Abstract
Background
The present study aimed at evaluating the effect of vitamin K (VK) supplementation on bone health of laying hens challenged by Salmonella Enteritidis.
Methods
A total of 80 32-week-old double negative salmonella-free brown-egg laying hens were randomly assigned to 4 treatments with 20 replicates each (1 bird per replicate) according to a 2 × 2 factorial design with 2 dietary VK supplementation levels [0 mg/kg (VK0) vs 2 mg/kg VK (VK2) and 2 challenge treatments [Salmonella Enteritidis (SE) vs physiological saline solution (PS)]. During the last 3 days of week 43 of age, birds of both VK treatments were either orally challenged with 1.0 mL suspension of 109 cfu/mL S. Enteritidis daily or received the same volume of PS.
Results
The laying rate, daily egg mass, tibia strength, CT, cOC and cOC/(cOC + ucOC) of VK2 treatment increased (P < 0.05) in contrast to VK0, however, the medullary area and ucOC of VK2 treatment decreased (P < 0.05) in contrast to VK0. Mortality, medullary area, serum Ca content of SE treatments increased (P < 0.05) in contrast to PS treatments. In both SE treatments, the decrease (P < 0.05) in birds’ tibia strength was associated with higher (P < 0.05) Ca levels in serum. There is an interaction (P < 0.05) between SE challenge and VK levels with regard to tibia strength and serum Ca levels. At week 42, serum CT was positively correlated with cOC (R = 0.99, P = 0.009); at week 44, tibia strength was positively correlated with BMD (R = 0.95, P = 0.045), but negatively correlated with medullary area (R = − 0.98, P = 0.018).
Conclusions
VK (2 mg/kg) supplementation to diets of laying hens can enhance bone strength under challenge situations with Salmonella Enteritidis. Medullary area has proven to be a sensitive biomarker for bone calcium loss caused by SE infection.
Keywords: Calcium, Laying hen, Salmonella Enteritidis, Vitamin K
Background
Some Salmonella serogroups are significant pathogens responsible for bone infections [1, 2]. Salmonella enterica serovar Enteritidis (Salmonella Enteritidis), a Gram-negative bacterium, is the prevalent egg-product-related foodborne pathogen [3]. Infections with Salmonella Enteritidis (SE) are known to affect the health conditions of chickens [4, 5] resulting in the loss of homeostasis which, in turn, will reduce chickens’ production performance.
Salmonella, well-recognized as an intracellular pathogen, has the ability to invade into and persist within osteoblasts [6]. Bacterial infections and their products have been described as potent stimulators of osteoclastogenesis and bone resorption [7].
Efforts aiming to maintain birds’ health comprise the use of various dietary supplements [8, 9] including vitamin K (VK). Vitamin K is an essential fat-soluble micronutrient. Besides its function in the blood coagulation pathway, VK has also been considered as important factor in bone health [10]. Bone health is orchestrated by dynamic balance of minerals, organic matrices and hormones. The skeleton is the major reservoir for providing calcium. Many functions of Ca are regulated by calcitonin (CT), parathyroid hormone (PTH), osteocalcin (OC), carboxylated osteocalcin (cOC, the active type of osteocalcin), undercarboxylated osteocalcin (ucOC, the inactive type of osteocalcin) [11]. According to recommendations both of the National Research Council (1994, NRC) and the Chinese Feeding Standard of Chicken (Ministry of Agriculture of People’s Republic of China, 2004), VK should be supplemented at a level of 0.5 mg/kg to the diet of laying hens. However, under practical conditions, both feed industry and producer prefer to supplement diets for laying hens with up to 2 mg VK/kg to optimize laying performance and bone health [12]. There are no reports so far on the effect of VK on bone health of laying hens exposed to SE. Thus, the objective of this work was to study in birds orally challenged with SE, if dietary supplementation of VK may protect bones against SE-induced bone injury.
Materials and methods
The experimental protocols were approved by the Animal Care and Use Committee of China Agricultural University (Beijing, China).
Experimental design, feeding and housing management
Prior to challenge, cloacal swab samples and serum samples were taken from all birds, and were tested for the presence of SE (Poultry Microbiological Lab, China Agricultural University, Beijing, China). Birds devoid of Salmonella, based on these 2 tests, were referred to as “double negative”. Cloacal samples were pre-enriched with tetrathionate broth (CM 203–01, Land Bridge Technology Ltd., Beijing, China) at 37 °C for 24 h, and then streaked on Bismuth sulphite agar (CM 207, Land Bridge Technology Ltd.) [13]. Serum samples were tested in replicates for SE specific antibodies using an enzyme-linked immunosorbent assays (ELISA) (Shanghai Yuanmu Biotechnology Co., Ltd) according to the manufacturer’s instruction.
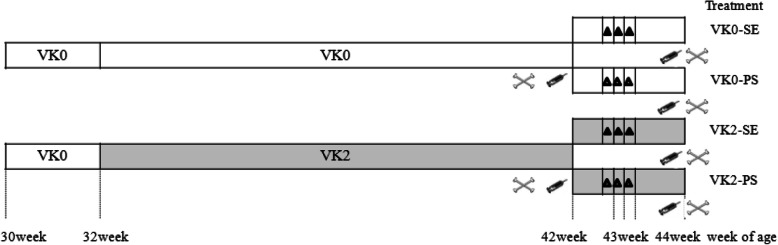
In total, 80 32-week-old double negative brown-egg laying hens (Beijing Yukou Poultry Co., Ltd., China) with similar laying rate (93.0 ± 1.4%) and body weight (1.52 ± 0.12 kg) were randomly allotted to 4 treatments with 20 replicates each (1 bird per replicate) according to a 2 × 2 factorial design with 2 dietary VK (menaphtone sodium bisulphite (MSB), purity 50%; Yunnan Luliang peacechem Technology Co., Ltd.) supplementation levels [0 mg/kg (VK 0) vs. 2 mg/kg VK (VK 2)] and 2 challenge treatments [SE vs. physiological saline solution (PS)]. Layers were caged individually (wire floored) from week 30 to 44. The 4 treatments were as follows (Fig. 1): VK0-PS (basal diet + 0 mg/kg VK + PS), VK0-SE (basal diet + 0 mg/kg VK + SE), VK2-PS (basal diet + 2 mg/kg VK + PS), VK2-SE (basal diet + 2 mg/kg VK + SE). Challenged and non-challenged birds were kept in 2 independent, mechanically ventilated, hen houses under the same environmental conditions to avoid cross-infection. The lighting schedule was 16-h-light throughout the experiment. Room temperature was maintained at 23 ± 2 °C. All birds had free access to diets and water. The basal diet was a corn-soybean meal-based mash diet (Table 1), which was formulated to meet the Chinese Feeding Standard of Chickens (Ministry of Agriculture of People’s Republic of China, 2004), except for VK, which was added to the basal diet according to the experimental design. The concentrations of VK3 in the basal diet (VK0) was not detected. The concentrations of VK3 in treatment diet (VK2) was 2.3 mg/kg in according to the HPLC method. The whole experiment comprised 14 weeks (from 210 to 308 days of age) consisting of 2 weeks adaptation period and 12 weeks experimental period. Before and during the experiment, the salmonella-free status both of diets and water was determined by using PCR extract bacterial DNA [14].
