Abstract
Iatrogenic malnutrition and underfeeding are ubiquitous in intensive care units (ICUs) worldwide for prolonged periods after ICU admission. A major driver leading to the lack of emphasis on timely ICU nutrition delivery is lack of objective data to guide nutrition care. If we are to ultimately overcome current fundamental challenges to effective ICU nutrition delivery, we must all adopt routine objective, longitudinal measurement of energy targets via indirect calorimetry (IC). Key evidence supporting the routine use of IC in the ICU includes (1) universal societal ICU nutrition guidelines recommending IC to determine energy requirements; (2) data showing predictive equations or body weight calculations that are consistently inaccurate and correlate poorly with measured energy expenditure, ultimately leading to routine overfeeding and underfeeding, which are both associated with poor ICU outcomes; (3) recent development and worldwide availability of a new validated, accurate, easy-to-use IC device; and (4) recent data in ICU patients with coronavirus disease 2019 (COVID-19) showing progressive hypermetabolism throughout ICU stay, emphasizing the inaccuracy of predictive equations and marked day-to-day variability in nutrition needs. Thus, given the availability of a new validated IC device, these findings emphasize that routine longitudinal IC measures should be considered the new standard of care for ICU and post-ICU nutrition delivery. As we would not deliver vasopressors without accurate blood pressure measurements, the ICU community is only likely to embrace an increased focus on the importance of early nutrition delivery when we can consistently provide objective IC measures to ensure personalized nutrition care delivers the right nutrition dose, in the right patient, at the right time to optimize clinical outcomes.
Keywords: critical illness, energy expenditure, indirect calorimetry, intensive care unit, malnutrition, nutrition support
INTRODUCTION
All existing intensive care unit (ICU) nutrition guidelines1,2 emphasize early nutrition delivery via enteral nutrition (EN) and/or parenteral nutrition (PN) as a key primary therapy leading to both nutrition and nonnutrition clinical outcome benefits. Unfortunately, iatrogenic malnutrition and underfeeding are virtually ubiquitous in ICUs worldwide for prolonged periods after ICU admission.3–5 A major driver leading to the lack of emphasis on nutrition therapy in the ICU is lack of objective data to guide nutrition care. As energy requirements are known to change throughout the course of critical illness, and given the recent availability of a new generation of indirect calorimeters,6 it is essential that we move to a culture of personalized, targeted nutrition delivery. ICU physicians would not deliver vasopressors without accurate blood pressure measurements from an arterial line or blood pressure cuff; thus, the ICU community has not embraced a focus on nutrition delivery being equally important to other care areas (ie, cardiovascular, respiratory, renal systems), owing to a lack of objective data to guide nutrition care. ICU nutrition care will not gain the respect it deserves until we are able to provide objective data to guide nutrition delivery. This is essential to address if we hope to bring nutrition care in line with other aspects of ICU care on ICU ward rounds. This is now more urgent than ever, as critical illness—and recently, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection that leads to the need for ICU care—is posing an ever-growing major healthcare challenge worldwide.7 The longitudinal metabolic phenotype and the measured resting energy expenditure (mREE) requirements of the critical care patients receiving current standards of ICU care are poorly understood, as very few recent longitudinal studies of IC exist. Further, the metabolic challenges that modern ICU care poses—and especially coronavirus disease 2019 (COVID-19) now poses as a new threat to human health—are poorly understood.
