Abstract
Natto, a traditional Japanese fermented soybean food, is well known to be nutritious and beneficial for health. In this study, we examined whether natto impairs infection by viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as well as bovine herpesvirus 1 (BHV-1). Interestingly, our results show that both SARS-CoV-2 and BHV-1 treated with a natto extract were fully inhibited infection to the cells. We also found that the glycoprotein D of BHV-1 was shown to be degraded by Western blot analysis and that a recombinant SARS-CoV-2 receptor-binding domain (RBD) was proteolytically degraded when incubated with the natto extract. In addition, RBD protein carrying a point mutation (UK variant N501Y) was also degraded by the natto extract. When the natto extract was heated at 100 °C for 10 min, the ability of both SARS-CoV-2 and BHV-1 to infect to the cells was restored. Consistent with the results of the heat inactivation, a serine protease inhibitor inhibited anti-BHV-1 activity caused by the natto extract. Thus, our findings provide the first evidence that the natto extract contains a protease(s) that inhibits viral infection through the proteolysis of the viral proteins.
Keywords: BHV-1, Glycoprotein D, SARS-CoV-2, Receptor binding domain, Bacillus subtilis, Antiviral effect
1. Introduction
There are no signs of an end to the ongoing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Reports regarding it have been dramatically accumulating worldwide since the first infection was reported over a year ago [1]. Vaccines are being rolled out at an unprecedented speed owing to the recent technological advancements. However, immunizing every individual in need of the vaccine will be a slow process. In addition, strains with mutated target epitopes of the vaccine have been reported [2]. Vaccination alone may not guarantee complete protection; therefore, it is important to develop other measures and treatments against SARS-CoV-2 infection.
Our aim in this study was to investigate whether natto has antiviral activities including SARS-CoV-2 and bovine herpesvirus 1 (BHV-1). BHV-1 causes respiratory disease to bovine. Natto is known to be one of the most traditional and unique foods in Japan and is produced by fermenting steamed soybeans with Bacillus subtilis. Traditionally, Japanese people have assumed that natto is beneficial for their health, and in recent years, research studies have revealed scientific evidence for this belief [[3], [4], [5]]. However, antiviral properties of natto were not well studied except for surfactin, a cyclic lipopeptide antibiotic [[6], [7], [8]]. In this study, we showed that protease activities in a natto extract degrade SARS-CoV-2 receptor-binding domain (RBD) and BHV-1's glycoprotein D, leading to inhibition of viral infections to cells.
2. Materials and methods
2.1. Preparation of natto extracts
We prepared natto extract using the methods described below. Ten grams of commercially available S903 natto (Takano Foods Co., Ltd., Ibaraki, Japan) was placed in a 50 mL centrifuge tube and stirred with a glass rod for 5 min at room temperature. Then, 10 mL of phosphate-buffered saline (PBS) was added. After mixing by inversion 50 times, the mixture was centrifuged at 3500 rpm for 10 min and the supernatant was filtered with a ϕ0.45 μm filter followed by a ϕ0.22 μm filter. The resultant soluble fraction, hereinafter called “natto extract” in this study, was kept at −30 °C until use. The natto extract was treated with or without heating at 100 °C for 10 min. Thus, two different samples were prepared, namely, heated-natto extract, unheated-natto extract.
2.2. Viruses and cells
BHV-1, Ishikawa strain (bovine/Japan/1988), was cultured using the MDBK cells. A strain of SARS-CoV-2 isolated from a patient who developed COVID-19 in the cruise ship Diamond Princess in Japan in February 2020 was obtained from the Kanagawa Prefectural Institute of Public Health (SARS-CoV-2/Hu/DP/Kng/19-027, LC528233) [9]. SARS-CoV-2 was cultured using Vero E6 cells expressing transmembrane protease serine 2 (TMPRSS2).
