Abstract
Objective
To evaluate the clinical utility of the multianalyte assay panel (MAP), commercially known as AVISE Lupus test (Exagen Inc.), in patients suspected of SLE.
Methods
A systematic review of medical records of ANA-positive patients with a positive (>0.1) or negative (<−0.1) MAP score was conducted when the MAP was ordered (T0), when the test results were reviewed (T1) and at a later time (T2, ≥8 months after T1). Confidence in the diagnosis of SLE and initiation of hydroxychloroquine (HCQ) were assessed.
Results
A total of 161 patient records from 12 centres were reviewed at T0 and T1. T2 occurred for 90 patients. At T0, low, moderate and high confidence in SLE diagnosis was reported for 58%, 30% and 12% patients, respectively. Confidence in SLE diagnosis increased for the MAP positive, while MAP negative made SLE less likely. Odds of higher confidence in SLE diagnosis increased by 1.74-fold for every unit of increase of the MAP score (p<0.001). Using the MAP-negative/anti-double-stranded DNA-negative patients as reference, the HR of assigning an International Classification of Diseases, Tenth Revision lupus code was 7.02-fold, 11.2-fold and 14.8-fold higher in the low tier-2, high tier-2 and tier-1 positive, respectively (p<0.001). The HR of initiating HCQ therapy after T0 was 2.90-fold, 4.22-fold and 3.98-fold higher, respectively (p<0.001).
Conclusion
The MAP helps increase the confidence in ruling-in and ruling-out SLE in patients suspected of the disease and informs on appropriate treatment decisions.
Keywords: biological products, lupus erythematosus, systemic, antirheumatic agents
Key messages.
What is already known about this subject?
The present study expands on previous data that demonstrated the clinical utility of a multianalyte assay panel (MAP, AVISE®) and shows that the results of the MAP test have three impacts on physician behavior, which are outlined below.
What does this study add?
Physician confidence in systemic lupus erythematosus (SLE) diagnosis increased with increasing test scores, and this was paralleled by a higher hazard of assigning a lupus ICD-10 code (M32) as the MAP score increased. A positive MAP was superior to anti-dsDNA antibodies in increasing the confidence in ruling-in SLE.
The test also increased the confidence in ruling-out SLE, as only 3 MAP negative patients (5%) were assigned a lupus ICD-10 code during the study, indicating a 95% probability of excluding the SLE diagnosis in MAP negative patients. In addition, more anti-dsDNA negative than MAP negative were at risk of M32 assignment, indicating that a negative MAP was superior to negative anti-dsDNA antibodies in excluding an SLE code.
The test also informed appropriate treatment decisions, as the hazard ratio of initiating hydroxychloroquine (HCQ) therapy after T0 increased with increasing MAP score.
How might this impact on clinical practice?
By systematically reviewing medical records of a large number of patients suspected of SLE this study builds on previous evidence that the results of the MAP test impact physician behavior by facilitating an SLE diagnosis in patients suspected of the disease and by guiding treatment decisions.
Introduction
The heterogeneity of the early signs and symptoms of SLE, which are often similar to those of other autoimmune and non-autoimmune diseases such as fibromyalgia,1–3 along with the low incidence and prevalence of the disease,4–6 makes the diagnosis challenging. Another challenge relates to the evolutive nature of the disease and the fact that many patients have only limited characteristics of lupus at presentation. These patients may be designated as incomplete, latent or probable lupus, and it may be difficult to predict who will go on to develop SLE and potentially organ damage.7 8 However, early diagnosis of SLE can lead to the institution of appropriate treatment (eg, hydroxychloroquine (HCQ)) to limit organ damage,9 lower flare rates and decrease healthcare utilisation costs.10
A plethora of biomarkers have been identified in SLE,11 however only traditional autoantibodies, such as ANA and antibodies to double-stranded DNA (anti-dsDNA), Smith (anti-Sm) or ribosomal P protein are routinely measured in current clinical practice. ANA is detectable in most patients with SLE at presentation12 and ANA, anti-dsDNA and anti-Sm are part of SLE classification criteria.13–15 However, these biomarkers have shortcomings. ANA is very sensitive but lacks specificity, while the other autoantibodies have high specificity but are negative in many patients with SLE. Also serum levels of the complement proteins C3 and C4 are normal in most patients with SLE or suspected of SLE.11 16
We have shown previously that complement activation, measured reliably by cell-bound complement activation products—especially C4d bound to erythrocytes (EC4d) and B lymphocytes (BC4d)—can be detected in SLE and in probable SLE with greater frequency than traditional biomarkers.16 17 The sensitivity of EC4d and BC4d is further improved when these biomarkers are part of a multianalyte assay panel (MAP)16 17 that combines EC4d and BC4d with eight lupus and non-lupus autoantibodies.18 The MAP is commercially known as AVISE Lupus and is available in North America through Exagen (Vista, California, USA), which has a clinical laboratory accredited by the College of American Pathologists and certified under the Clinical Laboratory Improvement Amendments. The MAP is also part of the AVISE CTD, which includes additional autoantibodies. These tests are currently approved by the Clinical Laboratory Evaluation Programme in the State of New York.18
