Abstract
Deleted in Liver Cancer 1 (DLC1) is a tumor suppressor gene deleted in many cancers, including angiosarcoma, an aggr malignancy of endothelial cell derivation. DLC1-deficiency in primary endothelial cells causes the loss of cell contact inhibition of growth through incompletely defined mechanisms. We report that DLC1 is a regulator of YAP, a transcriptional coactivator of proliferation-promoting and tumor-promoting genes; when confluent, active/nuclear YAP was significantly more abundant in DLC1-deficient endothelial cells compared with control cells. We also found that YAP is a required effector of the loss of cell contact inhibition of growth manifested by DLC1-deficient endothelial cells, as the silencing of YAP prevents this loss. Consistently, human angiosarcomas specimens contained a significantly greater proportion of DLC1− tumor cells with nuclear YAP compared with the DLC1+ normal cells in the adjacent tissue. Verteporfin, an inhibitor of YAP, significantly reduced angiosarcoma growth in mice. These results identify YAP as a previously unrecognized effector of DLC1 deficiency-associated loss of cell contact growth inhibition in endothelial cells and a potential therapeutic target in angiosarcoma.
Introduction
Angiosarcoma is a rare soft tissue sarcoma of endothelial cell derivation with an aggressive clinical course and poor prognosis [1–3]. Diverse genetic defects have been identified in a proportion of angiosarcomas, but no unifying driver mutations [4–7].
Recently, we have found that the tumor suppressor gene Deleted in Liver Cancer 1 (DLC1) is a physiological regulator of cell contact inhibition of proliferation in primary endothelial cells and that DLC1 expression is abnormally reduced in angiosarcoma, contributing to tumor growth [8]. Previously, DLC1 was recognized as a repressor of vascular endothelial growth factor-induced endothelial cell proliferation [9]. DLC1 is a rho-GTPase-activating protein that exerts tumor suppressive function by inactivating RhoA, -B, and -C and establishing functional interactions with focal adhesion protein family members [10–13]. In cancer cells, DLC1 expression is often low or lost due to genetic or epigenetic mechanisms, and reintroduction of DLC1 reduces cancer cell growth [10].
Cell contact inhibition of proliferation is a fundamental property of normal cells: when they occupy the entire space normally allocated to them, they stop growing, restricting cell number in normal tissues and organs [14, 15]. Cancer cells can escape cell contact inhibition of growth, which contributes to cancer cells’ ability to proliferate locally, infiltrate tissues and metastasize [16]. Nuclear YAP (yes-associated protein 1) and its paralog TAZ, transcriptional coactivators of proliferation-promoting and tumor-promoting genes, play a key role in the control of density-dependent regulation of cell growth [17, 18]. Liver-specific overexpression of YAP causes liver enlargement, which normalizes after cessation of YAP expression [19, 20]. YAP is active in many human cancers, where it is induced by a variety of pathways [21–25].
As DLC1, YAP, and TAZ control cell density and contact inhibition of growth, we investigated potential cross talk between the DLC1 and YAP signaling pathways in normal endothelial cells and angiosarcoma.
Results
Effects of DLC1 on YAP and TAZ expression
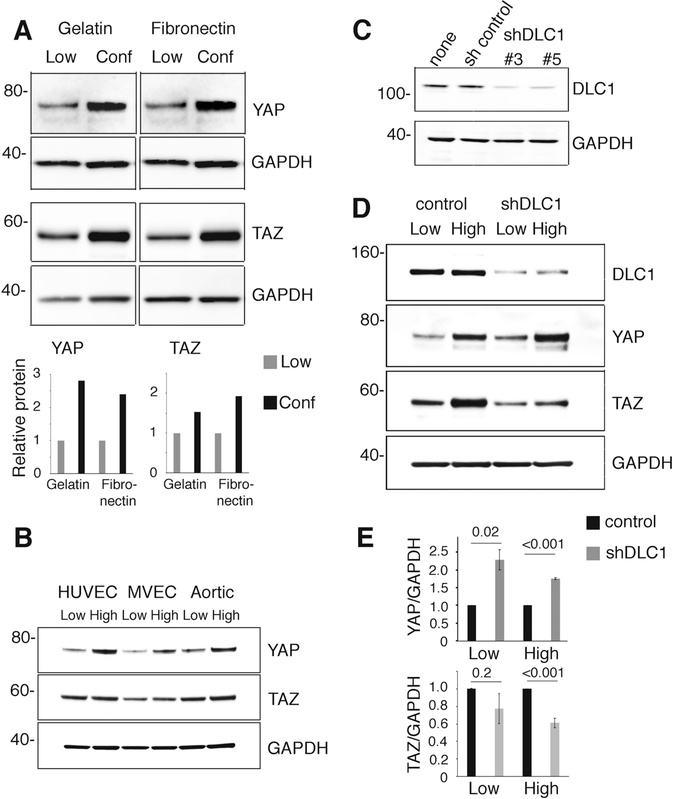
We found that YAP is generally more abundant in primary human umbilical vein endothelial cells (HUVEC) maintained as confluent monolayers compared with HUVEC propagated at a low cell density (Fig. 1a). Primary human microvascular endothelial cells (MVEC) and primary human aortic endothelial cells (Aortic) displayed a similar increase in YAP content when maintained at higher as opposed to lower cell densities (Fig. 1b). The YAP paralog, TAZ, less consistently displayed this density-dependent accumulation in endothelial cells (Fig. 1a, b). This density-dependent increase of YAP protein was associated with a relative increase of YAP mRNAs (Supplementary Fig. 1A) and with a redistribution of YAP and TAZ from the nucleus to the cytoplasm (Supplementary Fig. 1B). Previous studies have established that redistribution away from the nucleus limits YAP and TAZ cotranscriptional activity of proproliferative genes [17, 18].
Fig. 1.
DLC1 regulation of YAP and TAZ in primary endothelial cells. a, b Human umbilical vein endothelial cells (HUVEC), human microvascular endothelial cells (MVEC), and human aortic endothelial cells (Aortic) from low density (Low <35%), high density (High >75%) or confluent (Conf) cultures over gelatin or fibronectin-coated plates were tested for YAP, TAZ, and GAPDH protein content. Representative immunoblots and bands quantification in bar graphs. c Specific DLC1 shRNAs are used to silence DLC1 in HUVEC; controls include uninfected HUVEC (none) and HUVEC infected with nonmammalian shRNA control (sh control); representative immunoblotting. d and e YAP and TAZ protein content in DLC1-depleted HUVEC grown at low or high cell densities on gelatin-coated plates (top: representative immunoblots; bar graphs: cumulative quantitative results from three independent experiments expressed as relative means ± SDs). Statistical significance of group differences by two-tailed Student’s t test
DLC1 expression increases in primary endothelial cells maintained at confluency compared with cells propagated at lower cell densities [8] as do YAP and TAZ proteins. Consistent with this, RNA sequencing results from The Cancer Genome Atlas (TCGA) show a strong to moderate direct correlation between mRNA levels of DLC1 and those of YAP (Supplementary Fig. 2A), but not TAZ (Supplementary Fig. 2B) in normal tissues.
