Abstract
Background:
Although oral anticoagulants (OACs) have been shown to substantially reduce the risk of stroke and other thromboembolic events in patients with atrial fibrillation (AF), these medications are significantly underutilized in clinical practice. However, many studies showing underuse of OACs predated the advent of the non-vitamin K antagonist oral anticoagulants. We conducted this study to examine use of OACs in a large commercially insured population, which was enrolled in a randomized trial to address underuse of OACs.
Methods:
Administrative health care claims data from 5 research partners who participate in the FDA-Catalyst, a program of the Sentinel Initiative, were queried in September 2017 to identify patients ≥30 years old with ≥365 days of medical/pharmacy coverage, ≥2 diagnosis codes for AF, a CHA2DS2-VASc score ≥2, absence of selected conditions for which OAC use is contraindicated, and no evidence of OAC use in the 365 days prior to the index AF diagnosis. The identified cohort has been targeted for enrollment in the IMPACT-AFib trial, a randomized clinical trial evaluating the effect of patient and provider education interventions on the use of OACs.
Results:
A total of 241,044 AF patients met the cohort eligibility criteria prior to assessment of OAC treatment. In this cohort, 220,869 (92%) patients were ≥ 65 years old and 94,459 (39%) patients were ≥ 80 years old. Patients were randomized to early or delayed intervention. Among 120,522 patients randomized to the early intervention arm, 43,826 (36%) had no evidence of OAC use in the prior 12 months. Compared with patients with evidence of an OAC use in the prior 12 months, patients without OAC use were more likely to be 80 years of age or older, women, and residents of the Midwest region. Patients without OAC use were more likely to have a history of anemia (52% vs. 48%) and less likely to have diabetes (39% vs. 44%), a history of stroke or TIA (17% vs. 20%), and a history of heart failure (40% vs. 48%). The mean CHA2DS2-VASc score was 5 for both the OAC and no-OAC recipients; however, patients with no OAC use had a higher ATRIA score (39% vs. 35%).
Conclusions:
Data from a large privately insured population show that despite a high risk of stroke, over one third of patients with AF and no obvious contraindications to an OAC were not treated with an OAC in the prior 12 months. Thus, there is an unmet medical need for studies that develop evidence-based interventions that could lead to greater use of OACs in patients with AF who are at risk for stroke.
Atrial fibrillation (AF) affects more than 5 million Americans, and this number is increasing as the United States population ages.1–4 AF can result in substantial mortality and morbidity including thromboembolic events, myocardial infarction, and heart failure.1–7 Of the thromboembolic events that occur as a result of AF, stroke is by far the most common and serious. When stroke occurs as a complication of AF, it is more disabling and deadly than other strokes.1 Therefore, it is imperative to identify all patients with AF who are at risk of stroke, especially because this risk can be substantially reduced with oral anticoagulation. Identifying AF patients at risk for stroke can be accomplished by using well-established risk scores. One such risk score is the CHA2DS2-VASc score (C=congestive heart failure, H=hypertension, A=age, D=diabetes, S=prior stroke or transient ischemic attack (TIA), V=vascular disease, S=sex).2 The American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend using the CHA2DS2-VASc score in clinical practice and, in the absence of absolute contraindications, initiating an oral anticoagulant (OAC) promptly for a score of ≥ 2.2
Although oral anticoagulation is very effective for stroke prevention in AF, studies have shown that about half of AF patients with a CHA2DS2-VASc score of ≥ 2 are not on an OAC.8–11 However, most of these studies predated the non-vitamin K antagonist oral anticoagulants (NOACs).12–15 Our goal in this paper is therefore to examine current utilization of OACs for AF among privately insured individuals, including both commercially insured and Medicare Advantage populations. To that end, we used electronic health data from 5 large research partners, using a common data model and distributed querying methods developed under the FDA Sentinel Initiative. FDA-Catalyst is the Sentinel component that leverages the ability of Sentinel network partners to engage in interventions or interactions with health plan members and providers,16 and it provides the infrastructure for the IMplementation of a randomized controlled trial to imProve treatment with oral AntiCoagulanTs in patients with Atrial Fibrillation (IMPACT-AFib trial) that was launched in 2017. The main purpose of the IMPACT-AFib trial is to assess the effect of patient and provider education interventions on the use of OACs among patients with AF and with guideline-based indications for oral anticoagulation (CHA2DS2-VASc score of 2 or greater).17
Methods
Data sources
The data source for this FDA-Catalyst study was the Sentinel distributed network made up of electronic health data locally transformed to a Common Data Model. Each of the five participating research partners maintains a local version of its own data in a Common Data Model format in order to enable distributed queries.18 To ensure privacy and data protection, queries are distributed and results are returned through a secure portal. Health plan members older than 65 years of age include both commercially insured and Medicare Advantage beneficiaries.