Multiple studies have demonstrated the clinical validity and accuracy of the MAP in distinguishing SLE and probable SLE from a variety of other rheumatic diseases and fibromyalgia.16 17 19 20 The clinical utility of MAP has been shown in a small, case-control review of medical records21 and in a randomised prospective study that compared MAP with standard diagnosis laboratory testing (SDLT).22 These data show that MAP has clinical utility in facilitating SLE diagnosis and treatment decisions. In particular, the randomised study demonstrated that knowledge of a negative test result increased the physician confidence in ruling-out SLE, while knowledge of a positive test result makes the confidence higher, compared with knowledge of SDLT. In addition, a higher percentage of MAP-positive patients were prescribed prednisone or HCQ compared with MAP-negative patients if the rheumatologist knew the results of the test.22 Because of the low incidence and prevalence of SLE, a limitation of that study was the small number of patients with a positive test result.22 Therefore, the present study was designed to enrich the population of the MAP-positive patients and to generate additional data on the clinical utility of MAP. To this end, we carried out a systematic and longitudinal review of medical records of selected MAP-positive and MAP-negative patients for whom the test was ordered as part of their workup.
We hypothesised that the confidence in SLE as a likely or unlikely diagnosis would increase on review of the MAP results compared with prior to ordering the test. In addition, we hypothesised that initiation of HCQ therapy after review of the MAP result would be greater in the positive than negative patients. To gain a greater understanding of how positive test results change confidence in SLE diagnosis and treatment decisions, we performed reviews of medical records of patients in four groups: negative score, low positive tier-2 score, high positive tier-2 score and tier-1 positive.18
Methods
Study population
A systematic and longitudinal review of medical records was conducted by 12 rheumatology practices in the USA.
Patients for review of medical records were selected by the research group at Exagen by mining the company’s database. Selection was based on the ordering rheumatologist and the results of the MAP conducted between January 2018 and July 2020. The name MAP is used throughout this paper to refer to the AVISE Lupus or the AVISE CTD, as the MAP is included in both panels. The MAP score refers to the test algorithm, which can be negative (<−0.1), tier-2 positive (>0.1) or tier-1 positive. As previously described,18 anti-dsDNA antibodies measured by ELISA are not part of the algorithm to calculate the MAP score.
All selected patients were adult (age ≥18 on the day the test was performed). Selected patients were ANA positive by immunofluorescence (titre 1:80 or higher) because MAP’s intended use is for ANA-positive patients. Patients whose MAP score was indeterminate or equivocal were excluded. To decrease the risk of bias, sets of five possible eligible patients were selected for each site: in each set, two patients had a negative (<−0.1) and three had a positive (>0.1) score. Of the three positives, one was tier-1 positive; one had a tier-2 score >1 (high tier-2), and one had a tier-2 score >0.1 and≤1 (low tier-2) to maintain a ratio of 2:1:1:1. Positive and negative patients were matched by ordering rheumatologist and sex of the patients.
The cut-off of 1 for the high and low tier-2 positive was selected based on the likelihood ratio positive (LR+) of the test. In particular, in a cohort of 879 patients with positive and negative MAP score (excluding indeterminate and equivocal scores), comprising 462 patients with SLE and 417 patients with other rheumatic diseases, LR+ of high tier-2 (>1) and low tier-2 (>0.1 and ≤1) were 6.81 and 2.05, respectively. Assuming a pre-test probability of approximately 30%, post-test probability was approximately 75% for high tier-2 and 50% for low tier-2. We reasoned that these values of post-test probability made a cut-off of 1 adequate to differentiate the patient populations with high and low tier-2 score.
Study design
Each investigator received a list of patients potentially eligible for the review; each list included one or more sets of five patients per set. Medical records were not reviewed if patients met exclusion criteria at the time of the blood draw for the MAP (online supplemental material).
lupus-2021-000528supp001.pdf (323.4KB, pdf)
Investigators reviewed the records at three time points: when the AVISE test was ordered (T0), when the results were reviewed (T1) and, if available, at a later time point (T2). T2 was defined as the latest visit and could be included if it occurred at least 8 months after T1. In-person and non-in-person visits were allowed.
Investigators indicated the confidence in SLE diagnosis pre-test (T0) and post-test (T1 and T2) on a 3-point Likert scale (low, moderate or high) at T0 and 5-point Likert scale (very low, low, moderate, high or very high) at T1 and T2. They also provided the International Classification of Diseases, Tenth Revision (ICD-10) codes, the 1982/1997 American College of Rheumatology (ACR) classification criteria for SLE13 and the list of medications taken or prescribed. In addition, the investigators indicated at T0 the main reason for ordering the MAP.
