SUMMARY
Exploration of novel environments ensures survival and evolutionary fitness. It is expressed through exploratory bouts and arrests that change dynamically based on experience. Neural circuits mediating exploratory behavior should therefore integrate experience and use it to select the proper behavioral output. Using a spatial exploration assay, we uncovered an experience-dependent increase in momentary arrests in locations where animals arrested previously. Calcium imaging in freely exploring mice revealed a genetically and projection-defined neuronal ensemble in the basolateral amygdala that is active during self-paced behavioral arrests. This ensemble was recruited in an experience-dependent manner, and closed-loop optogenetic manipulation of these neurons revealed that they are sufficient and necessary to drive experience-dependent arrests during exploration. Projection-specific imaging and optogenetic experiments revealed that these arrests are effected by basolateral amygdala neurons projecting to the central amygdala, uncovering an amygdala circuit that mediates momentary arrests in familiar places but not avoidance or anxiety/fear-like behaviors.
In Brief
A genetically and projection-defined basolateral amygdala circuit mediates momentary arrests during exploration that emerge in an experience-dependent manner when mice familiarize themselves with a novel environment.
Graphical Abstract

INTRODUCTION
Exploration is highly conserved across species and can be constructed as the act of gathering information or resources from unknown surroundings (Fonio et al., 2009). It is therefore a self-paced process in which animals guide their behavior to learn about new environments. This behavior is fundamental for discovering new territories to gather food and water or find safe shelter; e.g., birds explore the territory before choosing a place to nest (Mills and Wood-Gush, 1985; Freire et al., 1996).
Many behavioral studies analyzing exploration in the lab use assays that depend on water- or food-deprived animals executing movements to ultimately find and consume food or water (Fonio et al., 2009; Benjamini et al., 2010; Vorhees and Williams, 2014). Less is known about the self-paced moment-to-moment actions that govern exploration of novel environments in the absence of explicit reinforcers (Tolman, 1948; Renner, 1990; Benjamini et al., 2011; Redish, 2016). Emergence exploratory paradigms were developed to study naturalistic exploratory behavior during transitions between familiar and novel environments (Fonio et al., 2009; Benjamini et al., 2011). These paradigms allow animals to explore a novel area in a self-paced manner, departing from a familiar one, and permit investigation of moment-to-moment exploratory dynamics during spatial familiarization as the animal performs defined trips from the familiar shelter. When facing novel surroundings and given freedom of movement, animals execute gradual and structured exploratory trips with development of a quantifiable behavioral gradient (Benjamini et al., 2011).
Several reports show that a wide variety of species use specific locations, sometimes called “home bases,” as strategic points of reference from which the animal begins and terminates exploratory trips (Eilam and Golani, 1989; Golani et al., 1993; Drai et al., 2000; Clark et al., 2006; Fonio et al., 2009; Benjamini et al., 2011). In exploration, home bases can be perceived as the origin of exploratory activity at the interface between known and unknown places, representing a key factor in understanding its general structure and organization (Eilam and Golani, 1989; Golani et al., 1993; Clark et al., 2006; Dvorkin et al., 2010). These locations are characterized by more frequent behavioral arrests than other areas explored by an animal (Eilam and Golani, 1989; Golani et al., 1993), indicating that exploratory motor dynamics are influenced by spatial knowledge. Spontaneous arrests are distinct from freezing responses because they are momentary, occur voluntarily in familiar places, and are not triggered by any apparent aversive stimuli (Phillips and LeDoux, 1992; Roelofs, 2017; Roseberry and Kreitzer, 2017). Instead, this self-initiated type of arrest may be critical for the deliberation process in familiar/safe places while the animal prepares for the next exploratory trip (Eilam and Golani, 1989; Clark et al., 2006; Redish, 2016).
Given that exploratory actions such as arrests depend on knowledge of the environment, the neural circuits mediating this behavior should integrate experience-dependent contextual information and use it to select a proper behavioral output. The main input structure of the amygdala, the basolateral nucleus (BLA), has been shown to be involved in the learning of contextual information related to appetitive and aversive experiences (Phillips and LeDoux, 1992; Herry et al., 2008; Janak and Tye, 2015; Namburi et al., 2015; Beyeler et al., 2016; Kim et al., 2016, 2017; Beyeler et al., 2018) as well as in expression of motor defensive behaviors via the central amygdala (CEA; Ciocchi et al., 2010; Botta et al., 2015; Tovote et al., 2016; Xu et al., 2016; Fadok et al., 2017; Terburg et al., 2018). Previous studies suggest that the amygdala plays a much broader role in integrating novel and familiar sensory stimuli in humans and monkeys; lesions of the BLA impair familiarity recognition (Wilson and Rolls, 1993; Schwartz et al., 2003; Mason et al., 2006; Farovik et al., 2011) and affect locomotor exploration in the open field (Jellestad and Bakke, 1985; Yim and Mogenson, 1989).
In the present study, we developed an emergence assay to investigate the dynamics of exploration in mice and, in particular, the neural mechanisms underlying momentary arrests during exploration. Using behavioral analyses, circuit mapping, single-cell calcium imaging, and closed-loop optogenetic approaches, we provide evidence of recruitment of a well-defined BLA neuronal population that mediates experience-dependent momentary arrests. Further analyses revealed that BLA neurons active during exploratory momentary arrests project to the CEA and that this neuronal population does not overlap beyond random with populations that are active during other moments of immobility, such as freezing. Activation and inhibition of the BLA neuronal population directly projecting to the CEA revealed that this projection mediates momentary behavioral arrests during exploration. Furthermore, we show that the role of the amygdala in modulating exploratory arrests was not directly associated with changes in the place value or place preference or anxiety/fear-like behavior.
RESULTS
A BLA Neuronal Ensemble Is Active during Self-Paced Behavioral Arrests
We investigated the progression of self-paced exploratory dynamics by analyzing the behavior of animals as they explored a 100-cm circular arena, departing from a familiar 20-cm arena, for 5 consecutive days (7 mice; Figure 1A; Figure S1). Although animals performed a constant number of trips from the small to the big arena, the duration of the trips and the area explored increased gradually during the 5 experimental days (Figure 1B; Figure S1; Videos S1A and S1B). As shown by our results and others (Benjamini et al., 2011), the complexity of the trajectories increased in late exploration (Figure S1). Our data confirm that the developed behavioral assay can be used to study gradual progression of exploration.
Figure 1. A BLA Neuronal Ensemble Encodes Self-Paced Behavioral Arrests.
(A) Top: behavioral protocol. Bottom: schematic of the dimensionality emergence assay.
(B) Daily area explored (percent) divided into quartiles, considering even portions of exploratory time.
(C) Left: brain section showing the unilateral AAV1-CAG-FLEX-Tdtomato injection into the BLA of the NL189-cre mouse line. Lines denote the injection site and amygdala borders. The central amygdala (CEA) is shown divided into the lateral (l) and medial (m) part. Right: heatmap of BLA soma density, defined as the number of cells within a 300-μm-diameter sphere.
(D) Top: scheme of the mini-endoscope mounted on top of the mouse’s head. Bottom: CNMF-E-processed video of the neurons located in the BLA, with neuronal calcium activity normalized by the max.
(E) Top: time course of the activity of single neurons recorded simultaneously in one animal during exploration of the large arena. Center: average population activity of the same animal. Bottom: movement speed during exploration.
(F) Time course of the cross-correlation (CC) for the entire neuronal population at different lags (range, −20 to 20 s).
(G) Pie chart showing the proportion of CC, anti-CC, and non-CC neurons (for all neurons/all animals; CC, n = 204; anti-CC, n = 670; non-CC, n = 561; total neurons, n = 1,435).
(H) Top: average speed of the arrest trials aligned to onset. Bottom: time course of the speed in all arrest trials for all the animals aligned to arrest onset. Time to arrest (0 s) is indicated by a dashed gray line and the purple dot.
(I) Top: average raw calcium trace of the entire neuronal NL189BLA population imaged (n = 1,435) at the time of arrest (in arbitrary units, a.u.). Bottom:, Z-score of all NL189BLA single-neuron activities at the time of arrest.
(J) Representative trace of a ROC-sorted arrest neuron showing average (top, black line) and single-arrest events (bottom, gray).
(K) Top: pie chart of Z-scored and ROC-sorted neurons; positive (+), negative (−), and non-modulated (ns). Bottom: time course of the peak neural activity versus time to arrest. Latency 0 s represents the time to arrest.
Data are presented as mean ± SEM.
To investigate the activity of excitatory pyramidal neurons in the BLA in this assay (Figure S1), we used a mouse line that expresses Cre recombinase in a population of those BLA neurons: the NL189 line (NL189BLA neurons; Figure 1C; Figure S2; Videos S1C and S1D; STAR Methods). After injecting a conditional AAV virus expressing GCamp6f in the BLA of NL189 Cre+ animals (Chen et al., 2013), we implanted a gradient index lens in seven mice (Figures S3A–S3E). Neuronal calcium activity was imaged from 1,435 NL189BLA neurons for 5 days (average neurons/animal/day = 41 ± 4.85; Figure S3E) using a miniaturized one-photon fluorescence microscope in freely exploring animals and processed with the constrained non-negative matrix factorization for endoscopic data (CNMF-E) algorithm (Figure 1D; Figures S3A–S3E; Video S1E; STAR Methods; Pnevmatikakis et al., 2016).
We observed that a large population of simultaneously recorded NL189BLA neurons had coincident, transient moments of increases in activity that corresponded to moments when the speed of the animals decreased (Figure 1E). Overall, the activity of the entire population of NL189BLA neurons was inversely cross-correlated with speed (Figure 1F; STAR Methods). A rather large proportion of single neurons was activated at low speed (negative cross-correlation [anti-CC]) compared with high-speed (positive cross-correlation [CC]) neurons (Figure 1G). Another analytical approach, the spike-triggering average, confirmed a large proportion of NL189BLA neurons to be active during decreased movement speed (Figures S3F and S3G). Using a segmentation algorithm (Benjamini et al., 2010; Figures S1G–S1I; STAR Methods), we were able to detect the transition between movements and momentary arrests (Figure 1H). Our analysis considered all spontaneous arrests of an animal in the small and big arenas. These arrests were momentary (average duration in the big arena, 1.11 ± 0.21 s; in the small arena, 2.2 ± 0.23 s; average latency to arrest, 0.68 ± 0.01 s; Figures S1J–S1L) and were usually followed by an increase in the angular speed of the head and a change in the direction of motion (Figures S1M–S1P; Videos S1F and S1G), suggestive of vicarious trial and error behavior (Muenzinger and Gentry, 1931; Tolman, 1939; Redish, 2016). In combination with the receiver operating characteristic (ROC) and Z-score analysis to sort neuronal activity between behavioral states, we found that a large proportion of NL189BLA neurons was positively modulated (active) during the transition between movement and spontaneous arrests compared with the negatively modulated (inhibited) neuronal population (Figures 1I–1K; STAR Methods). The majority of arrested NL189BLA neurons were active before arrest (latency of −0.29 ± 0.01 s; Figure 1K). Overall, these results show that a large subpopulation of BLA principal neurons, NL189BLA neurons, is active before and during behavioral arrests.
