Abstract
Hepatoid adenocarcinoma (HAC) is a rare tumour that produces an alpha-fetoprotein (AFP) mimicking hepatocellular carcinoma (HCC). Adrenal HAC is exceedingly rare. Here we report extremely high AFP-producing adrenal HAC, the first case in Thailand. A 47-year-old man presented with left flank pain and weight loss for 2 months. A palpably huge left flank mass was observed on physical examination. CT revealed a 7 cm enhanced mass involving the left adrenal gland and multiple contrast-enhanced hypodense masses in both liver lobes. The largest was a 3.7 cm at liver segment-VII without cirrhotic background, with an AFP level of 321 495 ng/mL. Both adrenal and liver biopsies were performed. This patient received a diagnosis of advanced adrenal HAC. Unfortunately, the tumour progressed, causing massive upper gastrointestinal bleeding and death. Adrenal HAC is challenging to diagnose, which multifocal HCC, pheochromocytoma and adrenocortical carcinoma should be excluded. Surgical resection is preferred among resectable patients. However, no systemic therapy has been standardised.
Keywords: urological cancer, endocrine cancer, hepatic cancer, pathology
Background
Hepatoid adenocarcinoma (HAC) originates from various organs, such as the ovaries, lungs, gall bladder, pancreas, duodenum and adrenal glands. The stomach is the most common site of the tumour.1 This is the first report of adrenal HAC with an extremely high alpha-fetoprotein (AFP) level.
Case presentation
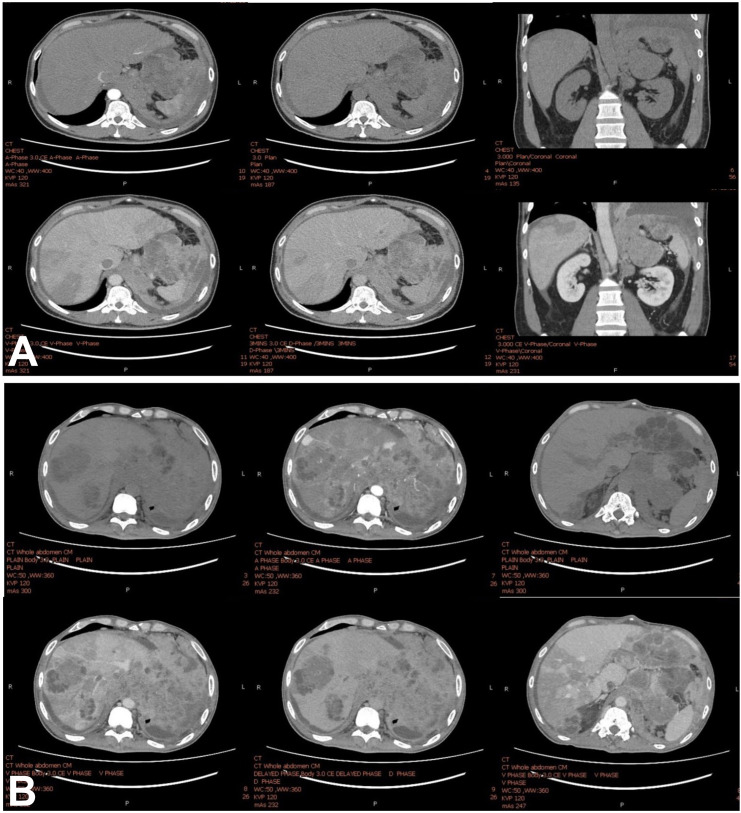
A 47-year-old man presented with severe left flank pain and progressive dyspnoea for 2 months. A CT scan of the chest and whole abdomen revealed a 5.5×7 cm enhanced mass involving the left adrenal gland and left hemidiaphragm, inferior vena cava (IVC) thrombosis and multiple hypodense liver lesions. Enlarged para-aortic, aortocaval and gastrohepatic lymph nodes were observed up to 3 cm and hypodense lesions scattered in both lobes of the liver. The largest was 3.7×3.3 cm at hepatic segment VII, for which all were contrast enhanced in the portovenous phase without a cirrhosis background (figure 1A).
Figure 1.
