Abstract
Transcranial magnetic stimulation (TMS)-induced silent periods provide an in vivo measure of human motor cortical inhibitory function. Cortical silent periods (cSP, also sometimes referred to as contralateral silent periods) and ipsilateral silent periods (iSP) may change with advancing age and disease and can provide insight into cortical control of the motor system. The majority of past silent period work has implemented largely varying methodology, sometimes including subjective analyses and incomplete methods descriptions. This limits reproducibility of silent period work and hampers comparisons of silent period measures across studies. Here, we discuss methodological differences in past silent period work, highlighting how these choices affect silent period outcome measures. We also outline challenges and possible solutions for measuring silent periods in the unique case of the lower limbs. Finally, we provide comprehensive recommendations for collection, analysis, and reporting of future silent period studies.
Keywords: cortical silent period, ipsilateral silent period, transcranial magnetic stimulation, noninvasive brain stimulation
1. Introduction
Transcranial magnetic stimulation (TMS) was first introduced in 1985 as a noninvasive method for stimulating the human brain (Barker et al., 1985). Barker et al. demonstrated that a single TMS pulse to the primary motor cortex could elicit responses in the muscles that received corticospinal input from the stimulated cortical region (Barker et al., 1985). Since this time, multiple TMS approaches including single pulse (e.g., Fling & Seidler, 2011; Swanson & Fling, 2018), paired pulse (e.g., Gagnon et al., 2011; Wittenberg et al., 2007), and repetitive TMS (e.g., Brunoni et al., 2017; Chou et al., 2015; Fitzgerald et al., 2006; Galhardoni et al., 2015) have been adopted and applied to a wide variety of tasks and patient populations.
Despite the growing popularity of TMS, there has been a lack of methodological studies for single pulse techniques, including testing of cortical and ipsilateral silent periods (cSPs and iSPs, respectively). TMS-induced silent periods present as a reduction of ongoing electromyography (EMG) activity and provide information regarding intracortical and interhemispheric inhibition during voluntary muscle contraction. Thus, they are particularly suited for studying how the central nervous system controls muscle activity. To date, silent period studies have used varying methodology and many papers fail to report complete methods. This has made it difficult to compare outcome measures across studies and has precluded meta-analyses among patient populations (Major et al., 2015) or in older age (Levin et al., 2014). For instance, older age has been associated with decreased upper limb cSP duration (Beynel et al., 2014; Davidson & Tremblay, 2013a; Oliviero et al., 2006; Sale & Semmler, 2005), no difference in cSP duration (Fujiyama et al., 2009, 2012; Hunter et al., 2008), and increased cSP duration (McGinley et al., 2010) across studies. Methodological differences between these studies make it difficult to understand how age relates to cSP duration.
In the present review, we address the potential impacts of methodological differences on silent period outcome variables and provide recommendations for future work. We begin with a discussion of the mechanisms underlying cSPs and iSPs as well as common silent period outcome measures (Section 2). Next, we outline methodological differences among past silent period work, which make inter-study comparisons difficult (Sections 3–5). Finally, we examine unique methodological considerations for measuring silent periods in the lower limbs (Section 6), and provide recommendations for collection, analysis, and reporting in future silent period studies (Section 7).
2. Transcranial Magnetic Stimulation Underlying Mechanisms
2.1. Overview of TMS in the Motor System
TMS induces currents in the brain via Faraday’s principle of electromagnetic induction. Ultimately, TMS depolarizes cerebral neurons and triggers action potentials. Descending corticospinal volleys induce glutamate release in cortico-motoneuronal synapses. Provided the volleys are strong enough to exceed the firing threshold an action potential is subsequently triggered in spinal motoneurons. These action potentials propagate along the peripheral motor axons to induce a muscle response. The resulting muscle responses can be recorded as motor-evoked potentials (MEPs), which are spikes in muscle activity due to the activation of corticospinal neurons. MEPs provide a direct measure of cortical and spinal motoneuron excitability. See Groppa et al. (2012) for a more detailed description of these TMS principles.
2.2. Overview of TMS-Induced Silent Periods
Silent periods represent the primary single pulse TMS method for assessing inhibitory function. Paired pulse methods (e.g., short- / long- latency intracortical inhibition and short- / long- latency interhemispheric inhibition) also provide metrics of cortical inhibition. However, these techniques typically do not measure the motor system during a sustained muscle contraction and are mediated by different underlying mechanisms than silent periods (Chen et al., 2003) and thus are beyond the scope of the present review. As silent periods measure inhibition of volitional motor activity, rather than inhibition of MEPs (as is the case for paired pulse methods), silent periods are particularly well suited for investigating the inhibitory effects of cortical and corticospinal control of voluntary motor output.
2.2.1. Cortical Silent Period (cSP)
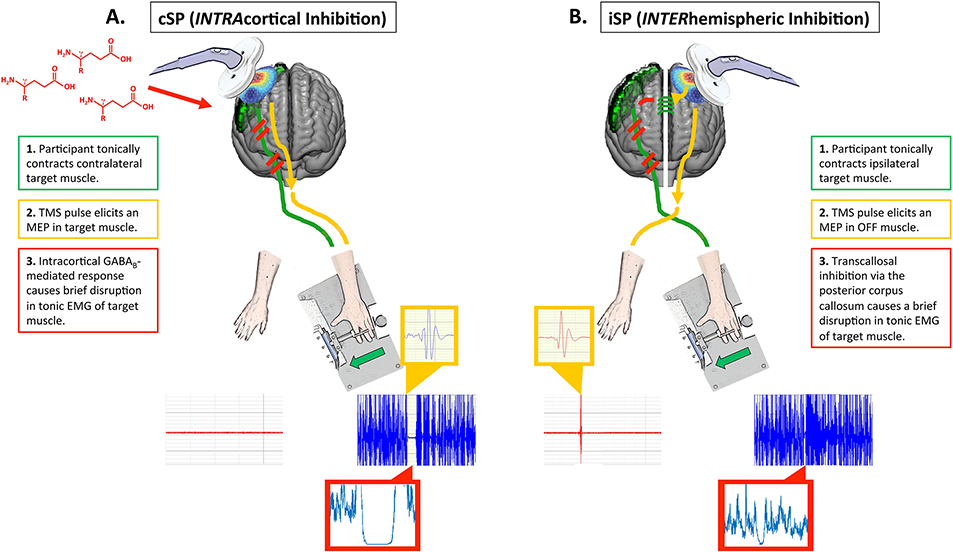
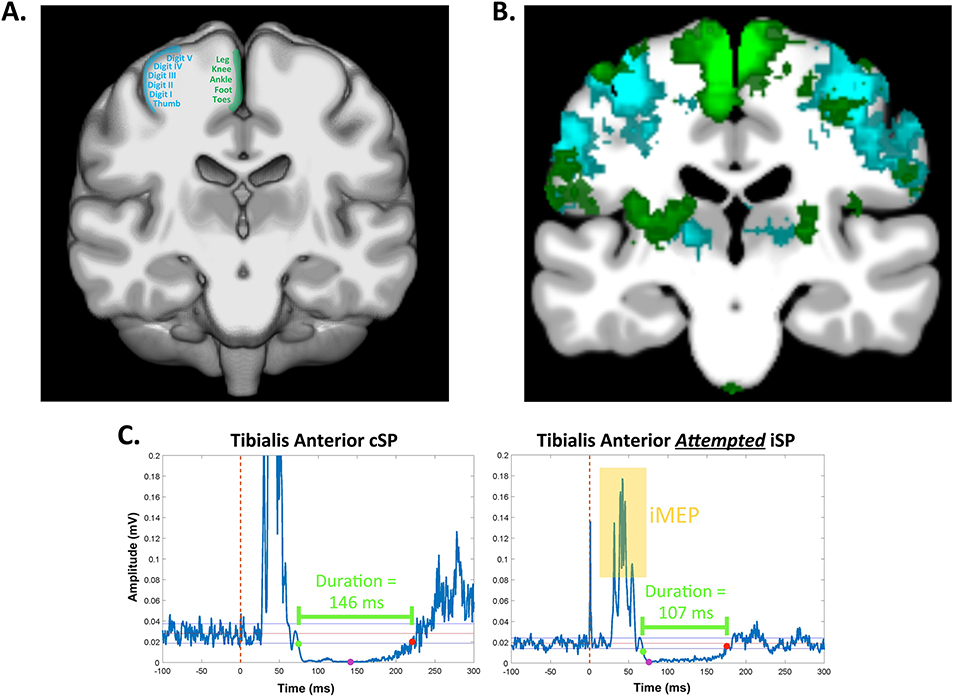
When TMS is applied to the primary motor cortex contralateral to the contracting target muscle, the resulting phenomenon is termed a cortical silent period (cSP; this effect is also sometimes referred to as a contralateral silent period; Fig. 1A). The TMS pulse typically causes a MEP in the target muscle, followed by a disruption or silence in the ongoing voluntary EMG activity for a period of up to several hundred milliseconds (Cantello et al., 1992). Of note, a cSP may not always be preceded by a MEP, as the threshold for inducing cSPs can sometimes be lower than the threshold required to elicit a MEP in certain target muscles. cSPs are typically quantified by their duration (Fig. 2), where longer cSP durations are interpreted as greater cortical inhibition. See Table 1 for a list of common silent period outcome measures.
Fig. 1. Cortical mechanism for cSPs and iSPs.
A. While both spinal (0–50 ms) and cortical mechanisms (50–200 ms) are thought to contribute to cSPs, here we depict the cortical mechanism, which dominates the cSP. A1. The primary motor cortex (green) subserves a tonic low-level contraction in the contralateral hand muscle. Here we depict a first dorsal interosseous (FDI) contraction elicited by asking the participant to push laterally against a plunger that presses against a force transducer. EMG from the active FDI is shown in blue; EMG from the opposite FDI which is resting is shown in red. A2. Figure-of-8 coil stimulation is delivered to the active primary motor cortex, resulting in a motor-evoked potential (MEP) in the target muscle (yellow inset box). A3. The cortical response then includes GABAB-receptor mediated intracortical inhibition, which causes a disruption of up to a couple hundred milliseconds in the target muscle (the unrectified silent period is visible in the blue EMG trace; the rectified silent period is visible in red inset box). B. iSPs are thought to be fully cortically mediated. B1. Similar to the cSP setup, the primary motor cortex subserves a tonic low-level contraction in the contralateral hand muscle (blue EMG trace), while the opposite hand is at rest (red EMG trace). B2. A TMS pulse is delivered to the primary motor cortex ipsilateral to the target muscle. This causes a MEP in the resting hand (yellow inset). B3. The TMS pulse results in excitation of glutamatergic transcallosal fibers which pass through the posterior corpus callosum. These fibers synapse onto inhibitory GABAergic interneurons. Excitation of these inhibitory interneurons then causes a brief disruption in descending corticospinal activation of the target muscle, which is visible as a brief silence (lasting only up to several dozen milliseconds) in the target muscle EMG (unrectified silent period visible in blue EMG trace; rectified silent period visible in red inset box).
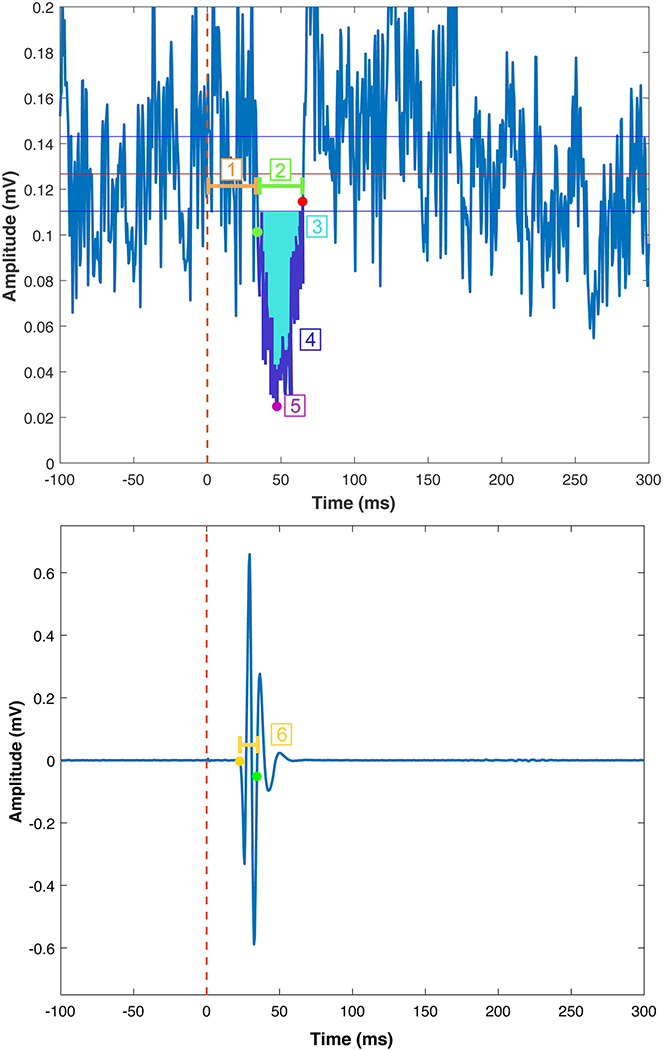
Fig. 2. Common silent period outcome metrics.
Here we depict example average rectified EMG data from the contracting (“ON”; top) and resting (“OFF; bottom) first dorsal interosseous muscles during an iSP trial. The TMS pulse occurred at time = 0 ms. The green and red points indicate the iSP onset and offset, respectively. The red line depicts the mean pre-stimulus EMG activity for 100 ms before the TMS pulse. The blue lines depict ± 0.89 * MCD reference lines for determining the time of iSP onset and offset, based on the MCD Threshold Method. 1. iSP Latency. The time elapsed between the TMS pulse and iSP onset. 2. iSP Duration. The time elapsed between the ISP onset and offset. 3. iSP Area. iSP area (bright blue shading) represents the area of the rectified EMG between the iSP onset and offset. See Tables 1–2 for information on calcuating the normalized iSP area. 4. Average iSP Depth. Calculation of average iSP depth involves taking the mean EMG signal for the entire iSP duration (i.e., the EMG signal colored in dark purple) and normalizing this depth to the average pre-stimulus EMG level. 5. Maximum iSP Depth. The maximum iSP depth is indicated by the pink point. Maximum iSP depth is typically normalized to the average pre-stimulus EMG level. 6. Transcallosal conduction time (TCT). The MEP onset for the OFF muscle is indicated by the yellow point. TCT is the time elapsed between this MEP onset and the iSP onset (indicated by the green point in both the top and bottom panels).
Table 1.
Common silent period outcome measures
| Outcome Measure | Description | Interpretation |
|---|---|---|
| Resting Motor Threshold (RMT) | ||
| RMT | Minimal stimulator intensity needed to reliably induce a MEP when a TMS pulse is applied to the motor hotspot. See Table 2 for common methods used to determine the RMT | Measure of cortical (i.e., corticospinal neuron) excitability; lower RMTs are interpreted as greater cortical excitability |
| Cortical Silent Period (cSP) | ||
| Duration | Time elapsed between the onset and offset of a cSPa | Duration of suppression of the contralateral EMG signal. Greater cSP duration is interpreted as greater intrahemispheric inhibition |
| MEP Amplitude | Spikes in the muscle activity resulting from the activation of corticospinal neurons. During cSP trials, a MEP (typically) precedes the silent period in the target (ON) muscle | Measure of cortical (i.e., corticospinal neuron) excitability. Greater MEP amplitude is interpreted as greater cortical excitability. Larger MEPs may predict longer cSPs (Orth & Rothwell, 2004). For this reason investigators should consider including the MEP : cSP ratio as an extra outcome variable |
| MEP : cSP Ratio | Ratio of MEP amplitude to the duration of the corresponding cSP | Intrahemispheric inhibition, controlling for cortical excitability (Orth & Rothwell, 2004). Provides a measure of the net excitability of the corticospinal tract (i.e., the balance between inhibition and excitability). If the cSP duration is greater in Group A than Group B and there is no difference in the corresponding MEP amplitudes, then Group A is exhibiting increased intrahemispheric inhibition compared to Group B. |
| Ipsilateral Silent Period (iSP) | ||
| Duration | Time elapsed between the onset and offset of an iSPa | Duration of suppression of the ipsilateral EMG signal. Greater duration is interpreted as greater interhemispheric inhibition |
| Depthb | Average or maximum EMG signal during the iSP, normalized to the pre-stimulus EMG level | Average or maximal amount of suppression of the ipsilateral EMG, accounting for the effect of the pre-stimulus muscle contraction level. Greater depth is interpreted as greater interhemispheric inhibition |
| Areab | Integral of the rectified EMG trace during the iSP (i.e., between the iSP onset and offset)a | Amount of suppression of the ipsilateral EMG. Laraer area is interpreted as greater interhemispheric inhibition |
| Normalized Areab | Area of the rectified EMG trace between the onset and offset of the iSP, normalized to the pre-stimulus EMG level | Amount of suppression of the ipsilateral EMG, accounting for the effect of the pre-stimulus muscle contraction level. Greater normalized iSP is interpreted as greater interhemispheric inhibition |
| Onset Latency | Time elapsed between the TMS pulse and the iSP onset | Speed of inter-hemispheric signal transmission. Shorter iSP onset latency is interpreted as faster interhemispheric signal transmission |
| Transcallosal conduction time (TCT) | Time elapsed between the contralateral MEP onset and the iSP onset | Speed of inter-hemispheric signal transmission. Shorter TCT is interpreted as faster interhemispheric signal transmission |
Table 1 Note. MEP = motor-evoked potential; TMS = transcranial magnetic stimulation; EMG = electromyography
See Section 5.2 and Table 2 for a discussion of common methods for determining silent period onsets and offsets
These metrics (depth, area, and normalized area) may also be calculated for cSPs and could provide useful additional outcome metrics for cSP studies. However, these metrics are less frequently reported for cSPs compared to iSPs
It is generally thought that both spinal and cortical mechanisms contribute to the cSP. Typically, the early portion (0–50 ms) of the cSP is attributed to spinal mechanisms (Cantello et al., 1992; Fuhr et al., 1991), including recurrent inhibition by Renshaw cell activation, motoneuron after-hyperpolarization, or disynaptic inhibition via Ia inhibitory interneurons (Cantello et al., 1992; Classen & Benecke, 1995; Fuhr et al., 1991; Inghilleri et al., 1993; Roick et al., 1993). The later portion (50–200 ms) is thought to be caused by intracortical suppression of corticospinal output (Cantello et al., 1992; Chen et al., 1999; Fuhr et al., 1991; Inghilleri et al., 1993; Schnitzler & Benecke, 1994). Given the larger assumed contribution of cortical (75%) versus spinal (25%) mechanisms, cSPs are said to be mainly due to activation of cortical inhibitory interneurons. However, this notion has been debated by some who argue that the spinal contributions are larger than once thought (Yacyshyn et al., 2016), as well as by some who argue that the cSP is generated in the primary motor cortex and thus is entirely of cortical origin (Roick et al., 1993; Schnitzler & Benecke, 1994). Given the above evidence, in the present review, we presume that the cSP has at least some cortical origin and therefore provides a measure of intracortical inhibition.
cSP inhibition is thought to be mediated by gamma-aminobutyric acid (GABA), particularly by GABAB receptors within the primary motor cortex (Siebner et al., 1998; Werhahn et al., 1999). Pharmacological evidence for this includes: (1) in healthy individuals, cSPs were prolonged following oral administration of the GABA reuptake inhibitor, tiagabine (Werhahn et al., 1999). (2) In a patient with dystonia, cSPs were prolonged following infusion of baclofen, a GABAB receptor agonist (Siebner et al., 1998). However, this notion is complicated by several studies that failed to show prolongation of cSPs after baclofen administration in healthy individuals (Inghilleri et al., 1996; Ziemann, Lönnecker, et al., 1996). While the doses used in these studies could have been insufficient for healthy individuals, this work still raises questions about the simplicity of the proposed relationship between GABAB and cSP duration. Positive modulators of GABAA receptor function (e.g., lorazepam) increase cSP durations at low stimulus intensities, but shorten cSP durations at higher stimulus intensities (Kimiskidis et al., 2006). Thus, with low-intensity stimulation, GABAA might make a direct contribution to the cSP, whereas for high-intensity stimulation, presynaptic GABAA receptors might suppress GABAB receptor function (Kimiskidis et al., 2006). This relationship is further complicated by other neuromodulators that have been found to affect cSP duration, including dopaminergic drugs, which may increase cSP duration (Priori et al., 1994; Ziemann, Bruns, et al., 1996). Thus, while cSPs are likely GABA-mediated, cSPs may also be influenced by dopaminergic transmission.
