNPs to enhancer regions that were important for Treg function (59). The results of epigenetic comparison among populations of Tregs and conventional T cells have shown that autoimmune SNPs were enriched in naive Treg-specific demethylated regions and, to a lesser extent, in activated Treg demethylated regions. These observations suggest that autoimmune SNPs are more consequential for tTreg development and function than for aberrant activation of autoimmune effector T cells.
It appears that in T1D several pathways important in Treg development, function, and lineage stability are impacted and can lead to Treg dysfunction. While studies using peripheral blood–derived Tregs provided evidence for altered Treg function in patients with T1D (2,60,61), it is unclear to what level peripheral blood can serve as an indicator of Treg function at the tissue site. Mouse models, in particular the NOD model, have provided important insights into the mechanisms that Tregs employ for suppression of islet autoimmunity. Through these studies, we have come to understand that some of the failures in Treg function are amplified at the tissue site and that Treg deficiencies are not always apparent from in vitro assays (62,63). It is likely that a combination of chronic inflammatory mediators, defects in IL-2 signaling pathway, and reduced TCR diversity, among other factors, converge in the pancreatic tissue to weaken Treg function (24,62,64–66). The optimal Treg-focused therapy should be designed to address a combination of defects.
Current and Future Therapeutic Treg-Based Therapies
Exogenous Treg Therapy
Treg-based therapeutic interventions are now an important focus for the treatment of allotransplantation and autoimmunity. Initial trials have shown promising results with an overall high safety index and positive indications for efficacy. Phase 1 clinical trials with expanded endogenous Tregs have proven the approach to be safe, and the next step is to test their efficacy (67). There are now more than 50 active and completed clinical trials for whole-organ transplantation, systemic lupus erythematosus, inflammatory bowel disorders, allergy, asthma, and T1D (68). However, several hurdles to the ultimate success of exogenous Treg therapy remain. An important consideration is whether certain subpopulations of Tregs are more appropriate or are more functional. While recent advances in Treg biology have underscored the functional specialization of these cells depending on the tissue site, it is unclear whether peripheral blood Tregs will be sufficient and/or appropriate for all situations.
Importantly, there are still lingering concerns regarding potential contamination of Tregs with effector T cells and functional stability of Tregs. FOXP3 can be upregulated in human activated effector T cells, and we still do not have cell surface markers that are unique to Tregs. On the other hand, Tregs can lose their lineage stability, as defined by loss of FOXP3, or they can lose their suppressive function while retaining FOXP3 expression (34,65,69,70). Inflammatory cytokines appear to be the main triggers for Treg instability and loss of function. Phase 1 clinical trials using deuterium-labeled infused Tregs have monitored Treg stability in peripheral blood over a year after transfer into patients and did not observe a loss of FOXP3 expression (67). The observations from the clinical study suggest that current methods for isolation and in vitro expansion of Tregs are sufficient to obtain lineage stability, at least in the circulation. However, whether the in vitro manipulation alters Treg longevity posttransfer is unknown. It is possible that robust expansion with anti-CD3/28 could push Tregs toward terminal differentiation and exhaustion, similar to what is observed with effector T cells. This is supported by the observations from GVHD studies where repeat infusion of Tregs was necessary to affect the GVHD symptoms, as the observed suppression was transient, suggesting loss of Treg numbers or functionality (71,72). Similarly, in the T1D clinical study where the Tregs were expanded in vitro and monitored with deuterium posttransfer, transferred Treg numbers progressively diminished over time (67). Alternative sources and methods for expansion of Tregs that maintain their intrapopulational heterogeneity and retention of CD62L-positive, less differentiated Tregs should be investigated. Interestingly, umbilical cord Tregs exhibit increased repertoire diversity and are better at maintaining Treg lineage stability (73). It is possible that the naive source of Tregs from umbilical cords and improved expansion methods could lead to longer-lasting Tregs that retain their suppressive capacity. Modulation of cell-extrinsic signals could also be used to promote Treg stability. As mentioned earlier, IL-2 is a critical factor necessary for Treg survival, and exogenous IL-2 has been explored extensively as a therapeutic approach for in vivo expansion of Tregs and suppression of autoimmune responses in T1D (55–57,74,75). An alternative approach might be to block inflammatory cytokines that drive Treg instability. Since a number of cytokines have been implicated as being damaging to β-cells and induction of Treg instability, it is hard to imagine that blocking any particular one would have a desired outcome.
