Abstract
Variable-temperature 15N solid-state NMR (SSNMR) spectroscopy is used to investigate the dynamics of three diamines within the pores of the metal–organic framework Mg2(dobpdc), which exhibit both bound and free nitrogen environments when coordinated to the framework open metal sites. From these experiments it is possible to quantify the rates and energetics for exchange between the two nitrogen environments, and the results can then be corroborated by density functional theory calculations and molecular dynamics simulations. The activation energy for the exchange also enables quantification of the metal–amine bond strength, which is correlated with the CO2 adsorption properties of the diamine-appended frameworks.
TOC GRAPHIC
Rising anthropogenic CO2 emissions remain the leading source of global climate change,1 and the capture and sequestration of CO2 from power plant flue gas emissions has been highlighted as a potentially promising emission mitigation strategy in recent years.2–4 Numerous approaches have been developed for the removal of CO2 from flue gas through the use of aqueous amine solutions,4 zeolites,5 metal−organic frameworks (MOFs),6 and covalent organic frameworks.7 Metal−organic frameworks with coordinatively-unsaturated metal centers such as the M2(dobdc) family (dobdc4− = 2,5-dioxido-1,4-benzenedicarboxylate; M = Mg, Mn, Fe, Co, Ni, Cu, Zn) have attracted considerable attention owing to the strong interaction that occurs between the metal centers and CO2 upon adsorption, which leads to a high affinity for CO2 adsorption and selectivity over N2. One drawback of these materials, however, is that their performance often diminishes under humid conditions.8–10 Recently, it was demonstrated that after grafting diamines onto the open metal sites of Mg2(dobpdc) (dobpdc4– = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate), an expanded form of Mg2(dobdc), the resulting material exhibits substantially improved CO2 adsorption properties arising from step-shaped adsorption isotherms (or isobars) that allow for high working capacities in temperature- or pressure-swing adsorption processes and robust performance under humid conditions.10–15 Notably, the step pressure (or temperature) can be tuned through variation of the appended diamine,11–15 and it is thus of fundamental importance to investigate in detail the influence of diamine structure on the CO2 adsorption properties of diamine-appended Mg2(dobpdc) materials.
The structures of diamine-appended variants of Mg2(dobpdc), such as the N,N′-dimethylethylenediamine (m-2-m)-appended framework (Figure 1), have one nitrogen coordinated to Mg2+, while the other amine group extends into the open MOF pore. Above a threshold temperature and pressure, CO2 inserts into the Mg−N bond, resulting in the formation of a carbamate group bound to magnesium center and an ammonium group formed at the other end of the diamine (Figure S1, Supporting Information). The interaction between the negative carbamate and a neighboring positive ammonium facilitates the synergistic formation of ammonium–carbamate chains along the framework channels, leading to the observed cooperative adsorption behavior.11,16 As a consequence, the metal–amine bond strength plays an important role in determining the pressure or temperature at which CO2 insertion occurs. This effect has been examined recently via single-crystal X-ray diffraction characterization of the isostructural diamine-appended Zn2(dobpdc) frameworks,13 which readily form single crystals. Even still, these single-crystal structures exhibit a large degree of diamine disorder within the framework channels at 100 K, and so we sought to develop additional approaches for assessing the structures, particularly for the diamine-appended Mg2(dobpdc) variants. We were specifically interested in exploring the dynamic behavior of the diamines within the MOF pores, as a potential means of guiding the design of new diamine-appended structures for CO2 capture.
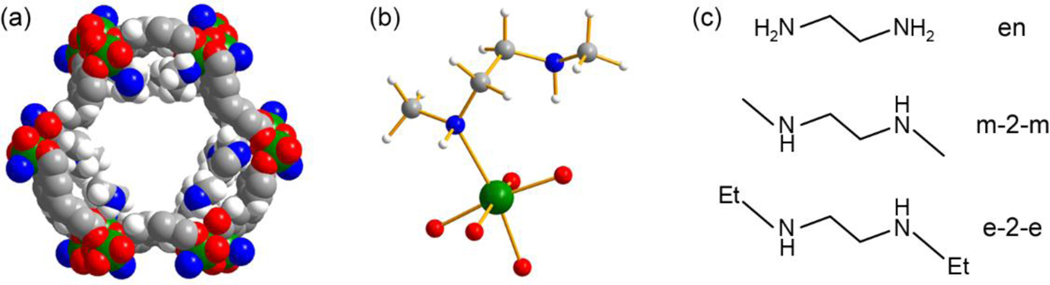
Figure 1.
