Abstract
BACKGROUND:
Negative urgency is a facet of impulsivity associated with negative affect and risky behavior that may involve the amygdala. The current study determined if social isolation during development alters negative urgency and c-Fos activity in the basolateral amygdala (BLA).
METHODS:
Female Sprague-Dawley rats were raised in an isolated condition (IC), a standard social condition (SC), or an enriched condition (EC), and then were tested for locomotor activity, novelty place preference and negative urgency using a reward omission task. Following performance on the reward omission task, brains were analyzed for c-Fos expression in Ca2+/calmodulin kinase II (CaMKII) and calbindin (CB) neurons, as well as in parvalbumin (PV) neurons associated with perineuronal nets (PNNs) in BLA.
RESULTS:
IC rats exhibited enhanced locomotion compared to both SC and EC rats, as well as enhanced novelty place preference compared to EC rats; only IC rats showed increased responding following omission of an expected reward (negative urgency). Following completion of the reward omission task, IC rats also displayed increased percent of c-Fos neurons in BLA associated with CaMKII, CB and PV neurons compared to SC and EC rats. In IC rats, c-Fos activation in BLA occurred following omission of an expected reward. Finally, IC rats displayed reduced PNN intensity associated with PV neurons compared to EC rats, but the percent of these neurons co-expressing c-Fos was greater in IC rats; SC rats were intermediate between IC and EC rats.
CONCLUSIONS:
Negative urgency was observed in IC rats, but not SC or EC rats. While multiple mechanisms are likely involved, this behavioral effect was associated with an isolation-induced increase in activity of excitatory neurons in BLA, as well as decreased PNN intensity surrounding GABAergic neurons in the same region.
Keywords: negative urgency, reward omission task, perineuronal nets, social isolation, basolateral amygdala, c-Fos
1. INTRODUCTION
Adverse early life experiences can have a significant impact on behavior and brain development. Clinical studies show that children raised in impoverished living conditions are at a greater risk for the development of psychiatric disorders such as anxiety and addiction (1). Likewise, rodents raised in social isolation are prone to greater drug self-administration compared to rats raised in socially enriched conditions (2–5). Socially isolated rats are also more sensitive to non-drug reinforcers such as visual novelty and palatable food (6, 7). In these preclinical studies, the differential rearing paradigm is typically used in which rats are raised in either an isolated condition (IC), a social condition (SC) or an enriched condition (EC) that consists of both social peers and novel objects (8, 9). EC rats are typically group-housed in large cages containing novel objects that provide animals with greater sensory, cognitive, and motor stimulation compared to IC rats that are housed individually without novel objects and are not handled during the rearing period. The absence of social cohorts, and the amount of handling are critical elements that singly or collectively alter the behavior and neurobiology of IC rats. Beyond the enhanced sensitivity to drug and non-drug reinforcers, social isolation produces a host of other neurobehavioral effects, including altered reactivity to stress, maladaptive social behavior, and changes in neurochemical and neuroendocrine systems (10). Indeed, animal models of early adverse experiences that utilize social isolation have been used to investigate the underlying mechanism through which decreased social, cognitive and sensory stimulation lead to changes in impulsivity-, stress- and reward-relevant brain areas, including the prefrontal cortex, orbitofrontal cortex, amygdala, and nucleus accumbens (11, 12).
The trait of impulsivity in humans, once thought to be a single construct, can be parsed into various facets, including urgency, lack of premeditation, lack of perseverance and sensation seeking (13). Among these facets, sensation-seeking may be most closely associated with the initiation of substance use (14, 15), whereas negative urgency has been most closely associated with problematic substance use (16, 17). Sensation seeking is a tendency to enjoy exciting and risky activities, and it has been modeled in laboratory rats by measuring locomotion in a novel environment or novelty place preference (18, 19). More importantly, in animal models of drug reward, individual differences in response to novelty have been linked to enhanced vulnerability to maladaptive behavior (18). Indeed, social isolation increases sensation seeking responses; however, the effects of social isolation on expression of negative urgency remains unknown. Negative urgency is a tendency to make rash or rapid choices when in an emotionally distressed state, and it has been modeled in laboratory animals using an operant-based reward omission task (20, 21). In the reward omission task, rats are trained in two different alternating components, a Pavlovian component in which a signal predicts food delivery and an operant component in which lever pressing leads to food delivery. After stable performance on the operant task is achieved, the food reward signaled by the Pavlovian cue is omitted on rare occasions and responding on the subsequent operant component is determined. Results show that omission of the expected reward produces enhanced operant responding for food. This reward omission effect translates into the trait of negative urgency when tested in a human laboratory setting (20). While these studies validate an animal model of negative urgency, it is not known if social isolation alters this facet of impulsivity, nor is it known what precise neurobiological mechanisms are involved. However, it has been hypothesized that the orbitofrontal cortex and the amygdala may play a critical role (21–23).
One recently identified neural substrate associated with reward processing and drug-seeking involves changes in extracellular matrix elements, specifically in perineuronal nets (PNNs). PNNs are extracellular matrix elements that form net-like structures surrounding the cell body and proximal dendrites of a subset of mainly fast-spiking, GABAergic interneurons within the central nervous system (24). Structural changes on PNN composition in cortical structures are associated with changes in learning and memory (25), drug seeking (26, 27) and sucrose seeking (7); however, relatively little is known about subcortical structures. In one relevant study, enzymatic digestion of PNNs in the basolateral amygdala (BLA) rendered fear memories susceptible to loss (28).
The purpose of the current study was to determine the effect of social isolation on performance in both locomotor and novelty place preference tests to model sensation seeking, as well as on performance in a reward omission task to model negative urgency, and to determine if isolation-induced changes in these facets of impulsivity are related to PNN expression BLA. It is already known that, compared to environmental enrichment, social isolation leads to a decrease in the number and intensity of PNN staining (7), but this work has been limited to cortical structures. Therefore, we sought to determine the impact of social isolation on PNN staining in the BLA because this region plays a pivotal role in reward- and emotion-based behaviors (29, 30). Both staining intensity and number of PNNs, as well as a c-Fos expression in BLA neurons phenotyped for Ca2+/calmodulin kinase II (CaMKII), calbindin (CB) and parvalbumin (PV) immunoreactivity, was examined in female rats. Females were used in the initial study because they display greater vulnerability to drug abuse than males (31, 32), thus allowing for greater translational value for understanding trait-based risk.
2. METHODS
2.1. Animals
Sixty-six adult female Sprague-Dawley rats at 21 days of age were obtained from Harlan Industries (Indianapolis, IN, USA) and were housed on a 12/12 hr light/dark cycle with ad libitum access to food and water as noted below. All experiments were conducted during the light phase. Animals were housed and handled one week prior to the beginning of behavioral testing. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and conformed to NIH guidelines.
2.2. Differential rearing paradigm
Upon arrival at 21 days of age, rats were divided randomly into an isolated condition (IC), standard condition (SC), or enriched condition (EC).
2.2.1. Isolated Condition (IC):
Thirty-two rats were housed individually in hanging stainless steel cages (17 × 24 × 20 cm) with wire mesh floors and front panels, as well as solid metal walls and top panels. IC rats were not handled until one week prior to the start of the behavioral procedures.
2.2.2. Standard Condition (SC):
Eighteen female rats were pair-housed in standard polycarbonate cages (26 × 48 × 20 cm) with a wire top and bedding. SC rats were also removed and handled briefly every day. The SC was chosen for comparison because it allowed social interaction without any novel objects, and it represented a standard NIH-guided housing condition for comparison.
2.2.3. Enriched Condition (IC):
Sixteen female rats were group-housed (n=8 per group) in large custom-built steel cages (122 × 61 × 45.5 cm) with wire mesh sides and solid steel floors covered with bedding that was changed weekly. EC rats were handled daily and given an assortment of 14 hard plastic objects (commercially available toys, plastic objects, containers, tubes, etc.). The objects varied in color, shape, and size. Rats remained in these conditions for the duration of the experiment. Seven objects were replaced daily, and objects were re-arranged to create a novel configuration each day.
All animals remained in these conditions throughout the duration of the experiments.
2.3. Behavioral Apparatus
2.3.1. Locomotor Activity Chamber
The chamber contained a horizontal 16 ×16 cm grid of photo beam sensors located 2.5 cm apart and 7 cm above the floor. The activity was recorded automatically using Versamax System software (AccuScan Instruments Inc., Columbus, OH).
