This cross-sectional study uses data from a dialysis organization in the US to assess the seroprevalence of SARS-CoV-2 antibodies among patients receiving dialysis and to estimate the seroprevalence among the adult population 1 year into the COVID-19 pandemic.
Key Points
Question
What is the SARS-CoV-2 seroprevalence among patients receiving dialysis and the adult population in the US as of January 2021?
Findings
In this cross-sectional study of 21 464 patients receiving dialysis in the US, who broadly represent persons most at risk of to SARS-CoV-2 infection (eg, older people, members of racial/ethnic minority groups), the SARS-CoV-2 seroprevalence was 18.9%. Seroprevalence varied little by US region. Younger persons, those living in communities with a majority Hispanic population, and those living in lower-income neighborhoods were the groups with the highest seroprevalence rates.
Meaning
After standardizing seroprevalence rates for patients receiving dialysis to the US adult population, results of this study suggest that most adults did not have evidence of natural SARS-CoV-2 infection by January 2021.
Abstract
Importance
Seroprevalence studies complement data on detected cases and attributed deaths in assessing the cumulative spread of the SARS-CoV-2 virus.
Objective
To estimate seroprevalence of SARS-CoV-2 antibodies in patients receiving dialysis and adults in the US in January 2021 before the widespread introduction of COVID-19 vaccines.
Design, Setting, and Participants
This cross-sectional study used data from the third largest US dialysis organization (US Renal Care), which has facilities located nationwide, to estimate SARS-CoV-2 seroprevalence among US patients receiving dialysis. Remainder plasma (ie, plasma that would have otherwise been discarded) of all patients receiving dialysis at US Renal Care facilities from January 1 to 31, 2021, was tested for SARS-CoV-2 antibodies. Patients were excluded if they had a documented dose of SARS-CoV-2 vaccination or if a residence zip code was missing from electronic medical records. Crude seroprevalence estimates from this sample (January 2021) were standardized to the US adult population using the 2018 American Community Survey 1-year estimates and stratified by age group, sex, self-reported race/ethnicity, neighborhood race/ethnicity composition, neighborhood income level, and urban or rural status. These data and case detection rates were then compared with data from a July 2020 subsample of patients who received dialysis at the same facilities.
Exposures
Age, sex, race/ethnicity, and region of residence as well as neighborhood race/ethnicity composition, poverty, population density, and urban or rural status.
Main Outcomes and Measures
The spike protein receptor-binding domain total antibody assay (Siemens Healthineers; manufacturer-reported sensitivity of 100% and specificity of 99.8%) was used to estimate crude SARS-CoV-2 seroprevalence in the unweighted sample, and then the estimated seroprevalence rates for the US dialysis and adult populations were calculated, adjusting for age, sex, and region.
Results
A total of 21 464 patients (mean [SD] age, 63.1 [14.2] years; 12 265 men [57%]) were included in the unweighted sample from January 2021. The patients were disproportionately older (aged 65-79 years, 7847 [37%]; aged ≥80 years, 2668 [12%]) and members of racial/ethnic minority groups (Hispanic patients, 2945 [18%]; non-Hispanic Black patients, 4875 [29%]). Seroprevalence of SARS-CoV-2 antibodies was 18.9% (95% CI, 18.3%-19.5%) in the sample, with a seroprevalence of 18.7% (95% CI, 18.1%-19.2%) standardized to the US dialysis population, and 21.3% (95% CI, 20.3%-22.3%) standardized to the US adult population. In the unweighted sample, younger persons (aged 18-44 years, 25.9%; 95% CI, 24.1%-27.8%), those who self-identified as Hispanic or living in Hispanic neighborhoods (25.1%; 95% CI, 23.6%-26.4%), and those living in the lowest-income neighborhoods (24.8%; 95% CI, 23.2%-26.5%) were among the subgroups with the highest seroprevalence. Little variability was observed in seroprevalence by geographic region, population density, and urban or rural status in the January 2021 sample (largest regional difference, 1.2 [95% CI, 1.1-1.3] higher odds of seroprevalence in residents of the Northeast vs West).
Conclusions and Relevance
In this cross-sectional study of patients receiving dialysis in the US, fewer than 1 in 4 patients had evidence of SARS-CoV-2 antibodies 1 year after the first case of SARS-CoV-2 infection was detected in the US. Results standardized to the US population indicate similar prevalence of antibodies among US adults. Vaccine introduction to younger individuals, those living in neighborhoods with a large population of racial/ethnic minority residents, and those living in low-income neighborhoods may be critical to disrupting the spread of infection.
