Abstract
Background
Diabetic nephropathy is the leading cause of chronic kidney disease. Observational studies suggest Roux-en-Y Bypass (RYGB) reduces progression of diabetic nephropathy.
Objectives
To unravel the mechanisms by which RYGB is beneficial and protective for diabetic nephropathy.
Setting
Academic laboratories
Methods
Forty-eight Zucker Diabetic Fatty (ZDF) rats were randomized to RYGB, sham surgery (SHAM) or pair-fed (PF) groups. An oral glucose tolerance test was performed at 25 days post-intervention and kidneys were harvested at 30 days. Primary outcome measures included expression of key genes and proteins in the glucose transport, oxidative stress, inflammation and fibrosis pathways.
Results
Thirty days post-intervention, RYGB rats weighed 349±8 g, which was lower than SHAM (436±14 g, P<0.001), but not PF (374±18 g) rats. RYGB rats had lower fasting glucose than PF animals.. These enhanced metabolic outcomes were accompanied by reduced sodium-glucose co-transporter 1 (Sglt1) gene expression (−23% vs PF, P=0.01) in the kidney of RYGB rats compared to SHAM rats. Expression of Sglt2, Glut1 or Glut2 mRNA, or oxidative stress and inflammation markers did not differ significantly. However, RYGB surgery induced a 19% lower expression of transforming growth factor (Tgfβ) mRNA (P=0.004) compared to SHAM treated animals. Notably, AMPK phosphorylation was increased (P=0.05) in kidneys of the RYGB surgery animals.
Conclusions
Remission of hyperglycemia after RYGB may reduce the glucose load on the kidney leading to a downregulation of specific glucose transporters. RYGB surgery may also attenuate kidney fibrosis through the AMPK/ TGFβ pathway.
Introduction
Diabetic nephropathy is the leading cause of chronic kidney disease, the prime indication for dialysis, and a major indicator of cardiovascular mortality risk1. As the incidence of obesity and type 2 diabetes (T2DM) has reached epidemic levels, the burden of diabetic nephropathy is also growing2. Modern medical therapies with glucagon-like peptide 1-receptor agonists or inhibitors of sodium/glucose co-transporter 2 can reduce hyperglycemia, albuminuria, and protect from further decline in glomerular filtration rate (GFR). However, there is an unmet need for specific treatments that counter development and progression of diabetic kidney disease3.
Metabolic surgery has emerged in the last decade as a powerful tool in the treatment paradigm for obesity and associated T2DM4,5. As such, Roux-en-Y gastric bypass (RYGB) surgery, results in significantly greater weight loss, reduction in glycated hemoglobin, and a lower incidence of microvascular and macrovascular complications in patients with T2DM and obesity when compared with nonsurgical medical management6,7. Metabolic surgery is also associated with a lower overall incidence of microvascular disease, including lower risk for neuropathy, nephropathy, and retinopathy than usual care8–10. A recent study showed a gain in GFR after surgery, independent of percentage of weight loss and type of surgery (not different for RYGB versus sleeve gastrectomy (SG)) 9. This suggests an alternate mechanism in the improvement of renal function other than weight loss alone. A potential pathway is the 5’ adenosine monophosphate-activated protein kinase (AMPK) / transforming growth factor beta (TGFβ) pathway, which has been identified as a critical pathway regulating both inflammation and pro-fibrotic pathways for both obesity-related kidney disease and diabetic kidney disease10,11.
Thus, in this experimental animal study, we sought to investigate the changes in kidney glucose transporters and nephropathy markers after RYGB surgery, and compare these to the effects of mere weight loss (caloric restriction by pair feeding) or sham surgery. We hypothesized that RYGB surgery would lead to downregulation of renal glucose transporters, and reversal of the cellular mechanisms that cause diabetic nephropathy.
Methods
Experimental set-up
Male Zucker Diabetic Fatty (ZDF) rats (Charles River Laboratories, Wilmington, MA), homozygous recessive for the leptin receptor (fa/fa), were randomized at age 12 weeks to RYGB (n=16), sham-operated (SHAM, n=16), or pair-fed (PF, n=16) groups. After intervention, the rats were followed for 30 days. The PF group did not undergo laparotomy and were fed the average daily intake of the rats that underwent RYGB. All rats had ad libitum access to water and the diabetogenic Purina 5008 diet with 56.4% carbohydrate (Research Diets, New Brunswick, NJ). Rats were housed in individual cages in the Cleveland Clinic Biological Resources Unit with temperature controlled to 22°C and a 12h-dark/light cycle. All animal experimental procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. The National Institute of Health Animal Use Guidelines were adhered to for this study. All efforts were made to minimize any suffering.
Animal surgical procedures and perioperative care
The surgical procedures have been detailed elsewhere12. Briefly, the stomach and distal esophagus were dissected free and a small proximal gastric pouch (20% of stomach) was created. The jejunum was transected 30 cm distal to the ligament of Treitz creating a 30-cm biliopancreatic limb. A 5 mm side-to-side gastrojejunostomy was made while an 8 mm side-to-side jejunojejunostomy was performed 10 cm below the gastrojejunostomy creating a 10 cm alimentary limb. For the sham operation, the terminal esophagus and stomach were likewise exposed and dissected free. A 5-mm gastrotomy was made on the anterior surface of the gastric fundus and closed. The jejunum was also divided 30 cm below the ligament of Treitz and then re-anastomosed.
