Summary
No approved medication exists for the treatment of eosinophilic esophagitis (EoE) in the United States, which forces patients to utilize off-label drugs and/or create their own formulations. We assessed the efficacy of a standardized compounded fluticasone suspension. To do this, we performed a retrospective cohort study identifying all EoE patients treated with compounded fluticasone. Compounded fluticasone was prescribed during routine clinical care and dispensed by a specialty compounding pharmacy. Clinical data were extracted from medical records. Outcomes (symptomatic, endoscopic, and histologic) were assessed after the initial and last compounded fluticasone treatment in our system. There were 27 included patients (mean age 34.2; 67% male; 96% white) treated for a mean length of 5.4 ± 4.4 months. The majority (89%) previously utilized dietary elimination or topical corticosteroids, and many (75%) had primary non-response or secondary loss of response to these treatments. After starting compounded fluticasone, symptoms and endoscopic findings improved [dysphagia (89 vs. 56%, P = 0.005), food impaction (59 vs. 4%, P = 0.003), heartburn (26 vs. 4%, P = 0.01), chest pain (26 vs. 8%, P = 0.05), white plaques (63 vs. 32%; P = 0.005), furrows (81 vs. 60%; P = 0.06), and edema (15 vs. 4%; P = 0.16)]. The median of the peak eosinophil counts decreased from 52 to 37 eos/hpf (P = 0.10) and 35% of patients achieved <15 eos/hpf. In conclusion, compounded fluticasone provided a significant improvement in symptoms and endoscopic findings, with more than a third achieving histologic response in a treatment refractory EoE population. Compounded fluticasone should be considered as an EoE management option.
Keywords: clinical pharmacy, eosinophilic esophagitis, fluticasone, outcomes research
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic immune and antigen-mediated clinicopathologic condition characterized by esophageal eosinophilia and symptoms of esophageal dysfunction. In adults, EoE manifests with dysphagia and food impaction, while in children, symptoms include regurgitation, vomiting, and food intolerance.1,2 Recognition of the disease first occurred in the 1970s3 and publications in the mid/late 1990s identified EoE as a distinct entity.4,5 Recent data suggest that this disease is rapidly increasing in prevalence,6 becoming a major cause of esophageal morbidity and source of healthcare costs.7,8
Corticosteroids represent the first-line medicinal therapy for EoE following proton pump inhibitor (PPI) non-response9 decreasing clinical symptoms and improving histological features of the disease.10,11 However, no current FDA-approved medications exist for the treatment of EoE in the United States.10,12 This forces the use of asthma-specific steroid preparations off-label, which can lead to untoward side effects, difficult delivery, less than ideal targeting of esophageal mucosa,13 as well as variable drug concentrations and administration.14
A previous study from our group explored the use of a specialty pharmacy prepared budesonide formulation in the treatment of EoE.15 We have shown that this represents an effective alternative to patient-prepared formulations. In this study, we aimed to evaluate the efficacy of a standardized, viscous fluticasone as an alternative modality for the treatment of EoE.
METHODS
We conducted a retrospective cohort study at the University of North Carolina (UNC) utilizing the UNC EoE Clinicopathologic database. The UNC EoE Clinicopathologic database has been previously described.15–19 Cases of EoE were diagnosed per consensus guidelines at the time that they were included in the database.9,20 The UNC Institutional Review Board approved this study.
Data were extracted for EoE patients who were treated with compounded viscous fluticasone during a 6-year period (2014–2019). In order to better isolate the effect of compounded fluticasone, data were extracted from treatment periods when patients received therapy without additional systemic corticosteroids, additional topical corticosteroids, or other second-line EoE pharmacologic therapies. The patients who previously received these modalities were not excluded from the analysis. Patients maintained on a stable food elimination diet or stable PPI dosing were included. Patient inclusion required endoscopy with biopsy both before and after compounded fluticasone therapy, providing baseline and follow-up data. The decision to utilize compounded fluticasone was made at the discretion of individual provider in the course of routine clinical practice.