Study Population
Administrative health care claims data from 5 research partners were queried in September 2017 to identify patients ≥30 years old with ≥365 days of medical/pharmacy coverage, ≥2 diagnosis codes for AF (1 within 365 days of research partners’ data availability end date), a CHA2DS2-VASc score ≥2, absence of specific conditions for which OAC use is contraindicated, and no OAC treatment in the 365 days prior to the research partners’ data abstraction end date. Contraindications to OAC use were intracranial hemorrhage, pregnancy, or hospitalization for bleeding within the prior 6 months. Patients were also excluded if they had a condition other than AF that requires anticoagulation such as deep vein thrombosis, pulmonary embolism, or a mechanical cardiac valve. Patients with dispensing of a P2Y12 antagonist (e.g. clopidogrel and prasugrel) within the 90 days prior to cohort identification were also excluded. Treatment was defined as at least 1 bill for dispensing of an OAC or ≥4 INR test results or procedure codes, to capture use of warfarin without a claim being made. Research partners’ end dates for data query ranged from June 2016 through November 2016.
The study protocol specified the method of identifying all patients eligible for treatment with an OAC and for randomizing them to early or delayed intervention. OAC exposure status was determined in the early intervention group. It will be determined in the delayed intervention group after 12 months, when the delayed intervention to the provider only will occur. Evidence of OAC use was defined as at least one dispensing of an OAC (warfarin, coumadin, dabigatran, rivaroxaban, apixaban or edoxaban) in the prior 365 days or at least four International Normalized Ratio (INR) test results based on procedure codes in the prior 365 days. INR testing is performed regularly in users of warfarin to determine whether the medication effect is in the therapeutic range, thus frequent INRs are another method to identify who is likely being treated with warfarin. Also, INRs were included because users of warfarin may not have dispensings recorded in their claims data due to low cost options that are paid out of pocket. Because individuals eligible for OAC treatment were randomized to the early and delayed intervention groups, analysis of the treatment status and other characteristics of the early intervention group are expected to be representative of the entire population of these health plans.
Statistical Analysis
Patients with evidence of OAC use in the prior 12 months were characterized and compared with patients with no OAC use in the prior 12 months. Categorical variables are presented as counts (percentages), and continuous variables as means (standard deviations [SD]). Categorical variables were compared using the Pearson’s chi-square or Fisher’s exact test, as appropriate. Continuous variables were compared using two-sample t-test. P-values <0.05 from two-sided tests were considered statistically significant. Adjustments were not made for multiple comparisons. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 309,859 patients were identified as having 2 or more diagnoses of AF with at least 1 diagnosis within the12 months prior to the last date used for cohort identification. Prior to assessing OAC use at baseline defined as any OAC use in the prior 12 months, 241,044 patients met guideline criteria for OAC therapy. Reasons for excluding patients (i.e. reasons for the drop from 309,859 to 241,044 patients) are shown in Figure 1 (some patients may have had more than 1 exclusion criterion). Table 1 shows the baseline characteristics of the 241,044 patients who met guideline criteria for OAC therapy. In the overall cohort of eligible AF patients, 220,869 (92%) were ≥ 65 years old with 94,459 (39%) patients ≥ 80 years old. Almost half of the cohort (112,500, 47%) were women. The most common comorbidities included hypertension (229,059, 95%), anemia (118,343, 49%), heart failure (108,588, 45%), diabetes (100,438, 42%), and a history of cerebrovascular or thromboembolic event (75,434, 31%). Prior stroke or TIA was present in 45,262 (19%) patients. There were 106,084 (44%) patients with CHA2DS2VASc scores of 4 and 5; the overall mean score was 5. Only 12% had a CHA2DS2VASc score of 2. A total of 120,642 (51%) patients had an intermediate or high Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) score for bleeding (score is based on prior stroke, age, sex, history of heart failure, hypertension, diabetes, proteinuria and renal function). The majority of patients (206,381, 86%) had not been hospitalized in the 6 months preceding cohort identification. As shown in Table 1, the baseline characteristics were well balanced between the early and delayed intervention groups.
