Abstract
Background
Patellofemoral pain syndrome (PFPS) is common among adolescents and young adults. It is characterised by pain behind or around the patella and crepitations, provoked by ascending or descending stairs, squatting, prolonged sitting with flexed knees, running and cycling. The symptoms impede function in daily activities or sports. Pharmacological treatments focus on reducing pain symptoms (non‐steroidal anti‐inflammatory drugs (NSAIDs), glucocorticosteroids), or restoring the assumed underlying pathology (compounds containing glucosamine to stimulate cartilage metabolism, anabolic steroids to increase bone density of the patella and build up supporting muscles). In studies, drugs are usually applied in addition to exercises aimed at building up supporting musculature.
Objectives
This review aims to summarise the evidence of effectiveness of pharmacotherapy in reducing anterior knee pain and improving knee function in people with PFPS.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group and Cochrane Rehabilitation and Related Therapies Field trials registers, the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 4, 2003), PEDro (up to January 2004) , MEDLINE (1966 to January 2004), EMBASE (1988 to January 2004), and CINAHL (1982 to January 2004).
Selection criteria
Controlled trials (randomised or not) comparing pharmacotherapy with placebo, different types of pharmacotherapy, or pharmacotherapy to other therapies for people with PFPS.
Data collection and analysis
The literature search yielded 780 publications. Eight trials were included, of which three were of high quality. Data were analysed qualitatively using best evidence synthesis, because meta‐analysis was impeded by differences in route of administration of drugs, care programs and outcome measures.
Main results
Four trials (163 participants) studied the effect of NSAIDs. Aspirin compared to placebo in a high quality trial produced no significant differences in clinical symptoms and signs. Naproxen produced significant short term pain reduction when compared to placebo, but not when compared to diflunisal. Laser therapy to stimulate blood flow in tender areas led to more satisfied participants than tenoxicam, though not significantly.
Two high quality RCTs (84 participants) studied the effect of glycosaminoglycan polysulphate (GAGPS). Twelve intramuscular injections in six weeks led to significantly more participants with a good overall therapeutic effect after one year, and to significantly better pain reduction during one of two activities. Five weekly intra‐articular injections of GAGPS and lidocaine were compared with intra‐articular injections of saline and lidocaine or no injections, all with concurrent quadriceps training. Injected participants showed better function after six weeks, though only the difference between GAGPS injected participants and non‐injected participants was significant. The differences had disappeared after one year.
One trial (43 participants) found that intramuscular injections of the anabolic steroid nandrolone phenylpropionate significantly improved both pain and function compared to placebo injections.
Authors' conclusions
There is only limited evidence for the effectiveness of NSAIDs for short term pain reduction in PFPS. The evidence for the effect of glycosaminoglycan polysulphate is conflicting and merits further investigation. The anabolic steroid nandrolone may be effective, but is too controversial for treatment of PFPS.
Plain language summary
Drugs used to treat the symptoms of patellofemoral syndrome have little evidence base
Patellofemoral pain syndrome is common among adolescents and young adults. The most common symptom is pain surrounding the kneecap when sitting with bent knees for prolonged periods of time or when performing activities like ascending or descending stairs, squatting or athletic activities. This review of pharmacological interventions showed that non‐steroidal anti‐inflammatory drugs (NSAIDs) may reduce pain in the short term, but overall pain did not improve after three months. There is conflicting evidence on the effect of glycosaminoglycan polysulphate. The anabolic steroid nandrolone may be effective, but associated risks demand extreme caution if used for patellofemoral pain syndrome, particularly in athletes.
Background
Patellofemoral pain syndrome (PFPS) is a common complaint in adolescents and young adults. The symptom most frequently reported is a diffuse peripatellar and retropatellar localised pain, typically provoked by ascending or descending stairs, squatting, and sitting with flexed knees for prolonged periods of time (the so called 'movie sign' or 'theatre sign'). Other common symptoms are crepitus and giving‐way (Cutbill 1997; Nissen 1998; Powers 1998; Thomee 1999; Zomerdijk 1998).
In the literature there is some agreement that PFPS is a term to be applied only to people with retropatellar pain in which no cartilage damage is evident (Arroll 1997; Cutbill 1997; Holmes 1998; Juhn 1999; Thomee 1999; Wilk 1998; ). However, retropatellar pain is generally thought of as a self‐limiting condition with a good prognosis, especially for people who are young (Kannus 1994), people who have unilateral complaints, and people in which crepitation is absent (Natri 1998). This means that people are usually managed in primary care and are rarely referred to specialist care (Bourne 1988). Therefore reliable diagnostic techniques for determining cartilage damage such as computed tomography (CT), magnetic resonance imaging (MRI) or arthroscopy (Cutbill 1997; Nissen 1998) are seldom applied. In fact a diagnosis based solely on symptoms and physical examination of the knee is not uncommon. Furthermore, Natri showed that neither the radiologic nor the MRI changes seen in affected knees showed a clear association with persistent symptoms seven years later (Natri 1998). This makes the distinction between chondromalacia patellae and PFPS theoretical rather than practical, so people with either chondromalacia patellae or PFPS will be included in this review.
Increased pressure on the patellofemoral joint seems to lie at the heart of the syndrome (Grelsamer 1998), either caused by increased activity levels, malalignment of the patella when moving through the femoral groove, muscle imbalance of the quadriceps or tight anatomical structures such as the retinaculum or iliotibial band. However, it remains elusive which structures or tissues cause the pain: several studies during the last two decades have shown a poor correlation between arthroscopic evidence of articular cartilage damage and retropatellar pain (Natri 1998; Thomee 1999; ). Furthermore, cartilage is not innervated, and so subchondral bone as well as peripatellar soft tissues may be involved. Depending on the presumed mechanism at work, different approaches can be taken when applying pharmacotherapy. Therefore a brief outline is given of the presumed mechanisms.
Increased patellofemoral joint reaction stress may cause microscopic damage to the patellar cartilage through friction. In this process proteolytic enzymes are released that cause further fragmentation of the cartilage matrix. Damage to the cartilage is countered with an increased production of proteoglycans and collagen, the building stones to make repairs (Bentley 1981). On the other hand, the damaged cartilage is less efficient in absorbing stresses and a vicious circle may be the result, in which the cartilage loses its ability to defer stresses from the subchondral bone. Increased intrapatellar pressure may also impede blood flow through the patella and cause subchondral bone degeneration, which may progress to the surface and ultimately result in chondral lesions (Arnoldi 1991; Goodfellow 1976). Increased physical activity or maltracking of the patella through the femoral groove may lead to peripatellar soft tissue irritation, so that retinacular nerve endings may generate the pain (Natri 1998; Nissen 1998; Thomee 1999; Witonski 1999). It is not very likely that pain arises from the synovium because in PFPS there is limited, if any, effusion.
When approaching PFPS as a cartilage problem, pharmacotherapy may focus on chemically disrupting the destructive enzymatic processes or aid constructive processes by providing nutrients for cartilage repair. Glycosaminoglycan polysulphate (GAGPS) inhibits proteolytic enzymes, which degrade proteoglycans and collagen in the cartilage (Kannus 1992). It has also been shown to increase the rate of synthesis and the degree of polymerisation of hyaluronic acid in the synovial fluid, which would benefit cartilage repair. Aspirin has been shown to inhibit destructive enzymatic processes in cartilage in animal studies (Bentley 1981).
However, when assuming that bone degeneration precedes chondral degeneration, reversal of bone density loss could be considered. The anabolic ester nandrolone phenylpropionate is used to increase bone density and also serves to build up muscles supporting normal patellofemoral glide.
When assuming that irritation of soft tissues causes the pain, suppression of inflammatory (or sub‐inflammatory) processes could be targeted, either through the use of NSAIDs or glucocorticosteroids.
Whatever the approach, pharmacotherapy is limited to the chemical processes that result from the increased pressure in the patellofemoral joint. Therefore it usually only plays an auxiliary role in pain reduction (NSAIDs), or reversing or limiting damage (glucosamine containing compounds, anabolic steroids), while at the same time tackling the ultimate cause with physical interventions. These physical interventions are usually conservative: refraining from pain provoking activities and training the knee extensor mechanism to build up muscles supporting normal patellofemoral glide, with or without the use of tape or braces to relieve pressure on the patellofemoral joint (Arroll 1997; Cutbill 1997; Juhn 1999; Thomee 1999). In the literature consensus has been reached that surgical interventions should be avoided, and should only be considered in very severe cases which have proven to be resistant to conservative treatment (Darracott 1973).
Objectives
This review was undertaken to assess the effectiveness of pharmacotherapy in the conservative treatment for patellofemoral pain syndrome, by:
comparing pharmacotherapy with placebo treatment or no treatment
comparing different types of pharmacotherapy
comparing pharmacotherapy with other conservative treatment or surgical treatment
using anterior knee pain, knee function and subjective assessments of recovery as clinically relevant outcome measures. Measurements up to one year follow‐up were considered short term outcomes, thereafter long term.
Methods
Criteria for considering studies for this review
Types of studies
Concurrent, randomised or quasi‐randomised (i.e. allocation of participants to treatment groups which are not strictly random, such as date of birth, alternation, etc.) controlled trials (RCTs) and concurrent controlled trials without randomisation (CCTs) on pharmacotherapy for patellofemoral pain were considered. Because CCTs are more likely to introduce bias, they were considered only for qualitative analyses, to give a complete overview of published data. Retrospective studies were excluded.
Types of participants
People suffering from patellofemoral pain syndrome (including anterior knee pain syndrome and chondromalacia patellae). Studies which specifically focus on other named knee pathologies such as Hoffa's disease, Osgood Schlatter disease, Sinding‐Larsen‐Johansson's disease, iliotibial band friction syndrome, tendinitis, neuromas, intra‐articular pathology including osteoarthritis, rheumatoid arthritis, traumatic injuries (such as injured ligaments, meniscal tears, patellar fractures and patellar luxation), plica syndromes, and more rarely occurring pathologies were excluded (Nissen 1998; Thomee 1999). No restrictions on age or setting were applied.
Types of interventions
Only controlled trials including at least one treatment arm consisting of pharmacotherapy for PFPS were included in this review. Oral, topical, intra‐articular or intramuscular administration of the following pharmaceutical agents were considered for this review:
non‐steroidal anti‐inflammatory drugs (NSAIDs)
analgesics (including opiates)
steroids
biological agents and dietary supplements such as glucosamine, capsaicin, hyaluronic acid, vitamin preparations or fish oil.
