Abstract
Background
Upon the COVID-19 pandemic emergence, safety concerns and logistic drawbacks stimulated the search for alternatives to pulse therapy at infusion centres to treat multiple sclerosis relapses.
Objective
To describe our experience treating multiple sclerosis relapses with a dilute injectable methylprednisolone powder orally administered, in a safe home-based environment and with totally virtual assessment and follow up via telemedicine.
Methods
Descriptive observational, retrospective, single-centre, open label, study in the real-world setting.
Results
Between August 2020 and March 2021, ten multiple sclerosis patients and one neuromyelitis optica spectrum disease patient, regularly assisted at our multiple sclerosis centre in Argentina, experienced twelve disease relapses (nine moderate/severe relapses and three mild relapses) and were treated with the oral dilute of injectable methylprednisolone powder pulses with good efficacy as well as adequate tolerance and safety profile.
Conclusions
The oral pulse therapy based on the methylprednisolone powder dilution we describe is simple and comfortable to administer and can be an option in countries like Argentina, where the oral methylprednisolone formulation is not marketed. In these pandemic times, a home based and virtually monitored pulse therapy could represent a safe and effective alternative to manage relapses while minimizing the patient's risk of exposure to SARS-CoV-2.
Keywords: Relapse treatment with oral methylprednisolone, MS, NMOSD, Telemedicine, COVID-19
1. Introduction
The pandemic scenario prompted us to outline new contingency plans to continue providing the best health care to our patients with demyelinating diseases, in the safest possible environment that preserved them from SARS-CoV-2 infection. The management of the relapses was one of many issues of great concern.
Intravenous methylprednisolone (ivMP) pulse is the most used regimen to treat Multiple Sclerosis (MS) and Neuromyelitis Optica Spectrum Disorder (NMOSD) relapses. However, in the current pandemic times, hospitalizing or admitting patients to day hospital facilities for infusions increases the risk of viral exposure.
Oral corticosteroids (oCS) have proven to be as effective as intravenous corticosteroids (ivCS) in the treatment of relapses (Lattanzi et al., 2017; Le Page et al., 2015; Liu et al., 2017; Ramo-Tello et al., 2014). They may be preferable to ivCS for some patients with an MS relapse (Burton et al., 2012) and could represent a remarkable option in order to spare our patients the risk of SARS-CoV-2 transmission (Brownlee et al., 2020; Segamarchi et al., 2020). Unfortunately, oral methylprednisolone (oMP) is not available in Argentina like in other countries.
Despite the pharmacokinetic differences between oral and intravenous formulations, the overall absorption and the systemic exposure of the injectable methylprednisolone (sodium succinate) powder orally administered are considered to reach clinically efficacious ranges. Furthermore, many MS expert neurologists have used such alternative in their clinical practice (Frohmann et al., 2007). In the literature, we found only one detailed publication of a trial with oral administration of MP injectable powder to treat MS relapses (Pascual et al., 2008) and few publications concerning this off-label route of the drug administration (Candel and Tejada Cifuentes, 2015).
The aim of this publication is to report our real-world experience treating MS and NMOSD exacerbations with an oral preparation of injectable MP powder dissolved in water, in a safe home-based environment and with close patient monitoring and follow up via telemedicine during COVID-19 pandemic.
2. Material and methods
This was a descriptive observational, retrospective, single-centre, open label, study.
Data were obtained from medical records of the MS and NMOSD patients regularly followed up at a single reference MS centre in Argentina, who developed a relapse between August 2020 and March 2021 and received pulse therapy with an oral preparation based on the injectable MP powder. The relapses were treated within 15 days of their onset. Baseline data including Expanded Disability Status Scale (EDSS) score (Kurtzke et al., 1983), demographic and clinical characteristics were documented for all patients.
Based on the adequate response and safety data from previous studies using oMP, and considering the restrictions in place during the pandemic, patients were asked to consider the possibility of a home-based therapy with oral MP pulses (Le Page et al., 2015; Pascual et al., 2008; Ramo-Tello et al., 2014).