Transient NL189BLA Neuron Activation Decreases Movement Speed and Triggers Momentary Arrest
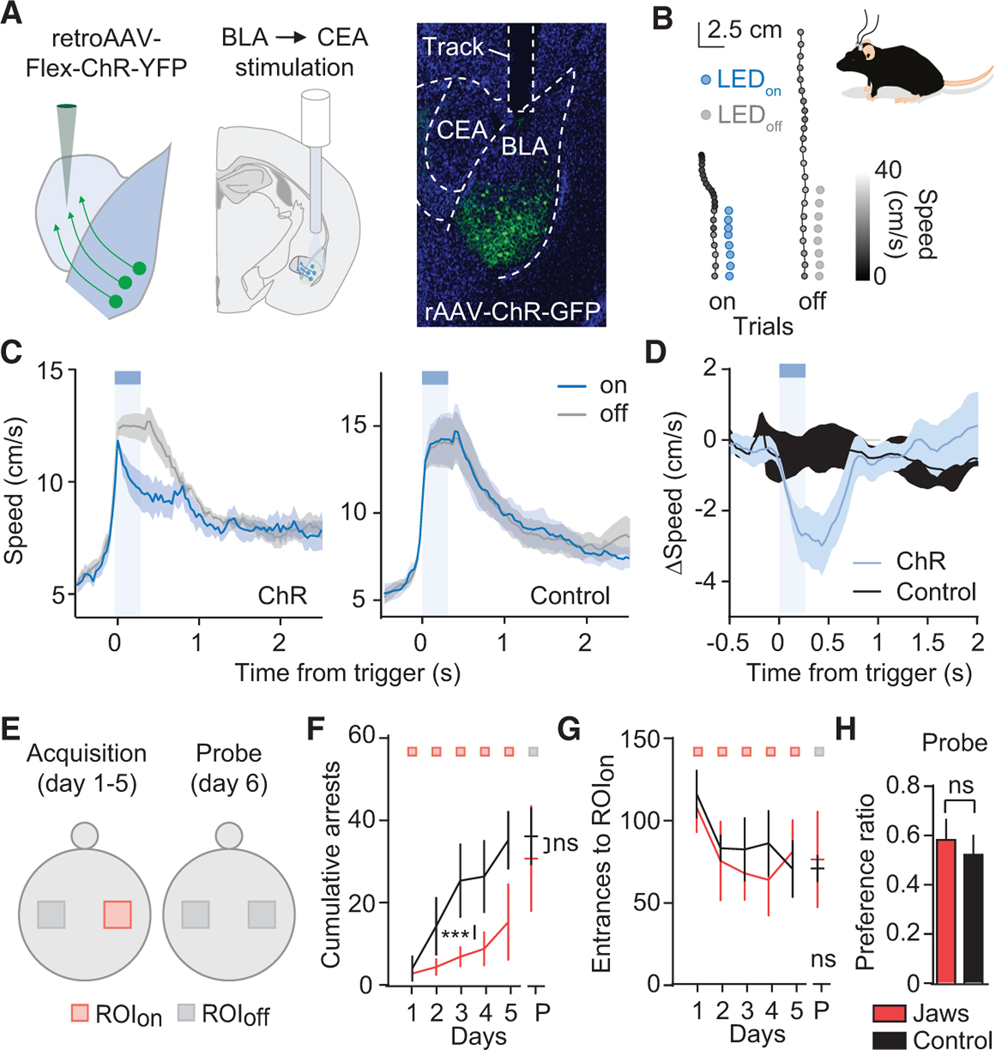
To test whether the activity of NL189BLA neurons is causally related to behavioral arrests, we injected a conditional AAV virus expressing channelrhodopsin hChR2(H134R [ChR]) and YFP into the BLA. Optogenetic stimulation of NL189BLA neurons using three different light patterns (1×, one 10-ms pulse; 2×, two 10-ms pulses at 20 Hz; 5×, five 10-ms pulses at 20 Hz; STAR Methods) showed a strong and reliable effect on eliciting neuronal activity in slice (Figures 2A and 2B; Figures S4A–S4D). Next we performed closed-loop optogenetic stimulation in which the LEDon was triggered ~33% of the time during bouts where the animal was moving and had accelerated for at least 100 ms in the ChR and control group (on trials; Figures 2C–2F; STAR Methods). The 66% of trials when light was not delivered served as within-animal controls (off trials; Figures 2F–2J). Acceleration-locked, closed-loop optogenetic activation of NL189BLA neurons using the three aforementioned optogenetic protocols decreased the speed of the animals tested in comparison with off trials and control animals (significant ~400–500 ms after stimulation onset; Figures 2F–2K; Figures S4E–S4H; Video S1H). The same stimulation protocol was ineffective in eliciting any evident change in speed in control animals (Figures 2G–2K; Figures S4E–S4H). Closed-loop optogenetic activation of NL189BLA neurons in the ChR group elicited a significant increase in behavioral arrests following stimulation (Figure 2M). The average duration of arrests resulting from the 1×, 2×, and 5× stimulation protocols was 0.57 ± 0.08 s, 0.64 ± 0.11 s, and 0.47 ± 0.08 s, respectively, suggesting that optogenetic activation of NL189BLA neurons triggered momentary arrests and not freezing (a more prolonged state; Herry et al., 2008; Tovote et al., 2016). To further clarify whether longer activation could lead to prolonged freezing-like arrest, we tested different stimulation protocols with various stimulation lengths (from 10 ms up to 2 s). Optogenetic activation of NL189BLA neurons using 1, 2, 5, 10, and 40 pulses (pulse duration, 10 ms) at 20 Hz (durations of 10 ms to 2 s) caused arrests with a duration of less than 1 s and no significant effect in arrest duration between protocols (Figure 2N), suggesting that activity in these neurons facilitates momentary arrests but not long periods of immobility or freezing. Importantly, there was no difference between the overall count of arrests throughout the behavioral session for the ChR versus the control group (Figure S4L), from which it can be deduced that the arrests were not caused by the animal freezing in other locations or generalization of immobility in that session (Figure S4L). It is important to note that stimulation was spatially sparse in the large arena and not triggered in every trial (~66% of the trials were light off). Additionally, classical analysis to detect anxiety-like behavior showed that the most intense optogenetic stimulation (5×) did not have a consistent effect on the speed at times other than during from LEDon stimulation, nor did it materially alter the average distance of the mouse from the center of the arena (Figure 2L; Figures S4I–S4K). In summary, direct optogenetic stimulation of NL189BLA neurons during ongoing movement reduces speed and promotes momentary arrests but does not have any effect on overall light-unlocked speed, arrest count, or anxiety-like behavior.
Figure 2. Transient NL189BLA Neuron Activation Decreases Movement and Promotes Arrest.
(A) Top left: schematic of patch-clamping in slice to test NL189BLA neurons expressing ChR and YFP. Top right and bottom: differential interference contrast (DIC), fluorescence, and merged image showing patching of a single NL189BLA neuron in slice.
(B) Top: 5× stimulation protocol. Bottom: effect of five 10-ms pulses at 20 Hz on neuronal firing (five trials) of a representative NL189BLA neuron. B’: the reliability to elicit firing with one, two, and five pulses at 20 Hz. Scale bars, 10 mV, 40 ms.
(C) Schematic injection of a conditional AAV virus expressing ChR2-YFP (top) followed by optical fiber implantation (bottom).
(D) Representative coronal section of a mouse injected bilaterally in the BLA with the conditional virus expressing ChR2-YFP and implanted with 200-μm optical fibers. Dashed lines show the fiber track.
(E) Acceleration-based closed-loop optogenetic activation protocol. The different colors denote that the animal has to be moving to trigger the trials.
(F) Top left: scheme of a mouse with bilateral optical fiber implantation attached to patch cords. Right: two-speed color-coded trajectories for the same time window in light on (left) and off (right, no light pulses) closed-loop trials (5× protocol). Aligned dots (blue for the on trials and gray for the off trials) show triggering of the LED for the 5× protocol (only on trials).
(G) Speed in closed-loop trials of two representative animals during light on (on, light blue) or off (off, gray) for the ChR (top) and control (bottom) groups. Scale bar, 4 cm/s.
(H–J) Speed in closed-loop trials using three stimulation protocols (1×, H; 2×, I; and 5×, J; n = 7 [ChR group] and n = 5 [control group]); two-way ANOVA repeated measures using the normalized speed. Multiple comparisons show that the start of the effect (on versus off) is well before 1 s with the 5× protocol (560 ms; black arrows show the statistical effect). Stimulation 2× caused a similar decrease in speed (from 500 ms to 1 s), whereas a 10-ms pulse caused a lesser but still significant effect on speed around 400 ms from the start of the stimulation.
(K) Left: Δspeed amplitude (on-off trial speed) elicited by the 5× stimulation protocol. Binning, 500 ms. Right: Δspeed amplitude (on-off trial speed during 2 s from starting the stimulation) elicited by the three stimulation protocols. The ChR group is shown in blue, whereas the control group is shown in black. *p < 0.05 and **p < 0.01 by unpaired t test for three different stimulation protocols (ChR versus control group).
(L) Binned raw speed in ChR and control animals during the 5× stimulation protocol.
(M) Left to right: cumulative arrests after 1×, 2×, and 5× stimulation in the ChR and control groups. The p values were calculated with unpaired two-tailed t test from 0–4 s.
(N) Optogenetic stimulation during movement using different pulse numbers at 20 Hz (n = 7 animals for 1×–5× pulses; n = 5 for 10×–40× pulses). The bottom x axis refers to the duration of stimulation. The top x axis shows the number of pulses of light (10-ms length) that were delivered. The inter-stimulation interval was about 60 s.
Data are presented as mean ± SEM.
Inhibition of NL189BLA Neurons Increases Movement Speed
Subsequently, to test loss of function, we adopted an optogenetic method using the inhibitory opsin Jaws (Chuong et al., 2014) to transiently silence NL189BLA neurons (Figures 3A–3C; Figure S4; STAR Methods). Contrary to the ChR experiments, speed- and acceleration-locked closed-loop optogenetic silencing of NL189BLA neurons (2 s of red light) revealed an increase in movement speed in comparison with off trials and control animals (Figures 3D–3G). To investigate whether NL189BLA neurons are important not only to trigger but also to maintain behavioral arrests, we designed a closed-loop optogenetic experiment where we applied 2 s of optogenetic inhibition when animals arrested for at least 500 ms (Figures 3H–3J). Similar to the manipulation of NL189BLA neurons during movements, we found that silencing triggered by 500-ms arrest causes an increase in starting speed compared with control conditions (Figures 3H and 3I). However, this silencing of NL189BLA neurons did not cause changes in arrest duration compared with the control condition (Figure 3J), indicating that their activity is related to inducing but not to maintaining immobility. This is consistent with our findings that arrest-related NL189BLA neurons are active before arrests and not during immobility and that their activation induces arrests but that prolonged activation does not lead to prolonged immobility, further suggesting that the activity of NL189BLA neurons induces momentary arrest during exploration.
Figure 3. Inhibition of NL189BLA Neurons Facilitates Movements.
(A) Top: 5× magnification of a slice in the patch-clamp chamber, held by a platinum anchor (black bar), showing expression of Jaws and Tdtomato in the BA. Bottom: 40× magnification showing the recording from a single NL189BLA neuron in slice. Blue lines show the patch pipette.
(B) Top: raster plot of firing elicited by a step of current in a NL189BLA neuron with 2 s of 630-nm light. Center: single firing trace from the raster plot. Bottom: average firing rate in 500-ms bins of light-induced inhibition (n = 6 neurons).
(C) Top inset: schematic of a mouse mounted with an nVoke mini-microscope to simultaneously image neuronal activity and elicit red-shifted opsin activation with 5 s of light and 15-s inter-trial interval (ITI). Center: representative neuronal calcium activity trials before, during, and after light inhibition in vivo. Scale bar, 1 Z-score. Bottom” area under the ROC (auROC) activity time course as shown in (B) but for in vivo imaging (n = 5 neurons).
(D) Representative coronal slice using a conditional AAV virus expressing Jaws and Tdtomato.
(E) Two-speed color-coded trajectories in on (top) and off (bottom) closed-loop trials. LEDon is shown in red circles. As shown in Figure 2, triggering of the light is caused by a moment of 100-ms acceleration (when animals are moving), with a variable ITI of ~15 s.
(F) Closed loop as for Figure 2 but triggering 2 s of 630-nm light for Jaws (n = 5) and control (n = 4).
(G) Change in speed between on (pink, F) and off trials (gray, F) for the Jaws (red) and control groups (gray). *p < 0.05, **p < 0.01 by two-way ANOVA with Sidak’s multiple comparison test between Jaws (red) and control (black).
(H) Speed profile for silencing NL189BLA neurons during arrest. Arrests with 500-ms duration trigger red LEDon for 2 s (λ = 630 nm; pink bar with red line) in the Jaws (n = 6) and control groups (n = 7). Time 0 represents the start of silencing.
(I) Total effect on speed under Jaws (n = 6) versus control conditions (n = 7; **p < 0.0016 by unpaired t test).
(J) Arrest fraction versus arrest duration in the control and Jaws groups.
Data are presented as mean ± SEM.
Arrest-Related NL189BLA Neurons Increase in an Experience-Dependent Manner
After assessing the direct involvement of NL189BLA neurons on movement speed and momentary behavioral arrests, we wanted to find out whether their activity changes as movement dynamics in exploration change with experience. As mentioned previously, in our emergence exploratory assay, animals freely explore a novel arena starting from a familiar home shelter in 5 sessions, one per day, from days 1–5 (Figures 1A and 1B). In line with a process of familiarization, exploratory arrests increased across days in the 100-cm arena, whereas their number is kept constant in the more familiar 20-cm-arena (Figures 4A–4C). The increase in exploratory arrests was related to the number of exploratory trips (Figure S5A). Voluntary arrests started to appear at the 26th exploratory trip, when 75.81% ± 7.485% of the area was explored (Figure S5A, gray dashed line).
Figure 4. NL189BLA Neurons Encoding Self-Paced Arrest Are Recruited in an Experience-Dependent Manner in Familiar Locations.
(A) Representative aerial view of a mouse’s trajectory super-imposed to arrests (purple circles) for all days.
(B) Same as (A) but for days 1 and 5.
(C) Arrest count in the small (left) and big (right) arena for each day.
(D) 4-Dimensional plot showing the arrest fraction of a representative animal for the 5 days. An ROIexample delineated by a dashed line was used in (E) and (F).
(E) Proportion of arrests at different distances. Inset: ROIexample showing the distribution of arrests (fraction).
