Abstract
Azoheteroarenes are an emerging class of photoswitchable compounds with unique photophysical properties and advantages over traditional azobenzenes. Therefore, methods to synthesize azoheteroarenes are highly desirable. Here, we utilize azide-alkyne click chemistry (AAC) to access arylazo-1,2,3-triazoles, a previously unexplored class of azoheteroarenes that exhibit high thermal stabilities and near quantitative bidirectional photoconversion. Controlling the catalyst or 1,3-dipole grants access to both regioisomeric arylazotriazoles as well as arylazoisoxazoles, highlighting the versatility of our approach.
Graphical Abstract

Photoswitchable compounds capable of toggling between two distinct isomeric states have applications ranging from materials science to biology owing to the precise spatial and temporal control that they can impart to a variety of systems.1,2,3,4,5,6,7,8 Azobenzenes have emerged as the most widely studied and utilized class of photoswitchable molecules due to their remarkable photostability, large and rapid geometric change following E → Z diazene isomerization, and highly tunable photophysical properties.9,10,11 While these properties make azobenzenes attractive photoswitches, significant challenges still exist in the design of optimized azobenzene-based photoswitches a priori.
Currently, complex asymmetric azobenzene photoswitches must be engineered on a case-by-case basis utilizing azologization or azoextension strategies,12,13 and they often require extensive optimization to achieve high degrees of bidirectional photoconversion, ideal thermal half-lives of the Z isomer, and acceptable photoswitching wavelengths for a given application. Optimization of any one of these parameters is often at the expense of another. For example, push-pull electronic systems are often utilized to red-shift the π → π* transition of the E isomer, but this can lead to greater overlap with the n → π* transition of the Z isomer causing incomplete photoswitching. As it is often difficult to predict how azobenzene modification might impact a range of photophysical properties, arduous empirical optimization is often required.13
Azoheteroarenes have sparked significant interest as alternative photochromic systems with many potential advantages over traditional azobenzenes.14 Of these, photoswitches comprised of five-membered nitrogenous heterocycles have shown the greatest promise (Figure 1). Herges and co-workers demonstrated that arylazoimidazoles can undergo near-quantitative E → Z photoconversion and exhibit thermal half-lives of several weeks. However, these molecules were unable to completely photoconvert back to the E isomer.15 Significant advancements came from Fuchter’s group with the development of arylazopyrazoles—compounds with unique photophysical properties including near quantitative bidirectional photoconversion and exceptionally long thermal half-lives (~1000 days).16 The vast majority of common nitrogenous heterocycles have been incorporated into azoheteroarenes and investigated in depth, with many possessing interesting photophysical characteristics.17,18 Additionally, arylazoisoxazole photoswitches were recently reported that can achieve >90% photoconversion to either the E or Z isomer and exhibit high Z isomer thermal stability, demonstrating the utility of oxygen heterocyclic photoswitches.19,20
Figure 1.

(A) Azoheteroarenes have emerged as attractive alternatives to traditional azobenzene photoswitches due to their useful photophysical properties. (B) Several modular photoswitch scaffolds are shown. The photoswitch module is depicted in blue and the appended structural motif in red.
Given the challenges associated with photoswitch optimization, several groups have developed methods for appending a pre-optimized photoswitchable module to any structural element of interest (Figure 1). Ravoo and co-workers added a level of modularity to previously reported arylazopyrazoles by installing an extended carboxylic acid linker as a tethering site on an existing azoheteroarene scaffold.21 Similarly, Yu, Li, and co-workers developed arylazopyrazole photoswitches with both phenol and ester tethering sites, enabling bifunctionalization.22 In 2019, our group developed a modular azobenzene scaffold that could be attached to a wide variety of groups via hydrazide click chemistry.23 Despite these advances, we still lack a general method for directly connecting any structural motif of interest to a photoswitchable module with pre-optimized photophysical properties.
Inspired by these discoveries, we decided to explore arylazo-1,2,3-triazoles—a previously unknown class of azoheteroarenes—as we reasoned that they might possess favorable photophysical properties while being readily accessed in a modular fashion through robust copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reactions. Arylazo-1,2,4-triazoles have been described previously,17 however the relative position of the three nitrogen atoms excludes them from the modularity and synthetic ease associated with CuAAC chemistry. We envisioned constructing the photoswitch through reaction of an arylazoalkyne-equivalent synthon with an azide pre-attached to any structure of interest. The robustness of CuAAC click chemistry and the associated wealth of methodology available for accessing azide-functionalized molecules24,25,26 made this strategy quite attractive. Moreover, we anticipated that the tethered group would be electronically decoupled from the photoswitchable module due to a lack of π-conjugation between the two components, and thus, would not impact the photochemistry. Unlike previous modular photoswitchable scaffolds, the tethered group of arylazotriazoles would be directly attached to the photoswitch, which would reduce the degrees of freedom and increase the likelihood that the two isomers would possess differential functional properties.