Fig. 1.
Experimental treatments and timeline of vitamin K supply and birds’ challenge. : blood sampling;
: blood sampling; : tibia sampling;
: tibia sampling; : Salmonella Enteritidis (SE) challenge;
: Salmonella Enteritidis (SE) challenge; : challenge physiological saline solution (PS);
: challenge physiological saline solution (PS); :dietary supplementation with 0 mg/kg vitamin K (VK0);
:dietary supplementation with 0 mg/kg vitamin K (VK0); :dietary supplementation with 2 mg/kg vitamin K (VK2)
:dietary supplementation with 2 mg/kg vitamin K (VK2)
Table 1.
Ingredient composition and nutrient content of basal diet (%, DM)
| Ingredient | % | Nutrient and energyc | |
|---|---|---|---|
| Corn | 66.45 | CP | 15.52% |
| Soybean meal | 22.80 | AME | 2,700, kcal/kg |
| Limestone | 8.20 | VK3 | 0 mg/kg |
| Dicalcium phosphate | 1.70 | Lys | 0.75% |
| Sodium chloride | 0.30 | Met | 0.37% |
| DL-Met | 0.12 | Met + Cys | 0.64% |
| Choline chloride | 0.10 | Thr | 0.57% |
| Vitamin premixa | 0.30 | Ca | 3.60% |
| Mineral premixb | 0.03 | Total P | 0.65% |
| Total | 100 | Available P | 0.39% |
aVitamin premix supplied (per kg of diet): vitamin A, 6,000 IU; vitamin D3, 1,500 IU; vitamin E, 15 IU; vitamin B1, 3 mg; vitamin B2, 10.2 mg; folic acid, 0.9 mg; calcium pantothenate, 15 mg; niacin, 45 mg; vitamin B6, 5.4 mg; vitamin B12, 24 μg; biotin, 150 μg
bMineral premix provided (per kg of diet): Cu (CuSO4·5H2O), 6.8 mg; Fe (FeSO4·7H2O), 66 mg; Zn (ZnSO4·7H2O), 83 mg; Mn (MnSO4·H2O), 80 mg; I (KI), 1 mg; Se (Na2SeO3), 0.3 mg
cContents of VK, CP and Ca were analyzed. Contents of other nutrients and energy content were calculated based on Feeding Standard of Chickens (Ministry of Agriculture of People’s Republic of China, 2004)
Salmonella Enteritidis inoculum and challenge
Salmonella enterica spp. enterica serovar Enteritidis (preservation number CVCC3377) was obtained from the China Institute of Veterinary Drug Control (Beijing, China). The frozen culture was recovered by using 10 mL of sterile tryptone soy broth and incubated at 37 °C with orbital shaking for 24 h. Subsequently, 5 mL of S. enteritidis pre-culture were transferred to 100 mL of tryptone soy broth and incubated with orbital shaking at 37 °C for 16–18 h. To determine the concentration of viable S. enteritidis in the culture, the inoculum was diluted with sterile phosphate buffer saline (PBS) (pH = 7.2), then plated on xylose lysine doxycholate (XLD) and incubated at 37 °C for 24 h. The stock culture was prepared in sterile PBS and adjusted to 1 × 109 cfu/ mL of S. enteritidis to be used as inoculum [15]. During the last 3 days of week 43 of age, birds were orally challenged with 1.0 mL suspension of 109 cfu/mL S. Enteritidis daily, whereas VK0-PS and VK2-PS treatments received the same volume of PS. A syringe with an attached flexible tube was used for the administration of the suspension and the physiological saline solution.
Laying performance
Egg weight and egg production were recorded daily in replicates. Daily egg mass was recorded and expressed on hen per day basis. Feed consumption was measured every week. Broken eggs and bird mortality were recorded daily in replicates.
Sample collection
Diets were analyzed for crude protein, calcium, total phosphorus and VK according to methods of Association of Official Analytical Chemists (AOAC, 1990). On the last day of week 44, 6 birds of each treatment were fasted for 12 h. Whole blood was collected via brachial vein, and kept in uncoated serum tubes at 12:00 h. Thereafter, serum was centrifuged at 1,000×g for 10 min at 4 °C and stored at − 20 °C.
Tibia was collected from left and right legs to determine fresh and dry tibia weight, tibia length, tibia breaking strength, bone mineral density, and bone Ca content.
Tibia collection and analysis
Tibia samples of the left and right legs were imaged using XR-36 Automatic Scanner (Norland Medical System. Inc. USA) to determine tibia length, bone mineral density (BMD) and medullary area (the section of BMD’s value under 0 was considered as medullary area, Fig. 2). The weight of fresh samples of the whole tibia was determined before samples were dried in an oven at 100 °C for 24 h until constant weight. Tibia strength was measured by means of a three-point bending test using the MTS-810 Material Test System (MTS Ltd. USA). The right tibia was cleaned and then dried at 105 °C for 24 h. Thereafter, the tibia was defatted for 7 d by using ether, and then dried again at 100 °C for 24 h and weighed. Bone samples were ashed at 600 °C overnight in a muffle furnace [16]. Bone calcium content was determined by ethylenediamine tetraacetic acid titration method described by Manobhavan [17].
