Abstract
Background
Cognitive reserve is most commonly measured using socio-behavioural proxy variables. These variables are easy to collect, have a straightforward interpretation, and are widely associated with reduced risk of dementia and cognitive decline in epidemiological studies. However, the specific proxies vary across studies and have rarely been assessed in complete models of cognitive reserve (i.e. alongside both a measure of cognitive outcome and a measure of brain structure). Complete models can test independent associations between proxies and cognitive function in addition to the moderation effect of proxies on the brain-cognition relationship. Consequently, there is insufficient empirical evidence guiding the choice of proxy measures of cognitive reserve and poor comparability across studies.
Method
In a cross-sectional study, we assessed the validity of 5 common proxies (education, occupational complexity, verbal intelligence, leisure activities, and exercise) and all possible combinations of these proxies in 2 separate community-dwelling older adult cohorts: The Irish Longitudinal Study on Ageing (TILDA; N = 313, mean age = 68.9 years, range = 54–88) and the Cognitive Reserve/Reference Ability Neural Network Study (CR/RANN; N = 234, mean age = 64.49 years, range = 50–80). Fifteen models were created with 3 brain structure variables (grey matter volume, hippocampal volume, and mean cortical thickness) and 5 cognitive variables (verbal fluency, processing speed, executive function, episodic memory, and global cognition).
Results
No moderation effects were observed. There were robust positive associations with cognitive function, independent of brain structure, for 2 individual proxies (verbal intelligence and education) and 16 composites (i.e. combinations of proxies). Verbal intelligence was statistically significant in all models. Education was significant only in models with executive function as the cognitive outcome variable. Three robust composites were observed in more than two-thirds of brain-cognition models: the composites of (1) occupational complexity and verbal intelligence, (2) education and verbal intelligence, and (3) education, occupational complexity, and verbal intelligence. However, no composite had larger average effects nor was more robust than verbal intelligence alone.
Conclusion
These results support the use of verbal intelligence as a proxy measure of CR in cross-sectional studies of cognitively healthy older adults.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-021-00870-z.
Keywords: Cognitive reserve, Cognitive ageing, Cognitive decline, Neuroimaging, Structural MRI
Background
Neuropathology and measures of brain structure do not fully explain cognitive decline [1] nor age-related variation in cognitive function [2]. This is evident in the finding of normal cognitive function in individuals who meet the diagnostic criteria for Alzheimer’s disease (AD) based on neuropathology [3, 4]. This well-established gap between brain and cognition may be explained by cognitive reserve (CR), wherein the effects of brain pathology or ageing on cognitive function are moderated by an individual’s ability to efficiently or flexibly use the brain’s resources to cope with task demands [5].
Accurate measurement of CR could improve the detection of, and risk assessments for, age-related cognitive decline and AD [6] and improve the measurement of intervention efficacy in clinical trials and intervention studies by enabling researchers to effectively statistically control for CR [7]. Difficulties in measuring CR [8], however, limit this potential. The most direct measures of CR are likely to be obtained using functional neuroimaging [8]. CR may be measured with functional MRI using resting-state and task-based functional connectivity. For example, a pattern of greater change in functional connectivity from resting-state in response to task demands is associated with better cognitive performance, above and beyond the effects of cortical thickness [9]. However, the considerable cost of MRI scanning [10] limits access to such measures, particularly in lower income countries [11]. As such, socio-behavioural variables reflecting the degree of exposure to, or engagement in, various lifetime experiences are often used as proxies of CR [8].
The rationale for using proxies is that greater exposure to certain lifetime experiences increases the adaptability of cognitive and functional brain processes, thereby enabling a greater ability to cope with brain changes or damage [8]. Considerable epidemiological evidence indicates a reduced risk and/or delayed onset of dementia and cognitive decline in individuals with greater educational attainment [12–14], occupational complexity/status [15–17], literacy and/or verbal intelligence [18–21], engagement in activities that were cognitively stimulating [22, 23], leisure-related [24, 25], physical [22, 26–28], and social [22, 23, 29]. Proxies also provide a single value with a simple interpretation—a higher degree of exposure reflects greater CR. Moreover, proxies are easy and inexpensive to obtain, and some, such as educational attainment, are routinely collected as part of most ageing studies. It is therefore not surprising that CR is most often measured using proxies [30].
Despite their advantages, the use of proxies to measure CR has been criticized. First, some proxies, such as educational attainment, are typically static measures [31] despite the fact that CR is considered to be a dynamic construct that can change over time [32]. Second, some argue that a single proxy fails to reflect the full CR construct which is thought to be influenced by a range of experiences [33, 34]. Finally, proxies may also be associated with cognitive decline via mechanisms other than reserve [35]. For instance, greater educational attainment is correlated with higher socioeconomic status [36] which is itself associated with slower cognitive decline [37] and reduced risk and prevalence of dementia [38, 39]. Low socioeconomic status is associated with various other factors, including stress and access to healthcare, which could exacerbate cognitive decline [38]. As such, the protective effect of education on cognitive decline and dementia (but cf. [40] for an alternative perspective) may be via mechanisms related to socioeconomic status, rather than CR [41].
The limitations of individual proxies may be mitigated by averaging (cf. transformation methods such as principal component analysis) multiple proxies to create a composite proxy measure that still provides a single summary value with a simple interpretation [42–46]. Composite proxies allow for a wider range of contributions to CR and enable the inclusion of dynamic proxies that can change over time, such as verbal intelligence or engagement in activities [31]. Furthermore, composite proxies may attenuate the issue of non-CR mechanisms of individual proxies because alternative mechanisms (e.g. socioeconomic status) might only be associated with some proxies, such as educational attainment, but not others like social engagement. Some composite-type approaches, including factor analytic and latent variable models, measure CR using inappropriate reflective measurement models, where the observed CR proxies are effectively considered to be reflective (i.e. caused by) the latent CR construct [35]. Composite proxies are a more appropriate formative measurement model, where the observed proxies are considered to form, or cause, CR. Moreover, this approach can reflect the unique additive contributions of individual proxies, whereas factor analytic models reflect only the shared variance across different proxies [8].
While the composite approach offers advantages over the use of single proxies, there is no agreed-upon gold-standard composite proxy [30] just as there is likewise no gold-standard individual proxy. Similarly, it is unclear which proxy should be used when assessing candidate neuroimaging measures of CR, as face validity is assessed via their association with CR proxies [47, 48]. The considerable variation [49, 50] and lack of coherence in the use of proxies means that there is poor comparability across studies, as an effect observed for one proxy (e.g. educational attainment) may not be observed to the same degree for another (e.g. occupational complexity), even though both putatively reflect CR. It also provides researchers in the field of CR with an additional “researcher degrees of freedom” [51] such that several different proxies could be examined but only statistically significant results are reported.
To assess the validity of a potential measure of CR, a complete model of CR is required, which includes 3 components: a measure of CR (e.g. a proxy), a measure of brain structure/pathology, and a measure of cognitive function [8, 52]. This enables the assessment of the cognitive benefit criterion [48]. This criterion can be satisfied via the observation of (1) an “independent effect” in which the candidate measure is positively associated with cognitive function, independent of brain structure, or (2) a “moderation effect” in which the candidate measure moderates the relationship between brain structure and cognitive function [8, 47]. The moderation effect is considered the ideal benchmark for CR, whereas the independent effect is considered a weaker level of evidence for a CR effect [8].
A systematic review of CR proxies from complete CR models reported inconclusive evidence for educational attainment, occupational complexity/status, and leisure activity as proxies of CR in cognitively healthy cohorts [53]. A single reviewed study provided evidence that greater engagement in cognitively stimulating activities in mid- and late-life provided CR effects [54]. Other proxies were not assessed in this systematic review, although individual studies have reported positive evidence for CR effects in complete CR models. Verbal intelligence has been positively associated with cognition, controlling for global AD neuropathology or hippocampal atrophy in cognitively healthy [55, 56] and cognitively impaired older adults [55]. Physical activity was positively associated with cognition in the presence of neuropathology [57] but not hippocampal atrophy [56]. Social engagement moderated the relationship between amyloid-beta deposition and cognitive decline [58]. The composite of verbal intelligence and education moderated the relationship of subcortical grey matter (GM) volume and cortical thickness with fluid reasoning but not memory or processing speed and attention [46]. This composite was also associated with memory controlling for GM volume [59] and global cognition controlling for a composite AD-biomarker [45]. Although other composites have been associated with cognition [50], there is very little empirical evidence regarding their effects within complete CR models.
There is currently no conclusive evidence for the best individual or composite proxy for measuring or validating neuroimaging measures of CR, particularly with respect to cognitively healthy older adults. A methodology for solving this problem is the use of hierarchical linear moderated regressions to systematically assess standard CR proxies and their composites in complete models, an approach that enables the examination of both moderation and independent effects within the same analysis framework. This is important because, although moderation effects should ideally be observed to validate a CR proxy or measure [8], they are typically small in real-world data [60], explaining 1–3% of the variance in the outcome [61]. Consequently, large sample sizes are required to detect typically small moderation effects [62]. This issue is further exacerbated when measurement error is present in either variable in the interaction term (e.g. the CR proxy and measure of brain structure) used to assess the moderation effect [63] or when either variable in the interaction term is associated with the outcome variable (e.g. cognitive function [64];). Given the noted difficulties in identifying moderation effects, it is important to also consider the independent effect when assessing the validity of CR proxies.
Hierarchical linear regressions allow the robustness (i.e. frequency of effects using different measures of brain structure and cognitive function) and magnitude of both moderation and independent effects of different proxies to be compared. Here, in two separate community-dwelling older adult cohorts, we examined five common putative CR proxies—education, occupational complexity, verbal intelligence, leisure activities, and exercise—and all of their possible combinations. We included three brain structure variables, mean cortical thickness, hippocampal volume, and grey matter volume, in each model. Our primary aim was to identify the CR proxies with the most robust and largest effects across two datasets. More formally, we define effective CR proxies as those variables that have a significant independent or moderation effect on measures of cognitive function and brain structure.
Method
Participants
The first dataset consisted of data from 313 community-dwelling adults (mean age = 68.90 years, SD = 6.75 years, range = 54–88 years; 50.48% female), a subset of The Irish Longitudinal Study on Ageing (TILDA), and a nationally representative longitudinal cohort study of older adults in Ireland [64, 65]. This data was collected during Wave 3 of the TILDA study [66]. All participants were screened for MRI contraindications, and study-specific inclusion criteria included no history of neurological conditions and available data for CR proxies and cognitive function.
