Abstract
Background
Osteoarthritis (OA) is the most common form of arthritis. Published guidelines and expert opinion are divided over the relative role of acetaminophen (also called paracetamol or Tylenol) and non‐steroidal anti‐inflammatory drugs (NSAIDs) as first‐line pharmacologic therapy. The comparative safety of acetaminophen and NSAIDs is also important to consider. This update to the original 2003 review includes nine additional RCTs.
Objectives
To assess the efficacy and safety of acetaminophen versus placebo and versus NSAIDs (ibuprofen, diclofenac, arthrotec, celecoxib, naproxen, rofecoxib) for treating OA.
Search methods
We searched MEDLINE (up to July 2005), EMBASE (2002‐July 2005), Cochrane Central Register of Controlled Trials (CENTRAL), ACP Journal Club, DARE, Cochrane Database of Systematic Reviews (all from 1994 to July 2005). Reference lists of identified RCTs and pertinent review articles were also hand searched.
Selection criteria
Published randomized controlled trials (RCTs) evaluating the efficacy and safety of acetaminophen alone in OA were considered for inclusion.
Data collection and analysis
Pain, physical function and global assessment outcomes were reported. Results for continuous outcome measures were expressed as standardized mean differences (SMD). Dichotomous outcome measures were pooled using relative risk (RR) and the number needed to treat (NNT) was calculated.
Main results
Fifteen RCTs involving 5986 participants were included in this review. Seven RCTs compared acetaminophen to placebo and ten RCTs compared acetaminophen to NSAIDs. In the placebo‐controlled RCTs, acetaminophen was superior to placebo in five of the seven RCTs and had a similar safety profile. Compared to placebo, a pooled analysis of five trials of overall pain using multiple methods demonstrated a statistically significant reduction in pain (SMD ‐0.13, 95% CI ‐0.22 to ‐0.04), which is of questionable clinical significance. The relative percent improvement from baseline was 5% with an absolute change of 4 points on a 0 to 100 scale. The NNT to achieve an improvement in pain ranged from 4 to 16. In the comparator‐controlled RCTs, acetaminophen was less effective overall than NSAIDs in terms of pain reduction, global assessments and in terms of improvements in functional status. No significant difference was found overall between the safety of acetaminophen and NSAIDs, although patients taking traditional NSAIDS were more likely to experience an adverse GI event (RR 1.47, (95% CI 1.08 to 2.00). 19% of patients in the traditional NSAID group versus 13% in the acetaminophen group experienced an adverse GI event. However, the median trial duration was only 6 weeks and it is difficult to assess adverse outcomes in a relatively short time period.
Authors' conclusions
The evidence to date suggests that NSAIDs are superior to acetaminophen for improving knee and hip pain in people with OA. The size of the treatment effect was modest, and the median trial duration was only six weeks, therefore, additional considerations need to be factored in when making the decision between using acetaminophen or NSAIDs. In OA subjects with moderate‐to‐severe levels of pain, NSAIDs appear to be more effective than acetaminophen.
Keywords: Humans; Acetaminophen; Acetaminophen/adverse effects; Acetaminophen/therapeutic use; Analgesics, Non‐Narcotic; Analgesics, Non‐Narcotic/adverse effects; Analgesics, Non‐Narcotic/therapeutic use; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/adverse effects; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Osteoarthritis; Osteoarthritis/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Acetaminophen for osteoarthritis
How well does acetaminophen work and compare to anti‐inflammatories to treat osteoarthritis and is it safe?
Fifteen studies of moderate to high quality were reviewed and provide the best evidence we have today. The studies tested almost 6000 people with osteoarthritis of the hip or knee. The studies compared people who took 4000 mg of acetaminophen (Tylenol, Paracetamol) a day to people who took a placebo (fake pill) or non‐steroidal anti‐inflammatory drugs (NSAIDs). Most studies lasted on average about 6 weeks. What is osteoarthritis and what drugs are used to treat it? Osteoarthritis (OA) is the most common form of arthritis that can affect the hands, hips, shoulders and knees. In OA, the cartilage that protects the ends of the bones breaks down and causes pain and swelling. There are two main types of drug treatments in OA: acetaminophen which is used to relieve pain but does not affect swelling; and NSAIDs, such as ibuprofen, diclofenac and cox IIs (celecoxib), which are used to decrease pain and swelling. It is not clear which type is best to use or which causes more side effects: high doses of acetaminophen may cause stomach problems, such as ulcers, and NSAIDs may cause stomach, kidney or heart problems. What did the studies show? Acetaminophen compared to placebo The studies show that people who took acetaminophen has less pain (when resting, moving, sleeping and overall) and felt better overall than people who took a placebo. Pain (when measured on a different scale), physical function and stiffness were about the same. • Pain decreased by 4 more points on a scale of 0‐100 for people who took acetaminophen instead of a placebo.
Background
Osteoarthritis (OA) is the result of a variety of patterns of joint failure, characterized by degeneration of articular cartilage, and simultaneous proliferation of new bone, cartilage, and connective tissue. OA is the most common form of arthritis, and it is often associated with significant disability and an impaired quality of life (Bradley 1991a, MMWR 1994, Scott 1993, Towheed 1998). An estimated 12.1% of Americans age 25 and older (nearly 21 million persons in 1990) have clinical signs and symptoms of OA (Lawrence 1998). Among American adults 30 years of age or older, symptomatic disease in the knee occurs in approximately 6% and symptomatic hip OA occurs in roughly 3% (Felson 1998, Hochberg 2000). OA of the hip and knee can be especially disabling to lower extremity functioning because the hip and knee are large weight‐bearing joints (Liang 1984). For example, OA accounts for more trouble with climbing stairs and walking than any other disease, especially in the elderly population (Guccione 1994). Advanced OA of the hip and knee is the most common reason for elective joint replacement (Hochberg 1996).
Although there are no curative therapies currently available for OA, individualized treatment programs are available to help relieve pain and stiffness, and to maintain and/or improve functional status (ACR 2000, Pendleton 2000, Tannenbaum 2000). Non‐pharmacological treatment, including physiotherapy, occupational therapy, weight loss and exercise, is currently the first line of treatment and often is successful. However, in many patients, these treatments are not sufficient and pharmacological therapy is required. Published systematic reviews and meta‐analyses have confirmed that the following pharmacological therapies have efficacy in the management of OA (Towheed 2002, Towheed 2002b): acetaminophen, non‐steroidal anti‐inflammatory drugs (NSAIDs), COX‐2 selective NSAIDs, topical capsaicin, topical NSAIDs, and chondroitin sulfate. A recent update of a systematic review of glucosamine failed to show benefit of glucosamine for pain and WOMAC function; however, the Rotta preparation did show benefit in improving pain and function compared to placebo (Towheed 2005).
Acetaminophen is a simple analgesic that has both analgesic and antipyretic actions (Clissold 1986). Acetaminophen is not generally considered to have potent anti‐inflammatory effects. In part, this can be explained on the basis that acetaminophen is a weak inhibitor of both cyclooxygenase (COX) isoenzymes, COX‐1 and COX‐2 (Warner 1999). Recent data suggests that acetaminophen selectively inhibits COX‐3, a variant of COX‐1, which is different from COX‐1 or COX‐2 (Swierkosz 2002, Chandrasekharan 2002, Graham 2005). Brand names for acetaminophen or paracetamol include Tylenol and Panadol.
The relative role of simple analgesics (acetaminophen or paracetamol) versus NSAIDs in the medical management of OA has been debated in recent years in the medical community (Brandt 2001, Brandt 2002, Courtney 2002, Felson 2001, Gotzsche 2000, Moskowitz 2001, Nikles 2005, Jawad 2005). Part of this debate stems from the fact that the pathogenesis of OA is complex and not well understood. For example, why a patient who has radiographic OA may or may not have pain and/or functional limitation is still unknown. In fact, the exact cause(s) of pain in OA is not well understood. These observations have suggested to some that OA is a mechanical pain syndrome, which unlike the case in rheumatoid arthritis (RA), may not necessarily benefit more by using NSAIDs than simple analgesics, such as acetaminophen. The 1996 ACR treatment guidelines for OA had also recommended acetaminophen as first line pharmacological therapy for OA. However, this recommendation was modified in the updated 2000 guidelines, which suggested that NSAIDs could also reasonably be considered as first line therapy for OA, especially in the patient with moderate to severe levels of pain. The change in the recommendation was based in part, on new evidence which suggested that NSAIDs were actually superior to acetaminophen in the treatment of OA related pain (Hochberg 2001b, Shamoon 2000,Shamoon 2001). The addition of the COX‐2 selective NSAIDs has also contributed to this change in recommendation.
Since the publication of the updated American College of Rheumatology (ACR) OA guidelines, considerable debate and controversy has been generated in the scientific literature (Hochberg 2001b). For example, a series of editorials in The Journal of Rheumatology outlined the key issues of this debate as presented by two opinion leaders in the field of OA (Brandt 2001,Moskowitz 2001). This review was conducted because of the great importance of the question as to what are the relative merits of acetaminophen versus NSAIDs, especially when being considered as an initial drug to treat the pain of OA. Our updated results will be compared with the results of four other published systematic reviews evaluating the same question (Eccles 1998, Lee 2004, Zhang 2004, Wegman 2004).
Objectives
1) To assess the efficacy and safety of acetaminophen (or paracetamol) versus placebo for treating participants with OA.
2) To assess the efficacy and safety of acetaminophen (or paracetamol) versus NSAIDs (including ibuprofen, diclofenac, arthrotec, celecoxib, naproxen, rofecoxib) for treating participants with OA.
Methods
Criteria for considering studies for this review
Types of studies
Two levels of criteria were used to identify all relevant studies for this review. The first criterion was used to screen all citations that involve acetaminophen (or paracetamol) in the management of OA. The second criterion was used to identify those studies that met the following additional requirements: 1) RCTs evaluating the efficacy and toxicity of acetaminophen in OA, 2) Both placebo‐based and/or comparative trials were eligible. However, the only comparative trials considered were those that directly compared acetaminophen to NSAIDs, 3) Both single blinded and double blinded trials were eligible. No unpublished studies were sought for this edition of the review.
Types of participants
All adults (age 18 years and older) with a diagnosis of either primary or secondary OA at any site, including the axial and peripheral skeleton. Primary OA is any OA where a definite etiology (cause) is not found. Secondary OA is where a definite cause can be found; for example trauma, obesity or hypermobility.
Types of interventions
Only studies that evaluated the efficacy and/or safety of acetaminophen were eligible. Two types of RCTs were specifically searched for: 1) RCTs that compared acetaminophen alone to placebo, and 2) RCTs that compared acetaminophen alone to NSAIDs.
Types of outcome measures
The four main outcome measures were chosen based on the core set of disease activity measures for OA clinical trials recommended by OMERACT (Outcome Measures in Rheumatology Clinical Trials). This core set of outcomes have been endorsed by expert committees (Altman 1996, Bellamy 1997). The main outcome measures reported in the included studies were pain (at rest, on motion, and using standardized, validated instruments, such as the Western Ontario and McMaster Universities osteoarthritis index (WOMAC) and the and Health Assessment Questionnaire (HAQ), functional assessments (50 foot walk time, WOMAC, HAQ), patient global assessments, and physician global assessments.
Efficacy Measures: Four main outcome measures: 1) Pain 2) Physical function ‐ both self‐reported measures of functional limitation (e.g. WOMAC, HAQ) and performance‐based measures of function (e.g., 50‐foot walk time) were included in this review 3) Patient global assessment 4) Functional assessment
The WOMAC is a questionnaire designed to assess OA related disability in the hip and/or knee. The HAQ is a self‐reported functional status (disability) measure used in many disease areas, including arthritis.