It is a critical research and clinical imperative to understand the metabolic consequences and nutrition needs of modern critically ill patients, including in severe COVID-19. This is essential to assist in improving the clinical and functional outcomes for the rapidly growing number of critically ill and COVID-19–related ICU survivors. To improve clinical and quality-of-life (QoL) outcomes in ICU survivors, an obvious therapeutic strategy is objective, targeted nutrition therapy to address preexisting and subsequent iatrogenic malnutrition, which commonly occurs in ICU patients. Preexisting malnutrition is highly prevalent in ICU patients, with up to 1 in 2 (30%–50%) patients with malnutrition at ICU admission.8 Unfortunately, clinical outcomes in patients with preexisting malnutrition are further complicated by the acute catabolic response that occurs in critical illness, leading to rapid loss of muscle mass and thus muscle weakness and functional disabilty.9–11
Unfortunately, as mentioned, iatrogenic malnutrition and underfeeding continue to be ubiquitous in ICUs worldwide for prolonged periods following ICU admission.3–5 Despite extensive efforts to improve ICU nutrition delivery, studies of current practice reveal that actual nutrition delivery in ICU patients is <50% of prescribed targets, even in the most malnourished patients.3 Alarmingly, US ICUs have the poorest track record of nutrition delivery compared with other world regions.3 In the present era of focus on medical error and patient safety, we and others have consistently document ICU patients only receive, on average, 40%–50% of their nutrition targets for prolonged periods (often >7–10 days) following ICU admission5,12–16. Further, it takes over 60 hours on average for any nutrition to be started in US ICUs.3 This is particularly concerning given that the average protein delivery (believed to be essential for muscle and functional QoL recovery) over the initial 12 days following ICU admission is only 0.6 g/kg/d,12 which is approximately one-third of current nutrition guidelines recommending 1.2–2.0 g/kg/d in the ICU.2 We believe a major barrier to overcoming this long-standing lack of emphasis on nutrition delivery is due to a lack of objective energy targets and other objective, measurable data to guide nutrition delivery as is present in virtually every other area of ICU management discussed on rounds. This presents an urgent patient safety crisis we must address in critical care.
The challenge of determining objective nutrition targets: We must do better!
Defining an ICU patient’s nutrition target is the essential first task of an ICU clinician prior to prescribing nutrition therapy. As stated, we believe a major driver of the lack of emphasis on improved nutrition therapy in the ICU and post-ICU period is the lack of objective energy target data. Our research group and many others have hypothesized that energy needs and targets change frequently throughout the course of critical illness and post-ICU recovery.11,17,18 However, this has not been validated with actual, measured, longitudinal REE measures. EE in critically ill patients is believed to be highly variable and dependent on a range of key features including initial illness/injury, illness severity, initial nutrition status, and current therapies.11 For example, older adult patients with reduced lean body mass (and/or sarcopenia) and increased fat mass have a reduced EE, which is difficult to accurately predict by using traditional equations for energy need. Similarly, patients with weight loss and cachexia related to surgery, cancer, anorexia nervosa, chronic obstructive pulmonary disease, chronic infections, or prolonged ICU stay or those with impaired muscle mass and function (ie, extensive muscle paralysis or muscle wasting, ICU-acquired weakness, sarcopenia) often have reduced EE, which is difficult to estimate. By contrast, younger ICU patients and those with acute infection, severe trauma, or obesity can have increases in EE that are quite difficult to accurately estimate.19,20 As stated, a range of published studies demonstrate that predictive equations developed to estimate EE in such patients are largely inaccurate at almost all time points in the course of critical illness and are commonly not clinically relevant,21–23 owing to the complex and dynamic metabolic alterations observed in critical illness.24,25 Given this, clinicians must regularly measure their patients’ EE to optimize the prescription of nutrition support and clinical outcomes,1,2 and IC is considered to be the gold standard for determination of EE in the ICU setting. Further emphasizing the need for routine, longitudinal IC measures, it has recently become clear that both overfeeding and underfeeding are associated with increased ICU mortality.1,2,26 To address accurate determination of EE and to optimize clinical outcomes, the most recent international ICU nutrition guidelines recommend routine use of IC to measure the EE in ICU patients to provide accurate determination of caloric needs.1,2 This is supported by data showing the use of metabolic cart (IC) data to optimize nutrition support has been associated with improved clinical outcomes from nutrition therapy.19,20