2.3.2. Place Preference Chamber
A 3-compartment apparatus (68 × 21 × 21 cm; ENV-013; MED Associates, St Albans, VT) located inside a sound-attenuating chamber (ENV-020M) was used to measure novelty place preference. The three compartments were separated by sliding guillotine doors. The middle compartment (12 × 21 × 21 cm) had gray walls with a smooth gray plastic floor. The end compartments (28 × 21 × 21 cm) provided different contexts, with one compartment having black walls with a stainless steel grid rod floor and the other end compartment having white walls with a stainless steel mesh floor. Recessed trays were located 2 cm below each compartment. A computer controlled the experimental session using Med-IV software. A series of infrared photobeams (6 beams in the black and white compartments and 3 beams in the gray compartment) was used to detect the rats’ presence in a particular compartment and record the amount of time spent in each compartment.
2.3.3. Operant Conditioning Chamber
The reward omission task was conducted in an operant conditioning chamber (28 × 24 × 21 cm; ENV-008CT; Med Associates, St. Albans, VT, USA), containing two retractable levers flanking a central magazine which dispensed food pellets. A cue light was located above each lever, and a house light was mounted over the center of the opposite wall.
2.4. Behavior
2.4.1. Locomotor activity
At 52 days of age, all animals were placed into the center of the locomotor activity chamber, and activity was recorded for 30 min.
2.4.2. Novelty Place Preference
At 53 days of age, each rat was tested for novelty place preference across 4 consecutive days. In the first session (pretest), the guillotine doors were opened, and rats were placed in the gray compartment and were allowed to explore all three compartments for 15 min. The duration spent in each compartment was recorded. Following the pretest, rats went through 2 days of habituation, in which rats were confined by the guillotine door to either the black or white compartment (counterbalanced across groups) for 30 min. On the next day (test), the guillotine doors were opened, and rats were allowed to explore all three compartments for 15 min. The time spent in each compartment was recorded, and a novelty place preference score was derived by dividing the total duration in the novel compartment by the total duration in both the novel and familiar compartments (33). Note that a score of 0.5 denoted no preference and a score above 0.5 denoted a preference for the novel compartment.
2.4.3. Reward Omission Task
Beginning on 56 days of age, rats were food restricted. Body weights were maintained at approximately 85% free feed throughout this task phase by providing 10–20 g of food to each rat at the end of each session. The experimental design was the following: Experiment 1, to determine if differentially housed rats showed a different operant response following reward omission, IC, SC and EC rats (n=16–18 per group) and Experiment 2, to determine if only reward or only omission is altered in IC rats (n=8 per group trial).
For the reward omission task, there were two different components, a Pavlovian component and an operant component, as described previously (20). The Pavlovian component consisted of 5 daily 30-min sessions of a light-food association. A white key light was illuminated on either the left or right side for 5 sec, followed by the delivery of one sucrose pellet (45 mg pellet, F0021, Bio-Serv, Flemington NJ). The side of the key light was counterbalanced across rats. Following a 2-sec dark delay, the house light was then illuminated for 10 sec (intertrial interval, ITI). Rats were given 32 trials per session for a total of 10 days (Supplement Figure 1A).
For establishing a stable baseline in the operant component, rats were given daily 32-trial sessions (60–80 min) in which the food pellet was earned by completing a fixed ratio (FR) response requirement. Two levers were presented, one of which was inactive (no programmed consequence) and the other, which was active (resulted in the delivery of food). The response requirement increased every two sessions from a FR 1, to 3, to 5, to 10. Rats remained in this phase until responding stabilized, defined by a difference of less than 20 lever presses on the active lever over three consecutive sessions on FR 10. Rats were given 32 2-min trials, separated by a 10-sec ITIs, for an average of 16–18 days.
Following the baseline phase on the operant component, rats were moved to a training phase in which the Pavlovian and operant components were presented alternately on each session. Each trial began with the Pavlovian component in which the cue light was illuminated for 5 sec, followed by immediate delivery of one food pellet. After the pellet was delivered, a 2-sec dark delay (no cue light) occurred to allow the rat to consume the pellet and to separate the two components of the trial. When the 2-sec delay ended, the operant component began. Two levers were presented, the active and inactive lever, for 2 min. Rats completed a FR 10 on the active lever to receive one pellet; there was no time-out period following reinforcement delivery. Rats could continually complete the FR 10 requirement to receive additional food pellets for each requirement completed within the 2-min operant component. A 10-sec ITI then occurred, signaled by illumination of the house light. Rats received 32 trials per session for 10 days, and they were moved into the test phase (Supplement Figure 1B).
To test for reward omission performance, the session consisted of 24 reward trials and 8 omission trials, randomly intermixed. Reward trials were identical to those presented in the baseline phase of the Pavlovian component. Omission trials were similar to reward trials, except that no pellet was delivered in the initial Pavlovian component. As before, operant components were 2-min in duration. In order to prevent habituation to the omission trials, a maintenance phase was presented 24 hr after the test, for a total of 3 reward omission tests, 3 maintenance sessions in between, and a final reward omission test followed by killing the rats for brain collection.
2.4. Cellular Analysis
For the cellular analysis a representative sample for each experiment and group was analyzed (n=8 per group)
2.4.1. Immunohistochemical Experiments
Immunofluorescent analysis of c-Fos and PNNs was performed on rats from the two separate experiments. In Experiment 1, to determine if differentially housing altered the cellular analysis, IC, SC and EC rats (n=8 per group) were perfused and brains extracted 45 min after the last reward omission session. In Experiment 2, to determine if reward omission per se altered the cellular analysis, a group of IC rats was trained as described above, except they were killed 45 min following the first omission test session (n=8) or following a reward test session prior to any omission testing (n=8).
Rats were deeply anesthetized with ketamine/xylazine cocktail and perfused transcardially with cold saline solution (0.9% NaCl) followed by cold 4% paraformaldehyde. After perfusion, brains were extracted and placed in a 4% paraformaldehyde solution overnight, followed by 30% sucrose solution for 48–72 hr or until immersion. Using a frozen medium (Histoprep, Fisher scientific), brains were then immersed under liquid nitrogen for 20 sec. Consecutive coronal sections at 40 ~m were obtained using a cryostat (Ag Protect Leica CM 1860) and stored in a freezing solution (30% ethylene glycol, 25% glycerol, 30% sucrose in PBS) at −20ºC.
2.4.2. Immunofluorescence
Free-floating sections were rinsed 7 times for 15 min each with 1x tris-PBS (TPBS; Tris–HCl 10 mM, sodium phosphate buffer 10 mM, 0.9% NaCl, pH 7.4), and incubated with the following primary antibodies: (1) rabbit polyclonal anti-c-Fos antibody (RPCA-c-Fos-AP, Encorbio, USA), diluted 1:500; (2) Wisteria floribunda agglutinin (WFA; L1516–2MG, Sigma Aldrich, USA), diluted 1:200; (3) mouse monoclonal anti-PV antibody (235, Swan, Swiss antibodies, Switzerland), diluted 1:1000; or (5) mouse monoclonal anti-CB antibody (300, Swan, Swiss antibodies, Switzerland) diluted 1:1500; (6) mouse monoclonal anti-CaMKII antibody (MA 1–048, Thermo Scientific, USA) diluted 1:50. Incubations were at 4º C for 48 hr in TPBS 0.1M Triton X-100 containing 3% of donkey serum (Santa Cruz Biotechnology sc-2044). After three 15-min TPBS rinses, tissue was incubated for 2 hr at room temperature protected from light with one of the following secondary antibodies with conjugated fluorochromes: (1) Alexa Fluor 488 donkey anti-rabbit (A-21206, Life Technologies, USA), diluted 1:500; (2) Alexa Fluor 647 donkey anti-mouse (715–605-150, Jackson Labs, USA), diluted 1:500; or (3) biotinylated goat anti-rabbit conjugated with streptavidin Texas red (Vector Labs, UK), diluted 1:500. Once the fluorescence reaction occurred, sections were mounted using Mowiol 4–88 reagent (475904–100GM, EMD Millipore, USA).
2.4.3. Neuronal counting
Three representative fluorescent-labeled sections for the BLA coordinates, approximately between −2.04 and −3.24 mm relative to Bregma (34), were examined using a confocal microscope (Nikon Eclipse-1C) by an observer who was not aware of the treatment condition for each section. Confocal images were taken in single XY planes, 1 μm thick, at a resolution of 1024 × 1024, and 100 Hz speed. Laser intensity, gain and offset were maintained constant for each image acquisition. Quantitative evaluations were made using Image J software (NIH sponsored image analysis software). In each of the 3 selected slices, c-Fos, CaMKII, CB and PV immunoreactivity were estimated with a 20x lens. In each section (n=6), all PV+ interneurons were assessed for presence of PNN by WFA immunoreactivity. Analysis of WFA staining intensity was performed on confocal images by Image J; brightness intensity (range 0–255) was assessed on 50 PNNs/section/animal (n=6/experimental condition). For each PNN, 15 densitometry points covering the PNN were collected (27). The background brightness, taken from a non-stained region of the cortical molecular layer, was subtracted from each brightness measurement. For each net, WFA intensity values <50% were categorized as low intensity PNN neurons and WFA intensity values >50% were categorized as high intensity PNN neurons. Nets touching the edges of the image were excluded. The densitometry analysis of the nets was performed in the soma area only (dendrites were not included). For all cellular parameters, immunofluorescence results are represented as the average of densitometry and cell counts collected from the 3 representative BLA sections.