(F) ROIexample showing the increase in proportion of arrests across days (from days 1–4).
(G) Probability of arrest (Parrest) at an ROI where animals arrest in relation to the number of visits to that ROI (black) and shuffled condition (gray).
(H) Inter-entrance interval (IEI; seconds) to ROIarrest (areas where mice arrested) or ROINon-Arrest (areas where mice did not arrest) in relation to the number of visits to those places.
(I) Top: representative animal trajectory in an early and late visit. A purple dot represents the arrest. Bottom: heatmap of calcium activity of a single neuron recorded from the same animal.
(J) Magnitude of neuronal activity of significant NL189BLA neurons (neurons in which the activity was positively modulated during arrest and inversely cross-correlated with speed) in relation to the number of visits to arrest areas. Early (1–5) and late (16–20) spatial experiences are compared.
(k) Heatmap of neuronal activity of significant NL189BLA neurons during early versus late arrests.
(L) Magnitude of neuronal calcium activity of significant NL189BLA neurons (auROC) during early (light purple) versus late arrests (dark purple).
(M) Magnitude of neuronal calcium activity of significant NL189BLA neurons during early versus late arrest. ****p < 0.0001 by paired t test.
(N) Fraction of significant NL189BLA neurons increases with spatial experience. ****p < 0.001 by paired t test, early versus late.
(O) Fraction of significant NL189BLA neurons in early (left) or late visits to ROIs (right) during arrest (arrest, light violet bar graph) or movement (move, white bar graph). *p < 0.01 by paired t test.
Data are presented as mean ± SEM.
These data indicate that a certain knowledge of the environment is required before voluntary exploratory arrests start occurring. We therefore investigated whether the onset of experience-dependent exploratory arrests was based on the number of visits to specific places. First we quantified the distance between the arrests’ coordinates (Figure 4E) and found that they accumulate within a distance of 4 cm (Figures 4D and 4E). Second, to understand whether such arrests emerged based on the experience at specific locations, we tracked the visits of the animal (referred to here as spatial experience) to a defined region of interest where the animal had arrested at least once (ROIarrest; Figure 4F). The diameter of the ROIarrest was chosen based on the most common distance between arrests, 4 cm (Figure 4E). Interestingly, the cumulative exploratory arrests as well as arrest duration increased upon entrance to these ROIarrest (Figure S5B and S5C; STAR Methods). Accordingly, the probability of arrest (Parrest) increased upon spatial experience in ROIarrest compared with other ROIs of similar size (shuffled data; Figure 4G). Furthermore, the inter-entrance interval (IEI; seconds) to ROIarrest decreased with the number of visits, but it remained constant in ROIs where the animal had never arrested before (ROINon-Arrest; Figure 4H). Additionally, the entrance preference to ROIarrest was higher compared with ROINon-Arrest (preference ratio: (ROIarrest − ROINon-Arrest)/(ROIarrest + ROINon-Arrest) = 0.3279 ± 0.04162). To understand whether visuospatial information is required for experience-dependent arrests, we performed the behavioral assay in the dark using infrared light versus light context (the same light intensity as for the rest of the behavioral experiments; Figures S5D–S5F). We found that, in the dark, animals arrest more, and, importantly, they did not arrest preferentially in places with which they were familiar with; they arrested as much in places where they visited 1–5 times as in places where they visited 16–20 times (Figures S5E and S5F). Therefore, the experience-dependent arrest is dependent on visual input. These results show that exploratory arrests depend on an interaction between spatial and behavioral experience because mice tend to momentarily arrest in places they visited previously and where they arrested previously.
Next we sought to understand whether the neuronal activity of NL189BLA neurons encoding self-paced arrests was modulated by spatial experience (Figures S5G–S5L; STAR Methods). First we used three separate analyses (ROC, CC, and event-triggering average) and observed an increase in neurons active during the deceleration phase or arrest from day 1 to day 5 (Figures S5G–S5K). The activity of arrest NL189BLA neurons was positively correlated with the daily arrest count (Figure S5K). Subsequently, we sorted pure arrest NL189BLA neurons by selecting them based on the positive ROC- and anti-CC-based analysis during exploration of the big and small arena (significant neurons; Figure S5L; STAR Methods). We found a substantial increase in the proportion of significant NL189BLA neurons encoding self-paced arrests and inversely cross-correlated with speed across days of exploration in the big arena (Figure S5L). To understand whether the arrest-related activity appeared based on fine spatial experience, we used two separate measures to track the overall activation of significant NL189BLA neurons during early (1–5) and late (16–20) entrances to ROIarrest (Figures 4I–4O; Figure S5M and S5N): (1) the magnitude of their calcium activity (Figures 4I–4M; Figure S5M) and (2) the fraction of significant neurons active at the time of spatial crossing (using a Z-score analysis; Figures 4N and 4O). We found that the magnitude of the neuronal activity of significant NL189BLA neurons increased with the number of visits to ROIarrest (the ROI where they arrest at least once; Figure 4J). The fraction of significant NL189BLA neurons and their probability to be active also increased with experience (Figure 4N). Importantly, we also calculated the same measures controlling for the same number of arrests in early and late visits (5) by considering only visits in which animals arrested at that location (Figures 4K–4M and 4O; Figure S5). Again, we found that significant neurons were more active in terms of peak calcium activity in late visits than early visits and that their proportion increased with experience (Figures 4K–4M and 4O; Figure S5). Overall, these data demonstrate that the number and the activity of arrest NL189BLA neurons increased when animals visited familiar locations where they arrested before during exploration (and did not reflect solely arrests in locomotion; Figure S5).
Overall, these findings reveal that NL189BLA neurons encoding self-paced behavioral arrests are recruited in an experience-dependent manner during exploration and tend to fire in familiar locations where animals arrested previously.
Amygdala Silencing Impairs Experience-Dependent Arrests during Exploration
To investigate the necessity of NL189BLA neurons for animals to arrest in locations that they visited and where they arrested before, we performed location-specific optogenetic silencing using Jaws in the emergence exploratory paradigm (Figure 5; STAR Methods). We observed that animals expressing Jaws in the BLA arrested less than the controls after crossing the ROIon (Figure 5C). Importantly, this was not because they avoided the area because the daily number of entrances to this ROI did not differ between the two experimental groups (Figure 5D). To understand whether this effect was spatially restricted to amygdala silencing, we measured cumulative arrests based on the spatial experience from crossing the ROIon and ROIoff (number of visits to ROIs; Figure 5E; Figure S6). In accordance with the previous results, cumulative arrests increased based on spatial experience (Figure 5E). However, cumulative arrests were decreased drastically at the entrance to the ROIon in the Jaws versus control group but remained unaffected in the ROIoff (Figure 5E; Figure S6). During a probe test (P) administered on day 6, where no light was turned on in the ROIon, we observed that the arrest count was initially higher in the control compared with the Jaws group but normalized after 25 visits (Figures S6P and S6Q). No significant difference was observed for the entrance to ROIoff between Jaws and control mice (Figure S6L). Furthermore, we calculated the preference ratio between the entrances to ROIon and ROIoff and found no significant difference between the control and Jaws group during the probe test in day 6 (Figure 5F), again suggesting that the manipulation that affected exploratory arrests based on spatial/behavioral experience did not affect the value of the ROI (Namburi et al., 2015; Beyeler, et al., 2016; Kim et al., 2016; Beyeler et al., 2018). No place avoidance or preference was induced by silencing NL189BLA neurons during exploration or performing a classical real-time preference assay (Figure S6H–S6S; Jain et al., 2012). Importantly, although the preference ratio for the duration and number of visits to the ROIon versus ROIoff did not differ between the Jaws and control groups in the place preference chamber, we observed a clear increase in speed at ROIon entrance during the conditioning day upon silencing of NL189BLA neurons (red LEDon at entrance of ROIon; Figure S6T), in line with our previous results.
Figure 5. Amygdala Silencing Impairs the Emergence of Experience-Dependent Exploratory Arrests.
(A) Location-based closed+--loop optogenetic silencing behavioral protocol at the entrance of ROIon. Upon entrance to ROIon, the LED is triggered for 2 s of 630-nm light delivery, followed by 4 s of refractory time.
(B) Schematic of ROI locations. Shown is the optogenetic protocol executed for the days of acquisition (days 1–5, inhibition in ROIon) and during the P (day 6, no inhibition). Control ROIoff is used to monitor the avoidance or preference to the ROIon.
(C) Cumulative arrests count from days 1–5 for the control (black; n = 7) and Jaws (red; n = 6) groups, followed by a probe test (P; no light) on day 6. *p < 0.05 and **p < 0.01 by unpaired t test between the two groups for all 5 days. Running a two-way ANOVA with repeated measures, we found an effect of time (for each time bin in the control but not in the Jaws group; F(5,50) = 2.811; p < 0.0001) and an interaction between time and treatment group (Jaws versus control) (interaction; F(5,50) = 7.644; p = 0.0259).
(D) Number of entrances to ROIon from days 1–5 followed by the probe test (P).
(E) Cumulative arrest number versus number of visits to ROIon during days 1–5.
(F) Preference ratio between ROIon and ROIoff of the number of entrances on day 6. ns, p > 0.05 by unpaired t test.
Data are presented as mean ± SEM.
The emergence assay helped us to clarify that location-specific silencing of NL189BLA neurons decreases the accumulation of arrest in a specific area. Because location-locked optogenetic silencing might have a general effect in the development of arrest preference, we also calculated the cumulative count of arrests in the entire big arena as well as during exploration of the big arena when the light was off. We found no difference in the number of arrests between Jaws and control animals (Figure S6O). Additionally, we performed an experiment to inhibit NL189BLA neurons in random locations and found no changes in cumulative arrests in both groups (Figure S6U).
Combined, these results show that NL189BLA neurons are fundamental for development of experience-dependent arrests in defined familiar places where animals have arrested before. On the other hand, this neuronal population does not affect place preference or have an effect on prolonged behavioral states.
CEA-Projecting NL189BLA Neurons Facilitate Momentary Arrests
Classical and recent studies demonstrated that electrical and optogenetic stimulation of the main output structure of the amygdala and CEA as well as direct optogenetic stimulation of different neuronal subtypes in this brain area induces unconditional immobility and a decrease in track length (Ciocchi et al., 2010; Li et al., 2013; Botta et al., 2015). Based on this, we investigated whether the specific projection of NL189BLA neurons to the CEA (CEA-projecting NL189BLA neurons) could encode and directly elicit behavioral arrests. We first confirmed that the fiber density of NL189BLA neurons was higher in the CEA than in other output structures, such as the bed nucleus of the stria terminalis (BST) and striatum (Str; Figures S7A–S7D). Using optogenetic stimulation in slice recordings, we found robust excitatory efferents from the BLA to different CEA neuronal populations that can be likely candidates to participate in triggering arrests (Figures S7E–S7M; STAR Methods; Roseberry et al., 2016, 2019; Roseberry and Kreitzer, 2017; Caggiano et al., 2018).
To investigate whether CEA-projecting NL189BLA neurons (NL189BLA-CEA neurons) are active during momentary arrests, we performed single-cell calcium imaging in seven freely moving animals during the exploratory emergence paradigm (n = 108 neurons; Figures 6A–6C; Figures S8A–S8D). After aligning the activity of these neurons with arrest onset (Figure 6B), we found that the majority of NL189BLA-CEA neurons were active during spontaneous arrests (fraction active = 0.713 ± 0.1; Figure 6C). Importantly, we found virtually no neurons with decreased activity at arrest onset, indicating that NL189BLA-CEA neurons are active during momentary arrests. In addition, bulk calcium imaging revealed that the overall activity of NL189BLA-CEA neurons transiently rises during arrest compared with movement onset (peak activity during arrest = 1.46 ± 0.27; peak activity during movement = 0.07 ± 0.32; *p = 0.0247 by paired two-tailed t test; n = 4 mice; Figures S8A–S8D). Importantly, bulk imaging of BLA neurons projecting to the dorsal Str (BLA-Str), an important region involved in voluntary movements, did not show a significant change in bulk fluorescence during arrest onset (Figure S8E). These experiments demonstrate that NL189BLA-CEA neurons are specifically active during momentary arrest onset.
Figure 6. NL189BLA Neurons Encode Self-Paced Arrest.
(A) Top left: injection of a retroconditional AAV virus expressing a floxed version of GCamp6f and Tdtomato. Bottom left: lens implantation scheme. Top right: CNMF-E-processed video of the neurons located in the BLA, with normalized neuronal calcium activity by the max. Bottom right: representative anatomical location of CEA-projecting NL189BLA neurons (BLA-CEA neurons) and lens implant.
(B) Calcium activity (Z-score) of all NL189BLA-CEA neurons imaged from one representative animal before and after arrest onset. A purple circle shows the arrest onset time (0 s).
(C) Sorted BLA-CEA neurons during arrest onset (+, active during arrest; −, inhibited during arrest).