Safety Measures: Data were sought for: 1) Total number of withdrawals due to adverse events (related to safety) 2) Total number of patients experiencing an adverse event in each treatment group 3) GI adverse events ‐ both a) total number of withdrawals due to GI adverse events and b) total number of patients experiencing an adverse GI event
Search methods for identification of studies
See: Collaborative Review Group Search Strategy.
Our previous systematic reviews evaluating pharmacological therapies for OA of the hip and knee were used to identify all relevant citations from the period of 1966 up to August 1994 (Towheed 1997a, Towheed 1997b). These reviews involved a very extensive MEDLINE search strategy and a thorough review of all pertinent articles.
In addition, for this 2005 version of the systematic review, an updated MEDLINE search including the years 1993 to July 2005 (inclusive) was performed to identify additional relevant citations (see below). MEDLINE Daily Update was searched as of July 19, 2005 for the year 2005. MEDLINE In‐Process and Other Non‐Indexed Citations was searched as of July 19, 2005 for the year 2005. Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, DARE, and the Cochrane Central Register of Controlled Trials Register (CENTRAL) was searched from 1994 and July 2005. EMBASE was searched from 2002 to July 2005. All retrieved citations were manually searched for additional references. The titles and abstracts identified by the searches were assessed, using an over inclusive approach, so as not to miss potentially relevant studies. See Appendix 1 for the full search strategy applied in MEDLINE/EMBASE/CDSR/ACP Journal Club/DARE/CENTRAL.
Data collection and analysis
Study identification (identifying citations that involve acetaminophen in the therapy of OA). Two reviewers (TT and MJ or LM) used the screening criteria to review all identified citations independently. All citations identified by either reviewer were retrieved and analysed for suitability. Authors of abstracts were contacted requesting the full manuscript, including the raw and final data incorporating the results. Authors of full publications were contacted to obtain statistical data necessary to perform meta‐analyses, when the data was not published in the trial report.
Study Selection (screening identified citations to see if they meet our additional criteria). Two reviewers (TT and MJ or LM) reviewed each relevant citation independently to see if it met the selection criteria described previously. At this stage, an emphasis was placed on selecting RCTs, and excluding non‐randomized trials. If the randomization status was not clear, the article was withheld, pending clarification from the principal author. In situations where authors were not available, a consensus was reached amongst the reviewers.
Data Extraction Two reviewers (TT and MJ or LM or MC) independently reviewed each RCT and extracted the trial raw data by using a standardized form. If outcome data were not reported in a form suitable for quantitative pooling in a meta‐analysis, the primary author was contacted for access to this information. For example, in order to calculate effect sizes using continuous data, the baseline means and standard deviations of the outcome variables are necessary. Data on adverse effects were also extracted from the RCTs.
Data Analysis For the quantitative outcome data, standardized mean differences (SMD) were used to pool across RCTs (Hedges 1985). The Hedges method of calculating effect size (standardized mean difference (SMD) with its 95% confidence interval) was used in this review. It is important to note that for the SMD calculations, we used the end of study data for the means and standard deviations. In the event that this was not available in the trial report, we made an attempt to contact the authors of the study for this missing information. For categorical outcome data, relative risks (RR) were calculated. To calculate clinical improvement, the NNT and NNH were calculated for dichotomous outcomes. NNT for continuous outcomes was calculated using Wells calculator ( Norman 2001;Tugwell 2004). Heterogeneity was tested with a chi‐square test. Fixed effects models were used unless heterogeneity was statistically significant, in which case the random effects model was used. Where possible, data from an intention‐to‐treat analysis were extracted.
For the qualitative review of the studies, the Jadad and Schultz assessment tools (Jadad 1996; Schulz 1995a; Schulz 1995b) were used to evaluate the quality of the RCT. This scores the trial on the basis of randomization, adequate concealment of randomization, degree of blinding, use of intention to treat analyses and description of withdrawals and dropouts. The scoring system is as follows: a report of randomization receives one point and an additional point is given if an appropriate method of randomization was described. A report of double‐blinding receives one point and if an appropriate description of the method of double‐blinding is provided an additional point is given. However, if the method is inappropriate then a point is deducted. A report of the number and reason for withdrawals and dropouts receives one point and no point if no statement is provided. Allocation concealment was assessed and rated as A (adequate or blind randomization) or B (inadequate and/or unclear method of randomization). Quality was assessed independently by two reviewers (TT and MJ or LM or MC) and any differences were resolved by consensus.
The two main comparisons of interest were acetaminophen vs placebo and acetaminophen vs NSAIDs. Several of the included studies in this systematic review were multi‐arm studies which included more than one type of NSAID compared to acetaminophen. To avoid double counting acetaminophen as the comparison group and to facilitate the comparison of NSAIDs to acetaminophen the following groupings were considered in the analysis: 1) NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs acetaminophen; 2) Acetaminophen vs placebo; 3) NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 25 mg, naproxen) vs acetaminophen; 4) NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs acetaminophen. All available data for these comparisons were entered into the table of comparisons and results were pooled where appropriate.
Results
Description of studies
Fifteen RCTs involving 5986 subjects are included in this review and are described in the table of included studies. Seven RCTs compared acetaminophen to placebo (Amadio 1983, ; Case 2003; Golden 2004; Miceli‐Richard 2004; Pincus a 2004; Pincus b 2004, Zoppi 1995). Ten RCTs involved a comparison of acetaminophen to an NSAID. Two RCTs were only reported in abstract form (Altman 1999; Shen 2004). The mean duration of the RCTs was 13.1 weeks (study duration ranged from 1 week to 104 weeks with a median of 6 weeks). However, when one removes the outlier study by Williams, which was 104 weeks, the mean trial duration was only 6.6 weeks. The mean age of the participants randomized was 62.2 years (69% female and 31% male). The mean number of participants randomized was 399, and the mean number completing the trials was 315 (79% of those randomized completed the trials). Eleven RCTs were performed in the United States. Two RCTs were performed in France, one RCT was performed in Italy and one RCT was performed in Switzerland. The oral dosage of acetaminophen that was used in 12 of the RCTs was 1000 mg four times daily. One trial used a lower dosage of acetaminophen (650 mg four times daily). Two RCTs used a dosage of 1000 mg three times daily Three RCTs clearly specified that only participants with primary OA were enrolled, one RCT enrolled both primary and secondary OA participants, and in 11 RCTs this was not specified. The knee joint was studied in all 15 RCTs, and five RCTs also included participants with OA of the hip. No other site of OA was studied in these trials such as the hand or spine. Only 4 RCTs used a standardized and validated classification of OA as a part of the inclusion criteria (Altman 1986). Eleven RCTs performed x‐rays of the target joints at baseline. Eleven RCTs were of parallel group design and four RCTs were of cross‐over design. Ten RCTs had some form of involvement with a pharmaceutical company.
Amadio 1983 reported a 6 week cross‐over, double‐blind RCT comparing acetaminophen (dosage of 1000 mg four times per day) to placebo in 25 participants with OA of the knee. All efficacy outcomes showed marked and consistent superiority of acetaminophen over placebo. Adverse effects in both groups were clinically insignificant and in no case required discontinuation of the drug.
Bradley 1991a and Bradley 1991b both represent the same study by Bradley in 1991. Bradley 1991a includes an ibuprofen 1200 mg group and Bradley 1991b represents an ibuprofen 2400 mg group. Bradley 1991b conducted the first RCT directly comparing acetaminophen to an NSAID (ibuprofen). This was a 4 week, parallel‐group, double‐blind RCT comparing acetaminophen (dosage of 1000 mg four times per day) with an analgesic dose of ibuprofen (1200 mg/day ‐ Bradley 1991a) and with an anti‐inflammatory dose of ibuprofen (2400 mg/day ‐ Bradley 1991b) in 184 participants with OA of the knee. All efficacy outcomes in the 3 groups were similar at the end of the trial, with the exception of rest pain, which favoured the NSAID groups over acetaminophen. Adverse effects were minor and similar in all 3 groups.
Williams 1993 reported a 2 year parallel group, double‐blind RCT comparing acetaminophen (650 mg four times per day) to naproxen (375 mg twice daily) in 178 participants with OA of the knee. The purpose of this study was to evaluate the two treatments in terms of radiographic progression and withdrawal from the trial due to lack of efficacy. There was a large number of drop outs at the two year endpoint (only 35% of those randomized completed the trial). For the purposes of this meta‐analysis, the 6 week efficacy outcome data was used to pool pain and functional status measures, whereas for the toxicity portion, the entire 2 year data was used.
Zoppi 1995 published a 7 day parallel‐group, double‐blind RCT comparing acetaminophen (dosage of 1000 mg three times per day) to placebo in 60 patients with OA of the knee or the hip. Analgesic efficacy was significantly better with acetaminophen versus placebo for the main outcome criteria (daily pain scores and patient global efficacy). At the end of the trial, 64% of the patients wished to continue acetaminophen treatment versus 37% for placebo. Adverse effects in both groups were not significantly different between the acetaminophen and placebo groups.
Altman 1999 was published only in abstract form, with limited information available on all aspects of the study. The study was presented at the American College of Rheumatology (ACR) annual meeting in 1999. The abstract describes a 5 day, parallel group, double‐blind RCT comparing ibuprofen (1200 mg/day), acetaminophen (4000 mg/day) and placebo in 693 patients with moderate OA of the knee. Ibuprofen was better than acetaminophen and both were significantly (p <0.05) better than placebo for reducing knee pain on walking, which was the primary efficacy variable. Adverse effects were uncommon, with fewer gastrointestinal events in the ibuprofen group than with acetaminophen. This RCT has not been published at this time, and efforts to obtain additional more detailed data have not been successful.
Pincus 2001 reported a 12 week, cross‐over, double‐blind RCT comparing acetaminophen (1000 mg four times per day) to arthrotec (75/200 twice daily) in 227 participants with OA of the knee (n=78% of participants) and hip (22% of participants). The NSAID (arthrotec) was significantly superior to acetaminophen in the primary outcomes of the WOMAC and the visual analog pain scale of the MHAQ. Adverse events were more common with arthrotec than with acetaminophen (54% vs 46%, respectively, p=0.046). This study was the first to compare the effects of both agents in the sub‐group of patients with more severe OA at baseline. Differences favouring arthrotec over acetaminophen were greater in patients with severe OA, defined on the basis of higher baseline pain scores, worse radiographic severity, or greater number of involved joints. Participants with milder severity of OA had a similar degree of improvement with both drugs. Participants were more likely to report that arthrotec was better or much better than acetaminophen (57% vs 20%), whereas, 22% of the participants reported no difference between the two drugs.
Geba 2002a, Geba 2002b, Geba 2002c represents a 6 week, parallel group, double‐blind RCT comparing acetaminophen (4000 mg/day), celecoxib (200 mg/day) ‐ Geba 2002c), rofecoxib (12.5 mg/day ‐ Geba 2002a) and rofecoxib (25 mg/day ‐ Geba 2002b) in 382 participants with OA of the knee. This trial is also referred to as the VACT‐1 study. Rofecoxib 25 mg/day was found to be overall superior in efficacy to acetaminophen, celecoxib, and rofecoxib 12.5 mg/day. All treatments were generally safe and well tolerated with a similar percentage of adverse events in all 4 groups. This study did not present efficacy data at baseline in the form of means and standard deviations which made it impossible to include in a meta‐analysis since SMD's could not be calculated. Efforts to obtain this information from the authors have not been successful.
