Summary
Hematopoietic cell transplantation (HCT) is established as a standard treatment approach for people living with HIV and poor prognosis hematologic malignancies. Studies with autologous HCT (autoHCT) and allogeneic HCT (alloHCT) suggest that HIV status does not adversely impact outcome. However, attention to possible drug-drug interactions is important. AlloHCT dramatically reduces the long-term HIV reservoir when complete donor chimerism is achieved. When CCR5Δ32 homozygous donors are used cure is possible.
Introduction
Hematopoietic cell transplantation (HCT) in people living with HIV (PLWH) and malignancy is of interest for two reasons. Transplantation is well established as a potentially curative intervention for patients with poor prognosis hematologic malignancies. As the population of PLWH grows and ages, the numbers of patients in need of HCT will grow. In addition, allogeneic HCT (alloHCT) offers the possibility of HIV cure. This review will focus on experience with autologous HCT (autoHCT) and allogeneic HCT (alloHCT) in PLWH with hematologic malignancies with regard to preparative regimens, complications, other special considerations, and oncologic outcomes as well as aspects of alloHCT that relate HIV reservoir(s) and the lessons learned with regard to the biology of cure.
AutoHCT for the treatment of hematologic malignancy
Autologous transplant with bone marrow or more recently with mobilized peripheral blood as a source of stem cells is a standard treatment for primary refractory or relapsed aggressive lymphomas such as those that occur among PLWH.1 Successes in PLWH with lymphoma followed the advent of leukocyte growth factors, Pneumocystis jirovecii prophylaxis, and combination antiretroviral therapy.2–10 Several reports indicated no adverse impact HIV status on outcome. 9,11 Other reports indicated that autoHCT did not impact HIV.12
The largest prospective multi-institutional trial was reported by the Bone Marrow Transplant Clinical Trials Network (BMT CTN) and AIDS Malignancy Consortium (AMC). These groups collaborated in a trial using carmustine, etoposide, cytarabine, and melphalan (BEAM) as the preparative regimen that enrolled 40 patients10. Patients were required to have had relapsed or primary refractory lymphoma (including Hodgkin lymphoma) that had responded to salvage therapy. Patients with active opportunistic infection or HIV deemed resistant to available antiretroviral agents were not entered into the trial. Organ function requirements were standard and the same as in previous CTN lymphoma transplant trials. Because of concerns that there might be drug interactions between the high dose chemotherapy regimen and antiretroviral agents, antiretroviral therapy was interrupted in all patients at the time of initiation of the preparative regimen and resumed at 7 days after completion of the preparative regimen or following recovery from transplant-related gastrointestinal toxicities. Median CD4+ T-cell counts returned to pretransplant levels by day +60 and were maintained for the first year, suggesting no long-term deterioration T-cell immunity following autoHCT. The 2-year progression-free survival (PFS) was 79.8%. To provide perspective, the outcomes were compared with results in patients without HIV in the Centers for International Bone Marrow Transplant Research (CIBMTR) database. Subjects were matched for age, performance score, disease, and disease stage. The hazard ratios for overall mortality and for malignancy treatment failure in the PLWH group were 0.67 (95% CI, 0.30–1.50; P = .33) and 0.52 (95% CI, 0.2927–1.03; P = .06), respectively.
A key aspect in the management of PLWH undergoing autoHCT is attention to drug interactions.13 In several studies antiretrovirals were stopped during high dose chemotherapy because of concerns with possible drug-drug interactions or intermittent or inconsistent dosing of antiretrovirals during periods of nausea and vomiting which might lead to the development of antiretroviral resistance.10 However, newer antiretrovirals have fewer risks of interaction with drugs in the preparative regimen.14 Integrase strand transferase inhibitors (INSTI) seem to be particularly safe in this setting. Among the drugs most likely to be problematic during the transplant preparative regimen and in the post-transplant period are the pharmacoenhancers ritonavir and cobicistat, both of which inhibit CYPP3A4.15 Potent CYP3A4 inducers such as older-generation non-nucleoside reverse transcriptase inhibitors (NNRTI) such as efavirenz can result in problematic drug-drug interactions.16 However second generation NNRTIs such as etravirine, rilpivirine and doravirine are only weak inducers of CYP3A4.15,17,18 Zidovudine is also relatively contraindicated because of its myelosuppressive properties. Furthermore, anti-emetics have improved so that oral therapies are less likely to be interrupted. Taken together the result is that most patients can remain on a multi-drug oral antiretroviral regimen during the transplant preparative regimen and thereafter.14
With regard to the preparative regimen itself, although there was an early report using a somewhat gentler than standard preparative regimen,19 more recent experiences have employed a variety of standard full dose preparative regimens and no particular HIV-specific issues have arisen.1
In terms of cancer indications, nearly all the autoHCT reported in PLWH and malignancy have been for the treatment of lymphoma, mainly diffuse large B cell lymphoma, Burkitt lymphoma, and Hodgkin lymphoma. very few patients with multiple myeloma have been reported14, and HIV-infected patients have generally been excluded from multiple myeloma autoHCT trials, but there are no indications that outcomes should differ from those in HIV uninfected patients and we anticipate that as clinical trial eligibility criteria are modernized, such patients will be included in future trials.20
In summary, present information suggests no long term adverse impact on CD4+ T-cell counts or HIV control as a consequence of standard autoHCT therapy and with attention to the antiretroviral regimen so as to minimize possible drug-drug interactions, and prophylaxis for Pneumocystis jiroveci, autoHCT for patients with HIV who meetis appropriate for PLWH and standard hematologic malignancy indications is appropriate.
