The effect of surges in COVID-19 caseload on COVID-19 survival rates is unclear, especially independent of temporal changes in survival. This retrospective cohort study used data from a large U.S. hospital database to study the association between caseload surges and risk-adjusted mortality in patients with COVID-19.
Visual Abstract. Association Between Caseload Surge and COVID-19 Survival in U.S. Hospitals.
The effect of surges in COVID-19 caseload on COVID-19 survival rates is unclear, especially independent of temporal changes in survival. This retrospective cohort study used data from a large U.S. hospital database to study the association between caseload surges and risk-adjusted mortality in patients with COVID-19.
Abstract
Background:
Several U.S. hospitals had surges in COVID-19 caseload, but their effect on COVID-19 survival rates remains unclear, especially independent of temporal changes in survival.
Objective:
To determine the association between hospitals' severity-weighted COVID-19 caseload and COVID-19 mortality risk and identify effect modifiers of this relationship.
Design:
Retrospective cohort study. (ClinicalTrials.gov: NCT04688372)
Setting:
558 U.S. hospitals in the Premier Healthcare Database.
Participants:
Adult COVID-19–coded inpatients admitted from March to August 2020 with discharge dispositions by October 2020.
Measurements:
Each hospital-month was stratified by percentile rank on a surge index (a severity-weighted measure of COVID-19 caseload relative to pre–COVID-19 bed capacity). The effect of surge index on risk-adjusted odds ratio (aOR) of in-hospital mortality or discharge to hospice was calculated using hierarchical modeling; interaction by surge attributes was assessed.
Results:
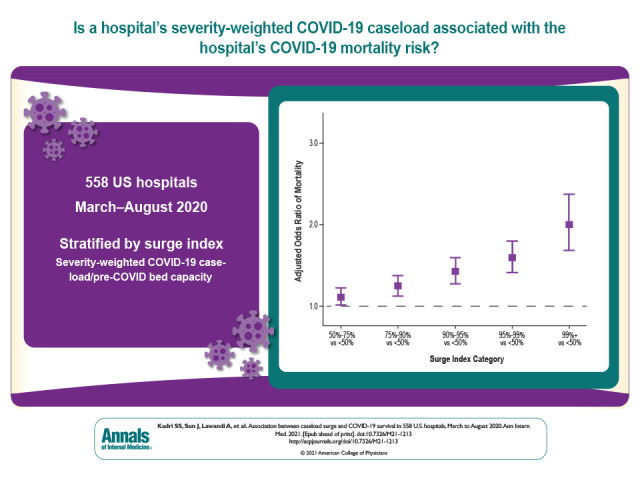
Of 144 116 inpatients with COVID-19 at 558 U.S. hospitals, 78 144 (54.2%) were admitted to hospitals in the top surge index decile. Overall, 25 344 (17.6%) died; crude COVID-19 mortality decreased over time across all surge index strata. However, compared with nonsurging (<50th surge index percentile) hospital-months, aORs in the 50th to 75th, 75th to 90th, 90th to 95th, 95th to 99th, and greater than 99th percentiles were 1.11 (95% CI, 1.01 to 1.23), 1.24 (CI, 1.12 to 1.38), 1.42 (CI, 1.27 to 1.60), 1.59 (CI, 1.41 to 1.80), and 2.00 (CI, 1.69 to 2.38), respectively. The surge index was associated with mortality across ward, intensive care unit, and intubated patients. The surge–mortality relationship was stronger in June to August than in March to May (slope difference, 0.10 [CI, 0.033 to 0.16]) despite greater corticosteroid use and more judicious intubation during later and higher-surging months. Nearly 1 in 4 COVID-19 deaths (5868 [CI, 3584 to 8171]; 23.2%) was potentially attributable to hospitals strained by surging caseload.
Limitation:
Residual confounding.
Conclusion:
Despite improvements in COVID-19 survival between March and August 2020, surges in hospital COVID-19 caseload remained detrimental to survival and potentially eroded benefits gained from emerging treatments. Bolstering preventive measures and supporting surging hospitals will save many lives.
Primary Funding Source:
Intramural Research Program of the National Institutes of Health Clinical Center, the National Institute of Allergy and Infectious Diseases, and the National Cancer Institute.
Many U.S. hospitals have contended with large surges in COVID-19 caseloads during the pandemic. Rapidly escalating demand relative to staff availability and burnout, space, supplies, and personal protective equipment might affect care (1–3) and survival (4). Decreased intensive care unit (ICU) bed availability (5) and increasing community case burden (6) have been implicated as risk factors for poor COVID-19 outcomes. A hypothesis-generating study reported that patients with COVID-19 admitted during periods of higher-than-usual ICU demand had higher case-fatality rates (7). However, the study's nearly all-male cohort from 88 Department of Veterans Affairs hospitals limits generalizability, and the absence of surging ICU caseloads in later study months suggests that temporal improvements could explain their findings (8).
Temporal improvements in hospital survival rates for COVID-19 have been widely reported (6, 9–13). Possible explanations include effective medications (14, 15) and better supportive care (13, 16, 17). However, wide variability in hospital survival reported even among contemporaneously admitted patients within (9, 18) and between (19, 20) regions suggests that differences in capacity and resources across hospitals and over time might have contributed to outcomes. We performed patient- and hospital-level analyses using a large U.S. hospital database to study the association between caseload surges and risk-adjusted mortality in patients with COVID-19.
Methods
Data Source
We performed a retrospective cohort study using the Premier Healthcare Database Special COVID-19 Release (release date 8 November 2020), an all-payer database of administrative data covering approximately 20% of overall U.S. hospitalizations at more than 800 hospitals across 48 states. Details about the database have been previously reported (21, 22). The study was based exclusively on deidentified data and was deemed to be exempt from institutional review board approval under the Revised Common Rule of the National Institutes of Health Office for Human Research Protections. Database curation steps (for example, quality control for delayed reporting due to near-real-time capture) are summarized in the Methods section of the Supplement, Supplement Table 1, and the Appendix Figure. Analyses were prespecified unless explicitly reported as post hoc; the study protocol was published on 30 December 2020 (ClinicalTrials.gov: NCT04688372), before analyses were done. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement guidelines (23) for reporting observational studies were followed (see the Supplement).