Patients were evaluated remotely according to the American Academy of Neurology's Telemedicine and COVID-19 Implementation Guide https://www.aan.com/siteassets/home-page/tools-and-resources/practicing-neurologist–administrators/telemedicine-and-remote-care/20-telemedicine-and-covid19-v103.pdf.
Relapses were evaluated in this telehealth context. Neurologic examination and tele-EDSS (Bove et al., 2019; Solà-Valls et al., 2019) were performed before and at Week 1 (W1) and Week 4 (W4) after oMP pulses during virtual follow-up appointments.
2.1. Relapse, relapse severity and treatment response definitions
A relapse was defined as any new or worsened pre-existing symptom attributable to MS that lasted more than 48 hours (Freedman et al., 2013) in the absence of fever or infection.
Relapse severity assessment was performed according to other author's definition that estimated the EDSS score increase from each patient's basal score to their first evaluation during the exacerbation: severe relapse > 2.5, moderate relapse 1.0 to 2.5, and mild relapse ≤ 0.5 increase (Nos et al., 2004; Ramo-Tello et al., 2014).
We considered to be a good response to oMP treatment an improvement of ≥ 1 EDSS point for moderate and severe exacerbations or a complete recovery to basal EDSS score for mild relapses, measured at W1 and W4 post oMP pulse.
2.2. Relapse treatment
A digital prescription of MP (sodium succinate) was sent to the patients, who obtained the drug in a nearby pharmacy.
Patients were instructed to dilute 1 gram of MP sodium succinate (only the powder for injectable use) in 500 ml (17.60 fl oz BIS) of mineral water. Sugar but not juice, could be added. Gastroprotection with proton pump inhibitors by oral route was indicated at least 2 h before the oral pulse.
We asked the patients to drink 100 ml of the solution (approximately 3-4 swallows) every hour, beginning early in the morning. Full dose intake (500 ml containing 1 gram of MP) was achieved in 4 h. The rationale for this dosage was the gradual administration of oMP -resembling a slow infusion rate, to mitigate the risk of adverse events (AEs) and to improve gastric tolerance. However, it is worth noting that corticosteroid-associated gastritis is not a result of direct effects on the gastric mucosa, but rather relates to systemic mechanisms which are independent of the route of administration (Barnes et al., 1997).
The oMP pulse was repeated for 3 days in moderate and severe relapses. According to the usual clinical practice at our centre, patients experiencing a mild relapse were prescribed a single day pulse when symptoms were bothersome enough to interfere with their daily-life activities.
Blood pressure and heart rate were monitored before and after each pulse administration by a home nursing service.
Clinical monitoring was performed twice daily via telehealth appointments during the course of pulse therapy: in the morning during oMP intake and in the afternoon once the daily dose was complete. Patients were advised to contact their treating neurologist (who would be available for video-calls on demand), in case of any AE, to warrant close clinical surveillance during all the process.
AEs were registered in the medical record during each virtual visit. A virtual neurological examination and EDSS evaluation were performed one, two and four weeks after relapse treatment completion.
2.3. Ethical aspects
All the patients gave their consent for the fully virtual assessment and follow up. This study was based on anonymized and de-identified data. Researchers ensured data confidentiality and all personal information was protected according to the Personal Data Protection Act of Argentina (Act #25,326 and regulations in force). The study was approved by the local Institutional Review Board.
3. Results
In total, 11 relapses in 10 relapsing-remitting MS patients –one of them experienced two relapses, and 1 relapse in an NMOSD patient were treated with oMP pulses at home under telemedicine supervision.
The clinical characteristics of MS patients are presented in Table 1 . Females represented 70% of MS patients and also the NMOSD patient. MS patient's mean age was 39 (± 7,6) years, NMOSD patient was 49 years old.
Table 1.