AlloHCT in PLWH and hematologic malignancy
The early alloHCT experience in PLWH demonstrated high mortality without benefit.21 With more effective antiretroviral therapy, outcomes began to improve. Beginning in 2005, anecdotal reports appeared in the literature indicating that alloHCT was feasible and beneficial in some PLWH with hematologic malignancies prompting a review of data in the Center for International Blood and Marrow Transplant Research database that showed improved survival after 1996 with 4 of these 9 patients surviving versus 2 of 14 those undergoing transplantation before 1996.
In a retrospective look at alloHCT in the United States using an all-payer database of hospitalizations, it was reported that there were 81 alloHCT between 1998 and 2012.22 There was no difference in inpatient mortality rates based on HIV status although there were higher rates of nontuberculous Mycobacterium and cytomegalovirus infection, but not of bacteremia, or graft-versus-host-disease (GvHD) in PLWH.
In the only prospective multi-institutional study, the BMT CTN and AMC evaluated the safety and efficacy of alloHCT in 17 PLWH with hematologic malignancies transplanted between 2012 and 2015. The study allowed myeloablative and non-myeloablative preparative regimens, as well as a variety of approaches to GvHD prophylaxis but was too small to allow any comparative analysis. The study was restricted to patients with matched related or matched-unrelated donors or 1-antigen mismatched donors. Cord blood and haploidentical donors were not permitted. T cell depletion and the use of anti-thymocyte globulin (ATG) as part of conditioning or GvHD prophylaxis were not permitted. Maintenance of the antiretroviral regimen throughout the transplant course including the preparative regimen was recommended but not required. The most common cause of treatment failure was relapse of malignancy as is true in the general population undergoing alloHCT for hematologic malignancy; however there was no suggestion of increased relapse risk for PLWH and the authors noted that there were no relapses after six months. There was no non-relapse mortality (NRM) at 6 months. The overall survival at one year was 59% with relapsed/progressive disease accounting for five deaths; and acute GvHD, adult respiratory distress syndrome and liver failure for one death each. Immunoglobulin levels and CD4+ T-cell counts returned to baseline post-transplant and there was no evidence to suggest that alloHCT was associated with a long term deterioration in humoral or cellular immune function. The study thus confirmed the safety and feasibility of alloHCT for PLWH with treatment responsive infection who meet standard transplant criteria.
These conclusions were reinforced by a retrospective Spanish study of 22 PLWH with high risk hematologic malignancies who underwent alloHCT23. In the report from Spain, overall survival with a median follow-up of 65 months was 46% and NRM at 12 months was 14%. Antiretroviral therapy was maintained for all patients although in two individuals severe toxicity attributed to drug interactions were noted.
Seattle investigators reported 8 consecutive PLWH with hematologic malignancies who underwent alloHCT14. There was one long term disease-free survivor. Six relapsed, and one died with severe GvHD and interstitial pneumonitis.
We have reported a series of patients with HIV and high risk hematologic malignancy from Johns Hopkins. All were enrolled on a trial combining post-transplant cyclophosphamide for GvHD prophylaxis with an optimized antiretroviral regimen that included enfuvirtide for days when the ability to maintain an oral regimen might be compromised by nausea, vomiting, mucositis. The end point of the trial was maintenance of antiretroviral therapy through 60 days post-transplant. With post-transplant cyclophosphamide which broadens the donor pool allowing haploidentical first and second degree relatives as well as unrelated partially matched individuals to serve as donors, it was possible to identify allogeneic donors for all seven transplant candidates (2 matched sibling, 2 matched unrelated, 2 haploidentical, and one single antigen mismatched unrelated). All patients maintained ART through day 60. None of the patients relapsed. Four are alive and tumor-free more than 2.5 years post-transplant. There were three deaths. One patient died with hepatic failure with hepatitis C and GvHD being possible contributing factors. A second patient died with GvHD and hepatic failure after having discontinued immunosuppression against medical advice. A third patient died with sepsis more than a year after transplant. Thus this report provides evidence that in a small number of patients, haploidentical and mismatched unrelated transplant can be beneficial for patients with poor prognosis hematologic malignancies.
Whereas autoHCT has mainly been used in PLWH with lymphoma, alloHCT has been used for patients with myeloid leukemias as well. In comparison with lymphoma, any increase in myeloid malignancies in PLWH is relatively modest—but as there are increasing numbers of people living with HIV, there are more and more patients with myeloid malignancies in need of alloHCT.24 The numbers of alloHCT in PLWH with lymphoma, acute leukemia or other myeloid malignancies remain small but to date there is no indication that the indications for alloHCT should differ in patients with HIV.
In summary, as with autoHCT, alloHCT is effective in a variety of poor risk hematologic malignancies and standard indications apply. Both myeloablative and non-myeloablative regimens have been successful in controlling malignancy. With post-transplant cyclophosphamide as GvHD prophylaxis and consideration of haploidentical donors, it should be possible to identify suitable donors for most patients in need of transplantation.
