Abstract
Fossil floras have been recovered from a unique deposit of early Permian age in North-Central Texas. The site, Kola Switch, preserves three distinct floras in different lithofacies, in a succession from a single outcrop. The sedimentary environment appears to be a floodplain channel fill of primarily siltstones and claystones. The lowermost flora, preserved in a kaolinitic siltstone, indicates active water flow. It is dominated by plants typical of well-drained substrates, dominated by Sphenopteris germanica, and contains no wetland elements. The middle flora is from a finely laminated carbonaceous claystone and is dominated by marattialean tree ferns, with no elements from habitats typical of seasonal moisture availability. It contains no roots and appears to have formed as a floating peat mat. The upper flora is a mixed assemblage of wetland taxa and those typical of well-drained soil environments or a seasonal rainfall regime. Unlike the two lower floras, it has a relatively even distribution of dominance and is the most diverse of the three assemblages. Palynofloras also were recovered from each of these beds. The palynofloras, although varying between and even within the beds, indicate a common background species pool during the time interval sampled, suggesting that these distinct floras reflect local changes in microhabitat conditions under a constant climatic background. The palynoflora from each bed has characteristics in common with the macroflora of that bed, but also distinct differences. Together, the macroflora and microflora provide an unusually broad picture of this site through time. Kola Switch compares favorably with the recently described flora from the nearby Sanzenbacher Ranch site of approximately the same age and also with floras of Rotliegend age from Central Europe.
Keywords: Texas, Wolfcampian, Archer city formation, Conifers, Sphenopteris germanica, Marattiales
Introduction
Our understanding of Permo-Carboniferous plants known from North-Central Texas is far from complete despite initial reports going back to the late ninteenth century. The pioneering efforts of David White (1862–1935), Charles B. Read (1907–1979), and Sergius H. Mamay (1921–2008) provided studies of novel taxa based largely upon their own field work in the region (e.g., White 1912; Read 1947; Mamay 1967, 1968, 1989, 1990; Mamay and Bateman 1991; Mamay et al. 2009). In their only published collaboration, Read and Mamay (1964) summarized the stratigraphic distribution of upper Paleozoic floras known from the conterminous United States. This influential contribution only hinted at the richness of the Texas record they knew first-hand from field work. With Mamay’s assistance, we started a multidisciplinary research program in the late 1980s to resample all the known plant-fossil deposits within the region. The goal was to obtain large samples that would form the basis of combined paleobotanical studies and paleoenvironmental analyses. This project built directly upon the efforts of White, Read, and Mamay, who likewise sought to answer questions beyond plant morphology and systematics.
We here report a series of three early Wolfcampian-age (299–280 Ma) floras from Kola Switch in southeastern Clay County, Texas, and compare these with the results of a similar analysis of collections from the nearby Sanzenbacher Ranch (DiMichele et al. 2018). These floras are from the same lithostratigraphic level and separated by only 8.6 km (Fig. 1). Although the plant assemblages appear to be drawn from the same species pool, they differ quantitatively, as do their sedimentological contexts. In combination, the Kola Switch and Sanzenbacher fossil deposits provide a measure of how variable megafloral and palynological records can be within an alluvial floodplain setting. Previous papers (DiMichele et al. 2005, 2006; Tabor et al. 2013; Looy and Hotton 2014) have outlined these variations but have not afforded a format to document the paleobotanical findings satisfactorily.
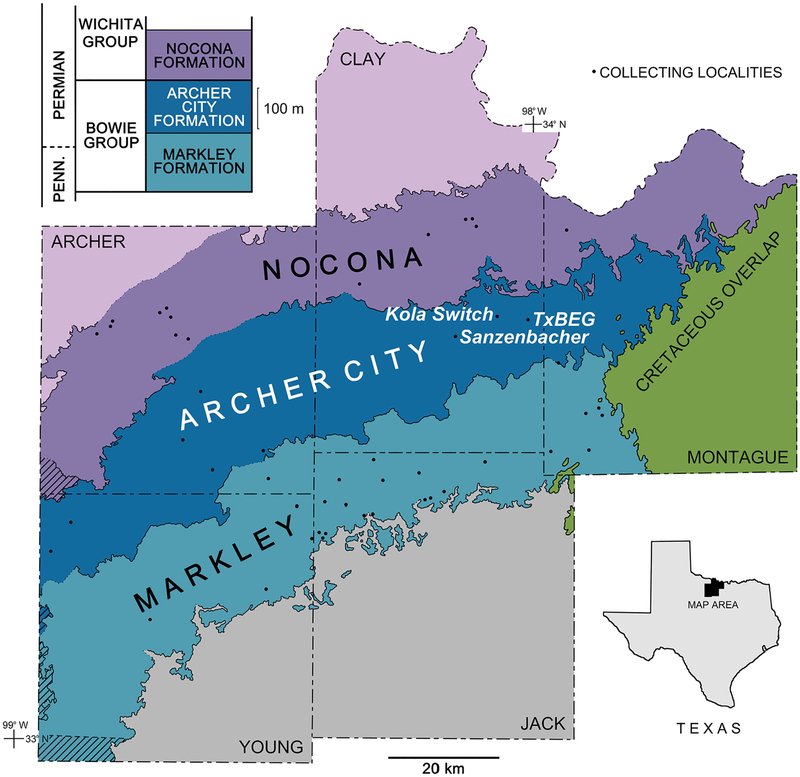
Fig. 1.
Generalized bedrock geology of Clay County and adjacent areas, North-Central Texas, with the plant localities of USNM/USGS Kola Switch and Sanzenbacher Ranch and that found by T.F. Hentz (labeled TxBEG) shown. Average formation thicknesses in stratigraphic column based on estimations of Hentz and Brown (1987). Formation contacts dotted in areas of surficial cover. Plant-collecting localities represented by US Geological Survey, the National Museum of Natural History, or the University of Texas collections represented by large dots. Marine-influenced rocks of the Albany and Cisco groups are marked by diagonal lines; these are coeval with continental rocks of the Wichita and Bowie groups, respectively. Inset shows map location within Texas. Geological mapping adapted from Hentz and Brown (1987), McGowen et al. (1991), and Brown and Goodson (1972). This is substantially the same map as that of DiMichele et al. (2018, fig. 1), with the addition of Kola Switch and TxBEG
In addition, we discuss the significance of our results in the context of a global understanding of Permo-Carboniferous floras, a theme to which Hans Kerp has contributed with distinction. The North-Central Texas floras, particularly those from the lower portions of the Permian section, such as that described herein, bear direct comparison with floras from strata of Lower Rotliegend age in Central Europe (e.g., Barthel 1976, 2009, 2016; Kerp 1982, 1984, 1996; Kerp and Fichter 1985; Kerp and Haubold 1988; Kerp et al. 1990, 2007; Barthel et al. 2010). The European floras come primarily from stratigraphic sequences lacking marine fossils. In contrast, the Texas sections can be correlated, to a variable degree, with international Permian standards, suggesting an Asselian age (299–295 Ma) for the Kola Switch and Sanzenbacher host strata.