Compounded fluticasone suspension was prescribed at a concentration of 1 mg/8 mL during the course of routine clinical care and both prepared and dispensed by a single specialty outpatient compounding pharmacy (Chapel Hill Compounding Pharmacy). The medication was formulated as a viscous suspension by mixing powdered fluticasone with a Methocel gel at a concentration of 1 mg/8 mL. Strict quality-control guidelines were used during preparation. Compounded fluticasone was initiated at a total mean daily dose of 2.5 mg ± 0.9 (range 1.5–4 mg) based on severity of disease and provider preference. The most common starting daily total dose was 2 mg, which was prescribed in 19 (70%) of patients. Following the initiation of compounded fluticasone, the standard of practice at UNC is to repeat an upper endoscopy to assess for endoscopic and histologic response following approximately 8 weeks of therapy, though this was also at the individual providers’ discretion. After assessment of initial response, attempts could be made to reduce doses as symptoms and histology allowed.
Once the cohort was assembled, data were extracted from UNC’s electronic medical records and the UNC Clinicopathologic database into a standardized data collection form. The standardized data collection form included demographics, symptoms, previous treatments, endoscopic findings, and outcomes (symptomatic global response [yes/no], endoscopic response [% with individual findings], and histologic response [absolute eosinophil count; % with <15 eos/hpf]).21,22 Symptoms were coded using dichotomous variables signifying their presence or absence [yes/no] and as a global symptomatic response [yes/no per patient perception of disease activity at follow-up appointments], as per previous reports;15,17 validated symptom metric data were not available. Data were extracted from three time points, including baseline, after initially starting therapy, and following the last compounded fluticasone treatment in our system. Patients were defined as having prior primary histologic non-response to alternative therapies (≥15 eos/hpf on post-treatment biopsy) or secondary loss of response (initial histological response that was later lost on subsequent biopsy without changing dose or adding/changing alternative treatments). Compliance with compounded fluticasone was assessed using an ordinal variable with three categories: good, partial, and poor that were based on clinical report from the patient and physician assessment. Good compliance represented complete compounded fluticasone adherence; partial compliance represented adherence to 50% or more doses; poor compliance represented adherence to <50% of doses.
For analysis, descriptive statistics described the cohort including the mean, median, standard deviation, and the shape of the distribution for all continuous variables; frequencies were tabulated for categorical variables. Bivariable statistics analyzed the relationship between baseline and post-treatment symptomatic, endoscopic, and histologic outcomes. As repeat measures were analyzed, McNemar's chi-squared test was used for dichotomous variables [symptomatic and endoscopic response] and paired Wilcoxon sign rank for continuous variables [peak eosinophil counts]. Pearson's chi-squared test was otherwise used when comparing non-paired categorical variables. The Wilcoxon rank-sum test was used for comparing non-parametric and non-paired continuous variables. All analyses were performed using Stata 14.2 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics and prior treatments
There were 27 patients with EoE that met the criteria for study inclusion. The mean age was 34.2 years old with 96% white and 67% male patients, 89% ≥ 18 years at diagnosis (Table 1). Atopic disease and IgE-mediated food allergy were common, present in 41 and 33% of patients, respectively. Patients had an average of 12.2 years of symptoms prior to diagnosis of EoE. At the time of this study, the mean length of treatment with compounded fluticasone was 5.4 ± 4.4 months (range: 2.0–19.2 months).
Table 1.
Patient demographics (N = 27)
| Age at diagnosis (median years; IQR*) | 33.1; 24.3–41.8 | ||
|---|---|---|---|
| Length of symptoms before diagnosis (median years; IQR) | 10.0; 6.0–15.7 | ||
| Adult ≥18 years (n, %) | 23 (85) | ||
| Male (n, %) | 18 (67) | ||
| White (n, %) | 26 (96) | ||
| Atopic disease diagnosis (n, %) | 11 (41) | ||
| Food allergy (n, %) | 9 (33) | ||
| Prior treatments | n (%) | ||
| Food elimination diet (FED) | 12 (44) | ||
| Proton pump inhibitor | 27 (100) | ||
| Fluticasone | 9 (33) | ||
| Budesonide | 17 (63) | ||
| Systemic Steroids | 2 (7) | ||
| Dilation | 20 (74) | ||
| Patients receiving FED or tCS2 before compounded fluticasone (n, %) | Patients receiving FED before compounded fluticasone (n, %) | Patients receiving tCS before compounded fluticasone (n, %) | 10 or 20 loss of response to tCS or FED3 (n, %) |
| 24 (89) | 12 (44) | 20 (74) | 20 (75) |
*IQR: Interquartile range; 2tCS: topical corticosteroid
The included EoE patients in this study were treatment experienced (Table 1). Nearly half (43%) of patients had attempted a food elimination diet (FED). Many had previous topical corticosteroid (tCS) use, including formulations of swallowed/inhaled fluticasone (37%) and oral viscous budesonide (60%). Only 30% were naïve to treatment with tCS. Dilation was performed in 74% of patients before compounded fluticasone. Only 7% had systemic steroids previously prescribed for their disease. When considering prior receipt of either FED or tCS for EoE, the majority of patients had received one or both of these modalities (89%). More than half of the patients (65%) experienced a primary non-response and a smaller percentage (10%) experienced a secondary non-response to either the FED or tCS modalities. Of those patients with prior loss of response, 30% (6 of 20) trialed an FED with 33% showing a histological response. However, adherence and continued symptoms led to tCS initiation. No difference was observed in the proportion of patients having a dilation prior to initial endoscopy based on prior treatment response (e.g. for primary, secondary, or treatment naïve: 69 vs. 100 vs. 100%; P = 0.26). In terms of previous tCS dosing, approximately half (45%) of patients had loss of response on stable dosing. Throughout the initial follow-up period, patients predominantly demonstrated partial to good adherence to a compounded fluticasone (88%).
