To the editor:
Patients with end-stage renal disease undergoing dialysis are at very high risk of death in case of coronavirus disease 2019.1 Growing evidence suggests that a relatively high proportion of dialysis patients develops anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies after vaccination, albeit to a lesser extent than healthy controls.2 Here, we investigated whether there are differences in anti–SARS-CoV-2 antibody levels (directed against the receptor binding domain of the S1 subunit of the Spike [S] protein, measured with the SARS-CoV-2 IgG II Quant assay [Abbott]) in patients on hemodialysis without a history of SARS-CoV-2 infection after full vaccination (2 doses) with 1 of the 2 currently available mRNA vaccines (BNT162b2, Pfizer–BioNtech; or mRNA-1273, Moderna) against SARS-CoV-2, three weeks after administration of the second vaccine dose. A patient flowchart, study methods, patient demographics, and additional analyses are shown in Supplementary Figure S1, the Supplementary Methods, Supplementary Table S1, and Supplementary Figures S2 and S3. In the entire cohort (N = 116), 3 patients (2.6%; 2 vaccinated with BNT162b2, 1 vaccinated with mRNA-1273) remained anti-S-antibody negative.
Patients vaccinated with mRNA-1273 showed significantly higher anti-S-antibody titers (median: 1507; interquartile range [IQR]: 612–3112 binding antibody units [BAU]/ml) than did patients vaccinated with BNT162b2 (median: 676; IQR: 197–1363 BAU/ml; P < 0.0013; Figure 1 ). After correction for age, sex, diabetes status, serum albumin, dialysis dose, previous kidney transplantation, ongoing immunosuppressive medication, and active malignancy, patients who were vaccinated with mRNA-1273 showed 2.98-fold higher anti-S-antibody titers than did patients vaccinated with BNT162b2 in a linear regression analysis (P < 0.0003). In a sensitivity analysis including only patients without ongoing immunosuppressive therapy (n = 102), anti-S-antibody titers of patients who were vaccinated with mRNA-1273 were 2.39-fold (P < 0.005) higher compared with patients who were vaccinated with BNT162b2.
Figure 1.
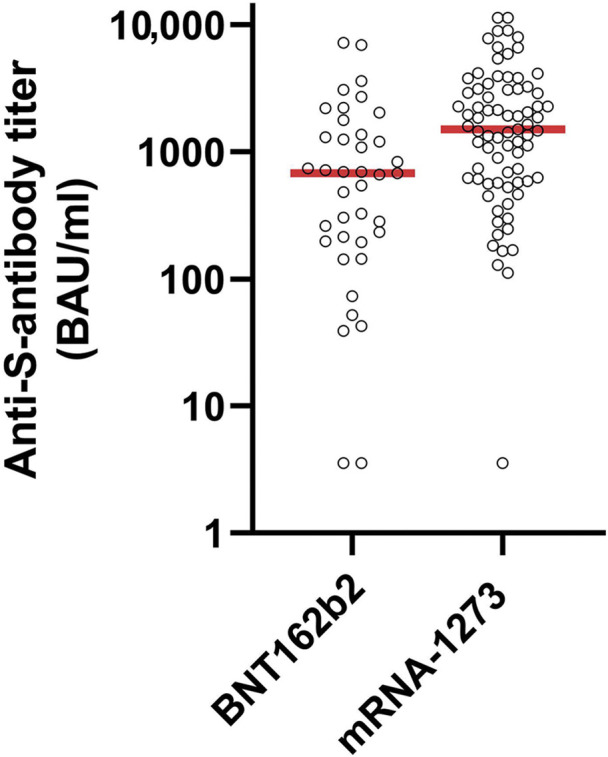
Anti-S-antibody titers (log-scale) in hemodialysis patients according to vaccine type. Open circles indicate anti-S-antibody titers of individual patients. Horizontal red lines indicate median antibody titers. BAU, binding antibody units.
In conclusion, patients on hemodialysis who were vaccinated with mRNA-1273 showed higher anti-S-antibody titers than did patients vaccinated with BNT162b2.
Editor’s Note.
In this issue of the Journal, several important preliminary data regarding the immunogenicity of mRNA vaccines in patients on maintenance dialysis are published. The Editors took option to report those data because there are accumulating questions regarding the “best vaccine” in terms of immune response and the sensitivity to vaccines of emerging variants now referred to as variants of concerns (VOCs). They also invite the readers to look at the paper devoted to the mechanisms of escape developed by these variants in the Journal Club of this issue of Kidney international. In a study comparing 2 mRNA vaccines, Kaiser et al. report that in a small and select cohort, hemodialysis patients vaccinated with mRNA-1273 (Moderna) showed higher anti-S antibody titers than patients vaccinated with BNT162b2 (Pfizer–BioNtech), but the cellular immune response was not analyzed. In another letter to the editor, Speer et al. investigated the neutralization of variants B.1.1.7 (alpha—first detected in the UK) and B.1.351 (beta—first detected in South Africa) using sera taken 3 weeks after the second BNT162b2 dose in 30 patients receiving maintenance hemodialysis and 18 healthy controls. While all healthy controls showed neutralizing activity against both the B.1.1.7 and B.1.351 variants, the ID50 (i.e., serum dilution that inhibits 50% of the infectivity) was lower in hemodialysis patients with neutralizing antibodies against the VOC B.1.351 variant detected in only 15 of the 24 immunized patients. Blazquez-Navarro et al. examined the cellular and humoral immunity toward SARS-CoV-2 reference and alpha and beta strains in COVID-19 convalescent (n = 18) and BNT162b2-vaccinated dialysis patients (n = 22). They showed a significantly higher number of humoral responders to VOC and titers of neutralizing antibodies to both SARS-CoV-2 and VOC in convalescent compared to fully vaccinated dialysis patients. Similar data were reported for cellular immune response. While these data should be interpreted with caution due to multiple limitations, such as small sample sizes, select cohorts, and limited adjustment for comorbidities, they also raise significant concern regarding ongoing risk for COVID-19 disease in vaccinated dialysis patients. Additional data will be crucial in coordinating future vaccination recommendations in this vulnerable patient population.
Footnotes
Supplementary Methods.
Figure S1. Patient flowchart.
Figure S2. Patients with a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (N = 8; mRNA-1273: N = 5, BNT-162b2: N = 3) showed higher anti-S-antibody titers (median: 9418; interquartile range [IQR]: 6114–11,360 binding antibody units [BAU]/ml) than patients without prior SARS-CoV-2 infection (median: 1200; IQR: 447–2275 BAU/ml).
Figure S3. Beta-coefficients ± 1.96 SEs of the multivariate linear regression analysis with anti-S-antibody titers as the dependent variable.
Table S1. Patient demographics.
Supplementary References.
Supplementary Material
References
- 1.Jager K.J., Kramer A., Chesnaye N.C. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon B., Rubey H., Treipl A. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared to healthy controls [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab179 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