2.3. Plaque formation assay
Ninety μL of each of two types of natto extract described above and 10 μL of BHV-1 or SARS-CoV-2 stock solution were mixed and incubated at 37 °C for 1 h. The mixture was then diluted at 10,000-fold or 5000-fold with minimum essential medium (MEM) and 500 μL of them was inoculated into MDBK cells for BHV-1 and VeroE6/TMPRSS2 cells for SARS-CoV-2 in a 12- or 6-well plate. After 1 h of adsorption, the cells were washed once with sterile PBS and layered with MEM containing carboxymethyl cellulose. After culturing at 37 °C for 3 days, plaque formation assay was conducted using crystal violet to visualize infectivity. Basically, for each test, at least two independent experiments were performed. The natto extracts alone tested did not cause any cytotoxic effects on the cells (data not shown).
2.4. Protease inhibitors
The 80 μL of natto extract diluted at 10-fold with PBS was incubated with 1 μL of Halt Protease Inhibitor Cocktail, EDTA-free (Thermo Fisher Science K.K., Tokyo, Japan) and 19 μL of PBS at 37 °C for 1 h. Ten μL of BHV-1 was added to the solution and plaque formation assay was performed as described above. The inhibitor cocktail contains six kinds of inhibitor. Aprotinin (Cayman Chemical, Michigan U.S.A.), AEBSF. hydrochloride (AdipoGen Life Sciences, Inc., San Diego, U.S.A.), Pepstatin A (Merck KGaA, Darmstadt, Germany), Leupeptin (Peptide Institute, Inc., Osaka, Japan), E-64 (Cayman Chemical), Bestatin hydrochloride (Sigma-Aldrich, Tokyo, Japan) were used.
2.5. Degradation analysis of viral proteins
2.5.1. Glycoprotein D of BHV-1
67.5 μL of BHV-1 stock solution was incubated with 7.5 μL of natto extract at 37 °C for 1 h. Western blot analysis was performed using an anti-BHV-1 glycoprotein D monoclonal antibody (1B8-F11 BHV-1/IBR gD-gIV; Veterinary Medical Research and Development, WA, U.S.A).
2.5.2. Receptor-binding domain (RBD) of SARS-CoV-2
A recombinant SARS-CoV-2 (S1 subunit protein) host cell receptor-binding domain (RBD) conjugated with a His-tag at its C-terminus (RayBiotech Life, GA, USA) was used in this study. The RBD protein was synthesized based on Wuhan-Hu-1 isolate (GenBank accession number QHD43416). We also used UK variant N501Y RBD-His recombinant protein (Sino Biological, PA, USA). The mixture of 4.5 μL of each type of natto extract and ∼2 μL of the SARS-CoV-2 RBD protein was incubated at 37 °C for 1 h. As controls, PBS was used accordingly. SDS-PAGE was performed under reducing conditions and the gel was stained with Coomassie Brilliant Blue after electrophoresis.