Fig. 2.

A photo illustrating the medullary area defined as section of BMD’s value under 0
Serum biochemical index
Serum Ca content was measured using a detection kit (Prodia diagnostics, Boetzingen, Germany) and a Hitachi 7600 automatic biochemical analyzer (Hitachi Co., Ltd., Tokyo, Japan). The serum CT, PTH, cOC, ucOC concentrations were determined by radioimmunoassay using commercial kits (Beijing North Institute of Biological Technology, Beijing, China). The ratio of cOC/(cOC + ucOC) was calculated to determine the percentage of active type of osteocalcin compared to the total of active and inactive types of osteocalcin.
Statistical analysis
Data were analyzed using SAS statistical software (2010, version 9.2, SAS Institute Inc., Cary, NC, USA). Data obtained before SE challenge were analyzed by a one-way ANOVA using the General Linear Model (GLM) procedure, whereas data obtained after SE challenge were analyzed according to a two-way ANOVA for a 2 × 2 factorial design using GLM procedure. The main effects of VK supplementation levels and SE challenge status as well as their interactions were determined. Tukey’s multiple comparison test was used to separate means when interactive effects differed significantly. Data of broken eggs and birds’ mortality were transformed to arcsine values prior to statistical analysis. Results were expressed as treatment means with their standard error of the mean (SEM). A P value of < 0.05 was considered to be statistically significant. Pearson’s correlation coefficient analysis was conducted to determine correlations between variables. The individual correlations were visualized by using the ‘corrplot’ package in R software.
Results
Laying performance
During week 32 to 42, no difference (P > 0.05) was observed in laying performance between treatments (Table 2). During week 43 to 44, laying rate and egg mass were affected (P < 0.05) by the main effect of VK supplementation. Laying rate and egg mass in VK2 treatment were higher (P = 0.046 and P = 0.032, respectively) in comparison to VK0 treatments (91.6 vs 85.8 and 55.21 vs 51.56, respectively). Mortality was affected (P < 0.05) by the main effect of SE challenge as mortality in SE treatments increased (P < 0.010) from 18.4% to 26.6%.
Table 2.
Effect of dietary vitamin K (VK) supplementation and Salmonella Enteritidis (SE) challenge on laying performance of laying hens (n = 20)
| VK, mg/kg | SEc | Laying rate, % | Daily egg mass, g | Egg weight, g | Daily feed intake, g/d/bird | Feed conversion, g/g | Broken eggs, % | Mortality, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 32–42 |
Week 43–44 |
Week 32–42 |
Week 43–44 |
Week 32–42 |
Week 43–44 |
Week 32–42 |
Week 43–44 |
Week 32–42 |
Week 43–44 |
Week 32–42 |
Week 43–44 |
Week 32–42 |
Week 43–44 |
||
| 0 | – | 92.6 | 87.1 | 55.24 | 52.49 | 59.62 | 60.23 | 116.91 | 101.20 | 2.11 | 1.93 | 3.16 | 0.00 | 11.9 | 18.4 |
| 0 | + | 84.5 | 50.64 | 59.99 | 99.32 | 1.96 | 0.00 | 26.6 | |||||||
| 2 | – | 94.5 | 92.4 | 56.75 | 55.92 | 60.06 | 60.49 | 114.83 | 105.23 | 2.03 | 1.89 | 2.08 | 3.45 | 5.0 | 18.4 |
| 2 | + | 90.8 | 54.49 | 60.08 | 106.78 | 1.96 | 0.00 | 26.6 | |||||||
| SEMd | 0.986 | 2.600 | 0.646 | 1.506 | 0.338 | 0.646 | 2.715 | 3.449 | 0.061 | 0.039 | 0.725 | 1.026 | 3.708 | 0.000 | |
| 0 | – | 85.8b | – | 51.56b | – | 60.11 | – | 100.26 | – | 1.95 | – | 0.00 | – | 22.5 | |
| 2 | – | 91.6a | – | 55.21a | – | 60.28 | – | 106.01 | – | 1.92 | – | 1.72 | – | 22.5 | |
| SEMd | – | 1.839 | – | 1.065 | – | 0.457 | – | 2.439 | – | 0.028 | – | 0.726 | – | 0.000 | |
| – | – | 89.8 | – | 54.21 | – | 60.36 | – | 103.21 | – | 1.91 | – | 1.72 | – | 18.4b | |
| + | – | 87.6 | – | 52.57 | – | 60.03 | – | 103.06 | – | 1.96 | – | 0 | – | 26.6a | |
| SEMd | – | 1.839 | – | 1.065 | – | 0.457 | – | 2.439 | – | 0.028 | – | 0.726 | – | 0.000 | |
| VK | 0.215 | 0.046 | 0.130 | 0.032 | 0.381 | 0.794 | 0.600 | 0.121 | 0.379 | 0.578 | 0.316 | 0.119 | 0.216 | – | |
| P-values3 | SE | – | 0.419 | – | 0.297 | – | 0.619 | – | 0.965 | – | 0.207 | – | 0.119 | – | < 0.010 |
| VK × SE | – | 0.846 | – | 0.890 | – | 0.901 | – | 0.627 | – | 0.578 | – | 0.119 | – | – | |
a, bMeans within a column with no common superscripts differ (P < 0.05)
cSE Salmonella Enteritidis; –, without SE challenge; +, with SE challenge
dSEM Pooled standard error of the mean
3P-values for main effect of VK, the main effect of SE challenge, and the interaction between the VK treatments and SE challenge
Tibia parameters
At week 42, supplementation of VK increased (P < 0.05) tibia strength (Table 3). Similarly, at week 44, the main effect of supplementation of VK resulted in higher (P < 0.05) tibia strength as well, whereas the main effect of SE challenge resulted in decreased (P < 0.05) tibia strength. There was an interaction (P < 0.05) between VK and SE on tibia strength. Compared to PS treatment, SE challenged hens fed the VK supplemented diet had a higher (P < 0.05) tibia strength. No difference (P > 0.05) was observed in medullary area at week 42 (Table 3). At week 44, the main effect of supplementation of VK resulted in lower (P < 0.05) medullary area, whereas the main effects of SE challenge increased (P < 0.05) medullary area. At week 42, CT was positively correlated with cOC (R = 0.99, P = 0.009). Values for PTH and cOC were positively correlated with ucOC (R = 0.96, P = 0.036 and R = 0.96, P = 0.039, respectively); PTH, OC, and ucOC were negatively correlated with cOC/(cOC + ucOC) (R = − 1.00, P = 0.001, R = − 0.95, P = 0.046 and R = − 0.96, P = 0.039, respectively). At week 44, tibia strength was positively correlated with BMD (R = 0.95, P = 0.045), but was inversely related to medullary area (R = − 0.98, P = 0.018). Values for PTH were negatively correlated with cOC (R = − 0.97, P = 0.035). Those for cOC were positively correlated with cOC/(cOC + ucOC) (R = 0.96, P = 0.039), and those for unOC were negatively correlated with cOC/(cOC + ucOC) (R = − 1.00, P = 0.004). (Table 4 and 5).