Baseline demographic and clinical characteristics of the patients.
| Patient # | Sex/Gender | Age (years) | Distance from MS centre (km) | Disease phenotype | Years on MS diagnosis | Comorbidities | EDSS before relapse | DMT | Time on last DMT (years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 49 | 59 | NMOSD | 0.2 | Hypothyroidism | 5 | No treatment | NA |
| 2 | F | 35 | 43 | RR MS | 13 | Chronic anaemia | 2 | Dimethyl fumarate | 3 |
| 3 | M | 50 | 149 | RR MS | 20 | No | 3 | Teriflunomide | 1 |
| 4 | M | 46 | 149 | RR MS | 15 | Hypertension/Obesity | 3.5 | Teriflunomide | 1 |
| 5 | M | 50 | 61 | RR MS | 20 | No | 3.5 | Teriflunomide | 3 |
| 6 | F | 36 | 77 | RR MS | 9 | Achondroplasia | 2.5 | Dimethyl fumarate | 5 |
| 7 | F | 31 | 435 | RR MS | 2 | No | 2 | Teriflunomide | 1 |
| 8 | F | 25 | 12 | RR MS | 5 | No | 2/2.5 | Teriflunomide | 3 |
| 9 | F | 39 | 110 | RR MS | 1 | Hypothyroidism | 2 | No treatment | NA |
| 10 | F | 39 | 149 | RR MS | 7 | No | 1.5 | Teriflunomide | 1.5 |
| 11 | F | 40 | 5 | RR MS | 7 | No | 2.5 | Dimethyl fumarate | 1 month |
DMT: disease modifying therapy; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; NA: not applicable; NMOSD: neuromyelitis optica spectrum disease; RR: relapsing remitting.
All the patients had medical insurance and with only one exception, all of them lived in cities distant 124 km in average from the MS infusion centre. During the peak of the SARS-CoV-2 pandemic in Argentina, severe restrictions were imposed on the use of individual car or public transport preventing travelling between cities, and also intermittent periods of social preventive isolation were mandatory.
Most of the patients living far away from the MS centre have a hospital with 24-hour ward in place at their home town and an emergency home service provided by each medical insurance.
All MS patients had a relapsing-remitting MS phenotype (Lublin et al., 2014) and the mean disease duration was 10 (± 6,5) years. The NMOSD patient (anti-A4 and anti-MOG Ab negative) had been recently diagnosed (2.5 months before the relapse). The median basal tele-EDSS (evaluated within 3 months before the relapse) was 2.5 (1.5-3.5) for MS patients and the NMOSD patient had a basal EDSS score of 5.0.
Comorbidities were present in 4 MS patients and in the NMOSD patient. For one patient with a history of obesity and hypertension, a comprehensive cardiologic evaluation was done before prescribing oMP, that included an electrocardiogram and the anti-hypertensive medication adjustment. Furthermore, a written authorization by the patient's local cardiologist was required before starting any medication.
Nine MS patients were on disease modifying therapy and had received their last MS therapy for a mean duration of 2 years; the NMOSD patient was not yet under therapy as had been recently diagnosed.
Nine of the 12 treated relapses –five of moderate severity and four severe, were treated with a 3-days course of high dose oMP without tapering; other 3 mild relapses were treated with an abbreviated course of one day only oMP pulse.
Fifty-five percent (n = 6) of MS relapses were polysymptomatic and the other 45% (n = 5) were monosymptomatic. The NMOSD patient experienced a severe polysymptomatic relapse. Table 2 shows the functional systems involved, being the motor and sensory pathways the most affected. Mean time from relapse onset to the oMP pulse was 8.25 (± 2.8) days. Median EDSS during the relapse was 4 (2-6.5) points for the MS patients and 8 points for the NMOSD patient.
Table 2.
Description of relapse severity, pulse therapy and outcomes of the MS and NMOSD patients who received home based pulse therapy with an oral preparation of methylprednisolone powder (for injection) under fully virtual assessment and follow up.