2.2.2. Ipsilateral Silent Period (iSP)
iSPs are elicited when TMS is applied to the hemisphere ipsilateral to a tonically contracting muscle (Fig. 1B). iSPs are thought to be a result of transcallosal inhibition via the posterior mid-body of the corpus callosum (Wassermann et al., 1991). That is, the proposed mechanism for iSPs is as follows. The TMS pulse results in excitatory (glutamatergic) transcallosal motor fibers synapsing on inhibitory (GABAergic) interneurons in the contralateral primary motor cortex (Ferbert et al., 1992; Meyer et al., 1995). This causes a net inhibitory effect and results in a brief depression in the descending corticospinal activity that is supporting the tonic muscle contraction (Ferbert et al., 1992; Meyer et al., 1995). This is visible as a short attenuation or interruption to the ongoing EMG activity in the contracting muscle. iSPs are typically quantified by duration, depth, and/or area, which each provide a measure of suppression of the ipsilateral EMG (Fig. 2). Greater depth, duration, and area are interpreted as greater interhemispheric inhibition (Table 1). Another common iSP measure includes transcallosal conduction time, which quantifies the speed of signal transmission through the posterior corpus callosum. Transcallosal conduction time is typically calculated as the time elapsed from the onset of the contralateral MEP to the onset of the iSP (Fig. 2; Table 1). To date, there are no studies that clearly explore how these measures relate within a single individual or whether these metrics quantify unique aspects of interhemispheric inhibition. Further, there are no studies which propose distinct physiologic mechanisms for iSP duration versus iSP depth or area. We suspect that the amount of EMG suppression (i.e., depth/area) compared to the duration of EMG suppression provides a unique metric of GABAergic inhibitory capacity and function; however, based on the current literature, such interpretations are not yet clear. We thus recommend that future work extract each of these measures, characterize whether and how these measures differ, and, where possible (e.g., in patient or drug studies), consider the physiologic mechanisms that may underlie these measures.
In contrast to cSPs, iSPs are thought to be completely of cortical origin. iSPs do not decrease H-reflex amplitude and thus are thought to not involve spinal contributions (Wassermann et al., 1991). Support for the transcallosal nature of iSPs includes absent or delayed iSPs in patients with agenesis or lesions of the posterior corpus callosum (Meyer et al., 1995, 1998) and callosal infarction (Li et al., 2012). The transcallosal route of iSPs is further supported by iSP abnormalities in patient populations with callosal pathologies, such as multiple sclerosis (Boroojerdi et al., 1998; Höppner et al., 1999; Lenzi et al., 2007; Schmierer et al., 2000) and schizophrenia (Bajbouj et al., 2004; Fitzgerald et al., 2002; Höppner et al., 2001). This transcallosal route is also supported by the absence of iSPs in children who do not have a fully developed corpus callosum, and typically have more prevalent physiologic mirroring (i.e., involuntary EMG activity in the resting limb during a unimanual movement; Heinen et al., 1998; Koerte et al., 2009).
3. Variations in Hardware Used for Silent Period Data Collection
In Sections 3–5, we discuss how methodological choices affect cSP and iSP outcome measures, which makes comparison across studies difficult and limits reproducibility. Many studies fail to comprehensively report their hardware settings, preventing replication of their work. Here we discuss some of the implications of various hardware settings that may be used for silent period testing.
3.1. Coil Type and Orientation
3.1.1. Coil Type
There are various TMS coils capable of eliciting neurophysiological responses in the form of cSPs and iSPs. Factors including loop diameter, number, and set angle of windings affect both depth of penetration and focality of stimulation (Deng et al., 2013). The original TMS coils were circular and induced a relatively broad non-focal electrical current, which was capable of superficial stimulation. To enhance penetration depth and focality, the figure-of-8 coil was developed in the mid 1990’s. This coil effectively uses two adjacent circular coils housed within a single encasement. The two circular loops produce current flow in opposing directions which greatly improves the focality of the induced electrical current (Deng et al., 2013). Improving the focality has been demonstrated in smaller loop diameters, although heat and stress ultimately limits these coils for practical use (Cohen & Cuffin, 1991; Yunokuchi & Cohen, 1991).
Further efforts have been made to enhance the penetration depth of stimulation. The figure-of-8 coil was modified so that the windings were secured at a set inward angle, commonly referred to as a butterfly (MagVenture) or double cone (MagStim) coil design. This angled design has enabled researchers to improve the depth of stimulation penetration, although at a cost of decreased focality compared to the original flat figure-of-8 coil design. This design has allowed researchers to investigate lower limb regions of the primary motor cortex which lie within the interhemispheric fissure. A note of caution: due to focality limitations for all coils in use with humans, it remains possible to unintentionally stimulate both hemispheres when targeting a muscle representation that is close to the midline of the brain. This could lead to unintended interhemispheric interactions if, for instance, a protocol is aiming to test cSP (i.e., intracortical inhibition) of a lower limb muscle (Di Lazzaro et al., 2004). See Section 6 for further discussion of this issue. For further details regarding coil characteristics, please see Deng et al. (2013).
Coil selection is largely based on the manufacturer of the TMS machine. Two of the most common manufacturers, MagStim and MagVenture, offer a variety of coil sizes and shapes depending on necessity. The most common coil for targeting the lower limbs is the angled figure-of-8 coil design. Both manufacturers offer versions of this coil design, the double cone coil and the butterfly coil (MagStim and MagVenture, respectively). While these coils are designed for similar purposes, they differ in coil size with the MagStim one averaging a larger winding diameter, theoretically reducing stimulation focality (described nicely in Deng et al 2013).
In practice, the angled figure-of-8 coil design is important for establishing specific stimulation parameters such as the resting motor threshold (RMT), or the minimum threshold needed to elicit a reliable MEP response (discussed in more detail in Section 4.2). For instance, one recent study found lower RMTs for a leg muscle using a MagStim double cone coil compared to a planar figure-of-8 or circular coil (Dharmadasa et al., 2019). Similarly, another recent study found lower RMTs with a MagVenture butterfly figure-of-8 coil compared to a planar figure-of-8 coil for both the first dorsal interosseous finger muscle and for the tibialis anterior leg muscle (Schecklmann et al., 2020). Silent period protocols typically base TMS intensity on the RMT (e.g., stimulations are delivered at 120% of the RMT); thus, coil selection may influence silent period characteristics, as detailed below.
Several studies have demonstrated that coil selection does directly affect silent periods. For instance, past work found that using a planar figure-of-8 versus a circular coil did not affect cSP variability (Badawy et al., 2011), but did reduce cSP duration (Badawy et al., 2011; Oozumi et al., 1992). The authors (Badawy et al., 2011) suggested that these results could be due in part to the circular coil stimulating a broader cortical area compared to the figure-of-8 coil. These authors suggested that the larger stimulation area of the circular coil may have enhanced the spinal contributions to the early portion of the cSP, which could prolong total cSP duration. Alternatively, or in addition, less focal stimulation may activate inhibitory pathways traveling from the supplementary motor or premotor cortices (Civardi et al., 2001), which could also lengthen the cSP. Of note, in one (Oozumi et al., 1992) of these two mentioned studies that compared the effects of coil choice on cSPs, the coil used was a prototype of modern day figure-of-8 coils and involved two 14.5-centimeter circular coils placed together. In comparison to a more recent study (Badawy et al., 2011), this coil configuration produced a more dramatic difference in cSP duration between the two coil types.
While coil selection should be determined based on the target muscles, use of different coil types across studies does make inter-study comparison difficult. Here our primary recommendation is to clearly report the coil type and brand so that others may replicate the coil selection in their future work.
3.1.2. Coil Orientation
In most cases, coil orientation and the resulting direction of the induced current for figure-of-8 coils is related to the coil handle, while for circular coils, the side of the coil that touches the head dictates the current direction. When using a figure-of-8 coil for stimulation, it is important to keep the handle orientation constant for each subject to ensure consistent stimulation conditions. The position of the coil greatly influences the direction of the induced current and affects a variety of factors, including the efficacy of stimulation (i.e., the intensity needed for corticospinal neurons to reach firing threshold), the types of neurons recruited (i.e., interneuron versus pyramidal; Brasil-Neto et al., 1992; Groppa et al., 2012; Rotenberg et al., 2014), and the site of neuronal depolarization (e.g., soma versus axon hillock; Fox et al., 2004; Niehaus et al., 2000; Thielscher et al., 2011). In addition, coil orientation has been shown to induce various patterns of descending volleys, such that lateral-to-medial induced currents have been shown to induce direct waves (“D-waves”) more easily compared to posteriorly or anteriorly oriented coils and currents (Rotenberg et al., 2014). Therefore, when targeting specific regions of the cortex, the anatomical orientation of the underlying neural tissues should be taken into consideration. Additional discussion of this topic is beyond the scope of the current review; for more details regarding coil orientation influences on induced currents, see Di Lazzaro et al. (2012).
Conventionally, when using a figure-of-8 coil, a posterior-to-anterior cortical current flow, with the coil positioned perpendicular to the central sulcus or angled at approximately 45 degrees with respect to the median longitudinal fissure, produces the lowest RMTs for upper limb muscles (Balslev et al., 2007; Brasil-Neto et al., 1992; Gomez-Tames et al., 2018; Laakso et al., 2014; see Chapter 5, pg. 81: Fig. 2 in Rotenberg et al. (2014) for a diagram of common figure-of-8 coil orientations). Work targeting the lower limbs with a figure-of-8 coil has found the medial-to-lateral coil orientation (i.e., the coil handle pointing laterally, to produce a lateral-to-medial induced current) to be more effective than the posterior-to-anterior coil orientation at activating corticospinal projections to the tibialis anterior muscle, by requiring lower stimulation intensities for achieving the same motor thresholds (Hand et al., 2020).
For the MagStim double cone coil, studies targeting lower limb cortical representations often recommend applying a posterior-to-anterior induced current, with the coil placed slightly posterior and lateral to the vertex (e.g., Madhavan et al., 2010; Mrachacz-Kersting et al., 2007). Double cone coils typically fit the head only if the windings are placed laterally. This limits the possible current directions that can be applied because the coil only fits onto the head in this manner.
One study specifically examined the effect of coil orientation on iSP duration by measuring iSPs in the first dorsal interosseous hand muscle using a MagStim planar figure-of-8 coil (Chen et al., 2003). This work found that an anterior-medial current direction produced longer iSP durations than a posterior-medial current direction, with no differences between the posterior-lateral or anterior-lateral directions (Chen et al., 2003; see Fig. 1 here for a diagram illustrating these current directions). Using a circular coil, within the lower limbs, one study suggested applying clockwise stimulation to the right motor cortex and counterclockwise stimulation to the left motor cortex for eliciting tibialis anterior iSPs (Lo & Fook-Chong, 2004). However, one important caveat is that these authors provided very few details regarding their methods for testing optimal coil orientation; thus, these recommendations should be interpreted with caution.
Overall, optimal coil orientation and direction of induced current will likely depend largely on the coil design and particular TMS paradigm. We have provided the above examples to highlight that coil orientation does influence responses within the motor system, including silent period outcome metrics. We therefore recommend that investigators clearly report the coil orientation and direction of induced current (ideally using a diagram that shows the coil positioning and direction of current flow in relation to the subject’s head) and any specific justification for selecting the reported coil orientation and current direction (e.g., pilot testing or past studies).
While most studies implement the same coil orientation for all subjects, other studies individualize coil orientation for each subject, adopting the one that induces the largest MEPs for that subject (e.g., Jung & Ziemann, 2006). This practice likely introduces greater between-subject variability to silent periods. If authors do elect to individualize coil orientation, we recommend that they clearly explain how the optimal coil orientation was determined for each subject (e.g., in steps of a certain number of degrees), as well as the duration required for this process (e.g., we performed X number of MEPs for each subject in each orientation; we selected the orientation which, on average, elicited the largest MEPs).
3.2. EMG Electrodes
Silent periods are typically obtained using surface EMG electrodes, such as Ag/AgCl cup electrodes. We were unable to identify a methodological study that systematically tested the influence of electrode features (e.g., size, placement, or shielding) on silent period outcome variables. However, many studies fail to report electrode characteristics such as size. Additionally, many studies fail to report whether any skin preparation was done prior to electrode placement and subsequently if impedance measures were obtained. As electrode size (Stegeman & Hermens, 2007), skin preparation (Merletti & Migliorini, 1998), and placement of recording and ground electrodes (Mesin et al., 2009; Stegeman & Hermens, 2007) can all influence EMG signal quality, our primary recommendation here is that authors report details of their EMG preparation. We further suggest adhering to all best practice recommendations set forth by the Surface EMG for the Non-Invasive Assessment of Muscles project (SENIAM; http://www.seniam.org). Of note, there are no widely implemented, standardized approaches for evaluating the quality of EMG data. However, in future work, we recommend that researchers consider calculating the signal-to-noise ratio (SNR) of collected EMG data. See Agostini & Knaflitz (2012) for a proposed EMG SNR calculation and Luki et al. (2020) for MatLab code implementing this calculation. Although, to our knowledge, SNR metrics have not previously been investigated for use as exclusion criteria or statistical covariates in TMS work, such quality control measures could prove useful if more widely used and tested.
4. Variations in Silent Period Data Collection Methods
Several variations in data collection methods influence silent period outcome measures and thus should be carefully described and justified when reporting methods.
4.1. Localization of the Motor Hotspot
Few studies provide a detailed description of the method by which they identified the motor hotspot (i.e., the optimal scalp location for eliciting MEPs in the target muscle). When describing hotspot localization procedures, many studies use broad language such as, “we determined the optimal spot for eliciting a MEP in the target muscle.” We suggest more detailed reporting of methods used to identify the hotspot. Both superficial current spread and overlapping muscle cortical representations often induce MEPs in several muscles at one time (see discussion in Kesar et al., 2018). A small MEP might still be visible in the first dorsal interosseous hand muscle, for instance, when the coil is not placed in the optimal location for eliciting the largest possible MEP for that digit.
We thus suggest that authors clearly report how they identify the motor hotspot, especially with patient populations, where long testing sessions may be uncomfortable and experimenters might be eager to use the first spot that elicits any MEP response. In particular, we recommend: (1) clearly indicating how the starting point for testing for the hotspot was determined (e.g., by measuring a certain distance in the anterior/posterior and lateral directions from the vertex of the head) and (2) indicating how locations for subsequent stimulations were determined to ensure that the best possible hotspot was identified (e.g., by testing 3–5 MEPs at 1 cm anterior, posterior, medial, and lateral to the measured starting spot).
We also suggest recording and reporting the number of stimulations required to identify the motor hotspot for each participant. Applying many subsequent stimulations could plausibly have a lasting effect on cortical excitability and could thus be a confounding variable if a greater number of stimulations is required to identify the motor hotspot for patient or aging subject groups. While there are no clear recommendations or methodology studies to date investigating an optimal interstimulus interval for identifying the motor hotspot or RMT (described below), “single pulse” TMS (as opposed to paired pulse or repetitive TMS) is typically defined as waiting at least 5–10 seconds between subsequent stimulations (Edwards et al., 2018; Rotenberg et al., 2014). We recommend this interval as a minimum safety standard for studies collecting only single pulse data.
4.2. Identification of Resting Motor Threshold (RMT)
Studies report multiple methods for identifying the RMT (listed in Table 2). The most commonly-used approach is the “Minimum Number at 50 μV Method” (Rossini et al., 1994). This method defines the RMT as the lowest stimulus intensity that induces a MEP with an amplitude of ≥ 50 microvolts in at least a certain percentage of trials (typically, 5 of 10 trials). Similar to this, we suggest the more systematic approach described by Groppa et al. (2012), who recommend: (1) gradually increasing intensity of stimulator output (e.g., in steps of 5%) until TMS consistently evokes MEPs with peak-to-peak amplitudes of ≥ 50 microvolts; (2) lowering the intensity in steps of 1% until less than 5/10 MEPs are ≥ 50 microvolts; (3) recording the RMT as this intensity +1%. As these approaches can be relatively time-consuming and may require many stimulations (e.g., as many as 75 stimulations; Tranulis et al., 2006), it might be suitable to use a smaller criterion such as 3/6 MEPs (e.g., McGinley et al., 2010), although a cut-off of fewer than 5/10 MEPs has not been validated (Groppa et al., 2012).
Table 2.
