Abstract
Current climate trends resulting in rapid declines in sea ice and increasing water temperatures are likely to expand the northern geographic range and duration of favorable conditions for harmful algal blooms (HABs), making algal toxins a growing concern in Alaskan marine food webs. Two of the most common HAB toxins along the west coast of North America are the neurotoxins domoic acid (DA) and saxitoxin (STX). Over the last 20 years, DA toxicosis has caused significant illness and mortality in marine mammals along the west coast of the USA, but has not been reported to impact marine mammals foraging in Alaskan waters. Saxitoxin, the most potent of the paralytic shellfish poisoning toxins, has been well-documented in shellfish in the Aleutians and Gulf of Alaska for decades and associated with human illnesses and deaths due to consumption of toxic clams. There is little information regarding exposure of Alaskan marine mammals. Here, the spatial patterns and prevalence of DA and STX exposure in Alaskan marine mammals are documented in order to assess health risks to northern populations including those species that are important to the nutritional, cultural, and economic well-being of Alaskan coastal communities. In this study, 905 marine mammals from 13 species were sampled including; humpback whales, bowhead whales, beluga whales, harbor porpoises, Northern fur seals, Steller sea lions, harbor seals, ringed seals, bearded seals, spotted seals, ribbon seals, Pacific walruses, and northern sea otters. Domoic acid was detected in all 13 species examined and had the greatest prevalence in bowhead whales (68%) and harbor seals (67%). Saxitoxin was detected in 10 of the 13 species, with the highest prevalence in humpback whales (50%) and bowhead whales (32%). Pacific walruses contained the highest concentrations of both STX and DA, with DA concentrations similar to those detected in California sea lions exhibiting clinical signs of DA toxicosis (seizures) off the coast of Central California, USA. Forty-six individual marine mammals contained detectable concentrations of both toxins emphasizing the potential for combined exposure risks. Additionally, fetuses from a beluga whale, a harbor porpoise and a Steller sea lion contained detectable concentrations of DA documenting maternal toxin transfer in these species. These results provide evidence that HAB toxins are present throughout Alaska waters at levels high enough to be detected in top predators and have the potential to impact marine mammal health in the Arctic marine environment.
1. Introduction
Harmful algal blooms (HABs) most commonly occur in temperate and tropical regions; however, current climate trends such as ocean warming and loss of seasonal sea ice, are likely to expand the geographic distribution and the duration of conditions that support blooms (Moore et al., 2008; Van Dolah, 2000), making HAB exposure potentially more common among marine mammals in Alaskan waters (Burek et al., 2008). Species of phytoplankton known to be toxic are not new to Alaskan waters. Diatoms of the genus Pseudo-nitzschia, have been documented as far north as the eastern Beaufort Sea (Bursa, 1963) and produce domoic acid (DA), the toxin responsible for amnesic shellfish poisoning (ASP). Domoic acid was first detected in low levels in razor clams in Kachemak Bay in July 1992 (RaLonde and Wright, 2011). Dinoflagellates of the genus Alexandrium produce toxins that cause paralytic shellfish poisoning (PSP) and have been well documented in Alaskan waters (Gessner and Middaugh, 1995; Gessner et al., 1997; Gessner and Schloss, 1996; Lewitus et al., 2012; Trainer et al., 2014). Saxitoxin (STX) is the most potent of the PSP causing toxins. Both DA and STX affect the central nervous system of vertebrates. Saxitoxin acts as a sodium channel blocker and prevents action potential activity in nerves causing paralysis primarily of the respiratory system (Cusick and Sayler, 2013). Domoic acid is an excitotoxin that over-stimulates glutamate receptors in the vertebrate central nervous system causing stimulation of nerves (Berman et al., 2002; Berman and Murray, 1997; Todd, 1993). While PSP has been documented in humans in Alaska since 1799, the only documented cases of ASP in humans occurred in Southeastern Canada in 1989 (Perl et al., 1990). Unlike temperate regions, no incidences of DA toxicosis and very few incidences of STX toxicosis have been documented in Alaskan marine mammals.
Ocean temperatures around Alaska are warming; shelf waters of the eastern Bering Sea have increased by almost 3°C during the past decade (Stabeno et al., 2007). The lowest sea ice extent measurements since satellite monitoring began in 1979 were recorded during 2007–2009 ((Stroeve et al., 2008) National Snow and Ice Data Center press release, October 6, 2009), until 2012, which is the record Arctic sea ice minimum documented to date. Loss of sea ice has allowed industrial maritime ship traffic across the Arctic to increase substantially. Ships can transport HAB species to new areas through ballast water discharge (Reeves et al., 2012), a process that is currently unregulated in the Arctic (Hallegraeff, 1998). Filter-feeding benthic invertebrates, zooplankton, and finfish can accumulate STX and DA and are well-known vectors of algal toxins to higher trophic level predators (Bargu et al., 2002; Costa et al., 2009; Lefebvre et al., 2002b; Wekell et al., 1994; White, 1986; Wohlgeschaffen et al., 1992).
The potential for health effects on Alaskan marine mammals may be high considering more than 40% of marine mammal unusual mortality events (UMEs) in the contiguous USA during the last 20 years have been attributed to algal toxin exposure (Flewelling et al., 2005; Gulland and Hall, 2007; Landsberg et al., 2014; Scholin et al., 2000; Torres De La Riva et al., 2009). The number of HAB-related strandings appears to be increasing in the contiguous USA as these events were relatively rare even in temperate regions only two decades ago (Landsberg et al., 2014). The negative impacts of algal toxins on marine mammal health have been well documented along the west coast of the USA. For example, the neurotoxic effects of DA were first reported in stranded California sea lions (Zalophus californianus) in 1998 through exposure from toxic planktivorous prey such as northern anchovies and Pacific sardines (Gulland, 2000; Lefebvre et al., 1999; Scholin et al., 2000). Clinical signs of acute DA poisoning in marine mammals include ataxia, head weaving, seizures or coma and/or death (Gulland et al., 2002). The frequency of DA-associated California sea lion strandings has increased since 1998 and strandings now occur annually, affecting hundreds of sea lions per year (Bargu et al., 2010). Additionally, a chronic neurological syndrome associated with repetitive sub-lethal exposure to the toxin is now recognized by behavioral changes, seizures, and atrophy of the hippocampal formation (Cook et al., 2015; Goldstein et al., 2008). Domoic acid has also been documented to cross the placenta of California sea lions and be present in milk, thus, neonates may be exposed in utero and after birth until weaning (Brodie et al., 2006; Rust et al., 2014). In addition to contributing to reproductive failure, in utero and lactational exposure to DA can result in developmental abnormalities leading to neurological and behavioral deficits in surviving offspring. Given that California sea lions and humans share a common prey base, sea lions serve as important food safety sentinels regarding the presence of HABs near California. The findings associated with DA exposure in California sea lions demonstrate the potential health effects for other marine mammal species, as well as the potential for marine mammals in other regions to be sentinels for public health threats. The effects of STX on marine mammals are not as well documented as they are for DA. The first reported STX-related mortality event involved humpback whales in the late 1980s when 14 humpback whales died near Cape Cod Bay after ingesting mackerel containing STX (Geraci et al., 1989). Saxitoxin was suspected (although not substantiated) to be a factor in 60 sea otter deaths in Alaska (Degange and Vacca, 1989) and in 117 Mediterranean monk seal (Monachus monachus) deaths in Western Sahara, Africa (Costas and Lopez-Rodas, 1998).
The goal of this study was to document the presence and extent of two algal toxins (DA and STX) in Alaskan marine mammals to identify emerging exposure risks in northern- ranging marine mammal populations, including those species that are important to people for subsistence purposes. Thirteen species were examined including: four cetaceans (humpback whales, Megaptera novaeangliae; bowhead whales, Balaena mysticetus; beluga whales, Delphinapterus leucas; and harbor porpoises, Phocoena phocoena), two otariids (northern fur seals, Callorhinus ursinus and Steller sea lions, Eumetopias jubatus), five phocids (harbor, Phoca vitulina; ringed P. hispida; bearded, Erignathus barbatus; spotted, P. largha and ribbon seals, Histriophoca fasciata), Pacific walruses (Odobenus rosmarus), and northern sea otters (Enhydra lutra).
1 2. Methods
1.1 2.1. Marine mammal sample collection:
A variety of samples (feces, stomach contents, intestinal contents, serum, milk, urine, amniotic fluid, bile, aqueous humor, and pleural, peritoneal and pericardial fluid) were collected from Alaskan marine mammals that were stranded, harvested for subsistence purposes, or captured for research. Samples were also collected during the Northern Alaska Pinniped Unusual Mortality Event (UME) (http://www.nmfs.noaa.gov/pr/health/mmume/events.html). Not all sample types were collected from each animal. The majority of the samples consisted of feces, urine, serum and stomach and intestinal contents. Samples were frozen as soon as possible after collection to prevent degradation, although some stranded animals had various levels of degradation. Samples were stored frozen until shipped to the Northwest Fisheries Science Center’s Wildlife Algal-Toxin Research and Response Network (WARRN-West) laboratory (Seattle, WA, USA) for algal toxin testing. All live and stranded animal handling was consistent with approved humane practices under the following permits: Marine Mammal Protection Act (MMPA) permit number MA041309-5, and National Marine Fisheries (NMFS) research permit numbers 358–1787, 15324, and 10091. A summary of the total number of animals, collection period, and locations is shown in Table 1. Additional detailed information on sample collection is provided in the sections below.
Table 1.
List of species, collection status, period and locations, and total number of animals for each species of the 905 marine mammals sampled in Alaska (AK).
| Species | Collection status | Collection period | Collection locations | Total # of animals |
|---|---|---|---|---|
| Humpback | Stranded | July 2007 to Sept. 2011 | Kodiak, The AK Peninsula, Southeast | 8 |
| Bowhead | Harvested | Spring & Fall 2006 to 2011 | Barrow | 25 |
| Beluga | Stranded & Harvested | Sept. 2005 to Oct. 2012 | Cook Inlet, Hooper Bay | 15 |
| Harbor porpoise | Stranded | Aug. 2008 to July 2011 | Cook Inlet | 5 |
| Northern fur seal | Harvested & Live Capture | 2010 | Saint George & Saint Paul Islands | 179 |
| Steller sea lion | Stranded | May 2004 to March 2013 | Gulf of AK | 42 |
| Harbor seal | Stranded | May 2008 to Aug. 2012 | Gulf of AK, Egegik | 9 |
| Ringed seal | Harvested | Nov. 2006 to Nov. 2012 | Barrow, Chukchi Sea, Bering Sea | 113 |
| Bearded seal | Harvested | Oct. 2007 to June 2013 | Barrow, Chukchi Sea, Bering Sea | 55 |
| Spotted seal | Harvested & Snow Urine | Nov. 2006 to Nov. 11 | Barrow, Chukchi Sea, Bering Sea | 158 |
| Ribbon seal | Harvested & Snow Urine | May 2009 to Oct. 2012 | Barrow, Chukchi Sea, Bering Sea, Yakutat | 21 |
| Pacific walrus | Harvested | May & June in 2012 & 2013 | Saint Lawrence Island | 82 |
| Northern sea otter | Stranded & Live Capture | April 2004 to May 2011 | Gulf of AK | 193 |
2.1.1. Humpback whale fecal, stomach and intestinal contents, aqueous humor, pleural fluid, and urine samples (n = 8 animals):
During 2007–2011, samples were collected from stranded humpback whales from Southeast Alaska (n = 5), Kodiak (n = 2) and The Alaska Peninsula (n = 1). Samples were stored frozen in Whirl-Pak® bags at −40 or −80 °C until analyzed for algal toxins.