AlloHCT and HIV cure
AlloBMT and zidovudine
Shortly after zidovudine was shown to be active as an antiretroviral and following the recognition that HIV was mainly in lymphocytes and monocytes/macrophages, alloHCT with bone marrow was carried out in a patient with aggressive B cell lymphoma with zidovudine in hopes of preventing infection of donor cells.25 As assessed by culture and PCR, HIV was cleared from peripheral blood mononuclear cells and bone marrow. The patient relapsed and died 47 days after transplant. Investigation of autopsy tissues showed no evidence of HIV. The investigators concluded that bone marrow ablative chemo-radiotherapy with zidovudine may have been able to prevent the establishment of HIV infection in donor hematopoietic-lymphoid cells. Several other patients underwent alloHCT with zidovudine with evidence of clearance of HIV in blood or autopsy tissues by PCR but died with transplant associated complications (reviewed in 26). In contrast, lymphocyte infusion and marrow transplantation from uninfected identical twins with zidovudine were not associated with evidence of viral clearance27.
AlloHCT and combination antiretroviral therapy
With the advent of combination antiretroviral therapy, HIV was converted from an almost uniformly fatal infection into a manageable chronic disease. Effective antiretroviral therapy stopped active replication of HIV and PLWH on antiretroviral achieved undetectable levels of HIV in blood. Initially this led to hopes that antiretroviral could cure HIV.28 However with interruption of therapy, HIV rebounded to pre-treatment levels.29 The source of this HIV rebound and the primary barrier to HIV cure is a stable, latent reservoir of virus primarily in resting CD4+T cells and perhaps in monocytes and macrophages.26,30–32 This reservoir can be measured long-term with minimal decay by a highly sensitive and specific quantitative viral outgrowth assay (qVOA), even in patients whose viral load is not detectable with standard clinical assays33.
As antiretroviral therapy improved and alloHCT improved, two patients (the “Boston patients”) who underwent non-myeloablative transplant were treated with combination antiretroviral therapy prior to and during the preparative regimen as well as thereafter and were long term survivors34. HIV DNA was readily detected in peripheral blood mononuclear cells prior to alloHCT but was no longer measurable post-transplant as full donor chimerism was established. Nor was HIV detectable in plasma.
Others also reported that with alloHCT, when antiretroviral therapy was continued throughout the preparative regimen and post-transplant period, the viral reservoir was markedly diminished or became unmeasurable35–37. The IciStem Consortium reported on an observational cohort and studied HIV reservoir dynamics in 6 patients surviving alloHCT beyond 2 years36. Five patients achieved full donor chimerism. In these patients proviral HIV DNA and quantitative viral outgrowth assay (qVOA) and HIV RNA analysis in plasma showed no evidence of HIV. All had received peripheral blood stem cells. In one patient longitudinal monitoring showed that the disappearance of the HIV reservoir corresponded in time to the appearance of full chimerism and GvHD. A sixth patient achieved only mixed chimerism and in that patient HIV was detected by each of these assays. That patient received transplantation using cord blood. The investigators also studied other sites and tissues including cerebrospinal fluid, bone marrow cells, lymph node and ileal biopsy specimens and did not detect HIV.
In the BMT CTN/AMC alloHCT trial cell-associated HIV DNA was undetectable at every time point after complete donor chimerism was achieved.37 In contrast, cell-associated HIV DNA was detected in patients who were mixed chimeras. In three patients who survived to one year, achieved complete donor T cell chimerism, and agreed to a large volume blood draw, inducible infectious virus was not detected by qVOA. In contrast, in two patients who demonstrated mixed chimerism at 1 year, infectious HIV remained detectable by qVOA. In the Johns Hopkins series, four patients achieved complete T-cell chimerism and HIV was undetectable by qVOA at all post-transplant time points except one, corresponding to an estimated 2–2.5 log reduction in the latent reservoir. For two patients with mixed chimerism, HIV remained detectable at stable levels when measured by qVOA.
AlloHCT and HIV rebound
With the observation that the “Boston patients” who had undergone alloHCT and achieved complete chimerism no longer had a measurable HIV reservoir, analytic treatment interruption (ATI) was undertaken to determine whether sustained, antiretroviral-free HIV remission could be achieved 38. Rebound of HIV was detected at 12 and 32 weeks after interruption of antiretroviral therapy. Severe symptoms of an acute retroviral syndrome developed in both patients, including meningitis in one. The investigators noted that in other settings, discontinuation of antiretrovirals typically leads to rebound in 2–3 weeks. Based on the delay in rebound, the investigators calculated that alloHCT in these patients was associated with at least a 3-log10 reduction in the number of circulating cells harboring proviral HIV DNA. Furthermore, on the basis of phylogenetic analyses, they inferred that only one or a few latent proviruses contributed to viral rebound. These authors suggested that the highly symptomatic aggressive rebound in patients achieving complete donor chimerism likely reflected the absence of donor cellular immunity to HIV.
In the Johns Hopkins experience we also had a case of post-alloHCT viral rebound. One patient missed many clinic and study visits after alloHCT and at approximately day 100 developed fevers and confusion prompting hospitalization. HIV was detected in plasma and CSF. Encephalopathy resolved with re-institution of antiretroviral therapy in parallel with fall in HIV in plasma and CSF. This case provides further evidence that interruption in antiretroviral therapy post-alloHCT could provoke a severe acute retroviral syndrome.
