Abstract
Purpose:
Laser-fenestrated thoracic endovascular aortic repair (LfTEVAR) in the aortic arch with covering of the left subclavian artery (LSA) orifice is challenging. To optimize fenestration, the so-called squid-capture technique has been introduced. We present here a modification to the technique that may help improve time-efficiency and safety.
Technique::
During the originally proposed squid-capture maneuver, the stent-graft is deployed in a preset snare wire loop, which is used to pull the stent graft toward the penetration device during in-situ fenestration. In preparation, the guidewire needs to be passed through the loop inside the aortic arch, which can be difficult and may predispose for embolic events. We propose here the creation of a “guidewire-through-snare-loop” configuration outside the body, which can then be reliably transferred into the aortic arch. The modified technique was successfully applied in a patient undergoing LfTEVAR for penetrating aortic ulcers.
Conclusion:
The proposed modification may help facilitate the squid-capture technique for LfTEVAR while saving time and resources. Given that LfTEVAR is becoming more frequently used, it is important to ensure technical success and safety of the procedure.
Keywords: fenestration, thoracic endovascular aortic repair, endovascular treatment/therapy, penetrating aortic ulcer, descending aorta
Introduction
Thoracic endovascular aortic repair (TEVAR) has evolved significantly during the past decade. It now allows treatment of various pathologies, including aortic dissection (AD), thoracic aortic aneurysm (TAA), and penetrating aortic ulcers (PAUs). When compared with open surgical repair (OSR), TEVAR has demonstrated superior morbidity and mortality rates.1–4 Although expertise throughout institutions is expanding continuously, TEVAR of the aortic arch remains challenging and complex aortic arch pathologies, which require covering of the left subclavian artery (LSA) orifice, are still a matter of concern. Here, fenestrated or branched stent grafts may offer a feasible option, but significant manufacturing delay and high cost limit their use. 5 Laser-fenestrated thoracic endovascular aortic repair (LfTEVAR) offers an off-label, off-the shelf solution for the aforementioned pathologies.6,7 The so-called squid-capture technique was introduced by Hongo et al 8 in 2014 to optimize contact between the penetration device and the stent graft fabric during fenestration by aligning and stabilizing the graft. 8
Here, a folded snare wire is introduced to a sheath to form a loop, which is placed into the aortic arch via the target supra-aortic vessel. A second wire is advanced over femoral artery access and passes the preset “squid”-wire loop. After the stent graft is placed inside the loop, the penetration device is introduced over a second wire. While pulling the squid wire during fenestration, this configuration helps secure positioning for precise penetration of the fabric. However, it can be challenging and time consuming to pass the guidewire through the preset squid-capture loop due to the lack of 3-dimensional imaging. Additionally, excessive wire manipulation inside the aortic arch may predispose for embolic events.
We report here a modification to the original squid-capture technique that allows to reliably set up a “guidewire-through-snare-loop” configuration outside the body. We applied the modified technique successfully in several patients and one example is presented in the following.
Technique
The procedure was performed in an 80-year-old woman diagnosed with thoracic PAU (30 mm maximum diameter, aortic segment III). In her medical history, she has had partial pancreatectomy in 2019 and right nephrectomy in 2003 for metastatic clear renal cell carcinoma. Additionally, the patient suffered from hypertension, type 2 diabetes and was treated with a direct oral anticoagulant (Apixaban) for pulmonary embolism. Furthermore, she underwent left-sided hemicolectomy for adenocarcinoma in 1994.
Location, morphology, and dimension of the PAU (Figure 1) required timely treatment. We offered an endovascular approach with LfTEVAR, as the patient was considered high risk for OSR. Informed consent was obtained prior to surgery and the patient gave written consent to publishing her case.