Introduction
In the 1-year period since the first SARS-CoV-2 case was reported in the US, COVID-19 cases, deaths, and hospitalizations overwhelmed health systems in the country. To date, more than 25 million cases and more than 500 000 deaths have been attributed to SARS-CoV-2 infection.1,2 Although the most intense burden of disease was experienced in and around the state of New York in the spring of 2020, nearly all regions in the US, including rural areas, faced threats to their hospital capacity, with the largest numbers of cases accruing between November and December 2020.2
Data suggest that symptomatic cases represent a fraction of persons infected with SARS-CoV-2.3 Most infected persons, however, whether asymptomatic or symptomatic, do mount a specific antibody response4,5,6; SARS-CoV-2 receptor-binding domain (RBD) immunoglobin (IgG) antibodies persist for at least 4 to 6 months after infection.4,7,8 Thus, seroprevalence estimates remain an essential measure of the extent of SARS-CoV-2 community spread.
A previous study proposed that patients receiving dialysis could serve as a sentinel population for SARS-CoV-2 seroepidemiology because the population is broadly representative of groups susceptible to SARS-CoV-2 infection, and these patients have routine requirements for monthly laboratory testing that facilitate surveillance.9 Serosurveillance in this patient population includes older persons, members of racial/ethnic minority groups, and people from low-income settings, without barriers related to community outreach or health care and insurance access.
Following the previously proposed strategy of performing repeated seroprevalence surveys among patients receiving dialysis,9 we conducted a cross-sectional seroprevalence analysis of 21 424 patients receiving dialysis in the US in January 2021. Our aim was to assess SARS-CoV-2 seroprevalence before the widespread introduction of COVID-19 vaccines. We also analyzed the data for the persistence of previously observed differences in seroprevalence by age, sex, race/ethnicity, geographic region, and other community-level strata of interest, including population density and neighborhood poverty. In addition, to assess for changes in diagnosis and treatment between July 2020 and January 2021,9 we compared case detection attributed to COVID-19 with the seroprevalence estimates at both time points.
Methods
This cross-sectional study was conducted from January 1 to January 31, 2021, and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. We tested for SARS-CoV-2 seroprevalence among patients receiving dialysis at facilities within a single dialysis network (US Renal Care), which is the third largest dialysis provider in the US and has more than 500 facilities located nationwide. Patients were excluded if they had a documented dose of SARS-CoV-2 vaccination or if a residence zip code was missing from the electronic medical record (eFigure 1 in the Supplement). Patients receiving dialysis undergo routine monthly laboratory testing for management of end-stage kidney disease and related complications. In partnership with a commercial laboratory (Ascend Clinical Laboratory) that received the laboratory samples, we used remainder plasma (ie, plasma samples that would have otherwise been discarded) from the January 2021 laboratory testing, replicating methods described elsewhere.9 The study received institutional review board approval from Stanford University. Because remainder plasma from blood draw for routine care was used in this study, the Stanford institutional review board waived the requirement for informed consent. We extracted data on patient demographic characteristics, residence, and vaccination status from electronic health records at Ascend Clinical Laboratory; the data were anonymized before transfer to Stanford University investigators. Most testing was performed during the first 2 weeks of January 2021.
Assay Characteristics
We used an RBD total antibody (Ig M and G) chemiluminescence assay (Siemens Healthineers), which has 100% sensitivity (≥14 days after positive polymerase chain reaction test results) and 99.8% specificity.10 An independent analysis from Public Health England confirmed the sensitivity and specificity rates.11 Remainder plasma was collected using lithium heparin gel plasma tubes and centrifuged within 2 hours at 3000 to 3400 rpm for 15 to 20 minutes. Following routine procedure, these specimens were stored and transported at 2 to 8 °C until analysis. If specimens were not tested within 2 days, then they were frozen at −20 °C until analysis could be performed.
Statistical Analysis
We determined crude seroprevalence estimates and 95% CIs for the unadjusted sample from January 2021. We then standardized seroprevalence estimates, adjusting for age, sex, and region, by using the distribution of the US dialysis population as available through the US Renal Data System12 and the distribution for the US adult population as available through the 2018 American Community Survey 1-year estimates.13 Next, we examined differences in seroprevalence by the following strata: age group, sex, region, self-reported race/ethnicity, neighborhood race/ethnicity composition, neighborhood income level, and urban or rural status.9 Income level was defined as the proportion of people living below the federal poverty level in the zip code tabulation area.
Using logistic regression adjusted by age group (18-44 years, 45-64 years, 65-79 years, ≥80 years), we also compared January 2021 data with July 2020 data9 to assess for differences in seroprevalence by patient and neighborhood characteristics and region of residence strata. In the July 2020 study, samples were obtained at multiple dialysis networks from patients residing in 46 states (n = 28 503). To ensure accurate comparisons with January 2021 data, we limited the comparator July 2020 sample used in the present analysis to patients receiving dialysis at the same US Renal Care facilities (11 746 of 28 503 [41%] tested in July 2020) (eTable 1 in the Supplement).