Based on these results, we investigated potential interactions between DLC1 and YAP/TAZ. We found that sustained depletion of DLC1 in HUVEC increases YAP, but not TAZ, protein content (Fig. 1c–e), suggesting that DLC1 is a negative regulator of YAP. This observation prompted an analysis of the mechanisms by which DLC1 regulates YAP in endothelial cells and examined the functional consequences of this YAP regulation.
Analysis of DLC1 regulation of YAP
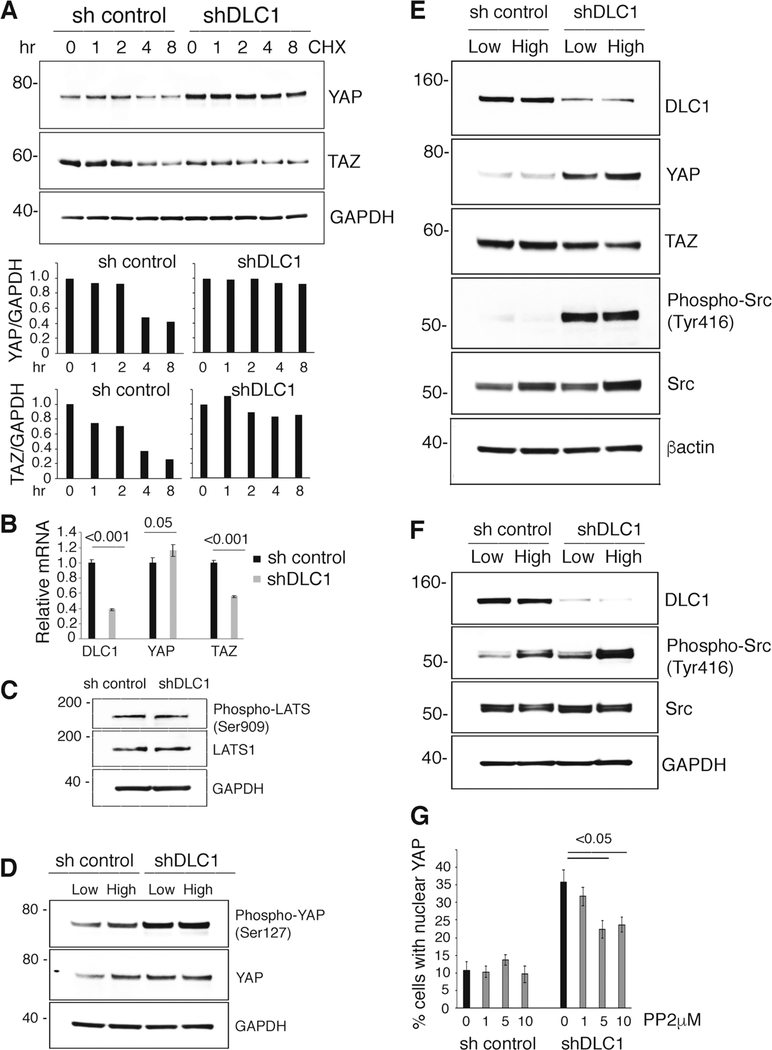
Since posttranslational regulation, particularly degradation, is critical to YAP control [21], we examined if DLC1 regulates YAP protein levels by modulating YAP protein stability. We found that YAP and TAZ protein stability is increased in DLC1-depleted HUVEC compared with control, particularly YAP protein stability (Fig. 2a). In addition, we found that YAP mRNA levels are increased in DLC1-depleted HUVEC, whereas TAZ mRNA levels are decreased (Fig. 2b).
Fig. 2.
Mechanisms of DLC1 regulation of YAP and TAZ in primary endothelial cells. a Analysis of YAP and TAZ protein stability in HUVEC. Cycloheximide (CHX, 25 μM) was added for the indicated time intervals to control (sh control) and DLC1-depleted HUVEC (shDLC1). Representative immunoblotting results and quantification in the bar graph; relative protein values are normalized to sh control and shDLC1. b Relative mRNA levels of DLC1, YAP, and TAZ in control and DLC1-depleted HUVEC; means of triplicate wells (±SDs). c Cell content of phosphorylated (Ser909) LATS and total LATS in control and DLC1-depleted HUVEC. d Cell content of phosphorylated (Ser127) YAP and total YAP in control and DLC1-depleted HUVEC. e Effects of DLC1 silencing on DLC1, YAP, TAZ, phosphorylated (Tyr416) Src, and total Src in HUVEC cultured at low or high cell density. f Effects of DLC1 silencing on phosphorylated (Tyr416) Src and total Src in MVEC cultured at high or low cell density. g Control (sh control) and DLC1-silenced (shDLC1) HUVEC were treated with PP2 (0–10 μM) or diluent only. The % cells with nuclear YAP was measured by quantitative immunofluorescence imaging (results of triplicate cultures ± SDs). Statistical significance of group differences by two-tailed Student’s t test
We found little or no change in serine phosphorylation of Large Tumor Suppressors (LATS1/2 Ser909) (Fig. 2c) and YAP (Ser127) (Fig. 2d) after DLC1 silencing in HUVEC. Phosphorylated (ser909) LATS kinases are regulators of YAP stability as they phosphorylate YAP (at serine residue 127) thereby inducing YAP translocation from the nucleus to the cytoplasm where it is degraded [26].
We examined the activity status of Src kinases in DLC1-depleted primary endothelial cells because active Src kinases promote YAP stability and YAP nuclear localization [27, 28]. In addition, DLC1 deficiency is often associated with increased Src activity in cancer cells [29, 30]. We found that Src kinases are significantly more active in DLC1-depleted HUVEC (Fig. 2e) and MVEC (Fig. 2f and Supplementary Fig. 3A) compared with control, as reflected by increased Src phosphorylation at tyrosine 416. Additional experiments showed that the Src inhibitor PP2 reduces YAP protein content (Supplementary Fig. 3B) and nuclear YAP in DLC1-silenced HUVEC (Fig. 2g and Supplementary Fig. 3C). This suggested Src dependency of DLC1 regulation of YAP stability in endothelial cells.
We also found that DLC1 silencing increases the activity of the focal adhesion kinase FAK (Tyr397) in endothelial cells (Supplementary Fig. 3D). This observation is consistent with previous studies showing that DLC1 is a negative regulator of FAK tyrosine phosphorylation at Tyr397 [12, 31]. Since phosphorylated FAK (Tyr397) is an activator of Src (Y416) [32–34], we examined the role of active FAK in the regulation of Src activity in endothelial cells. We found that the FAK inhibitor, FAK14 [35] markedly reduces Src activity in DLC1-silenced HUVEC (Supplementary Fig. 3D). These results indicate that active FAK promotes Src activation in DLC1-silenced endothelial cells. All together, these results show that DLC1 is an indirect regulator of YAP protein stability and nuclear location in endothelial cells.