3. Results and discussion
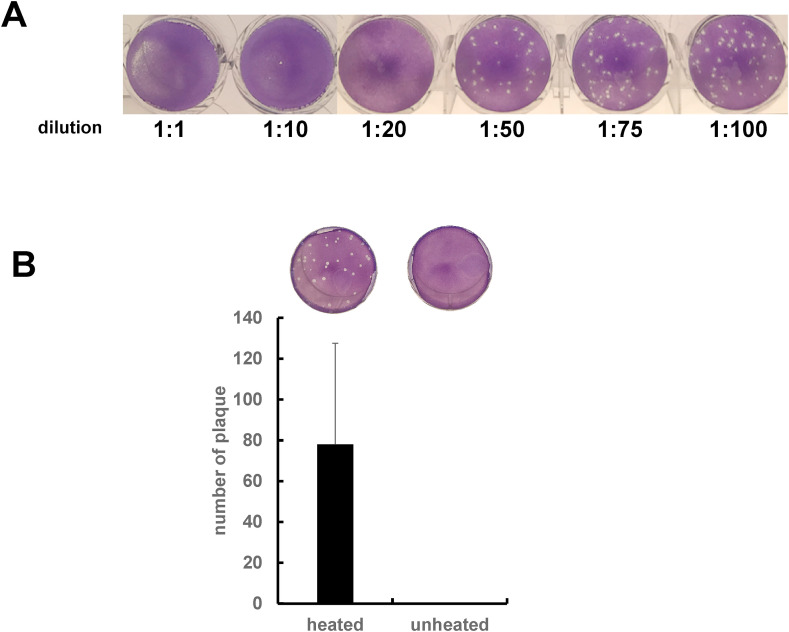
To investigate whether the natto extract has antiviral activity, BHV-1 was incubated with the natto extract and performed plaque formation assay. As shown in Fig. 1 A, 1-, 10- and 20-fold dilution of natto extract inhibited BHV-1 infection to MDBK cells. Further dilution of the natto extract, such as 50-, 75-, and 100-fold, clearly produced plaques in a dose-dependent manner. This result indicated that the natto extract contains a dose-dependent antiviral activity. Interestingly, when the natto extract was heated at 100 °C for 10 min, such antiviral activity was lost (Fig. 1B), suggesting an inhibitory factor(s) in the extract is heat-sensitive. Surfactin that is produced by Bacillus subtilis as a surfactant is known to have an antiviral activity [[6], [7], [8]]. As expected, our additional experiments demonstrated that 0.1 mg/mL of surfactin inhibited BHV-1 infection when BHV-1 was incubated with unheated surfactin (data not shown). However, BHV-1 kept infecting the MDBK cells when the virus was incubated with heated surfactin that was heated at 100 °C for 10 min (data not shown). Hence, surfactin carrying the antiviral activity in our hand is heat-resistant, while the putative inhibitory factor(s) in the natto extract that is discovered in this study is heat-sensitive. In addition, when the natto extract was incubated with BHV-1 at 4 °C, the anti-BHV-1 activity was not observed (data not shown). Thus, taken together, these results led us to hypothesize that the antiviral activity in the natto extract is probably due to an enzyme(s) such as a protease(s) but not surfactin.
Fig. 1.
Inhibitory effects of natto extract on BHV-1 infection. (A) BHV-1 was incubated with a series of the diluted natto extract from 1-fold to 100-fold dilution and the plaque formation assay was then performed. (B) BHV-1 was incubated with either 1-fold dilution of heated or unheated natto extract. The plaque formation assay was then performed and number of plaques was shown in the graph (mean ± SE, n = 3).
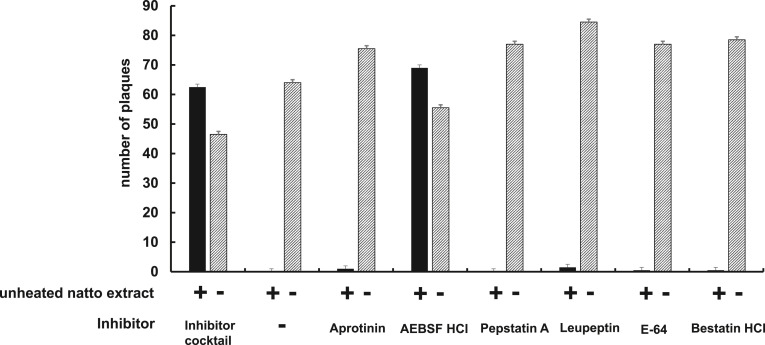
To confirm our hypothesis, natto extract was treated with protease inhibitors. As shown in Fig. 2 , when the natto extract was treated with a protease inhibitor cocktail, BHV-1 was apparently able to infect the cells. This result strongly suggested that a protease(s) in the natto extract was the heat-sensitive inhibitory factor(s). Furthermore, plaques were observed when one of the protease inhibitors, AEBSF HCl (4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride) which is an irreversible serine-protease inhibitor, was treated (Fig. 2). Hence, this result reveals that a serine-protease(s) in the natto extract is one of candidates for the antiviral activity.