2.1.2. Bowhead whale fecal samples (n = 25 animals):
During 2006–2011, fecal samples from bowhead whales harvested for subsistence purposes were collected during the spring and fall in Barrow, Alaska. Sections of colon were cut and fecal matter was removed using plastic spoons. Samples were stored frozen in Whirl-Pak® bags at −20 °C until analyzed for algal toxins.
2.1.3. Beluga whale fecal, stomach contents, amniotic fluid, pericardial fluid, and urine samples (n = 15 animals):
During 2007–2012, samples were collected from stranded Cook Inlet beluga whales. Three females were pregnant and samples were collected from both the mother and the fetus in all three cases. Stomachs from two belugas harvested for subsistence purposes at Hooper Bay were also collected. Samples were stored frozen in Whirl-Pak® bags at −40 or −80 °C until analyzed for algal toxins.
2.1.4. Harbor porpoise fecal, stomach and intestinal contents, aqueous humor, and urine (n = 5 animals):
During 2010–2013, samples were collected from five stranded harbor porpoises from Cook Inlet, with both mother and fetus analyzed in one case. Samples included feces (n = 2), aqueous humor (n = 1), stomach (n = 1) and intestinal contents (n = 1), and urine (n = 2) with some animals having multiple sample types analyzed. Samples were stored in Whirl-Pak® bags at −40 or −80 °C until analyzed for algal toxins.
2.1.5. Northern fur seal fecal and serum samples (n = 179 animals):
Between 7–15 October 2010, serum samples were collected from 131 live-captured adult female northern fur seals with pups on Saint George Island (Pribilof Islands) and fecal samples were collected from 48 northern fur seals harvested on Saint Paul Island (Pribilof Islands) for subsistence purposes. Samples were frozen in cryovials or Whirl-Pak® bags and stored at −20 °C until analyzed for algal toxins.
2.1.6. Steller sea lion fecal, stomach and intestinal contents, amniotic, pleural, peritoneal and pericardial fluid, bile, and urine samples (n = 42 animals):
During 2004–2013, samples were collected from 42 stranded Steller sea lions, some of which were rookery pups and aborted fetuses on rookeries. Stranded animals were sampled across Alaska from southeast Alaska through Prince William Sound and through the Aleutian Islands. Samples were collected in amber vials (bile), Whirl-Pak® bags (feces, and stomach and intestinal contents) and cryovials (urine, and amniotic, pleural, peritoneal and pericardial fluid) and stored at −80 °C until analyzed for algal toxins.
2.1.7. Harbor seal bile, feces, aqueous humor, placenta, and urine samples (n = 9 animals):
During 2008–2012, samples were collected from nine stranded harbor seals; three in Southeast Alaska (Bartlett Cove in Glacier Bay, Lynn Canal, and Sitka), four in Southcentral Alaska (Kachemak Bay, Resurrection Bay, Kenai, and Cook Inlet), one in Southwest Alaska (Izembek Lagoon), and one in Bristol Bay (Egegik). Samples were collected in amber vials (bile), Whirl-Pak® bags (feces and placenta) or cryovials (aqueous humor and urine) and stored at −60°C until analyzed.
2.1.8. Ice seals (ringed (n = 113), bearded (n = 55), spotted (n = 158), and ribbon seals (n = 21)) stomach/intestinal contents, fecal, and urine samples:
During 2006–2013, samples from ice seals harvested during spring and fall for subsistence purposes were collected from the coastal communities of Hooper Bay, Savoonga, Gambell, Little Diomede, Shishmaref, Kotzebue, Point Hope, Wainwright, and Barrow in the Bering Strait region and Chukchi Sea. Whole stomachs or a piece of intestine were collected in Ziploc® bags. Urine was collected in a centrifuge tube. All samples were frozen and shipped to the Alaska Department of Fish and Game (ADF&G) laboratory in Fairbanks and stored at −20°C. Stomachs and intestines were thawed and 5 ml of content was removed from each, placed in centrifuge tubes with screw caps, and refrozen. During May of 2009–2010, live captures were conducted by the National Marine Mammal Lab (Alaska Fisheries Science Center’s Polar Ecosystems Program) and samples were obtained from ice floes and collected using a metal shovel to scoop the urine soaked ice which was placed in Whirl-Pak® bags and frozen at −80°C until analyzed for algal toxins.
2.1.9. Pacific walrus stomach and intestinal content samples (n = 82 animals):
During May 2012–2013, stomach and intestinal contents were collected from walruses harvested for subsistence purposes from the coastal communities of Gambell and Savoonga on Saint Lawrence Island. Hunters collected the samples in situ and brought them to shore where they were frozen on site at −18°C and shipped to the ADF&G laboratory in Fairbanks and subsequently stored at −20°C until algal toxin analysis. Stomachs and intestines were thawed and 5 ml of content was removed and placed in centrifuge tubes with screw tops and refrozen.
2.1.10. Sea otter urine, pericardial fluid, and maternal milk samples (n = 193 animals):
From 2004–2011, samples were collected from Northern sea otter carcasses (n=172) recovered in the Gulf of Alaska and Aleutian Islands - notably Sitka, Juneau, Glacier Bay, Yakutat Bay, Prince William Sound, lower Kenai Peninsula, lower Cook Inlet, Kodiak, eastern Aleutians, and Cold Bay. Additionally, urine samples (n=21) were collected during 2011 from live-captured otters from the northern end of Kuiu Island in the southern Gulf of Alaska. Samples were collected and stored at −20°C until analyzed for algal toxins.
1.1.1 2.2. Sample extraction for toxin analysis :
All samples were thawed at room temperature. Depending on the amount of sample available, 1–4 g was weighed out or 1–4 ml was aliquoted into a 15 ml polypropylene screw-cap tube (Falcon-BD). The initial extraction step was carried out by adding 50% aqueous methanol (for DA extraction) or 80% ethanol (for STX extraction) to the sample in a 1:4 wt/wt ratio (1 part sample, 3 parts solvent) and thoroughly vortexing the sample. For fecal material, stomach contents and intestinal contents, samples were homogenized for at least 60 seconds using an Omni ES homogenizer. The homogenized sample was then centrifuged at 10,000 × g (Sorvall RC 5C Plus centrifuge) for 20 minutes at 4°C. The supernatant was then filtered through a 0.22 μm membrane microcentrifuge tube filter (Millipore Ultrafree-MC centrifugal concentration device, Durapore membrane, 0.22 μm pore size) and spun in a desk-top microcentrifuge (Eppendorf model 5415C) for at least 10 minutes at a setting of 14. For urine, serum and other body fluids, samples were sonicated (Branson Sonifier 450) at 50% pulse for 45 seconds at a setting of 5. Samples were then centrifuged at 10,000 × g for 20 minutes at 4°C. The supernatant was then filtered through a 25 mm diameter, 0.45 μm pore size syringe filter (Pall Gelman Acrodisc PSF G × F with GHP membrane). All sample extracts were stored at 4°C until analysis by ELISA.
1.1.2 2.3. Quantification of algal toxins in marine mammal extracts:
Algal toxins were quantified using commercially available enzyme-linked immunosorbent assay (ELISA) kits; Biosense® DA ELISA for DA and Abraxis saxitoxin ELISA for STX, following the instruction protocol supplied by the manufacturer (Biosense® Laboratories, Bergen, Norway and Abraxis LLC, Warminster, PA) with slight modifications based on sample matrix. These kits were originally developed for testing shellfish rather than marine mammal samples. Consequently, testing in order to determine matrix effects for feces, stomach and intestinal contents, urine, bile, aqueous humor, serum, and milk were performed in a previous study (Lefebvre et al., 2010). For DA ELISAs, the minimum dilutions of the 1:4 50% MeOH extracts required to eliminate all matrix effects were 1:100 for feces and bile, 1:50 for milk and stomach and intestinal contents, and 1:10 for urine, aqueous humor and serum, using our extraction methods and ELISA kits. For STX ELISAs, a 1:50 dilution of the 1:4 80% ethanol extracts was sufficient to eliminate matrix effects in all sample types. Additionally, the Abraxis ELISA kit is designed to measure only STX (with some limited cross-reactivity to several other PSP toxins, as listed in the Abraxis product documents), consequently all PSP levels are listed as STX equivalents and as such, may underestimate the presence of other congeners. With these minimum dilutions, the detection limits for DA in sample material were, 4 ng/g or ml for feces and bile, 2 ng/g or ml for stomach and intestinal contents and milk, and 0.4 ng/ml urine, aqueous humor and serum. The detection limit for STX in all sample matrices was 3 ng/ml.
2 3. Results
Algal toxins were detected in at least one animal from all 13 species of marine mammals sampled (n = 905 total animals; Tables 2 and 3). In addition, 46 individuals contained detectable concentrations of both DA and STX, including three of eight humpbacks, six of 25 bowheads, five of 110 ringed seals, three of 44 bearded seals, and 20 of 82 walruses tested for both toxins (Table 4). Saxitoxin and DA were present in marine mammals sampled throughout our study area, from the southeastern Gulf of Alaska to the eastern Beaufort Sea (Fig. 1). Domoic acid was detected in more animals (Table 2) and species (all 13) than STX (10 species; Table 3).
Table 2.
Summary of the number of domoic acid-positive individuals from 13 species of Alaskan marine mammals, including the sample matrix with the highest concentration.
| Species | Number of animals | Number positive | % Positive | Max conc. (ng/g or ml) | Sample matrix |
|---|---|---|---|---|---|
| Cetaceans | |||||
| Humpback whale | 8 | 3 | 38% | 51 | F |
| Bowhead whale | 25 | 17 | 68% | 359 | F |
| Beluga whale | 15 | 2 | 13% | 7 | SC |
| Harbor porpoise | 5 | 2 | 40% | 15 | F |
| Otariids | |||||
| Northern fur seal | 179 | 8 | 5% | 14 | S |
| Steller sea lion | 44 | 12 | 27% | 7 | SC |
| Phocids | |||||
| Harbor seal | 9 | 6 | 67% | 8 | F |
| Ringed seal | 113 | 19 | 17% | 127 | F |
| Bearded seal | 55 | 14 | 25% | 48 | IC |
| Spotted seal | 158 | 5 | 3% | 40 | SC |
| Ribbon seal | 21 | 5 | 24% | 7 | F |
| Odobenids | |||||
| Pacific walrus | 82 | 34 | 41% | 6,457 | SC |
| Mustelids | |||||
| Northern sea otter | 172 | 43 | 25% | 162 | U |
| Total number | 886 | 188 | 21% |
F = Feces, SC = Stomach Contents, S = Serum, IC = Intestinal Contents, U = Urine.
Table 3.