Comparison of common analysis methods for obtaining silent period outcome measures
| Outcome Measure and Method Name | Source(s) Describing Methoda | Description | Involves Subjective Judgment? |
|---|---|---|---|
| Resting Motor Threshold (RMT) | |||
| Adaptive Threshold Huntingb | (Mishory et al. 2004; Awiszus 2003) | Models the relationship between the TMS intensity and the probability of eliciting a MEP. After each trial, the model suggests what TMS intensity should be used next by selecting an intensity that has a 50% chance of evoking a MEP. This method requires a computer program for modeling and selecting TMS intensities (e.g. the Motor Threshold Assessment Tool, https://www.clinicalresearcher.org/software.htm) | No |
| Minimum Number at 50 μV | (Rossini et al. 1994) | Lowest intensity that evokes MEPs with a peak-to-peak amplitude >50 μV in at least: • 3 of 5 consecutive trials (Fujiyama et al. 2009, 2012) • 3 of 6 consecutive trials (McGinley et al. 2010) • 5 of 10 consecutive trials (Rossini et al. 1994) • 10 of 15 consecutive trials (Sale and Semmler 2005) |
No |
| Median Threshold | (Mills & Nithi 1997) |
1) Define lower threshold: starting at a suprathreshold intensity, stimulation intensity is decreased by 1% until no MEPs are evoked for 10/10 consecutive trials 2) Define upper threshold: intensity is increased by 1% until identification of the minimum intensity that evokes MEPs with peak-to-peak amplitude >50 μV in at least 5 of 10 consecutive trials 3) Define RMT: take the median intensity between the lower and upper threshold values |
No |
| Cortical Silent Period (cSP) Duration | |||
| Automated Methods | |||
| Visualize EMG TMS Analyze (VETA) | (Jackson & Greenhouse 2019) |
cSP onset: MEP offset cSP offset: “inflection point” after the onset (i.e., “where the mean of the rectified signal starts to increase”); Automatic MatLab-based algorithm: https://github.com/greenhouselab/Veta |
No |
| CortEX Tool | (Harquel et al. 2013) |
cSP onset: “beginning of the induced muscular atonia” cSP offset: “end of the induced muscular atonia” Automatic algorithm based on the “thresholding of the first derivative of the epoched signal.” cSP start and end are defined based on when the EMG signal falls above and below this threshold |
No |
| Automated Method from the TMS Pulse to the End of EMG Silence | (Tazoe et al. 2007) |
cSP onset: TMS pulse cSP offset: recurrence of continuous EMG, defined by calculating the root mean square of the EMG signal for 100 ms before the TMS pulse and then identifying the point at which the post-stimulus EMG signal first exceeds 2 standard deviations of the pre-stimulus level |
No |
| Automated Method from the Start of the MEP to the End of EMG Silence | (Silbert et al. 2006) |
cSP onset: start of the MEP, defined as the first point equal to the mean value from a sample of the 310 ms before the TMS pulse cSP offset: the first point after the MEP equal to the mean value from a sample of the 310 ms before the TMS pulse |
No |
| Standard Deviation Thresholding | (Goodall et al., 2010) |
cSP onset: TMS pulse cSP offset: time at which the post-stimulus EMG exceeds 2 standard deviations above the pre-stimulus EMG for at least 100 ms |
No |
| Visual Inspection Methods | |||
| Visual Inspection from the Start of the MEP | (Damron et al. 2008; Davidson & Tremblay 2013) |
cSP onset: MEP onset (determined by visual inspection); initial deflection of the MEP cSP offset: first sign of EMG recovery (determined by visual inspection); specifically, the first positive or negative deflection of the EMG signal associated with the resumption of the voluntary EMG signal |
Yes |
| Visual Inspection from the End of the MEP | (Oliviero et al. 2006) |
cSP onset: end of the MEP (determined by visual inspection) cSP offset: resumption (“at any level”) of sustained EMG activity (determined by visual inspection) |
Yes |
| Visual Inspection from the TMS Pulse | (Sale & Semmler 2005) |
cSP onset: onset of the TMS pulse (determined by visual inspection) cSP offset: resumption of consistent EMG to pre-stimulus levels (determined by visual inspection) |
Yes |
| Ipsilateral Silent Period (iSP) Duration | |||
| Automated Methods | |||
| Pre-Stimulus EMG 10 s Method | (Strauss et al. 2019) |
iSP onset: when the post-stimulus EMG falls below the pre-stimulus EMG for at least 10 ms iSP offset: when the post-stimulus EMG activity resumes for at least 10 ms |
No |
| Average Rectified EMG Threshold | (Spagnolo et al. 2013) |
iSP onset: the time at which the average rectified post-stimulus EMG activity becomes smaller than the average pre-stimulus (between −60 and −10 ms before the stimulus) EMG level for at least 10 ms iSP offset: first point after the iSP onset at which the post-stimulus EMG activity “regains the baseline activity” for at least 10 ms |
No |
| Percentage of Pre-Stimulus EMG | (McGregor et al. 2011) |
iSP onset: first of five consecutive data points after the contralateral MEP that shows a minimum decrease of 80% from the mean EMG 20 ms before the stimulus iSP offset: first of five data points that shows a return to >20% of the pre-stimulus mean EMG level |
No |
| MCD Threshold | (Garvey et al. 2001) |
iSP onset: first of five consecutive data points to fall below the lower mean consecutive difference (MCD) threshold (e.g., MCD × 2.66, equivalent to 3 standard deviations) iSP offset: first data point to fall above the lower MCD threshold if 50% or more of data in following 5 ms window also fall above this threshold See Section 5.2 for a full description of this method |
No |
| Visual Inspection Methods | |||
| Boundary Visual Method | (Petitjean & Ko 2013) | iSP onset and offset: “sharp boundaries of the electrical silent period” | Yes |
| Stimulus Artifact Visual Method | (Cacchio et al. 2009) |
iSP onset: start of the stimulus artifact (i.e., approximately at the time of the TMS pulse) iSP offset: when the EMG amplitude is “comparable” to the pre-stimulus EMG level |
Yes |
| iSP Depth | |||
| Average iSP Depth | (Jung and Ziemann 2006; Strauss et al. 2019) | Mean EMG during the iSP, expressed as a percentage of the mean pre-stimulus EMG: Depth % = 100 − [(Mean EMGiSP / Mean EMGpre) × 100%] |
No |
| Maximum iSP Depth | (Jung & Ziemann 2006) | Maximum amount of EMG suppression during the iSP, expressed as a percentage of the mean pre-stimulus EMG (similar to average iSP depth formula, except the max instead of mean EMGiSP is used) | No |
| iSP Area | |||
| Normalized iSP Area | (Coppi et al. 2014) | iSP area, normalized by the amount of pre-stimulus EMG signal (to account for between-subject variability in pre-stimulus EMG levels) Normalized Area = [((iSPamplitude × iSPduration)−(Pre-stimulusArea)) / (Pre-stimulusArea × 100)] × 100% |
No |
| iSP Area | (Davidson & Tremblay 2013) |
iSP onset: first time point where the EMG signal “clearly fell under the mean level observed before the cortical stimulus” iSP offset: first time point after the iSP onset at which the EMG level “returned to the mean level” iSP area: after rectifying the EMG, the iSP area is calculated as the integral of the area between the iSP onset and offset |
Yes |
| iSP Onset Latency | |||
| iSP Onset Latency | (Davidson & Tremblay 2013) | Time interval from the TMS pulse until the “first sign of sustained decline in EMG levels (>25% of mean EMG level for at least 5 ms)” | Yes |
| Transcallosal Conduction Time (TCT) | |||
| TCT | (Petitjean & Ko 2013) | Calculated by subtracting the contralateral muscle MEP latency (i.e., the time of onset of the MEP) from the iSP onset latency (i.e., the time from the TMS pulse to the start of the iSP) TCT = iSPonset − contralateral MEP latency |
Unsure |
Table 2 Note. Here we intend to provide an overview of common methods for analyzing silent period data, with the goal of highilghting discrepancies between these methods. This is not meant to be a comprehensive list of all possible approaches for analyzing silent period data
TMS = transcranial magnetic stimulation; MEP = motor-evoked potential; EMG = electromyography; ms = milliseconds.
Here we aim to list key studies that either originally defined each of these methods or adapted and implemented these methods. This is not meant to provide a comprehensive list of all studies that have utilized each method. Rather, we intend to point readers to published work using each method so that readers may refer to these studies themselves for further details
Several other similar adaptive threshold hunting modeling methods have been introduced (e.g., Qi et al. 2011; Awiszus 2011) and may substantially reduce time and number of stimulations required to identify the RMT. However, these methods have not yet been widely implemented or validated, so we do not discuss these in detail here
In all cases, cSP and iSP duration = onset - offset (although onset and offset are defined differently depending on the method)
As calculating TCT requires identification of iSP onset time and contralateral MEP latency, this metric could involve subjective measurements if investigators use visual methods to identify either the iSP onset time or the contralateral MEP latency
Other past work has suggested similar methods based on amplitude criteria (e.g., Mills & Nithi, 1997), as well as newer adaptive modeling methods (e.g., Awiszus, 2003; 2011; Mishory et al., 2004; Qi et al., 2011). Such adaptive modeling methods function by estimating the probability of eliciting a MEP at a given stimulus intensity. These approaches require additional (often freely available) software (e.g., the Motor Threshold Assessment Program, https://www.clinicalresearcher.org/software.htm). Although these methods may substantially reduce the time required to determine the RMT (Awiszus, 2011; Qi et al., 2011), they have not yet been widely implemented. Our recommendation for RMT identification is transparency in how the RMT was determined across subjects. Further, we recommend that investigators ensure that an unbiased method was implemented in order to reduce any subjectivity that may be associated with RMT determination, especially for clinical or aging population studies.
4.3. Silent Period Trial Parameters and Force Task
4.3.1. Minimum Number of Stimulations
Typically, investigators elicit multiple silent periods per subject and average across individual trials to then calculate measures such as average silent period duration. Some recommendations suggest averaging 5 to 6 trials for silent period testing (see Groppa et al., 2012; Rossini et al., 2015), although we believe this recommendation to be too few. Garvey et al. (2001) systematically tested the influence of the number of trials on cSP duration. They found no statistically significant differences between averaging 10, 20, 30, 40, or 50 trials for cSP analysis, suggesting that fewer trials may still provide a reliable indication of cSP metrics (Garvey et al., 2001). However, a caveat to Garvey et al. (2001) is that this study included only 13 individuals (8 children and 5 young adults), making these findings difficult to generalize to other populations. In contrast, as iSPs are shorter, shallower, and more difficult to elicit, we recommend collecting and averaging a greater number of trials when testing iSPs. As recent work has found that a minimum of at least 20–30 trials is needed to accurately estimate MEP amplitude (Brownstein et al., 2018; Cuypers et al., 2014; Goldsworthy et al., 2016), short-interval intracortical inhibition (Brownstein et al., 2018), and intracortical facilitation (Brownstein et al., 2018), with no added benefits after 30 trials for MEP amplitude (Goldsworthy et al., 2016), we recommend using a similar number for all silent period testing. The number of trials averaged to calculate silent period outcome measures and the reasoning for this selection should be clearly noted so that others may replicate it.
4.3.2. Force Level
As depicted in Fig. 1, to elicit a silent period, the participant must be holding a tonic contraction. However, past work has implemented widely varying parameters for these tonic contractions. For instance, some studies have used force goals as low as 15–20% of one’s maximal voluntary contraction (MVC; Fling & Seidler, 2012; Fling & Seidler, 2011; Swanson & Fling, 2018), while others have used maximal contractions (i.e., 100% of MVC; Giovannelli et al., 2009; Jung & Ziemann, 2006). There is no consensus on whether the intensity of the target muscle contraction influences cSP duration. Some studies have found that background EMG has little effect on cSP duration (Säisänen et al., 2008; Taylor et al., 1997; Yasuo Terao & Ugawa, 2002). For instance, past work found cSP duration to be independent of target muscle activation level for contractions ranging from 0–75% (Taylor et al., 1997) and 20–80% (Säisänen et al., 2008) of MVC. However, others have found that target muscle activation level does affect cSP duration for forces ranging from 10–100% of MVC (Mathis et al., 1998; Matsugi, 2019; Stĕtkárová et al., 1994). Some studies have found that increasing force level relates to shorter cSPs (Mathis et al., 1998; Matsugi, 2019). Other studies have found that increasing force level relates to shorter or longer cSPs, depending on the method used for defining cSP onset and offset (Stĕtkárová et al., 1994). For instance, Stĕtkárová et al. (1994) found that defining the cSP offset as “relative” (i.e., the “return of uninterrupted EMG activity”) versus “absolute” (i.e., the period of “complete EMG silence”) yielded opposite results. Greater force levels related to longer cSPs when considering the relative offset, but shorter cSPs when considering the absolute offset. Thus, as demonstrated in this example by Stĕtkárová et al. (1994), it is possible that a reason for these varied findings could be due to study-specific methods for calculating the cSP duration (see Section 5.2).
In contrast to cSPs, to be elicited reliably, iSPs appear to require greater contraction intensity of the target muscle than cSPs (Ferbert et al., 1992; Giovannelli et al., 2009; Jung & Ziemann, 2006). Some past work suggests that iSPs should be tested during short maximal contractions (i.e., 100% of MVC; Davidson & Tremblay, 2013b; Giovannelli et al., 2009; McGregor et al., 2013; Perez et al., 2014). However, we have demonstrated that upper limb iSPs may be elicited even at low (e.g., 20% MVC) contraction levels (Fling & Seidler, 2012; Fling & Seidler, 2011). No study to date has clearly examined differences in silent period outcome measures when using low-level sustained contractions versus short bursts of maximal contraction.
Some past work suggests that varying the contraction intensity of the target muscle between 30%, 50%, and 100% of MVC does not affect iSP duration of the abductor pollicis brevis hand muscle (Kuo et al., 2017). However, other past iSP work suggests that the contraction level of the contralateral hand affects iSP duration (Giovannelli et al., 2009). That is, some protocols involve contraction of both the target muscle (i.e., ipsilateral to the TMS stimulation) and the opposite hand (i.e., contralateral to the TMS stimulation). This work has found that only the contraction level of the contralateral hand influences iSP duration (Giovannelli et al., 2009).
Given that force level may affect silent period outcomes, when selecting force parameters, care should be taken to avoid fatiguing the target muscle. Thus, we recommend that participants either sustain a low-level contraction (e.g., 15–20% MVC) for the entire duration of the trial (Fling & Seidler, 2012; Fling & Seidler, 2011; Swanson & Fling, 2018), or alternatively, that participants perform short, near-maximal contraction bursts with standard inter-trial rest intervals between each subsequent stimulation (Davidson & Tremblay, 2013b; Giovannelli et al., 2009; McGregor et al., 2013; Perez et al., 2014). The latter option may function better for patient or aging populations who are more susceptible to muscle fatigue.
We also recommend checking for possible signs of fatigue in the EMG of the target muscle, such as an increase in the amplitude of the EMG signal for silent period paradigms that use sustained, submaximal contractions (for review, see Enoka & Duchateau (2008)). In this case, it is possible for an experimenter to visually observe increased EMG amplitude in real-time during a silent period collection; for an example of visually increasing EMG amplitude due to fatigue, see Fig. 4B in Enoka & Duchateau (2008). During post-processing of data, investigators may wish to calculate the average amplitude or root mean square of the EMG signal and quantify whether these metrics change significantly across the course of the trial. One could then test whether such EMG metrics of fatigue differed between a patient and control group to rule out fatigue as a potential cause of group differences in silent period metics. Furthermore, in watching for signs of fatigue during data collection, we also recommend that investigators clearly monitor force output to ensure that participants achieve and maintain the target force level throughout each trial.
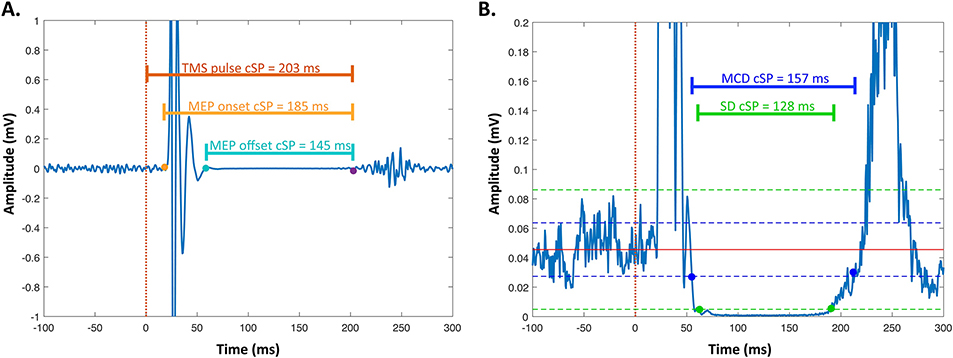
Fig. 4. Varying methods for coding the silent period onset and offset.
Data here are shown for a healthy young adult cSP (average of 20 individual cSPs). A. This panel shows several common methods for coding the silent period onset and offset based on the MEP. The dotted line (time = 0 ms) marks the time of the TMS pulse, the yellow point marks the onset of the MEP, the blue point marks the offset of the MEP, and the purple point marks the offset of the silent period. Horizontal lines show the resulting cSP durations depending on which events are used for the duration calculation. B. This panel shows several common methods for coding the silent period onset and offset based on the rectified EMG signal. The blue dots and blue solid horizontal line indicate the cSP duration calculated based on the MCD Threshold Method (Garvey et al., 2001). The blue dotted lines depict ± 2.66*MCD around the mean pre-stimulus EMG. The green dots and green solid horizontal line indicate the cSP duration calculated based on the standard deviation method. The green dotted lines depict ± 3 standard deviations around the mean pre-stimulus EMG.
Fatigue increases corticospinal excitability, as evidenced by increased MEP amplitude as muscle fatigue develops in the upper limbs (Benwell et al., 2006; Yoon et al., 2012) and in the knee extensors (Kennedy et al., 2016; Vernillo et al., 2018). Fatigue also increases cSP duration in the upper (Hunter et al., 2008; McKay et al., 1996; Yoon et al., 2012) and lower (Goodall et al., 2018; Kennedy et al., 2016; Vernillo et al., 2018) limbs. For instance, Goodall et al (2018) recently identified that cSP duration increases following multiple fatiguing contractions in the lower limbs, suggesting that investigators should control for fatigue in cSP analyses. Although some paradigms that employ only low target force levels (e.g., 15% MVC) may not induce fatigue, it is still important to be aware of potential fatigue effects, especially when using higher force levels or collecting multiple subsequent trials.
Taken together, the different levels and patterns of muscle contraction used make it difficult to compare silent period outcome measures across studies. However, we acknowledge that it may be difficult to avoid this issue, depending on the primary aims of future work. Thus, future studies should strive to clearly report: (1) how MVCs were obtained; (2) the percentage of MVC used for the force production task; (3) the reasoning behind each of these choices; and (4) the methods used to quantify or account for muscle fatigue.
4.3.3. Force Task
In addition to the level of force produced, the type of force task can also influence silent periods. For instance, (Tinazzi et al., 2003) identified shorter first dorsal interosseous cSP durations for pincer and power grips than for index finger abduction. This was potentially the case because motor cortical neurons become more excited during complex manual tasks that require the activation of multiple adjacent synergistic muscles (e.g., pincer and power grips) than during an isolated movement of one digit (e.g., index finger abduction; Hess et al., 1986, 1987). During isolated movements, muscles that are not involved in the task are likely inhibited, which may lengthen cSP duration (Tinazzi et al., 2003).