Postoperatively, the rats were kept nil per os for 24h. During this period, hydration was maintained with 50mL/kg saline injected subcutaneously. Feeding was resumed after 24 hours of the respective procedure with ad libitum liquid diet of Boost (Nestle, Buffalo Grove, IL) and tap water. On the postoperative day (POD) 5, Purina 5008 diet was resumed until POD 30.
Food intake and body weight was recorded daily. An Oral Glucose Tolerance Test (OGTT) was performed after an overnight fast in awake animals 25 days after randomization. Briefly, fasting blood glucose was determined (by glucometer on tail vein blood). Then, glucose (2 g/kg body weight) was oral gavaged, followed by measurement of glycemia levels at 10, 30, 60, and 120 min.
Tissue harvesting and serum analyses
On POD 30, the rats were euthanized by exsanguination under isoflurane anesthesia. Blood was collected by cardiac puncture and blood samples were centrifuged at 2000x g for 10 minutes at 4°C and then the plasma was stored at −80°C for subsequent analysis. Fasting insulin levels were determined using commercially available enzyme-linked immunosorbent assay kits for rat plasma (Linco Research, St. Charles, MO). Both kidneys were collected and snap-frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
RNA extraction and RT-qPCR
One part of whole kidney tissue was frozenly grounded and total RNA was extracted from 20 mg of grounded tissue using commercial guadinium thiocyanate-phenol reagent, Trizol (Life Technologies, Beverly, MA). Isolated RNA was further cleaned using the Qiagen RNA isolation kit, and genomic DNA was digested using RNase free-DNase (Qiagen, Germantown, MD). The RNA concentration and purity was determined by measuring the absorbance at 230, 260 and 280 nm using NanoDrop ND-1000 Spectrophotomoter (Thermo Scientific, Wilmington, DE). Isolated RNA was aliquoted and stored at −80°C. One microgram of complementary DNA (cDNA) was reverse transcribed using an iScript cDNA synthesis kit (BioRad, Hercules, CA) following manufacturer’s instruction, and cDNA was stored at −20°C.
Determination of relative mRNA expression was performed in duplicate as previously described 13. Rat glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal standard for sample normalization, whose expression was not influenced by experimental conditions. The relative changes in mRNA abundance were calculated using the comparative ΔΔCt method.
Gene-specific primers for qRT-PCR analysis are: catalase (Cat), monocyte chemoattractant protein-1 (Mcp1), glutathione peroxidase 1 (Gpx1), facilitative glucose transporter 1 and 2 (Glut1 and Glut2), sodium/glucose co-transporter 1 and 2 (Sglt1 and Sglt2), superoxide dismutase 1 (Sod1), and Tgfβ. Primer sequences are listed in Supplementary Table 1 and were obtained from PrimerBank (pga.mgh.harvard.edu/primerbank). All primers were checked for specificity to the genes of interest by conducting Blast analysis.
Protein extraction and Western Blotting
Kidney homogenates were prepared by grinding the tissue (20 mg) with ice-cold cell extraction buffer (Invitrogen) with protease and phosphatase inhibitor as previously explained13. Protein concentration was measured using a Protein BCA assay kit (Thermo Scientific, Waltham, MA). The protein samples were separated in 4–20% Novex TrisGlycine SDS PAGE (Life Technologies, Camarillo, CA), followed by transferring onto polyvinylidene fluoride membrane (Biorad, Hercules, CA). Membranes were then incubated overnight with primary antibodies for AMPKα (Cell Signaling, #2532), Phospho-AMPKα (Thr172, Cell Signaling, #4188), Sirtuin 1 (SIRT1, Abcam, #ab32441) or TGFβ (Abcam, #ab66043) and actin (Santa Cruz Biotechnology; #sc–1616) was used as loading control. Membranes were washed with PBS-T and incubated with secondary horseradish peroxidase-conjugated antibodies, with anti-rabbit or anti-mouse (GE Healthcare, Piscataway, NJ) and anti-goat (Santa Cruz Biotechnology). Immunoreactive proteins were visualized by enhanced chemiluminescence reagent (ECL Prime; GE Healthcare, Piscataway, NJ) and quantified by densitometric analysis using ImageJ software.
Statistical Analysis
Statistical analyses were performed with GraphPadPrism 8.0.2 for Windows (GraphPad Software, San Diego, CA). Normal distribution was confirmed by Kolmogorov-Smirnov test, prior to data comparison using One-way Analysis of variance (ANOVA) and post hoc analysis with Tukey’s multiple-comparison test. For correlation analysis, a Pearson correlation coefficient was calculated. Data are presented as mean±SEM. Differences were considered significant at P≤0.05.