Fig. 2.
Effects of protease inhibitors on anti-BHV-1 activity by the natto extract. BHV-1 was incubated with heated or unheated natto extract at 10-fold dilution after treatment with a protease inhibitor cocktail, Aprotinin, AEBSF HCl, Pepstatin A, Leupeptin, E-64, or Bestatin HCl. The plaque formation assay was then performed and number of plaques was shown in the graph (mean ± SE, n = 3).
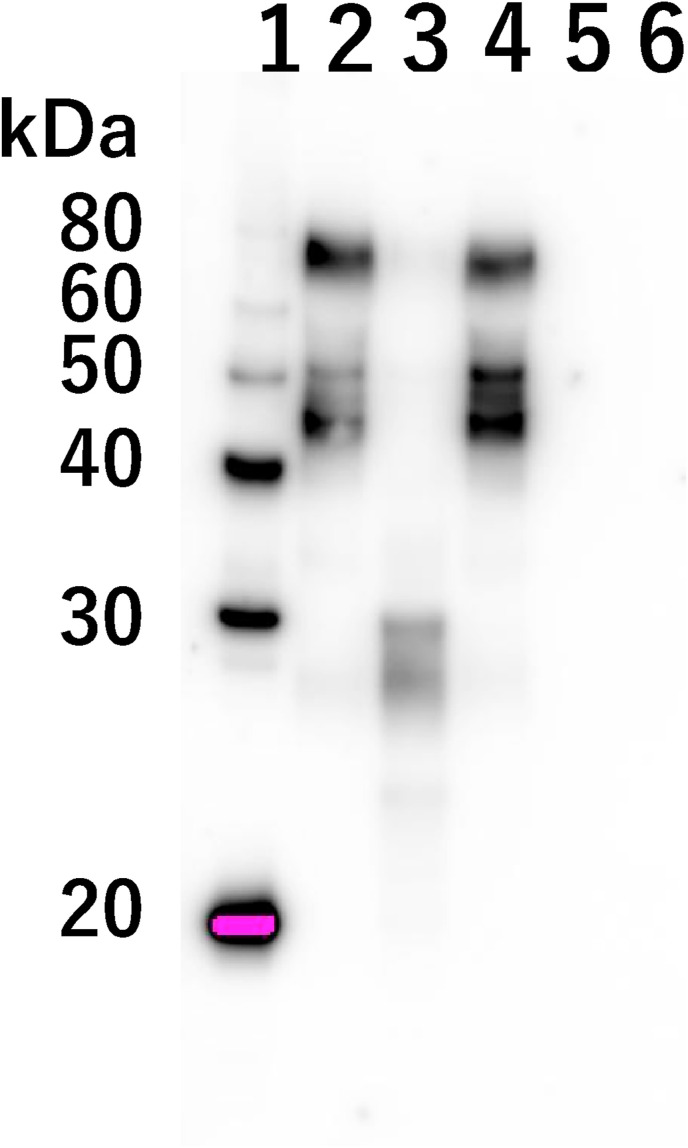
To investigate whether the putative protease(s) digests a surface protein of BHV-1 particle in vitro, Western blot analysis using the anti-BHV-1 glycoprotein D monoclonal antibody was performed. The glycoprotein D plays an important role in virus envelope-cell membrane fusion [10]. The glycoprotein D appears as broad bands at approximately 45–77 kDa when BHV-1 was incubated with the heated natto extract (Fig. 3 , lane 2). In contrast, incubation of BHV-1 with the unheated natto extract induced proteolytical degradation of glycoprotein D (Fig. 3, lane 3). Bands detected by the glycoprotein D antibody were apparently lower (around 30 kDa) compared to those in lane 3. Therefore, these results imply that the putative protease(s) inhibits BHV-1 infection by the digestion of glycoprotein D.
Fig. 3.