DLC1 regulation of YAP nuclear and cytoplasmic distribution
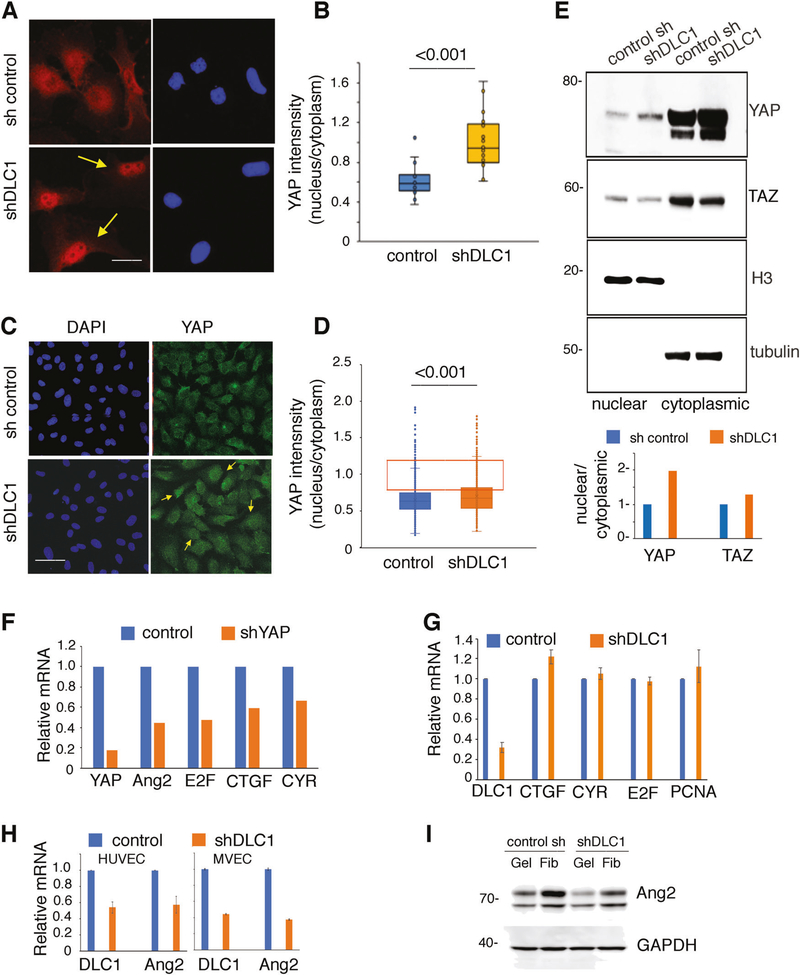
Since YAP nuclear localization is required for YAP co-transcriptional activity [19, 36, 37], we directly analyzed the effects of DLC1 silencing on YAP nuclear and cytoplasmic localization. A quantitative imaging technique [38] showed that DLC1 silencing promotes a significant redistribution of YAP to the nucleus in MVEC (Fig. 3a, b) and HUVEC (Fig. 3c, d) compared with the control. Cell fractionation into nuclear and cytoplasmic fractions also showed a relative nuclear accumulation of YAP in DLC1-depleted HUVEC compared with control HUVEC (Fig. 3e).
Fig. 3.
Effect of DLC1 silencing on YAP nuclear localization. a–d Control and DLC1-silenced MVEC (a, b) HUVEC (c, d) seeded at low or high densities on gelatin were stained with specific antibodies to YAP (red in a, green in c). DAPI (blue) identifies cell nuclei. Representative images (a, c); yellow arrows point to nuclear YAP. Quantification of YAP subcellular localization is expressed as a ratio of fluorescence intensity in nucleus/cytoplasm (b, d). Box and whiskers graphs showing the distribution of data points from individual cells; horizontal line within the colored boxes: median; box bottom: first quartile; box top: third quartile; whiskers: lowest and highest data points within 1.5 times the interquartile range. The orange-lined rectangles in d limit data points with intensity range 0.8–1.2 reflective of nuclear+ cytoplasmic fluorescence. Statistical significance of group differences by one-way ANOVA. e Cell lysates from control and DLC1-silenced HUVEC were separated into nuclear and cytoplasmic fractions documented by the enrichment of histone H3 (H3) or tubulin. YAP relative nuclear/cytoplasmic distribution is quantified from immunoblotting results. f Gene expression in YAP-silenced HUVEC. Results expressed as mRNA levels relative to control. g Gene expression in DLC1-silenced HUVEC. Ang2: angiopoietin-2; CTGF: connective tissue growth factor; CYR: cysteine rich angiogenic inducer 61; E2F: E2F1 transcription factor 1; PCNA: proliferating cell nuclear antigen. h, i Ang2 mRNA (h) and protein (i) levels in DLC1-silenced HUVEC; gelatin (Gel) or fibronectin (Fib). Scale bars: 10 μm (a) 50μm (b)
We examined the impact of this subcellular redistribution on the co-transcriptional activity of YAP with TEADs, Smads, E2F, and other transcription factors [36, 37]. YAP silencing in HUVEC expectedly reduced expression of the known YAP target genes CTGF [17], CYR61 [39], and E2F [36, 40] (Fig. 3f) whereas DLC1 silencing did not (Fig. 3g). However, DLC1 silencing reduced mRNA (Fig. 3g) and protein (Fig. 3h) levels of the YAP-target gene Ang2 [40] in endothelial cells, suggesting that YAP and DLC1 regulate Ang2 expression through distinct pathways.
YAP contribution to DLC1 regulation of cell contact inhibition of growth
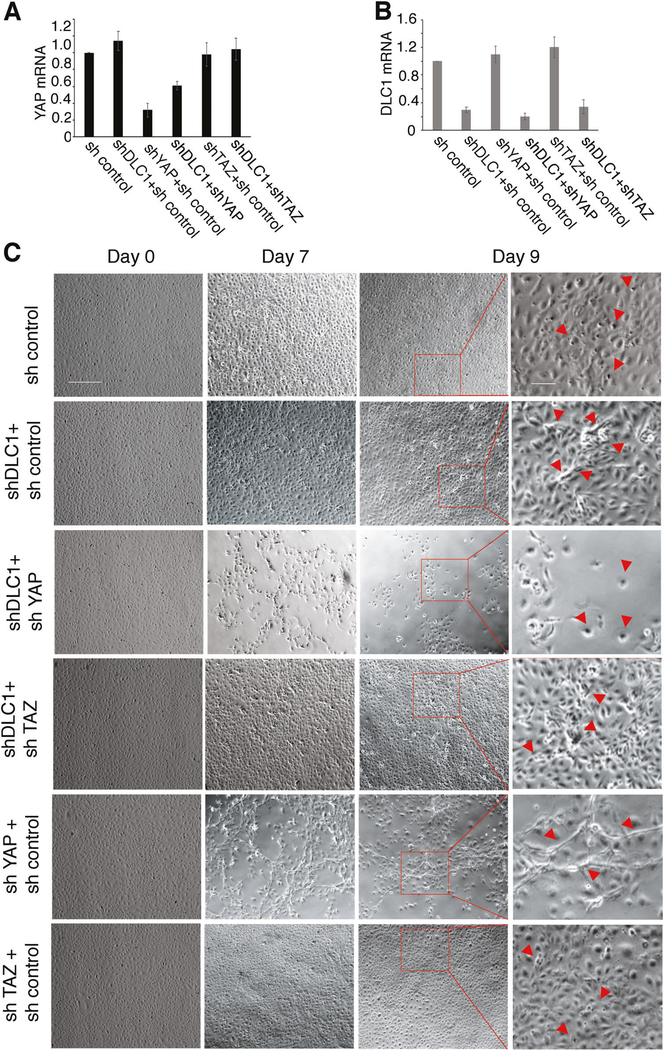
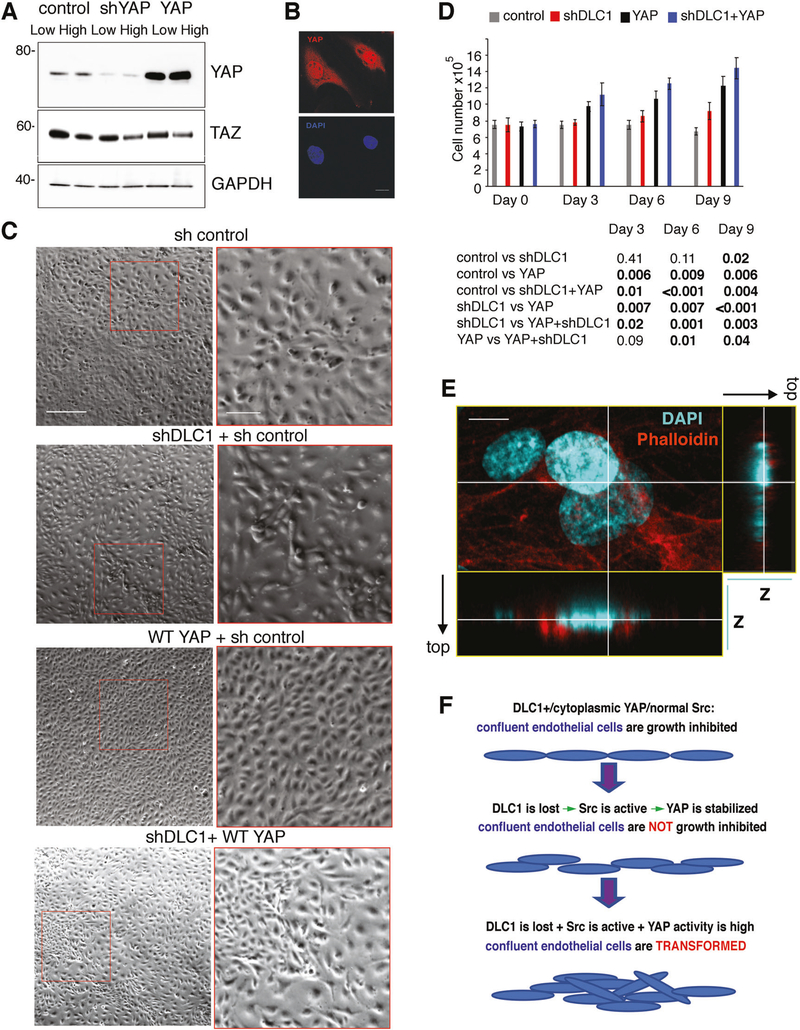
Cell-contact inhibition of growth is deregulated in DLC1-depleted endothelial cells, as evidenced by cell piling at confluency [8]. To test if YAP contributes to this deregulation, we silenced YAP individually or with DLC1 (Supplementary Fig. 4A–D and Fig. 4a, b). We then monitored confluent HUVEC cultures for 9 days (Fig. 4c). Confirming our previous observations [8], DLC1-silenced HUVEC displayed some cell piling when maintained at confluency, whereas control HUVEC maintained monolayer status, in part through increased cell death (Fig. 4c). Instead, silencing DLC1 plus YAP caused a progressive loss of the original HUVEC monolayer; by day 9, only clusters or individual cells adhered to the well (Fig. 4c). This phenotype differed from the phenotype from YAP-only silencing, which caused the appearance of a network of cord-like structures and some reduction of monolayer density (Fig. 4c).