Figure 1:
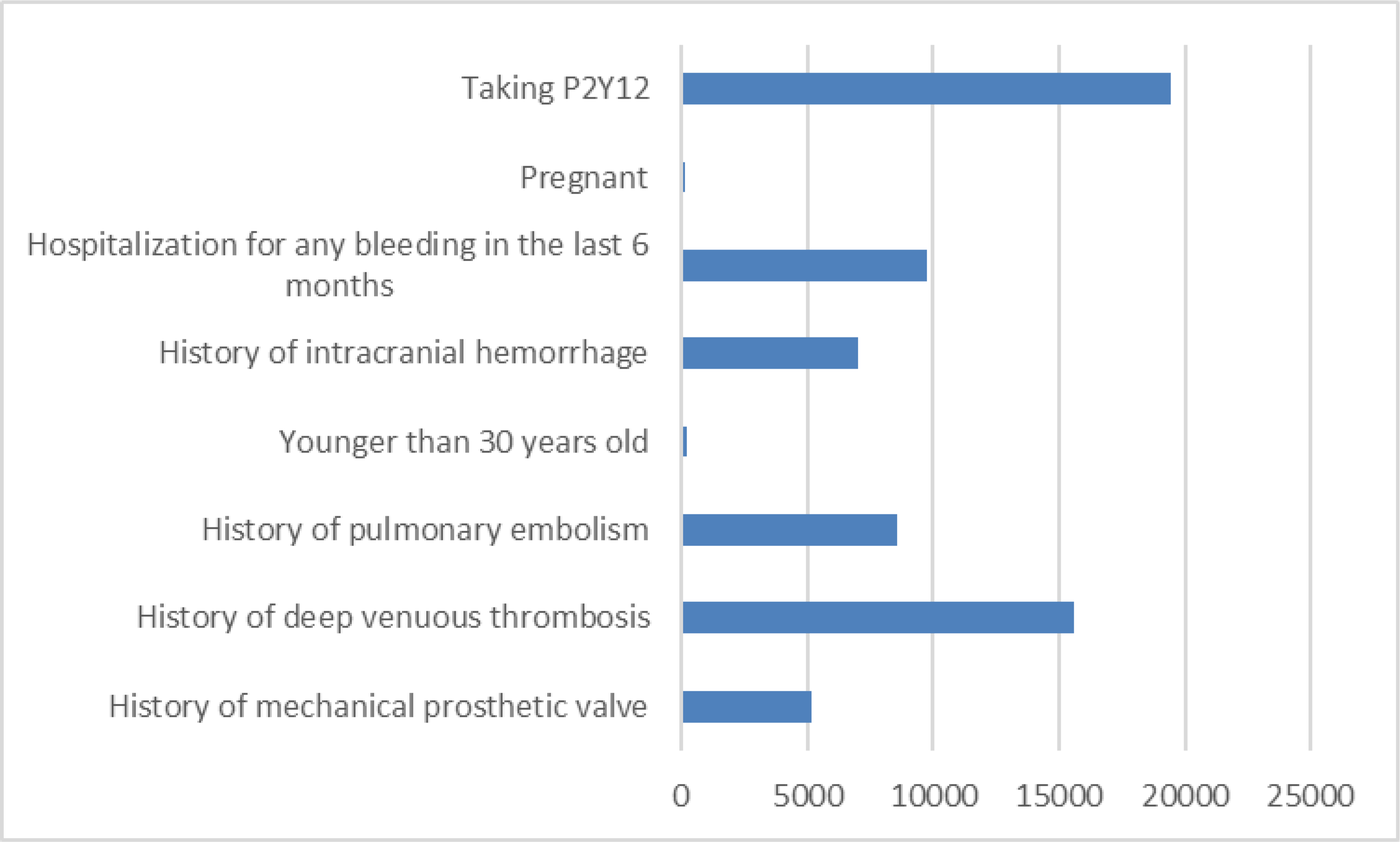
Reasons for excluding patients (some patients may have had more than 1 reason)
Table 1:
Baseline characteristics of privately insured patients with atrial fibrillation and CHA2DS2-VASC scores ≥2, by randomization status to early and delayed intervention cohorts
| All members meeting inclusion criteria with CHA2DS2-VASC scores ≥2 | Randomized to delayed intervention | Randomized to early intervention | |||||
|---|---|---|---|---|---|---|---|
| Totals | 241,044 | 120,522 | 120,522 | ||||
| N | % | N | % | N | % | P-value | |
| Age | 0.69 | ||||||
| <30–44 | 646 | 0.3 | 338 | 0.3 | 308 | 0.3 | |
| 45–64 | 19,529 | 8.1 | 9,747 | 8.1 | 9,782 | 8.1 | |
| 65–74 | 74,774 | 31.0 | 37,372 | 31.0 | 37,402 | 31.0 | |
| 75–80+ | 146,095 | 60.6 | 73,065 | 60.6 | 73,030 | 60.6 | |
| Female sex | 112,500 | 47 | 56,085 | 47 | 56,415 | 47 | 0.18 |
| Geographic Region | 0.09 | ||||||
| New England | 9,778 | 4 | 4,931 | 4 | 4,847 | 4 | |
| Mid-Atlantic | 12,543 | 5 | 6,290 | 5 | 6,253 | 5 | |
| South-Atlantic | 130,707 | 54 | 65,064 | 54 | 65,643 | 55 | |
| Midwest | 59,317 | 25 | 29,923 | 25 | 29,394 | 24 | |
| Mountain | 11,702 | 5 | 5,903 | 5 | 5,799 | 5 | |
| Pacific | 16,941 | 7 | 8,385 | 7 | 8,556 | 7 | |
| Unknown | 56 | 0 | 26 | 0 | 30 | 0 | |
| Anemia | 118,343 | 49 | 59,076 | 49 | 59,267 | 49 | 0.44 |