Antigen-Specific Treg Therapies
Studies in the NOD mouse model show that antigen-specific Tregs are superior in their ability to control pancreatic autoimmunity compared with polyclonal peripheral Tregs (31,32). This is consistent with the important role antigen recognition plays in recruitment and accumulation of T cells in the pancreatic tissue, as well as Treg dependence on continuous TCR signaling for their suppressive function (49,76,77). Since Treg suppressive mechanisms depend on cell-to-cell contact or close proximity to target cells, Tregs need to home to the appropriate tissue niche to exert their function. These observations suggest that therapeutic approaches focused on pancreatic antigen-specific Tregs will provide a more efficacious result. Therefore, significant effort has been applied to develop protocols for generation of antigen-specific Tregs.
DCs are central in the generation of thymically derived Foxp3+ Tregs, which maintain dominant self-tolerance (78,79). Indeed, studies have shown a correlation between a reduction in DC numbers and residual β-cell function in T1D subjects (80), while in the NOD mouse there is an overall reduction in DCs (81,82), suggesting a relationship between self-tolerance and the absolute number of DCs. Supplementing DCs has been shown to have beneficial effects on diabetes in part through the expansion of Tregs (83). Generation of thymic regulatory T cells by antigen-presenting mTECs and DCs early in life (neonatal) is critical for maintaining life-long tolerance to self (84,85). However, not all peripheral antigens are expressed by mTECs, and therefore negative selection must also rely on peripheral antigen retrieval and delivery to the thymus by DCs. For instance, DEC205+ pDCs from the periphery can migrate to the thymus to present antigen (79,86), and both CD8+ DCs and pDCs express the Clec9a C-type Lectin-like receptor (87,88). DEC205 and Clec9a are internalized upon binding their ligands, and are targeted intracellularly to endocytic vesicles, feeding directly into the antigen processing and presentation pathway (86,87). It is possible these endocytic receptors could be therapeutically targeted by fusion antibodies to generate thymic or peripheral Tregs. Indeed, DCs targeted with anti-DEC205 fused to a strong agonist insulin mimotope prevented the onset of T1D in mice, in part because of the generation of Foxp3+ Treg cells (89). However, it is still unknown to what extent peptide-bearing APCs can influence the development of autoreactive T cells and protective T regulatory cells in the thymus and the periphery. This new area of investigation is allowing for exciting opportunities to study the development of Tregs specific to β-cell native antigens and posttranslationally modified neo-antigens.
Several approaches could be considered to redirect polyclonal Treg population to the correct anatomical location. Of these, the chimeric antigen receptor (CAR) approach might be most promising since it can be used “off the shelf,” does not depend on the HLA haplotype of the recipient, and has the potential for high specificity. The advent of CAR T cells has been one of the major breakthroughs in anticancer immunotherapy, and these successes have propelled the research for their use in treatment of autoimmunity. The CAR consists of an extracellular domain, typically a single-chain antibody variable fragment (scFV), which recognizes the antigen of interest. The intracellular portion is more akin to the TCR signaling motifs, and often contain the signal 1-CD3 ζ chain and its 3-immunoreceptor tyrosine–based activation motifs and signal 2-costimulatory CD28 or 4-1BB domains. Utilization of CARs specific for tissue antigens has become an area of interest to redirect exogenous Tregs to the target tissue in GVHD and autoimmunity, including T1D (68,90). Autoantigen-specific CAR-Tregs are capable of specific and bystander suppression of pathogenic T cells at the targeted site (31,32,91). However, optimizing CAR-Tregs is still an ongoing process, especially as it pertains to Treg stability and longevity. Because the generation of CAR-Tregs requires significant manipulation, further exacerbated by robust CAR signaling in vivo, there is an increased chance of terminal differentiation, loss of longevity, and Treg exhaustion (68,92). While the switch from CD28 to 4-1BB domain has improved conventional CAR T-cell longevity, CD28 is an important costimulus for Tregs, and 4-1BB domain does not seem to be functional in CAR-Tregs (93). Further CAR signaling modifications will be necessary to balance increased CAR-Treg function with CAR-Treg longevity. It is possible that downstream signaling domains of costimulatory molecules associated with Treg function could serve as candidates in CAR modification.