In addition, we compared case detection and infection fatality rates from July 2020 with those from January 2021. We computed case detection rates as the proportion of detected cases per 100 0002 from a denominator totaling seroprevalence estimates per 100 000 (standardized to the US adult population) and deaths per 100 000 from January 1 to June 31, 2020, for the July 2020 estimates and from July 1 to December 31, 2020, for the January 2021 estimates. We adjusted the numerator of detected cases to reflect adults only by using the US Centers for Disease Control and Prevention (CDC) data on the proportion of overall cases occurring in adults (approximately 92% in June 202014 and approximately 89% in December 202015). We computed the infection fatality rate as the proportion of deaths per 100 000 from a denominator of seroprevalent persons per 100 000 in the corresponding time periods.
Results
A total of 21 464 patients (mean [SD] age, 63.1 [14.2] years; 12 265 men [57%]) were included in the sample and underwent laboratory testing in the first 2 weeks of January 2021. The patients were disproportionately older (aged 65-79 years, 7847 [37%]; aged ≥80 years, 2668 [12%]) and members of racial/ethnic minority groups (Hispanic patients, 2945 [18%]; non-Hispanic Black patients, 4875 [29%]) (Table 1). Although patients resided in a total of 43 states, 33 states contributed 30 or more patients to the sample (eFigure 1 in the Supplement). Persons from the South and West regions of the US were modestly overrepresented. Neighborhood race/ethnicity composition closely matched that of the US dialysis population.
Table 1. Characteristics of the Sample, US Dialysis, and US Adult Populations, January 2021a.
| Patient characteristics | No. of individuals (%) | ||
|---|---|---|---|
| Sample population | US adult dialysis population | US adult population | |
| No. of individuals | 21 464 | 499 150 | 253 815 197 |
| Age, y | |||
| 18-44 | 2314 (11) | 60 540 (12) | 117 499 477 (46) |
| 45-64 | 8635 (40) | 207 022 (41) | 83 892 606 (33) |
| 65-79 | 7847 (37) | 174 341 (35) | 39 949 825 (16) |
| ≥80 | 2668 (12) | 57 247 (11) | 12 473 289 (5) |
| Sex | |||
| Male | 12 265 (57) | 285 281 (57) | 123 578 869 (49) |
| Female | 9199 (43) | 213 869 (43) | 130 236 328 (51) |
| Race/ethnicityb | |||
| Hispanic | 2945 (18) | 87 611 (18) | 60 861 275 (19) |
| Non-Hispanic White | 6311 (37) | 203 421 (41) | 197 202 727 (60) |
| Non-Hispanic Black | 4875 (29) | 173 190 (35) | 39 717 152 (12) |
| Non-Hispanic otherc | 2834 (17) | 34 928 (7) | 28 493 202 (9) |
| ZCTA majority race/ethnicityd,e | |||
| Non-Hispanic White | 6481 (30) | 206 678 (41) | 189 968 192 (58) |
| Non-Hispanic Black | 1504 (7) | 54 999 (11) | 12 550 083 (4) |
| Hispanic | 4023 (19) | 52 953 (11) | 26 310 796 (8) |
| Hispanic and Black | 2321 (11) | 43 396 (9) | 17 238 911 (5) |
| Integrated | 7135 (33) | 140 781 (28) | 80 206 374 (25) |
| Geographic region | |||
| Northeast | 2113 (10) | 78 619 (16) | 44 519 465 (18) |
| South | 10 857 (50) | 214 974 (43) | 96 250 597 (38) |
| Midwest | 2055 (10) | 94 490 (19) | 52 876 708 (21) |
| West | 6439 (30) | 111 067 (22) | 60 168 427 (24) |
Abbreviation: ZCTA, zip code tabulation area.
US adult population in 2018, US adult patients on dialysis population as of January 1, 2017. Race/ethnicity and ZCTA majority race/ethnicity computed for total US 2018 population (N = 326 274 356).
Proportions are reported for persons with available data; 904 (7.7%) in the sample were missing race/ethnicity.
Other comprises Asian, Native American, Pacific Islander, or persons reporting “other” race/ethnicity.
343 People in the US Renal Data System had missing data on ZCTA majority race/ethnicity due to missing zip code.
The ZCTA majority is defined as population in ZCTA ≥60% Hispanic persons, non-Hispanic Black persons, or non-Hispanic White persons; if among the remaining ZCTAs the Hispanic and Black population exceeded 60%of the population, the ZCTA is defined as “majority Hispanic and Black” or else as “integrated.”
In January 2021, SARS-CoV-2 RBD assay seroprevalence estimates were 18.9% (95% CI, 18.3%-19.5%) in the unweighted sample of 21 464 patients (ranging from 15.3% in the Northeast to 20.8% in the South), 18.7% (95% CI, 18.1%-19.2%) standardized to the US dialysis population, and 21.3% (95% CI, 20.3%-22.3%) standardized to the US adult population (Table 2). In the unweighted sample, younger age groups (18-44 years, 25.9%; 95% CI, 24.1%-27.8%), persons who self-identified as Hispanic or living in Hispanic neighborhoods (25.1%; 95% CI, 23.6%-26.4%), and those living in the lowest-income neighborhoods (24.8%; 95% CI, 23.2%-26.5%) were among the subgroups with the highest seroprevalence.