Unfortunately, recent studies demonstrate that current commercially available IC devices are often inaccurate27,28 and the inconvenience and challenge of IC measurements (ie, long warm-up duration and challenging calibration, complex maintenance, large device size, etc) have significantly limited IC use in clinical practice.29,30 These comparative studies raising concern for the accuracy of currently available IC technology include a study by Sundstrom et al in which 3 currently available IC devices were compared and conflicting estimates of REE, respiratory quotient, and expiratory minute volume were observed between devices in mechanically ventilated patients.27 A second study by Graf et al compared 3 currently available IC devices, and differences in REE between devices were observed, which authors concluded were not acceptable for clinical practice. The authors indicate new indirect IC technology was in need of development.28 Further, a comprehensive study of a large number of recent existing IC devices showed quite variable validation, accuracy, and reliability by device.31 Specifically, via testing of 12 current IC devices, accuracy was shown at only 1 of the 2 study sites for each of the devices tested and not for all variables tested.31 Most clinical studies using IC utilize a device developed ~35 years ago (Deltatrac Metabolic Monitor, Datex). The production of this device was discontinued 10 years ago, and very few working units remain in use. Difficulties in IC conduct, calibration, and interpretation of results have also continued to limit the use of IC in ICU patients.29,30 Further limitations of existing IC technology in the ICU are also discussed in the accompanying point-counterpoint paper (Reference per NCP publisher when available) in this issue. Given the consistent inaccuracy of the estimation of caloric need by equations and the unreliable results from the calorimeters on the market, the development of an accurate and reliable calorimeter has been urgently needed. The ideal new IC device must be convenient and simple to use, require minimal calibration and maintenance, and have a reasonable cost to allow for the routine, longitudinal IC measurements that are needed in ICU patients.
A NEW-GENERATION INDIRECT CALORIMETER: AN OPPORTUNITY FOR OBJECTIVE, LONGITUDINAL NUTRITION TARGET ASSESSMENT
To address the urgent need for a new-generation metabolic cart (IC), an ambitious endeavor was recently undertaken, uniting leaders in the critical care nutrition field with industry leadership to address this essential deficiency in ICU nutrition care. The group, the International Multicentric Study Group for Indirect Calorimetry (ICALIC), championed a project to develop an accurate, cost-affordable, reliable, and user-friendly indirect calorimeter to measure EE in the ICU and other patients. The result of this project was the development of the next-generation Q-NRG indirect calorimeter device, which is now US Food and Drug Administration (FDA) approved and available worldwide.32
The new Q-NRG (Baxter and COSMED, Inc) device was extensively validated vs mass spectroscopy (gold standard) to assure accuracy and evaluate analytical performance. This new IC device was found to be accurate at FIO2 ranges up to 70%, extending the traditional range of IC Fraction of Inspired Oxygen (FiOs) measurements beyond 60%.33 The performance of the new-generation IC device in clinical practice and vs existing IC devices was recently published in the new ICALIC project paper.34 This multicenter study evaluated the ease of clinical use of the new IC device in critical care patients in a wide range of ICU settings. The study examined real-world device performance in 6 international academic ICU centers on 3 continents. The results of the study demonstrate the Q-NRG IC required a much shorter time (with reliable, steady-state measurements in ~10 minutes) to determine EE in mechanically ventilated ICU patients vs other existing IC devices. A summary of critical differences between the new-generation Q-NRG IC device and previous IC devices is shown in Table 1. This new IC device allows accurate measurements in a much broader range of patients, including FIO2 up to 70% and higher positive end-expiratory pressure (PEEP) settings, and as described in recent publications on extracorporeal membrane oxygenation35 and potentially during continuous renal replacement therapy (CRRT).36 We as the authors concluded the new Q-NRG provides accurate EE and IC measures in an efficient and timely fashion. It fills a long-standing void in ICU and clinical nutrition care as the only commercially available IC device tested against mass spectrometry to ensure gas accuracy while being easy to use for longitudinal IC measures in a range of settings in and out of the ICU. These characteristics should allow for a much broader use of IC to optimize the prescription of nutrition support by objectively determining energy targets to limit poor clinical outcomes due to the risk of underfeeding or overfeeding.