Collections
The name Kola Switch has never appeared on government-issued topographic maps for Clay County, but the name Kola does appear on a 1924 Texas General Land Office map of the county. Kola was located at the junction of the Fort Worth and Denver Railroad (now part of the Burlington Northern Santa Fe system) and a local ranch road approximately 6 km northwest of Bellevue. That Kola was known locally as Kola Switch suggests that a railroad switch and related siding were once there to serve the cattle trade, agricultural interests, and the oil and gas industry. The actual collecting site is approximately 1 km east of Kola Switch in the Texas and New Orleans Railroad Survey section 3 (A-861) (Fig. 1).
The earliest record of collecting in this specific area is that of Adolph H. Witte (1895–1983), who headed the Clay County Unit of the State-Wide Paleontological-Mineralogical Survey of the Work Projects Administration (or WPA; see DiMichele et al. 2018, for additional historical data and sources). In a field report dated January 1, 1941, Witte noted “various fragmentary bones” collected from the surface of the “Sidney Webb pasture” east of Kola Switch. Although he assigned WPA Clay County locality number 16 to the site, and a period map of the WPA collecting sites in the region is annotated with “Webb 16—Plants,” no specimens from this locality are known to have survived in the WPA collections of either the Vertebrate Paleontology or the Nonvertebrate Paleontology Laboratories at The University of Texas at Austin. It seems certain, however, that Witte found significant plant fossils on the property in the early 1940s because on Sunday, March 12, 1961, he took Mamay and his field assistant Arthur Watt to a locality that Mamay referred to as Kola Switch in Mamay’s notes. The site yielded exceptionally well-preserved plants from two separate, lithologically distinct beds (USGS 9998A and C). In addition, although not recorded in his field notes, the USNM holds a second collection made by Mamay, according to a note in the collection drawers, 1.6 km (1 mile) to the east of the main Kola Switch collecting site (USGS 9998B). All of these collections originally were given the same USGS locality number (USGS 9998).
Nearly 30 years later, on Saturday, April 21, 1990, Mamay led a field party consisting of Kenneth W. Craddock, William A. DiMichele, Robert W. Hook, Nicholas Hotton, III, and Louis Todd to Kola Switch. Three separate macrofossil collections were made from distinct fossil-bearing lithologies (USNM 40069, 40070, and 40071) during that visit (Fig. 2). As detailed below, the macrofloras of these lithologies differ significantly. In addition to macrofloras, macerations revealed the presence of common to abundant palynomorphs in all three lithologies and particularly well-preserved material in two of the three beds. We have since concluded, based on the lithological characteristics of the different beds, that USGS 9998A and B are from the same stratigraphic horizon as USNM 40069, although, as noted above, USGS 9998B is from a different site than USNM 40069; USGS 9998C is from the same horizon as USNM 40070. Mamay and his 1961 party did not collect from the horizon of USNM 40071.
Fig. 2.
Kola Switch outcrop. Plant collections were made from mudrocks exposed in three vertically successive layers, numbered 1–3 (lower, middle, and upper beds). Sandstone blocks have rolled downhill from the ridgetop, Archer City Formation informal sandstone member 8 of Hentz and Brown (1987). Personnel, l–r: Nicholas Hotton, Sergius Mamay, Louis Todd, Kenneth Craddock
An additional plant-bearing deposit was found in 1984 by Tucker F. Hentz (Bureau of Economic Geology, The University of Texas at Austin) approximately 5 km east of, and along strike with, the original Kola Switch collecting site (Fig. 1). Hentz brought this discovery to the attention of Theodore Delevoryas (Department of Botany, The University of Texas at Austin), who subsequently obtained a collection that, decades later, was given to the Division of Paleobotany, Biodiversity Institute of the University of Kansas (KU). Although time constraints have prevented us from studying this material, information provided by Hentz and KU paleobotany collection manager Rudolph Serbet lead us to conclude that the geology and plants of this deposit are very similar to those of Kola Switch USGS 9998A and B, and USNM 40069. The significance of this additional record is considered below.
Geology
General geology
The bedrocks of Clay County consist of the Permo-Carboniferous Bowie Group and the Permian Wichita Group (Fig. 1). These mudrock-dominated, entirely continental units are exposed poorly in a low-relief landscape of prairies and grasslands. Thus, the current lithostratigraphy of the region is based mainly upon interpretation of stereoscopic aerial photographs (Hentz 1988, 1989; Hentz and Brown 1987). Although some of the existing lithostratigraphic divisions have been regarded as unacceptable on a regional scale (Lucas 2006), the mapping of Hentz and Brown (1987) has been found to be sound on the outcrop scale and also indispensable during our many years of field work in the region. The intraformational divisions mapped by Hentz and Brown (1987) in Clay County are alluvial sandstone bodies up to approximately 15 m in thickness that usually cap low ridges. These units, termed “informal sandstone members” by Hentz and Brown, allow for lithostratigraphic correlations between areas of discontinuous exposure, which is the case between Kola Switch and Sanzenbacher.
Both Kola Switch and Sanzenbacher occur below informal sandstone member 8 of the Archer City Formation, the upper formation of two that comprise the Bowie Group (Fig. 1). Whereas the underlying Markley Formation has a number of organic-rich intervals and even once-mineable coals, such deposits do not occur in the Archer City Formation. At Sanzenbacher, plants occur in a coarsening-upward floodplain pond or lake deposit that consists mainly of silty claystones and clayey siltstones. In contrast, the geological section at Kola Switch (Fig. 3) superficially resembles a condensed version of certain organic-rich Markley Formation deposits that have been broadly interpreted as floodplain facies and that hosted floras within both well-drained and swampy habitats (Tabor et al. 2013).