Statistical analysis
Statistical analysis (R software, V.4.0.3) consisted of Fisher’s exact test, Kaplan-Meier survival analysis with Cox proportional HR and ordered logistic regression, as appropriate. For the Cox proportional hazard model, we used the likelihood ratio test for overall model significance with alpha <0.05. With the exception of fibromyalgia (ICD-10 code M79.7), ICD-10 codes were grouped based on the first three characters (one alpha and two numeric) for analysis, regardless of the additional numeric or alpha characters indicated by the investigators (eg, all ICD-10 codes corresponding to SLE were grouped into the M32 section).
Results
Study population
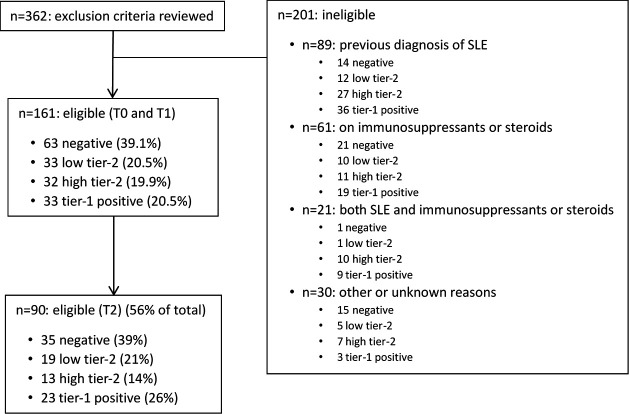
A total of 161 patients (63 negative, 98 positive (33 low tier-2, 32 high tier-2, 33 tier-1)) from 12 sites were included in the study (figure 1). For all patients, records were reviewed at the T0 and T1 time points, corresponding to when the MAP was ordered and when the results were reviewed, respectively.
Figure 1.
Flow chart of patients included or not in the study. Negative patients had a multianalyte assay panel (MAP) score <−0.1; low tier-2 had a score >0.1 and ≤1; high tier-2 had a score >1; for the tier-1 positives, the score was not calculated as they met the criteria of tier-1 positivity.
The demographic characteristics at the time of blood draw (T0) of the patients included in the study are reported in table 1. Race/ethnicity distribution was similar in the four MAP score groups (online supplemental table 1). At T0, a small number of patients (n=21, 13%) fulfilled the 1982/1997 ACR classification criteria of SLE. Additional methodological details are in the online supplemental material.
Table 1.
Demographic characteristics at T0
| Number of patients | 161 |
| Age (years) | |
| Average | 51.7 |
| Median (IQR) | 53 (39–63) |
| Minimum | 19 |
| Maximum | 89 |
| Female sex (n, %) | 156 (97%) |
| Race/Ethnicity (n, %) | |
| White | 114 (70.8%) |
| Black | 34 (21.1%) |
| Asian | 3 (1.9%) |
| Hispanic | 4 (2.5%) |
| Native American | 1 (0.6%) |
| Other | 5 (3.1%) |
Values represent the number and percentage (%) in each category.
Confidence in SLE diagnosis and ICD-10 codes
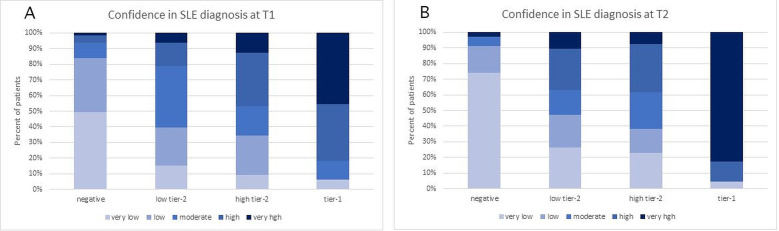
At each time point, the investigators estimated the confidence in SLE diagnosis. At T0, physician confidence in SLE diagnosis was low for 93 (58%), moderate for 49 (30%) and high for 19 (12%) patients. Confidence in SLE diagnosis changed significantly during the study. Ordered logistic regression analysis showed that the odds of higher confidence in SLE diagnosis increased by 1.74-fold for every unit of increase of the MAP score (p<0.001). At T1, confidence of SLE was very low for 49% and low for 35% of patients with negative MAP, while confidence was high for 36% and very high for 45% with tier-1 positive MAP. Intermediate values were observed with low tier-2 and high tier-2 MAP scores (figure 2A). At T2, the differences between groups were even more evident, with very low confidence in the SLE diagnosis for 74% with negative MAP and very high confidence for 83% with tier-1 positive MAP. The differences in the other two groups were less striking, however the majority (62%) of the high tier-2 positive MAP continued to be in the moderate/high/very high range and the majority (63%) of the low tier-2 positive MAP in the very low/low/moderate range also at T2 (figure 2B).