Types of outcome measures
The primary outcome was knee pain intensity, measured on a visual analogue scale, numerical rating scale or pain index. Secondary outcomes focus on functional disability level and subjective assessments of recovery. Questionnaires focusing on knee function and the ability to perform tests were considered measures for functional disability (e.g. Lysholm scale for characteristics of knee function, Tegner scale for activity levels, or the ability to perform jumps or squats). Measures of recovery include ordinal rating scales (improved, no change, worse) or percentage ratings (subjective percentage improvement, where each patient estimates his/her own improvement from ‐100% (deterioration) to 100% (full recovery)).
Adverse effects like increased pain levels were taken into consideration as well. As changes on impairment level alone (i.e. range of motion, muscle strength, etc.) do not directly represent changes in the symptoms of patellofemoral pain or the resulting disability, we will not base conclusions on effectiveness on these outcome measures in this review.
Search methods for identification of studies
We searched the Cochrane Bone, Joint and Muscle Trauma Group and Cochrane Rehabilitation and Related Therapies Field trials registers, the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 4, 2003), PEDro (http://www.pedro.fhs.usyd.edu.au to January 2004), MEDLINE (1966 to January 2004), EMBASE (1988 to January 2004), CINAHL (1982 to January 2004), and reference lists of articles. We also searched the proceedings of the American Academy of Orthopedic Surgeons (AAOS) from 1989 to 2004. No language restriction was applied.
In MEDLINE (OVID WEB), the search strategy was combined with all phases of the optimal trial search strategy (Alderson 2004a) and was modified for use in other databases (seeAppendix 1).
Data collection and analysis
Selecting trials for inclusion Two reviewers (SB, MB) independently selected the trials, initially based on title and abstract. For the selected references a final decision about inclusion was made based on the full article, using a standardised form listing the inclusion criteria. Disagreements on inclusion were resolved by discussion.
Methodological quality assessment The methodological quality was assessed by two reviewers independently (BK, JV). They used the criteria list recommended by the Cochrane Bone, Joint and Muscle Trauma Group, combined with the Delphi list (Verhagen 1998) and one additional question adapted from the Maastricht‐Amsterdam consensus list for Methodological Quality Assessment (van Tulder 1997). Special emphasis was placed on the items that are included in the Delphi list (Verhagen 1998). Disagreements were solved in a consensus meeting.
In Table 1 items beginning with 'D' denote items from the Delphi‐list, while those beginning with 'M' denote items taken from the Cochrane Bone, Joint and Muscle Trauma Group methodological quality assessment tool and 'T' denotes the item from the Maastricht‐Amsterdam consensus list. In view of the complexity of the diagnosis of anterior knee pain, one more item was added for scoring whether a predefined set of diagnostic criteria was provided in the study. This criterion is denoted with 'X'. For items that were not originally in the Cochrane Bone, Joint and Muscle Trauma Group methodological quality assessment tool appropriate scores were made up instead of 'yes' 'don't know' or 'no'.
1. Methodological quality assessment scheme.
| Items | Scores | Notes |
| D1. Was a method of randomisation performed? | 2 = yes, clearly described method of randomisation 1 = unclear whether treatment allocation was truly random 0 = no, prospective study or other design without (quasi‐)random assignment | |
| M‐A. (D2) Was the assigned treatment adequately concealed prior to allocation? | 2 = method did not allow disclosure of assignment 1 = small but possible chance of disclosure of assignment or unclear 0 = quasi‐randomised or open list/tables | Cochrane code (Alderson 2004b): Clearly Yes = A; Not sure = B; Clearly No = C. |
| M‐B. (D9) Were the outcomes of patients who withdrew described and included in the analysis (intention to treat)? | 2 = withdrawals well described and accounted for in analysis 1 = withdrawals described and analysis not possible 0 = no mention, inadequate mention or obvious differences and no adjustment | |
| M‐C. (D5) Were the outcome assessors blinded to treatment status? | 2 = effective action taken to blind assessors 1 = small or moderate chance of unblinding of assessors 0 = not mentioned or not possible | |
| M‐D. (D3) Were the treatment and control group comparable at entry? | 2 = good comparability of groups, or confounding adjusted for in analysis 1 = confounding small; mentioned but not adjusted for 0 = large potential for confounding, or not discussed | |
| M‐E. (D7) Were the patients blind to assignment status after allocation? | 2 = effective action taken to blind patients 1 = small or moderate chance of unblinding patients 0 = not possible, or not mentioned (unless double‐blind), or possible but not done | |
| M‐F. (D6) Were the treatment providers blind to assignment status after allocation? | 2 = effective action taken to blind treatment providers 1 = small or moderate chance of unblinding of treatment providers 0 = not possible, or not mentioned (unless double‐blind), or possible but not done | |
| M‐G. Were care programmes, other than the trial options, identical? | 2 = care programmes clearly identical 1 = clear but trivial differences 0 = not mentioned or clear and important differences in care programmes | |
| M‐H. (D4) Were the inclusion and exclusion criteria clearly defined? | 2 = clearly defined 1 = inadequately defined 0 = not defined | |
| M‐I. Were the interventions clearly defined? | 2 = clearly defined interventions are applied with a standardised protocol 1 = clearly defined interventions are applied but the application protocol is not standardised 0 = intervention and/or application poorly or not defined | |
| M‐J. Were the outcome measures used clearly defined? | 2 = clearly defined 1 = inadequately defined 0 = not defined | |
| M‐K. Were diagnostic tests used in outcome assessment clinically useful? (by outcome) | 2 = optimal 1 = adequate 0 = not defined, not adequate | |
| M‐L. Was the surveillance active and of clinically appropriate duration? | 2 = active surveillance and appropriate duration (> three weeks) 1 = active surveillance, but inadequate duration (< three weeks) 0 = surveillance not active or not defined. | |
| D8. Were point estimates and measures of variability presented for the primary outcome measures? | 2 = point estimates and measures of variability presented 1 = point estimates, but no measures of variability presented 0 = only vague descriptions of outcome measures presented | |
| T. Was the compliance rate in each group unlikely to cause bias? | 2 = compliance well described and accounted for in analysis 1 = compliance well described but differences between groups not accounted for in analysis 0 = compliance unclear | |
| X. Was a predefined set of diagnostic criteria provided for the included patients? | 2 = clear description of diagnosis as well as diagnostic criteria were provided, or clear diagnostic exclusion criteria were provided (e.g. 'chondromalacia patellae', defined by the presence of lesions in patellar cartilage determined at arthroscopy) 1 = only diagnosis without criteria was provided (e.g. 'chondromalacia patellae') and no clear diagnostic exclusion criteria were provided 0 = neither clear diagnosis nor criteria or symptoms were provided (e.g. 'anterior knee pain') |
Scoring of each quality item of the included studies is presented in Figure 1. High quality is defined as: presenting an adequate or concealed randomisation procedure and adequate blinding (Cochrane code A), or a positive score on five or more Delphi items.
1.
Methodological quality of included studies
Data extraction Two reviewers (EH, RB) independently extracted the data regarding the interventions, type of outcome measures, follow‐up, loss to follow‐up, and outcomes. The various outcome measures were presented separately. The results of each RCT are expressed as relative risks (RR) with corresponding 95 per cent confidence intervals (CI) for dichotomous data and standardised mean differences (SMD) and 95 per cent confidence intervals for continuous data. The statistical analysis component of RevMan (RevMan 2003), was used to analyse the data.
Analysis As the included studies were heterogeneous with respect to pharmaceutical agent and/or administration mode, quantitative analysis of pooled results was not possible. A summary is given of all clinically relevant outcome measures. A further analysis was performed, using a rating system with levels of evidence based on the overall quality, and the outcome of the studies (van Tulder 1997; van Tulder 2004). The rating criteria are listed here:
strong evidence ‐ provided by generally consistent findings in multiple high quality RCTs;
moderate evidence ‐ provided by generally consistent findings in one high quality RCT and one or more lower quality RCTs, or by generally consistent findings in multiple low quality RCTs;
limited evidence ‐ provided by only one RCT (either high or low quality) or generally consistent findings in CCTs;
conflicting evidence ‐ inconsistent findings in multiple RCTs and CCTs;
no evidence ‐ no CCTs or RCTs.
Results
Description of studies
The included studies covered a wide area of pharmaceutical agents. The studies are presented here arranged by type of pharmaceutical agent under investigation. NSAIDs Bentley 1981 High quality randomised trial with 30 participants comparing aspirin to placebo. Patients for whom isometric exercising was unsuccessful for a period of three months were submitted to arthroscopy to confirm the clinical diagnosis of chondromalacia patellae and to grade chondral lesions prior to drug administration. Aspirin or placebo was administered for a period of 10 weeks. Doses and frequency of administration were not mentioned, though serum salicylate levels were monitored weekly at a constant time approximately 4 hours after ingestion of a dose to check whether the level was effective. Sixteen participants received aspirin, 13 placebo and there was one drop‐out for which the treatment was not mentioned. Arthroscopy was repeated after three months (end of follow‐up period) to establish the healing effects of aspirin on chondral lesions. Chondral lesions, clinical signs and symptoms were both rated on a three point scale: improved, no change, worse. No conflicts of interest were reported.
Marchese 1998 Low quality randomised trial with 35 participants, performed by a physiotherapist comparing the NSAID tenoxicam, administered as 20 mg once daily for 15 days, with 15 sessions of laser therapy, three times weekly. Laser therapy is thought to increase blood circulation in tender areas, intended to facilitate healing processes. Only women suffering from chondromalacia patellae for at least three months were included. Tendinopathy, patella alta, recent trauma and lesions to meniscus and ligaments were excluded based on physical examination, though pain to ligaments and positive tests for meniscal lesions (Appley's test) were reported. Follow‐up continued up to 60 days and included several pain measurements, presence of crepitations and patient satisfaction. No conflicts of interest were reported.
Suter 1998 Low quality double blind randomised trial with 42 participants comparing NSAID naproxen with placebo to study the effect on muscle inhibition and knee extensor moments in people with unilateral anterior knee pain syndrome. Muscle inhibition is thought to cause muscle atrophy, which predisposes to further injury. Participants were randomly assigned to either 550 mg naproxen or placebo twice daily for 7 days. Pain levels were determined using a visual analogue scale before and during maximal voluntary muscle contractions. The average of those measurements was used to determine statistical differences between baseline and seven days follow‐up.