Data are presented as mean ± SEM.
CEA has been implicated in freezing (Ehrlich et al., 2009; Ciocchi et al., 2010). Therefore, we tested whether the NL189BLA-to-CEA neurons active before momentary arrests during exploration were the same as the ones active during freezing by performing single-cell calcium imaging in freely moving animals during exploration in the emergence assay and fear conditioning in the same animals (STAR Methods; Figures S8F–S8J). One day after fear conditioning, we were able to reliably elicit freezing bouts with 30-s CS+ (n = 7; Figure S8G). To allow the same statistical power, we sorted arrest and freezing neurons using the same number of arrest and freezing bout onsets during exploration and fear tests. Less than 50% of the NL189BLA-CEA neurons were active at the onset of freezing bouts, and about 30% were negatively modulated by freezing (Figure S8I), contrasting the results obtained during exploratory arrests, where the majority of BLA-CEA neurons were positively modulated and there were virtually no negatively modulated neurons (Figure 6C). NL189BLA-CEA neurons that increased activity during arrest did not correspond to neurons showing increased activity during freezing (Figure S8J). The Parrest NL189BLA-CEA neurons being activated during freezing, inhibited during freezing, or not modulated by freezing was similar; the overlap between these populations was no different than expected by chance (Figure S8J; p > 0.05 by Monte Carlo simulations; 10,000 samples were used to generate a distribution of the number of overlapping neurons for arrest and freezing, assuming random assignment).
Next we targeted NL189BLA neurons projecting to the CEA during speed- and acceleration-locked closed-loop optogenetic stimulation (Figure 7A). Similar to the optogenetic stimulation of the entire population of NL189BLA neurons, CEA-projecting NL189BLA neurons induced a transient decrease in speed in the ChR group (Figures 7B–7D), whereas their transient inhibition facilitated movement (Jaws versus control group; Figures S8R–S8T). This change in movement speed was not observed in the control group (Figures 6F and 6G) or by stimulating neurons projecting to the Str (Figures S8K, S8L, S8R, and S8T).
Figure 7. Moment-to-Moment Arrest Is Mediated by CEA-Projecting NL189BLA Neurons.
(A) Left: injection of a retroconditional AAV virus expressing a floxed version of ChR and YFP. Center: fiber implantation scheme. Right: anatomical location of CEA-projecting NL189BLA neurons and optical fiber implant.
(B) Two-speed color-coded trajectories in on (left) and off (right) closed-loop trials. The entire stimulation duration (5×) is denoted by blue circles (centroid of the animal during stimulation with the 5× protocol). Scale bar, 2.5 cm.
(C) Speed during the closed-loop stimulation protocol upon crossing the acceleration threshold for the ChR (left; n = 7) and control (right; n = 6) groups during light-on and -off trials.
(D) Change in speed between the ChR (light blue) and control (dark) groups in triggering trials.
(E) Closed-loop location-locked optogenetic inhibition behavioral protocol in acquisition (days 1–5, inhibition in ROIon) and during the P (day 6, no inhibition).
(F) Cumulative arrest count from days 1–5 for the control (black; n = 6) and Jaws (red; n = 9) groups, followed by day 6 (probe test [P]). ***p < 0.001 by unpaired t test between the two groups for all 5 days. Two-way ANOVA with repeated measures: time (F(4,48) = 12.09; p < 0.0001), interaction (F(4,48) = 3.333; p < 0.0173).
(G) Number of entrances to ROIon from days 1–5, followed by the probe test (P).
(H) Preference ratio between the entrances to ROIon and ROIoff on day 6. ns, p > 0.05 by unpaired t test.
Data are presented as mean ± SEM.
We also assessed whether location-locked closed-loop optogenetic stimulation of NL189BLA-CEA neurons was able to affect the development of experience-dependent exploratory arrests. We found that the number of arrests increased during the acquisition days in the ChR versus the control group (Figures S8M–S8Q). Again, this manipulation did not change the number of entrances to ROIon (Figure S8P). In addition, no differences were observed in cumulative arrests, entrances to the ROIoff previously ROIon and preference ratio between ChR and control animals during the probe test (probe, P) (Figures S8O–S8Q). Additionally, location-locked closed-loop silencing of NL189BLA-CEA neurons significantly decreased cumulative arrests during acquisition without impairing the number of visits to the ROIon (Figures 7E–7G) and the preference ratio between ROIon and ROIoff on the probe test (Figure 7H). Our findings demonstrate that CEA-projecting NL189BLA neurons are essential for triggering self-paced behavioral arrests independent of long-term effects on locomotion or place preference.
DISCUSSION
The results presented here show that a genetically and projection-defined amygdala neuronal ensemble in the BLA mediates momentary arrests during exploration that emerge in an experience-dependent manner when animals familiarize themselves with a novel environment. A combination of behavioral analyses, calcium imaging, and state-locked closed-loop optogenetic manipulations revealed that NL189BLA neurons control movement speed and momentary arrests via their projection to the CEA. The recruitment of this neuronal population gradually increased in an experience-dependent manner as exploration progressed, primarily in specific areas that animals had visited multiple times and where they had arrested before. Transient loss of function of NL189BLA neurons led to suppression of momentary arrests, highlighting the fundamental role of this population in experience-dependent arrest. Importantly, these arrests were not associated with valence or anxiety/fear-related behaviors. These results unveil a dual role of the amygdala as a novelty/familiarity detector and as an effector circuit with the ability to drive or suppress spontaneous movements based on spatial experience during exploratory behavior.
Exploration is required to gain knowledge about a new environment. Unrestrained spatial exploratory behavior can be structured into defined trips toward the unknown (Fonio et al., 2009). Our behavioral findings show that animals choose to momentarily arrest in specific locations with which they became familiar and where they arrested previously after exploration. This was confirmed by the fact that spatial experience-dependent accumulation of behavioral arrests is influenced by the dimension of the environment (faster in the small versus the big arena; Figure S5) and impaired in the absence of visual inputs (dark versus light condition; Figure S5). These findings are consistent with the novelty management model (Gordon et al., 2014). Momentary arrests in familiar places can potentially be decision points underlying a vicarious trial and error behavior (Redish, 2016; Thompson et al., 2018), given that animals initiate and terminate exploratory trips from these locations and changed head and trajectory orientation after the arrest (Figure S1). In agreement with this hypothesis, it has been shown that a variety of hippocampal neurons encode past, current space, and upcoming spatial trajectory sequences during periods of immobility in an open field (Kay et al., 2016; Redish, 2016).
One of the challenges in understanding neural implementation of experience-dependent behavioral exploration was to identify neuronal circuits that could simultaneously participate in processing of novelty/familiarity inputs and also shape movement dynamics. Although it has been hypothesized that the hippocampal formation and other brain regions comprising the medial temporal lobe are fundamental in familiarity recognition (Yonelinas, 2002; Eichenbaum et al., 2007, 2010; Squire et al., 2007; Sauvage, 2010), they do not seem to be involved in direct modulation of movements. On the other hand, other independent studies have shown that the amygdala is directly involved in contextual learning, novelty, and familiarity detection but also that it is tightly connected to brain areas involved in defensive freezing responses (Yim and Mogenson, 1989; Wilson and Rolls, 1993; Schwartz et al., 2003; Ciocchi et al., 2010; Farovik et al., 2011; Freeze et al., 2013; Roseberry et al., 2016; Tovote et al., 2016; Roseberry and Kreitzer, 2017). Our study provides extensive evidence that a BLA neuronal population can operate as a neuronal detector-effector circuit, able to integrate experience-dependent contextual information and mediate behavioral arrests.
Inputs into the BLA carrying spatial or spatial familiarity signals could potentially originate from the medial temporal lobe, such as the hippocampus and perirhinal cortex (Fortin et al., 2004; Eichenbaum et al., 2007; Squire et al., 2007; Farovik et al., 2011; Tomás Pereira et al., 2016), which are known to send robust efferents to the BLA. The hippocampus and perirhinal cortex could contribute to exploratory behavior, allowing animals to acquire information about novel surroundings based on self-motion (Fortin et al., 2004; Hines and Whishaw, 2005). BLA neurons may integrate inputs encoding spatial familiarity to guide behavior and even link spatial experience and the appropriate behavioral output via plasticity; for example, via spike-timing long-term potentiation (Jung et al., 2010). The latter could explain why we observed a delayed location-specific arrest formation during probe test in day 6, after NL189BLA neurons were inhibited for 5 days upon entrance to the area (Figure S6). Our data demonstrating that long-term recruitment of an arrest-encoding BLA circuit originated in familiar locations where animals had arrested before suggest that recognition of spatial familiarity levels can be integrated and processed in the amygdala and used to guide a specific type of exploratory behavior: momentary arrests. These data could also provide a framework to understand the neuronal mechanisms underlying the process of spatial latent learning, through which spatial learning occurs with no apparent reinforcers (e.g., food pellets) or associations (e.g., acoustic cues) (Tolman, 1948).
Although it has been found that stimulation of CEA-projecting BLA neurons induces appetitive and defensive behaviors (Beyeler et al., 2016; Kim et al., 2016), we did not observe that manipulations of the NL189BLA neuronal ensemble caused short- or long-term avoidance or preference behavior using two types of behavioral assays. This apparent discrepancy may be caused by the genetic background of the NL189BLA neuronal population that differs from previous studies. Alternatively or in combination, our specific state-locked optogenetic manipulation differs from the common optogenetic stimulation adopted previously (Beyeler et al., 2016; Kim et al., 2017). However, when silencing NL189BLA neurons during a classical real-time place preference paradigm, we only observed an increase in speed with entrance to the ROIon rather than occurrence of short- or long-term avoidance or preference. Overall, these data suggest that NL189BLA neurons are not involved in signaling spatial avoidance or preference in an exploratory state.
The implication of the amygdala in mediating experience-dependent behavioral arrests may seem improbable given the attention it has received in relation to positive/negative valence and anxiety/fear-related behaviors. However, previous independent studies demonstrate that pharmacological administration of N-methyl-d-aspartic acid (NMDA; an NMDA receptor agonist) to the BLA triggers stopping behavior in a dose-dependent manner, whereas its lesioning decreases immobility (Jellestad and Bakke, 1985; Yim and Mogenson, 1989). Additionally, the amygdala has been revealed to participate in execution of ongoing actions in humans (Sagaspe et al., 2011). CEA-projecting NL189BLA neurons were found to be active during spontaneous arrest but not during movement onset, and their efferents to the CEA (but not to the dorsal Str) are responsible for the decrease in movement speed and triggering behavioral arrests (Figure 7; Figure S8). In addition, our data show that arrest-related NL189BLA neurons projecting to the CEA only partially overlap with neurons active or inhibited during freezing. Our results and other groups have found that BLA principal neurons form direct excitatory projections onto a variety of CEA neurons (Samson and Paré, 2005; Ciocchi et al., 2010; Li et al., 2013; Tovote et al., 2016; Figure S8). Fine-tuning of the direct BLA glutamatergic inputs onto defined CEA neurons could elicit arresting behavior via a feedforward inhibition to midbrain structures known to modulate movement, such as ventrolateral periaqueductal gray matter (vlPAG; Tovote et al., 2016) as well as the mesencephalic locomotor region (MLR) (Roseberry et al., 2016, 2019; Bouvier et al., 2015; Caggiano et al., 2018). Our connectivity studies reveal that NL189BLA neurons have a strong bias to contact MLR-projecting CEA neurons compared with those projecting to the vlPAG (Figure S8). This raises the interesting hypothesis of a differential role of parallel circuits for distinct types of behavioral arrest targeted by different contextual inputs. For example, although the vlPAG would be recruited by CEA inputs involved in generation of strong defensive and learned freezing responses (Tovote et al., 2016), the connection between BLA and MLR-projecting CEA neurons could control momentary arrests during exploration, unrelated to fear (Roseberry et al., 2019).
Our imaging data show that a large proportion of BLA-CEA neurons active during momentary arrests do not correspond to those active during freezing. Therefore, our results suggest that the output circuits mediating momentary arrests and freezing are different because (1) the arrests are momentary and not prolonged (Figure S5), (2) they occur spontaneously in familiar places and are not associated with negative valence (Figures 2, 3, 4, 5, and 6; Figures S5, S6), (3) they are associated with a change in heading and motion direction (Figure S1), (4) activation of NL189BLA neurons occurs at the onset of arresting but does not influence the duration of arrest (Figures 3H–3J), (5) even prolonged stimulation of NL189BLA neurons fails to produce long-lasting immobility or freezing—they always lead to a momentary arrest of approximately the same duration (Figure 2N), and (6) a large neuronal population of NL189BLA neurons projecting to the CEA was found to be active only during arrest and not active during freezing (not modulated or even inhibited during freezing; Figure 6; Figure S8).