Case and colleagues (Case 2003) published a 12 week, parallel group, double‐blind RCT comparing acetaminophen (4000 mg/day), diclofenac (75 mg twice per day) and placebo in 82 subjects with OA of the knee. At 2 and 12 weeks, clinically and statistically significant improvements were seen in the diclofenac treated group; however, no significant improvements were seen in the acetaminophen treated group. The primary outcome measure was the WOMAC and the secondary outcome measure was the Lequesne Index. The efficacy of acetaminophen was indistinguishable from placebo. This is the first published evidence reporting that the efficacy of acetaminophen is not better than placebo in subjects with OA at any site.
Shen 2004 recently published an abstract of a small (N=20 patients) 3 month RCT evaluating acetaminophen (doses up to 4 gm/day) versus rofecoxib (25 mg/day). This work was also presented at the 2003 EULAR meeting. At the end of 3 months, both acetaminophen and rofecoxib alleviated pain significantly when assessed by the visual analogue scale (VAS). Although the data is not presented in the abstract, there would appear to be no statistically significant differences in improvements in pain at the end of the 3 month trial.
Golden 2004 published pooled results of two identical 7 day, multicenter, double‐blind, parallel group RCTs comparing naproxen sodium (660 mg/day), acetaminophen (4 gm/day) or placebo in 465 patients with OA of the knee. The data from both studies were consistent in showing that naproxen and acetaminophen are superior to placebo in terms of efficacy. The study data also showed that naproxen was more effective than acetaminophen in reducing the symptoms of day and night pain and pain on weight bearing. Naproxen and acetaminophen had similar safety profiles to placebo in both seven day studies. The authors concluded that naproxen is an alternative in the initial treatment of OA and may be preferred to acetaminophen as first‐line therapy in patients with moderate or severe pain.
Miceli‐Richard 2004 published a 6 week, double‐blind, parallel group RCT comparing acetaminophen (4 gm/day) versus placebo in 779 patients with symptomatic OA of the knee. A statistically significant symptomatic effect of oral acetaminophen over placebo was not found. No serious adverse events were attributable to treatment.
Boureau 2004 published a 14 day, double‐blind, parallel group RCT comparing acetaminophen (3 gm/day) versus ibuprofen (1.2 gm/day) in 222 patients with symptomatic OA of the knee or hip. The analgesic efficacy of both single and multiple doses of ibuprofen was compared with that of acetaminophen. Ibuprofen 400 mg at a single and multiple doses (1.2 gm/day) for 14 days is more effective than acetaminophen, either as a single dose of 1000 mg or a multiple dose (3 gm/day). Both agents had similar tolerability. This prompted the authors to conclude that the efficacy/tolerability of ibuprofen is better than that of acetaminophen over a 14 day period.
Pincus 2004 recently published results of two separate double‐blind, placebo controlled, crossover RCTs in patients with OA of the knee or hip (referred to as the PACES‐a and PACES‐b trials). Results from both studies, which were of identical design, were presented separately in the same publication (Pincus a 2004; Pincus b 2004). After wash out of treatment, subjects were randomized to 6 weeks of celecoxib (200 mg/day), acetaminophen (4 gm/day) or placebo. After a second wash out, subjects crossed over to 6 weeks of a second treatment arm. A total of 524 patients were randomized in the PACES‐a trial and 556 patients were randomized in the PACES‐b trial. Celecoxib was more effective than acetaminophen in both periods and in both studies. Acetaminophen was generally more effective than placebo. No clinically or statistically significant differences were seen in adverse events or tolerability among the three treatment groups.
Schnitzer 2005a published the results of the VACT‐2 RCT which compared rofecoxib (12.5 mg/day) versus rofecoxib (25 mg/day), acetaminophen (4000 mg/day), celecoxib (200 mg/day). This was a parallel group, double‐blind RCT which enrolled a total of 1578 patients with symptomatic OA of the knee. The VACT‐2 RCT was conducted to confirm the results of the earlier VACT‐1 RCT (Geba 2002a) in a larger patient population using the same clinical endpoints. Patients were evaluated over days 1 to 6 and at 6 weeks with the patient global response to therapy outcome as well as with the WOMAC. The results showed that rofecoxib and celecoxib provided a superior efficacy to acetaminophen. There was a rapid and greater response with rofecoxib 25 mg/day than celecoxib 200 mg/day. Rofecoxib 12.5 mg/day demonstrated a greater efficacy than celecoxib 200 mg/day over the first 6 days, but was similar over 6 weeks. All study were generally well tolerated with no significant differences between treatment groups in the percentage of patients who experienced a clinical adverse event.
Risk of bias in included studies
Generally, these RCTs were of good methodological quality. The mean overall quality score (excluding Altman's study and Shen's study which were only available in abstract form) was 3.6 out of a possible 5. All RCTs were randomized and fourteen were double‐blinded. Four RCTs had clear evidence for allocation concealment, but in eleven RCTs, either the trials either were inadequate in this regard and/or the information necessary to make this judgement was not available in the trial report. Eleven RCTs either had no withdrawals or used an intention‐to‐treat analysis. Sample size calculations were presented in nine RCTs.
Amadio 1983 study received an overall score of 3/5 (lacking the description of withdrawals and dropouts), and no description was provided in order to evaluate whether the randomization methodology was appropriate in terms of allocation concealment (rating B). The Bradley (Bradley 1991a, Bradley 1991b) study received an overall score of 4/5, with an allocation concealment rating of B. The Williams (Williams 1993) study received an overall score of 5/5, with an allocation concealment rating of B. The Altman (Altman 1999) study received an overall score of 2/5 and an allocation concealment rating of B. However, this study was only reported in abstract form and this precludes a thorough review of its methodological quality. The Zoppi 1995 study received an overall quality score of 2/5, (lacking a description of the method of randomization and lacking a description of withdrawals) an allocation concealment rating of B. The Pincus (Pincus 2001) study received an overall quality score of 4/5, and an excellent allocation concealment rating of A. The Geba 2002a study received an overall quality score of 4/5, and an allocation concealment rating of B. The Case 2003 study received an overall quality score of 3/5, (lacking a description of withdrawals and dropouts) and an allocation concealment rating of B. The abstract by Shen 2004 could not be adequately scored because there was a lack of methodological detail available from the abstract. The Boureau 2004 study received an overall quality score of 4/5, lacking the description of randomization, and an allocation concealment rating of B. The Miceli‐Richard 2004 study received an overall quality score of 3/5, with randomization, double‐blinding and withdrawals and dropouts reported, and an allocation concealment rating of B (unclear). The Golden 2004 study received an overall quality score of 4/5 (lacking a description of the method of randomization) and an allocation concealment rating of B (unclear). The Pincus a 2004 study received an overall quality score of 3/5 (lacking a description of the method of randomization and a statement on withdrawals and dropouts) and an allocation concealment rating of A. The Pincus b 2004 study received an overall quality score of 3/5 for the same reasons as Pincus a 2004 and an allocation concealment rating of A. The Schnitzer 2005a study received an overall quality score of 5/5 and an allocation concealment rating of A.
Effects of interventions
1. ACETAMINOPHEN COMPARED TO PLACEBO
1.1 EFFICACY Seven RCTs compared acetaminophen to placebo (Amadio 1983, Case 2003; Golden 2004; Miceli‐Richard 2004; Pincus a 2004; Pincus b 2004; Zoppi 1995). Five RCTs found that acetaminophen was superior to placebo Amadio 1983; Golden 2004; Pincus a 2004; Pincus b 2004; Zoppi 1995), whereas, two RCTs failed to show a benefit of acetaminophen over placebo (Case 2003; Miceli‐Richard 2004). In the meta‐analyses, acetaminophen was statistically significantly better (P < 0.05) than placebo in the following outcomes: Pain response, pain on motion, physician global assessment, patient global assessment, day pain, modified HAQ pain, night pain and for overall pain (comprising pain as measured by multiple methods). However, there was no difference in efficacy in terms of the WOMAC and Lequesne outcomes. For dichotomous outcomes from one study (Amadio 1983), the NNTs (95% CI ) were: pain response 4 (2, 24), pain on motion 5 (2, 24), physician global assessment 2 (2, 11), patient global assessment 2 (2, 13) [Table 1]. For overall pain from five studies (Case 2003; Golden 2004; Miceli‐Richard 2004; Pincus a 2004; Pincus b 2004), the SMD was ‐0.13 (95% CI, ‐0.22 to ‐0.04) with an NNT of 16.
1. Number needed to benefit (Group2) Acetaminophen vs Placebo (dichotomous; 1 study.
| Outcome | Plac.: % Improvement | Acet.: % Improvement | RR of Impr. w/ Acet | Abs. Risk Reduction% | NNT (95% CI) w/ Ac |
| Rest Pain | 2/22 (4%) | 16/22 (72%) | 8.00 (2.08, 30.73) | 64% (41, 86) | 4 (2,24) |
| Pain on Motion | 4/22 (9%) | 15/22 (66%) | 3.75 (1.48, 9.52) | 50% (25, 75) | 5 (2,24) |
| Physician Global Assessment | 1/21 (5%) | 20/21 (95%) | 20.00 (2.95, 135.76) | 90% (78, 103) | 2 (2,11) |
| Patient Global | 1/19 (5%) | 18/19 (95%) | 18 (2.66, 121.26) | 89% (75, 104) | 2 (2,13) |
1.2 SAFETY For the toxicity outcome of total number of patients reporting any adverse event, the RR comparing acetaminophen to placebo was 1.02 (95% CI, 0.89 to 1.17). For the toxicity outcome of total number of withdrawals due to toxicity, the RR comparing acetaminophen versus placebo was 1.24 (95% CI, 0.87 to 1.77). Thus, there was no significant differences in toxicity between acetaminophen and placebo in these RCTs [Table 2].
2. Number Needed to Harm (Group 2) Acetaminophen (Acet.) vs Placebo (Plac.) (one st.
| Outcome | % w/ Plac. | % w/ Acet. | Acet: RR Outcm (95 | AR Increase (95% CI) | NNH (95% CI) |
| Adverse Events (all clinically insignificant and did not require discontinuation of drug) | 318/1239 26% | 290/1146 25% | 1.02 (0.89, 1.17) | 1% (3,4) | Harm not estabished |
2. ACETAMINOPHEN COMPARED TO NSAIDS 2.1 EFFICACY Twelve RCTs compared acetaminophen to NSAIDs (Altman 1999, Bradley 1991a, Boureau 2004; Bradley 1991b, Case 2003; Geba 2002a, Geba 2002b, Geba 2002c, Golden 2004;Pincus 2001, Pincus a 2004; Pincus b 2004; Schnitzer 2005a; Shen 2004; Williams 1993). Three separate groups of NSAIDS compared acetaminophen are examined in this systematic review (see table of comparisons for more details). NSAIDS that were compared to acetaminophen included groupings of ibuprofen, diclofenac, arthrotec, celecoxib, naproxen and rofecoxib. In the comparator‐controlled RCTs, acetaminophen was less effective overall than NSAIDs in terms of pain reduction, WOMAC pain, stiffness, function, and total scales, global assessments (patient and investigator) and functional status.
Five types of outcome variables were reported in this systematic review : pain, function, patient global assessment, physician global assessment and safety.