Table 3.
Effect of dietary vitamin K (VK) supplementation and Salmonella Enteritidis (SE) challenge on tibia parameters of laying hens (n = 12)
| VK, mg/kg | SEc | Tibia length, cm | Fresh tibia weight, g | Dry tibia weight, g | Tibia strength, N | Total BMDd, g/cm2 | Medullary area, cm2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | ||
| 0 | – | 11.53 | 11.67 | 9.73 | 9.97 | 4.99 | 5.13 | 183.25b | 235.50b | 0.29 | 0.28 | 1.42 | 1.59 |
| 0 | + | 11.43 | 9.39 | 4.98 | 209.44b | 0.28 | 1.91 | ||||||
| 2 | – | 11.66 | 11.46 | 9.46 | 9.61 | 5.00 | 5.08 | 221.14a | 364.00a | 0.28 | 0.32 | 1.52 | 0.87 |
| 2 | + | 11.58 | 9.75 | 5.25 | 220.75b | 0.29 | 1.65 | ||||||
| SEMe | 0.079 | 0.101 | 0.248 | 0.253 | 0.131 | 0.190 | 12.320 | 28.053 | 0.033 | 0.023 | 0.312 | 0.193 | |
| 0 | – | 11.55 | – | 9.68 | – | 5.06 | – | 222.47b | – | 0.28 | – | 1.76a | |
| 2 | – | 11.53 | – | 9.69 | – | 5.17 | – | 287.60a | – | 0.30 | – | 1.26b | |
| SEMe | – | 0.072 | – | 0.182 | – | 0.137 | – | 20.185 | – | 0.016 | – | 0.140 | |
| – | – | 11.57 | – | 9.80 | – | 5.11 | – | 295.47a | – | 0.30 | – | 1.19b | |
| + | – | 11.51 | – | 9.57 | – | 5.11 | – | 215.09b | – | 0.29 | – | 1.77a | |
| SEMe | – | 0.072 | – | 0.182 | – | 0.137 | – | 20.185 | – | 0.016 | – | 0.140 | |
| VK | 0.260 | 0.781 | 0.446 | 0.984 | 0.923 | 0.573 | 0.049 | 0.017 | 0.851 | 0.306 | 0.836 | 0.017 | |
| P-values4 | SE | – | 0.571 | – | 0.400 | – | 0.963 | – | 0.004 | – | 0.531 | – | 0.009 |
| VE × SE | – | 0.094 | – | 0.168 | – | 0.418 | – | 0.045 | – | 0.589 | – | 0.253 | |
a, bMeans within a column with no common superscripts differ (P < 0.05)
cSE Salmonella Enteritidis; −, without SE challenge; +, with SE challenge
dTotal BMD Total bone mineral density
eSEM Pooled standard error of the mean
4P-values for main effect of VK, the main effect of SE challenge, and the interaction between the VK treatments and SE challenge
Table 4.
Pearson’s correlation coefficients between tibia strength, BMD, medullary area and serum biochemical indices at week 42
| Week 42 | Tibia strength | BMDa | Medullary area | Serum Cab | CTc | PTHd | cOCe | ucOCf | cOC/(cOC + ucOC) |
|---|---|---|---|---|---|---|---|---|---|
| Tibia strength | 1.00 | ||||||||
| BMDa | −0.43 | 1.00 | |||||||
| medullary area | 0.11 | −0.52 | 1.00 | ||||||
| Serum Cab | −0.63 | 0.39 | −0.19 | 1.00 | |||||
| CTc | −0.72 | 0.66 | −0.51 | 0.94 | 1.00 | ||||
| PTHd | −0.35 | 0.75 | −0.58 | 0.82 | 0.89 | 1.00 | |||
| cOCe | −0.40 | 0.55 | −0.52 | 0.93 | 0.99* | 0.94 | 1.00 | ||
| ucOCf | −0.42 | 0.58 | −0.38 | 0.93 | 0.93 | 0.96* | 0.96* | 1.00 | |
| cOC/(cOC + ucOC) | 0.38 | −0.78 | 0.61 | −0.82 | − 0.90 | −1.00* | −0.95* | − 0.96* | 1.00 |
aBMD Bone mineral density
bSerum Ca Serum calcium
cCT Calcitonin
dPTH Parathyroid hormone
ecOC Carboxylated osteocalcin
fucOC Undercarboxylated osteocalcin
*Correlation is significant at P < 0.05
Note: The correlation is based on the 6 samples, n = 6/treatment, the tibia parameters were the average values of both sides
Table 5.