Prior to the initiation of fluticasone therapy, EoE-related clinical features were commonplace among the study population (Table 2). The majority of patients (89%) experienced dysphagia. Food impaction was another common complication, reported by 59% of the cohort. The next most common symptoms included heartburn (26%), chest pain (26%), weight loss (19%), and abdominal pain (22%). Similarly, typical endoscopic findings of EoE were found prior to the initial of compounded fluticasone with 85 and 81% of patients have rings and furrows, respectively. Baseline EREFS scores showed a total of 4.88 +/− 2.12 (edema: 0.92 +/− 0.74, rings: 1.23 +/− 0.86, exudates: 0.92 +/− 0.48, furrows: 0.96 +/− 0.60, stricture: 0.73 +/− 0.45). Prior to treatment initiation, the median eosinophil count was 52 eos/hpf (IQR: 35–80).
Table 2.
Patient symptoms, endoscopic, and histological findings
| Symptoms | Baseline (n, %) | After start of treatment (n, %) | Baseline vs initial response McNemar’s X2 | |
|---|---|---|---|---|
| % Dysphagia | 24 (89) | 15 (56) | 0.005 | |
| % Food impaction | 16 (59) | 1 (4) | 0.003 | |
| % Heartburn | 7 (26) | 1 (4) | 0.01 | |
| % Chest pain | 7 (26) | 2 (8) | 0.05 | |
| % Nausea | 3 (11) | 1 (4) | 0.16 | |
| % Vomiting | 3 (12) | 0 (0) | 0.03 | |
| % Weight loss | 5 (19) | 1 (4) | 0.05 | |
| % Food intolerance | 5 (19) | 1 (4) | 0.10 | |
| % Abdominal pain | 6 (22) | 2 (8) | 0.05 | |
| % Global improvement | n/a | 13 (48) | n/a | |
| Endoscopic findings | Baseline (n, %) | After start of treatment (n, %) | Baseline vs initial response McNemar’s X2 | |
| % Normal | 1 (4) | 3 (12) | 0.31 | |
| % Rings | 23 (85) | 22 (80) | 0.65 | |
| % Stricture | 21 (78) | 19 (72) | 0.65 | |
| % Narrowing | 9 (33) | 6 (24) | 0.56 | |
| % Furrows | 22 (81) | 16 (60) | 0.06 | |
| % White plaques | 17 (63) | 9 (32) | 0.005 | |
| % Edema | 4 (15) | 1 (4) | 0.16 | |
| % Dilated | 20 (74) | 18 (68) | 0.65 | |
| % Candida | 2 (7) | 1 (4) | 0.32 | |
| Histology | Baseline Eos/HPF (median; IQR) | After treatment start Eos/HPF (median; IQR) | % < 15 eos/hpf (n, %) | Wilcoxon sign rank |
| 52; 35–80 | 37; 9–70 | 9 (35) | 0.10 |
The initiation of compounded fluticasone treatment was either a once or twice daily dose at the discretion of the provider. There were 19 (70%) patients with once daily dosing and 8 (30%) with twice daily prescription. The most common starting daily total dose was 2 mg, which was prescribed in 19 (70%) of patients. The mean starting dose of compounded fluticasone was 2.5 mg ± 0.9 (range 1.5–4 mg).