To access arylazotriazoles with diverse electronic properties, we first coupled di-tert-butyl hydrazodiformate (1) with a variety of aryl iodides under copper catalysis (Scheme 1).27 The resulting bis-Boc arylhydrazines proved challenging substrates for a variety of alkynylation reactions including standard ynamide-forming cross-coupling reactions with silyl-protected bromoalkynes28 and a recently reported CuCl2-catalyzed silyl-acetylene oxidative ynamide coupling.29 Fortunately, we were able to access 4a–4d in good to excellent yields through a very robust 2-step sequence involving dichlorovinylation followed by alkyl lithium-mediated elimination and lithium halogen exchange (Scheme 1).30
Scheme 1. Synthesis of a masked arylazoalkyne module.

Reagents and conditions. (a) aryl iodide, copper (I) iodide, 1,10-phenanthroline, Cs2CO3, DMF, 80°C. (b) NaH, DMF, then trichloroethylene. (c) n-BuLi, THF, −78°C to room temperature, then n-BuLi, −78°C followed by H2O. (d) N-(4-iodophenyl)acetamide, copper (I) iodide, 1,10-phenanthroline, Cs2CO3, DMF, 90°C. (e) TBAF, THF, 0°C.
The method used to synthesize 4a–d was not amenable to the synthesis of 4e, so we took an alternative approach. The addition of lithiated silyl-acetylene to di-tert-butyl azodicarboxylate produced 5 in 48% yield.31 Copper-catalyzed cross-coupling of 5 with N-(4-iodophenyl) acetamide yielded 6 in moderate yield, which was readily deprotected with TBAF (Scheme 1). We successfully employed this method with other aryl halides, and thus, it seems to be general. However, we favor the dichlorovinylation/elimination sequence in most scenarios given its simplicity and robustness.
To access a series of model arylazotriazoles, we reacted compounds 4a–4e with benzyl azide under CuAAC conditions followed by Boc group removal and oxidation of the hydrazine (Scheme 2). We opted to take the bis-protected hydrazines into the cycloaddition reaction given that unprotected ynamines are known to be relatively unstable and readily tautomerize to reactive ketenimines which undergo rapid hydration to form the corresponding hydrazides.32,33 Cascade deprotection-oxidation strategies using acid were unsuccessful given that acid can catalyze the rearrangement of hydrazo compounds to benzidenes, diphenylines, and semidines.34 Thermal deprotection of the Boc groups only produced the desired azo compounds in low yields. Ultimately, we found that Boc group deprotection with TMSI35,36 followed by hydrolysis of the resulting trimethylsilyl carbamates in an oxygenated atmosphere afforded the desired benzyl substituted arylazo-1,2,3-triazoles 8a–e in good overall yields (Scheme 2).
Scheme 2.

Preparation of model arylazotriazoles
Next, we performed constant illumination NMR experiments23,37 to establish the PSSs of these electronically diverse model arylazotriazoles under a variety of wavelengths (Figure 2A–B). The electron withdrawn photoswitch 8a underwent near near-quantitative conversion to the Z isomer when irradiated with 365 nm light but was unable to fully isomerize back to the E isomer under any wavelength tested. The unsubstituted photoswitch 8b also did not possess ideal photophysical properties as it was unable to reach highly enriched E or Z PSSs under any wavelength tested and displayed a diminished extinction coefficient in the UV-Vis spectrum, as compared to other arylazotriazole photoswitches.
Figure 2.