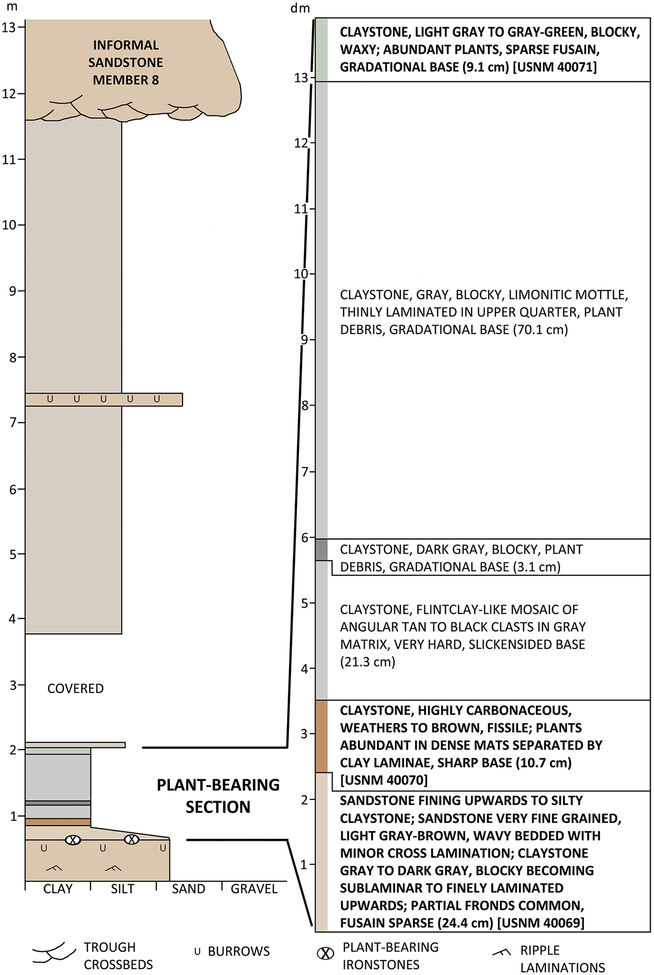
Fig. 3.
Stratigraphic section at Kola Switch. Left column is overall section in meters. Right column expanded section of the approximately 1.4-m-thick plant-bearing interval, graduated in decimeters
The fossiliferous interval
The plant-bearing interval at Kola Switch measures nearly 1.4 m in thickness (Fig. 3). Plants occur throughout most of this section, but identifiable and abundant remains are limited to two beds at the bottom and one at the top (Table 1). The fossiliferous interval is underlain by a very fine-grained, ripple-bedded and burrowed sandstone. Iron-stone nodules containing well-preserved plant foliage, identical to that of the lower bed, occur in the uppermost part of the basal sandstone.
Table 1.
Macrofossil raw quadrat counts
| USNM 40071 | USNM 40070 | USGS 9998C | USNM 40069 | USGS 9998A | USGS 9998B | |
|---|---|---|---|---|---|---|
| Informative quadrats | 90 | 125 | 67 | 182 | 633 | 24 |
| Barren quadrats | 13 | 0 | 1 | 29 | 101 | 2 |
| Sampling level | Top bed | Middle bed | Middle bed | Lower bed | Lower bed | Lower bed |
| Aphlebia erdmannii | 33 | 16 | ||||
| Lepidophylloides spp. | 2 | |||||
| Calamitalean stems | 15 | 2 | 3 | |||
| Asterophylites sp. | 2 | |||||
| Annularia carinata | 26 | |||||
| Calamostachys sp. | 1 | |||||
| Palaeostachya sp. | 1 | |||||
| Sphenophyllum miravallis | 3 | 7 | ||||
| Sphenophyllum angustifolium | 1 | |||||
| Sphenophyllum cf. thonii | 32 | |||||
| Sphenophyllum reproductive organ | 1 | |||||
| Sphenopteris small fern | 1 | 1 | 1 | |||
| Pecopteris cf. jongmansii | 18 | 98 | 49 | |||
| Pecopteris cf. cyathea | 7 | |||||
| Marattialean: lobatopterid 1 | 1 | 10 | ||||
| Marattialean: lobatopterid 2 | 2 | |||||
| Marattialean: polymorphopterid | 26 | 7 | ||||
| Marattialean: large, Ad-obscure | 4 | |||||
| Marattialean: large, sparse veins | 1 | |||||
| Marattialean: Asterotheca | 1 | 13 | ||||
| Diplazites unitus | 3 | |||||
| Indeterminate neuropterid | 2 | 1 | ||||
| Neuropteris cf. ovata | 1 | |||||
| cf. Neurodontopteris auriculata | 1 | 1 | ||||
| Odontopteris cf. readii | 12 | 37 | ||||
| Odontopteris subcrenulata | 69 | 4 | ||||
| Reticulopteris sp. | 1 | |||||
| Autunia conferta | 4 | 5 | 49 | 1 | ||
| Rhachiphyllum sp. | 1 | 2 | ||||
| Autunia naumannii | 1 | 3 | 2 | |||
| Arnhardtia scheibeii | 1 | |||||
| Autunia repro organ | 1 | 2 | ||||
| cf. Peltaspermum sp. | 3 | |||||
| Cordaites sp. | 3 | 3 | ||||
| Walchia sp. | 6 | |||||
| Walchia piniformis | 12 | 34 | 1 | |||
| Walchia cf. schneideri | 35 | 136 | 2 | |||
| Sphenopteris germanica | 9 | 151 | 473 | 19 | ||
| S. germanica repro organs | 3 | 1 | ||||
| Charliea or Yuania | 1 | 5 | ||||
| UN foliage cf. odontopterid | 1 | 1 | ||||
| Seeds | 9 | 1 | 7 | 49 | 1 | |
| Axes | 34 | 98 | 56 | 50 | 165 | 5 |
| Roots | 1 | |||||
| Comminuted plant debris | 1 | 28 | ||||
| Charcoal | 3 | 3 | 11 | |||
| Conchostracans | 1 | 4 | ||||
| Shark coprolite | 1 |
USNM and USGS collections
The lowermost collecting interval (USNM 40069, USGS 9998A) is an approximately 25-cm-thick, fining-upward transition from a wavy-bedded, very fine-grained sandstone to a finely laminated silty claystone. Plant remains are common and include partial fronds. Some foliage is draped or folded in small-scale recumbent beds. The contact between the top of collecting-interval one and the base of collecting-interval two is sharp. The lithological character of USGS 9998B is identical to the USNM and USGS collections made from collecting interval 1, although its broader geological context is not known.