Degradation of glycoprotein D of BHV-1 treated with natto extract. BHV-1 was incubated with the heated or unheated natto extracts. Western blot analysis was conducted using anti-glycoprotein D monoclonal antibody. Lane 1: protein marker, Lane 2: BHV-1 treated with PBS, Lane 3: BHV-1 treated with unheated-natto extract, Lane 4: BHV-1 treated with heated-natto extract, Lane 5: PBS treated with unheated-natto extract, Lane 6: PBS treated with heated-natto extract.
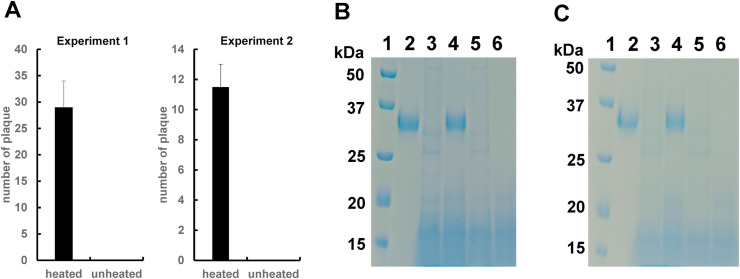
Next, we tested whether the natto extract was able to inhibit infection of SARS-CoV-2 to Vero E6 cells. After mixing SARS-CoV-2 with the unheated-natto extract, plaque formation assay was performed. As shown in Fig. 4 A, plaques were formed when SARS-CoV-2 was incubated with the unheated natto extract, but the heated natto extract lost such capability (Fig. 4A). These results were consistent with those with BHV-1 (Fig. 1B), suggesting that the putative inhibitory factor(s) in the natto extract is heat-sensitive protease(s). The receptor binding domain (RBD) of spike protein in SARS-CoV-2 plays an important role for attachment to the ACE2 receptor of the host cell during the early stages of infection [11]. As shown in Fig. 4B, the 33 kDa recombinant RBD protein was apparently degraded by incubation with unheated-natto extract (lane 3). On the other hand, the RBD protein was intact when the heated natto extract was used (lane 4). Taken together, these findings support the notion that the inhibition of SARS-CoV-2 to the cells shown in Fig. 4A was due to RBD degradation by the natto extract. Recently, emergence of mutant SARS-CoV-2 is a huge threat. The N501Y mutant in RBD has become predominant in England [12]. Fig. 4C indicated that RBD protein carrying a point mutation, N501Y, was degraded. In addition, evolutionary analysis of the SARS-CoV-2 RBD protein indicated that V367F is one of important mutations for higher binding affinity toward ACE2 receptor [13]. The RBD protein carrying V367Y is also degraded by treatment with the natto-extract (data not shown). These results strongly suggested that the natto extract has potential to digests many types of mutations, which are occurring now and will occur in future.
Fig. 4.
Anti-SARS-CoV-2 activity caused by the natto extract. (A) SARS-CoV-2 was incubated with the heated and unheated natto extracts (1-fold dilution). The plaque assay was carried out three times (experiment 1, 2 and 3). Each plaque assay was performed in duplicate. Experiment 1 and 2 were shown in the panel A. A similar result was obtained in Experiment 3 (data not shown). Number of plaques in each examination was average of duplicate. (B) The recombinant RBD protein of SARS-CoV-2 was incubated with the heated or unheated natto extract. The samples were loaded on gels for SDS-PAGE. Lane 1: protein marker, Lane 2: RBD protein treated with PBS, Lane 3: RBD protein treated with unheated-natto extract, Lane 4: RBD protein treated with heated-natto extract, Lane 5: PBS treated with unheated-natto extract, Lane 6: PBS treated with heated-natto extract. (C) The recombinant RBD protein of SARS-CoV-2 carrying a point mutation (N501Y) was incubated with the heated or unheated natto extract. The samples were loaded on gels for SDS-PAGE. Lane 1: protein marker, Lane 2: RBD protein (N501Y) treated with PBS, Lane 3: RBD protein (N501Y) treated with unheated-natto extract, Lane 4: RBD protein (N501Y) treated with heated-natto extract, Lane 5: PBS treated with unheated-natto extract, Lane 6: PBS treated with heated-natto extract.