Appendix Figure. Flowchart depicting cohort selection, 558 U.S. hospitals, March to August 2020.
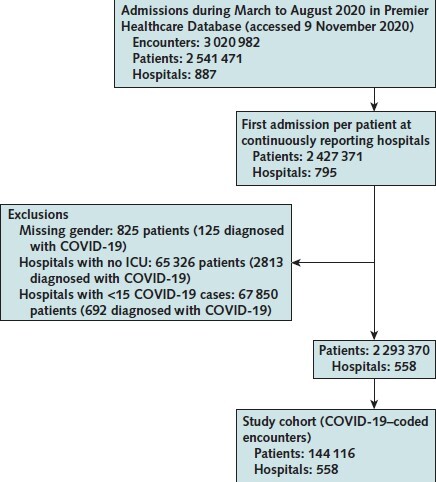
ICU = intensive care unit.
Study Population
Adult (aged ≥18 years) inpatient encounters with admission between 1 March and 31 August 2020 and discharge dispositions through 31 October 2020 were identified at U.S. hospitals that continuously reported encounter data for each of the 6 months. Only the first admission per patient over the study period was included. Among inpatients admitted between April and August 2020, those with COVID-19 were identified using the COVID-19–specific diagnosis code (U07.1) from the International Classification of Diseases, 10th Revision (ICD-10). This strategy captures inpatients who are positive for SARS-CoV-2 on polymerase chain reaction testing with sensitivity of 98%, specificity of 99%, and a positive predictive value of 92% (24). Those admitted in March 2020 (before release of the U07.1 code) were identified using the ICD-10 code for generic coronaviruses (B97.29; Appendix Table 1) as recommended by the Centers for Disease Control and Prevention (CDC). To preserve statistical reliability, only hospitals with 15 or more unique COVID-19–coded inpatient encounters during the study period were included in the primary cohort (5).
Appendix Table 1.
ICD-10 Code–Based Algorithms

Variables and Risk Adjustment
Surge Index (Exposure Variable)
An index was created to capture both the quantitative and qualitative burden in each hospital-month due to surging COVID-19 caseload relative to baseline bed capacity. Inpatient COVID-19 counts for each hospital-month were incrementally weighted as follows: any need for invasive mechanical ventilation (weight, 5×) versus ICU without invasive ventilation or non-ICU setting with noninvasive positive-pressure ventilation (NIPPV) (weight, 2×) versus neither (weight, 1×). The relative weight of 2× was based on the increased intensity of nursing needs in ICUs and advanced respiratory units (optimal nurse-to-patient ratio, 1:2) compared with care on the ward (optimal nurse-to-patient ratio, 1:4). A weight of 5× for invasive ventilation encounters was based on the product of 2× (the aforementioned escalation in nurse-to-patient ratio from 1:4 to 1:2) and 2.5× (representing an escalation in the optimal respiratory therapist-to-patient ratio from 1:10 to 1:4 between routine ICU patients not receiving ventilation vs. those receiving invasive ventilation). Optimal staffing ratios are not federally regulated and were therefore based on staffing mandates exclusively laid out by the State of California (25). The numerator count was multiplied by 10 for ease of reporting and was divided by pre–COVID-19 bed capacity:
Surge index (per hospital-month) = ([(n without ICU, NIPPV, or mechanical ventilation) + 2 × (n with NIPPV or ICU) + 5 × (n with mechanical ventilation)] / pre–COVID-19 bed capacity) × 10
In this calculation, n was the number of unique COVID-19 inpatient encounters; those requiring NIPPV or ICU care did not also need mechanical ventilation; and ICU, NIPPV, and MV could be received at any time during hospitalization.
As an example, consider hospitals A and B, each of which had 20 COVID-19 admissions in June 2020. Hospital A is a 100-bed hospital where zero patients with COVID-19 required ICU admission, NIPPV, or intubation. Hospital B is a 50-bed hospital where all 20 patients with COVID-19 were intubated. Despite identical June caseloads (n = 20), the June surge index for hospital B was 20, or 10 times the surge index of 2 for hospital A.
Patient-Level Covariates
Covariates included age, sex, race/ethnicity, underlying conditions defined by the CDC to carry poor prognosis for COVID-19 (Appendix Table 2), insurance status, point of origin (for example, nursing facility), COVID-19 treatments with potential benefit (systemic corticosteroids, remdesivir) or potential harm (hydroxychloroquine plus azithromycin) (15, 26, 27), and baseline treatment limitations (do-not-resuscitate status present on admission).
Appendix Table 2.
High-Risk ICD-10 Codes
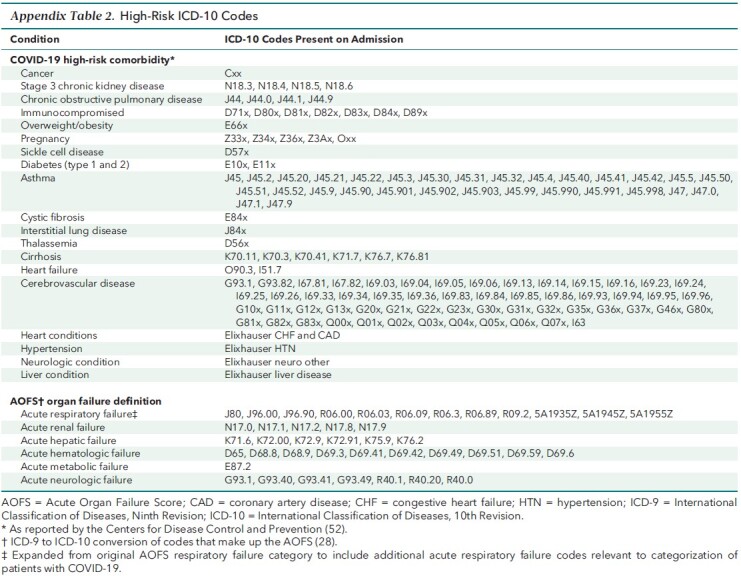
Severity of acute illness on presentation was controlled for using admission acuity (emergent or urgent vs. elective) and evidence of acute organ failure present on admission (Appendix Table 2). We stratified COVID-19–related acute respiratory failure on admission (+1 day) in descending order: mechanical ventilation; ICU admission or NIPPV; present-on-admission coding for acute respiratory failure without need for invasive ventilation, NIPPV, or ICU admission; or no indicators. Shock was defined as need for vasopressors on admission (+1 day). Acute hepatic, renal, neurologic, hematologic, and metabolic failures were identified using corresponding Acute Organ Failure Score (28) domains formulated by crosswalking present-on-admission diagnosis codes from ICD-9 to ICD-10.