Table 2. Estimated Seroprevalence of SARS-CoV-2 Antibodies in the January 2021 Study Sample and Samples Standardized to US Dialysis and US Adult Populationa.
| Variable | Unweighted sample | Standardized to US adult dialysis population, seropositive % (95% CI) | Standardized to US adult population, seropositive % (95% CI) | Seropositive persons per 100 000 personsb | |
|---|---|---|---|---|---|
| No. of individuals | Seropositive % (95% CI) | ||||
| Age, yc | |||||
| 18-44 | 591 | 25.5 (23.6-27.7) | 25.5 (23.7-27.5) | 25.3 (23.3-27.4) | 23 328 |
| 45-64 | 1824 | 21.1 (20.2-22.1) | 20.3 (19.4-21.2) | 19.9 (18.9-20.8) | 19 861 |
| 65-79 | 1276 | 16.3 (15.4-17.2) | 15.9 (15.0-16.7) | 15.9 (15.0-16.8) | 15 884 |
| ≥80 | 370 | 13.9 (12.5-15.4) | 14.1 (12.7-15.6) | 14.5 (13.1-16.2) | 14 527 |
| Sexd | |||||
| Male | 2250 | 18.3 (17.6-19.1) | 18.1 (17.4-18.9) | 20.7 (19.5-22.0) | 19 934 |
| Female | 1811 | 19.7 (18.8-20.6) | 19.3 (18.5-20.2) | 21.9 (20.5-23.5) | 21 189 |
| Race/ethnicityc | |||||
| Hispanic | 763 | 25.9 (24.1-27.8) | 26.2 (24.5-27.9) | 29.0 (26.2-31.8) | 15 495 |
| Non-Hispanic White | 1084 | 17.2 (16.2-18.2) | 16.6 (15.6-17.6) | 17.6 (16.0-19.2) | 6189 |
| Non-Hispanic Black | 885 | 18.2 (17.0-19.4) | 18.1 (17.0-19.3) | 21.0 (18.9-23.1) | 32 439 |
| Othere | 374 | 13.2 (11.9-14.6) | 13.3 (12.0-14.7) | 17.0 (14.3-19.7) | 16 315 |
| ZCTA majority race/ethnicityf | |||||
| Hispanic | 1010 | 25.1 (23.6-26.4) | 25.2 (23.9-26.6) | 27.4 (25.3-29.7) | 7450 |
| Non-Hispanic White | 993 | 15.3 (14.4-16.3) | 15.0 (14.1-16.0) | 16.4 (14.8-18.1) | 41 150 |
| Non-Hispanic Black | 306 | 20.3 (18.3-22.4) | 20.4 (18.2-22.6) | 26.5 (22.2-30.9) | 40 574 |
| Hispanic and Black | 540 | 23.3 (21.5-25.0) | 23.8 (22.1-25.7) | 26.4 (23.5-29.5) | 37 928 |
| Integrated | 1212 | 17.0 (16.1-17.9) | 17.5 (16.5-18.5) | 20.8 (19.0-22.5) | 19 574 |
| Geographic regionc | |||||
| Northeast | 323 | 15.3 (13.8-16.8) | 15.6 (14.1-17.1) | 19.0 (16.1-21.9) | 18 370 |
| South | 2257 | 20.8 (20.0-21.6) | 21.0 (20.2-21.8) | 24.2 (22.9-25.4) | 23 290 |
| Midwest | 341 | 16.6 (15.0-18.2) | 16.7 (15.1-18.4) | 19.9 (17.0-22.8) | 19 199 |
| West | 1140 | 17.7 (16.7-18.6) | 17.9 (17.0-18.9) | 19.8 (18.2-21.3) | 19 086 |
| ZCTA poverty levelc,g | |||||
| <10% | 776 | 13.3 (12.4-14.2) | 13.5 (12.6-14.5) | 15.6 (13.9-17.5) | 8135 |
| 10% to <20% | 1517 | 18.8 (17.9-19.6) | 18.8 (17.9-19.7) | 21.0 (19.4-22.6) | 16 364 |
| 20% to <30% | 1101 | 22.7 (21.5-23.9) | 22.4 (21.1-23.6) | 26.0 (23.9-28.2) | 27 489 |
| ≥30% | 666 | 24.8 (23.2-26.5) | 23.8 (22.1-25.5) | 26.1 (23.3-29.0) | 38 123 |
| ZCTA rural or urban statusd | |||||
| Dense urban | 3332 | 18.7 (18.1-19.4) | 18.5 (17.9-19.2) | 21.4 (20.3-22.5) | 17 773 |
| Metropolitan | 269 | 16.7 (14.8-18.8) | 15.7 (14.0-17.6) | 16.8 (13.7-19.9) | 10 007 |
| Micropolitan | 247 | 23.0 (20.3-26.1) | 21.2 (18.8-23.9) | 21.6 (17.7-25.5) | 9142 |
| Small town or rural | 205 | 22.0 (19.2-25.2) | 22.2 (19.4-25.3) | 25.1 (20.1-30.0) | 12 196 |
| Overall | 4061 | 18.9 (18.3-19.5) | 18.7 (18.1-19.2) | 21.3 (20.3-22.3) | 20 578 |
Abbreviation: ZCTA, zip code tabulation area.