Fig. 4.
Roles of DLC1, YAP, and TAZ in defective endothelial cell contact inhibition. Relative YAP (a) and DLC1 (b) mRNA expression levels in HUVEC after silencing with the indicated shRNAs; results from qPCR (triplicate determinations). c HUVEC control (sh control), depleted of DLC1 (shDLC1 + sh control), depleted of YAP (shYAP + sh control), depleted of TAZ (sh TAZ+ sh control), depleted of DLC1+ YAP (shDLC1 + shYAP) or depleted of DLC1 + TAZ (shDLC1 + shTAZ) were seeded to generate a confluent monolayer on gelatin-coated dishes. Culture morphology after cell attachment (time 0) on day 7 and day 9 after seeding was captured by phase contrast microscopy. The square areas limited by red ink on day 9 images are magnified on the adjacent most right panels. The arrow heads point to distinguishing features. Scale bars 150 μm; 50 μm (magnified panels)
We hypothesized that Angiopoietin-2 (Ang2) deficiency was responsible for the loss of monolayer integrity upon the silencing of DLC1 and YAP in endothelial cells since we found that the combined silencing of DLC1 and YAP reduced Ang2 expression more substantially than the individual silencing of DLC1 or YAP (Supplementary Fig. 4E). AMG386 (10 μM), a peptide-Fc fusion protein inhibitor of Ang2 binding to its Tie receptor [41], caused a deterioration of the endothelial cell monolayer associated with cell rounding and detachment, similar in morphology to the combined silencing of DLC1 and YAP (Supplementary Fig. 4F). These results suggest that Ang2 depletion underlies the loss of endothelial monolayer integrity in YAP + DLC1-silenced endothelial cells. TAZ-silenced confluent HUVEC were similar to control HUVEC, and DLC1 plus TAZ silenced HUVEC were similar to DLC1-only silenced confluent HUVEC (Fig. 4c). Overall, these results show that YAP is a necessary mediator of DLC1 regulation of cell contact inhibition of growth in endothelial cells, whereas TAZ is not.
Next, we examined the effect of forced expression of YAP in this system (Fig. 5a, b). YAP-overexpressing HUVEC (Fig. 5c) reached a significantly higher than normal (P = 0.006; day 9; Fig. 5d) saturation density while maintaining a monolayer distribution. The combination of DLC1-depletion and YAP overexpression caused multilayer growth of HUVEC associated with a greater saturation density than achieved by DLC1-deficient (P = 0.003; day 9; Fig. 5d) or YAP-overexpressing (P = 0.04) HUVEC (Fig. 5d). Volume reconstructions of Z-stack images acquired by confocal microscopy confirmed the occurrence of endothelial cell piling after DLC1 depletion and YAP overexpression (Fig. 5e and Supplementary Fig. 4G). These results suggest a potential sequence of events in endothelial transformation (Fig. 5f), where the loss of the tumor suppressor gene DLC1, which induces Src activation and YAP stabilization, disrupts cell contact inhibition of growth, and this phenotype is amplified by increased YAP activity. Supporting this model, sequencing results from TCGA show that cancers with abnormally low expression of DLC1 display a substantial expression of YAP (Supplementary Fig. 5A–C). This differs from normal tissues adjacent to these tumors where mRNA levels of DLC1 and YAP mRNA are directly correlated (Supplementary Fig. 2A, B).
Fig. 5.