Hospital-Level Covariates
Static covariates included teaching hospital status (29), urban location, U.S. region, the ratio of patients to attending physicians, the proportion of overall admissions that were Medicaid beneficiaries or uninsured in 2019 to 2020, and the proportion that received mechanical ventilation in 2019. A 4-level technological index stratified hospitals on existing infrastructure for patients with COVID-19 (level 1: equipped with extracorporeal membrane oxygenation; level 2: multiple ICUs; level 3: single ICU with continuous renal replacement therapy; level 4: single ICU without continuous renal replacement therapy).
By-month covariates included proportions of patients with COVID-19 who were intubated, required ICU admission, and were tested for SARS-CoV-2 using polymerase chain reaction on admission (+1 day); availability of remdesivir (30); and admission month.
Statistical Analysis
To avoid making parametric assumptions about the relationship between outcomes and the right-skewed surge index, hospital-months were ranked by their surge indices and grouped into prespecified shrinking strata to capture effects at extremes (<50th percentile [reference; “nonsurging”], 50th to 75th percentile, 75th to 90th percentile, 90th to 95th percentile, 95th to 99th percentile, and >99th percentile). Violin plots were constructed to compare probability density, caseload, and regional distributions of hospital-months at different surge index values.
Hierarchical (patient- and hospital-level) generalized linear models were used to determine the effect of hospital-month surge index on the risk-adjusted odds ratio (aOR) of in-hospital mortality or discharge to hospice (primary outcome) among patients with COVID-19. All variables were prespecified and selected on the basis of potential for confounding. A random effect for the hospital was included to account for within-hospital correlation.
Crude mortality rates were plotted by month and surge index category for visual comparison. The surge index was log-transformed to enable slope difference comparisons. First, a prespecified interaction by period of admission (March to May vs. June to August) was tested on the relationship between the log surge index and the log odds of mortality. Given evidence of a significant quantitative interaction by admission period, we elected post hoc to examine remaining interactions separately within each period. These included prespecified (surge index in the previous month) and post hoc (region, severity indicators of COVID-19–related respiratory failure, and non–COVID-19 caseload) interactions.
Mortality risk differences were derived for each surge category in each period using logistic regression models with generalized estimating equations (31). These risk differences were calculated as the difference between the marginally adjusted mortality risk for a given surge category and the corresponding nonsurging category. The product of the risk difference and population at risk (number of patients in each category) yielded estimates of surge-attributable deaths for each stratum–period combination. Ninety-five percent CIs for attributable deaths were similarly calculated using CIs for corresponding risk differences.
In sensitivity analyses, models were reestimated as follows: 1) using alternative parameterizations of the surge index (unweighted, log-transformed, deciles above median), 2) using the Elixhauser Comorbidity Index (32) in lieu of CDC-defined high-risk conditions, 3) excluding COVID-19 medications (given concerns about confounding by indication), 4) imputing discharges to hospice as alive, and 5) imputing tracheotomy recipients and patients transferred to other acute care hospitals as dead to examine the effect of discharge bias. To focus on within-hospital (longitudinal) associations, the primary and all sensitivity analyses using the continuous (log) surge index were repeated post hoc, this time including the hospital's mean surge index as a covariate (33), which potentially mitigated unmeasured between-hospital confounding. All analyses were performed using SAS, version 9.4 (SAS Institute), and R, version 4.0.2 (R Foundation for Statistical Computing). Additional details and the GitHub link for the statistical code are provided in the Methods section of the Supplement.
Role of the Funding Source
The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Results
Of 795 continuously reporting hospitals, 558 recording 144 116 index COVID-19 inpatient encounters met inclusion criteria. The study cohort had a greater representation of larger, urban hospitals compared with all U.S. hospitals (Appendix Figure; Table 1). Exclusion criteria collectively excluded fewer than 3% of patients with COVID-19 (Methods section of the Supplement). Overall, 35 883 (24.9%) were admitted to the ICU, 19 583 (13.6%) received mechanical ventilation, and 25 344 (17.6%) died.
Table 1.