The approximately 11-cm-thick middle bed, collecting interval 2 (USNM 40070, USGS 9998C), is marked by an abrupt change to very dark gray to brown carbonaceous laminae, an absence of silt, and paper-like fissility. Plants in the second interval occur in dense carbonized mats separated by clay laminae. As in the underlying plant bed and basal sandstone, no roots or evidence of rooting are present.
The bed immediately overlying the second collecting interval is an approximately 0.2-m-thick claystone that is devoid of megascopic plant remains and exceedingly hard. Comprising a mosaic of angular, buff to dark gray to black “clasts” in a siliceous gray matrix, this bed very closely resembles a flint clay, a kaolinite-rich rock type that occurs in close association with coals and freshwater carbonates in the Appalachian coalfields. Because the overlying two claystone intervals of approximately 0.7 m in total thickness yielded only sparse and indeterminate plant remains, they were not sampled for palynological analysis. The uppermost or third collecting interval (USNM 40071) is a light gray to gray-green, blocky and waxy but firm claystone, approximately 9 cm thick. Plants are abundant, well preserved, and accompanied by sparse fusain.
The plant-bearing interval is truncated by a siltstone that is overlain by a mostly poorly exposed mudstone section of approximately 7.5 m. Much of this mudstone interval has weakly developed pedogenic features that are common in mudstones of the Archer City Formation (see Tabor and Montañez 2004), but it was not excavated for detailed description. The uppermost bed at Kola Switch is the erosive-based informal sandstone member 8, a major alluvial channel.
Methods
Macrofossils
Both USGS and USNM macrofossil collections were made without conscious collection bias: all specimens that contained identifiable plant remains were collected. Collections from the three USNM collecting intervals were kept separate at the time the collections were made. The entire USGS collection appears to have been made, however, without distinguishing the beds as subcollections. However, the significant difference in the matrices and fossil content of the USNM collections from collecting intervals 1 and 2 permitted us to make a similar division of the USGS materials.
Identifications were made with a hand lens and, where necessary, a compound dissecting microscope. Specimen photographs were taken under natural light and, if needed, enhanced in Photoshop to increase brightness and contrast. Macrofossil collections were quantified using the method of Pfefferkorn et al. (1975), modified as explained by Bashforth and Nelson (2015). In this method, each hand-specimen surface is counted as a sampling quadrat. Barren surfaces are noted. Counterparts are tabulated only once. All taxa or objects (e.g., axes) are noted on each quadrat and counted only once, regardless of how many individual specimens are present. The result, therefore, is a frequency of occurrence within the full suite of quadrats sampled. Final tabulation of percentage occurrence does not take into account barren surfaces.
Palynology
Palynological samples were taken from the matrix of one hand specimen from the USNM 40069 collection and two hand specimens of the USNM 40071 collection. Matrix collected specifically for palynological analysis was used for USNM 40070. Approximately 10 g of matrix per sample was processed at the Laboratory of Palynology and Palaeobotany, Utrecht University, using standard techniques (digestion using hydrofluoric and hydrochloric acid, heavy liquid separation, no oxidation, and sieving over 15-micron mesh). One sample (USNM 40070) was stained with Bismarck Brown in an attempt to darken exines. The yield was good for all four samples, but only sample USNM 40069 contained well-preserved palynomorphs. All residues were strew-mounted in glycerine jelly, and a 300-grain count was made for each sample. Additional slides were scanned for rare taxa, to a total of approximately 2000 grains for each sample. Specimens were observed with a Nikon Eclipse 80i microscope and imaged with a DXMF 1200 camera through a Plan Apo objective using Differential Interference Contrast or a Leica DM2500 microscope and Nikon DS-Fi1 Digital Camera. Extended Depth of Field micrographs were created using Photoshop. Position of imaged and referenced grains was indicated with the slide number and England Finder reticule coordinates. Illustrated specimens are stored in the Paleobotanical Type and Illustrated Collections under the USNM catalog numbers 723079–723100.
The variable preservation of the palynomorph samples hindered precise identification, especially at the species level, so many were left at the generic level or treated as “cf.” Distinguishing species of Potonieisporites was particularly difficult. One of the assemblages (USNM 40069) contained specimens of Potonieisporites displaying wide diversity in size, shape of the central body and saccus, length and shape of the aperture and apertural thickenings, folds around the central body and other traits. Although many of these features have been used to circumscribe species, they are likely to be caused, at least in part, by developmental variability and taphonomy. In the absence of a detailed morphometric analysis, we refrain from recognizing distinct species of Potonieisporites and instead treat them as a complex.
Results
Macrofloras
The floras collected from the Kola Switch outcrop are summarized in Table 1. These include three separate USNM collections and two USGS collections from the main Kola Switch site. We include the small collection from the additional site discovered by Mamay in 1961, which appears to be from the same lithology as that enclosing the lower flora at the main Kola Switch site.
Lower flora, collecting interval 1
A comparison of the three collections made from the lower bed at or in the vicinity of Kola Switch is summarized in Fig. 4a. These are minimum diversity estimates. Reproductive organs, for example, have been removed from the compilation if vegetative remains attributable to the same plant or plant group also were present. Some categories of unidentifiable material, such as axes or comminuted plant debris also have been removed, and some plant categories have been combined (e.g., Odontopteris species, the distinction between which was uncertain). The macroflora is illustrated in Figs. 5, 6, 7, and 8, including representatives of as many of the identified elements as possible.