| Hypertension | 229,059 | 95 | 114,470 | 95 | 114,589 | 95 | 0.26 |
| Diabetes | 100,438 | 42 | 49,941 | 41 | 50,497 | 42 | 0.02 |
| Peripheral vascular disease | 72,396 | 30 | 36,172 | 30 | 36,224 | 30 | 0.82 |
| Prior cerebrovascular or thromboembolic event | 75,434 | 31 | 37,835 | 31 | 37,599 | 31 | 0.30 |
| Prior cerebrovascular event | 54,495 | 23 | 27,206 | 23 | 27,289 | 23 | 0.69 |
| Stroke or TIA | 45,262 | 19 | 22,620 | 19 | 22,642 | 19 | 0.91 |
| TIA | 34,948 | 15 | 17,495 | 15 | 17,453 | 15 | 0.81 |
| Stroke | 22,954 | 10 | 11,438 | 10 | 11,516 | 10 | 0.59 |
| Heart failure | 108,588 | 45 | 54,426 | 45 | 54,162 | 45 | 0.28 |
| Kidney disease | 16,913 | 7 | 8,392 | 7 | 8,521 | 7 | 0.30 |
| Dialysis | 7,813 | 3 | 3,917 | 3 | 3,896 | 3 | 0.81 |
| History of MI | 27,070 | 11 | 13,495 | 11 | 13,575 | 11 | 0.61 |
| History of CABG | 34,230 | 14 | 17,062 | 14 | 17,168 | 14 | 0.54 |
| CHA2DS2-VASC | 0.36 | ||||||
| 0–1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 24,413 | 10 | 12,209 | 10 | 12,204 | 10 | |
| 3 | 44,148 | 18 | 22,133 | 18 | 22,015 | 18 | |
| 4 | 56,459 | 23 | 28,154 | 23 | 28,305 | 24 | |
| 5 | 49,625 | 21 | 24,847 | 21 | 24,778 | 21 | |
| 6 | 33,941 | 14 | 17,121 | 14 | 16,820 | 14 | |
| 7 | 19,385 | 8 | 9,570 | 8 | 9,815 | 8 | |
| 8 | 10,256 | 4 | 5,105 | 4 | 5,151 | 4 | |
