Abstract
The study aimed to explore chronotype-specific effects of two versus four consecutive morning or night shifts on sleep-wake behavior. Sleep debt and social jetlag (a behavioral proxy of circadian misalignment) were estimated from sleep diary data collected for 5 weeks in a within-subject field study of 30 rotating night shift workers (29.9 ± 7.3 years, 60% female). Mixed models were used to examine whether effects of shift sequence length on sleep are dependent on chronotype, testing the interaction between sequence length (two vs. four) and chronotype (determined from sleep diaries). Analyses of two versus four morning shifts showed no significant interaction effects with chronotype. In contrast, increasing the number of night shifts from two to four increased sleep debt in early chronotypes, but decreased sleep debt in late types, with no change in intermediate ones. In early types, the higher sleep debt was due to accumulated sleep loss over four night shifts. In late types, sleep duration did not increase over the course of four night shifts, so that adaptation is unlikely to explain the observed lower sleep debt. Late types instead had increased sleep debt after two night shifts, which was carried over from two preceding morning shifts in this schedule. Including naps did not change the findings. Social jetlag was unaffected by the number of consecutive night shifts. Our results suggest that consecutive night shifts should be limited in early types. For other chronotypes, working four night shifts might be a beneficial alternative to working two morning and two night shifts. Studies should record shift sequences in rotating schedules.
Keywords: night work, shift scheduling, shift rotation, sleep regularity, social jetlag, sleep deprivation, rotation speed
Work schedules are often tight corsets for sleep-wake behavior, requiring workers to get up or go to sleep at times that may or may not be in synch with their biological rhythms. This is especially true in rotating shift schedules, where shift workers need to cope with changing work hours, including night shifts. As a result, sleep in shift workers is often shortened, mistimed, and of poor quality (Boivin and Boudreau, 2014). To reduce adverse effects on health, social life, and safety, guidelines on night and shift work offer several recommendations for shift scheduling, including fast and forward rotating schedules, avoiding early morning starts, and limiting the number of consecutive night shifts to three or less (Burgess, 2007; Knauth, 1993). Most studies on the effects of consecutive shifts have focused on sleepiness, cognitive performance, and occupational injuries during night shifts, with mixed results: several studies reported less cognitive impairment after four compared with two consecutive night shifts (Chang et al., 2014, 2017), and saw peak impairments after the third night shift (Behrens et al., 2019; Lamond et al., 2004), whereas others have found no or limited evidence for adaptation effects (Ganesan et al., 2019; Magee et al., 2016; McHill and Wright, 2019; Fischer, Lombardi, Folkard, et al., 2017). However, even in studies that observed improvements, performance levels were still lower than those during day shifts. One factor for these inconsistencies might be the context in which consecutive shifts occur: if night shift performances are assessed in isolation from other shift types (i.e., surrounded by days off or in laboratory simulations), a very different picture could emerge, as compared with measures that are embedded into a rotational schedule (where, for example, quick changes between early and night shifts can be observed). Shift schedules are often characterized as fast or slowly rotating, determined by how many shifts of the same type are scheduled in a row. For instance, fast rotations may consist of two morning shifts, followed by two evening and two night shifts, whereas a slow rotation may have a weekly or monthly rhythm for each shift type, only separated by work-free days. The distinction may not always be straightforward: In some schedules, each type of shift has its own periodicity (e.g., six morning, two evening, three night shifts). That periodicity may also change within a rotation cycle, that is, working two night shifts the first week, but four night shifts the week after. Detailed analyses of sleep-wake behavior in shift sequences, instead of characterizing entire shift schedules as fast or slow, are needed to evaluate current recommendations for the number of consecutive shifts and potentially add to guidelines.
A majority of both day and shift workers experience sleep debt: They accumulate sleep loss during the workweek, which they partially compensate for by sleeping longer on days off (Paine and Gander, 2016; Roenneberg et al., 2012; Seo et al., 2021). Similarly, sleep on workdays occurs usually earlier compared with work-free days, resulting in a misalignment of sleep times that has been coined “social jetlag” (Roenneberg et al., 2019; Wittmann et al., 2006). Social jetlag has been associated with adverse health outcomes in both day and shift workers (Henderson et al., 2019, Parsons et al., 2015, Roenneberg et al., 2012). There is a growing body of evidence suggesting that chronotype (i.e., the behavioral manifestation of underlying circadian rhythms) modulates sleep-wake behavior in day and shift schedules (Kervezee et al., 2021; Korsiak et al., 2018; Razavi et al., 2019; Roepke and Duffy, 2010; van de Ven et al., 2016). For example, cross-sectional questionnaire and performance data collected in rotational shift workers demonstrated that late chronotypes on average perform better, sleep longer, and experience less circadian misalignment on night shifts compared with early chronotypes (Juda et al., 2013a; Vetter et al., 2012). For morning shifts, with an early start time of 0600 h, the opposite pattern has been described, with early types sleeping longer and better, experiencing less circadian misalignment and performing better than late types.
Here, we compared sleep-wake behavior across two versus four consecutive morning and night shifts and their respective work-free days in a within-subject field study of 30 shift workers. We used interaction analysis between our sleep-wake behavior outcomes and chronotype to examine whether shift sequences might have differential effects depending on an individual’s chronotype. We hypothesized that increasing the number of consecutive night shifts (from two to four) will result in an increase of sleep debt and social jetlag for early chronotypes but a decrease for late types. Increasing the number of consecutive morning shifts, in turn, was expected to result in an increase of sleep debt and social jetlag for late chronotypes but a decrease for early types.
Methods
All participants provided written informed consent, and study design and materials were approved by the ethical committee of the Ludwig-Maximilian-University Munich as well as the workers’ council at the respective industry plant.
Study Design
Data were collected in 2008 in Germany as part of a field study examining cognitive performance in shift workers, who were employed at an electronics manufacturing plant with homogeneous work units, in terms of product, work processes, and supervision (for more details on the study, refer to Vetter et al., 2012). Thirty-four participants filled out daily sleep logs over 5 weeks, including time of preparing to fall asleep, minutes required to fall asleep, time of awakening, time of getting up, use of alarm clocks (yes/no), and sleep quality (1 = poorest, 10 = best). Information about shift schedules were provided by the management and verified by participants in the sleep logs, noting any deviations from their regular schedules (i.e., sick days, fill-ins). Participants worked a forward-rotating, 3 × 8 h system with transition times of 0600 h (morning shift), 1400 h (evening shift), and 2200 h (night shift; Suppl. A, Fig. S1).
Shift Sequences
Shift Sequences of Different Length (2- vs. 4-Day Blocks)
The shift schedule was divided into “blocks” of consecutive workdays, with either a 2- or a 4-day morning shift block (2M vs. 4M), a block of four evening shifts (4E), and either a 2- versus 4-day night shift block (2N vs. 4N). Each sequence ended on the first day off after a block of workdays.
Shift Sequences of Equal Length (5-Day Blocks)
Effects of consecutive shifts can be biased by comparing different numbers of workdays. To increase comparability between night shift sequences, we post hoc determined sequences of equal length: five consecutive days including one work-free day and four workdays (2M + 2N vs. 4N), followed by first day off.
Data Processing
Exposure Variables
The primary exposure variables were chronotype and the consecutive number of morning (2M vs. 4M) and night shifts (2N vs. 4N). Our primary interest was the interaction between chronotype and number of consecutive shifts. The secondary exposure variable was the order of shifts (first, second, etc.).