Effects of YAP overexpression in confluent endothelial cells. a YAP protein levels after silencing and overexpression of YAP in HUVEC propagated at low or high cell densities; representative immunoblotting. b Representative confocal images of HUVEC transduced with WT-YAP (red); nuclei are detected with DAPI (blue). c Phase contrast images of HUVEC control, DLC1 silenced, transduced with WT-YAP or DLC1 silenced and transduced with WT-YAP. Confluent cultures were imaged on day 9 after been established as confluent cultures. d Viable cell numbers in the indicated confluent cultures were measured after attachment (time 0) and on days 3, 6, and 9; the results reflect the means ± SD (triplicate counts). Statistical significance of group differences is measured by two-tailed Student’s t test. e Confocal imaging of DLC1-depleted adherent HUVEC showing evidence of cell piling. Z-stack images on one plane (larger rectangle; nuclei/DAPI: cyan; cytoplasm/phalloidin: red) were reconstructed in 3-D. The vertical and horizontal lines through the Z-stack images (smaller rectangles) show the relative spatial location of the cells in 3D. f Schematic representation of endothelial cell transformation. Scale bars: b 10 μm; c 150 μm; 50 μm (magnified panels); e 10 μm
DLC1 and YAP in angiosarcoma
These experiments predicted that malignant endothelial cells would be DLC1-deficient and contain active YAP. Angiosarcoma tissues are DLC1-deficient [8], and were reported to often contain nuclear YAP [25]. We now examined the relationship between DLC1 deficiency and YAP activity in angiosarcoma cells within the tumor tissues.
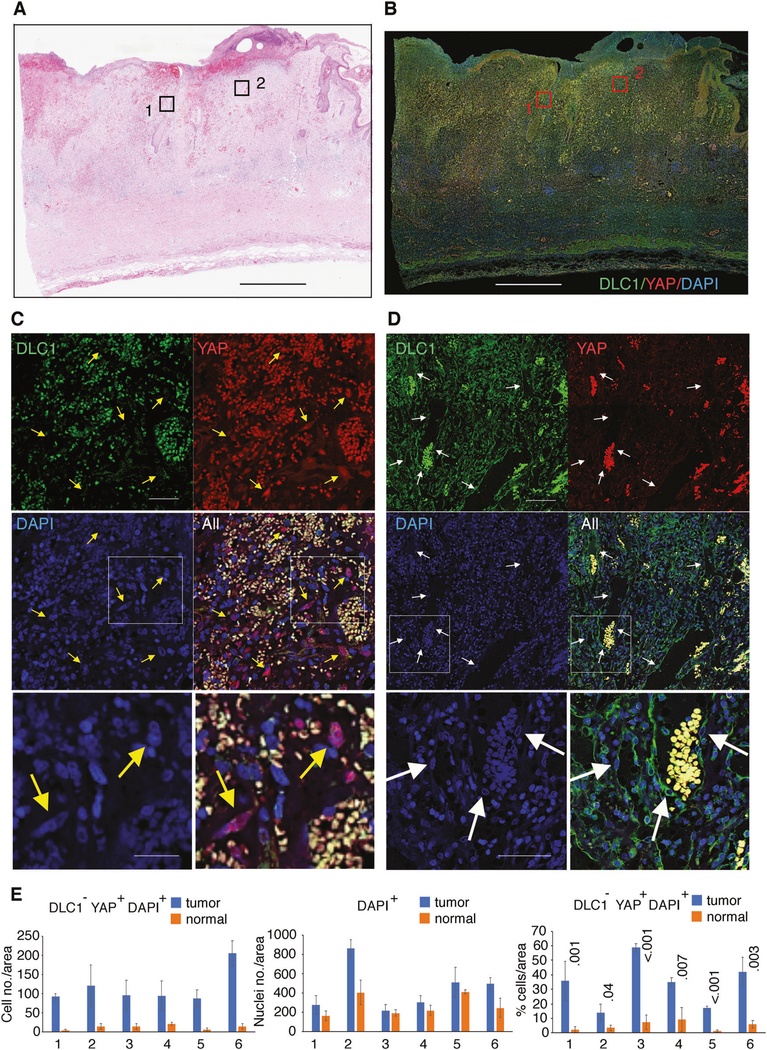
We evaluated biopsies from six aggressive angiosarcomas (Fig. 6a, b). Immunofluorescent staining of DLC1 and YAP in conjunction with DAPI (for nuclei identification) detected numerous DLC1− cells containing nuclear YAP in the tumor tissue (Fig. 6c). In contrast, DLC1−/YAP+ cells were infrequent in the endothelial cells lining blood vessels and other cells in the adjacent normal tissue, which were mostly DLC1+ with little or no nuclear YAP (Fig. 6d; Supplementary Fig. 6A–C).
Fig. 6.
DLC1 and YAP in angiosarcoma. Angiosarcoma biopsy specimen; histology by hematoxylin and eosin staining (a) and immunofluorescence detection of DLC1 (green) and YAP (red); DAPI (blue) visualizes cell nuclei (b); Scale bars: 1 mm. c Magnifications of section 1 from b display a tumor area highly infiltrated with autofluorescent nonnucleated red cells (yellow) and YAP+ nucleated cells (purple: red + blue) indicated by the yellow arrows. d Magnifications of section 2 from b display capillaries (containing autofluorescent red cells) lined by DLC1+ nucleated endothelial cells indicated by white arrows. The boxed areas in c and d are magnified in the lowest panels. Scale bars: a, b 2.6 mm; panels c, 50μm (top 4 panels) and 25 μm (bottom 2 panels); panels d 120 μm (top 4 panels) and 60 μm (bottom 2 panels). e Quantification of DLC1−/YAP+/DAPI+ cells in six angiosarcoma specimens containing tumor areas and adjacent normal areas. Each specimen was evaluated by measuring three areas each of tumor and normal tissue (each area = 340 μm2/20002 pixels). The results reflect the average (±SD) number of DLC1−/YAP+ cells (left panel); number of nucleated cells (middle panel); and the % DLC1−YAP+ nuclei (right panel) in each tumor (blue bars) and normal (orange bars) areas in each angiosarcoma specimen (numbered 1–6). Statistical significance measured by two-tailed Student’s t test
Quantitative imaging of all six angiosarcoma biopsies, each of which included tumor and normal looking-areas, showed a significant difference in the number of DLC1−/nuclear-YAP+ cells in the tumor cells compared with the normal cells included in the biopsy (Fig. 6e). Thus, angiosarcoma cells, unlike normal cells, are frequently DLC1-deficient and have nuclear/active YAP.
