Abstract
OBJECTIVES:
Hypercoagulability may be a key mechanism for acute organ injury and death in patients with severe coronavirus disease 2019, but the relationship between elevated plasma levels of D-dimer, a biomarker of coagulation activation, and mortality has not been rigorously studied. We examined the independent association between D-dimer and death in critically ill patients with coronavirus disease 2019.
DESIGN:
Multicenter cohort study.
SETTING:
ICUs at 68 hospitals across the United States.
PATIENTS:
Critically ill adults with coronavirus disease 2019 admitted to ICUs between March 4, 2020, and May 25, 2020, with a measured D-dimer concentration on ICU day 1 or 2.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The primary exposure was the highest normalized D-dimer level (assessed in four categories: < 2×, 2–3.9×, 4–7.9×, and ≥ 8× the upper limit of normal) on ICU day 1 or 2. The primary endpoint was 28-day mortality. Multivariable logistic regression was used to adjust for confounders. Among 3,418 patients (63.1% male; median age 62 yr [interquartile range, 52–71 yr]), 3,352 (93.6%) had a D-dimer concentration above the upper limit of normal. A total of 1,180 patients (34.5%) died within 28 days. Patients in the highest compared with lowest D-dimer category had a 3.11-fold higher odds of death (95% CI, 2.56–3.77) in univariate analyses, decreasing to a 1.81-fold increased odds of death (95% CI, 1.43–2.28) after multivariable adjustment for demographics, comorbidities, and illness severity. Further adjustment for therapeutic anticoagulation did not meaningfully attenuate this relationship (odds ratio, 1.73; 95% CI, 1.36–2.19).
CONCLUSIONS:
In a large multicenter cohort study of critically ill patients with coronavirus disease 2019, higher D-dimer levels were independently associated with a greater risk of death.
Keywords: anticoagulant, cohort study, coronavirus disease 2019, critical care, D-dimer, mortality
As of October 15, 2020, the coronavirus disease 2019 (COVID-19) pandemic has infected over 38 million individuals globally (1). Emerging data suggest a hypercoagulable state exists in patients with COVID-19, particularly in those who are critically ill, which may contribute to the high morbidity and mortality observed in this population (2–4). These observations have led to intense research efforts into the clinical application of hemostasis biomarkers as risk factors for severe disease and therapeutic anticoagulation as a potential intervention to improve outcomes (5, 6). Understanding the clinical implications of abnormal markers of hemostasis independent of other risk factors in patients with COVID-19 remains an important knowledge gap, especially in the context of ongoing clinical trials of therapeutic anticoagulation (7, 8).
D-dimer is generated by the lysis of cross-linked fibrin monomers, and a high plasma concentration is indicative of repeated coagulation and fibrinolysis. Clinically, a high D-dimer is usually considered a marker of coagulation activation but can also represent pathologic fibrinolysis (9). High D-dimer concentration has been previously described in many severe illnesses, including other viral infections (10, 11), and is similarly associated with death in critically ill patients (12, 13).
Prior studies have suggested that high D-dimer level is a risk marker for mortality and thromboembolic events in patients with COVID-19 (14–16), and several investigators have hypothesized that elevated D-dimer levels can be used to help guide interventions such as therapeutic anticoagulation (17, 18). Despite these findings, the prognostic role of D-dimer in patients with severe illness from COVID-19 is not established. No study has rigorously assessed whether higher D-dimer levels are independently associated with mortality in patients with COVID-19 after accounting for other risk factors, including acute severity of illness and anticoagulation status. Additionally, prior studies were limited by modest sample size, limited generalizability (due to being single center), heterogeneous patient populations that included both critically ill and noncritically ill patients, and failure to consider the important differences between D-dimer assays used at different institutions (4, 14, 15, 19, 20).
We used data from a large multicenter cohort study of critically ill patients with COVID-19 to examine whether higher D-dimer levels are independently associated with a higher risk of death. These analyses aim to provide urgently needed context to the assessment of D-dimer elevation in patients with severe illness from COVID-19.
MATERIALS AND METHODS
Study Design and Data Collection
We used data from the Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19 (STOP-COVID), a multicenter cohort study that enrolled consecutive adults with laboratory-confirmed COVID-19 admitted to participating ICUs at 68 hospitals across the United States (21). A list of all STOP-COVID participating sites is provided in Table S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/G206). Data were collected by study staff by detailed chart review and entered into a standardized electronic data collection form (Research Electronic Data Capture). All data were validated through a series of automated and manual checks. Variable definitions are further described in the Supplemental Methods (Supplemental Digital Content 1, http://links.lww.com/CCM/G206). STOP-COVID was approved with a waiver of informed consent by the Institutional Review Board (IRB) at each participating site (protocol number 2007000003 for the Mass General Brigham IRB).
Study Population
We included patients admitted to participating ICUs between March 4, 2020 and May 25, 2020, with at least one measured D-dimer within 2 days following ICU admission. No formal sample size calculation was performed. For the primary analysis, patients were followed until the first of hospital discharge, death, or 28 days from ICU admission. Patients who were discharged alive from the hospital prior to 28 days were considered to be alive at 28 days (we tested the validity of this assumption in a subset of patients, described in the Supplemental Methods, Supplemental Digital Content 1, http://links.lww.com/CCM/G206). In the secondary analysis, patients were followed until the first of hospital discharge, death, or 90 days from ICU admission.