(a) Space-filling model of Mg2(dobpdc) appended with N,N′-dimethylethylenediamine, m-2-m–Mg2(dobpdc), and (b) Ball-and-stick model of an m-2-m-appended Mg2+ site within the framework, with bound and free nitrogen environments. The models are taken from the DFT-optimized structures reported in this work. Green, red, blue, grey, and white spheres represent Mg, O, N, C, and H atoms, respectively; all other H atoms are omitted for clarity. (c) The structures of three diamines studied in this work.
Solid-state NMR (SSNMR) spectroscopy is complementary to X-ray diffraction as a characterization technique and is extremely sensitive to the local environment around the observed nucleus. The subtle difference between the bound and free nitrogen environments of diamines should in particular be detectable by 15N SSNMR spectroscopy.17,18 Although the 15N SSNMR spectrum of m-2-m–Mg2(dobpdc) was previously found to exhibit a single 15N peak at room temperature,11 a recent 25Mg SSNMR study indicated that the two nitrogen environments undergo rapid exchange, yielding an averaged environment on the NMR time scale.19 Herein, we analyze variable-temperature (VT) 15N SSNMR data to probe the nitrogen environments and dynamics of three diamines appended to Mg2(dobpdc), namely ethylenediamine (en), N,N′-dimethylethylenediamine (m-2-m), and N,N′-diethylethylenediamine (e-2-e). In each case, the NMR data enable elucidation of the diamine dynamics within the framework, yielding activation energies for diamine exchange that provide a measure of the metal−amine bond strength. When combined with density functional theory (DFT) calculations and molecular dynamics (MD) simulations, these results provide insights into correlations between diamine structure and the CO2 adsorption properties of these materials.
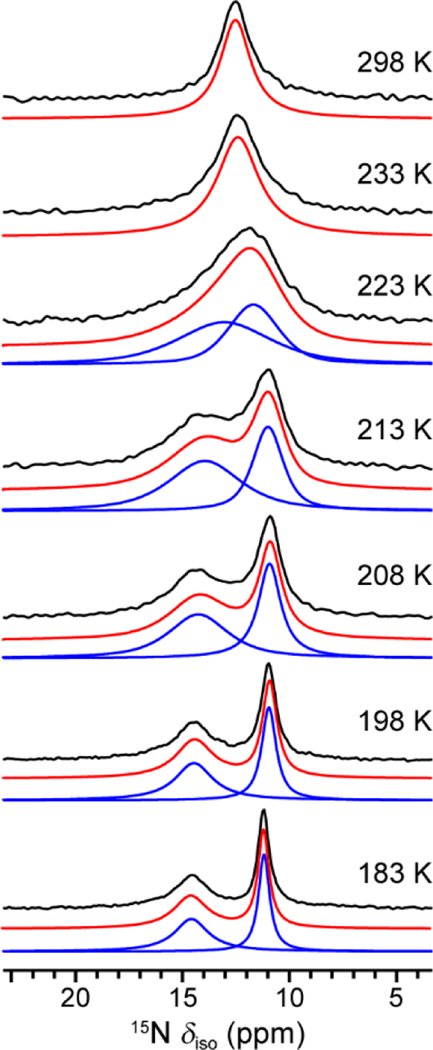
The variable-temperature 15N SSNMR spectra of en–Mg2(dobpdc) are shown in Figure 2. At 298 K, a symmetric 15N peak arises at 12.7 ppm. This peak broadens considerably upon cooling to 233 K and then becomes asymmetric at 223 K, eventually separating into two peaks upon further cooling. The separation between these two new peaks continues to increase with decreasing temperature, while their characteristic line widths (full width at half maximum) decrease. At the lowest measurement temperature of 183 K, the two peaks are observed at 11.4 and 14.8 ppm (relative to neat NH3), respectively. This overall trend as a function of temperature is consistent with the rapid exchange between two nitrogen environments.20–24 We assigned the peaks at 14.8 and 11.4 ppm to the metal-bound and free amine nitrogen environments, respectively, considering that nitrogen will be deshielded by the Mg2+ cation and experience a larger 15N chemical shift value. This assignment was verified by DFT calculations (Figure S6).