Pearson’s correlation coefficients between tibia strength, BMD, medullary area and serum biochemical indices at week 44
| Week 44 | Tibia strength | BMDa | Medullary area | Serum Cab | CTc | PTHd | cOCe | ucOCf | cOC/(cOC + ucOC) |
|---|---|---|---|---|---|---|---|---|---|
| Tibia strength | 1.00* | ||||||||
| BMDa | 0.95* | 1.00 | |||||||
| medullary area | −0.98 | − 0.95 | 1.00 | ||||||
| Serum Cab | −0.04 | 0.13 | 0.17 | 1.00 | |||||
| CTc | −0.43 | −0.23 | 0.53 | 0.91 | 1.00 | ||||
| PTHd | −0.06 | −0.31 | 0.18 | −0.03 | −0.15 | 1.00 | |||
| cOCe | 0.29 | 0.50 | −0.42 | −0.10 | − 0.06 | −0.97* | 1.00 | ||
| ucOCf | −0.53 | −0.73 | 0.61 | −0.11 | − 0.01 | 0.87 | − 0.94 | 1.00 | |
| cOC/(cOC + ucOC) | 0.45 | 0.67 | −0.54 | 0.11 | 0.05 | −0.91 | 0.96* | −1.00* | 1.00 |
aBMD Bone mineral density
bSerum Ca Serum calcium
cCT Calcitonin
dPTH Parathyroid hormone
ecOC Carboxylated osteocalcin
fucOC Undercarboxylated osteocalcin
*correlation is significant at P < 0.05
Note: the correlation is based on 6 samples, n = 6 per treatment, the tibia parameters were the average values of both sides
Bone calcium concentration
There were no significant differences (P > 0.05) among all treatments with regard to bone calcium indices (Table 6).
Table 6.
Effect of dietary vitamin K (VK) supplementation and Salmonella Enteritidis (SE) challenge on tibia bone calcium (Ca) concentration of laying hens (n = 6)
| VK, mg/kg | SEa | Based on defatted dry matter | Based on total ash | ||
|---|---|---|---|---|---|
| Cab, % | Cab, % | ||||
| Week 42 | Week 44 | Week 42 | Week 44 | ||
| 0 | – | 23.56 | 21.17 | 38.74 | 38.39 |
| 0 | + | 21.86 | 37.58 | ||
| 2 | – | 24.36 | 21.32 | 40.58 | 38.82 |
| 2 | + | 21.49 | 37.08 | ||
| SEMc | 0.739 | 0.0259 | 1.021 | 0.763 | |
| 0 | – | 21.51 | – | 37.98 | |
| 2 | – | 21.41 | – | 37.74 | |
| SEMc | 0.190 | – | 0.549 | ||
| – | – | 21.24 | – | 38.60 | |
| + | – | 21.68 | – | 37.08 | |
| SEMc | – | 0.190 | – | 0.549 | |
| VK | 0.472 | 0.693 | 0.225 | 0.688 | |
| P-values4 | SE | – | 0.114 | – | 0.055 |
| VE × SE | – | 0.347 | – | 0.346 | |
aSE Salmonella Enteritidis; −, without SE challenge; +, with SE challenge
bCa Calcium
cSEM Pooled standard error of the mean
4P-values for main effect of VK, the main effect of SE challenge, and the interaction between the VK treatments and SE challenge
Serum biochemical indices
At week 42, there were no differences (P > 0.05) in serum biochemical indices between treatments (Table 7). At week 44, the main effect of VK2 treatment revealed higher (P < 0.05) CT and cOC levels, as well as cOC/(cOC + ucOC), but lower (P < 0.05) ucOC concentrations compared to the VK0 treatment. There were interactions (P < 0.05) between VK supplementation and SE challenge for Ca. However, at week 44, hens without supplemental VK had a higher (P < 0.05) serum Ca content compared to the PS treatment.
Table 7.
Effect of dietary vitamin K (VK) supplementation and Salmonella Enteritidis (SE) challenge on serum biochemical indices of laying hens (n = 6)
| VK, mg/kg | SEc | Cad, mmol/L | CTe, pg/mL | PTHf, pg/mL | cOCg, ng/mL | ucOCh, ng/mL | cOC/(cOC + ucOC), % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | Week 42 | Week 44 | ||
| 0 | – | 5.83 | 3.47b | 321.53 | 231.04 | 102.19 | 98.26 | 2.38 | 2.07 | 2.10 | 2.43 | 57.92 | 44.33 |
| 0 | + | 5.40a | 330.98 | 99.39 | 1.87 | 2.46 | 43.90 | ||||||
| 2 | – | 5.03 | 4.66ab | 270.89 | 262.15 | 67.43 | 92.20 | 2.32 | 2.45 | 1.54 | 1.45 | 59.75 | 63.70 |
| 2 | + | 4.57ab | 301.62 | 81.93 | 2.76 | 1.39 | 67.64 | ||||||
| SEMi | 0.973 | 0.406 | 29.193 | 25.980 | 15.255 | 8.219 | 0.301 | 0.100 | 0.400 | 0.285 | 0.731 | 2.539 | |
| 0 | – | 4.37 | – | 246.60b | – | 98.64 | – | 1.99b | – | 2.44a | – | 44.16b | |
| 2 | – | 4.62 | – | 314.20a | – | 88.78 | – | 2.52a | – | 1.43b | – | 64.69a | |
| SEMi | – | 0.287 | – | 17.770 | – | 5.897 | – | 0.073 | – | 0.179 | – | 2.273 | |
| – | – | 4.06b | – | 281.01 | – | 95.23 | – | 2.67 | – | 2.01 | – | 54.02 | |
| + | – | 4.98a | – | 284.71 | – | 90.66 | – | 2.76 | – | 1.92 | – | 51.81 | |
| SEMi | – | 0.287 | – | 17.770 | – | 5.811 | – | 0.071 | – | 0.178 | – | 2.244 | |
| VK | 0.622 | 0.661 | 0.345 | 0.022 | 0.248 | 0.20 | 0.898 | < 0.001 | 0.424 | 0.005 | 0.220 | 0.001 | |
| P-values8 | SE | – | 0.032 | – | 0.973 | – | 0.60 | – | 0.662 | – | 0.959 | – | 0.611 |
| VK × SE | – | 0.019 | – | 0.260 | – | 0.513 | – | 0.051 | – | 0.861 | – | 0.530 | |
a, bMeans within a column with no common superscripts differ (P < 0.05)
cSE Salmonella Enteritidis; −, without SE challenge; +, with SE challenge
dCa Calcium
eCT calcitonin
fPTH Parathyroid hormone
gcOC Carboxylated osteocalcin
hucOC Undercarboxylated osteocalcin
iSEM Pooled standard error of the mean
8P-values for main effect of VK, the main effect of SE challenge, and the interaction between the VK treatments and SE challenge
Discussion
According to the results of the present study, dietary supplementation of 2 mg/kg VK maintained higher laying rate, egg mass, and tibial strength of hens at week 42 of age.