Chronotype assessment
Chronotype was calculated from sleep diaries as midsleep on work-free days after evening shifts, corrected for potential sleep loss on workdays (MSFEsc; Juda et al., 2013b). It was used as a continuous variable in regression models but as a categorical variable for visualization purposes. Cut-offs for categories were chosen in line with previous studies (Fischer, Lombardi, Marucci-Wellman, and Roenneberg, 2017): early chronotypes MSFEsc < 0330h intermediate types MSFEsc 0330 h to 0530 h, and late types MSFEsc > 0530 h.
Outcome variables
Sleep variables were determined from sleep logs, including sleep onset, offset, midsleep, duration, and quality (subjective scale 1-10). The two main outcome variables were sleep debt and social jetlag, calculated as the relative difference between average sleep duration and timing on workdays and sleep on the first day off. We specifically chose the first day off to estimate acute effects of sleep deprivation after a block of work shifts. Sleep deprivation or restriction is followed by “recovery sleep” (e.g., longer than usual sleep) that gradually reverts to habitual sleep after one or more nights. As such, sleep on the first day off (instead of, for example, average sleep duration) was considered a more direct assessment of acute effects of sleep loss than average sleep duration. Sleep debt and social jetlag were formulated so that negative values signified shorter (for duration) or earlier (for timing) sleep on workdays compared with the first day off after a given shift sequence:
| (1) |
| (2) |
where is the number of consecutive workdays, is sleep duration on workdays, is sleep duration on the first day off, is midsleep on workdays, and is midsleep on the first day off. We furthermore calculated relative differences between sleep duration and timing on each shift (first, second, etc.) within a given shift block and first day off. The goal of these secondary outcome variables was to examine how sleep changed over time in a given shift sequence.
In post hoc analyses, we also estimated cumulative sleep loss (SLc) for each shift sequence by calculating the difference between average workday sleep duration in that sequence and average sleep duration on work-free days after evening shifts (as a proxy for individual sleep need, given that this sleep episode is typically the least restricted one in shift workers [Juda et al., 2013b]), multiplied by the number of workdays:
| (3) |
where is the number of workdays, is the average sleep duration on workdays, and is the average sleep duration on work-free days after evening shifts (proxy for sleep need).
Covariates
We included between-subject variables age and sex even though they remain stable across repeated measurements to improve generalizability and to provide estimates that are also useful in the context of studies with samples different to ours in age and sex composition.
Statistical Analyses
Mixed models with random intercept (by participant) were calculated separately for morning shifts and night shifts. Models included the following fixed effects: number of consecutive shifts (two vs. four) or the order of consecutive shifts (first, second, etc.); chronotype (continuously, MSFEsc); interaction term for number or order of consecutive shifts with chronotype; age (continuously); and sex. We used Q-Q plots and Shapiro-Wilk tests to test for normal distribution of model residuals; results showed no assumption violation. We verified heteroscedasticity using Breusch-Pagan tests and by plotting residuals versus fitted values. Spearman’s rank correlations were calculated due to non-linear relationships between chronotype and sleep duration variables. Analyses involving SLc or sequences of equal length (5-day blocks) as exposure variables were conducted post hoc. Level of significance was corrected for multiple comparisons and set to 0.001 for effects not included in our hypotheses (two-sided testing, that is, sleep quality, onsets, offsets) and to 0.002 for hypothesis-driven effects (one-sided testing, that is, sleep duration and timing). Data processing and analyses were conducted in R (version 3.6.3).
Sensitivity and Additional Secondary Analyses
In sensitivity analyses, we excluded all first days off with use of alarm clocks to test the independence of effects from forced wakeups. Additional secondary analyses tested the robustness of our findings using alternate calculations of average sleep duration (i.e., including naps to calculate an average 24 h sleep duration) and alternate approaches to calculating sleep debt (i.e., using the average sleep duration on work-free days after evening shifts and an average of subsequent days off after a given sequence [i.e., first and second vs. first, second, and third day off] as reference for the calculation of sleep debt, instead of only the first day off).
Results
Full model descriptions including random effects can be found in Supplement A, Tables S1 to S11.
Sample Description
Of initially 34 participants, 4 were not included due to incomplete observations, for example, they worked two, but not four, consecutive shifts during the study period. Table 1 shows sample characteristics, and Tables 2 to 4 show descriptives of sleep variables. The early chronotype group was on average older than intermediate and late chronotypes (χ2 = 23.6, p = 0.05; Table 1).
Table 1.
Sample characteristics.
| Variable | Chronotype (MSFEsc) | |
|---|---|---|
| Age a (years) | Total | 29.9 (7.3) |
| Early (<0330 h) | −40.3 (2.5) | |
| Intermediate (0330 h-0530 h) | 29.1 (6.1) | |
| Late (>0530 h) | 28.4 (7.8) | |
| Sex (female) | Total | 18 (60%) |
| Early (<0330 h) | 2 (66%) | |
| Intermediate (0330 h-0530 h) | 9 (60%) | |
| Late (>0530 h) | 7 (58%) | |
| Nappers (%) | Total | 25 (83%) |
| Early (<0330 h) | 3 (100%) | |
| Intermediate (0330 h-0530 h) | 12 (80%) | |
| Late (>0530 h) | 10 (83%) |
Abbreviation: MSFEsc = chronotype proxy (midsleep on work-free days after evening shifts, corrected for potential sleep loss during evening shifts). Values are expressed as mean (standard deviation), respectively, number (percentage). Total N = 30. Chronotype: early n = 3 (MSFEsc < 0330 h), intermediate n = 15 (MSFEsc 0330 h-0530 h), late n = 12 (MSFEsc > 0530 h).
Early chronotypes were on average older than the other chronotype groups (Kruskal-Wallis χ2 = 23.6, p = 0.05).
Table 2.
Sleep variables I.
| Variable | Chronotype (MSFEsc) | Difference Between 2N and First Day Off After 2N | Difference Between 4N and First Day Off After 4N |
|---|---|---|---|
| ΔSD (h) | Total | −2.6 (1.7) | −1.8 (1.6) |
| Early (<3:30) | −3.3 (1.2) | −4.8 (1.4) | |
| Intermediate (3:30-5:30) | −2.5 (1.8) | −2.2 (1.4) | |
| Late (>5:30) | −2.8 (1.8) | −0.9 (0.8) | |
| ΔMS (h) | Total | 4.3 (1.9) | 4.8 (2.1) |
| Early (<3:30) | 6.3 (0.5) | 7.2 (0.7) | |
| Intermediate (3:30-5:30) | 4.6 (1.7) | 5.6 (1.1) | |
| Late (>5:30) | 3.1 (1.6) | 3.2 (2.2) |
Abbreviations: MSFEsc = chronotype proxy (midsleep on work-free days after evening shifts, corrected for potential sleep loss during evening shifts); 2N = two consecutive night shifts; 4N = four consecutive night shifts; ΔSD = proxy of sleep debt; calculated as difference in sleep duration between workdays and first day off; ΔMS = proxy of circadian misalignment (“social jetlag”); calculated as difference in midsleep timing between workdays and first day off. Values are expressed as mean (standard deviation). Note that negative values represent shorter sleep (ΔSD), respectively, earlier sleep (ΔMS) on workdays than on days off.
Table 4.