Results
Body weight evolution and glucose parameters
2 RYGB and 1 SHAM rats died before the anticipated end of the experiment, and 3 PF animals had to be excluded because of missing RYGB animal (2) or tissue processing error (1). At the end of the study, 30 days after randomization, RYGB rats weighed 349±8 g, which was similar to PF rats (374±18 g, P=0.41) (Fig. 1). SHAM surgery rats weighed 15–20% more (436± 14 g) than RYGB (P<0.001) or PF (P=0.01) rats.
Figure 1.
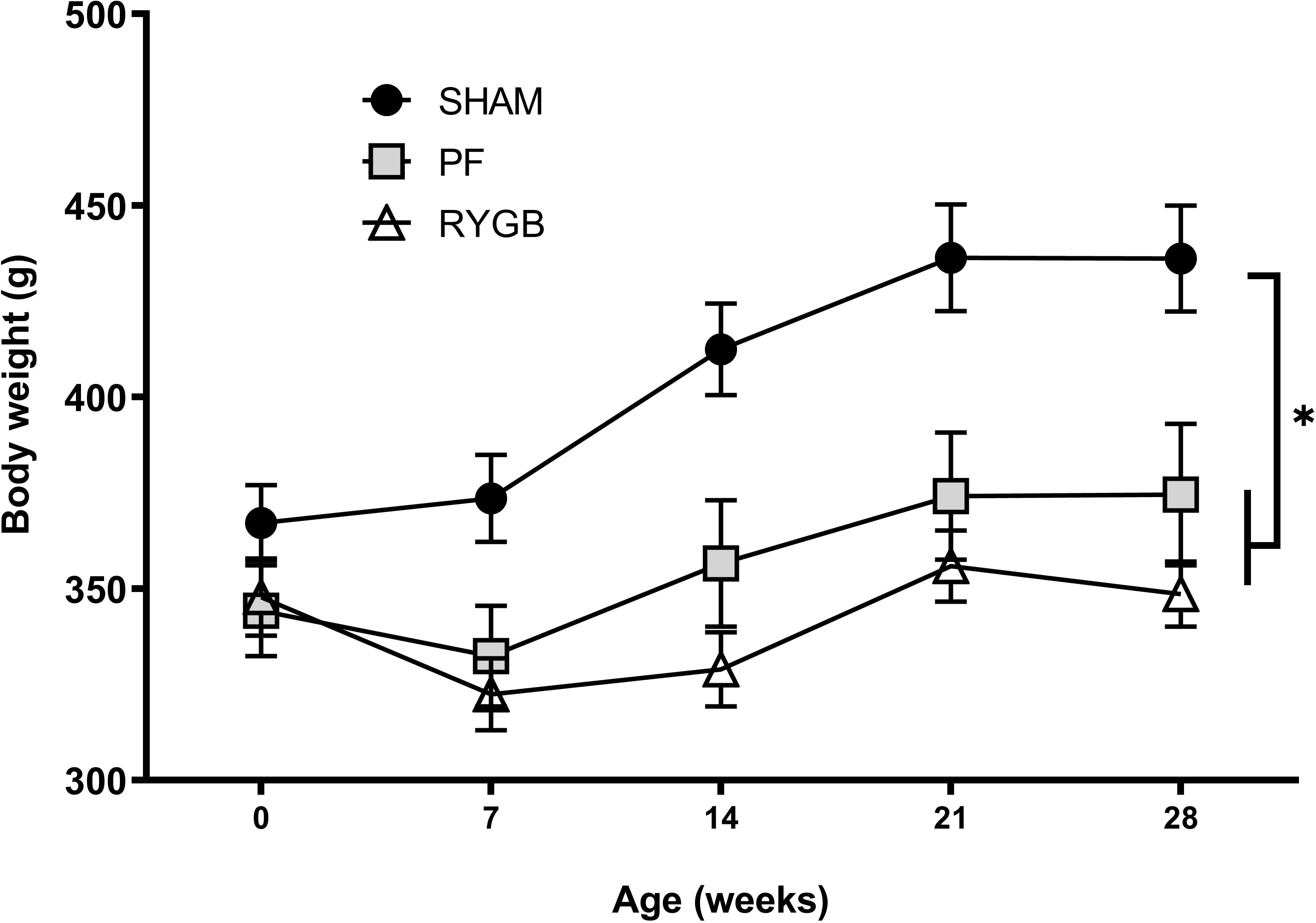
Bodyweight evolution during 4 weeks follow up after randomization: RYGB (△, n=14), SHAM (●, n=15) and pair-fed (PF, ▦, n=13) rats. Data are presented as mean±SEM. * denotes P≤0.05 ANOVA with Tukey post-test at age 30 days.
RYGB rats had a lower fasting blood glucose level than PF rats (168±9 vs 259±.37 mg/dl, P=0.05) and there was a similar trend compared to SHAM rats, but that did not reach statistical significance (251±26 mg/dl, P=0.06) (Fig 2A). Fasting insulin was 184±26 pmol/L in RYGB rats, which was 2-fold lower than PF (P=0.003) and 1.8-fold lower than in SHAM rats (P=0.03). When combined into HOMA-IR calculation, the index was 11.4±1.7 for RYGB rats, which was significantly reduced compared to PF (32.6±2.6, P<0.001) and SHAM rats (27.9±3.1, P<0.001). There were no significant differences in serum insulin concentration or HOMA-IR between PF and SHAM (Fig 2B).
