Abstract
Faithful tumor mouse models are fundamental research tools to advance the field of immuno-oncology (IO). This is particularly relevant in diseases with low incidence, as in the case of pediatric malignancies, that rely on pre-clinical therapeutic development. However, conventional syngeneic and genetically engineered mouse models fail to recapitulate the tumor heterogeneity and microenvironmental complexity of human pathology that are essential determinants of cancer-directed immunity. Here, we characterize a novel mouse model that supports human natural killer (NK) cell development and engraftment of neuroblastoma orthotopic patient-derived xenograft (O-PDX) for pre-clinical antibody and cytokine testing. Using cytotoxicity assays, single-cell RNA-sequencing, and multi-color flow cytometry, we demonstrate that NK cells that develop in the humanized mice are fully licensed to execute NK cell cytotoxicity, permit human tumor engraftment, but can be therapeutically redirected to induce antibody-dependent cell-mediated cytotoxicity (ADCC). Although these cells share phenotypic and molecular features with healthy controls, we noted that they lacked an NK cell subset, termed activated NK cells, that is characterized by differentially expressed genes that are induced by cytokine activation. Because this subset of genes is also downregulated in patients with neuroblastoma compared to healthy controls, we hypothesize that this finding could be due to tumor-mediated suppressive effects. Thus, despite its technical complexity, this humanized patient-derived xenograft mouse model could serve as a faithful system for future testing of IO applications and studies of underlying immunologic processes.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02713-6) contains supplementary material, which is available to authorized users.
Keywords: Pediatric oncology, Natural killer cells, Neuroblastoma, Antibody therapy
Introduction
Pre-clinical animal models have emerged as useful research tools and led to major drug discoveries in the field of cancer research [1]. Particularly in pediatric oncology, where only a few patients are diagnosed each year and available for clinical trials, therapeutic advancements rely on pre-clinical testing. Although drug efficacy studies in mouse model systems closely correlate with clinical outcomes in patients [2], their application in immuno-oncology (IO) studies encounters several limitations. In syngeneic murine models, immunocompetent inbred mouse strains are inoculated with murine-derived tumor cell lines [3] or exposed to carcinogens to provoke tumor formation [4]. Despite yielding highly reproducible drug testing results, these tumors fail to recapitulate the genetic and microenvironmental complexity found in humans that are critical in modulating the immunologic response [5]. Genetically engineered mouse models (GEMM) give rise to autochthonous tumors with the native microenvironment, but they lack the mutational burden that defines many cancers and determines their immunogenicity [6, 7].
Orthotopic patient-derived xenograft (O-PDX) models closely reproduce human pathology and have found broad applicability in studies of chimeric antigen receptor (CAR) T cell therapies and antibody-based treatment modalities [1, 8]. For example, athymic nude or Rag2null/Il2Rγcnull mice injected with O-PDX cells are a useful tool for antibody studies, because they have intact innate immunity, including natural killer (NK) cells that are the main effector cell in antibody-dependent cell-mediated cytotoxicity (ADCC), while being T cell-depleted [9]. In profoundly immunocompromised NOD/scid/Il2Rγcnull (NSG) mice that lack functional B, T, and NK cells, immune reconstitution occurs via adoptive effector cell transfer (ACT), such as CAR T cells, or CD34+ hematopoietic progenitor cell (HPC) injection to induce the development of functional B and T cells [10, 11]. However, the major drawback of humanized NSG mice is that they are unable to establish innate immunity [12, 13] due to the limited cross-reactivity between mouse and human cytokines [14].
To overcome these limitations, M-CSFh/hIL-3/GM-CSFh/hSIRPAh/mTPOh/hRag2null/Il2Rγcnull (MISTRG) mice were developed that promote the endogenous development of human myeloid and NK cells, thus, constituting a suitable animal model to examine the effects of therapeutic cytokines and antibodies [15]. Here, we validate humanized MISTRG mice as a unique experimental model to study NK cell biology and ADCC against neuroblastoma in vivo. Using functional assays and single-cell RNA sequencing (scRNA-seq), we compare the cytotoxic and molecular properties of NK cells derived from neuroblastoma-bearing MISTRG mice with that from patients with neuroblastoma and healthy adults and demonstrate that this model system can be adopted for future studies of IO therapies and NK cell biology.
Methods
Animals
Animal studies with NSG and MISTRG mice (Jackson Laboratory, Bar Harbor, ME) were approved and performed under the St. Jude Children's Research Hospital Animal Care and Use Committee.
Human NK cells and patient tumor volumes
NK cells from patients with newly diagnosed neuroblastoma, consented to our institutional phase II trial NB2012 (NCT01857934), and healthy adults were isolated [16]. The study was approved by our institutional review board. NK cells were grown in culture in RPMI 1640 medium (Lonza) supplemented with 10% fetal bovine serum (Biowest), 100 IU/mL penicillin, 100 µg/mL streptomycin, 2 mM l-glutamine (Gibco media), and IL-2 (50 IU/mL) or IL-15 (10 ng/mL) when indicated (Biological Resource Branch at the National Cancer Institute).
Human hematopoietic progenitor cells and bone marrow transplantation procedures
CD34+HPCs were isolated from commercially obtained umbilical cord blood products (Key Biologics) with the CliniMACS device (MiltenyiBiotec), yielding > 90% purity (Fig. S1).
Pups were exposed to irradiation (Cesium irradiator) with 100 cGy (NSG) or 150 cGy (MISTRG or MITRG) within the first 2 postnatal days. Subsequently, 100,000 CD34+HPCs were injected into the liver. Adult 4- to 6-week-old MISTRG mice were irradiated with 250 cGy and received 100,000 CD34+HPCs as intraperitoneal injection. Successful engraftment was defined as > 10% and calculated as: % = .