(A) CT scan with four-phase contrast shows an irregular infiltrative heterogeneous enhanced mass involving the left hemidiaphragm and left adrenal gland mass with several hypodense lesions scattered throughout the lobes of the liver. No radiological evidence supports liver cirrhosis. (B) The CT study shows an interval increase in the left adrenal gland mass, now measuring about 11.2×15.2×5.5 cm. Interval increased extension of necrotic soft tissue and multiple matted necrotic nodes involving the left hemidiaphragm, pericardial fat pad, pericardium, periaortic, gastrohepatic and peripancreatic regions, which are unprecedented. This lesion shows the direct invasion of the distal oesophagus and gastric cardia, causing proximal oesophageal dilation. Increased extension of tumour thrombus in inferior vena cava extending into the right hepatic vein ascends to the right atrium and extension in the left inferior pulmonary vein reaching into the left atrium. These conditions progress within 3 weeks after the first CT study.
The serum AFP level was 321 495 ng/mL and otherwise, within normal limits. The 24-hour urine normetanephrine was investigated for preoperative evaluation before the left adrenal gland biopsy and to exclude pheochromocytoma condition. A non-significant elevation was observed of the 24-hour urine normetanephrine level.
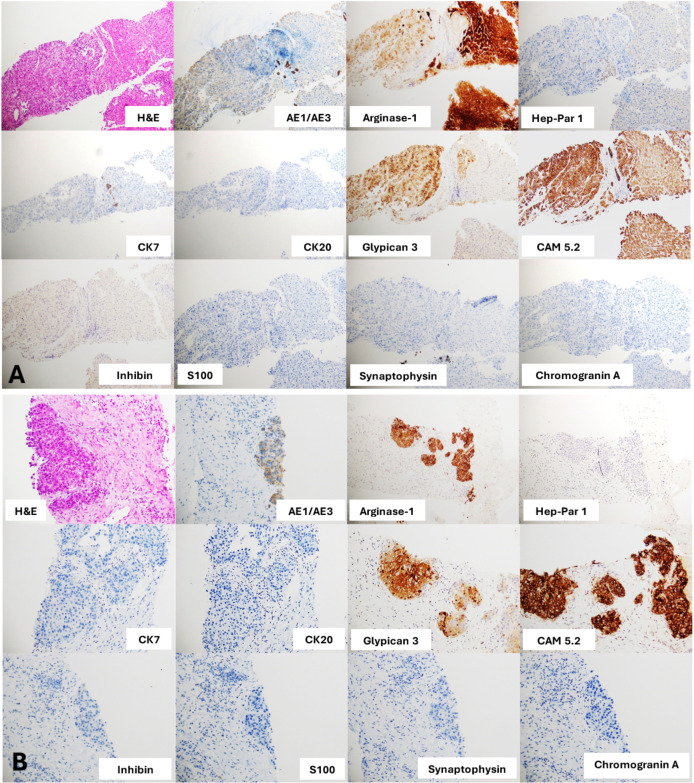
Biopsies of the left adrenal and liver mass were performed. The pathological report showed carcinoma with hepatocytic differentiation. The immunohistochemistry (IHC) studies showed immunoreactive with CAM5.2, arginase-1 and glypican-3; focally weakly positive for AE1/AE3 but negative for CK7, CK20, CK19, inhibin A, chromogranin A, synaptophysin, S100 and HepPar-1 (figure 2). Therefore, this patient received a diagnosis of advanced adrenal HAC with multiple liver metastases regarding clinical presentation, imaging, histology and IHC staining.
Figure 2.
(A) Sections of liver nodule core biopsies show an epithelial neoplasm consisting of the proliferated polygonal epithelial cells arranged in 6–7 cell thick trabeculae and nested separately by flat endothelial lining sinusoidal spaces. The neoplastic cells contain vesicular and slightly pleomorphic irregular thick nuclear membrane nuclei, prominent nucleoli and rare mitoses. Intranuclear cytoplasmic inclusions are noted. Adjacent normal liver parenchyma is present on the right side of the picture. These biopsies appear to have positive stain for AE1/AE3, arginase-1, glypican-3 and CAM5.2, but a negative stain for CK7, CK20, inhibin, S100, synaptophysin or chromogranin A. Hepatocellular carcinoma or hapatoid adenocarcinoma is suggested from these results. (B) Sections of adrenal mass core biopsies show an epithelial neoplasm within a desmoplastic stroma. The adrenal mass shares similar histological features to the liver nodule; positive stain for AE1/AE3, arginase-1, glypican-3 and CAM5.2 and negative stain for CK7, CK20, inhibin, S100, synaptophysin or chromogranin A. No residual non-neoplastic adrenal tissue was present in the core biopsies. Thus, we can conclude the resemblance of both liver nodules and adrenal mass.