TABLE 1.
Comparison of barriers to use in existing IC vs new-generation IC device
| Barriers | Past/existing IC technologya | New-generation IC deviceb |
|---|---|---|
| Setup and measurement time | 45→60 minutes | ~10–15 minutes |
| Calibration time | Extensive calibration time Complicated external calibration often required | Minimal calibration timeSelf-calibrating |
| Size | Bulky, large device | Lightweight, portable, easy to transport |
| - | Variable validation, accuracy, and reliability by device---27,28,31 | Accuracy validated via mass spectrometry33,34 |
| Patient-use flexibility | Typically limited to ventilated patients in ICU only | Use in ventilated and spontaneously breathing patients in and out of ICU |
| FIO2 limits of accuracy | ≤60% FiO2 | ≤70% FIO2 |
| Potential PEEP limits | Suggested: <10–12 | <16 (per Longitudinal Energy Expenditure and Metabolic Effects in Patients With COVID-19 [LEEP-COVID] data)37 |
FIO2, fraction of inspired oxygen; FiOs, xxxx; IC, indirect calorimetry; ICU, intensive care unit; LEEP-COVID, xxxx; PEEP, positive end-expiratory pressure.
Some data from QNRG+Technical Brochure- (COSMED, Inc). (website: https://www.cosmed.com/hires/Q-NRG+_brochure_A3_C04672-22-93_EN_web.pdf) Accessed 03/04/2021
USE OF IC IN COVID-19 ICU PATIENTS
In response to the recent worldwide COVID-19 pandemic,7 the new-generation Q-NRG IC device was utilized to conduct the first longitudinal study of the metabolic phenotype and mREE in this novel pandemic illness. To address defining the metabolic phenotype of COVID-19, the LEEP-COVID study group recently published data37 demonstrating first that longitudinal IC measures can be efficiently and routinely obtained in mechanically ventilated COVID-19 ICU patients. The LEEP-COVID results show that during the first ICU week in intubated COVID-19 patients, mREE fell between 15 and 20 kcal/kg (for actual body weight in BMI < 30 and adjusted body weight in obese patients).1 Markedly increased hypermetabolism and wider variability in mREE values were observed following the first ICU week. Unlike data from smaller studies in other ICU populations,38 the hypermetabolism observed in COVID-19 patients persisted and, in fact, increased during the second and third ICU week (mean mREE = 150%, predicted REE [pREE] in third ICU week). In fact, some patients exhibited resting metabolic rates >2 times that predicted by the Harris-Benedict equation (HBE). Consistent with previous studies showing the inaccuracy of predictive equations throughout ICU stay,23 the HBE significantly underpredicted mREE consistently following the first ICU week and, in fact, often overpredicted need in the first ICU week in COVID-19 patients. This further emphasizes that current predictive equations do not appear to accurately predict energy targets and that currently utilized predictive equations likely lead to significant overfeeding and underfeeding. We found observed changes in mREE do not demonstrate a significant relationship to organ failure severity and are only minorly affected by prone positioning/paralysis, as over the study period the use of these therapies was not significantly different. This is consistent with previously published data showing that paralysis appears to have only a minor effect on mREE.39 Our data strongly suggest that personalization of nutrition delivery via routine, longitudinal IC use1,34 should be considered as the new standard of care to accurately assess EE, help guide nutrition therapy in COVID-19 (and, likely, other ICU patients), and improve patient care overall.
SUMMARY: KEY EVIDENCE SUPPORTING ROUTINE USE OF IC IN THE ICU AND CLINICAL IC PROTOCOL RECOMMENDATIONS
If we are to ultimately overcome the fundamental challenges and perceptions in delivering nutrition in the ICU, we must all adopt routine objective, longitudinal measurement of energy targets and nutrition requirements.