Sleep variables III.
| Variable | Chronotype (MSFEsc) | Seq1: 2M + 2N | Seq2: 4N |
|---|---|---|---|
| ΔSD (h) | Total | −2.2 (1.5) | −1.4 (1.4) |
| Early (<3:30) | −1.9 (0.7) | −3.9 (1.0) | |
| Intermediate (3:30-5:30) | −1.7 (1.4) | −1.5 (1.3) | |
| Late (>5:30) | −2.9 (1.7) | −0.6 (0.6) | |
| Sleep duration (h) | Total | 6.4 (0.9) | 7.0 (0.9) |
| Early (<3:30) | 5.8 (1.2) | 5.5 (1.1) | |
| Intermediate (3:30-5:30) | 6.7 (0.8) | 6.9 (0.8) | |
| Late (>5:30) | 6.3 (0.8) | 7.4 (0.7) | |
| Sleep quality (1-10) |
Total | 6.7 (1.7) | 6.8 (1.6) |
| Early (<3:30) | 6.2 (2.6) | 5.3 (2.5) | |
| Intermediate (3:30-5:30) | 6.3 (1.8) | 6.5 (1.6) | |
| Late (>5:30) | 7.4 (1.1) | 7.4 (1.1) | |
| Cumulative sleep loss (SLc) (h) |
Total | 8.6 (5.4) | 6.4 (3.8) |
| Early (<3:30) | 7.9 (7.3) | 9.7 (4.3) | |
| Intermediate (3:30-5:30) | 8.3 (5.8) | 6.6 (3.7) | |
| Late (>5:30) | 9.2 (4.9) | 5.0 (3.8) |
Abbreviations: MSFEsc = chronotype proxy (midsleep on work-free days after evening shifts, corrected for potential sleep loss during evening shifts); Seq1: 2M + 2N = shift sequence including two morning shifts, one day off, and two night shifts; Seq2: 4N = shift sequence including one day off and four night shifts; ΔSD = proxy of sleep debt; calculated as difference in sleep duration between the shift sequence and first day off (negative values = shorter sleep on shift days); SLc = cumulative sleep loss; calculated as sum of hours of sleep lost across several workdays. Values are expressed as mean (standard deviation). Values of timing-related sleep variables ΔMS (“social jetlag”), midsleep, sleep onset, and sleep offset are not provided for sequences of equal length (“Seq1: 2M + 2N” and “Seq2: 4N”), since averaging clock times across morning and night shifts is not meaningful.
Table 3.
Sleep variables II.
| Variable | Chronotype (MSFEsc) | 2N | First Day Off After 2N | 4N | First Day Off After 4N |
|---|---|---|---|---|---|
| Sleep duration (h) | Total | 6.0 (1.3) | 8.6 (1.5) | 6.4 (1.2) | 8.3 (1.3) |
| Early (<3:30) | 4.4 (1.7) | 7.7 (0.6) | 4.6 (1.7) | 9.4 (0.5) | |
| Intermediate (3:30-5:30) | 6.0 (1.2) | 8.4 (1.6) | 6.3 (0.8) | 8.4 (1.6) | |
| Late (>5:30) | 6.4 (1.0) | 9.2 (1.5) | 7.1 (1.1) | 7.9 (0.7) | |
| Midsleep (hh: mm) | Total | 10:18 (0.9) | 6:02 (2.0) | 10:38 (0.8) | 5:50 (2.4) |
| Early (<3:30) | 9:27 (0.4) | 3:08 (0.9) | 9:37 (0.5) | 2:27 (0.7) | |
| Intermediate (3:30-5:30) | 10:11 (0.8) | 5:32 (1.5) | 10:34 (0.7) | 4:58 (0.9) | |
| Late (>5:30) | 10:41 (1.0) | 7:34 (1.7) | 10:58 (0.9) | 7:46 (2.4) | |
| Sleep onset (hh: mm) | Total | 7:17 (0.7) | 1:43 (2.2) | 7:25 (0.6) | 1:40 (2.7) |
| Early (<3:30) | 7:14 (0.6) | 23:16 (0.8) | 7:19 (0.6) | 21:46 (0.9) | |
| Intermediate (3:30-5:30) | 7:11 (0.6) | 1:19 (1.9) | 7:25 (0.6) | 0:45 (1.4) | |
| Late (>5:30) | 7:29 (0.8) | 2:59 (2.2) | 7:26 (0.7) | 3:48 (2.4) | |
| Sleep offset (hh: mm) | Total | 13:17 (1.4) | 10:22 (2.1) | 13:51 (1.3) | 9:59 (2.3) |
| Early (<3:30) | 11:40 (1.2) | 7:00 (1.1) | 11:55 (1.2) | 7:08 (0.5) | |
| Intermediate (3:30-5:30) | 13:11 (1.3) | 9:46 (1.4) | 13:42 (0.9) | 9:11 (1.0) | |
| Late (>5:30) | 13:53 (1.4) | 12:09 (1.6) | 14:31 (1.3) | 11:43 (2.4) | |
| Sleep quality (1-10) |
Total | 6.9 (2.1) | 7.1 (2.5) | 6.5 (1.9) | 6.4 (2.9) |
| Early (<3:30) | 5.3 (3.8) | 4.5 (4.9) | 5.0 (3.3) | 6.0 (3.5) | |
| Intermediate (3:30-5:30) | 6.4 (1.7) | 6.9 (2.2) | 6.2 (1.8) | 5.9 (3.15) | |
| Late (>5:30) | 7.1 (1.3) | 7.0 (2.6) | 8.1 (1.6) | 7.8 (2.4) | |
| Cumulative sleep loss (SLc) (h) |
Total | 3.8 (2.3) | N/A | 6.4 (3.9) | N/A |
| Early (<3:30) | 5.4 (2.8) | 10.1 (5.5) | |||
| Intermediate (3:30-5:30) | 4.1 (2.4) | 6.8 (3.6) | |||
| Late (>5:30) | 3.1 (2.1) | 5.0 (3.6) |
Abbreviations: MSFEsc = chronotype proxy (midsleep on work-free days after evening shifts, corrected for potential sleep loss during evening shifts); 2N = two consecutive night shifts; 4N = four consecutive night shifts; SLc = cumulative sleep loss; calculated as sum of hours of sleep lost across several workdays. Values are expressed as mean (standard deviation). Values of cumulative sleep loss (SLc) are provided for workdays only.
Increasing the Number of Consecutive Morning Shifts Had No Significant Effects on Sleep
Morning-shift sleep showed the known chronotype-dependency: Sleep before morning shifts occurred later (b = 0.4, p < 0.001) and was shorter (b = −0.7, p < 0.001) the later the chronotype. However, sleep was similar in the 2M versus 4M block, with no significant chronotype interaction. This means that while sleep before morning shifts was modulated by chronotype, the chronotype-specific pattern was overall comparable for 2M versus 4M.
Increasingthe Number of Consecutive Night Shifts Increased Sleep Debt for Early Chronotypes, but Decreased Sleep Debt for Late Chronotypes
As was the case for morning shifts, chronotype affected night shift sleep: Sleep after night shifts occurred significantly later (b = 0.4, p = 0.002) the later the chronotype (Table 5). When night shifts increased from 2N to 4N, sleep debt increased for early types, but decreased for late types (Figure 1a; significant interaction effect between shift sequence and chronotype; b = 0.7, p < 0.001, Table 5). Secondary analyses using different reference days to calculate sleep debt showed the same patterns, demonstrating robustness of our findings (Figure 2 and Suppl. B, Fig. S2). We did not observe an effect of shift sequence or an interaction effect of shift sequence and chronotype on any other sleep variable (i.e., social jetlag, sleep onset, offset, midsleep, and quality).
Table 5.