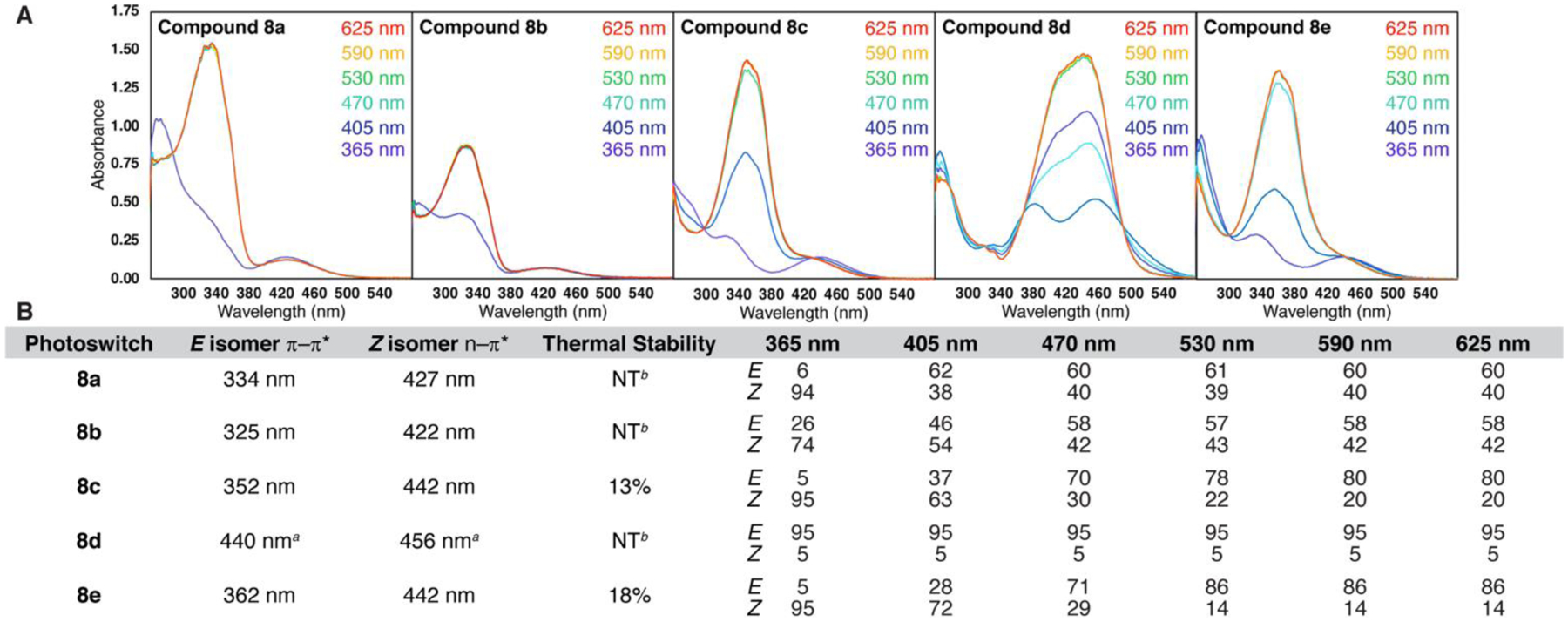
(A) UV-Vis spectra of model arylazotriazole photoswitches (50 μM in DMSO) under various wavelengths of light. (B) Photophysical properties as determined via UV-Vis spectroscopy (electronic transitions) and constant illumination NMR (thermal stability and PSSs). Thermal stability indicates the percentage of Z isomer that reverts to the E isomer after 1 week in the dark as measured by 1H NMR. aThe π → π* and n → π* transitions for the E and Z isomers, respectively, overlap. NT = not tested. bNot determined due to an inability to achieve highly enriched PSS states.
Fortunately, the methoxy substituted 8c can access a PSS quantitatively enriched in the Z isomer under 365 nm light with longer wavelengths achieving approximately 80% photoconversion to the E isomer (Figure 2B). Increasing the electron donating ability of the para substituent further as in 8d appeared to be detrimental, likely due to rapid thermal relaxation due to the strong push-pull effect. Compound 8e, chosen for its electronic similarity to 8c, achieved near-quantitative conversion to the Z isomer at 365 nm, and >85% photoconversion to the E isomer at longer wavelengths. Interestingly, unlike push-pull azobenzenes which typically experience rapid thermal relaxation, compounds 8c and 8e are exceptionally stable as their Z isomers. Their thermal stabilities, defined as the percentage of Z to E relaxation in the dark after 1 week measured via 1H NMR integration ratios, were observed to be 13% and 18%, respectively. The observed Z isomer stability may be rationalized through a favorable H-π interaction between the triazole and phenyl component, as proposed by Fuchter in regards to the Z isomer stability of similar azoheteroarenes.17
Due to the desirable photophysical properties of 8c and 8e, we reacted a variety of alkyl and aryl azides with 4c and 4e followed by deprotection/oxidation to produce arylazo-1,2,3-triazole photoswitches (note: care must be taken when working with organic azides as they are potentially explosive). Ambient temperature was suitable for a majority of the deprotection sequences, but the reactions of aromatic tethered compounds 10–13 were low yielding under these conditions. This issue was resolved by using extended reaction times and/or heating during the deprotection step. As expected, the tethered group had minimal impact on the photophysical properties of the photoswitchable module with all arylazotriazoles achieving high bidirectional photoconversion (Figure 3). Additionally, we investigated the UV-Vis spectral properties of photoswitches based on 4c with various alkyl and aromatic functional groups and found that the electronic transitions were comparable regardless of direct aryl or alkyl attachment to the photoswitch scaffold (Figure S1). Photoswitches derived from 4c were preferred to those derived from 4e given that 4c can easily be prepared on gram scale.