KEY EVIDENCE SUPPORTING ROUTINE IC USE IN THE ICU
The following list highlights key evidence for supporting routine IC use in the ICU:
Universal guideline recommendations calling for use of IC to determine energy requirements in ICU1,2
Data showing that pREE from predictive equations or body weight calculations is consistently inaccurate and correlates poorly with mREE, ultimately leading to routine overfeeding and/or underfeeding, which are both associated with poor ICU outcomes1
The development and worldwide availability of IC devices that are validated, accurate, and easy to use, maintain, and interpret32–34
Recent data in COVID-19 ICU patients from LEEP-COVID study showing progressive hypermetabolism throughout ICU stay and the variability in metabolic responses in different forms of critical illness and emphasizing inaccuracy of predictive equations with marked day-to-day variability in nutrition needs37
New metabolic cart technology (Q-NRG device) that is simple to use and maintain allows for a range of disciplines and healthcare professionals to perform IC testing (ie, registered dietitians [RDs]) and develop IC teams
Thus, given these key findings, the use of this new-generation metabolic cart device provides an opportunity for IC to be the new standard of care for objective delivery of all nutrition, including EN, PN, and oral nutrition in the ICU and post-ICU patient.
A structured approach to the use of routine, longitudinal IC measurements to guide evidence-based ICU nutrition delivery is essential, as it is utilized in other areas of critical care. Key recommendations for guidelines for patient use of the new IC are summarized in Table 2. Additionally, a suggested personalized IC-guided ICU nutrition algorithm derived from a range of recent evidenced-based ICU nutrition reviews is presented in Figure 1.11,17 Another excellent algorithm is described in a recently published landmark paper (the Effect of early nutritional support on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial [EFFORT] trial), supporting the essential role of an RD-driven nutrition pathway. This large, multicenter randomized trial in acutely ill hospitalized patients at high malnutrition risk40 found that a structured nutrition pathway led to significant reductions in complications at 30 days and in mortality and significantly improved recovery of functional independence and QoL as measured by EQ-5D at 30 days after hospitalization. Importantly, this nutrition pathway can easily be adapted for both care of the ICU patient and post-ICU care.
TABLE 2.
New-Generation (Q-NRG) IC measurement guidelines
| ✓ | Patient not agitated and sedation and analgesia drug doses stable, neuromuscular blockade are acceptable for measures and has minimal effect on REE measure |
| ✓ | Ideally, for best measures:
|
| ✓ | Establish FIO2 of <70%, the maximum expected for clinical accuracy for new QNRG Device accuracy |
| ✓ | Preferred PEEP: ≤16 cm H2O, and peak airway pressure: ≤30 cm H2O |
| ✓ | No air leaks (ie, no chest tubes) and patient has stable ventilator settings for ≥30 minutes |
| ✓ | Perform IC 4 hours before/after CRRT use. Perform IC before or >4 hours after intermittent hemodialysis |
| ✓ | Ideally, wait ≥60 minutes after painful procedure or changes in catecholamine, sedative, or analgesic dose |
Recommendations taken from Oshima et al,6 Kaviani et al,31 and Uehara et al38 and QNRG operating manual (COSMED, Inc).
ABG, arterial blood gas; CRRT, continuous renal replacement therapy; IC, indirect calorimetry; FIO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; REE, resting energy expenditure.
FIGURE 1.