Effects of verteporfin on experimental angiosarcoma
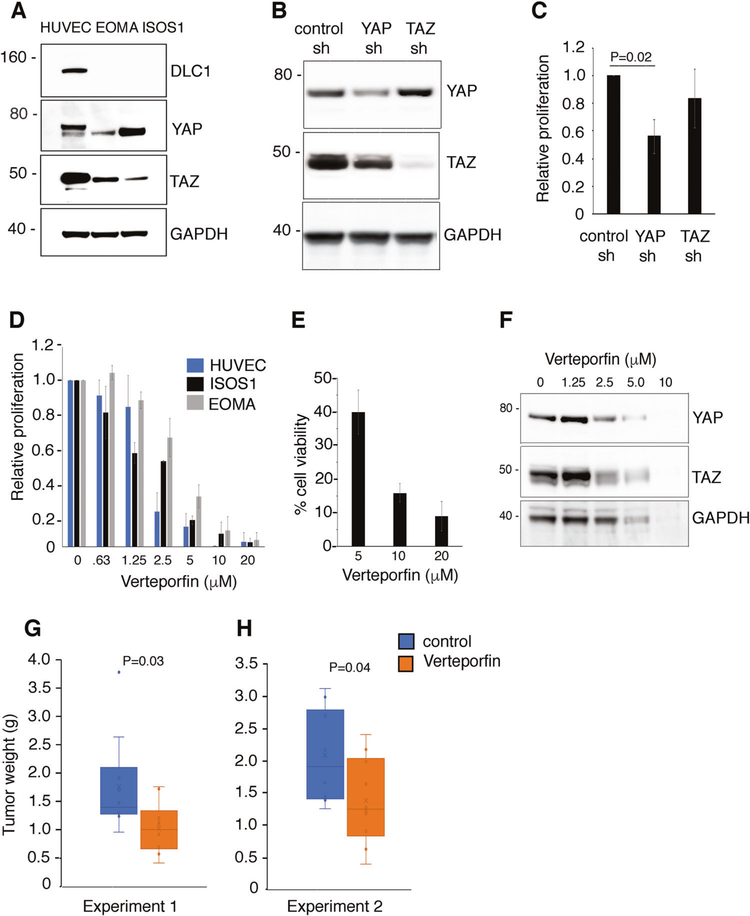
To test the antitumor activity of YAP targeting, we selected the ISOS1 [42] and EOMA mouse angiosarcoma cell lines [43] from a pool of six because they are tumorigenic in syngeneic immunocompetent mice (BALB/c and 129P3/J, respectively) and the tumors resemble histologically human angiosarcoma. ISOS1 and EOMA cells are DLC1-deficient but contain YAP and TAZ proteins (Fig. 7a). We silenced YAP or TAZ in ISOS1 cells (Fig. 7b). In proliferation assays, we found that the silencing of YAP, but not TAZ, reduces ISOS1 cell proliferation (Fig. 7c).
Fig. 7.
Effects of a YAP inhibitor on angiosarcoma cell growth in vitro and in mice. a Protein levels of DLC1, YAP and TAZ in HUVEC and in the mouse angiosarcoma cell lines EOMA and ISOS-1. b, c Effects of YAP or TAZ depletion on the proliferation of ISOS-1 cells. Results of 3H thymidine incorporation expressed as mean ± SD relative values (three independent experiments). d Relative proliferation of HUVEC, ISOS-1 and EOMA cells after 72 h incubation with 0.63–20 μM verteporfin. Results of 3H thymidine incorporation are expressed as mean ± SD relative values (3–5 independent experiments). e Viability of ISOS1 cells after 72 h incubation with verteporfin. f Dose-dependent effects of verteporfin on protein levels of YAP and TAZ in ISOS1 cells. g, h Effects of verteporfin treatment on ISOS-1 tumors established in syngeneic mice. Tumor weight at harvest is displayed by individual dots (10 mice/group; g; and 11 mice/group; h). The box and whisker plots reflect the distribution of data points; the horizontal bar indicates the median; the bottom box reflects the first quartile and the top box reflects the third quartile; lowest and highest data points are within 1.5 the interquartile range; whiskers: lowest and highest data points within 1.5 times the interquartile range. Statistical significance of differences in each experiment is calculated by two-tailed Student’s t test
The porphyrin verteporfin, which disrupts interaction between YAP/TAZ and TEAD transcription factors thereby inhibiting YAP co-transcriptional activity [44], reduced YAP-induced liver overgrowth in mice [44], and tumor growth in mice [4, 45]. Independent of YAP/TEAD blocking, Verteporfin is a photosensitizer for the photodynamic treatment of macular degeneration [46]. In addition, verteporfin displays “cell toxicity”, which is in part related to inhibition of essential YAP functions and to disruption of the proteosomal degradation pathway [22, 47]. We found that verteporfin dose-dependently inhibits ISOS1, EOMA, and HUVEC cell proliferation (Fig. 7d) promoting cell death (Fig. 7e), attributable to the essential roles of YAP in sustaining physiological cell growth. In ISOS1 tumor cells, verteporfin dose-dependently reduced protein levels of YAP, TAZ, and GAPDH (Fig. 7f), attributable to the previously recognized effect of verteporfin on proteins clearance in cancer cells [47].
We tested the impact of verteporfin on the growth of EOMA tumors in syngeneic 129P3/J mice. No valuable information could be drawn from these experiments because the tumors resemble blood-replete sponges that shrink when touched expelling blood, thereby preventing accurate measurements of tumor size and tumor weight. We subsequently generated subcutaneous ISOS1 tumors in syngeneic female BALB/c mice. When all the tumors were measurable (0.04–0.06 cm3), groups of ten mice were assigned to receive intraperitoneal injections of vehicle or verteporfin (100 mg/kg, 3 times/week). This regimen was well tolerated; no adverse events or weight loss were observed. All mice were sacrificed when the first mouse had a tumor measuring 2000 mm3. The average weight [standard deviation] of tumors from mice treated with verteporfin (1.02 g [0.46]) was significantly lower (P = 0.03) than the weight of tumors in the vehicle control group (1.76 g [0.85]) (Fig. 7g). These results were confirmed in a repeat experiment (11 mice/group) (Fig. 7h); the weight of tumors from mice treated with verteporfin (1.38 g [0.66]) was significantly (P = 0.04) lower than the weight of the control group (2.08 g [0.74]).
As noted previously [47], verteporfin-treated tumors, but not tissue from lung and spleen (not shown), displayed extensive tissue necrosis, which was mostly absent in vehicle-only treated tumors (Supplementary Fig. 7A–D). Thus, the targeting of YAP may be useful for the treatment of DLC1-deficient angiosarcoma where YAP is active.
Discussion
The current study provides novel insights into endothelial cell contact inhibition of growth and endothelial tumorigenesis. We made three observations. First, we discovered that the tumor suppressor protein DLC1 is a previously unrecognized regulator of the transcriptional coactivator YAP. We find that DLC1 regulates YAP transcription and, through Src, YAP stability. As a result, when DLC1 is depleted from confluent endothelial cells, YAP activity is abnormally high. This observation extends the spectrum of DLC1 capabilities beyond its Rho-GAP dependent and independent functions that sustain DLC1’s tumor suppressive roles [8, 10–13]. Previously, mutant KRAS and APC oncogenes and the LKB1 tumor-suppressor gene were reported to activate YAP, recruited as an oncogenic effector [48–50]. We now report that deficiency of a different tumor suppressor gene, DLC1, enhances YAP stability and function, contributing to endothelial cell dysfunction.
The second discovery we made is that YAP is an essential mediator of the loss of cell contact inhibition of growth in DLC1-depleted endothelial cells. YAP was previously recognized as a regulator of cell contact inhibition of growth in certain cell types [18, 22, 37]. We now extend this function of YAP to include endothelial cells, thereby broadening the spectrum of YAP functions in vascular biology. The endothelial-specific deletion of YAP in mice using the Tie2-Cre transgenic line is embryonically lethal attributed to defective endothelial-to-mesenchymal transformation in the heart [51]. After birth, YAP regulates retinal vascularization by promoting the transcription of angiopoietin-2 [40] and is required during vascular sprouting by coupling mechanical signals with Notch and BMP signaling [38]. Here, we provide evidence that YAP is a mediator of postangiogenic events rather than vascular sprouting and may contribute to endothelial cell "transformation" and tumorigenesis.