Figure 2.
Confidence of SLE diagnosis at T1 (panel A) and T2 (panel B) for patients who had a negative, low tier-2, high tier-2 or tier-1 multianalyte assay panel (MAP) score.
The most common ICD-10 section at T0 was abnormal immunological findings in serum (R76, n=73, 45%).
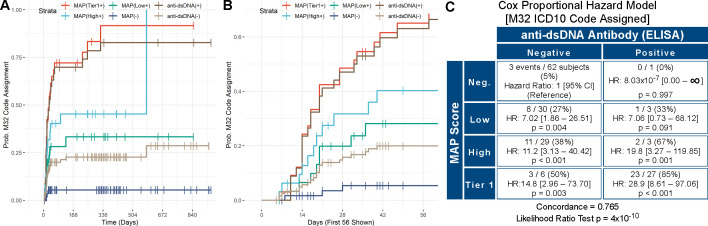
The ICD-10 section for SLE (M32) was not reported at T0, while it was indicated 48 times (30%) at T1 and in three additional patients at T2. As ICD-10 codes may be used as proxy for diagnoses,4 we evaluated the association of the MAP score with the assignment of a certain ICD-10 section during the study. Using the MAP-negative patients as reference, the hazard of assigning of the M32 section was 7.2-fold higher in the low tier-2 MAP group (HR 7.2, 95% CI 1.9 to 26.6), 12.3-fold higher in the high tier-2 MAP group (HR 12.3, 95% CI 3.5 to 43.5) and 26.6-fold higher in the tier-1 MAP group (HR 26.6, 95% CI 8.0 to 88.7) (p<0.001) (data not shown). Only 3 of 63 (5%) MAP-negative patients were assigned an M32 code during the study.
Anti-dsDNA antibodies have low sensitivity in SLE,14 16 however are highly specific23 and are used as a standard-of-care marker to rule-in SLE.22 We compared the results of the MAP score versus anti-dsDNA antibodies measured by ELISA at Exagen’s laboratory (figure 3A and 3B). Of all the 127 patients who were anti-dsDNA negative, 22 (17%) were MAP positive. Positive MAP was superior to anti-dsDNA at increasing the confidence in SLE diagnosis. In fact, HRs for assigning M32 in anti-dsDNA-negative patients were 7.02, 11.2 and 14.8 for low tier-2, high tier-2 and tier-1, respectively (p<0.001) (figure 3C). M32 was assigned to 22 of 65 MAP-positive patients (34%) who were anti-dsDNA negative. MAP negative was also superior to anti-dsDNA negative at excluding an SLE code, as more anti-dsDNA-negative than MAP-negative patients were at risk of M32 assignment (63% vs 52%) in the first 56 days in the study (data not shown). When all the MAP-positive patients were combined in one group, the HR for assignment of the M32 section was 9.35-fold higher in MAP-positive/anti-dsDNA-negative compared with MAP-negative/dsDNA-negative patients (HR 9.35, 95% CI 2.79 to 31.32, p<0.001) (online supplemental figure 1).
Figure 3.
Survival analysis (M32). (Panels A and B) Kaplan-Meier time-to-event curves for assignment of the M32 International Classification of Diseases, Tenth Revision (ICD-10) section over time. Curves show the per cent probability of assignment of the M32 section after T0 for the four study groups (tier-1 (multianalyte assay panel (MAP)(Tier1+)), high tier-2 (MAP(High+)), low tier-2 (MAP(Low+)), MAP negative (MAP(−)), together with anti-double-stranded DNA positive (anti-dsDNA(+)) and negative (anti-dsDNA(−)) patients throughout the study. The x-axis reports the number of days since T0. Panels A and B report the same data analysis, with panel B allowing better visualisation of the initial portion of the survival curves. (Panel C) Cox proportional hazard model comparing the MAP score (Neg.: negative; Low: low tier-2; High: high tier-2, Tier 1: tier-1 positive) versus anti-dsDNA antibodies for assignment of the M32 section. In each quadrant, the numerators represent the number of subjects that were assigned an M32 section after T0 (events, n=51 in total) while the denominators represent all subjects in that quadrant. Concordance and p value of the likelihood ratio test are also reported. For data analysis, we used the date of the visit when the M32 section was recorded in the ICD-10 list.
Interestingly, an M32 designation had a negative correlation with age (HR for age 0.93, 95% CI 0.90 to 0.96, p<0.001), consistent with SLE being more likely in younger patients.3 9
Data on hazard of assignment of other ICD-10 sections were not significant and are reported in the online supplemental material.