We planned to start palliative chemotherapy combining cisplatin and etoposide. Unfortunately, the patient developed massive upper gastrointestinal (GI) bleeding 3 weeks after the biopsy. CT of the abdomen demonstrated an increase in the size of the left adrenal gland invading the stomach (figure 1B). His clinical status deteriorated rapidly developing hypovolaemic shock from severe upper GI bleeding and he died 2 days later.
An autopsy report demonstrated that a gross tumour originated from the left adrenal gland and directly invaded the stomach and adjacent organs, causing massive GI bleeding. The microscopic examination showed the micro-invasion of metastatic disease in the stomach and adjacent organs, disrupting the normal tissue layers. The tumour metastasised to the lungs, pleura, liver and peritoneum.
Investigations
CT of the chest and whole abdomen revealed a 5.5×7 cm enhanced mass at the left adrenal gland involving the left hemidiaphragm, multiple hypodensity lesions with contrast enhanced in portovenous phase at both lobes of the liver, the largest mass was 3.7 cm in diameter at liver segment VII, and IVC thrombosis. Moreover, multiple lymphadenopathies were observed at para-aortic, aortocaval and gastrohepatic lymph nodes, up to 3 cm (figure 1). The same characteristics from related reports showed a hypodensity lesion, with contrast enhancing in the venous phase on the CT.2–4 This contrasted with typical hepatocellular carcinoma (HCC) imaging based on the American Association for the Study of Liver Diseases 2018 and Liver Imaging Reporting and Data System five criteria, referring to more than 1 cm liver mass arterial hyperenhancement with rapid washout on the portovenous phase in the cirrhotic liver background.5
Extremely high-serum AFP was observed, namely, 321 ng/mL and 495 ng/mL. However, liver and adrenal gland biopsies should be performed regarding atypical features of HCC using CT imaging. The 24-hour urine normetanephrine was investigated for preoperative evaluation before performing biopsy at the left adrenal gland and to exclude pheochromocytoma condition. A non-significant elevation of the 24-hour urine normetanephrine level was noted; therefore, left adrenal and liver biopsies were performed. Pathological reports revealed carcinoma with hepatocytic differentiation. IHC studies showed immunoreactivity with CAM5.2, arginase-1 and glypican-3, which was focally weakly positive for AE1/AE3 but negative for CK7, CK20, CK19, inhibin A, chromogranin A, synaptophysin, S100 and HepPar-1, (figure 2) similar to related reports (table 1).
Table 1.
Patient demographics and clinicopathological and treatment characteristics
| No | Authors | Sex | Age, years | Tumour location | Size | AFP, ng/mL | Treatment | Immunohistochemistry | Outcome |
| 1 | Current authors | M | 47 | Left adrenal gland | 11.2×15.2×5.5 cm3 | 321 495 | Supportive treatment | AE1/AE3 ⨁, arginase-1 ⨁, CAM5.2 ⨁, glypican-3 ⨁, HepPar1 ⨁ and Ki-67 20%, alpha-inhibin ⊖, chromogranin A ⊖, S100 ⊖, synaptophysin ⊖ | Death at 3 weeks after diagnosis |
| 2 | Yoshioka et al6 | M | 57 | Left adrenal gland | 8×5 cm2 | 30 500 | Thoracoabdominal nephro-adrenalectomy | N/A | N/A |
| 3 | Yi et al *13 | M | 57 | Left adrenal gland | 3.5×2.2×2 cm3 | 570 | Surgery | HepPar1⨁, AFP ⨁, ferritin ⨁, CEA ⨁, CK8 ⨁, CK18 ⨁, α1-ACT, α1-AT ⨁ | N/A |
| 4 | Malya et al11 | F | 48 | Right adrenal gland | 4×5 cm2 | 3900 | Surgery+radiotherapy+5FU and gemcitabine | AFP ⨁, glipan ⨁, CK8 ⨁, HepPar1⨁, CK17⨁, CK19⨁, luminal/focal ⨁, polygonal CEA ⨁ | N/A |
| 5 | Jing et al*14 | M | 53 | Left adrenal gland | 13×10×8 cm3 | 31 353 | Oxaliplatin+capecitabine | HepPar1 ⨁, CK ⨁, AFP ⨁, Ki-67 30%, CD34 ⨁ | Alive at 7 months of follow-up |
| 6 | Liu et al2 | M | 60 | Right adrenal gland | 5×7 cm2 | 6.39 | Surgery | HepPar1 ⨁, glapican-3 ⨁, CD34 ⨁, CK ⨁, AFP ⊖, α-inhibin ⊖, CgA ⊖ and CEA ⊖ | Alive at 30 months of follow-up |