D-dimer Measurement and Normalization
D-dimer testing was ordered as part of routine clinical care, and measurements were performed according to each site’s standard laboratory procedures. To limit potential variability induced by differing practice patterns across hospitals, extreme values assessed by serial dilution were set to greater than or equal to the maximum value of assay linear range (Table 1).
TABLE 1.
D-dimer Assays and Assay-Specific Results
| D-dimer Assay | Units | Linear Range | ULN | Upper Limit of Linear Assay Detection/ULN | No. of Patients | No. of Sites | Median (IQR) Fold-Difference From ULN |
|---|---|---|---|---|---|---|---|
| Stago STA Liatest | ng/mL FEU | 270–4,000 | 500 | 8.0 | 1,111 | 21 | 4.7 (2.7–8.0) |
| HemosIL HS | ng/mL D-dimer units | 150–3,680 | 243 | 15.1 | 769 | 13 | 3.3 (1.7–10.6) |
| Siemans Innovance | ng/mL FEU | 120–4,460 | 500 | 8.9 | 723 | 11 | 3.3 (1.7–8.3) |
| HemosIL HS500 | ng/mL FEU | 215–7,650 | 500 | 15.3 | 542 | 16 | 3.4 (1.8–10.2) |
| bioMérieux Vidas | ng/mL FEU | 45–10,000 | 500 | 20.0 | 151 | 3 | 2.6 (1.7–4.9) |
| Roche Tina-quant | ng/mL FEU | 150–9,000 | 500 | 18.0 | 122 | 2 | 3.5 (1.8–10.3) |
FEU = fibrinogen equivalence units, IQR = interquartile range, ULN = upper limit of normal defined as the assay manufacturer specified venous thromboembolism cutoff.
To compare D-dimer measurements across assays, values were normalized by dividing the assay result by the assay-specific upper limit of normal (ULN) (Table 1). The unitless multiples of ULN were then analyzed as categories, guided by clinical recommendations, previous findings, and assay ranges: less than 2× ULN (reference group), 2–3.9× ULN, 4–7.9× ULN, and greater than or equal to 8× ULN (7, 15).
Outcomes
The primary outcome was 28-day mortality. The secondary outcome was 90-day mortality.
Statistical Analysis
Primary Analysis.
Categorical variables are expressed as count and percentage, continuous variables are expressed as median and interquartile range. Multivariable logistic regression with complete case analysis was used to examine the association between D-dimer and 28-day mortality. D-dimer was assessed as the highest value within 2 days following ICU admission. Our multivariable adjustment strategy was hierarchical and based on biological and clinical plausibility of covariates as potential confounders of the association between D-dimer and death. Model 1 was unadjusted. Model 2 was adjusted for the following demographics and comorbidities: age, sex, race, hypertension, diabetes mellitus, body mass index, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, current smoking status, and active malignancy. Model 3 was further adjusted for time from symptom onset to ICU admission, as well as each of the following acute severity of illness covariates assessed within 2 days following ICU admission: lymphopenia (defined as lymphocyte count < 1,000 per mm3), shock (defined as requirement for ≥ 2 vasopressors), receipt of invasive mechanical ventilation, and the highest renal, liver, and coagulation Sequential Organ Failure Assessment (SOFA) scores. Model 4 was further adjusted for medications impacting hemostasis, including receipt of therapeutic anticoagulants at home, as well as receipt of therapeutic anticoagulants, aspirin, and steroids within 2 days following ICU admission. Detailed definitions of model covariates are provided in the Supplemental Methods (Supplemental Digital Content 1, http://links.lww.com/CCM/G206).
Data Completeness.
Data on body mass index were missing in 3.6% of patients and were not imputed. Lymphocyte count measurement was missing in 8.6% of patients and was modeled with a separate term, as these data may not have been missing at random. Data pertaining to at least one SOFA component score were missing in 5.4% of patients and categorized as 0 (22– 24). All other data were complete.
Secondary Analysis.
We conducted similar analyses as above but extended the endpoint from 28- to 90-day mortality.
Sensitivity Analyses.
In a sensitivity analysis, we only included patients with D-dimer measured by the most common assay. In this analysis, D-dimer was considered both categorically, as above, and continuously. Restricted cubic splines were also used to examine the potential for a nonlinear relationship between D-dimer and death.
Mortality Risk Stratification.
In order to provide clinical context to these findings, we explored the ability of D-dimer to assist in risk stratification of death using a series of hypothetical patients. To do so, we fit additional logistic regression models to the cohort data and examined mortality risk with varying patient characteristics. Descriptions of these methods may be found in the Supplementary Appendix (Supplemental Digital Content 1, http://links.lww.com/CCM/G206). Analyses were performed using R (Version 3.4.6, R Foundation, Vienna, Austria).
RESULTS
Patient Characteristics
The initial study population included 4,949 patients from 68 centers, of whom 3,418 (69.1%) from 66 centers had at least one D-dimer measured in the first 2 days following ICU admission. Patients with D-dimer measured had similar baseline characteristics as those without D-dimer measured but had higher rates of invasive mechanical ventilation and shock within 2 days of ICU admission (Table S2, Supplemental Digital Content 1, http://links.lww.com/CCM/G206).