Prescription of hydroxychloroquine
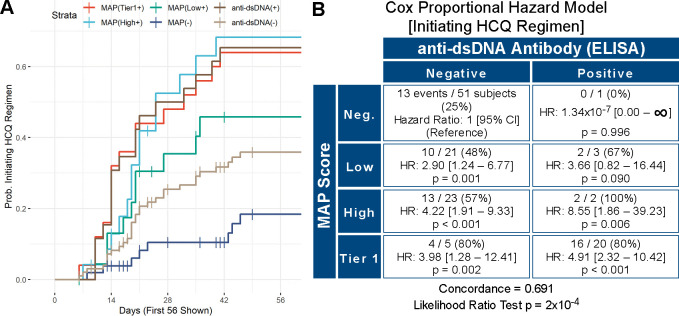
At each time point, the investigators indicated the medications that the patient was taking and those that were prescribed at that visit. As HCQ is often used in SLE, we evaluated the use of HCQ during the study. Of the 161 patients, 35 were taking HCQ at T0. Thus, we evaluated the use of HCQ in the remaining 126 patients. Using the MAP negative as reference, HCQ therapy was initiated after T0 more frequently in the low tier-2 MAP (HR 2.4; 95% CI 1.3 to 4.4), high tier-2 MAP (HR 2.8; 95% CI 1.6 to 5.1) and tier-1 MAP group (HR 3.1; 95% CI 1.8 to 5.4) (p<0.001) (data not shown). Comparison of the four MAP groups with anti-dsDNA-positive and anti-dsDNA-negative patients was also performed. Kaplan-Meier curves are reported in figure 4A. Cox proportional HRs using MAP-negative/anti-dsDNA-negative patients as reference showed that HRs of initiating HCQ therapy in anti-dsDNA-negative patients were 2.90 (p=0.001) for low tier-2 MAP, 4.22 (p<0.001) for high tier-2 MAP and 3.98 (p=0.002) for tier-1 MAP (figure 4B). In addition, MAP negative was superior to anti-dsDNA negative at avoiding initiation of HCQ prescription (58% vs 45%, data not shown). When all the MAP-positive patients were combined in one group, the hazard of HCQ therapy initiation was 3.58-fold higher in MAP-positive/anti-dsDNA-negative compared with MAP-negative/dsDNA-negative patients (HR 3.58, 95% CI 1.80 to 7.12, p<0.001) (online supplemental figure 2).
Figure 4.
Survival analysis (hydroxychloroquine (HCQ)). (Panel A) Kaplan-Meier time-to-event curves for use of HCQ over time. Curves show the per cent probability of using HCQ after T0 for the four study groups (tier-1 (multianalyte assay panel (MAP)(Tier1+)], high tier-2 (MAP(High+)), low tier-2 (MAP(Low+)), MAP negative (MAP(−)), together with anti-double-stranded DNA positive (anti-dsDNA(+)) and negative (anti-dsDNA(−)) patients throughout the study. TThe x-axis reports the number of days since T0 and is truncated at 56 days to allow better visualisation of the initial portion of the survival curves. (Panel B) Cox proportional hazard model comparing the MAP score (Neg.: negative; Low: low tier-2; High: high tier-2, Tier 1: tier-1 positive) versus anti-dsDNA antibodies for use of HCQ. In each quadrant, the numerators represent the number of subjects on HCQ after T0 (events, n=60 in total) while the denominators represent all subjects in that quadrant. Concordance and p value of the likelihood ratio test are also reported. For data analysis, we used the date of the visit when HCQ use was recorded in the medication list.
Reason for ordering the MAP
The reasons for ordering the MAP are reported in table 2. In addition, the investigators indicated that they chose to order the out-of-network MAP instead of an in-network test mainly because of greater trust in the reliability of these results compared with those from other laboratories (n=99, 61.5%).
Table 2.
Reason for ordering the MAP
| Rule-in SLE n (%) |
Rule-out SLE n (%) |
Differential diagnosis n (%) |
|
| Low confidence, n=93 | 5 (5.4) | 51 (54.8) | 37 (39.8) |
| Moderate confidence, n=49 | 18 (36.7) | 14 (28.6) | 17 (34.7) |
| High confidence, n=19 | 15 (78.9) | 0 (0) | 4 (21.1) |
At T0, the investigators indicated what was the main reason for ordering the MAP and could choose between ruling-in SLE, ruling-out SLE or making a differential diagnosis of connective tissue diseases.
Values represent the number and percentage (%) in each category. Per cent values were calculated based on the total number of patients for whom confidence in SLE diagnosis was low, moderate or high (93, 49 and 19, respectively).
Discussion
Rheumatologists often rely on traditional biomarkers, such as autoantibodies and complement levels, to facilitate the differential diagnosis of SLE, even if these biomarkers have suboptimal diagnostic accuracy. The superior clinical validity and accuracy of novel diagnostic tests over traditional biomarkers is insufficient to satisfy the scrutiny from stakeholders in the healthcare system, including payors, clinicians and patients.24 Thus, new diagnostic technologies need to demonstrate their clinical utility in improving patient outcome.