Summary of the number of saxitoxin-positive individuals from 13 species of Alaskan marine mammals, including the sample matrix with the highest concentration.
| Species | Number of animals | Number positive | % Positive | Max conc. (ng/g or ml) | Sample Matrix |
|---|---|---|---|---|---|
| Cetaceans | |||||
| Humpback whale | 8 | 4 | 50% | 62 | F |
| Bowhead whale | 25 | 8 | 32% | 63 | F |
| Beluga whale | 12 | 1 | 8% | 4 | F |
| Harbor porpoise | 5 | 0 | 0% | na | na |
| Otariids | |||||
| Northern fur seal | 179 | 8 | 5% | 42 | F |
| Steller sea lion | 42 | 4 | 10% | 7 | F |
| Phocids | |||||
| Harbor seal | 8 | 0 | 0% | na | na |
| Ringed seal | 110 | 15 | 14% | 172 | F |
| Bearded seal | 44 | 6 | 14% | 15 | IC |
| Spotted seal | 145 | 1 | 1% | 3 | SC |
| Ribbon seal | 7 | 0 | 0% | na | na |
| Odobenids | |||||
| Pacific walrus | 82 | 23 | 28% | 240 | IC |
| Mustelids | |||||
| Northern sea otter | 163 | 37 | 23% | 45 | U |
| Total number | 830 | 107 | 13% |
F = Feces, SC = Stomach Contents, IC = Intestinal Contents, U = Urine; na = not applicable.
Table 4.
Summary of animals (N = 46) that tested positive for both domoic acid (DA) and saxitoxin (STX) and mean (± sd) toxin values (ng/g or ml).
| Species | N | DA | STX | Matrix | Collection Locations |
|---|---|---|---|---|---|
| Humpback whale | 3 | 21 ± 26 | 30 ± 28 | F | Southeast Alaska |
| Bowhead whale | 6 | 83 ± 137 | 48 ± 11 | F | Barrow |
| Ringed seal | 5 | 6 ± 2 | 41 ± 73 | F/SC | Chukchi Sea, Bering Sea |
| Bearded seal | 3 | 11 ± 14 | 8 ± 6 | F/SC/U | Chukchi Sea, Bering Sea |
| Pacific walrus | 20 | 524 ± 1,432 | 64 ± 80 | IC | St. Lawrence Island |
| Northern sea otter | 9 | 2 ± 2 | 7 ± 3 | U | Kachemak Bay, Juneau, Glacier Bay |
| Total number | 46 |
Sample matrix includes: F= Feces, SC = Stomach Contents, IC = Intestinal Contents, and U = Urine.
Fig. 1.
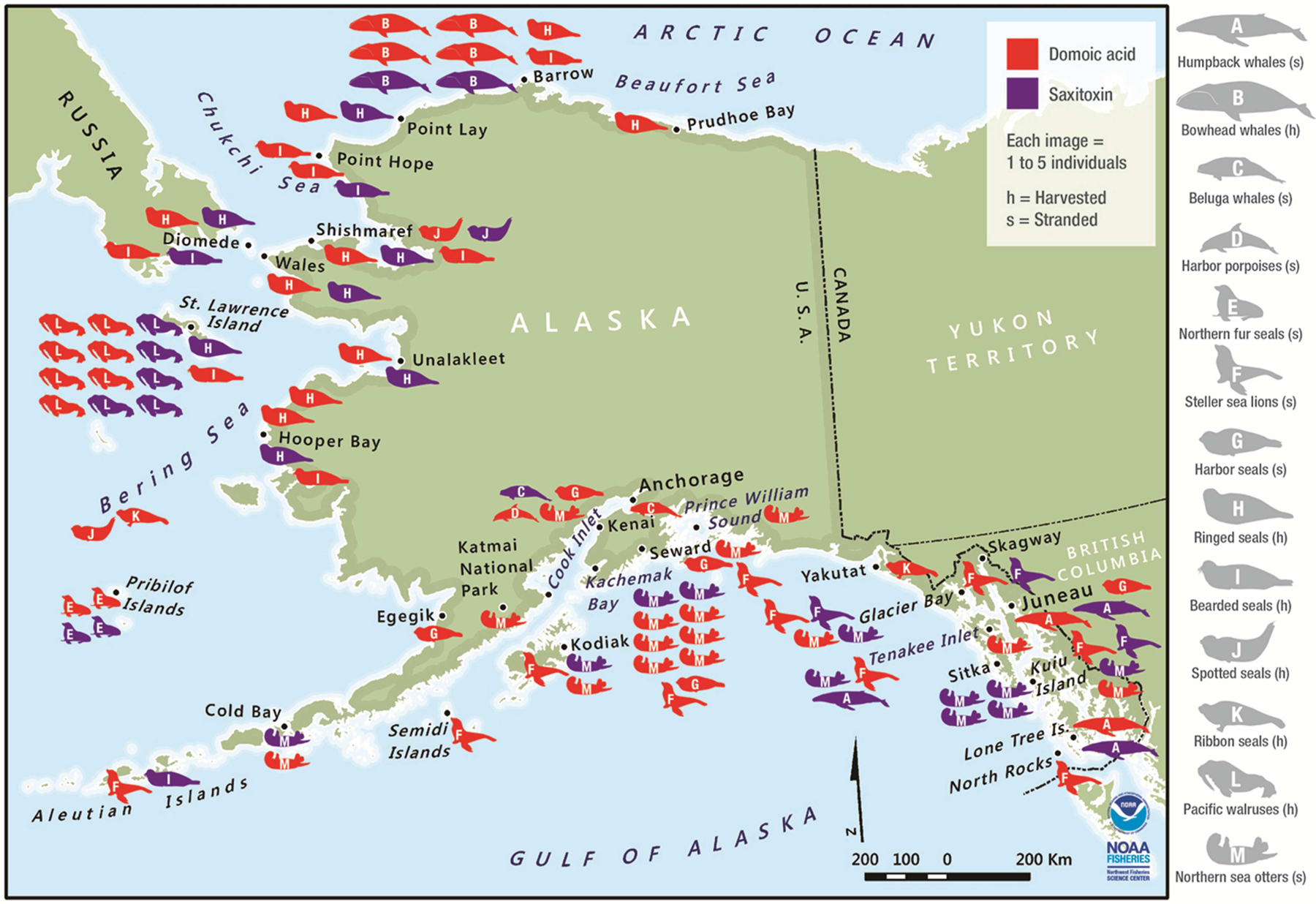
Locations where algal toxins were detected in stranded (s) and harvested (h) marine mammals. Red images represent species positive for domoic acid (DA) and purple images represent species positive for saxitoxin (STX). Marine mammal species are listed as follows; A) Humpback whales, B) Bowhead whales, C) Beluga whales, D) Harbor porpoises, E) Northern fur seals, F) Steller sea lions, G) Harbor seals, H) Ringed seals, I) Bearded seals, J) Spotted seals, K) Ribbon seals, L) Pacific walruses, and M) Northern sea otters.
3.1. Domoic acid
Bowhead whales had the greatest prevalence of DA (68%), followed by harbor seals (67%), walruses (41%), harbor porpoises (40%), humpback whales (38%), Steller sea lions (27%), bearded seals (25%), northern sea otters (25%), ribbon seals (24%), ringed seals (17%), beluga whales (13%), northern fur seals (5%), and spotted seals (3%; Table 2). The highest concentrations were found in feces, stomach contents, intestinal contents, and urine (Table 2). The maximum DA concentration detected was from the intestinal contents of a 15-year-old female walrus (6,457 ng/g) from the northern Bering Sea. Domoic acid was also detected in three fetuses (one beluga, one harbor porpoise and one Steller sea lion). Fig. 2 shows all DA positive fecal, gastrointestinal, and urine samples for all species examined. Additionally, DA concentrations in feces and urine from ten California sea lions (CSLs) sampled from the central California coast that were exhibiting clinical signs of DA toxicosis (seizures) are shown in red in Fig. 2 for comparison. These CSLs were selected from Appendix A, Table 1 of a report on toxin detection methods for marine mammals where both urine and feces were analyzed for comparison in multiple animals (Frame and Lefebvre, 2012). The identification numbers were used to access the Marine Mammal Center Database to determine which animals were observed to have seizures.
Fig. 2.
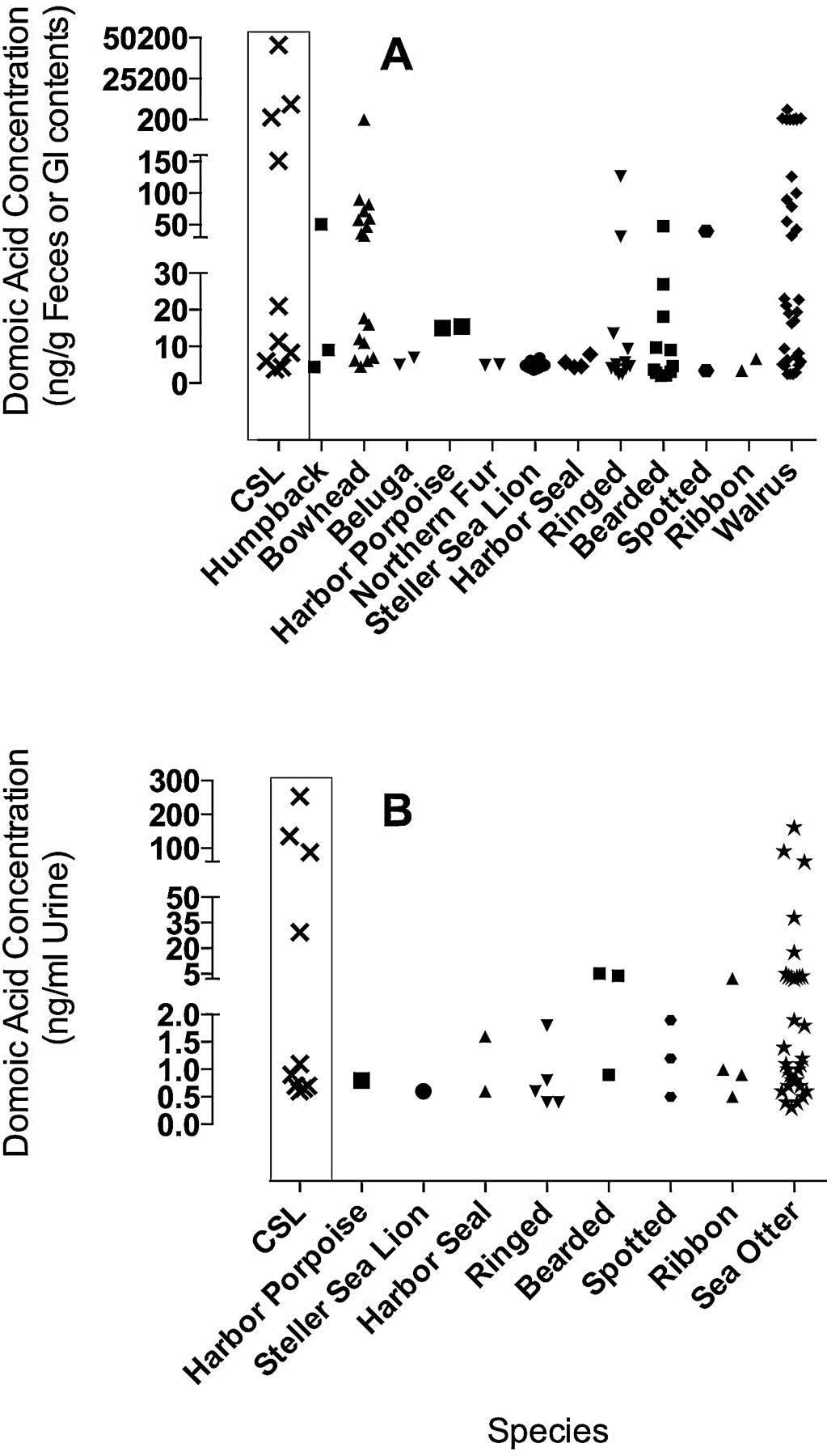
Domoic acid (DA) concentrations quantified in A) feces and gastrointestinal (GI) contents, and B) urine for all Alaskan species sampled. Domoic acid concentrations detected in 10 California sea lions (CSL) exhibiting signs of DA toxicosis (seizures) are included for reference and shown in the box.