The second dataset consisted of data from 234 community-dwelling adults (mean age = 64.49 years, SD = 7.42 years, range = 50–80 years; 51.28% female) selected from participants in the Cognitive Reserve/Reference Ability Neural Network (CR/RANN) studies [67–69]. Participants were screened for MRI contraindications, hearing and visual impairments, medical or psychiatric conditions, and dementia or MCI. Participants selected for the current analyses were aged 50 years or older with data available for CR proxies, cognitive function, and MRI.
Measures: CR proxies
Data was available for 5 socio-behavioural proxies in both datasets: educational attainment, occupational complexity, verbal intelligence, leisure activities, and physical activity. In TILDA, further data was available for the proxies: cognitively stimulating activities and social engagement.
Educational attainment was measured using years of formal education in both datasets. In TILDA, participants were asked to indicate the age at which they first left continuous full-time education. This information was missing for 4 participants in the final sample (1.28%), so it was imputed using educational qualification, father’s education, age, sex, and rural residence during childhood as previously described [70].
Occupational complexity was measured using the complexity of work in the dimensions of data, people, and things [71] using ratings obtained from an online catalogue of the Dictionary of Occupational Tiles (DOT: www.occupationalinfo.org). Ratings for each dimension were reversed (such that higher scores reflected greater complexity) and then summed to create a total occupational complexity score, with scores ranging from 0 (minimal complexity) to 21 (maximal complexity). This was obtained for each participant’s current occupation or last occupation before retirement in TILDA and for participant’s occupation of longest duration of their lifetime in CR/RANN.
Verbal intelligence was measured using the total number of correctly pronounced words on the National Adult Reading Test (NART; Nelson and Willinson [72]) in TILDA and the American National Adult Reading Test (AMNART; Grober and Sliwinski [73]) in CR/RANN. In TILDA, a stress/anxiety-preventative and time-saving measure [74] was employed such that participants only completed the second half of the NART if they scored greater than 20 on the first half. A correction procedure was employed whereby scores of 0–11 were retained as full scores, but scores of 12–20 in participants who did not complete the second half were corrected using a conversion table outlined by Beardsall and Brayne [75, 76]. Possible scores on the NART, in TILDA, ranged from 0 to 50 and on the AMNART, in CR/RANN, from 0 to 45. While the NART is often used to provide a measure of premorbid intelligence, we have labelled NART scores here as verbal intelligence in line with previous cognitive reserve studies [42, 77]. The NART is “effectively a test of knowledge acquisition” [78] that may reflect the exposure to various educational and cognitive experiences across the lifespan [79–82].
Leisure activities were assessed in TILDA by participants rating their current frequency of engagement on an 8-point Likert scale (0 = never to 7 = daily/almost daily) in 9 activities: watching television, going to films/plays/concerts, travel, listening to music/radio, going to the pub, eating out, sports/exercise, visiting/talking on phone, and volunteering. In CR/RANN, participants rated their frequency of engagement over the preceding 6 months on a 3-point Likert scale (1 = never to 3 = often) in 17 activities: television/radio, cards/games, reading, lectures/concerts, theatre/movies, travel, walks/rides, crafts/hobbies, music, visiting, sports/dancing/exercise, cooking, group membership, collecting, religious activities, and volunteering. For both datasets, total scores were created by summing individual responses and possible scores ranged from 17 to 51.
Physical activity was assessed in TILDA by calculating the total metabolic minutes arising from self-reported physical activity over the last week using the International Physical Activity Questionnaire-Short Form (IPAQ-SF; Craig et al. [83]; Lee et al. [84]). This questionnaire assessed the time spent in 3 categories: vigorous, moderate, and walking. Responses were converted to metabolic equivalent minutes [83] and summed. In CR/RANN, physical activity was calculated using total metabolic hours arising from physical activity in an average week. The Godin leisure-time exercise questionnaire [85] assessed the frequency of activity sessions lasting > 15 min in 3 categories: strenuous, moderate, and mild exercise. Responses were then weighted by the average estimated duration of activity in each category (0.5, 0.75, and 1 h, respectively) and their metabolic equivalent values (9, 5, 3; Ogino et al., [28]; Scarmeas et al. [86]).
Cognitively stimulating activities were assessed in TILDA with a questionnaire where participants rated their frequency of engagement on an 8-point Likert scale (0 = never to 7 = daily/almost daily) in 5 activities: attending classes and lectures, working in the garden/home or on a car, reading books/magazines, spending time on hobbies/creative activities, and playing cards/bingo/games. Total scores were created by summing individual responses and possible scores ranged from 0 to 35.
Social engagement was measured in TILDA using the Social Network Index [87] which provides a total score, ranging from 0 to 4, reflecting an individual’s degree of social connection [88].
Composite proxies were created by first standardizing (z-scoring) individual proxies. Next, every unique combination of proxies was generated and the composite proxy was the average of those proxies. For TILDA, this produced 120 unique composite proxies. For CR/RANN, this resulted in 26 composite proxies.
To summarize, for TILDA, there were 127 proxies in total (individual and composite) and 31 in total for CR/RANN. To attenuate possible effects of outliers, all proxies were Winsorized using a robust technique based on the median absolute deviation [89]. Outliers were identified as values greater than a threshold of 3 median absolute deviations from the median. Identified outliers were replaced by the median ± 3 median absolute deviations.
Measures: cognitive function
Verbal fluency was assessed using the total score on the Animal Naming Test which measures the ability to spontaneously produce the name of animals in 1 min [74]. The total number of animals named was used as the total score in both datasets.
Processing speed was measured using the time to complete the Colour Trails Task 1 (CTT 1; D’Elia et al. [90]) in TILDA and the Trail Making Task A (TMT A; Reitan [91]) in CR/RANN. The CTT is considered a cross-culturally valid form of the TMT [74]. Scores were reversed coded, such that higher scores reflected greater cognitive performance.
Executive function was assessed using the CTT 2 (D’Elia et al. [90]) in TILDA and the TMT B (Reitan [91]) in CR/RANN. Both measures reflect the multi-dimensional executive function construct [92, 93], specifically visual attention and cognitive flexibility with contributions from processing speed as well [74]. The time taken to complete both tasks was used as the outcome measure. Scores were reverse coded such that higher scores reflected greater cognitive performance.
Episodic memory was measured in both datasets with a composite measure created using the average of standardized and Winsorized immediate and delayed recall variables. In TILDA, immediate and delayed recall were measured using a 10-item word list [94] as used originally in the Health and Retirement Study [95]. The word list was assessed over 2 trials in TILDA and the average score for immediate and delayed recall from both trials was used. In CR/RANN, immediate and delayed recall were measured using the total and delayed recall scores from the Selective Reminding Test (SRT; Buschke and Fuld [96]).
Global cognition was measured using a composite measure of all 5 cognitive variables in each dataset: verbal fluency, processing speed, executive function, episodic memory (immediate recall), and episodic memory (delayed recall). Cognitive variables were Winsorized and standardized prior to creation of the composite. The composite variable was then Winsorized and standardized itself.
Measures: brain structure
T1-weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) scans were acquired in both datasets using a 3-T scanner (Achieva, Philips Medical Systems, The Netherlands). TILDA parameters: FOV = 240 × 240 × 162 mm3, matrix size = 288 × 288, slice thickness/gap = 0.9/0 mm, TR/TE = 6.7/3.1 ms. CR/RANN parameters: FOV = 256 × 256 × 180 mm3, matrix size = 256 × 256, slice thickness/gap = 1/0 mm, TR/TE = 6.5/3 ms.
T1-MRIs were inspected and processed in TILDA and CR/RANN using FreeSurfer v6.0 and v5.1 [97], respectively, as described previously [68, 98]. Total GM volume and hippocampal volume were obtained from Freesurfer and both were divided by Freesurfer’s estimated total intracranial volume. Brain images were parcellated using the Desikan Killiany atlas, with 34 cortical regions of interest (ROIs) per hemisphere [99]. The mean cortical thickness of each cortical ROI was calculated. Overall cortical thickness was calculated as the mean over cortical ROIs. All variables were standardized and Winsorized (based on z-scores > |3|). These three measures were selected based on their availability across both datasets and because they have been used in previous studies, with complete CR models, to represent brain structure: GM volume [100, 101], hippocampal volume [102, 103], and mean cortical thickness [9, 43, 104].
Analysis
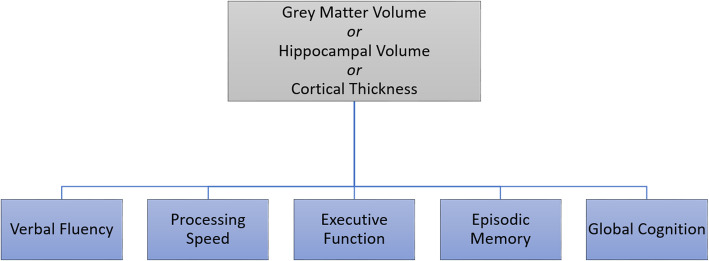
Fifteen individual brain structure-cognitive function models were created for each combination of brain structure and cognitive function variable, where one brain structure variable was selected as an independent variable and one cognitive function variable was selected as an outcome variable (Fig. 1). A moderated hierarchical regression (Fig. 1) was conducted within each brain structure-cognitive function model (n = 15) for each unique proxy (TILDA = 127; CR/RANN = 31). In step 1, a cognitive measure was regressed on age, sex, and a measure of brain structure. In step 2, a proxy variable (Fig. 2) was included as an independent variable. In step 3, the interaction term for brain structure and the proxy was added.
Fig. 1.
Schematic of basic brain structure-cognitive function models created for analysis
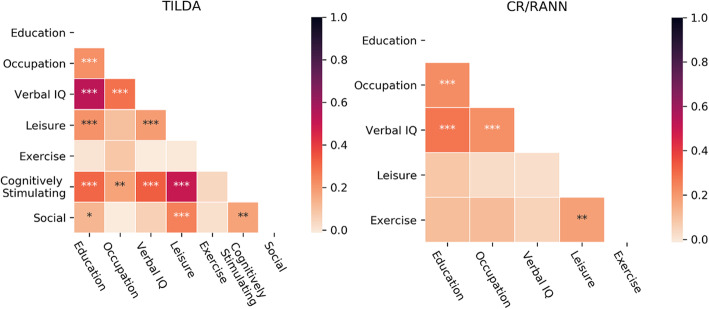
Fig. 2.