This study strengthens previous data that demonstrated the clinical utility of the MAP test.21 22 By reviewing medical records of a large number of patients suspected of SLE—with positive and negative MAP scores—at three time points over the course of approximately 1 year, this study demonstrates that the MAP test has a favourable impact on physician behaviour. In particular, a positive test increased the confidence in ruling-in SLE while a negative test increased the physician confidence that SLE was an unlikely diagnosis. Importantly, the MAP also informed appropriate treatment decisions.
Virtually all patients with SLE are ANA positive at presentation.23 Because the study aimed to evaluate physician behaviour in patients for whom the rheumatologist had a suspicion of SLE, we included in the study only ANA-positive patients as determined at Exagen laboratory. As SLE has low incidence and prevalence even in rheumatology practices,25 the majority of patients for whom the MAP is ordered obtain a negative score.18 Thus, to evaluate physician behaviour based on both positive and negative test results, the patient selection was conducted to enrich the study for patients with positive results (n=98, 61%). The ability to rule-out SLE in symptomatic patients is equally important, as misdiagnosis can cause stress in patients without autoimmune diseases and can lead to unnecessary rheumatology consultations, further testing and inappropriate treatment. Thus, the study also included 63 patients (39%) with a negative score (<−0.1). To decrease risk of bias, positive and negative patients were selected from the same practice and 12 rheumatology practices throughout the USA participated in the study. Overall, the patient population included in the study was typical of patients suspected of SLE, with ANA, arthritis and cutaneous manifestations being the most common ACR classification criteria at study entry (online supplemental material).8 16 22 26
A positive MAP result (especially tier-1 and high tier-2) increased physician confidence, while a negative MAP result decreased the confidence in SLE diagnosis on review of the test results (T1) and, even more strikingly, at a later time point (T2) (figure 2). These data indicate that the MAP supplemented the clinical evaluation, aided the physicians to make a correct assessment of diagnosis early on (T1) and predicted clinical diagnosis over time (T2). Similar conclusions were reached when analysing the data according to the ICD-10 section of M32, which can be used as a surrogate for diagnosis of SLE.4 Compared with MAP negative, risk of assigning a lupus ICD-10 code over time was highest for the tier-1 positive followed by high tier-2 and low tier-2. Only three (5%) MAP-negative patients were assigned an M32 code during the study, indicating a 95% probability that a negative MAP supported the physician clinical judgement that SLE was an unlikely diagnosis.
We could not compare the MAP with ANA because all patients selected for the study were ANA positive (see above). However, it is well established that ANA is extremely sensitive for SLE but has low specificity, being positive in many patients with conditions other than SLE.27 Comparison of the MAP test with another standard-of-care biomarker of SLE, anti-dsDNA antibodies measured by ELISA, showed that the MAP test was superior to anti-dsDNA antibodies in increasing confidence in both ruling-in and in ruling-out SLE. In particular, M32 was assigned to 22 of 65 (34%) MAP-positive patients who were anti-dsDNA negative; these patients would have been diagnosed incorrectly as not having SLE if anti-dsDNA antibodies had been the only test performed. In addition, a negative MAP result was superior to negative anti-dsDNA antibodies in increasing the confidence that SLE was an unlikely diagnosis (figure 3).
Taken together with previous studies,21 22 these data demonstrate the superiority of the MAP score in increasing confidence that SLE is a more likely or less likely diagnosis compared with standard-of-care lupus biomarkers in isolation.
The MAP helped make a correct diagnosis—both early on and during the course of the study—and informed appropriate treatment decisions. Immunosuppressant use in this patient population was minimal (two, five and nine patients at T0, T1 and T2, respectively), which is expected given that these patients were early in the course of their disease. On the contrary, HCQ was heavily used in this patient population. HCQ treatment was initiated for 35 patients on or before T0, that is, before the results of the MAP were reviewed, which is not surprising given that HCQ is used in various rheumatic diseases—and even when diagnosis is uncertain—to control inflammatory signs and symptoms with minimal toxicity.28–30 After T0, initiation of HCQ therapy was greater in the MAP positive than negative patients during the study (figure 4), indicating that the test results informed treatment decisions. Although this study did not address patient outcome in the long term, it is well established that HCQ therapy has numerous beneficial effects and reduces flares and organ damage over time.9 31–33 In addition, it has been demonstrated that patient-reported outcome measures improve in the 2 years following diagnosis34 and with HCQ therapy,35 suggesting that early diagnosis and appropriate treatment have a positive impact on physical and mental function and on overall quality of life. Taken together, our results suggest that the MAP, by facilitating early diagnosis and appropriate treatment decisions in patients suspected of SLE, may improve patient outcome and decrease healthcare costs.10 36
As expected, the test was ordered mainly to rule-in SLE if suspicion of SLE was high and to rule-out SLE if suspicion was low (table 2). The MAP test is intended to be used in patients suspected of SLE, however 110 patients could not be included in the study because of a previous diagnosis of SLE (figure 1). Fifty per cent of these patients were from one site; for the other sites, percentages of patients with a previous SLE diagnosis varied from 0% to 14%, indicating that most investigators used the test for the intended patient population in most cases. This study did not investigate why the MAP was ordered for patients already diagnosed with SLE; however, it is possible that the test was used to confirm the SLE diagnosis, to monitor biomarker levels or to evaluate positivity of various lupus and non-lupus autoantibodies, also consistent with the high level of trust in the test.