Interaction effects between chronotype and number of consecutive night shifts on sleep duration variables.
| Exposure | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| ΔSD | SDw | SD1stf | SLc | |||||
| Estimate (SE, 95% CI) |
p Value | Estimate (SE, 95% CI) |
p Value | Estimate (SE, 95% CI) |
p Value | Estimate (SE, 95% CI) |
p Value | |
| Shift sequences of different length | ||||||||
| 4N (ref: 2N) |
−2.6 (0.9, −4.5, −0.7) |
0.011 | 0.1 (0.7, −1.2, 1.4) |
0.844 | 2.8 (1.5, 1.0, 4.6) |
0.005 | 5.7 (1.8, 2.2, 9.3) |
0.004 |
| MSFEsc | 0.1 (0.2, −0.3, 0.4) |
0.631 | 0.3 (0.1, 0.1, 0.6) |
0.023 | 0.2 (0.2, −0.1, 0.6) |
0.216 | −0.7 (0.4, −1.5, 0.1) |
0.095 |
| MSFEsc × 4N | 0.7 (0.2, 0.3, 1.0) |
0.001 | 0.1 (0.1, −0.2, 0.3) |
0.619 | −0.6 (0.2, −1.0, −0.3) |
0.002 | −0.6 (0.4, −1.3, 0.1) |
0.082 |
| Shift sequences of equal length | ||||||||
| Seq2: 4N (ref: Seq1: 2M + 2N) |
−4.2 (1.1, −6.3, −2.1) |
<0.001 | −1.4 (0.6, −2.6, −0.2) |
0.036 | 2.8 (1.1, 0.7, 4.9) |
0.013 | 3.4 (3.6, −3.6, 10.4) |
0.353 |
| MSFEsc | −0.4 (0.2, −0.7, −0.1) |
0.022 | −0.2 (0.1, −0.4, 0.0) |
0.118 | 0.2 (0.2, −0.1, 0.6) |
0.234 | 0.1 (0.7, −1.2, 1.4) |
0.877 |
| MSFEsc × Seq2: 4N | 1.0 (0.2, 0.6, 1.4) |
<0.001 | 0.4 (0.1, 0.1, 0.6) |
0.002 | −0.6 (0.2, −1.0, −0.2) |
0.001 | −1.2 (0.7, −2.5, 0.2) |
0.108 |
Abbreviations: Estimate = age- and sex-adjusted unstandardized regression coefficient b; ΔSD = proxy of sleep debt; calculated as difference in sleep duration between workdays and first day off; SDw = average sleep duration on workdays; SD1stf = sleep duration on first day off; SLc = cumulative sleep loss; calculated as sum of hours of sleep lost across several workdays; SE = standard error; CI = confidence interval; 4N = four consecutive night shifts; 2N = two consecutive night shifts; MSFEsc = chronotype proxy (midsleep on work-free days after evening shifts, corrected for potential sleep loss during evening shifts). Seq2: 4N = shift sequence including one day off and four night shifts; Seq1: 2M + 2N = shift sequence including two morning shifts, one work-free day, and two night shifts. Note that the significance threshold was set to 0.001 for effects not included in hypotheses (i.e., sleep quality, onsets, offsets) and to 0.002 for hypothesis-driven effects (i.e., sleep duration and timing).
Figure 1.
Chronotype-specific sleep debt for two versus four consecutive night shifts: (a) Sleep debt, calculated such that negative values indicate shorter sleep on workdays than first day off. (b) Average sleep duration after night shifts. (c) Average sleep duration on first day off after night shifts. (d) and (e) Sleep duration by order of consecutive night shifts. (f) Sleep onsets and offsets after night shifts. Abbreviations: ΔSleep duration = proxy for sleep debt; 2N = two consecutive night shifts; 4N = four consecutive night shifts. Note that chronotype was used as a continuous variable in regression models and cut-offs are used for illustration purposes only.
*Significant (p ≤ 0.002) interaction effect “chronotype × number of consecutive shifts.”
Figure 2.
Alternate approaches to calculating sleep debt. Different ways of calculating sleep debt were compared: the difference in sleep duration between (a) the average on workdays and the first day off (primary outcome of this study); (b) the average on workdays and the average across the first, second, and third day off; (c) the average on free days and the first day off; and (d) the average on workdays and the average on free days. Panels a-c show robust results for the effect of chronotype and shift sequences (2N vs. 4N) on sleep debt. While the more typical calculation of sleep debt as illustrated in panel d shows the known chronotype-dependency in sleep debt, it does not capture the otherwise observed interaction between chronotype and shift sequence. This calculation shows similar patterns for 2N and 4N, even though sleep loss accumulates over consecutive workdays. The similar patterns are due to the fact that average sleep duration across all free days is used as the reference, being the same for 2N and 4N. Abbreviations: 2N = two consecutive night shifts; 4N = four consecutive night shifts.
DoesLower Sleep Debt After 4N Mean Late Chronotypes Sleep Longer on Workdays When Working More Nights?
Increasing the number of consecutive night shifts from two to four increased sleep duration on workdays for late chronotypes by 40 min on average (vs. 11 min and 17 min in early and intermediate types, respectively; Figure 1b). Despite this clinically relevant effect size, this increase for late types was not statistically significant (interaction: b = 0.61, p = 0.619, Table 5). The number of night shifts did, however, significantly affect sleep duration on the first day off: after 4N, late chronotypes slept 77 min less than after 2N, whereas early types slept 99 min longer (Figure 1c). When comparing between chronotype groups, unexpectedly, sleep duration on the first day off after 2N was substantially longer in late types (9.2 h) than in early ones (7.7 h; interaction: b = 0.6, p = 0.010), suggesting that late types needed more recovery sleep after 2N than early chronotypes.
Analyzing sleep duration on each individual shift (first, second, etc.) showed no significant differences: Sleep was already longer for late types after the first night shift in the 4N versus 2N sequence, and did not gradually increase over consecutive night shifts (Figure 1d and 1e). The longer recovery sleep in early types on the first day off after 4N versus 2N was driven by an earlier sleep onset (interaction: b = 0.6, p = 0.010), while the shorter recovery sleep observed in late types resulted from both a later onset and earlier offset after 4N versus 2N (Figure 1f). We thus tested whether the shorter sleep on first day off after 4N in late types was due to forced wakeups, and repeated analyses excluding work-free days where participants indicated waking up with alarm clocks (excluding 8% of first days off). Our results were robust, suggesting that forced wakeups were not driving the chronotype-specific pattern of sleep we observed on the first day off. Taken together, the findings suggest that sleep debt on the shift sequences 2N and 4N did not account for the recovery sleep pattern observed on the first day off. In post hoc analyses, we therefore examined (1) cumulative sleep loss and (2) shift sequences containing the same number of workdays.
Late Chronotypes Accumulate Less Sleep Loss Across Night Shifts Than Earlier Types
To account for effects of chronically curtailed sleep that accumulates over successive workdays, we calculated cumulative sleep loss (SLc) in post hoc analyses. Figure 3c shows that for both 2N and 4N, sleep loss was lower in late chronotypes than in early or intermediate ones. As for sleep debt, sleep loss across night shifts did not explain the duration of recovery sleep on the first day off (compare Figure 3c and 3d). Specifically, sleep loss after 2N was lower in late types (SLc = 3.1 h) than in intermediate (4.1 h) and early ones (5.4 h); yet, despite late types having lower sleep loss, their recovery sleep on the first day off was longer (9.2 h) than that of intermediate (8.4 h) and early types (7.7 h). This finding indicates that neither sleep debt nor cumulative sleep loss on night shift sequences explained why recovery sleep after 2N was longer in late types than in early ones.