| Patient # | Relapse # | Relapse date | System affected | Relapse severity | EDSS before relapse | EDSS during relapse | Time from relapse to oMP pulse (days) | Days on oMP at home | Recovery | Time to recovery | EDSS at W1 post oMP* | EDSS at W4 post oMP* | AEs | Infection after relapse | MRI during relapse | MRI after relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 31/08/2020 | Motor, cerebellar, sphincter | Severe | 5.0 | 8 | 10 | 3 | Partial | 2 weeks | 7.5 | 6.5 | Metallic taste | Not done | Yes: no activity | |
| 2 | 2 | 19/08/2020 | Motor | Moderate | 2 | 3.5 | 7 | 3 | Ad integrum | 2 weeks | 3.0 | 2 | Metallic taste | Not done | ||
| 3 | 3 | 19/08/2020 | Motor | Severe | 3 | 6 | 4 | 3 | Ad integrum | 4 weeks | 5.5 | 3 | Tachycardia, lower limb oedema, mild blood pressure elevation, metallic taste | Not done | ||
| 4 | 4 | 08/09/2020 | Motor, cerebellar | Severe | 3.5 | 6.5 | 8 | 3 | Partial | 4 weeks | 5 | 4.5 | Metallic taste | New lesions and Gd+ | ||
| 5 | 5 | 19/02/2021 | Motor, sensory | Moderate | 3.5 | 5 | 10 | 3 | Partial | 2 weeks | 4,5 | 4 | Stomach-ache, pyrosis, tachycardia, headache (resolved within 12 hours) | Not done | ||
| 6 | 6 | 04/01/2021 | Motor, cerebellar, sensory | Severe | 2.5 | 6 | 15 | 3 | Partial | 2 weeks | 5,5 | 3 | None | New lesions and Gd+ | ||
| 7 | 7 | 01/10/2020 | Sensory, visual (optic neuritis) | Mild | 2 | 2.5 | 7 | 1 | Ad integrum | 2 weeks | 2 | 2 | Metallic taste | New lesions and Gd+ | ||
| 8 | 8 | 09/09/2020 | Sensory (myelitis) | Moderate | 2 | 3.5 | 7 | 3 | Partial | 4 weeks | 2,5 | 2.5 | Metallic taste | Urinary infection (22/09/2020) | New lesions | |
| 9 | 04/12/2020 | Sensory | Mild | 2.5 | 3 | 6 | 1 | Ad integrum | 2 weeks | 2,5 | 2.5 | Metallic taste | COVID-19 (11/01/21) Positive nasal swab | New lesions and Gd+ | ||
| 9 | 10 | 07/03/2021 | Sensory, motor, other | Moderate | 2 | 4 | 10 | 3 | Ad integrum | 2 weeks | 2 | 2 | Metallic taste, anxiety, euphoria | Not done | ||
| 10 | 11 | 17/09/2020 | Sensory | Mild | 1.5 | 2 | 5 | 1 | Ad integrum | 2 weeks | 1,5 | 1.5 | Metallic taste, anxiety | Not done | ||
| 11 | 12 | 13/03/2021 | Sensory, motor | Moderate | 2.5 | 4 | 10 | 3 | Ad integrum | 1 week | 2,5 | 2.5 | Metallic taste, insomnia | Not done |
AEs: adverse events; EDSS: Expanded Disability Status Scale; Gd+: positive gadolinium enhancement; MRI: magnetic resonance imaging; oMP: oral methylprednisolone; W4: week 4 after pulse therapy.
All the cases were outpatients. They received pulse therapy at home and were fully virtually assessed and followed up. All the patients received gastric protection with an oral proton pump inhibitor, either omeprazole, esomeprazole or pantoprazole.
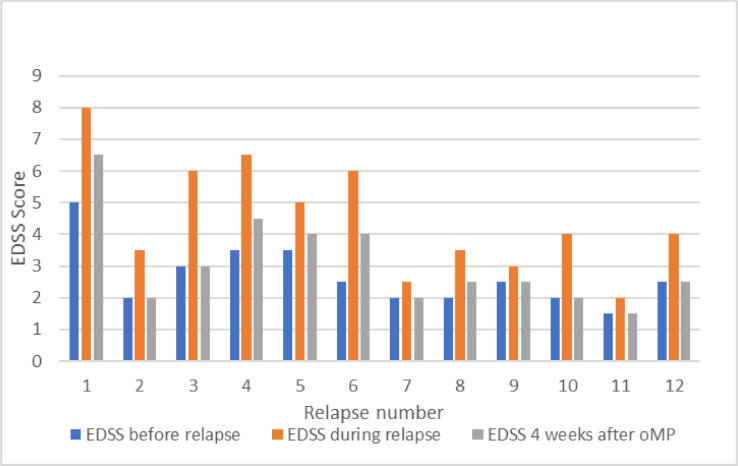
Median EDSS score in MS patients was 2.5 (1.5-5.5) at W1 following oMP pulse and 2.5 (1.5-4.5) at W4; for the NMOSD patient, EDSS score was 7.5 and 6.5 at W1 and W4, respectively. All the patients with a moderate or severe relapse had improved by at least 1 EDSS point at W4. Seven relapses fully recovered by W4 -including the 3 mild relapses treated with a single day oMP pulse (Fig. 1 ).