Among the 3,418 patients with a measured D-dimer, the median age was 62 years (interquartile range, 51–71 yr), 2170 (63%) were male, and 1,315 (38%) were White (Table 2). Those with higher D-dimer levels were more likely to be male, non-White, and to have a prior diagnosis of hypertension and coronary artery disease compared with those in lower D-dimer categories. Patients with higher D-dimer levels also had higher rates of invasive mechanical ventilation and shock and were more likely to have higher coagulation, renal, and liver SOFA scores on ICU admission compared with patients with lower D-dimer levels. Fewer patients with higher D-dimer levels were receiving therapeutic anticoagulants prior to hospitalization. However, in regard to in-hospital COVID-19 treatment, more received therapeutic anticoagulants and steroids within 2 days following ICU admission (Table 2).
TABLE 2.
Baseline Characteristics by D-dimer Category
| D-dimer, Fold-Difference Above Upper Limit of Normal | |||||
|---|---|---|---|---|---|
| < 2× | 2–3.9× | 4–7.9× | ≥ 8× | ||
| Characteristics | All Patients (n = 3,418) | (n = 938) | (n = 875) | (n = 582) | (n = 1,023) |
| Demographics | |||||
| Age (yr), median (IQR) | 62 (51–71) | 59 (49–68) | 62 (51–71) | 64 (54–73) | 63 (51–71) |
| Male sex, n (%) | 2,170 (63.1) | 581 (61.9) | 626 (60.0) | 372 (63.9) | 692 (67.6) |
| Race, n (%) | |||||
| White | 1,315 (38.5) | 403 (43.0) | 336 (38.4) | 223 (38.3) | 353 (34.5) |
| Black | 1,030 (30.1) | 244 (26.0) | 292 (33.4) | 154 (26.5) | 340 (33.2) |
| Asian | 190 (5.6) | 57 (6.1) | 49 (5.6) | 41 (7.0) | 43 (4.2) |
| Other | 880 (25.7) | 233 (24.8) | 197 (22.5) | 164 (28.2) | 286 (28.0) |
| Hispanic, n (%) | 858 (25.1) | 280 (29.9) | 204 (23.3) | 126 (21.6) | 248 (24.2) |
| Body mass index, median (IQR) | 30 (27–36) | 31 (27–36) | 30 (26–36) | 30 (26–35) | 30 (26–35) |
| Pregnant, n (%) | 34 (1.0) | 13 (1.4) | 16 (1.8) | 3 (0.5) | 2 (0.2) |
| Coexisting conditions, n (%) | |||||
| Diabetes mellitus | 1,444 (42.2) | 366 (39.0) | 402 (45.9) | 250 (43.0) | 1,444 (42.2) |
| Hypertension | 2,089 (61.1) | 519 (55.3) | 531 (60.7) | 386 (66.3) | 653 (63.8) |
| Chronic obstructive pulmonary disease | 272 (8.0) | 91 (9.7) | 72 (8.2) | 49 (8.4) | 60 (5.9) |
| Current smoker | 160 (4.7) | 48 (5.1) | 38 (4.3) | 31 (5.3) | 43 (4.2) |
| Coronary artery disease | 436 (12.8) | 110 (11.7) | 123 (14.1) | 82 (14.1) | 121 (11.8) |
| Congestive heart failure | 332 (9.7) | 89 (9.5) | 72 (8.2) | 49 (8.4) | 60 (5.9) |
| Active malignancy | 142 (4.2) | 34 (3.6) | 39 (4.5) | 26 (4.5) | 43 (4.2) |
| ≤ 3 d from symptoms to ICU admission, n (%) | 726 (21.2) | 201 (21.4) | 184 (21.0) | 133 (22.9) | 208 (20.3) |
| Severity of illness within 2 d after ICU admission, n (%) | |||||
| Invasive mechanical ventilation | 2,359 (69.0) | 517 (55.1) | 563 (64.3) | 452 (77.7) | 827 (80.8) |
| Shock | 609 (17.8) | 106 (11.3) | 136 (15.5) | 107 (18.4) | 260 (25.4) |
| Lymphocyte count < 1,000 per mm3 | 1,501 (43.9) | 266 (28.4) | 318 (36.3) | 277 (47.6) | 640 (62.6) |
| Coagulation component of SOFA score | |||||
| 0 | 2,684 (78.5) | 726 (77.4) | 690 (78.9) | 455 (78.2) | 813 (79.5) |
| 1 | 533 (15.6) | 165 (17.6) | 142 (16.2) | 86 (14.8) | 140 (13.7) |
| ≥ 2 | 201 (5.9) | 47 (5.0) | 43 (4.9) | 41 (7.0) | 70 (6.8) |
| Liver component of SOFA score | |||||
| 0 | 2,933 (85.8) | 863 (92.0) | 777 (88.8) | 495 (85.1) | 798 (78.0) |
| 1 | 330 (9.7) | 54 (5.8) | 67 (7.7) | 62 (10.7) | 147 (14.4) |
| ≥ 2 | 155 (4.5) | 21 (2.2) | 31 (3.5) | 25 (4.3) | 78 (7.6) |
| Renal component of SOFA score | |||||
| 0 | 1,353 (39.6) | 495 (52.8) | 366 (41.8) | 214 (36.8) | 278 (27.2) |
| 1 | 641 (18.8) | 199 (21.2) | 170 (19.4) | 98 (16.8) | 174 (13.7) |
| ≥ 2 | 1,423 (41.7) | 244 (26.0) | 339 (38.7) | 270 (46.4) | 571 (55.8) |
| Medications affecting hemostasis, n (%) | |||||
| Therapeutic anticoagulation prior to hospital admission | 292 (8.5) | 100 (10.7) | 81 (9.3) | 55 (9.5) | 56 (5.5) |
| Therapeutic anticoagulation within 2 d after ICU admission | 916 (26.8) | 164 (17.5) | 194 (22.2) | 158 (27.1) | 400 (39.1) |
| Aspirin within 2 d after ICU admission | 527 (15.4) | 134 (14.3) | 143 (16.3) | 97 (16.7) | 153 (15.0) |
| Steroids within 2 d after ICU admission | 858 (25.1) | 146 (15.6) | 188 (21.5) | 169 (29.0) | 355 (34.7) |
IQR = interquartile range, SOFA = Sequential Organ Failure Assessment.