US adult population in 2018, US adult population receiving dialysis as of January 1, 2017.
Seropositivity per 100 000 persons calculated as (standardized count/category n) × 100 000.
Different at P < .001 for the sample population.
Different at P < .05 for the sample population.
Other comprises Asian, Native American, Pacific Islander, or persons reporting “other” race/ethnicity.
The ZCTA majority is defined as population in ZCTA ≥60% Hispanic, non-Hispanic Black persons, or non Hispanic White persons; if among the remaining ZCTAs the Hispanic and Black population exceeded 60% of the population, the ZCTA is defined “majority Hispanic and Black” else as “integrated.”
Percentage of persons living below the federal poverty line in the ZCTA.
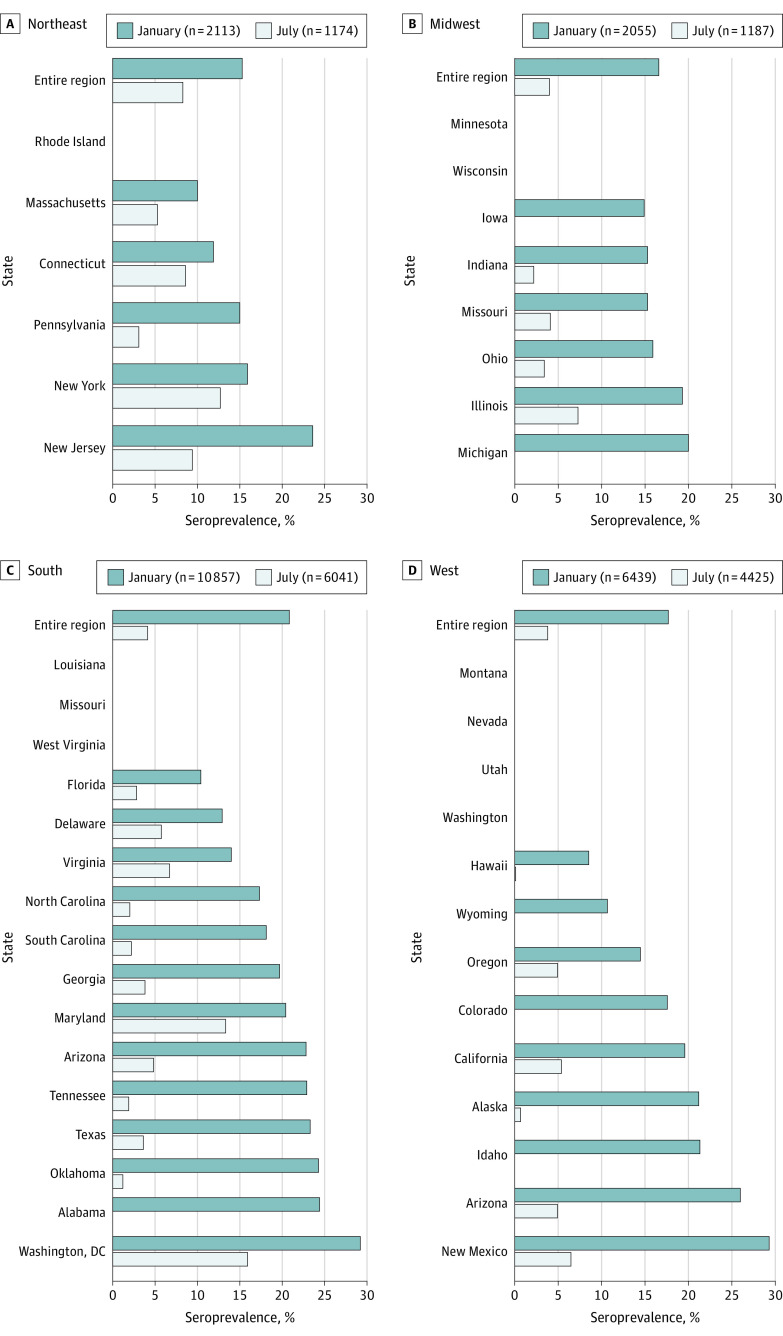
We compared these values with observations from patients within the US Renal Care network who underwent laboratory testing in July 2020.9 In this subset from July 2020, the unweighted sample SARS-CoV-2 seroprevalence rates were 4.4% (95% CI, 4.0%-4.8%), the dialysis-adjusted rates were 4.7% (95% CI, 4.3%-5.2%), and the US population–adjusted seroprevalence rates were 5.4% (95% CI, 4.6%-6.2%) (eTable 2 in the Supplement). Compared with the seroprevalences from July 2020, the January 2021 seroprevalences were 1.8-fold (95% CI, 1.5-2.3) higher in the Northeast, 4.1-fold (95% CI, 3.0%-5.7%) higher in the Midwest, and 5.1-fold (95% CI, 3.9%-5.5%) higher in the West (Figure). The largest increase in seroprevalence was observed in the South (5.1-fold; 95% CI, 4.5-5.8). Regional and rural vs urban seroprevalences in the present study varied less than those in the previous study9 (eFigure 2 in the Supplement). The largest regional difference observed was 1.2-fold (95% CI, 1.1-1.3) higher odds of seroprevalence in residents of the South vs the West in January 2021 compared with 2.3-fold (95% CI, 1.8-3.0) higher odds of seroprevalence in residents of the Northeast versus West in July 2020. Differences remained in SARS-CoV-2 seroprevalence stratified by race/ethnicity and neighborhood income level. Seroprevalence was 1.7-fold (95% CI, 1.6-1.9) higher among persons living in neighborhoods with a majority Hispanic population compared with neighborhoods with a majority White population, and 2.0-fold (95% CI, 1.8-2.3) higher among persons living in neighborhoods with 30% or more vs less than 10% of residents living at the federal poverty level (eFigure 2 in the Supplement).
Figure. SARS-CoV-2 Seroprevalence by US Region in January 2021 With Comparison to Sampled States in July 2020.
Overall seroprevalence was between 15%-21% across the Northeast (A), Midwest (B), South (C), and West (D), with the highest seroprevalence in the South. The 42 states (and Washington, DC) in which patients resided are listed in the figure but data are presented only for states with at least 30 persons in the January sample (n = 33).
Compared with data from July 2020, estimated SARS-CoV-2 case detection rates increased from 14% (741 detected cases per 100 000 adults of 5291 seroprevalent cases per 100 000 adults) to 23% (4676 detected cases per 100 000 adults of 20 578 seroprevalent cases per 100 000 adults) in January 2021. Infection fatality rates decreased from 0.7% (39 deaths per 100 000 adults of 5291 seroprevalent cases per 100 000 adults) in July 2020 to 0.3% (66 deaths per 100 000 adults of 20 578 seroprevalent cases per 100 000 adults) in January 2021 (eFigure 3 in the Supplement).