Pain Three separate types of pain variables were analyzed in the RCTs including rest pain, pain on motion, and pain as measured by the validated HAQ, WOMAC and Lequesne questionnaires. An overall pain variable comprising pain as measured by multiple methods was also analyzed. NSAIDs were superior to acetaminophen for rest pain, overall pain and HAQ pain. For rest pain, the effect sizes as measured by the standardized mean difference (SMD) were ‐0.20 (95% CI, ‐0.36 to ‐0.03) and ‐0.19 (95%CI, ‐0.35 to ‐0.03). For overall pain, the effect sizes as measured by the SMD were ‐0.25 (95%CI, ‐0.33 to ‐0.17) and ‐0.31 (95% CI, ‐0.40 to ‐0.21). For HAQ pain, the SMD's was ‐0.26 (95% CI, ‐0.45 to ‐0.07). For pain on motion, the SMD's were not statistically significant: 0.04 (95% CI, ‐ 0.20 to 0.28) and ‐0.03 (95% CI, ‐0.27 to 0.21). Both WOMAC pain and WOMAC total outcomes showed that NSAIDs were superior to acetaminophen: for WOMAC pain (SMD = ‐0.24; 95% CI, ‐0.38 to ‐0.09 and SMD=‐0.37; 95% CI, ‐0.50 to ‐0.24), and for WOMAC total (SMD = ‐0.46, 95% CI, ‐0.73 to ‐0.19 and SMD = ‐0.25 (95%CI, ‐0.39 to ‐0.11). The magnitude of these effect sizes correspond to a small to modest treatment effect (Cohen 1988). There were no differences in the Lequesne pain index.
Stiffness The WOMAC stiffness scale showed a superiority of NSAIDs over acetaminophen: (SMD=‐0.20; 95% CI, ‐0.34 to ‐0.05 and SMD=‐0.38; 95%CI, ‐0.51 to ‐0.25).
Physical Function Two separate physical function assessments were analyzed. Fifty foot walk time, a performance‐based measures of function, and function as measured by the validated HAQ, WOMAC and Lequesne questionnaires. Neither the 50' foot walk time, the HAQ or the Lequesne function index scale showed a superiority of NSAIDs over acetaminophen. For the 50 foot walk time, the SMD's were 0.02 (95% CI, ‐0.14 to 0.19) and 0.03 (95% CI, ‐0.14 to 0.19). For the HAQ disability index, the SMD's were ‐0.20 (95% CI, ‐0.41 to 0.01) and ‐0.14 (95% CI, ‐0.36 to 0.07). For the Lequesne function index, the SMD's was ‐0.07 (95% CI,‐0.60 to 0.47). The WOMAC function scale showed a superiority of NSAIDs over acetaminophen: (SMD=‐0.25; 95%CI, ‐0.40 to ‐0.11 and SMD=‐0.40; 95%CI, ‐0.53 to ‐0.27)
Global Assessment Patient global assessment of overall efficacy was measured and reported two ways: one was measured using a continuous scale and the other used a dichotomous rating. Three outcomes showed that NSAIDs were superior to acetaminophen and one did not show any difference in efficacy. The three significant measures in favour of NSAIDs had RR's of 1.23 (95% CI, 1.06 to 1.43), 1.50 (95%CI, 1.27 to 1.76) and 1.44 (95% CI, 1.14 to 1.82).
Similarly, physician global assessment of overall efficacy was also measured and reported using both a continuous measure and a dichotomous measure. One comparison out of the three favoured NSAIDs, whereas the other two did not show a difference in efficacy. In the significant comparison favouring NSAIDs, the SMD was ‐0.33 (95%CI, ‐0.54 to ‐0.12).
2.2 SAFETY Two safety measures were reported separately and compared the safety of NSAIDs versus acetaminophen (total number of withdrawals due to adverse events and the total number of patients experiencing any adverse event). On these two measures of safety, there were no significant differences between NSAIDs and acetaminophen. For the comparison of the total number of adverse effects, the relative risk ratios were 1.01 (95% CI, 0.92 to 1.11), 1.02 (95% CI, 0.92 to 1.14) and 1.04 (95%CI, 0.93 to 1.17). For the comparison of total number of withdrawals due to adverse effects, the relative risk ratios were 0.79 (95% CI, 0.59 to 1.05), 0.94 (95% CI, 0.68 to 1.30) and 0.89 (95%CI, 0.62 to 1.26) for the three NSAIDS vs acetaminophen groups.
GI adverse events are of particular interest and importance when considering the use of NSAIDs vs acetaminophen for treating people with OA. We therefore examined the RR for i) regular NSAIDs vs acetaminophen and ii) coxib NSAIDs vs acetaminophen for two GI outcomes: a) total number of participants who withdrew from treatment due to GI events and b) the total number of participants who experienced a GI related event or symptom (e.g diarrhea, nausea, heartburn, and/or abdominal pain). For GI withdrawals there was a statistically significant difference between the traditional NSAIDs and acetaminophen groups, the RR was 2.00 (95% CI, 1.05 to 3.81), indicating participants taking NSAIDS (ibuprofen, diclofenac or naproxen) were more likely to withdraw due to GI events. For GI adverse events, there was no significant difference in the number of patients reporting events when combined NSAIDS (traditional and coxib NSAIDS) were compared to acetaminophen (combined NSAIDS vs acetaminophen RR was 1.11 (95% CI, 0.94 to 1.31). When coxib NSAIDS were compared to acetaminophen, the RR was 0.98 (95% CI, 0.80 to 1.20). However, when traditional NSAIDS were compared to acetaminophen the RR was 1.47 (95% CI, 1.08 to 2.00), indicating that participants taking traditional NSAIDS (ibuprofen or naproxen) were more likely to experience a GI adverse event than those taking acetaminophen [Table 3].
3. Number needed to harm ‐ GI events Acetaminophen vs NSAIDs.
| Intervention | % w NSAID | % w Acetaminophen | RR (95%CI) | ARD(95%CI) | NNH (95% CI) |
| Traditional NSAID | 91/484 (19%) | 51/407(13%) | 1.47 (1.08,2.00) | 6%(1%, 11%) | 12 (6,66) |
| Coxib NSAIDs | 303/2320 (13%) | 118/994(12%) | 0.98(0.80, 1.20) | 0%(‐1%, 4%) | NA |
| Combined traditional & Coxib | 394/2804(14%) | 169/1401 (12%) | 1.11 (0.94, 1.31) | 1%(‐1%,3%) | NA |
| Legend | ARD=absolute risk difference NA=not applicable ARD=Absolute risk difference NA=not applicable |
Serious safety outcomes, including serious gastrointestinal, renal and cardiovascular safety, were not identified to be more common with NSAIDs versus acetaminophen. However, this is a very difficult assessment to make since these adverse outcomes are relatively rare, and are often not appreciated in a relatively short term RCT, that enrolls a relatively small number of participants for a relatively short period of time (6 days to 2 years).
Discussion
The results of this review support the notion that acetaminophen is an effective and relatively safe treatment modality for OA. This conclusion held true in five of the seven RCTs that compared acetaminophen to placebo. However, acetaminophen is not as effective as NSAIDs in terms of pain reduction, global assessments of efficacy (patient and investigator) and improvements in functional status. In terms of safety, acetaminophen appears to be as safe as placebo and safer than traditional NSAIDs in terms of GI adverse events.
The results of our review will now be compared to results obtained by other published systematic reviews. Our results are similar to those obtained by Eccles (Eccles 1998) in their meta‐analysis comparing acetaminophen to NSAIDs in OA. For example, they found a statistically significantly increased efficacy of NSAIDs over acetaminophen for the outcomes of pain at rest and pain on motion, with similar magnitudes of effect size. Measures of function (50 foot walk time) and quality of life did not differ between the two treatments. The conclusion of the Eccles (Eccles 1998) review was that initial treatment for OA pain should be acetaminophen (paracetamol) followed by an NSAID (ibuprofen). The meta‐analysis by Lee and colleagues (Lee 2004) compared the efficacy and safety of NSAIDs versus acetaminophen in the treatment of symptomatic OA of the hip and knee based on seven RCTs. NSAIDs were statistically superior in reducing rest and walking pain compared with acetaminophen. Safety, measured by discontinuation due to adverse events, was not statistically different between NSAID and acetaminophen treated groups. The review by Lee et al (Lee 2004)did not compare acetaminophen to placebo. The meta‐analysis by Zhang and colleagues (Zhang 2004) included ten RCTs including 1712 patients with symptomatic OA of the knee and/or hip. The results showed that acetaminophen was more effective than placebo in relieving pain due to OA. NSAIDs were superior to paracetamol for pain relief. The number of patients who preferred NSAIDs was more than twice that preferred acetaminophen. However, NSAIDs were associated with more frequent gastrointestinal discomfort than paracetamol (RR=1.35, 95% CI, 1.05 to 1.75). The authors concluded that although NSAIDs are clearly superior to acetaminophen in OA, there are clinically important differences in terms of gastrointestinal safety, which forms the basis for their recommendation that acetaminophen should still be the first line therapy for OA pain. The meta‐analysis by Wegman and colleagues (Wegman 2004) included five RCTs which compared acetaminophen to NSAIDs in OA. NSAIDs were found to be superior to acetaminophen in terms of pain reduction. The review by Wegman et al (Wegman 2004) did not compare acetaminophen versus placebo.
The relative superiority of NSAIDs over acetaminophen appears to be more marked in those OA participants having more severe levels of baseline pain. For example, the study by Pincus 2001 found that the efficacy differences between an NSAID (diclofenac/misoprostol) and acetaminophen were negligible in patients with mild disease, whether mild was defined by symptoms, number of involved joints, or by radiographic severity. However, there was a marked difference in efficacy favouring the NSAID in those OA patients who had more severe levels of baseline disease. In the study by Altman 1999, the NSAID (ibuprofen) was superior to acetaminophen in participants having moderately severe or severe baseline pain. The evidence suggesting that baseline levels of OA pain predict the relative responsiveness to NSAIDs versus acetaminophen is not entirely consistent. In a hypothesis generating post‐hoc analysis of their earlier RCT, Bradley and colleagues (Bradley 2001) concluded that acetaminophen and ibuprofen were comparably effective in treating OA pain, despite the levels of baseline pain severity. It is also not unknown with certainty whether the subgroup of OA participants with clinical evidence of joint inflammation will respond better to an NSAID than to acetaminophen. A hypothesis generating post hoc analysis by Bradley 1992 found that baseline inflammatory features of knee OA did not predict a greater degree of response from an NSAID versus acetaminophen. In summary, on balance, there is evidence from RCTs that participants with moderate‐to‐severe OA pain will do better with an NSAID than with acetaminophen. However, in participants with mild OA pain, the two agents appear to be more similar in efficacy. The determination of pain severity, as described in this context, is essentially a combination of that reported by the patient and a clinical judgement made by the treating physician.
The safety results of this review suggest that there is no overall significant differences between acetaminophen and NSAIDs in terms of two separate measures of safety: total number of patients reporting any adverse reaction, and total number of withdrawals due to adverse events. However, it needs to be noted that the RCTs were relatively of short duration and studied a relatively small number of highly selected participants with OA (number of patients in the studies ranged from 20 to 1578). This would preclude the ability to detect the less common, but much more significant, adverse effects. This especially applies to the NSAIDs which have been clearly documented to have the potential for serious gastro‐intestinal, renal, and cardio‐vascular toxicities (Singh 2000). It can therefore be concluded that in the realm of the short term RCTs, which enrolled highly selected patient populations, with a relatively low baseline risk for drug related adverse events, one does not find any significant overall difference in safety between acetaminophen and NSAIDs. This conclusion may not be generalizable to the clinical setting where a heterogeneous patient population (some of whom who may have significant baseline risk factors for NSAID toxicity) is being managed. Risk factors for upper GI bleeding in patients treated with NSAIDs include age > = 65 years, history of peptic ulcer disease or of upper GI bleeding, concomitant use of oral glucocorticoids or anticoagulants, presence of comorbid conditions (Gabriel 1991, Lanza 1998). Risk factors for NSAID‐induced reversible renal failure in patients with intrinsic renal disease include age > = 65 years, hypertension and/or congestive heart failure, and concomitant use of diuretics and angiotensin‐converting enzyme inhibitors (Garell 1984). A recent review by Benson and colleagues summarized the clinically relevant concerns regarding the use of acetaminophen in patients with liver disease (Benson 2005). They recommended that acetaminophen, when used in currently recommended doses, can be used safely in patients with liver disease and is a preferred analgesic/antipyretic because of the absence of the platelet impairment, gastrointestinal toxicity, and nephrotoxicity associated with NSAIDs.