Figure 2.
15N direct-polarization SSNMR spectra of en–Mg2(dobpdc) at 20.0 T as a function of temperature. Black, blue, and red profiles are experimental, deconvoluted, and the summation of deconvoluted spectra, respectively. The signal intensities are normalized for clarity.
Deconvolution of the SSNMR spectra enables determination of the frequency separation between the two 15N peaks and their line widths, and from this information it is possible to calculate a chemical exchange rate constant. For example, in the range where the peak positions exhibit a strong temperature dependence (223–208 K), the exchange rate constant was calculated using the equation , where is the frequency separation between two peaks (in Hz) without exchange and is the separation with exchange.20–24 The exchange rate constant was found to be 3.16 × 102 s−1 at 213 K, and the calculation details for all values are shown in Supporting Information Section S5 (Table S3).
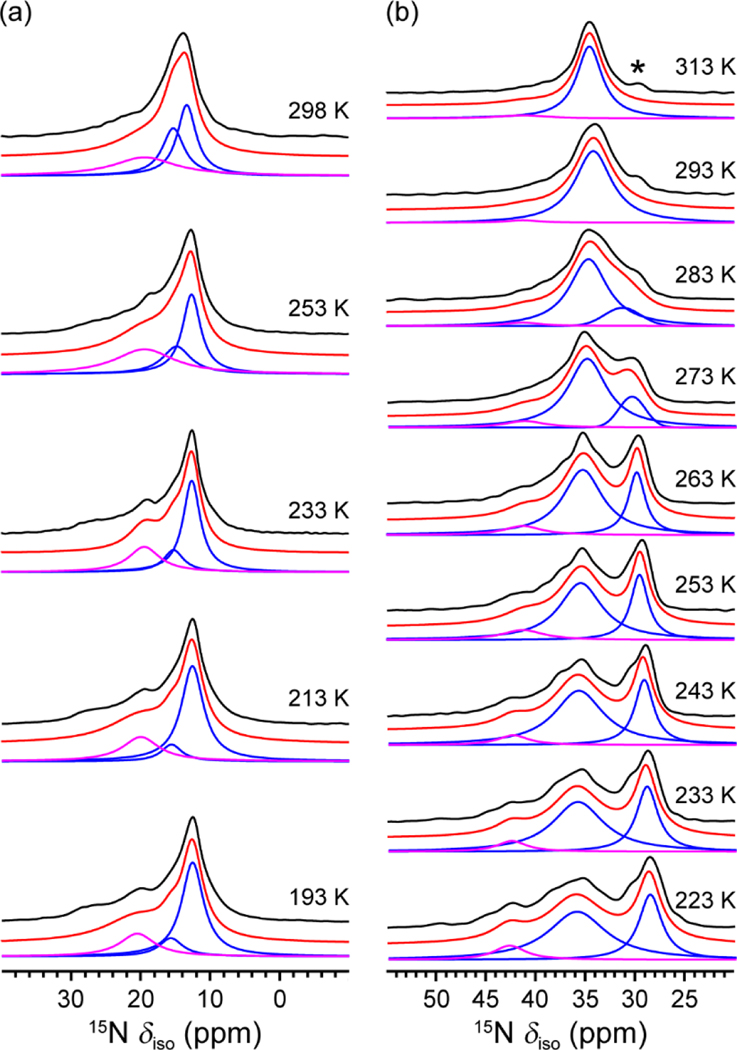
The same variable-temperature analysis was used to assess the dynamics of m-2-m and e-2-e appended to Mg2(dobpdc). At 293 K, the spectrum of m-2-m consists of an asymmetric peak at 13.3 ppm and a weak shoulder at ~20 ppm (Figure 3a). Upon cooling, the asymmetric peak narrows and shifts to the more shielded region of the spectrum (lower ppm values). This peak can be deconvoluted into two components at all temperatures, and at 193 K the deconvoluted peaks occur at 16.7 and 15.7 ppm for the bound and free nitrogen environments, respectively. The chemical shift value of the shoulder is close to that of the single 15N resonance observed for free N,N′-dimethylethylenediamine in toluene, and was assigned to the unbound amine trapped in the MOF pores. Characterization of a second m-2-m–Mg2(dobpdc) sample verified the above peak assignments and the changes in peak position as a function of temperature (Figures S12 and S14, Supporting Information).