These and other experiences with HIV rebound following interruption of antiretroviral therapy prompted an editorial counseling caution in analytic therapeutic interruptions 39. Similarly, it led to interest in the investigation of an approach to generating HIV-specific cellular response in HIV-naïve donors with an eye toward the possibility that such cells might one day be used to supplement donor cells in the context of alloHCT to protect against such aggressive rebound40.
CCR5Δ32 homozygous donors
Homozygous inactivating deletions in the CCR5 receptor gene inhibits HIV entry into CD4 T cells by R5 trophic virus and confer natural resistance to HIV infection as was recognized early in the epidemic.41 This 32-bp deleted variant allele (CCR5Δ32) is homozygous in approximately 1% of Caucasians but is much less prevalent in people of African or Asian descent. In 2006, a PLWH later known as the “Berlin patient” was diagnosed with acute myeloid leukemia (AML) and required an alloHCT for curative treatment. An unrelated donor search was initiated and 80 potential HLA-identical donors were identified.42 Sixty two were screened to identify one CCR5Δ32 homozygote. The patient received myeloablative conditioning and antithymocyte globulin (ATG) before transplant. Antiretroviral therapy was stopped before stem cell infusion but no HIV rebound was detected. The patient received cyclosporine and mycophenolate as GvHD prophylaxis. The patient relapsed with AML almost one year later and was re-induced, and received a second transplant from the same donor following a single fraction of low dose of total body irradiation (200 cGy). Since that time the patient has remained in remission and off antiretroviral therapy43. Complete chimerism was achieved in peripheral blood and numbers of donor-derived peripheral CD4 T cells increased until the normal range was reached. In addition, evaluation of the gut mucosa showed donor derived CD4 T cells without any sign of HIV infection. HIV remained undetectable in peripheral blood mononuclear cells and in plasma. With time, HIV specific antibodies also became undetectable. A neurologic event led to brain biopsy and HIV was not detected in brain tissue. Similarly, HIV was not detected in liver tissue. Furthermore, there was no expression of CCR5 in these tissues consistent with the possibility that donor cells replaced host microglial cells and Kupffer cells. This experience generated great interest in the possibility of HIV cure. And the possibility was confirmed many years later in a patient with Hodgkin lymphoma transplanted in London with a CCR5Δ32 homozygous donor.44
Several other patients have received transplants from CCR5Δ32 homozygous donors and not fared as well. As in alloHCT in general, patients have relapsed with malignancy or succumbed to GvHD or other consequences of alloHCT so as to preclude long term assessment of the impact of the procedure on the long term reservoir. In one case, there was a shift in tropism with emergence of CXCR4 HIV variants after transplantation.45 Deep sequencing revealed that CXCR4-tropic HIV variant replicating after alloHCT was present more than three months before transplant. 46
The decade between the cases of cure in the Berlin and London patients reflects in part the rarity of patients with HIV and hematologic malignancies appropriate for transplant, the rarity of suitable HLA-matched donors, and the difficulties in identifying such donors. Allogeneic bone marrow transplantation remains a procedure associated with substantial morbidity and mortality. There is little enthusiasm among transplant physicians to treat patients without life threatening malignancies for which allogeneic transplant has been shown to have a potentially life-saving role.
Finding an HLA matched donor has in the past been a barrier to transplant. Various unrelated transplant donor registries can identify HLA-matched unrelated donors for a majority of Caucasian patients, but identifying donors for patients of African descent remains more challenging. This reflects the greater complexity and diversity of the HLA locus in patients of African descent. While approximately 1% of Caucasian donors are homozygous for the CCR5Δ32 variant, the polymorphism is not found in other populations. Thus identifying a matched donor who was homozygous for CCR5Δ32 was like being struck twice by lightening—a rare event. Furthermore, until recently, genetic analysis of the CCR5 locus was not a standard part of the evaluation of donors so that even when a patient with many potential matched unrelated donors were identified, the process of evaluating these potential donors to identify those who were HIV-resistant was time consuming.
In the BMT CTN/AMC trial of alloHCT, we cared for a patient with aggressive lymphoma and many hundreds of HLA-matched donors, but counseled the patient that it was in his interest to proceed with the first available unrelated donor rather than await testing to identify a CCR5Δ32 homozygous donor. The patient is alive and well today, but must continue on antiretrovirals. Now it is routine for new potential donors to unrelated donor networks to be evaluated not only at the HLA locus but at the CCR5 locus as well and a rare patient with many matched unrelated donors could expect to have a CCR5Δ32 homozygous donor speedily identified.
An alternative source of CCR5Δ32 homozygous hematopoietic stem cells for alloHCT is cord blood.47–49 Cord blood banks have been studied to identify potential donors and several PLWH have received CCR5Δ32 homozygous grafts. Thus far there are no reports of long term survivors off antiretroviral therapy, although it seems likely there will be in the future.
What are the requirements for cure?
With only two successful transplant-related HIV cures reported, the numbers of patients are inadequate to address the issue with any certainty. However, reports consistently show that the reservoir as measured in peripheral blood disappears with the achievement of complete chimerism and is not significantly reduced in cases of mixed chimerism. Complete chimerism and disappearance of the measured peripheral blood HIV reservoir can be achieved with or without a myeloablative regimen, and with or without ATG. Virtually all preparative regimens have include some total body irradiation although only low dose total body irradiation has been used in non-myeloablative preparative regimens. It would appear that the minimum requirement for cure is for complete chimerism and that any preparative regimen that achieves complete chimerism is adequate. A graft versus host effect is probably necessary to achieve complete donor chimerism.