Initial clinical, endoscopic, and histologic response to compounded fluticasone
Following the initial course of compounded fluticasone, approximately half of patients (48%) reported a global improvement in EoE-related symptoms (Table 2). Of the 48% with global improvement, 8 were prior non-responder, 0 were prior responders, and 4 were treatment naïve. There was a 33% absolute reduction in dysphagia, dropping from 89 to 56% (P = 0.005). The complaint of dysphagia in patients with and without dilation did not differ following initiation of compounded fluticasone (56 vs. 57%; P = 0.94). Food impactions also decreased significantly, from 59 to 4% (P < 0.001). The proportion of patients with a food impaction following initiation of compounded fluticasone did not differ by dilation status (0 vs. 14%; P = 0.10). Clinically significant improvements were also recorded for heartburn (26 to 4%, P = 0.01), chest pain (26 to 8%, P = 0.05), weight loss (19 to 4%, P = 0.05), and vomiting (22 to 0%, P = 0.03).
Several endoscopic findings improved with administration of compounded fluticasone. Furrows decreased from 81 to 60% (P = 0.06); white plaques decreased from 63 to 32% (P = 0.005); esophageal edema trended toward improvement, decreasing from 15 to 4% (P = 0.16). EREFS also saw significant improvement in total score to 3.43 +/− 2.31 (P = 0.004) (edema: 0.5 +/− 0.60, P = 0.01; rings: 1.0 +/− 0.67, P = 0.18; exudates: 0.59 +/− 0.59, P = 0.03; furrows: 0.66 +/− 0.56, P = 0.06; stricture: 0.68 +/− 0.48, P = 0.65). Endoscopic findings of rings, strictures, and diffuse esophageal narrowing did not substantially change following therapy (Table 2). The proportion of patients undergoing dilation remained stable between baseline EGD (74%) and post-treatment (68%) (P = 0.65). Histologically, there was a trend towards reduction of eosinophils observed on biopsy with the median number decreasing from 52 eos/HPF down to 37 eos/HPF following treatment initiation (P = 0.10) (Table 2). This was associated with 35% of patients achieving a complete histological response (<15 eos/hpf) to compounded fluticasone therapy.
Dose changes were rare during the follow-up period. In two patients, doses were increased from 2 mg daily to 2 mg twice per day after an initial histologic non-response. Follow-up endoscopy for these patients showed peak eosinophil counts of 45 and 56 eos/hpf, respectively. A third patient had a follow-up examination after decreasing their starting dose from 2 mg daily to 0.6 mg daily with a continued histologic response (10 eos/hpf followed by 3 eos/hpf). Patients who were started on a daily dose of 1.5 or 2.0 mg were compared those with a starting dose of 4.0 mg. At initial follow-up, follow-up peak eosinophil counts differed among these groups, 28.5 versus 105.5 eos/hpf (P = 0 0.02). Symptoms and endoscopic findings did not show significant difference after compounded fluticasone initiation between these cohorts.
Last clinical, endoscopic, and histologic response to compounded fluticasone
After a follow-up time of 5.5 +/− 4.4 months (range: 2.0–19.2) on compounded fluticasone, the majority (57%) of the cohort remained on the medication. The most common reason for discontinuing compounded fluticasone was primary histologic non-response (27%), followed by relocation out of UNC’s practice network (7%) and medication expense (3%). A single patient’s medication (3%) was stopped secondary to CMV ulcer development, which occurred on high-dose compounded fluticasone following an increase to 2 mg twice daily. Another additional patient (3%) self-discontinued the medication given personal preference to stop all medicinal therapies.