2.5. Statistics
All statistical analyses were conducted using STATISTICA 7 software package (Statsoft, Inc., Tulsa, OK, USA). Data were expressed as the mean and standard error of the mean (SEM) and were analyzed using either parametric or non-parametric statistics. For parametric statistics, ANOVAs with posthoc Tukey HSD tests were used; a t-test for used when only 2 groups are involved. For WFA intensity, a MANOVA was used, followed by Tukey HSD. In all cases, the level of significance was set at p< 0.05.
3. RESULTS
3.1. Locomotor activity
There was a main effect of rearing environment on locomotor activity across the 30-min test [one-way ANOVA; F (2, 21) = 11.46, p < .001; Figure 1A]. Tukey’s HSD tests revealed that activity in IC rats was significantly higher than both SC and EC rats (each p <0.05). Activity in SC rats was also significantly higher than EC rats (p < 0.05).
Figure 1.
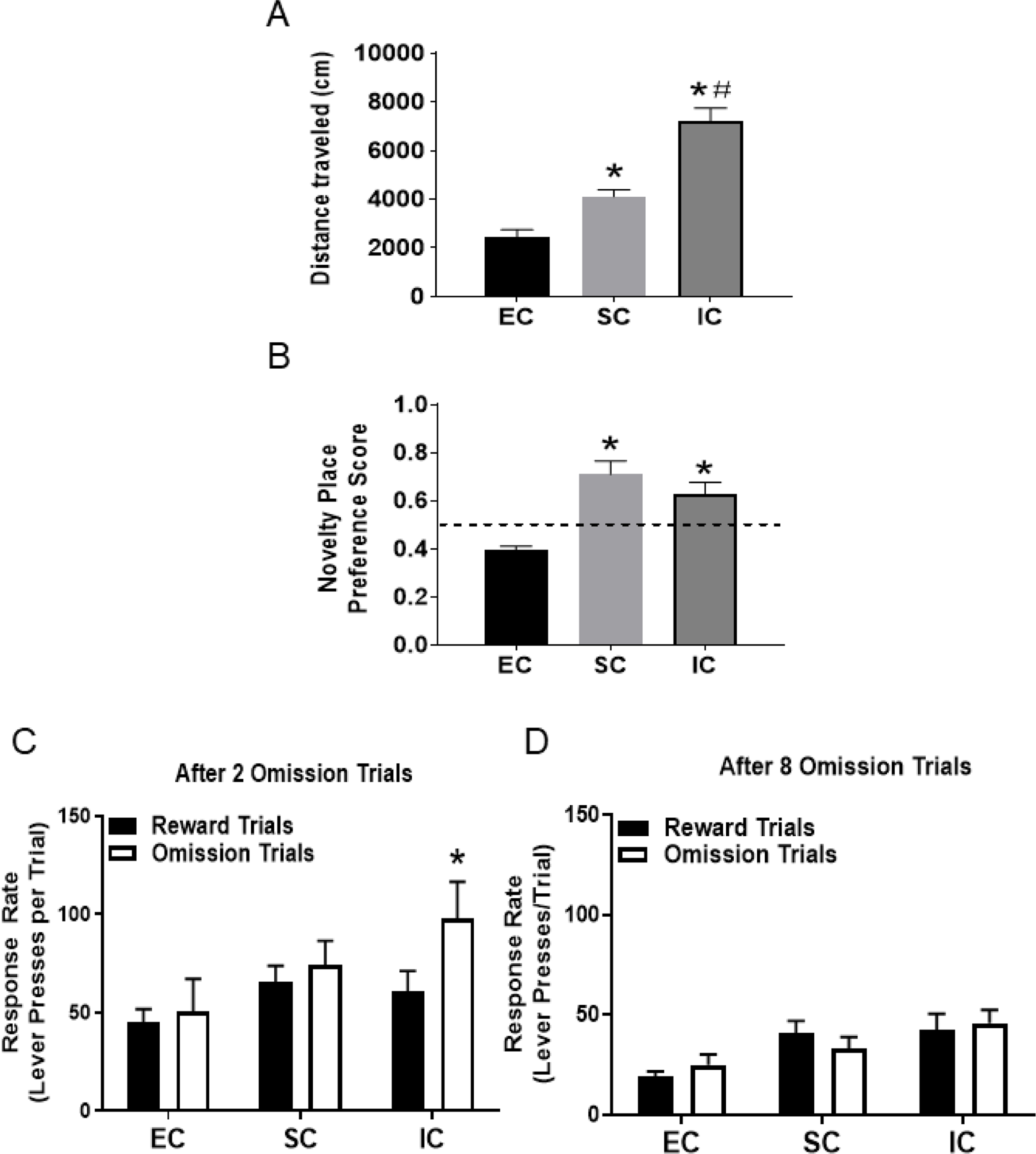
A. Mean (±SEM) distance traveled in EC, SC, and IC rats. *Represents a significant difference from EC. #Represents significant difference from SC. B. Mean (±SEM) novelty place preference score in EC, SC and IC rats. *Represents a significant difference from EC. C. Mean (±SEM) response rate (number of lever presses/trial) for sucrose pellets after 2 omission trials. *Represents a significant difference from reward trials. D. Mean (±SEM) response rate (number of lever presses/trial) for sucrose pellets after 8 omission trials.
3.2. Novelty place preference
There was a main effect of rearing environment on the novelty place preference score [one-way ANOVA; F (2, 21) = 12.67, p < .001; Figure 1B]. Both IC and SC rats spent more time on the novel compartment compared to EC rats (each Tukey HSD test, p<0.01). EC failed to show a novelty place preference and trended toward an aversion.
3.3. Reward Omission Task
For the reward omission task, we analyzed the data after both 2 and 8 reward omission trials within the test sessions. After 2 omission trials, a 2 × 3 ANOVA showed a significant main effect for trial-type [F (2, 42)=13.15, p<0.0001], environment [F(2,42)=19.59, p<0.0001] and an interaction effect [F (2, 42) = 8.49, p <0.0001]. Further analysis revealed that the operant response rate was significantly higher after omission trials than after reward trials in IC rats (p<0.05), but not in SC or EC rats (see Figure 1C). In contrast, after 8 omission trials, a 2 × 3 ANOVA found only a significant main effect for the environment [F(2,42)=6.99, p<0.001], with no significant effect of trial type, indicating an overall higher response rate in both IC and SC rats compared to EC rats regardless of trial type. In addition, there was no interaction effect between environment and trial type, indicating that the reward omission effect observed in IC rats following 2 trials (Figure 1C) dissipated after 8 trials (Figure 1D).
3.4. Effects of differential rearing on activation of phenotypically distinct neuronal subpopulations in basolateral amygdala (BLA)
In Experiment 1, to determine the effects of differential housing on c-Fos and PNNs within the BLA following the reward omission test, we analyzed: (1) the total number of c-Fos+ neurons; (2) the number c-Fos+ neurons phenotyped by CaMKII, CB or PV immunoreactivity; and (3) PNN number and intensity, including double-labelled WFA+/PV+ neurons co-expressing c-Fos.
In BLA, c-Fos expression varied across differential housing conditions. A one-way ANOVA revealed a main effect of the environment [F (2, 21) = 16.26, p<0.001; Figure 2A and 2B]. Pairwise comparisons revealed that IC rats displayed a significant increase in the number of c-Fos+ neurons when compared to either SC or EC rats (each Tukey HSD p<0.001); there was no significant difference between SC and EC rats.
Figure 2.