Fulkerson 1986 Low quality randomised trial with 56 participants comparing the NSAIDs diflunisal and naproxen, sponsored by the producer of diflunisal. Most of the participants with anterior knee pain in this trial were diagnosed with chondromalacia patellae or PFPS. Though there were many participants with multiple diagnoses, diagnostic methods were not reported. For example, swelling is unusual for people with PFPS, though it was reported for 12 out of 20 people in the diflunisal group, and 9 out of 16 people in the naproxen group. Symptoms were mild to moderate at the start of the study. Reduction of pain and swelling were reported for the follow‐up period of five days. Administration of the drugs far exceeded this period, but criteria for continuation are not mentioned.
Glucocorticoid steroids Antich 1986 Low quality trial with 64 participants comparing four treatment modalities: a combination of ultrasound alternated with ice application, ice application alone, 1 cc Hexadrol (dexamethasone) and 1 cc of 4% Xylocaine (lidocaine) applied topically and driven into the tender area of the knee with either phonophoresis or iontophoresis. Iontophoresis and phonophoresis are both techniques to drive the topical drug into the soft tissue surrounding the patella, to reduce irritation or inflammation in these tissues. Fifty eight people were randomised, but six participants were not randomised because lack of tender areas presented a problem with the area to be targeted with treatment. These six participants were treated with ice application. The number of participants per treatment was not specified, only the number of knees of participants that had completed the 10 day treatment period (end of follow‐up period). Participants were diagnosed with chondromalacia patellae, peripatellar pain or patellar tendinitis, by physical examination. The relative frequencies of these diagnoses were not reported and patients who came to the clinic only for evaluation and instruction in a home program were excluded. The reported outcomes were subjective percentage improvement and strength increases in quadriceps and hamstrings. No conflicts of interest were reported. The unit of analysis (knees) did not equal the unit of randomisation (participants) making the results suspect.
Anabolic steroids Darracott 1973 Low quality randomised trial with 43 participants comparing intramuscular administration of an anabolic ester nandrolone phenylpropionate (Durabolin) 25 mg to intramuscular administration of placebo, both administered weekly for six weeks. The population consisted of military recruits with unilateral, severe disabling symptoms of chondromalacia patellae. Both groups performed concurrent graduated exercises to reverse quadriceps inhibition: minimal "flicking" quadriceps contractions until onset of aching 8 to 10 times a day for first three weeks, followed by gradual build up of exercise regime through functional active exercises with the patient's confidence and within pain tolerance until full circuit and battle training was possible, usually at 6 to 8 weeks. Outcome measures were clinical improvement of symptoms and bone density of the patella, determined by X‐ray after 6 to 8 weeks. No conflicts of interest were reported.
Glycosaminoglycan polysulphate (GAGPS) Kannus 1992 High quality randomised trial with 53 participants comparing five weekly intra‐articular injections of 50 mg GAGPS and 10 mg lidocaine with injections of saline and lidocaine, or no pharmacotherapy. Concomitant use of the NSAID piroxicam (20 mg daily) for 20 days as well as open kinetic chain exercising to strengthen quadriceps musculature were prescribed. All participants suffered from chronic chondromalacia patellae. Follow‐up was performed after six weeks and six months. Outcome measures cover quadriceps strength, pain (VAS), Lysholm and Tegner scales to assess function of the knee, symptoms during function tests, symptoms and signs on the patella tests, and complete recovery, as determined by the physician. A follow‐up after 7 years does not give separate results for each treatment and will therefore only be mentioned in the discussion. No conflicts of interest were reported.
Raatikainen 1990 High quality randomised trial with 31 participants with PFPS, comparing 12 intramuscular 50 mg GAGPS injections in six weeks with placebo injections. Patellar cartilage damage was evaluated arthroscopically at baseline (31 participants) and after one year (28 participants). Paracetamol was allowed as an analgesic. After the first six weeks standardised quadriceps exercises were started. Outcome measures covered pain on patella tests, pain during daily functions, hindrance to normal life and sports and overall therapeutic effect, all assessed after 6 and 10 weeks. No conflicts of interest were reported.
Risk of bias in included studies
Two reviewers (BK, JV) independently determined the methodological quality of the eight selected studies. Consensus was reached after a meeting between both reviewers. The methodological quality of the selected studies as determined during the consensus meeting is shown in Figure 1. Cohen's Kappa and % agreement were calculated for the initial scores given by each reviewer independently. Agreement between the reviewers ranged from 50% to 100% per item. If the number of Delphi items that received a maximum score exceeded 50% (i.e. at least 5 positive items), the study was labelled as 'high quality'. If the assigned treatment was adequately concealed prior to allocation (item M‐A/D2) the study received Cochrane code A, which was used as an alternative criterion for high quality of a study and is listed in the table with study characteristics. Of the three studies marked as high quality, only Bentley 1981 and Kannus 1992 received the Cochrane code A, whereas the study by Raatikainen 1990 received Cochrane code B.
Effects of interventions
Of the 780 titles and abstracts identified by the systematic search of the literature, two reviewers (SB, MB) selected eight studies that met the inclusion criteria. The methodological quality assessment of these studies, as described in the previous chapter, yielded three studies rated as 'high quality', i.e. at least five items on the Delphi list received the maximum score. The remaining two reviewers (EH, RB) extracted results from the publications. Quantitative meta‐analysis of pooled high quality studies was impossible due to the heterogeneity of the interventions used for comparison, heterogeneity of gathered outcome measures and applied instruments.
The outcome measures pain, function and clinical improvement are represented in the graphs. Outcomes that represent clinical patella tests, swelling, muscle strength, cartilage damage or bone density are only mentioned in the text below, and will not be taken into account for the best evidence synthesis.
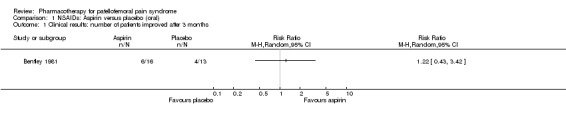
NSAIDs Bentley 1981 performed a high quality study comparing the effects of aspirin to that of placebo. Subjective clinical evaluations revealed no significant difference; 6 out of 16 participants in the aspirin group versus 4 out of 13 participants in the placebo group showing improvement of symptoms and signs (see Graphs: comparison 01.01). Due to side effects or non‐cooperation four participants in the aspirin group did not maintain effective levels of 15 to 25 mg/100 ml blood during 10 weeks. None of these participants improved, and one deteriorated. If these people are left out of the analysis, the difference between the groups is still not significant. No people in the placebo group deteriorated. Comparison of changes in cartilage injuries observed during arthroscopy at baseline and after 13 weeks revealed no improvements and one deterioration (effective level) in the aspirin group versus one improvement and two deteriorations in the placebo group.
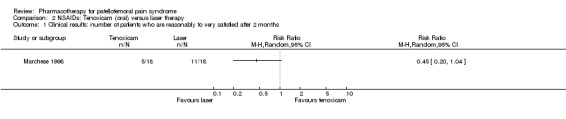
Marchese 1998 performed a low quality study comparing the NSAID tenoxicam with laser therapy alternated with ice application. In the text of the article the author claims a significant difference in pain reduction in favour of the laser therapy group (mean follow‐up value visual analogue scale 2.93 versus 4.52 for the tenoxicam group), though lack of baseline information and variability measures makes verification impossible. Other outcome measures were not reported adequately. Only the patient's satisfaction with the treatments is reported satisfactorily, and amounts to 61% (11 out of 18 participants) in the group receiving laser therapy versus 29% (5 out of 18) in the group receiving tenoxicam. The relative risk 0.45 (95%CI 0.20 to 1.04) does not reveal a significant difference (see Graphs: comparison 02.01).
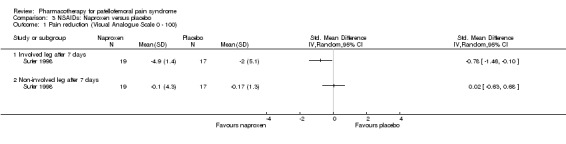
Suter 1998 performed a low quality study comparing the NSAID naproxen with placebo. Pain scores were obtained by averaging Visual Analogue Scale scores before and during maximal knee extensor contraction for each leg. Reported mean differences between baseline and 7 days follow‐up for both the involved and the non‐involved leg were used for statistical comparison of placebo and naproxen. Pain reduction was significantly greater for naproxen than for placebo in the involved leg (SMD ‐0.78, 95% CI ‐1.46 to ‐0.10), but not in the non‐involved leg (SMD 0.02, 95% CI ‐0.63 to 0.68) (see Graphs: comparison 03.01).
Fulkerson 1986 performed a low quality study in which no baseline or follow‐up values for pain have been reported, and no definition is supplied for the term "significant pain relief". No significant differences in "significant pain relief" were detected when comparing naproxen (10 out of 16 participants) with diflunisal (11 out of 20 participants) after a period of five days (RR 0.88; 95%CI 0.51 to 1.52) (see Graphs: comparison 04.01). Differences in swelling reduction were almost significant when comparing naproxen (4 out of 9 participants) with diflunisal (2 out of 12 participants) after a period of five days (RR 0.27; 95%CI 0.07 to 1.00).
Glucocorticoid steroids Antich 1986 performed a low quality study and reports mean values for "subjective improvement" which each patient was asked to rate as a percentage relative to the baseline situation. No measures of variability were supplied and no statistical analyses were performed. The highest mean value for "subjective improvement" was 47% for the group treated with alternating ultrasound and ice application, followed by phonophoresis of dexamethazone/lidocaine (Hexadrol/Xylocaine) with 32%, iontophoresis of dexamethazone/lidocaine (Hexadrol/Xylocaine) with 24% and application of ice bags with 22%. The respective strength increases of quadriceps and hamstrings were 28% and 34%, 13% and 0%, 15% and 15%, and 5% and 15%. Percentages are stated for knees without mention of the number of participants. Furthermore, no variability measures or statistical analyses are given. Therefore, conclusions about relative effectiveness are impossible.