At the time of last follow-up encounter, favorable symptom responses were recorded. Most (70%) patients at this point in treatment reported a sustained global improvement in symptoms. Compared to baseline, individual symptoms of dysphagia (89 vs. 40%; P = 0.03), food impaction (59% vs. 0; P = 0.008), heartburn (26 vs. 10%; P = 0.16), and chest pain (26 vs. 10%; P = 0.31) trended toward sustained improvements. Only a minority (6; 22%) of patients underwent a second endoscopy with biopsies while continuing on longer-term compounded fluticasone treatment. EREFS scores for second follow-up endoscopy down trended to 4.00 +/− 2.12 (edema: 1.10 +/− 0.55, P = 0.48; rings: 0.90 +/− 0.74, P = 0.34; exudates: 0.60 +/− 0.55, P = 0.16; furrows: 0.80 +/− 0.45, P = 0.32; stricture: 0.60 +/− 0.55, P = 1.0) compared to baseline score of 5.6 +/− 1.3 (P = 0.09) (edema: 1.40 +/− 0.89; rings: 1.40 +/− 0.89; exudates: 1 +/− 0; furrows: 1 +/− 0; stricture: 0.6 +/− 0.55). Reasons for a second follow-up upper endoscopy included new epigastric discomfort (1 patient), re-evaluation after patient recorded poor initial medication compliance (1 patient), evaluation after dose increase (3 patients), and evaluation following seasonal allergy exacerbation (1 patient). Limited numbers restricted interpretation of endoscopic findings from baseline at this point in therapy. The median eosinophil count at last follow-up was 34 eos/hpf, which associated with a 25% histologic response (median baseline 52 vs. 34; P = 0.08).
Treatment response among patients with a prior non-response to topical corticosteroid and/or food elimination diet
Within our cohort, there were 15/20 (75%) patients who had previously not responded or lost response to a tCS and/or FED (mean age at diagnosis: 30.6 years old; 67% male; 100% white) (Table 1). Despite being treatment experienced, approximately half of these patients (53%) reported a global improvement in their symptoms. After viscous fluticasone, individual symptoms of dysphagia (87 vs. 60%; P = 0.05), chest pain (33 vs. 13%; P = 0.08), and reported food impactions (53 vs. 7%; P = 0.008) significantly improved or trended toward improvement (Fig. 1). Some endoscopic features also improved in this subset, including the proportion with normal endoscopies (0 vs. 20%; P = 0.08), white plaques (67 vs. 33%; P = 0.03), decreased vascularity (13 vs. 7%; P = 0.32), rings (80 vs. 80%; P = 1), and furrows (87 vs. 67%; P = 0.18) (Fig. 2). The proportion requiring dilation (73 vs. 67%; P = 0.56) did not meaningfully change following the initiation of compounded fluticasone. Median eosinophils decreased following initiation of therapy (55 vs. 30 eos/hpf; P = 0.06).
Fig. 1.
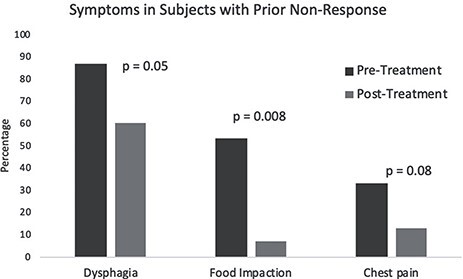
Symptom response in subjects with prior non-response, pre-treatment versus post-treatment.
Fig. 2.
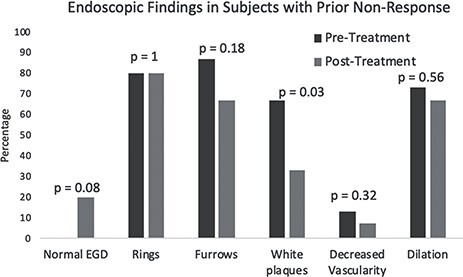
Endoscopic response in subjects with prior non-response, pre-treatment versus post-treatment.
Patients with a prior non-response and/or loss of response to tCS or FED were also compared to those without a prior non-response and/or loss of response to treatment. Some notable differences were observed (Table 3). Specifically, at initial follow-up, mean eosinophils were 30 eos/hpf in the prior non-responders versus 46 eos/hpf in those without prior non-response (P = 0.48). A post-treatment normal EGD was seen more often in prior non-responders (20% vs. 0; P = 0.40) after compounded fluticasone. Furthermore, it appears that a global response to therapy was more common in those with a prior non-response and/or loss of response compared to those without a prior non-response (53% vs. 0; P = 0.09).
Table 3.
Selected outcomes for prior non-response versus no prior non-response
| Outcome | Prior non-response | No prior non-response | P-value |
|---|---|---|---|
| Initial follow-up eosinophils (median; IQR) | 37; 9–75 | 46; 37–70 | 0.44 |
| Normal EGD (n, %) | 3 (20) | 0 (0) | 0.40 |
| Global response (n, %) | 8 (53) | 0 (0) | 0.09 |
DISCUSSION
Topical corticosteroids represent an effective first-line treatment modality for patients with EoE.9,11,14,23,24 However, as no FDA-approved medication exists for EoE in the US, patients must utilize off-label steroid formulations primarily intended for respiratory diseases. This is not ideal, as the utilization of patient-prepared corticosteroid formulations leads to variable drug concentrations and poor administration.13 As previously described by our group, pharmacy-prepared compounded steroids help standardize dosing, simplify clinical application, and may improve outcomes.15 In this study, we aimed to evaluate the efficacy of compounded fluticasone in a cohort of treatment experienced EoE patients, a preparation that has not been extensively reported.