Tumor cells and orthotopic tumor injections
The GD2+ human leukocyte antigen (HLA)-deficient O-PDX line SJNBL046_X was used to reconstitute animals with orthotopic neuroblastoma(Fig. S2). Characteristics of this xenograft at an earlier passage were previously reported [17] and revealed MYCN amplification and gain on chromosome 17 by fusion to chromosomes 13 and 22, gain on chromosome 1 by fusion to chromosome 7, and gain on chromosome 7 and 15 by fusion to chromosome 1. Four- to 6-week-old mice underwent ultrasound-guided orthotopic injection with 1 × 106 tumor cells. Adult mice undergoing double transplantation with CD34+HPCs and tumor cells had a 72-h recovery period in between procedures. First signs of tumors engraftment were noted on ultrasound imaging as early as 4 weeks after injection. Mice were euthanized at the end of two chemo-immunotherapy cycles (6 weeks) or when they reached humane endpoints, such as moribund appearance, or abdominal distention due to tumor growth causing physical impairment or indicating pain and distress in the animal.
Monoclonal therapeutic antibody
The anti-GD2 antibody hu14.18K322A was provided by Merck Serono and manufactured by Children's GMP, LLC and used in patients receiving therapy on NB2012 as well as in all shown experiments.
GD2 detection in tumor tissues
NSG animals were injected via tail vein with 4 μg/kg hu14.18K322A. At predetermined time points, tumor tissues were non-enzymatically processed into single-cell suspensions, counterstained with an anti-human IgG Fc antibody (HP6017; Biolegend), and analyzed by flow cytometry.
Flow Cytometry and fluorescence-activated cell sorting(FACS)
We identified human NK cells as hCD45+ (HI30) CD3− (UCHT1) CD56+ cells (NCAM16.2; BD), and mouse lymphocytes as mCd45+ cells (30-F11; Biolegend). NKp46 and HLA status were recognized by W6/32 and 9E2, respectively (Biolegend). For blocking, the human FcR binding inhibitor (Thermo Fisher) and anti-mouse CD16/CD32 (2.4G2; Tonbo Bioscience) were used.
In vivo therapy
Male or female animals with tumor volumes ≥ 10 mm3confirmed by ultrasound were randomized to receive chemotherapy during week 1 of each cycle as daily intraperitoneal irinotecan (1.6 mg/kg) and oral temozolomide (16.5 mg/kg) for five days. Doses were pharmacokinetically guided, corresponding to human doses [1]. Immunotherapy was administered on week 2 of each cycle as hu14.18K322A (100 μg per animal per day) via tail vein and intraperitoneal recombinant human IL-2 (10,000 IU per animal per day) for five days. ACT was administered as 5 × 106 human NK cells on day 1 of immunotherapy as an intraperitoneal injection after stimulating these cells for 24 h with IL-2 (50 IU/mL) in culture and determining that cytotoxicity against K562 cells exceeded 90% tumor lysis.
In vitro kill assay
NK cells were co-cultured with yellow fluorescent protein-expressing K562 cells for four hours. Because of the limited amount of blood samples, effector-to-target (E:T) ratio titration was not feasible. Therefore, an E:T ratio of 5:1 was chosen for all experiments to distinguish the differences in NK cytotoxic activity among donors and NK cell activation status, as described previously [16]. After completion of the assay, residual tumor cells were quantified by flow cytometry, and results were normalized with counting particles (Spherotech).
Single-Cell RNA Sequencing (scRNA-seq)
Isolated NK cells from the spleens of double-transplanted MISTRG mice and peripheral blood of patients with neuroblastoma were processed as outlined (Fig. S3) and compared to publicly available scRNA-seq datasets from the peripheral blood and spleens of healthy adults [18]. hCD45+ CD56+ CD3– NK cells (> 90% viability) were partitioned into droplets with barcoded beads (10 × Genomics), and barcoded libraries were generated. Samples were sequenced to an average depth of 25,000–60,000 reads per cell on an Illumina HiSeq 4000 sequencer. Output was processed using the 10 × Genomics Cell Ranger pipeline (v3.0.2) and aligned to the GRCh37 (Hg19) human reference genome.
The analysis was performed in R using the Seurat package version 3.1.1 [19]. Initial quality control steps removed (1) genes that were detected in less than three cells, (2) cells with less than 200 or greater than 5000 detected genes, (3) cells with greater than 10% mitochondrial or 50% ribosomal RNA content. The data were normalized and scaled prior to integration and analysis using scTransform [20]. Cells were clustered using the Seurat command Find Neighbors. Contaminating subpopulations when lacking NKG7 expression and presence of CST3 (dendritic cells), MS4A7 (macrophages), or CD79A (B cells) were removed. NK cell subsets were assigned to NK1 or NK2 based on the expression of GZMB or GZMK [18]. Differential gene expression was performed by Wilcoxon rank-sum testing within Seurat. Datasets (normal human spleen with MISTRG double-transplanted spleen and normal human blood with neuroblastoma patient) were combined using Seurat's integration features, setting the normal human datasets as reference. To generate module scores for NK subsets, we used the AddModuleScore command in Seurat. The genes used to score each subset were based on a previous report [21].
Statistics
We applied D'Agostino-Pearson omnibus and Shapiro–Wilk normality tests to determine the distribution of variables. We compared normally distributed groups with the two-tailed Student's t test (two groups) and the one-way analysis of variance and Tukey's multiple comparison for post hoc analysis (multiple groups). If data were not normally distributed, we applied the unpaired Mann–Whitney test (two groups) and the unpaired Kruskal–Wallis test with Dunn's multiple comparison (multiple groups). All statistical analyses were performed with GraphPad Prism 8. A P value < 0.05 was considered statistically significant.