Figure 3.
(a) 1H–15N cross-polarization (CP) SSNMR spectra of m-2-m–Mg2(dobpdc) as a function of temperature. (b) 1H–15N CP SSNMR spectra of e-2-e–Mg2(dobpdc) as a function of temperature. Black profiles are experimental spectra. Blue and pink profiles are deconvoluted spectra. Red profiles are the summation of deconvoluted spectra. Pink profiles represent the 15N signals that were assigned to unbound diamines trapped in the MOF pores (Figure S14). All signal intensities are normalized for clarity. The asterisk in the 313 K data indicates signal arising from the transmitter artifact. 1H–15N CP experiments were performed at 20.0 T with a contact time of 5 ms for m-2-m–Mg2(dobpdc) and at 16.4 T with a contact time of 0.5 ms for e-2-e–Mg2(dobpdc).
The variable-temperature spectra of e-2-e–Mg2(dobpdc) are likewise shown in Figure 3b. At 313 K, a single 15N peak is apparent at 34.6 ppm and grows asymmetric at 283 K, eventually splitting into two peaks upon further cooling. The peak separation increased with decreasing temperature, and at the lowest measured temperature (223 K), the two resulting peaks were at 36.0 and 28.7 ppm for the bound and free nitrogen environments, respectively. Similar to m-2-m–Mg2(dobpdc), a weak shoulder was present at ~40 ppm in all spectra and can be assigned to unbound N,N′-diethylethylenediamine in the pores (Figure S14). For both frameworks, these diamines were not observed to participate in the exchange (see Figure S15 and the following discussion). The separation of the coordinated diamine nitrogen peaks increased significantly even at the lowest measured temperature for both materials, and thus it was not possible to determine and the precise exchange rate constants for m-2-m and e-2-e appended frameworks. It was possible to calculate a potential range of values, however, and this data (Table S3) along with additional discussions can be found in Supporting Information Section S5.
Using the Arrhenius equation and the rate constants obtained from SSNMR data, it was possible to determine the activation energy for free and bound nitrogen exchange in each material (Table 1). A much larger activation energy was found for en (37 ± 2 kJ·mol−1) than for m-2-m and e-2-e (ranging from 0.67 ± 0.06 to 6.4 ± 1.5 kJ·mol−1 and from 0.83 ± 0.05 to 10.9 ± 0.9 kJ·mol−1, respectively), indicating that the substitution of an amine hydrogen with an alkyl group (i.e., changing the bound amine from primary to secondary) significantly weakens the metal–amine bond. The result is consistent with single-crystal X-ray diffraction data for the Zn analogues,13 which confirms that primary/secondary and primary/tertiary diamines preferentially coordinate to the Zn2+ centers through the primary amine.
Table 1.
Activation energies of three diamines appended to Mg2(dobpdc).