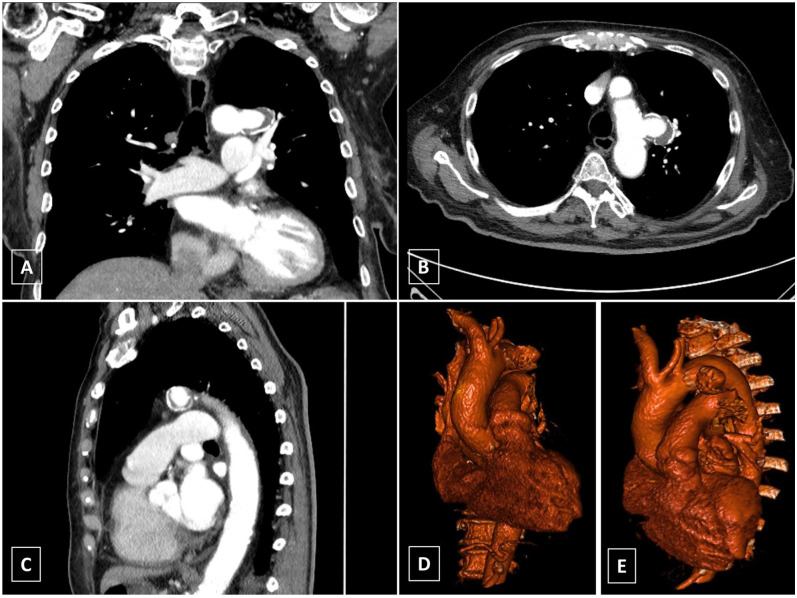
Figure 1.
Aortic computed tomography imaging of the Patient. An 80-year-old woman was diagnosed with thoracic penetrating aortic ulcer of 30 mm maximum diameter in aortic segment III. Because of the short proximal sealing zone, covering of the left subclavian artery orifice was necessary for treatment with laser-assisted in situ fenestrated thoracic endovascular aortic repair (A-C). Three-dimensional volume rendered reconstruction (D-E).
The procedure was performed in a hybrid operating room (OR) under general endotracheal anesthesia. Left femoral artery (LFA) access was obtained percutaneously and a hydrophilic-coated Glidewire (Terumo, Eschborn, Germany) was advanced into the descending aorta through an 8F sheath (Cook Medical, Bloomington, IN, USA). A second Glidewire was advanced through a 10F sheath (Cook Medical, Bloomington, IN, USA), placed in the left axillary artery (LAA) into the descending aorta. Here it was snared from the left groin and retrieved, allowing through-and-through access from the LSA to the LFA (Figures 2A and 3A).
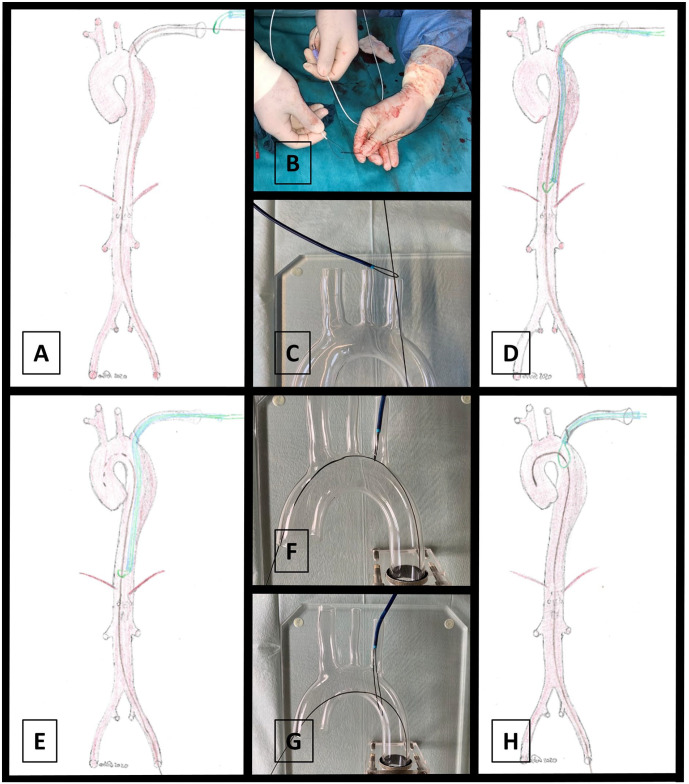
Figure 2.
Description of the squid-capture maneuver and modification. Through-and-through access from the left subclavian artery (LSA) to the left femoral artery (LFA) is established (A). A 0.018 roadrunner wire (Cook Medical, Bloomington, IN, USA) is folded in two to form a loop at the tip of a snare catheter sheath (B). The through-and through wire is passed through the snare loop outside the patient (C). Next, the loop-mounted sheath is advanced into the descending aorta along with the through-and-through wire via LSA access (D). The through-and-through wire is retrieved into the aortic arch and then advanced into the ascending aorta (E). Now, the snare catheter sheath is retrieved and the loop positioned at the LSA takeoff (F and G).