Selective Approach to Treg Therapy
It is now well appreciated that T1D is a heterogeneous disease that varies in the age of onset, rate of progression, environmental triggers, and primary antigens, among other distinctions (94). It is generally accepted that immunomodulatory therapies have a better chance of making a difference at an early stage post-diagnosis, when there is still sufficient residual β-cell mass that can recover and provide at least partial insulin independence. However, whether Treg therapy alone or in combination with another approach would be applicable in all cases is currently unclear. Current and future clinical trials utilizing adoptive Treg therapy could benefit from incorporation of known Treg biomarkers or susceptible SNP analyses, such as IL2RA SNPs (95), to assess whether Treg functionality prior to treatment can be used to stratify responders and nonresponders. Additionally, antigen-specific therapies including low-dose tolerance induction or high-dose peripheral deletion of antigen-specific T cells could be performed in concordance with islet autoantibody positivity (96,97). Treg therapy may also be beneficial in situations where there is no specific defect identified in the Treg population (98). Expansion of the functional Treg population or increasing Treg functional potential could sway the balance toward tolerance irrespective of the underlying immune dysfunction that triggered autoimmunity.
Conclusion
Results obtained from genome-wide association studies and transcriptional and functional studies converge on the conclusion that alterations in Foxp3+ Tregs is a key immunological defect in T1D. Based on our current understanding of disease etiology, Treg-based therapies could rebalance the system and reverse autoimmunity. However, the complexity of Treg functions and limited information about tissue-infiltrating Tregs in T1D are puzzles that need to be solved for Treg therapy to reach its full potential. Future studies focused on disease-specific anatomical sites will be able to unequivocally address suspected Treg failure in pancreatic autoimmunity and draw the connections between global changes in Treg function to tissue-specific effects. Such studies are currently underway using samples collected by the Network of the Pancreatic Organ Donors with Diabetes program (nPOD) and will inevitably provide important insights for the role of Treg failure in T1D.
Article Information
Funding. This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases 1R01-DK-19352-01 (M.L.B.), the National Institute of Allergy and Infectious Diseases 1R01-AI-125301 (M.B.) and 7R01-AI-136963 (M.L.B.), and JDRF 1-INO-2020-920-A-N (M.L.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1. Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest 2002;109:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TIM. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes 2005;54:92–99 [DOI] [PubMed] [Google Scholar]
- 3. Brusko T, Wasserfall C, McGrail K, et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes 2007;56:604–612 [DOI] [PubMed] [Google Scholar]
- 4. Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 [DOI] [PubMed] [Google Scholar]
- 5. Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia 2017;60:1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med 2007;204:2039–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuster C, Jonas F, Zhao F, Kissler S. Peripherally induced regulatory T cells contribute to the control of autoimmune diabetes in the NOD mouse model. Eur J Immunol 2018;48:1211–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med 2012;209:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holohan DR, Van Gool F, Bluestone JA. Thymically-derived Foxp3+ regulatory T cells are the primary regulators of type 1 diabetes in the non-obese diabetic mouse model. PLoS One 2019;14:e0217728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bettini ML, Vignali DAA. Development of thymically derived natural regulatory T cells. Ann N Y Acad Sci 2010;1183:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol 2016;37:803–811 [DOI] [PubMed] [Google Scholar]
- 12. Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004;113:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanda S, Roep BO, von Herrath M. Islet antigen specific IL-10+ immune responses but not CD4+CD25+FoxP3+ cells at diagnosis predict glycemic control in type 1 diabetes. Clin Immunol 2008;127:138–143 [DOI] [PubMed] [Google Scholar]
- 14. Clemente-Casares X, Blanco J, Ambalavanan P, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016;530:434–440 [DOI] [PubMed] [Google Scholar]
- 15. Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med 2004;200:1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng MH, Shum AK, Anderson MS. What’s new in the Aire? Trends Immunol 2007;28:321–327 [DOI] [PubMed] [Google Scholar]
- 17. Yu W, Jiang N, Ebert PJR, et al. Clonal deletion prunes but does not eliminate self-specific αβ CD8+ T lymphocytes. Immunity 2015;42:929–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGinty JW, Chow I-T, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes 2014;63:3033–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bettini M, Scavuzzo MA, Liu B, et al. A critical insulin TCR contact residue selects high-affinity and pathogenic insulin-specific T cells. Diabetes 2020;69:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee T, Sprouse ML, Banerjee P, Bettini M, Bettini ML. Ectopic expression of self-antigen drives regulatory T cell development and not deletion of autoimmune T cells. J Immunol 2017;199:2270–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest 2005;115:2268–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol 2001;2:501–507 [DOI] [PubMed] [Google Scholar]
- 23. Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997;15:289–292 [DOI] [PubMed] [Google Scholar]