Figure 3.

Synthesis and properties of arylazotriazoles. PSSs at 365 nm (most Z-enriched) and 530 nm (most E-enriched) are shown. Quantification of a photostationary state consisting of >95% of a single isomer is challenging due to the signal-to-noise ratio of 1H NMR. Therefore, a 95:5 ratio represents a maximally enriched photostationary state. aPhotoswitch 15 achieves the most E-enriched PSS (78%) at 470 nm, not 530 nm.
Having demonstrated the exceptional photophysical properties of 1,4-substituted triazoles derived from 4c, we were interested in accessing the regioisomeric 1,5-substituted triazoles. Fortunately, we were able to access 18, simply by switching from copper(I)- to ruthenium(II)-catalysis38 (Figure 4). Like its regioisomer 8c, 18 was able to achieve high bidirectional photoconversion. The ability to access two structural distinct photoswitches from the same starting materials is a hallmark of our approach and should prove exceptionally useful when optimizing for functional properties. Finally, we constructed arylazoisoxazole photoswitch 19 through dipolar cycloaddition of 4c with a nitrile oxide derived from benzyl hydroximoyl chloride (Figure 4).39 While 4-arylazoisoxazole photoswitches have been described recently,19,20 this new class of 5-arylazoisoxaole can only be accessed via the specialized arylynehydrazides presented here. Like the arylazotriazoles, compound 19 achieved high bidirectional photoconversion (>90% enrichment of either the E or Z isomer). However, the thermal stabilities of 8c, 18, and 19 were quite different. While 8c was so stable that we were unable to reliably measure a half-life (13% conversion in 1 week as measured by 1H NMR), the half-lives of 18 and 19 were measured to be 35.2 h and 25.5 min, respectively (as measured by UV-Vis).
Figure 4.

(A) Synthesis of 1,5-substituted triazole 18. (B) Synthesis of 3,5-substituted isoxazole 19. PSSs at 365–625 nm are shown. UV-Vis spectra for 18 and 19 are shown to the right (50 μM in DMSO) under various wavelengths of light. Half-lives (t1/2) were measured by UV-Vis spectroscopy.
In conclusion, we have discovered arylazo-1,2,3-triazoles—a new class of azoheteroarenes that possess exceptional photophysical properties and can be easily prepared in a modular fashion. Furthermore, our strategy enables the synthesis of both arylazotriazole regioisomers as well as arylazoisoxazoles simply by controlling the catalyst or 1,3-dipole. Given the availability of their azide precursors, the predictability of their photophysical properties, and the ease of their synthesis, we anticipate that arylazotriazoles will find great utility as light-responsive molecular switches for a variety of applications.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997 to DEO; T32GM113770 to RJT), a Provost’s Undergraduate Fellowship (NY), an R. Bryan Miller Graduate Fellowship (RJT), and a Francesca Miller Undergraduate Research Award (NY). Funding for NMR spectrometers was provided by the National Science Foundation (Grants DBI0079461 and DBI-0722538). Analysis for this project was performed in the UC Davis Campus Mass Spectrometry Facilities, with instrument funding provided by the NIH (Grant 1S10OD025271).