3.2. Saxitoxin
Humpback whales had the greatest prevalence of STX (50%), followed by bowhead whales (32%), walruses (28%), northern sea otters (23%), ringed seals (14%), bearded seals (14%), Steller sea lions (10%), beluga whales (8%), northern fur seals (5%), and spotted seals (1%; Table 3). The highest STX concentrations were detected in feces, stomach and intestinal contents, and urine (Table 3). The maximum STX concentration was detected from the intestinal contents of a 21-year-old male walrus (240 ng STX equiv./g; Table 3) from the northern Bering Sea. Additionally, this walrus also contained a high concentration of DA (991 ng/g) in the intestinal contents sample.
4. Discussion
The number of species and the extensive geographic range in which DA and STX were detected demonstrates that HABs are present throughout Alaska’s marine environment and thus the potential for health effects due to exposure is present for all 13 Alaskan marine mammal species tested in this study. For adult marine mammals, DA and STX are being ingested through prey, however, in the case of DA, fetuses and suckling young can also be exposed through amniotic fluid and milk (Rust et al., 2014). Maternal transfer of DA has been well documented by laboratory studies and natural environmental exposures with California sea lions (Maucher and Ramsdell, 2005; Maucher and Ramsdell, 2007; Ramsdell and Zabka, 2008). In the present study, one beluga whale fetus, one harbor porpoise fetus, and one Steller sea lion fetus contained detectable concentrations of DA in stomach contents and feces, further documenting the risk of maternal transfer of toxins from pregnant females with environmental exposures to these biotoxins. Additionally, several sea otter pups and harbor seal neonates contained detectable concentrations of DA, however whether they were actively nursing is unknown. The diets of the 13 species tested in this study are varied due to their diverse marine mammal life histories and range from zooplankton, to benthic invertebrates, to finfish.
4.1. Cetaceans
4.1.1. Humpback whales:
In Alaska, humpback whales seasonally range from the southern Gulf of Alaska to the Chukchi Sea during the summer months. During winter they migrate south to Mexico, Baja California and the Hawaiian Islands to breed and calve. Feeding in cooler Alaskan waters typically occurs during the spring, summer and fall months (Baker et al., 1986). There may be resident populations of humpback whales in the southeastern Gulf of Alaska. In Alaska, their diet consists of krill and many different kinds of fish including herring (Clupea pallasii) and capelin (Mallotus villosus); all of which are planktivorous and therefore likely vectors of DA and STX (Bargu et al., 2002; Doucette et al., 2005; Lefebvre et al., 2002a). A lower percentage of humpbacks tested positive for DA (38%, highest concentration = 51 ng/g feces) (Table 2) than STX (50%, highest concentration = 62 ng/g) (Table 3). The highest DA and STX concentrations were found in an individual that died from a ship strike, which may not be a coincidence because STX and DA intoxication have been suggested to be a factor in the loss of ability to avoid ships and to be a cause of stranding (Geraci et al., 1989).
4.1.2. Bowhead whales:
The entire population of western Arctic bowhead whales ranges through Arctic Alaskan waters from the central Bering Sea to the Canadian Beaufort Sea during their annual migration cycle. Bowhead whales are an important subsistence species for western and northern Alaska providing more than ten villages with substantial meat and blubber each year. This stock of bowhead whales is listed as endangered under the Endangered Species Act (ESA), however the population is increasing (3% annually) and believed to have recovered substantially (George et al., 2004; Gerber et al., 2007; Givens et al., 2013; Zeh and Punt, 2005), suggesting that the current reduction in sea ice has had no detectable negative effects on population growth. Bowheads feed on small zooplankton consisting mainly of calanoid copepods and euphausiids, both of which consume phytoplankton (Moore et al., 2010) and are likely the source of the high occurrence rates of DA (68%) and STX (32%) in fecal samples.
4.1.3. Beluga whales:
In Alaska, there are four stocks of beluga whales, which range from the Bering Sea to the Canadian Beaufort Sea. These stocks are abundant and support subsistence harvests. In addition, there is a fifth stock in Cook Inlet, a tidal estuary located in the northern Gulf of Alaska. All but two of the animals sampled were part of the Cook Inlet stock. This stock is the most genetically isolated (O’Corry-Crowe et al., 2002), was listed in 2008 as “endangered” under the ESA, and is not currently showing signs of recovery; no harvest is currently allowed. Generally, beluga whales prey on a wide variety of fish, crustaceans, and cephalopods. In Cook Inlet, primary prey species consist of at least three species of Pacific salmon (Chinook, Oncorhynchus tshawytscha; chum, O. keta; and coho, O. kisutch), which have been found in beluga stomachs, however it is likely that sockeye (O. nerka) and pink salmon (O. gorbuscha) are also eaten when they are available (Quakenbush et al., 2015). In addition, eulachon (Thaleichthys pacificus), Pacific cod (Gadus macrocephalus), walleye pollock (Theragra chalcogramma), saffron cod (Eleginus gracilis), starry flounder (Platichthys stellatus) and yellowfin sole (Limanda aspera) have also been found in the stomachs of Cook Inlet belugas. Seven types of invertebrates were found in Cook Inlet beluga stomachs, with the frequency of occurrence in non-empty stomachs being highest for shrimp (39%), followed by polychaetes and amphipods (Quakenbush et al., 2015). Invertebrates appear to be much less important to Cook Inlet beluga diet compared to the other stocks. Therefore, analysis of HABs in other beluga stocks would be of interest (Moore et al., 2000).
The two belugas sampled from the Eastern Bering stock did not have detectable levels of DA and were not tested for STX. A relatively low percentage of Cook Inlet beluga whales we examined were positive for DA (13%) and fewer for STX (8%). In addition, all concentrations of both toxins were low with the highest level of DA at 7 ng/g from stomach contents of one animal and STX at 4 ng/g feces in another, the only STX positive beluga (Tables 2 and 3). This may be because beluga prey consists of fewer planktivorous fish and invertebrate species. Although DA has been shown to be widely distributed in fish species in California, the non-planktivorous fish species contained lower concentrations of toxin compared to planktivorous species such as anchovies and sardines (Lefebvre et al., 2002a; Lefebvre et al., 2002b). This is similarly true for STX in that PSP toxins can be found in several of the prey species including Pacific cod and chum salmon, but at lower levels. Crabs and polychaetes are known to concentrate STX (Deeds et al., 2008).
4.1.4. Harbor porpoises:
Harbor porpoises are widespread in the Northern Hemisphere, found in most cool temperate and subpolar waters (Jefferson et al., 1993), including coastal and inland waters, and are seldom found in waters with an annual average temperature above 17°C (Read, 1999). They generally forage on small, pelagic schooling fish in waters less than 200 m deep (Shelden et al., 2014). Harbor porpoises are not harvested for food in Alaska. In Alaskan waters, harbor porpoise stock structure is unclear, but three stocks are currently recognized for management purposes: Southeast Alaska, Gulf of Alaska, and Bering Sea (Shelden et al., 2014), all of which belong to the subspecies P.p. yomerina. Harbor porpoises eat a wide variety of fish, cephalopods and benthic invertebrates with the main prey items varying by region and season (Culik, 2004; Jefferson et al., 1993; Reyes, 1991). In Cook Inlet, harbor porpoises feed on schooling planktivorous fish such as smelt (Family Osmeridae) and Pacific herring (Clupea pallasii pallasii) (Shelden et al., 2014), which are known to accumulate DA. Harbor porpoise samples were collected primarily from Cook Inlet with one animal each from Prince William Sound and Kachemak Bay. The highest concentration of DA was 15.3 ng/g, which occurred in the feces of one animal and the intestinal contents of another animal. One of these was pregnant and the fetus also contained DA at 8 ng/g in its feces documenting maternal transfer of algal toxins. Saxitoxin was not detected in any of the harbor porpoise samples. The higher prevalence of DA is likely a result of a planktivorous fish diet.
4.2. Otariids
4.2.1. Northern fur seals:
Northern fur seals breed and give birth on the Pribilof Islands of Alaska in the southern Bering Sea during the summer (June-August) and pups remain dependent until mid-November when the rookeries are abandoned for the winter and weaning occurs abruptly. Immature males are harvested for food in summer. In Alaska, the majority of northern fur seals winter in the North Pacific and return to Alaskan waters the following spring. Movements from seals tagged with satellite-linked transmitters indicate that all sex and age classes can migrate thousands of kilometers as far west as Kamchatka and east to the west coast of the USA (Baker, 2007; Lea et al., 2009; Pelland et al., 2014; Ream et al., 2005; Sterling et al., 2014). Newly weaned pups may remain south of the Aleutian chain for two or more years before returning to the Pribilofs to breed. Northern fur seals in Alaska predominantly forage on schooling fish and gonatid squid with walleye pollock representing approximately 40–75% of the prey observed in scat collections (Ream et al., 2005; Sinclair et al., 1994). Little is known about the recent winter diet since pelagic sealing and the collection of seals for scientific purposes has ceased. Collections made during 1958–1974 in the eastern Bering Sea, however, indicated that in addition to pollock, northern fur seals consumed capelin, Pacific herring, and squid (Perez and Big, 1986). The serum samples for this study were collected from adult female fur seals with young pups. Provisioning pups with milk every few days limits the distance that females can forage to relatively local waters near the Pribilof Islands and may explain the relatively low occurrence of DA and STX (both at 5% of the animals tested; Tables 2 and 3).
4.2.2. Steller sea lions:
Steller sea lions range throughout the Pacific Rim from southern California, across the Gulf of Alaska, to Northern Honshu Island in Japan, and north into the Bering Strait (Lander et al., 2009). In Alaska, they are harvested for food and managed as two stocks, the eastern distinct population segment (DPS) and western DPS. From 1980 to 2000 there was a greater than 80% population decline in the western DPS, which included Russian and Alaskan waters of the Gulf of Alaska, North Pacific Ocean, and Bering Sea, leaving fewer than 55,000 individuals (Lander et al., 2009). Steller sea lions were listed as threatened under the ESA in 1990, and the western portion of the population was reclassified as endangered in 1997. The cause of the decline is unknown. The population has stabilized in the Gulf of Alaska and the eastern DPS, but continues to decline in the western and central Aleutian Islands. Adult Steller sea lions eat a wide variety of fish, with either walleye pollock or Atka mackerel (Pleurogrammus monopterygius) predominant in most areas. Other prey consists of schooling fish, including Pacific herring and salmon (Oncorhynchus spp.), with smaller numbers of Pacific sand lance (Ammodytes hexapterus), capelin, eulachon, Pacific cod, Pacific hake (Merluccius productus), flatfish, demersal fish, and cephalopods (Merrick et al., 1997). Several of these prey species are planktivorous including herring, juvenile chum salmon, walleye pollock and sand lance.