Anabolic steroids Darracott 1973 performed a low quality study and presented individual results determined after 6‐8 weeks. A significant difference in the number of participants that improved clinically was observed: 1 out of 20 participants in the placebo group improved clinically compared to 20 out of 23 in the nandrolone group (RR 17.39; 95%CI 2.56 to 118.26) (see Graphs: comparison 06.01). Patellar bone density measurements also revealed a significantly better result for the nandrolone group: bone density increased in 1 out of 20 participants in the placebo group, compared to 16 out of 20 in the nandrolone group (RR 13.91; 95%CI 2.02 to 95.79). Glycosaminoglycan polysulphate (GAGPS) Kannus 1992 performed a high quality study and found that after a treatment period of six weeks two thirds of the participants receiving either GAGPS or placebo injections into the knee showed excellent recovery from PFPS symptoms, as determined by subjective, functional and clinical assessments. When comparing participants receiving intra‐articular GAGPS injections with the group receiving no injections (Kannus 1992) the number of people without symptoms during a full squat differed significantly after 6 weeks (RR 2.20; 95%CI 1.03 to 4.68), but this difference was no longer observed after six months (see Graphs: comparison 08.03). When comparing means and standard deviations using the analysis tool in RevMan 2003, there was a significant difference between scores on the Lysholm functional scale between the groups receiving GAGPS injections and the group receiving no injections after 6 weeks (SMD 0.93; 95%CI 0.19 to 1.66) (see Graphs: comparison 08.05). However, the author did not find a significant difference using repeated measurements analysis that takes individual changes into account. The Tegner activity scores differed significantly between GAGPS injected participants and non‐injected participants after six weeks (SMD 1.12; 95%CI 0.37 to 1.88) and after six months (SMD 0.74; 95%CI 0.02 to 1.46) (see Graphs: comparison 08.06). Based on the physician's assessment, the number of people who were fully recovered at six weeks was greater in the injection groups than the group without injections, though the difference was never significant. At six months, three quarters of the participants reported excellent recovery, though there was no significant difference between the groups. Patella tests were performed and differed significantly between the injection and no injection groups after six weeks. Muscle strength relative to the healthy limb improved in all groups and no significant differences were observed. Overall, no beneficial effect of glycosaminoglycan polysulphate was observed.
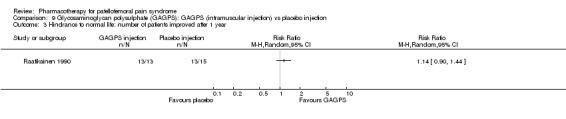
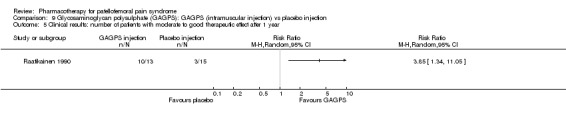
Raatikainen 1990 performed a high quality study and found that pain while going down stairs was significantly less in people receiving intramuscular injections of GAGPS compared to people receiving placebo injections (RR 1.85; 95%CI 1.07 to 3.19; NNT: 3). However, pain when squatting did not reveal a significant difference (RR 1.38; 95%CI 0.73 to 2.62) and neither did hindrance to normal life (RR 1.15; 95%CI 0.95 to 1.41) or hindrance to sports activities (RR 1.79; 95%CI 0.91 to 3.52). Nevertheless, the number of people with moderate to good therapeutic effect assessed by the physician after one year was significantly higher in the group receiving GAGPS injections (10 out of 13 in the GAGPS group and 3 out of 15 in the placebo group: RR 3.85; 95%CI 1.34 to 11.05; NNT: 2). Re‐arthroscopy was used to determine the improvement in cartilage lesions and revealed improvement in 3 out of 13 participants in the placebo group and in 8 out of 13 participants in the GAGPS group. This difference was not significant (RR 2.79; 95%CI 0.90 to 7.86). Overall, some very limited beneficial effects of glycosaminoglycans were observed (see Graphs: comparisons 09.01 to 09.05).
Best evidence synthesis There is limited evidence from one high quality study that aspirin is not more effective than placebo for improving clinical symptoms and signs in people with chondromalacia patellae (Bentley 1981).
There is limited evidence from one low quality study that tenoxicam is less effective than laser therapy for reducing pain in people with chondromalacia patellae and that patient satisfaction is not different between these treatments (Marchese 1998).
There is limited evidence from one low quality study that naproxen is more effective than placebo for reducing pain in people with anterior knee pain syndrome in the short term (Suter 1998).
There is limited evidence from one low quality study that diflunisal and naproxen do not differ in reducing pain in people with anterior knee pain in the short term (Fulkerson 1986).
There is limited evidence from one low quality study that nandrolone phenylpropionate is more effective than placebo for improving clinical symptoms and signs in people with chondromalacia patellae ( Darracott 1973).
There is conflicting evidence from two high quality studies for the effectiveness of glycosaminoglycan polysulphate compared with placebo in people with PFPS (Kannus 1992) or chondromalacia patellae ( Raatikainen 1990). One high quality study found marginally better results from its administration for some outcomes, but the other study found no statistically significant difference between groups.
There is no evidence to support the claim from one low quality study that alternating ultrasound and ice massage improves subjective symptoms more than topical Hexadrol and Xylocaine in people with knee extensor mechanism disorders (chondromalacia patellae, infrapatellar tendinitis, or peripatellar pain) (Antich 1986).
Discussion
The literature search resulted in a very small number of trials studying pharmacotherapy for PFPS or chondromalacia patellae. This in itself is remarkable if one considers the widespread use of NSAIDs for pain reduction in people with PFPS or chondromalacia patellae.
NSAIDs The study of Bentley 1981 (data from 29 participants were analysed) shows that aspirin is not more effective for treating symptoms of chondromalacia patellae than placebo. The anticipated reduction of cartilage lesions was also not observed. Therefore, the hypothesised pathways by which aspirin was expected to influence cartilage metabolism could not be demonstrated. Marchese 1998 (data from 35 participants were analysed) found that tenoxicam is significantly less effective than laser therapy for treating pain in people with chondromalacia patellae, but pain levels were reported poorly and this claim cannot be verified. Suter 1998 found that the short term pain reduction in people with anterior knee pain syndrome was significantly higher for naproxen than for placebo (data from 36 participants were analysed). However, although pain ratings ranged from 0 to 57 at baseline, mean values were only 11 ± 13 for the involved leg. The average pain reduction remained below 5 mm for the involved leg, and questions as to the clinical relevance of this reduction are not addressed by Suter 1998. The short term "significant" pain reduction in people with anterior knee pain reported by Fulkerson 1986 (data from 36 participants were analysed) was not measured on a visual analogue scale and can therefore not be compared to the results of Suter 1998. Furthermore, the term "significant" pain reduction is not defined, preventing insight into the clinical relevance of this outcome. So, although the use of NSAIDs as analgesics in people with PFPS is already widespread, our systematic review of the literature has produced only limited evidence that NSAIDs are effective for pain reduction and clinical relevance of this evidence remains unclear.
Glucocorticoid steroids Antich 1986 used topical Hexadrol (dexamethasone), a class 1 corticosteroid with anti‐inflammatory and vasoconstrictive action, in combination with topical Xylocaine (lidocaine), which has an analgetic effect. Iontophoresis and phonophoresis are both techniques to drive the topical drug into the soft tissue surrounding the patella, to reduce irritation or inflammation in these tissues, thought by some to cause the pain in PFPS. Though the trial does not provide statistical evidence, the drug does not seem to give good results (data from 67 knees of 51 participants were reported). Whether this lack of result reflects that the mechanism causing pain resides in other tissues will have to remain a point of speculation.
Anabolic steroids Darracott 1973 used nandrolone phenylpropionate, a steroid which has been shown to have significant anabolic effect at dose levels below the threshold for androgenic response. The use of an anabolic ester yields rather impressive results (data from 43 participants were analysed) when clinical improvement is considered. Whether this clinical improvement may be due to the reversal of patellar osteoporosis, or to the muscular hypertrophy it induces, cannot be derived from these results. Although successful, application of nandrolone is not likely to be widely accepted as anabolic esters are included in international doping lists and have significant side effects, such as premature closure of epiphyses, virilisation, liver insufficiency and heart failure. Its use in the treatment of PFPS should therefore be considered with great care.
Glycosaminoglycan polysulphate (GAGPS) Both the studies by Kannus 1992 (data from 49 participants were analysed) and Raatikainen 1990 (data from 27 participants were analysed) are of high methodological quality. Though the pharmaceutical agent is the same, the route and frequency of administration is not. Moreover, the design of both studies differs greatly from the timing of start of exercises to the methods used for outcome assessment. Raatikainen 1990 performs repeated arthroscopies to evaluate the appearance of the patellar cartilage. Kannus 1992 however, views the administration of GAGPS as additional to the conservative treatment that has gained a strong foothold in clinical practice: a combination of reducing activities that cause symptoms, strengthening the quadriceps muscles, and prescribing NSAIDs or other analgesics to facilitate exercising. It should be noted that although no differences were found between the three treatment strategies in the study by Kannus 1992, three quarters of all participants were deemed clinically recovered after six months. This was reduced to two thirds at seven years follow‐up. Raatikainen 1990 found that three quarters of the participants in the GAGPS group showed a moderate to good therapeutic effect, versus only 20% of the controls; a significant difference. Because of the different approaches it is impossible to say whether these conflicting results reflect a difference in the effectiveness of the drug, in the route of administration, the frequency of administration, the presence of cartilage damage, or the additional treatment components. For example, it could be argued that the training program used by Kannus 1992 gives such good results that GAGPS does not substantially add to the positive effect of training.
Choice of outcomes and assessment techniques The severity of symptoms and of patellar cartilage damage at inclusion varies from study to study. The weight given to the extent of the patellar cartilage lesions has been reconsidered in the previous decade. This is due to changing insights into the nature of retropatellar pain. Pain and crepitations have repeatedly been shown to be poorly correlated with visible cartilage damage (Arnoldi 1991; Goodfellow 1976; Royle 1991). The gradual acceptance of these insights is reflected in the dates of the studies that employed the technique of arthroscopy for determining cartilage damage (Bentley 1981; Raatikainen 1990). Recent developments in MRI techniques provide non‐invasive techniques to determine cartilage damage that are risk free for the patient. Furthermore MRI techniques enable quantification of cartilage volumes and surface area measurements (Eckstein 2002). Kannus 1992 used such MRI measurements to determine the cartilage thickness and abnormalities in his 7 year follow‐up, and found no abnormalities in 81% of the participants. This is more than the two thirds that were still fully recovered. This is another indication that cartilage damage is not the most relevant outcome measure for PFPS.