Results
Xeno-transplantation in adult MISTRG mice permits extended double-engraftment of human immune cells and tumor
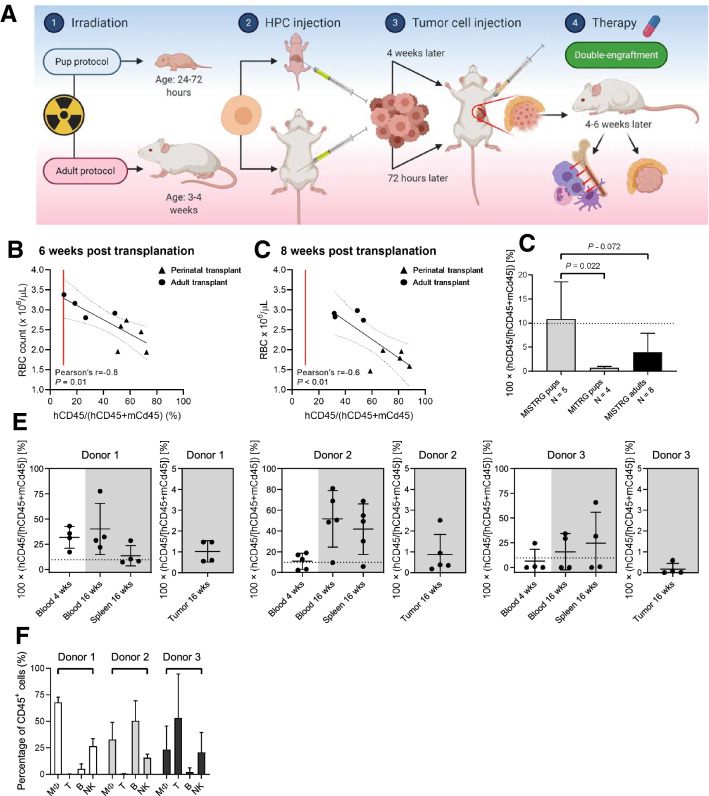
To create a humanized mouse model for IO testing, were constituted MISTRG mice with human hematopoiesis and O-PDX cells (double transplantation model; Fig. 1a). When we conducted CD34+HPC transplantation in the perinatal phase and O-PDX inoculation at 4 weeks of age (perinatal protocol), the longevity of these double-transplanted MISTRG mice was limited by severe anemia evident by 6 weeks post-transplant, precluding extended IO studies (Fig. 1b, c). To mitigate the mortality from anemia, we developed a modified transplantation protocol, in which we performed consecutive hematopoietic transplantation and tumor injections at 4 weeks of age (adult protocol; Fig. 1a). At 4 weeks post-transplantation, human CD45+ cells reached significantly higher levels in the blood of perinatally transplanted MISTRG mice than in MITRG mice (P = 0.022) and marginally higher levels compared to adult transplantations (P = 0.072; Fig. 1d). Importantly, mice transplanted using the adult protocol exhibited increased and sustained blood and organ engraftment at 16 weeks post-transplantation (Fig. 1e), comprising CD14+ monocytes, CD56+ CD3− NK cells, and CD19+ B cells (Fig. 1f). As expected in this O-PDX line, tumor-infiltrating hCD45+ cells were detectable at only low levels (Fig. 1e). Thus, the adult protocol permits modeling of long-term human hematopoiesis and O-PDX engraftment within an experimental animal system.
Fig. 1.
NK cell engraftment in MISTRG mice with a refined adult transplantation protocol. a Animals aged 24–72 h (pup protocol) or 4 weeks (adult protocol) were sublethally irradiated and intraperitoneally injected with CD34+ HPCs. Para-adrenal O-PDX cell injections were performed about 4 weeks (pups) or 72 h after transplantation (adults). Double-engraftment occurred 4 to 6 weeks later. b, c The red blood cell count and human CD45+ cell engraftment defined as human CD45+ cells of human and mouse CD45+ cells are inversely correlated. Already at 6 weeks post-transplantation, all animals have ≥ 10% human engraftment. Higher engraftment levels and more severe anemia is observed in mice transplanted as pups than as adults. d Engraftment is shown for MISTRG and MITRG mice that received transplantation of HPCs from one donor according to the perinatal and adult transplantation protocols. Early engraftment levels at 4 weeks after transplantation is significantly higher in MISTRG mice receiving transplants as pups than in MITRG mice (P = 0.022). This difference was marginally higher when compared with that of adult MISTRG animals (P = 0.07; one-way analysis of variance and Tukey's multiple comparisons test). e Three groups of animals were reconstituted with CD34+ cells from three different donors and reconstituted with the same O-PDX SJNBL046_X, per the adult transplantation protocol. Early engraftment levels at 4 weeks in blood and late levels at 16 weeks in blood, spleen, and tumors demonstrate that adult MISTRG mice can sustain long-term engraftment after double-transplantation. f Peripheral blood hCD45+ cells at 16 weeks post-transplantation comprised CD14+ monocytes, CD19+ B cells, and CD56+ CD3− NK cells but lacked CD3+ T cells
NK cells from double-transplanted MISTRG mice exhibit intact NK cell cytotoxicity against K562 cells and ADCC
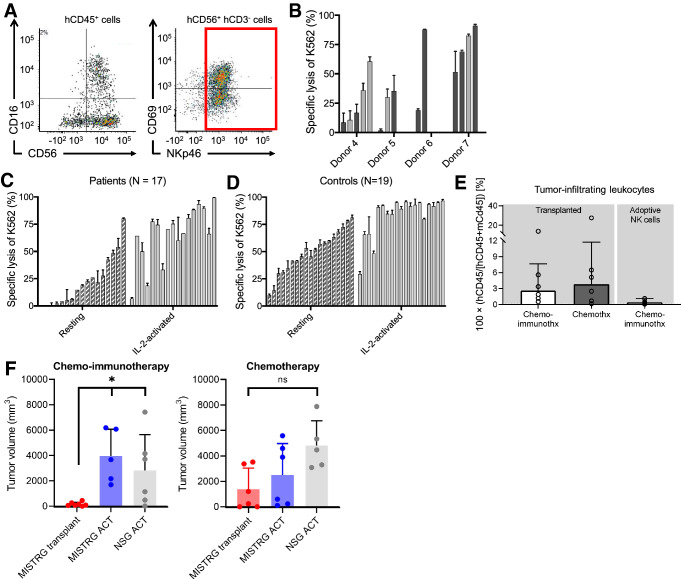
Phenotypically mature human CD3− CD56+NKp46+NK cells were FACS-isolated from dissociated spleens of CD34+HPC transplanted mice to quantitate NK cell cytotoxicity against K562 cells (Fig. 2a). NK cell cytotoxicity refers to the ability of NK cells to eliminate targets without prior sensitization [22]. This contrasts with ADCC, which is induced by antibody-labeling of the target cell. After sorting, NK cells were stimulated overnight with IL-2 (50 IU/mL) in culture and subsequently tested against MHC I-deficient K562 cells ex vivo (Fig. 2b). A subset of the NK cells effectively eliminated K562 cells, but tumor lysis ranged from 2 to 91%, varied among donors, and was independent of tumor status (P > 0.05; Fig. 2b). Such large variability was also noted in resting and IL-2–activated peripheral blood NK cells from patients with neuroblastoma at the time of diagnosis (7 to 99%; Fig. 2c) and healthy adult controls (10 to 81%; Fig. 2d), indicating that NK cells from MISTRG mice span a comparable spectrum of cytotoxic activity.
Fig. 2.