On the other hand, it is clear that the contextual input to this circuit (i.e., the input to NL189BLA neurons that makes them increase activity during an arrest in familiar places) is related to spatial experience and not to threatening situations. NL189BLA neurons are not as active when animals arrest in areas they never visited before (Figures 4K–4O) or in areas to which they had been strongly habituated (Figure S5N). This activity seems to reflect a mechanism underlying detection of familiarity based on visual input. Furthermore, our data reveal that many of these neurons are not activated during the presentation of CSs that predict shocks and lead to freezing.
In summary, the data presented here suggest that a BLA-CEA axis acts as novelty/familiarity detector-effector circuitry for generating self-paced behavioral arrests based on the familiarity of a spatial location. They also reveal circuits mediating a latent learning strategy fundamental to performing efficient and safe exploration of novel surroundings by which animals move through space to acquire novel information at the interface between the familiar and the unknown.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for material and codes should be directed to and will be fulfilled by the Lead Contact, Rui M. Costa (rc3031@columbia.edu).
Materials Availability
Further information and requests for reagents and mice should be directed to and will be fulfilled by the Lead Contact and it is found in the Key Resources Table. This study did not generate new unique reagents or mice.
KEY RESOURCE TABLE
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| GFP Tag polyclonal 488 | Molecular probes | A-21311 |
| DAPI | Sigma | D9542 |
| Virus strains | ||
| AAV5-CAG-Flex-GCaMP6f-WPRE.SV40 | Chen et al., 2013 | 100835-AAV5 (Addgene) |
| AAV1-CAG-Flex-Jaws-KGC-Tdtomato-ER2 | Chuong et al., 2014 | 84446 (Addgene) |
| AAV1-CAG-FLEX-Tdtomato | Oh et al., 2014 | 51503-AAV1 (Addgene) |
| AAV1-EF1a-DIO-YFP | Deisseroth lab | 27056-AAV1 (Addgene) |
| AAV1-EF1a-DIO-hChR2(H134R)-eYFP-WRE-hGH | Deisseroth lab | 20298-AAV1 (Addgene) |
| AAV1-EF1a-DIO-hChR2(H134R)-mCherry-WPRE-HGHpA | Deisseroth lab | 20297-AAV1 (Addgene) |
| AAV-EF1a-DIO-mCherry-WPRE-HGHpA | Deisseroth lab | 20299-AAVrg (Addgene) |
| AAV-EF1a-DIO-hChR2(H134R)-mCherry-WPRE-HGHpA | Deisseroth lab | 20297-AAVrg (Addgene) |
| AAV pCAG-FLEX-tdTomato-WPREv | Deisseroth lab | 51503-AAVrg (Addgene) |
| rAAV2-CamKII-eYFP | UNC | N/A |
| retroAAV-CAG-Tdtomato | Boyden Lab | 59462-AAVrg |
| Experimental model | ||
| Tg(Arhgef6-cre)NL189Gsat/Mmucd | UCD | 034805-UCD |
| Software and algorithms | ||
| MATLAB | The MathWorks | https://www.mathworks.com/ |
| Fiji (ImageJ) | NIH | https://fiji.sc/ |
| Ilastik 1.3.2 | Sommer et al., 2011) | https://www.ilastik.org/ |
| Elastix | Elastix for image registration | https://elastix.lumc.nl/ |
| Imaris for 3D visualization and rendering | Oxford Instruments | https://imaris.oxinst.com/ |
| R for statistical computing | R project | https://www.r-project.org/ |
| BrainJ for image processing, registration, and analysis | This manuscript | https://www.cellularimaging.org/ |
| NIS-Elements for image acquisition | Nikon Instruments | https://www.microscope.healthcare.nikon.com/products/software/nis-elements |
| Illustrator | Adobe | https://www.adobe.com/products/illustrator.html |
| Constrained Non-negative Matrix Factorization (CNMF) | Pnevmatikakis et al., 2016 | N/A |
| Mosaic version 1.1.2.0 | Inscopix | https://www.inscopix.com |
| Mosaic version 1.2.0 | Inscopix | https://www.inscopix.com |
| Bonsai (x64) | Bonsai | https://bonsai-rx.org/ |
| Lowess algorithm | (Drai et al., 2000) | N/A |
| ROC analysis | (Cohen et al., 2012) | N/A |
| Clampex | Clampfit 10 | Molecular Devices | https://www.moleculardevices.com/ |
| GraphPad Prism 6 | GraphPad Prism | https://www.graphpad.com:443/ |
| Pipeline for image processing | Cellular Imaging, Zuckerman Institute | https://github.com/lahammond/BrainJ |
Data and Code Availability
The pipeline for image processing of fixed brain slice were processed using can be found here: https://github.com/lahammond/BrainJ. Further information and requests should be directed to and will be fulfilled by the Lead Contact and it is found in the Key Resources Table.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All experimental protocols were approved by the Columbia University Institutional Animal Care and Use Committee. All experimental animals were 3 to 5 month-old male and female mice individually housed on a 12 hr light/dark cycle with ad libitum access to food and water. BAC Transgenic mice expressing Cre recombinase under the control of Arhgef6 (Tg(Arhgef6-cre)NL189Gsat/Mmucd, 034805-UCD) were used. The line has been backcrossed onto C57Bl6/J mice for at least 8 generations. Sample size is detailed in the Results or figure legends.
METHOD DETAILS
Viral injection
Surgeries were performed under sterile conditions and isoflurane (1%–5%, plus oxygen at 1–1.5 l/min) anesthesia on a motorized stereotactic frame (David Kopf Instruments, Model 900SD). Throughout each surgery, mouse body temperature was maintained at 37◦C using an animal temperature controller (ATC2000, World Precision Instruments) and afterward, each mouse was allowed to recover from the anesthesia in its homecage on a heating pad. Before starting the surgery animals were subcutaneously injected with Buprenorphine SR (0.5 – 1 mg/Kg). The mouse head was shaved, cleaned with 70% alcohol and iodine, an intradermic injection of bupivacaine was administered and the skin on the skull was removed to allow for aligning of the head, drilling the hole for the injection site and performing the implantation. For the calcium imaging, each animal was unilaterally injected with 500 nL of AAV5-CAG-Flex-GCaMP6f-WPRE.SV40 (titer: 7×1012 vg/mL; Addgene) into the left hemisphere of basolateral amygdala (AP: 1.2 mm, ML: 3.4 mm, DV: 4.2 mm) using a Nanojet III Injector (Drummond Scientific, USA) at a pulse rate of 2.6 nL per second, 9.9 nL per pulse every 5 s. To avoid the 500 nL of volume being delivered only in one location, potentially causing tissue damage, all basolateral amygdala injections were performed moving the tip of the pipette 50 μm deeper than the DV coordinate and injecting a volume of about 100nL every 10 μm. After each 10um, the pipette was slowly retracted until reaching the DV coordinate. The injection pipette was left in place for 10 min post-injection before it was slowly removed (rate 200 μm/s).
For calcium imaging combined with optogenetic inhibition, the virus AAV5-CAG-Flex-GCaMP6f-WPRE.SV40 was simultaneously injected with AAV-CAG-Flex-Jaws-KGC-tdTomato-ER2 (titer: 1.2×1013 vg/mL; Addgene; ratio 1:1). For anatomical studies to assess CamKII and cre co-expression, each animal was unilaterally injected with 500nL rAAV2-CamKII-eYFP (titer: 4×1012 vg/mL; UNC) and AAV1-CAG-FLEX-tdTomato (titer: 7.8×1012 vg/mL; Addgene) as described above (ratio 1:1; 4×1012 vg/mL;). For patch-clamp experiments to characterize the input-output curve, basolateral amygdala was bilaterally injected using AAV1-CAG-FLEX-tdTomato. For connectivity patch-clamp studies, BLA was injected with AAV1-EF1a-DIO-hChR2-YFP (titer: 2.3×1013 vg/mL; Addgene) to express ChR. To target vlPAG-, MLR-projecting CEA neurons and Mc-projecting vlPAG neurons we used a retro AAV-CAG-TdTomato (titer: 1.01×1013 vg/mL; Addgene) injected either vlPAG (AP: lamda, ML: 0.6 mm, DV: 2.35 mm), MLR (AP: 4.6 mm, ML: 1.2 mm, DV: 3.6 mm) or Mc (AP: 6.4 mm, ML: 0.95 mm, DV: 5.6 mm). For anatomical experiments in central amygdala, each animal was unilaterally injected (AP: 1.1 mm, ML: 2.5 mm, DV: 4.2 mm) into the left hemisphere using 100nL of a retro AAV virus AAV1-EF1a-DIO-YFP (titer: 3.5×1012 vg/mL; Addgene) at a rate of 2.6nL per second, 4.6nL per pulse every 10 s. For optogenetic experiments, bilateral injections into basolateral amygdala were performed using 500nL conditional AAV viruses AAV1-EF1a-DIO-hChR2-YFP (titer: 2.3×1013 vg/mL; Addgene; ChR group), AAV1-EF1a-DIO-YFP (titer: 3.5×1012 vg/mL; Addgene; Control group), AAV-CAG-Flex-Jaws-KGC-tdTomato-ER2 (titer: 7.8×1012 vg/mL; Jaws group), AAV1-CAG-Flex-tdTomato (titer: 5.9×1013 vg/mL; Addgene; Control group). Using retrograde AAV viruses for the optogenetic experiments to specifically modulate the activity of central-projecting basolateral amygdala neurons, the bilateral injection in central amygdala of AAV-EF1a-DIO-hChR2(H134R)-mCherry-WPRE-HGHpA (titer: 1×1013 vg/mL; Addgene; ChR group), AAV-EF1a-DIO-mCherry-WPRE-HGHpA (titer: 6.2×1012 vg/mL; Addgene; Control group for ChR), pAAV-CAG-FLEX-Jaws-KGC-GFP-ER2 (titer: 1.3×1013 vg/mL; Addgene; Jaws group), AAV pCAG-FLEX-mCherry-WPREv (titer: 6.2×1012 vg/mL; Addgene; Jaws-Control group) were performed using the volume of 100nL as stated above. For imaging experiments of NL189BLA-CEA, we injected a retrograde AAV-EF1a-DIO-GCaMP6f-P2A-nls-dTomato (titer: 8×1012 vg/mL; Addgene 51083 - AAVrg; 200nl; Figures 6A–6C) in CEA. For all the anatomical and patch-clamp experiments, after the injection, the skull was cleaned with saline and the skin sealed with Vetbond tissue adhesive (3M, USA) and stitches (MYCO Medical, USA).
Chronic lens and optogenetic implantation
Following the same surgical procedures, about two weeks after viral injection, a 500-nm-diameter gradient index (GRIN) lens (1050–002183, length ~8.4, NA 0.5, pitch 2; Inscopix, Palo Alto, CA, USA) was implanted in the left mouse amygdala above the injection site (AP: 1.2 mm, ML: 3.4 mm, DV: 4.2 mm). Once in place, the lens was secured to the skull using a combination of black Ortho-Jet powder and liquid acrylic resin site (Lang Dental, USA) as well as super glue (Loctite, LOC1255800). Care was taken to minimize scratches, moreover, the lens was covered with paper/tape to protect its surface. Three weeks after the GRIN lens implantation, the microendoscope baseplate (Inscopix) was mounted onto the head of the mouse under visual guidance using the attached microscope to determine the best field of view. The imaging field of view was inspected and allowed to clear for at least five days prior to imaging and behavioral experiments. For optical stimuli delivery, fiber optics (200 μm diameter, NA = 0.66) were implanted 200 um above the site of injection using super glue (Loctite, LOC1255800) and cement (as described above; Methods S1). For the NL189BLA-CEA imaging, GRIN lenses of 0.6mm-diameter secured in BLA were placed using slightly different coordinates (AP: −1.50mm; ML: −2.90 ~−3.00mm (left side); DV: −4.20mm).
Calcium imaging in freely-moving animals
Mice were briefly anaesthetized using a mixture of isoflurane and oxygen and the mini-epifluorescence microscope was attached to the baseplate. A period of 20–30 min was allowed to recover in the home cage before experiments started. Fluorescence images were acquired at 20 Hz and the LED power was set at 10%–20% (0.1–0.2 mW) with a gain of 4 [excitation: blue light-emitting diode (LED); excitation filter: 475/10 nm, 0.24–0.6 mW/mm2; emission filter: 535/50 nm; Inscopix, Palo Alto, CA]. Image acquisition parameters were set to the same values between sessions to be able to compare the activity recorded. Seven GCaMP6f-expressing NL189-Cre mice were imaged during the emergence exploratory paradigm and fear test paradigm. The procedure and optical parameters were the same also for the nVoke mini-endoscope (Inscopix) with the only exception that the animal was head-fixed to perform the optogenetic inhibition in basolateral amygdala with Jaws delivering 120 pulses every 10 s of 630nm light.