Figure 2.
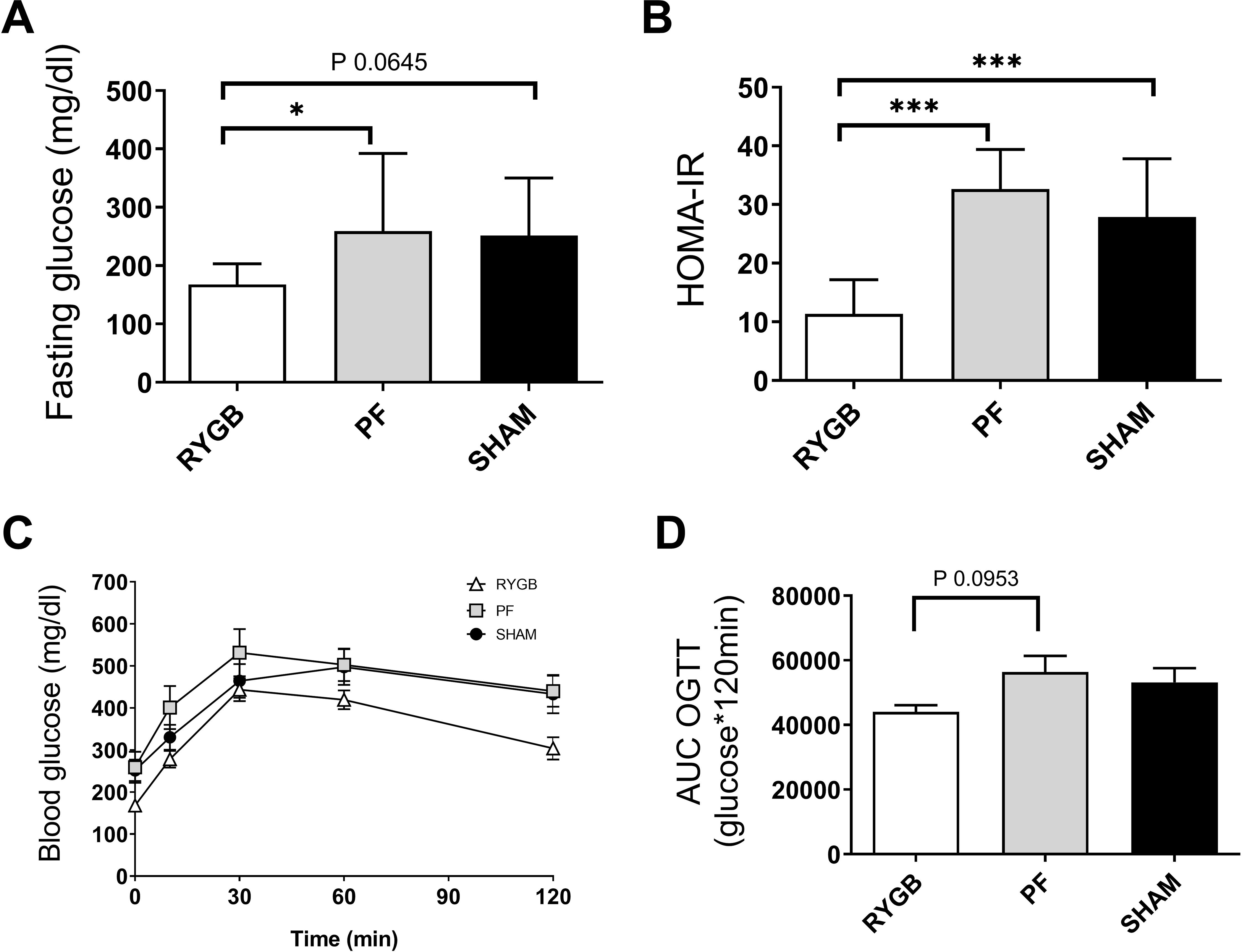
Glucose homeostasis parameters 25–30 days after randomization. A. Fasting plasma glucose. B. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). C. Blood glucose during oral glucose tolerance test (OGTT) 25 days after randomization. D. Area under the curve (AUC) for OGTT in RYGB (white, △ and bars), SHAM (black, ● and bars) and pair-fed (PF, grey ▦ and bars) rats (n = 13–15 per group). Data are presented as mean±SEM. * denotes P≤0.05 ANOVA with Tukey post-test at age 30 days.
The glucose area under the curve (AUC) during OGTT demonstrated a similar profile between PF and SHAM group (P=0.84, Fig 2C), despite the lower bodyweight of the PF rats. Oral glucose tolerance test showed a similar profile with no difference in glucose tolerance between PF and SHAM (56390±4980 vs 53165±4434 mg/dl glucose*120 min, P=0.84), despite the lower bodyweight of the PF rats (Fig 2C). While the AUC OGTT for RYGB rats (44062±2026 mg/dl glucose*120 min) was 23% and 17% lower numerically than for PF or SHAM rats respectively, statistical significance was not reached (P=0.09 for RYGB vs PF, P=0.24 for RYGB vs SHAM). The OGTT AUC correlated well with both body weight (Pearson r=−0.40, P=0.009) and fasting plasma glucose (r=0.88, P <0.001).