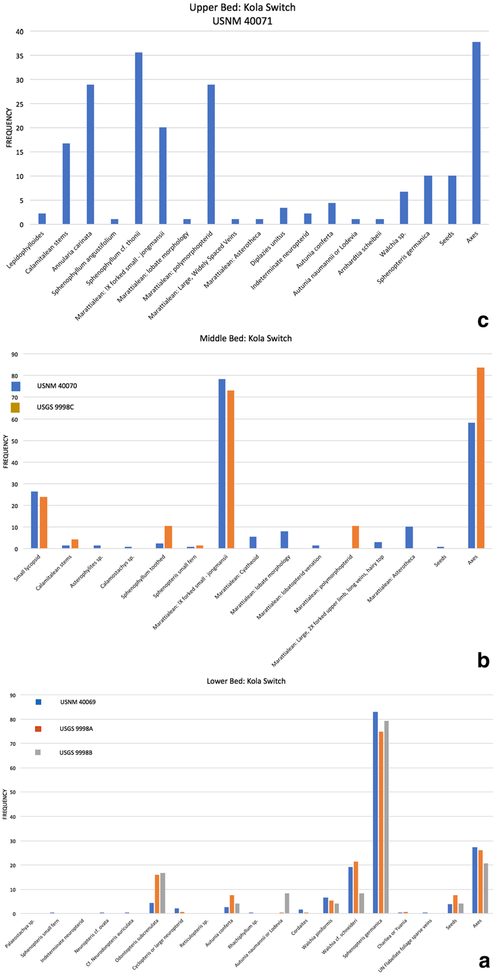
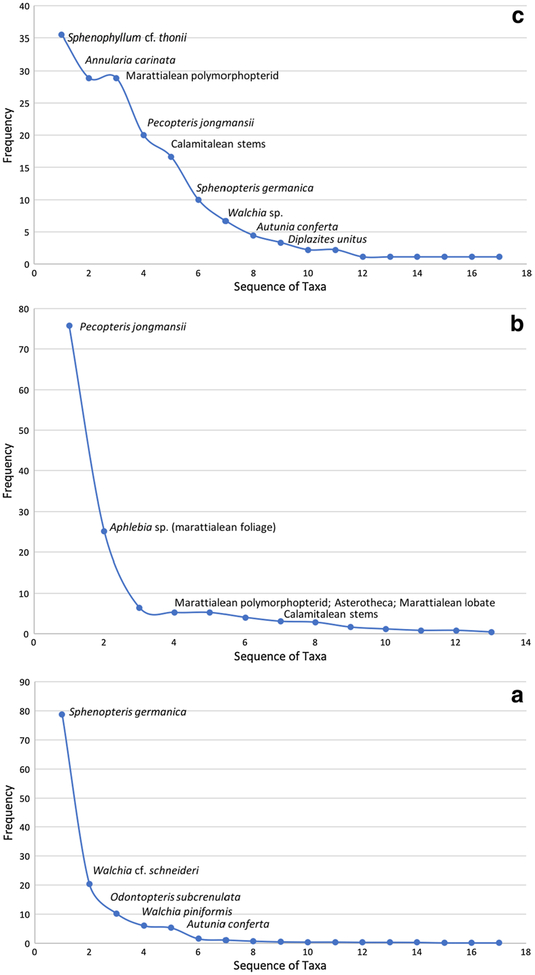
Fig. 4.
Quantitative taxonomic composition of collections from the three Kola Switch collecting levels. a Lower bed, two collections from principle Kola Switch outcrop (USNM 40069 and USGS 9998A), one (USGS 9998B) 1.6 km east. b Middle bed, two collections (USGS 9998C and USNM 40070). c Upper bed, one collection (USNM 40071)
Fig. 5.
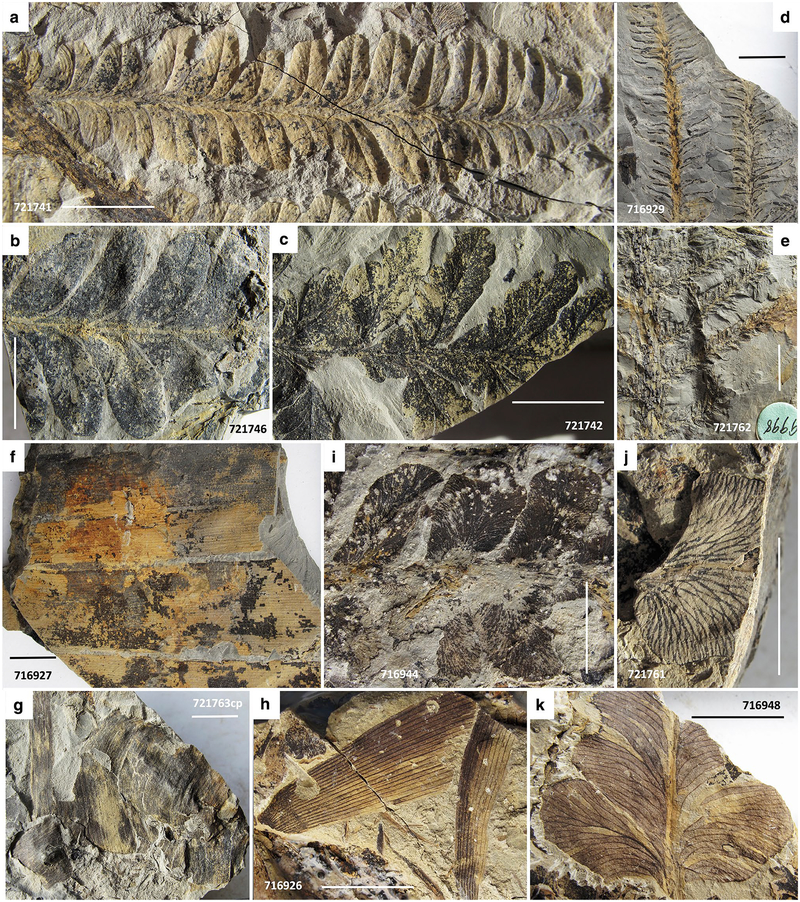
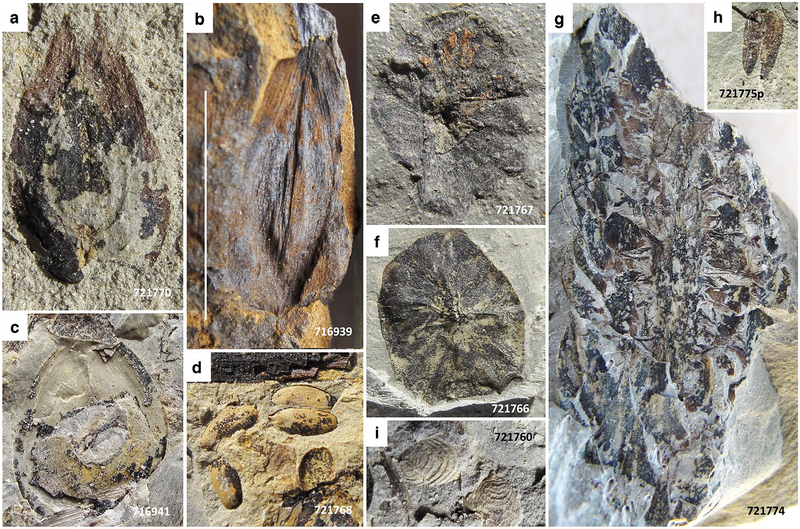
Macroflora of the lower bed. a–f Sphenopteris germanica. a Sterile foliage. USNM716921. b, c Main fork of axis with distinctive horizontal sclerotic plates. USNM716918, 716919. d, e Telangiopsis-type, H-branched (white arrows in d) reproductive axes similar to those illustrated by Mamay (1992) from Late Pennsylvanian age from Kinney Quarry, New Mexico, associated with similar vegetative foliage. USNM721773, 716925p. f Foliage angularly disposed to bedding. USNM716923. g Unidentified vegetative foliage, possibly of a small fern, or perhaps a callipterid. USNM721764. USNM specimen number also indicated on photograph. Scale bars 1 cm
Fig. 6.