Functional evaluation of NK cells from MISTRG mice, patients with neuroblastoma, and healthy controls. a Gating strategy of FACS-isolation of CD3− CD56+ NKp46+ NK cells. b FACS-isolated NK cells from the spleens of transplanted MISTRG mice were stimulated with IL-2 (50 IU/mL) for 12 to 18 h and tested in a natural killer cytotoxicity assay against K562 cells. Each bar represents one animal. Tumor-bearing mice are marked by dark grey and tumor-free animals (because of tumor engraftment failure) by light grey bars. c Ex vivo lysis of K562 cells by peripheral blood NK cells from 17 patients with newly diagnosed and untreated neuroblastoma and d 19 healthy adult controls. e Residual tumor-infiltrating human CD45+ cells at the end of therapy are shown for mice with endogenous human hematopoiesis and contrasted to mice that received adoptive NK cell injections. Transplanted animals had significantly more infiltrating cells (P = 0.01; Mann–Whitney test). f Experimental cohorts were grouped by intervention (chemo-immunotherapy and chemotherapy) and separated by the
source of effector cells. Transplanted MISTRG mice (red) are compared with MISTRG (blue) and NSG (grey) mice that received ACT of NK cells from one donor. Among mice receiving chemo-immunotherapy, transplanted MISTRG mice (N = 7) had more significant tumor growth suppression than did MISTRG and NSG mice that received adoptive NK cells with therapy (N = 5 and 6; P = 0.003; one-way ANOVA). No significant differences occurred between these groups treated with chemotherapy (each MISTRG groups N = 6, NSG group N = 5)
In pre-clinical antibody studies, NSG mice are commonly reconstituted via ACT of IL-2–activated human NK cells to target neuroblastoma in vivo [23]. We compared ADCC in CD34+HPC transplanted MISTRG mice with MISTRG and NSG mice that underwent ACT. Adult 4- to 6-week-old MISTRG mice assigned to the transplantation group were irradiated with 250 cGy and received 100,000 CD34 + cells as an intraperitoneal injection. Animals in the adoptive NK cell group received 5 × 106 human NK cells on day 1 of immunotherapy as an intraperitoneal injection but were not transplanted. All animals were injected with 1 × 106 PDX tumor cells. Mice with established tumor (≥ 10 mm3) were randomized to receive either chemotherapy (daily irinotecan and temozolomide for 5 days) or chemo-immunotherapy (chemotherapy followed by daily hu14.18K322A and IL-2 for 5 days of the following week) [9]. Drug doses were based on pharmacokinetic studies [1], and antibody saturation in tumor tissue was confirmed (Fig. S4). Respective animals underwent ACT of 5 × 106 human NK cells from one donor with each course of immunotherapy. NK cells were activated in culture with IL-2 (50 IU/mL) for 24 h prior to ACT and exhibited > 90% specific lysis of K562 after stimulation (data not shown). Animals that underwent ACT were not treated with IL-2 unless they received chemo-immunotherapy. We modeled these therapies according to current regimens in the clinic that use ACT of NK cells [24–26].
CD34+ HPC transplanted MISTRG mice had higher levels of tumor-infiltrating human CD45+ cells at the end of therapy than did mice who underwent ACT (P = 0.01; Fig. 2e; Fig. S5). All mice had macroscopic tumors after 2 courses of treatment (6 weeks). The PDX used in our experiments grows very aggressively, and we have not been able to cure animals in humanized animal studies. Therefore, it was not surprising that none of the mice eliminated the tumor. Tumor growth was different after two courses (i.e., at the 6-week time point) of chemo-immunotherapy in CD34 + HPC transplanted MISTRG mice compared to MISTRG or NSG that had undergone ACT (P = 0.003; Fig. 2f). When we performed this experiment with a different CD34 + HPC donor for the purpose of obtaining weekly imaging under general anesthesia, we noted no longer a difference in these methodologies (P = 0.06; Fig. S6A and B). We suspect that this discrepancy in efficacy is related to the different donor sources given that we have also noted donor-dependent variability in the ex vivo kill assays against K562 (Fig. 2b). Among the animals given chemotherapy alone, no difference in tumor size occurred between the two groups. Altogether, our findings suggest that better tumor control was achieved in the double-transplantation model than in the ACT model in vivo. In the transplantation model, human NK cells permit O-PDX engraftment, but were capable of being therapeutically redirected to induce ADCC against the tumor.