Heatmaps showing Pearson’s correlations between individual proxies in each dataset. *p < .05, **p < .01, ***p < .001
To protect against violations of linear regression assumptions, the analysis was repeated using a robust regression, specifically an iteratively reweighted least squares regression with Tukey’s biweight function and median absolute deviation scaling. Effects within each dataset were only considered significant if they were statistically significant in both the linear regression and robust regression. To control for multiple comparisons and to ensure generalizability of findings, effects were only considered significant if they were statistically significant across both datasets. The analysis was conducted with customized Python code (available here: https://github.com/rorytboyle/hierarchical_regression) which used the statsmodels module [105]. The change in R2 (i.e. amount of variance explained) from step 1 to step 2 and from step 2 to step 3 in linear regression models was used to assess the size of the independent and moderation effects of CR proxies, respectively. Where significant effects were observed, the mean R2 change across both datasets was calculated to assess the average additional variance explained by the proxy and its interaction with brain structure.
Results
Demographics
In TILDA, some data were missing for mean cortical thickness (N = 34) and CTT 2 and Global Cognition (N = 2). In CR/RANN, the same N was used (N = 234) in all models. Consequently, different Ns were used across models within TILDA (see Table 1).
Table 1.
Demographics for each hierarchical regression model
| Dataset | Brain structure | Cognition | N | Mean age (SD, range) | Sex (M/F) |
|---|---|---|---|---|---|
| TILDA | Grey matter volume, hippocampal volume | Verb Flu, Proc Speed, Epi Mem | 313 | 68.90 (6.75, 54–88) | 155/158 |
| Grey matter volume, hippocampal volume | Exec Func, Glob Cog | 311 | 68.91 (6.77, 54–88) | 154/157 | |
| Mean cortical thickness | Verb Flu, Proc Speed, Epi Mem | 279 | 69.16 (6.64, 54–88) | 137/142 | |
| Mean cortical thickness | Exec Func, Glob Cog | 277 | 69.18 (6.66, 54–88) | 136/141 | |
| CR/RANN | All | All | 234 | 64.49 (7.42, 50–80) | 114/120 |
SD standard deviation, M male, F female, Verb Flu verbal fluency, Proc Speed processing speed, Epi Mem episodic memory, Exec Func executive function, Glob Cog global cognition
Step 1: Brain-cognition relationships
Models in step 1 of the hierarchical regression (i.e. containing a brain structure measure, sex, and age) were significantly associated with cognitive measures across both datasets (see Tables 2 and 3), except for two models in CR/RANN (hippocampal volume-executive function, and hippocampal volume-episodic memory). Sex was independently associated with cognitive function in 40% and 20% of brain-cognition models in TILDA and CR/RANN, respectively. In TILDA, females had higher cognitive function than males, on average, with other variables (i.e. brain structure and age) being equal. In CR/RANN, females had lower cognitive function than males, on average, with other variables being equal. Age was negatively associated with cognitive function, independent of brain structure and sex, in 100% and 40% of models in TILDA and CR/RANN, respectively.
Table 2.
Step 1 of hierarchical regression models in TILDA
| Cognition | Model statistics | Brain structure | Sex | Age | |||
|---|---|---|---|---|---|---|---|
| n | R2 | f | Variable | β | β | β | |
| Verb Flu | 313 | .043 | 4.597** | Grey matter volume | .042 | −.030 | −.205** |
| Proc Speed | 313 | .129 | 15.320**** | .041 | .084 | −.360**** | |
| Exec Func | 311 | .143 | 17.070**** | .048 | .052 | −.383**** | |
| Epi Mem | 313 | .079 | 8.780**** | .021 | .352** | −.207** | |
| Glob Cog | 311 | .159 | 19.400**** | .048 | .217* | −.373**** | |
| Verb Flu | 313 | .042 | 4.475** | Hippocampal volume | −.005 | −.004 | −.229** |
| Proc Speed | 313 | .129 | 15.226**** | −.025 | .120 | −.394**** | |
| Exec Func | 311 | .143 | 17.010**** | −.041 | .101 | −.428**** | |
| Epi Mem | 313 | .080 | 8.902**** | .044 | .341** | −.195** | |
| Glob Cog | 311 | .158 | 19.171**** | .002 | .243* | −.396**** | |
| Verb Flu | 279 | .051 | 4.898** | Mean cortical thickness | .103 | .002 | −.192** |
| Proc Speed | 279 | .173 | 19.217**** | .122* | .042 | −.370**** | |
| Exec Func | 277 | .195 | 22.040**** | .090 | .065 | −.428**** | |
| Epi Mem | 279 | .091 | 9.202**** | −.036 | .414** | −.216*** | |
| Glob Cog | 277 | .195 | 22.105**** | .065 | .251* | −.391**** | |
Verb Flu verbal fluency, Proc Speed processing speed, Exec Func executive function, Epi Mem episodic memory, Glob Cog global cognition
*p < .05, **p < .01, ***p < .001, ****p < .0001
Table 3.
Step 1 of hierarchical regression models in CR/RANN
| Cognition | Model statistics | Brain | Sex | Age | |||
|---|---|---|---|---|---|---|---|
| n | R2 | f | Variable | β | β | β | |
| Verb Flu | 234 | .087 | 7.320*** | Grey matter volume | .258*** | −.073 | −.062 |
| Proc Speed | 234 | .087 | 7.344*** | .218** | −.296* | −.120 | |
| Exec Func | 234 | .047 | 3.762* | .175* | −.247* | −.063 | |
| Epi Mem | 234 | .061 | 4.998** | .221** | .070 | −.072 | |
| Glob Cog | 234 | .130 | 11.498**** | .330**** | −.148 | −.117* | |
| Verb Flu | 234 | .043 | 3.449* | Hippocampal volume | .078 | <−.001 | −.111* |
| Proc Speed | 234 | .061 | 5.014** | .034 | −.225 | −.173** | |
| Exec Func | 234 | .030 | 2.339 | .026 | −.190 | −.107 | |
| Epi Mem | 234 | .033 | 2.608 | .032 | .142 | −.127* | |
| Glob Cog | 234 | .069 | 5.671*** | .061 | −.044 | −.195** | |
| Verb Flu | 234 | .065 | 5.303** | Mean cortical thickness | .166** | −.024 | −.098 |
| Proc Speed | 234 | .073 | 6.063*** | .129 | −.252* | −.152* | |
| Exec Func | 234 | .048 | 3.834* | .153* | −.226 | −.077 | |
| Epi Mem | 234 | .053 | 4.281** | .159* | .106 | −.098 | |
| Glob Cog | 234 | .109 | 9.401**** | .231*** | −.092 | −.158** | |
Verb Flu verbal fluency, Proc Speed processing speed, Exec Func executive function, Epi Mem episodic memory, Glob Cog global cognition
*p < .05, **p < .01, ***p < .001, ****p < .0001
In TILDA, only one brain structure variable, mean cortical thickness, was independently positively associated with cognitive function (processing speed). In CR/RANN, grey matter volume was independently positively associated with all cognitive measures and cortical thickness was independently positively associated with all cognitive measures except for processing speed. Hippocampal volume was not independently associated with any measure of cognition in either dataset.
Step 2a: Independent effects
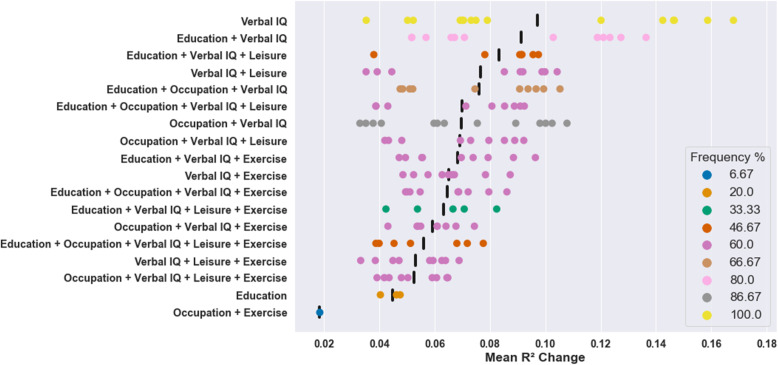
Significant positive independent effects were observed for 18 proxies, including 2 individual proxies and 16 composites, across the 15 models in both datasets (see Additional file 1 for significant independent effects across both datasets; see Additional file 2 for all significant independent effects in TILDA; see Additional file 3 for all significant independent effects in CR/RANN). The proxy with the largest average independent effect was verbal intelligence (mean R2 change = 0.10; see Fig. 3). Verbal intelligence was the most robust proxy: independent effects were replicated across both datasets in 100% of models. The largest average independent effects were observed for verbal intelligence on global cognition where it explained a mean additional 16.80% (hippocampal volume), 15.87% (grey matter volume), and 14.66% (mean cortical thickness) of the variance after accounting for age, sex, and brain structure (for scatter plots of proxies with 10 largest average independent effects, see Additional file 4, Fig. S1). Education was the only other individual proxy with reproducible independent effects (mean R2 change = 0.05), which were observed in 20% of models, all of which contained executive function.
Fig. 3.
Mean R2 change across datasets in all models for proxies with significant effects. + indicates composite proxies (e.g. Education + Verbal IQ = composite of educational attainment and verbal intelligence). Black vertical bars represent the mean of significant R2 change values across all models for that proxy. All models were adjusted for brain structure, age, and sex
The most robust composite proxy was comprised of occupational complexity and verbal intelligence (mean R2 change = 0.07) which was replicated in 86.67% of models. The composite proxy with the largest average effect was educational attainment and verbal intelligence (mean R2 change = 0.09) which was replicated in 80% of models. Only one composite with reproducible independent effects—occupational complexity and physical activity—did not include verbal intelligence. This was the least robust composite as it was replicated in a single model and had the smallest average effect (mean R2 change = 0.02).