- 24. Baker RL, Jamison BL, Wiles TA, et al. CD4 T cells reactive to hybrid insulin peptides Are indicators of disease activity in the NOD mouse. Diabetes 2018;67:1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu B, Hood JD, Kolawole EM, et al. A hybrid insulin epitope maintains high 2D affinity for diabetogenic T cells in the periphery. Diabetes 2020;69:381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 2009;10:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cipolletta D, Feuerer M, Li A, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012;486:549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan TG, Mathis D, Benoist C. Singular role for T-BET+CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc Natl Acad Sci USA 2016;113:14103–14108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood 2005;105:2220–2226 [DOI] [PubMed] [Google Scholar]
- 30. Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood 2005;106:3300–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 2004;199:1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004;199:1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall AO, Beiting DP, Tato C, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 2012;37:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Overacre-Delgoffe AE, Chikina M, Dadey RE, et al. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell 2017;169:1130–1141.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang N, Schröppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009;30:458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sprouse ML, Scavuzzo MA, Blum S, et al. High self-reactivity drives T-bet and potentiates Treg function in tissue-specific autoimmunity. JCI Insight 2018;3:e97322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li C, DiSpirito JR, Zemmour D, et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-Treg phenotype. Cell 2018;174:285–299.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miragaia RJ, Gomes T, Chomka A, et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 2019;50:493–504.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pesenacker AM, Wang AY, Singh A, et al. A regulatory T-cell gene signature is a specific and sensitive biomarker to identify children with new-onset type 1 diabetes. Diabetes 2016;65:1031–1039 [DOI] [PubMed] [Google Scholar]
- 40. Pesenacker AM, Chen V, Gillies J, et al. Treg gene signatures predict and measure type 1 diabetes trajectory. JCI Insight 2019;4:123879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol 2009;21:612–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature 2015;528:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalekar LA, Cohen JN, Prevel N, et al. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Sci Immunol 2019;4:eaaw2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen J, Ganguly A, Mucsi AD, et al. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. J Exp Med 2017;214:327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akkaya B, Oya Y, Akkaya M, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol 2019;20:218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004;21:589–601 [DOI] [PubMed] [Google Scholar]
- 47. Gondek DC, Lu L-F, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 2005;174:1783–1786 [DOI] [PubMed] [Google Scholar]
- 48. Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 2007;27:635–646 [DOI] [PubMed] [Google Scholar]
- 49. Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol 2014;15:1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bakay M, Pandey R, Hakonarson H. Genes involved in type 1 diabetes: an update. Genes (Basel) 2013;4:499–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Todd JA, Walker NM, Cooper JD, et al. Genetics of Type 1 Diabetes in Finland; Wellcome Trust Case Control Consortium. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007;39:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Long SA, Cerosaletti K, Wan JY, et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4+ T cells. Genes Immun 2011;12:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garg G, Tyler JR, Yang JHM, et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol 2012;188:4644–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang JHM, Cutler AJ, Ferreira RC, et al. Natural variation in interleukin-2 sensitivity influences regulatory T-cell frequency and function in individuals with long-standing type 1 diabetes. Diabetes 2015;64:3891–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Long SA, Rieck M, Sanda S, et al.; Diabetes TrialNet and the Immune Tolerance Network . Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 2012;61:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sockolosky JT, Trotta E, Parisi G, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 2018;359:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trotta E, Bessette PH, Silveria SL, et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med 2018;24:1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ward NC, Lui JB, Hernandez R, et al. Persistent IL-2 receptor signaling by IL-2/CD25 fusion protein controls diabetes in NOD mice by multiple mechanisms. Diabetes 2020;69:2400–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ohkura N, Yasumizu Y, Kitagawa Y, et al. Regulatory T cell-specific epigenomic region variants are a key determinant of susceptibility to common autoimmune diseases. Immunity 2020;52:1119–1132.e4 [DOI] [PubMed] [Google Scholar]