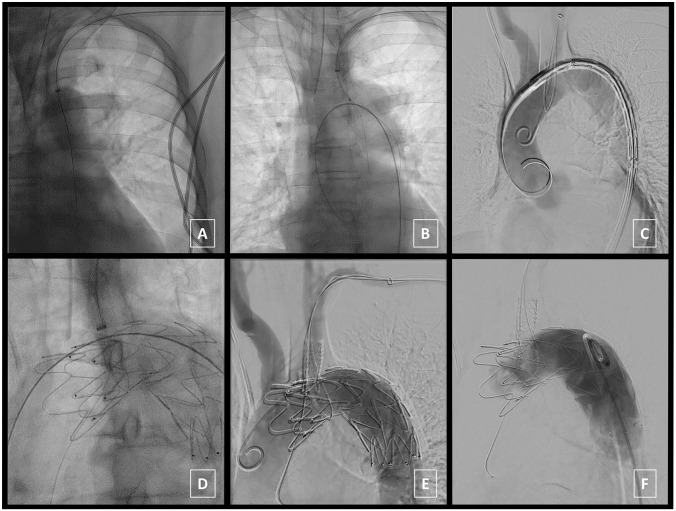
Figure 3.
In situ imaging during procedure. Through-and-through access from the left subclavian artery (LSA) to the left femoral artery (LFA) is established (A). A 0.018 roadrunner wire (Cook Medical, Bloomington, IN, USA) is folded in two to form a loop at the tip of a snare catheter sheath, transferred inside the aorta and widened (B). An extra-stiff Lunderquist wire (Cook Medical, Bloomington, IN, USA) is introduced through the LFA access to deliver the thoracic stent graft [34 mm diameter/113 mm length (Zenith Alpha Thoracic Endovascular Graft, Cook Medical, Bloomington, IN, USA)] through the snare wire loop and position it distal to the LSA takeoff (C). Next, a Turbo Elite laser catheter [2 mm diameter (Spectranetics, Colorado Springs, CO, USA)] is guided onto the stent-graft over a second 0.018 roadrunner wire. Laser-assisted in situ fenestration is performed while pulling both ends of the squid snare loop wire to maximize contact between stent graft fabric and laser catheter (D). The 0.018 roadrunner wire was advanced through the laser catheter into the ascending aorta and angioplasty balloons are used to expand the fenestration. Next, a covered stent graft [10 mm diameter/37 mm length (BeGraft, Bentley, Hechingen, Germany)] is deployed into the fenestration (E). A completion angiogram confirms exclusion of the penetrating aortic ulcer (PAU) without endoleak and sufficient flow through the stented in situ fenestration to the LSA (F).
Now, a 0.018 roadrunner wire (Cook Medical, Bloomington, IN, USA) was folded in two to form a loop at the tip of a snare catheter sheath. In contrast to the originally described squid-capture technique, the through-and through wire was passed through the snare loop outside the patient (Figure 2B). Next, the loop-mounted sheath was advanced into the descending aorta along with the through-and-through wire through the LSA access (Figure 2C and D). The through-and-through wire was carefully retrieved into the aortic arch and then advanced into the ascending aorta (Figure 2E). Now the snare catheter sheath was retrieved to position the loop just at the LSA takeoff (Figures 2F-H and 3B). The Glidewire was replaced with an extra-stiff Lunderquist wire (Cook Medical, Bloomington, IN, USA) through the LFA access. Then, we used a second rail to introduce another Glidewire over the LAA access to be positioned in the ascending aorta, which was then replaced with a pigtail-catheter for digital subtraction angiography (DSA).
Now, the thoracic stent graft [34 mm diameter/113 mm length (Zenith Alpha Thoracic Endovascular Graft, Cook Medical, Bloomington, IN, USA)] was delivered through the LFA access, advanced through the snare wire loop and positioned distal to the LSA takeoff (Figure 3C). DSA confirmed correct positioning prior to deployment.
Next, a Turbo Elite laser catheter [2 mm diameter (Spectranetics, Colorado Springs, USA)] was guided onto the stent-graft over a second 0.018 roadrunner wire. Laser-assisted in situ fenestration was performed while pulling both ends of the squid snare loop wire to maximize contact between stent graft fabric and laser catheter (Figure 3D). Technical success of stent graft fabric fenestration was achieved in first attempt. Following fenestration, the 0.018 roadrunner wire was advanced through the laser catheter into the ascending aorta. The laser catheter was withdrawn and replaced for 4- and 6-mm angioplasty balloons to expand the fenestration. Next, the 0.018 roadrunner wire was replaced with a Glidewire to deploy the covered stent graft [10 mm diameter/37 mm length (BeGraft, Bentley, Hechingen, Germany)] into the fenestration (Figure 3E).