Is graft vs host disease necessary? Complete chimerism is commonly achieved without any clinical evidence of GvHD and this has been documented in several PLWH as well. However, at a first approximation, a graft vs. host response (not clinical disease) is necessary to achieve complete chimerism and in the treatment of PLWH this is a graft versus reservoir effect i.e. donor cells play an important role in eradicating host cells including those that harbor HIV (Fig. 1).36,50 Although gene therapy strategies to protect hematopoietic stem cells from HIV infection are emerging and have been applied in the autoHCT and alloHCT setting, the graft vs reservoir effect seems likely to be critical to achieving cure. 51–53
Figure 1.
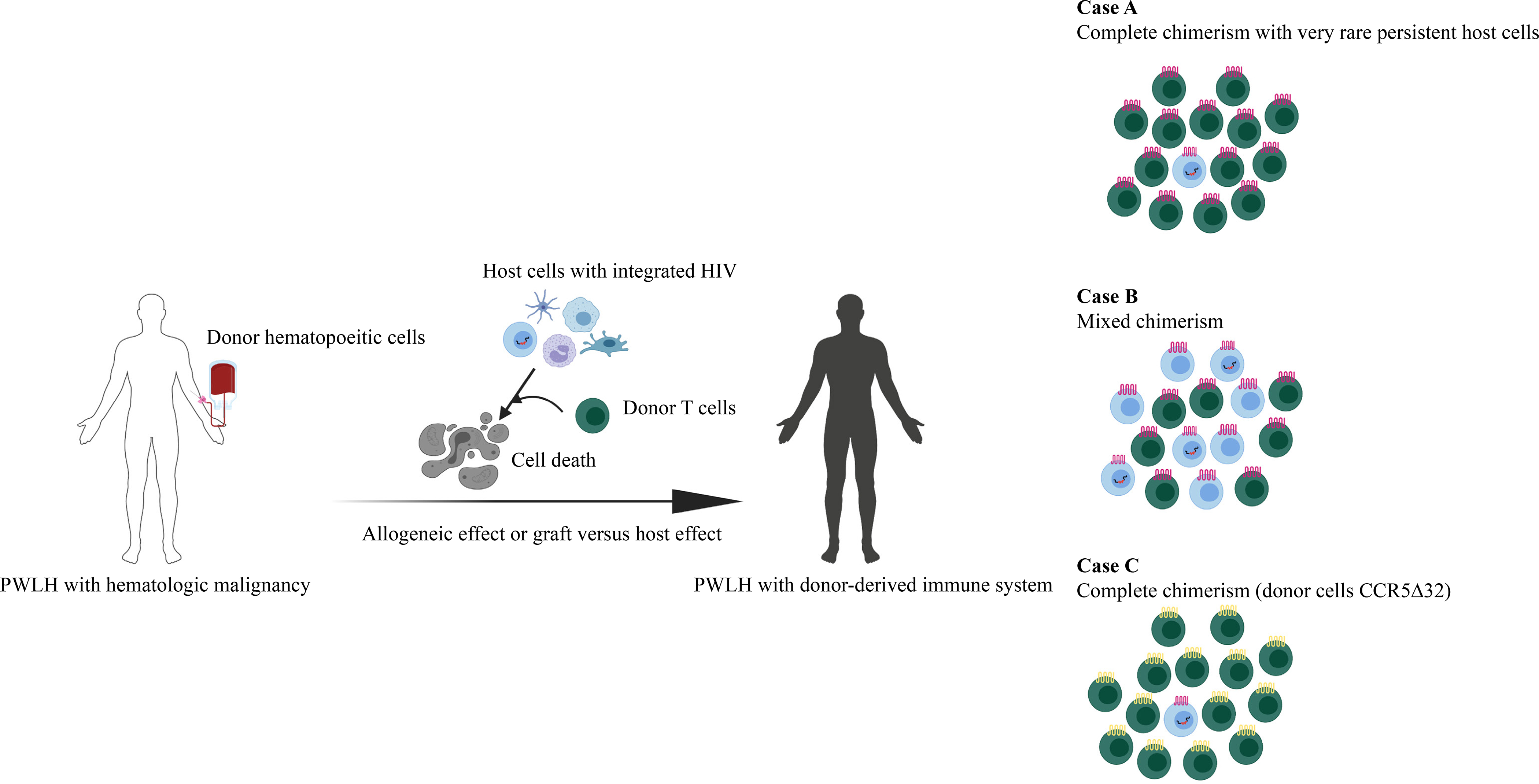
With alloHCT, host cells (blue) are eliminated by the allogeneic effect of donor lymphocytes (green) which provide graft-versus-tumor effects and graft-versus-reservoir effects. The extent of the graft-versus-reservoir effect depends on the extent of donor cell replacement. (A) In the case of apparent complete chimerism as determined in studies of peripheral blood, the reservoir appears undetectable but very rare persistent host cells harboring latent HIV may lead to rebound as in the Boston patients. (B) In mixed chimeras, the reservoir is little changed. (C) With a CCR5Δ32 homozygous donor, even if rare persistent host cells reactivate, if those cells harbor R5 tropic HIV, the virus cannot infect the new donor cells and cure may be achieved.