There are no reports so far on the effect of supplemental VK on performance of laying hens challenged with SE, but similar to our findings in non-challenged birds [18] did not observe any effect of 10 mg/kg VK addition to the diet of LSL White Leghorn laying hens on birds’ egg production. If birds were challenged with SE, however, a higher mortality rate was obtained in the present study which is in agreement with observations in Arbor Acre broilers [5].
Bones as dynamic organs are constantly remodelled by osteoclasts and osteoblasts [19], and VK plays an important role in bone development and quality [20]. Thus, long-term VK deficiency may result in the loss of bone mass [21]. In the present study, VK supplementation increased the tibia strength at week 42. This is in agreement with results of a study by Guo [22], where dietary supplementation of 0.5 and 4.0 mg/kg of VK increased tibia breaking strength of male broilers on d 21. Following SE challenge, lower tibia strength points towards the decrease of BMD [23]. Similarly, Higgins et al. [24] and Ikejiri K et al. [25] reported bone deformity and bone infections, respectively, when birds were challenged with SE, and humans were infected with SE. In the present study, the main effect of supplementation of VK resulted in increased tibia strength, however, the main effect of SE challenge resulted in decreased tibia strength. This finding is in support of previous work, where dietary VK has shown not only to improve bone formation, but also to inhibit bone degradation [8]. Furthermore, tibia strength was positively correlated with BMD (R = 0.95, P = 0.045) and negatively correlated with medullary area (R = − 0.98, P = 0.018) at week 44, which is in support of the observation that tibia strength might be influenced by minerals [2]. However, it remains open, why supplemental VK improved tibia strength of non-challenged birds, but did not alleviate tibia strength upon SE challenge. More studies are needed to address the relationship between dietary VK and tibia strength under SE challenge conditions.
Homeostasis of Ca is an important driving force in the maintenance of bone strength as Ca is released into the blood stream during bone resorption and deposited into bones during bone formation [11]. In the present study, SE challenge increased serum levels of Ca suggesting SE may induce a defect in Ca homeostasis. The serum Ca content of challenged birds was similar to levels determined in non-challenged birds fed a diet supplemented with 2 mg/kg VK, however, it was higher than in non-challenged birds fed a diet devoid of supplemental VK. This phenomenon points towards the release of Ca from the bone into the blood. In addition, VK serves as a cofactor of endoplasmic reticulum resident γ-glutamyl carboxylase (GGCX), which carboxylates any selected glutamate residues on the target proteins and enables these proteins such as cOC to bind to Ca. Due to this function, VK may increase the affinity of cOC for Ca ions and hydroxyapatite crystals, and hence facilitate bone formation [26].
Calcitonin (CT) inhibits bone resorption and stimulates bone growth by rapidly lowering serum Ca levels and decreasing Ca released from bone, respectively [27–29]. In the present study, supplementation of 2 mg/kg VK to the diet of SE challenged birds increased serum CT levels, indicating that VK might be able to ameliorate SE-induced bone damage as well as maintain serum Ca balance.
Osteoblasts present in bones are known to synthesize cOC as precursor, which is then γ-carboxylated in the presence of VK, followed by incorporation into bone matrix, where cOC is involved in bone calcification. In the present study, VK supplementation increased the cOC levels in serum in comparison to the treatment devoid of supplemental VK in the diet. It appears that VK has a beneficial effect on bone formation, thus contributing to improved tibia strength. Previously, cOC has been described as a marker of bone formation [30], and as the most sensitive known serum marker for VK status in bone tissue [31]. In addition, OC has 3 γ-carboxyglutamic acid residues [32], and can bind with Ca and hydroxyapatite. In addition, cOC regulates the rate of mineral maturation through expanding hydroxyapatite crystal size [33]. Therefore, it is assumed that cOC plays a vital role in skeletal development, and is considered as marker of bone formation [34–36]. As coenzyme for γ-carboxylase [37], VK plays an important role in bone metabolism as well [38].
During deficiency of VK, OC does not undergo complete γ-carboxylation, and ucOC is released from osteoblasts into the circulating blood [39]. In this context, ucOC has been described as potential risk factor for bone fracture [10, 35]. Therefore, serum levels of ucOC may reflect birds’ nutritional status with regard to VK intake. Moreover, ucOC appears to be a negative regulator of bone formation [40]. These findings are in support of the present results, where VK supplementation decreased serum levels of ucOC and increased the affinity of Ca to the bone matrix [41] which, in turn, contributed to increased tibia strength. Consequently, the greater tibia strength due to VK supplementation might reflect improved balance between bone formation markers such as cOC and fracture risk factors such as ucOC.
The skeleton comprises compact bone and medullary bones [42], but infection with SE may initiate bone resorption from the medullary bones only, without affecting the compact bones. As a result, osteoblastic bone formation can be impaired and bone resorption will be enhanced, thereby contributing to increased bone fragility and fracture risk. According to the World Health Organization (WHO), porous bones in association with deteriorated bone tissue result in low BMD values, also referred to as osteoporosis. In this study, supplementation of VK decreased in SE-challenged birds the medullary area due to beneficial effects of VK on bone formation. Without dietary VK supplementation, however, SE challenge caused an increase of medullary area as bone resorption was enhanced.
Conclusions
Dietary supplementation of 2 mg/kg VK may mitigate the Ca homeostasis disruptions caused by SE challenge, and can be used as dietary intervention for control of Salmonella infection in laying hens through strengthening of birds’ skeleton. Medullary area has proven to be a sensitive biomarker for bone calcium loss caused by SE infection. Further knowledge of the various metabolic functions of VK may aid in developing novel feeding strategies to secure both performance and health of laying hens.