The third observation we made relates to the identification of YAP as a potential new pharmacologic target for therapy in angiosarcoma, a rapidly lethal malignancy of endothelial cell derivation where DLC1 is usually abnormally low [1–3, 8]. We now report that active YAP is significantly enriched in DLC1− angiosarcoma compared with the adjacent normal tissue, and that the YAP inhibitor verteporfin significantly reduces experimental angiosarcoma growth, causing extensive tumor necrosis. Verteporfin is a well-documented inhibitor of YAP binding to TEAD transcription factors thereby inhibiting YAP cotranscriptional activity [44], and a photosensitizer already approved by the U.S. Food and Drug Administration for systemic administration for photodynamic therapy of abnormal blood vessels in the eye in age-related macular degeneration [46, 52]. Preclinical studies have shown that verteporfin has anticancer activity [53–55], but there has been little investigation of the value of verteporfin in clinical oncology [56], in part because it has poor solubility. Renewed efforts at targeting YAP for cancer therapy by blocking the mevalonate metabolic pathway, which indirectly limits YAP activity [57] and inhibiting YAP–TEAD interaction with vestigial-like family member 4 (VGLL4)-mimicking peptides [58] hold promise for human angiosarcoma where YAP is active.
Since DLC1 deficiency and increased activity of YAP are expected to sustain unrestrained endothelial cell proliferation, endothelial tumorigenesis, and angiosarcoma growth, the current results disclose novel approaches for reversing this process. DLC1 depletion from endothelial cells causes activation of Src family kinases which activate YAP cotranscriptional activity. Thus, tyrosine kinase inhibitors targeting Src family kinases and YAP inhibitors hold promise for the pharmacologic treatment of currently untreatable angiosarcoma.
Materials and methods
Cells and cell function
Primary HUVEC (Lifeline Cell Technology, Frederick MD; FC-0003), human MVEC (ATCC, Manassas VA; CRL2922) and human aortic endothelial cells derived in our laboratory as described [59] were propagated on gelatin-coated surfaces up to passage 8–10 as described [8, 60]. The mouse angiosarcoma cell lines EOMA [43] (ATCC, CRL-2586, Manassas, VA) and ISOS-1 ([42], a gift of the originator Dr. Kato) were propagated up to passage 10 as described in the Supplementary Methods. All cell lines tested mycoplasma-negative by qPCR (Frederick National Laboratory, Frederick, MD). Cell proliferation was measured by 3H-thymidine incorporation, as described [60]; viable and dead cells were counted using dual fluorescence automated cell counter (Luna Logos Biosystems, Annandale, VA) and flow cytometry after staining with propidium iodide and Hoechst, as described [60] and detailed in Supplementary Methods.
Gene expression
Lentiviral particles for gene silencing, overexpression and control shRNA were prepared using a third-generation system as described [8, 60] and detailed in Supplementary Methods. Control shRNA (SHC002); DLC1-silencing shRNA (SHCLNG-NM_006094; TRCN0000047823 and −26; selected from a pool of 6 [8]), YAP-silencing shRNA (SHCLNG-NM_006106; TRCN0000107266, −67 and −68) and TAZ-silencing shRNA (SHCLNG-NM_015472; TRCN0000370007, −08 and −09) were from MilliporeSigma (St. Louis, MO); WT-YAP lentivirus (YAP1-V5, plasmid #42555, cat. no. 42555) was from Addgene (Waterton, MA). All cells infected with control or silencing lentiviruses were selected with puromycin (1 μg/ml, ThermoFisher, A11138) over at least 10 days to ensure continued gene silencing. WT-YAP transduced cells were selected in blasticidin (1 μg/ml, InvivoGen, ant-bl-05) over at least 7 days. RNA purification, cDNA synthesis, quantitative polymerase chain reaction (qPCR), and primers used (Supplementary Table 1) are described in the Supplementary Methods.
Immunoblotting, protein stability, and subcellular fractionation
Specific proteins were detected with appropriate primary (Supplementary Table 2) and secondary antibodies: sheep anti-mouse-HRP; donkey anti-rabbit-HTP, NA934V (GE, Pittsburg, PA); and donkey anti-goat IgG-HRP (Santa Cruz Biotechnology, sc-2020; Santa Cruz, CA). Bands were visualized using ECL prime kit (GE LifeSciences, RPN2232; Issaquah, WA) or the SuperSignal West Femto substrate (ThermoFisher Scientific, 34095; Logan, VT). Images were acquired using a LAS 4000 imager (GE) and quantified by ImageJ (NIH, Bethesda, MD). For determination of protein stability, control- or DLC1-silenced HUVEC were seeded (1 × 105 cells/well); 26 h after seeding, cells were treated with cycloheximide (25 μM) for 0, 1, 2, 4, or 8 h. Proteins were evaluated by immunoblotting. Nuclear and cytoplasmic protein extracts were prepared using NE-PER kit (Nuclear and cytoplasmic extraction reagents, ThermoFisher, 78833) according to the manufacturer’s instructions.
Immunofluorescence staining and quantification
Anonymized angiosarcoma specimens were obtained from six patients under institutionally approved protocols (Kyoto University, Japan) with written informed consent (Supplementary Table 3) and from mouse tumors. HUVEC were seeded on gelatin or fibronectin-coated coverslips (5 × 104 or 20 × 104). Details of cell and tissue fixation, processing and immunostaining are described in Supplementary Methods. Primary antibodies and their dilutions are listed in Supplementary Table 2. Images were acquired using confocal microscopes (LSM710 or LSM780; Carl Zeiss, Oberkochen, Germany) at ×20 or ×63 magnifications. Quantitative measurements of immunostaining, subcellular distribution and analysis of confocal z-stack images were performed as described [38, 60] and detailed in the Supplementary Methods.
Mouse experiments
All mouse experiments were conducted in adherence to the NIH Guide for Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee of CCR. BALB/c (Charles River Laboratories; Horsham, PA) and 129P3/J (the Jackson Laboratory; stock 000690) female mice (age 6–8 weeks) were injected subcutaneously (s.c.) with 1 × 106 ISOS1 cells/mouse (BALB/c) or 5 × 106 EOMA cells/mouse (129P3/J). When all mice developed measurable tumors, mice were allocated (based on having similar tumor sizes) to receive intraperitoneal (i.p.) injections of vehicle only (DMSO/PBS) or verteporfin (100 mg/kg; Selleck Chemicals, S1786; Houston, TX) 3 times/week. The investigator was blinded to the group allocation and to assessing the tumor weight outcome of the experiment. Group sample size (n = 10 or 11) was chosen based on our experience that established average and ranges of tumor growth after injection of the ISOS1 cell line in BALB/c female mice age 6–8 weeks. All mice were euthanized when any tumor reached the estimated size of 2000 mm3 (V = 1/2 × D × d2, where “D” and “d” are the longest and shortest perpendicular tumor diameters). Mouse experiments are detailed in the Supplementary Methods.