Fig. 1.
Changes in EDSS score in twelve treated relapses. Scores were obtained before the relapse, during the relapse and before oMP pulse, and four weeks after oMP pulse.
Five patients underwent an MRI scan showing activity during the relapse. The NMOSD patient was the only one who underwent a control MRI scan after relapse recovery that showed no activity at that time.
All AEs were mild. The most frequent AE was metallic taste during the dilute oMP administration (n = 10); two patients experienced tachycardia and two, referred anxiety. There were two episodes of infection in one patient (see description below). The following AEs were reported once each: high blood pressure, lower limbs oedema, stomach-ache, pyrosis, headache, insomnia and euphoria. (Table 2)
A female MS patient had 2 relapses. After the first oMP course, she experienced a urinary tract infection. Almost 3 months after the previous one, the patient developed a new relapse which was mild and was treated with a single day oMP pulse. A mild COVID-19 disease was confirmed by nasal swab test within a month after the second oMP pulse. She experienced dry cough and loss of smell and taste (no fever nor other symptoms) and made a full recovery within a week.
4. Discussion
To our knowledge, this is the first publication of a case series concerning the oral use of an injectable MP sodium succinate powder dilution to treat MS and NMOSD relapses in Argentina. There are anecdotal reports of a so called “smoothie-medrol” administered to treat MS relapses (Frohmann et al., 2007), besides personal communications from world experts in MS and a few publications about this off-label route of administration of MP (Pascual et al., 2008). In the available clinical trials, oMP was prepared at the hospital's pharmacy in the form of tablets and dispensed to patients (Le Page et al., 2015; Ramo-Tello et al., 2014).
The effectiveness and the safety of high-dose corticosteroids orally administered to treat MS relapses has been well documented in many studies (Alam et al., 1993; Le Page et al., 2015; Martinelli et al., 2009; Morrow et al., 2018; Ramo-Tello et al., 2014). In recent reviews and meta-analyses, authors identified clinical trials providing evidence that high doses of oMP have similar efficacy as ivMP in reducing EDSS score and gadolinium enhancing lesions in the MRI scan of MS patients (Lattanzi et al., 2017; Liu et al., 2017; Martinelli et al., 2009; Segamarchi et al., 2020). In our study, we included MS patients and also one NMOSD patient with a severe relapse.
Unfortunately, oMP is not available in Argentina like in many other countries. In a recent publication, some Argentine authors suggested, on the basis of their experience and according to the results of a literature review they performed, that the use of oral prednisone 1,250 mg/daily or dexamethasone 160 mg/daily could replace oMP. However, considering the currently available formulations in Argentina, such dosage would imply the administration of 25 tablets of prednisone 50 mg or their equivalent dose –e.g., 40 tablets of dexamethasone 4 mg (Segamarchi et al., 2021).
Some authors have used bioequivalent doses to 1,250 mg of oMP (Ramo-Tello et al., 2014). The results of different studies demonstrated the non-inferiority of equal doses, as comparable efficacy and safety profiles were observed when comparing oMP 1,000 mg versus ivMP 1,000 mg –the most used dose in clinical practice (Le Page et al, 2015; Liu et al., 2017; Pascual et al., 2008). Based on these results, we decided to use an identical dose scheme and the oral route of administration to treat our patients’ exacerbations during COVID-19 pandemic. As has been mentioned above, the patient's safety and the logistic drawbacks are important considerations in pandemic times, especially for those patients living in distant places from the MS centre.