Double-transplanted MISTRG mice develop NK1 and NK2 subpopulations in similar proportions like healthy controls
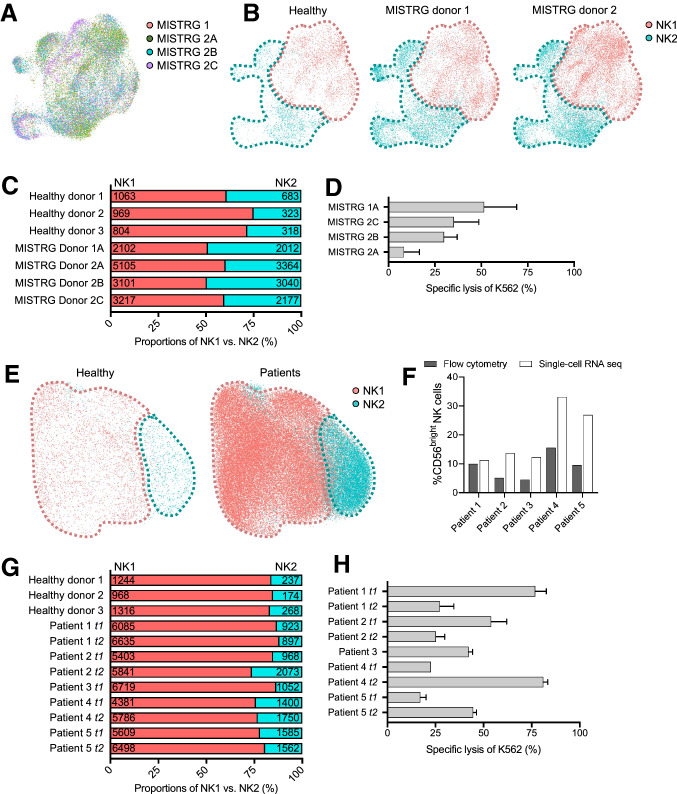
To compare molecularly distinct subsets of NK cells among neuroblastoma-bearing MISTRG mice, patients with neuroblastoma, and healthy controls, we applied scRNA-seq (Fig. S3). We conducted the analysis with four murine samples from two umbilical cord blood donors (adult protocol: MISTRG 1, 2A, and 2C; perinatal protocol: MISTRG 2B). Targeting 10,000 NK cells per sample, we analyzed a total of 20,906 splenic NK cells and detected a median number of 1072 genes. Peripheral blood NK cells from five patients with high-risk neuroblastoma were used for comparison. Targeting 10,000 NK cells per sample, we analyzed a total of 33,359 cells and detected a median number of 701 genes. Finally, we retrieved published raw single-cell transcriptomic datasets of peripheral blood and splenic NK cells from three healthy adults for additional comparison [18].
Integrating the murine data, we found that population density and distribution overlapped between MISTRG 2B and 2C, whereas MISTRG 1 and 2A separated into distinct clusters (Fig. 3a). Integrating the murine and splenic data from healthy controls, we identified the CD56dim NK1 and CD56bright NK2 cluster (Fig. 3b) [18], of which NK2 accounted for 25 to 40% (Fig. 3c). When compared to NK2, the NK1 cluster was enriched for transcripts that encoded proteins involved in cytotoxicity (e.g., GNLY, FGFBP2, GZMB, and GZMH), cytokine-mediated processes (e.g., CX3CR1 and TCF7), and NK cell regulation (e.g., KLF2, LAIR2, and FAM65B). Enriched transcripts in NK2 were associated with NK cytotoxicity (e.g., GZMA, GZMK, and KLRB1), NK cell regulation (e.g., CEBPD, CD160, SH2D1A, and TIGIT), and cytokine production (e.g., CCL3, ALOX5AP, CCL4, and CXCR6). The tested NK cells showed intact NK cell cytotoxicity (Fig. 3d).
Fig. 3.