Statistical analysis
Results are presented as means or medians with standard deviations, standard errors or variance. Unpaired or paired two-tailed Student’s t-test and Mann–Whitney U test were used for analysis of two groups with normal or homoscedastic distribution. One-way or two-way ANOVA was used for statistical analysis of differences comparing three or more groups. A P value of less than 0.05 was considered statistically significant. Correlation between two sets of data was measured by the Pearson Product Moment Correlation (PPMC). Correlation coefficients are expressed as “r” values; strong positive/negative correlation: 1.0 to 0.5/−1.0 to −0.5; moderate correlation: 0.5 to 0.3/−0.5 to −0.3; weak correlation 0.3 to 0.1/−0.3 to −0.1); strength of r values is expressed as P value.
Supplementary Material
Acknowledgements
We thank Michael Kruhlak, Langston Lim, and Andy Tran for helping with confocal imaging and images quantification; Ms. Luowei Li, M. DiPrima, Drs. R. Yarchoan, D. Sanchez-Martin, H. Ohnuki, M. Potente, H. Gerhard and Jing-xin Feng; and members of the Laboratory of Cellular Oncology for contributing in various aspects of this work. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (DSM, XQ, DRL, GT) and by the Japan Society for the Promotion of Science KAKENHI, Grants-in-Aid for Scientific Research 15H05790 (AO, KK).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary information The online version of this article (https://doi.org/10.1038/s41388-019-0944-x) contains supplementary material, which is available to authorized users.
References
- 1.Pang A, Carbini M, Maki RG. Contemporary therapy for advanced soft-tissue sarcomas in adults: a review. JAMA Oncol. 2016;2:941–7. [DOI] [PubMed] [Google Scholar]
- 2.Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan JA, Maki RG, Ravi V. Pathologic angiogenesis of malignant vascular sarcomas: implications for treatment. J Clin Oncol. 2018;36:194–201. [DOI] [PubMed] [Google Scholar]
- 4.Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet. 2014;46:376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimozono N, Jinnin M, Masuzawa M, Masuzawa M, Wang Z, Hirano A, et al. NUP160-SLC43A3 is a novel recurrent fusion oncogene in angiosarcoma. Cancer Res. 2015;75:4458–65. [DOI] [PubMed] [Google Scholar]
- 6.da Costa A, Bonner M, Arbiser JL. Comprehensive profiling of H-Ras signalling in angiosarcoma endothelium. Clin Exp Dermatol. 2017;42:645–7. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Martin D, Otsuka A, Kabashima K, Ha T, Wang D, Qian X, et al. Effects of DLC1 deficiency on endothelial cell contact growth inhibition and angiosarcoma progression. J Natl Cancer Inst. 2018;110:390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih YP, Liao YC, Lin Y, Lo SH. DLC1 negatively regulates angiogenesis in a paracrine fashion. Cancer Res. 2010;70:8270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durkin ME, Yuan BZ, Zhou X, Zimonjic DB, Lowy DR, Thorgeirsson SS, et al. DLC-1: a Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med. 2007;11:1185–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao YC, Lo SH. Deleted in liver cancer-1 (DLC-1): a tumor suppressor not just for liver. Int J Biochem Cell Biol. 2008;40:843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Du X, Vass WC, Papageorge AG, Lowy DR, Qian X. Full activity of the deleted in liver cancer 1 (DLC1) tumor suppressor depends on an LD-like motif that binds talin and focal adhesion kinase (FAK). Proc Natl Acad Sci USA. 2011;108:17129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun AC, Olayioye MA. Rho regulation: DLC proteins in space and time. Cell Signal. 2015;27:1643–51. [DOI] [PubMed] [Google Scholar]
- 14.Eagle H, Levine EM. Growth regulatory effects of cellular interaction. Nature. 1967;213:1102–6. [DOI] [PubMed] [Google Scholar]
- 15.Stoker MG. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973;246:200–3. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. [DOI] [PubMed] [Google Scholar]
- 20.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–312. [DOI] [PubMed] [Google Scholar]
- 22.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards DN, Ngwa VM, Wang S, Shiuan E, Brantley-Sieders DM, Kim LC, et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci Signal. 2017;10:eaan4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20:888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuneki M, Kinjo T, Mori T, Yoshida A, Kuyama K, Ohira A, et al. Survivin: a novel marker and potential therapeutic target for human angiosarcoma. Cancer Sci. 2017;108:2295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian X, Li G, Vass WC, Papageorge A, Walker RC, Asnaghi L, et al. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell. 2009;16:246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripathi BK, Anderman MF, Qian X, Zhou M, Wang D, Papageorge AG, Lowy DR. SRC and ERK cooperatively phosphorylate DLC1 and attenuate its Rho-GAP and tumor suppressor functions. J Cell Biol. 2019; 10.1083/jcb.201810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TY, Lee JW, Kim HP, Jong HS, Kim TY, Jung M, et al. DLC-1, a GTPase-activating protein for Rho, is associated with cell proliferation, morphology, and migration in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2007;355:72–7. [DOI] [PubMed] [Google Scholar]
- 32.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC 2nd, Schaller MD. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–83. [DOI] [PubMed] [Google Scholar]
- 34.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–35. [DOI] [PubMed] [Google Scholar]
- 35.Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, et al. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 2015;11:e1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neto F, Klaus-Bergmann A, Ong YT, Alt S, Vion AC, Szymborska A, et al. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. Elife. 2018;7:e31037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008;68:2789–94. [DOI] [PubMed] [Google Scholar]
- 40.Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. [DOI] [PubMed] [Google Scholar]
- 41.Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuzawa M, Fujimura T, Tsubokawa M, Nishiyama S, Katsuoka K, Terada E, et al. Establishment of a new murine-phenotypic angiosarcoma cell line (ISOS-1). J Dermatol Sci. 1998;16:91–8. [DOI] [PubMed] [Google Scholar]
- 43.Obeso J, Weber J, Auerbach R. A hemangioendothelioma-derived cell line: its use as a model for the study of endothelial cell biology. Lab Invest. 1990;63:259–69. [PubMed] [Google Scholar]
- 44.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, et al. ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell. 2015;27:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziemssen F, Heimann H. Evaluation of verteporfin pharmakokinetics–redefining the need of photosensitizers in ophthalmology. Exp Opin Drug Metab Toxicol. 2012;8:1023–41. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Ramakrishnan SK, Triner D, Centofanti B, Maitra D, Gyorffy B, et al. Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci Signal. 2015;8:ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–70. [DOI] [PubMed] [Google Scholar]
- 49.Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Y, Zhang W, Han X, Li F, Wang X, Wang R, et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat Commun. 2014;5:4629. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, et al. Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J Biol Chem. 2014;289:18681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu L, Murphy RP. Photodynamic therapy: a new approach to the treatment of choroidal neovascularization secondary to age-related macular degeneration. Curr Opin Ophthalmol. 1999;10:217–20. [DOI] [PubMed] [Google Scholar]
- 53.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74: 4170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110: 1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–66. [DOI] [PubMed] [Google Scholar]
- 58.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80. [DOI] [PubMed] [Google Scholar]
- 59.Murga M, Yao L, Tosato G. Derivation of endothelial cells from CD34- umbilical cord blood. Stem Cells. 2004;22:385–95. [DOI] [PubMed] [Google Scholar]
- 60.Salvucci O, Ohnuki H, Maric D, Hou X, Li X, Yoon SO, et al. EphrinB2 controls vessel pruning through STAT1-JNK3 signalling. Nat Commun. 2015;6:6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.