Macroflora of the lower bed. a Autunia conferta. USNM721741. b Rhachiphyllum schenkii. USNM721746. c Probably Autunia naumannii. USNM721742. d Walchia cf. schneideri. USNM716929. e Walchia piniformis. USNM721762. f Cordaites sp. USNM716927. g Cf. Taeniopteris sp, ravaged by decay and transport. USNM721763cp. h Unidentified noeggerathialean foliage, possibly Yuania taeniata. USNM716926. i Neuropteris cf. ovata. USNM716944. j Reticulopteris sp. USNM721761. k Unidentified foliage of odontopterid aspect. USNM716948. USNM specimen number also indicated on photograph. Scale bars 1 cm
Fig. 7.
Macroflora of the lower bed. a, b Odontopteris subcrenulata. USNM713330, 721749. c–g Odontopteris similar to O. readii (Stull et al. 2013), from the Padgett site in Young County, TX, also below Sandstone 8 of the Archer City Formation. USNM721754, 721753, 721759, 721755, 716947. h Odontopteris schlotheimii with sparse ultimate venation. USNM721757. USNM specimen number also indicated on photograph. Scale bars 1 cm
Fig. 8.
Macroflora and invertebrates from the lower bed. a, b Platyspermic winged seeds. USNM721770, 716939. c Platyspermic seed with narrow compression border. USNM716941. d Radiospermic seeds. USNM721768. e, f Possible Peltaspermum, reproductive organs of callipterids; however, these are rather large and could be synangia of Remia, although no vegetative foliage of this plant was identified. USNM721767, 721766. g Unidentified strobilus suspected to be of conifer origin. USNM721774. h Valvate structure, suspected pollen organ. USNM721775p. i Conchostracans, a rarely encountered example. USNM721760. USNM specimen number also indicated on photograph. Scale bar in image b 1 cm, all specimens of same magnification
The USNM 40069 lower flora is well preserved and includes fragments of plant material over 15 cm in length. This flora is dominated by Sphenopteris germanica (Fig. 5a–f), which occurs at a frequency of 83% among the 182 informative, hand-sample surfaces. Much less abundant constituents include Walchia cf. schneideri (19.2%) (Fig. 6d), W. piniformis (6.6%) (Fig. 6e), Odontopteris subcrenulata (4.4%) (Fig. 7a, b), and Autunia conferta (2.7%) (Fig. 6a). These all consist of relatively large, well-preserved specimens, which suggests that the parent plants lived near the site of deposition. The remaining seven taxa are singletons with the exception of cordaitalean foliage (1.6%), which may be underrepresented because of the difficulty of separating fragmentary leaf remains from those of longitudinally striate axes. Only one of these singleton taxa is suspected to be a pteridophyte, a small fragment of tiny foliage that may be of filicalean affinity (Fig. 5g). Fusain is present on a small number of hand samples (1.6%), and there are no indicators of brackish to marine conditions, which, as a rule, are not found in Clay County. A non-spinescent myriapod whose otherwise poor preservation does not allow identification was found in this bed.
The USGS lower flora, USGS 9998A, is similar to that collected by the USNM, but is three and a half times larger at 633 informative surfaces. Just like in the USNM collection, the most abundant taxon is Sphenopteris germanica (74.7% frequency of occurrence). Walchia cf. schneideri is the second most frequently occurring taxon (21.5%). There is some shifting of those taxa that occur at lower abundances compared with their order of importance in the USNM collection, but the same three taxa make up this group. Odontopteris subcrenulata and O. osmundaeformis, which may be the same taxon in the collections, have a combined frequency of 16%, thus switching position with both Autunia conferta (7.7%) and Walchia piniformis (5.4%). No other elements, as in the USNM flora with the exception of Cord-aites (Fig. 6f), occur at > 1%, and there is a notable paucity of plants thought to have a propensity to occupy wet substrates or humid microenvironments—no small ferns and only one tentatively identified calamitalean strobilus. Fusain is present at 1.7%, about the same frequency as in the USNM collections, a most interesting result given the much larger size of the USGS collection. Conchostracans occur on four hand samples (0.6%).
The USGS flora collected by Mamay and Watt 1 mile east of Kola Switch, is virtually identical floristically to the larger USNM and USGS collections. Consisting of only 24 informative surfaces, the recovered flora is, naturally, less diverse than that of the other sites. Sphenopteris germanica occurs at a frequency of 79.2%. There is some variation in frequency of less common taxa, all but one of which is found in the other two collections. These include, in order of abundance, Odontopteris subcrenulata (16.7%), Walchia cf. schneideri (8.3%), cf. Autunia naumannii (8.3%), Autunia conferta (8.2%), and Walchia piniformis (4.2%). There are no other plants in the assemblage.