Our data indicate that compounded fluticasone results in significant improvement in EoE clinical and endoscopic findings, with trend towards improvement in eosinophil counts. This is notable because in this cohort, where three-quarters of those previously receiving first-line therapies had no response to or loss of response to a tCS and/or FED, 35% achieved histological remission (<15 eos/hpf) with compounded fluticasone treatment. Compounded fluticasone also led to a global symptomatic improvement in approximately half of the population at initial follow-up, which was largely sustained by the end of available follow-up in patients remaining on treatment. Upon sub-analysis of the portion of the cohort with prior non-response to tCS or FED modalities, we noted improvements in symptomatic, endoscopic, and histologic features. Interestingly, those with prior non-response reported greater global improvement than those without prior non-response. We acknowledge that some of this symptom improvement could also be attributed to high rates of esophageal dilation; however, no significant difference was observed in the symptom of dysphagia or food impaction between those dilated and those not dilated prior to fluticasone initiation. The proportion dilated among the treatment experienced and treatment naïve also did not differ. Overall, this preparation of fluticasone appears to represent an effective induction therapy for patients with EoE.
The current study utilizes a novel viscous fluticasone formulation, which has not been previously described in the literature. We note that Kia et al. performed a similar study using the powder extracted from the blister packs inside of a fluticasone disk device that is placed on the tongue and directly swallowed rather than mixed into a suspension.25 They found similar improvements in histological, endoscopic, and symptomatic response. The fluticasone formulation for our study was pharmacy-prepared as a viscous suspension and prescribed with strict protocols allowing for ease of dosing and administration.
Based on our findings, compounded fluticasone represents a therapeutic option to induce remission in treatment naïve as well as treatment-experienced patients. Clinical improvement in this latter group of patients is particularly notable. The treatment-experienced group had a trend toward lower eosinophil counts at initial follow-up compared to treatment naïve patients. Higher histologic eosinophils observed in those without previous non-response may be subject to seasonality,26 random variation,27,28 or a potential sign of ongoing worsening of disease, and clinical significance is unclear. Yet, prior work underscores the importance of the formulation utilized in topical corticosteroid administration.10,13,29,30 For example, in a study by our group evaluating pharmacy compounded viscous budesonide, 28% of the treatment group had undergone previous tCS therapy without histological response. However, 47% were able to gain histological remission (<15 eos/hpf) with the compounded formulation, which is the budesonide preparation comparable to the fluticasone utilized in this study.29 This supports prior work showing that viscous topical compounds are more effective than nebulized.13 Furthermore, a phase 1b/2a randomized clinical trial utilizing a fluticasone propionate orally disintegrating tablet showed efficacy, with histological response at 8 weeks in 75 and 63% of patients using 1.5 mg BID an 3.0 mg BID, respectively.31 Similar results were seen after 12 weeks of treatment in the follow-up phase 2b study.32 These response rate were within range of previously published clinical trials (e.g. typically 19–89%) and emphasizes the importance of formulation and drug delivery.33,34
Maintaining tight disease control is important longitudinally for patients with EoE, as ongoing histologic response associates with decreased deleterious outcomes including esophageal strictures and food bolus impactions.8,6,35 However, data also indicate that patients may lose response to formally effective steroid treatments. For example, prior work suggests that histologic response may be lost in as many as 50 and 75% of patients at 1.5 years and 2.5 years, respectively.18 These observations support the role for and necessity of additional options in the armamentarium of EoE therapeutics. Data on comparisons of inhaled glucocorticoids have shown fluticasone to be more potent than budesonide on a microgram to microgram basis and have higher receptor affinity, which raises the possibility that fluticasone may behave similarly in EoE therapy.36,37 As 35% of the patients included in this study obtained histologic response, despite 75% of the cohort having had no response or loss of response to prior tCS and/or FED therapies, there may be utility into switching to a different tCS, such as compounded fluticasone, in patients who previously lost control of their disease.