| 7 | Zhang and Hua*12 | M | 57 | Left adrenal gland | 13×10×9 cm3 | >13 000 | Surgery+oxaliplatin+gemcitabine | EMA ⨁, CK8 ⨁, AFP ⨁, hepatocyte ⨁, CK18 ⨁, CD10 ⨁ | Alive at 7 months of follow-up |
| 8 | Lin et al3 | M | 64 | Left adrenal gland | 9.3×8.9×9.7 cm3 | 2.75 | mFOLFOX6×4 cycles, transcatheter arterial chemoembolisation, apatinib | TTF-1 ⊖, CK5/6 ⊖, P63 ⊖, NSE ⊖, synaptophysin ⊖, chromogranin A ⊖, CK8/18 ⨁, CK19 ⨁, CK7 ⨁, CD 20 ⊖, hepatocyte ⨁, vimentin ⊖, HepPar1⨁ | Death at 9 months after diagnosis |
| 9 | Deng et al4 | M | 83 | Left adrenal gland | 13×8.7×11 cm3 | >24 200 | Surgery+sorafenib | HepPar1⨁, glypican-3 ⨁, AFP ⨁, arginase-1 ⨁, Ki-67 50% | Death at 9 months after diagnosis |
*Published in Chinese.
AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CK, cytokeratin; EMA, epithelial membrane antigen; 5FU, 5-fluorouracil; N/A, not available; NSE, neuron specific enolase.
Differential diagnosis
Distinguishing between adrenocortical carcinoma (ACC) with multiple liver metastases and adrenal HAC is difficult using clinical presentation and CT imaging. Both ACC and adrenal HAC are rare and similar in age distribution, the fourth to fifth decade of life. However, men frequently appear to develop HAC more often than women but without sufficient confirmatory evidence. Nevertheless, HAC mostly produces high-serum AFP, ranging from 4730 ng/mL to 700 000 ng/mL as reported in retrospective studies.3 Moreover, liver metastasis at first diagnosis is the most common presentation in HAC. HCC was excluded for this patient due to atypical diagnostic criteria from dynamic CT imaging. In addition, IHC staining proved to be a crucial guide for diagnosis. For epithelial neoplasm negative for CK7 and CK20, ACC and HCC are included. However, ACC should occasionally be shown positive for HepPar-1 staining. Moreover, glypican-3 staining maintains a high positive rate in HAC. Therefore, IHC stains for the patient were compatible with primary adrenal HAC.
Treatment
Surgery is the mainstay of treatment for localised disease. Additionally, HAC is chemotherapy and radiation resistant. Therefore, no survival benefit is derived from adjuvant chemotherapy. For systemic treatment among patients with advanced-stage cancer, some case reports showed marginal benefits from chemotherapy such as an oxaliplatin-based regimen (mFOLFOX, or capecitabine+oxaliplatin),2–4 gemcitabine+oxaliplatin,5 or gemcitabine monotherapy.3 In addition, vascular endothelial growth factor-targeting tyrosine kinase inhibitors (apatinib, sorafenib) were reported for treatment.2 6
Outcome and follow-up
The estimated overall survival is 7–9 months.3 However, AFP-producing HAC has a worse prognosis than non-AFP-producing HAC. Our patient did not receive chemotherapy due to his clinical deterioration. The deceased patient developed severe upper GI bleeding from the progression of the primary tumour, invading the stomach before death.
Discussion
The incidence of HAC is about 1.3%–1.5% worldwide.1 Only eight cases have been reported for adrenal HAC since 1994. The first case was reported by Yoshioka et al.6 The clinical characteristics were more common among men; age ranging from 47 to 83 years and involving the left adrenal gland. Elevated serum AFP level was common and related to a poor prognosis. The median level of serum AFP was 2235 ng/mL (2.75–30 500 ng/mL).
Multifocal HCC with adrenal gland metastasis is more common than primary adrenal HA, and distinguishing between them would be difficult. Multifocal HCC could have multicentric origination or intrahepatic metastases. Some studies have proposed the pathophysiology of multifocal HCC.7 8 Alternatively, multifocal HCC is predominantly used to describe only intrahepatic lesions. Thus, HAC may mimic and be indistinguishable from HCC, especially when liver metastasis occurs. IHC study may not be useful in this circumstance.9 10 Hence, a related study showed that comprehensive gene profiling might be helpful in selected cases.3 However, clinical presentation and imaging may be of major help to determine the primary site of cancer.