Baseline Characteristics of COVID-19–Coded Inpatient Encounters, by Admission Period and Surge Index Category, 558 U.S. Hospitals, March to August 2020
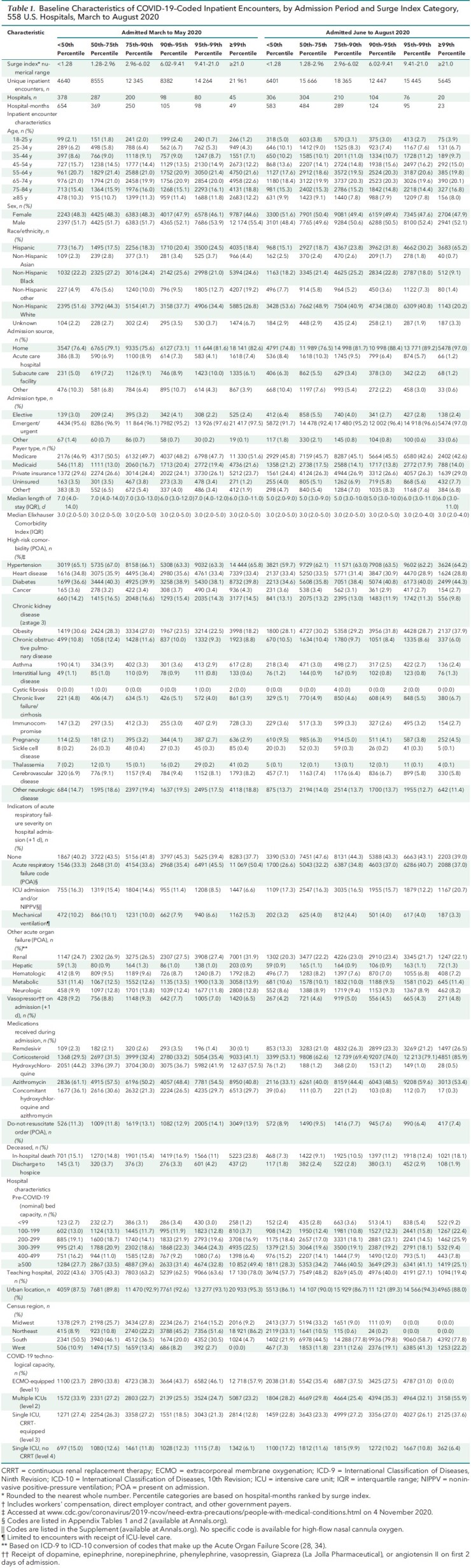
There was marked regional and temporal variation in COVID-19 caseload across hospitals; the surge index showed a right-skewed distribution over 3348 hospital-months (median, 1.28 [interquartile range, 0.44 to 2.96]; mean, 3.52 [SD, 3.90]; range, 0 to 44.6). Clusters of hospitals with extremely high surge indices were observed in the Northeast in April and in the South and West in July (Figure 1). The within-hospital distribution of the surge index by month is presented using a heat map (Supplement Figure 1). In nonsurging months, 11 041 (7.7%) patients with COVID-19 were admitted across 378 of 558 (67.7%) hospitals, whereas hospitals experiencing surges admitted 78 144 (54.2%) and 27 606 (19.2%) patients while in the top decile and 99th percentile of surge index, respectively. Forty-nine hospitals entered the 99th percentile of surge index between March and May 2020, and 20 hospitals entered this category between June and August 2020. Within the 99th percentile category between March and May 2020, Hispanic patients represented 18% of cases and 16% of deaths; this increased to 65% of cases and 71% of deaths between June and August 2020 (Supplement Figure 2). The median age was younger in June through August (61 years [interquartile range, 47 to 74 years]) than in March through May (64 years [interquartile range, 52 to 76 years]). Demographic and comorbidity distributions were otherwise similar (Table 1).
Figure 1. Distribution of U.S. hospital-months' surge indices by admission month and hospital census region, 558 U.S. hospitals, March to August 2020.
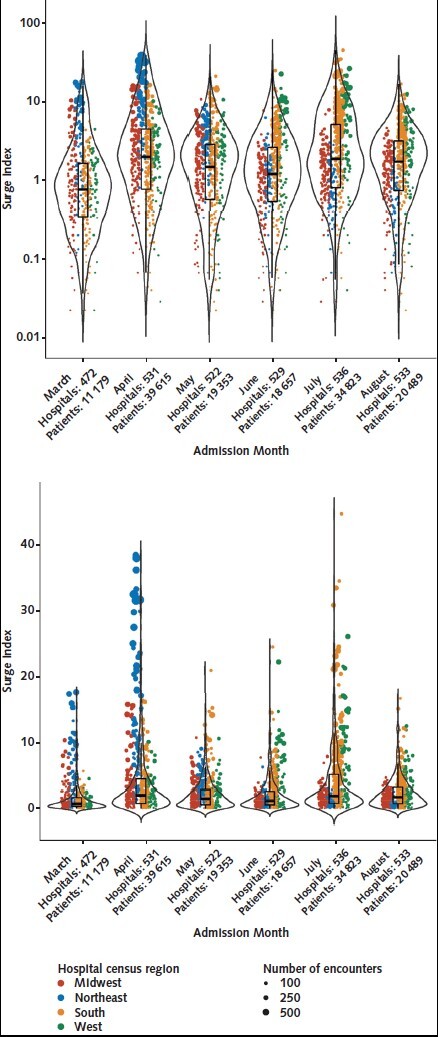
These violin plots show the distribution of patients within each hospital-month, stratified by admission month and log surge index (top) and surge index (bottom), with colors indicating the hospital census region. The overlaid box plots indicate the median, interquartile range, and 95% CI for each month's distribution. The size of each dot represents the total number of encounters in each hospital-month. Peak surges can be observed in the Northeast in April and in the South and West in July.
Rates of ICU admission and intubation decreased over time (Supplement Figure 3). Use of NIPPV emerged only in later months and remained infrequent. During March through May, intubation on admission (+1 day) occurred in 10% of patients in hospitals not experiencing surges and 4.3% in the greater than 99th percentile category, and this remained low (range, 3.2% to 4.3%) across surge index categories among admissions in June through August. Admissions in March through May showed higher frequency of age-stratified do-not-resuscitate orders that were present on admission than admissions in June through August (Supplement Table 3). Corticosteroid use increased after May in all surge and severity strata, with greater use among the highest surge indices (Supplement Figure 4A); a pattern was also seen for remdesivir use (Supplement Figure 4B). Nearly three quarters of patients received hydroxychloroquine in March as shown previously (34), but use decreased sharply thereafter and stayed near zero in June through August (Supplement Figure 4C).