Figure 3.
Chronotype-specific cumulative sleep loss (SLc): (a) and (b) 28-day shift schedule with morning shifts (light gray boxes), evening shifts (gray boxes), night shifts (dark gray boxes), and work-free days (white boxes). Striped boxes indicate first days off following a given shift sequence. Panel a shows 2-and 4-day blocks of consecutive night shifts (2N, 4N). Panel b shows 5-day blocks, including four workdays and one free day (2M + 2N, 4N). (c) Chronotype-specific SLc for shift sequences 2N versus 4N. (d) Sleep duration on first day off after 2N versus 4N. (e) Chronotype-specific SLc for 5-day shift sequences 2M + 2N versus 4N. (f) Sleep duration on first day off after 5-day shift sequences 2M + 2N versus 4N. Abbreviations: 4N = four consecutive night shifts; 2N = two consecutive night shifts; Seq2: 4N = shift sequence including one work-free day and four night shifts; Seq1: 2M + 2N = shift sequence including two morning shifts, one work-free day, and two night shifts. Note that chronotype was used as a continuous variable in regression models and cut-offs are used for illustration purposes only.
*Significant (p ≤ 0.002) and #marginally significant (p < 0.10) interaction effect “chronotype × number of consecutive shifts.”
Late Chronotypes Accumulate More Sleep Loss Than Earlier Types When Night Shifts Are Preceded by Morning Shifts
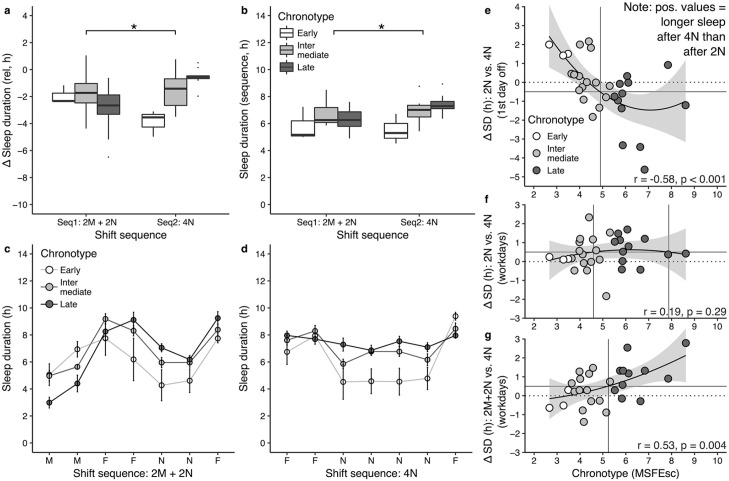
To account for the fact that shift sequences are not isolated occurrences but embedded into schedules of rotational work hours, we also analyzed 5-day blocks of shift sequences (i.e., four workdays, one day off) in post hoc analyses, taking into account preceding workdays. In the present shift schedule, 2N were preceded by two morning shifts (2M) and one day off in-between. Sequence 1 (“2M + 2N”) thus included 2M, one work-free day, and 2N. Sequence 2 (“4N”) included one work-free day and 4N. As before, sleep debt decreased for late types and increased for early types when night shifts in a sequence of 5 days increased from 2N to 4N (interaction: b = 1.0, p < 0.001; Figure 4a). Unlike before, the change in sleep debt was due to significant changes in sleep duration on both the first day off and during the sequence (Figure 4b-4d): sleep duration increased on average by 66 min in late types when comparing the 2M + 2N with the 4N sequence (interaction: b = 0.4, p = 0.002). On the first day off, sleep decreased by 77 min in late types and increased by 99 min in early types (interaction: b = −0.6, p = 0.001). Examining cumulative sleep loss, SLc now perfectly mirrored recovery sleep on the first day off (compare Figure 3e and 3f), although effects were not significant (interaction: b = −1.2, p = 0.108). Specifically, sleep loss after 2M + 2N was higher in late chronotypes than in early types (9.2 h vs. 5.0 h), being in line with recovery sleep on the first day off after 2M + 2N that was longer in late types than in early ones (9.2 h vs. 7.7 h). Results did not change when sleep duration estimates included napping (see Suppl. C, Figs. S3-S5). The finding indicates that, by taking into account morning shifts that preceded night shifts in this schedule, cumulative sleep loss on workdays accounted for chronotype differences in recovery sleep observed on the first day off.
Figure 4.
Chronotype-specific sleep in shift sequences including preceding morning shifts: (a) Sleep debt, calculated such that negative values indicate shorter sleep on workdays than first day off. (b) Average sleep duration in 5-day shift sequences: “Seq1: 2M + 2N” includes two morning shifts, one work-free day, and two night shifts; “Seq2: 4N” includes one work-free day and four night shifts. (c) and (d) Sleep duration by sleep episode in the shift sequence. Note that five workdays equal six associated sleep episodes, due to the night shift, for example, for the 3-day sequence “morning shift—work-free day—night shift,” there are four sleep episodes: one before the morning shift, a second after the morning shift and onto the work-free day, a third after the work-free day onto the first night shift, and a fourth the next day after the first night shift. (e) Difference in sleep duration on the first day off for 2N versus 4N. (f) Difference in sleep duration on workdays for 2N versus 4N. (g) Difference in sleep duration for 2M + 2N versus 4N. Positive values in panels e-g indicate more sleep for sequences with four night shifts than for sequences with two night shifts (2N, 2M + 2N). Abbreviations: Seq1: 2M + 2N = shift sequence including two morning shifts, one work-free day, and two night shifts; Seq2: 4N = shift sequence including one work-free day and four night shifts; 2N = two consecutive night shifts; 4N = four consecutive night shifts; MSFEsc = chronotype proxy. Note that chronotype was used as a continuous variable in regression models and cut-offs are used for illustration purposes only.
*Significant (p ≤ 0.002) interaction effect “chronotype × number of consecutive shifts.”
Late Chronotypes Are Most Likely to Benefit From Consecutive Night Shifts but the Extent Depends on Preceding Shifts
We defined “benefitting” from a schedule by a reduction in sleep debt, specifically longer sleep on workdays and shorter sleep on the first day off. Seventy-seven percent (n = 23) in our sample slept longer on workdays when working four versus two night shifts; yet, the absolute increase was rather small (of those 23, 9 had an increase < 0.5 h; Figure 4f). The same pattern was observed for recovery sleep on the first day off (Figure 4e): 63% (n = 19) slept less after 4N versus 2N, but the absolute decrease was rather small (<0.5 h) for 7 of the 19 shift workers. The difference between individuals, however, was large: three late types slept as much as 4.6 h less on the first day off after 4N versus 2N (Figure 4e). The large decrease in some late types was driven by very long sleep durations on the first day off after 2N, with one participant, for example, sleeping 12.7 h after 2N and 8.1 h after 4N. The sleep duration observed after 4N in this case was equivalent to this participant’s average sleep duration on work-free days. When analyses took into account preceding morning shifts, all but two late chronotypes (83%) benefited from working 4N compared with 2M + 2N (Figure 4g). The result was similar for intermediate types: 64% (n = 9 out of 14) slept longer working 4N than 2M + 2N, though for 3 of the 9 (33%) the increase was less than 0.5 h. Cut-off values based on fitted curves (Figure 4e and 4f) suggested that shift workers with a chronotype later than ~5:00 might benefit from working 4N versus 2N in terms of sleep duration. R2 values were larger for polynomial than linear fits, indicating a saturation effect such that additional benefits for extremely late chronotypes (MSFEsc > 8:00) might be limited (Figure 4f). However, when comparing 4N with 2M + 2N, even extremely late types benefited (Figure 4g), suggesting a linear relationship when morning shifts were involved (r = 0.53, p = 0.004).