Limitations of this study include its retrospective nature and the potential risk of bias, although this was mitigated by including 12 rheumatology practices and by requiring that each rheumatologist reviewed records of patients with different MAP scores. However, we cannot exclude that the recalled pre-test confidence in SLE diagnosis was partially influenced by knowledge of the results of the test, as this was not a blinded study. However, this potential bias is irrelevant for the estimate of the confidence in SLE diagnosis on review of results (T1) and after approximately 1 year of follow-up (T2) and, more importantly, for initiation of HCQ therapy.
In conclusion, this study demonstrates that the MAP test leads to increased confidence that SLE is a more likely or less likely diagnosis in patients suspected of the disease and, importantly, informs appropriate treatment decisions in this patient population.
Acknowledgments
We wish to acknowledge all the investigators who performed the medical record reviews: Drs Daniel J. Wallace, Olga Kromo, James Mossell, Augusto C. Posadas, Ananta Subedi, Kavitta Allem, Cory Conniff, Wambui Machua, Andrew Sharobeem, Wajeeha Yousaf and Jack Waxman. We also thank the research coordinators who participated in the study; Tyler O’Malley for technical assistance in the identification of the patients to be included in the study and critical reading of the manuscript; Rory Block for statistical analysis support; the personnel of Exagen’s clinical laboratory for performing laboratory testing; Tami Powell for input on the study design; Debra J. Zack and Anja Kammesheidt for input on the study design and critical reading of the manuscript.
Footnotes
Contributors: Study conception and design: RVA, SR and AW. Acquisition of data: RVA, SR, VD, MA and JQ. Analysis and interpretation of data: RVA, JC and AW.
Funding: This study was funded by Exagen Inc.
Competing interests: VD, MA and JQ have received research grants from Exagen. RVA, SR and JC are employed by Exagen. AW is consultant to Exagen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Internal review boards (Advarra IRB and WIRB) approved the study and informed consent was waived.
References
- 1.Atzeni F, Cazzola M, Benucci M, et al. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol 2011;25:165–71. 10.1016/j.berh.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 2.Di Franco M, Guzzo MP, Spinelli FR, et al. Pain and systemic lupus erythematosus. Reumatismo 2014;66:33–8. 10.4081/reumatismo.2014.762 [DOI] [PubMed] [Google Scholar]
- 3.Lam GKW, Petri M. Assessment of systemic lupus erythematosus. Clin Exp Rheumatol 2005;23:S120–32. [PubMed] [Google Scholar]
- 4.Lim SS, Bayakly AR, Helmick CG, et al. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: the Georgia lupus registry. Arthritis Rheumatol 2014;66:357–68. 10.1002/art.38239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the centers for disease control and prevention national lupus registries. Arthritis Rheumatol 2021;73:991–6. 10.1002/art.41632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers EC, Marder W, Cagnoli P, et al. Population-Based incidence and prevalence of systemic lupus erythematosus: the Michigan lupus epidemiology and surveillance program. Arthritis Rheumatol 2014;66:369–78. 10.1002/art.38238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson JM, James JA. Preclinical systemic lupus erythematosus. Rheum Dis Clin North Am 2014;40:621–35. 10.1016/j.rdc.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alarcón GS, McGwin G, Roseman JM, et al. Systemic lupus erythematosus in three ethnic groups. XIX. natural history of the accrual of the American College of rheumatology criteria prior to the occurrence of criteria diagnosis. Arthritis Rheum 2004;51:609–15. 10.1002/art.20548 [DOI] [PubMed] [Google Scholar]
- 9.Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 2019;96:1–13. 10.1016/j.jaut.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oglesby A, Korves C, Laliberté F, et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl Health Econ Health Policy 2014;12:179–90. 10.1007/s40258-014-0085-x [DOI] [PubMed] [Google Scholar]
- 11.González LA, Ugarte-Gil MF, Alarcón GS. Systemic lupus erythematosus: the search for the ideal biomarker. Lupus 2021;30:181–203. 10.1177/0961203320979051 [DOI] [PubMed] [Google Scholar]