| Diamine | (kJ·mol−1) | |
|---|---|---|
| Experimental | Calculated | |
| en | 37 ± 2 | |
| m-2-m | 0.67 ± 0.06–6.4 ± 1.5 | 7.93 ± 1.41 |
| e-2-e | 0.83 ± 0.05–10.9 ± 0.9 | 10.5 ± 1.3 |
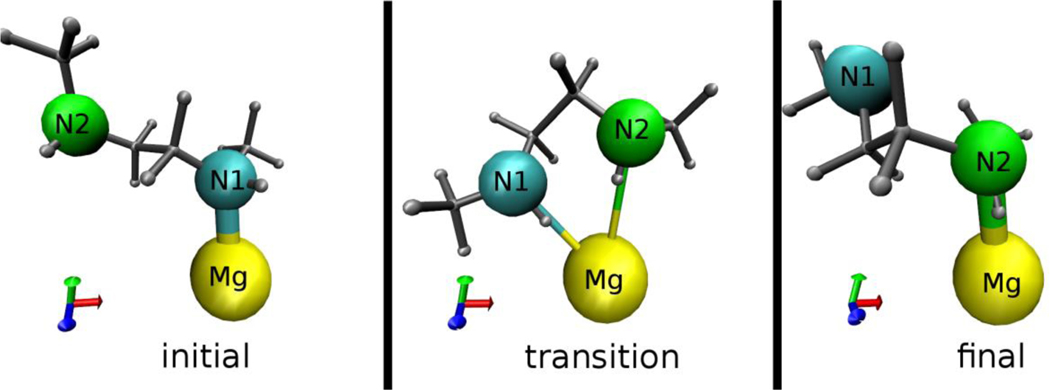
Molecular dynamics (MD) simulations have been used extensively to study flexibility and guest dynamics in metal−organic frameworks,19,25–29 and we used this approach to further examine the dynamics and compute exchange rates for the diamines appended to Mg2(dobpdc). In order to capture the bond breaking and formation in the exchange event, we employed the ReaxFF, a reactive force field based on bond order.25,30 The simulation results confirm exchange between the bound and free nitrogen atoms (see Figure 4, Figure S7, and the movie in the Supporting Information) and enable identification of a possible exchange mechanism, with a transition state consisting of both nitrogen atoms of the diamine molecule weakly bound to the same Mg2+ center. The calculated kinetic activation energies () in Table 1 have similar magnitudes and display the same trend as the experimental values: the activation energy for m-2-m is smaller than that of e-2-e, and both values are smaller than the experimental value for en.
Figure 4.
Still frames from the MD trajectory illustrating representative initial, transition, and final states for the amine nitrogen atoms in an m-2-m exchange event. In the transition state, the nitrogen that is initially unbound (N2) coordinates to the Mg2+ center, and in the final state the initially bound nitrogen (N1) becomes free.
As previously elucidated, the CO2 adsorption mechanism in these diamine-appended materials involves the insertion of CO2 into the metal−amine bonds and the synergistic formation of ammonium−carbamate chains (Figure S1).11,16 Because the metal−amine bonds are broken upon CO2 adsorption, stronger bonds and therefore higher activation energies for nitrogen exchange are expected to correlate with higher adsorption step pressures (or lower step temperatures). The opposite trend was observed experimentally, with step pressure decreasing in the order m-2-m > e-2-e > en, despite the larger activation barrier toward exchange for en compared to m-2-m and e-2-e.11–14 Therefore, our NMR data and MD simulations confirm that the metal−amine bond strength is not the only important factor in determining the adsorption step pressure. Other factors can also influence the thermodynamics of adsorption,13 such as the carbamate enthalpy of formation and the loss of rotational and vibrational degrees of freedom upon ammonium−carbamate chain formation (i.e., large negative entropy change).
In conclusion, we used variable-temperature 15N SSNMR spectroscopy to characterize the coexistence of bound and free nitrogen environments in three diamine-appended variants of the Mg2(dobpdc) metal−organic framework, and further identified a likely transition state involved in nitrogen exchange from MD simulations. The magnitude of the activation energies extracted from both SSNMR experiments and MD simulations afforded a measure of metal−amine bond strength, indicating that m-2-m– and e-2-e–Mg2(dobpdc) materials have similar bond strengths that are both weaker than the metal−amine bond strength for en–Mg2(dobpdc). The trends in the experimental data were verified by DFT calculations and MD simulations, and all together the data confirm that the metal−amine bond strength is not the only important determiner of CO2 adsorption step pressure. The approach described herein can be importantly extended to other diamine-appended metal−organic frameworks to understand diamine dynamics and CO2 adsorption toward the design of improved carbon capture materials.