Optogenetic manipulation
The power from the optical fibers was 6–7 mW for the 465nm blue light for ChR and 3–4mW for the 630nm red light for Jaws. Before starting the series of behavioral experiments, optogenetic manipulation was tested using slice electrophysiology for ChR and Jaws as well in vivo for the case of Jaws. Regarding the Jaws experiments during exploration, we used 6 Jaws animals and 7 Control (3 out of 7 Control animals did not express the fluorophore). Regarding the experiments using the conditional AAV virus expressing ChR2 in BLA, all controls expressed the fluorophore YFP. Regarding the six Control animals in retro-ChR experiments, four expressed the fluorophore mCherry in BLA. All Control animals of retro-Jaws experiments expressed the fluorophore mCherry in BLA. All the experimental groups bilaterally expressed either Jaws or ChR (Methods S1).
Histology
After completion of the behavioral experiments, mice were transcardially perfused with saline and 4% paraformaldehyde in PBS. Brains were removed for histological analysis and coronal slices were sectioned at 50 μm (Leica vibratome VT1000). For calcium imaging, immunohistochemistry was performed for GCaMP6 expression by incubating the sections with a GFP antibody (GFP Tag polyclonal antibody, Alexa Fluor 488 conjugate, Molecular Probes #A-21311) diluted at 1:1000 in 0.4% Triton-PBS overnight at room temperature. DAPI was used as counterstaining for all the experiments.
Imaging
Automated high-throughput imaging of tissue sections was performed on a custom-built automated slide scanner using a AZ100 microscope equipped with a 4× 0.4NA Plan Apo objective (Nikon Instruments Inc) and P200 slide loader (Prior Scientific), controlled by NIS-Elements using custom acquisition scripts (Nikon Instruments Inc.). This approach provided images with a lateral resolution of 1mm/pixel.
Image processing and analysis
Images of tissue sections were processed and reconstructed in ImageJ/Fiji (Schindelin et al., 2012) using BrainJ, a collection of custom tools developed to facilitate automated whole brain analysis of tissue sections in a manner similar to serial two photon tomography (Kim et al., 2017) and light sheet microscopy (Renier et al., 2016). Multi-channel tissue section images are first arranged in an anterior to posterior order and processed to remove external fluorescence and neighboring objects and/or tissue sections. Sub-sequently, sections are centered and oriented to facilitate a 2D rigid body registration (Thévenaz et al., 1998), which ultimately yields a 3D brain volume. In order to detect cell bodies and projections (axons/dendrites), a machine learning based pixel classification approach was employed using Ilastik (Sommer et al., 2011) on images that had background subtracted via a rolling ball filter. Several images for each brain were pooled and used for training on cell bodies, projections, and background pixels. The resulting probability images were further processed in ImageJ for segmentation and analysis of cells and projections.
To analyze the resulting data within the context of the Allen Brain Atlas Common Coordinate Framework (CCF), 3D image registration was performed as previously described (Ragan et al., 2012) using Elastix (Klein et al., 2010). In this case, DAPI labeling of cell nuclei was used to register the brains to the reference brain using a 3D affine transformation with 4 resolution levels, followed by a 3D B-spline transformation with 3 resolution levels, using Mattes Mutual information to calculate similarity. Following registration, coordinates of detected cells, along with raw image data and segmented projection datasets were transformed into the CCF, allowing analysis of brain region based cell densities, measurements of intensity, and projection densities. Cell plots, density heatmaps, overlays of projections, brain region specific extraction, and subsequent outputs and visualizations were generated using BrainJ and Imaris (Oxford Instruments). The pipeline can be found here: https://github.com/lahammond/BrainJ.
Slice electrophysiology
Standard procedures were used to prepare 300-μM-thick coronal slices from 12- to 20-week-old NL189–cre and NL189 x Ai9 mice. Briefly, the brain was dissected in high-magnesium (10 mM) ice-cold artificial cerebrospinal fluid (ACSF), mounted on a plate and sliced with a vibrating-blade microtome (V1200S, Leica, USA) at 4°C. Slices were maintained for 45 min at 37°C in an interface chamber containing ACSF equilibrated with 95% O2/5% CO2 and containing the following (in mM): 124 NaCl, 2.7 KCl, 2 CaCl2, 1.3 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 18 glucose, 0.79 ascorbate. After incubation, slices were left for at least 30 min at room temperature. Recordings were performed with ACSF in a recording chamber at a temperature of 35°C at a perfusion rate of 1–2 ml/min. Neurons were visually identified with infrared video microscopy using an upright microscope equipped with a 40 × objective (Olympus, Tokyo, Japan). Patch electrodes (3–5 MΩ) were pulled from borosilicate glass tubing (G150F-3, Warner Instrument). For current clamp experiments, patch electrodes were filled with a solution containing the following (in mM): 123 potassium gluconate, 12 KCl, 10 HEPES, 10 phosphocreatine, 4 Mg-ATP and 0.3 Na-GTP (pH adjusted to 7.25 with KOH, 295 mOsm). Optogenetic stimulations in slice were performed with a wavelength of 630 nm for Jaws or 465 nm for ChR2 using an optical fiber connected to a PlexBright LD-1 Single Channel LED Driver (Plexon, USA) or using the CoolLED system (pE-300white; CoolLED Ltd, UK), respectively. Whole-cell patch-clamp recordings were excluded if the access resistance exceeded 20 MΩ or changed more than 20% during the recordings. Seal resistance, for cell-attached recordings, was around 20 to 50 MΩ and data were excluded if it changed more that 20% from the initial value. Connectivity was assessed by stimulating the axons of BLA for 10ms at 100% power ( ~35mW) with 465nm-light for 10ms. Recordings were in presence of TTX (1 μM) and 4-aminopyridine (4AP, 100 μM). oEPSCs were blocked by further bath-application of the AMPA-receptor antagonist, NBQX (10 μM). Successful connected neurons were defined to have oEPSCs > = 10pA with a success rate larger than 50% for all the stimulation trials. We used a conservative approach to avoid including false positives and ensure enough separation from baseline noise (about 2–5pA). As mentioned in the main text, we have included all the tested connections and found an overall significant difference in optogenetically evoked excitatory postsynaptic current (oEPSC) amplitude between NL189-BLA to vlPAG- and MLR-projecting CEA neurons (Figure S8; p < 0.01 by unpaired t test). Data were recorded with a MultiClamp 700B (Molecular Devices) amplifier and digitized at 10 kHz with a digidata 1550A (Molecular Devices). Lowpass Filter of 0.2 kHz was applied for V-Clamp experiments. Data were acquired and analyzed with Clampex 10.0, Clampfit 10.0 (Molecular Devices) and in-house MATLAB codes. All chemicals for the internal and external solutions were purchased from Fluka/Sigma (Buchs, Switzerland). Glutamatergic blockers were purchased from Tocris Bioscience (Bristol, UK).
Behavioral setup
Exploratory behavioral paradigm
The dimensionality emergence assay consists of two opaque white cylindrical arenas of 20 cm and 100 cm of diameter with walls 30 cm of height. The opacity avoids reflection of light ensuring a better tracking of the animal without background contaminations. The arenas are interconnected with a 6 cm wide opening that can be closed with an automatic gate moving at 90 degrees. The setup is located inside a sound-attenuating box (2 × 2 × 2 m; skeleton in aluminum, Item 24; 25mm 75% Sound-Absorbing foam Sheet, McMaster; built in-house) that also confers the ability to control the general light intensity (~6 Lux) without creating obvious shades inside the open field. The arenas were cleaned with 70% ethanol and an odor killer solution at the end of each session to remove olfactory cues. The locomotion of the animal was monitored with a camera (Grasshopper3, GS3-U3–41C6M, Flycapture) with wide-field objective (MVL8M1 – 8 mm EFL, f/1.4, for 1” C-Mount Format Cameras, THORLABS) mounted about 150cm above the two arenas. Spatial resolution was 1 cm per 5 pixels and temporal resolution was 30 frames per second. The animal’s center coordinates (centroid) were acquired online with the open source visual language BONSAI (https://bonsai-rx.org/). The triggering of closed-loop optogenetic stimulation or inhibition was also performed with BONSAI via a standard Arduino MEGA 2560 board.
Mice were given four weeks to recover from surgery. Afterward they were handled for two consecutive days (five min each day) using a dummy endoscopic that mimics the weight of the miniscope or with optical patch cables to habituate them to the calcium imaging or optogenetic behavioral procedures, respectively.
Calcium imaging.
For the calcium imaging experiments, the emergence assay exploratory procedure was performed in five days. On day 1, the animal was placed in the 20cm-arena for 30 minutes to promote familiarization (7 mice). Afterward, the automatic gate opened giving the animal the possibility to explore the 100cm-arena for 20 minutes. From day 2 to 5, the gate opened after ten minutes as familiarization to this area had already occurred on day 1.
Optogenetic manipulation.
In the emergence assay, the location-specific optogenetic manipulation was made by setting one region of interest (ROI; 20 × 20 cm) in the 100cm-arena. The side of the ROI was counterbalanced between animals and selected to trigger the PlexBright optogenetic stimulation system LED module (Plexon, USA) to illuminate 465 nm blue light or 630 nm red light in basolateral amygdala. For the first five days (acquisition), the position of the ROI was maintained constant to ensure the acquisition of spatial memories. On day 6 (probe test), the entrance to the previous ROIon would not trigger the LED. Inhibition occurred for 2 s upon entrance to the ROI with a refractory time of 4 s in which the optogenetic LED could not be triggered. Regarding location-locked closed-loop optogenetic stimulation, it occurred using five 10ms pulses at 20 Hz followed by a refractory time of 4 s. In the speed-and acceleration-locked closed-loop experiments, optogenetic stimulation (20 Hz, 5 pulses of 10 ms; 250 ms length) or inhibition (2 s constant inhibition) triggered by animals’ acceleration was performed in the 100cm-arena. In the acceleration protocol, to make sure the animal was moving prior optogenetic stimulation, LEDs were triggered with a probability of about 33% only if the animal accelerated for at least 100 ms with a speed higher than 2 pixels per second (0.2 cm/s). The optogenetic manipulations were repeated for 50 trials. There was a refractory time of 20 s in between optogenetic light stimulation or inhibition. Control trials (trial off: ~66%) were triggered by the acceleration but did not induce any light-emission.
Fear conditioning paradigm
Fear conditioning was elicited on day 1 (fear acquisition) in the fear conditioning chamber, a rectangular box with a metallic shock grid floor with the dimensions of 20 × 24 × 14 cm. After a period of 2min baseline, mice were exposed to ten 4kHz constant tones (30 s; ~60dB; CS+) co-terminating with 2 s footshock (0.7mA) with a random interval of 90 and 180 s. Sound of 4kHz was elicited with a sound buzzer WINGONEER 5PCS New Version Passive Buzzer Module for Arduino (about 60dB by iPhone app). Footshock was elicited using a generator MED associates Inc. (ENV-414S). On day 2, after performing calcium imaging in the exploration assay, we moved the mice to the fear test chamber (round and white open field: 38cm of diameter and 30cm of height). To assess fear learning, after a period of 2min baseline, mice were exposed to five CS+ (no shock) with a random inter-tone interval between 90 and 180 s. Tones and footshocks were triggered with an arduino code written by Erica J. Rodriguez from Salzman lab at Columbia University. The two days of fear conditioning paradigm occurred after five days of exploration in the emergence assay combined with calcium imaging. The mini-edondoscope was still mounted between the exploration and fear test chambers to allow the tracking of the same neurons in two different paradigms. The fear chambers for the acquisition and test were placed in a sound-attenuated box that allowed as well the separation between experimenter and mouse. In order to create a unique olfactory context never experienced before, we placed a 50mL beaker inside the sound-attenuated box containing 1% acetic acid and 10% ethanol for the fear acquisition and test, respectively. We cleaned and removed escrements after each animal using 1% acetic acid and 10% ethanol for fear acquisition and test, respectively. The locomotion of the animal was monitored with a camera mounted above the fear acquisition and test chambers. Synchronization between animal’s movements and the onset and offset of the calcium imaging and tone was achieved with two LEDs, completely isolated from the animal’s view but visible with the camera mounted on top. Spatial resolution was 1 cm per 17.2105 pixels and temporal resolution was 30 frames per second.
Real-time place preference paradigm
We allowed animals to explore a rectangular open field (height: 24cm, length: 66cm) and perform an habituation, conditioning and test sessions in three consecutive days for 30min in each session. Only on day 2 (conditioning) the red LED was triggered upon entrance and stay of the mouse in one side area of the arena (28 × 24cm). We randomly counterbalanced the side of light inhibition to avoid innate preference for one side.