For COVID-19 admissions in March through May, crude mortality decreased with each subsequent month across every surge index category (Figure 2). However, this trend seemed to plateau for patients admitted between June and August, during which time higher surge indices showed higher crude mortality. When surge index deciles were compared in the model, risk-adjusted mortality increased exponentially only at higher deciles (Figure 3, A). However, when shrinking percentile strata were used (Figure 3, B; Supplement Table 4), the aOR of mortality showed a stepladder increase. Compared with hospitals not having surges (<50th percentile), aORs in the 50th to 75th, 75th to 90th, 90th to 95th, 95th to 99th, and greater than 99th percentiles were 1.11 (95% CI, 1.01 to 1.23), 1.24 (CI, 1.12 to 1.38), 1.42 (CI, 1.27 to 1.60), 1.59 (CI, 1.41 to 1.80), and 2.00 (CI, 1.69 to 2.38), respectively. The aOR of mortality was 1.22 (CI, 1.18 to 1.27) per unit increase in the log-transformed surge index.
Figure 2. Crude mortality rate for categorical parameterizations of the surge index, 558 U.S. hospitals, March to August 2020.
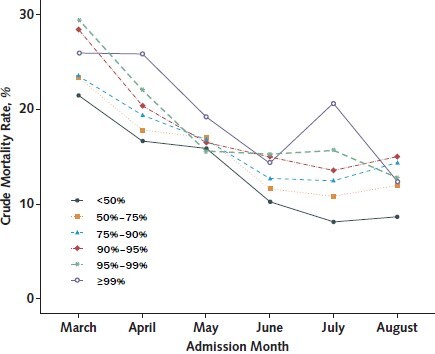
Crude mortality rates across admission month, stratified by shrinking surge index categories, enable visualization of secular patterns beyond the relationship between surge index and crude mortality.
Figure 3. Adjusted odds of mortality for categorical parameterizations of the surge index, 558 U.S. hospitals, March to August 2020.
![Figure 3. Adjusted odds of mortality for categorical parameterizations of the surge index, 558 U.S. hospitals, March to August 2020. Risk-adjusted odds ratios of mortality were calculated using surge index deciles above the median (A) and shrinking percentile categories (B) for the primary study cohort (admissions in March to August 2020). In panel B, the shrinkage distribution is applied to evince the prognostic effect in categories of extremely high surge index. Panels C and D illustrate effect modification of the relationship between surge index and mortality by period of admission. The slopes in the relationship between log surge index and log odds of mortality (see Supplement Figure 5) for June through August versus March through May intersect (slope difference, 0.10 [95% CI, 0.033 to 0.16]), indicating a significant quantitative interaction by period of admission.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3e4a/8276718/7c5ec939063a/aim-olf-M211213-M211213ff3.jpg)
Risk-adjusted odds ratios of mortality were calculated using surge index deciles above the median (A) and shrinking percentile categories (B) for the primary study cohort (admissions in March to August 2020). In panel B, the shrinkage distribution is applied to evince the prognostic effect in categories of extremely high surge index. Panels C and D illustrate effect modification of the relationship between surge index and mortality by period of admission. The slopes in the relationship between log surge index and log odds of mortality (see Supplement Figure 5) for June through August versus March through May intersect (slope difference, 0.10 [95% CI, 0.033 to 0.16]), indicating a significant quantitative interaction by period of admission.
The surge index remained associated with the aOR of mortality within each admission period when shrinking percentile strata (Figure 3, C and D) or log-transformed surge index (aORs of 1.19 [CI, 1.14 to 1.25] for March through May and 1.31 [CI, 1.25 to 1.38] for June through August) were used. This relationship was stronger during June through August than in March through May (slope difference, 0.10 [CI, 0.033 to 0.16]) (Supplement Figure 5). A detrimental relationship between log surge index and mortality was observed across patients who received mechanical ventilation or NIPPV, ICU patients, and ward patients with or without respiratory failure codes present on admission (Supplement Table 5). However, during March through May, this detrimental effect was greater for intubated patients than for ward patients without respiratory failure codes (Supplement Tables 5 and 6). During June through August, the detrimental effect of surge index was greater at hospitals that had surges (>50th percentile) in the prior month. Differences in non–COVID-19 caseload did not seem to affect the relationship between COVID-19 surge index and mortality, but 82.6% of hospital-months showed lower non–COVID-19 caseloads compared with the corresponding month in 2019 (Supplement Figures 6A and 6B). Results of all sensitivity analyses resembled those of the primary analysis when both categorical and log surge index were used (Supplement Tables 7 and 8). The OR for the hospital mean log surge index was 0.99 (CI, 0.92 to 1.07) in the primary analysis, and the CI for this variable crossed 1 in all sensitivity analyses as well, collectively suggesting that there was no significant cross-sectional or between-hospital unmeasured confounding (33). This was reinforced by the similarity in effect estimates with and without adjustment for the hospital mean log surge index (Supplement Table 8).
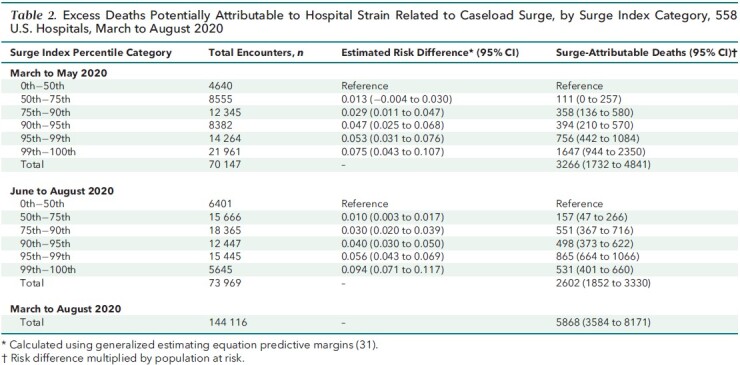
Of 25 344 total COVID-19 deaths, an estimated 5868 (CI, 3584 to 8171; 23.2%) were potentially attributable to hospital caseload surge (Table 2).
Table 2.