Supplementary Material
ACKNOWLEDGMENT
This work was supported through the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under Award DE-SC0001015. The 20.0 T variable-temperature 15N SSNMR experiments were performed at the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research. DFT calculations were performed on the Dino cluster in the Molecular Graphics and Computation Facility at UC Berkeley, which is funded by the U.S. National Institutes of Health under Award S10OD023532. The MD simulations were carried out at the National Energy Research Scientific Computing Center, a User Facility supported by the Office of Science of the U.S. Department of Energy under Contract DE-AC02–05CH11231. We thank the NSF Graduate Research Fellowship program for support of Y.M.L. We thank the National Institute of General Medical Sciences of the National Institutes of Health for a postdoctoral fellowship for P.J.M. (F32GM120799). We thank Prof. Gina L. Hoatson and Dr. Rony Kalfarisi for use of a 750 MHz spectrometer in College of William and Mary, United States, during initial attempts to collect 15N variable-temperature SSNMR data. We thank Dr. Jeffrey Martell for experimental assistance and fruitful discussions, and Dr. Katie Meihaus for editorial assistance.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Additional information including elucidated CO2 adsorption mechanism, sample preparation, characterizations, theoretical calculations, rate constant calculations and additional discussions. DFT-optimized structures. Movie of diamine exchange. The following files are available free of charge via the Internet at http://pubs.acs.org. Electronic Supporting Information (PDF)
DFT-optimized Structure (CIF)
Movie of diamine exchange (MPG)
The authors declare the following competing financial interest(s): T.M.M. and J.R.L. have a financial interest in Mosaic Materials, Inc., a start-up company working to commercialize metal–organic frameworks for gas separations. UC Berkeley has applied for a patent on some of the materials discussed herein, on which T.M.M. and J.R.L. are included as inventors.
REFERENCES
- (1).IPCC. Contributions of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2013: The Physical Science Basis; Stocker TF, Qin D, Plattner G-K, Tignor MMB, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, Eds.; Cambridge University Press: Cambridge, United Kingdom and New York, 2013. [Google Scholar]
- (2).IPCC IPCC Special Report on Carbon Dioxide Capture and Storage, Cambridge University Press: Cambridge, United Kingdom and New York, 2005. [Google Scholar]
- (3).Bhown AS; Freeman BC Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [DOI] [PubMed] [Google Scholar]
- (4).Schimming V; Hoelger C-G; Buntkowsky G; Sack I; Fuhrhop J-H; Rocchetti S; Limbach H-H Evidence by 15N CPMAS and 15N−13C REDOR NMR for Fixation of Atmospheric CO2 by Amino Groups of Biopolymers in the Solid State. J. Am. Chem. Soc. 1999, 121, 4892–4893. [Google Scholar]
- (5).Choi S; Drese JH; Jones CW Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [DOI] [PubMed] [Google Scholar]
- (6).Sumida K; Rogow DL; Mason JA; McDonald TM; Bloch ED; Herm ZR; Bae T-H; Long JR Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [DOI] [PubMed] [Google Scholar]
- (7).Zeng Y; Zou R; Zhao Y. Covalent Organic Frameworks for CO2 Capture. Adv. Mater. 2016, 28, 2855–2873. [DOI] [PubMed] [Google Scholar]
- (8).Kizzie AC; Wong-Foy AG; Matzger AJ Effect of Humidity on the Performance of Microporous Coordination Polymers as Adsorbents for CO2 Capture. Langmuir 2011, 27, 6368–6373. [DOI] [PubMed] [Google Scholar]
- (9).Yu J; Balbuena PB Water Effects on Postcombustion CO2 Capture in Mg-MOF-74. J. Phys. Chem. C 2013, 117, 3383–3388. [Google Scholar]
- (10).Mason JA; McDonald TM; Bae T-H; Bachman JE; Sumida K; Dutton JJ; Kaye SS; Long JR Application of a High-Throughput Analyzer in Evaluating Solid Adsorbents for Post-Combustion Carbon Capture via Multicomponent Adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [DOI] [PubMed] [Google Scholar]