Similar to the case for NSAIDs, acetaminophen has also been linked to an increased risk for serious upper gastrointestinal complications. Garcia Rodriguez (GarciaRodriguez 2001) reviewed the data on the risk of upper gastrointestinal (GI) complications associated with NSAIDs and acetaminophen. They noted that published epidemiological data on the association between acetaminophen use and risk for serious upper gastrointestinal safety are limited and inconsistent (pooled relative risk [RR], 1.4; 95% confidence interval [CI], 1‐2). However, two studies evaluated the effect of dose, and both reported an increased risk of serious upper gastrointestinal toxicity with acetaminophen usage at high doses. These authors also evaluated the safety of acetaminophen by conducting a well designed nested case‐control study using the UK General Practice Research Database (2,105 cases age and gender were matched to 11,500 controls). Current users of acetaminophen had an overall significantly increased risk of serious upper GI complications (RR, 1.3; 95% CI, 1.1‐1.5). There was a clear dose‐response relationship observed. At daily doses of less than 2000 mg/day, acetaminophen was not associated with an increased risk for upper GI complications (RR, 0.9;95% CI, 0.8‐1.1). Acetaminophen at a dosage of 2000 mg/day was associated with an increased risk (RR, 1.9;95% CI, 1.4‐2.6). Acetaminophen at dosages greater than or equal to 2000 mg/day were associated with the highest risk (RR, 3.7;95% CI, 2.6‐5.1). Surprisingly, the RR estimates were quite similar to those obtained for low/medium and high doses of NSAIDs of 2.4 (1.9‐3.1) and 4.9 (4.1‐5.8). There was also evidence of a very strong interaction between the use of acetaminophen at doses of > = 2000 mg/day and NSAIDs (RR, 16.6;95%CI, 11‐24.9). This important study provides for the first time evidence that high dose acetaminophen (> 2gm/day) may not be as safe as previously thought. The mechanism of acetaminophen's apparent upper GI safety may be related to its ability to act as a weak inhibitor of the cyclo‐oxygenase 1 enzyme (Warner 1999). This type of adverse effect is quite rare, and therefore, it was not observed in the relatively short term clinical trials. In order to determine the long term adverse effects of acetaminophen observational databases, such as the Arthritis Rheumatism and Aging Medical Information System (ARAMIS) database, could potentially be very useful. Further confirmation of the results of this important study are still necessary to better delineate the risk‐benefit ratio of high dose acetaminophen in OA. Fries and Bruce (Fries 2003) studied 5692 patients with rheumatoid arthritis and 3124 patients with OA from 12 databank centers, with 36,262 patient‐years of observation, who had taken one of three study analgesics, and examined the frequency of serious gastrointestinal events requiring hospitalization. The three analgesics compared were aspirin, acetaminophen and ibuprofen. They found that in over the counter doses, there were no significant differences in gastrointestinal toxicity among the three analgesics
There are at least 3 published guidelines for the medical management of OA originating from content experts: American College of Rheumatology guidelines (ACR 2000), European League Against Rheumatism Guidelines (Pendleton 2000), and the Canadian Consensus Guidelines (Tannenbaum 2000). The relative role of acetaminophen versus NSAIDs has been addressed in each of these guidelines. The ACR (ACR 2000) guidelines recommend that acetaminophen be considered as a reasonable initial therapy in those with mild‐moderate OA pain, however, in those with moderate to severe OA pain, NSAIDs may be considered as an alternative initial therapeutic approach. The EULAR guidelines (Pendleton 2000) recommend that acetaminophen should be first choice therapy in OA, and that NSAIDs should be reserved for those patients who are unresponsive to acetaminophen. The Canadian guidelines (Tannenbaum 2000) recommend that NSAIDs should be the pharmacological agents of choice for the symptomatic treatment of moderate to severe OA. Acetaminophen may also be considered for those with mild OA pain. Therefore, although all three guideline statements acknowledge that both agents are effective in OA, they differ in the strength of their recommendation as to what agent should be considered to be first line therapy for symptomatic OA. A number of recent editorials written by content experts in OA have highlighted the controversies in this area (Brandt 2001, Courtney 2002 , Felson 2001, Moskowitz 2001).
There have been two patient preference survey studies reported in the recent literature that are pertinent to the results of this review. Both surveys convincingly demonstrate that patients are far more likely to prefer NSAIDs than acetaminophen in the treatment of their OA symptoms. Pincus (Pincus 2000) conducted a 15 minute telephone survey with 300 patients (172 with confirmed OA) with the objective of analyzing results of treatment with acetaminophen and NSAIDs. The results included the following: 1) Patients take many different drugs for OA, most of which are not continued beyond 2 years, 2) Many patients take both acetaminophen and NSAIDs, 3) Most patients who identified a drug as "most helpful" named an NSAID rather than acetaminophen or an analgesic drug (80% named an NSAID and 20% named acetaminophen or another analgesic), 4) Only 16% of patients named acetaminophen as being the most helpful drug, and 5) acetaminophen was significantly less likely to be discontinued because of safety than NSAID. The survey by Wolfe (Wolfe 2000) also found a considerable and statistically significant preference for NSAIDs, compared to acetaminophen among 3 groups of rheumatic disease patients (OA, rheumatoid arthritis, and fibromyalgia). The authors concluded: "if safety and cost are not issues, there would hardly ever be a reason to recommend acetaminophen over NSAIDs, since patients generally preferred NSAIDs and fewer than 14% preferred acetaminophen" (Wolfe 2000). Of interest though, this study did find that 38% of patients reported acetaminophen to be as effective or more effective than NSAIDs.
Cohen (Cohen 1988) has provided guidelines for the interpretation of effect sizes, and these suggest that an effect size of 0.2 to 0.5 represents a small or modest treatment effect. Therefore, the magnitude of the effect sizes obtained in this meta‐analysis suggest that the advantage of NSAIDs over acetaminophen is relatively small and modest. For these reasons, the final choice of whether to use acetaminophen or an NSAID as a first line agent in OA will need to consider a number of additional important factors. Additional considerations include: cost considerations and accessibility (acetaminophen is the least costly treatment choice in the management of mild to moderate OA pain (Holzer 1996), clinical judgement based in part on relative risks for safety from the two agents (for example, is the patient at risk for gastrointestinal, renal or cardiovascular safety?) and severity of OA pain (for example, those with moderate to severe pain are more likely to respond to an NSAID than to acetaminophen), and patient preferences (for example, taking into account cost, risks etc).
Authors' conclusions
Implications for practice.
Evidence available to date supports the efficacy of both acetaminophen and NSAIDs in the management of OA. There is also evidence that NSAIDs are superior to acetaminophen in terms of pain reduction and improvements in patient and physician global assessments and functional status. The relative superiority of NSAIDs over acetaminophen is most marked in those with moderate to severe levels of pain. The benefits of NSAIDs over acetaminophen are relatively modest, and therefore, additional factors are still important to consider in the decision to use these drugs. These factors include patient preferences, prescriber's clinical judgement, cost considerations and accessibility, and the comparative safety risks from both acetaminophen and NSAIDs in the individual patient.
Implications for research.
Additional RCTs are necessary to help identify the patient with OA who is most likely to benefit from NSAIDs, as opposed to acetaminophen. Moderate to severe levels of baseline OA pain may be associated with a better response to NSAIDs compared to acetaminophen; this requires further study. A strategy of combination NSAID and acetaminophen, depending on the severity of OA symptoms, may be more helpful than either drug alone, and this also needs to be studied systematically. Acetaminophen at doses greater than or equal to 2000 mg/day may also be associated with a significantly increased risk for upper GI complications and this unexpected finding requires confirmation from additional studies.
What's new
| Date | Event | Description |
|---|---|---|
| 17 September 2008 | Amended | Converted to new review format. CMSG ID: C095‐R |
History
Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 1 November 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Ashley Nicholson for her assistance with final preparation of the manuscript for this review. Dr. John Kirwan and Dr. Peter Tugwell provided valuable feedback during the peer review process.
Appendices
Appendix 1. Full search strategy
1. (acetaminophen or paracetamol).ti,ab. 2. tylenol,ab. 3. or/1‐2 4. exp *arthritis/ 5. (arthrit$ or osteoarthrit$).ti,ab. 6. 4 or 5 7. exp osteoarthritis/ 8. (degenerative adj2 arthritis). tw. 9. osteoarthr$.tw. 10. or/7‐9 11. 10 or 6 12. 3 and 11 13. limit 12 to humans
Data and analyses
Comparison 1. Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rest Pain | 3 | 573 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.36, ‐0.03] |
| 2 HAQ Pain | 4 | 1075 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.45, ‐0.07] |
| 3 Pain on motion (walking) | 2 | 270 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.20, 0.28] |
| 4 HAQ Disability | 2 | 349 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.41, 0.01] |
| 5 50 Foot Walk Time | 3 | 571 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.14, 0.19] |
| 6 Physician Global (dichotomous) | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.63, 1.58] |
| 7 Physician Global (means) | 2 | 359 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.54, ‐0.12] |
| 8 Patient Global (mean) | 2 | 280 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.44, 0.03] |
| 9 Patient Global (dichotomous) | 2 | 967 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.06, 1.43] |
| 10 Toxicity 1 (Total Number of Patients with any AE) | 7 | 3168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 11 Toxicity 2 (Withdrawals due to toxicity) | 8 | 2793 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 12 WOMAC Pain | 2 | 832 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
| 13 WOMAC Stiffness | 2 | 828 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.34, ‐0.05] |
| 14 WOMAC Function | 2 | 832 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.40, ‐0.11] |
| 15 WOMAC Total | 3 | 780 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.39, ‐0.11] |
| 16 Lequesne Pain | 1 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.66, 0.41] |
| 17 Lequesne Walking | 1 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.73, 0.35] |
| 18 Lequesne Function | 1 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.60, 0.47] |
| 19 Lequesne Total | 1 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.67, 0.40] |
| 20 Overall Pain (multiple methods) | 8 | 2358 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
1.1. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 1 Rest Pain.
1.2. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 2 HAQ Pain.
1.3. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 3 Pain on motion (walking).
1.4. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 4 HAQ Disability.
1.5. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 5 50 Foot Walk Time.
1.6. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 6 Physician Global (dichotomous).
1.7. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 7 Physician Global (means).
1.8. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 8 Patient Global (mean).
1.9. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 9 Patient Global (dichotomous).
1.10. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 10 Toxicity 1 (Total Number of Patients with any AE).
1.11. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 11 Toxicity 2 (Withdrawals due to toxicity).
1.12. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 12 WOMAC Pain.
1.13. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 13 WOMAC Stiffness.
1.14. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 14 WOMAC Function.
1.15. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 15 WOMAC Total.
1.16. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 16 Lequesne Pain.
1.17. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 17 Lequesne Walking.
1.18. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 18 Lequesne Function.
1.19. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 19 Lequesne Total.