Shock is defined as need for ≥ 2 vasopressors. Data on body mass index and lymphocyte count were missing in 3.6% and 8.6% of patients, respectively. Data pertaining to at least one SOFA component score were missing in 5.4% of patients and categorized as 0. All other data are complete. The coagulation SOFA component score was calculated using the lowest measured platelet count within 2 d after ICU admission as follows: 0 points, ≥ 150 × 103/μL; 1 point, 100–149 × 103/μL; ≥ 2 points, < 100 × 103/μL. The liver SOFA component score was calculated using the highest bilirubin level within 2 d after ICU admission as follows: 0 points, < 1.2 mg/dL; 1 point, 1.2–1.9 mg/dL; ≥ 2 points, ≥ 2 mg/dL. The renal SOFA component score was calculated using the highest measured serum creatinine value, the lowest 24 hr urine output in the same time frame, and/or receipt of renal replacement therapy within 2 d after ICU admission, or end-stage renal disease status as follows: 0 points, creatinine < 1.2 mg/dL and urine output ≥ 500 mL/d; 1 point, creatinine 1.2–1.9 mg/dL and urine output ≥ 500 mL/d; ≥ 2 points, creatinine ≥ 2.0 mg/dL, urine output < 500 mL/d, receipt of acute renal replacement therapy, or end-stage renal disease. If urine output was missing, the category was assigned according to the serum creatinine.
D-dimer Distribution and Univariate Association With Mortality
D-dimer was measured by six common assays (Table 1). D-dimer levels were elevated in most patients, with only 221 patients (6.4%) having values below the ULN (Fig. 1A). Extreme D-dimer elevation was common, with 821 patients (24.0%) having values greater than or equal to the maximum value of the assay’s linear range.
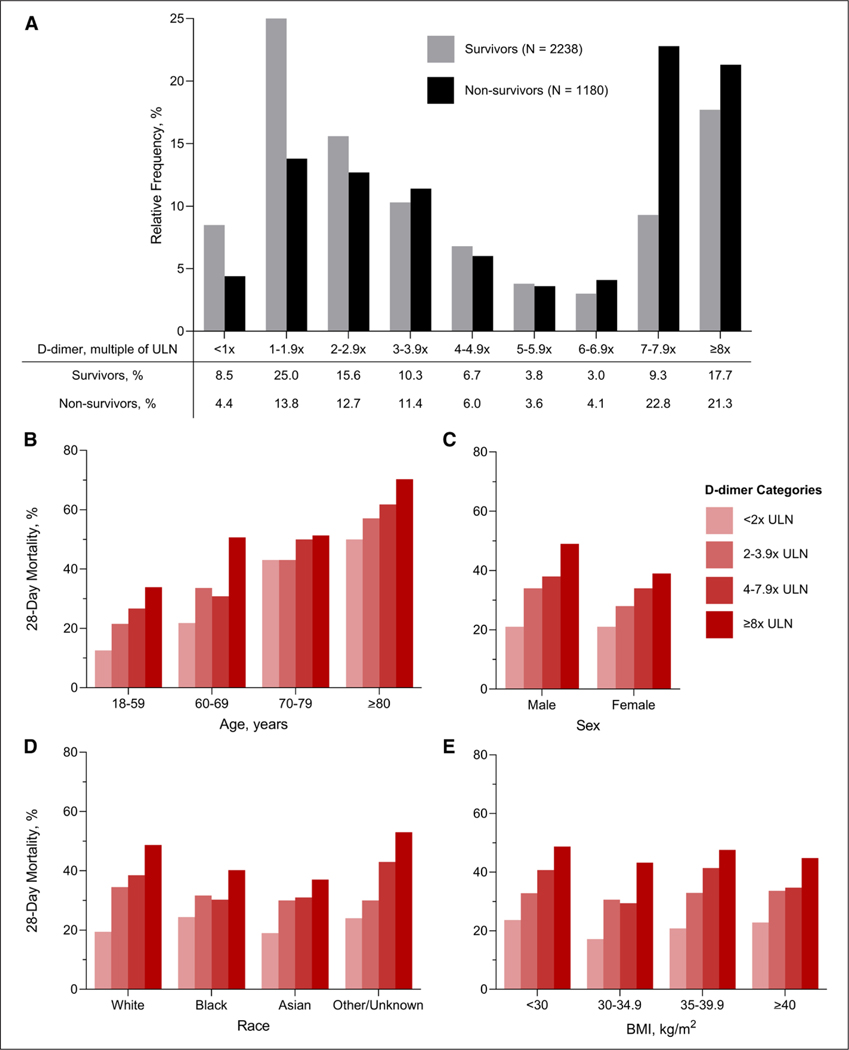
Figure 1.