Conclusions
The safety and efficacy of HCT transplant strategies for the treatment of hematologic malignancies is established. In the alloHCT setting, interruption of antiretroviral therapy after transplant poses a risk for a severe acute retroviral syndrome. AlloHCT with CCR5Δ32 homozygous donors also offers an opportunity for HIV cure. The opportunities for cure are increasing as potential donors are typed at the CCR5 locus and HLA-matching requirements are relaxed with post-transplant cyclophosphamide or cord blood transplants.
Search strategy and selection criteria
We identified references for this Viewpoint through searches of PubMed and Google Scholar databases, using the search terms “HIV”, “transplantation”, “autologous“, and “allogeneic”. We included articles that were published from Jan 1, 1986, to February 1, 2020. We only considered results from papers published in English.
Table.
Approaches to Monitoring the HIV Reservoir
| Assay | Description | Advantages | Disadvantages |
|---|---|---|---|
| PCR-based assays to measure cell-associated proviral DNA54 | Variety of quantitative PCR assays that can be used to measure both integrated55 and unintegrated56 HIV using a variety of platforms (real-time PCR, digital droplet PCR57 etc) | Rapid. Can be applied to a variety of cell types and tissue.58 Requires small volume of sample. | Measures both replication competent, infectious viruses as well as defective, mutated viruses. Since defective viruses account for the majority of proviral DNA,59 it has very low signal to noise ratio and may miss clinically significant reductions in the replication competent reservoir. |
| Single copy assay60 | Quantitative polymerase chain reaction to measure residual HIV plasma RNA in patients on antiretroviral therapy. | Has been used as an indirect measure of HIV persistence in several clinical trials of antiretroviral intensification.61–63 Requires less sample volume that culture-based assays. | Most patients on antiretroviral therapy have only 1–3 copies HIV RNA/mL of plasma. Thus, this assay has poor dynamic range. |
| Quantitative viral outgrowth assay (qVOA)30 | Culture-based assay where patient CD4 T cells (or other cells types) are plated in limiting dilution, stimulated to produce virus which is amplified by donor CD4 T cells or a transformed CD4+ T cell line expressing CCR5. | Considered the gold-standard for measuring the minimal size of the replication competent latent viral reservoir. Can detect viral outgrowth from a single cell and is highly specific for infectious virus. | Time and labor-intensive, also requires large volume sample. May underestimate the size of the reservoir as some cells may not be stimulated to produce virus on single-round stimulation.64 |
| Intact proviral detection assay (IDPA)65 | Droplet digital PCR using discriminatory probes for common deleted or hypermutated proviruses to distinguish from intact proviruses | Rapid. Requires fewer cells/sample volume than culture-based assays. Considered both sensitive and specific for replication competent viruses as it | New assay that has not yet been validated in clinical trials. |
Panel 1.
Approaches to Transplant
-
Autologous [high dose cytotoxic chemotherapy and sometimes radiation]
Bone marrow
Peripheral blood stem cell
-
Allogeneic
-
Preparative regimens
High dose cytotoxic chemotherapy and sometimes radiation
Non-myeloablative cytotoxic chemotherapy and sometimes radiation
-
Stem cell source
Bone marrow
Mobilized peripheral blood
Cord blood
-
HLA matching
HLA matched related
Haplo matched related
HLA matched unrelated
HLA mismatched unrelated bone marrow
-
Syngeneic [has not been effective clearing the HIV latent reservoir]
Panel 2:
Recommendations for allogeneic transplantation
HLA matched or haplo matched or unrelated matched or unrelated 1 antigen mismatched are all associated with good outcomes
No evidence that the impact on the HIV reservoir is different between myeloablative or high dose and non-myeloablative approaches
No evidence that ATG plays a special role in the prep for HIV transplants
Funding
RFA reports grants from NCI P01CA015396, P01 CA225618-01A1, P30 AI094189. CMD reports grants from from amfAR, The Foundation for AIDS Research (Mathilde Krim Fellow108707-54-RKRL), the National Cancer Institute (K23CA177321-01A1) and the National Institute of Allergy and Infectious Diseases (Johns Hopkins University Center for AIDS Research; P30AI094189). CMD serves on a grant review committed for Gilead Sciences.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- 1.Alvarnas JC, Zaia JA, Forman SJ. How I treat patients with HIV-related hematological malignancies using hematopoietic cell transplantation. Blood, The Journal of the American Society of Hematology 2017; 130(18): 1976–84. [DOI] [PubMed] [Google Scholar]
- 2.Gabarre J, Azar N, Autran B, Katlama C, Leblond V. High-dose therapy and autologous haematopoietic stem-cell transplantation for HIV-1-associated lymphoma. The Lancet 2000; 355(9209): 1071–2. [DOI] [PubMed] [Google Scholar]
- 3.Molina A, Krishnan AY, Nademanee A, et al. High dose therapy and autologous stem cell transplantation for human immunodeficiency virus‐associated non‐Hodgkin lymphoma in the era of highly active antiretroviral therapy. Cancer 2000; 89(3): 680–9. [PubMed] [Google Scholar]
- 4.Re A, Cattaneo C, Michieli M, et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2003; 21(23): 4423–7. [DOI] [PubMed] [Google Scholar]
- 5.Gabarre J, Marcelin A-G, Azar N, et al. High-dose therapy plus autologous hematopoietic stem cell transplantation for human immunodeficiency virus (HIV)-related lymphoma: results and impact on HIV disease. Haematologica 2004; 89(9): 1100–8. [PubMed] [Google Scholar]
- 6.Krishnan A, Molina A, Zaia J, et al. Durable remissions with autologous stem cell transplantation for high-risk HIV-associated lymphomas. Blood 2005; 105(2): 874–8. [DOI] [PubMed] [Google Scholar]