4.3. Harbor seals
In Alaska, harbor seals are primarily found in coastal waters throughout the Gulf of Alaska, Aleutian Islands, and Southeastern Bering Sea where they are harvested for food (Small et al., 2003). They are found in diverse habitats including glacial and non-glacial areas and are generally non-migratory (Bigg, 1981). Harbor seals mainly forage on fish including Pacific herring, rainbow smelt (Osmerus mordax), salmon (Salmonidae), walleye pollock, Pacific cod, greenling (Hexagrammidae), sculpins (Cottidae), Pacific sand lance, and flatfish (Pleuronectidae) (Pitcher, 1980a, 1980b). Invertebrates such as octopus, squid, and shrimp are also consumed (Pitcher, 1980a, 1980b). The importance of these prey items varies by location. In the Bering Sea and Gulf of Alaska, pollock and octopus are the most common prey items, whereas shrimp and capelin are most common in the southeastern Gulf of Alaska (Pitcher, 1980b). No harbor seals tested positive for STX, however six of nine animals tested positive for DA, although the maximum concentration was low; 8 ng/g feces (Table 2). Harbor seals had a much higher percentage of individuals that tested positive (67%) than spotted seals (3%) even though they consume similar fish species. This could be due to the more southern range of harbor seals compared to the spotted seal’s more northerly range in the Bering Sea. Most harbor seals sampled were from the Gulf of Alaska, which has warmer waters and as such are more likely to be exposed to HABs, although no data are available on HAB or shellfish toxicity for verification. Additionally, the samples sizes tested were vastly different (n = 9 for harbor seals and n = 158 for spotted seals) making direct comparisons difficult.
4.4. Ice seals (Ringed, Bearded, Spotted and Ribbon)
Ringed, bearded, and spotted seals are sea ice-associated seals that range throughout the Bering, Chukchi, and Beaufort seas in Alaska (Burns, 1970). Of these species, only ringed seals are currently ESA listed. Bearded seals were listed under ESA, but a court overturned the decision, which is being appealed by the National Marine Fisheries Service (NMFS). Ribbon seals are also ice-associated and occur throughout the Bering and Chukchi seas, but are not often found in the Beaufort Sea (Burns, 1981). Although movements in winter months are restricted by sea ice, these seals move widely in spring, summer, and fall (Burns, 1970; Burns, 1981; Crawford et al., 2012; Harwood et al., 2012a; Harwood et al., 2012b; Lowry et al., 2000). Ringed and bearded seals tend to inhabit areas that are seasonally ice covered and are found in heavy pack ice. Spotted and ribbon seals are less ice dependent at certain times of the year and can be found near the ice edge, in the broken pack ice, of the Bering Sea in winter and spring. The distribution of spotted seals shifts northward and toward the coasts as sea ice recedes in May and June and many spotted seals enter bays and rivers and haul out on sand bars and barrier islands (Burns et al., 1981). The distribution of ribbon seals also shifts northward as sea ice recedes in May and June. When sea ice melts, however, the majority of the ribbon seal population likely becomes pelagic in the North Pacific and the central Bering Sea, although some seals follow receding ice into the Chukchi Sea (Burns et al., 1981). All four species are harvested for food, mostly in spring and fall.
The diets of ringed, bearded, spotted, and ribbon seals vary widely. Ringed seals feed mostly in the water column on pelagic and semi-demersal fish (including arctic cod, Boreogadus saida; saffron cod, walleye pollock, and sculpins) and invertebrates (including mysids, amphipods, shrimps and echiurids) (Crawford et al., 2015; Dunbar, 1941; Fedoseev, 1965; Johnson et al., 1966; Lowry et al., 1980; McLaren, 1958). Bearded seals feed on a wide variety of benthic invertebrates (including bivalves, gastropods, cephalopods, isopods, amphipods, shrimp, crab, echiurids and polychaetes) and fish (including arctic and saffron cod; sculpins; snailfish (Liparidae); pricklebacks (Stichaeidae); Pacific sandlance, and flatfish) (Antonelis et al., 1994; Burns, 1981; Chapskii, 1938; Crawford et al., 2015; Dunbar, 1941; Quakenbush et al., 2011; Smith, 1981). Spotted seals eat mostly pelagic fish including arctic and saffron cod; Pacific herring; and smelt (Bukhtiyarov et al., 1984; Frost and Lowry, 1981; Quakenbush et al., 2009). Ribbon seal diet is less well documented because most have empty stomachs when they are harvested in the late spring. But their diet is most similar to spotted seals and includes fish (arctic and saffron cod and pollock), shrimp (Crangonid and Pandalid species) and octopus (Dehn et al., 2007; Frost and Lowry, 1980; Quakenbush and Citta, 2008).
Bearded (25%) and ribbon seals (24%) were similar in the percent sampled that contained DA. Fewer ringed seals (17%) were positive, but a female ringed seal pup had the highest concentration of DA (127 ng/g) of any of the ice seals. Spotted seals were the lowest (3% positive). Bearded and ringed seals were both 14% positive for STX and again ringed seals had the higher concentration (172 ng/g feces). Spotted seals were lower at 1% and STX was not detected in any ribbon seals. We would expect bearded seals, as benthic feeders, to be most vulnerable to STX. We would also expect spotted and ribbon seals, as fish-eaters, to be least vulnerable to STX. The higher values for both DA and STX for ringed seals may be due to some individuals consuming larger volumes of mysids, euphausiids, or amphipods (all of which eat algae and detritus) and may explain their higher exposure to HABs.
4.5. Pacific walrus
Pacific walruses are migratory, following the southern margins of the pack ice from the Bering Sea to the Chukchi Sea in the spring, where foraging is optimal in the relatively shallow shelf waters (Estes and Gilbert, 1978; Fay, 1982; Gilbert, 1989). Walruses are harvested for subsistence and in addition to walrus tissues, clams found in the stomach during butchering are also eaten by harvesters. The Pacific walrus population is currently a candidate species under the ESA due to concern regarding the species’ response to changes in summer sea ice habitat (Robards and Garlich-Miller, 2013; USFWS, 2011). Walruses feed primarily on benthic invertebrates including marine worms (e.g., polychaetes, sipunculids, echiurids priapulids), mollusks (e.g., bivalves and gastropods), and crustaceans (e.g., amphipods, shrimp, crabs) (Born et al., 2003; Bowen and Siniffand, 1999; Dehn et al., 2007; Fay, 1982; Sheffield et al., 2001; Sheffield and Grebmeier, 2009) although fish and other vertebrates (including seals) are also occasionally reported (Fay, 1982; Seymour et al., 2014; Sheffield et al., 2001; Sheffield and Grebmeier, 2009). Walruses are not physiologically adapted for deep diving and concentrate foraging efforts in shallower waters, typically using the sea ice as a resting platform between feeding trips (Fay, 1982). Since 2007, walruses summering in the Chukchi Sea have been hauling out in large numbers at two terrestrial haulout sites on the eastern Chukchi Sea (Icy Cape and Point Lay) beginning in late summer when sea ice over the Continental Shelf disappears (Robards and Garlich-Miller, 2013).
Stomach contents from walruses sampled near St. Lawrence Island had the highest measured concentrations of both DA and STX of any species examined in this study (Tables 2 and 3). That 41% and 28% of walruses sampled contained elevated concentrations of DA and STX, respectively, is surprising due to the sampling location. These walruses were sampled in the northern Bering Sea during May, as they were moving northward with the receding sea ice (Fay, 1982). Water temperatures with sea ice present are not considered favorable to support HABs, although the DA and STX could have come from invertebrates eaten farther south. The elevated toxin concentrations in walruses suggest that DA and STX producing phytoplankton are well established in seasonally ice covered waters to accumulate in clams within the foraging range of walruses. That the highest concentrations of both DA and STX in this study came from walruses and that the walrus with the highest concentration of STX also had relatively high DA is cause for continued monitoring and investigation. The DA concentrations detected in walruses are similar to those detected in California sea lions suffering from DA toxicosis, although hunters did not report abnormal behavior in any of the sampled walruses (Fig. 2; (Lefebvre et al., 1999; Scholin et al., 2000).
Walruses and bearded seals are typically benthic feeders with overlapping ranges, but the percent positive and maximum concentration for both DA and STX was higher for walruses than bearded seals. This could be because bearded seals are more generalist foragers than walruses.
4.6. Sea otters
Three stocks of northern sea otters are recognized in Alaska: southeast, southcentral, and southwest (Gorbics and Bodkin, 2001). The southeast and southcentral stocks are considered to be increasing. The southwest stock, however, was listed as threatened under the ESA in 2005, but is currently believed to have stabilized (USFWS 2014).
The primary prey of sea otters in the Gulf of Alaska (Southeast Alaska, Prince William Sound, Kachemak Bay and Kodiak Island) are clams, such as butter clams (Saxidomus giganteus) (Calkins, 1978; Doroff and Bodkin, 1994; Doroff and Degange, 1994; Hoyt et al., 2014; Kvitek et al., 1993). In contrast, the diet in the Aleutian Islands is dominated by sea urchins and a variety of finfish, including those in the families of Hexagrammidae, Gadidae, Cottidae, Cyclopteridae, and Scorpaenidae (Estes et al., 1982; Kenyon, 1969). The majority of the sea otter carcasses recovered and sampled for this study were from the northern Gulf of Alaska (i.e. Kachemak Bay) where clams are an important prey item (Doroff et al., 2012; Newsome et al., 2015).
Given that clams are the predominant prey items for sea otters in Alaska, the percentage of sea otters containing detectable concentrations of DA and STX were lower than expected (Tables 2 and 3) and in the case of STX, may be due to avoidance behaviors. Although sea otters are not immune to PSP toxins, they can detect and avoid lethal amounts of toxic prey (Kvitek and Bretz, 2004; Kvitek, 1991). However, acute toxicosis from STX may have contributed to at least two sea otters being struck and killed by boats in November 2009 in the Kodiak boat harbor (Gill et al. in prep). Urine from these two otters were included in this study and had the highest concentrations of STX (45 and >100 ng/g) for all otters tested. Their behavior prior to being hit by the boats was suggestive of intoxication as they were lethargic and non-reactive at the surface of the water.
Additionally, between May 12–28, 2011 sea otters (n=21) were live-captured around Kuiu Island in Southeast Alaska (Fig. 1). Urine was collected and they were implanted with VHF transmitters and released (Hoyt et al., 2014). Twenty of these otters contained detectable concentrations of STX (3.0–28.4 ng/ml urine). During capture and handling, none of these animals exhibited any clinical signs (i.e. paralysis, difficulty breathing) associated with STX toxicity. After being released they were relocated and feeding was observed between August 2011 and May 2013. Tagged sea otters consumed a total of 32 unique prey types (Hoyt et al., 2014). In terms of biomass, the three most common prey items were clams (primarily Saxidomus giganteus) followed by green urchins (Strongylocentrotus droebachiensis) and Dungeness crab (Metacarcinus magister). Continuing to track DA and STX concentrations in sea otters will be important in understanding threats to their populations especially in the ESA-listed stock. As otters are a nearshore, highly visible species that do haul out on land in some human inhabited Alaskan locations, it may be prudent to set up protocols for documenting and reporting signs of HAB-related toxicosis.
4.7. Toxic Exposure Levels and Data Limitations
An understanding of how the concentrations reported here relate to those known to cause clinical signs of toxicity (behavioral neuroexcitotoxicity for DA and paralysis for STX) in mammals from other regions is needed in order to assess health risks to Alaskan marine mammals. Data on STX concentrations quantified in marine mammals experiencing toxicosis are lacking, however, data for DA concentrations are prevalent due to the regular occurrence of DA toxicosis in California sea lions along the central California coast, USA. Fig. 2 compares concentrations of DA quantified in feces and urine of ten acutely exposed California sea lions exhibiting seizures with concentrations quantified in Alaskan species. These data suggest that Alaskan marine mammals may already be near toxic exposures particularly in humpback and bowhead whales, ringed, bearded and spotted seals, Pacific walruses and sea otters (Fig. 2A & B).