On a similar note, Mathis et al. (1998) found that cSP duration depended on the instructions provided to the participant regarding how they should react to the TMS pulse. At the start of all trials, subjects held a tonic contraction of the biceps brachii muscle. In one condition, subjects were instructed to perform an additional voluntary contraction of the biceps brachii muscle “immediately after” the TMS pulse. This instruction resulted in shorter cSP durations compared to maintaining a constant force level (Mathis et al., 1998). Contrarily, instructing subjects to relax their biceps brachii muscle “immediately after” the TMS pulse resulted in longer cSP durations compared to maintaining a constant force level (Mathis et al., 1998). These effects were more pronounced at lower stimulation intensities and lower force levels (Mathis et al., 1998). Further, this group found cSPs up to 130% longer in duration for “maintain-position” contractions (i.e., holding the same arm position against a load force) compared to “maintain-force” contractions (i.e., maintaining the same arm force output) of the biceps brachii and brachioradialis muscles (Mathis et al., 1999). Of note, the force level of the contraction was held constant between both of these conditions, permitting comparison of contraction type effects on cSP (controlling for force level). Together, these studies highlight the need for consistent instructions for careful selection of force tasks and consistent participant instructions, as well as the difficulties associated with comparing across studies that have implemented differing force tasks.
One confounding factor here is that different tasks may elicit different absolute forces and, as discussed in Section 4.3.2, force level may affect the silent period. For instance, while Tinazzi et al. (2003) elicited cSPs as subjects completed a pincer grip, power grip, or index finger abduction at 20% of their MVC for each of these tasks, the MVC differed by task. Consequently, the absolute force produced in each condition was different. Thus, investigators should consider and justify both the force level and the motor task when designing silent period experiments.
4.3.4. Variability of EMG and Force Output
Few studies have examined whether silent period outcome metrics vary with EMG or force output variability (i.e., how the subject’s EMG signal or force output varies around their mean level; both EMG and force variability have similar interpretations). EMG variability in the target muscle does not appear to significantly influence cSP duration (Garvey et al., 2001). This was noted when comparing healthy adults to children (i.e., who showed greater EMG variability; Garvey et al., 2001), in addition to analyzing a single subject who was asked to purposely vary his or her EMG activity during a cSP trial (Garvey et al., 2001). This lack of relationship removes a potentially confounding variable in cSP work, given that older adults (e.g., Deutsch & Newell, 2001; Sosnoff & Newell, 2011; Vaillancourt et al., 2003) and many patient populations (e.g., Sheridan & Flowers, 1990; Vaillancourt et al., 2002) tend to show increased force variability compared to healthy young adults. However, as the effects of motor output variability on silent period outcomes have only been examined in several studies using small sample sizes, this warrants further investigation. We thus recommend reporting basic EMG and/or force variability measures (e.g., coefficient of variation of the background EMG of the target muscle and/or coefficient of variation of the force output of the target muscle) when comparing silent periods for two groups or pre-/post-intervention. Although not investigated in silent period studies to date, investigators may also wish to calculate a measure of force accuracy (e.g., root mean square error) around the target force level to report a more complete subject performance profile and to assess whether and how force accuracy affects silent period outcome metrics.
4.3.5. Stimulator Intensity
Past work has used a wide variety of stimulation intensities (Table 3). Greater stimulation intensity is associated with longer cSPs (Devanne et al., 1997; Inghilleri et al., 1993; Kimiskidis et al., 2006; Säisänen et al., 2008; Wilson et al., 1993) and longer iSPs (Chen et al., 2003; Kimiskidis et al., 2005), until a plateau occurs at very high stimulation intensities for both cSPs (Kimiskidis et al., 2005) and iSPs (Chen et al., 2003). For instance, iSP area and duration have been found to increase from intensities of 45% of stimulator output to 60% stimulator output, but to plateau at intensities of 75% to 90% (Chen et al., 2003); however, this may not be a fully representative example, as this study included only 10 healthy young adults who may have had differing levels of corticospinal excitability (e.g., different RMTs). Similarly, Meyer and colleagues (Meyer et al., 1995) found iSP duration to plateau after an intensity of 60%; see Fig. 5D in Meyer et al., (1995) for an example of this plateau. Similarly, using visual identification methods, Meyer and colleagues (1995) found iSP latency (i.e., the time interval from the TMS pulse to the onset of the iSP) to increase with increasing stimulation intensities of 50–70%, and plateau at 80%–100% stimulator output. This is in contrast to other work using automated methods to quantify iSP latency, which have not found an effect of stimulator intensity on iSP latency (Chen et al., 2003).
Table 3.
Comparison of methods used in example upper limb cSP studies comparing healthy young and older adults
| Study | Subjs. | Coil, Brand, Current Dir | Muscle Tested | Hemi. Tested | RMT Methoda | ISIb | # of Trials Avg. / Muscle | Force Task % of MVC | % of RMT or SO | Samp. Rate, Amp., Filter | DVs and cSP ID Methoda | Primary Aging-Related cSP Findingc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies reporting reduced cSP duration in older age | ||||||||||||
| (Beynel et al. 2014) |
YA:
n = 20 6M; 14F 26.4 ± 7.9 yrs OA: n = 19 7M; 12F 63.7 ± 1.7 yrs |
Figure-of-8, MagVenture Current direction not reported |
FDI | ??d | Adaptive Threshold Hunting (Awiszus 2003) | ??d | 10 stims | 50% | 120% RMT |
Samp: 12000 Hz Amp: “1–10 K” BP: 1000 – 6000 Hz |
Duration CortEx Tool |
Reduced cSP duration for OAs |
| (Davidson & Tremblay 2013) |
YA:
n = 13 9M; 4F 22.4 ± 3.0 yrs OA: n = 17 6M; 11F 73.0 ± 7.6 yrs |
Figure-of-8, MagStim Current direction not reported |
FDI | Both | Median Threshold | 10–15s between stims | 5 stims / hemi | 100% MVC in IPSI hand. ∼15% MVC in CONTRA hande | 120% RMT |
Samp: 2000 Hz Amp: “Amplified” LP: 1000 Hz; time constant = 10 ms |
Duration Visual Inspection from MEP Onset |
Reduced cSP duration for OAs |
| (Oliviero et al. 2006) |
YA:
n = 20 9M; 11F 26.0 ± 4.0 yrs OA: n = 22 8M; 14F 71.0 ± 6.0 yrs |
Figure-of-8, MagStim PA current |
FDI | Right | Minimum Number at 200 μVf (5/10 trials) | ??d | 5 stims | 50% | 150% active MTf |
Samp: ??d Amp: ??d BP: ??d |
Duration; cSP dur. : MEP amplitude ratio Visual Inspection from End of MEP |
Reduced cSP duration for OAs No age differences in cSP duration : MEP amplitude ratio |
| (Sale & Semmler 2005) |
YA:
n = 10 5M; 5F 26.6 ± 1.3 yrs OA: n = 10 5M; 5F 67.6 ± 2.3 yrs |
Circular, MagStim Clockwise current flow for right M1. Counter-clockwise current flow for left M1. |
FDI | Both | Minimum Number at 50 μV (10/15 trials) | TMS pulses delivered at ∼0.2 Hz | 4 hand tasksg × 3 intensities = 12 trials per hemi 15 stims/trial |
5% of Max. EMG (goal based on EMG not on MVC) | 80, 100, 120% RMTg |
Samp: 2000 Hz Amp: 100–1000× BP: 13–1,000 Hz |
Duration Visual Inspection from TMS Pulse |
Reduced cSP duration for OAs cSP duration shorter for L versus R hand for OAs but not YAs Largest task-dependent changes in cSP for R hand for OAs |
| Studies reporting no difference in cSP duration with older age | ||||||||||||
| (Fujiyama et al. 2012) | See full description of this study below. | No age differences in cSP duration at baseline | ||||||||||
| (Fujiya ma et al. 2009) | See full description of this study below. | No age differences in cSP duration in baseline conditions | ||||||||||
| (Hunter et al. 2008) |
YA:
n = 17 9M; 6F 25.5 ± 3.6 yrs OA: n = 7 5M; 2F 73.0 ± 3.3 yrs |
Circular, MagStim Current direction not reportedh |
Biceps Brachii | Dominant | Threshold based on biceps brachii muscle action potentialsi |
Baseline: 5 s between stims; 120 s between trials Fatiaue: 10–20 s break between each stim Recovery: 10 stims at 15 sec - 10 min after the fatigue protocol |
Baseline: 5 sets of 2–3 s contracts; 1 stim during each contract Fatigue: 6 stims (22 s contracts; 1 stim at beginning and 1 at end of each contract) Recovery: 10 stims |
100%,60%, 80% MVC | 100% of threshold (based on biceps brachii muscle action potentials) |
Samp: 2000 Hz Amp: 100–300× BP: 16–1,000 Hz |
Duration Visual Inspection from TMS Pulse |
No age difference in cSP duration at baseline or during recovery from fatiguing contractionsj cSP duration increased less for OAs than YAs during the fatigue task |
| Studies reporting increased cSP duration in older age | ||||||||||||
| (McGinley et al. 2010) |
YA:
n = 21 10M; 11F 21.4 ± 0.8 yrs OA: n = 9 5M; 4F 70.9 ± 1.8 yrs |
Figure-of-8, MagStim PA current |
Flexor Carpi Radialis | Non-dominant | Minimum Number at 50 μV (3/6 trials) | ??d | 6 stims | 15% MVC | 130% active MTk |
Samp: 5000 Hz Amp: ?? BP: 10–500 Hz |
Duration Visual Inspection from MEP Onset |
Increased cSP duration for OAs |
| Studies supporting reduced ability to modulate cSP duration in older age | ||||||||||||
| (Fujiyama et al. 2012) |
YA:
n = 15l 7M; 8F 21.1 ± ??d yrs OA: n = 15l 5 M; 9F 61.9 ± ??d yrs |
Figure-of-8, MagStim PA current |
Extensor Carpi Radialis | Both | Minimum Number at 50 μV (3/5 trials) | ≥ 5 s between stims |
Exp. 1: 3 80 s / task for 4 tasks + baseline; 14–16 stims / task Exp. 2: 80 s / task for 5 tasks; 14–16 stims / task |
N/Am | 130% RMT |
Samp: 2000 Hz Amp: 1000× BP: 10–500 Hz |
Duration Automated Method from TMS Pulse to End of EMG Silence |
YAs had increased cSP duration during IPSI limb tasks and decreased cSP duration during CONTRA limb tasks. OAs had reduced cSP duration only during opposite direction IPSI limb tasks. OAs showed little modulation of cSP duration across conditions |
| (Fujiyama et al. 2009) |
YA:
n = 15 6M; 9F 21.9 ± ??d yrs OA: n = 15 6M; 9F 66.7 ± ??d yrs |
Circular, MagStim PA current |
Extensor Carpi Radialis | Left | Minimum Number at 50 μV (3/5 trials) | ≥ 5 s between stims | 2 trials / task × 5 tasks = 10 trials 5–6 stims / per 30 s trial |
N/Am | 140% RMT |
Samp: 2000 Hz Amp: 1000× BP: 10–500 Hz |
Duration Automated Method from TMS Pulse to End of EMG Silence |
YAs had longer cSP durations during the most difficult tasks (e.g., moving IPSI limb pairs in opposite directions). OAs had no differences in cSP durations across conditions (i.e., suggesting impaired modulation of cortical inhibition to meet task demands) |
Table 3 Note. This table is not meant to provide a comprehensive list of upper limb cSP aging studies. Instead, we have selected example studies that have used varying methods and reported conflicting results regarding how cSP duration changes with aging. Our purpose here is to highlight how methodological differences influence study outcomes and complicate comparison across studies. Studies are sorted by date, with the most recent listed first in each category
RMT = resting motor threshold; ISI = interstimulus interval; MVC = maximal voluntary contraction; SO = stimulator output; DVs = dependent variables; cSP = cortical silent period; ID = identification; YA = young adult; OA = older adult; M = male; F = female; PA = posterior to anterior current direction; FDI = first dorsal interosseous muscle; Samp = sampling rate; Amp = amplification; BP = band pass filter; LP = low pass filter; M1 = primary motor cortex; IPSI = ipsilateral limb to TMS stimulation; CONTRA = contralateral limb to TMS stimulation; Exp = experiment
RMT and silent period identification (ID) methods are described in detail in Table 2
ISI refers to the time between subsequent TMS pulses during cSP trials (if reported)
We do not list all of the results from each study in this column. Instead, we list only those results pertaining to age group differences in cSP parameters, in order to highlight how methodological differences affect conclusions regarding age differences in cSP metrics
We use “??” to indicate when the study did not mention the indicated characteristics: interstimulus interval (ISI), EMG sampling rate (samp. rate), signal amplification (amp.), signal filtering parameters, or standard deviation of participant ages
This study (Davidson & Tremblay 2013) measured cSP and iSP concurrently. Each TMS pulse was delivered while the participant maintained a light contraction (∼15% MVC) of the CONTRA hand (i.e., the hand contralateral to the TMS stimulation) and a maximal contraction (100% MVC) of the IPSI hand (i.e., the hand ipsilateral to the TMS stimulation)
Here the investigators (Oliviero et al. 2006) based stimulator intensity during the cSP trials on active rather than resting motor threshold. They defined the active motor threshold similarly to RMT: the minimum intensity that produces MEPs of 200 μV in 50% of trials (Rossini et al. 1994)
For this study (Sale & Semmler 2005), the four tasks used included: isolated index finger abduction, power grip (i.e., grasping a cylinder with the hand), precision grip (i.e., pressing the thumb and index finger against a staple remover), and scissor grip (i.e., pressing the thumb and index finger against spring-loaded gardening shears). TMS was delivered at 80%, 100%, and 120% of RMT, but cSPs were only calculated for the trials at 100% and 120% of RMT. The longest cSPs were observed for the scissor grip for both YAs and OAs
Here (Hunter et al. 2008) the authors did not report a current direction but they did indicate that “the direction of current flow in the coil preferentially activated the motor cortex in the hemisphere that innervated the dominant arm”
In this study (Hunter et al. 2008), the investigators used a less common approach for determining individual stimulation intensity to use for silent period trials. They identified an intensity that produced a “large MEP” in the biceps muscle (i.e., a minimum of 50–60% of the maximal compound muscle action potential, based on electrical stimulation of the brachial plexus) during brief MVCs of the elbow flexor muscles (Todd et al., 2004)
This study (Hunter et al. 2008) found no difference in the biceps brachii cSP duration at baseline or during recovery from fatiguing contractions. The authors did not report whether there were significant age differences in cSP duration during the fatigue protocol (i.e., in which participants completed six 22-second maximal contractions and cSP was tested at the beginning and end of each maximal contraction), so it is unknown whether cSP duration was statistically different between the two age groups for these six cSP trials (see Fig. 6 in Hunter et al. (2008) for a plot of all cSPs by age group). Instead, the authors report only a significant age by time interaction for the fatigue protocol, which revealed that cSP duration increased less for old compared to younger adults across the fatigue protocol
The investigators here (McGinley et al. 2010) based stimulator intensity during the cSP trials on active rather than resting motor threshold. They defined the active motor threshold as the lowest TMS intensity required to evoke 3 out of 6 consecutive MEPs with a peak-to-peak amplitude ≥ 2 times that of the EMG during ∼6 voluntary contractions at 15% MVC without TMS (i.e., instead of using an amplitude cut-off of e.g., 300 μV, which is a more common approach).
This study (Fujiyama et al. 2012) excluded data from two younger and two older participants in the group-level analyses “due to unclear cSP duration”
Participants in these two studies (Fujiyama et al. 2009, 2012) did not complete isotonic contractions during TMS stimulation. Instead, participants received stimulations during cyclic iso- and non-isodirectional movements with ipsilateral and contralateral limb pairs
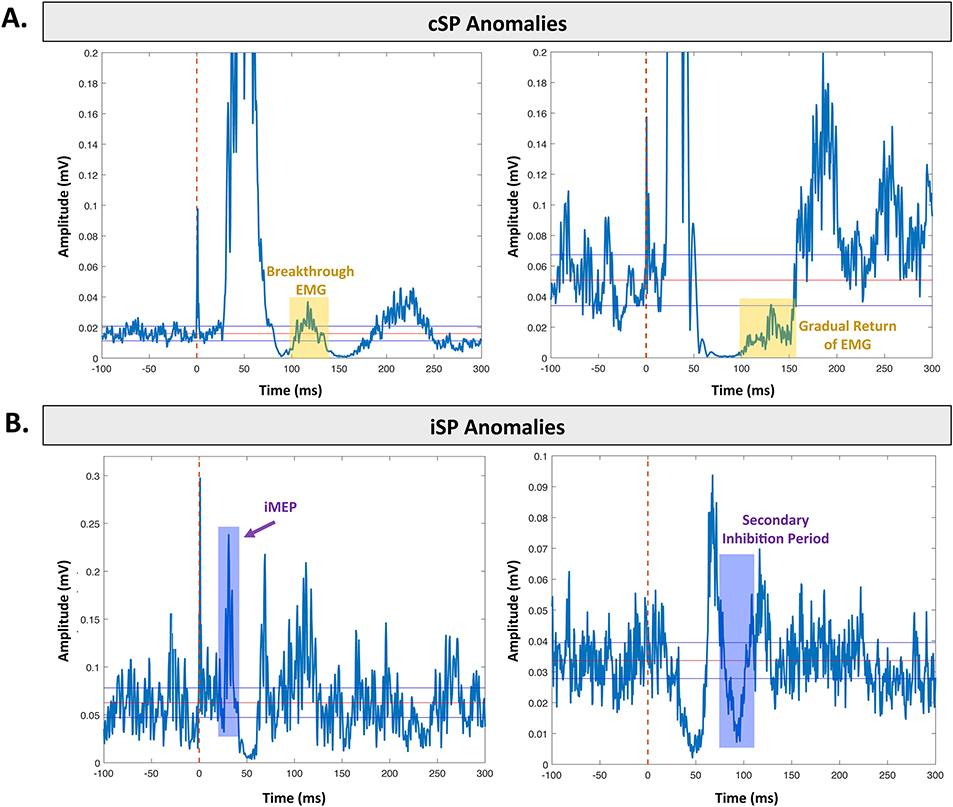
Fig. 5. Common anomalies in silent periods.
Each example depicts an average silent period for a healthy young adult. A. Common anomalies in cSP data. Left. Breakthrough EMG signal (yellow shading) for the tibialis anterior leg muscle. Right. Gradual return of the EMG signal (yellow shading) for the first dorsal interosseous hand muscle. B. Common anomalies in iSP data. Left. iMEP (purple shading) elicited in the first dorsal interosseous hand muscle. Right. Secondary inhibition period (purple shading) elicited in the first dorsal interosseous hand muscle.
Further complicating matters, although most studies (e.g., Swanson & Fling, 2018) use an individualized stimulation intensity for each subject (i.e., a certain percentage of their RMT), some studies have applied the same stimulation intensity across participants (e.g., Jung & Ziemann, 2006). Jung and Ziemann (2006) justified applying the same stimulation intensity of 80% to all subjects because of the plateau in iSP outcome measures at intensities of greater than ~60%–80% identified by Meyer et al. (1995). Despite this, we do not recommend applying the same stimulation intensity to all subjects. Presuming that RMT is calculated in an unbiased and systematic manner, failure to individualize stimulation intensity to percentage of RMT might risk eliciting shorter or shallower silent periods in individuals with reduced cortical excitability. This would be especially problematic for the case of aging (e.g., Bhandari et al., 2016; Oliviero et al., 2006) or patient (e.g., Bütefisch et al., 2001; Schippling et al., 2009) studies where the groups of interest may have altered cortical excitability.
