Key Points
Severe exacerbation of underlying hematologic conditions can occur within 1 to 4 days after dose 2 of a 2-dose SARS-CoV-2 vaccine series.
A mild exacerbation after dose 1 and/or a history of vaccine-related adverse events may portend a more serious event after dose 2.
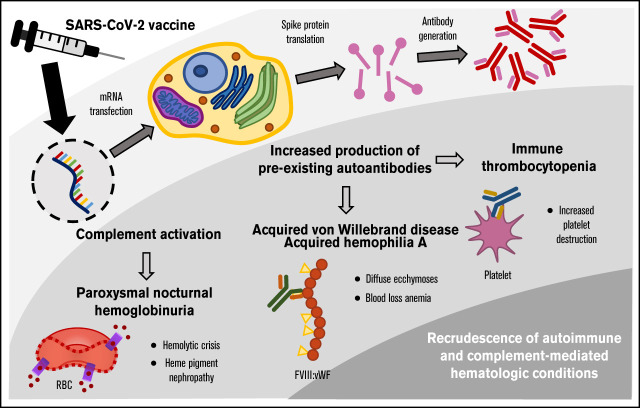
Visual Abstract
Abstract
A variety of autoimmune disorders have been reported after viral illnesses and specific vaccinations. Cases of de novo immune thrombocytopenia (ITP) have been reported after SARS-CoV-2 vaccination, although its effect on preexisting ITP has not been well characterized. In addition, although COVID-19 has been associated with complement dysregulation, the effect of SARS-CoV-2 vaccination on preexisting complementopathies is poorly understood. We sought to better understand SARS-CoV-2 vaccine-induced recurrence of autoimmune- and complement-mediated hematologic conditions. Three illustrative cases were identified at the University of Washington Medical Center and the Seattle Cancer Care Alliance from January through March 2021. We describe the recrudescence of 2 autoimmune conditions (ITP and acquired von Willebrand Disease [AvWD]/acquired hemophilia A) and 1 complementopathy (paroxysmal nocturnal hemoglobinuria [PNH]). We report the first known case of AvWD/acquired hemophilia A, and describe the first PNH exacerbation in the absence of complement inhibition after SARS-CoV-2 vaccination. Although SARS-CoV-2 vaccine-induced ITP is a known concern, our case clearly depicts how thrombocytopenia in the setting of preexisting ITP can sequentially worsen with each vaccine dose. Based on our experiences and these examples, we provide considerations for how to monitor and assess risk in patients with underlying autoimmune- and complement-mediated hematologic conditions.
Introduction
The etiology of autoimmune disorders is largely attributable to an individual’s genetic risk factors, exposure to environmental triggers, and underlying immune dysregulation.1 Whereas a variety of autoimmune disorders have been reported after vaccination, specific vaccines elicit unique autoimmune pathology.2 For example, secondary immune thrombocytopenia (ITP) is strongly linked to the measles, mumps, and rubella vaccine3,4 and is associated with hepatitis A and B vaccines.5 Yet other vaccines, such as the quadrivalent human papilloma virus vaccine, demonstrate no autoimmune safety signal.6
SARS-CoV-2 infection has been shown to effect sustained dysregulation of adaptive and innate immunity.7 The immunomodulatory effects of SARS-CoV-2 vaccination, however, are poorly understood. In a recent commentary, 20 cases of secondary ITP were reported after vaccination with both Pfizer and Moderna SARS-CoV-2 vaccines, 17 of which had no history of thrombocytopenia. Exacerbations of preexisting ITP have been suspected, but not confirmed.8 A letter to the editor of Blood recently described 4 patients with paroxysmal nocturnal hemoglobinuria (PNH), all treated with terminal complement inhibition (eg, eculizumab and ravulizumab) and all of whom developed breakthrough hemolysis after vaccination with both Pfizer and Moderna SARS-CoV-2 vaccines.9 However, increased hemolysis in the absence of complement inhibition has not been described.
The effect of SARS-CoV-2 vaccination on patients with preexisting autoimmune hematologic conditions is poorly understood, but is of considerable interest to direct patient care and public health. To this end, we present 3 illustrative cases of hematologic vaccine-related adverse events after SARS-CoV-2 vaccination and provide considerations for postvaccination surveillance and management.