- 7.Re A, Michieli M, Casari S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation as salvage treatment for AIDS-related lymphoma: long-term results of the Italian Cooperative Group on AIDS and Tumors (GICAT) study with analysis of prognostic factors. Blood, The Journal of the American Society of Hematology 2009; 114(7): 1306–13. [DOI] [PubMed] [Google Scholar]
- 8.Balsalobre P, Díez-Martín JL, Re A, et al. Autologous stem-cell transplantation in patients with HIV-related lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009; 27(13): 2192–8. [DOI] [PubMed] [Google Scholar]
- 9.Diez-Martin JL, Balsalobre P, Re A, et al. Comparable survival between HIV+ and HIV- non-Hodgkin and Hodgkin lymphoma patients undergoing autologous peripheral blood stem cell transplantation. Blood 2009; 113(23): 6011–4. [DOI] [PubMed] [Google Scholar]
- 10.Alvarnas JC, Le Rademacher J, Wang Y, et al. Autologous hematopoietic cell transplantation for HIV-related lymphoma: results of the BMT CTN 0803/AMC 071 trial. Blood 2016; 128(8): 1050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan A, Palmer JM, Zaia JA, Tsai NC, Alvarnas J, Forman SJ. HIV status does not affect the outcome of autologous stem cell transplantation (ASCT) for non-Hodgkin lymphoma (NHL). Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2010; 16(9): 1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cillo AR, Krishnan A, Mitsuyasu RT, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. Journal of acquired immune deficiency syndromes (1999) 2013; 63(4): 438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudek MA, Ambinder RF, Flexner CW, Deeken JF. Systemic therapy for malignancy in patients on anti retroviral medications. In: Basow D, editor. UpToDate. Waltham, MA: UpToDate; 2013. [Google Scholar]
- 14.Johnston C, Harrington R, Jain R, Schiffer J, Kiem HP, Woolfrey A. Safety and Efficacy of Combination Antiretroviral Therapy in Human Immunodeficiency Virus-Infected Adults Undergoing Autologous or Allogeneic Hematopoietic Cell Transplantation for Hematologic Malignancies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2016; 22(1): 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid E, Suneja G, Ambinder RF, et al. Cancer in people living with HIV, version 1.2018, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2018; 16(8): 986–1017. [DOI] [PubMed] [Google Scholar]
- 16.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. The lancet oncology 2011; 12(9): 905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berretta M, Caraglia M, Martellotta F, et al. Drug–drug interactions based on pharmacogenetic profile between highly active antiretroviral therapy and antiblastic chemotherapy in cancer patients with HIV infection. Frontiers in pharmacology 2016; 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalilieh SG, Yee KL, Sanchez RI, et al. Doravirine and the potential for CYP3A-mediated drug-drug interactions. Antimicrobial agents and chemotherapy 2019; 63(5): e02016–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer Thomas R, Ambinder Richard F, Lee Jeannette Y, et al. Dose-reduced busulfan, cyclophosphamide, and autologous stem cell transplantation for human immunodeficiency virus-associated lymphoma: AIDS Malignancy Consortium study 020. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008; 14(1): 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uldrick TS, Ison G, Rudek MA, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research HIV Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(33): 3774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta V, Tomblyn M, Pedersen TL, et al. Allogeneic hematopoietic cell transplantation in human immunodeficiency virus-positive patients with hematologic disorders: a report from the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2009; 15(7): 864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta K, Im A, Rahman F, Wang H, Veldkamp P. Epidemiology and Outcomes of Hematopoietic Stem Cell Transplantation in Human Immunodeficiency Virus-Positive Patients From 1998 to 2012: A Nationwide Analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018; 67(1): 128–33. [DOI] [PubMed] [Google Scholar]
- 23.Kwon M, Bailén R, Balsalobre P, et al. Allogeneic stem-cell transplantation in HIV-1-infected patients with high-risk hematological disorders. Aids 2019; 33(9): 1441–7. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. The lancet HIV 2017; 4(11): e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland HK, Saral R, Rossi JJ, et al. Allogeneic bone marrow transplantation, zidovudine, and human immunodeficiency virus type 1 (HIV-1) infection. Studies in a patient with non-Hodgkin lymphoma. Annals of internal medicine 1989; 111(12): 973–81. [DOI] [PubMed] [Google Scholar]
- 26.Kuritzkes DR. Hematopoietic stem cell transplantation for HIV cure. The Journal of clinical investigation 2016; 126(2): 432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane HC, Zunich KM, Wilson W, et al. Syngeneic bone marrow transplantation and adoptive transfer of peripheral blood lymphocytes combined with zidovudine in human immunodeficiency virus (HIV) infection. Annals of internal medicine 1990; 113(7): 512–9. [DOI] [PubMed] [Google Scholar]
- 28.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997; 387(6629): 188–91. [DOI] [PubMed] [Google Scholar]
- 29.Davey RT, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proceedings of the National Academy of Sciences 1999; 96(26): 15109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278(5341): 1295–300. [DOI] [PubMed] [Google Scholar]
- 31.Chun T-W, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences 1997; 94(24): 13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278(5341): 1291–5. [DOI] [PubMed] [Google Scholar]