A completion angiogram was performed that confirmed exclusion of the PAU without endoleak and sufficient flow through the stented in situ fenestration to the LSA (Figure 3F).
LFA access was closed using Vascular ProGlide devices (Abbott, Chicago, IL, USA) while LAA access was closed by suturing. Total procedure time was 232 minutes, total use of contrast agent was 55 mL, dose area product (DAP) was 36.7 Gy·cm2, and total fluoroscopy time was 39 minutes. The patient was extubated in the hybrid OR and transferred to the intensive care unit for surveillance. The patient was transferred to ward the following day without complications and discharged at day 7 postsurgery.
Discussion
The squid-capture technique, first introduced by Hongo et al 8 in 2014, allows precise TEVAR fenestration to maintain perfusion, if covering of one or more supra-aortic trunks is inevitable. By ensuring direct contact between the penetration device and the stent graft fabric, the squid-capture maneuver facilitates in-situ fenestration without need for prior carotid-subclavian bypass surgery or custom-made fenestrated stent-grafts.
Although the technique is straightforward and does not require special equipment, technical issues may arise when trying to pass the preset squid snare loop inside the aortic arch. This can be aggravated due to lack of 3-dimensional imaging, wide aortic arch dimensions and emergency settings.
We modified the technique by passing the squid snare loop with a through-and-through wire outside the patient. This configuration can reliably be transferred into the aortic arch, and therefore promises a substantial improvement of the squid-capture technique. We feel that this potentially time- and fluoroscopy-saving modified approach may augment the application range to a variety of clinical scenarios while requiring minimal additional material. It may also reduce the risk for embolic events such as stroke, since intra-aortic wire manipulations are minimized, although thorough evaluation in a larger patient cohort is warranted to validate these potential advantages.
We used laser to create the fenestration in the stent-graft fabric, while several other authors, including the pioneers of the technique, used needle puncture.8–10 We prefer laser-assisted fenestration as the vaporizing laser energy deposition creates a clean cutting margin, while needle puncturing may result in rather frayed fabric fibers. This hypothesis is supported by bench-top studies that found enhanced fraying of the fenestration perimeter following needle-based penetration techniques.11,12 Additionally, 2 small-sized case series found low complication rates applying laser-assisted fenestration, suggesting a safe use with high patient benefit.6,7 In our study, a 2-mm diameter laser catheter was used due to availability, but smaller-sized laser catheters might also be applicable.
Noteworthy, a recent metanalysis summarized outcomes of 44 patients from 16 case series and found a technical success rate as high as 95.6%. The authors reported 2 failed attempts during 46 fenestrations. Notably, one reported failure was due to a tortuous subclavian artery with acute angle, which compromised contact between laser catheter and stent graft. 13
The largest and most recent study to this point analyzed outcomes of 148 patients undergoing LfTEVAR for acute and subacute aortic arch diseases. 14 In their cohort, the authors reported 4 cases with technical failure during laser-assisted fenestration. Again, all of these were due to acute angle between laser device and thoracic stent graft. The authors proposed the use of tip-adjustable sheaths or multiview fluoroscopy as a potential solution; however, the squid-capture technique may enable similar corrections during the procedure while being more cost- and time-effective. Worth mentioning, there are alternative techniques, such as inflating a compliant balloon inside the graft as counter bearing during fenestration, which also aim to ensure stable contact between the penetration device and the stent graft fabric. Commonly, all these techniques are particular important in in type II and III aortic arches, where the laser catheter tends to slip forward to the ascending aorta. 7
In a more recent report, Hongo et al 15 presented midterm outcomes in 17 patients that underwent LfTEVAR with use of the squid-capture technique during in situ fenestration. No endoleaks, aneurysm progression, or reintervention were reported, suggesting good durability of this innovative approach. 15
LfTEVAR holds a lot potential for treatment of various pathologies of the aortic arch. Maneuvers like the squid-capture technique and growing expertise throughout institutions help make it safer and establish its use in elective settings. While recent studies suggest good short-term results with acceptable complication rates, long-term outcomes are pending thorough evaluation.