| 9 | 2,817 | 1 | 1,383 | 1 | 1,434 | 1 | |
| Mean | 5 | NA | 5 | NA | 5 | NA | >0.99 |
| SD | 1.7 | NA | 1.6 | NA | 1.7 | NA | |
| Atria score | 0.44 | ||||||
| <= 3 (low) | 120,402 | 50 | 60,312 | 50 | 60,090 | 50 | |
| 4 (intermediate) | 33,558 | 14 | 16,677 | 14 | 16,881 | 14 | |
| 5 (high) | 87,084 | 36 | 43,533 | 36 | 43,551 | 36 | |
| Hospitalizations in the prior 6 months | 0.54 | ||||||
| 0 | 206,381 | 86 | 103,208 | 86 | 103,173 | 86 | |
| 1 | 26,934 | 11 | 13,451 | 11 | 13,483 | 11 | |
| 2 | 5,490 | 2 | 2,715 | 2 | 2,775 | 2 | |
| ≥3 | 2,239 | 1 | 1,148 | 1 | 1,091 | 1 | |
OAC use at baseline was defined as any OAC use in the prior 12 months.
Table 2 shows the baseline characteristics of the 120,552 patients randomized to the early intervention group. Among these, 43,826 (36%) did not have evidence of OAC use in the prior 12 months. Compared with patients with OAC use in the prior 12 months, patients without OAC use were more likely to be 80 years of age or older, women, and residents of the Midwest. Patients without evidence of an OAC in the prior 12 months were more likely to have a history of anemia and less likely to have diabetes, a history of stroke or TIA, and a history of heart failure. The mean CHA2DS2-VASc score for both groups was 5; however, more patients with no OAC use in the prior 12 months had a high ATRIA score.