A major limitation in the assessment of health risks is that toxin concentrations in marine mammal samples are not directly related to the magnitude of an animal’s exposure. For example, not all California sea lions with acute behavioral signs of toxicity (e.g., seizures) have high concentrations of DA in feces and urine because elimination rates are rapid (Lefebvre et al., 1999)(Fig. 2). Passage rates for captive sea lions fed Pacific herring averaged less than 5 hours (Helm, 1984) and laboratory studies have reported 99% of algal toxin is eliminated through urine within 24 hours of dosing (Suzuki and Hierlihy, 1993). Therefore, the concentrations presented here provide proof of exposure risk and evidence for potential neurotoxic impacts to several marine mammal species in Alaska.
5. Conclusions
Our results demonstrate that the algal toxins DA and STX are present in Alaskan Subarctic and Arctic ecosystems and have the potential to affect most marine mammal species in USA waters farther north than expected. Given the current trend of decreasing sea ice and warming ocean waters that will extend the open water season favorable to HABs, the prevalence and concentrations of DA and STX documented in this study are expected to increase creating a greater risk to marine mammals. Clinical signs of neurotoxicity were not confirmed in the present study, however many of the animals were dead when sampled. Additionally, toxin effects could contribute to an increase in ship strikes for large cetaceans and increased vulnerability to subsistence harvested seals, walruses and whales, both of which would be difficult to detect due to concurrent increases in ship traffic and changes in ice and weather patterns that affect hunting. Sea lions along the central California coast provide a cautionary example of increasing HAB impacts on marine mammal health. This threat to marine mammals was first recognized in 1998, and now has a major impact on sea lions annually. Recent studies have suggested that HABs are also affecting large cetaceans in southern latitudes such as the Minke whale (Balaenoptera acutorostrata) (Fire et al., 2010). This study documents the presence of HAB toxins in top predators from southeast Alaska to the Arctic Ocean revealing a potentially growing exposure risk to northern marine mammal populations. Unless unknown factors inhibit HABs in northern waters, warming water temperatures and increased light availability due to loss of sea ice are likely to support more blooms increasing toxin concentrations and the health risks they present for northern marine mammal species as they have for southern species.
Acknowledgements:
Without the efforts and support of the coastal communities of northern and western Alaska allowing us to examine their marine mammals, we would not have the information presented in this manuscript regarding Arctic species. We greatly appreciate the willingness of subsistence hunters to share their harvested seals, walrus, bowhead and belugas for research. We thank the whaling captains of Barrow, Alaska and the Alaska Eskimo Whaling Commission for allowing the sampling of subsistence harvested bowhead whales. Samples were collected under NMFS Permit 17350-00 issued to the North Slope Borough Department of Wildlife Management. We would like to thank the Native Villages of Gambell and Savoonga and all of the subsistence hunters who donated walrus samples, and the subsistence hunters and Tribal government of St. Paul Island for their assistance collecting Northern fur seal samples. We would also like to thank Mark Nelson, Louise Foster, Sheena Anningayou, Harold Kiyuklook, and Alaska Department of Fish and Game (ADF&G) college interns for their help with sample collection and processing. We would like to thank the volunteer Alaska Marine Mammal Stranding Network and the Alaska SeaLife Center for helping with sample collection. Additionally, we express gratitude to Kristin Worman at the US Fish and Wildlife Service (USFWS) for helping with sea otter samples. Sea otter samples were collected under MMPA permit No. MA041309-5 issued to USFWS Marine Mammals Management office. Other stranded marine mammal samples were collected under NMFS permits 932-1489 and 932-1905 and some of the work was funded through John H. Prescott Marine Mammal Rescue Assistance grants NA09NMF4390236 and NA12NMF4390162. Sample collection was funded by the USFWS, ADF&G and NOAA’s National Marine Fisheries Service (NMFS). Samples contributed by ADF&G were collected and analyzed under NMFS research permit #s 358-1787, 15324 and 10091 and USFWS collection letters issued to ADF&G. Samples of adult female northern fur seals were collected under authorization of NMFS permit #14327 issued to the Marine Mammal Laboratory. Data for intoxicated California sea lions shown in Fig. 2 came from the US West Coast Wildlife Algal Toxin Research and Response Network (WARRN-West) and the Marine Mammal Center databases. This project was funded by the North Pacific Research Board (NPRB project #1113, publication #575). Additional support for research staff and toxin analyses came from grants from the National Institute of Health (NIH) R01 ES021930 and the National Science Foundation (NSF) OCE-1314088. We thank Dr. Linda Rhodes and Dr. Walt Dickhoff of the NWFSC, Dr. Teri Rowles of the Office of Protected Resources (NOAA) and Dr. Peter Boveng of the Polar Ecosystems Program (NOAA/MML) for careful review of the manuscript. Special thanks goes to Su Kim and Damon Holzer of the NWFSC for help creating the Fig. 1 map.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service and NOAA Fisheries.Mention of trade names or commercial products is solely for providing specific information and does not imply recommendation or endorsement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Antonelis GA, Melin SR, Bukhtiyarov YA, 1994. Early spring feeding habits of bearded seals (Erignathus barbatus) in the Central Bering Sea, 1981. Arctic 47(1), 74–79. [Google Scholar]
- Baker CS, Herman LM, Perry A, Lawton WS, Straley JM, Wolman AA, Kaufman GD, Winn HE, Hall JD, Reinke JM, Ostman J, 1986. Migratory movement and population structure of humpback whales (Megaptera novaeangliae) in the central and eastern North Pacific. Mar Ecol Prog Ser 31, 105–119. [Google Scholar]
- Baker JD, 2007. Post-weaning migration of northern fur seal Callorhinus ursinus pups from the Pribilof Islands, Alaska. Mar Ecol Prog Ser 341, 243–255. [Google Scholar]
- Bargu S, Powell CL, Coale SL, Busman M, Doucette GJ, Silver MW, 2002. Krill: A potential vector for domoic acid in marine food webs. Marine Ecology-Progress Series 237, 209–216. [Google Scholar]
- Bargu S, Silver M, Goldstein T, Roberts K, Gulland F, 2010. Complexity of domoic acid-related sea lion strandings in Monterey Bay, California: foraging patterns, climate events, and toxic blooms. Marine Ecology-Progress Series 418, 213–222. [Google Scholar]
- Berman FW, LePage KT, Murray TF, 2002. Domoic acid neurotoxicity in cultured cerebellar granule neurons is controlled preferentially by the NMDA receptor Ca(2+) influx pathway. Brain Res 924(1), 20–29. [DOI] [PubMed] [Google Scholar]
- Berman FW, Murray TF, 1997. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J Neurochem 69(2), 693–703. [DOI] [PubMed] [Google Scholar]
- Bigg MA, 1981. Harbour Seals Phoca vitulina Linnaeus, 1758 and Phoca largha Pallas, 1811. Academic Press, New York, NY. [Google Scholar]
- Born EW, Rysgaard S, Ehlmé G, Sejr M, Acquarone M, Levermann N, 2003. Underwater observations of foraging free-living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biol 26, 348–357. [Google Scholar]
- Bowen D, Siniffand DB, 1999. Distribution, population biology, and feeding ecology of marine mammals, In: J.E. R, Rommel SA (Eds.), Biology of Marine Mammals. Smithsonian Institution Press, Washington, DC, pp. 423–484 [Google Scholar]
- Brodie EC, Gulland FMD, Greig DJ, Hunter M, Jaakola J, St Leger J, Leighfield TA, Van Dolah FM, 2006. Domoic acid causes reproductive failure in california sea lions (Zalophus californianus). Mar Mammal Sci 22(3), 700–707. [Google Scholar]
- Bukhtiyarov YA, Frost KJ, Lowry LF, 1984. New information on foods of the spotted seal, Phoca largha, in the Bering sea in Spring, In: Fay FH, Fedoseev GA (Eds.), Soviet-America cooperative research on marine mammals, U.S. Department of Commerce, pp. 55–59. [Google Scholar]
- Burek KA, Gulland FM, O’Hara TM, 2008. Effects of climate change on Arctic marine mammal health. Ecol Appl 18(2 Suppl), S126–134. [DOI] [PubMed] [Google Scholar]
- Burns JJ, 1970. Remarks on the distribution and natural history of pagophilic pinnipeds in the Bering and Chukchi seas. Journal of Mammalogy 51, 445–454. [Google Scholar]
- Burns JJ, 1981. Ribbon seal Phoca fasciata Zimmermann, 1783. Academic Press, New York, NY. [Google Scholar]
- Burns JJ, Shapiro LH, Fay FH, 1981. Ice as marine mammal habitat in the Bering Sea. U.S. Department of Commerce, NOAA, U.S. Department of Interior, Office of Marine Pollution Assessment, and Bureau of Land Management, Washington, D.C. [Google Scholar]
- Bursa A, 1963. Phytoplankton in coastal waters of the Arctic Ocean at Point Barrow, Alaska. Arctic 16, 239–262. [Google Scholar]
- Calkins DG, 1978. Feeding-Behavior and Major Prey Species of Sea Otter, Enhydra-Lutris, in Montague Strait, Prince-William-Sound, Alaska. Fish B-Noaa 76(1), 125–131. [Google Scholar]
- Chapskii KK, 1938. The Bearded Seal (Erignathus barbatus Fabr.) of the Kara and Barents Sea. Trudy Arktichoskogo Instituta; 123, 7–70. [Google Scholar]
- Cook PF, Reichmuth C, Rouse AA, Libby LA, Dennison SE, Carmichael OT, Kruse-Elliott KT, Bloom J, Singh B, Fravel VA, Barbosa L, Stuppino JJ, Van Bonn WG, Gulland FM, Ranganath C, 2015. Algal toxin impairs sea lion memory and hippocampal connectivity, with implications for strandings. Science 350(6267), 1545–1547. [DOI] [PubMed] [Google Scholar]
- Costa PR, Baugh KA, Wright B, Ralonde R, Nance SL, Tatarenkova N, Etheridge SM, Lefebvre KA, 2009. Comparative determination of paralytic shellfish toxins (PSTs) using five different toxin detection methods in shellfish species collected in the Aleutian Islands, Alaska. Toxicon 54(3), 313–320. [DOI] [PubMed] [Google Scholar]