Case description
Case 1
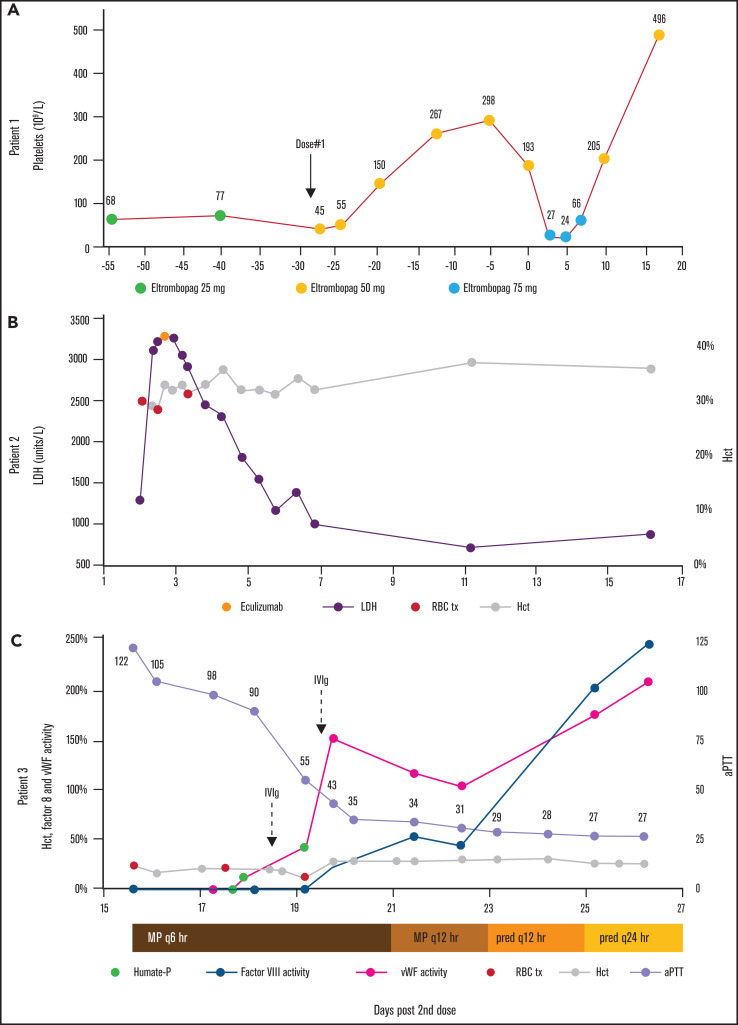
Patient 1 was a 72-year-old woman with chronic ITP on maintenance eltrombopag 25 mg daily. Recent SARS-CoV-2 semiquantitative total antibody testing was negative. Before dose 1 of the Moderna SARS-CoV-2 vaccine, her platelet baseline was 65 × 109/L to 80 × 109/L (Figure 1A). Approximately 13 hours after receiving dose 1 of the Moderna SARS-CoV-2 vaccine, her platelet count fell to 45 × 109/L (42% decrease). The eltrombopag dose was increased to 50 mg daily, with an increase in platelet count to a peak of 298 × 109/L. Approximately 1 hour before receiving dose 2, the platelet count was 193 × 109/L. Three days later, the platelet count fell to 27 × 109/L (86% decrease), prompting an increase in eltrombopag to 75 mg daily. In response to 3 doses, the platelet count increased to 205 × 109/L.
Figure 1.
Disease parameters relative to dose 2 of the SARS-CoV-2 vaccine series. (A) The platelet count of patient 1 exhibited a mild decrease after dose 1 and a marked decrease after dose 2. The eltrombopag daily dose was adjusted to compensate for dynamic platelet destruction. (B) In patient 2, the hemolysis marker, LDH, rapidly increased after dose 2. Eculizumab was administered on day 3, with excellent response. (C) In patient 3, factor VIII and vWF activities were initially undetectable, but increased in response to methylprednisolone (MP) and prednisone (pred), Humate-P, and IVIg. aPTT improved concurrently. Red blood cell transfusion and hematocrit are noted.