This study does have limitations. One of the main limitations would be its retrospective nature, and because of this, the symptoms were assessed subjectively. However, individual symptoms were rigorously sought during the medical record review in this study and recorded onto standardized collection forms. Moreover, as this study was retrospective, we were not able to use validated patient reported outcomes for variable assessment. However, objective endoscopic and histologic findings are reported, and trend in the same direction as the global symptom response measures. This study also has several strengths. It uses a retrospective cohort design that studies a formulation of fluticasone that has not yet been evaluated in the literature. In addition, the population in the study is similar to that in clinical practice, with a large proportion of the patients having undergone previous therapies or loss of response to specific treatments.
In conclusion, our novel compounded fluticasone formulation led to improvement of clinical, endoscopic, and histological features in a cohort of EoE patients that were largely refractory to prior treatments. This adds an additional treatment option, and if future prospective studies have similar results, this formulation may help achieve longer-term histological remission and the prevention of long-term sequelae of disease in those who lost histologic response to formerly utilized treatments. Therefore, compounded fluticasone represents an effective alternative therapy for patients with EoE.
Grant Support
This research was supported by NIH Awards R01 DK101856.
Conflict of interest
Dr. Dellon is a consultant for Abbott, Adare, Aimmune, Allakos, Arena, AstraZeneca, Biorasi, Calypso, Celgene/Receptos, Eli Lilly, EsoCap, Gossamer Bio, GSK, Parexel, Regeneron, Robarts, Salix, and Shire/Takeda; receives research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire/Takeda; and has received an educational grant from Allakos, Banner, and Holoclara. None of the other authors report and have potential conflict of interest with this study.
Contributor Information
Corey J Ketchem, Center for Esophageal Diseases and Swallowing, and Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Craig C Reed, Center for Esophageal Diseases and Swallowing, and Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Zoe Stefanadis, Chapel Hill Compounding Pharmacy, Chapel Hill, NC, USA.
Evan S Dellon, Center for Esophageal Diseases and Swallowing, and Center for Gastrointestinal Biology and Disease, Division of Gastroenterology and Hepatology, Department of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
References
- 1. Straumann A, Katzka D A. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology 2018; 154: 346–59. [DOI] [PubMed] [Google Scholar]
- 2. Furuta G T, Liacouras C A, Collins M H et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133: 1342–63. [DOI] [PubMed] [Google Scholar]
- 3. Landres R T, Kuster G G, Strum W B. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978; 74: 1298–301. [PubMed] [Google Scholar]
- 4. Attwood S E, Smyrk T C, Demeester T R, Jones J B. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38: 109–16. [DOI] [PubMed] [Google Scholar]
- 5. Straumann A, Spichtin H P, Bernoulli R, Loosli J, Vögtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr 1994; 124: 1419–29. [PubMed] [Google Scholar]
- 6. Dellon E S, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018; 154: 319–332 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jensen E T, Kappelman M D, Martin C F, Dellon E S. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015; 110: 626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaheen N J, Mukkada V, Eichinger C S, Schofield H, Todorova L, Falk G W. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus 2018; 31: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dellon E S, Liacouras C A, Molina-Infante J et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 2018; 155: 1022–1033 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucendo A J, Miehlke S, Schlag C et al. Efficacy of budesonide orodispersible tablets as induction therapy for eosinophilic esophagitis in a randomized placebo-controlled trial. Gastroenterology 2019; 157: 74–86 e15. [DOI] [PubMed] [Google Scholar]
- 11. Cotton C C, Eluri S, Wolf W A, Dellon E S. Six-food elimination diet and topical steroids are effective for eosinophilic esophagitis: a meta-regression. Dig Dis Sci 2017; 62: 2408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirano I, Spechler S, Furuta G, Dellon E S, White Paper A G A. Drug development for eosinophilic esophagitis. Clin Gastroenterol Hepatol 2017; 15: 1173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dellon E S, Sheikh A, Speck O et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012; 143: 321–4 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dellon E S, Woosley J T, Arrington A et al. Efficacy of budesonide vs fluticasone for initial treatment of eosinophilic esophagitis in a randomized controlled trial. Gastroenterology 2019; 157: 65–73 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed C C, Fan C, Koutlas N et al. Compounded oral viscous budesonide is effective and provides a durable response in eosinophilic esophagitis. HSOA J Gastroenterol Hepatol Res 2018; 7: 2509–15. [PMC free article] [PubMed] [Google Scholar]