Discussion
In this within-subject field study of 30 shift workers, we examined chronotype-specific effects of consecutive shifts on sleep using 5 weeks of continuous sleep-wake reports. This allowed us to examine two specific blocks of shifts, namely, two versus four consecutive morning and night shifts. We calculated the difference in sleep duration and timing between workdays and the first day off to estimate sleep debt and social jetlag. Unexpectedly, sleep debt and social jetlag did not change with an increased number of consecutive morning shifts. However, sleep debt after night shifts increased in early, but decreased in late chronotypes, with no meaningful change in intermediate chronotypes. In early types, the increase in sleep debt from 2N to 4N was attributable to accumulated sleep loss over four consecutive night shifts. Sleep on workdays was consistently short (<5 h) resulting in catch-up sleep on the first day off, that was on average ~2 h longer after 4N compared with 2N. In late types, sleep debt decreased from 2N to 4N, but interestingly, sleep duration did not increase over successive night shifts, suggesting that adaptation might not be the reason for the observed decrease in sleep debt. Rather, sleep debt was unexpectedly high in late types after 2N, which were, in this schedule, preceded by two early morning shifts. In fact, the combination of morning and night shifts within a 5-day timeframe led to an overall decreased sleep duration on workdays for late chronotypes. Late types compensated for this by sleeping in more on the first day off following 2N, ~1.5 h longer than following 4N. Our results were robust, independent of alarm clock usage and napping. Results were also similar when using different calculations of sleep debt, indicating that effects of sleep restriction as a function of shift sequence are consistently dependent on chronotype.
While night shifts represent the most disruptive and strenuous shifts for all shift workers, if they are necessary, increasing night shifts from two to four may result in less sleep debt for late chronotypes, especially when the alternative is a combination of morning and night shifts. Correlational cut-off values indicated that a workers’ chronotype would need to be later than (MSFEsc) ~5:00 to likely see benefits for their sleep-wake behavior when working four successive night shifts, which is close to the cut-off we chose for defining a late chronotype (>5:30). Improvements were only apparent in sleep duration and did not translate to improvements in other sleep variables (i.e., sleep quality), likely because the absolute benefits were rather small. A recent study found large inter-individual variability in the direction and magnitude of phase shifts in urinary 6-sulphatoxymelatonin (aMT6s), a melatonin metabolite, after exposure to three to four consecutive night shifts (Stone et al., 2018). Including chronotype improved their model prediction of individual phase shifts and explained more variance than other factors, with late types showing the largest phase delays. Their findings provide further support that individual chronotype is a determining factor for adaptation to consecutive night shifts. However, both our findings and those presented by Stone and colleagues describe large inter-individual variability that went beyond categories of chronotype, suggesting that other factors, such as behavioral patterns and light-dark exposure, may also play a role.
In line with a recent study by Garde et al. (2020), we saw no adaptation effects: Sleep duration did not significantly increase over the course of 2N or 4N. Studies using physiological markers (i.e., melatonin, cortisol) reported progressive delays of circadian phase over the course of successive night shifts (Jensen, Hansen, et al., 2016) but no adaptation (Jensen, Garde, et al., 2016). Full circadian adaptation to night shifts seems limited even in permanent night shift workers (Folkard, 2008). Importantly, night shifts often do not occur in isolation from other shift types (day/morning or evening shifts). When we included two morning shifts that preceded the two night shifts in this schedule, we observed that late types experienced a higher cumulative sleep loss than after four night shifts. This high level of sleep loss then resulted in catch-up sleep on the first day off that was longer than catch-up sleep after 4N. Our findings highlight two important factors: (1) Chronotype effects are often not straightforward but most likely involve interactions with other exposure variables (i.e., number and type of shift), and (2) shift sequences such as 2N and 4N are often embedded in rotational schedules with alternating shift times. Findings for consecutive night shifts in permanent schedules are likely to be different from those for night shifts in the context of day/morning and evening shifts. Our results furthermore suggest that any number of night shifts regardless of chronotype may be best scheduled between (several) days off.
The finding that late chronotypes appeared to have carried over sleep loss from preceding morning shifts also suggests that one day off in-between morning and night shifts may not be sufficient for recovery. Studies using polysomnographic assessment of sleep suggest that one night of recovery sleep may be enough in terms of slow-wave sleep and sleep stages (Åkerstedt et al., 2009; Hennecke et al., 2019). However, studies on self-report variables, performance measures, and physiological factors suggest higher numbers of recovery days: >2 days for metabolic dysregulation (Depner et al., 2019), ≥3 days for fatigue and subjective health (Haluza et al., 2019; Lekander et al., 2013), and >7 days for cognitive lapses (Axelsson et al., 2008).
Our findings showed that early types accumulated tremendous amounts of sleep loss over consecutive night shifts (66% lost > 5 h of sleep over 2N). Previous studies have reported early types to frequently nap before night shifts, attenuating sleep loss to such an extent that the relationship between a later chronotype and longer sleep for night shifts was no longer statistically significant (Fischer et al., 2016; Kervezee et al., 2021). We observed a similar napping pattern in our study; yet, while naps did mitigate sleep loss during workdays, early types still had longer recovery sleep on their first day off than late types, suggesting that napping did not fully compensate for cumulative sleep loss. A limitation of our study is the fact that we had only three early chronotypes in our sample (MSFEsc < 0330 h), hampering generalizability. However, it is well-established that night shifts carry on average a higher risk for early types, in terms of sleep (Fischer et al., 2016), health (Vetter et al., 2015), and occupational safety (Del Rio-Bermudez et al., 2014). Taken together, we argue that even a few night shifts must be regarded as potentially hazardous. This suggests that night work should be limited for early chronotypes, in particular successive night shifts.
Contrary to our hypotheses, we did not see significant interaction effects between chronotype and the number of morning shifts. Late types seemed to gradually advance their sleep onset over 4M resulting in slightly (but non-significantly) increased sleep duration on workdays (data not shown). Previous studies have reported more irregular sleep-wake behavior in late types (Fischer et al., 2016; Phillips et al., 2017), which may reflect the ability to sleep at various circadian phases, thereby facilitating adaption. Even so, the slight increase in sleep duration for late types was not enough to offset their sleep loss accumulated over consecutive morning shifts.
Strength and Limitations
Our study has several strengths, but also limitations. One strength is the within-subject design of our study, which minimized confounding. A recent study on the impact of consecutive nights on sleep duration and quality (Garde et al., 2020) considered the fact that they had a washout period of 7 days before each block of night shifts as an advantage of their study, having minimized carry-over effects. We see it as a strength of our study to not have had such washout intervals: Many shift schedules involve rotating shift times, and detailed analyses of how consecutive shifts are embedded in the wider schedule are highly informative and much needed. Thus, our study provides highly granular insights of ecological validity.