- 60. McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 2011;186:3918–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Okubo Y, Torrey H, Butterworth J, Zheng H, Faustman DL. Treg activation defect in type 1 diabetes: correction with TNFR2 agonism. Clin Transl Immunology 2016;5:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008;28:687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bettini ML, Pan F, Bettini M, et al. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity 2012;36:717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hundhausen C, Roth A, Whalen E, et al. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med 2016;8:356ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009;10:1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferreira C, Palmer D, Blake K, Garden OA, Dyson J. Reduced regulatory T cell diversity in NOD mice is linked to early events in the thymus. J Immunol 2014;192:4145–4152 [DOI] [PubMed] [Google Scholar]
- 67. Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Raffin C, Vo LT, Bluestone JA. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020;20:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014;20:62–68 [DOI] [PubMed] [Google Scholar]
- 70. Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013;39:949–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Trzonkowski P, Bieniaszewska M, Juścińska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol 2009;133:22–26 [DOI] [PubMed] [Google Scholar]
- 72. Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011;117:1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Motwani K, Peters LD, Vliegen WH, et al. Human regulatory T cells from umbilical cord blood display increased repertoire diversity and lineage stability relative to adult peripheral blood. Front Immunol, 15 April 2020 Available from 10.3389/fimmu.2020.00611 [DOI] [PMC free article] [PubMed]
- 74. Manirarora JN, Wei C-H. Combination therapy using IL-2/IL-2 monoclonal antibody complexes, rapamycin, and islet autoantigen peptides increases regulatory T cell frequency and protects against spontaneous and induced type 1 diabetes in nonobese diabetic mice. J Immunol 2015;195:5203–5214 [DOI] [PubMed] [Google Scholar]
- 75. Karakus U, Sahin D, Mittl PRE, Mooij P, Koopman G, Boyman O. Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species. Sci Transl Med 2020;12:eabb9283. [DOI] [PubMed] [Google Scholar]
- 76. Lennon GP, Bettini M, Burton AR, et al. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity 2009;31:643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang J, Tsai S, Han B, Tailor P, Santamaria P. Autoantigen recognition is required for recruitment of IGRP206–214-autoreactive CD8+ T cells but is dispensable for tolerance. J Immunol 2012;189:2975–2984 [DOI] [PubMed] [Google Scholar]
- 78. Lin J, Yang L, Silva HM, et al. Increased generation of Foxp3+ regulatory T cells by manipulating antigen presentation in the thymus. Nat Commun 2016;7:10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hadeiba H, Lahl K, Edalati A, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity 2012;36:438–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Galgani M, Nugnes R, Bruzzese D, et al. Meta-immunological profiling of children with type 1 diabetes identifies new biomarkers to monitor disease progression. Diabetes 2013;62:2481–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Prasad SJ, Goodnow CC. Cell-intrinsic effects of non-MHC NOD genes on dendritic cell generation in vivo. Int Immunol 2002;14:677–684 [DOI] [PubMed] [Google Scholar]
- 82. Feili-Hariri M, Morel PA. Phenotypic and functional characteristics of BM-derived DC from NOD and non-diabetes-prone strains. Clin Immunol 2001;98:133–142 [DOI] [PubMed] [Google Scholar]
- 83. Lo J, Xia C-Q, Peng R, Clare-Salzler MJ. Immature dendritic cell therapy confers durable immune modulation in an antigen-dependent and antigen-independent manner in nonobese diabetic mice. J Immunol Res 2018;2018:5463879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 2015;348:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Scharschmidt TC, Vasquez KS, Truong H-A, et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. immunity 2015;43:1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tel J, Benitez-Ribas D, Hoosemans S, et al. DEC-205 mediates antigen uptake and presentation by both resting and activated human plasmacytoid dendritic cells. Eur J Immunol 2011;41:1014–1023 [DOI] [PubMed] [Google Scholar]
- 87. Caminschi I, Proietto AI, Ahmet F, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 2008;112:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem 2008;283:16693–16701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med 2011;208:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mohseni YR, Tung SL, Dudreuilh C, Lechler RI, Fruhwirth GO, Lombardi G. The future of regulatory T cell therapy: promises and challenges of implementing CAR technology. Front Immunol 2020;11:1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wright GP, Notley CA, Xue S-A, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S A 2009;106:19078–19083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fritsche E, Volk H-D, Reinke P, Abou-El-Enein M. Toward an optimized process for clinical manufacturing of CAR-Treg cell therapy. Trends Biotechnol 2020;38:1099–1112 [DOI] [PubMed] [Google Scholar]
- 93. Boroughs AC, Larson RC, Choi BD, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 2019;5:126194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol 2019;15:635–650 [DOI] [PubMed] [Google Scholar]
- 95. Marwaha AK, Panagiotopoulos C, Biggs CM, et al. Pre-diagnostic genotyping identifies T1D subjects with impaired Treg IL-2 signaling and an elevated proportion of FOXP3+IL-17+ cells. Genes Immun 2017;18:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007;148:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med 2012;2:a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Visperas A, Vignali DAA. Are regulatory T cells defective in type 1 diabetes and can we fix them? J Immunol 2016;197:3762–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]