Although pain is the symptom that will prompt a patient to seek medical advice, several studies (Antich 1986; Bentley 1981; Darracott 1973) did not report pain as a separate outcome. instead they reported the outcome measure "clinical symptoms and signs" or "percentage change in condition" (ranging from ‐100% to +100%). This may well include pain, but definitions of these outcome measures have not been reported. Similarly, Fulkerson 1986 does not provide a definition of the main outcome measure: "significant pain reduction". This makes interpretation of the results rather difficult. Methodology Because no meta‐analysis was performed and because the ranking of high and low quality of the trials did not influence the best‐evidence synthesis, analysis of the cut‐off point for discrimination between the high and low methodological quality was redundant. We encountered severe problems with the interpretation of the trials because of the low quality of certain studies. Bias could ensue from any of the items listed in the criteria used for determination of methodological quality. Apart from those issues, there are some other aspects that severely impede the interpretation of the results.
Antich 1986 did not report the number of participants per treatment arm, the inevitable correlation between results of knees of bilaterally afflicted patients was not taken into account and their distribution over treatments was not reported, and no statistical analyses were performed. The study therefore serves only as an example for possible applications of pharmacotherapy, as it is not suitable for presentation of evidence. Most results from Marchese 1998 cannot be used in this review because the baseline and follow‐up measures for each treatment were not reported. Therefore, only "patient satisfaction" remains for evaluation.
In general, the number of participants in each trial is very limited, which seriously reduces the power of the included studies. The relevance of statistical evaluations then becomes questionable, as differences between treatments will be hard to detect and individuals with deviating outcomes can have an enormous effect. Therefore, the reported estimates and confidence intervals should be interpreted with great caution. On the other hand statistical significance does not always reflect clinical relevance. This is demonstrated by the small reduction of pain levels found by Suter 1998, which is nonetheless statistically significant, resulting in the qualification 'limited evidence' for pain reduction.
Patient characteristics The studies of Fulkerson 1986 and Antich 1986 included participants with diagnoses other than PFPS or chondromalacia patellae in their study populations. Although clinicians may (in part) prescribe the same therapies for all diagnoses, patients with different diagnoses may show very different responses to these therapies and should therefore have been reported separately. In spite of this severe shortcoming, we decided to include these trials into this review, to give the reader a full scope of the scant literature available on the subject of pharmacotherapy for PFPS. The excluded patients (Antich 1986) who attended the clinic for instruction in a home program may have had less severe symptoms and may have reacted differently to the treatments.
Both Bentley 1981 and Raatikainen 1990 only included patients in which cartilage damage had been detected at arthroscopy. Marchese 1998 included patients with at least one radiological sign of femoropatellar dysplasia, and Darracott 1973 only included patients with severely debilitating symptoms. Of the 53 patients included by Kannus 1992 17 had a previous arthroscopy but this detected cartilage damage in only eight cases.
As most pharmaceutical agents are evaluated in only one study, no observations could be made whether the presence of cartilage damage influences the results. The only exception is GAGPS: Raatikainen 1990 included only participants in which cartilage damage was observed, and Kannus 1992 included participants with and without cartilage damage. The fact that the effectiveness of GAGPS seems greater in the study in which the participants had more cartilage damage, suggests that either cartilage damage is not a predictive factor for recovery, or GAGPS works better when cartilage damage is evident.
The strength of this review is that it gives an overview of the available evidence for pharmacotherapy for PFPS. The poor methodological quality of some of the included studies does not subtract from that, because it emphasises the poverty of the available evidence. That the methodologically poor studies are mentioned in this review may serve to emphasise the need for qualitatively sound research. The fact that we also found some high quality trials indicates that it certainly is possible to conduct valid RCTs in this field. Future trials should pay more attention to the methodological aspects of design and reporting as well as the number of subjects included in the study.
Authors' conclusions
Implications for practice.
Despite widespread application of NSAIDs for PFPS there is only limited evidence for their effectiveness in reducing pain and the evidence is limited to the short term only (up to one week). If the use of NSAIDs is considered in spite of that, the drug with the least possible side effects and lowest costs should be first choice for use in people with PFPS, as there is no evidence that one kind of NSAID is superior to another.
The evidence for the effect of GAGPS is contradictory and merits further investigation. There is limited evidence that the anabolic steroid nandrolone may be effective, but the drug is too controversial for use in the treatment of PFPS.
Implications for research.
The limited evidence for the effectiveness of NSAIDs and the lack of insight into the clinical relevance of this evidence could constitute an area for medical cost reduction. Therefore, further research on the effectiveness of NSAIDs should be obtained through trials in which NSAIDs are compared to placebo for at least several weeks, with a follow‐up period to assess long term effects. The NSAIDs may be given either in addition to other interventions or not. The comparison of NSAIDs to other treatments of which the effectiveness is unknown is undesirable.
The effectiveness of GAGPS would merit further research, to investigate the contradictory results of Kannus 1992 and Raatikainen 1990.
To gather more evidence for the influence of cartilage damage on recovery, future researchers may consider the use of imaging techniques to determine the extent of cartilage damage to use it for stratification of treatment groups and possible subgroup analysis.
Any further research should pay attention to methodological aspects of design and reporting. Power calculations should be provided to ensure that the number of participants is sufficient to obtain both clinically and statistically significant outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 15 July 2008 | Amended | Converted to new review format. |
Notes
This study will serve as the basis for a PhD thesis in 'Non traumatic knee injuries in adolescents' at The Institute of General Practice at the Erasmus University Rotterdam by E.M. Heintjes (MSc) and will be supervised by Dr. M.Y. Berger (MD) and Dr. S.M.A. Bierma‐Zeinstra (PhD).
Acknowledgements
We thank the following for helpful comments at editorial review: Prof William Gillespie, Prof Marc Swiontkowski, Prof Rajan Madhok, Peter Herbison, Dr. Janet Wale, Lesley Gillespie, Dr. Bruce Arroll, Dr. Arianne Verhagen and Leeann Morton.
Furthermore we thank Petra Zeeuwe for help in assessing the methodological quality of the trial by Marchese (Italian).
Appendices
Appendix 1. Search strategy for MEDLINE (OVID WEB)
1. Arthralgia/ 2. Knee Joint/ or Knee/ or Patella/ 3. and/1‐2 4. anterior knee pain.tw. 5. ((patell$ or femoropatell$ or femoro‐patell$ or retropatell$) adj2 (pain or syndrome or dysfunction)).tw. 6. ((lateral compression or lateral facet or lateral pressure or odd facet) adj syndrome).tw. 7. ((chondromalac$ or chondropath$) adj2 (knee$1 or patell$ or femoropatell$ or femoro‐patell$ or retropatell$)).tw. 8. or/4‐7 9. or/3,8 10. (dt or tu or ad).fs 11. and/9‐10
Data and analyses
Comparison 1. NSAIDs: Aspirin versus placebo (oral).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical results: number of patients improved after 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 NSAIDs: Aspirin versus placebo (oral), Outcome 1 Clinical results: number of patients improved after 3 months.
Comparison 2. NSAIDs: Tenoxicam (oral) versus laser therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical results: number of patients who are reasonably to very satisfied after 2 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 NSAIDs: Tenoxicam (oral) versus laser therapy, Outcome 1 Clinical results: number of patients who are reasonably to very satisfied after 2 months.
Comparison 3. NSAIDs: Naproxen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain reduction (Visual Analogue Scale 0 ‐ 100) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Involved leg after 7 days | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐involved leg after 7 days | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 NSAIDs: Naproxen versus placebo, Outcome 1 Pain reduction (Visual Analogue Scale 0 ‐ 100).
Comparison 4. NSAIDs: Diflunisal versus naproxen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients reporting significant pain reduction after 5 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.1. Analysis.
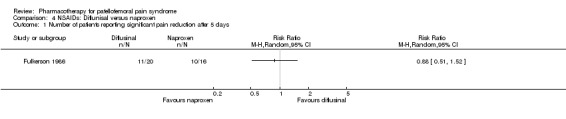
Comparison 4 NSAIDs: Diflunisal versus naproxen, Outcome 1 Number of patients reporting significant pain reduction after 5 days.
Comparison 5. Glucocorticoid steroids: Dexamethasone and lidocaine (topical, by two methods) vs ultrasound + ice vs ice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average individual subjective % improvement in knee status after 10 days | Other data | No numeric data |
5.1. Analysis.
Comparison 5 Glucocorticoid steroids: Dexamethasone and lidocaine (topical, by two methods) vs ultrasound + ice vs ice, Outcome 1 Average individual subjective % improvement in knee status after 10 days.
| Average individual subjective % improvement in knee status after 10 days | ||||
|---|---|---|---|---|
| Study | Iontophoresis | Phonophoresis | Ultrasound / Ice | Ice |
| Antich 1986 | 24% improved 21 knees | 32% improved 9 knees | 47% improved 13 knees | 22% improved 16 knees |
Comparison 6. Anabolic steroids: Nandrolone phenylpropionate versus placebo (by intramuscular injection).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical results: number of patients improved after 6 to 8 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
6.1. Analysis.
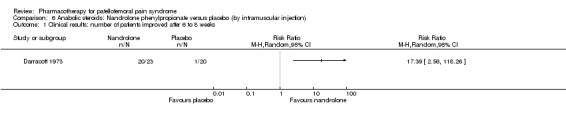
Comparison 6 Anabolic steroids: Nandrolone phenylpropionate versus placebo (by intramuscular injection), Outcome 1 Clinical results: number of patients improved after 6 to 8 weeks.
Comparison 7. Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (Visual Analogue Scale 0 ‐ 100 mm) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 After 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Function ‐ return to physical activity (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Function ‐ full squat (N symptom free) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Function ‐ one leg jumping (N symptom free) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Function (Lysholm Scale) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 After 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 After 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Function (Tegner Scale) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 After 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 After 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Complete recovery (N) determined by physician | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Subjective overall assessment rated as "excellent" (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
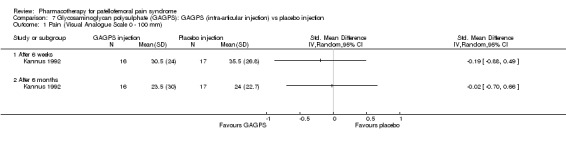
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 1 Pain (Visual Analogue Scale 0 ‐ 100 mm).