Step 2b: Additional independent effects
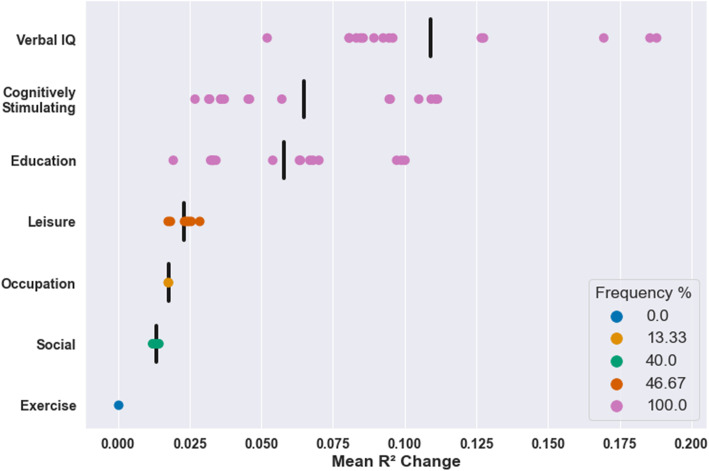
Data was only available for cognitively stimulating activities and social engagement in TILDA. Consequently, these effects could not be assessed in terms of their reproducibility. However, within TILDA, positive independent effects of cognitively stimulating activities on cognition were observed in 100% of models and this proxy had the second largest average independent effect of all individual proxies (mean R2 change = 0.065, see Fig. 4). In contrast, positive independent effects of social activities on cognition were observed in only 40% of models and this proxy had the second smallest average independent effect of all individual proxies (mean R2 change = 0.013). The only individual proxy with smaller effects than social engagement was the physical activity which did not have significant effects in any model.
Fig. 4.
Mean R2 change of significant effects in all TILDA models for individual proxies. Black vertical bars represent the mean of significant R2 change values across all models for that proxy. All models were adjusted for brain structure, age, and sex
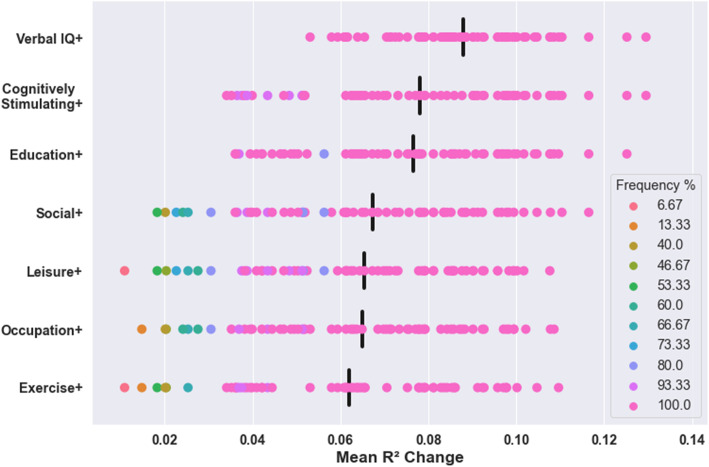
Composite proxies including verbal intelligence had the largest average effects, followed by cognitively stimulating activities, and then education (see Fig. 5). Composites including verbal intelligence had significant effects in all models in TILDA. The composite with the largest effect in TILDA was verbal intelligence and cognitively stimulating activities (mean R2 change = 0.13). The only composite proxy which was not significant in any model was social engagement and physical activity.
Fig. 5.
Mean R2 change of significant effects in all TILDA models for composite proxies. Each row refers to all composites including that proxy (e.g. Verbal IQ+ refers to all composites including verbal intelligence). Black vertical bars represent the mean of significant R2 change values across all models for all composites containing that proxy. All models were adjusted for brain structure, age, and sex
Step 3: Moderation effects
There were no significant moderation effects, in either dataset for any proxy, on the association between brain structure—as measured by GM volume, hippocampal volume, or mean cortical thickness—and cognition. Negative moderation effects are consistent with the CR hypothesis because they reflect weaker associations between brain structure and cognition in individuals with higher CR, suggesting that individuals with higher CR are less reliant on brain structure to sustain cognitive function. Thirty-one non-replicated negative moderation effects (i.e. consistent with the CR hypothesis) were observed in TILDA (see Additional file 4, Table S1), but none survived correction for multiple comparisons (Bonferroni-adjusted alpha = 0.0004: alpha [0.05]/comparisons per model [106]). 61.29% of these effects were observed for composite proxies including cognitively stimulating activities, which was not available in CR/RANN. No negative moderation effects were observed in CR/RANN.
Positive moderation effects contradict the CR hypothesis as they reflect stronger associations between brain structure and cognition in individuals with higher CR, suggesting that individuals with higher CR are more reliant on brain structure to sustain cognitive function. Non-replicated positive moderation effects (i.e. contradicting the CR hypothesis) were observed in both datasets (see Additional file 4, Table S2), but none survived correction for multiple comparisons. Eight effects were observed in TILDA (Bonferroni-adjusted alpha = 0.0004) and seven effects were observed in CR/RANN (Bonferroni-adjusted alpha = 0.0016: alpha [0.05]/comparisons per model [31]). The Bonferroni corrections for multiple comparisons applied here are liberal as they correct for the number of proxies compared per brain-cognition model (TILDA 127, CR/RANN 31) rather than the number of total comparisons across all proxies and all brain-cognition models (TILDA 1905; CR/RANN 465).
Discussion
The reproducibility and magnitude of moderation and independent effects of 33 CR proxies, comprised of 5 standard individual proxies and all their unique combinations, were assessed across 2 datasets to investigate their validity as measures of CR. No moderation effects of CR proxies on the association between brain structure—as measured by GM volume, hippocampal volume, or mean cortical thickness—and cognition were observed across both datasets. Replicated independent effects—positive associations with cognitive function, independent of brain structure—were observed for 2 individual proxies (verbal intelligence and educational attainment) and 16 composites. The most robust and largest effects on cognition were found for verbal intelligence, which satisfied the independent effect criterion in all 15 brain-cognition models across both datasets. Educational attainment satisfied the independent effect criterion in 3 brain-cognition models. No composite proxy had larger or more robust independent effects on cognition than verbal intelligence alone. Our results support the use of verbal intelligence as a proxy measure of CR in cross-sectional studies of cognitively healthy older adults.
Verbal intelligence had larger and more robust effects on cognition than educational attainment
We found that verbal intelligence had the largest and most robust independent effects on cognition. Unlike previous studies, due to the availability of two large neuroimaging datasets, we could demonstrate that independent effects of verbal intelligence on cognition were present in several brain-cognition models and were replicable. This validation of verbal intelligence as a CR proxy supports previous, narrower, associations between verbal intelligence and cognitive function in the presence of hippocampal atrophy [56], a neuropathological ‘residual’ measure of CR [55], a functional connectivity measure of CR based on task potency [9], and a possible neuromarker of CR, locus coeruleus signal intensity [107].
Aside from verbal intelligence, the only other individual proxy with replicable independent effects on cognition was educational attainment. These replicable effects were only observed in brain-cognition models where executive function was the cognitive outcome variable. While education has been previously positively associated with executive function, without accounting for brain structure, in cognitively healthy older adults [108] and in a systematic review [50], our results show that this association is independent of GM volume, hippocampal volume, or mean cortical thickness. Notably, the effects of education were less robust than verbal intelligence, as positive associations were not seen across both datasets for verbal fluency, processing speed, episodic memory, and global cognition. As such, these results suggest that educational attainment is not a reliable individual proxy of CR in cognitively healthy older adults. This conclusion is supported by previous findings including a systematic review which found positive evidence for education in only 38% of complete models with cognitively healthy samples [53] and a non-significant association between education (when considered separately from other possible CR proxies) and a neuropathological residual measure of CR [54]. Based on their findings using ex-vivo neuropathological measures, Reed et al. [54] concluded that the observed effects of education on cognition should not be simply considered as reserve effects. Our results further show that this conclusion is valid when using in-vivo neuroimaging measures of GM volume, hippocampal volume, or mean cortical thickness.
The general finding that verbal intelligence had larger and more robust CR effects than educational attainment convincingly supports an argument favouring the use of verbal intelligence over education [79]. This argument was previously broadly supported by evidence that, compared to educational attainment, verbal intelligence was a stronger predictor of cognitive function/decline [109, 110] and had greater protective effects on the onset of clinical symptoms of MCI/AD [43, 111]. More specifically, Malek-Ahmadi et al. [31] directly compared educational attainment and verbal intelligence in a mixed autopsy sample, consisting of adults with diagnoses of no cognitive impairment, MCI, and AD. In complete CR models, including neuropathological indices and measures of episodic memory and executive function, positive evidence was found for verbal intelligence, but not education, as a CR proxy, leading to the conclusion that verbal intelligence measures are superior to educational attainment as CR proxies. Here, we have shown that verbal intelligence is also a superior CR proxy when using in-vivo measures of GM volume, hippocampal volume, or mean cortical thickness and when assessed in respect to additional cognitive outcome measures, including verbal fluency, processing speed, and global cognition. Importantly, our results show that this conclusion holds when tested across two separate samples of cognitively healthy older adults.
The larger and more robust effects of verbal intelligence on cognition reported here and elsewhere could be explained by 2 key factors. Firstly, verbal intelligence may be a closer reflection of the quality, benefit, or outcomes of educational attainment [112] than years of education, which simply reflects the quantity of educational attainment. Quality of education can differ greatly among individuals with the same quantity of education due to various socioeconomic and systemic factors [113], such as class size [114], and also due to individual-level factors such as intrinsic learning motivation and academic self-efficacy [115]. Secondly, measures of verbal intelligence may reflect wider lifetime educational and cognitive experiences as compared to years of education which is generally restricted to early-life formal education [79–82] and typically neglects to consider later-life education which has been positively associated with cognitive function [116, 117]. In this sense, verbal intelligence could be considered a dynamic CR proxy which can change over time [118, 119], as it may increase from young to mid-adulthood before decreasing in older adulthood [120]. In contrast, years of education may be considered a static CR proxy [31]. Despite the widespread use of educational attainment as an individual CR proxy, our results suggest that it should only be used as an individual proxy where verbal intelligence is not available.
Composite proxies had smaller and less robust effects on cognition than verbal intelligence
We found significant positive independent effects of 16 different composite proxies on cognition across both datasets. Three of these composites had significant effects on cognition in at least two-thirds of the brain-cognition models assessed: occupational complexity and verbal intelligence (86.67% of models); education and verbal intelligence (80% of models); and education, occupational complexity, and verbal intelligence (66.67% of models). This is a novel finding as the most robust composite—occupational complexity and verbal intelligence—has never (to the best of our knowledge) been used previously as a CR proxy, likely due to the predominant use of education both as an individual proxy and in composites. The next most robust composite of education and verbal intelligence has been widely used [42, 43, 45, 46, 59, 77, 111] and our results support a previous positive association between this composite and episodic memory, controlling for GM volume [59]. A speculative explanation for the greater robustness of occupational complexity and verbal intelligence as a composite may be that occupational complexity and verbal intelligence are less strongly correlated with each other than educational attainment and verbal intelligence (see Fig. 2).