- (11).McDonald TM; Mason JA; Kong X; Bloch ED; Gygi D; Dani A; Crocella V; Giordanino F; Odoh SO; Drisdell WS et al. Cooperative Insertion of CO2 in Diamine-Appended Metal-Organic Frameworks. Nature 2015, 519, 303–308. [DOI] [PubMed] [Google Scholar]
- (12).McDonald TM; Lee WR; Mason JA; Wiers BM; Hong CS; Long JR Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [DOI] [PubMed] [Google Scholar]
- (13).Siegelman RL; McDonald TM; Gonzalez MI; Martell JD; Milner PJ; Mason JA; Berger AH; Bhown AS; Long JR Controlling Cooperative CO2 Adsorption in Diamine-Appended Mg2(dobpdc) Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 10526−10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lee WR; Hwang SY; Ryu DW; Lim KS; Han SS; Moon D; Choi J; Hong CS Diamine-Functionalized Metal-Organic Framework: Exceptionally High CO2 Capacities from Ambient Air and Flue Gas, Ultrafast CO2 Uptake Rate, and Adsorption Mechanism. Energ. Environ. Sci. 2014, 7, 744–751. [Google Scholar]
- (15).Milner PJ; Siegelman RL; Forse AC; Gonzalez MI; Runčevski T; Martell JD; Reimer JA; Long JR A Diaminopropane-Appended Metal–Organic Framework Enabling Efficient CO2 Capture from Coal Flue Gas via a Mixed Adsorption Mechanism. J. Am. Chem. Soc. 2017, 139, 13541−13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Vlaisavljevich B; Odoh SO; Schnell S; Dzubak AL; Lee K; Planas N; Neaton J; Gagliardi L; Smit B. CO2 Induced Phase Transitions in Diamine-Appended Metal Organic Frameworks. Chem. Sci. 2015, 6, 5177–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Witanowski M; Stefaniak L; Webb GA Nitrogen NMR Spectroscopy. Annu. Rep. NMR Spectrosc. 1993, 25, 1–480. [Google Scholar]
- (18).Mason J. Nitrogen NMR. In Encycl. NMR; Harris RK, Wasylishen RE, Eds.; John Wiley & Sons, Ltd, 2012; Vol. 5. [Google Scholar]
- (19).Xu J; Blaakmeer ESM; Lipton AS; McDonald TM; Liu YM; Smit B; Long JR; Kentgens APM; Reimer JA Uncovering the Local Magnesium Environment in the Metal–Organic Framework Mg2(dobpdc) Using 25Mg NMR Spectroscopy. J. Phys. Chem. C 2017, 121, 19938−19945. [Google Scholar]
- (20).McConnell HM Reaction Rates by Nuclear Magnetic Resonance. J. Chem. Phys. 1958, 28, 430–431. [Google Scholar]
- (21).Abragam A. The Principles of Nuclear Magnetism, Clarendon Press: Oxford, 1961. [Google Scholar]
- (22).Bain AD Chemical Exchange in NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2003, 43, 63–103. [Google Scholar]
- (23).Alexander S. Exchange of Interacting Nuclear Spins in Nuclear Magnetic Resonance. II. Chemical Exchange. J. Chem. Phys. 1962, 37, 974–980. [Google Scholar]
- (24).Limbach H-H; Wehrle B; Schlabach M; Kendrick R; Yannoni CS CPMAS Polarization Transfer Methods for Superposed Chemical Exchange and Spin Diffusion in Organic Solids. J. Magn. Reson. 1988, 77, 84–100. [Google Scholar]
- (25).Huang L; Joshi KL; Duin A. C. T.v.; Bandosz TJ; Gubbins KE ReaxFF Molecular Dynamics Simulation of Thermal Stability of a Cu3(BTC)2 Metal-Organic Framework. Phys. Chem. Chem. Phys. 2012, 14, 11327−11332. [DOI] [PubMed] [Google Scholar]
- (26).Getman RB; Bae Y-S; Wilmer CE; Snurr RQ Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 703–723. [DOI] [PubMed] [Google Scholar]
- (27).Lin L-C; Kim J; Kong X; Scott E; McDonald TM; Long JR; Reimer JA; Smit B. Understanding CO2 Dynamics in Metal–Organic Frameworks with Open Metal Sites. Angew. Chem. Int. Ed. 2013, 52, 4410–4413. [DOI] [PubMed] [Google Scholar]
- (28).Boyd PG; Moosavi SM; Witman M; Smit B. Force-Field Prediction of Materials Properties in Metal-Organic Frameworks. J. Phys. Chem. Lett. 2017, 8, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Jawahery S; Simon CM; Braun E; Witman M; Tiana D; Vlaisavljevich B; Smit B. Adsorbate-Induced Lattice Deformation in IRMOF-74 Series. Nat. Commun. 2017, 8, 13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).van Duin ACT; Dasgupta S; Lorant F; Goddard WA ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.