7.2. Analysis.
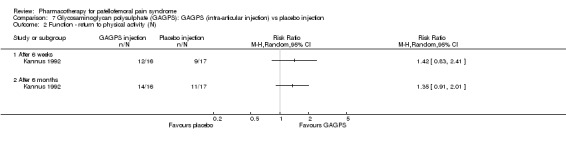
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 2 Function ‐ return to physical activity (N).
7.3. Analysis.
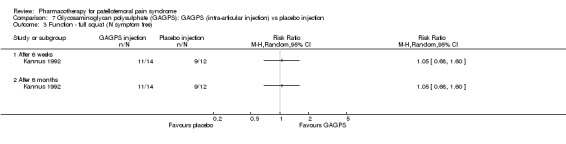
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 3 Function ‐ full squat (N symptom free).
7.4. Analysis.
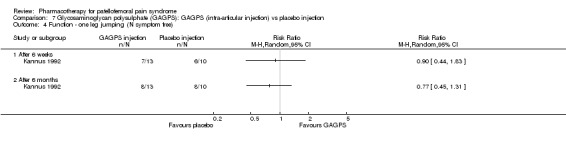
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 4 Function ‐ one leg jumping (N symptom free).
7.5. Analysis.
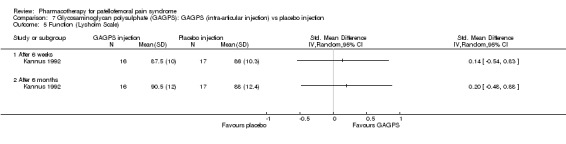
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 5 Function (Lysholm Scale).
7.6. Analysis.
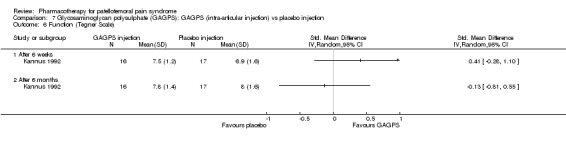
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 6 Function (Tegner Scale).
7.7. Analysis.
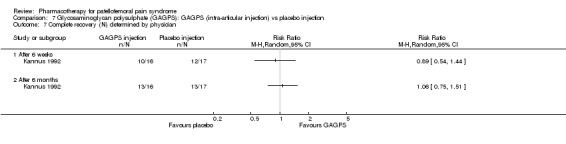
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 7 Complete recovery (N) determined by physician.
7.8. Analysis.
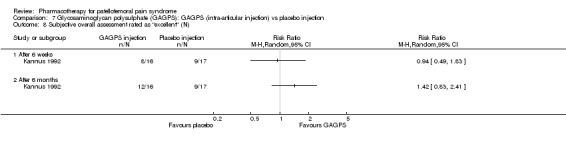
Comparison 7 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs placebo injection, Outcome 8 Subjective overall assessment rated as "excellent" (N).
Comparison 8. Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (Visual Analogue Scale 0 ‐ 100 mm) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 After 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Function ‐ return to physical activity (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Function ‐ full squat (N symptom free) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Function ‐ one leg jumping (N symptom free) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Function (Lysholm Scale) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 After 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 After 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Function (Tegner Scale) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 After 6 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 After 6 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Complete recovery (N) determined by physician | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Subjective overall assessment rated as "excellent" (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 After 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 After 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
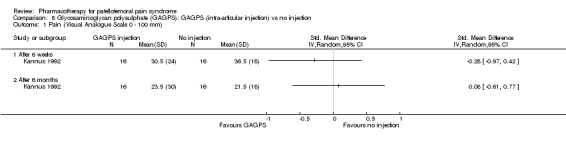
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 1 Pain (Visual Analogue Scale 0 ‐ 100 mm).
8.2. Analysis.
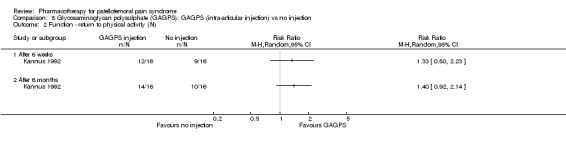
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 2 Function ‐ return to physical activity (N).
8.3. Analysis.
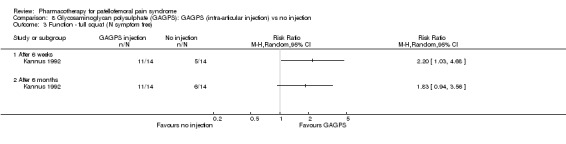
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 3 Function ‐ full squat (N symptom free).
8.4. Analysis.
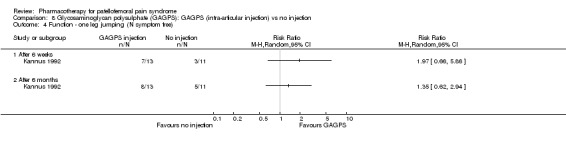
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 4 Function ‐ one leg jumping (N symptom free).
8.5. Analysis.
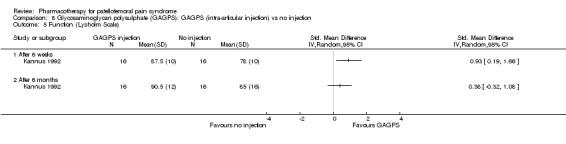
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 5 Function (Lysholm Scale).
8.6. Analysis.
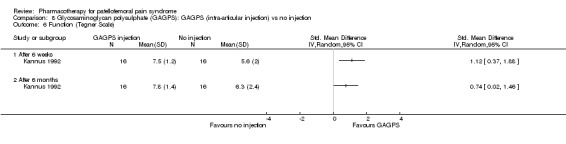
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 6 Function (Tegner Scale).
8.7. Analysis.
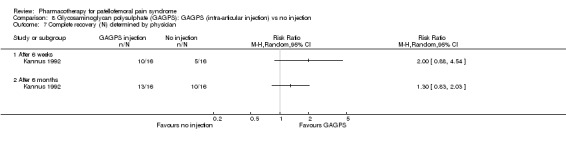
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 7 Complete recovery (N) determined by physician.
8.8. Analysis.
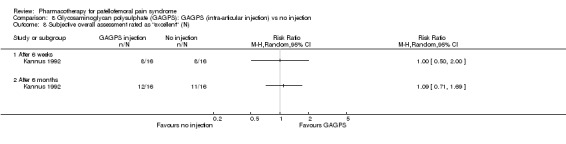
Comparison 8 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intra‐articular injection) vs no injection, Outcome 8 Subjective overall assessment rated as "excellent" (N).
Comparison 9. Glycosaminoglycan polysulphate (GAGPS): GAGPS (intramuscular injection) vs placebo injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain going downstairs: number of patients improved after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Pain when squatting: number of patients improved after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Hindrance to normal life: number of patients improved after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Hindrance to sports activities: number of patients improved after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Clinical results: number of patients with moderate to good therapeutic effect after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
9.1. Analysis.
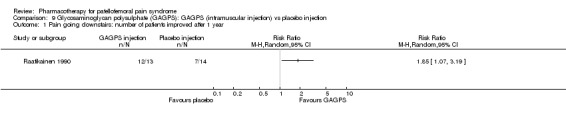
Comparison 9 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intramuscular injection) vs placebo injection, Outcome 1 Pain going downstairs: number of patients improved after 1 year.
9.2. Analysis.
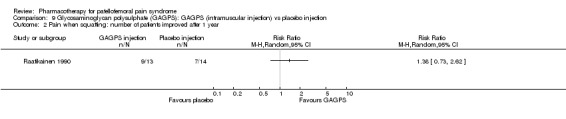
Comparison 9 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intramuscular injection) vs placebo injection, Outcome 2 Pain when squatting: number of patients improved after 1 year.
9.3. Analysis.
Comparison 9 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intramuscular injection) vs placebo injection, Outcome 3 Hindrance to normal life: number of patients improved after 1 year.
9.4. Analysis.

Comparison 9 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intramuscular injection) vs placebo injection, Outcome 4 Hindrance to sports activities: number of patients improved after 1 year.
9.5. Analysis.
Comparison 9 Glycosaminoglycan polysulphate (GAGPS): GAGPS (intramuscular injection) vs placebo injection, Outcome 5 Clinical results: number of patients with moderate to good therapeutic effect after 1 year.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antich 1986.
| Methods | RCT, randomisation method not described, but partially dependent on screening outcomes Low quality study: Delphi score 0 | |
| Participants | Patients with knee extensor mechanism disorders: chondromalacia patella (more than half of patients), infrapatellar tendinitis, peripatellar pain. Patients who only attended the clinic for evaluation and instruction in a home program were excluded. 64 patients randomised, 22 bilateral 6 patients without clear palpable tender area's were assigned to the only treatment not requiring specific localisation: ice bags 51 patients concluded 10 day trial period, 16 bilateral, 5 without palpable tender areas | |
| Interventions | 4 treatment sessions in 7‐8 days
All groups received quadriceps training exercises: hip adduction, quadriceps setting, modified straight leg raises and short arc quadriceps exercises with weights (1) Iontophoresis, 1 cc Hexadrol (dexamethazone) and 1 cc 4% xylocaine 20 min (n=21 knees after 10 days) (2) Phonophoresis, 1 cc Hexadrol and 1 cc 4% xylocaine 7 min (n=9 knees after 10 days) (3) Ultrasound/ice bags, 3 times alternating heat (ultrasound 3 min) and cold (ice 2 min) (n=13 knees after 10 days) (4) Ice bags, 10 min after exercises (n=16 tender knees, n=8 non tender knees in 5 patients after 10 days) 29 patients took NSAIDs as concomitant medication |
|
| Outcomes | Subjective % improvement estimated by each patient % increase of muscle strength in quadriceps and hamstrings | |
| Notes | Number of patients per diagnosis unclear Number of patients and drop‐outs per group unclear as only knees at follow‐up are mentioned. (6 patients started out with non tender knees, 8 knees of 5 patients are presented, one drop‐out in this group) No measures of variability are given for the outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Bentley 1981.