1.20. Analysis.

Comparison 1 Group 1: NSAID (ibuprofen 2400 mg, diclofenac, arthrotec, celecoxib, naproxen) vs Acetaminophen, Outcome 20 Overall Pain (multiple methods).
Comparison 2. Group 2: Acetaminophen vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Response (dichotomous) | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 8.00 [2.08, 30.73] |
| 2 Pain on Motion (dichotomous) | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [1.48, 9.52] |
| 3 Physician Global Assessment (dichotomous) | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 20.00 [2.95, 135.75] |
| 4 Patient Global Assessment (dichotomous) | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 18.00 [2.66, 121.63] |
| 5 Toxicity 1 (Total number of Patients with any AE) | 6 | 2385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 6 WOMAC Pain | 1 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.49, 0.55] |
| 7 WOMAC Stiffness | 1 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.41, 0.63] |
| 8 WOMAC Function | 2 | 829 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.18, 0.10] |
| 9 WOMAC Total | 3 | 767 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.26, 0.02] |
| 10 Lequesne Pain | 1 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.68, 0.36] |
| 11 Lequesne Walking | 1 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.35, 0.69] |
| 12 Lequesne Function | 1 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.34, 0.71] |
| 13 Lequesne Total | 1 | 57 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.52, 0.52] |
| 14 Pain at rest | 1 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.39, 0.06] |
| 15 Pain on passive motion | 1 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.33, 0.12] |
| 16 Pain Response (continuous) | 3 | 1411 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.01] |
| 17 Stiffness at rest | 1 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.44, 0.02] |
| 18 Day pain | 1 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.52, ‐0.06] |
| 19 Night pain | 1 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.51, ‐0.05] |
| 20 50 foot walk time | 1 | 294 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 21 MDHAQ VAS Pain | 2 | 710 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.33, ‐0.03] |
| 22 Toxicity 2 (Withdrawals due to toxicity) | 6 | 2146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.87, 1.77] |
| 23 Overall Pain (multiple methods) | 5 | 1835 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.22, ‐0.04] |
| 24 Patient Global Assessment (continuous) | 1 | 776 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.23, 0.05] |
2.1. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 1 Pain Response (dichotomous).
2.2. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 2 Pain on Motion (dichotomous).
2.3. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 3 Physician Global Assessment (dichotomous).
2.4. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 4 Patient Global Assessment (dichotomous).
2.5. Analysis.
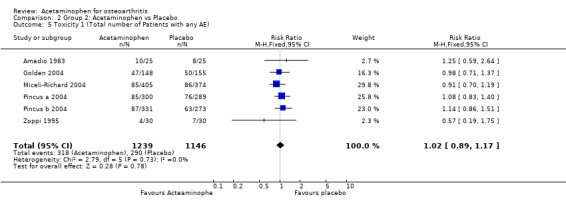
Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 5 Toxicity 1 (Total number of Patients with any AE).
2.6. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 6 WOMAC Pain.
2.7. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 7 WOMAC Stiffness.
2.8. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 8 WOMAC Function.
2.9. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 9 WOMAC Total.
2.10. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 10 Lequesne Pain.
2.11. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 11 Lequesne Walking.
2.12. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 12 Lequesne Function.
2.13. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 13 Lequesne Total.
2.14. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 14 Pain at rest.
2.15. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 15 Pain on passive motion.
2.16. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 16 Pain Response (continuous).
2.17. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 17 Stiffness at rest.
2.18. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 18 Day pain.
2.19. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 19 Night pain.
2.20. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 20 50 foot walk time.
2.21. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 21 MDHAQ VAS Pain.
2.22. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 22 Toxicity 2 (Withdrawals due to toxicity).
2.23. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 23 Overall Pain (multiple methods).
2.24. Analysis.

Comparison 2 Group 2: Acetaminophen vs Placebo, Outcome 24 Patient Global Assessment (continuous).
Comparison 3. Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rest pain | 4 | 594 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.35, ‐0.03] |
| 2 HAQ Pain | 2 | 341 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.77, 0.33] |
| 3 Pain on motion (walking) | 2 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.27, 0.21] |
| 4 50 Foot Walk Time | 3 | 571 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.14, 0.19] |
| 5 HAQ disability index | 2 | 341 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.36, 0.07] |
| 6 Physician Global (Dichotomous) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.75, 1.77] |
| 7 Patient Global (Dichotomous) | 3 | 1190 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.27, 1.76] |
| 8 Toxicity 1 (Total number of Patients with any AE) | 6 | 2039 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 9 Toxicity 2 (Withdrawals due to toxicity) | 6 | 1908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 10 WOMAC Pain | 2 | 1000 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.50, ‐0.24] |
| 11 WOMAC Stiffness | 2 | 998 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.51, ‐0.25] |
| 12 WOMAC Function | 2 | 1000 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.53, ‐0.27] |
| 13 WOMAC Total | 1 | 217 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.73, ‐0.19] |
| 14 Overall Pain (multiple methods) | 7 | 1812 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.40, ‐0.21] |
3.1. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 1 Rest pain.
3.2. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 2 HAQ Pain.
3.3. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 3 Pain on motion (walking).
3.4. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 4 50 Foot Walk Time.
3.5. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 5 HAQ disability index.
3.6. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 6 Physician Global (Dichotomous).
3.7. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 7 Patient Global (Dichotomous).
3.8. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 8 Toxicity 1 (Total number of Patients with any AE).
3.9. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 9 Toxicity 2 (Withdrawals due to toxicity).
3.10. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 10 WOMAC Pain.
3.11. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 11 WOMAC Stiffness.
3.12. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 12 WOMAC Function.
3.13. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 13 WOMAC Total.
3.14. Analysis.

Comparison 3 Group 3: NSAIDS (ibuprofen 1200 mg,arthrotec, rofecoxib 25 mg, naproxen) vs Acetaminophen, Outcome 14 Overall Pain (multiple methods).
Comparison 4. Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient Global (Dichotomous) | 3 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.14, 1.82] |
| 2 Toxicity 1 (Total Number of Patients with any AE) | 6 | 1772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.93, 1.17] |
| 3 Toxicity 2‐ Withdrawals due to toxicity | 6 | 1641 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.26] |
| 4 WOMAC Pain | 1 | 520 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.48, ‐0.13] |
| 5 WOMAC Stiffness | 1 | 518 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.43, ‐0.08] |
| 6 WOMAC Function | 1 | 520 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.52, ‐0.17] |
4.1. Analysis.

Comparison 4 Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen, Outcome 1 Patient Global (Dichotomous).
4.2. Analysis.

Comparison 4 Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen, Outcome 2 Toxicity 1 (Total Number of Patients with any AE).
4.3. Analysis.

Comparison 4 Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen, Outcome 3 Toxicity 2‐ Withdrawals due to toxicity.
4.4. Analysis.

Comparison 4 Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen, Outcome 4 WOMAC Pain.
4.5. Analysis.

Comparison 4 Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen, Outcome 5 WOMAC Stiffness.
4.6. Analysis.

Comparison 4 Group 4: NSAIDS (ibuprofen 1200 mg, arthrotec, rofecoxib 12.5 mg, naproxen) vs Acetaminophen, Outcome 6 WOMAC Function.
Comparison 5. NSAIDs vs Acetaminophen ‐ GI outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events ‐ GI events | 13 | 4205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.94, 1.31] |
| 1.1 Traditional NSAIDs vs acetaminophen | 5 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.08, 2.00] |
| 1.2 Coxib NSAIDs vs acetaminophen | 8 | 3314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.20] |
| 2 GI withdrawals | 5 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.05, 3.81] |
| 2.1 Traditional NSAIDs vs acetaminophen | 5 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.05, 3.81] |
5.1. Analysis.

Comparison 5 NSAIDs vs Acetaminophen ‐ GI outcomes, Outcome 1 Adverse events ‐ GI events.
5.2. Analysis.

Comparison 5 NSAIDs vs Acetaminophen ‐ GI outcomes, Outcome 2 GI withdrawals.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altman 1999.
| Methods | Randomized, double‐blind, parallel group trial of 6 days duration. | |
| Participants | Adults with moderate OA of the knees. | |
| Interventions | Acetaminophen 4000 mg/day versus ibuprofen 1200 mg/day versus placebo. | |
| Outcomes | Knee pain on walking, knee pain on weight‐bearing, WOMAC, limitation of activity, overall efficacy evaluation, daily knee pain evaluation. | |
| Notes | This study is still only available in abstract form. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Amadio 1983.
| Methods | Randomized, double‐blind, cross‐over, placebo controlled trial of 6 weeks duration. | |
| Participants | Adults having radiographic evidence of typical OA of the knee. Median age 64 years. 88% female. Most likely enrolled those with primary (idiopathic) OA. | |
| Interventions | Acetaminophen (1000 mg po qid) versus placebo | |
| Outcomes | Tenderness, pain at rest, pain on motion, swelling, heat, 50' walk time, physician global, patient global, toxicity | |
| Notes | This represents the first published randomized controlled trial that compares acetaminophen to placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Boureau 2004.
| Methods | Randomized, double‐blind, parallel group trial of 14 days duration. | |
| Participants | Adults with symptomatic OA of the knee or hip. | |
| Interventions | Acetaminophen 3000 mg/day versus ibuprofen 1200 mg/day | |
| Outcomes | Pain (after single doses and multidoses), patient global assessment, sleep quality, WOMAC. | |
| Notes | First study to compare the efficacy of single doses of ibuprofen and acetaminophen in OA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bradley 1991a.
| Methods | Randomized, double‐blind, parallel group trial of 4 weeks duration | |
| Participants | Adults having radiographic OA of the knee. Mean age 56.5 years. 75% female. Both primary and secondary (post‐traumatic) OA enrolled. | |
| Interventions | Acetaminophen (1000 mg po qid) versus ibuprofen 1200 mg/day versus ibuprofen 2400 mg/day | |
| Outcomes | HAQ pain, walking pain score, rest pain score, walking distance score, 50' walk time, HAQ disability, physician global, knee tenderness, knee range of motion, knee swelling, toxicity | |
| Notes | This study compared acetaminophen to both an analgesic and an anti‐inflammatory dose of ibuprofen. Additional data was obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bradley 1991b.
| Methods | see Bradley 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Case 2003.
| Methods | Randomized, double‐blind, parallel group trial of 12 weeks duration. | |
| Participants | Adults (aged 40‐75 years) having clinical and radiographic OA of the knee. Mean age 62.2 years. 50% female. Primary OA of the knee enrolled. | |
| Interventions | Acetaminophen (1000 mg po qid) versus diclofenac (75 mg twice per day) versus placebo. | |
| Outcomes | WOMAC, Lequesne, patient global assessment, physican global assessment. | |
| Notes | This represents the first published study which finds no difference in efficacy between acetaminophen and placebo. Additional data was obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Geba 2002a.
| Methods | Randomized, double‐blind, parallel group trial of 6 weeks duration. | |
| Participants | Adults (> = 40 years) with primary OA of the knee that was previously treated with NSAIDs or acetaminophen. Mean age 62.6 years. 68% female. ACR criteria for OA of the knee was used. | |
| Interventions | Acetaminophen (1000 mg po qid) versus celecoxib 200 mg/day versus rofecoxib 12.5 mg/day versus rofecoxib 25 mg/day | |
| Outcomes | WOMAC composite pain, WOMAC composite stiffness, WOMAC composite function, WOMAC night pain, WOMAC walking pain, WOMAC morning stiffness, patient global, toxicity | |
| Notes | Also referred to as the VACT‐1 trial. The first published trial that has compared acetaminophen to the coxibs (celecoxib and rofecoxib) in subjects with OA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Geba 2002b.