Discussion
In this cross-sectional study in January 2021 of nationwide seroprevalence of SARS-CoV-2 among US patients receiving dialysis, our results suggest that, despite national surges in cases and almost half a million deaths, fewer than 1 in 4 US adults had evidence of SARS-CoV-2 antibodies. Compared with data from July 2020, the spread of SARS-CoV-2 throughout the US became more uniform across geographic regions and between urban and rural areas. The largest differences in seroprevalences occurred between younger and older age groups, residents of neighborhoods with a majority Hispanic vs White population, and residents of low-income vs more affluent neighborhoods. SARS-CoV-2 case detection improved but approximately 3 in 4 cases remained undiagnosed. The data suggest the critical need for widespread vaccination because, as our evaluation suggests, much of the population still remains vulnerable to SARS-CoV-2 infection as of January 2021.
To our knowledge, minimal up-to-date data exist on SARS-CoV-2 seroprevalence from countries with a high number of cases and deaths from COVID-19. In the US, the CDC implemented nationwide serosurveillance using remnant sera from commercial clinical laboratories.16 Our July 2020 seroprevalence estimates were similar to those reported using the CDC strategy.9 The latest available data from the CDC, however, are from November 2020, before the largest surge of cases in the US. Outside of the US, more than 15 000 home visits were performed in India in 700 communities that tested 29 000 people for plasma nucleocapsid IgG between August and September 2020; the estimated seroprevalence was 7% among adults, at a time when the country had recorded roughly 6 million cases.17 In the UK, the Real-Time Assessment of Community Transmission (REACT) study mailed lateral flow immunoassays to randomly selected UK residents.18,19 This ongoing study had a wide reach (>350 000 participants reached by fall 2020) and has been amenable to repeat serosurveillance. In the last iteration before the introduction of the vaccine, the authors observed a decline in seroprevalence over time, possibly attributable to decay in the qualitative measure of the antispike antibody, limiting the study’s ability to estimate cumulative infection rates in the UK.18 Brazilian investigators using blood donor samples in Manaus, Brazil, created a seroprevalence model that incorporated a parameter for antibody decay.20 Using this strategy, they concluded that 75% in Manaus had been infected by October 2020. A later surge of infections in Manaus, however, has raised questions about the model’s assumptions.21
Ease of replication, unbiased sampling, and high-quality immunoassays—all features of dialysis sampling in the US—are crucial to rapid and informative seroprevalence. Nonetheless, our strategy may also have modestly underestimated SARS-CoV-2 cumulative infections for 2 reasons. First, the level of SARS-CoV-2 antibodies may decrease over time. However, several large studies performed in this population indicate that the RBD domain antibody typically persists for at least 4 to 6 months,4,5 longer than the period indicated by the nucleocapsid assays used in other seroprevalence studies.17,20 Second, our current sample underrepresents New York City, a high-risk area, perhaps contributing to a modestly lower seroprevalence than might be otherwise expected both in the Northeast and nationally.