Reliably eliciting iSPs requires higher stimulation intensity than eliciting cSPs. However, iSPs have been reported to occur with stimulation intensities as low as 110% of RMT (Davidson, 2016). Unpublished thesis work reported that iSPs only occur about 57% of the time at stimulation intensities of 110% RMT, about 80% of the time at 120% RMT, and plateau at about 97% of the time at 130% RMT and 95% of the time at 140% RMT (Davidson, 2016). Thus, this group has suggested that 130% RMT represents the lowest optimal intensity for reliability eliciting iSPs.
Some groups have used a stimulation intensity as high as 160% of RMT for eliciting iSPs (Petitjean & Ko, 2013; Sommer et al., 2006). While this may be feasible in healthy young adults, such a high threshold would become problematic in certain populations (e.g., older adults) with high RMTs (Bhandari et al., 2016), such that 160% of RMT could be greater than maximum stimulator output (i.e., >100% of stimulator output). In such cases, investigators would need to exclude all individuals with RMTs that are too high to use the same relative stimulation intensity for all subjects. Using high stimulation also reduces the focality of the stimulation and increases the likelihood of stimulating other nearby motor cortical representations and thus should be avoided.
Past work has successfully elicited cSPs at stimulator intensities varying from 80% RMT (Säisänen et al., 2008) to 140% RMT (Fujiyama et al., 2009; although 80% RMT failed to elicit cSPs in one subject included in (Säisänen et al., 2008)). Säisänen et al. (2008) tested how stimulator intensities ranging from 80% to 120% of RMT influenced cSP characteristics for the abductor pollicis brevis muscle in 10 healthy young adults. This group found the lowest intra-individual variability (i.e., coefficient of variation) for stimulator intensities of 120% RMT and thus recommends using this intensity for cSP tasks (Säisänen et al., 2008).
Given the above work, we recommend using an intensity of 130% RMT for upper limb iSP trials and 120% RMT for upper limb cSP trials. If investigators select to use other intensity levels (e.g., because 130% RMT is too high for a certain patient population), then justification for this choice should be provided. Methodological work is needed to determine whether optimal stimulator intensities for eliciting silent periods in the lower limbs differ from those needed for the upper limbs.
4.3.6. Ordering of the Protocol
It is also presently unknown whether single pulse TMS induces cumulative effects on the primary motor cortex. That is, studies have not been conducted to determine if it would be optimal to incorporate breaks into a testing session instead of running several silent period trials subsequently, or if the stimulations required to locate the motor hotspot and determine the RMT influence the parameters of a silent period trial, if these procedures are completed directly before collecting silent periods. Additional studies are thus warranted. At a minimum, conditions should always be counterbalanced across participants and this should be reported.
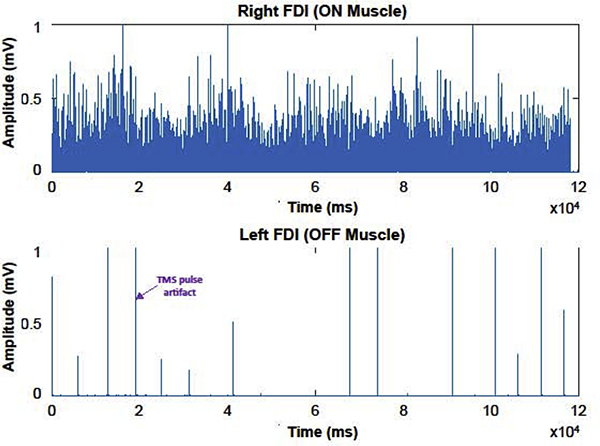
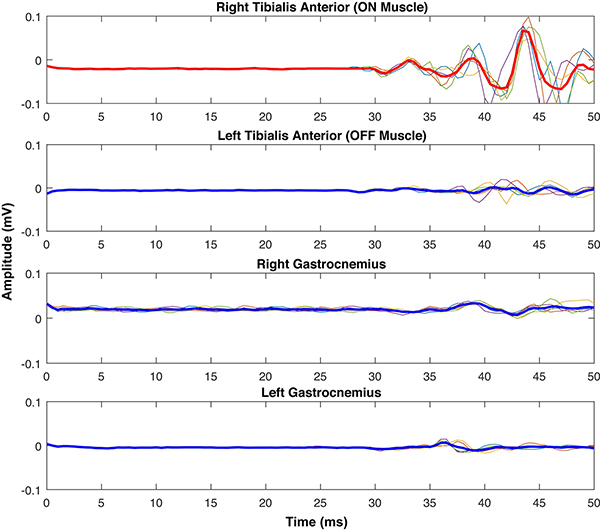
4.4. Relaxation of the OFF Muscle During iSP Trials
It is necessary to keep the contralateral homologous muscle (i.e., the “OFF” muscle) completely relaxed during silent period trials. In the case of iSPs, it has been shown that contracting the OFF muscle (even at low levels, but also at one’s MVC) or even imagining contracting the OFF muscle enhances iSP area, potentially via enhancing interhemispheric motor inhibition of the contralateral primary motor cortex (Giovannelli et al., 2009). To avoid (or at a minimum, quantify) this confound, we recommend collecting EMG data from the target muscle and from the OFF muscle. Examining the EMG activity from the target and OFF muscles (Fig. 3) serves as a quick quality check to visually assess whether any notable EMG occurred in the OFF muscle, and to provide a visual estimation of whether fatigue has occurred across the trial. Some studies have used real time feedback (e.g., acoustic feedback; Giovannelli et al., 2009) to allow participants to know whether they are fully relaxing the OFF muscle and to make adjustments if necessary. Instructions should be given to the participant to fully relax prior to starting each trial (and, if needed, during the trial), and it should be ensured that participants have a comfortable position in which to rest their OFF muscle during the trials.
Fig. 3. Rectified EMG for an iSP trial.
Rectified EMG trace for the target (ON) FDI muscle and contralateral (OFF) FDI muscle during an iSP trial. These data were collected during a 2-minute iSP trial, with a TMS pulse applied to the right hemisphere at 110% of the subject’s RMT approximately every 10 seconds, with 20 stimulations total. Spikes in the OFF muscle indicate timing of TMS pulses. Little to no EMG signal is evident in the OFF muscle here. The EMG signal remains relatively steady throughout the trial for the ON muscle, indicating that no fatigue occurred over the course of the trial. This represents acceptable EMG signal for an iSP EMG recording. As OFF muscle activation can influence iSP outcome metrics (Giovanelli et al., 2009), investigators should demonstrate that the OFF muscle was fully at rest during silent period trials. If the OFF muscle was not at rest, the OFF muscle EMG signal should be examined and quantified, for instance for evidence of motor overflow.
If it is still found that the OFF muscle has not remained at rest during silent period trials (particularly, iSP trials), the OFF muscle EMG activity should be analyzed for motor overflow (Fling & Seidler, 2011). In such a case, it could be that the brain is experiencing difficulty suppressing activity in the OFF muscle. Motor overflow can be assessed by calculating the rectified integral (i.e., area) of the OFF muscle EMG between each subsequent TMS pulse and normalizing this to the “baseline” EMG level for the same hand (i.e., the EMG level immediately before each stimulation; Carey et al., 1983; Fling & Seidler, 2012; Fling & Seidler, 2011). This is equivalent to expressing motor overflow as a percentage of the baseline EMG, to account for any inter-subject variability due to differences in skin-electrode impedance, noise, or arousal. We previously found that reduced iSP depth predicted greater motor overflow (Fling & Seidler, 2012), supporting the notion that those with poorer transcallosal inhibitory capacity also have reduced ability to suppress OFF muscle EMG. Given that motor overflow tends to increase with more challenging motor tasks, higher cognitive load, fatigue, and older age (for review see Cincotta & Ziemann, 2008), measuring motor overflow during silent period trials can add valuable data for interpretation.
5. Post-Processing and Analysis of Silent Period Data
5.1. EMG Signal Filtering
Many studies report band pass filtering EMG data collected during silent period trials; however, many studies have failed to report filtering parameters used. Current recommendations suggest band pass filtering of 1 Hz to 2,000 Hz (Groppa et al., 2012). However, settings may need to be adjusted for individual EMG systems. We have found a 10–1000 Hz band pass filter to be optimal for data collected in our laboratory (Fling & Seidler, 2012; Fling & Seidler, 2011). We have noted past work using band pass filters with cutoffs ranging from high pass: 2 Hz (Goodall et al., 2018) to 1,000 Hz (Beynel et al., 2014) and low-pass: 500 Hz (Fujiyama et al., 2009) to 10,000 Hz (Silbert et al., 2006). The high pass threshold (e.g., 1 Hz) will ideally shorten the duration of the stimulus artifact, and the low pass threshold (e.g., ~2,000 Hz) should be determined based on a value that falls well above the maximal frequency spectrum of the EMG signal (Groppa et al., 2012). Based on the Nyquist theorem, the low pass threshold (and also the sampling rate itself) needs to be at least twice that of the highest frequency in the signal of interest. Our primary recommendation here is to clearly report bandpass filtering cut-off values, so that others may replicate the same filtering processes in future work.
5.2. Identification of Silent Period Onsets and Offsets
There are widely varied definitions of onset and offset for silent periods (and thus widely different methods used to calculate the silent period duration; Table 2; Fig. 4). Some studies have defined silent period onset as the onset of the TMS pulse (e.g., Tazoe et al., 2007), others have defined it as the MEP onset (e.g., Davidson & Tremblay, 2013a), while others have defined it as the MEP offset (e.g., Oliviero et al., 2006). Further complicating matters, many previous studies have provided only a vague explanation of the silent period offset, such as “the resumption (at any level) of sustained EMG activity” (Oliviero et al., 2006), paired with only a brief description of how this was determined (although presumably in such cases, the offset was determined using visual inspection methods). As depicted in Fig. 4, each method results in a different cSP duration. This makes it impossible to compare across studies and to conduct robust meta-analyses.
Past work has employed many different methods to determine the onset and offset of silent periods (and thus to calculate the silent period duration; Table 2). These methodological differences are particularly concerning because many studies have used subjective visual methods for identifying onsets and offsets (e.g., Damron et al., 2008; McGinley et al., 2010; Petitjean & Ko, 2013). While multiple studies have argued that such visual methods are reliable (Damron et al., 2008; Petitjean & Ko, 2013) and produce high inter-rater reliability (e.g., ICC = 0.99 for all iSP parameters, (Petitjean & Ko, 2013), we argue that the benefits of automated methods (described below) outweigh the ease of visual inspection, especially for complex situations such as breakthrough EMG (Section 5.3) or secondary inhibition periods (Section 5.4). Several studies have found cSP duration to vary by over 20 ms when two separate investigators from the same group analyzed them using a visually-guided manual method (Garvey et al., 2001; Nilsson et al., 1997). This is a notable difference, as variations of similar magnitude have been reported as significant differences in silent period duration between younger versus older adults (e.g., Beynel et al., 2014; McGinley et al., 2010; Sale & Semmler, 2005) and patients versus controls (e.g., Ziemann et al., 1997). This issue is contentious because others (e.g., as previously mentioned, Damron et al., 2008; Petitjean & Ko, 2013) have found high inter-rater reliability of visual inspection methods; it may that rater training and experience plays a role. However, we argue that the best (i.e., most transparent and reproducible) approach is implementing an objective analytical method (described below) so that subjective raters are not needed.
We recommend using only objective methods and do not suggest visual inspection for identifying cSP and iSP onsets and offsets. In particular, we recommend the Mean Consecutive Difference (MCD) Threshold Method (Garvey et al., 2001), given that it is simple, easy to implement, and based on a systematic methodological study. This method is described in detail in (Garvey et al., 2001) with the appendix detailing step-by-step directions regarding calculation of the MCD; we briefly describe it here. (1) All silent period trials are rectified (i.e., the absolute value is taken) and averaged. (2) The MCD of 100 ms of pre-stimulus EMG is calculated. MCD is the mean successive difference between individual data points; smaller differences between sequential data points equate to a smaller MCD, while larger differences between sequential data points push the MCD further from the mean. That is, instead of using thresholds based on the average pre-stimulus EMG, this method creates thresholds based on the variability in the pre-stimulus EMG. (3) Thresholds are set at: ± MCD x 2.66 (blue dotted lines in Fig. 4B). This covers 99.76% of possible pre-stimulus EMG data points, which is equivalent to 3 standard deviations. (4) Silent period onset is determined as the point at which the post-stimulus EMG falls below the variation threshold (i.e., -MCD x 2.66) for five consecutive data points. As random data points fall outside 99.76% variation limits less than 1% of the time, five consecutive points of post-stimulus EMG can be considered different from the pre-stimulus mean (Pfadt & Wheeler, 1995). (5) The silent period offset is determined as the point at which the post-stimulus EMG returns above the variation threshold (i.e., -MCD x 2.66) for five consecutive data points.
We have implemented the MCD Threshold Method in our previous work (Fling & Seidler, 2012; Fling & Seidler, 2011), and it has been widely used by others (e.g., Giovannelli et al., 2009; McGregor et al., 2011). As described by Garvey et al. (2001), we have found that narrower variation limits are required for correctly identifying iSP trials (e.g., MCD x 1.77; Fling & Seidler, 2012; Fling & Seidler, 2011) compared to cSP trials (e.g., MCD x 2.66) because iSPs are shorter and less pronounced than cSPs. Thus, individual studies should employ the MCD Threshold Method, but should be aware that thresholds may need to be adjusted (and reported) depending on whether iSPs or cSPs are being tested. Alternatively, investigators may wish to test and report whether and how their primary results differ when using varying MCD thresholds.
We do not recommend using a standard deviation threshold instead of an MCD threshold (Garvey et al., 2001), although some have done this (e.g., Goodall et al., 2010). Calculation of a standard deviation threshold assumes that each data point is independent, which is not the case for time series data such as EMG. Further, as shown in Fig. 4B, similar to the findings of Garvey et al. (2001), when using three standard deviations as the threshold for identification of cSP onsets and offsets compared to ± MCD x 2.66, only the most dramatic suppression is quantified as part of the cSP and the cSP duration is substantially shorter. Thus, we do not recommend using the standard deviation to set threshold lines.
Newer options are currently in development to encourage further automation of silent period identification (Table 3). For instance, the freely available Visualize EMG TMS Analyze (VETA) MatLab toolbox (https://github.com/greenhouselab/Veta) has recently been released and described (Jackson & Greenhouse, 2019). VETA is designed to interface with EMG and TMS systems to facilitate collection and visualization of EMG data, as well as automatic detection of cSPs. This software makes specific assumptions (e.g., it defines the cSP onset as the MEP offset time and the cSP offset as the “inflection point” after onset “where the mean of the rectified signal starts to increase”). While the VETA data collection features are currently only supported for certain EMG vendors, future releases of the VETA toolbox may represent a promising avenue for streamlining collection and analysis procedures in silent period studies.
5.3. Breakthrough EMG Activity
Use of automated methods for identifying silent period onsets and offsets also circumvents issues that may arise with abnormal silent period tracings such as breakthrough EMG (Fig. 5A). That is, the TMS pulse sometimes induces two periods of EMG silence which are interrupted by a short burst of EMG activity (i.e., “breakthrough” EMG). Multiple studies have reported the presence of breakthrough EMG (e.g., Butler et al., 2012; Chen et al., 2003; Garvey et al., 2001; Jung & Ziemann, 2006; Lixandrão et al., 2020). Some authors have suggested that this breakthrough EMG arises from contributions by ipsilateral cortical or subcortical structures (Holmgren et al., 1990). Others have hypothesized that breakthrough EMG is mediated by spinal reflex mechanisms (Lixandrão et al., 2020). That is, muscle force drops quickly following the TMS pulse (during the muscle silence). This leads to muscle lengthening, which increases muscle spindle firing and ultimately triggers the firing of spinal alpha motor neurons and results in the visible EMG breakthrough activity (Burke et al., 2013; Li & Francisco, 2015). This notion is supported by previous work which found decreased EMG breakthrough activity during shortening muscle contractions (Butler et al., 2012) and with joint immobilization (Burke et al., 2013; i.e., two conditions in which muscle lengthening was prevented during the cSP).
Few studies directly report how they may have quantified breakthrough EMG. In many cases breakthrough should be easily identified (such as in Fig. 5A) and could be ignored when determining the silent period offset. However, if the occurrence of breakthrough activity is less clear in any instances (e.g., breakthrough combines with a gradual return of EMG activity, Fig. 5B) using an objective analytical method rather than a visual method prevents the experimenter from needing to subjectively determine whether the breakthrough should be considered as the offset of the EMG activity. This thus removes a level of subjectivity out of silent period duration measurements and allows for better future reproducibility.
Additionally, we and others (e.g., Fritz et al., 1997; Fuhr et al., 1991) have encountered situations where the EMG activity returns more gradually (Fig. 5A). In such cases, an objective analytical method is also recommended because, in such a situation, it would be quite difficult to subjectively determine where the offset point should be placed within the yellow shaded box in Fig. 5A. Taken together, regardless of past reports of high inter-rater reliability with visual methods, such subjective approaches fail to circumvent the problem of special situations such as breakthrough EMG and gradual return of EMG activity.
In general, we recommend that breakthrough EMG should not be counted as the offset of the silent period, due to the potentially non-cortical origins of this activity. We recommend that breakthrough EMG be counted as part of the silent period and included as part of the whole cSP duration. When calculating metrics such as silent period depth in cases of breakthrough EMG, authors should carefully report how they handled these scenarios (e.g., by keeping versus removing only the breakthrough portions or any trials that included breakthrough EMG for depth calculations). Additionally, we recommend that authors clearly report the number of trials that included any breakthrough EMG for each participant. Such reporting would allow future investigators to know whether to expect breakthrough EMG for certain muscles or subject populations. Further, such reporting would allow for future work designed to examine possible underlying mechanisms of breakthrough EMG.
5.4. The Secondary Inhibition Period and Ipsilateral MEPs
5.4.1. The Secondary Inhibition Period
iSP trials may produce another potentially confounding factor—a secondary inhibition period (Fig. 4B; Jung & Ziemann, 2006; Meyer et al., 1995). This secondary inhibition period does not seem to occur reliably for every iSP trial for a given subject, but does seem to occur more frequently in certain muscles. For instance, one study found more frequent secondary inhibition periods for the first dorsal interosseous muscle compared to the abductor pollicis brevis muscle (i.e., 40% of subjects for the first dorsal interosseous but only 5% of subjects for the abductor pollicis brevis; Jung & Ziemann, 2006).
Special consideration should be given to this secondary inhibition period, as evidence suggests that it does not represent transcallosal inhibition (which is the intended measurement of iSP; Jung & Ziemann, 2006): (1) This secondary inhibition period was evident in some patients with complete agenesis of the corpus callosum, while the initial iSP was absent in these individuals (Meyer et al., 1995). (2) The H reflex was not altered during the iSP, suggesting that ipsilateral descending cortical pathways may underlie iMEPs, rather than spinal contributions (Jung & Ziemann, 2006). Thus, it is suspected that secondary inhibition phases are mediated by ipsilateral corticospinal pathways (Jung & Ziemann, 2006) such as the corticoreticulospinal or corticopropriospinal pathways.