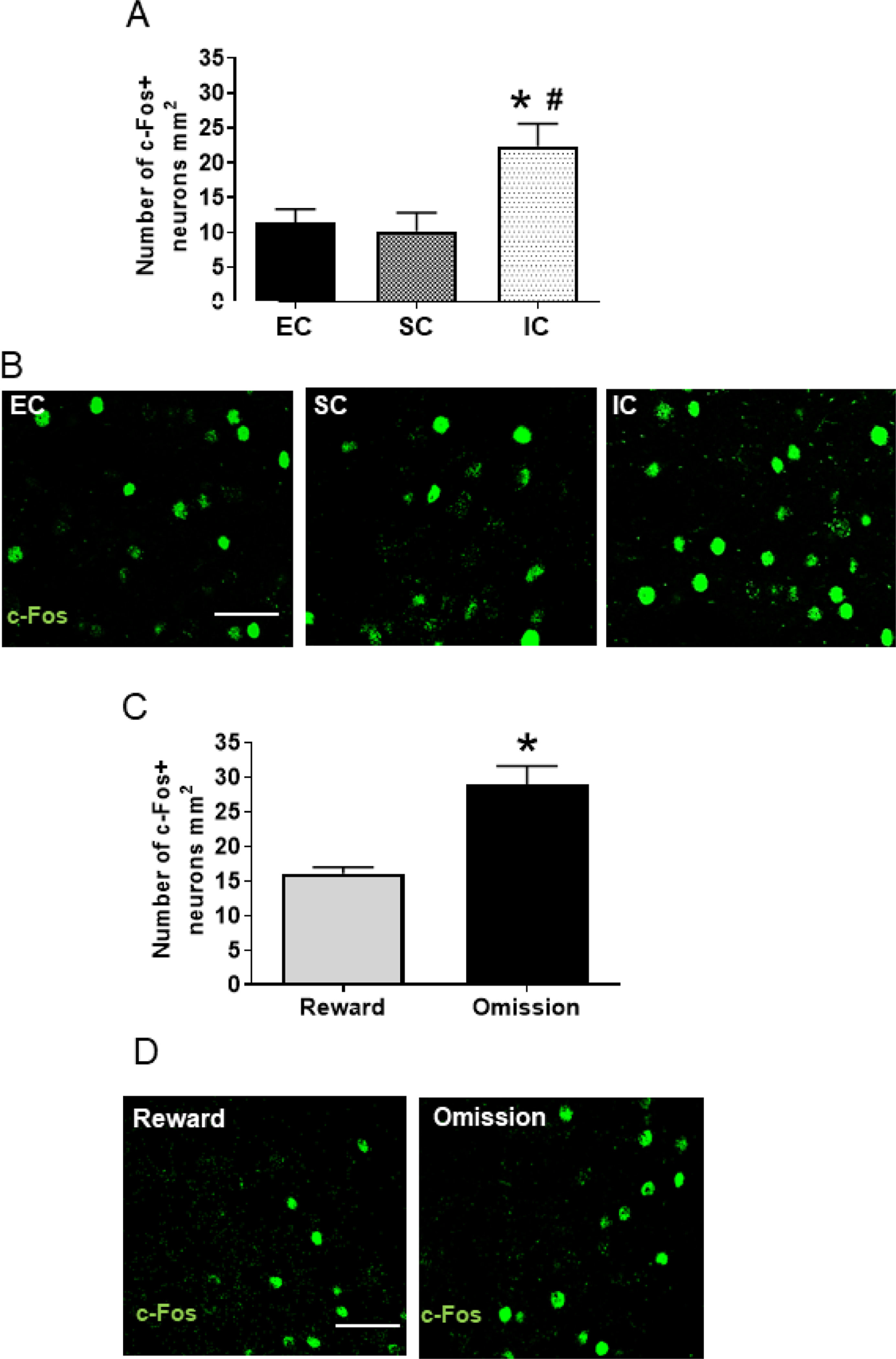
A. Mean (±SEM) number of neurons expressing c-Fos after the last reward omission test session in EC, SC, and IC rats. *Represents a significant difference from EC. #Represents a significant difference from SC. B. Photos are representative confocal images of c-Fos expression in an EC, SC and IC rat; white bar represents 15 um. C. Mean (±SEM) number of neurons expressing c-Fos in IC rats after a baseline reward session or a session consisting of 2 omission trials. *Represents a significant difference from reward trials. D. Photos are representative confocal images of c-Fos expression in an IC rat after a baseline reward session or a session consisting of 2 omission trials; white bar represents 15 um.
In Experiment 2, because IC rats showed a reward omission effect after 2 omission trials, but not after 8 omission trials, a separate group of IC rats was used to evaluate cellular changes in BLA after only 2 omission trials or after a baseline reward session. In this experiment, a t-test found a significant effect of trial type on the number of c-Fos+ neurons in BLA [t(7) =2.71, p<0.05], with the number of c-Fos+ neurons higher following the reward omission session compared to a baseline reward session (Figure 2C and 2D).
3.4.1. Ca2+/calmodulin kinase II (CaMKII)
CaMKII+ multipolar neurons of pyramidal or piriform shape consistent with a glutamatergic phenotype were detected, with a size and density similar to that reported previously (35). In Experiment 1, a one-way ANOVA revealed a significant main effect of environment on the total number of CaMKII neurons [F (2, 21) = 5.89, p<0.05]. Pairwise comparisons showed that IC rats displayed more CamKII+ neurons compared to both SC and EC rats (each Tukey HSD p<0.05; Figure 3A). A one-way ANOVA also revealed a significant main effect of the environment on CaMKII+ neurons co-expressing c-Fos [F (2, 21) = 3.90, p<0.05]. Pairwise comparisons revealed that IC rats had an increased percentage of CaMKII+ neurons co-expressing c-Fos compared to both SC and EC rats [each Tukey HSD p<0.05; Figure 3B and 3D]; there was no significant difference between SC and EC rats. In Experiment 2 with IC rats, while there was no significant difference in number of CaMKII+ neurons, the percentage of CaMKII+ neurons co-expressing c-Fos was higher following a reward omission session compared to a baseline reward session [t (7) = 2.70, p<0.05; Figure 3C].
Figure 3.
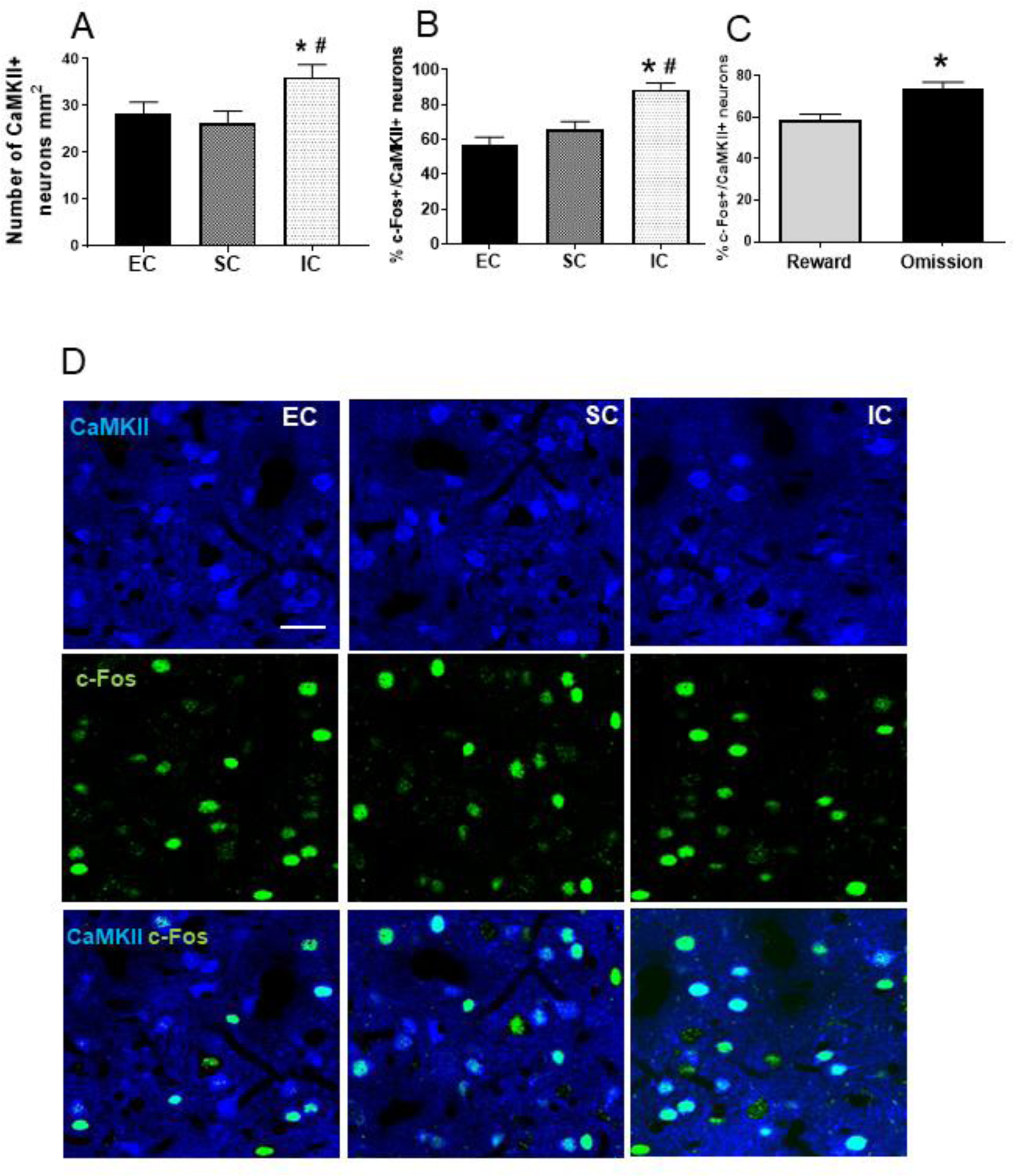
A. Mean (±SEM) number of CaMKII+ neurons in EC, SC, and IC rats. *Represents a significant difference from EC. #Represents a significant difference from SC. B. Mean (±SEM) percent of CaMKII+ neurons co-expressing c-Fos in EC, SC, and IC rats. *Represents a significant difference from EC. #Represents a significant difference from SC. C. Mean (±SEM) percent of CaMKII+ neurons co-expressing c-Fos in IC rats after a baseline reward session or a session consisting of 2 omission trials. *Represents a significant difference from reward trials. D. Photos are representative confocal images of CaMKII+ neurons co-labelled with c-Fos in EC, SC and IC rats.