Table 2:
Baseline characteristics by evidence of use of OACs among privately insured members with atrial fibrillation and CHA2DS2-VASC scores ≥2
| Patients Not Using an OAC at Baseline* | Patients Using an OAC at Baseline* | P-value | ||||
|---|---|---|---|---|---|---|
| Totals | 43,826 | 76,696 | ||||
| N | % | N | % | |||
| Age | <0.0001 | |||||
| <30–44 | 150 | 0.3 | 158 | 0.2 | ||
|
45–64 |
3,375 | 7.7 | 6,407 | 8.4 | ||
|
65–74 |
13,343 | 30.4 | 24,059 | 31.4 | ||
|
75–80+ |
26,958 | 61.5 | 46,072 | 60.1 | ||
| Female sex | 21,171 | 48 | 35,244 | 46 | <0.0001 | |
| Geographic Region | <0.0001 | |||||
| New England | 1,245 | 3 | 3,602 | 5 | ||
|
Mid-Atlantic |
1,879 | 4 | 4,374 | 6 | ||
|
South-Atlantic |
23,488 | 54 | 42,155 | 55 | ||
|
Midwest |
12,204 | 28 | 17,190 | 22 | ||
|
Mountain |
2,074 | 5 | 3,725 | 5 | ||
|
Pacific |
2,921 | 7 | 5,635 | 7 | ||
|
Unknown |
15 | 0 | 15 | 0 | ||
| Anemia | 22,702 | 52 | 36,565 | 48 | <0.0001 | |
| Hypertension | 41,247 | 94 | 73,342 | 96 | <0.0001 | |
| Diabetes | 17,172 | 39 | 33,325 | 44 | <0.0001 | |
| Peripheral vascular disease | 13,253 | 30 | 22,971 | 30 | 0.29 | |
| Prior cerebrovascular or thromboembolic event | 14,127 | 32 | 23,472 | 31 | <0.0001 | |
| Prior cerebrovascular event | 9,075 | 21 | 18,214 | 24 | <0.0001 | |
| Stroke or TIA | 7,493 | 17 | 15,149 | 20 | <0.0001 | |
| TIA | 5,850 | 13 | 11,603 | 15 | <0.0001 | |
| Stroke | 3,547 | 8 | 7,969 | 10 | <0.0001 | |
| Heart failure | 17,694 | 40 | 36,468 | 48 | <0.0001 | |
| Kidney disease | 3,328 | 8 | 5,193 | 7 | <0.0001 | |
| Dialysis | 1,498 | 3 | 2,398 | 3 | 0.006 | |
| History of MI | 5,405 | 12 | 8,170 | 11 | <0.0001 | |
| History of CABG | 6,529 | 15 | 10,639 | 14 | <0.0001 | |
| CHA2DS2-VASC | <0.0001 | |||||
| 0–1 | 0 | 0 | 0 | 0 | ||
|
2 |
5,264 | 12 | 6,940 | 9 | ||
|
3 |
8,240 | 19 | 13,775 | 18 | ||
|
4 |
10,179 | 23 | 18,126 | 24 | ||
|
5 |
8,620 | 20 | 16,158 | 21 | ||
|
6 |
5,798 | 13 | 11,022 | 14 | ||
|
7 |
3,374 | 8 | 6,441 | 8 | ||
|
8 |
1,809 | 4 | 3,342 | 4 | ||
|
9 |
542 | 1 | 892 | 1 | ||
|
Mean |
5 | NA | 5 | NA | >0.99 | |
|
SD |
1.7 | NA | 1.6 | NA | ||
| Atria score | <0.0001 | |||||
| <= 3 (low) | 20,852 | 48 | 39,238 | 51 | ||
|
4 (intermediate) |
6,021 | 14 | 10,860 | 14 | ||
|
5 (high) |
16,953 | 39 | 26,598 | 35 | ||
| Hospitalizations in the prior 6 months | 0.15 | |||||
| 0 | 37,382 | 85 | 65,791 | 86 | ||
|
1 |
5,007 | 11 | 8,476 | 11 | ||
|
2 |
1,032 | 2.4 | 1,743 | 2.3 | ||
|
≥3 |
405 | 0.9 | 686 | 0.9 | ||
OAC use at baseline was defined as any OAC use in the prior 12 months.
Discussion
This study has three main findings. First, despite the morbidity burden with a high CHA2DS2-VASc score and the fact that patients with a recent significant bleeding event were excluded from this analysis, the number of patients with AF and with no evidence of OAC use in the prior 12 months was high (36%). Second, in univariable analyses, clinical and geographic differences exist between AF patients treated with an OAC use in the prior 12 months and those not treated. Third, patients identified for potential enrollment in the IMPACT-AFib trial were relatively old and had a high burden of morbidity.