In this case, the primary tumour was located in the epicentre of the left adrenal gland with multiple liver metastases, and the clinical presentation mainly occurred from compressive symptoms from a primary tumour. The functional adrenal tumours, including pheochromocytoma, paraganglioma and hormone-producing adrenal carcinoma, should be excluded before diagnosing ACC or adrenal HAC.
From related reports, surgical resection was the primary treatment. Several systemic therapies were reported, for example, 5-fluorouracil-based regimen,3 11 gemcitabine-based regimen,11 12 targeted therapy (sorafenib or apatinib)3 4 and transarterial embolisation.3 The median overall survival ranged from 7 to 30 months.
Learning points.
Adrenal hepatoid adenocarcinoma is an extremely rare cancer not easily diagnosed for which multifocal hepatocellular carcinoma, paraganglioma and pheochromocytoma should be excluded.
Surgical resection is preferred for resectable conditions.
There is no standard treatment established for systemic therapy.
Acknowledgments
We acknowledge our patient and his family, the Phramongkutklao Hospital (PMK) Tumor Board, Asst Prof Kasan Seetalarom; Senior Medical Oncologist of Medical Oncology Division, Dr Nattapol Sathavarodom; Endocrinologist of Endocrinology Division, and Clinical Nutrition Division, Department of Internal Medicine, Dr Chatwadee Limpaiboon; Department of Radiology, and Department of Pathology for collaborating to diagnose and treat this patient.
Footnotes
Twitter: @ttawasapon
NP and SS contributed equally.
Contributors: Manuscript writing, review, diagnosis and treatment—TT. Manuscript writing, diagnosis and treatment—NP and SS. Manuscript writing and dagnosis—KS. Final approval—TT, NP and SS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Next of kin consent obtained.
References
- 1.Su J-S, Chen Y-T, Wang R-C, et al. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol 2013;19:321–7. 10.3748/wjg.v19.i3.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Man Q, Ding K. A rare case of hepatoid adenocarcinoma of the adrenal gland. Int J Clin Exp Pathol 2016;9:4247–50. [Google Scholar]
- 3.Lin J, Cao Y, Yu L, et al. Non-α-fetoprotein-producing adrenal hepatoid adenocarcinoma: a case report and literature review. Medicine 2018;97:e12336. 10.1097/MD.0000000000012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng X, Jin Y, Yang W, et al. An adrenal Hepatoid adenocarcinoma with left renal vein thrombosis extending into the inferior vena cava. Urol J 2019;16:511–4. 10.22037/uj.v0i0.5250 [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723–50. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka M, Ihara H, Shima H, et al. [Adrenal hepatoid carcinoma producing alpha-fetoprotein: a case report]. Hinyokika Kiyo 1994;40:411–4. [PubMed] [Google Scholar]
- 7.Nomoto S, Kinoshita T, Kato K, et al. Hypermethylation of multiple genes as clonal markers in multicentric hepatocellular carcinoma. Br J Cancer 2007;97:1260–5. 10.1038/sj.bjc.6604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Xia C-Y, Lau W-Y, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg 2013;217:1054–62. 10.1016/j.jamcollsurg.2013.07.402 [DOI] [PubMed] [Google Scholar]
- 9.Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol 2003;27:1302–12. 10.1097/00000478-200310000-00002 [DOI] [PubMed] [Google Scholar]
- 10.Kashani A, Ellis JC, Kahn M, et al. Liver metastasis from hepatoid adenocarcinoma of the esophagus mimicking hepatocellular carcinoma. Gastroenterol Rep 2017;5:67–71. 10.1093/gastro/gov021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malya FU, Bozkurt S, Hasbahceci M, et al. A rare tumor in a patient with hepatic hydatic cyst: adrenal hepatoid adenocarcinoma. Case Rep Med 2014;2014:1–4. 10.1155/2014/824574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang R, Hua J. One case of left adrenal hepatoid adenocarcinoma. Chin J Endocrinol 2016;6:527–8. [Google Scholar]
- 13.Yi L, Jiangyang L, Xiaohong W. Clinicopathological observation of adrenal hepatoid adenocarcinoma. Diagn Pathol J 2009. [Google Scholar]
- 14.Jing L, Rui Z, Ping Z. Clinical and pathological observation of adrenal Hepatoid adenocarcinoma practical cancer journal. 02, 2015: 194–7. [Google Scholar]