D-dimer distribution and correlates with mortality. A, The relative frequencies of D-dimer levels, defined as the highest value measured within two days following ICU admission, by 28-day mortality status. B-E, Correlation of D-dimer categories with death by age, sex, race, and body mass index (BMI) categories, respectively. ULN = upper limit of normal.
Within 28 days of ICU admission, 1,180 patients (34.5%) died, 1,341 (29.2%) were discharged alive, and 897 (26.3%) remained hospitalized. A greater proportion of survivors had D-dimer values less than 2× ULN compared with nonsurvivors (33.5% and 18.2%, respectively), whereas a greater proportion of nonsurvivors had D-dimer values greater than or equal to 8× ULN compared with survivors (21.3% and 17.7%, respectively) (Fig. 1A). Higher D-dimer category was associated with a greater risk of death across categories of age, sex, race, and body mass index (Fig. 1B–E).
A total of 1,485 patients (43.4%) had measured D-dimer values on both ICU days 1 and 2, including 149 patients (10.0%) with a higher value on day one, 367 patients (24.7%) with a higher value on day 2, and 969 patients (65.3%) with unchanging levels when assessed categorically. Crude mortality rates did not appreciably vary with rising D-dimer category (31.9%), falling D-dimer category (30.2%), or unchanging D-dimer category (31.5%).
Multivariable Association Between D-dimer and Mortality
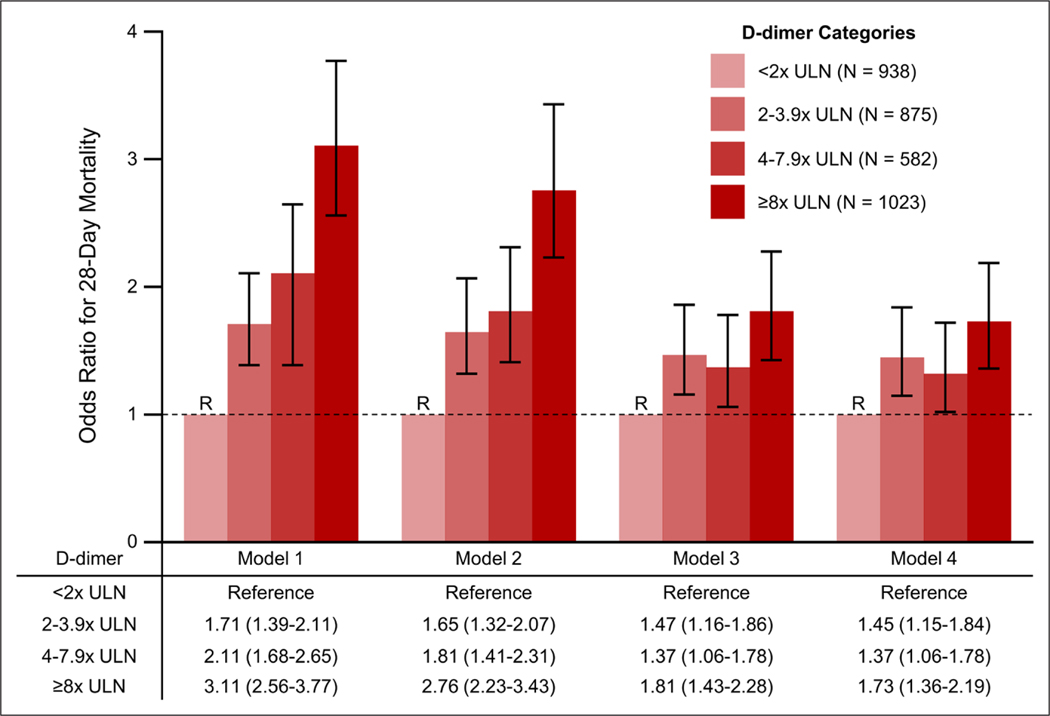
Results of sequential logistic regression models examining the relationship between D-dimer and 28-day mortality are shown in Figure 2. In unadjusted analyses (Model 1), higher D-dimer category was associated with a higher risk of death (odds ratio [OR], 3.11; 95% CI, 2.56–3.77 for highest vs lowest D-dimer category). Adjustment for demographics and comorbidities (Model 2) slightly attenuated these associations (OR, 2.76; 95% CI, 2.23–3.43 for highest vs lowest D-dimer category). Additional adjustment for acute severity of illness (Model 3) further attenuated this relationship (OR, 1.81; 95% CI, 1.43–2.28 for highest vs lowest D-dimer category). Additional adjustment for medications impacting hemostasis, including therapeutic anticoagulation (Model 4), only minimally altered this relationship (OR, 1.73; 95% CI, 1.36–2.19 for highest vs lowest D-dimer category). The associations between each of the covariates included in the final model and death are shown in Figure S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/G206).