Middle flora, collecting interval 2
Marattialean ferns dominate both the USNM and USGS collections from collecting interval 2; a comparison between these collections is shown in Fig. 4b. Elements of the macro-flora are illustrated in Figs. 9 and 10. The USNM collection consists of 125 informative hand-sample surfaces. A considerable number of specimens was excavated and examined in the field and, because it revealed such low diversity, a relatively small representative collection was considered then to be an adequate representation of the flora. One species of marattialean fern foliage, tentatively identified as Pecopteris jongmansii, dominates the flora (78.4% frequency of occur-rence) (Fig. 4b). Four other morphological forms of marattialean foliage also are found in this collection. Three of the forms may simply be parts of P. jongmansii and reflect intrafrond variability: a Pecopteris cyathea-like form; a form with lobatopterid venation; and a third consisting of tiny pinnules that lack clear midveins, seemingly representative of a part of a marattialean frond with highly lobate pinnae/pinnules. The fourth form, which has large pinnules with scales or hairs on the adaxial surface, appears distinct but is represented only by a single specimen. Fertile marattialean specimens, Asterotheca (10.4%), also are present (Fig. 10a, b). Second in importance are flabellate structures with broad central surfaces and a finger-like fringe, further edged with small projections (Fig. 10c). The surfaces are covered with small angular raised areas that are, at a glance, close to helically arranged but that, on closer observation, are clearly not in a regular pattern. We interpret these remains as aphlebiae associated with marattialean fern fronds and assign them to Aphlebia erdmannii. They occur at a frequency of 26.4%. Together, the foliage and aphlebiae comprise extreme marattialean fern dominance of the macrofossil flora. Other elements of the flora include small numbers of calamitaleans (Calamites stems, Asterophyllites foliage, and a Calamostachys cone) (Fig. 10d–f), isolated leaves of Sphenophyllum sp. (Fig. 9c), possibly S. miravallis, and a single specimen of possible small fern foliage. No foliage of pteridosperms was recorded, and seeds (Fig. 10g) occur at < 1% frequency. Importantly, for interpretation of the nature of this deposit, no roots were noted among the plant remains, despite the fact that roots are a major element of marattialean fern stem architecture. Fusain and animal remains are absent.
Fig. 9.
Macroflora of the middle bed, marattialean ferns (“Pecopteris” cf. jongmansii) and Sphenophyllum cf. miravallis. a Marattialean pinna fragment showing transition from large pinnules with complex, candelabra-form venation near apex, transitioning to lobed margins and nearly free pinnules nearer base. USNM713340. b Marattialean pinna fragment showing rapid transition from large pinnules apically to lobed pinnules to nearly free pinnules basally. USNM713343. c Marattialean pinnae with small, candelabra-veined pinnules. S, at arrow = Sphenophyllum cf. miravallis leaf. USNM721733. d, e Marattialean pinnules from different parts of the same pinna. d From pinna base, e from pinna apical area; note difference in degree of lobation, but similarity in venation. USNM721737. f–h Series of pinnules of decreasing size with once to twice forked lateral veins, likely from parts of the P. jongmansii frond. USNM721732, 713349, 713359. i Sphenophyllum stem. USNM721736. USNM specimen number also indicated on photograph. Scale bars 1 cm, Scale of f and e the same as adjacent images
Fig. 10.
Macroflora of the middle bed. a, b Fertile marattialean pinna fragments, cf. Asterotheca. USNM713353, 713352. c Aphlebia erdmannii marattialean fern foliage. USNM713346. d Asterophyllites equisetiformis. USNM716947. e Calamitalean stem. USNM713362. f Calamitalean strobilus, probably Calamostachys, given evidence of sporangiophore attachment scars between whorls of sterile bracts. USNM713356. g Platyspermic, winged seed. USNM713357. USNM specimen number also indicated on photograph. Scale bars 1 cm
The USGS organic shale collection consists of 67 informative surfaces, thus about one-half the size of the NMNH collection, and is compositionally very similar. Marattialean fern foliage of the Pecopteris jongmansii type dominates the assemblage (73.1%). Small numbers of other marattialean morphotypes also are present, but we suspect these simply to be forms of the dominant tree-fern foliage, which reflects the complex intra-frond variability of lobate forms. Remains of marattialean aphlebiae, specifically Aphlebia erdmannii, are second in importance (23.9%). These dominant elements occur in approximately the same proportions as they do in the USNM collection. Less abundant but paralleling their occurrence in the NMNH collection are isolated leaves of Sphenophyllum sp.; this wedge-shaped form has rounded apical margins lined with blunt teeth and is similar to S. miravallis. As with the NMNH collection, the USGS samples also contain small amounts of Sphenopteris ferns (1.5%, twice that of the NMNH collection) and calamitalean remains (stems only, 4.5%) at higher frequency than in the NMNH collections. However, like the NMNH collections, no pteridosperm remains, roots, or fusain were noted. Both the USGS and USNM collections have an abundance of striate axes, which are most likely petioles or rachises of marattialean fronds.
Upper flora, collecting interval 3
Of the three Kola Switch floras, the uppermost contains the highest diversity with 14 forms based on a conservative estimate of whole-plant assemblages (Fig. 4c; elements of the flora are illustrated in Figs. 11, 12, 13). The most commonly encountered remains (Fig. 11a–e) are those of the sphenopsids. The most abundant are those of Sphenophyllum cf. thonii (35.6%), a species that, like many other sphenophyll species known from compression, likely had a scrambling to thicket-forming growth habit, including facultative climbing (Batenburg 1981; Bashforth and Zodrow 2007). Calamitaleans also are common (Annularia carinata, 26.6%; Calamites stems, 16.7%). These are followed in abundance by remains of marattialean ferns (Fig. 11f–j), the most prominent being a form with polymorphopterid venation (28.9%) and Pecopteris jongmansii (20%). Less frequent are a number of distinct forms, including Diplazites unitus (Fig. 12j), a morphotype with large pinnules and widely spaced veins, and the fertile form Asterotheca (Fig. 11f–g). Other typical wetland elements include lycopsid leaves and indeterminate neuropteroid pinnule fragments (each 2.2%). The remainder of the flora consists of taxa more typically found in seasonally moisture-deficient habitats (Fig. 12b–g), including the callipterids Autunia conferta (4.4%), and single specimens of Autunia naumanii and cf. Arnhardtia scheibei (1.1% each), the conifer Walchia sp. (6.7%), and the probable seed fern Sphenopteris germanica (10%). Reproductive organs, particularly seeds (Fig. 13), are rare. Given that seeds are adapted for dispersal, and that such seeds as were found are primarily winged forms, hence wind-dispersed, their paucity seems noteworthy in an otherwise allochthonous fossil assemblage. The upper plant-fossil-bearing bed contains conchostracans. In addition, three hand samples with fusain were recovered.
Fig. 11.
Macroflora of the upper bed. a Annularia carinata. USNM538995. b Calamitalean stem. USNM713312. c Calamitalean stem preserved as charcoal. USNM713313. d, e Sphenophyllum thonii. USNM713315, 713314. f Fertile marattialean foliage, cf. Asterotheca. USNM713326. g Marattialean foliage of uncertain affinity, possibly part of the “Pecopteris” jongmansii lobate pinnule series. USNM713331. h–j “Pecopteris” jongmansii lobate pinnule series, i and j showing transition from lobed to unlobed pinnae. USNM538997, 713332, 713335. USNM specimen number also indicated on photograph. Scale bars 1 cm
Fig. 12.