Conclusion
In summary, our proposed modification to the squid-capture technique for LfTEVAR may help with reliable and fast positioning of the guidewire through the snare loop. Because the through-and-through wire passes the snare loop ex vivo, it may minimize intra-aortic manipulation. Since LfTEVAR may become more popular for treatment of the aortic arch and descending aorta, the squid-capture technique may be applied more frequently, which in turn underlines the need to ensure high technical success and safety of the procedure.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by DFG to JM, HS, and MUW (TRR259, Project A07).
ORCID iDs: Joscha Mulorz  https://orcid.org/0000-0002-7435-6558
https://orcid.org/0000-0002-7435-6558
Markus Udo Wagenhäuser  https://orcid.org/0000-0002-6597-1364
https://orcid.org/0000-0002-6597-1364
References
- 1. Cambria RP, Crawford RS, Cho JS, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255–1264.e1-4. doi: 10.1016/j.jvs.2009.07.104 [DOI] [PubMed] [Google Scholar]
- 2. Tespili M, Banfi C, Valsecchi O, et al. Endovascular treatment of thoracic aortic disease: mid-term follow-up. Catheter Cardiovasc Interv. 2007;70:595–601. doi: 10.1002/ccd.21262 [DOI] [PubMed] [Google Scholar]
- 3. Brandt M, Mussel K, Walluscheck KP, et al. Stent-graft repair versus open surgery for the descending aorta: a case-control study. J Endovasc Ther. 2004;11:535–538. doi: 10.1583/04-1219.1 [DOI] [PubMed] [Google Scholar]
- 4. Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–377. doi: 10.1016/j.jtcvs.2006.07.040 [DOI] [PubMed] [Google Scholar]
- 5. Michel M, Becquemin JP, Clément MC, et al. Editor’s Choice. Thirty day outcomes and costs of fenestrated and branched stent grafts versus open repair for complex aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;50:189–196. doi: 10.1016/j.ejvs.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 6. Redlinger RE, Ahanchi SS, Panneton JM. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J Vasc Surg. 2013;58:1171–1177. doi: 10.1016/j.jvs.2013.04.045 [DOI] [PubMed] [Google Scholar]
- 7. Qin J, Zhao Z, Wang R, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. J Am Heart Assoc. 2017; 6:e004542. doi: 10.1161/JAHA.116.004542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hongo N, Miyamoto S, Shuto R, et al. “Squid-capture” modified in situ stent-graft fenestration technique for aortic arch aneurysm repair. Cardiovasc Intervent Radiol. 2014; 37:1093–1098. doi: 10.1007/s00270-014-0933-y [DOI] [PubMed] [Google Scholar]
- 9. Hongo N, Miyamoto S, Shuto R, et al. Endovascular aortic arch reconstruction using in situ stent-graft fenestration in the brachiocephalic artery. J Vasc Interv Radiol. 2011;22: 1144–1148. doi: 10.1016/j.jvir.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 10. Sonesson B, Resch T, Allers M, et al. Endovascular total aortic arch replacement by in situ stent graft fenestration technique. J Vasc Surg. 2009;49:1589–1591. doi: 10.1016/j.jvs.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 11. Riga CV, Bicknell CD, Basra M, et al. In vitro fenestration of aortic stent-grafts: implications of puncture methods for in situ fenestration durability. J Endovasc Ther. 2013;20:536–543. doi: 10.1583/12-4175.1 [DOI] [PubMed] [Google Scholar]
- 12. Saari P, Manninen H. Fenestration of aortic stent graftsin vitro tests using various device combinations. J Vasc Interv Radiol. 2011;22:89–94. doi: 10.1016/j.jvir.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 13. Crawford SA, Sanford RM, Forbes TL, et al. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J Vasc Surg. 2016;64:244–250. doi: 10.1016/j.jvs.2016.03.445 [DOI] [PubMed] [Google Scholar]
- 14. Li C, Xu P, Hua Z, et al. Early and midterm outcomes of in situ laser fenestration during thoracic endovascular aortic repair for acute and subacute aortic arch diseases and analysis of its complications. J Vasc Surg. 2020;72:1524–1533. doi: 10.1016/j.jvs.2020.01.072 [DOI] [PubMed] [Google Scholar]
- 15. Hongo N, Miyamoto S, Wada T, et al. Total endovascular aortic arch reconstruction (zone 0 thoracic endovascular aortic repair) for aortic arch aneurysm using in situ stent graft fenestration: midterm outcomes. J Vasc Surg. 2018;67:e59. doi: 10.1016/j.jvs.2018.03.033 [DOI] [Google Scholar]