- 16. Dellon E S, Gibbs W B, Fritchie K J et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009; 7: 1305–13 quiz 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reed C C, Fan C, Koutlas N T, Shaheen N J, Dellon E S. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2017; 46: 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eluri S, Runge T M, Hansen J et al. Diminishing effectiveness of long-term maintenance topical steroid therapy in PPI non-responsive eosinophilic esophagitis. Clin Transl Gastroenterol 2017; 8: e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Runge T M, Eluri S, Cotton C C et al. Causes and outcomes of Esophageal perforation in eosinophilic esophagitis. J Clin Gastroenterol 2017; 51: 805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liacouras C A, Furuta G T, Hirano I et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20 e6 quiz 21-2. [DOI] [PubMed] [Google Scholar]
- 21. Wolf W A, Cotton C C, Green D J et al. Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J Gastroenterol Hepatol Res 2015; 4: 1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reed C C, Wolf W A, Cotton C C et al. Optimal histologic cutpoints for treatment response in patients with eosinophilic esophagitis: analysis of data from a prospective cohort study. Clin Gastroenterol Hepatol 2018; 16: 226–233 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rank M A, Sharaf R N, Furuta G T et al. Technical review on the Management of Eosinophilic Esophagitis: a report from the AGA Institute and the joint task force on allergy-immunology practice parameters. Gastroenterology 2020; 158: 1789–1810 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirano I, Chan E S, Rank M A et al. AGA Institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020; 158: 1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kia L, Nelson M, Zalewski A et al. Oral delivery of fluticasone powder improves esophageal eosinophilic inflammation and symptoms in adults with eosinophilic esophagitis. Dis Esophagus 2018; 31: 1–6. [DOI] [PubMed] [Google Scholar]
- 26. Dowling P J, Neuhaus H, Polk B I. The role of the environment in eosinophilic esophagitis. Clin Rev Allergy Immunol 2019; 57: 330–9. [DOI] [PubMed] [Google Scholar]
- 27. Shah A, Kagalwalla A F, Gonsalves N, Melin-Aldana H, Li B U K, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol 2009; 104: 716–21. [DOI] [PubMed] [Google Scholar]
- 28. Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao M S, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006; 64: 313–9. [DOI] [PubMed] [Google Scholar]
- 29. Dellon E S, Katzka D A, Collins M H et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology 2017; 152: 776–786 e5. [DOI] [PubMed] [Google Scholar]
- 30. Miehlke S H P, Von Arnim U, Madisch A et al. Two new budesonide formulations are highly efficient for treatment of active eosinophilic esophagitis: results from a randomized, double-blind, double-dummy, placebo-controlled multicenter trial. Gastroenterology 2014; 146(5): S-16. [Google Scholar]
- 31. Hirano I, Safroneeva E, Roumet M C et al. Randomised clinical trial: the safety and tolerability of fluticasone propionate orally disintegrating tablets versus placebo for eosinophilic oesophagitis. Aliment Pharmacol Ther 2020; 51: 750–9. [DOI] [PubMed] [Google Scholar]
- 32. Dellon E S, Katzka D A, Collins M H et al. Safety and efficacy of budesonide oral suspension maintenance therapy in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019; 17: 666–673 e8. [DOI] [PubMed] [Google Scholar]
- 33. Dellon E S, Gonsalves N, Hirano I et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108: 679–92 quiz 693. [DOI] [PubMed] [Google Scholar]
- 34. Chang J W, Yeow R Y, Waljee A K, Rubenstein J H. Systematic review and meta-regressions: management of eosinophilic esophagitis requires histologic assessment. Dis Esophagus 2018; 31: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoepfer A M, Safroneeva E, Bussmann C et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145: 1230–6 e1-2. [DOI] [PubMed] [Google Scholar]
- 36. Hubner M, Hochhaus G, Derendorf H. Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin North Am 2005; 25: 469–88. [DOI] [PubMed] [Google Scholar]
- 37. Nielsen L P, Dahl R. Therapeutic ratio of inhaled corticosteroids in adult asthma. A dose-range comparison between fluticasone propionate and budesonide, measuring their effect on bronchial hyperresponsiveness and adrenal cortex function. Am J Respir Crit Care Med 2000; 162: 2053–7. [DOI] [PubMed] [Google Scholar]