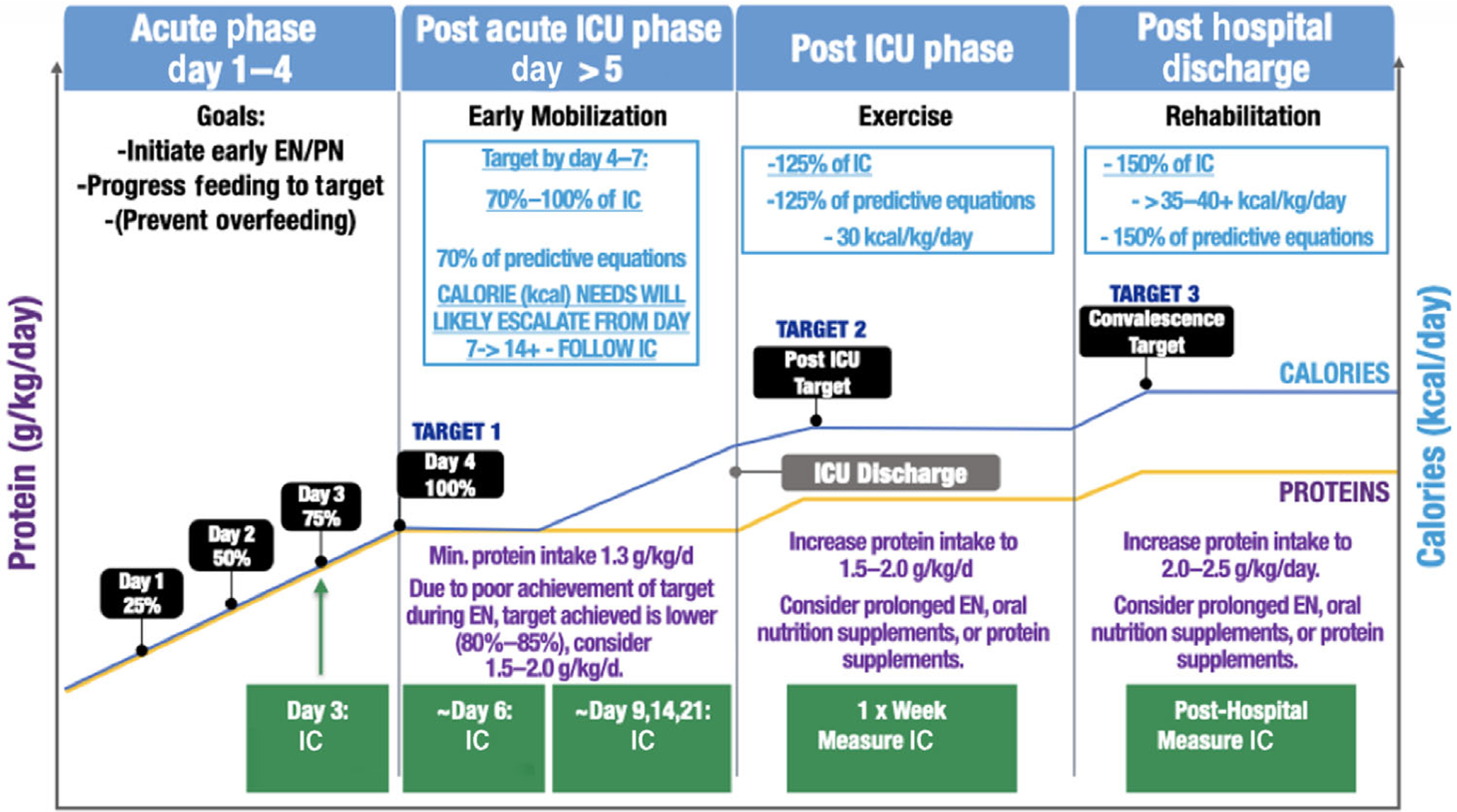
Personalized IC-guided ICU nutrition algorithm (derived from recent evidenced-based ICU nutrition reviews11,17). Please note that suggested IC measurement days are meant as general guidelines to create consistency in measurement throughout patient stay. IC should ideally be performed 2–3 times per week or when there is a significant clinical change in patient status. EN, enteral nutrition; IC, indirect calorimetry; ICU, intensive care unit; PN, parenteral nutrition
In conclusion, it is essential that longitudinal IC measures before, during, and after ICU care become the new worldwide standard of care to guide nutrition care and become as ubiquitous in their reporting on rounds as blood pressure values and heart rates are reported to guide vasopressor and other ICU care. It is only with continued implementation of objective nutrition data, such as longitudinal IC measures and ultrasound-derived muscle mass measures,41 that we will ensure each ICU patient receives personalized nutrition care that delivers the right nutrition, in the right patient, at the right time to optimize clinical outcomes.