In this study, we showed that the natto extract inhibited infectious ability of BHV-1 and SARS-CoV-2, to the cells and degraded the glycoprotein D of BHV-1 and the RBD protein of SARS-CoV-2. However, this inhibitory effect of the natto extract was impaired upon heating as well as treating with the serine-protease inhibitor. Hence, it is conceivable that this inhibitory effect was due to the putative heat-sensitive serine-protease(s). This is further supported by the evidence that the protease(s) was able to digest multi-sites of glycoprotein D and RBD protein. Therefore, our study suggests that the putative serine-protease(s) in natto may impair the infectious function of BHV-1 and SARS-CoV-2, probably through the proteolysis of its glycoprotein D and the spike protein, respectively. Future research must focus on identifying the proteases and locating the cleavage sites. Purified protease(s) in the natto extract is worth to be further investigated as antiviral drug(s). Finally, our findings open a future avenue towards new application of natto as a raw material.
Declaration of competing interest
This study was funded by Takano Foods Co., Ltd. We used S903 natto of the company in this study.
Acknowledgments
This study was supported by a fund from Takano Foods Co., Ltd.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2021.07.034.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., et al. Novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382:727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisblum Y., Schmidt F., Zhang F., et al. Escape from neutralizing antibodies 1 by SARS-CoV-2 spike protein variants. Elife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagata C. Soy intake and chronic disease risk: findings from prospective cohort studies in Japan. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-020-00744-x. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Nozue M., Shimazu T., Charvat H., et al. Fermented soy products intake and risk of cardiovascular disease and total cancer incidence: the Japan Public Health Center-based Prospective study. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-020-00732-1. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Pabich M., Materska M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients. 2019;11:1660. doi: 10.3390/nu11071660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson B.A., Hage A., Kalveram B., et al. Peptidoglycan-associated cyclic lipopeptide disrupts viral infectivity. J. Virol. 2019;93:1–15. doi: 10.1128/jvi.01282-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M., Mück D., Grossmann L., et al. Surfactin from Bacillus subtilis displays promising characteristics as O/W-emulsifier for food formulations. Colloids Surf. B Biointerfaces. 2021;203:111749. doi: 10.1016/j.colsurfb.2021.111749. [DOI] [PubMed] [Google Scholar]
- 8.Yuan L., Zhang S., Wang Y., et al. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J. Virol. 2018;92:1–19. doi: 10.1128/jvi.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallapaty S. What the cruise-ship outbreaks reveal about COVID-19. Nature. 2020;580:18. doi: 10.1038/d41586-020-00885-w. [DOI] [PubMed] [Google Scholar]
- 10.Levings R.L., Collins J.K., Patterson P.A., et al. Virus, strain, and epitope specificities of neutralizing bovine monoclonal antibodies to bovine herpesvirus 1 glycoproteins gB, gC, and gD, with sequence and molecular model analysis. Vet. Immunol. Immunopathol. 2015;164:179–193. doi: 10.1016/j.vetimm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Smaoui M.R., Yahyaoui H. Unraveling the stability landscape of mutations in the SARS-CoV-2 receptor-binding domain. Sci. Rep. 2021;11:pp1–pp13. doi: 10.1038/s41598-021-88696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung K., Shum M.H.H., Leung G.M., et al. MedRxiv.; 2020. Early Empirical Assessment of the N501Y Mutant Strains of SARS-CoV-2 in the United Kingdom; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S. Evolutionary and structural analysis elucidates mutations on SARS-CoV2 spike protein with altered human ACE2 binding affinity. Biochem. Biophys. Res. Commun. 2021;538:97–103. doi: 10.1016/j.bbrc.2021.01.035. 10.1016/j.bbrc.2021.01.035 PMID: 33602511 PMCID: PMC7883683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.