| Methods | see Geba 2002a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Geba 2002c.
| Methods | see Geba 2002a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Golden 2004.
| Methods | Two identical randomized, double‐blind, placebo‐controlled multidose, parallel group studies. Data analysis presented in aggregate for both studies. Study duration was 7 days. | |
| Participants | Adults aged over 25 years with at least moderate pain in the knee from OA. Radiographic confirmation of OA diagnosis. | |
| Interventions | Naproxen sodium 220 mg po tid versus acetaminophen 1000 mg po qid versus placebo for 7 days. | |
| Outcomes | Pain, functional assessments, quality of life questionnaire‐a modified version of the AIMS‐2, patient and investigator global assessments. | |
| Notes | This study evaluated an over the counter dosage and preparation of naproxen. Additional data was obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miceli‐Richard 2004.
| Methods | Randomized, double‐blind, parallel group, placebo controlled trial of 6 weeks duration. | |
| Participants | Adults with symptomatic OA of the knee. | |
| Interventions | Acetaminophen 4 gm/day versus placebo for 6 weeks. | |
| Outcomes | Pain, WOMAC, patient global assessments, OARSI responder criteria. | |
| Notes | This is the second published study which fails to show that acetaminophen is better than placebo in OA. Additional data was obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pincus 2001.
| Methods | Randomized, double‐blind, cross‐over clinical trial of 6 weeks duration. | |
| Participants | Adults (age > 40 years) with radiographic OA of the knee or hip. Mean age 61.5 years. 71% female. | |
| Interventions | Acetaminphen (1000 mg po qid) versus diclofenac/misoprostol (75/200 po bid) | |
| Outcomes | WOMAC target joint, MDHAQ VAS pain, SF‐36 pain, MDHAQ GI distress, MDHAQ basic ADL, MDHAQ patient global, physician global, toxicity, physician global | |
| Notes | Additional data was obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pincus a 2004.
| Methods | Two randomized, double‐blind, placebo controlled, crossover trials in patients with OA of the hip or knee. Data is presented for both trials. | |
| Participants | Adults (age >= 45 years) with symptomatic, radiographically confirmed OA of the knee or hip. | |
| Interventions | Celecoxib 200 mg/day versus acetaminophen 1000 mg po qid, versus placebo. Each intervention was given for 6 weeks. | |
| Outcomes | WOMAC, MDHAQ, SF‐36, patient and investigator global assessments, patient preference, pain. | |
| Notes | Also referred to as the PACES‐a trial. Additional data for meta‐analysis was obtained from website. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pincus b 2004.
| Methods | see Pincus a 2004 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Also referred to as the PACES‐b trial. Additional data for meta‐analysis was obtained from website and from Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schnitzer 2005a.
| Methods | Randomized, parallel group, multicentre, double‐blind trial of 6 weeks duration. | |
| Participants | Adults (aged > = 40 years) meeting ACR criteria for symptomatic OA of the knee. | |
| Interventions | Acetaminophen 4000 mg/day versus celecoxib 200 mg/day versus rofecoxib 12.5 mg/day versus rofecoxib 25 mg/day for 6 weeks duration. | |
| Outcomes | WOMAC, Patient global assessment to therapy. | |
| Notes | This is also known as the VACT‐2 study and is similar in design to the Geba 2001 study which is referred to as the VACT‐1 study. This paper pools the outcome data for both VACT trials. Additional outcome data was obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schnitzer 2005b.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Schnitzer 2005c.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Shen 2004.
| Methods | Randomized, parallel group trial of 3 months duration. | |
| Participants | 20 patients with symptomatic OA of the knee. Lacking other details. | |
| Interventions | Acetaminophen (up to 4 gms/day) versus rofecoxib 25 mg/day. | |
| Outcomes | VAS pain scores, changes in serum levels of serotonin, substance P, beta‐endorphin, and opioid receptor kappa. | |
| Notes | This is still only available as an abstract, and was identified through the meta‐analysis published by Zhang et al, 2004. The abstract was presented at the 2003 EULAR meeting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Williams 1993.
| Methods | Randomized, double‐blind, cross‐over clinical trial of 2 years' duration. | |
| Participants | Adults with radiographic OA of the knee. Mean age 59.6 years. 75% female. | |
| Interventions | Acetaminophen 650 mg po qid versus naproxen 375 mg po bid. | |
| Outcomes | Pain at rest, pain on motion, 50' walk time, physician global, patient global, knee effusion, knee crepitus, knee range of motion, radiographic progression, toxicity, pain on motion or tenderness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zoppi 1995.
| Methods | Randomized, double‐blind, parallel group trial of 7 days duration. | |
| Participants | Adults with radiographic OA of the knee. Mean age 56 years. 62% female. | |
| Interventions | Acetaminophen 1000 mg po tid versus placebo. | |
| Outcomes | Pain, functional assessments, patient global assessments, number of awakenings during the night. | |
| Notes | This study was identified only through the meta‐analysis published by Zhang et al, 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Contributions of authors
TT completed the literature review, reviewed references for suitability, extracted data from included studies, performed methodological quality review of included studies, completed the analyses, and wrote the text portion.
LM reviewed the literature search strategy and results, verified the data abstraction forms, checked the accuracy of the analyses, was involved in writing revisions of the text of the review, and helped with the editing and entry of the final version in Rev Man.
MJ reviewed the literature search strategy and results, verified the data abstraction forms, checked the accuracy of the analyses, was involved in writing revisions of the text of the review, and helped with the editing and entry of the final version in Rev Man.
MC extracted data from included studies and performed methodological quality review of included studies.
MH reviewed and contributed to the review by providing expert interpretation of the findings and also provided critical comments on the final version.
GW reviewed and contributed to the statistical methodology and analyses.
Sources of support
Internal sources
Queen's University ‐A Canadian Cochrane Network Site, Canada.
Institute for Population Health, University of Ottawa, Canada.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Altman 1999 {published data only}
- Altman RD. Ibuprofen, acetaminophen and placebo in osteoarthritis of the knee: A six‐day double‐blind study. Arthritis Rheum. 1999; Vol. September Supplement:S403.
Amadio 1983 {published data only}
- Amadio P, Cummings DM. Evaluation of acetaminophen in the management of osteoarthritis of the knee. Curr Ther Res 1983;34(1):59‐66. [Google Scholar]
Boureau 2004 {published data only}
- Boureau F, Schneid H, Zeghari N, Wall R, Bourgeois P. The IPSO study: ibuprofen, paracetamol study in osteoarthritis. A randomized comparative clinical study comparing the efficacy and safety of ibuprofen and paracetamol analgesic treatment of osteoarthritis of the knee or hip. Ann Rheum Dis 2004;63:1028‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 1991a {published data only}
- Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. NEJM 1991;325:87‐91. [DOI] [PubMed] [Google Scholar]
Bradley 1991b {published data only}
- Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. New England Journal of Medicine 1991;325:87‐91. [DOI] [PubMed] [Google Scholar]
Case 2003 {published data only}
- Case JP, Baliunas AJ, Block JA. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis. Arch Intern Med 2003;163:169‐178. [DOI] [PubMed] [Google Scholar]
Geba 2002a {published data only}
- Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ, for the VACT Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in ostearthritis of the knee. JAMA 2002;287(1):64‐71. [DOI] [PubMed] [Google Scholar]
Geba 2002b {published data only}
- Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ, for the VACT Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in ostearthritis of the knee. JAMA 2002;287(1):64‐71. [DOI] [PubMed] [Google Scholar]
Geba 2002c {published data only}
- Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ, for the VACT Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in ostearthritis of the knee. JAMA 2002;287(1):64‐71. [DOI] [PubMed] [Google Scholar]
Golden 2004 {published data only}
- Golden HE, Moskowitz RW, Minic M. Analgesic efficacy and safety of nonprescription doses of naproxen sodium compared with acetaminophen in the treatment of osteoarthritis of the knee. American Journal of Therapeutics 2004;11:85‐94. [DOI] [PubMed] [Google Scholar]
Miceli‐Richard 2004 {published data only}
- Miceli‐Richard C, Bars M, Schmidely N, Dougados M. Paracetamol in osteoarthritis of the knee. Ann Rheum Dis 2004;63:923‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 2001 {published data only}
- Pincus T, Koch GG, Sokka T, Lefkowith J, Wolfe F, Jordan JM, et al. A randomized, double‐blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rheum 2001;44(7):1587‐1598. [DOI] [PubMed] [Google Scholar]
Pincus a 2004 {published data only}
- Pincus T, Koch G, Lei H, Mangal B, Sokka T, Moskowitz R, Wolfe F, Gibofsky A, Simon L, Zlotnick S, Fort JG. Patient preference for placebo, acetaminophen or celecoxib efficacy studies (PACES): two randomized, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis 2004;63:931‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus b 2004 {published data only}
- Pincus T, Koch G, Lei H, Mangal B, Sokka T, Moskowitz R, Wolfe F, Gibofsky A, Simon L, Zlotnick S, Fort JG. Patient preference for placebo, acetaminophen or celecoxib efficacy studies (PACES): two randomized, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis 2004;63:931‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnitzer 2005a {published data only}
- Schnitzer TJ, Weaver AL, Polis AB, Petruschke RA, Geba GP, for the VACT‐1 and VACT‐2 study groups. Efficacy of rofecoxib, celecoxib, and acetaminophen in patients with osteoarthritis of the knee. A combined analysis of the VACT studies. J Rheumatol 2005;32:1093‐1105. [PubMed] [Google Scholar]
Schnitzer 2005b {published data only}
- Schnitzer TJ, Weaver AL, Polis AB, Petruschke RA, Geba GP, for the VACT‐1 and VACT‐2 study groups. Efficacy of rofecoxib, celecoxib, and acetaminophen in patients with osteoarthritis of the knee. A combined analysis of the VACT studies. Journal of Rheumatology 2005;32:1093‐1105. [PubMed] [Google Scholar]
Schnitzer 2005c {published data only}
- Schnitzer TJ, Weaver AL, Polis AB, Petruschke RA, Geba GP, for the VACT‐1 and VACT‐2 study groups. Efficacy of rofecoxib, celecoxib, and acetaminophen in patients with osteoarthritis of the knee. A combined analysis of the VACT studies. Journal of Rheumatology 2005;32:1093‐1105. [PubMed] [Google Scholar]
Shen 2004 {published data only}
- Shen H, Sprott H, Aeschlimann A, Gay R, Uebelhart D, Michel BA, Gay S. Primary analgesic action of acetaminophen and rofecoxib in osteoarthritis. Ann Rheum Dis. 2003; Vol. 62 (Suppl 1):258.