3.4.2. Calbindin (CB).
The immunofluorescence pattern observed for CB+ neurons resulted in a uniform staining pattern confined to the cytoplasm of the neuron that labeled an oval spheroid pattern ranging in size from 12 to 18 ~m, consistent with a Ca2+-binding phenotype (35). The total number of CB+ neurons was not affected by housing conditions (Experiment 1) or reward omission in IC rats (Experiment 2); however, a one-way ANOVA revealed a significant effect of environment on the percentage of CB+ neurons co-expressing c-Fos [F (2, 21) = 16.26, p<0.001]. Pairwise comparisons revealed that IC rats had greater percentage of CB+ neurons co-expressing c-Fos compared to both EC and SC rats [each Tukey HSD, p<0.001; Figure 4A and 4C]; there was no significant difference between SC and EC rats. In Experiment 2 with IC rats, while there was no significant difference in the number of CB+ neurons, the percentage of CB+ neurons co-expressing c-Fos was higher following a reward omission session compared to a baseline reward session [t (7) = 3.22, p<0.01; Figure 4B].
Figure 4.
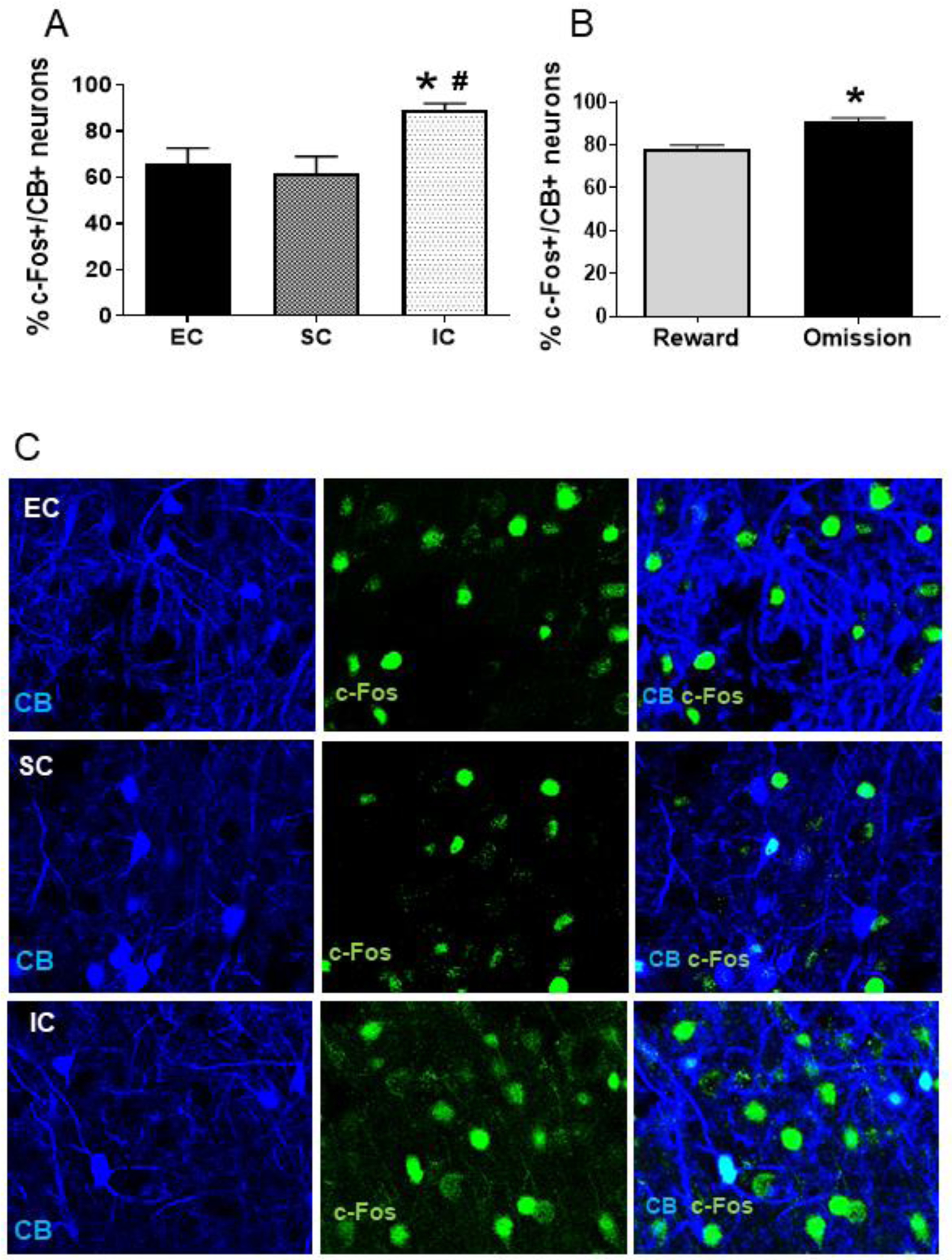
A. Mean (±SEM) percent of CB+ neurons co-expressing c-Fos in EC, SC and IC rats. *Represents a significant difference from EC. #Represents a significant difference from SC. B. Mean (±SEM) percent of CB+ neurons co-expressing c-Fos in IC rats after a baseline reward session or a session consisting of 2 omission trials. *Represents a significant difference from reward trials. C. Photos are representative confocal images of CB+ neurons co-labeled with c-Fos in EC, SC and IC rats.
3.4.3. Parvalbumin (PV).
The staining pattern observed in PV+ neurons was consistent with the size and shape GABA-PV+ neurons (35). In Experiment 1, differential rearing did not produce significant changes in the number of PV+ neurons. However, the percentage of PV+ neurons co-expressing c-Fos was higher in both IC and SC rats compared to EC rats (each Tukey HSD p<0.001; Figure 5A). In Experiment 2 with IC rats, the percentage of PV+ neurons co-expressing c-Fos did not differ significantly between a reward omission session vs. baseline reward session.
Figure 5.