Kidney glucose transporters
Gene expression of the Sglt1 in RYGB rats was 23% lower than PF rats (P=0.012) but did not differ from SHAM rats (Fig 3A). Sglt2 mRNA was not different between groups (Fig 3B). There was a trend to increased Glut1 mRNA expression for RYGB compared toPF (119%, P=0.07) and SHAM rats (120%, P=0.06)(Fig 3C). Glut2 mRNA, on the other hand, was similar between RYGB PF, and SHAM rats (Fig 3D). We could not detect significant correlations between glucose transporter gene expression (Sglt1, Sglt2, Glut1, Glut2) and body weight, fasting plasma glucose, OGTT AUC, OGTT 60 min or OGTT 120 min.
Figure 3.
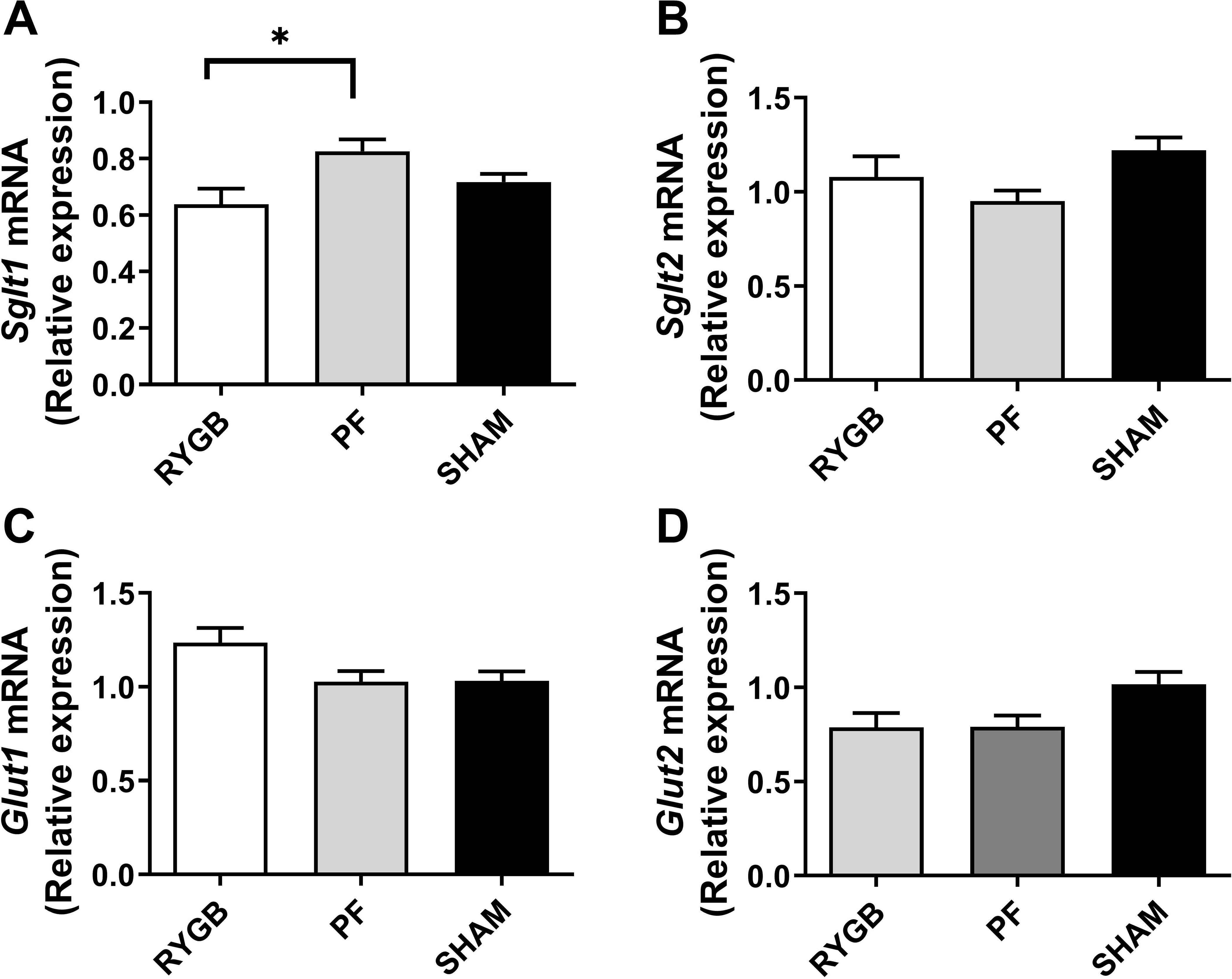
Kidney glucose transporters mRNA expression 30 days after randomization in RYGB (white bars), SHAM (black bars) and pair-fed (PF, grey bars) rats (n = 13–15 per group). Sodium-glucose cotransporter 1 (Sglt1, A) and 2 (Sglt2, B) and Glucose transporter 1 (Glut1, C) and 2 (Glut2, D). Data are presented as mean±SEM. * denotes P≤0.05 ANOVA with Tukey post-test.