Given the probable non-transcallosal origins, the secondary inhibition period should likely not be counted as part of the iSP. In our past work, the MCD Threshold Method performed well in avoiding capturing secondary inhibition periods as part of the iSP; however, future investigators should verify this in their work. One study found that, in some cases, the iSP merged with the secondary inhibition period (which would make the iSP duration quite long; Jung & Ziemann, 2006). In such cases, care should be taken to address these instances, by, for example, removing trials where this happens, or by reporting the number of trials where this occurred.
5.4.2. Ipsilateral MEPs
Typically, a MEP would only be expected in the contralateral muscle during an iSP trial (Giovannelli et al., 2009; Wassermann et al., 1991; Ziemann et al., 1999). An ipsilateral MEP (iMEP) occurs when there is a noticeable MEP in the ipsilateral muscle. Similar to secondary inhibition periods, iMEPs are likely not of transcallosal origin (Chen et al., 2003). As iMEPs are visible in patients with complete corpus callosum agenesis (Ziemann et al., 1999), it has been suggested that direct descending oligosynaptic pathways from ipsilateral motor cortex are more likely to mediate iMEP responses than transcallosal interhemispheric mechanisms.
There is no widely accepted definition for an iMEP; one study defined an iMEP as occurring if the averaged rectified post-stimulus EMG signal exceeded 120% of the mean background EMG levels for at least 5 ms (Giovannelli et al., 2009). Another study defined iMEPs as present if the post-stimulus EMG exceeded the pre-stimulus mean EMG by >1 standard deviation for ≥5 ms (Chen et al., 2003). Our primary recommendation in handling iMEPs is to clearly report the criteria used to classify them and to report metrics such as the percentage of trials in which iMEPs occurred, whether there were group differences in iMEPs, and if iMEP prevalence correlates with experimental variables of interest such as silent period duration. Additionally, if iMEPs occur in a small enough percentage of trials, investigators might consider using iMEP presence as an exclusion criterion.
Although past work has not identified correlations between iMEP amplitude and iSP duration (Jung & Ziemann, 2006), this work suggests that the occurrence of an iMEP is linked to the occurrence of a secondary inhibition period. This study found that the secondary inhibition period occurred for 6 of 8 subjects after an iMEP and for only two subjects without an iMEP (Jung & Ziemann, 2006). We thus recommend that—particularly if measuring the first dorsal interosseous muscle, for which secondary inhibition periods may be more likely to occur—investigators qualitatively check for and report the presence of iMEPs.
iMEP prevalence may also depend on individual characteristics, such as handedness. We previously reported that less lateralized individuals (i.e., those who rely less on one dominant hand) were more likely to show iMEPs during TMS applied to the hand motor cortex (Bernard et al., 2011). Given these findings, we thus recommend testing and reporting participant limb dominance in all silent period studies.
Of note, one study found that higher stimulation intensities (e.g., ~60% stimulator output and above on their set-up) caused iMEPs in the majority of subjects (Chen et al., 2003). This should thus also be considered when choosing stimulation intensity parameters for iSP studies.
5.5. Accounting for MEP Amplitude in cSP Calculations
cSP duration may be strongly correlated with MEP size (Orth & Rothwell, 2004). That is, the larger the evoked MEP in the contralateral muscle, the longer the cSP. If this is the case, intracortical inhibition should only be considered greater if the cSP duration increases without concurrent increase in MEP amplitude (Orth & Rothwell, 2004). Given that motor cortical excitability and, consequently MEP amplitude, change with certain pathologies (e.g., Huntington’s disease (Schippling et al., 2009), stroke (Bütefisch et al., 2001), and aging (Bhandari et al., 2016; Oliviero et al., 2006)), it is recommended that the ratio of cSP duration to MEP size be included as an additional outcome variable. This allows for the analysis of cSPs to rule out possible contributions of differences in motor cortical excitability and MEP size to cSP duration. Importantly, past work has found that group differences (e.g., age differences) disappear when correcting cSP duration for MEP amplitude (Orth & Rothwell, 2004). We thus suggest reporting both the corrected and uncorrected cSP duration and computing any between group or behavioral performance correlation statistics using both of these metrics.
5.6. Silent Period Depth and Area
We also suggest including average and maximal silent period depth as other measures of inhibition. We have found iSP depth to be more sensitive for delineating between young and older adults than iSP duration (Fling & Seidler, 2011). Despite these findings for iSP depth, cSP depth is not frequently reported; it could be that cSPs tend to reach a higher level of inhibition than iSPs (Garvey et al., 2001), making cSP depth less variable (i.e., as it would be close to 100% for most people) and thus making it less likely for group differences or associations with behavioral performance to emerge.
Silent period area and normalized area are also reported less frequently compared to silent period duration. As outlined in Table 1, silent period area is typically calculated as the integral of the rectified EMG trace in the region between the onset and offset of the iSP. Normalized area is then calculated by normalizing this area to the average pre-stimulus EMG level. The benefit of calculating normalized iSP area is that this takes the pre-stimulus muscle contraction level into account (Coppi et al., 2014; Kuo et al., 2017). As discussed in Section 4.3.2, as silent periods may be affected by contraction level, this represents a reproducible way to account for contraction level. In 25 healthy young adults, Kuo and colleagues (2017) found normalized iSP area to be the most consistent measurement (determined by a homogeneity of variance test and by the coefficient of variation). Normalized iSP area was consistent across all contraction levels (i.e., 30%, 50%, and 100% of MVC; Kuo et al., 2017). Thus, Kuo and colleagues (2017) recommend normalized iSP area over other iSP metrics for future work. To our knowledge, past work has not reported normalized cSP area, although this would be possible to calculate and could also have less measurement variability than other possible outcome metrics. Finally, as noted in Section 2.2.2, a major caveat to the discussion of silent period depth and area is that no studies to our knowledge propose distinct physiologic mechanisms for silent period duration versus depth or area; the functional interpretation of the depth and area of EMG suppression, compared to the duration, remains unclear.
5.7. Transcallosal Conduction Time (TCT)
Finally, we recommend testing transcallosal conduction time (TCT) when measuring the iSP. This is typically calculated as the time from the onset of the contralateral MEP to the time of the onset of the iSP (Petitjean & Ko, 2013). TCT may be a more between-group measurement than iSP duration alone. For instance, (Davidson & Tremblay, 2013a) found that TCT but not iSP duration was significantly different between young and older adults.
As discussed in Section 2.2.2, there are no studies that report how silent period duration, depth, area, and TCT relate. Thus, we recommend that future work extract each of these measures and attempt to clarify how these metrics might quantify different aspects of cortical inhibition.
5.8. Removal of Trials
It is imperative to report any removals of trials. As discussed in Section 4.3.5, past work has found that iSPs only occur about 57% of the time at stimulation intensities of 110% RMT, about 80% at 120% RMT, and plateau at about 97% at 130% RMT (Davidson, 2016). This means that, when investigators stimulate at 120% RMT to induce silent periods, trials are more than likely being excluded when calculating average silent period metrics, or, if these trials are included in the average, they artificially suppress silent period metrics. Despite this, few studies report exclusions of silent period trials. Others, perhaps concerningly, allude to excluding trials but do not specify how many trials were excluded. For instance, Petitjean and Ko (2013) noted that, “stimulation was applied so as to obtain 9 consecutive iSPs (defined as true electrically silent period, i.e. without any detectable EMG activity).” This implies that these investigators delivered some TMS pulses that did not elicit an iSP; however, they did not report how many trials were excluded and whether the percentage of excluded trials differed for their young versus older adults.
6. Special Considerations for Lower Limb Muscles
There are several unique challenges with collecting silent period data for the lower limbs. A recent review details general considerations for applying TMS to lower limb muscles (Kesar et al., 2018). Here we discuss several challenges specific to eliciting silent periods in lower limb muscles.
6.1. Stimulation Intensity and Coil Type
General challenges of applying TMS to lower limb muscles include the deep anatomical location of the lower limb motor cortical representations. The lower limb motor cortical representations locations are folded into the interhemispheric fissure of the brain, about 3–4 cm below the surface of the scalp (Fig. 6A). This makes it more challenging for TMS to induce MEPs in this cortex, as the strength of the induced electric field diminishes the further the target is from the scalp (Deng et al., 2013).
Fig. 6. Silent period testing within the lower limbs.
A. Schematic indicating the approximate locations of upper (blue) and lower (green) limb motor cortical representations. These locations are overlaid onto a 3D-rendered template brain. B. Average functional brain activation (i.e., fMRI activation) during upper (blue) and lower (green) limb tasks. These fMRI maps were obtained from Neurosynth (http://neurosynth.org/) and overlaid onto a 3D-rendered template brain. The lower limb activation was obtained from an automated meta-analysis (association test) of 83 studies using the search term “foot”; the upper limb activation was obtained from an automated meta-analysis (association test) of 83 studies using the search term “finger movements.” C. Left. cSP in the right tibialis anterior for a young adult subject. Right. Attempted iSP for the right tibialis anterior muscle in the same young adult subject. We believe that this is actually a hybrid iSP-cSP due to superficial current spread, as this trace contains several characteristics of a cSP, including a long duration (>100 ms) and an ipsilateral MEP (iMEP, shaded in yellow). Large iMEPs are not typical for iSPs. In both of these cases, we show the rectified average of 20 silent periods elicited with a MagStim double cone coil while the participant dorsiflexed at 15% of their maximal contraction. The TMS pulse was delivered at time = 0 ms, and the onset onset, offset, and maximum depth are indicated by green, red, and purple points, respectively.
In comparison to upper extremity muscles, lower extremity muscles are controlled by larger corticospinal neurons with higher activation thresholds (Smith et al., 2017). Axon orientations also make these neurons more difficult to stimulate trans-synaptically (Groppa et al., 2012). Further, the longer central conduction distance for leg muscles results in less optimal summation of the descending volley, making it more difficult to elicit lower limb MEPs (Groppa et al., 2012). Together, these factors make it more difficult to elicit lower limb MEPs compared to upper limb muscles; higher stimulation intensities are necessary (Smith et al., 2017). Typically, specialized coils (e.g., double cone or angled butterfly; Section 3.1.1) are needed to target these deeper cortical regions.
6.2. Localization of Lower Extremity Muscles
When targeting lower limb muscles with TMS, it may be difficult compared to the upper limbs to find the hotspot for the muscle of interest. The primary motor cortex representations of the lower extremity muscles are within close physical proximity and overlap, which makes it difficult to stimulate only one muscle at a time (Kesar et al., 2018; Fig. 6). This is not a major concern for testing cSPs. However, during cSP testing, the TMS pulses may cause simultaneous activation of corticospinal neurons that innervate agonist, antagonist, and synergist muscles, which could make it more difficult for participants to sustain a tonic lower extremity contraction during a cSP trial (Kesar et al., 2018).
The close anatomical proximity of the left and right leg motor cortical representations is of greater concern for testing iSPs. When attempting to elicit leg iSPs, it can be difficult to position the TMS coil in a way that avoids superficial current spread and induces only a unilateral response. To our knowledge, there is only one study that has reported iSPs in the lower limbs (Lo & Fook-Chong, 2004). This group used a circular coil. While we have successfully elicited cSPs in the tibialis anterior leg muscle using a double cone coil, we have not been able to elicit iSPs using a double cone coil in this muscle (Fig. 6C). We suspect that the double cone coil stimulation is not focal enough and reaches the bilateral motor cortical representation, causing a “weak” cSP in the target muscle which covers up any iSP that may have occurred (Fig. 6C). We have found that such stimulation elicits a silent period far too long to be an iSP, as well as a large iMEP, which suggests that a cSP, not an iSP, has occurred (Fig. 6C). Thus, we do not recommend using a double cone coil for testing iSPs in the lower limbs. We recommend that future lower limb iSP work attempt to replicate the (Lo & Fook-Chong, 2004) study with a circular coil. If positioned optimally, the medial side of a circular coil could potentially be used to stimulate only the leg representation better than a double cone coil (although the lateral side of the circular coil may cause concurrent stimulation of more lateral motor representations, such as arm and hand areas). Despite this, since Lo and Fook-Chong (2004) supposedly elicited lower limb iSPs with a circular coil and recommended a circular over a double cone coil for this purpose, we suggest that future investigators consider using a circular coil for eliciting iSPs in the lower limbs. Future studies may also wish to instead implement a MagVenture angled butterfly coil for eliciting iSPs in the leg muscles, as the angled butterfly coil can stimulate at increased depths but with more focality than the circular or double cone coils.
Our other primary recommendation for testing lower limb silent periods is to record from multiple muscles. As demonstrated in Fig. 2 in Kesar et al. 2018 and in Fig. 7, recording from multiple muscles will allow for demonstration that you have found the motor hotspot to the best of your ability for the lower limb muscle of interest. Particularly a double cone coil will likely induce activity in multiple muscles; however, it is possible to localize a spot that elicits the best response in the muscle of interest and only minimal activity in other muscles. We recommend using as many EMG channels as possible—ideally at least four channels—to confirm the hotspot location. Four EMG channels allows for recording of the target muscle, the homologous contralateral muscle, and two control muscles.
Fig. 7. EMG recording from multiple muscles.
Here we depict the EMG trace during five MEP trials in the bilateral tibialis anterior and bilateral medial gastrocnemius muscles. These MEPs were elicited with a double cone coil and stimulation intensity set at the subject’s resting motor threshold. In the top panel, the average MEP is plotted in red; this panel depicts the target (ON) muscle. In the bottom three panels, the average MEP is plotted in blue; these panels depict the non-targeted (OFF) muscles. Here, the EMG traces reveal clear MEPs in ON muscle, and some, but generally minimal EMG signal in OFF muscles. These EMG traces highlight the importance of recording from multiple muscles when identifying the motor hotspot, especially for lower limb muscles.
7. Recommendations for Future Reporting and Work
Based on our review of the literature, we have compiled our list of best practices for silent period experiments (Table 4). Additionally, we report power analyses in Table 5 for the aging studies detailed in Table 3. Table 5 suggests that between 2–33,484 per group is required to observe age differences in cSP duration at 0.80 power and alpha p < 0.05. Table 5 serves an example for future work (which, if possible, should justify sample size using a power analysis). Following the comprehensive guidelines outlined in Table 4 and adequately powering studies will increase reproducibility of silent period experiments, especially as future work applies this technique to clinical populations and moves towards more silent period experiments in the lower limbs.
Table 4.
Recommendations of best practices for reporting silent period methods.
| Variables to Report | Details to Report |
|---|---|
| 1. Study design | |
| Sample size | |
| Power analysis or other sample size justification | • Full details on power analyis used to determine sample size (e.g., details on past work or pilot study used to calculate power, statistical program used for power calculation, and thresholds (alpha level, power) used for calculation |
| • If a power analysis was not used, provide details regarding how the study sample size was determined | |
| 2. Hardware | |
| Coil | |
| Coil type | • Brand, size, and type (e.g., Magstim, 70 mm, double cone coil) |
| Coil orientation | • Direction of current flow (e.g., posterior to anterior) |
| • Orientation of the coil with respect to participant head (e.g., 45-degree angle from the midline) | |
| • Whether the coil orientation was individualized for each participant or kept standard between participants. If the orientation was individualized for each participant, provide a clear description of the process for determining the optimal orientation | |
| Stimulator | |
| Details on TMS unit | • Brand and model (e.g., MagVenture, MagPro X100) |
| Navigation (if applicable) | |
| Details on set-up | • Brand and model of the navigation system (e.g., Rogue Research Brainsight), details regarding the set-up of the participant for navigation and calibration of the navigation system |
| Structural MRI details, if collected | • Brand and model of the MRI scanner (e.g., 3T Siemens MAGNETOM Prisma), MRI scan parameters, time (e.g, # of days) between the MRI scan and TMS session |
| EMG | |
| Details on EMG unit | • Brand and model (e.g., BIOPAC Systems Inc., EMG100C) |
| In-unit analog filtering | • Analog high and low-pass filtering settings, if applicable |
| Sampling rate | • Sampling rate used for data collection in Hz; indication of whether the EMG data were down-sampled for offline analyses |
| Electrodes | |
| Details on electrodes used | • Brand, model, size, whether or not cables were shielded (e.g., BIOPAC Systems Inc., Ag/AgCl electrodes: 11 mm diameter, 95 mm2 conductive contact area; shielded cables were used for the recording electrodes; an unshielded cable was used for the ground electrode) |
| Skin preparation | • All skin preparation steps (e.g., hair shaving procedures, abrasive gel used, taping of electrode wires into loops and/or to avoid wires touching, etc.) |
| Electrode locations | • Figure indicating placements of all electrodes, including the ground electrode if applicable |
| Head Cap (if applicable) | |
| Details on cap used for head | • Whether or not a cap was used; if so, note brand, sizing, and material (e.g., cotton vs. lycra) |
| 3. Hotspot and RMT Determination | |
| Motor Hotspot | |
| Procedures for localizing the motor hotspot | • How the starting point for hotspot testing was determined (e.g., by measuring X cm anterior/posterior and X cm lateral from the vertex) • How the locations for subsequent stimulations were determined to ensure that an optimal hotspot location was found (e.g., by testing 3–5 MEPs at 1 mm anterior, posterior, medial, and lateral to the starting spot) |
| Number of stimulations required to find the motor hotspot | • # of TMS pulses delivered |
| Indication of whether a visible twitch was seen in the target muscle | • # of participants for whom a muscle twitch was visible in the appropriate ON muscle in response to TMS stimulations at the motor hotspot |
| Multiple EMG channels showing clear localization of hotspotb | • EMG trace for an exemplar subject (Fig. 7) to demonstrate that the hotspot showed a clear MEP in the ON muscle, no activity in the contralateral OFF muscle, and minimal or no activity in other muscles with nearby motor cortical representations |
| Location of the motor hotspot, reported as raw values and normalized to head sizea | • Raw values: (1) Distance anterior or posterior to Cz (2) Distance lateral from Cz • Normalized to head size: (1) [(Anterior/posterior distance from Cz) / (Total nasion to inion distance)] × 100% (2) [(Lateral distance from Cz) / (Total tragus to tragus distance)] × 100% |
| RMT | |
| Method used | • See Table 1 for descriptions of common RMT identification methods |
| Statistics for participant RMT values | • Mean, standard deviation, and range of RMT values across participants |
| Number of stimulations required to identify the RMT | • Mean, standard deviation, and range for number of TMS pulses required to identify the RMT across participants |
| 4. Silent Period Trials | |
| Maximal Force Testing | |
| Method for obtaining the target muscle MVC | • Description of force recording device, visual feedback (if provided), and the method used for obtaining MVCs (e.g., total number of MVC trials collected, duration of the rest period between trials) |
| TMS Parameters | |
| Intensity of stimulator output for silent period trials | • % of RMT used and justification for selection. We recommend using 120% RMT for cSP trials and 130% RMT for iSP trials |
| Total number of TMS pulses | • Total number of TMS pulses per trial • Mean and standard deviation if the number of TMS pulses was different between participants |
| Interstimulus interval between subsequent TMS pulses | • Interstimulus interval (s) |
| Length of each trial and number of trials | • Total length (s) of each trial, total number of trials, and ordering of trials • Description of counterbalancing of different trial conditions between subjects |
| Hemisphere(s) tested | • Hemisphere(s) tested, whether hemisphere was on dominant or non-dominant side (based on dominance of muscle being tested), what measures were taken to determine dominance (e.g., Edinburgh Handedness Inventory or Waterloo Footedness Questionnaire), breakdown of dominance across the sample (e.g., was TMS applied to the left hemisphere for all participants, or to the dominant hemisphere for all participants?) |
| Force Task and Feedback | |
| % of MVC used for force contraction during silent period trials | • % of MVC used for force goal (and justification for this choice) |
| Method for providing subject feedback on force production | • Complete description of the force task and the hardware/software used to collect force data • Specifications of feedback given to the participant to maintain desired force level (e.g., visual feedback) |
| Participant Withdrawal | |
| Number of subjects who withdrew because of their inability to tolerate TMS | • Whether any subjects could not tolerate the stimulation intensity and/or number of stimulations during the experiment and consequently terminated testing early |
| 5. Post-Processing of Data | |
| Filtering of EMG | • Bandpass filtering cut-off values (e.g., 1–2,000 Hz). Information on any other filters applied to the EMG data |
| Rectifying of EMG | • Whether the EMG data were rectified (i.e., absolute value taken) and if silent period metrics were calculated using the rectified or un-rectified EMG data |
| 6. Silent Period Analysis | |
| Method for identifying silent period onsets and offsets | • See Table 1 for a list of common approaches for identifying silent period onsets and offset times |
| Definitions for all silent period metrics calculated | • See Table 1 for details regarding common silent period outcome metrics for cSPs and iSPs. We recommend reporting the metrics used and a clear definition (and formula, if applicable) used for calculating these metrics. As the majority of silent period studies report only duration, we recommend calculating other metrics in addition to duration (e.g., normalized area and depth) and testing whether and how each of these metrics relate |
| Removal of silent period trials | • Whether any silent period trials were removed. If so, report the number of subjects with removed trials and the mean percentage and standard deviation of removed trials across participants |
| 7. Quality Controls | |
| EMG and/or Force Variability; Force Accuracy | • Variability metric (e.g., coefficient of variation) of the background EMG of the target muscle and/or of the force output for a defined time period pre- and post-TMS pulses • Investigators may also wish to report force accuracy (e.g., root mean square error) around the target force level |
| EMG in the OFF Muscle | • Any quality control approaches for minimizing muscle activity in the OFF muscle during silent period trials (e.g., visual or auditory feedback to the participant of the OFF muscle EMG activity) • Any methods for quantifying EMG activity in the OFF muscle (e.g., calculation of motor overflow in the OFF muscle) |
| Muscle Fatigue | • Any subjective (e.g., participant report) or objective methods for recording participant fatigue throughout silent period trails. Objective methods for quantifying fatigue could include examining whether the mean EMG activity (e.g., root mean square of the EMG) changed over the course of the trial and whether the participants were able to maintain the desired force throughout the trial |
| Secondary Inhibition Period | • Whether secondary inhibition periods occurred; if so, in what percentage of trials secondary inhibition periods occurred • If applicable, report the percentage of trials in which the secondary inhibition period merged with the silent period and how this was handled when calculating the average silent period for each participant |
| iMEPs | • Whether iMEPs occurred during iSP trials (e.g., percentage of trials per participant where iMEPs were evident) • Whether iMEP presence associated with any iSP outcome metrics (e.g., if the presence of an iMEP associated with longer iSP duration) to assess how iMEP presence may have affected iSP outcomes |
| Breakthrough EMG | • Information regarding any breakthrough EMG activity and how this was handled in calculating silent period outcome measures Report the percentage of trials per participant that included EMG breakthrough. Report whether automated analysis methods characterized breakthrough activity as a silent period offset / how this was handled. Report whether any breakthrough activity was removed or included in silent period area or depth measures |
The location of motor hotspot should be reported as the peak location of stimulation; that is, reporting should take into account the distance from the edge to the center of the coil.