| Methods | RCT, randomisation by pharmacist High quality: Delphi score 7 | |
| Participants | Chondromalacia patellae diagnosed by arthroscopy performed in patients that did not improve with 3 months treatment with isometric quadriceps exercises 30 patients, 2 bilateral, worst knee is treated 8 male, 22 female Age 15‐35 Many athletic patients | |
| Interventions | Baseline arthroscopy for grading of cartilage damage, then randomisation (1) Aspirin (Aloxiprin) (n=16) (2) Placebo (n=13) 10 week period, during which serum levels of aspirin were monitored Repeated arthroscopy after 12 weeks |
|
| Outcomes | Subjective change in symptoms (improved, unchanged, worse) at 12 weeks Arthroscopic grading of cartilage damage at baseline and 12 weeks Arthroscopic changes (improved no change worse) at 12 weeks | |
| Notes | 4 of 16 patients did not maintain effective blood levels of between 15 and 25 mg/100 ml, due to side effects or non‐cooperation 1 drop out refused repeated arthroscopy, treatment group is not mentioned patients were not allowed aspirin during 3 month exercise period previous to aspirin treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Darracott 1973.
| Methods | RCT, double blind, method of randomisation not specified Low quality: Delphi score 3 | |
| Participants | 43 military patients with severe unilateral painful patella, fulfilling diagnostic criteria of chondromalacia patellae 40 male, 3 female Age 17‐34 (mean 22, SD 4) Duration of symptoms 4 ‐ 36 months, onset due to trauma in 12 patients, to surgery in 11, to patellar subluxation, 2 to severe exercise, 1 to kneeling and 16 spontaneous | |
| Interventions | All patients performed gradually increasing exercises to reverse quadriceps inhibition for 6‐8 weeks until return to full circuit and battle training, combined with: (1) Intramuscular administration of nandrolone phenylpropionate 25 mg weekly for 6 weeks (anabolic ester in arachis oil) (n=23) (2) Intramuscular administration of placebo weekly for 6 weeks (arachis oil) (n=20) |
|
| Outcomes | Subjective change in clinical symptoms Change in bone density in skyline radiographs (using healthy knee for calibration) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fulkerson 1986.
| Methods | RCT, randomisation through odd ‐ even assignment, not blind Low quality: Delphi score 0 | |
| Participants | 56 patients enrolled, 36 patients with anterior knee pain of mild to moderate severity completed the study 28 women, 8 men, mean age 32.3 years multiple diagnoses per patient: 42% chondromalacia patellae, 39% internal derangement, 30%tendonitis/sprains, 8% inflamed plica, 5% degenerative joint disease, 1% loose body, 1% Sindig‐Larsen‐Johansson disease | |
| Interventions | (1) NSAID diflunisal initial dose 1000 mg, followed by 500 mg BID (mean duration 19.8 days) (n=20) (2) NSAID naproxen initial dose 500 mg, followed by 250 mg QID (mean duration 15.2 days) (n=16) |
|
| Outcomes | Severity of pain and swelling at baseline and 1, 2, and 5 days after start of therapy Adverse reactions: common but mild Diflunisal: headache (2), gastric distress (2), tinnitus, nausea, indigestion, urinary retention, rash, cramps, fever Naproxen: drowsiness (2) pounding in head, cramps, inability to concentrate, mild depression, euphoria | |
| Notes | The high prevalence of chondromalacia and patellofemoral malalignment and the limited number of trials available for this review have led us to include this study into the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kannus 1992.
| Methods | RCT, randomisation using sealed envelopes, double blind assessments with respect to injections of saline and glucosaminoglycan polysulphate High quality: Delphi score 5 | |
| Participants | 53 patients attending the Tampere Research Station of Sports Medicine with chronic patellofemoral pain syndrome: duration of symptoms mean 16, SD 19 months, all unilateral 17 previous arthroscopy, 8 with pathologic changes in patellar cartilage, no correlation with symptoms 25 men 28 women Age: mean 27, SD 9 | |
| Interventions | Conservative treatment: no symptom‐producing activities for 6 weeks, isometric quadriceps tensioning exercises and straight leg raises, ice packs on patella if necessary, 20 mg piroxicam (NSAID) for 20 days (1) Conservative treatment only (n=16) (2) Conservative treatment and 5 weekly intra‐articular injections with saline and lidocaine (10 mg) (n=17) (3) Conservative treatment and 5 weekly intra‐articular injections with GAGPS (50 mg) and lidocaine (10 mg) (n=16) |
|
| Outcomes | Follow‐up assessments took place at 6 weeks and 6 months and again after 7 years subjective assessment: ‐ Pain and discomfort during activities on 0‐100 mm Visual Analogue Scale (0 = none, 100 = extremely intense pain) ‐ Overall evaluation of condition of the knee on 5 point scale (1 = asymptomatic, 5 = worse than before treatment) ‐ Level of physical activity on 4 point scale (1 = higher than before knee problem and 4 = unable to perform any physical activities) Combined subjective and functional evaluations of the knee: ‐ Lysholm scale (0‐100 point scale, 0 = worst score, 100 = best score) ‐ Tegner scale (0‐10 point scale, 0 = no physical activity possible, 10 = all kinds of activities possible) Symptoms on tests of function: ‐ Hopping on affected leg (4 point scale, 1 = no problem, 4 = unable) ‐ Duck walking (4 point scale, 1 = no problem, 4 = unable) ‐ 25 repetition full squat (1 = no squat without pain, 2 = 1‐5 without pain, 3 = 6‐10 without pain, 7 = > 25 squats without pain) Clinical evaluation: ‐ Pain during compression test (0‐4 point scale, 0 = none, 4 = intense) ‐ Crepitation during compression test (0‐4 point scale, 0 = none, 4 = intense) ‐ Pain during apprehension test (0‐4 point scale, 0 = none, 4 = intense) ‐ Physician's opinion of overall therapeutic effect (5 point scale, 1 = complete subjective, functional and clinical recovery and 5 = overall status worse than before treatment) Muscle strength: ‐ Isometric quadriceps muscle strength in 60 degrees of flexion using and isometric dynamometer Radiological signs after 7 years ‐ MRI and radiographs of both knees after 7 years, to look for both early and advanced signs of osteoarthritis in the patellofemoral joint ‐ Bone density of the distal part of the femur, the patella and proximal part of the tibia to determine whether PFPS had caused bone loss or osteopenia in the affected knee compared with the unaffected knee: a 5% deficit was deemed clinically important | |
| Notes | 2 patients were excluded after enrolment because they had osteoarthritis (group 1 and 3) 1 patient of group 3 never returned 1 patient of group 2 was excluded due to an adverse event: intense aseptic effusion (reactive synovitis) following first injection 45 of the 49 patients of the original study were reassessed after 7 years (Kannus 1999). Because analysis of the data showed no differences among the groups at 6 months the results of all groups were pooled for analysis at 7 years. The results of the 7 year follow‐up are therefore only included in the text of this review. MRI and radiograph assessment was blind as to which knee was affected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Marchese 1998.
| Methods | RCT, method not specified Low quality study: Delphi score 0 | |
| Participants | Anterior knee pain (chondromalacia patellae) for at least 3 months, no recent trauma, tendinopathy or osteochondrosis of the knee 35 female patients Age 13.8 ‐ 15.8 years 29 patients performed sports activities, none of them at a competitive or amateur level | |
| Interventions | (1) Laser therapy for 15 sessions (n=18) (2) 20 mg tenoxicam once daily for 15 days (NSAID) (n=17) |
|
| Outcomes | Clinical results: patient satisfaction with the results of the treatment Other outcomes (VAS and palpation tests for pain, crepitation, Kujala scale for function) were assessed at baseline, 15 days and 60 days, though the reporting of these outcomes was too poor to be understandable. | |
| Notes | The time frame of the laser therapy is not entirely clear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Raatikainen 1990.
| Methods | RCT, randomisation method not defined, double blind High quality: Delphi score 5 | |
| Participants | 31 consecutive patients with patellofemoral pain attending sports clinic at Deaconess Institute 22 men and 7 women Inclusion criteria: resisted patellar movement causes pain, arthroscopy revealed patellar cartilage lesions Exclusion criteria: other pathology at arthroscopy, corticosteroid use or glucosaminoglycan polysulphate within 40 days, NSAIDs within 7 days of commencement of trial, pregnant or nursing mothers, age under 18 years, contraindication for GAGPS. | |
| Interventions | All patients received standardised quadriceps training after 6 week injection period. Paracetamol allowed as an escape analgesic.
Baseline arthroscopy (1) 12 intramuscular injections of 50 mg glucosaminoglycan polysulphate (Arteparon) (n=15) (2) Placebo ‐ 12 intramuscular injections with physiologic saline (n=14) Arthroscopy repeated after 1 year |
|
| Outcomes | All outcomes were assessed at baseline, 6, 10 and 58 weeks on a 4 point scale (0=none, 1=slight, 2=moderate, 3=severe or extensive) Pain on palpation, pain on pressing patella against femur, pain during apprehension test, pain going downstairs, pain when squatting, hindrance to normal life, hindrance to sports activities, overall therapeutic effect, change from initial damage assessed by arthroscopy | |
| Notes | 1 patient was excluded due to trauma after 6 injections (treatment unknown), 1 patient dropped out after 8 injections (treatment unknown) 1 patient glucosamine group lost to follow‐up at final assessment, 2 in placebo group refused re‐arthroscopy (1 asymptomatic and 1 with moderate to severe clinical symptoms) Clinical results were dichotomised by the authors to indicate "good clinical results" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Suter 1998.
| Methods | RCT, randomisation method not defined, double blind. Low quality: Delphi score 4 | |
| Participants | 42 patients, identified through patient database at the University Sports Medicine Center 29 men and 13 women Age: mean 35.6, SD 8.4 Inclusion criteria: unilateral anterior knee pain syndrome of varying duration, no history of knee surgery or knee instability, quadriceps injury, or bone, meniscus or ligament injury, no history of peptic ulceration, no concurrent disease of other organ systems. | |
| Interventions | (1) NSAID naproxen, 550 mg twice daily for 7 days (n=20) (2) placebo, twice daily for 7 days (n=22) |
|
| Outcomes | Pain reductions of averaged VAS scores before and during testing maximal contraction of knee extensors Muscle inhibition at 30 and 60 degrees of knee flexion Knee extensor moments at 30 and 60 degrees of knee flexion All outcomes were determined for the involved and the noninvolved leg | |
| Notes | One man and two women dropped out because of drug intolerance Three men dropped out because of time constraints Analyses were performed with remaining 36 patients (no intention to treat) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ABBREVIATIONS BID: twice a day (from Latin: bis in die) GAGPS: glycosaminoglycan polysulphate NSAID: non‐steroidal anti‐inflammatory drug PFPS: patellofemoral pain syndrome QID: four times a day (from Latin: quarter in die) VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berenfeld 1991 | Only 26% of all patients suffered from PFPS, the rest suffered from OA. Results were not reported separately for each diagnosis. |
| Braham 2003 | Patients with OA. |
| Dahlberg 1994 | Patients with OA and in two thirds with a history of previous major knee trauma. Of the patients with patellar pain on palpation none had a history of patellar pain syndrome. |
| Noble 1981 | Only 10% patients with PFPS, the rest was diagnosed with injuries that are excluded in this review. |
| Wang 1997 | Non‐controlled trial. |
The studies by Antich 1986 and Fulkerson 1986 also included patients with diagnoses mentioned in the exclusion criteria, but because the majority of patients suffered from PFPS these studies were included. ABBREVIATIONS OA: osteoarthritis PFPS: patellofemoral pain syndrome
Contributions of authors
Edith Heintjes: data extraction, data analysis and text of the review Marjolein Berger: primary support, study selection, feedback on clinical aspects Sita Bierma: study selection and feedback and textual feedback Roos Bernsen: data extraction and statistical feedback Jan Verhaar: methodological scoring and textual feedback Bart Koes: methodological scoring and textual feedback
Sources of support
Internal sources
Department of General Practice of the Erasmus University Rotterdam, Netherlands.