Kidney damage markers
There were no significant differences in expression of oxidative stress-related genes Gpx1, Cat, and Sod1 (Fig 4A–C). Furthermore, there was no difference in Mcp1 mRNA (Fig 4D) or Sirtuin 1 protein expression (Fig 4E), but RYGB rat kidneys had a 19% lower Tgfβ mRNA expression than SHAM rats (P= 0.004, Fig 4G). Consistent with Tgfβ gene downregulation, we noted an increased AMPK phosphorylation in RYGB rats compared to SHAM animals (P=0.04, Fig 4F), and less intensive bands for the 14kDa active monomer form of TGFβ protein expression in the RYGB compared to the SHAM group upon Western Blotting, although the numerical quantification did not reach statistical significance (Fig 4H–I).
Figure 4.
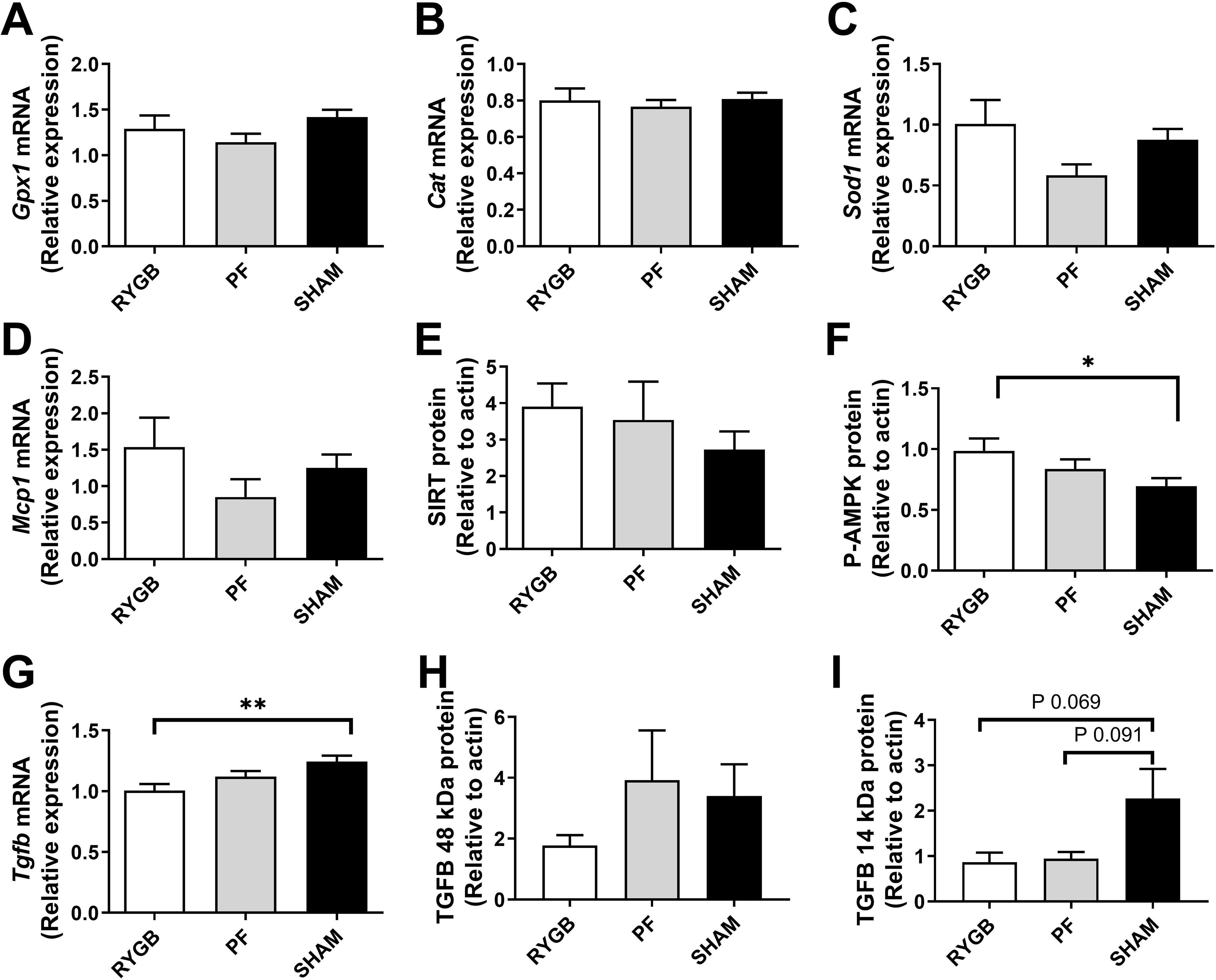
Kidney damage markers 30 days after randomization in RYGB (bars), SHAM (black bars) and pair-fed (PF, grey bars) rats (n = 13–15 per group). mRNA expression of Glutathione Peroxidase 1 (Gpx1, A) Catalase (Cat, B), superoxide dismutase (Sod1, C), Monocyte chemoattractant protein 1 (Mcp1, D) and Transforming growth factor beta (Tgfβ, G). Protein expression, normalized to actin, of Sirtuin (SIRT, E) phospho-5’ AMP-activated protein kinase (P-AMPK, F) and TGFβ 48 kDa and 14 kDa (H,I). Data are presented as mean±SEM. * denotes P≤0.05 ** denotes P <0.01 ANOVA with Tukey post-test.