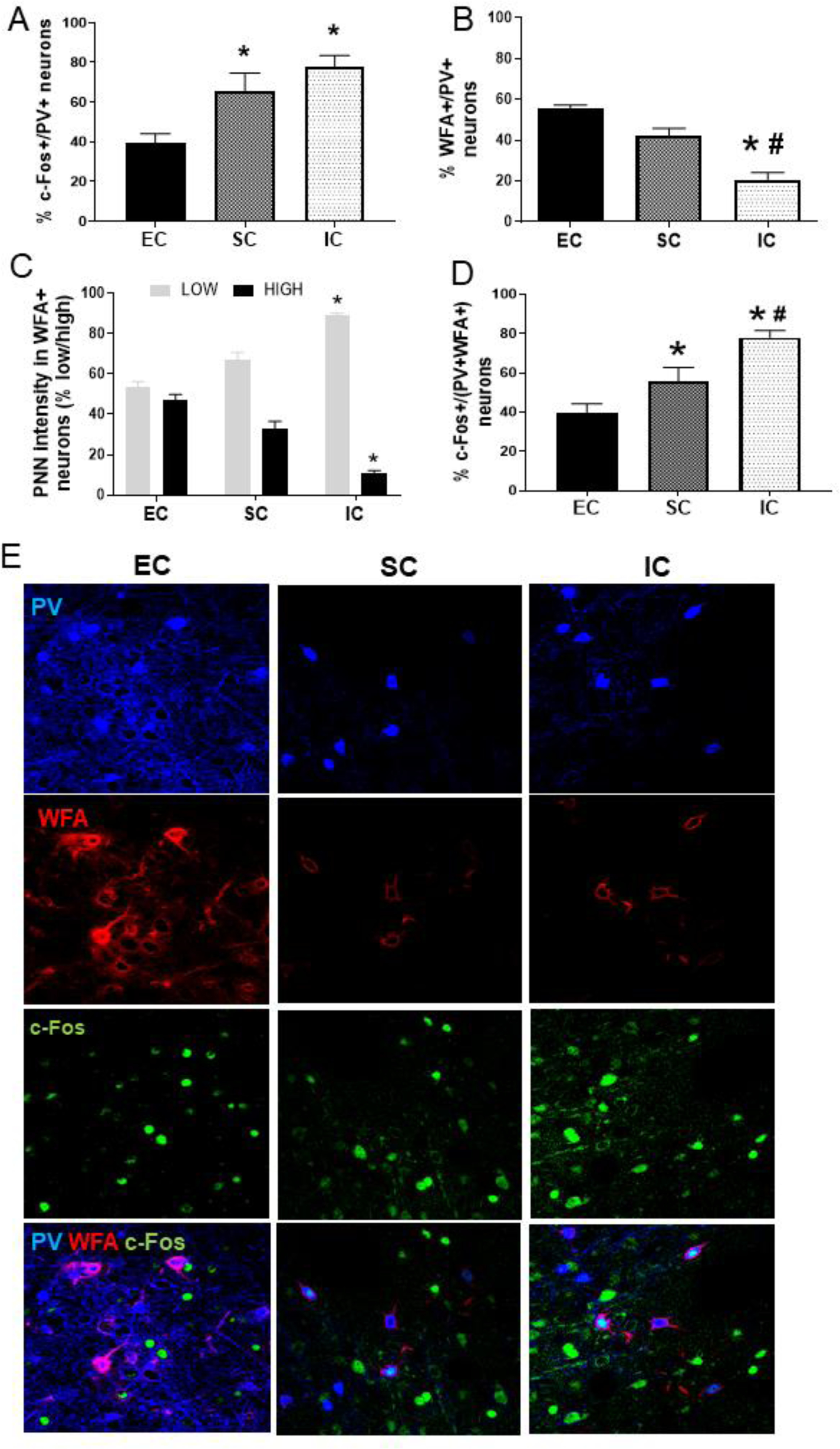
A. Mean (±SEM) percent of PV+ neurons co-expressing c-Fos in EC, SC and IC rats. *Represents a significant difference from EC. B. Mean (±SEM) percent of PV+ neurons co-expressing WFA in EC, SC and IC rats. *Represents a significant difference from EC. #Represents a significant difference from SC. C. Mean (±SEM) percent of WFA+ neurons with low intensity staining and with high intensity staining in EC, SC and IC rats. *Represents significant difference from EC. D. Mean (±SEM) percent of double-labeled WFA+/PV+ neurons co-expressing c-Fos in EC, SC and IC rats. *Represents a significant difference from EC. #Represents a significant difference from SC. E. Photos are representative confocal images of PRV+ neurons co-expressing WFA and c-Fos in EC, SC and IC rats.
3.4.4. Perineuronal Nets (PNNs)
PNN analysis in BLA was similar to that described previously in other brain regions (36). The majority (>90%) of PV+ neurons in BLA co-expressed WFA. In addition, among those neurons expressing WFA, approximately 6% were CB+ neurons, whereas WFA staining was not observed around any CaMKII+ neurons (Supplement Figure 1C). Thus, PNN expression in BLA between the bregma coordinates approximately −2.56 and −3.30 was associated primarily with PV+ GABAergic neurons, with relatively sparse localization to some CB+ neurons.
While differential rearing had no effect on total number of PV+ neurons (see previous section), a one-way ANOVA revealed an effect of differential rearing on the percentage of PV+ neurons co-expressing WFA [F (2, 21) = 7.14, p<0.05]. Pairwise comparisons revealed that IC rats had a decreased percentage of PV+ neurons co-expressing WFA compared to both SC and EC rats (each Tukey HSD, p<0.01); there was no significant difference between EC and SC rats (Figure 5B). In addition, a MANOVA used to analyze WFA staining intensity (low vs. high) revealed an effect of environment [Wilks= 0.18 (4, 38)= 12.9, p< 0.001]. Subsequent univariate statistics showed an effect of environment in the percentage of WFA+ neurons with low intensity staining [F(2,20)= 45.37, p<0.001] and with high intensity staining [F(2,20)= 55.67, p<0.001]. Pairwise comparisons revealed that IC rats displayed reduced high intensity WFA+ staining compared to SC (Tukey HSD, p<0.05) and EC rats (Tukey HSD, p<0.01); conversely, IC rats displayed increased low intensity WFA+ staining compared to both SC and EC rats (each Tukey HSD, p<0.01; Figure 5C). As for c-Fos co-expression in differentially housed rats, a one-way ANOVA showed a significant main effect of the environment [F(2,43)= 10.46, p<0.001]. Subsequent analysis revealed that the percentage of double-labelled PV+/WFA+ neurons co-expressing c-Fos was higher in IC rats than both SC and EC rats (Tukey HSD p<0.001); SC rats were also higher than EC rats (Tukey, HSD, p<0.05; Figure 5D and 5E).
DISCUSSION
The current study examined the effect of social isolation on behavioral performance in tasks modeling sensation seeking (locomotor activity and novelty place preference) and negative urgency (reward omission task), as well as determining if these behavioral changes were related to changes in neuronal activity and PNN expression of BLA neurons phenotyped for CaMKII, CB or PV immunoreactivity. The main behavioral finding is that IC rats exhibited higher levels of locomotion, novelty place preference, and operant responding following 2 reward omission trials compared to EC rats. The response of SC rats on each of these measures was variable, with SC rats showing locomotion intermediate between IC and EC rats, novelty place preference similar to IC rats, and a lack of reward omission responding similar to EC rats. Regardless of the variation in the outcome for SC rats, the difference between IC and EC rats is consistent with previous work showing isolation-induced increases in locomotion (37, 38). The current results extend this finding to novelty place preference and operant-based responding following reward omission. As back-translational models of sensation seeking and negative urgency, these latter findings are important because both of these traits have been strongly associated with drug abuse (15, 39). Consistent with this, previous results show that IC rats have enhanced vulnerability to drug self-administration and reinstatement compared to EC and SC rats (40).
An unexpected finding from the NPP test is that, while both IC and SC rats showed a novelty preference, EC rats did not, instead trending toward a novelty aversion. This pattern of results indicates that the presence of the novel objects, rather than social peers per se, was responsible for the loss of the novelty preference. Since the NPP apparatus consisted of either a wire grid or rod floor in the end compartments, one might consider this as a potential factor in the group differences obtained because a grid floor was used in the IC home cage, whereas a smooth floor covered with bedding was used in the SC and EC home cages. Indeed, one report found that rats raised in a home cage with a metal grid floor come to prefer that floor texture (41). However, different floor textures likely played little role in the lack of novelty preference in EC rats, as the novel compartment (grid vs rod) was counterbalanced across rats and SC rats showed a novelty preference despite having a solid floor in the home cage similar to EC rats. Regardless of the explanation, the loss of novelty preference in EC rats corroborates previous work showing that environmental enrichment reduces incentive motivation for a novel visual stimulus (42).
The reward omission task used here is thought to model negative urgency in humans and rats (20). As predicted, the behavioral results showed greater operant responding for sucrose following the omission of an expected reward than following the delivery of reward. However, this increase in response was observed only in IC rats and only after 2, but not after 8 omission trials. The reward omission effect observed here appeared weaker in magnitude to that reported previously from our laboratory (20, 21). Direct comparison across studies should be avoided, however, as the current study used females, while the previous studies used males. Regardless of potential sex differences, similar to the current report, those previous reports housed rats in single cages and the results showed some diminution of the reward omission effect across repeated testing. Together, these results suggest that the reward omission effect is transient and subject to change with differential social housing.