Case 2
Patient 2 was an 81-year-old woman with a long-standing history of steroid-responsive, classic PNH (granulocyte clone size, 98%), with a flare 2 years earlier, triggered by her second Shingrix vaccine. She had never been treated with terminal complement inhibition. Approximately 8 hours after receiving dose 2 of the Moderna SARS-CoV-2 vaccine, she developed flu-like symptoms (Figure 1B). One day after dose 2, she developed dark urine, generalized weakness, abdominal pain, and odynophagia. She presented to hematology clinic, where laboratory results demonstrated severe hemolysis: lactate dehydrogenase (LDH), 1272 U/L; total bilirubin, 1.8 mg/dL; hemoglobin (Hb), 10.0 g/dL; hematocrit (Hct), 30%; creatinine (Cr), 0.91 mg/dL; and a negative direct antibody test. She received a transfusion of 2 U red blood cells (RBCs), was sent to the emergency department and then was admitted for further management of a PNH flare. Admission reverse transcription polymerase chain reaction for SARS-CoV-2 was negative.
Overnight, she received a transfusion of 1 additional unit of RBCs. She received 1× methylprednisolone 1 g and 1× eculizumab 600 mg IV. On day 2, LDH peaked at 3282 U/L. She received a transfusion of 1 additional unit of RBCs to suppress erythropoiesis. Hemolysis levels rapidly improved. Her course was complicated by heme pigment–associated acute kidney injury (peak Cr, 1.69 mg/dL; baseline, 0.79 mg/dL), which responded to treatment with aggressive IV fluids. She received meningococcal prophylaxis with meningococcal B and ACWY vaccines without complications. She was discharged on day 6 and continued on terminal complement inhibition with ravulizumab in the outpatient setting.
Case 3
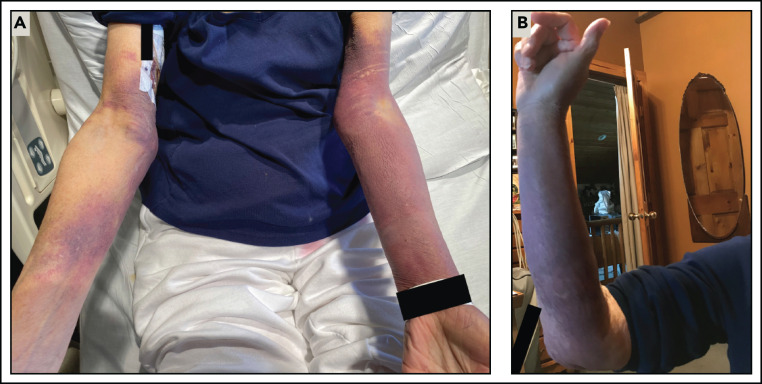
Patient 3 was a 76-year-old woman with a history of asthma; Raynaud’s phenomenon; multiple episodes of large, upper extremity ecchymoses; and a paraesophageal hernia status post recent uncomplicated Nissen fundoplication. She tolerated dose 1 of the Moderna SARS-CoV-2 vaccine. After dose 2, on day 4 she developed large ecchymoses covering most of her upper extremities (Figure 2A). The lesions were similar to those that had spontaneously developed about 1 year earlier (Figure 2B), at which time laboratory testing demonstrated abnormal ristocetin-induced platelet aggregation consistent with vWD and mildly prolonged activated partial thromboplastin time (aPTT), although no definitive diagnosis was made. On day 7, she developed persistently melanotic stool. On day 15 she presented to the emergency department with presyncope and was admitted for blood loss anemia. Initial laboratory results were notable for international normalized ratio, 1.5; aPTT, 122 s; von Willebrand factor (vWF) antigen, 5%; vWF activity, <3%; and factor VIII activity, <3% (Figure 1C). Admission SARS-CoV-2 reverse transcription polymerase chain reaction was negative. Monoclonal protein was not detected by serum protein electrophoresis. A vWF multimer analysis showed a normal pattern and distribution of bands, consistent with a quantitative vWF deficiency. A factor VIII inhibitor was detected and quantified as 11.2 Bethesda units (BUs). The patient was diagnosed with recurrent AvWD and acquired hemophilia A, presumed to be due to an antibody directed against the FVIII/vWF complex.
Figure 2.
Ecchymoses due to acquired vWD/acquired hemophilia A. (A) In patient 3, left greater than right upper extremity ecchymoses developed on day 4 after dose 2 of the Moderna SARS-CoV-2 vaccine. (B) A similar right upper extremity ecchymosis had spontaneously developed about 1 year earlier. Although laboratory testing at this time was notable for abnormal ristocetin-induced platelet aggregation and a mildly prolonged aPTT, a definitive diagnosis of acquired vWD/acquired hemophilia A was not made until her hospital presentation, as described in case 3.