While composite proxies purportedly provide advantages over individual proxies, our results show that their independent effects on cognition are less robust (i.e. less frequently observed across brain-cognition models) and smaller in magnitude than those found for verbal intelligence alone. This may be explained by the large individual effects of verbal intelligence on cognition and its strong correlation with other proxies (see Fig. 2) considering that all composite proxies with replicated effects contained verbal intelligence, except for the composite with the least robust effects, occupational complexity and physical activity. While adding another proxy to verbal intelligence to form a composite should have an additive effect, this could also add noise to an already strong proxy measure as well as shared variance in situations where the proxies are correlated. Consequently, the overall effect of the composite may then be smaller than verbal intelligence alone. Alternative methods to creating composites, such as principal component analysis, could potentially mitigate this issue but may not be theoretically appropriate [35], and incorporating this method within the analysis framework used here would have significantly increased the complexity of the analysis. Of all composites considered here, our results especially support the use of education and verbal intelligence as well as occupational complexity and verbal intelligence as composite proxies where multiple proxies are available. However, using composites may lead to more type II errors than using verbal intelligence alone, given the more robust and larger effects of verbal intelligence. As such, our results suggest that researchers should use, or at least repeat analyses using, verbal intelligence alone, in cross-sectional studies of cognitively healthy older adults.
Occupational complexity, leisure activities, and physical activity did not show robust effects on cognition
We did not find any evidence for robust independent effects of 3 individual proxies on cognition across both datasets. Occupational complexity was not positively associated with any domain of cognitive function, adjusting for GM volume, hippocampal volume, or mean cortical thickness. This suggests that the small positive associations between this proxy and cognition, as reported in a meta-analysis [50], may not be independent of these measures of brain structure. Unlike the detailed nature of the occupational complexity measure used here, occupational complexity has been typically measured using government classification codes that are effectively a socioeconomic classification of occupations (e.g. the UK’s Office Of Population Statistic classification as in Staff et al. [121]). As such, previously reported effects for occupational complexity may have in fact reflected the effect of socioeconomic status, which can support cognitive health via greater access to resources and healthcare, among many other mechanisms [35]. While Chapko et al. [53] concluded that the evidence for this proxy in complete CR models using cognitively healthy samples was inconclusive, our results, do not support the use of occupational complexity as a proxy measure of CR in cross-sectional studies of cognitively healthy older adults.
As with occupational complexity, we did not find robust evidence to support the use of leisure activities as an individual CR proxy. Although it has been associated with a reduced risk of dementia and AD ([122], but cf. [123]), few studies have rigorously tested this proxy in a complete CR model. One study found a moderation effect for midlife leisure activities, but in line with our findings, they did not find evidence of either a moderation or independent effect for later-life leisure activities [124]. Future research is warranted to clarify which specific leisure activities should be included in measures for this proxy given that only a few activities have been associated with cognition in mid-/old-age samples, albeit without adjusting for brain structure [116, 125]. However, our results do not support the use of later-life leisure activities as a proxy measure of CR in cross-sectional studies of cognitively healthy older adults.
Finally, our results do not support the use of physical activity as an individual CR proxy. While this proxy has been previously associated with cognitive function in older adults without controlling for brain structure [106, 126], our results show that these associations are not independent of GM volume, hippocampal volume, or mean cortical thickness. This supports previous findings of non-significant associations from the few complete CR models assessing this proxy adjusting for brain structure using GM volume and hippocampal atrophy [56, 100]. The disparity in the observed associations when brain structure is accounted for could be because the protective effects of exercise may be exerted via improved brain maintenance, i.e. the relative preservation of brain structural health [8, 127], rather than improved CR [128]. This is supported by the finding that the protective effects of exercise on cognition were mediated by increases in prefrontal cortex volume [129] and also by associations of greater physical activity with lower brain-predicted age difference scores [130], which reflects better brain maintenance [131], and greater cortical thickness [132] and regional GM volumes [133, 134]. Setting aside a possible contribution of physical activity to brain maintenance, our results suggest that it does not contribute to greater CR and therefore do not support the use of physical activity as a proxy measure of CR in cross-sectional studies of cognitively healthy older adults.
Lack of evidence for moderation effects of CR proxies
Robust moderation effects of CR proxies on the association between brain structure—as measured by GM volume, hippocampal volume, or mean cortical thickness—and cognition were not identified here. This lack of evidence is in line with previously reported non-significant moderation effects on the relationship between episodic memory and GM volume [59] and right hippocampal volume [102] but conflicts with previous evidence of significant moderation effects reported for CR proxies in similar brain-cognition models [46, 124, 135]. However, the evidence for moderation is largely inconsistent as highlighted by the finding of moderation effects reported on 1 measure, but not on 2 other measures, of episodic memory within the same study [135] and even findings of a positive moderation effect, which contradicts the CR hypothesis, on the relationship between left hippocampal volume and episodic memory [102]. It is likely that our non-significant effects highlight the general difficulties in detecting CR moderation effects.
The ability to detect a moderation effect here may have been impaired because the participants were cognitively and neurologically healthy and therefore had a relatively restricted range of cognitive function and brain atrophy in comparison to cognitively and/or neurologically impaired individuals. The relatively restricted range of the predictor variable of brain structure restricts the range of the interaction term [136] which can substantially reduce statistical power to detect a moderation effect [137]. This is exacerbated by the fact that neuroimaging variables explain a relatively small amount (20%) of variance in healthy older adults’ cognition [2], which effectively constrains the size of the moderation effect [62]. While the present study was designed using pre-existing data from two cognitively and neurologically healthy cohorts, an experimental approach where individuals with extremely low or high scores on measures of cognitive reserve and brain structure are oversampled may be better able to detect the existence of a moderation effect for these proxies [136].
Promising evidence for cognitively stimulating activities but not social engagement as proxies but replication required
We were unable to assess the reproducibility of the effects of cognitively stimulating activities and social engagement on cognition across datasets as we only had sufficient data in TILDA for these proxies. Within TILDA, cognitively stimulating activities was highly robust as it had positive independent effects on cognition in all brain-cognition models, and had the largest average independent effect on cognition after verbal intelligence. This finding supports associations between this proxy and neuropathological ‘residual’ measures of CR [54, 55] and suggests that previously reported consistent positive associations [49, 50] can be observed with several cognition domains when controlling for brain structure, as measured by GM volume, hippocampal volume, and mean cortical thickness. Social engagement was less robust as it had positive independent effects on cognition in only 40% of brain-cognition models and had the second smallest average independent effect on cognition of all individual proxies. This inconsistent evidence emphasizes a need for further study of social engagement in complete CR models. While mixed evidence of moderation effects has been reported to date for this proxy controlling for neuropathology [58, 138], this is the first attempt to assess it in a complete CR model including neuroimaging variables. As our focus was on replication across datasets rather than single dataset findings requiring correction for multiple comparisons and because this proxy was only available in a single dataset, these findings remain speculative until they can be replicated. With this in mind, while we cannot make definitive conclusions, we can tentatively suggest that cognitively stimulating activities may be a reasonable choice of CR proxy where verbal intelligence is not available and that social engagement should not be used as an individual proxy.
Limitations
The present study provides data-driven evidence supporting the use of specific proxies to measure CR in cross-sectional studies of cognitively healthy older adults. Nonetheless, there are some limitations which, if addressed in future research, could further strengthen these recommendations and provide additional insights. The main limitation of the present results is that they are cross-sectional. As such, we cannot make solid inferences about the casual direction of the relationships between the robust proxies and cognitive function. Similarly, while CR is supposed to protect against cognitive decline, our analysis only provides information about its association with individual differences in cognitive function, not decline. Future analyses after further waves of data collection will be necessary to assess whether the effects of these proxies are consistent when assessed in the context of cognitive decline.
Another limitation is that the CR models used here were limited to three brain structure variables: GM volume, hippocampal volume, and mean cortical thickness. Aside from hippocampal volume, the CR models did not contain regional measures such as parietotemporal cortical thickness or measures of WM microstructural integrity, WM hyperintensity volume, or AD-related neuropathology. As CR proxies have been previously reported to moderate the relationship between these measures and cognition [43, 82, 139–142], future studies could assess proxies in complete CR models containing these brain structure variables to extend the conclusions made here to a wider spectrum of brain-cognition relationships. Furthermore, there were differences in the relationship between age and cognition across both datasets. Age was negatively associated with cognition in 100% of brain-cognition models in TILDA, but only in 40% of models in CR/RANN. Tentative explanations for these differences may have been the larger sample size and older age of the TILDA brain-cognition models. Finally, some CR proxies, namely leisure activities and physical activity were measured differently in both datasets. Differences in these measures or in the specific activities included in each measure may have contributed to differing effects across both datasets. This may be particularly pertinent for leisure activities as its relationship with cognitive function can vary based on the specific leisure activities assessed [116]. However, this variability across the two datasets reflects the typical variability in the measurement of CR with proxies.
Conclusions
Despite the discussed limitations, the present findings are informative for researchers using proxies as measures of CR. We built on previous meta-analyses and systematic reviews of CR proxies by assessing a wider set of standard proxies, including their composites, and evaluating their effects across complete and theoretically consistent models of CR and in multiple brain-cognition relationships. Our analysis framework enabled the comparison of the robustness and magnitude of effects. Furthermore, the reported findings are stringent, robust, and replicable, as they were only considered statistically significant if they were replicated in a robust regression and across two datasets.
The present study is the first systematic investigation of the validity of different proxies, and their composites, in complete CR models. Verbal intelligence was associated with better cognitive function in all variables assessed, controlling for mean cortical thickness, GM volume, and hippocampal volume. The independent effects on cognition of education and composite proxies, including verbal intelligence and occupational complexity as well as verbal intelligence and education, were smaller and less robust. Our results suggest that, in cross-sectional studies of cognitively healthy older adults, verbal intelligence should be used as a CR proxy, over other proxies including education, occupational complexity, leisure activities, exercise, and composites including all possible combinations of these proxies. While no robust moderation effects of CR proxies on the association between brain structure—as measured by GM volume, hippocampal volume, or mean cortical thickness—and cognition were found here, this may be due to the considerable statistical difficulties in detecting such effects in normal healthy ageing samples. In sum, the finding of robust independent effects across all brain-cognitive domains assessed provides strong evidence for the use of verbal intelligence as a CR proxy.
Supplementary Information
Additional file 1. Significant independent effects across both datasets.
Additional file 2. All significant independent effects in TILDA.
Additional file 3. All significant independent effects in CR/RANN.
Additional file 4 Table S1 Negative moderation effects of cognitive reserve proxies within TILDA. Table S2 Positive moderation effects of cognitive reserve proxies within both datasets. Figure S1: Association between proxies and cognition, adjusting for brain structure, age, and sex.