Single-cell transcriptomic analysis of NK cells from humanized MISTRG mice and patients with neuroblastoma. a Integrated scRNA-seq data sets that combine splenic NK cells from four MISTRG mice using t-distributed stochastic neighbor embedding. The four mice received CD34+ HPCs from two donors (MISTRG 1 and MISTRG 2A, B, C). b Visualization of CD56dim NK1 and CD56bright NK2 cluster of combined splenic NK cells from four MISTRG mice and three healthy adult controls. c Relative percentages of CD56dim NK1 and CD56bright NK2 cells per mouse and control. The absolute numbers of cells are plotted for each cluster. d Lysis of K562 cells ex vivo by NK cells from MISTRG mice. e Visualization of CD56dim NK1 and CD56bright NK2 cluster of combined peripheral blood NK cells from five patients with neuroblastoma using t-distributed stochastic neighbor embedding. f Percentage of CD56bright NK cells identified by flow cytometry (grey bar) or scRNA-seq (white bar). g Relative percentages of CD56dim NK1 and CD56bright NK2 cells per patient. The absolute numbers of cells are plotted for each cluster. t1 and t2 refer to sampling time points pre-therapy and after two courses of chemo-immunotherapy. h Lysis of K562 cells ex vivo by the patient
Analysis of the patient samples also identified the two major cell CD56dim NK1 and CD56bright NK2 clusters (Fig. 3e). The relative proportion of CD56bright NK2 cells in the patients was more conservatively defined by single cell-RNA sequencing than by flow cytometry (Fig. 3f) and accounted for approximately 10 to 25% in both patients and healthy controls (Fig. 3g). While the NK1-to-NK2 ratio remained relatively stable after two courses of chemo-immunotherapy and surgery (t2) when tumor volumes were markedly decreased (148 ± 102 mm3 versus 14 ± 14 mm3), we noted that the NK cell cytotoxic capacity of total NK cells fluctuated independently from that (Fig. 3h). When compared to NK2, the NK1 cluster differentially expressed transcripts that encoded membrane-bound (e.g., KLRB1 and FCGR3A) and secreted proteins (e.g., FGFBP2, GZMH, and GZMB) involved in NK cytotoxicity and pathogen defense, and proteins involved in chemotaxis (e.g., CCL3, CCL4,and CCL5)(Fig. 2e). Enriched transcripts in NK2 were associated with cytotoxicity (e.g., GZMK, CD2), chemotaxis (e.g., XCL1 and XCL2), and NK cell regulation (e.g., LTB, IL7R, and TCF7; Fig. 2f). Collectively, we found that the major two NK cell populations, NK1 and NK2, were equally represented in the NK cell repertoire of patients and mice with neuroblastoma when compared to healthy controls.
Patients and MISTRG mice with neuroblastoma lack a subset of active NK cells compared to healthy controls
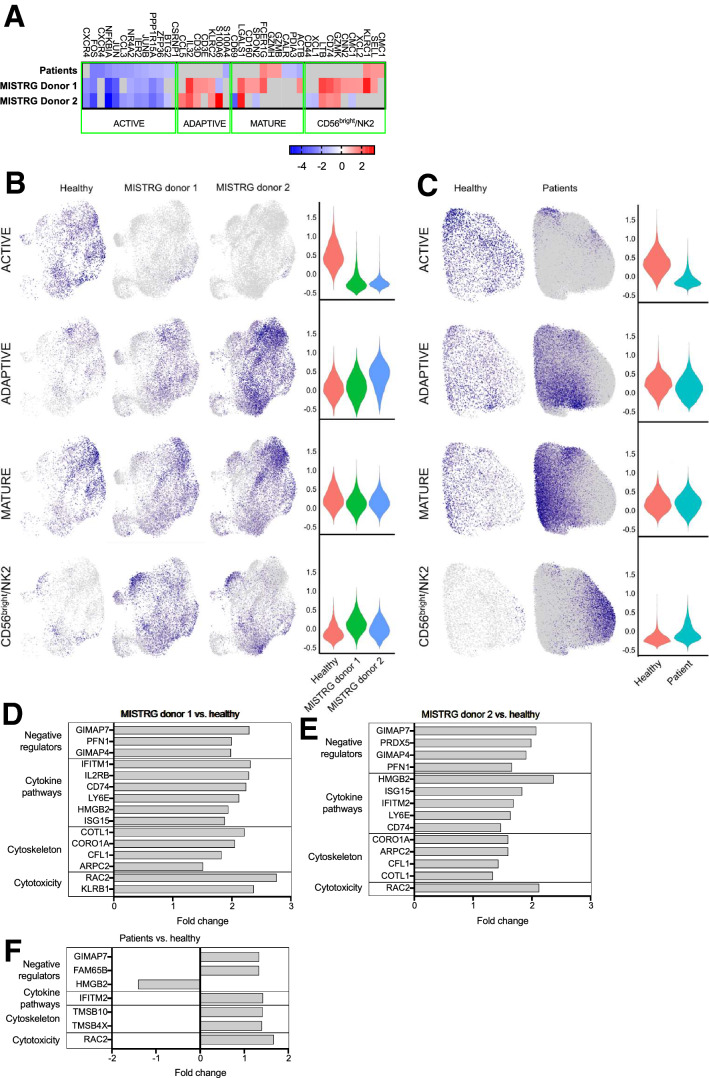
Beyond the major NK1 and NK2 cluster, it has been recently shown that single-cell transcriptomic analysis distinguishes five separate subpopulations in peripheral blood NK cells, namely CD56bright/NK2, transitional, active, mature, and terminal NK cells [21]. We examined the differential expression of genes characteristic for each population by comparing our murine and patient data set with that of the healthy controls. Genes specific to the adaptive, mature, and CD56bright/NK2 subsets were mostly upregulated in NK cells from patients and MISTRG mice compared to controls (Fig. 4a). However, genes that defined active NK cells were uniformly down-regulated in NK cells derived from both MISTRG donors (Fig. 4b) and patients (Fig. 4c). These genes have been reported to be rapidly inducible by cytokine stimulation (e.g., NR4A2, FOSB, FOS, JUN, and JUNB) [27]. Separate from the cluster-specific genes, enriched transcripts were associated with cytotoxicity (e.g., RAC2, KLRB1), cytoskeletal remodeling that is critical for cytotoxicity and degranulation (e.g., CORO1A, COTL1, and CFL1)[28], cytokine pathways (e.g., ITIM2 and IL2RB), and negative regulation of NK cells (e.g., GIMAP4 and GIMAP7) (Fig. 4d, e, f). Altogether, single-cell transcriptomic analysis revealed that compared to healthy controls, mice and patients lacked a certain subset of NK cells, termed active NK cells, that is characterized by differential expression of genes that are inducible by cytokine activation. Genes associated with conventional NK cell functions and regulation were upregulated and corroborate our observation that these cells have intact effector function.
Fig. 4.