Acknowledgements
National Science Foundation of China (Grant No.31772627) was funding for the design of the study and collect sample. A Special Fund for China Agricultural Research System program (CARS-40-K08) was funding for writing the manuscript. Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, 328017493/GRK 2366), and National Key Research and Development Program of China (2017YFD0500500) were funding for analysis the sample.
Abbreviations
- BMD
Bone mineral density
- Ca
Calcium
- cOC
Carboxylated osteocalcin
- CT
Calcitonin
- ELISA
Enzyme-linked immunosorbent assays
- OC
Osteocalcin
- PBS
Phosphate buffer saline
- PS
Physiological saline solution
- PTH
Parathyroid hormone
- SE
Salmonella Enteritidis
- ucOC
Undercarboxylated osteocalcin
- VK
Vitamin K
- XLD
Xylose lysine doxycholate
Authors’ contributions
The authors’ responsibilities were as follows: QGM obtained financial support and designed the study. YJL conducted the experiments, analyzed the sample and wrote the manuscript. JYZ and LHZ collected and analyzed the experiment data. RM and CJ helped with revisiting and reviewing the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Science Foundation of China (Grant No.31772627), a Special Fund for China Agricultural Research System program (CARS-40-K08), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, 328017493/GRK 2366), and National Key Research and Development Program of China (2017YFD0500500).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
All animal protocols used in this study were approved and carried out according to the guidelines for the ethical treatment of animals by the institutional Animal Care and Use Committee of China Agricultural University (Beijing, China). (NO. AW13301202–1-6).
Consent for publication
All the authors read and agree to the content of this paper and its publication.
Competing interests
The authors declare that there is no conflict of interest.
Contributor Information
Yaojun Liu, Email: yaojunliu@outlook.com.
Rainer Mosenthin, Email: rainer.mosenthin@uni-hohenheim.de.
Lihong Zhao, Email: zhaolihongcau@cau.edu.cn.
Jianyun Zhang, Email: jyzhang@cau.edu.cn.
Cheng Ji, Email: jicheng@cau.edu.cn.
Qiugang Ma, Email: maqiugang@cau.edu.cn.
References
- 1.Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res. 2004;30(3):291–308. doi: 10.1385/IR:30:3:291. [DOI] [PubMed] [Google Scholar]
- 2.Shao Y, Sun G, Cao S, Lu L, Zhang L, Liao X, Luo X. Bone phosphorus retention and bone development of broilers at different ages. Poult Sci. 2019;98(5):2114–2121. doi: 10.3382/ps/pey565. [DOI] [PubMed] [Google Scholar]
- 3.Li ZY, Guo RX, Wang F, Geng SZ, Kang XL, Meng C, Gu D, Jiao X, Pan Z. Inactivation of Salmonella Enteritidis on eggshells by lactic acid spray. Food Control. 2019;104:201–207. doi: 10.1016/j.foodcont.2019.04.046. [DOI] [Google Scholar]
- 4.Liu YJ, Zhao LH, Mosenthin R, Zhang JY, Ji C, Ma QG. Protective effect of vitamin E on laying performance, antioxidant capacity, and immunity in laying hens challenged with Salmonella Enteritidis. Poult Sci. 2019;98(11):5847–5854. doi: 10.3382/ps/pez227. [DOI] [PubMed] [Google Scholar]
- 5.Gan L, Fan H, Mahmood T, Guo Y. Dietary supplementation with vitamin C ameliorates the adverse effects of Salmonella Enteritidis-challenge in broilers by shaping intestinal microbiota. Poult Sci. 2020;99(7):3663–3674. doi: 10.1016/j.psj.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bost KL, Bento JL, Ellington JK, Marriott I, Hudson MC. Induction of colony-stimulating factor expression following Staphylococcus or Salmonella interaction with mouse or human osteoblasts. Infect Immun. 2000;68(9):5075–5083. doi: 10.1128/IAI.68.9.5075-5083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64(7):2371–2380. doi: 10.1128/IAI.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming RH, McCormack HA, Whitehead CC. Bone structure and strength at different ages in laying hens and effects of dietary particulate limestone, vitamin K and ascorbic acid. Br Poult Sci. 1998;39(3):434–440. doi: 10.1080/00071669889024. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Li D, Wang F, Dong T. Effects of dietary vitamin K levels on bone quality in broilers. Arch Anim Nutr. 2003;57(3):197–206. doi: 10.1080/0003942031000136620. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Olleros Rodriguez C, Diaz CM. Vitamin K and bone health: a review on the effects of vitamin K deficiency and supplementation and the effect of non-vitamin K antagonist oral anticoagulants on different bone parameters. J Osteoporos. 2019;2019:2069176. doi: 10.1155/2019/2069176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song L. Calcium and bone metabolism indices. Adv Clin Chem. 2017;82:1–46. doi: 10.1016/bs.acc.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhen W, Shao Y, Wu Y, Li L, Pham VH, Abbas W, Wan Z, Guo Y, Wang Z. Dietary yeast beta-glucan supplementation improves eggshell color and fertile eggs hatchability as well as enhances immune functions in breeder laying hens. Int J Biol Macromol. 2020;159:607–621. doi: 10.1016/j.ijbiomac.2020.05.134. [DOI] [PubMed] [Google Scholar]
- 13.Arafat N, Eladl AH, Mahgoub H, El-Shafei RA. Effect of infectious bursal disease (IBD) vaccine on Salmonella Enteritidis infected chickens. Vaccine. 2017;35(29):3682–3689. doi: 10.1016/j.vaccine.2017.04.076. [DOI] [PubMed] [Google Scholar]
- 14.Fan S, Zheng J, Duan Z, Yang N, Xu G. The influences of SE infection on layers’ production performance, egg quality and blood biochemical indicators. J Anim Sci Biotechnol. 2014;5(1):4. doi: 10.1186/2049-1891-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen W, Shao Y, Gong X, Wu Y, Geng Y, Wang Z, Guo Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult Sci. 2018;97(8):2654–2666. doi: 10.3382/ps/pey119. [DOI] [PubMed] [Google Scholar]