- 12.Pisetsky DS, Spencer DM, Lipsky PE, et al. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis 2018;77:annrheumdis-2017-212599–-2013. 10.1136/annrheumdis-2017-212599 [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 14.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aringer M, Costenbader K, Daikh D, et al. 2019 European League against Rheumatism/American College of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. 10.1002/art.40930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey-Goldman R, Alexander RV, Massarotti EM, et al. Complement activation in patients with probable systemic lupus erythematosus and ability to predict progression to American College of Rheumatology-Classified systemic lupus erythematosus. Arthritis Rheumatol 2020;72:78–88. 10.1002/art.41093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putterman C, Furie R, Ramsey-Goldman R, et al. Cell-Bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med 2014;1:e000056. 10.1136/lupus-2014-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dervieux T, Conklin J, Ligayon J-A, et al. Validation of a multi-analyte panel with cell-bound complement activation products for systemic lupus erythematosus. J Immunol Methods 2017;446:54–9. 10.1016/j.jim.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 19.Wallace DJ, Silverman SL, Conklin J, et al. Systemic lupus erythematosus and primary fibromyalgia can be distinguished by testing for cell-bound complement activation products. Lupus Sci Med 2016;3:e000127. 10.1136/lupus-2015-000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalunian KC, Chatham WW, Massarotti EM, et al. Measurement of cell-bound complement activation products enhances diagnostic performance in systemic lupus erythematosus. Arthritis Rheum 2012;64:4040–7. 10.1002/art.34669 [DOI] [PubMed] [Google Scholar]
- 21.Mossell J, Goldman JA, Barken D, et al. The Avise lupus test and cell-bound complement activation products aid the diagnosis of systemic lupus erythematosus. Open Rheumatol J 2016;10:71–80. 10.2174/1874312901610010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace DJ, Alexander RV, O'Malley T, et al. Randomised prospective trial to assess the clinical utility of multianalyte assay panel with complement activation products for the diagnosis of SLE. Lupus Sci Med 2019;6:e000349. 10.1136/lupus-2019-000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 2020;16:565–79. 10.1038/s41584-020-0480-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bossuyt PMM, Reitsma JB, Linnet K, et al. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem 2012;58:1636–43. 10.1373/clinchem.2012.182576 [DOI] [PubMed] [Google Scholar]
- 25.Jokar M, Jokar M. Prevalence of inflammatory rheumatic diseases in a rheumatologic outpatient clinic: analysis of 12626 cases. Rheum Res 2018;3:21–7. 10.22631/rr.2017.69997.1037 [DOI] [Google Scholar]
- 26.Bodolay E, Csiki Z, Szekanecz Z, et al. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD). Clin Exp Rheumatol 2003;21:313–20. [PubMed] [Google Scholar]
- 27.Pisetsky DS. Antinuclear antibody testing - misunderstood or misbegotten? Nat Rev Rheumatol 2017;13:495–502. 10.1038/nrrheum.2017.74 [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. 10.1136/ard.2008.101766 [DOI] [PubMed] [Google Scholar]
- 29.Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med 2020;12:1–17. 10.15252/emmm.202012476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dima A, Jurcut C, Arnaud L. Hydroxychloroquine in systemic and autoimmune diseases: where are we now? Joint Bone Spine 2021;88:p. 105143:105143. 10.1016/j.jbspin.2021.105143 [DOI] [PubMed] [Google Scholar]
- 31.Petri M, Konig MF, Li J, et al. Association of higher hydroxychloroquine blood levels with reduced thrombosis risk in systemic lupus erythematosus. Arthritis Rheumatol 2021;73:997–1004. 10.1002/art.41621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durcan L, Petri M. Immunomodulators in SLE: clinical evidence and immunologic actions. J Autoimmun 2016;74:73–84. 10.1016/j.jaut.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhavan PS, Su J, Lou W, et al. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol 2013;40:831–41. 10.3899/jrheum.120572 [DOI] [PubMed] [Google Scholar]
- 34.Urowitz M, Gladman DD, Ibañez D, et al. Changes in quality of life in the first 5 years of disease in a multicenter cohort of patients with systemic lupus erythematosus. Arthritis Care Res 2014;66:1374–9. 10.1002/acr.22299 [DOI] [PubMed] [Google Scholar]
- 35.Jolly M, Sehgal V. P173 is use of hydroxychloroquine associated with better patient reported outcomes in lupus? Lupus Sci Med 2020. 10.1136/lupus-2020-eurolupus.215 [DOI] [Google Scholar]
- 36.Clarke AE, Weinstein A, Piscitello A, et al. Evaluation of the economic benefit of earlier systemic lupus erythematosus (SLE) diagnosis using a multivariate assay panel (MAP). ACR Open Rheumatol 2020;2:629–39. 10.1002/acr2.11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2021-000528supp001.pdf (323.4KB, pdf)
Data Availability Statement
Data are available on reasonable request.