- 33.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine 2003; 9(6): 727–8. [DOI] [PubMed] [Google Scholar]
- 34.Henrich TJ, Hu Z, Li JZ, et al. Long-Term Reduction in Peripheral Blood HIV Type 1 Reservoirs Following Reduced-Intensity Conditioning Allogeneic Stem Cell Transplantation. The Journal of infectious diseases 2013; 207(11): 1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koelsch KK, Rasmussen TA, Hey-Nguyen WJ, et al. Impact of allogeneic hematopoietic stem cell transplantation on the HIV reservoir and immune response in three HIV infected individuals. Journal of acquired immune deficiency syndromes (1999) 2017; 75(3): 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salgado M, Kwon M, Gálvez C, et al. Mechanisms That Contribute to a Profound Reduction of the HIV-1 Reservoir After Allogeneic Stem Cell Transplant. Annals of internal medicine 2018. [DOI] [PubMed] [Google Scholar]
- 37.Ambinder RF, Wu J, Logan B, et al. Allogeneic hematopoietic cell transplant for HIV patients with hematologic malignancies: the BMT CTN-0903/AMC-080 trial. Biology of Blood and Marrow Transplantation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-Free HIV-1 Remission and Viral Rebound After Allogeneic Stem Cell Transplantation: Report of 2 Cases. Annals of internal medicine 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugarman J, Lewin SR, Henrich TJ, Rasmussen TA. Ethics of ART interruption after stem-cell transplantation. Lancet HIV 2016; 3(1): e8–10. [DOI] [PubMed] [Google Scholar]
- 40.Patel S, Lam S, Cruz CR, et al. Functionally Active HIV-Specific T Cells that Target Gag and Nef Can Be Expanded from Virus-Naive Donors and Target a Range of Viral Epitopes: Implications for a Cure Strategy after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2016; 22(3): 536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996; 382(6593): 722–5. [DOI] [PubMed] [Google Scholar]
- 42.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. The New England journal of medicine 2009; 360(7): 692–8. [DOI] [PubMed] [Google Scholar]
- 43.Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 2011; 117(10): 2791–9. [DOI] [PubMed] [Google Scholar]
- 44.Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 2019; 568(7751): 244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kordelas L, Verheyen J, Beelen DW, et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. The New England journal of medicine 2014; 371(9): 880–2. [DOI] [PubMed] [Google Scholar]
- 46.Verheyen J, Thielen A, Lübke N, et al. Rapid rebound of a preexisting CXCR4-tropic HIV variant after allogeneic transplantation with CCR5 delta32 homozygous stem cells. Clinical Infectious Diseases 2018. [DOI] [PubMed] [Google Scholar]
- 47.Petz LD, Redei I, Bryson Y, et al. Hematopoietic cell transplantation with cord blood for cure of HIV infections. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2013; 19(3): 393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte RF, Salgado M, Sanchez-Ortega I, et al. CCR5 Delta32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. Lancet HIV 2015; 2(6): e236–42. [DOI] [PubMed] [Google Scholar]
- 49.Rothenberger M, Wagner JE, Haase A, et al. Transplantation of CCR5Δ 32 homozygous umbilical cord blood in a child with acute lymphoblastic leukemia and perinatally acquired HIV infection. Open forum infectious diseases; 2018: Oxford University Press; US; 2018. p. ofy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon M, Balsalobre P, Serrano D, et al. Single cord blood combined with HLA-mismatched third party donor cells: comparable results to matched unrelated donor transplantation in high-risk patients with hematologic disorders. Biology of Blood and Marrow Transplantation 2013; 19(1): 143–9. [DOI] [PubMed] [Google Scholar]
- 51.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. New England Journal of Medicine 2014; 370(10): 901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiGiusto DL, Cannon PM, Holmes MC, et al. Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells. Mol Ther Methods Clin Dev 2016; 3: 16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L, Wang J, Liu Y, et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. New England Journal of Medicine 2019; 381(13): 1240–7. [DOI] [PubMed] [Google Scholar]
- 54.Massanella M, Richman DD. Measuring the latent reservoir in vivo. The Journal of clinical investigation 2016; 126(2): 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y, Lassen K, Monie D, et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. Journal of virology 2004; 78(12): 6122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clinical infectious diseases 2010; 50(6): 912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrich TJ, Gallien S, Li JZ, Pereyra F, Kuritzkes DR. Low-level detection and quantitation of cellular HIV-1 DNA and 2-LTR circles using droplet digital PCR. Journal of virological methods 2012; 186(1–2): 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durand CM, Ghiaur G, Siliciano JD, et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. The Journal of infectious diseases 2012; 205(6): 1014–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho Y-C, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155(3): 540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. Journal of clinical microbiology 2003; 41(10): 4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandhi RT, Coombs RW, Chan ES, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999) 2012; 59(3): 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS medicine 2010; 7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. The Journal of infectious diseases 2011; 203(7): 960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155(3): 540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566(7742): 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