Department of Orthopaedics of the University Hospital Rotterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Antich 1986 {published data only}
- Antich TJ, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities. Journal of Orthopaedic and Sports Physical Therapy 1986;8(5):255‐9. [DOI] [PubMed] [Google Scholar]
Bentley 1981 {published data only}
- Bentley G, Leslie IJ, Fischer D. Effect of aspirin treatment on chondromalacia patellae. Annals of Rheumatic Disease 1981;40(1):37‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darracott 1973 {published data only}
- Darracott J. Treatment of the painful knee fulfilling diagnostic criteria for 'chondromalacia patellae'. Current Medical Research and Opinion 1973;1(7):412‐22. [DOI] [PubMed] [Google Scholar]
Fulkerson 1986 {published data only}
- Fulkerson JP, Folcik MA. Comparison of diflunisal and naproxen for relief of anterior knee pain. Clinical Therapeutics 1986;9 Suppl C:59‐61. [PubMed] [Google Scholar]
Kannus 1992 {published data only}
- Kannus P, Natri A, Niittymäki S, Järvinen M. Effect of intraarticular glycosaminoglycan polysulfate treatment on patellofemoral pain syndrome ‐ A prospective, randomized double‐blind trial comparing glycosaminoglycan polysulfate with placebo and quadriceps muscle exercises. Arthritis and Rheumatism 1992;35(9):1053‐61. [DOI] [PubMed] [Google Scholar]
- Kannus P, Natri A, Paakkala T, Järvinen M. An outcome study of chronic patellofemoral pain syndrome. Seven‐year follow‐up of patients in a randomized, controlled trial. Journal of Bone and Joint Surgery. American Volume 1999;81(3):355‐63. [DOI] [PubMed] [Google Scholar]
Marchese 1998 {published data only}
- Marchese A, Cherubini M, Forte R. Laser therapy versus pharmacological in anterior knee pain [Laser terapia versus terapia farmacologica nel dolore anteriore del ginocchio]. La Riabilitazione 1998;31(4):189‐98. [Google Scholar]
Raatikainen 1990 {published data only}
- Raatikainen T, Väänänen K, Tamelander G. Effect of glycosaminoglycan polysulfate on chondromalacia patellae ‐ a placebo‐controlled 1‐year study. Acta Orthopaedica Scandinavia 1990;61(5):443‐8. [DOI] [PubMed] [Google Scholar]
Suter 1998 {published data only}
- Suter E, Herzog W, Souza K, Bray R. Inhibition of the quadriceps muscles in patients with anterior knee pain. Journal of Applied Biomechanics 1998;14(4):360‐73. [Google Scholar]
References to studies excluded from this review
Berenfeld 1991 {published data only}
- Berenfeld A, Bessing WD, Bisplinghoff U, Bittner G, Matthiesen HO, Tilscher H, et al. Gonalgia: treatment with therapeutic local anesthesia. Results of a controlled comparative study versus diclofenac [Gonalgie: Behandlung met der therapeutischen Lokalanästhesie. Ergebnisse einer kontrollierten Vergleichsstudie versus Diclofenac]. Fortschritte der Medizin 1991;109(35):724‐8. [PubMed] [Google Scholar]
Braham 2003 {published data only}
- Braham R, Dawson B, Goodman C. The effect of glucosamine supplementation on people experiencing regular knee pain. British Journal of Sports Medicine 2003;37(1):45‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlberg 1994 {published data only}
- Dahlberg L, Lohmander LS, Ryd L. Intraarticular injections of hyaluronan in patients with cartilage abnormalities and knee pain. A one‐year double‐blind, placebo‐controlled study. Arthritis and Rheumatism 1994;37(4):521‐8. [DOI] [PubMed] [Google Scholar]
Noble 1981 {published data only}
- Noble C. Fenbufen in the treatment of over‐use injuries in long‐distance runners. South African Medical Journal 1981;60(10):387‐8. [PubMed] [Google Scholar]
Wang 1997 {published data only}
- Wang L. A report of 76 cases of chondromalacia patellae treated with an ointment. Journal of Traditional Chinese Medicine 1997;17(1):40‐3. [PubMed] [Google Scholar]
Additional references
Alderson 2004a
- Alderson P, Green S, Higgins JPT, editors. MEDLINE highly sensitive search strategy for OVID‐MEDLINE. Cochrane Reviewers' Handbook 4.2.1 [updated December 2003]; Appendix 5b2. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Alderson 2004b
- Alderson P, Green S, Higgins JPT, editors. Assessment of study quality. Cochrane Reviewers' Handbook 4.2.1 [updated December 2003]; Section 6. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Arnoldi 1991
- Arnoldi CC. Patellar pain. Acta Orthopaedica Scandinavica. Supplementum 1991;(244):1‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arroll 1997
- Arroll B, Ellis‐Pegler E, Edwards A, Sutcliffe G. Patellofemoral pain syndrome. A critical review of the clinical trials on nonoperative therapy. American Journal of Sports Medicine 1997;25(2):207‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bourne 1988
- Bourne MH, Hazel WA Jr, Scott SG, Sim FH. Anterior knee pain. Mayo Clinic Proceedings 1988;63(5):482‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cutbill 1997
- Cutbill JW, Ladly KO, Bray RC, Thorne P, Verhoef M. Anterior knee pain: a review. Clinical Journal of Sport Medicine 1997;7(1):40‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eckstein 2002
- Eckstein F, Muller S, Faber SC, Englmeier KH, Reiser M, Putz R. Side differences of knee joint cartilage volume, thickness, and surface area, and correlation with lower limb dominance‐‐an MRI‐based study. Osteoarthritis & Cartilage 2002;10(12):914‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodfellow 1976
- Goodfellow J, Hungerford DS, Woods C. Patello‐femoral joint mechanics and pathology. 2. Chondromalacia patellae. Journal of Bone & Joint Surgery. British Volume 1976;58(3):291‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grelsamer 1998
- Grelsamer RP, Klein JR. The biomechanics of the patellofemoral joint. Journal of Orthopaedic and Sports Physical Therapy 1998;28(5):286‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holmes 1998
- Holmes SW, Glancy WG. Clinical classification of patellofemoral pain and dysfunction. Journal of Orthopaedic and Sports Physical Therapy 1998;28(5):299‐306. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juhn 1999
- Juhn MS. Patellofemoral pain syndrome: a review and guidelines for treatment. American Family Physician 1999;60(7):2012‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Kannus 1994
- Kannus P, Niittymaki S. Which factors predict outcome in the nonoperative treatment of patellofemoral pain syndrome? A prospective follow‐up study. Medicine & Science in Sports & Exercise 1994;26(3):289‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Natri 1998
- Natri A, Kannus P, Jarvinen M. Which factors predict the long‐term outcome in chronic patellofemoral pain syndrome? A 7‐yr prospective follow‐up study. Medicine & Science in Sports & Exercise 1998;30(11):1572‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nissen 1998
- Nissen CW, Cullen MC, Hewett TE, Noyes FR. Physical and arthroscopic examination techniques of the patellofemoral joint. Journal of Orthopaedic and Sports Physical Therapy 1998;28(5):277‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Powers 1998
- Powers CW. Rehabilitation of patellofemoral joint disorders: a critical review. Journal of Orthopaedics and Sports Physical Therapy 1998;28(5):345‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Royle 1991
- Royle SG, Noble J, Davies DR, Kay PR. The significance of chondromalacic changes on the patella. Arthroscopy 1991;7(2):158‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomee 1999
- Thomee R, Augustsson J, Karlsson J. Patellofemoral pain syndrome: a review of current issues. Sports Medicine 1999;28(4):245‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Tulder 1997
- Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane collaboration back review group for spinal disorders. Spine 1997;22(20):2223‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Tulder 2004
- Tulder MW, Jellema P, Poppel MNM, Nachemson AL, Bouter LM. Lumbar supports for prevention and treatment of low back pain (Cochrane review). Cochrane Database of Systematic Reviews 2004, Issue 1. [CD001823] [DOI] [PubMed] [Google Scholar]
Verhagen 1998
- Verhagen AP, Vet HC, Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology 1998;51(12):1235‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilk 1998
- Wilk KE, Davies GJ, Mangine RE, Malone TR. Patellofemoral disorders: A classification system and clinical guidelines for nonoperative rehabilitation. Journal of Orthopaedic & Sports Physical Therapy 1998;28(5):307‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Witonski 1999
- Witonski D, Wagrowska‐Danielewicz M. Distribution of substance‐P nerve fibers in the knee joint in patients with anterior knee pain syndrome. A preliminary report. Knee Surgery, Sports Traumatology, Arthroscopy 1999;7(3):177‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zomerdijk 1998
- Zomerdijk TE, Beetsma AJ, Dekker R, Wijck R, Horn JR. Conservative treatment of the Patellofemoral Pain syndrome ‐ a systematic review of literature [Conservatieve behandeling van het patellofemoraal pijnsyndroom ‐een systematisch literatuuronderzoek]. Nederlands Tijdschrift voor Fysiotherapie 1998;108(4):95‐102. [Google Scholar]