Figure 2.
Logistic regression models for 28 d mortality by D-dimer category. Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, body mass index, diabetes mellitus, hypertension, coronary artery disease, chronic obstructive pulmonary disease, current smoking status, and active malignancy. Model 3 is further adjusted for receipt of invasive mechanical ventilation, shock, and the renal, coagulation, and liver components of the Sequential Organ Failure Assessment score, each assessed within the first 2 d following ICU admission. Model 4 is further adjusted for home anticoagulation as well as receipt of therapeutic anticoagulation, aspirin, and steroids in the first 2 d following ICU admission. Covariates are further defined in the Supplemental Methods (Supplemental Digital Content 1, http://links.lww.com/CCM/G206). ULN = upper limit of normal.
Secondary Analyses—90-Day Mortality
Results were similar for models assessing mortality at 90 days after ICU admission, by which time 1,353 patients (39.6%) died, 2,029 (59.4%) were discharged alive, and 36 (1.0%) remained hospitalized (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/G206).
Sensitivity Analyses
Interpretations were similar in models restricted to the 1,111 patients with D-dimer measured with the most common assay (STA Liatest [Diagnostica Stago SAS, Asnières sur Seine, France]; fully adjusted OR, 1.65; 95% CI, 1.06–2.57 for highest vs lowest D-dimer category) (Table S4, Supplemental Digital Content 1, http://links.lww.com/CCM/G206). When considering D-dimer continuously in these patients, each 500 ng/ mL fibrinogen equivalence units increase (corresponding with the ULN for this assay) was associated with a 1.05-fold increased odds of death (95% CI, 0.99–1.11) in a fully adjusted model (Table S5, Supplemental Digital Content 1, http://links.lww.com/CCM/G206). Restricted cubic spline plots did not reveal a nonlinear relationship between D-dimer and mortality (Fig. S2, Supplemental Digital Content 1, http://links.lww.com/CCM/G206).
D-dimer and Mortality Risk Stratification
Figure S3 (Supplemental Digital Content 1, http://links.lww.com/CCM/G206) shows the predicted risk of mortality in hypothetical patients. In four hypothetical patients with characteristics prespecified based on clinical knowledge (Fig. S3A, Supplemental Digital Content 1, http://links.lww.com/CCM/G206), the added value of D-dimer in risk stratifying patients was most apparent for the intermediate (moderate and high risk) patients, whereas only a modest effect was observed for the extreme (low and very high risk) patients.
These trends were also observed when risk was predicted in 10,000 hypothetical patients with randomly generated characteristics (Figure S3B, Supplemental Digital Content 1, http://links.lww.com/CCM/G206). D-dimer elevation (highest vs lowest category) was associated with an absolute mortality risk increase of up to 20.5% in intermediate-risk patients, whereas it was less useful in predicting death in patients with very low- or very high–pretest probability.
DISCUSSION
In this multicenter cohort study of 3,418 critically ill patients with COVID-19, D-dimer elevation was common, with over 90% of patients having plasma concentrations above the ULN on ICU days 2 or 2. Higher D-dimer levels were independently associated with an increased risk of death, even after adjustment for many baseline and severity of illness characteristics and medications, including receipt of therapeutic anticoagulation. The added value of D-dimer in risk stratifying patients for death was most apparent for intermediate-risk patients as compared to low- or very high–risk patients.
Several studies have described elevated D-dimer levels in patients with severe illness from COVID-19, including the finding that higher D-dimer levels associate with worse outcomes (3, 4, 14, 15, 19, 25, 26). Most of these studies, however, were single center and had only a modest sample size. Additionally, many of these studies did not report the basic characteristics of the D-dimer assay used, including the name of the manufacturer, its ULN and upper limit of detection cutoff values, and the units of measurement. These limitations make it nearly impossible to harmonize the results reported across studies, a topic that has been described in detail elsewhere (27, 28). Most importantly, few studies have examined whether D-dimer is independently associated with mortality after comprehensively accounting for severity of illness, as we did here.
It is unknown whether elevated D-dimer levels reflect a hypercoagulable state unique to COVID-19, since elevated D-dimer levels have been similarly observed in other respiratory infections (11, 29) and in critical illness in general (10). However, the assessment of D-dimer has become particularly common in COVID-19, as elevated levels have been used to justify initiation of therapeutic anticoagulation or dose adjustment of prophylactic/intermediate-dose anticoagulation (14, 30). The benefit of therapeutic anticoagulation in patients with COVID-19 is currently unclear and awaits the results of ongoing randomized controlled trials, several of which rely upon D-dimer elevation as an inclusion criterion (ClinicalTrials.gov NCT04401293, NCT04359277, and NCT04377997).
Our results indeed show that higher D-dimer levels are independently associated with a greater risk of death, but adjustment for early initiation of therapeutic anticoagulation did not attenuate risk. There are several possible explanations for this finding. Higher D-dimer levels may be a general marker of disease severity rather than reflective of a unique pathophysiology driving mortality. Alternatively, elevated D-dimer levels could be indicative of a hypercoagulable state, but initiation of therapeutic anticoagulation with an elevated D-dimer may be too late to alter the pathologic process. Finally, given the observational design, it is possible that the decision to initiate (or not initiate) therapeutic anticoagulation could have been confounded by other factors that were unmeasured or unaccounted for in our analyses, which could have obscured our ability to detect benefit (or harm) from therapeutic anticoagulation.