Several limitations are noteworthy. Additional descriptive data (in particular, the use of sleep aids) were not available for this sample. Early chronotypes were on average older than other chronotype groups. The relationship between early chronotype and older age is well-established (Roenneberg et al., 2004; Fischer, Lombardi, Marucci-Wellman, and Roenneberg, 2017; van de Ven et al., 2016); as such, we do not regard it as a limitation but a representation of the real world. Despite this between-group difference, it is unlikely that it confounded our results significantly in view of the within-subject design. Our chronotype groups were not equally distributed. In fact, we only had three participants in the earliest category. It is noteworthy that we used chronotype as a continuous variable in all our regression models. In view of the on average higher health and safety risk that night shifts carry for early types, we do not expect our findings to change in studies with a more balanced chronotype distribution. The sole outcome of our study was sleep-wake behavior assessed from sleep diaries. Even though we did not measure performance or fatigue, we would expect the changes in sleep to translate to changes in performance, given the vast evidence that sleep deprivation leads to cognitive impairments (Van Dongen et al., 2003; Elmenhorst et al., 2018). The exact magnitude may depend on additional factors, including task load, light environment, monotony, and so on. Time awake was not considered in regression models because shift workers frequently napped before night shifts, ranging in duration from 15 min to 5 h. Future investigations should explore the link between naps, time awake, and main sleep-wake behavior on different shift sequences. Our calculated measures sleep debt and cumulative sleep loss are rather crude. Effects of sleep loss on subsequent recovery sleep are not a zero-sum game (−1 h on workdays = + 1 h on days off); sleep quality and architecture (i.e., NREM REM cycles) play an important role, but could not be assessed in this field study setting. Using the duration of sleep without information on sleep architecture or circadian timing will always be limited in assessing the “true” amount of sleep debt. First, the relationship between accumulated sleep loss and subsequent recovery sleep is non-linear due to ceiling effects. For example, an extended 11 h recovery sleep after 4 night shifts may not extend further after >4 night shifts. Second, sleep duration is also constrained by a circadian process (e.g., Daan et al., 1984), for example, sleep may end prematurely despite an elevated homeostatic sleep pressure. In shift work, the alignment of sleep relative to the circadian cycle may be different between workdays and recovery days. Hence, the circadian constraint on sleep duration may also differ, which might introduce a confound in sleep debt assessments from sleep duration alone. This could affect early and late chronotypes differently, such that a difference in sleep debt could instead be a difference in circadian timing. With increasing availability of wearable devices to track sleep architecture (e.g., Winnebeck et al., 2018, Walch et al., 2019), and techniques to assess circadian phase in free-living individuals (e.g., Laing et al., 2017, Braun et al., 2018; Wittenbrink et al., 2018), future studies in shift workers may be able to overcome the limitations of behavioral actigraphy and sleep log methods. While we did not assess circadian timing based on a physiological measure (i.e., dim light melatonin onset [DLMO]), we aimed to limit the impact of this potential confound by adjusting our statistical models for midsleep timing, a behavioral proxy for differences in circadian timing between chronotypes (Kantermann et al., 2015, Ghotbi et al., 2020). Last, what might be beneficial for sleep might not also result in positive effects for other factors, such as social and family life. Evening shifts are a prominent example: They interfere least with sleep-wake behavior but limit social participation.
Concluding Remarks
To the best of our knowledge, this is the first study to address chronotype-specific effects of consecutive shifts on sleep. Our results suggest that the number of consecutive night shifts should be limited in early chronotypes, and that increasing the number of consecutive night shifts reduces sleep debt in late chronotypes, especially if the alternative would be to include early morning shifts within a shift block that includes night shifts. These results add to the growing body of evidence that chronotype should be considered a relevant factor when designing shift schedules. Future studies should record exact shift sequences, together with chronotype.
Supplemental Material
Supplemental material, sj-pdf-1-jbr-10.1177_07487304211006073 for Chronotype-specific Sleep in Two Versus Four Consecutive Shifts by Dorothee Fischer, Till Roenneberg and Céline Vetter in Journal of Biological Rhythms
Acknowledgments
We thank all participants and Dr. Myriam Juda for her help in recruiting participants and collecting data. This work was supported by a scholarship of the Hanns-Seidl-Foundation to D.F.
Footnotes
Conflict of Interest Statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.F. and T.R. declare no conflicts of interest. C.V., during the part of this work, received research support from the NIH, the Colorado Clinical and Translational Sciences Institute, and the University of Colorado Boulder; was an unpaid scientific advisory board member of Circadian Light Therapy Inc. and Chronosulting; and served as a paid consultant to the U.S. Department of Energy and NIOSH outside of the submitted work. Study design, data processing and analysis, and result presentation and interpretation were independent from third parties.
ORCID iDs: Dorothee Fischer  https://orcid.org/0000-0002-2122-3938
https://orcid.org/0000-0002-2122-3938
Céline Vetter  https://orcid.org/0000-0002-3752-1067
https://orcid.org/0000-0002-3752-1067
Supplementary material is available for this article online.
References
- Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. (2009) Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep 32:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, Kecklund G, Åkerstedt T, Donofrio P, Lekander M, Ingre M. (2008) Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int 25:297-308. [DOI] [PubMed] [Google Scholar]
- Behrens T, Burek K, Pallapies D, Kösters L, Lehnert M, Beine A, Wichert K, Kantermann T, Vetter C, Brüning T, et al. (2019) Decreased psychomotor vigilance of female shift workers after working night shifts. PLoS ONE 14:e0219087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, Boudreau P. (2014) Impacts of shift work on sleep and circadian rhythms. Pathol Biol 62:292-301. [DOI] [PubMed] [Google Scholar]
- Braun R, Kath WL, Iwanaszko M, Kula-Eversole E, Abbott SM, Reid KJ, Zee PC, Allada R. (2018) Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci U S A 115:E9247-E9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PA. (2007) Optimal shift duration and sequence: recommended approach for short-term emergency response activations for public health and emergency management. Am J Pub Health 97:S88-S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-S, Chen H-L, Wu Y-H, Hsu C-Y, Liu C-K, Hsu C. (2014) Rotating night shifts too quickly may cause anxiety and decreased attentional performance, and impact prolactin levels during the subsequent day: a case control study. BMC Psychiatry 14:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-S, Wu Y-H, Chen H-L, Hsu C-Y. (2017) Four night shifts have a degree of performance adaptation. Human Factors 59:925-936. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. (1984) Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol 246:R161-R183. [DOI] [PubMed] [Google Scholar]
- Del Rio-Bermudez C, Diaz-Piedra C, Catena A, Buela-Casal G, Di Stasi LL. (2014) Chronotype-dependent circadian rhythmicity of driving safety. Chronobiol Int 31:532-541. [DOI] [PubMed] [Google Scholar]