Acknowledgements
The authors would like to thank all participants for the time and effort they generously gave to participate in the TILDA and CR/RANN studies. IHR thanks The Atlantic Philanthropies for their grant to the Global Brain Health Institute. MRI data collection in TILDA was supported by Prof. James Meaney and Dr. Jason McMorrow in the National Centre for Advanced Medical Imaging (CAMI) at St. James’ Hospital, Dublin.
Abbreviations
- AD
Alzheimer’s disease
- AMNART
American National Adult Reading Test
- CR
Cognitive reserve
- CR/RANN
The Cognitive Reserve/Reference Ability Neural Network Study
- CTT
Colour Trails Test
- DOT
Dictionary of Occupational Titles
- FOV
Field of view
- GM
Grey matter
- IPAQ-SF
International Physical Activity Questionnaire-Short Form
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- MPRAGE
Magnetization-prepared rapid acquisition with gradient echo
- NART
National Adult Reading Test
- ROI
Region of interest
- TILDA
The Irish Longitudinal Study on Ageing
- TMT
Trail Making Test
- TR/TE
Repetition time/echo time
- WM
White matter
Authors’ contributions
Study conception and design: RB and RW, in consultation with IHR and YS; TILDA data collection, preparation, and co-ordination: CDL, SPK, SS, and RAK; CR/RANN data collection and co-ordination: YS; statistical analysis, data interpretation, and drafting the manuscript: RB and RW. All authors provided feedback and revisions on the manuscript and approved the final version for submission.
Funding
RB is supported by the Irish Research Council under grant number EPSPG/2017/277. YS is supported by NIA RF1 AG038465 and R01 AG026158. SPK is supported by Science Foundation Ireland grant number 18/FRL/6188. The TILDA MRI study was funded by the Health Research Board grant number HRA-PHR-2014-667. TILDA is funded by core grants from the Health Research Board, Atlantic Philanthropies and Irish Life. The National Centre for Advanced Medical Imaging (CAMI) is grant-funded by the Health Research Board. The funding agencies had no involvement in the conduct of the research or preparation of the article.
Availability of data and materials
The TILDA dataset analysed in this study is available from TILDA upon reasonable request. The procedures to gain access to TILDA data are specified at https://tilda.tcd.ie/data/accessing-data/.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74(3):478–489. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, Buckner RL. Multiple brain markers are linked to age-related variation in cognition. Cereb Cortex. 2014;26(4):1388–1400. doi: 10.1093/cercor/bhu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 6.Tucker AM, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res. 2011;8(4):354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondini S, Madella I, Zangrossi A, Bigolin A, Tomasi C, Michieletto M, et al. Cognitive reserve in dementia: implications for cognitive training. Front Aging Neurosci. 2016;8:84. doi: 10.3389/fnagi.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, and the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020;16(9):1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Loenhoud AC, Habeck C, van der Flier WM, Ossenkoppele R, Stern Y. Identifying a task-invariant cognitive reserve network using task potency. Neuroimage. 2020;210:116593. doi: 10.1016/j.neuroimage.2020.116593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarracanie M, Lapierre CD, Salameh N, Waddington DEJ, Witzel T, Rosen MS. Low-cost high-performance MRI. Sci Rep. 2015;5(1):1–9. doi: 10.1038/srep15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogbole GI, Adeyomoye AO, Badu-Peprah A, Mensah Y, Nzeh DA. Survey of magnetic resonance imaging availability in West Africa. Pan Afr Med J. 2018;30:240. doi: 10.11604/pamj.2018.30.240.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HX, Gustafson DR, Kivipelto M, Pedersen NL, Skoog I, Windblad B, et al. Education halves the risk of dementia due to apolipoprotein ε4 allele: a collaborative study from the Swedish Brain Power initiative. Neurobiol Aging. 2012;33(5):1007.e1–1007.e7. doi: 10.1016/j.neurobiolaging.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, et al. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53:3113–3123. doi: 10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]
- 14.Dekhtyar S, Wang H-X, Fratiglioni L, Herlitz A. Childhood school performance, education and occupational complexity: a life-course study of dementia in the Kungsholmen Project. Int J Epidemiol. 2016;45(4):1207–1215. doi: 10.1093/ije/dyw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kröger E, Andel R, Lindsay J, Benounissa Z, Verreault R, Laurin D. Is complexity of work associated with risk of dementia?: The Canadian Study of Health and Aging. Am J Epidemiol. 2008;167(7):820–830. doi: 10.1093/aje/kwm382. [DOI] [PubMed] [Google Scholar]
- 16.Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B, et al. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. J Gerontol Ser B. 2005;60(5):P251–P258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- 17.Potter GG, Helms MJ, Burke JR, Steffens DC, Plassman BL. Job demands and dementia risk among male twin pairs. Alzheimer’s Dement. 2007;3(3):192–199. doi: 10.1016/j.jalz.2007.04.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaup AR, Simonsick EM, Harris TB, Satterfield S, Metti AL, Ayonayon HN, et al. Older adults with limited literacy are at increased risk for likely dementia. J Gerontol Ser A. 2013;69(7):900–906. doi: 10.1093/gerona/glt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervilla JA, Prince M, Joels S, Lovestone S, Mann A. Long-term predictors of cognitive outcome in a cohort of older people with hypertension. Br J Psychiatry. 2000;177(JUL):66–71. doi: 10.1192/bjp.177.1.66. [DOI] [PubMed] [Google Scholar]
- 20.Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22(4):367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- 21.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25(5):680–690. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- 22.Marioni RE, Proust-Lima C, Amieva H, Brayne C, Matthews FE, Dartigues JF, Jacqmin-Gadda H. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study Chronic Disease epidemiology. BMC Public Health. 2015;15(1):1089. doi: 10.1186/s12889-015-2426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 24.Akbaraly TN, Portet F, Fustinoni S, Dartigues J-F, Artero S, Rouaud O, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- 25.Paillard-Borg S, Fratiglioni L, Winblad B, Wang HX. Leisure activities in late life in relation to dementia risk: principal component analysis. Dement Geriatr Cogn Disord. 2009;28(2):136–144. doi: 10.1159/000235576. [DOI] [PubMed] [Google Scholar]
- 26.Bowen ME. A prospective examination of the relationship between physical activity and dementia risk in later life. Am J Heal Promot. 2012;26(6):333–340. doi: 10.4278/ajhp.110311-QUAN-115. [DOI] [PubMed] [Google Scholar]
- 27.Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 28.Ogino E, Manly JJ, Schupf N, Mayeux R, Gu Y. Current and past leisure time physical activity in relation to risk of Alzheimer’s disease in older adults. Alzheimer’s Dement. 2019;15(12):1603–1611. doi: 10.1016/j.jalz.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Wang P, Fang Y. Social engagement and its change are associated with dementia risk among Chinese older adults: a longitudinal study. Sci Rep. 2018;8(1):1–7. doi: 10.1038/s41598-017-17879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern Y, Barulli D. Chapter 11 - cognitive reserve. In: Dekosky ST, Asthana SBT-H of CN, editors. Geriatric neurology. Amsterdam: Elsevier; 2019. p. 181–190.
- 31.Malek-Ahmadi M, Lu S, Chan Y, Perez SE, Chen K, Mufson EJ. Static and dynamic cognitive reserve proxy measures: interactions with Alzheimer’s disease neuropathology and cognition. J Alzheimer’s Dis Park. 2017;07(06):390. [DOI] [PMC free article] [PubMed]
- 32.Bettcher BM, Gross AL, Gavett BE, Widaman KF, Fletcher E, Dowling NM, Buckley RF, Arenaza-Urquijo EM, Zahodne LB, Hohman TJ, Vonk JMJ, Rentz DM, Mungas D. Dynamic change of cognitive reserve: associations with changes in brain, cognition, and diagnosis. Neurobiol Aging. 2019;83:95–104. doi: 10.1016/j.neurobiolaging.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahodne LB, Manly JJ, Brickman AM, Siedlecki KL, Decarli C, Stern Y. Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. J Int Neuropsychol Soc. 2013;19:1–9. doi: 10.1017/S1355617713000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kartschmit N, Mikolajczyk R, Schubert T, Lacruz ME. Measuring cognitive reserve (CR) - a systematic review of measurement properties of CR questionnaires for the adult population. PLoS One. 2019;14(8):e0219851. doi: 10.1371/journal.pone.0219851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17(04):593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev Educ Res. 2005;75(3):417–453. [Google Scholar]
- 37.Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol. 2017;186(7):805–814. doi: 10.1093/aje/kwx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;19:347. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer C, Yeung E, Hansen T, Gibbons S, Fornazzari L, Ringer L, Schweizer TA. Impact of socioeconomic status on the prevalence of dementia in an inner city memory disorders clinic. Int Psychogeriatrics. 2009;21(6):1096–1104. doi: 10.1017/S1041610209990846. [DOI] [PubMed] [Google Scholar]
- 40.Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM. Education and cognitive functioning across the life span. Psychol Sci Public Interes. 2020;21(1):6–41. doi: 10.1177/1529100620920576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology. 2015;29(4):649–657. doi: 10.1037/neu0000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleck JI, Kuti J, Mercurio J, Mullen S, Austin K, Pereira O. The impact of age and cognitive reserve on resting-state brain connectivity. Front Aging Neurosci. 2017;9(DEC):392. [DOI] [PMC free article] [PubMed]
- 43.Pettigrew C, Soldan A, Zhu Y, Wang MC, Brown T, Miller M, et al. Cognitive reserve and cortical thickness in preclinical Alzheimer’s disease. Brain Imaging Behav. 2017;11(2):357–367. doi: 10.1007/s11682-016-9581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettigrew C, Soldan A, Zhu Y, Cai Q, Wang MC, Moghekar A, Miller MI, Singh B, Martinez O, Fletcher E, DeCarli C, Albert M. Cognitive reserve and rate of change in Alzheimer’s and cerebrovascular disease biomarkers among cognitively normal individuals. Neurobiol Aging. 2020;88:33–41. doi: 10.1016/j.neurobiolaging.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soldan A, Pettigrew C, Cai Q, Wang J, Wang MC, Moghekar A, Miller MI, Albert M, BIOCARD Research Team Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging. 2017;60:164–172. doi: 10.1016/j.neurobiolaging.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffener J, Barulli D, Habeck C, O’Shea D, Razlighi Q, Stern Y. The role of education and verbal abilities in altering the effect of age-related gray matter differences on cognition. Chao L, editor. PLoS One. 2014;9(3):e91196. doi: 10.1371/journal.pone.0091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern Y, Habeck C. Deriving and testing the validity of cognitive reserve candidates. In: Perneczky RG, editor. Neuromethods. New York: Humana Press; 2018. pp. 63–70. [Google Scholar]