Although patients we identified were at a high risk of stroke and the cohort was derived after excluding patients with a prior intracranial hemorrhage and patients with a hospitalization for bleeding in the past 6 months, a significant number of patients (43,826; 36%) were not using an OAC in the prior 12 months. Underuse of OACs in AF patients has been reported previously with rates exceeding 50% in some studies.8–10 Reasons for underutilization of vitamin K antagonists (an OAC) in patients with AF were examined in the AVERROES (Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trial. Although non-compliance was among the main reasons for not using a vitamin K antagonist, the majority of patients were deemed not to be candidates for a VKA based on multiple reasons.19
Prior to this analysis, in the United States, factors associated with underuse of OACs in clinical practice had not been examined among the large population of individuals with commercial or Medicare Advantage coverage. Although several studies have investigated patterns of use of OACs, these studies did not report on reasons for underuse of OACs.8–10 One study in the United States investigated reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) database and found the most common reasons were physician preference, patient refusal, and bleeding events.20 Most studies that have examined factors associated with underutilization of OACs in clinical practice were conducted outside the United States. One retrospective cross-sectional study was conducted in Sweden and aimed to identify reasons for underutilization of OACs in patients with AF. Among 2,274 patients with AF, 1187 (52%) were not treated with an OAC. Of the untreated patients, 19% either had a CHA2DS2-VASc score < 2 or had declined or had experienced an adverse event other than bleeding on warfarin therapy. The most common reason (38% of patients) for not using an OAC was presence of risk factors for bleeding.21 One study used the Global Anticoagulant Registry in the FIELD (GARFIELD), a database of patients with newly diagnosed non-valvular AF. Patients were enrolled between December 2009 and October 2011 at 540 sites in 19 countries in Europe, Asia-Pacific, Central/South America, and Canada. Sites participating in this registry were representative of the distribution of AF care settings in each country. The analysis included 10,614 adults (≥18 years) diagnosed with AF within the previous 6 weeks, with ≥1 investigator-defined stroke risk factor. Overall, 38% of patients with a CHADS2 score ≥2 did not receive an OAC. This underuse resulted from physician refusal on the basis of bleeding risk, fall risk, and concern over patient non-compliance in 48% of patients. Other reasons were not clearly defined.22
The current study adds to the body of information on factors associated with underuse of OACs. Patients with no OAC use in the prior 12 months in this study differed significantly from patients with an OAC use in that they were more likely to be 80 years of age or older, women, and residents of the Midwest region of the United States and to have a history of anemia and a high Atria score. Underutilization of OACs in older patients and women has been reported previously; this study shows these trends in practice have persisted and calls for individualized approaches to addressing these disparities.8,9,23 To that end, it will be important to obtain information from a diverse group of patients by age, sex, and race about what drives their decisions regarding use of an OAC and strategies that may work or not work in specific subgroups of patients.
The large size of the source population for IMPACT-Afib trial will ensure generalizability of this trial’s findings to patients in the general community. Indeed, the vast majority of the patients we identified (92%) were at least 65 years old, and 39% were at least 80 years old. The morbidity burden in these patients was relatively high with 31% having had a prior history of cerebrovascular or thromboembolic event and 19% having had a prior stroke or TIA. The prevalence of hypertension was very high, and an appreciable number of patients had heart failure and diabetes. Therefore, it is not surprising that the mean CHA2DS2-VASc score was 5, a score that portends a high risk of stroke ranging from an annual risk of 7% to 15%.24
This study has some limitations. Data quality is dependent on the accuracy and completeness of documentation and coding in administrative claims data. We did not assess the days covered with an OAC or whether users in the last year had ongoing OAC use at baseline, and due to the lack of patient-level data, we were not able to create a multivariable model to evaluate factors independently associated with underuse of OACs.
Conclusions
Although patients in this study had a high CHA2DS2-VASc score, and therefore were at a high risk of stroke, the number of patients with AF and with no evidence of OAC use in the prior 12 months was high. Clinical and geographic differences exist between AF patients with and those without OAC use in the prior 12 months that may inform future initiatives aimed at improving utilization of OACs in eligible patients. These gaps in care underscore the importance of trials to address this problem like IMPACT-AFib, and it is indeed reassuring that patients enrolled in the IMPACT-AFib trial were relatively old and had a high burden of morbidity. This profile will likely ensure generalizability of the trial’s findings to patients seen in clinical practice.