As it is standard practice, we prescribed the oMP pulses during three days for moderate or severe relapses. In the case of mild relapses, patients received a single day oMP pulse according to the local clinical practice at our centre. It has been our experience that patients presenting with symptoms that interfere with their daily living activities (Freedman et al., 2004) -even if the relapse is mild, obtain clear benefit from the abbreviated pulse therapy.
The ingestion speed of the MP dilution was fractionated hourly in order to minimize AEs and to improve oral tolerance. Overall, patients had good tolerance and our results were in line with other publications.
The improvement of at least 1 point in the EDSS score assessed at W1 and W4 after oMP pulse was comparable to the reported in other publications (Table 3 ) (Le Page et al., 2015; Pascual et al., 2008; Ramo-Tello et al., 2014).
Table 3.
Comparative improvement in EDSS score between the present study and other oMP published studies.
| Author | Number of relapses | Percentage of patients with at least 1 point improvement in EDSS score | |
|---|---|---|---|
| Week 1 post oMP | Week 4 post oMP | ||
| Our study* | 9 | 43 | 100 |
| Ramo Tello et al., 2014 | 25 | 46 | 68 |
| Pascual et al., 2008 | 21 | 33.4 | 85.7 |
| Le Page et al., 2015 | 82 | Not available | 81 |
* For comparative purposes, only the moderate and severe relapses are included in this table, while the three mild relapses are excluded.
Adverse events were all mild and transitory, the most frequent of which was metallic taste during the oral preparation ingestion. In a thorough and comprehensive systematic review and meta-analysis, insomnia was found significantly associated with the oral route of administration of corticosteroids (Lattanzi et al., 2017). In our study, only one patient had insomnia, this low frequency could have been related to the small number of cases or, alternatively to the administration of oMP in the early morning. Other reported AEs were present in 10-20% of cases, comparable to other publications. There were no serious adverse events in our series. Only one patient, who had two relapses and received an oral 3-days pulse for the first episode and a single day pulse for the second and mild relapse, developed COVID-19 which course was mild and recovered completely within a week. It has been described an increased risk for Covid-19 worse outcome in patients who received high doses of MP during the month previous (Salter et al., 2021; Sormani et al., 2021), for this reason we should be cautious and indicate pulses only if the if the benefits outweigh the risks. Fortunately, in our patient the infection was mild.
Regarding the ethical concerns that may arise about this type of administration, it is noteworthy that off-label drug prescription is common in the clinical practice (e.g., when no approved drugs are available for certain patient population or in many special situations). The administration of drugs via any route that is different from the approved one for an indication falls into the off-label category. There are a number of publications about injectable drugs that can be orally or enterally administered under certain circumstances -i.e. when the oral formulation is not available, and that are safe and well tolerated (Lozada et al., 2012; Toledo et al., 2015). The ivMP powder is one of the examples (Candel GR et al, 2015; Pascual et al, 2008). Legally, off-label drugs use is not an issue as clinicians are allowed to prescribe a marketed drug for an unapproved indication if it is relevant in the patient's best interest according to the professional's judgment. Off- label use of drugs is not forbidden by the regulatory agencies in many countries. The Argentine regulatory agency (ANMAT) had made a statement on the issue http://www.anmat.gov.ar/comunicados/indicaciones_de_medicamentos_fp.pdf.
Because of COVID-19 pandemic, patients are exposed to an increased risk of SARS-CoV-2 infection if they are hospitalized or admitted at day hospital facilities to receive therapy for an MS or NMOSD relapse (Brownlee et al., 2020). Some authors have hypothesized that high dose oCS therapy could be started at home and be followed up via telemedicine (Segamarchi et al., 2021). Our patients were closely and safely monitored via a telehealth system during the whole process: with frequent virtual visits to evaluate the relapse from its beginning, during the oMP administration period, and with close post therapy monitorization and follow up. To our best knowledge, this is the first case series that describes a fully telehealth management of MS and NMOSD exacerbations during a pandemic.
The oral regimen allows rapid access to treatment; it is comfortable for the patient, simple to administer, and cost effective to treat MS exacerbations (Frohmann et al., 2007; Lattanzi et al., 2017; Le page et al., 2015). Furthermore, in these challenging pandemic times, it emerges as a safe and effective option to treat MS and NMOSD relapses while minimizing the risk of exposure to SARS-CoV-2.