Williams 1993 {published data only}
- Williams HJ, Ward JR, Egger MJ, Neuner R, Brooks RH, Clegg DO, et al. Comparison of naproxen and acetaminophen in a two‐year study of treatment of osteoarthritis of the knee. Arthritis Rheum 1993;36(9):1196‐1206. [DOI] [PubMed] [Google Scholar]
Zoppi 1995 {published data only}
- Zoppi M, Peretti G, Boccard E. Placebo‐controlled study of the analgesic efficacy of an effervescent formulation of 500 mg paracetamol in arthritis of the knee or the hip. Eur J Pain 1995;16:42‐48. [Google Scholar]
Additional references
ACR 2000
- American College of Rheumatology (ACR) Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum 2000;43:1905‐1915. [DOI] [PubMed] [Google Scholar]
Altman 1986
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039‐49. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman R, Brandt K, Hochberg M, Moskowitz R. Design and conduct of clinical trials in patients with osteoarthritis: Recommendations from a task force of the Osteoarthritis Research Society. Osteoarthritis and Cartilage 1996;4:217‐43. [DOI] [PubMed] [Google Scholar]
Badley 1995
- Badley E. The effect of osteoarthritis on disability and health care use in Canada. J Rheumatol 1995;22 (suppl 43):19‐22. [PubMed] [Google Scholar]
Bellamy 1997
- Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis: Consensus development at OMERACT III. J Rheumatol 1997;24(4):799‐802. [PubMed] [Google Scholar]
Benson 2005
- Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther 2005;12(2):133‐41. [DOI] [PubMed] [Google Scholar]
Bradley 1992
- Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal anti‐inflammatory drug or pure analgesic. J Rheumatol 1992;19:1950‐54. [PubMed] [Google Scholar]
Bradley 2001
- Bradley JD, Katz BP, Brandt KD. Severity of knee pain does not predict a better response to an antiinflammatory dose of ibuprofen than to analgesic therapy in patients with osteoarthritis. J Rheumatol 2001;28:1073‐6. [PubMed] [Google Scholar]
Brandt 2001
- Brandt KD, Bradley JD. Should the initial drug be used to treat osteoarthritis pain be a nonsteroidal antiinflammatory drug. J Rheumatol 2001;28(3):467‐73. [PubMed] [Google Scholar]
Brandt 2002
- Brandt KD. Simple analgesics versus NSAIDs. J Rheumatol 2002;29(3):640‐2. [PubMed] [Google Scholar]
Chandrasekharan 2002
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL: COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen, other analgesic/antipyretic drugs: cloning. structure, expression. Proc Natl Acad Sci USA 2002. 99(21):13926‐13931. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;21(99):13926‐13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clissold 1986
- Clissold SP. Paracetamol and phenacetin. Drugs 1986;32 (suppl 4):46‐59. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Courtney 2002
- Courtney P, Doherty M. Key questions concerning paracetamol and NSAIDs for osteoarthritis. Ann Rheum Dis 2002;61(9):767‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 1998
- Eccles M, Freemantle N, Mason J, for the North of England Non‐Steroidal Anti‐Inflammatory Drug Guideline Development Group. North of England evidence based guideline development project: summary guideline for non‐steroidal anti‐inflammatory drugs versus basic analgesia in treating the pain of degenerative arthritis. BMJ 1998;317:526‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felson 1998
- Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998;41(8):1343‐55. [DOI] [PubMed] [Google Scholar]
Felson 2001
- Felson DT. The verdict favors nonsteroidal antiinflammatory drugs for treatment of osteoarthritis and a plea for more evidence on other treatments. Arthritis Rheum 2001;44(7):1477‐80. [DOI] [PubMed] [Google Scholar]
Fries 2003
- Fries JF, Bruce B. Rates of serious gastrointestinal events from low dose use of acetylsalicylic acid, acetaminophen, and ibuprofen in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol 2003;30:2226‐33. [PubMed] [Google Scholar]
Gabriel 1991
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti inflammatory drugs: a meta‐analysis. Ann Intern Med 1991;115:787‐796.. [DOI] [PubMed] [Google Scholar]
GarciaRodriguez 2001
- Garcia Rodriguez LA, Hernandez‐Diaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti‐inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res 2001;3:98‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garell 1984
- Garell S, Matarese RA. Renal effects of prostaglandins and clinical adverse effects of nonsteroidal anti‐inflammatory agents. Medicine (Baltimore) 1984;63:165‐181.. [DOI] [PubMed] [Google Scholar]
Gotzsche 2000
- Gotzsche PC. Non‐steroidal anti‐inflammatory drugs. BMJ 2000;320:1058‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2005
- Graham GG, Scott KF. Mechanism of action of paracetamol. Americal Journal of Therapeutics 2005;12(1):46‐55. [DOI] [PubMed] [Google Scholar]
Guccione 1994
- Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW. The effects of specific medical conditions on functional limitations of elders in the Framingham study. Am J Public Health 1994;84:351‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedges 1985
- Hedges L, Olkin I. Statistical methods for meta‐analysis. San Diego, CA: Academic Press, 1985. [Google Scholar]
Hochberg 1996
- Hochberg MC, Perlmutter D, Hudson J, et al. Preferences in the management of osteoarthritis of the hip and knee: Results of a survey of community‐based rheumatologists in the United States. Arthritis Care Res 1996;9(3):170‐76. [DOI] [PubMed] [Google Scholar]
Hochberg 2000
Hochberg 2001
- Hochberg MC, Dougados M. Pharmacological therapy of osteoarthritis. Best Practice and Research Clinical Rheumatology 2001;15(4):583‐93. [DOI] [PubMed] [Google Scholar]
Hochberg 2001b
- Hochberg MC. What a difference a year makes: Reflections on the ACR recommendations for the medical management of osteoarthritis. Current Rheumatology Reports 2001;3:473‐78. [DOI] [PubMed] [Google Scholar]
Holzer 1996
- Holzer SS, Cuerdon T. Development of an economic model comparing acetaminophen to NSAIDs in the treatment of mild‐to‐moderate osteoarthritis. The American Journal of Managed Care 1996;II, Supplement:S15‐S26. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carrol D, Jenkinson C. Assessing the quality of reports of randomized controlled trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jawad 2005
- Jawad AS. Analgesics and osteoarthritis: are treatment guidelines reflected in clinical practice?. American Journal of Therapeutics 2005;12(1):98‐103. [DOI] [PubMed] [Google Scholar]
Lanza 1998
- Lanza FL, and the Members of the Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. A guideline for the treatment and prevention of NSAID‐induced ulcers. Am J Gastroenterol 1998;93:2037‐2046. [DOI] [PubMed] [Google Scholar]
Lawrence 1998
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41(5):778‐99. [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee C, Straus WL, Balshaw R, Barlas S, Vogel S, Schnitzer TJ. A comparison of the effficacy and safety of nonsteroidal antiinflammatory agents versus acetaminophen in the treatment of osteoarthritis: a meta‐analysis.. Arthritis Rheum 2004;51(5):746‐54. [DOI] [PubMed] [Google Scholar]
Liang 1984
- Liang M, Larsen M, Thomson M, et al. Costs and outcomes in rheumatoid arthritis and osteoarthritis. Arthitis Rheum 1984;27(5):522‐29. [DOI] [PubMed] [Google Scholar]
MMWR 1994
- Centers for Disease Control. Arthritis prevalence and activity limitations‐United States, 1990. MMWR 1994;43:433‐38. [PubMed] [Google Scholar]
Moskowitz 2001
- Moskowitz RW. Osteoarthritis: simple analgesics versus nonsteroidal antiinflammatory drugs. J Rheumatol 2001;28(5):932‐4. [PubMed] [Google Scholar]
Nikles 2005
- Nikles CJ, Yelland M, Mar C, Wilkinson D. The role of paracetamol in chronic pain: an evidence‐based approach.. American Journal of Therapeutics 2005;12(1):80‐91. [DOI] [PubMed] [Google Scholar]
Norman 2001
- Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution‐ and anchor‐based approaches in interpretation of changes in health‐related quality of life. Medical Care 2001;39(10):1039‐47. [DOI] [PubMed] [Google Scholar]
Pendleton 2000
- Pendleton A, Arden N, Dougados M, et al. EULAR recomendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000;59:936‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 2000
- Pincus T, Swearingen C, Cummings P, Callahan LF. Preference for nonsteroidal antiinflammatory drugs versus acetaminophen and concomitant use of both types of drugs in patients with osteoarthritis. J Rheumatol 2000;27:1020‐27. [PubMed] [Google Scholar]
Schulz 1995a
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 1995b
- Schulz KF. Subverting randomization in controlled trials. JAMA 1995;274(18):1456‐8. [PubMed] [Google Scholar]
Scott 1993
- Scott JC, Hochberg MC. Arthritic and other musculoskeletal diseases. In: Brownson RC, Remington PL, Davis JR editor(s). Chronic Disease Epidemiology and Control. Washington, DC: American Public Health Association, 1993. [Google Scholar]
Shamoon 2000
- Shamoon M, Hochberg MC. Treatment of osteoarthritis with acetaminophen: efficacy, safety, and comparison with nonsteroidal anti‐inflammatory drugs. Curr Rheumatology Rep 2000;2:454‐58. [DOI] [PubMed] [Google Scholar]
Shamoon 2001
- Shamoon M, Hochberg MC. The role of acetaminophen in the management of patients with osteoarthritis. Am J Med 2001;110(3A):46S‐49S. [DOI] [PubMed] [Google Scholar]
Singh 2000
- Singh, G. Gastrointestinal complications of prescription and over‐the‐counter nonsteroidal anti‐inflammatory drugs: a view from the ARAMIS database. Arthritis, Rheumatism, and Aging Medical Information System. Am J Ther. March 2000 Mar;7(2):115‐21. Mar;7(2):115‐21. Mar;7(2):115‐21.;7(2):115‐21. [DOI] [PubMed] [Google Scholar]
Swierkosz 2002
- Swierkosz TA, Jordan L, McBride M, McGough K, Devlin J, Botting RM. Actions of paracetamol on cyclooxygenases in tissue and cell homogenates of mouse and rabbit. Med Sci Monit 2002;8(12):496‐503. [PubMed] [Google Scholar]
Tannenbaum 2000
- Tannenbaum H, Peloso PM, Russell AS, Marlow B. An evidence‐based approach to prescribing NSAIDs in the treatment of osteoarthritis and rheumatoid arthritis: the second Canadian Consensus Conference. Canadian Journal of Clinical Pharmacology 2000;7 (suppl A):4A‐16A. [PubMed] [Google Scholar]
Towheed 1997a
- Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the hip. J Rheumatol 1997;24:349‐57. [PubMed] [Google Scholar]
Towheed 1997b
- Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Semin Arth Rheum 1997;26:755‐70. [DOI] [PubMed] [Google Scholar]
Towheed 1998
- Towheed TE. The impact of musculoskeletal disorders in Canada. Annals of the Royal College of Physicians and Surgeons of Canada 1998;31(5):229‐232. [Google Scholar]
Towheed 2002
- Towheed TE. Published meta‐analyses of pharmacological therapies for osteoarthritis. Osteoarthritis and Cartilage In Press: November 2002. [DOI] [PubMed] [Google Scholar]
Towheed 2002b
- Towheed T, Shea B, Wells G, Hochberg M. Analgesia and non‐aspirin, non‐steroidal anti‐inflammatory drugs for osteoarthritis of the hip. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000517.pub2] [DOI] [PubMed] [Google Scholar]
Towheed 2005
- Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD002946. DOI: 10.1002/14651858.CD002946.pub2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tugwell 2004
- Tugwell P, Shea B, Boers M, Brooks P, Simon L, Strand V, Wells G. Introduction. Evidence Based Rheumatology. BMJ Books, 2004:xxvii. [Google Scholar]
Warner 1999
- Warner TD, Giuliao F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo‐oxygenase‐1 rather than cyclo‐oxygenase‐2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA 1999;96:7563‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wegman 2004
- Wegman A, Windt D, Tulder M, Stalman W, Vries T. Nonsteroidal antiinflammatory drugs or acetaminophen for osteoarthritis of the hip or knee? A systematic review of evidence and guidelines. J Rheumatol 2004;31(2):344‐54. [PubMed] [Google Scholar]
Wolfe 2000
- Wolfe F, Zhao D, Lane N. Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum 2000;43:378‐85. [DOI] [PubMed] [Google Scholar]
Zhang 2004
- Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta‐analysis of randomized controlled trials. Ann Rheum Dis 2004;63(8):901‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]