Discussion
In this study, we compared the effects of RYGB surgery on glucose homeostasis and kidney damage pathways by comparing rats that underwent sham surgery or calorie restriction by pair feeding. Overall, our results suggest that RYGB induces a downregulation of SGLT1 and a reduction in TGFβ production.
As expected in this model, RYGB weighed less than SHAM animals, but had a similar body weight as PF rats. Importantly,, RYGB rats had a lower fasting glycemia, lower HOMA-IR levels and a trend to a decreased AUC at OGTT compared to PF rats.The fasting glucose and oral glucose tolerance of RYGB and SHAM rats were not statistically different in the current study, and in contrast with the significantly lower HOMA-IR in RYGB compared to SHAM rats. Indeed, a previous study by our group with a similar model did show a significant difference in AUC OGTT between RYGB and SHAM groups, with less variation in the SHAM group. 14
We evaluated the gene expression of renal glucose transporters. We observed a significant downregulation of Sglt1 mRNA in RYGB compared to PF rats. This suggests an improvement in whole body glucose homeostasis after metabolic surgery. Indeed, SGLT1 is a high affinity, low capacity luminal transporter in the proximal collecting tubules, known to be upregulated in T2DM as a compensatory mechanism for increased glucose filtration15. Previous mechanistic studies have shown that SGLT1 is not regulated by insulin16, but the exact feedback loop remains unknown. The SGLT2 transporter is also on the luminal membrane in the proximal collecting tubules, but it is a low affinity, high capacity for glucose. Its expression was unchanged in the experimental groups of the current study. While Norton et al. have indeed found that the expression of SGLT2 is unchanged in T2DM patients15, our findings are in contrast to an earlier study that reported a down-regulation of renal SGLT2 expression after Duodenal Jejunal Bypass in Wistar rats17. We did not detect significant changes in the mRNA levels of Glut1 and Glut2, suggesting that these passive glucose transporters on the vascular membrane are unchanged by RYGB or weight loss. This also fits the observation of unchanged GLUT1 and GLUT2 expression in individuals with T2DM compared to controls15.
Contrary to our hypothesis, there were no significant differences in expression of oxidative stress-related genes Gpx1, Cat, Sod1, nor the inflammatory marker Mcp1. Ramos et al. have reported that increased adiposity may amplify the oxidative stress and inflammation that accompany moderate to severe CKD, as they found a correlation between adiposity and oxidative stress markers F2-isoprostanes, protein thiols, and CRP in a cross-sectional study of 184 patients with moderate to severe CKD18. While such negative effects may subsequently contribute to the decline in renal function and increased cardiovascular disease in obesity, the data from the current rat model do not support a decrease in key regulatory genes of the oxidative stress pathway.
On the other hand, Tgfβ mRNA was significantly downregulated in RYGB rats compared to SHAM, and its upstream inhibitor AMPK was activated by phosphorylation19. TGFβ is thought to be a major driver of fibrosis and key mediator of the hypertrophic and prosclerotic changes in diabetic nephropathy20, and thus downregulation seems to be a potential favorable effect in our gastric bypass model. We have to keep in mind though, that previous treatment attempts with addition of a monoclonal antibody against TGFβ1 added to renin-angiotensin system inhibitors did not slow progression of diabetic nephropathy 21.
Although the findings of reduced Sglt1 and Tgfβ expression after RYGB intervention are positive and reassuring, our study has its limitations. First, as in any animal study, the translatability of our findings in the current rat study to human patients is uncertain. Even though the ZDF rat model is a validated and widely used model for T2DM, with or without metabolic surgery, our data should ideally be confirmed in human patients. Secondly, we choose to assess the markers of kidney disease at a relatively short time after surgery (30 days after surgery) at a time of maximal weight discrepancy between RYGB and SHAM groups. However, this time point might be too early to see the full extent of effect of weight loss, improved glucose handling and restorative signals to various tissues, including the kidney. Finally, we did not perform morphological evaluation of the glomeruli. A previous report in ZDF rats showed that RYGB and weight matching can induce a 30–50% reduction in sclerotic lesions compared to SHAM animals, 13 weeks after the surgery22.
Conclusion
Overall, our study helps to unravel the mechanisms by which metabolic surgery is beneficial and protective for diabetic nephropathy. Namely, the remission of hyperglycemia in RYGB versus PF rats, is associated with restoration of the renal SGLT1 expression and the body weight reduction in RYGB rats compared to SHAM rats, is associated with a reduced fibrotic TGFβ signal. These findings warrant prospective human studies on the role of metabolic surgery in prevention and regression of diabetic nephropathy.
Supplementary Material
Funding - acknowledgements
The study was supported by the funding from the Research Program Committee (RPC) of the Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio (RPC # 2014-1072).
RV was supported by a fellowship of the Fulbright Commission and a fellowship of the Belgian American Educational Foundation.