Our study has limitations: it is a single centre study; the design is retrospective; there is no control group; the sample size is small, and the follow up time is short. However, the follow up duration was sufficient to assess the patients’ outcomes.
The results of our real-world experience suggests that the oral administration of an injectable MP powder dilution provides a safe and effective means to treat MS and NMOSD relapses, that avoids unnecessary hospitalizations and also overcomes the discomfort for patients having to ingest a large number of tablets to achieve corticosteroid equivalent doses. Even in countries where oMP is available, the number/size of tablets can be troublesome for some patients, making the dilute preparation suggested in this study a more convenient option.
Our results support and add to the evidence on the use of this easily self-prepared and home administered oral pulse, especially in countries where MP tablets are not available. This work also adds to the real-world experience with a fully telehealth assessment, treatment and follow up of MS and NMOSD relapses, sparing our patients the risk of SARS-CoV-2 infection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
CRediT authorship contribution statement
Geraldine G. Luetic: Conceptualization, Methodology, Validation, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. María Laura Menichini: Writing – review & editing. Óscar Fernández: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
GL has received: research support; educational, advisory boards and consultation fees; travel reimbursements and grants for congresses from: Bayer, Biogen, Merck, Novartis, Synthon-Bagó, Teva and Tuteur.
MLM has no conflict of interest.
OF has received honoraria as a consultant on advisory boards, and as a moderator or speaker at meetings, and has also participated in clinical trials and other research projects promoted by Biogen - Idec, Bayer - Schering; Merck - Serono, Teva, Novartis, Actelion, Almirall, Genzyme, Roche, Allergan, Orizon and Araclon
Acknowledgements
We would like to thank Dr. Juan I. Rojas for his continued support, the critical review of the manuscript and comments. We also thank Dr. Carla D'Angelo for her writing assistance. Finally, we would like to thank all the patients who participated in this study.
References
- Alam S.M., Kyriakides T., Lawden M., Newman P.K. Methylprednisolone in multiple sclerosis: a comparison of oral with intravenous therapy at equivalent high dose. J. Neurol. Neurosurg. Psychiatry. 1993;56(11):1219–1220. doi: 10.1136/jnnp.56.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Hughes R.A., Morris R.W., Wade-Jones O., Brown P., Britton T., et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet. 1997;349(9056):902–906. doi: 10.1016/s0140-6736(96)06453-7. [DOI] [PubMed] [Google Scholar]
- Bove R., Bevan C., Crabtree E., Zhao C., Gomez R., Garcha P., et al. Toward a low-cost, in-home, telemedicine-enabled assessment of disability in multiple sclerosis. Mult. Scler. 2019;25(11):1526–1534. doi: 10.1177/1352458518793527. [DOI] [PubMed] [Google Scholar]
- Brownlee W., Bourdette D., Broadley S., Killestein J., Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- Burton J.M., O'Connor P.W., Hohol M., Beyene J. Cochrane Libr [Internet]; 2012. Oral Versus Intravenous Steroids for Treatment of Relapses in Multiple Sclerosis. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candel G.R., Tejada Cifuentes F. Que inyectables pueden ser administrados por vía oral o enteral? Art. Esp. Rev. Clín. Med. Fam. 2015;8(2):119–124. [Google Scholar]
- Freedman M.S., Patry D.G., Grand'Maison F., Myles M.L., Paty D.W., Selchen D.H., Canadian MS Working Group Treatment optimization in multiple sclerosis. Can. J. Neurol. Sci. 2004;31(2):157–168. doi: 10.1017/s0317167100053804. May. [DOI] [PubMed] [Google Scholar]
- Freedman M.S., Selchen D., Arnold D.L., Prat A., Banwell B., Yeung M., Morgenthau D., Lapierre Y. Canadian Multiple Sclerosis Working Group. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can. J. Neurol. Sci. 2013;40(3):307–323. doi: 10.1017/s0317167100014244. May. [DOI] [PubMed] [Google Scholar]