QUANTIFICATION AND STATISTICAL ANALYSES
Method to confirm the nature of NL189BLA neurons as principal cells in BLA
We targeted NL189 cre neurons (NL189BLA neurons) with an adeno-associated virus (AAV) expressing tdTomato in a cre-dependent manner to quantify their location in BLA (Figure 1C; Videos S1C and S1D). Anatomical analysis and automated quantification of cell populations reveal that NL189BLA neurons are primarily located in the lateral-posterior part of the basal amygdala and ventral-lateral part of the lateral amygdala (Figure 1C; Figure S2; Video S1D). Double injection of AAV viruses, one expressing tdTomato in a cre dependent manner and one expressing yellow fluorescence protein (YFP) in a CamKII-dependent manner, showed an overlap of ~80% between NL189BLA neurons and CamKII-expressing neurons (Figures S2H and S2I). Using a clustering analysis based on three parameters collected with the input-output function of NL189BLA neurons (first inter-spike interval distribution, spike adaptation and maximum firing rate), we estimated that the majority are regular firing (Figures S2J–S2N). Together these results confirm the nature of NL189BLA neurons as principal cells in BLA (pyramidal; Faber et al., 2001; Likhtik et al., 2006; Sosulina et al., 2006; Fonio et al., 2009; Ehrlich et al., 2012).
Connectivity studies
Using optogenetic stimulation in slice recordings, we tested the direct connectivity between BLA and the CEA neurons located in the medial subdivision (CEm) that project to the ventral-lateral periaqueductal gray matter (vlPAG) (known to be involved in eliciting immobility; Ciocchi et al., 2010; Tovote et al., 2016; Figure S7). Confirming previous results assessed with electrical stimulation of BLA to CEA (Samson and Paré, 2005), we found that 54.5% of vlPAG-CEm neurons (12 out of 22) receive direct glutamatergic inputs from NL189BLA neurons (Figures S7F–S7I). As recent studies show that the mesencephalic locomotor region (MLR), fundamental in locomotor initiation, is targeted by afferents from CEA (Roseberry et al., 2016, 2019; Roseberry and Kreitzer, 2017; Caggiano et al., 2018), we examined whether MLR-projecting CEA neurons can receive glutamatergic inputs from NL189BLA neurons using the same optogenetic approach to target vlPAG-projecting in slices. Not only did we find a direct glutamatergic connection from NL189BLA neurons to MLR-projecting CEA neurons located in CEm, but also identified that this connection was significantly stronger than that of vlPAG-projecting CEA neurons (Figures S7F–S7I). We were able to elicit optogenetically-evoked excitatory postsynaptic currents (oEPSCs) onto MLR-projecting CEA neurons with a success rate of 100% (n = 22; Figure S7F). To avoid any bias in our connectivity analysis (see STAR Methods), we included all the tested connections and found an overall significant difference in oEPSC amplitude between NL189BLA to vlPAG- and MLR-projecting CEA neurons (Figure S7G; p < 0.01 by unpaired t test). The oEPSCs amplitude recorded from this neuronal population was significantly higher than the oEPSCs recorded from vlPAG-projecting neurons (Figure S7G). We also tested whether NL189BLA neurons could directly project to vlPAG, specifically to the neurons known to send their afferents to a pontine motor area (Medulla, Mc) important for freezing behavior (Tovote et al., 2016). As no significant connections were found for all 28 neurons tested (Figure S7G), these results provide further evidence that there is no strong projection between BLA to vlPAG that could explain the arrests (Tovote et al., 2016; Xu et al., 2016). On the other hand, the excitatory efferents from BLA to different CEA neuronal populations were found to be quite robust and be likely candidates to participate in the triggering of arrests (Figure S7; Roseberry et al., 2016, 2019; Roseberry and Kreitzer, 2017; Caggiano et al., 2018).
Testing of inhibitory opsin Jaws
We first tested the photocurrent elicited by light-activation of Jaws compared to eNpHR3.0 using whole-cell somatic patch-clamp recordings in slice (red-light, λ = 630nm; powermax = 3.3mW; Figures 3A and 3B; Figure S4). As we found that Jaws was much stronger in eliciting light-inducing hyperpolarizing photocurrent compared to eNpHR3.0 (Figure S4N), we used this inhibitory opsin to further screen its effect on neuronal excitability (Figures 3A–3C; Figure S4). The firing of NL189BLA neurons elicited by a step-injection of somatic depolarizing current was completely inhibited by a 2 s red-light pulse and immediately restored after the light was switched off (Figures 3A and 3B). Additionally, inhibition caused a shift of the input-output function of NL189BLA neurons toward more hyperpolarized values (Figures S4O–S4R). Jaws-induced NL189BLA neurons inhibition rapidly and reliably blocked single action potentials elicited by 10ms-current step (Figures S4S and S4T). Furthermore, by co-injecting two conditional AAV viruses expressing Jaws and GCamp6f (1:1), we demonstrated the transient silencing of NL189BLA neurons by combining optogenetic inhibition with in vivo calcium imaging of NL189BLA neurons using the newly developed nVoke miniaturized microscope (Owen et al., 2018; Figure 3C).
Arrest detection
Segmentation of the smoothed path into progression segments and lingering episodes was done using the EM algorithm with a two-gaussians mixture model as described (Golani et al., 1993; Drai et al., 2000; Drai and Golani, 2001). Briefly, we estimated the amount of motion within a temporal window of 500 ms and determined a threshold value under which a data point will be counted as arrest. We therefore computed the standard deviation (SD) of the distances between the data points and their mean using a sliding window of 0.5 s. This value was computed assuming the minimum amount of arrest that the animal can do (Drai et al., 2000). The arrests are found by studying the statistical distribution of SD and determining a threshold value. To estimate the distribution of the log max SD values of all the episodes in a session, a density estimator was used. Based on the notion that arrests segments are characterized by low SD and estimated background noise, we used the cut-off of three SDs to have a clear-cut between arrests and movement segments. Latency to arrest was the time of a maximum speed within 1 s before arrest onset relative to time of arrest onset (0 s). For the bulk imaging of BLA-Str neurons, we used one animal to average the calcium response at the onset of 256 arrests.
Freezing bout detection
Segmentation of the smoothed path into progression segments and lingering episodes was done using exactly the same EM algorithm explained above (Arrest detection section). We qualify freezing onset as the start of complete immobility occurring during the 30 s CS+.
Spatial analysis of arrest areas in the big arena
We considered arrests occurring only in the 100cm-arena to study their formation in novel environments. For the mouse’s xy coordinates collected during calcium imaging studies, we combined the position of the animal across five days of exploration. Second, a spatial analysis was used to detect the first arrests occurring in specific region-of-interests (ROIs) of the big arena. Each first arrest was assigned to a defined round ROI (10 pixel radius, 2 cm radius). If arrests occurred within that radius range from the first arrests, they were counted as belonging to the same area. The script detected multiple spatially-segregated arrest areas that we could follow in time with the advantage that entrances, number of arrests and neuronal activity into these areas could be studied. If the entrances occurred less than 3 s apart, they were excluded to avoid a misleading overlap of neuronal activity from two consecutive visits. We excluded first arrests occurring less than 2cm from the border of an adjacent arrest area. Novel entrances were considered from the visit 1 to 5 while familiar entrances from 16 to 20. To calculate angles between past and future trajectories and the gate direction, the 5 most visited arrest areas of each day were analyzed. For each arrest inside those arrest areas the entry and exit direction of the animal’s trajectory was determined using the intersection of the trajectory with a circle of 2cm radius around the arrest position. Angles were calculated between entry and exit, and exit and gate direction for each trajectory. Angle histograms were normalized within animal and day and the normalized counts were averaged across animals and days to generate the polar histograms.
Arrest detection in the small arena
In order to tested whether momentary arrests in the small arena were associated with similar exploratory dynamics. We found that the arrest duration is slightly higher than in the large arena (Figure S1L). In addition, the number of arrests was initially low but increased within a couple of minutes after placing the animal for the first time in the small arena (Figure S5O). This strategy is similar to what was observed in the large arena, but the familiarization occurs much faster, as the space to explore is smaller (cumulative arrests in small versus big arena; Figure S5P). We further analyzed the imaging data of NL189BLA neurons in the small area in two phases: the first four (early phase; arrest 1–4) and the following four arrests (late phase; arrest 5–8) on the first day in the small arena (Figures S5Q–S5T). Similar to what was observed in the large arena, we found that the fraction of arrest neurons and their calcium transient magnitude increased from the early phase compared to the late phase (Figures S5Q–S5T). Similar to what was observed in the large arena, we found that the fraction of arrest neurons and their calcium transient magnitude increased from the early phase compared to the late phase (Figures S5I–S5T; see STAR Methods).
Preference analysis
Place preference was calculated either using the number of entrances or duration to the ROIon (20 × 20 cm square; LED switched on upon entrance during acquisition) versus the ROIoff (20 × 20 cm square; located in the opposite site from the ROIon; the LED was off upon entrance), using the following formula: (ROIon-ROIoff) (ROIon+ROIoff). The same formula was applied for assessing the place preference in the real-time place preference paradigm. To further clarify whether silencing of NL189BLA neurons might cause spatial avoidance or preference, we performed the classical real-time place preference assay where these neurons where silenced when the animal entered a specific half of the chamber (Figure S6S). No place avoidance or preference were induced by silencing NL189BLA neurons during the conditioning day, or in the subsequent test day (day 3; Figure S6S).
Computational analysis of neuronal activity
All calcium movies were initially preprocessed in Mosaic (v.1.1.2, Inscopix) for spatial binning (4 × 4 pixels) and motion correction and subsequently analyzed using MATLAB. One-photon imaging is known to contain significant background signals arising from out-of-focal plane light and neuropil (Pnevmatikakis et al., 2016; Klaus et al., 2017) due to the fluorescence excitation of a relatively large three-dimensional volume compared to, for example, two-photon imaging. A constrained non-negative matrix factorization for endoscopic data (CNMF-E; Pnevmatikakis et al., 2016) was employed to correct somatic calcium transients. It allowed a superior estimation of calcium transients by eliminating the contaminated background fluorescence as a result of the modeling of spatial and temporal background statistics superimposed to the neuronal signals.
Cross-correlation analysis
We calculated the speed of the animal using animal coordinates and converted for time (30Hz) and space resolution (1cm is 5pixels).
In order to understand the relationship between neuronal activity and speed, we computed the cross-correlation using the following MATLAB function as described (Costa et al., 2006) either using the mean neuronal activity from each animal or single cell:
where CC is the cross-correlation of two discrete-time sequences, x and y; lag is a vector with the lags at which the correlations are computed; x is the normalized z-score of the neuronal activity from single neurons or from the entire population; y is the normalized z-score of the animal’s speed; maxlag represents the time-lag range from -maxlag to maxlag (20 s). The cross-correlation function was considered statistically significant when more than nine consecutive bins within the [−5 s, 5 s] interval lay outside the [minimum, maximum] bound of its values at intervals [26 s, 40 s] and [0 s, −14 s].
Activity sorting analysis for arrest and freezing bouts
For the area under the receiver operating characteristic curve (auROC) analysis, we used a method similar to the one described previously (Cohen et al., 2012; da Silva et al., 2018). To produce the ROC curves, we compared the raw calcium activity average during the transition from movement to arrests of each 100-ms bin to the baseline calcium activity (−5 to −1 s before arrest) across trials. The auROC for each bin was calculated using trapezoidal numerical integration. In order to select arrest-responsive neurons, each auROC bin-values were compared to the baseline. Significance was established if at least three consecutive bin-values between the time range 1–2 s exceeded 1.5 SD (arrest active neurons) or were less than −1.5 SD (arrest inhibited neurons) from baseline average. After the selection of neurons using two methods, auROC and cross-correlation analysis, neurons active during arrest and anti-CC with speed were considered the “pure” arrest neurons, or significant neurons, and used the spatial analysis of arrest areas. Neuronal activity of neurons active during arrest and anti-CC with speed was averaged for each animal and the peak response (difference between the peak between 5 to 7 s and baseline range, −5 to −1 s) was tested during entrance to the novel and familiar areas. Latency to calcium activity onset was the time of a significant peak calcium response relative to time of arrest onset (0 s).
Together with the auROC analysis, we used a z-score sorting analysis to understand which neurons were active during arrest and freezing bouts onset. To calculate z-score values, we compared the raw calcium activity average during the transition from movement to arrests, or to freezing, to the baseline calcium activity (−5 to −1 s before arrest) across trials. Significance was established if at least three consecutive values between the time range 1–2 s exceeded 2.567 SD (arrest active neurons) or were less than −2.567 SD (arrest inhibited neurons) from baseline average.
Statistical analysis
Mean ± standard error of the mean (SEM) was used to report statistics if not indicated otherwise. For all within-subject quantifications, we calculated the average across all 5 recording sessions. Statistical tests used and the sample size for each analysis is listed in the Results or figure legends. Both parametric and non-parametric tests were used wherever appropriate. Hypothesis testing was done at a significance level of p = 0.05. No statistical methods were used to predetermine sample size. All analyses were performed in MATLAB (MathWorks) while the graphs and statistical tests with GraphPad Prism 6 (GraphPad software, USA). Experimental data were excluded based on imaging quality due to movement artifact, a lack of cells, a bad focal plane, lack of viral infection in the targeted areas and misplacement of the optical fibers.