In our view and based on available data, D-dimer elevation alone may not be an appropriate indication for initiation of therapeutic anticoagulation in patients with severe illness from COVID-19. Although anticoagulation reduces the mortality of pulmonary emboli (31), the role of anticoagulation in microangiopathic hemolytic anemias seen in other infections and autoimmune diseases is not established. Additionally, randomized trials prior to the COVID-19 pandemic did not shown a clear benefit for therapeutic anticoagulation in critically ill patients with elevated D-dimer levels (32). D-dimer elevation is clearly a risk marker for mortality in multiple patient populations, but whether D-dimer reflects a thrombotic pathophysiology where intervention can reduce mortality is uncertain.
Our results are consistent with and expand on previous findings that elevated D-dimer concentrations are associated with increased mortality in critically ill patients with COVID-19. However, it remains unclear whether elevated concentrations of D-dimer are simply indicative of overall severity of illness or if they reflect a unique pathophysiology related to a hypercoagulable state leading to mortality. The distinction is of critical importance, since the latter can potentially be targeted therapeutically. Our models indicate that D-dimer and illness severity are indeed correlated, as noted by the attenuation in the strength of association between D-dimer and death after adjustment for a large number of severity of illness factors. Nonetheless, D-dimer remained independently and strongly associated with death despite comprehensive adjustment for severity of illness, consistent with elevated D-dimer representing an underlying pathophysiology contributing to adverse outcomes. D-dimer elevation may reflect underlying hypercoagulability, pathologic fibrinolysis, inflammatory processes, or may itself be pathogenic (9, 11, 13, 33–35). This study represents a step toward understanding the prognostic role of D-dimer in COVID-19, but further basic science/translational mechanistic work, along with ongoing clinical trials, will provide additional answers to fundamental questions related to D-dimer pathophysiology.
Although our findings support a role for D-dimer as a risk marker for COVID-19 mortality, it is important to consider for whom D-dimer results may be most useful. Our results show D-dimer level was not particularly helpful in predicting mortality risk for low- or very high–risk patients, whereas it had modest utility in predicting mortality in intermediate-risk patients. As with all tests, consideration of baseline risk and pretest probability is likely very important in determining D-dimer’s utility in this patient population.
Interpretation of D-dimer levels, both in COVID-19 and other contexts, has been particularly affected by heterogeneity between assays. These differences are often incompletely considered, as evidenced by the numerous issues noted in the current COVID-19 literature on D-dimer (27, 28). We propose a novel methodology to compare D-dimer results across assays by standardizing the results with the ULN. Although our primary findings closely aligned with results limited to the most commonly used D-dimer assay, there is no single standard approach to harmonizing data from different D-dimer assays.
This study has several strengths. First, we used granular data from a large cohort of consecutive ICU patients admitted to geographically diverse hospitals across the United States. Second, all data were collected by manual chart review rather than reliance on administrative or billing codes, which have well-described limitations (36). Finally, we attempted to normalize D-dimer values to limit interassay variability in results.
We also note several limitations. First, D-dimer results are not readily comparable between assays, and there are few established procedures for normalizing D-dimer data (9). However, our normalized results corrected for differing dilution measurement practices across six assays closely matched the analyses limited to the most commonly used assay in our cohort. Second, our results are limited to patients who had a D-dimer measured for routine clinical purposes. However, we found that patients with D-dimer measured had overall similar baseline characteristics as those without D-dimer measured (Table S2, Supplemental Digital Content 1, http://links.lww.com/CCM/G206). Additionally, our models are not adjusted for other markers of abnormal hemostasis, such as fibrinogen, prothrombin time, partial thromboplastin time, and international normalized ratio, due to data missingness. Finally, clinical use of D-dimer may have varied across sites and over time, with the potential for increasing use of therapeutic anticoagulation over time. This likely did not impact our results, however, as adjustment for therapeutic anticoagulation did not affect the observed association between D-dimer and mortality.
CONCLUSIONS
In conclusion, in this multicenter cohort study of critically ill adults with COVID-19 admitted to ICUs across the United States, higher D-dimer was independently associated with a greater risk of death. The added value of D-dimer in risk stratifying patients for death was most apparent for intermediate-risk patients as compared to low- or very high–risk patients. These data should not be used to justify anticoagulation as a means to reduce mortality in this population, but as further evidence that D-dimer is a risk marker for mortality. Further studies are needed to examine whether therapies targeting the proposed hypercoagulable state in critically ill patients with COVID-19 can reduce the high mortality observed in this population.
Supplementary Material
Acknowledgments
A full list of the STOP-COVID investigators is provided in the Supplemental Appendix (Supplemental Digital Content 1, http://links.lww.com/CCM/G206).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the following grants from the National Institutes of Health: F32DC017342 (to Dr. Gupta); R01HL153384 (to Dr. Hayek); R01HL144566 and R01DK125786 (to Dr. Leaf); K23DK120811(to Dr. Srivastava); K08GM134220, and R03AG060179 (to Dr. Shaefi); and R01HL141290 (to Dr. Zakai).