- 48.Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M. Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology. 2017;88(11):1054–1061. doi: 10.1212/WNL.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison SL, Sajjad A, Bramer WM, Ikram MA, Tiemeier H, Stephan BCM. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol. 2015;37(3):253–264. doi: 10.1080/13803395.2014.1002759. [DOI] [PubMed] [Google Scholar]
- 50.Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Aging, Neuropsychol Cogn. 2016;23(1):40–60. doi: 10.1080/13825585.2015.1041450. [DOI] [PubMed] [Google Scholar]
- 51.Wicherts JM, Veldkamp CLS, Augusteijn HEM, Bakker M, van Aert RCM, van Assen MALM. Degrees of freedom in planning, running, analyzing, and reporting psychological studies: a checklist to avoid p-hacking. Front Psychol. 2016;7(NOV):1832. doi: 10.3389/fpsyg.2016.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen H, Anstey KJ, Leach LS, Mackinnon AJ. The handbook of aging and cognition. 3. New York: Psychology Press; 2008. Intelligence, education, and the brain reserve hypothesis; pp. 133–188. [Google Scholar]
- 53.Chapko D, McCormack R, Black C, Staff R, Murray A. Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia–a systematic literature review. Aging Mental Health. 2018;22:915–926. doi: 10.1080/13607863.2017.1348471. [DOI] [PubMed] [Google Scholar]
- 54.Reed BR, Dowling M, Tomaszewski Farias S, Sonnen J, Strauss M, Schneider JA, Bennett DA, Mungas D. Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc. 2011;17(4):615–624. doi: 10.1017/S1355617711000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Negash S, Wilson R, Leurgans S, Wolk D, Schneider J, Buchman A, Bennett D, Arnold S. Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr Alzheimer Res. 2013;10(8):844–851. doi: 10.2174/15672050113109990157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topiwala A, Suri S, Allan C, Valkanova V, Filippini N, Sexton CE, et al. Predicting cognitive resilience from midlife lifestyle and multi-modal MRI: a 30-year prospective cohort study. Ginsberg SD, editor. PLoS One. 2019;14(2):e0211273. doi: 10.1371/journal.pone.0211273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchman AS, Yu L, Wilson RS, Lim A, Dawe RJ, Gaiteri C, Leurgans SE, Schneider JA, Bennett DA. Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology. 2019;92(8):E811–E822. doi: 10.1212/WNL.0000000000006954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biddle KD, d’Oleire Uquillas F, Jacobs HIL, Zide B, Kirn DR, Rentz DM, et al. Social engagement and amyloid-β-related cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27(11):1247–1256. doi: 10.1016/j.jagp.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwak S, Shin M, Kim H, Cho B, Ha J, Han G, et al. Moderating effect of cognitive reserve on the association between grey matter atrophy and memory varies with age in older adults. Psychogeriatrics. 2020;20(1):87–95. doi: 10.1111/psyg.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris JH, Sherman JD, Mansfield ER. Failures to detect moderating effects with ordinary least squares-moderated multiple regression. Some reasons and a remedy. Psychol Bull. 1986;99(2):282–288. [Google Scholar]
- 61.Champoux JE, Peters WS. Form, effect size and power in moderated regression analysis. J Occup Psychol. 1987;60(3):243–255. [Google Scholar]
- 62.Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. J Fam Psychol. 2005;19(1):111–120. doi: 10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
- 63.Dunlap WP, Kemery ER. Effects of predictor intercorrelations and reliabilities on moderated multiple regression. Organ Behav Hum Decis Process. 1988;41(2):248–258. [Google Scholar]
- 64.Whelan BJ, Savva GM. Design and methodology of the Irish longitudinal study on ageing. J Am Geriatr Soc. 2013;61:S265–S268. doi: 10.1111/jgs.12199. [DOI] [PubMed] [Google Scholar]
- 65.Kearney PM, Cronin H, O’Regan C, Kamiya Y, Savva GM, Whelan B, et al. Cohort profile: the Irish longitudinal study on ageing. Int J Epidemiol. 2011;40(4):877–884. doi: 10.1093/ije/dyr116. [DOI] [PubMed] [Google Scholar]
- 66.Donoghue OA, McGarrigle CA, Foley M, Fagan A, Meaney J, Kenny RA. Cohort profile update: the Irish longitudinal study on ageing (TILDA) Int J Epidemiol. 2018;47(5):1398–1398 l. doi: 10.1093/ije/dyy163. [DOI] [PubMed] [Google Scholar]
- 67.Stern Y, Gazes Y, Razlighi Q, Steffener J, Habeck C. A task-invariant cognitive reserve network. Neuroimage. 2018;178:36–45. doi: 10.1016/j.neuroimage.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habeck C, Gazes Y, Razlighi Q, Steffener J, Brickman A, Barulli D, Salthouse T, Stern Y. The reference ability neural network study: life-time stability of reference-ability neural networks derived from task maps of young adults. Neuroimage. 2016;125:693–704. doi: 10.1016/j.neuroimage.2015.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stern Y, Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, Shaked D, Salthouse T. The reference ability neural network study: motivation, design, and initial feasibility analyses. Neuroimage. 2014;103:139–151. doi: 10.1016/j.neuroimage.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henretta JC, McCrory C. Childhood circumstances and mid-life functional mobility. J Aging Health. 2016;28(3):440–459. doi: 10.1177/0898264315594135. [DOI] [PubMed] [Google Scholar]
- 71.Smart EL, Gow AJ, Deary IJ. Occupational complexity and lifetime cognitive abilities. Neurology. 2014;83(24):2285–2291. doi: 10.1212/WNL.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson HE, Willinson J. The National Adult Reading Test (NART): test manual. Windsor: NFER: Nelson; 1982. [Google Scholar]
- 73.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 74.Strauss EH, Sherman EMS, Spreen O. A compendium of neuropsychological tests; administration norms and commentary. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 75.Beardsall L, Brayne C. Estimation of verbal intelligence in an elderly community: a prediction analysis using a shortened NART. Br J Clin Psychol. 1990;29(1):83–90. doi: 10.1111/j.2044-8260.1990.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 76.Boyle R, Jollans L, Rueda-Delgado LM, Rizzo R, Yener GG, McMorrow JP, et al. Brain-predicted age difference score is related to specific cognitive functions: a multi-site replication analysis. Brain Imaging Behav. 2020;15:327–45. [DOI] [PMC free article] [PubMed]
- 77.Oosterman JM, Jansen MG, Scherder EJA, Kessels RPC. Cognitive reserve relates to executive functioning in the old–old. Aging Clin Exp Res. 2020;6:1–6. doi: 10.1007/s40520-020-01758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hatch SL, Feinstein L, Link BG, Wadsworth MEJ, Richards M. The continuing benefits of education: adult education and midlife cognitive ability in the British 1946 birth cohort. Journals Gerontol Ser B Psychol Sci Soc Sci. 2007;62(6):S404–S414. doi: 10.1093/geronb/62.6.s404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perneczky R, Kempermann G, Korczyn AD, Matthews FE, Ikram MA, Scarmeas N, et al. Translational research on reserve against neurodegenerative disease: consensus report of the International Conference on Cognitive Reserve in the Dementias and the Alzheimer’s Association Reserve, Resilience and Protective Factors Professional Interest Ar. BMC Med. 2019;17. [DOI] [PMC free article] [PubMed]
- 80.Schwartz CE, Rapkin BD, Healy BC. Reserve and reserve-building activities research: key challenges and future directions. BMC Neurosci. 2016;17(1):62. doi: 10.1186/s12868-016-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh H, Razlighi QR, Stern Y. Multiple pathways of reserve simultaneously present in cognitively normal older adults. Neurology. 2018;90(3):E197–E205. doi: 10.1212/WNL.0000000000004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baker LM, Laidlaw DH, Cabeen R, Akbudak E, Conturo TE, Correia S, Tate DF, Heaps-Woodruff JM, Brier MR, Bolzenius J, Salminen LE, Lane EM, McMichael AR, Paul RH. Cognitive reserve moderates the relationship between neuropsychological performance and white matter fiber bundle length in healthy older adults. Brain Imaging Behav. 2017;11(3):632–639. doi: 10.1007/s11682-016-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sport Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 84.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Activ. 2011;8:1–11. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci J Can des Sci Appl au Sport. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 86.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 88.McCrory C, Finucane C, O’Hare C, Frewen J, Nolan H, Layte R, Kearney PM, Kenny RA. Social disadvantage and social isolation are associated with a higher resting heart rate: evidence from the Irish longitudinal study on ageing. Journals Gerontol Ser B. 2016;71(3):463–473. doi: 10.1093/geronb/gbu163. [DOI] [PubMed] [Google Scholar]
- 89.Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol. 2013;49(4):764–766. [Google Scholar]
- 90.D’Elia LF, Satz P, Uchiyama CL, White T. Color Trials Test. Professional manual. Odessa: Psychological Assessment Resources; 1996. [Google Scholar]
- 91.Reitan RM. The relation of the Trail Making Test to organic brain damage. J Consult Psychol. 1955;19(5):393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 92.Oosterman JM, Vogels RLCC, Van Harten B, Gouw AA, Poggesi A, Scheltens P, et al. Assessing mental flexibility: neuroanatomical and neuropsychological correlates of the trail making test in elderly people. Clin Neuropsychol. 2010;24(2):203–219. doi: 10.1080/13854040903482848. [DOI] [PubMed] [Google Scholar]
- 93.Mohapatra B, Marshall RS. Performance differences between aphasia and healthy aging on an executive function test battery. Int J Speech Lang Pathol. 2019;22:1–11. [DOI] [PubMed]
- 94.Feeney J, O’Sullivan M, Kenny RA, Robertson IH. Change in perceived stress and 2-year change in cognitive function among older adults: the Irish Longitudinal Study on Ageing. Stress Heal. 2018;34(3):403–410. doi: 10.1002/smi.2799. [DOI] [PubMed] [Google Scholar]
- 95.Wallace RB, Herzog AR. Overview of the health measures in the health and retirement study. J Hum Resour. 1995;30:S84. [Google Scholar]