On days 17 to 19, vWF/FVIII replacement therapy was provided with Humate-P 2290 U every 12 hours ×4 doses. Reduction of factor inhibitors was achieved using IV immunoglobulin (IVIg) and corticosteroids as follows: IVIg 1 g/kg on days 18 and 19; and methylprednisolone 125 mg every 6 hours on days 15 through 20, tapered to 125 mg 2 times daily on days 21 and 22; and prednisone 50 mg 2 times daily on days 23 and 24, then 50 mg daily starting on day 25. On day 21, upper endoscopy revealed mild oozing near the folds of the Nissen fundoplication. On day 25, vWF and factor VIII activity levels began spontaneously uptrending. On days 19 and 26, factor VIII inhibitor levels were 1.6 BUs and <0.5 BUs, respectively. She was discharged on day 28.
Methods
Patients who completed a SARS-CoV-2 vaccine series from January through March 2021 with development of vaccine-related adverse events were identified at the University of Washington Medical Center and Seattle Cancer Care Alliance. Three illustrative cases were selected where vaccination was thought to have triggered a flare of an underlying autoimmune- or complement-mediated hematologic condition.
Results and discussion
ITP, acquired hemophilia A, and AvWD are mediated by autoantibodies directed against platelet glycoproteins IIb/IIIa and Ib/Ix,10 coagulation factor VIII (FVIII),11,12 and vWF, respectively. All are associated with autoimmune disorders and malignancies.13,14 ITP and acquired hemophilia A can be triggered by vaccines10,15-18 and viral infections, including SARS-CoV-2.19,20 The pathogenesis of postviral de novo ITP is thought to involve molecular mimicry and circulating immune complexes.21 Flares of preexisting ITP, however, result from an augmentation of a prior immune response.
Hematologic autoimmune disease recrudescence after SARS-CoV-2 may involve stimulation of autoantibody production from preexisting B cells. As SARS-CoV-2 infection and vaccine are both capable of triggering autoimmunity, a shared element such as the spike protein is perhaps a necessary pathogenic component. Notably, whereas cases of de novo ITP primarily occurred with a first vaccine dose,8 case 1 demonstrates how the exacerbation of preexisting ITP can sequentially increase in severity with each of the 2 doses. The platelet count fell by 86% with the second dose compared with 42% with the first, consistent with a cumulative effect on the underlying humoral response. In case 3, the prolonged duration of coagulation abnormalities illustrates a durable immune response and highlights the utility of corticosteroids in eliminating an autoantibody inhibitor. The rapid normalization of FVIII and vWF activity after administration of IVIg is suggestive of an IgG antibody-mediated process against endogenous and exogenous FVIII/vWF complex.
PNH is not mediated by the adaptive immune system and instead results from complement-mediated lysis of glycosylphosphatidylinositol-deficient RBCs. Vaccine-induced PNH flares are not typical, although 1 case has been reported after influenza vaccination.22 In addition, breakthrough hemolysis was reported in 4 patients on terminal complement inhibition after vaccination with Pfizer or Moderna SARS-CoV-2 vaccines.9 Among patients with severe COVID-19, complement activation has been demonstrated in the lungs, blood vessels, and kidneys, and unrestrained complement activation has been implicated in the development of multiorgan failure. One proposed mechanism by which this occurs involves stimulation of the lectin pathway via binding of the SARS-CoV-2 spike protein to mannose-binding lectin.23 As the Pfizer and Moderna vaccines deliver mRNA encoding the SARS-CoV-2 spike protein,24,25 the vaccine-generated spike protein may exacerbate underlying PNH through inadvertent stimulation of the lectin pathway. However, as the SARS-CoV-2 spike protein subunit 1 is unable to induce lysis of PNH erythrocytes in vitro,9 and our patient 2 had previously developed a similarly severe PNH exacerbation after dose 2 of the Shingrix vaccine series, the pathogenesis may simply involve nonspecific adjuvant-induced inflammation.