CONFLICT OF INTEREST
P. E. Wischmeyer has received grant funding related to this work from the National Institutes of Health, Canadian Institutes of Health Research, Abbott, Baxter, Fresenius, Nutricia, and Takeda. P. E. Wischmeyer serves as a consultant to Abbott, Fresenius, Baxter, Nutricia, and Takeda for research related to nutrition in surgery and ICU care; received unrestricted gift donation for surgical and critical care nutrition research from MuscleSound and COSMED; and received honoraria or travel expenses for CME lectures on improving nutrition care in surgery and critical care from Abbott, Baxter, Nutricia, and Fresenius.
Footnotes
FINANCIAL DISCLOSURE
None.
References
- 1.Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. [DOI] [PubMed] [Google Scholar]
- 2.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 3.Cahill NE, Dhaliwal R, Day AG, Jiang X, Heyland DK. Nutrition therapy in the critical care setting: what is “best achievable” practice? An international multicenter observational study. Critical Care Med. 2010;38(2):395–401. [DOI] [PubMed] [Google Scholar]
- 4.Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in canadian ICUs–opportunities for improvement? J Parenteral Enteral Nutri. 2003;27(1):74–83. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124(1):297–305. [DOI] [PubMed] [Google Scholar]
- 6.Oshima T, Delsoglio M, Dupertuis YM, et al. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clin Nutr. 2020;39(10):3105–3111. [DOI] [PubMed] [Google Scholar]
- 7.Berlin D, Gulick R, Martinez F. Severe Covid-19. N Engl J Med. 383(25): 2020. [DOI] [PubMed] [Google Scholar]
- 8.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. [DOI] [PubMed] [Google Scholar]
- 9.Dinglas VD, Aronson Friedman L, Colantuoni E, et al. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45(3):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wischmeyer PE. Are we creating survivors…or victims in critical care? Delivering targeted nutrition to improve outcomes. Curr Opin Crit Care. 2016;22(4):279–284. [DOI] [PubMed] [Google Scholar]
- 11.Wischmeyer PE. Nutrition therapy in sepsis. Crit Care Clin. 2018;34(1):107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125(4):1446–1457. [DOI] [PubMed] [Google Scholar]
- 13.Binnekade JM, Tepaske R, Bruynzeel P, Mathus-Vliegen EM, de Hann RJ. Daily enteral feeding practice on the ICU: attainment of goals and interfering factors. Crit Care. 2005;9(3):R218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jonghe B, Appere-De-Vechi C, Fournier M, et al. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered? Crit Care Med. 2001;29(1):8–12. [DOI] [PubMed] [Google Scholar]
- 15.Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in canadian ICUs–opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27(1):74–83. [DOI] [PubMed] [Google Scholar]
- 16.Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32(2):350–357. [DOI] [PubMed] [Google Scholar]
- 17.van Zanten ARH, De Waele E, Wischmeyer PE. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. 2019;23(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wischmeyer PE. Tailoring nutrition therapy to illness and recovery. Crit Care. 2017;21(S3):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidegger CP, Berger MM, Graf S, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–393. [DOI] [PubMed] [Google Scholar]
- 20.McClave SA, Martindale RG, Rice TW, Heyland DK. Feeding the critically ill patient. Crit Care Med. 2014;42(12):2600–2610. [DOI] [PubMed] [Google Scholar]
- 21.Fraipont V, Preiser JC. Energy estimation and measurement in critically ill patients. JPEN J Parenter Enteral Nutr. 2013;37(6):705–713. [DOI] [PubMed] [Google Scholar]
- 22.Guttormsen AB, Pichard C. Determining energy requirements in the ICU. Curr Opin Clin Nutr Metab Care. 2014;17(2):171–176. [DOI] [PubMed] [Google Scholar]