Excess Deaths Potentially Attributable to Hospital Strain Related to Caseload Surge, by Surge Index Category, 558 U.S. Hospitals, March to August 2020
Discussion
In a cohort of 558 U.S. hospitals that included approximately 1 of every 7 COVID-19 deaths reported in the United States (35), we found an association between surge index (a severity-weighted metric of COVID-19 caseload adjusted for baseline hospital capacity) and escalating COVID-19 mortality risk. This association was robust to multiple parameterizations of the surge index and several sensitivity analyses. Importantly, nearly 1 in 4 COVID-19 deaths in our cohort might have been attributable to hospital strain related to COVID-19 caseload. Although baseline inpatient COVID-19 survival improved over the study period, after adjustment for changing case mix and treatment patterns and other temporal hospital factors, mortality risk associated with hospitals experiencing surges was found to increase even more in later study months.
The surge index was constructed with the intention of capturing the aggregate severity burden of COVID-19 at a hospital and applying it to a large database of hospitals to enable comparisons of burden and effect across and within hospitals over time. The surge index not only enabled capture of the potential detrimental effects (36) of overburdened staff (2) during a surge but also highlighted ongoing needs for specific care settings (for example, ICU) and supplies (such as respiratory support devices). Although we are unable to establish causal inferences, our findings suggest potential value in prioritizing staffing, inventory, and logistical support early, especially to select hospitals approaching concerning surge index thresholds. Doing so might prepare these hospitals to better manage patients with COVID-19 in the event of even greater and more prolonged surges. This is suggested by the scale of benefit achievable by preempting surge-attributable deaths clustered within few hospitals with very high caseloads.
Our data raise the question of whether there may be a role for earlier diversion of patients with COVID-19 from emergency departments of hospitals experiencing surges. Preemptive engagement of relief health care (“shock absorber”) facilities is already occurring. Medical operations coordination cells (37) are enabling these triage efforts to cross state lines, especially when neighboring hospitals are also experiencing surges. However, the risks and benefits of transporting patients with COVID-19 must be carefully studied (38) and calibrated to individual hospitals' capacity, infrastructure, and resources.
Decreasing non–COVID-19 caseload further when it is already below prepandemic levels may not affect prognosis of patients with COVID-19. However, the secondary effect of surges on non–COVID-19 patient outcomes requires further study. It is important that vaccination and basic, low-cost, and highly effective preventive strategies remain the primary focus to decrease the chances of surges occurring in the first place.
Standards of care for patients hospitalized with COVID-19 have evolved during the pandemic with growing evidence, experiential learning, and availability of new therapies. This likely contributed to temporal improvements in COVID-19 survival reported in previous studies (6, 9–11, 13) and observed across surge index strata in our study. However, mortality burden remains high; COVID-19 has become a leading cause of death in the United States (39). Hence, we evaluated the relationship between surges and outcomes in the context of changing treatment patterns over time (13). Corticosteroids and remdesivir were increasingly used during later study months after publication of influential randomized trials (14, 15) and guideline recommendations (40). Hydroxychloroquine use decreased sharply as lack of associated benefit and potential for harm (especially when used with azithromycin) were recognized (41). More patients were intubated on presentation early in the pandemic. The detrimental effect of this early practice pattern has been suggested previously (13); we showed that it may have accentuated the detrimental association between caseload surge and survival. More selective intubation after growing confidence in high-flow oxygen (16) and diminishing concerns about acquiring aerosolized virus with adequate personal protective equipment, coupled with the growing tracheotomy use and fewer code status limitations assigned on admission (42) that we observed in our study, may have contributed to temporal improvements. Notwithstanding early temporal improvements, survival seemed to be dampened by the negative effect of surges—more so in later study months despite increasing use of corticosteroids and access to remdesivir. In fact, medication use patterns generally indicated adherence to practice guidelines (40) regardless of hospitals' surge status. As such, maximal benefit from emerging therapies (14, 15) and refinements in supportive care may be more readily achievable under routine (nonsurging) working conditions. Our study also identified a need to control for caseload surges in future COVID-19 outcome studies.
Our study has important public health implications. Despite a downtrend in the third pandemic wave and recent acceleration in mass vaccination efforts, surges continue to pose a serious threat. Some countries where a substantial portion of the population has been vaccinated continue to experience surges in cases (43). Furthermore, the emergence and rapid spread of SARS-CoV-2 variants of concern have made our findings more relevant. Highly transmissible variants (44) could cause health care systems to be overwhelmed if many patients are infected and could drive surge-based mortality independent of intrinsic virulence relative to wild-type virus. In our study, more than half the patients were clustered in hospital-months in the top surge index decile, and these hospital-months had a substantial portion of surge-attributable deaths. The disproportionate preponderance of Hispanic patients among COVID-19 admissions and deaths at the most overburdened hospitals, especially between June and August, likely tracks with the geographic evolution of the pandemic but also indicates that surging caseload may accentuate existing health disparities.
Our study has limitations. The findings may not be generalizable to all U.S. hospitals. Residual confounding may have occurred due to social determinants of health, prone positioning, and finer differences in severity of acute illness that were uncaptured in administrative data. There is currently no billing code specific to high-flow nasal cannula oxygen, and present-on-admission coding for acute respiratory failure in the absence of coding for continuous positive airway pressure or bilevel positive airway pressure may have been an imperfect proxy. The extent to which oxygen delivery exclusively via high-flow nasal cannula was captured in our NIPPV variable is unclear. However, administrative codes reliably capture COVID-19 cases (24), and our administrative data models offered good discrimination for mortality, emphasizing the strong prognostic influence of age (45) and underlying comorbidities in COVID-19. Residual confounding may have occurred at the hospital level (46); deidentification restricted data on dates to month, precluding daily assessments of ICU census, expanded capacity, and staffing. Information on outcomes beyond discharge was not available, and readmissions were not assessed, but a sensitivity analysis imputing death for everyone who received tracheotomy and/or was transferred out yielded similar findings. Changing ward and ICU admission thresholds to manage dynamic caseloads may have introduced collider bias (47). Notably, several hospitals experienced an onslaught of acutely ill patients with COVID-19, leaving little room for discretion in triage and prompting them to use tiered staffing (48) and expand bed capacity (49). We were unable to identify patients who received intermediate care unit–level services on the ward. However, we analyzed all hospitalized (in lieu of only ICU) patients, utilized ICU charges to capture patients receiving ICU-level care at alternative care sites (such as a cafeteria or parking lot) (50), and controlled for monthly proportions of patients with COVID-19 who were admitted to the ICU and/or intubated on admission. Our conservative estimate of excess surge-related COVID-19 deaths does not account for patients missing COVID-19 diagnosis labels and indirect effects (for example, deaths at home due to avoidance of hospitals, or altered resuscitation policies).