Ideally EMG for four or more muscles should be recorded. This includes the ON and OFF muscles and at least two “control” muscles with motor cortical representations located near to the ON muscle, in order to demonstrate specificity of the identified hotspot
Table 5.
Power analyses based on cSP aging studies
| Study | YA n | OA n | YA mean cSP duration (ms) ± SD | OA mean cSP duration (ms) ± SD | Effect Size | Estimated Sample Sizea |
|---|---|---|---|---|---|---|
| Studies reporting reduced cSP duration in older age | ||||||
| (Beynel et al., 2014)b | 20 | 19 | 76.1 ± 4.0 | 54.7 ± 1.4 | 7.14 | n = 2 / group |
| 76.1 ± 17.9 | 54.7 ± 6.1 | 1.60 | n = 8 / group | |||
| (Davidson & Tremblay, 2013): Right Hand | 13 | 17 | 141.5 ± 34.8 | 115.9 ± 24.2 | 0.85 | n = 23 / group |
| (Davidson & Tremblay, 2013): Left Hand | 148.9 ± 37.9 | 115.3 ± 26.0 | 1.03 | n = 16 / group | ||
| (Oliverio et al., 2006) | 20 | 22 | 147 ± 39 | 87 ± 29 | 1.75 | n = 7 / group |
| (Sale & Semmler, 2005)c | 10 | 10 | 173.9 ± 9.5 | 150.3 ± 9.2 | 2.52 | n = 4 / group |
| Studies reporting no difference in cSP duration with older age | ||||||
| (Fujiyama et al., 2009)c,d: Baseline Tonic | 15 | 15 | 150.08 ± 19.25 | 150.58 ± 26.38 | 0.02 | n = 33,484 / group |
| (Fujiyama et al., 2009)c,d: Baseline Phasic | 132.55 ± 26.30 | 139.47 ± 33.69 | 0.23 | n = 301 / group | ||
| (Fujiyama et al., 2012)e | 15 | 15 | 140.47 ± 17.20 | 134.16 ± 12.38 | 0.42 | n = 90 / group |
| Studies reporting increased cSP duration in older age | ||||||
| (McGinley et al., 2010)b | 21 | 9 | 112.5 ± 6.5 | 84 ± 3.9 | 5.32 | n = 3 / group |
| 112.5 ± 29.8 | 84 ± 11.7 | 1.26 | n = 11 / group | |||
Table 5 Note. Here we present power analyses for each of the studies detailed in Table 3. We intend for this table to provide an example for estimating sample size for future studies. When possible, future studies should use past work or pilot data to calcluate power and provide sample size justifcation.
We used G*Power 3.1 (Faul et al., 2007) to estimate sample size for future work using the effect size of young vs. older adult differences in cSP duration from the studies listed in Table 3. We used the following G*Power parameters for these sample size estimations: t-test, difference between two independent means (two groups), tails = two, alpha = 0.05, power = 0.80, allocation ratio N2/N1 = 1
In these cases (Beynel et al., 2014; McGinley et al., 2010), we include two estimations of sample size because it was unclear whether the reported errors represent standard deviation or standard error. For these cases, the top row assumes that the errors reported in text represent standard deviations. The bottom row assumes that the errors reported in text represent standard errors; here we used the standard errors to calculate the standard deviations using the formula: SD = (standard error) * sqrt(group size)
In these cases (Sale & Semmler, 2005; Fujiyama et al., 2009) the authors reported standard error instead of standard deviation. We have thus used standard error to calculate standard deviation using the formula described in footnote b
Here (Fujiyama et al., 2009) we report estimated sample size for only the two baseline conditions (i.e., tonic and phasic contractions of the extensor carpi radialis muscle), for which the authors identified no differences in silent period duration between young and older adults. Of note, the primary goal of this study was to compare silent period duration between coordination conditions for each age group, and not to compare young versus older adult cSP duration differences for the baseline conditions
Here (Fujiyama et al., 2012) we report estimated sample size for only two baseline condition, for which the authors identified no differences in silent period duration between young and older adults. Of note, similar to Fujiyama et al. (2009), the primary goal of this study was to compare silent period duration between coordination conditions for each age group, and not to compare young versus older adult cSP duration differences for the baseline condition. The authors provided the 95% confidence interval instead of the standard deviation; thus, we calculated the standard deviation as follows: SD = sqrt(group size)*[(95% confidence interval)/4.29]. The divisor 4.29 was calcuated using the sample size for each group (n = 15) and the Excel formula: =(TINV(1-0.95,15-1))*2
Highlights.
Muscle silent periods provide a valuable in vivo measurement of cortical inhibitory function in the human brain and can be leveraged to characterize how advancing age and disease impact the cortical control of movement.
Past silent period studies have implemented varying methodology, including subjective analyses and lack of detail in methods descriptions, limiting comparison across studies and reproducibility.
Here, we review in detail the impact of methodological choices on silent period outcome measures, including considerations for the unique case of collecting lower limb silent periods.
We conclude with comprehensive recommendations to improve the consistency of data collection, analysis, and reporting in future silent period studies.
Acknowledgments
During completion of this work K.H. was supported by a National Science Foundation (NSF) Graduate Research Fellowship under Grant nos. DGE-1315138 and DGE1842473, as well as a training grant T32-NS082128 from the National Institute of Neurologic Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostini V, & Knaflitz M (2012). An algorithm for the estimation of the signal-to-noise ratio in surface myoelectric signals generated during cyclic movements. IEEE Transactions on Bio-Medical Engineering, 59(1), 219–225. [DOI] [PubMed] [Google Scholar]
- Awiszus F (2003). TMS and threshold hunting. Supplements to Clinical Neurophysiology, 56, 13–23. [DOI] [PubMed] [Google Scholar]
- Awiszus F (2011). Fast estimation of transcranial magnetic stimulation motor threshold: Is it safe? Brain Stimulation, 4(1), 58–59. [DOI] [PubMed] [Google Scholar]
- Badawy RAB, Tarletti R, Mula M, Varrasi C, & Cantello R (2011). The routine circular coil is reliable in paired-TMS studies. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 122(4), 784–788. [DOI] [PubMed] [Google Scholar]
- Bajbouj M, Gallinat J, Niehaus L, Lang UE, Roricht S, & Meyer B-U (2004). Abnormalities of inhibitory neuronal mechanisms in the motor cortex of patients with schizophrenia. Pharmacopsychiatry, 37(02), 74–80. [DOI] [PubMed] [Google Scholar]
- Balslev D, Braet W, McAllister C, & Miall RC (2007). Inter-individual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. Journal of Neuroscience Methods, 162(1–2), 309–313. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, & Freeston IL (1985). Non-invasive magnetic stimulation of human motor cortex. The Lancet, 325(8437), 1106–1107. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, & Thickbroom GW (2006). Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: A central adaptation to fatigue? Experimental Brain Research, 170(2), 191–198. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Taylor SF, & Seidler RD (2011). Handedness, dexterity, and motor cortical representations. Journal of Neurophysiology, 105(1), 88–99. [DOI] [PubMed] [Google Scholar]
- Beynel L, Chauvin A, Guyader N, Harquel S, & Marendaz C (2014). Age-related changes in intracortical inhibition are mental-cognitive state-dependent. Biological Psychology, 101, 9–12. [DOI] [PubMed] [Google Scholar]
- Bhandari A, Radhu N, Farzan F, Mulsant BH, Rajji TK, Daskalakis ZJ, & Blumberger DM (2016). A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 127(8), 2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Hungs M, Mull M, Töpper R, & Noth J (1998). Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control, 109(3), 230–237. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, & Hallett M (1992). Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 9(1), 132–136. [PubMed] [Google Scholar]
- Brownstein CG, Ansdell P, Škarabot J, Howatson G, Goodall S, & Thomas K (2018). An optimal protocol for measurement of corticospinal excitability, short intracortical inhibition and intracortical facilitation in the rectus femoris. Journal of the Neurological Sciences, 394, 45–56. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, & Carvalho AF (2017). Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry, 74(2), 143–152. [DOI] [PubMed] [Google Scholar]
- Burke D, Wissel J, & Donnan GA (2013). Pathophysiology of spasticity in stroke. Neurology, 80(3), S20–S36. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Netz J, Seitz RJ, Schicks WT, & Hömberg V (2001). Remote cortical excitability changes after stroke. NeuroImage, 13(6), 1140.11352619 [Google Scholar]
- Butler JE, Petersen NC, Herbert RD, Gandevia SC, & Taylor JL (2012). Origin of the low-level EMG during the silent period following transcranial magnetic stimulation. Clinical Neurophysiology, 123(7), 1409–1414. [DOI] [PubMed] [Google Scholar]
- Cacchio A, Cimini N, Alosi P, Santilli V, & Marrelli A (2009). Reliability of transcranial magnetic stimulation-related measurements of tibialis anterior muscle in healthy subjects. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(2), 414–419. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, & Mutani R (1992). Magnetic brain stimulation: The silent period after the motor evoked potential. Neurology, 42(10), 1951–1951. [DOI] [PubMed] [Google Scholar]
- Carey JR, Allison JD, & Mundale MO (1983). Electromyographic study of muscular overflow during precision handgrip. Physical Therapy, 63(4), 505–511. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, & Ashby P (1999). Mechanism of the silent period following transcranial magnetic stimulation evidence from epidural recordings. Experimental Brain Research, 128(4), 539–542. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, & Li J-Y (2003). Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. Journal of Neurophysiology, 89(3), 1256–1264. [DOI] [PubMed] [Google Scholar]
- Chou Y-H, Hickey PT, Sundman M, Song AW, & Chen N-K (2015). Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurology, 72(4), 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincotta M, & Ziemann U (2008). Neurophysiology of unimanual motor control and mirror movements. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 119(4), 744–762. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, & Rothwell JC (2001). Transcranial Magnetic Stimulation Can Be Used to Test Connections to Primary Motor Areas from Frontal and Medial Cortex in Humans. NeuroImage, 14(6), 1444–1453. [DOI] [PubMed] [Google Scholar]
- Classen J, & Benecke R (1995). Inhibitory phenomena in individual motor units induced by transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control, 97(5), 264–274. [DOI] [PubMed] [Google Scholar]
- Cohen D, & Neil Cuffin B (1991). Developing a More Focal Magnetic Stimulator. Part I. Journal of Clinical Neurophysiology, 8(1), 102–111. [DOI] [PubMed] [Google Scholar]
- Coppi E, Houdayer E, Chieffo R, Spagnolo F, Inuggi A, Straffi L, Comi G, & Leocani L (2014). Age-related changes in motor cortical representation and interhemispheric interactions: A transcranial magnetic stimulation study. Frontiers in Aging Neuroscience, 6, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Thijs H, & Meesen RLJ (2014). Optimization of the transcranial magnetic stimulation protocol by defining a reliable estimate for corticospinal excitability. PloS One, 9(1), e86380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron LA, Dearth DJ, Hoffman RL, & Clark BC (2008). Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. Journal of Neuroscience Methods, 173(1), 121–128. [DOI] [PubMed] [Google Scholar]
- Davidson T (2016). The ipsilateral silent period as a measure of transcallosal inhibition: an investigation of individual and methodological factors influencing interhemispheric inhibition between motor cortices (Tremblay F (ed.)) [Doctor of Philosophy in Human Kinetics]. University of Ottawa. [Google Scholar]
- Davidson T, & Tremblay F (2013a). Age and hemispheric differences in transcallosal inhibition between motor cortices: An ispsilateral silent period study. BMC Neuroscience, 14(1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T, & Tremblay F (2013b). Hemispheric differences in corticospinal excitability and in transcallosal inhibition in relation to degree of handedness. PloS One, 8(7), e70286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z-D, Lisanby SH, & Peterchev AV (2013). Electric field depth–focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimulation, 6(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch KM, & Newell KM (2001). Age differences in noise and variability of isometric force production. Journal of Experimental Child Psychology, 80(4), 392–408. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, & Capaday C (1997). Input-output properties and gain changes in the human corticospinal pathway. Experimental Brain Research, 114(2), 329–338. [DOI] [PubMed] [Google Scholar]
- Dharmadasa T, Matamala JM, Howells J, Simon NG, Vucic S, & Kiernan MC (2019). The effect of coil type and limb dominance in the assessment of lower-limb motor cortex excitability using TMS. Neuroscience Letters, 699, 84–90. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, & Rothwell JC (2004). The physiological basis of transcranial motor cortex stimulation in conscious humans. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(2), 255–266. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, & Pilato F (2012). I-wave origin and modulation. Brain Stimulation, 5(4), 512–525. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Fried PJ, Davila-Pérexz P, Horvath JC, Rotenberg A & Pascual-Leone A (2018). A practical manual for transcranial magnetic stimulation. Intensive Course in Transcranial Magnetic Stimulation (TMS). Harvard Medical School, Berenson-Allen Center for Noninvasive Brain Stimulation. [Google Scholar]
- Enoka RM, & Duchateau J (2008). Muscle fatigue: What, why and how it influences muscle function. The Journal of Physiology, 586(1), 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, & Marsden CD (1992). Interhemispheric inhibition of the human motor cortex. The Journal of Physiology, 453(1), 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, DeCastella A, & Kulkarni J (2002). A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophrenia Research, 56(3), 199–209. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, & Daskalakis ZJ (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(12), 2584–2596. [DOI] [PubMed] [Google Scholar]
- Fling BW, & Seidler RD (2011). Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. Cerebral Cortex, 22(11), 2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, & Seidler RD (2012). Task-dependent effects of interhemispheric inhibition on motor control. Behavioural Brain Research, 226(1), 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Sandoval H, Fox SP, Kochunov P, Lancaster JL (2004). Column-based model of electric field excitation of cerebral cortex. Human Brain Mapping, 22, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz C, Braune HJ, Pylatiuk C, & Pohl M (1997). Silent period following transcranial magnetic stimulation: A study of intra- and inter-examiner reliability. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control, 105(3), 235–240. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, & Hallett M (1991). Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 81(4), 257–262. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Levin O, Swinnen SP, & Summers JJ (2009). Age-related differences in inhibitory processes during interlimb coordination. Brain Research, 1262, 38–47. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Garry MI, & Summers JJ (2012). Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiology of Aging, 33(7), 1484–e1. [DOI] [PubMed] [Google Scholar]
- Gagnon G, Schneider C, Grondin S, & Blanchet S (2011). Enhancement of episodic memory in young and healthy adults: A paired-pulse TMS study on encoding and retrieval performance. Neuroscience Letters, 488(2), 138–142. [DOI] [PubMed] [Google Scholar]
- Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, Marcolin MA, Bouhassira D, Teixeira MJ, & de Andrade DC (2015). Repetitive transcranial magnetic stimulation in chronic pain: A review of the literature. Archives of Physical Medicine and Rehabilitation, 96(4), S156–S172. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Becker DA, Barker CA, & Bartko JJ (2001). New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 112(8), 1451–1460. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, & Ziemann U (2009). Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. The Journal of Physiology, 587(22), 5393–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, & Ridding MC (2016). Minimum number of trials required for within-and between-session reliability of TMS measures of corticospinal excitability. Neuroscience, 320, 205–209. [DOI] [PubMed] [Google Scholar]
- Gomez-Tames J, Hamasaka A, Laakso I, Hirata A, & Ugawa Y (2018). Atlas of optimal coil orientation and position for TMS: A computational study. Brain Stimulation, 11(4), 839–848. [DOI] [PubMed] [Google Scholar]
- Goodall S, Howatson G, & Thomas K (2018). Modulation of specific inhibitory networks in fatigued locomotor muscles of healthy males. Experimental Brain Research, 236(2), 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Ross EZ, & Romer LM (2010). Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee-extensor contractions. Journal of Applied Physiology, 109(6), 1842–1851. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, & Thickbroom GW (2012). A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 123(5), 858–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand BJ, Opie GM, Sidhu SK, & Semmler JG (2020). TMS coil orientation and muscle activation influence lower limb intracortical excitability. Brain Research, 147027. [DOI] [PubMed] [Google Scholar]
- Harquel S, Bécu M, Beynel L, Chauvin A, Guyader N, & Marendaz M (2013). CortExTool: A signal processing toolbox for cortical excitability by transcranial magnetic stimulation.