Supplementary Material
Highlights.
A genetically and projection-defined amygdala circuit mediates exploratory arrests
Arrest-related BLA neurons are recruited in an experience-dependent manner
Their activity drives experience-dependent arrests during exploration
Arrests in familiar places are effected via CEA-projecting BLA neurons
ACKNOWLEDGMENTS
We acknowledge M. Angelhuber and J. Bauto for the initial consultation regarding behavioral analysis. We thank H.F.M. Rodrigues for building the behavioral box and fear conditioning paradigm implementation, A. Vaz and M. L. Correia for mouse maintenance, and the Costa lab for helpful feedback. We thank E.J. Rodriguez for advice regarding implementing the fear conditioning paradigm and for sharing codes and materials. We thank A. Azinheira for the video concatenation. A special note of thanks goes to Dr. Akira Fushiki in appreciation of all the support and effort put into the project, especially to finish critical experiments for revision during the COVID-19 pandemic. This research project was supported in part by the Zuckerman Institute Cellular Imaging Platform, Swiss National Science Foundation early postdoctoral mobility, an EMBO long-term postdoctoral fellowship and NIH K99 NS107721 (to P.B.), SNSF postdoc mobility fellowships P2EZP3-172128 and P400PM_183904 (to A.M.), and European Research CouncilConsolidator (COG 617142) and U19 Brain Initiative (5U19NS104649) grants (to R.M.C.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.09.023.
REFERENCES
- Benjamini Y, Lipkind D, Horev G, Fonio E, Kafkafi N, and Golani I. (2010). Ten ways to improve the quality of descriptions of whole-animal movement. Neurosci. Biobehav. Rev 34, 1351–1365. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Fonio E, Galili T, Havkin GZ, and Golani I. (2011). Quantifying the buildup in extent and complexity of free exploration in mice. Proc. Natl. Acad. Sci. USA 108, 15580–15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, and Tye KM (2016). Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron 90, 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Chang CJ, Silvestre M, Lévêque C, Namburi P, Wildes CP, and Tye KM (2018). Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala. Cell Rep. 22, 905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, Lu T, Poe MM, Xu L, Cook JM, et al. (2015). Regulating anxiety with extrasynaptic inhibition. Nat. Neurosci 18, 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, and Kiehn O. (2015). Descending Command Neurons in the Brainstem that Halt Locomotion. Cell 163, 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, and Kiehn O. (2018). Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong AS, Miri ML, Busskamp V, Matthews GAC, Acker LC, Sørensen AT, Young A, Klapoetke NC, Henninger MA, Konandaramaiah SB, et al. (2014). Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci 17, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Hamilton DA, and Whishaw IQ (2006). Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: Modification of movement distribution, distance, location, and speed. Physiol. Behav 87, 805–816. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, and Uchida N. (2012). Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Lin S-C, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, and Nicolelis MAL (2006). Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 52, 359–369. [DOI] [PubMed] [Google Scholar]
- da Silva JA, Tecuapetla F, Paixão V, and Costa RM (2018). Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248. [DOI] [PubMed] [Google Scholar]
- Drai D, and Golani I. (2001). SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neurosci. Biobehav. Rev 25, 409–426. [DOI] [PubMed] [Google Scholar]
- Drai D, Benjamini Y, and Golani I. (2000). Statistical discrimination of natural modes of motion in rat exploratory behavior. J. Neurosci. Methods 96, 119–131. [DOI] [PubMed] [Google Scholar]
- Dvorkin A, Szechtman H, and Golani I. (2010). Knots: attractive places with high path tortuosity in mouse open field exploration. PLoS Comput. Biol 6, e1000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, and Lüthi A. (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771. [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, and Rainnie DG (2012). Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J. Physiol 590, 4819–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, and Ranganath C. (2007). The medial temporal lobe and recognition memory. Annu. Rev. Neurosci 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin N, Sauvage M, Robitsek RJ, and Farovik A. (2010). An animal model of amnesia that uses Receiver Operating Characteristics (ROC) analysis to distinguish recollection from familiarity deficits in recognition memory. Neuropsychologia 48, 2281–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, and Golani I. (1989). Home base behavior of rats (Rattus norvegicus). Behav. Brain Res 34, 121–199. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Callister RJ, and Sah P. (2001). Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J. Neurophysiol 85, 714–723. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller K, Kovacevic A, et al. (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100. [DOI] [PubMed] [Google Scholar]
- Farovik A, Place RJ, Miller DR, and Eichenbaum H. (2011). Amygdala lesions selectively impair familiarity in recognition memory. Nat. Neurosci 14, 1416–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonio E, Benjamini Y, and Golani I. (2009). Freedom of movement and the stability of its unfolding in free exploration of mice. Proc. Natl. Acad. Sci. USA 106, 21335–21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, and Eichenbaum H. (2004). Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature 431, 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, and Kreitzer AC (2013). Control of basal ganglia output by direct and indirect pathway projection neurons. J. Neurosci 33, 18531–18539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire R, Appleby MC, and Hughes BO (1996). Effects of nest quality and other cues for exploration on pre-laying behaviour. Appl. Anim. Behav. Sci 48, 37–46. [Google Scholar]
- Golani I, Benjamini Y, and Eilam D. (1993). Stopping behavior: constraints on exploration in rats (Rattus norvegicus). Behav. Brain Res 53, 21–33. [DOI] [PubMed] [Google Scholar]
- Gordon G, Fonio E, and Ahissar E. (2014). Emergent exploration via novelty management. J. Neurosci 34, 12646–12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, and Lüthi A. (2008).Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606. [DOI] [PubMed] [Google Scholar]
- Hines DJ, and Whishaw IQ (2005). Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur. J. Neurosci 22, 2363–2375. [DOI] [PubMed] [Google Scholar]
- Jain A, Dvorkin A, Fonio E, Golani I, and Gross CT (2012). Validation of the dimensionality emergence assay for the measurement of innate anxiety in laboratory mice. Eur. Neuropsychopharmacol 22, 153–163. [DOI] [PubMed] [Google Scholar]
- Janak PH, and Tye KM (2015). From circuits to behaviour in the amygdala. Nature 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellestad FK, and Bakke HK (1985). Passive avoidance after ibotenic acid and radio frequency lesions in the rat amygdala. Physiol. Behav 34, 299–305. [DOI] [PubMed] [Google Scholar]
- Jung S-Y, Kim J, Kwon OB, Jung JH, An K, Jeong AY, Lee CJ, Choi YB, Bailey CH, Kandel ER, Kim JH, et al. (2010). Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc. Natl. Acad. Sci. USA 107, 4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay K, Sosa M, Chung JE, Karlsson MP, Larkin MC, and Frank LM (2016). A hippocampal network for spatial coding during immobility and sleep. Nature 531, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, and Tonegawa S. (2016). Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci 19, 1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, and Tonegawa S. (2017). Basolateral to Central Amygdala Neural Circuits for Article Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Martins GJ, Paixao VB, Pengcheng Z, Paninski L, and Costa RM (2017). The Spatiotemporal Organization of the Striatum Encodes Action Space. Neuron 95, 1171–1180.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Staring M, Murphy K, Viergever MA, and Pluim JP (2010). elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 29, 196–205. [DOI] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, and Li B. (2013).Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 16, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Popescu AT, and Paré D. (2006). Identification of basolateral amygdala projection cells and interneurons using extracellular recordings. J. Neurophysiol 96, 3257–3265. [DOI] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, and Amaral DG (2006). Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion 6, 73–81. [DOI] [PubMed] [Google Scholar]
- Mills AD, and Wood-Gush DG (1985). Pre-laying behaviour in battery cages. Br. Poult. Sci 26, 247–252. [DOI] [PubMed] [Google Scholar]
- Muenzinger KF, and Gentry E. (1931). Tone discrimination in white rats. J. Comp. Psychol 12, 195–206. [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, et al. (2015). A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. (2014). A mesoscale connectome of the mouse brain. Nature 508, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Berke JD, and Kreitzer AC (2018). Fast-Spiking Interneurons Supply Feedforward Control of Bursting, Calcium, and Plasticity for Efficient Learning. Cell 172, 683–695.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, and Svoboda K. (2009). The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, and LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Pnevmatikakis EA, Soudry D, Gao Y, Machado TA, Merel J, Pfau D, Reardon T, Mu Y, Lacefield C, Yang W, et al. (2016). Simultaneous Denoising, Deconvolution, and Demixing of Calcium Imaging Data. Neuron 89, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan T, Kadiri LR, Venkataraju KU, Bahlmann K, Sutin J, Taranda J, Arganda-Carreras I, Kim Y, Seung HS, and Osten P. (2012). Serial two-photon tomography for automated ex vivo mouse brain imaging. Nat. Methods 9, 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD (2016). Vicarious trial and error. Nat. Rev. Neurosci 17, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, et al. (2016). Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell 165, 1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MJ (1990). Neglected aspects of exploratory and investigatory behavior. Psychobiology 18, 16–22. [Google Scholar]
- Roelofs K. (2017). Freeze for action: Neurobiological mechanisms in animal and human freezing. Philos. Trans. R Soc. Lond. B Biol. Sci 372, 20160206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry T, and Kreitzer A. (2017). Neural circuitry for behavioural arrest. Philos. Trans. R Soc. Lond. B Biol. Sci 372, 20160197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, and Kreitzer AC (2016). Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell 164, 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry TK, Lalive AL, Margolin BD, Kreitzer AC, et al. (2019). Locomotor suppression by a monosynaptic amygdala to brainstem circuit. bioRxiv. 10.1101/724252. [DOI] [Google Scholar]
- Sagaspe P, Schwartz S, and Vuilleumier P. (2011). Fear and stop: a role for the amygdala in motor inhibition by emotional signals. Neuroimage 55, 1825–1835. [DOI] [PubMed] [Google Scholar]
- Samson RD, and Pare D. (2005). Activity-Dependent Synaptic Plasticity in the Central Nucleus of the Amygdala. J. Neurosci 25, 1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM (2010). ROC in animals: uncovering the neural substrates of recollection and familiarity in episodic recognition memory. Conscious. Cogn 19, 816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, and Rauch SL (2003). Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol. Psychiatry 53, 854–862. [DOI] [PubMed] [Google Scholar]
- Sommer C, Straehle CN, Köthe U, and Hamprecht FA (2011). Ilastik: Interactive learning and segmentation toolkit. IEEE, 230–233. [Google Scholar]
- Sosulina L, Meis S, Seifert G, Steinhäuser C, and Pape HC (2006). Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Mol. Cell. Neurosci 33, 57–67. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, and Clark RE (2007). Recognition memory and the medial temporal lobe: a new perspective. Nat. Rev. Neurosci 8, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D, et al. (2018). The Basolateral Amygdala Is Essential for Rapid Escape : A Human and Rodent Study Article The Basolateral Amygdala Is Essential for Rapid Escape : A Human and Rodent Study. Cell 175, 723–735.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, and Unser M. (1998). A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process 7, 27–41. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Berkowitz LE, and Clark BJ (2018). Behavioral and neural subsystems of rodent exploration. Learn. Motiv 61, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC (1939). Prediction of vicarious trial-and-error by means of the schematic sow-bug. Psychol. Rev 46, 318–336. [Google Scholar]
- Tolman EC (1948). The Psychological Review Cognitive Maps in Rats and Men. Psychol. Rev 55, 189–208. [DOI] [PubMed] [Google Scholar]
- Tomás Pereira I, Agster KL, and Burwell RD (2016). Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. I. afferents. Hippocampus 26, 1189–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SB, Ramakrishnan C, Fenno L, Deisseroth K, et al. (2016). Midbrain circuits for defensive behaviour. Nature 534, 206–212. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, and Williams MT (2014). Assessing spatial learning and memory in rodents. ILAR J. 55, 310–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FAW, and Rolls ET (1993). The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Exp. Brain Res 93, 367–382. [DOI] [PubMed] [Google Scholar]
- Xu C, Krabbe S, Gründemann J, Botta P, Fadok JP, Osakada F, Saur D, Grewe BF, Schnitzer MJ, Callaway EM, and Lüthi A. (2016). Distinct Hippocampal Pathways Mediate Dissociable Roles of Context in Memory Retrieval. Cell 167, 961–972.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim CY, and Mogenson GJ (1989). Low doses of accumbens dopamine modulate amygdala suppression of spontaneous exploratory activity in rats. Brain Res. 477, 202–210. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP (2002). The nature of recollection and familiarity: A review of 30 years of research. J. Mem. Lang 46, 441–517. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pipeline for image processing of fixed brain slice were processed using can be found here: https://github.com/lahammond/BrainJ. Further information and requests should be directed to and will be fulfilled by the Lead Contact and it is found in the Key Resources Table.