Among patients with autoimmune hematologic conditions, we advise close monitoring in the 1 to 4 days after each dose of a 2-dose SARS-CoV-2 vaccine series. If disease-associated abnormalities arise after dose 1, this may portend a more severe vaccine-related adverse event after dose 2 and should prompt consideration of delaying or deferring this dose. A history of vaccine-related adverse events may pose increased risk with SARS-CoV-2 vaccinations.
Authorship
Contribution: A.J.P. wrote the manuscript with support from C.S.; T.G., R.K.-J., and J.A. provided oversight; and the final manuscript was reviewed and approved by all authors.
Conflict-of-interest disclosure: T.G. has received consultancy fees from Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Andrew Jay Portuguese, University of Washington, 1100 Fairview Ave N, D5-100, Seattle, WA 98109; e-mail: aportugu@uw.edu.
References
- 1.Ehrenfeld M, Tincani A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AD, Shoenfeld Y.. Vaccine-induced autoimmunity. J Autoimmun. 1996;9(6):699-703. [DOI] [PubMed] [Google Scholar]
- 3.France EK, Glanz J, Xu S, et al. ; Vaccine Safety Datalink Team. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics. 2008;121(3):e687-e692. [DOI] [PubMed] [Google Scholar]
- 4.O’Leary ST, Glanz JM, McClure DL, et al. The risk of immune thrombocytopenic purpura after vaccination in children and adolescents. Pediatrics. 2012;129(2):248-255. [DOI] [PubMed] [Google Scholar]
- 5.Meyboom RH, Fucik H, Edwards IR.. Thrombocytopenia reported in association with hepatitis B and A vaccines. Lancet. 1995;345(8965):1638. [DOI] [PubMed] [Google Scholar]
- 6.Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271(2):193-203. [DOI] [PubMed] [Google Scholar]
- 7.Files JK, Boppana S, Perez MD, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest. 2021;131(1):e140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber GF, Yuan X, Yu J, et al. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria [letter]. Blood. 2021;DOI: 10.1182/blood.2021011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchette V, Bolton-Maggs P.. Childhood immune thrombocytopenic purpura: diagnosis and management. Pediatr Clin North Am. 2008;55(2):393-420, ix. [DOI] [PubMed] [Google Scholar]
- 11.Hay CR. Acquired haemophilia. Baillieres Clin Haematol. 1998;11(2):287-303. [DOI] [PubMed] [Google Scholar]
- 12.Collins P, Macartney N, Davies R, Lees S, Giddings J, Majer R.. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol. 2004;124(1):86-90. [DOI] [PubMed] [Google Scholar]
- 13.Franchini M, Gandini G, Di Paolantonio T, Mariani G.. Acquired hemophilia A: a concise review. Am J Hematol. 2005;80(1):55-63. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Pruthi RK, Nichols WL.. Acquired von Willebrand disease. Mayo Clin Proc. 2002;77(2):181-187. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Chen Z, Cheng X, et al. A previously treated severe haemophilia A patient developed high-titre inhibitor after vaccinations. Int J Immunopathol Pharmacol. 2020;34:2058738420934618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moulis G, Pugnet G, Bagheri H, et al. Acquired factor VIII haemophilia following influenza vaccination. Eur J Clin Pharmacol. 2010;66(10):1069-1070. [DOI] [PubMed] [Google Scholar]
- 17.Perricone C, Ceccarelli F, Nesher G, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res. 2014;60(2-3):226-235. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt N, Maitland H.. Acute Immune Thrombocytopenia following administration of Shingrix recombinant zoster vaccine. Am J Hematol. 2021;96(5):E136-E137. [DOI] [PubMed] [Google Scholar]
- 19.Franchini M, Glingani C, De Donno G, et al. The first case of acquired hemophilia A associated with SARS-CoV-2 infection. Am J Hematol. 2020;95(8):E197-E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahévas M, Moulis G, Andres E, et al. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190(4):e224-e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B.. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620-632. [DOI] [PubMed] [Google Scholar]
- 22.Green H, Eliakim-Raz N, Zimra Y, Gafter-Gvili A.. Paroxysmal nocturnal hemoglobinuria diagnosed after influenza vaccine: coincidence or consequence? Isr Med Assoc J. 2014;16(2):122-124. [PubMed] [Google Scholar]
- 23.Noris M, Benigni A, Remuzzi G.. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses [published correction appears in Nature. 2021;590(7844):E17]. Nature. 2020;586(7830):594-599. [DOI] [PubMed] [Google Scholar]