- Costas E, Lopez-Rodas V, 1998. Paralytic phycotoxins in monk seal mass mortality. Veterinary Record 142(23), 643–644. [DOI] [PubMed] [Google Scholar]
- Crawford JA, Frost KJ, Quakenbush LT, Whiting A, 2012. Different habitat use strategies by subadult and adult ringed seals (Phoca hispida) in the Bering and Chukchi seas. Polar Biol 35(2), 241–255. [Google Scholar]
- Crawford JA, Quakenbush LT, Citta JJ, 2015. A comparison of ringed and bearded seal diet, condition and productivity between historical (1975–1984) and recent (2003–2012) periods in the Alaskan Bering and Chukchi seas. Progress in Oceanography. [Google Scholar]
- Culik BM, 2004. Review of small cetaceans. Distribution, Behaviour, Migration and Threats, Regional Seas Reports and Studies. [Google Scholar]
- Cusick KD, Sayler GS, 2013. An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions. Mar Drugs 11(4), 991–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeds JR, Landsberg JH, Etheridge SM, Pitcher GC, Longan SW, 2008. Non-traditional vectors for paralytic shellfish poisoning. Marine Drugs 6(2), 308–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degange AR, Vacca MM, 1989. Sea Otter Mortality at Kodiak Island, Alaska, during Summer 1987. Journal of Mammalogy 70(4), 836–838. [Google Scholar]
- Dehn LA, Sheffield GG, Follmann EH, Duffy LK, Thomas DL, O’Hara TM, 2007. Feeding ecology of phocid seals and some walruses in the Alaskan and Canadian Arctic as determined by stomach contents and stable isotope analysis. Polar Biol 30(2), 167–181. [Google Scholar]
- Doroff A, Badajos O, Corbell K, Jenski D, Beaver M, 2012. Assessment of sea otter (Enhydra lutris kenyoni) diet in Kachemak Bay, Alaska (2008–2010). IUCN Otter Spec Group Bull 29, 15–23. [Google Scholar]
- Doroff AM, Bodkin JL, 1994. Sea otter foraging behavior and hydrocarbon levels in prey. Academic Press, San Diego, CA. [Google Scholar]
- Doroff AM, Degange AR, 1994. Sea Otter, Enhydra-Lutris, Prey Composition and Foraging Success in the Northern Kodiak Archipelago. Fish B-Noaa 92(4), 704–710. [Google Scholar]
- Doucette GJ, Turner JT, Powell CL, Keafer BA, Anderson DM, 2005. Trophic accumulation of PSP toxins in zooplankton during Alexandrium fundyense blooms in Casco Bay, Gulf of Maine, April-June 1998. I. Toxin levels in A-fundyense and zooplankton size fractions. Deep-Sea Research Part Ii-Topical Studies in Oceanography 52(19–21), 2764–2783. [Google Scholar]
- Dunbar MJ, 1941. On the food of seals in the Canadian eastern Arctic. Canadian Journal of Research 19, 150–155. [Google Scholar]
- Estes JA, Gilbert JR, 1978. Evaluation of an Aerial Survey of Pacific Walruses (Odobenus-Rosmarus Divergens). J Fish Res Board Can 35(8), 1130–1140. [Google Scholar]
- Estes JA, Jameson RJ, Rhode EB, 1982. Activity and Prey Election in the Sea Otter - Influence of Population Status on Community Structure. Am Nat 120(2), 242–258. [Google Scholar]
- Fay FH, 1982. Ecology and biology of the Pacific walrus, Odobenus rosmarus divergens illiger, U.S. Fish and Wildlife Service, North American Fauna, Washington, D. C., p. 279. [Google Scholar]
- Fedoseev GA, 1965. Food of the ringed seal. Izvestiya TINRO 59, 216–223. [Google Scholar]
- Fire SE, Wang Z, Berman M, Langlois GW, Morton SL, Sekula-Wood E, Benitez-Nelson CR, 2010. Trophic Transfer of the Harmful Algal Toxin Domoic Acid as a Cause of Death in a Minke Whale (Balaenoptera acutorostrata) Stranding in Southern California. Aquatic Mammals 36(4), 342–350. [Google Scholar]
- Flewelling LJ, Naar JP, Abbott JP, Baden DG, Barros NB, Bossart GD, Bottein MY, Hammond DG, Haubold EM, Heil CA, Henry MS, Jacocks HM, Leighfield TA, Pierce RH, Pitchford TD, Rommel SA, Scott PS, Steidinger KA, Truby EW, Van Dolah FM, Landsberg JH, 2005. Brevetoxicosis: red tides and marine mammal mortalities. Nature 435(7043), 755–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame ER, Lefebvre KA, 2012. ELISA methods for domoic acid quantification in multiple marine mammal species and sample matrices Northwest Fisheries Science Center, NOAA Fisheries, Seattle, WA. [Google Scholar]
- Frost KJ, Lowry LF, 1980. Feeding of Ribbon Seals (Phoca-Fasciata) in the Bering Sea in Spring. Can J Zool 58(9), 1601–1607. [Google Scholar]
- Frost KJ, Lowry LF, 1981. Ringed, Baikal and Caspian seals—Phoca hispida, Phoca sibirica, and Phoca caspica. Academic Press, New York, NY. [Google Scholar]
- George JCC, Zeh J, Suydam R, Clark C, 2004. Abundance and population trend (1978–2001) of western Arctic bowhead whales surveyed near Barrow, Alaska. Mar Mammal Sci 20(4), 755–773. [Google Scholar]
- Geraci JJ, Anderson DM, Timperi RJ, Staubin DJ, Early GA, Prescott JH, Mayo CA, 1989. Humpback whales (Megaptera novaeangliae) fatally poisoned by dinoflagellate toxin. Can. J. Fish. Aquat. Sci 46(11), 1895–1898. [Google Scholar]
- Gerber LR, Keller AC, DeMaster DP, 2007. Ten thousand and increasing: Is the western Arctic population of bowhead whale endangered? Biological Conservation 137(4), 577–583. [Google Scholar]
- Gessner BD, Middaugh JP, 1995. Paralytic shellfish poisoning in Alaska: a 20-year retrospective analysis. Am J Epidemiol 141(8), 766–770. [DOI] [PubMed] [Google Scholar]
- Gessner BD, Middaugh JP, Doucette GJ, 1997. Paralytic shellfish poisoning in Kodiak, Alaska. West J Med 167(5), 351–353. [PMC free article] [PubMed] [Google Scholar]
- Gessner BD, Schloss M, 1996. A population-based study of paralytic shell fish poisoning in Alaska. Alaska Medicine 38(2), 54–58. [PubMed] [Google Scholar]
- Gilbert JR, 1989. Aerial Census of Pacific Walruses in the Chukchi Sea, 1985. Mar Mammal Sci 5(1), 17–28. [Google Scholar]
- Givens GHG, Edmonson SL, George JC, Suydam R, Charif RA, Rahaman A, Hawthorne D, Tudor B, De Long RA, Clark CW, 2013. Estimate of 2011 Abundance of the Bering-Chukchi-Beaufort Seas Bowhead Whale Population, Annual Report to the Scientific Committee of the International Whaling Commission, Colorado State University, Dept. of Statistics, Alaska, p. 30. [Google Scholar]
- Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM, 2008. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc Biol Sci 275(1632), 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbics CS, Bodkin JL, 2001. Stock structure of sea otters (Enhydra lutris kenyoni) in Alaska. Mar Mammal Sci 17(3), 632–647. [Google Scholar]
- Gulland F, 2000. Domoic acid toxicity in California sea lions (Zalophus californianus) stranded along the central california coast, may-october 1998. Report to the National Marine Fisheries Service Working group on unusual marine mammal Mortality Events., In: U.S. Department of Commerce, N.t.m. (Ed.). Silver Spring, Maryland, p. 45p. [Google Scholar]
- Gulland FM, Haulena M, Fauquier D, Langlois G, Lander ME, Zabka T, Duerr R, 2002. Domoic acid toxicity in Californian sea lions (Zalophus californianus): clinical signs, treatment and survival. Vet Rec 150(15), 475–480. [DOI] [PubMed] [Google Scholar]
- Gulland FMD, Hall AJ, 2007. Is marine mammal health deteriorating? Trends in the global reporting of marine mammal disease. Ecohealth 4(2), 135–150. [Google Scholar]
- Hallegraeff GM, 1998. Transport of toxic dinoflagellates via ships’ ballast water: Bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar Ecol Prog Ser 168(0), 297–309. [Google Scholar]
- Harwood LA, Smith TG, Auld JC, 2012a. Fall Migration of Ringed Seals (Phoca hispida) through the Beaufort and Chukchi Seas, 2001–02. Arctic 65(1), 35–44. [Google Scholar]
- Harwood LA, Smith TG, Melling H, Alikamik J, Kingsley MCS, 2012b. Ringed Seals and Sea Ice in Canada’s Western Arctic: Harvest-Based Monitoring 1992–2011. Arctic 65(4), 377–390. [Google Scholar]
- Helm RC, 1984. Rate of digestion in three species of pinnipeds. Canadian Journal of Zoology 62(9), 1751–1756. [Google Scholar]
- Hoyt ZN, Eckert G, Gill VA, Rice A, 2014. Sea otter recolonization and interactions with commercially important macroinvertebrates in southeast Alaska, North Pacific Research Board Final Report, Anchorage, AK, p. 82. [Google Scholar]
- Jefferson TA, Leatherwood S, Webber WA, 1993. FAO Species identification guide. UNEP/FAO, Rome. [Google Scholar]
- Johnson ML, Fiscus CH, Ostenson BT, Barbour ML, 1966. Marine mammals. U.S. Atomic Energy Commission, Oak Ridge, TN. [Google Scholar]
- Kenyon KW, 1969. The sea otter in the eastern Pacific Ocean. North American Fauna 68, 352. [Google Scholar]
- Kvitek R, Bretz C, 2004. Harmful algal bloom toxins protect bivalve populations from sea otter predation. Mar Ecol Prog Ser 271, 233–243. [Google Scholar]
- Kvitek RG, 1991. Paralytic shellfish toxins sequestered by bivalves as a defense against siphon-nipping fish. Marine biology 111(3), 369–374. [Google Scholar]
- Kvitek RG, Bowlby CE, Staedler M, 1993. Diet and Foraging Behavior of Sea Otters in Southeast Alaska. Mar Mammal Sci 9(2), 168–181. [Google Scholar]
- Lander ME, Loughlin TR, Logsdon MG, VanBlaricom GR, Fadely BS, Fritz LW, 2009. Regional differences in the spatial and temporal heterogeneity of oceanographic habitat used by Steller sea lions. Ecol Appl 19(6), 1645–1659. [DOI] [PubMed] [Google Scholar]
- Landsberg JH, Lefebvre KA, Flewelling LJ, 2014. Effects of Toxic Microalgae on Marine Organisms, In: Rossini GP (Ed.), Toxins and Biologically Active Compounds from Microalgae. CRC Press, Boca Raton, pp. 379–449. [Google Scholar]
- Lea MA, Johnson D, Ream R, Sterling J, Melin S, Gelatt T, 2009. Extreme weather events influence dispersal of naive northern fur seals. Biology Letters 5(2), 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, Silver MW, 2002a. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon 40(7), 971–977. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Powell CL, Busman M, Doucette CJ, Moeller PDR, Sliver JB, Miller PE, Hughes MP, Singaram S, Silver MW, Tjeerdema RS, 1999. Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Natural Toxins 7(3), 85–92. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Robertson A, Frame ER, Colegrove CM, Nance S, Baugh KA, Wiedenhoft A, Gulland FMD, 2010. Clinical signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of toxin detection methods. Harmful Algae 9(4), 374–383. [Google Scholar]
- Lefebvre KA, Silver MW, Coale SL, Tjeerdema RS, 2002b. Domoic acid in planktivorous fish in relation to toxic Pseudo-nitzschia cell densities. Marine biology 140(3), 625–631. [Google Scholar]