Footnotes
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, characterization data, and 1H and 13C NMR spectra (PDF)
REFERENCES
- 1.Tochitsky I; Kienzler MA; Isacoff E; Kramer RH Restoring Vision to the Blind with Chemical Photoswitches. Chem. Rev 2018, 118, 10748–10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szymański W; Beierle JM; Kistemaker HAV; Velema WA; Feringa BL Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev 2013, 113 6114–6178. [DOI] [PubMed] [Google Scholar]
- 3.Browne WR; Feringa BL Light Switching of Molecules on Surfaces. Annu. Rev. Phys. Chem 2009, 60, 407–428. [DOI] [PubMed] [Google Scholar]
- 4.Fehrentz T; Schönberger M; Trauner D Optochemical Genetics. Angew. Chem. Int. Ed 2011, 50, 12156–12182. [DOI] [PubMed] [Google Scholar]
- 5.Russew M; Hecht S Photoswitches: From Molecules to Materials. Adv. Mater 2010, 22, 3348–3360. [DOI] [PubMed] [Google Scholar]
- 6.Velema WA; Szymanski W; Feringa BL Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc 2014, 136, 2178–2191. [DOI] [PubMed] [Google Scholar]
- 7.Hüll K; Morstein J; Trauner D In Vivo Photopharmacology. Chem. Rev 2018, 118, 10710–10747. [DOI] [PubMed] [Google Scholar]
- 8.Pianowski ZL Recent Implementations of Molecular Photoswitches into Smart Materials and Biological Systems. Chem. Eur. J 2019, 25, 5128–5144. [DOI] [PubMed] [Google Scholar]
- 9.Bandara HMD; Burdette SC Photoisomerizaiton in Different Classes of Azobenzene. Chem. Soc. Rev 2012, 41, 1809–1825. [DOI] [PubMed] [Google Scholar]
- 10.Beharry AA; Woolley GA Azobenzene Photoswitches for Biomolecules. Chem. Soc. Rev 2011, 40, 4422–4437. [DOI] [PubMed] [Google Scholar]
- 11.Oscurato SL; Salvator M; Maddalena P; Ambrosio A From Nanoscopic to Macroscopic Photo-Driven Motion in Azobenzene-Containing Materials. Nanophotonics 2018, 7, 1387–1422. [Google Scholar]
- 12.Morstein J; Awale M; Reymond J; Trauner D Mapping the Azolog Space Enables the Optical Control of New Biological Targets. ACS Cent. Sci 2019, 5, 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briochhagen J; Frank JA; Trauner D A Roadmap to Success in Photopharmacology. Acc. Chem. Res 2015, 48, 1947–1960. [DOI] [PubMed] [Google Scholar]
- 14.Crespi S; Simeth NA; König B Heteroaryl Azo Dyes as Molecular Photoswitches. Nat. Rev. Chem 2019, 3, 133–146. [Google Scholar]
- 15.Wendler T; Schütt C; Näther C; Herges R Photoswitchable Azoheterocycles via Coupling of Lithiated Imidazoles with Benzenediazonium Salts. J. Org. Chem 2012, 77, 3284–3287. [DOI] [PubMed] [Google Scholar]
- 16.Weston CE; Richardson RD; Haycock PR; White AJP; Fuchter MJ Arylazopyrazoles: Azoheteroarene Photoswitches Offering Quantitative Isomerization and Long Thermal Half-Lives. J. Am. Chem. Soc 2014, 136, 11878–11881. [DOI] [PubMed] [Google Scholar]
- 17.Calbo J; Weston CE; White AJP; Rzepa HS; Contreras-García J; Fuchter MJ Tuning Azoheteroarene Photoswitch Performance Through Heteroaryl Design. J. Am. Chem. Soc 2017, 139, 1261–1274. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y; Gao C; Andréasson J; Grøtl M Synthesis and Photophysical Characterization of Azoheteroarenes. Org. Lett 2018, 20, 4875–4879. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P; Srivastava A; Sah C; Devi S; Venkataramani S Arylazo-3,5-dimethylisoxazoles: Azoheteroarene Photoswitches Exhibiting High Z-isomer Stability, Solid-State Photochromism, and Reversible Light-Induced Phase Transition. Chem. Eur. J 2019, 25, 11924–11932. [DOI] [PubMed] [Google Scholar]
- 20.Kortekaas L; Simke J; Kurka DW; Ravoo BJ Rapid Photoswitching of Low Molecular Weight Arylazoisoxazole Adhesives. ACS Appl. Mater. Interfaces 2020, 12, 32054–32060. [DOI] [PubMed] [Google Scholar]