Another finding from the current report is that IC and SC rats responded more for palatable reward overall than EC rats, regardless of whether it was a reward omission session or a reward session. While it is possible that IC and SC rats may have simply had a higher motivation for sucrose reward than EC rats (43), this is unlikely in the current study because EC rats outperform IC rats when responding for sucrose reward under food restricted conditions similar to those used here (44). Regardless of the interpretation, however, our study provides evidence for the first time that the reward omission effect as a model of negative urgency is boosted by social isolation and that this effect is not simply due to enhanced motivation for palatable reward because both IC and SC showed similar enhancement in overall responding for sucrose reward, but only IC rats showed a reward omission effect. Perhaps the reward omission effect in IC rats observed here may offer a translation model of distress-based overeating in humans, as clinical evidence indicates that females who are high in negative urgency are more likely to binge on palatable foods when emotionally distressed (17). In any case, IC rats may be characterized as having augmented incentive motivation following loss of reward compared to both SC and EC rats.
There were also several key cellular findings from BLA in the current study. First, in IC rats given 2 reward omission trials, the overall number of neurons expressing c-Fos+ was increased relative to the baseline reward session, indicating that the loss of an expected reward activates BLA neurons. Second, when assessed after the last reward omission session, the total number of neurons expressing c-Fos was greater in IC rats compared to both EC and SC rats. Third, social isolation increased the overall number of CaMKII+ neurons, but not CB+ or PV+ neurons. Fourth, across all phenotyped neurons (CaMKII, CB and PV), co-expression of c-Fos in each of these phenotyped BLA neurons was greater in IC rats compared to both SC and EC rats. Fifth, there was an environment-induced change in PNN density and intensity, with IC rats showing a lower percent of PV+ neurons associated with WFA+, as well as an attenuated WFA intensity, compared to both SC and EC rats. Finally, the percentage of doubled-labelled PV+WFA+ neurons that co-expressed c-Fos was greater in IC rats than either SC or EC rats. Thus, compared to SC and EC rats, IC rats show greater activation of glutamatergic (CaMKII+) and calcium-binding (CB+) phenotypic neurons in BLA, as well as greater activation of GABAergic (PV+) phenotypic neurons in the same region, with this latter effect linked to reduced intensity of PNNs (WFA+).
In the BLA, CaMKII represents approximately 85% of the total neurons, and these neurons appear to have an essential role in learning and emotion-based memory (45). The current results extend these results to show enhanced CaMKII+ glutamatergic activity associated with mood-based impulsivity. Previous work has shown that individuals with mood-related disorders display altered activity and function in the glutamatergic projections from the BLA to the medial prefrontal cortex (46). Moreover, CaMKII knockout mice are more likely to express anxiety-like behaviors (47) and other work shows that social isolation in a post-traumatic stress disorder model (predator exposure) significantly increases the levels of CaMKII (48). Thus, the negative urgency response observed in IC rats may be mediated, at least in part, by an increased density and activation of CaMKII glutamatergic neurons. Similarly, while not examined, it is also possible that this neural change may be associated with the locomotor and novelty place preference changes observed in IC rats.
With regard to PNNs, this extracellular matrix component was examined because of its role in neuroplasticity and addiction (49, 50) and because it has been shown to be affected by different environmental conditions in prefrontal cortex (7) and striatum (51). Our study is the first to report that social isolation alters PNN structure and intensity in BLA. IC rats displaying higher levels of negative urgency also showed an attenuated intensity of PNNs within BLA. Although this finding is correlational, it is possible that isolation rearing alters the state of plasticity within BLA to alter negative urgency. In any case, the current isolation-induced decrease in PNNs is consistent with a previous study showing a similar effect in prefrontal cortex (7).
Consistent with previous reports (25–27), we found that the majority of GABAergic neurons (PV+) were associated with surrounding PNNs (WFA immunoreactivity) in BLA. In addition, we found a population of PNNs surrounding calcium-binding (CB+) neurons in this same region, although this population was more sparse than that reported previously in male mice (47). Regardless, PNN intensity is known to be highly correlated with the intensity of PV staining of GABAergic interneurons (52) and is highly influenced by glutamatergic input (25). This suggests that the decreased PNN density and intensity in IC rats in the present study may be due to elevated glutamatergic input to PNN-surrounded GABAergic interneurons, which could then increase GABAergic output to pyramidal neurons and thus result in the expression of emotion-based responding. This interpretation is consistent with the current finding that IC rats showed an increased percent of double-labelled PV+/WFA+ neurons co-expressing c-Fos, as well as with previous reports suggesting that activation of a distinct neuronal population contributes to negative affect (53) and that BLA is involved in encoding the predictive relationship between positive or negative valences involved in fine tuning glutamatergic and GABAergic neurons (54, 55). Future studies should determine if isolation-induced the changes in BLA neurons are linked to plasticity in other parts of the brain involved in mood-based impulsivity.
Supplementary Material
Supplement Figure 1
A. Schematic for baseline acquisition in reward omission task. B. Schematic for testing in reward omission task. C. Photos are representative confocal images co-labeled with CaMKII+ and WFA+ illustrating absence of colocalization of glutamatergic neurons and PNNs.
ACKNOWLEDGEMENTS
This work was supported by: NIH grants P50 DA05312 (MB) and R01 DA12964 (MB) AND Start Funds DVS-Oklahoma State University Center for Health and Sciences. We thank Emily Denehy and Seth Mayfield for technical support. We thank Drs. Mark Prendergast and James Pauly for allowing us the use of equipment.
Footnotes
CONFLICT OF INTEREST: The authors of the present manuscript declare no conflict of Interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.JAFFEE SR, HANSCOMBE KB, HAWORTH CM, DAVIS OS, PLOMIN R. Chaotic homes and children’s disruptive behavior: a longitudinal cross-lagged twin study, Psychological science 2012: 23: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GIPSON CD, BECKMANN JS, EL-MARAGHI S, MARUSICH JA, BARDO MT Effect of environmental enrichment on escalation of cocaine self-administration in rats, Psychopharmacology 2011: 214: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BARDO MT, KLEBAUR JE, VALONE JM, DEATON C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats, Psychopharmacology 2001: 155: 278–284. [DOI] [PubMed] [Google Scholar]
- 4.GREEN TA, GEHRKE BJ, BARDO MT Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules, Psychopharmacology 2002: 162: 373–378. [DOI] [PubMed] [Google Scholar]
- 5.BARDO MT, DWOSKIN LP Biological connection between novelty- and drug-seeking motivational systems, Nebraska Symposium on Motivation Nebraska Symposium on Motivation 2004: 50: 127–158. [PubMed] [Google Scholar]
- 6.CAIN ME, GREEN TA, BARDO MT Environmental enrichment decreases responding for visual novelty, Behavioural processes 2006: 73: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SLAKER M, BARNES J, SORG BA, GRIMM JW Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats, PloS one 2016: 11: e0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VAN PRAAG H, KEMPERMANN G, GAGE FH Neural consequences of environmental enrichment, Nature reviews Neuroscience 2000: 1: 191–198. [DOI] [PubMed] [Google Scholar]
- 9.HOFFORD RS, CHOW JJ, BECKMANN JS, BARDO MT Effects of environmental enrichment on self-administration of the short-acting opioid remifentanil in male rats, Psychopharmacology 2017: 234: 3499–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MUMTAZ F, KHAN MI, ZUBAIR M, DEHPOUR AR Neurobiology and consequences of social isolation stress in animal model-A comprehensive review, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2018: 105: 1205–1222. [DOI] [PubMed] [Google Scholar]
- 11.CHAUVET C, LARDEUX V, JABER M, SOLINAS M. Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment, Neuroscience 2011: 184: 88–96. [DOI] [PubMed] [Google Scholar]
- 12.GRIMM JW, BARNES JL, KOERBER J, GLUECK E, GINDER D, HYDE J. et al. Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats, Brain structure & function 2016: 221: 2817–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHITESIDE SP, LYNAM DR Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale, Experimental and clinical psychopharmacology 2003: 11: 210–217. [DOI] [PubMed] [Google Scholar]