We encourage future investigations into drivers of the relationship between surges and mortality that were not fully discernible in our study. Furthermore, the treatment paradigm for COVID-19 is rapidly evolving, and hospitals have had growing situational awareness, lead time for planning, and federal and state support over time. As such, our findings might not represent surge–mortality relationships observed in the third U.S. pandemic wave. We encourage ongoing tracking of the burden and dynamic effect of caseload surges in more recent data and validation using other data sets enriched with additional key elements (for example, daily census or expanded bed capacity). In certain hard-hit, non-U.S. regions where even basic treatment modalities like oxygen have been in short supply (51), detrimental effects of surging caseload, although not quantified to date, are likely to be substantial. International studies on this topic are critically needed. The surge index framework could also be used to study the effect of caseload surge on other acute conditions not related to COVID-19 and in future pandemics.
In conclusion, among patients admitted with COVID-19 at 558 U.S. hospitals between March and August 2020, mortality risk increased with escalating severity-weighted COVID-19 caseload; approximately 1 in every 4 COVID-19 deaths was potentially attributable to surges in caseload at hospitals. This volume–outcome relationship was stronger in later pandemic months despite greater use of corticosteroids and more selective intubation in later and higher-surging months. Many COVID-19 deaths may be preventable through prudent public health and health care organizational interventions that minimize the effect of surges.
Supplementary Material
Footnotes
This article was published at Annals.org on 6 July 2021.
* Dr. Demirkale and Ms. Warner contributed equally to this work.
References
- 1. Cleveland Manchanda EC, Sanky C, Appel JM.. Crisis standards of care in the USA: a systematic review and implications for equity amidst COVID-19. J Racial Ethn Health Disparities. 2020. [PMID: ] doi: 10.1007/s40615-020-00840-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lasater KB , Aiken LH , Sloane DM , et al. Chronic hospital nurse understaffing meets COVID-19: an observational study. BMJ Qual Saf. 2020. [PMID: ] doi: 10.1136/bmjqs-2020-011512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Medicare & Medicaid Services. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Updated 24 May 2021. Accessed at www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf on 28 June 2021.
- 4. Perez S , Innes GK , Walters MS , et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions—New Jersey, February–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1827-31. [PMID: ] doi: 10.15585/mmwr.mm6948e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta S , Hayek SS , Wang W , et al; STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1436-47. [PMID: ] doi: 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asch DA , Sheils NE , Islam MN , et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181:471-8. [PMID: ] doi: 10.1001/jamainternmed.2020.8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bravata DM , Perkins AJ , Myers LJ , et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4:e2034266. [PMID: ] doi: 10.1001/jamanetworkopen.2020.34266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubinson L . Intensive care unit strain and mortality risk among critically ill patients with COVID-19—there is no “me” in COVID. JAMA Netw Open. 2021;4:e2035041. [PMID: ] doi: 10.1001/jamanetworkopen.2020.35041 [DOI] [PubMed] [Google Scholar]
- 9. Horwitz LI , Jones SA , Cerfolio RJ , et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16:90-2. [PMID: ] doi: 10.12788/jhm.3552 [DOI] [PubMed] [Google Scholar]
- 10. Auld SC , Caridi-Scheible M , Robichaux C , et al; Emory COVID-19 Quality and Clinical Research Collaborative. Declines in mortality over time for critically ill adults with coronavirus disease 2019 [Letter]. Crit Care Med. 2020;48:e1382-e1384. [PMID: ] doi: 10.1097/CCM.0000000000004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ledford H . Why do COVID death rates seem to be falling. Nature. 2020;587:190-2. [PMID: ] doi: 10.1038/d41586-020-03132-4 [DOI] [PubMed] [Google Scholar]
- 12. Acosta AM , Mathis AL , Budnitz DS , et al. COVID-19 investigational treatments in use among hospitalized patients identified through the US Coronavirus Disease 2019–Associated Hospitalization Surveillance Network, March 1–June 30, 2020. Open Forum Infect Dis. 2020;7:ofaa528. [PMID: ] doi: 10.1093/ofid/ofaa528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doidge JC , Gould DW , Ferrando-Vivas P , et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med. 2021;203:565-74. [PMID: ] doi: 10.1164/rccm.202008-3212OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sterne JAC , Murthy S , Diaz JV , et al; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-41. [PMID: ] doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beigel JH , Tomashek KM , Dodd LE , et al; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-26. [PMID: ] doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demoule A , Vieillard Baron A , Darmon M , et al. High-flow nasal cannula in critically ill patients with severe COVID-19 [Letter]. Am J Respir Crit Care Med. 2020;202:1039-42. [PMID: ] doi: 10.1164/rccm.202005-2007LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson AE , Ranard BL , Wei Y , et al. Prone positioning in awake, nonintubated patients with COVID-19 hypoxemic respiratory failure. JAMA Intern Med. 2020;180:1537-9. [PMID: ] doi: 10.1001/jamainternmed.2020.3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goyal P , Choi JJ , Pinheiro LC , et al. Clinical characteristics of Covid-19 in New York City [Letter]. N Engl J Med. 2020;382:2372-4. [PMID: ] doi: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Auld SC , Caridi-Scheible M , Blum JM , et al; and the Emory COVID-19 Quality and Clinical Research Collaborative. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e799-e804. [PMID: ] doi: 10.1097/CCM.0000000000004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson S , Hirsch JS , Narasimhan M , et al; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052-9. [PMID: ] doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenthal N , Cao Z , Gundrum J , et al. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3:e2029058. [PMID: ] doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Premier Applied Sciences. Premier Healthcare Database White Paper: Data That Informs and Performs. 2 March 2020.