- 16.Kolakshyapati M, Flavel RJ, Sibanda TZ, Schneider D, Welch MC, Ruhnke I. Various bone parameters are positively correlated with hen body weight while range access has no beneficial effect on tibia health of free-range layers. Poult Sci. 2019;98(12):6241–6250. doi: 10.3382/ps/pez487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manobhavan M, Elangovan AV, Sridhar M, Shet D, Ajith S, Pal DT, et al. Effect of super dosing of phytase on growth performance, ileal digestibility and bone characteristics in broilers fed corn-soya-based diets. J Anim Physiol Anim Nutr (Berl) 2016;100(1):93–100. doi: 10.1111/jpn.12341. [DOI] [PubMed] [Google Scholar]
- 18.Fleming RH, McCormack HA, McTeir L, Whitehead CC. Effects of dietary particulate limestone, vitamin K3 and fluoride and photostimulation on skeletal morphology and osteoporosis in laying hens. Br Poult Sci. 2003;44(5):683–689. doi: 10.1080/00071660310001643688. [DOI] [PubMed] [Google Scholar]
- 19.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25(1):629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 20.Fu X, Moreines J, Booth SL. Vitamin K supplementation does not prevent bone loss in ovariectomized Norway rats. Nutr Metab (Lond) 2012;9(1):12. doi: 10.1186/1743-7075-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jie KG, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K status and bone mass in women with and without aortic atherosclerosis: a population-based study. Calcif Tissue Int. 1996;59(5):352–356. doi: 10.1007/s002239900139. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, Xv J, Li Y, Bi Y, Hou Y, Ding B. Interactive effects of dietary vitamin K3 and Bacillus subtilis PB6 on the growth performance and tibia quality of broiler chickens with sex separate rearing. Animal. 2020;1:1–9. doi: 10.1017/S1751731120000178. [DOI] [PubMed] [Google Scholar]
- 23.Sadeghi AA. Bone mineralization of broiler chicks challenged with Salmonella enteritidis fed diet containing probiotic (Bacillus subtilis) Probiotics Antimicrob Proteins. 2014;6(3–4):136–140. doi: 10.1007/s12602-014-9170-6. [DOI] [PubMed] [Google Scholar]
- 24.Higgins WA, Christiansen JB, Schroeder CH. A Salmonella enteritidis infection associated with leg deformity in turkeys. Poult Sci. 1944;23(4):340–341. doi: 10.3382/ps.0230340. [DOI] [Google Scholar]
- 25.Ikejiri K, Suzuki K, Ito A, Yasuda K, Shindo A, Ishikura K, Imai H. Invasive Salmonella Enteritidis infection complicated by bacterial meningitis and vertebral osteomyelitis shortly after influenza a infection in an immunocompetent young adult. J Infect Chemother. 2020;26(2):269–273. doi: 10.1016/j.jiac.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Akbari S, Rasouli-Ghahroudi AA. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int. 2018;2018:4629383. doi: 10.1155/2018/4629383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallach S, Rousseau G, Martin L, Azria M. Effects of calcitonin on animal and in vitro models of skeletal metabolism. Bone. 1999;25(5):509–516. doi: 10.1016/S8756-3282(99)00200-8. [DOI] [PubMed] [Google Scholar]
- 28.Copp DH, Cheney B. Calcitonin - a hormone from the parathyroid which lowers the calcium-level of the blood. Nature. 1962;193(4813):381–382. doi: 10.1038/193381a0. [DOI] [PubMed] [Google Scholar]
- 29.Martin TJ. Drug and hormone effects on calcium release from bone. Pharmacol Ther. 1983;21(2):209–228. doi: 10.1016/0163-7258(83)90073-6. [DOI] [PubMed] [Google Scholar]
- 30.Rossi M, Battafarano G, Pepe J, Minisola S, Del Fattore A. The endocrine function of osteocalcin regulated by bone resorption: a lesson from reduced and increased bone mass diseases. Int J Mol Sci. 2019;20(18):4052. doi: 10.3390/ijms20184502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evenepoel P, Claes K, Meijers B, Laurent M, Bammens B, Naesens M, Sprangers B, Pottel H, Cavalier E, Kuypers D. Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res. 2019;34(2):262–269. doi: 10.1002/jbmr.3608. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Zhang H, Yang C, Li Y, Dai Z. An overview of osteocalcin progress. J Bone Miner Metab. 2016;34(4):367–379. doi: 10.1007/s00774-015-0734-7. [DOI] [PubMed] [Google Scholar]
- 33.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23(3):187–196. doi: 10.1016/S8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 34.Kruse K, Kracht U. Evaluation of serum osteocalcin as an index of altered bone metabolism. Eur J Pediatr. 1986;145(1–2):27–33. doi: 10.1007/BF00441848. [DOI] [PubMed] [Google Scholar]
- 35.Minisola S, Romagnoli E. Undercarboxylated osteocalcin (ucOC) level should be considered a marker of the risk of hip fracture. Bone. 1996;19(5):565. doi: 10.1016/S8756-3282(96)00249-9. [DOI] [PubMed] [Google Scholar]
- 36.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture: a three year follow-up study. Bone. 1996;18(5):487–488. doi: 10.1016/8756-3282(96)00037-3. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 2010;29(6):761–765. doi: 10.1016/j.clnu.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom. 2013;16(4):409–413. doi: 10.1016/j.jocd.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 40.Bugel S. Vitamin K and bone health. Proc Nutr Soc. 2003;62(4):839–843. doi: 10.1079/PNS2003305. [DOI] [PubMed] [Google Scholar]
- 41.Je SH, Joo NS, Choi BH, Kim KM, Kim BT, Park SB, Cho DY, Kim KN, Lee DJ. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old. J Korean Med Sci. 2011;26(8):1093–1098. doi: 10.3346/jkms.2011.26.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonucci E. Bone mineralization. Front Biosci (Landmark Ed) 2012;17:100–128. doi: 10.2741/3918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.