- 96.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 97.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carey D, Nolan H, Kenny RA, Meaney J. Cortical covariance networks in ageing: cross-sectional data from the Irish Longitudinal Study on Ageing (TILDA) Neuropsychologia. 2019;122:51–61. doi: 10.1016/j.neuropsychologia.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 100.Casaletto KB, Rentería MA, Pa J, Tom SE, Harrati A, Armstrong NM, Rajan KB, Mungas D, Walters S, Kramer J, Zahodne LB. Late-life physical and cognitive activities independently contribute to brain and cognitive resilience. J Alzheimer’s Dis. 2020;74(1):363–376. doi: 10.3233/JAD-191114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Groot C, van Loenhoud AC, Barkhof F, Van Berckel BNM, Koene T, Teunissen CC, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90(2):e149–e156. doi: 10.1212/WNL.0000000000004802. [DOI] [PubMed] [Google Scholar]
- 102.Resende E d PF, Rosen HJ, Chiang K, Staffaroni AM, Allen I, Grinberg LT, et al. Primary school education may be sufficient to moderate a memory-hippocampal relationship. Front Aging Neurosci. 2018;10:381. doi: 10.3389/fnagi.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Belleville S, Mellah S, Cloutier S, Dang-Vu TT, Duchesne S, Maltezos S, Phillips N, Hudon C, CIMA-Q group Neural correlates of resilience to the effects of hippocampal atrophy on memory. NeuroImage Clin. 2021;29:102526. doi: 10.1016/j.nicl.2020.102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee DH, Lee P, Seo SW, Roh JH, Oh M, Oh JS, Oh SJ, Kim JS, Jeong Y. Neural substrates of cognitive reserve in Alzheimer’s disease spectrum and normal aging. Neuroimage. 2019;186:690–702. doi: 10.1016/j.neuroimage.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 105.Seabold S, Perktold J. Proceedings of the 9th Python in Science Conference. 2010. Statsmodels: econometric and statistical modeling with Python. [Google Scholar]
- 106.Tsai HJ, Chang FK. Associations of exercise, nutritional status, and smoking with cognitive decline among older adults in Taiwan: results of a longitudinal population-based study. Arch Gerontol Geriatr. 2019;82:133–138. doi: 10.1016/j.archger.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 107.Clewett DV, Lee TH, Greening S, Ponzio A, Margalit E, Mather M. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging. 2016;37:117–126. doi: 10.1016/j.neurobiolaging.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laubach M, Lammers F, Zacharias N, Feinkohl I, Pischon T, Borchers F, Slooter AJC, Kühn S, Spies C, Winterer G, BioCog Consortium Size matters: grey matter brain reserve predicts executive functioning in the elderly. Neuropsychologia. 2018;119:172–181. doi: 10.1016/j.neuropsychologia.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Appl Neuropsychol. 2004;11(1):37–46. doi: 10.1207/s15324826an1101_5. [DOI] [PubMed] [Google Scholar]
- 110.Manly JJ, Schupf N, Tang M-X, Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol. 2005;18(4):213–217. doi: 10.1177/0891988705281868. [DOI] [PubMed] [Google Scholar]
- 111.Pettigrew C, Soldan A, Li S, Lu Y, Wang MC, Selnes OA, Moghekar A, O’Brien R, Albert M, Research Team the BIOCARD Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Cogn Neurosci. 2013;4(3–4):136–142. doi: 10.1080/17588928.2013.831820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- 113.Chin AL, Negash S, Xie S, Arnold SE, Hamilton R. Quality, and not just quantity, of education accounts for differences in psychometric performance between African Americans and white non-Hispanics with Alzheimer’s disease. J Int Neuropsychol Soc. 2012;18(2):277–285. doi: 10.1017/S1355617711001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ehrenberg RG, Brewer DJ, Gamoran A, Willms JD. Class size and student achievement. Psychol Sci Public Interest. 2001;2(1):1–30. doi: 10.1111/1529-1006.003. [DOI] [PubMed] [Google Scholar]
- 115.Hsieh TL. Motivation matters? The relationship among different types of learning motivation, engagement behaviors and learning outcomes of undergraduate students in Taiwan. High Educ. 2014;68(3):417–433. [Google Scholar]
- 116.Anatürk M, Suri S, Smith S, Ebmeier K, Sexton C. Mid-life and late life activities and their relationship with MRI measures of brain structure and functional connectivity in the UK Biobank cohort. bioRxiv. 2020; 2020.04.10.035451. [DOI] [PMC free article] [PubMed]
- 117.Peeters G, Kenny RA, Lawlor B. Late life education and cognitive function in older adults. Int J Geriatr Psychiatry. 2020;35:gps.5281. [DOI] [PubMed]
- 118.Deary IJ, Starr JM, MacLennan WJ. Is age kinder to the initially more able?: differential ageing of a verbal ability in the healthy old people in Edinburgh study. Intelligence. 1998;26(4):357–375. [Google Scholar]
- 119.McHutchison CA, Chappell FM, Makin S, Shuler K, Wardlaw JM, Cvoro V. Stability of estimated premorbid cognitive ability over time after minor stroke and its relationship with post-stroke cognitive ability. Brain Sci. 2019;9(5):117. doi: 10.3390/brainsci9050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giambra LM, Arenberg D, Zonderman AB, Kawas C, Costa PT. Adult life span changes in immediate visual memory and verbal intelligence. Psychol Aging. 1995;10(1):123–139. doi: 10.1037//0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- 121.Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- 122.Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. J Gerontol - Ser B Psychol Sci Soc Sci. 2003;58(5):P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- 123.Sommerlad A, Sabia S, Livingston G, Kivimäki M, Lewis G, Singh-Manoux A. Leisure activity participation and risk of dementia: an 18-year follow-up of the Whitehall II Study. Neurology. 2020;95:e2803–e2815. doi: 10.1212/WNL.0000000000010966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chan D, Shafto M, Kievit R, Matthews F, Spink M, Valenzuela M, Henson RN. Lifestyle activities in mid-life contribute to cognitive reserve in late-life, independent of education, occupation, and late-life activities. Neurobiol Aging. 2018;70:180–183. doi: 10.1016/j.neurobiolaging.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park S, Choi B, Choi C, Kang JM, Lee JY. Relationship between education, leisure activities, and cognitive functions in older adults. Aging Ment Heal. 2019;23(12):1651–1660. doi: 10.1080/13607863.2018.1512083. [DOI] [PubMed] [Google Scholar]
- 126.Lipnicki DM, Makkar SR, Crawford JD, Thalamuthu A, Kochan NA, Lima-Costa MF, et al. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: a COSMIC collaboration cohort study. PLoS Med. 2019;16(7):12. doi: 10.1371/journal.pmed.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nilsson J, Lövdén M. Naming is not explaining: future directions for the cognitive reserve and brain maintenance theories. Alzheimers Res Ther. 2018;10(1):34. doi: 10.1186/s13195-018-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng ST. Cognitive reserve and the prevention of dementia: the role of physical and cognitive activities. Curr Psychiatry Rep. 2016;18:1. doi: 10.1007/s11920-016-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, Wojcicki TR, Mailey E, McAuley E, Kramer AF, Erickson KI. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26(5):811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steffener J, Habeck C, O’Shea D, Razlighi Q, Bherer L, Stern Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging. 2016;40:138–144. doi: 10.1016/j.neurobiolaging.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Habeck C, Razlighi Q, Gazes Y, Barulli D, Steffener J, Stern Y. Cognitive reserve and brain maintenance: orthogonal concepts in theory and practice. Cereb Cortex. 2017;27(8):3962–3969. doi: 10.1093/cercor/bhw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stern Y, Mackay-Brandt A, Lee S, McKinley P, McIntyre K, Razlighi Q, et al. Effect of aerobic exercise on cognition in younger adults: a randomized clinical trial. Neurology. 2019;92(9):E905–E916. doi: 10.1212/WNL.0000000000007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arenaza-Urquijo EM, de Flores R, Gonneaud J, Wirth M, Ourry V, Callewaert W, Landeau B, Egret S, Mézenge F, Desgranges B, Chételat G. Distinct effects of late adulthood cognitive and physical activities on gray matter volume. Brain Imaging Behav. 2017;11(2):346–356. doi: 10.1007/s11682-016-9617-3. [DOI] [PubMed] [Google Scholar]
- 134.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35:383–90 Elsevier Inc. [DOI] [PMC free article] [PubMed]
- 135.Vuoksimaa E, Panizzon MS, Chen CH, Eyler LT, Fennema-Notestine C, Fiecas MJA, Fischl B, Franz CE, Grant MD, Jak AJ, Lyons MJ, Neale MC, Thompson WK, Tsuang MT, Xian H, Dale AM, Kremen WS. Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia. 2013;51(6):1124–1131. doi: 10.1016/j.neuropsychologia.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 1993;114(2):376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- 137.Aguinis H, Stone-Romero EF. Methodological artifacts in moderated multiple regression and their effects on statistical power. J Appl Psychol. 1997;82(1):192–205. [Google Scholar]
- 138.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 139.Dufouil C, Alpérovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60(5):831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- 140.Zahodne LB, Mayeda ER, Hohman TJ, Fletcher E, Racine AM, Gavett B, Manly JJ, Schupf N, Mayeux R, Brickman AM, Mungas D. The role of education in a vascular pathway to episodic memory: brain maintenance or cognitive reserve? Neurobiol Aging. 2019;84:109–118. doi: 10.1016/j.neurobiolaging.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Joannette M, Bocti C, Dupont PS, Lavallée MM, Nikelski J, Vallet GT, et al. Education as a moderator of the relationship between episodic memory and amyloid load in normal aging. J Gerontol Ser A. 2019;75(10):1820–1826. doi: 10.1093/gerona/glz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rentz DM, Mormino EC, Papp KV, Betensky RA, Sperling RA, Johnson KA. Cognitive resilience in clinical and preclinical Alzheimer’s disease: the association of amyloid and tau burden on cognitive performance. Brain Imaging Behav. 2017;11(2):383–390. doi: 10.1007/s11682-016-9640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Significant independent effects across both datasets.
Additional file 2. All significant independent effects in TILDA.
Additional file 3. All significant independent effects in CR/RANN.
Additional file 4 Table S1 Negative moderation effects of cognitive reserve proxies within TILDA. Table S2 Positive moderation effects of cognitive reserve proxies within both datasets. Figure S1: Association between proxies and cognition, adjusting for brain structure, age, and sex.
Data Availability Statement
The TILDA dataset analysed in this study is available from TILDA upon reasonable request. The procedures to gain access to TILDA data are specified at https://tilda.tcd.ie/data/accessing-data/.