- 23. von Elm E, Altman DG, Egger M, et al; STROBE Initiative.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24. Kadri SS , Gundrum J , Warner S , et al. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. JAMA. 2020;324:2553-4. [PMID: ] doi: 10.1001/jama.2020.20323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cal. Code of Regulations tit. 22 div. 5 (2012).
- 26. Horby P , Lim WS , Emberson JR , et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. [PMID: ] doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fiolet T , Guihur A , Rebeaud ME , et al. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19-27. [PMID: ] doi: 10.1016/j.cmi.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Courtright KR , Halpern SD , Bayes B , et al. Adaptation of the Acute Organ Failure Score for use in a Medicare population. Crit Care Med. 2017;45:1863-70. [PMID: ] doi: 10.1097/CCM.0000000000002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landon BE , Normand SL , Lessler A , et al. Quality of care for the treatment of acute medical conditions in US hospitals. Arch Intern Med. 2006;166:2511-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 30. Crutchfield P , Gibb TS , Redinger MJ , et al. Ethical allocation of remdesivir. Am J Bioeth. 2020;20:84-6. [PMID: ] doi: 10.1080/15265161.2020.1779395 [DOI] [PubMed] [Google Scholar]
- 31. Bieler GS , Brown GG , Williams RL , et al. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;171:618-23. [PMID: ] doi: 10.1093/aje/kwp440 [DOI] [PubMed] [Google Scholar]
- 32. Quan H , Sundararajan V , Halfon P , et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 33. Begg MD , Parides MK . Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22:2591-602. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 34. Kadri SS , Demirkale CY , Sun J , et al. Real-world inpatient use of medications repurposed for coronavirus disease 2019 in United States hospitals, March–May 2020. Open Forum Infect Dis. 2021;8:ofaa616. [PMID: ] doi: 10.1093/ofid/ofaa616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. COVID Data Tracker. Accessed at https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendsdeaths on 16 January 2021.
- 36. Tarnow-Mordi WO , Hau C , Warden A , et al. Hospital mortality in relation to staff workload: a 4-year study in an adult intensive-care unit. Lancet. 2000;356:185-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 37. NRCC Healthcare Resilience Task Force. Medical Operations Coordination Cells Toolkit. First Edition. Hospital Team. 2020.
- 38. Choi HK , Shin SD , Ro YS , et al. A before- and after-intervention trial for reducing unexpected events during the intrahospital transport of emergency patients. Am J Emerg Med. 2012;30:1433-40. [PMID: ] doi: 10.1016/j.ajem.2011.10.027 [DOI] [PubMed] [Google Scholar]
- 39. Woolf SH , Chapman DA , Lee JH . COVID-19 as the leading cause of death in the United States. JAMA. 2021;325:123-4. [PMID: ] doi: 10.1001/jama.2020.24865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. National Institutes of Health. COVID-19 Treatment Guidelines. Accessed at www.covid19treatmentguidelines.nih.gov/critical-care on 16 January 2021. [PubMed]
- 41. Hernandez AV , Roman YM , Pasupuleti V , et al. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020;173:287-96. [PMID: ]. doi: 10.7326/M20-2496 [DOI] [PubMed] [Google Scholar]
- 42. Chao TN , Harbison SP , Braslow BM , et al. Outcomes after tracheostomy in COVID-19 patients. Ann Surg. 2020;272:e181-e186. [PMID: ] doi: 10.1097/SLA.0000000000004166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hart R. Covid Surges in 4 of 5 Most Vaccinated Countries—Here's Why the U.S. Should Worry. Forbes. 11 May 2021.
- 44. Washington NL , Gangavarapu K , Zeller M , et al. Genomic epidemiology identifies emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. med. Rxiv. 2021. [PMID: ] doi: 10.1101/2021.02.06.21251159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raschke RA , Agarwal S , Rangan P , et al. Discriminant accuracy of the SOFA score for determining the probable mortality of patients with COVID-19 pneumonia requiring mechanical ventilation. JAMA. 2021;325:1469-70. [PMID: ] doi: 10.1001/jama.2021.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hatfield KM , Dantes RB , Baggs J , et al. Assessing variability in hospital-level mortality among U.S. Medicare beneficiaries with hospitalizations for severe sepsis and septic shock. Crit Care Med. 2018;46:1753-60. [PMID: ] doi: 10.1097/CCM.0000000000003324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Griffith GJ , Morris TT , Tudball MJ , et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11:5749. [PMID: ] doi: 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris GH , Baldisseri MR , Reynolds BR , et al. Design for implementation of a system-level ICU pandemic surge staffing plan. Crit Care Explor. 2020;2:e0136. [PMID: ] doi: 10.1097/CCE.0000000000000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abir M, Nelson C, Chan EW, et al. Instructions for Using the RAND Critical Care Surge Response Tool: An Excel-Based Model for Helping Hospitals Respond to the COVID-19 Crisis. April 2020.
- 50. Centers for Medicare & Medicaid Services. The Centers for Medicare & Medicaid Services (CMS) Fact Sheet for State and Local Governments: CMS Programs & Payment for Care in Hospital Alternate Care Sites. 26 May 2020.
- 51. The Lancet . India's COVID-19 emergency [Editorial]. Lancet. 2021;397:1683. [PMID: ] doi: 10.1016/S0140-6736(21)01052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention. People with Certain Medical Conditions. Accessed at www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html on 28 June 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