Funding
This work was funded by the US FDA through the Department of Health and Human Services contract number HHSF223201400030I.
References:
- 1).Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 017;135:000–000. DOI: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. DOI: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 3).Lopes RD, Crowley MJ, Shah BR, Melloni C, Wood KA, Chatterjee R, Povsic TJ, Dupre ME, Kong DF, de Barros e Silva PGM, dos Santos MHH, Armaganijan LV, Katz M, Kosinski A, McBroom AJ, Chobot MM, Gray R, Sanders GD. Stroke Prevention in Atrial Fibrillation. AHRQ Comparative Effectiveness Reviews. 2013. [PubMed] [Google Scholar]
- 4).Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112(8):1142–7. DOI: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 5).Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014. February;129(8):837–47. DOI: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991. August;22(8):983–988. [DOI] [PubMed] [Google Scholar]
- 7).Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 8).Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010. July;123(7):638–645.e4. DOI: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 9).Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson ED, Fonarow GC; Get With The Guidelines Steering Committee and Hospitals. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009. September 29;54(14):1280–9. DOI: 10.1016/j.jacc.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 10).Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE Registry. JAMA Cardiol. 2016. April 1;1(1):55–62. DOI: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
- 11).Suarez J, Piccini JP, Liang L, Atherton JJ, Hayward CS, Krum H, Fonarow GC, Lopes RD, Hernandez AF. International variation in use of oral anticoagulation among heart failure patients with atrial fibrillation. Am Heart J 2012;163, Issue 5,804–811. DOI: 10.1016/j.ahj.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. DOI: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 13).Granger CB, Alexander JH, McMurray JV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. DOI: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 14).Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. DOI: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 15).Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013. November 28;369(22):2093–104. DOI: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 16).https://www.sentinelinitiative.org/sentinel/about, accessed 10/25/18.
- 17).https://www.sentinelinitiative.org/FDA-catalyst/projects/implementation-randomized-controlled-trial-improve-treatment-oral-anticoagulants-patients, accessed 10/25/18.
- 18).Cocoros NM, Pokorney SD, Haynes K, Garcia C, Al-Khalidi HR, Al-Khatib SM, Archdeacon P, Goldsack JC, Harkins T, Lin ND, Martin D, McCall D, Nair BPharm V, Parlett L, Temple R, Walraven C, Granger CB, Platt R. FDA-Catalyst – using FDA’s Sentinel Initiative for large scale pragmatic randomized trials. Clin Trials. 2018:1740774518812776. doi: 10.1177/1740774518812776. [DOI] [PubMed] [Google Scholar]
- 19).Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011. March 3;364(9):806–17. DOI: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 20).O’Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, Thomas LE, Ezekowitz MD, Mahaffey KW, Chang P, Piccini JP, Peterson ED. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2014;168(4):487–94. doi: 10.1016/j.ahj.2014.07.002. Epub 2014 Jul 11. [DOI] [PubMed] [Google Scholar]
- 21).Johansson C, Hägg L, Johansson L, Jansson JH. Characterization of patients with atrial fibrillation not treated with oral anticoagulants. Scand J Prim Health Care. 2014; 32(4): 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY, Mantovani LG, Turpie AG, van Eickels M, Misselwitz F, Rushton-Smith S, Kayani G, Wilkinson P, Verheugt FW; GARFIELD Registry Investigators. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013. May 21;8(5):e63479. DOI: 10.1371/journal.pone.0063479. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Lee S, Je NK. Antithrombotic therapy underutilization in patients with atrial flutter. J Cardiovasc Pharmacol Ther. 2018. May;23(3):237–243. doi: 10.1177/1074248418757010. Epub 2018 Feb 26. [DOI] [PubMed] [Google Scholar]
- 24).Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010. February;137(2):263–72. DOI: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]