- Heinen F, Glocker F, Fietzek U, Meyer B, Lücking C, & Korinthenberg R (1998). Absence of transcallosal inhibition following focal mangnetic stimulation in preschool children. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 43(5), 608–612. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, & Murray NM (1986). Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neuroscience Letters, 71(2), 235–240. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, & Murray NM (1987). Responses in small hand muscles from magnetic stimulation of the human brain. The Journal of Physiology, 388(1), 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren H, Larsson LE, & Pedersen S (1990). Late muscular responses to transcranial cortical stimulation in man. Electroencephalography and Clinical Neurophysiology, 75(3), 161–172. [DOI] [PubMed] [Google Scholar]
- Höppner J, Kunesch E, Buchmann J, Hess A, Groβmann A, & Benecke R (1999). Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 110(4), 748–756. [DOI] [PubMed] [Google Scholar]
- Höppner J, Kunesch E, Großmann A, Tolzin C, Schulz M, Schläfke D, & Ernst K (2001). Dysfunction of transcallosally mediated motor inhibition and callosal morphology in patients with schizophrenia. Acta Psychiatrica Scandinavica, 104(3), 227–235. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Todd G, Butler JE, Gandevia SC, & Taylor JL (2008). Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. Journal of Applied Physiology, 105(4), 1199–1209. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, & Manfredi M (1993). Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. The Journal of Physiology, 466(1), 521–534. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, & Manfredi M (1996). Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Experimental Brain Research, 109(3), 467–472. [DOI] [PubMed] [Google Scholar]
- Jackson N, & Greenhouse I (2019). VETA: An Open-Source Matlab-Based Toolbox for the Collection and Analysis of Electromyography Combined With Transcranial Magnetic Stimulation. Frontiers in Neuroscience, 13, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, & Ziemann U (2006). Differences of the ipsilateral silent period in small hand muscles. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 34(4), 431–436. [DOI] [PubMed] [Google Scholar]
- Kennedy DS, McNeil CJ, Gandevia SC, & Taylor JL (2016). Effects of fatigue on corticospinal excitability of the human knee extensors. Experimental Physiology, 101(12), 1552–1564. [DOI] [PubMed] [Google Scholar]
- Kesar TM, Stinear JW, & Wolf SL (2018). The use of transcranial magnetic stimulation to evaluate cortical excitability of lower limb musculature: Challenges and opportunities. Restorative Neurology and Neuroscience, 36(3), 333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, Kazis A, & Mills KR (2006). Lorazepam-induced effects on silent period and corticomotor excitability. Experimental Brain Research, 173(4), 603–611. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Sotirakoglou K, Kazis DA, Kazis A, & Mills KR (2005). Silent period to transcranial magnetic stimulation: Construction and properties of stimulus–response curves in healthy volunteers. Experimental Brain Research, 163(1), 21–31. [DOI] [PubMed] [Google Scholar]
- Koerte I, Heinen F, Fuchs T, Laubender RP, Pomschar A, Stahl R, Berweck S, Winkler P, Hufschmidt A, & Reiser MF (2009). Anisotropy of callosal motor fibers in combination with transcranial magnetic stimulation in the course of motor development. Investigative Radiology, 44(5), 279–284. [DOI] [PubMed] [Google Scholar]
- Kuo Y-L, Dubuc T, Boufadel DF, & Fisher BE (2017). Measuring ipsilateral silent period: Effects of muscle contraction levels and quantification methods. Brain Research, 1674, 77–83. [DOI] [PubMed] [Google Scholar]
- Laakso I, Hirata A, & Ugawa Y (2014). Effects of coil orientation on the electric field induced by TMS over the hand motor area. Physics in Medicine and Biology, 59(1), 203–218. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P, Caramia F, Inghilleri M, Pozzilli C, & Pantano P (2007). Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: A functional and anatomical study. Human Brain Mapping, 28(7), 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, & Summers JJ (2014). Aging and motor inhibition: A converging perspective provided by brain stimulation and imaging approaches. Neuroscience and Biobehavioral Reviews, 43, 100–117. [DOI] [PubMed] [Google Scholar]
- Li J-Y, Lai P-H, & Chen R (2012). Transcallosal inhibition in patients with callosal infarction. Journal of Neurophysiology, 109(3), 659–665. [DOI] [PubMed] [Google Scholar]
- Li S, & Francisco GE (2015). New insights into the pathophysiology of post-stroke spasticity. Frontiers in Human Neuroscience, 9, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lixandrão MC, Stinear JW, Rich T, Chen C-Y, Feyma T, Meekins GD, & Gillick BT (2020). EMG breakthrough during cortical silent period in congenital hemiparesis: a descriptive case series. Brazilian Journal of Physical Therapy, 24(1), 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YL, & Fook-Chong S (2004). A transcranial magnetic stimulation study of the ipsilateral silent period in lower limb muscles. Neuroscience Letters, 368(3), 337–340. [DOI] [PubMed] [Google Scholar]
- Luki w (2020). EMG_SignalToNoiseRatio (https://www.mathworks.com/matlabcentral/fileexchange/61830-emg_signaltonoiseratio), MATLAB Central File Exchange. Retrieved August 9, 2020.
- Madhavan S, Rogers LM, & Stinear JW (2010). A paradox: After stroke, the nonlesioned lower limb motor cortex may be maladaptive. The European Journal of Neuroscience, 32(6), 1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major BP, Rogers MA, & Pearce AJ (2015). Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: a systematic review. Clinical and Experimental Pharmacology and Physiology, 42(4), 394–405. [DOI] [PubMed] [Google Scholar]
- Mathis J, de Quervain D, & Hess CW (1998). Dependence of the transcranially induced silent period on the ìnstruction set’ and the individual reaction time. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control, 109(5), 426–435. [DOI] [PubMed] [Google Scholar]
- Mathis J, de Quervain D, & Hess CW (1999). Task-dependent effects on motor-evoked potentials and on the following silent period. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 16(6), 556–565. [DOI] [PubMed] [Google Scholar]
- Matsugi A (2019). Changes in the cortical silent period during force control. Somatosensory & Motor Research, 36(1), 8–13. [DOI] [PubMed] [Google Scholar]
- McGinley M, Hoffman RL, Russ DW, Thomas JS, & Clark BC (2010). Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Experimental Gerontology, 45(9), 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor KM, Nocera JR, Sudhyadhom A, Patten C, Manini T, Kleim JA, Crosson B, & Butler AJ (2013). Effects of aerobic fitness on aging-related changes of interhemispheric inhibition and motor performance. Frontiers in Aging Neuroscience, 5, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor KM, Zlatar Z, Kleim E, Sudhyadhom A, Bauer A, Phan S, Seeds L, Ford A, Manini TM, & White KD (2011). Physical activity and neural correlates of aging: a combined TMS/fMRI study. Behavioural Brain Research, 222(1), 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay WB, Stokic DS, Sherwood AM, Vrbova G, & Dimitrijevic MR (1996). Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle & Nerve, 19(8), 1017–1024. [DOI] [PubMed] [Google Scholar]
- Merletti R, & Migliorini M (1998). Surface EMG electrode noise and contact impedance.
- Mesin L, Merletti R, & Rainoldi A (2009). Surface EMG: the issue of electrode location. Journal of Electromyography and Kinesiology: Official Journal of the International Society of Electrophysiological Kinesiology, 19(5), 719–726. [DOI] [PubMed] [Google Scholar]
- Meyer B, Röricht S, & Woiciechowsky C (1998). Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Annals of Neurology, 43(3), 360–369. [DOI] [PubMed] [Google Scholar]
- Meyer B-U, Röricht S, Von Einsiedel HG, Kruggel F, & Weindl A (1995). Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain: A Journal of Neurology, 118(2), 429–440. [DOI] [PubMed] [Google Scholar]
- Mills KR, & Nithi KA (1997). Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 20(5), 570–576. [DOI] [PubMed] [Google Scholar]
- Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, Stroud Z, Nahas Z, & George MS (2004). The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. The Journal of ECT, 20(3), 160–165. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Fong M, Murphy BA, & Sinkjaer T (2007). Changes in excitability of the cortical projections to the human tibialis anterior after paired associative stimulation. Journal of Neurophysiology, 97(3), 1951–1958. [DOI] [PubMed] [Google Scholar]
- Niehaus L, Meyer BU, Weyh T (2000). Influence of pulse configuration and direction of coil current on excitatory effects of magnetic motor cortex and nerve stimulation. Clinical Neurophysiology, 111, 75–80. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Panizza M, & Arieti P (1997). Computer-aided determination of the silent period. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 14(2), 136–143. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, & Di Lazzaro V (2006). Effects of aging on motor cortex excitability. Neuroscience Research, 55(1), 74–77. [DOI] [PubMed] [Google Scholar]
- Oozumi T, Ito Y, Tsuji S, & Murai Y (1992). Inhibitory period following motor potentials evoked by magnetic cortical stimulation. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 85(4), 273–279. [DOI] [PubMed] [Google Scholar]
- Orth M, & Rothwell JC (2004). The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(5), 1076–1082. [DOI] [PubMed] [Google Scholar]
- Perez MA, Butler JE, & Taylor JL (2014). Modulation of transcallosal inhibition by bilateral activation of agonist and antagonist proximal arm muscles. Journal of Neurophysiology, 111(2), 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean M, & Ko JYL (2013). An age-related change in the ipsilateral silent period of a small hand muscle. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 124(2), 346–353. [DOI] [PubMed] [Google Scholar]
- Pfadt A, & Wheeler DJ (1995). Using statistical process control to make data-based clinical decisions. Journal of Applied Behavior Analysis, 28(3), 349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Accornero N, & Manfredi M (1994). Motor cortical inhibition and the dopaminergic system: pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain: A Journal of Neurology, 117(2), 317–323. [DOI] [PubMed] [Google Scholar]
- Qi F, Wu AD, & Schweighofer N (2011). Fast estimation of transcranial magnetic stimulation motor threshold. Brain Stimulation, 4(1), 50–57. [DOI] [PubMed] [Google Scholar]
- Richter L, Neumann G, Oung S, Schweikard A, & Trillenberg P (2013). Optimal coil orientation for transcranial magnetic stimulation. PloS One, 8(4), e60358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roick H, Von Giesen HJ, & Benecke R (1993). On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Experimental Brain Research, 94(3), 489–498. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, & Lücking CH (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91(2), 79–92. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, ... & Hallett M (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clinical Neurophysiology, 126(6), 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Horvath JC, & Pascual-Leone A (Eds.). (2014). Transcranial magnetic stimulation. New York, NY: Springer. [Google Scholar]
- Säisänen L, Pirinen E, Teitti S, Könönen M, Julkunen P, Määttä S, & Karhu J (2008). Factors influencing cortical silent period: optimized stimulus location, intensity and muscle contraction. Journal of Neuroscience Methods, 169(1), 231–238. [DOI] [PubMed] [Google Scholar]
- Sale MV, & Semmler JG (2005). Age-related differences in corticospinal control during functional isometric contractions in left and right hands. Journal of Applied Physiology, 99(4), 1483–1493. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Schmaußer M, Klinger F, Kreuzer PM, Krenkel L, & Langguth B (2020). Resting motor threshold and magnetic field output of the figure-of-8 and the double-cone coil. Scientific Reports, 10(1), 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippling S, Schneider SA, Bhatia KP, Münchau A, Rothwell JC, Tabrizi SJ, & Orth M (2009). Abnormal Motor Cortex Excitability in Preclinical and Very Early Huntington’s Disease. Biological Psychiatry, 65(11), 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Niehaus L, Röricht S, & Meyer B-U (2000). Conduction deficits of callosal fibres in early multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 68(5), 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, & Benecke R (1994). The silent period after transcranial magnetic stimulation is of exclusive cortical origin: Evidence from isolated cortical ischemic lesions in man. Neuroscience Letters, 180(1), 41–45. [DOI] [PubMed] [Google Scholar]
- Sheridan MR, & Flowers KA (1990). Movement variability and bradykinesia in Parkinson’s disease. Brain, 113(4), 1149–1161. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, & Conrad B (1998). Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine, 21(9), 1209–1212. [DOI] [PubMed] [Google Scholar]
- Silbert LC, Nelson C, Holman S, Eaton R, Oken BS, Lou JS, & Kaye JA (2006). Cortical excitability and age-related volumetric MRI changes. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(5), 1029–1036. [DOI] [PubMed] [Google Scholar]
- Smith M-C, Stinear JW, Alan Barber P, & Stinear CM (2017). Effects of non-target leg activation, TMS coil orientation, and limb dominance on lower limb motor cortex excitability. Brain Research, 1655, 10–16. [DOI] [PubMed] [Google Scholar]
- Sommer M, Alfaro A, Rummel M, Speck S, Lang N, Tings T, & Paulus W (2006). Half sine, monophasic and biphasic transcranial magnetic stimulation of the human motor cortex. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(4), 838–844. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, & Newell KM (2011). Aging and motor variability: A test of the neural noise hypothesis. Experimental Aging Research, 37(4), 377–397. [DOI] [PubMed] [Google Scholar]
- Spagnolo F, Coppi E, Chieffo R, Straffi L, Fichera M, Nuara A, Gonzalez-Rosa J, Martinelli V, Comi G, & Volontè MA (2013). Interhemispheric balance in Parkinson’s disease: a transcranial magnetic stimulation study. Brain Stimulation, 6(6), 892–897. [DOI] [PubMed] [Google Scholar]
- Stegeman D, & Hermens H (2007). Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede: Roessingh Research and Development, 108–112. [Google Scholar]
- Stĕtkárová I, Leis AA, Stokić DS, Delapasse JS, & Tarkka IM (1994). Characteristics of the silent period after transcranial magnetic stimulation. American Journal of Physical Medicine & Rehabilitation / Association of Academic Physiatrists, 73(2), 98–102. [DOI] [PubMed] [Google Scholar]
- Strauss S, Lotze M, Flöel A, Domin M, & Grothe M (2019). Changes in Interhemispheric Motor Connectivity Across the Lifespan: A Combined TMS and DTI Study. Frontiers in Aging Neuroscience, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CW, & Fling BW (2018). Associations between gait coordination, variability and motor cortex inhibition in young and older adults. Experimental Gerontology, 113, 163–172. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, & Gandevia SC (1997). Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Experimental Brain Research, 117(3), 472–478. [DOI] [PubMed] [Google Scholar]
- Tazoe T, Endoh T, Nakajima T, Sakamoto M, & Komiyama T (2007). Disinhibition of upper limb motor area by voluntary contraction of the lower limb muscle. Experimental Brain Research, 177(3), 419–430. [DOI] [PubMed] [Google Scholar]
- Terao Y, & Ugawa Y (2002). Basic mechanisms of TMS. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 19(4), 322–343. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Hanajima R, Machii K, Furubayashi T, Mochizuki H, Enomoto H, Shiio Y, Uesugi H, Iwata NK, & Kanazawa I (2000). Predominant activation of I1-waves from the leg motor area by transcranial magnetic stimulation. Brain Research, 859(1), 137–146. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Opitz A, Windhoff M (2011). Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. NeuroImage, 54, 234–243. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Farina S, Tamburin S, Facchini S, Fiaschi A, Restivo D, & Berardelli A (2003). Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Experimental Brain Research, 150(2), 222–229. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, & Gandevia SC (2004). Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. Journal of Applied Physiology, 97(1), 236–242. [DOI] [PubMed] [Google Scholar]
- Tranulis C, Guéguen B, Pham-Scottez A, Vacheron MN, Cabelguen G, Costantini A, Valero G, & Galinovski A (2006). Motor threshold in transcranial magnetic stimulation: comparison of three estimation methods. Neurophysiologie Clinique = Clinical Neurophysiology, 36(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, & Newell KM (2003). Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiology of Aging, 24(1), 25–35. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, & Newell KM (2002). Inter-digit Individuation and Force Variability in the Precision Grip of Young, Elderly, and Parkinson’s Disease Participants. Motor Control, 6(2), 113–128. [DOI] [PubMed] [Google Scholar]
- Vernillo G, Temesi J, Martin M, & Millet GY (2018). Mechanisms of Fatigue and Recovery in Upper versus Lower Limbs in Men. Medicine and Science in Sports and Exercise, 50(2), 334–343. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Fuhr P, Cohen LG, & Hallett M (1991). Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology, 41(11), 1795–1795. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, & Classen J (1999). Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. The Journal of Physiology, 517(2), 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SA, Lockwood RJ, Thickbroom GW, & Mastaglia FL (1993). The muscle silent period following transcranial magnetic cortical stimulation. Journal of the Neurological Sciences, 114(2), 216–222. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, & Pons TP (2007). Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. Neurorehabilitation and Neural Repair, 21(6), 568–573. [DOI] [PubMed] [Google Scholar]
- Yacyshyn AF, Woo EJ, Price MC, & McNeil CJ (2016). Motoneuron responsiveness to corticospinal tract stimulation during the silent period induced by transcranial magnetic stimulation. Experimental Brain Research, 234(12), 3457–3463. [DOI] [PubMed] [Google Scholar]
- Yoon T, Schlinder-Delap B, Keller ML, & Hunter SK (2012). Supraspinal fatigue impedes recovery from a low-intensity sustained contraction in old adults. Journal of Applied Physiology, 112(5), 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunokuchi K, & Cohen D (1991). Developing a More Focal Magnetic Stimulator. Part II. Journal of Clinical Neurophysiology, 8(1), 112–120. [PubMed] [Google Scholar]
- Ziemann Paulus, & Rothenberger. (1997). Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. The American Journal of Psychiatry, 154, 1277–1284. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Bruns D, & Paulus W (1996). Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: Evidence from transcranial magnetic stimulation. Neuroscience Letters, 208(3), 187–190. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, & Wassermann EM (1999). Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. The Journal of Physiology, 518(3), 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]