Our data reliably assessed differences in SARS-CoV-2 seroprevalence by patient- and community-level characteristics over time. We noted the rapid spread of the infection throughout the breadth of the US. Although July 2020 seroprevalence rates varied from less than 1% to 34% by state, in January 2021, nearly all sampled states in the US had seroprevalence estimates exceeding 10%. Among the 4 geographic regions, the range of seroprevalence was relatively narrow (15.3%-20.8%). These patterns fit with other descriptions of SARS-CoV-2 prevalence from other countries; for example in Brazil, international travel first brought the pandemic to major cities with rapid spread in densely populated areas, but eventual spread of SARS-CoV-2 occurred throughout the country.22
In the present study, estimates for SARS-CoV-2 case detection and infection fatality, with infection fatality exceeding 1% in the Northeast after the first wave in the spring, matched modeled estimates reported by Yang et al.23 The improvement in both parameters since July 2020 indicate better detection and treatment and, in general, improvements were uniformly spread throughout the US. In an analysis by Streeck et al,24 in which the authors studied postcarnival seroepidemiology in a small town in Germany, 16% of patients had evidence of infection as measured by reverse transcriptase–polymerase chain reaction or SARS-CoV-2 serology, although only 3% were reported as cases. After taking into account the entire seroprevalent population, the infection fatality rate was 0.36%, which matched our estimate. In a seroepidemiology-based estimate of the infection fatality rate from Spain, after the first intense wave of COVID-19 in the spring and summer of 2020, the authors estimated that 0.8% of people infected with SARS-CoV-2 died overall.25 The overall infection fatality rate, however, masks the high burden of death experienced in groups with higher risks. In a systematic review referenced by the US Centers for Disease Control and Prevention for its pandemic modeling scenarios, Levin et al26 illustrated the exponential rise in infection fatality rate with age, with mortality rates less than 0.5% in persons younger than 55 years but reaching greater than 25% in persons 85 years or older.
Strengths and Limitations
Strengths of the present study include an unbiased sampling of hard-to-reach populations that is reflective of people most at risk of SARS-CoV-2, and the use of a highly specific and sensitive assay for an antibody for which data indicate a prolonged persistence in most infected people infected with SARS-CoV-2.4,5,27 This pragmatic approach enabled rapid and repeated evaluations. Limitations of this study include the lack of data on SARS-CoV-2 reverse transcriptase–polymerase chain reaction testing or COVID-19 symptoms and relative undersampling from the Northeast geographic region. In addition, there were unmeasured differences among patients receiving dialysis and the general population, specifically with respect to health status and employment. Although the sample of patients receiving dialysis was free of other biases that are typically introduced in serosurveys of the general community (eg, in outreach to low-income populations in community-based household surveys, or in insurance/health care access in commercial laboratory surveys), patients receiving dialysis are generally older, with a higher comorbidity burden, and less able to shelter in place because most require in-center hemodialysis. Estimates of SARS-CoV-2 infection from the dialysis population may have overestimated general population exposure. Alternatively, patients on dialysis are less likely to be employed and are more likely to die from SARS-CoV-2 infection, factors that may have contributed to underestimation of SARS-CoV-2 seroprevalence when extrapolated to the general population. The prior analysis in July 2020, which relied on seroprevalence in US patients receiving dialysis extrapolated to the general population, closely matched CDC estimates for seroprevalence in the general population.9,16
Conclusions
Results of this cross-sectional study suggest that, in the US, fewer than 1 in 4 patients receiving dialysis and adults overall had evidence of SARS-CoV-2 antibodies in January 2021, which is well below the level needed to confer herd immunity. Residents of neighborhoods with a large population of racial/ethnic minority groups, those from low-income neighborhoods, and individuals from younger age groups had a substantially higher prevalence of SARS-CoV-2 antibodies. The results suggest that, because these subpopulations overlap with people who express high levels of vaccine hesitancy in the US,28,29 vaccination campaigns may need to engage these high-risk groups to achieve sufficient penetration and reach community-level protection against SARS-CoV-2.