- Frohmann E., Shah A., Eggenberger E., Metz L., R Z., Stuve O. Corticosteroids for multiple sclerosis: i. application for treating exacerbations. Neurotherapeutics. 2007;4(4):618–626. doi: 10.1016/j.nurt.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lattanzi S., Cagnetti C., Danni M., Provinciali L., Silvestrini M. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J. Neurol. 2017;264:1697–1704. doi: 10.1007/s00415-017-8505-0. [DOI] [PubMed] [Google Scholar]
- Le Page E., Veillard D., Laplaud D.A., Hamonic S., Wardi R., Lebrun C., et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386:974–981. doi: 10.1016/S0140-6736(15)61137-0. [DOI] [PubMed] [Google Scholar]
- Liu S., Liu X., Chen S., Xiao Y., Zhuang W. Oral versus intravenous methylprednisolone for the treatment of multiple sclerosis relapses: A meta-analysis of randomized controlled trials. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada Y., Falcone M., Granero R. Administración oral de preparado parenteral de vitamina K en anticoagulación excesiva por warfarina [Oral administration of intravenous preparation of Vitamin K for excessive anticoagulation due to warfarin. Medicina (B Aires. 2012;72(2) Spanish. [PubMed] [Google Scholar]
- Lublin F.D., Reingold S.C., Cohen J.A., Cutter G.R., Soelberg S.P., Thompson A.J., et al. Defining the Clinical Course of Multiple Sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli V., Rocca M.A., Annovazzi P., Pulizzi A., Rodegher M., Martinelli B.F., et al. A short-term randomized MRI study of high-dose oral vs intravenous methylprednisolone in MS. Neurology. 2009;73(22):1842–1848. doi: 10.1212/WNL.0b013e3181c3fd5b. [DOI] [PubMed] [Google Scholar]
- Morrow S.A., Fraser J.A., Day C., Bowman D., Rosehart H., Kremenchutzky M., et al. Effect of treating acute optic neuritis with bioequivalent oral vs intravenous corticosteroids: a randomized clinical trial. JAMA Neurol. 2018;75(6):690. doi: 10.1001/jamaneurol.2018.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nos C., Sastre-Garriga J., Borràs C., Río J., Tintoré M., Montalban X., et al. Clinical impact of intravenous methylprednisolone in attacks of multiple sclerosis. Mult. Scler. 2004;10(4):413–416. doi: 10.1191/1352458504ms1068oa. [DOI] [PubMed] [Google Scholar]
- Pascual A.M., Boscá I., Escutia M., Bernat A., Coret F., Casanova B. Prospective assessment of the treatment of multiple sclerosis relapses with oral high-dose methylprednisolone: response and tolerability data. Neurologia. 2008;23(2):73–77. Spanish. [PubMed] [Google Scholar]
- Ramo-Tello C., Grau-López L., Tintoré M., Rovira A., Ramió i Torrenta L., Brieva L., et al. A randomized clinical trial of oral versus intravenous methylprednisolone for relapse of MS. Mult. Scler. 2014;20(6):717–725. doi: 10.1177/1352458513508835. [DOI] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. [Internet] 2021 doi: 10.1001/jamaneurol.2021.0688. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segamarchi C., Silva B., Saidon P., Garcea O., Alonso R. Would it be recommended treating multiple sclerosis relapses with high dose oral instead intravenous steroids during the COVID-19 pandemic? Yes. Mult. Scler. Relat. Disord. 2020;46(102449) doi: 10.1016/j.msard.2020.102449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solà-Valls N., Vicente-Pascual M., Blanco Y., Solana E., Llufriu S., Martínez-Heras E., et al. Spanish validation of the telephone assessed Expanded Disability Status Scale and Patient Determined Disease Steps in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2019;27:333–339. doi: 10.1016/j.msard.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., et al. Disease-modifying therapies and Coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo A., Amato C.S., Clarke N., Reitz R.E., Salo D. Injectable dexamethasone sodium phosphate administered orally? A pharmacokinetic analysis of a common emergency department practice. J. Pediatr. Pharmacol. Ther. 2015;20(2):105–111. doi: 10.5863/1551-6776-20.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]