- Depner CM, Melanson EL, Eckel RH, Snell-Bergeon JK, Perreault L, Bergman BC, Higgins JA, Guerin MK, Stothard ER, Morton SJ, et al. (2019) Ad libitum weekend recovery sleep fails to prevent metabolic dysregulation during a repeating pattern of insufficient sleep and weekend recovery sleep. Curr Biol 29:957-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst E-M, Elmenhorst D, Benderoth S, Kroll T, Bauer A, Aeschbach D. (2018) Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A 115:8009-8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Folkard S, Willetts J, Christiani DC. (2017) Updating the “risk index”: a systematic review and meta-analysis of occupational injuries and work schedule characteristics. Chronobiol Int 34:1423-1438. [DOI] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. (2017) Chronotypes in the US: influence of age and sex. PLoS ONE 12:e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Vetter C, Roenneberg T. (2016) A novel method to visualise and quantify circadian misalignment. Sci Rep 6:38601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Vetter C, Oberlinner C, Wegener S, Roenneberg T. (2016) A unique, fast-forwards rotating schedule with 12-h long shifts prevents chronic sleep debt. Chronobiol Int 33:98-107. [DOI] [PubMed] [Google Scholar]
- Folkard S. (2008) Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int 25:215-224. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Magee M, Stone JE, Mulhall MD, Collins A, Howard ME, Lockley SW, Rajaratnam SMW, Sletten TL. (2019) The impact of shift work on sleep, alertness and performance in healthcare workers. Sci Rep 9:4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garde AH, Nabe-Nielsen K, Aarrebo Jensen M, Kristiansen J, Sørensen JK, Hansen ÅM. (2020) The effects of the number of consecutive night shifts on sleep duration and quality. Scand J Work Environ Health 46:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghotbi N, Pilz LK, Winnebeck EC, Vetter C, Zerbini G, Lenssen D, Frighetto G, Salamanca M, Costa R, Montagnese S, et al. (2020) The µMCTQ: an ultra-short version of the Munich ChronoType Questionnaire. J Biol Rhythms 35:98-110. [DOI] [PubMed] [Google Scholar]
- Haluza D, Schmidt V-M, Blasche G. (2019) Time course of recovery after two successive night shifts: a diary study among Austrian nurses. J Nurs Manag 27:190-196. [DOI] [PubMed] [Google Scholar]
- Henderson SEM, Brady EM, Robertson N. (2019) Associations between social jetlag and mental health in young people: a systematic review. Chronobiol Int 36:1316-1333. [DOI] [PubMed] [Google Scholar]
- Hennecke E, Elmenhorst D, Mendolia F, Putzke M, Bauer A, Aeschbach D, Elmenhorst E-M. (2019) Reestablishment of individual sleep structure during a single 14-h recovery sleep episode after 58 h of wakefulness. J Sleep Res 28:e12641. [DOI] [PubMed] [Google Scholar]
- Jensen MA, Garde AH, Kristiansen J, Nabe-Nielsen K, Hansen ÅM. (2016) The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): a systematic review of field studies. Int Arch Occ Env Health 89:531-545. [DOI] [PubMed] [Google Scholar]
- Jensen MA, Hansen ÅM, Kristiansen J, Nabe-Nielsen K, Garde AH. (2016) Changes in the diurnal rhythms of cortisol, melatonin, and testosterone after 2, 4, and 7 consecutive night shifts in male police officers. Chronobiol Int 33:1280-1292. [DOI] [PubMed] [Google Scholar]
- Juda M, Vetter C, Roenneberg T. (2013. a) Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms 28:141-151. [DOI] [PubMed] [Google Scholar]
- Juda M, Vetter C, Roenneberg T. (2013. b) The Munich ChronoType Questionnaire for shift-workers (MCTQShift). J Biol Rhythms 28:130-140. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Sung H, Burgess HJ. (2015) Comparing the morningness-eveningness questionnaire and Munich ChronoType Questionnaire to the dim light melatonin onset. J Biol Rhythms 30:449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervezee L, Gonzales-Aste F, Boudreau P, Boivin DB. (2021) The relationship between chronotype and sleep behavior during rotating shift work: a field study. Sleep 225. [DOI] [PubMed] [Google Scholar]
- Knauth P. (1993) The design of shift systems. Ergonomics 36:15-28. [DOI] [PubMed] [Google Scholar]
- Korsiak J, Tranmer J, Leung M, Borghese MM, Aronson KJ. (2018) Actigraph measures of sleep among female hospital employees working day or alternating day and night shifts. J Sleep Res 27:e12579. [DOI] [PubMed] [Google Scholar]
- Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN, Dijk D-J. (2017) Blood transcriptome based biomarkers for human circadian phase. eLife 6:e20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond N, Dorrian J, Burgess H, Holmes A, Roach G, McCulloch K, Fletcher A, Dawson D. (2004) Adaptation of performance during a week of simulated night work. Ergonomics 47:154-165. [DOI] [PubMed] [Google Scholar]
- Lekander M, Nixon Andreasson A, Kecklund G, Ekman R, Ingre M, Åkerstedt T, Axelsson J. (2013) Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain Behav Immun 34:43-46. [DOI] [PubMed] [Google Scholar]
- Magee M, Sletten TL, Ferguson SA, Grunstein RR, Anderson C, Kennaway DJ, Lockley SW, Rajaratnam SMW. (2016) Associations between number of consecutive night shifts and impairment of neurobehavioral performance during a subsequent simulated night shift. Scan J Work Env Health 42:217-227. [DOI] [PubMed] [Google Scholar]
- McHill AW, Wright KP. (2019) Cognitive impairments during the transition to working at night and on subsequent night shifts. J Biol Rhythms 34:432-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine S-J, Gander PH. (2016) Differences in circadian phase and weekday/weekend sleep patterns in a sample of middle-aged morning types and evening types. Chronobiol Int 33:1009-1017. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, Caspi A. (2015) Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obesity 39:842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, Czeisler CA. (2017) Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep 7:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi P, Devore EE, Bajaj A, Lockley SW, Figueiro MG, Ricchiuti V, Gauderman WJ, Hankinson SE, Willett WC, Schernhammer ES. (2019) Shift work, chronotype, and melatonin rhythm in nurses. Cancer Epi Prev Biomarkers 28:1177-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. (2012) Social jetlag and obesity. Curr Biol 22:939-943. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. (2004) A marker for the end of adolescence. Curr Biol 14:R1038-R1039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. (2019) Chronotype and social jetlag: a (self-) critical review. Biology 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke SE, Duffy JF. (2010) Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat Sci Sleep 2:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Park J, Kim S, Jung H, Sohn M, Kim Y-H. (2021) Obesity and sleep mismatch between weekends and weekdays in the Korean population according to working status. Maturitas 144:87-92. [DOI] [PubMed] [Google Scholar]
- Stone JE, Sletten TL, Magee M, Ganesan S, Mulhall MD, Collins A, Howard M, Lockley SW, Rajaratnam SMW. (2018) Temporal dynamics of circadian phase shifting response to consecutive night shifts in healthcare workers: role of light-dark exposure. J Physiol 596:2381-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven HA, van der Klink JJL, Vetter C, Roenneberg T, Gordijn M, Koolhaas W, de Looze MP, Brouwer S, Bültmann U. (2016) Sleep and need for recovery in shift workers: do chronotype and age matter? Ergonomics 59:310-324. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. (2003) The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26:117-126. [DOI] [PubMed] [Google Scholar]
- Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. (2015) Mismatch of sleep and work timing and risk of type 2 diabetes. Diab Care 38:1707-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C, Juda M, Roenneberg T. (2012) The influence of internal time, time awake, and sleep duration on cognitive performance in shiftworkers. Chronobiol Int 29:1127-1138. [DOI] [PubMed] [Google Scholar]
- Walch O, Huang Y, Forger D, Goldstein C. (2019) Sleep stage prediction with raw acceleration and photoplethysmography heart rate data derived from a consumer wearable device. Sleep 42:zsz180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnebeck EC, Fischer D, Leise T, Roenneberg T. (2018) Dynamics and ultradian structure of human sleep in real life. Curr Biol 28:49-59.e5. [DOI] [PubMed] [Google Scholar]
- Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A, et al. (2018) High-accuracy determination of internal circadian time from a single blood sample. J Clin Investigation 128:3826-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. (2006) Social jetlag: misalignment of biological and social time. Chronobiol Int 23:497-509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jbr-10.1177_07487304211006073 for Chronotype-specific Sleep in Two Versus Four Consecutive Shifts by Dorothee Fischer, Till Roenneberg and Céline Vetter in Journal of Biological Rhythms