- 21.Stricker L; Fritz E; Peterlechner M; Doltsinis NL; Ravoo BJ Arylazopyrazoles as Light-Responsive Molecular Switches in Cyclodextrin-Based Supramolecular Systems. J. Am. Chem. Soc 2016, 138, 4547–4554. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z; He Y; Zhou Z; Yu C; Han L; Li T Pyrazolylazophenyl Ether‐Based Photoswitches: Facile Synthesis, (Near‐)Quantitative Photoconversion, Long Thermal Half‐Life, Easy Functionalization, and Versatile Applications in Light‐Responsive Systems. Chem. Eur. J 2019, 25, 13402–13410. [DOI] [PubMed] [Google Scholar]
- 23.Zhu JS; Larach JM; Tombari RJ; Gingrich PW; Bode SR; Tuck JR; Warren HT; Fettinger JC; Haddadin MJ; Tantillo DJ; Kurth MJ; Olson DE A Redox Isomerization Strategy for Accessing Modular Azobenzene Photoswitches with Near Quantitative Bidirectional Photoconversion. Org. Lett 2019, 21, 8765–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moses JE; Moorhouse AD The Growing Applications of Click Chemistry. Chem. Soc. Rev 2007, 36, 1249–1262. [DOI] [PubMed] [Google Scholar]
- 25.Meng G; Guo T; Ma T; Zhang J; Shen Y; Barry KB, Dong J Modular Click Chemistry Libraries for Functional Screens Using a Diazotizing Reagent. Nature 2019, 574, 86–89. [DOI] [PubMed] [Google Scholar]
- 26.Hein CD; Liu X; Wang D Click Chemistry, a Powerful Tool for Pharmaceutical Sciences. Pharm. Res 2008, 25, 2216–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolter M; Klapars A; Buchwald SL Synthesis of N-Aryl Hydrazides by Copper-Catalyzed Coupling of Hydrazides with Aryl Iodides. Org. Lett 2001, 3, 3803–3805. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y; Hsung RP; Tracey MR; Kurtz KCM; Vera EL Copper Sulfate-Pentahydrate-1,10-Phenanthroline Catalyzed Amidations of Alkynyl Bromides. Synthesis of Heteoraromatic Amine Substituted Ynamides. Org. Lett 2004, 6, 1151–1154. [DOI] [PubMed] [Google Scholar]
- 29.Hamada T; Ye X; Stahl S Copper-Catalyzed Aerobic Oxidative Amidation of Terminal Alkynes: Efficient Synthesis of Ynamides. J. Am. Chem. Soc 2008, 130, 833–835. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield SJ; Campbell CD; Jones MW; Anderson EA A Robust and Modular Synthesis of Ynamides. Chem. Commun 2015, 51, 3316–3319. [DOI] [PubMed] [Google Scholar]
- 31.Beveridge ER; Batey RA Terminal Alkyne Addition to Diazodicarboxylates: Synthesis of Hydrazide Linked Alkynes (Ynehydrazides). Org. Lett 2012, 14, 540–543. [DOI] [PubMed] [Google Scholar]
- 32.Dodd R; Cariou K Ketenimines Generated from Ynamides: Versatile Building Blocks for Nitrogen-Containing Scaffolds. Chem. Eur. J 2018, 24, 2297–2304. [DOI] [PubMed] [Google Scholar]
- 33.Huang H; Tang L; Xi Y; He G; Zhu H Metal-Free Hydration of Ynamides: Convenient Approach to Amides. Tetrahedron Lett 2016, 57, 1873–1876. [Google Scholar]
- 34.Yang Z; Hou S; He W; Cheng B; Jiao P; Xu J Regioselectivity of the Ortho- and Para-Semidine and Diphenyline Rearrangements. Tetrahedron 2016, 72, 2186–2195. [Google Scholar]
- 35.Jung ME, Martinelli MJ, Olah GA, Surya Prakash GK and Hu J Encyclopedia of Reagents for Organic Synthesis Chichester, UK: John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 36.Chang JW; Cognetta AB; Niphakis MJ; Cravatt BF Proteome-Wide Reactivity Profiling Identifies Diverse Carbamate Chemotypes Tunes for Serine Hydrolase Inhibition. ACS Chem. Biol 2013, 8, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banghart MR; Trauner DA 1H NMR Assay for Measuring the Photostationary States of Photoswitchable Ligands. Methods Mol. Biol 2013, 995, 107–120. [DOI] [PubMed] [Google Scholar]
- 38.Johansson JR; Somfai-Beke T; Stålsmeden AS; Kann N Ruthenium-Catalyzed Azide Alkyne Cycloaddition Reaction: Scope, Mechanism, and Applications. Chem. Rev 2016, 116, 14726–14768. [DOI] [PubMed] [Google Scholar]
- 39.Roscales S; Plumet J Metal-Catalyzed 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides. Org. Biomol. Chem 2018, 16, 8446–8461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