- 14.WILLS TA, VACCARO D, MCNAMARA G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory, Journal of substance abuse 1994: 6: 1–20. [DOI] [PubMed] [Google Scholar]
- 15.BARDO MT, DONOHEW RL, HARRINGTON NG Psychobiology of novelty seeking and drug seeking behavior, Behavioural brain research 1996: 77: 23–43. [DOI] [PubMed] [Google Scholar]
- 16.TRAN J, TEESE R, GILL PR UPPS-P facets of impulsivity and alcohol use patterns in college and noncollege emerging adults, The American journal of drug and alcohol abuse 2018: 44: 695–704. [DOI] [PubMed] [Google Scholar]
- 17.CYDERS MA, SMITH GT Emotion-based dispositions to rash action: positive and negative urgency, Psychological bulletin 2008: 134: 807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BECKMANN JS, MARUSICH JA, GIPSON CD, BARDO MT Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat, Behavioural brain research 2011: 216: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DELLU F, PIAZZA PV, MAYO W, LE MOAL M, SIMON H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man, Neuropsychobiology 1996: 34: 136–145. [DOI] [PubMed] [Google Scholar]
- 20.GIPSON CD, BECKMANN JS, ADAMS ZW, MARUSICH JA, NESLAND TO, YATES JR et al. A translational behavioral model of mood-based impulsivity: Implications for substance abuse, Drug and alcohol dependence 2012: 122: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.YATES JR, DARNA M, GIPSON CD, DWOSKIN LP, BARDO MT Dissociable roles of dopamine and serotonin transporter function in a rat model of negative urgency, Behavioural brain research 2015: 291: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UM M, WHITT ZT, REVILLA R, HUNTON T, CYDERS MA Shared Neural Correlates Underlying Addictive Disorders and Negative Urgency, Brain sciences 2019: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CYDERS MA, DZEMIDZIC M, EILER WJ, COSKUNPINAR A, KARYADI KA, KAREKEN DA Negative Urgency Mediates the Relationship between Amygdala and Orbitofrontal Cortex Activation to Negative Emotional Stimuli and General Risk-Taking, Cerebral cortex (New York, NY : 1991) 2015: 25: 4094–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PIZZORUSSO T, MEDINI P, BERARDI N, CHIERZI S, FAWCETT JW, MAFFEI L. Reactivation of ocular dominance plasticity in the adult visual cortex, Science (New York, NY) 2002: 298: 1248–1251. [DOI] [PubMed] [Google Scholar]
- 25.SLAKER M, CHURCHILL L, TODD RP, BLACKTOP JM, ZULOAGA DG, RABER J. et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory, The Journal of neuroscience : the official journal of the Society for Neuroscience 2015: 35: 4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VAZQUEZ-SANROMAN DB, MONJE RD, BARDO MT Nicotine self-administration remodels perineuronal nets in ventral tegmental area and orbitofrontal cortex in adult male rats, Addiction biology 2017: 22: 1743-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VAZQUEZ-SANROMAN D, CARBO-GAS M, LETO K, CEREZO-GARCIA M, GIL-MIRAVET I, SANCHIS-SEGURA C. et al. Cocaine-induced plasticity in the cerebellum of sensitised mice, Psychopharmacology 2015: 232: 4455–4467. [DOI] [PubMed] [Google Scholar]
- 28.GOGOLLA N, CARONI P, LUTHI A, HERRY C. Perineuronal nets protect fear memories from erasure, Science (New York, NY) 2009: 325: 1258–1261. [DOI] [PubMed] [Google Scholar]
- 29.BURGOS-ROBLES A, KIMCHI EY, IZADMEHR EM, PORZENHEIM MJ, RAMOS-GUASP WA, NIEH EH et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment, Nature neuroscience 2017: 20: 824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WASSUM KM, IZQUIERDO A. The basolateral amygdala in reward learning and addiction, Neuroscience and biobehavioral reviews 2015: 57: 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LYNCH WJ Modeling the development of drug addiction in male and female animals, Pharmacology, biochemistry, and behavior 2018: 164: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ANKER JJ, CARROLL ME Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones, Current topics in behavioral neurosciences 2011: 8: 73–96. [DOI] [PubMed] [Google Scholar]
- 33.MARUSICH JA, DARNA M, CHARNIGO RJ, DWOSKIN LP, BARDO MT A Multivariate Assessment of Individual Differences in Sensation Seeking and Impulsivity as Predictors of Amphetamine Self-Administration and Prefrontal Dopamine Function in Rats, Experimental and clinical psychopharmacology 2011: 19: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PAXINOS G, WATSON C. The Rat Brain in Stereotaxic Coordinates Amsterdam: Academic Press; 2016. [Google Scholar]
- 35.REZNIKOV LR, REAGAN LP, FADEL JR Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress, The Journal of comparative neurology 2008: 508: 458–472. [DOI] [PubMed] [Google Scholar]
- 36.THOMPSON EH, LENSJO KK, WIGESTRAND MB, MALTHE-SORENSSEN A, HAFTING T, FYHN M. Removal of perineuronal nets disrupts recall of a remote fear memory, Proceedings of the National Academy of Sciences of the United States of America 2018: 115: 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ELLIOTT BM, GRUNBERG NE Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats, Behavioural brain research 2005: 165: 187–196. [DOI] [PubMed] [Google Scholar]
- 38.BOWLING SL, BARDO MT Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats, Pharmacology, biochemistry, and behavior 1994: 48: 459–464. [DOI] [PubMed] [Google Scholar]
- 39.SMITH GT, CYDERS MA Integrating affect and impulsivity: The role of positive and negative urgency in substance use risk, Drug and alcohol dependence 2016: 163 Suppl 1: S3–s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.STAIRS DJ, BARDO MT Neurobehavioral effects of environmental enrichment and drug abuse vulnerability, Pharmacology, biochemistry, and behavior 2009: 92: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MCALLISTER DE, MCALLISTER WR, ZELLNER DK Preference for Familiar Stimuli in the Rat, Psychological Reports 1966: 19: 868–870. [Google Scholar]
- 42.CAIN ME, GREEN TA, BARDO MT Environmental enrichment decreases responding for visual novelty, Behav Processes 2006: 73: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GRIMM JW, SAUTER F. Environmental enrichment reduces food seeking and taking in rats: A review, Pharmacology, biochemistry, and behavior 2020: 190: 172874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.GREEN TA, ALIBHAI IN, ROYBAL CN, WINSTANLEY CA, THEOBALD DE, BIRNBAUM SG et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens, Biol Psychiatry 2010: 67: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ROWNIAK M, BOGUS-NOWAKOWSKA K, ROBAK A. The densities of calbindin and parvalbumin, but not calretinin neurons, are sexually dimorphic in the amygdala of the guinea pig, Brain research 2015: 1604: 84–97. [DOI] [PubMed] [Google Scholar]
- 46.CHEN Y, JIANG Y, YUE W, ZHOU Y, LU L, MA L. Chronic, but not acute morphine treatment, up-regulates alpha-Ca2+/calmodulin dependent protein kinase II gene expression in rat brain, Neurochemical research 2008: 33: 2092–2098. [DOI] [PubMed] [Google Scholar]
- 47.MORIKAWA S, IKEGAYA Y, NARITA M, TAMURA H. Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval, Scientific reports 2017: 7: 46024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LIU XH, ZHU RT, HAO B, SHI YW, WANG XG, XUE L. et al. Norepinephrine Induces PTSD-Like Memory Impairments via Regulation of the beta-Adrenoceptor-cAMP/PKA and CaMK II/PKC Systems in the Basolateral Amygdala, Frontiers in behavioral neuroscience 2019: 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.SLAKER M, BLACKTOP JM, SORG BA Caught in the Net: Perineuronal Nets and Addiction, Neural plasticity 2016: 2016: 7538208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SORG BA, BERRETTA S, BLACKTOP JM, FAWCETT JW, KITAGAWA H, KWOK JC et al. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity, The Journal of neuroscience : the official journal of the Society for Neuroscience 2016: 36: 11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’CONNOR AM, BURTON TJ, MANSURI H, HAND GR, LEAMEY CA, SAWATARI A. Environmental Enrichment From Birth Impacts Parvalbumin Expressing Cells and Wisteria Floribunda Agglutinin Labelled Peri-Neuronal Nets Within the Developing Murine Striatum, Front Neuroanat 2019: 13: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.YAMADA J, OHGOMORI T, JINNO S. Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus, The European journal of neuroscience 2015: 41: 368–378. [DOI] [PubMed] [Google Scholar]
- 53.CALU DJ, ROESCH MR, HANEY RZ, HOLLAND PC, SCHOENBAUM G. Neural correlates of variations in event processing during learning in central nucleus of amygdala, Neuron 2010: 68: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SHARPE MJ, SCHOENBAUM G. Back to basics: Making predictions in the orbitofrontal-amygdala circuit, Neurobiology of learning and memory 2016: 131: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.IORDANOVA MD, DEROCHE ML, ESBER GR, SCHOENBAUM G. Neural correlates of two different types of extinction learning in the amygdala central nucleus, Nature communications 2016: 7: 12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1
A. Schematic for baseline acquisition in reward omission task. B. Schematic for testing in reward omission task. C. Photos are representative confocal images co-labeled with CaMKII+ and WFA+ illustrating absence of colocalization of glutamatergic neurons and PNNs.