eTable 1. Comparison of Sampled Population, US Adult Dialysis Population, and US Adult Population (July US Renal Sample)
eTable 2. Seroprevalence of SARS-CoV2 Antibodies in Patients Receiving Dialysis in the US (July US Renal Sample)
eFigure 1. Counties of Residence of Patients Included in Study and Flowchart of Patients Included in the Study
eFigure 2. Differences in SARS-CoV-2 Seroprevalence by Neighborhood Characteristics in January 2021, With Comparisons to July 2020
eFigure 3. Case Detection and Infection Fatality Rates in January 2021, With Comparison to July 2020
References
- 1.Centers for Disease Control and Prevention . CDC COVID data tracker. Accessed January 24, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724-1734. doi: 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227-1230. doi: 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595-1606. doi: 10.1016/S0140-6736(20)32137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duysburgh E, Mortgat L, Barbezange C, et al. Persistence of IgG response to SARS-CoV-2. Lancet Infect Dis. 2021;21(2):163-164. doi: 10.1016/S1473-3099(20)30943-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;S0140-6736(20)32009-2. doi: 10.1016/S0140-6736(20)32009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food & Drug Administration. EUA authorized serology test performance. Accessed January 24, 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 11.Public Health England . Evaluation of Sensitivity and Specificity of Four Commercially Available SARS-CoV-2 Antibody Immunoassays. PHE Publications; 2020. [Google Scholar]
- 12.USRDS . Annual data report. Accessed June 15 2020. https://www.usrds.org/annual-data-report/
- 13.US Census Bureau . Explore Census data. Accessed July 10, 2020. https://data.census.gov/cedsci/
- 14.Sisk B, Cull W, Harris JM, Rothenburger A, Olson L. National trends of cases of COVID-19 in children based on US state health department data. Pediatrics. 2020;146(6):e2020027425. doi: 10.1542/peds.2020-027425 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention . Demographic trends of COVID-19 cases and deaths in the US reported to CDC: cases by race/ethnicity; deaths by race/ethnicity; cases by age group; deaths by age group; cases by sex; deaths by sex. December 22, 2020. Accessed February 6, 2021. https://stacks.cdc.gov/view/cdc/99332
- 16.Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2021;181(4):450-460. doi: 10.1001/jamainternmed.2020.7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murhekar MV, Bhatnagar T, Selvaraju S, et al. ; ICMR Serosurveillance Group . SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9(3):e257-e266. doi: 10.1016/S2214-109X(20)30544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward H, Cooke G, Atchison C, et al. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. 2020:2020.2010.2026.20219725.
- 19.Ward H, Atchison C, Whitaker M, et al. Antibody prevalence for SARS-CoV-2 following the peak of the pandemic in England: REACT2 study in 100,000 adults. medRxiv. 2020:2020.2008.2012.20173690.
- 20.Buss LF, Prete CA Jr, Abrahim CMM, et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288-292. doi: 10.1126/science.abe9728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabino EC, Buss LF, Carvalho MPS, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452-455. doi: 10.1016/S0140-6736(21)00183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candido DS, Claro IM, de Jesus JG, et al. ; Brazil-UK Centre for Arbovirus Discovery, Diagnosis, Genomics and Epidemiology (CADDE) Genomic Network . Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369(6508):1255-1260. doi: 10.1126/science.abd2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W, Kandula S, Huynh M, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect Dis. 2021;21(2):203-212. doi: 10.1016/S1473-3099(20)30769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streeck H, Schulte B, Kümmerer BM, et al. Infection fatality rate of SARS-CoV2 in a super-spreading event in Germany. Nat Commun. 2020;11(1):5829. doi: 10.1038/s41467-020-19509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastor-Barriuso R, Pérez-Gómez B, Hernán MA, et al. ; ENE-COVID Study Group . Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371:m4509. doi: 10.1136/bmj.m4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123-1138. doi: 10.1007/s10654-020-00698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ripperger TJ, Uhrlaub JL, Watanabe M, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53(5):925-933.e4. doi: 10.1016/j.immuni.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szilagyi PG, Thomas K, Shah MD, et al. National trends in the US public’s likelihood of getting a COVID-19 vaccine—April 1 to December 8, 2020. JAMA. 2020;325(4):396-398. doi: 10.1001/jama.2020.26419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS-CoV-2 control. JAMA. 2020;324(20):2095-2096. doi: 10.1001/jama.2020.20892 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of Sampled Population, US Adult Dialysis Population, and US Adult Population (July US Renal Sample)
eTable 2. Seroprevalence of SARS-CoV2 Antibodies in Patients Receiving Dialysis in the US (July US Renal Sample)
eFigure 1. Counties of Residence of Patients Included in Study and Flowchart of Patients Included in the Study
eFigure 2. Differences in SARS-CoV-2 Seroprevalence by Neighborhood Characteristics in January 2021, With Comparisons to July 2020
eFigure 3. Case Detection and Infection Fatality Rates in January 2021, With Comparison to July 2020