Subset analysis using single-cell transcriptome. a Heat map of cluster-specific genes characterizing active, adaptive, mature, and CD56brigh/NK2 cells. Visualization and violin plots of NK cell subsets in MISTRG mice b and children with neuroblastoma c. Differential gene expression analysis of other functional NK cell genes comparing d MISTRG donor 1, e MISTRG donor 2, and f patient NK cells with healthy controls
Discussion
In the rapidly evolving field of IO, a major impediment toward progress is the lack of accurate murine models to conduct mechanistic and therapeutic studies in the laboratory. Conventional syngeneic and GEMM only partially reproduce the tumor heterogeneity and immunologic response. To address this unmet need, the development of next-generation humanized mouse models aims at reconstituting mice with a human immune system and human tumor and has been successful for a limited number of hematologic malignancies [29, 30]. For the first time, we report a neuroblastoma patient-derived xenotransplantation model with MISTRG mice that permits simultaneous engraftment of tumor and NK cells for an extended period of time for the study of antibody and cytokine therapy. Because NK cells are thought to be one of the main effector cells in ADCC, we conducted a careful analysis of NK cell function, single-cell transcriptome, and phenotype in NK cells from neuroblastoma-bearing MISTRG mice and compared these data with patient NK cells and healthy controls.
We confirmed preserved effector cell function and phenotypic similarities with patients and healthy controls; however, tumor-bearing mice and patients lacked a subset of NK cells, termed active NK cells. This subgroup was recently described in the peripheral blood [21] and in the murine spleen [18] and exhibited transcriptional activation, activation of signaling the KRAS-MAPK and TRAF6-NF-κB pathways, and a state of activation by TNF, IL-2, TLR, or GPCR stimulation [21]. Therefore, it has been hypothesized that the active NK cell cluster evolves during homeostatic activation of NK cells. The absence of active NK cells in transplanted MISTRG mice and patients with neuroblastoma raises the question if this could be due to suppressive effects by the tumor. Alternatively, because the control NK cells were retrieved from healthy adult donors (as were the samples in the study by Yang et al.), it is also possible that our observation reflects a physiologic state during the aging of NK cells, a developmental process that is yet to be fully elucidated. To further elucidate the underlying mechanisms, we propose single-cell transcriptomic analysis in tumor-free animals and in healthy children in the future.
In our experiments, we used tumor mismatched CD34+ HPCs to conduct bone marrow transplantation in MISTRG mice. Although we have demonstrated that NK cells allowed tumor engraftment to occur in the mice, it is uncertain if the mismatched status influences ADCC and tumor surveillance in this model. In this regard, the therapy course for neuroblastoma offers a unique opportunity to obtain autologous tumor matched CD34+ HPCs. This is because the standard of care of patients with high-risk disease involves autologous double-transplantation as a consolidative measure [31]. In preparation, patients undergo peripheral blood CD34+ mobilization and harvest during the first courses of induction chemotherapy and may allow the donation of small samples for research purposes in patients from whom an O-PDX has been established.
In contrast to existing literature [23], ACT of NK cells was unexpectedly ineffective in our experiment, despite NK cell activation with IL-2 and lack of HLA expression on the xenograft that have rendered tumor cells susceptible to NK cytotoxicity in other studies [16, 32]. Although we modeled these therapies according to current regimens in the clinic that use ACT of NK cells, the lack of supplementary IL-2 in vivo may have contributed to less efficacy of adoptive NK cells in the NSG model in our experiments. Because our experiment was performed with only one allogeneic donor, it is possible that the lack of anti-tumor activity was due to donor-related factors or an insufficient cell number despite intact cytotoxic activity in vitro. Nevertheless, our observation can be reconciled with findings from pediatric trials that tested ACT of NK cells for acute myeloid leukemia and failed to show an event-free survival benefit with infused NK cells [24] especially with cell doses that were less than 12.5 × 106 NK cells per kg body weight, despite clear evidence that killer-cell immunoglobulin-like receptors (KIR)–HLA-mismatched transplants can induce long-term tumor control [33].
Our animal studies were performed with only one previously characterized O-PDX line. Future studies should employ multiple different O-PDXs and HPC donors to get a broader understanding of NK cell development in MISTRG mice in the presence and absence of neuroblastoma. Although current data suggest that the NK cell transcriptome is organ-specific and conserved within humans and mice [18], future studies with more patient and mice samples may also help determine the donor- or animal-dependent variations in the context of neuroblastoma and immunotherapy.
Taken together, we present a faithful neuroblastoma O-PDX mouse model for the study of ADCC and cytokine therapy in vivo. We demonstrated that NK cells have preserved cytotoxic function and phenotypic characteristics. While the cause of absent active NK cells should be further elucidated, this finding may be a reflection of tumor-modulating effects on NK cells in neuroblastoma that could be overcome with therapeutic cytokine administration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Asa Karlstrom, Ph.D., for regulatory support; Nisha Badders, Ph.D., ELS, for editing the manuscript; Mitchell J. Weiss, MD Ph.D., for providing HPCs; William E. Janssen, Ph.D., for providing purity data on HPCs; Jennifer Peters, Ph.D., for acquiring images of the stained tumor slides; Thomas Confer for assisting with the ultrasound studies; Merck Sorono and Children's GMP, LLC for providing hu14.18K322A for our studies; and the NCI Biological Resource Branch for providing IL-2 and IL-15. We thank Adeline Crinier, Bertrand Escalier, and Eric Vivier, Ph.D., for providing the biocomputational datasets of healthy controls. This work was supported, in part, by Cancer Center Support (CA21765) from the National Cancer Institute and grants to RN from the Conquer Cancer ASCO Foundation (12822), to WJA from the National Institutes of Health (R50CA211481), and to MAD from the National Institutes of Health (EY014867 and EY018599 and CA168875). This research was supported by Howard Hughes Medical Institute.