- 23.Zusman O, Kagan I, Bendavid I, Theilla M, Cohen J, Singer P. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin Nutr. 2019;38(3):1206–1210. [DOI] [PubMed] [Google Scholar]
- 24.Oshima T, Graf S, Heidegger CP, Genton L, Pugin J, Pichard C. Can calculation of energy expenditure based on CO2 measurements replace indirect calorimetry? Crit Care. 2017;21(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graf S, Pichard C, Genton L, Oshima T, Heidegger CP. Energy expenditure in mechanically ventilated patients: The weight of body weight! Clin Nutr. 2017;36(1):224–228. [DOI] [PubMed] [Google Scholar]
- 26.Ridley EJ, Tierney A, King S, et al. Measured energy expenditure compared with best-practice recommendations for obese, critically ill patients-a prospective observational study. JPEN J Parenter Enteral Nutr. 2020;44(6):1144–1149. [DOI] [PubMed] [Google Scholar]
- 27.Sundstrom M, Tjader I, Rooyackers O, Wernerman J. Indirect calorimetry in mechanically ventilated patients. A systematic comparison of three instruments. Clin Nutr. 2013;32(1):118–121. [DOI] [PubMed] [Google Scholar]
- 28.Graf S, Karsegard VL, Viatte V, et al. Evaluation of three indirect calorimetry devices in mechanically ventilated patients: which device compares best with the Deltatrac II((R))? A prospective observational study. Clin Nutr. 2015;34(1):60–65. [DOI] [PubMed] [Google Scholar]
- 29.De Waele E, Spapen H, Honore PM, et al. Introducing a new generation indirect calorimeter for estimating energy requirements in adult intensive care unit patients: feasibility, practical considerations, and comparison with a mathematical equation. J Crit Care. 2013;28(5):884 e881–886. [DOI] [PubMed] [Google Scholar]
- 30.Oshima T, Berger MM, De Waele E, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clinical Nutrition. 2017;36(3):651–662. [DOI] [PubMed] [Google Scholar]
- 31.Kaviani S, Schoeller DA, Ravussin E, et al. Determining the Accuracy and Reliability of Indirect Calorimeters Utilizing the Methanol Combustion Technique. Nutr Clin Pract. 2018;33(2):206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima T, Berger MM, De Waele E, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 2017;36(3):651–662. [DOI] [PubMed] [Google Scholar]
- 33.Delsoglio M, Dupertuis YM, Oshima T, van der Plas M, Pichard C. Evaluation of the accuracy and precision of a new generation indirect calorimeter in canopy dilution mode. Clin Nutr. 2020;39(6):1927–1934. [DOI] [PubMed] [Google Scholar]
- 34.Oshima T, Delsoglio M, Dupertuis YM, et al. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clinical Nutrition. 39(10): 2020. [DOI] [PubMed] [Google Scholar]
- 35.De Waele E, Jonckheer J, Pen JJ, et al. Energy expenditure of patients on ECMO: A prospective pilot study. Acta Anaesthesiol Scand. 2019;63(3):360–364. [DOI] [PubMed] [Google Scholar]
- 36.Jonckheer J, Spapen H, Malbrain M, Oschima T, De Waele E. Energy expenditure and caloric targets during continuous renal replacement therapy under regional citrate anticoagulation. A viewpoint. Clin Nutr. 2020;39(2):353–357. [DOI] [PubMed] [Google Scholar]
- 37.Whittle J, Molinger J, MacLeod D, Haines K, Wischmeyer PE; LEEP-COVID Study Group. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med. 1999;27(7):1295–1302. [DOI] [PubMed] [Google Scholar]
- 39.Koekkoek WAC, Menger YA, van Zanten FJL, van Dijk D, van Zanten ARH. The effect of cisatracurium infusion on the energy expenditure of critically ill patients: an observational cohort study. Critical Care. 2020;24(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–2321. [DOI] [PubMed] [Google Scholar]
- 41.Molinger J, Pastva AM, Whittle J, Wischmeyer PE. Novel approaches to metabolic assessment and structured exercise to promote recovery in ICU survivors. Curr Opin Crit Care. 2020;26(4):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]