- Lewitus A, Horner RA, Caron DA, Garcia-Mendoza E, Hickey BM, Hunter M, Huppert DD, Kudela RM, Langlois G, Largier JL, 2012. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 19, 133–159. [Google Scholar]
- Lowry LF, Frost KJ, Burkonov VN, Simpkins MA, Davis R, DeMaster DP, Suydam R, Springer A, 2000. Habitat use and habitat selection by spotted seals (Phoca largha) in the Bering Sea. Canadian Journal of Zoology 78, 1959–1971. [Google Scholar]
- Lowry LF, Frost KJ, Burns JJ, 1980. Variability in the diet of ringed seals, Phoca hispida, in Alaska. Canadian Journal of Fisheries and Aquatic Sciences 37, 2254–2261. [Google Scholar]
- Maucher JM, Ramsdell JS, 2005. Domoic acid transfer to milk: Evaluation of a potential route of neonatal exposure. Environmental Health Perspectives 113(4), 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucher JM, Ramsdell JS, 2007. Maternal-fetal transfer of domoic acid in rats at two gestational time points. Environmental Health Perspectives 115(12), 1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren IA, 1958. The Biology of the Ringed Seal (Phoca hispida Schreber) in the Eastern Canadian Arctic. Bulletin Fisheries Research Board of Canada 118, 97. [Google Scholar]
- Merrick RL, Chumbley MK, Byrd GV, 1997. Diet Diversity of Steller Sea Lions (Eumetopias jubatus) and their Population Decline in Alaska: a Potential Relationship. Canadian Journal of Fisheries and Aquatic Sciences 54, 1342–1348. [Google Scholar]
- Moore SE, George JC, Sheffield G, Bacon J, Ashjian CJ, 2010. Bowhead Whale Distribution and Feeding near Barrow, Alaska, in Late Summer 2005–06. Arctic 63(2), 195–205. [Google Scholar]
- Moore SE, Shelden KE, Litzky LK, Mahoney BA, Rugh DJ, 2000. Beluga, Delphinapterus leucas, habitat associations in Cook Inlet, Alaska. Marine Fisheries Review 62(3), 60–80. [Google Scholar]
- Moore SK, Trainer VL, Mantua NJ, Parker MS, Laws EA, Backer LC, Fleming LE, 2008. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ Health 7 Suppl 2, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome SD, Tinker MT, Gill VA, Hoyt ZN, Doroff A, Nichol L, Bodkin JL, 2015. The interaction of intraspecific competition and habitat on individual diet specialization: a near range-wide examination of sea otters. Oecologia 178(1), 45–59. [DOI] [PubMed] [Google Scholar]
- O’Corry-Crowe GM, Dizon AE, Suydam R, Lowry LF, 2002. Molecular Genetics Studies of Population Structure and Movement Patterns in a Migratory Species: The Beluga Whale, Delphinapterus leucas, in the Western Nearctic, In: Pfeiffer CJ (Ed.), Molecular and Cell Biology of Marine Mammals. Krieger Publishing Company, Malabar, Florida. [Google Scholar]
- Pelland NA, Sterling JT, Lea MA, Bond NA, Ream RR, Lee CM, Eriksen CC, 2014. Fortuitous Encounters between Seagliders and Adult Female Northern Fur Seals (Callorhinus ursinus) off the Washington (USA) Coast: Upper Ocean Variability and Links to Top Predator Behavior. Plos One 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Big MA, 1986. Diet of northern fur seals, Calorhinus ursinus, off western North America. Fish B-Noaa 84, 957–971. [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS, 1990. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med 322(25), 1775–1780. [DOI] [PubMed] [Google Scholar]
- Pitcher KW, 1980a. Food of the Harbor Seal, Phoca-Vitulina-Richardsi, in the Gulf of Alaska. Fish B-Noaa 78(2), 544–549. [Google Scholar]
- Pitcher KW, 1980b. Stomach Contents and Feces as Indicators of Harbor Seal, Phoca-Vitulina, Foods in the Gulf of Alaska. Fish B-Noaa 78(3), 797–798. [Google Scholar]
- Quakenbush LT, Citta JJ, 2008. Biology of the ribbon seal in Alaska, Report to National Marine Fisheries Service, p. 46. [Google Scholar]
- Quakenbush LT, Citta JJ, Crawford JA, 2009. Biology of the spotted seal (Phoca largha) in Alaska from 1962 to 2008, Preliminary report to the National Marine Fisheries Service, p. 66. [Google Scholar]
- Quakenbush LT, Citta JJ, Crawford JA, 2011. Biology of the Bearded Seal (Erignathus barbatus) in Alaska from 1962 to 2009, Preliminary report to National Marine Fisheries Service, p. 71. [Google Scholar]
- Quakenbush LT, Suydam RS, Bryan AL, Lowry LF, Frost KJ, Mahoney BA, 2015. Diet of beluga whales, Delphinapterus leucas, in Alaska from stomach contents, March-November. Marine Fisheries Review 77(1), 70–84. [Google Scholar]
- RaLonde R, Wright BA, 2011. Using Blue Mussels as an Indicator Species for Testing Domoic Acid Toxicity in Subsistance Bivalve Harvest, NPRB Final Report. North Pacific Research Board, Anchorage, AK. [Google Scholar]
- Ramsdell JS, Zabka TS, 2008. In utero domoic acid toxicity: A fetal basis to adult disease in the california sea lion (Zalophus californianus). Marine Drugs 6(2), 262–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AJ, 1999. Harbour porpoise - Phocoena phocoena (Linnaeus, 1758), Handbook of Marine Mammals: The second book of dolphins and porpoises, pp. 323–356. [Google Scholar]
- Ream RR, Sterling JT, Loughlin TR, 2005. Oceanographic features related to northern fur seal migratory movements. Deep-Sea Research Part Ii-Topical Studies in Oceanography 52(5–6), 823–843. [Google Scholar]
- Reeves R, Rosa C, George JC, Sheffield G, Moore M, 2012. Implications of Arctic industrial growth and strategies to mitigate future vessel and fishing gear impacts on bowhead whales. Marine Policy 36, 454–462. [Google Scholar]
- Reyes RC, 1991. The conservation of small cetaceans: a review, Report prepared for the Secretariat of the Convention on the Conservation of Migratory Species of Wild Animals. [Google Scholar]
- Robards M, Garlich-Miller J, 2013. Workshop on assessing Pacific walrus population attributes from coastal haul-outs, In: Service, U.S.F.a.W. (Ed.), Marine Mammals Management, Anchorage, Alaska. [Google Scholar]
- Rust L, Gulland F, Frame E, Lefebvre K, 2014. Domoic acid in milk of free living California marine mammals indicates lactational exposure occurs. Mar Mammal Sci 30(3), 1272–1278. [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R 3rd, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM, 2000. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403(6765), 80–84. [DOI] [PubMed] [Google Scholar]
- Seymour J, Horstmann-Dehn L, Wooller MJ, 2014. Proportion of higher trophic-level prey in the diet of Pacific walruses (Odobenus rosmarus divergens). Polar Biol 37(7), 941–952. [Google Scholar]
- Sheffield G, Fay FH, Feder H, Kelly BP, 2001. Laboratory digestion of prey and interpretation of walrus stomach contents. Mar Mammal Sci 17(2), 310–330. [Google Scholar]
- Sheffield G, Grebmeier JM, 2009. Pacific walrus (Odobenus rosmarus divergens): Differential prey digestion and diet. Mar Mammal Sci 25(4), 761–777. [Google Scholar]
- Shelden KEW, Agler BA, Brueggeman JJ, Cornick IA, Speckman SG, Prevel-ramos A, 2014. Harbor porpoise, Phocoena phocoena vomerina, in Cook Inlet, Alaska. Mar. Fish. Rev 76, 22–50. [Google Scholar]
- Sinclair E, Loughlin T, Pearcy W, 1994. Prey Selection by Northern Fur Seals (Callorhinus-Ursinus) in the Eastern Bering Sea. Fish B-Noaa 92(1), 144–156. [Google Scholar]
- Small RJ, Pendleton GW, Pitcher KW, 2003. Trends in abundance of Alaska harbor seals, 1983–2001. Mar Mammal Sci 19(2), 344–362. [Google Scholar]
- Smith TG, 1981. Notes on the Bearded Seal, Erignathus barbatus, in the Canadian Arctic. Canadian Technical Report of Fisheries and Aquatic Sciences 1042, 49. [Google Scholar]
- Stabeno PJ, Bond NA, Salo SA, 2007. On the recent warming of the southeastern Bering Sea shelf. Deep-Sea Research Part Ii-Topical Studies in Oceanography 54(23), 2599–2618. [Google Scholar]
- Sterling JT, Springer AM, Iverson SJ, Johnson SP, Pelland NA, Johnson DS, Lea MA, Bond NA, 2014. The Sun, Moon, Wind, and Biological Imperative-Shaping Contrasting Wintertime Migration and Foraging Strategies of Adult Male and Female Northern Fur Seals (Callorhinus ursinus). Plos One 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeve J, Serreze M, Drobot S, Gearheard S, Holland M, Maslanik J, Meier W, Scambos T, 2008. Arctic Sea Ice Extent Plummets in 2007. Eos, Transactions American Geophysical Union 89(2), 13. [Google Scholar]
- Suzuki CA, Hierlihy SL, 1993. Renal clearance of domoic acid in the rat. Food Chem Toxicol 31(10), 701–706. [DOI] [PubMed] [Google Scholar]
- Todd ECD, 1993. Domoic acid and amnesic shellfish poisoning: A review. Journal of Food Protection 56(1), 69–83. [DOI] [PubMed] [Google Scholar]
- Torres De La Riva G, Johnson CK, Gulland FMD, Langlois GW, Heyning JE, Rowles TK, Mazet JAK, 2009. Association of an unusual marine mammal mortality event with Pseudo-nitzschia spp. blooms along the southern California coastline. Journal of Wildlife Diseases 45(1), 109–121. [DOI] [PubMed] [Google Scholar]
- Trainer V, Sullivan K, Eberhart BTL, Shuler A, Hignutt E Jr, Kiser J, Eckert GL, Shumway SE, Morton SL, 2014. Enhancing Shellfish Safety in Alaska through Monitoring of Harmful Algae and Their Toxins. Journal of Shellfish Research 33(2), 531–539. [Google Scholar]
- USFWS, 2011. Endangered and threatened wildlife and plants; 12-month finding on a petition to list the Pacific walrus as endangered or threatened, In: Service, U.S.F.a.W. (Ed.), Federal Register 76, pp. 7634–7679. [Google Scholar]
- Van Dolah FM, 2000. Marine algal toxins: Origins, health effects, and their increased occurrence. Environmental Health Perspectives 108, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekell JC, Gauglitz EJ, Barnett HJ, Hatfield CL, Eklund M, 1994. The Occurrence of Domoic Acid in Razor Clams (Siliqua-Patula), Dungeness Crab (Cancer-Magister), and Anchovies (Engraulis-Mordax). Journal of Shellfish Research 13(2), 587–593. [Google Scholar]
- White AW, 1986. High toxin content in the dinoflagellate Gonyaulax excavata in nature. Toxicon 24(6), 605–610. [DOI] [PubMed] [Google Scholar]
- Wohlgeschaffen GD, Mann KH, Subba Rao DV, Pocklington R, 1992. Dynamics of the phycotoxin domoic acid: Accumulation and excretion in two commercially important bivalves. Journal of Applied Phycology 4, 297–310. [Google Scholar]
- Zeh JE, Punt AE, 2005. Updated 1978–2001 abundance estimates and their correlations for the Bering-Chukchi-Beaufort Seas stock of bowhead whales. Journal of Cetacean Research Management 7(2), 169–175. [Google Scholar]