Abbreviations
- ACT
Adoptive cell transfer
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- CAR
Chimeric antigen receptor
- E.T
Effector-to-target
- FACS
Fluorescence-activated cell sorting
- GD2
Disialoganglioside
- GEMM
Genetically engineered mouse model
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HLA
Human leukocyte antigen
- HPCs
Hematopoietic progenitor cells
- IL
Interleukin
- IO
Immuno-oncology
- KIR
Killer-cell immunoglobulin-like receptors
- MISTRG
M-CSFH/hIL-3/GM-CSFh/hSIRPAh/mTPOh/hRag2null/Il2Rγcnull
- MITRG
M-CSFH/hIL-3/GM-CSFh/hTPOh/hRag2null/Il2Rγcnull
- NK
Natural killer
- NSG
NOD/scid/Il2RγcNull
- O-PDX
Orthotopic patient-derived xenograft
- scRNA-seq
Single-cell RNA-sequencing
Author contributions
RN designed and conducted all experiments, analyzed and interpreted all data, and drafted and reviewed the manuscript; AGP analyzed and interpreted the scRNA-seq data and reviewed the manuscript; LMG maintained the MISTRG colony and supplied the mice and reviewed the manuscript; EAS maintained the MISTRG colony, conducted murine experiments requested in revisions, reviewed the manuscript; JD maintained the MISTRG colony, conducted murine experiments requested in revisions, reviewed the manuscript; JH performed flow cytometry analysis and reviewed the manuscript; MJ and WJA performed tumor injections and ultrasound-based tumor measurements and reviewed the manuscript; WLF: provided patient samples and clinical data and reviewed the manuscript; MAD generated the hypothesis, designed, funded, supervised the study, interpreted data, and reviewed and edited the draft.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rosa Nguyen and Anand G. Patel have contributed equally.
References
- 1.Stewart E, Federico SM, Chen X, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature. 2017;549:96–100. doi: 10.1038/nature23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu Z, Jiang J, Yan Y, et al. Evaluation of the correlations between patient-derived xenograft (PDX) model-based mouse trials and cancer patient-based clinical trials. J Clin Oncol. 2017;35:e23140-e. doi: 10.1200/JCO.2017.35.15_suppl.e23140. [DOI] [Google Scholar]
- 3.Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, Berns A. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. 2011;19:244–256. doi: 10.1016/j.ccr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18:5160–5162. doi: 10.1158/1078-0432.CCR-12-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen R, Moustaki A, Norrie JL, Brown S, Akers WJ, Shirinifard A, Dyer MA. Interleukin-15 enhances anti-GD2 antibody-mediated cytotoxicity in an orthotopic PDX model of neuroblastoma. Clin Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-19-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 11.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 12.Strowig T, Chijioke O, Carrega P, Arrey F, Meixlsperger S, Ramer PC, Ferlazzo G, Munz C. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116:4158–4167. doi: 10.1182/blood-2010-02-270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gille C, Orlikowsky TW, Spring B, et al. Monocytes derived from humanized neonatal NOD/SCID/IL2Rgamma(null) mice are phenotypically immature and exhibit functional impairments. Hum Immunol. 2012;73:346–354. doi: 10.1016/j.humimm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rongvaux A, Willinger T, Martinek J, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen R, Houston J, Chan WK, Finkelstein D, Dyer MA. The role of interleukin-2, all-trans retinoic acid, and natural killer cells: surveillance mechanisms in anti-GD2 antibody therapy in neuroblastoma. Cancer Immunol Immunother. 2018;67:615–626. doi: 10.1007/s00262-017-2108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart E, Shelat A, Bradley C, et al. Development and characterization of a human orthotopic neuroblastoma xenograft. Dev Biol. 2015;407:344–355. doi: 10.1016/j.ydbio.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crinier A, Milpied P, Escaliere B, et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity. 2018;49(971–86):e5. doi: 10.1016/j.immuni.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296. doi: 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, Siebert JR, Burns R, et al. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat Commun. 2019;10:3931. doi: 10.1038/s41467-019-11947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–4486. [PubMed] [Google Scholar]
- 23.Barry WE, Jackson JR, Asuelime GE, et al. Activated natural killer cells in combination with anti-GD2 antibody dinutuximab improve survival of mice after surgical resection of primary neuroblastoma. Clin Cancer Res. 2019;25:325–333. doi: 10.1158/1078-0432.CCR-18-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen R, Wu H, Pounds S, et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J Immunother Cancer. 2019;7:81. doi: 10.1186/s40425-019-0564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen R, Sahr N, Sykes A, et al. Longitudinal NK cell kinetics and cytotoxicity in children with neuroblastoma enrolled in a clinical phase II trial. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furman WL, Federico SM, McCarville MB, et al. A phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res. 2019;25:6320–6328. doi: 10.1158/1078-0432.CCR-19-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahrami S, Drablos F. Gene regulation in the immediate-early response process. Adv Biol Regul. 2016;62:37–49. doi: 10.1016/j.jbior.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, Forbes LR, Watkin LB, Orange JS. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol. 2014;92:245–255. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Rongvaux A, Taylor A, et al. A highly efficient and faithful MDS patient-derived xenotransplantation model for pre-clinical studies. Nat Commun. 2019;10:366. doi: 10.1038/s41467-018-08166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wunderlich M, Chou FS, Link KA, Mizukawa B, Perry RL, Carroll M, Mulloy JC. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24:1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JR, Kreissman SG, London WB, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: a randomized clinical trial. JAMA. 2019;322:746–755. doi: 10.1001/jama.2019.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung W, Handgretinger R, Iyengar R, Turner V, Holladay MS, Hale GA. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539–542. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.