Dr. Gupta’s institution received funding from the Foundation for the National Institutes of Health (NIH) 5 F32 DC 17342–2; she received funding from GlaxoSmithKline; she is a scientific coordinator for the Anemia Studies in CKD: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat (ASCEND) trial (GlaskoSmithKline). Dr. Srivastava received funding from CVS Caremark, AstraZeneca, Horizon Therapeutics, PLC, and Tate & Latham. Dr. Shaefi’s institution received funding from the National Institute on Aging/NIH R03AG060179 and the National Institute of General Medical Sciences/NIH K08GM134220; he received support for article research from the NIH. Dr. Bagchi’s institution received funding from the American Heart Association 20IPA35360009. He received funding from Lungpacer Medical, Inc. Dr. Al-Samkari received funding from Agios, Dova, Rigel, Argenx, and Amgen. Dr. Zakai’s institution received funding from the NIH and Centers for Disease Control and Prevention. Dr. Leaf received research support from BioPorto. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Home: Johns Hopkins Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/. Accessed October 15, 2020
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. : Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Ni Z, Hu Y, et al. : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goshua G, Pine AB, Meizlish ML, et al. : Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol 2020; 7:e575–e582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay D, van Gerwen M, Alsen M, et al. : Impact of anticoagulation prior to COVID-19 infection: A propensity score-matched cohort study. Blood 2020; 136:144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paranjpe I, Fuster V, Lala A, et al. : Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76:122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thachil J, Cushman M, Srivastava A: A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost 2020; 4:731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Madhavan MV, Gupta A, et al. : Pharmacological agents targeting thromboinflammation in COVID-19: Review and implications for future research. Thromb Haemost 2020;120:1004–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favresse J, Lippi G, Roy PM, et al. : D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci 2018; 55:548–577 [DOI] [PubMed] [Google Scholar]
- 10.Shitrit D, Izbicki G, Shitrit AB, et al. : Prognostic value of a new quantitative D-dimer test in critically ill patients 24 and 48 h following admission to the intensive care unit. Blood Coagul Fibrinolysis 2004; 15:15–19 [DOI] [PubMed] [Google Scholar]
- 11.Salluh JIF, Rabello LSCF, Rosolem MM, et al. : The impact of coagulation parameters on the outcomes of patients with severe community-acquired pneumonia requiring intensive care unit admission. J Crit Care 2011; 26:496–501 [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Wang X, Chen Q, et al. : D-dimer is a predictor of 28-day mortality in critically ill patients receiving continuous renal replacement therapy. Arch Med Res 2016; 47:356–364 [DOI] [PubMed] [Google Scholar]
- 13.Shorr AF, Thomas SJ, Alkins SA, et al. : D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest 2002; 121:1262–1268 [DOI] [PubMed] [Google Scholar]
- 14.Tang N, Li D, Wang X, et al. : Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Yan X, Fan Q, et al. : D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020; 18:1324–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso-Fernández A, Toledo-Pons N, Cosío BG, et al. : Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: A prospective study. PLoS One 2020; 15:e0238216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. : Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 2020; 192:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu B, Li X, Chen J, et al. : Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: A retrospective analysis. J Thromb Thrombolysis 2020; 50:548–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Y, Cao J, Wang Q, et al. : D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J Intensive Care 2020; 8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Hayek SS, Wang W, et al. : Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raith EP, Udy AA, Bailey M, et al. ; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE): Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 2017; 317: 290–300 [DOI] [PubMed] [Google Scholar]
- 23.Jentzer JC, Bennett C, Wiley BM, et al. : Predictive value of the sequential organ failure assessment score for mortality in a contemporary cardiac intensive care unit population. J Am Heart Assoc 2018; 7:e008169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aakre C, Franco PM, Ferreyra M, et al. : Prospective validation of a near real-time EHR-integrated automated SOFA score calculator. Int J Med Inform 2017; 103:1–6 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Zhang S, Wu Z, et al. : Clinical outcomes of COVID-19 in Wuhan, China: A large cohort study. Ann Intensive Care 2020;10:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naymagon L, Zubizarreta N, Feld J, et al. : Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res 2020; 196:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favaloro EJ, Thachil J: Reporting of D-dimer data in COVID-19: Some confusion and potential for misinformation. Clin Chem Lab Med 2020; 58:1191–1199 [DOI] [PubMed] [Google Scholar]
- 28.Thachil J, Longstaff C, Favaloro EJ, et al. : The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on fibrinolysis. J Thromb Haemost 2020; 18:2408–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmers JD, Singanayagam A, Scally C, et al. : Admission D-dimer can identify low-risk patients with community-acquired pneumonia. Ann Emerg Med 2009; 53:633–638 [DOI] [PubMed] [Google Scholar]
- 30.Kollias A, Kyriakoulis KG, Dimakakos E, et al. : Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol 2020; 189:846–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SB, Geske JB, Maguire JM, et al. : Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest 2010; 137:1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thachil J, Iba T: The application of anticoagulant therapy to sepsis. J Intensive Care 2017; 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levi M, Thachil J, Iba T, et al. : Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020; 7:e438–e440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. : COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020; 136:489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Tang H: Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol 2016; 13:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Walraven C, Bennett C, Forster AJ: Administrative database research infrequently used validated diagnostic or procedural codes. J Clin Epidemiol 2011; 64:1054–1059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.