Footnotes
Disclosure statement
The authors have no conflicts of interest or financial ties to disclose.
References
- 1.Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol [Internet] 2017. [cited 2019 Mar 25];12(12):2032–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28522654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabète FI du. Eighth edition 2017. 2017. [Google Scholar]
- 3.Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 Inhibition for the Prevention and Treatment of Diabetic Kidney Disease: A Review. Am J Kidney Dis [Internet] 2018. [cited 2019 Mar 25];72(2):267–77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29866460 [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes — 5-Year Outcomes. N Engl J Med [Internet] 2017;376(7):641–51. Available from: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wexler DJ, Davies MJ, Mathieu C, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia [Internet] 2018;61(12):2461–98. Available from: 10.1007/s00125-018-4729-5 [DOI] [PubMed] [Google Scholar]
- 6.Sjöström L, Peltonen M, Jacobson P, et al. Association of Bariatric Surgery With Long-term Remission of Type 2 Diabetes and With Microvascular and Macrovascular Complications. JAMA [Internet] 2014. [cited 2019 Mar 25];311(22):2297. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24915261 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Corsino L, Shantavasinkul PC, et al. Gastric Bypass Surgery Leads to Long-term Remission or Improvement of Type 2 Diabetes and Significant Decrease of Microvascular and Macrovascular Complications. Ann Surg [Internet] 2016. [cited 2019 Mar 25];263(6):1138–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26599565 [DOI] [PubMed] [Google Scholar]
- 8.O’Brien R, Johnson E, Haneuse S, et al. Microvascular Outcomes in Patients With Diabetes After Bariatric Surgery Versus Usual Care. Ann Intern Med [Internet] 2018. [cited 2019 Mar 25];169(5):300. Available from: 10.7326/M17-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb CN, Goss LE, Almehmi A, Grams JM, Corey BL. Bariatric surgery is associated with renal function improvement. Surg Endosc [Internet] 2018. [cited 2019 Mar 25];32(1):276–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28664440 [DOI] [PubMed] [Google Scholar]
- 10.Epstein FH, Border WA, Noble NA. Transforming Growth Factor β in Tissue Fibrosis. N Engl J Med [Internet] 1994. [cited 2019 Aug 19];331(19):1286–92. Available from: 10.1056/NEJM199411103311907 [DOI] [PubMed] [Google Scholar]
- 11.Sharma K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl [Internet] 2014. [cited 2019 Aug 19];4(1):113–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25401040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, Aminian A, Hassan M, et al. Gastric Bypass Surgery Improves the Skeletal Muscle Ceramide/S1P Ratio and Upregulates the AMPK/ SIRT1/ PGC-1α Pathway in Zucker Diabetic Fatty Rats. Obes Surg [Internet] 2019. [cited 2019 Mar 25];1–8. Available from: 10.1007/s11695-019-03800-z [DOI] [PubMed] [Google Scholar]
- 13.Mulya A, Haus JM, Solomon TPJ, et al. Exercise training-induced improvement in skeletal muscle PGC-1α-mediated fat metabolism is independent of dietary glycemic index. Obesity [Internet] 2017. [cited 2019 Aug 9];25(4):721–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28349667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mocanu AO, Mulya A, Huang H, et al. Effect of Roux-en-Y Gastric Bypass on the NLRP3 Inflammasome in Pancreatic Islets from Zucker Diabetic Fatty Rats. Obes Surg 2017;26(12):3076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton L, Shannon CE, Fourcaudot M, et al. Sodium-glucose co-transporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes, Obes Metab [Internet] 2017. [cited 2019 Mar 25];19(9):1322–6. Available from: 10.1111/dom.13003 [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi C, Wright EM. Regulation of the human Na + -dependent glucose cotransporter hSGLT2. Am J Physiol Physiol [Internet] 2012. [cited 2019 Mar 25];303(3):C348–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22673616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elina Akalestou, Carolina Bebi, Laurent Genser, Francesco Villa, Katharine Hunt, Geltrude Mingrone, Roger Williams, Amiel Stephanie RF. Down-regulation of Renal SGLT-2 Expression after Duodenal Jejunal Bypass: Evidence for a Gut-Kidney Axis in Glucose Metabolism (. In: Conference: American Diabetes Association. 2017. p. 1138–50. [Google Scholar]
- 18.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol [Internet] 2008. [cited 2019 Mar 25];19(3):593–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18256365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem [Internet] 2008. [cited 2019 Mar 25];283(16):10461–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18250161 [DOI] [PubMed] [Google Scholar]
- 20.Meng X, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol [Internet] 2016;12:325. Available from: 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 21.Voelker J, Berg PH, Sheetz M, et al. Anti–TGF-β1 Antibody Therapy in Patients with Diabetic Nephropathy. J Am Soc Nephrol [Internet] 2017. [cited 2019 Mar 25];28(3):953–62. Available from: https://jasn.asnjournals.org/content/28/3/953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neff KJ, Elliott JA, Corteville C, et al. Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in the Zucker diabetic fatty rat. Surg Obes Relat Dis [Internet] 2017;13(1):21–7. Available from: 10.1016/j.soard.2016.08.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