Macroflora of the upper bed. a Diplazites unitus, marattialean fern foliage. USNM713330. b, c Callipterid Autunia conferta. USNM713337. d Callipterid Rhachiphyllum schenkii. USNM538996. e Callipterid Arnhardtia scheibei. USNM713338. f Pteridosperm Sphenopteris germanica. USNM713319. g Conifer Walchia cf. schneideri. USNM713322. USNM specimen number also indicated on photograph. Scale bars 1 cm
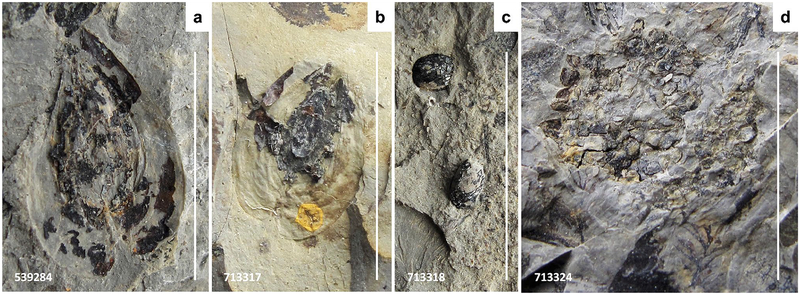
Fig. 13.
Macroflora of the upper bed. a, b Platyspermic winged seeds. USNM539284, 713317. c Radiospermic seeds. USNM713318. d Megaspore mass of uncertain affinity, probably representing a megasporangium. USNM713324. USNM specimen number also indicated on photograph. Scale bars 1 cm
Palynofloras
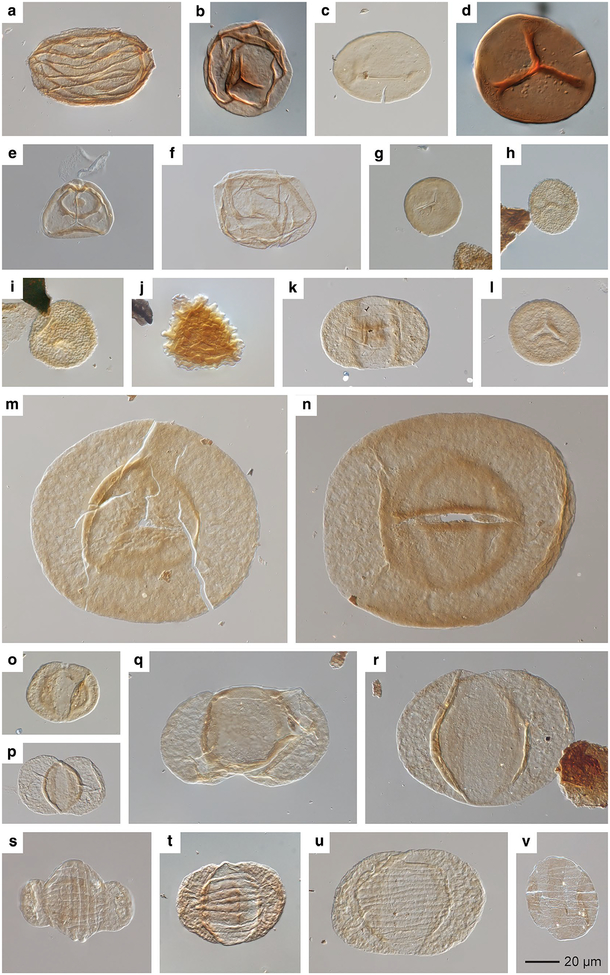
Palynomorph quantitative data are presented in Table 2. All three Kola Switch palynomorph assemblages (Fig. 14) are typical of other early Permian Texas palynofloras in the abundance of Potonieisporites sp. (Fig. 14n) and cf. Nuskoisporites sp. (Fig. 14m), as well as Pseudovesicaspora splendens and Vesicaspora spp. (Fig. 14o), and the absence or rarity of typical Late Pennsylvanian wetland taxa (Looy and Hotton 2014). Bisaccate genera, such as Pityosporites communis (Fig. 14q) and Alisporites plicatus (Fig. 14r), are present at moderate abundance. Characteristic Permian taeniate pollen, such as Hamiapollenites spp. (Fig. 14s, t), Striatoabeites richteri (Fig. 14u), and Vittatina lata (Fig. 14v), are present but rare (1–2%). A few Pennsylvanian holdovers are present, such as Convolutispora florida (Fig. 14j) and Illinites unicus (Fig. 14k). Rarely, certain Pennsylvanian forms are present in great abundance, such as the sphenophyll spore Columinisporites ovalis (Fig. 14a) and Laevigatosporites spp. (Fig. 14c). Other sphenopsids, such as Calamospora breviradiata (Fig. 14b), are very rare. Lycopsids are represented by a single taxon, Cadiospora magna (Fig. 14d). Taxa of uncertain affinity are also sporadically present, sometimes in abundance (see below).
Table 2.
Raw palynomorph counts
| USNM 40069-1 | USNM40070-BB-7 | USNM40071-24CP1-2 | USNM40071-41-1 | |
|---|---|---|---|---|
| Lycopodiopsida | ||||
| Cadiospora magna | 14 | 1 | 9 | 2 |
| Equisetidae | ||||
| Calamitales | ||||
| Calamospora breviradiata | 1 | 1 | 1 | |
| Sphenophyllales | ||||
| Laevigatosporites minor | 4 | 22 | ||
| Laevigatosporites medius | 6 | |||
| Columinisporites ovalis | 104 | 7 | 16 | 148 |
| Marattiidae | ||||
| Cyclogranisporites aureus | 15 | |||
| Cyclogranisporites minutus | 12 | |||
| Cyclogranisporites sp. cf. C. orbicularis | 1 | 1 | ||
| Laevigatosporites globosus | 1 | |||
| Punctatisporites aerarius | 2 | 1 | 1 | |
| Punctatisporites/Punctatosporites minutus complex | 2 | 24 | 12 | |
| Punctatisporites obliquus | 9 | |||
| Punctatisporites sp. | 4 | |||
| Thymospora sp. | 5 | |||
| Polypodiidae | ||||
| Convolutispora florida | * | |||
| Triquitrites sp. | 1 | |||
| Spores incertae sedis | ||||
| Dictyotriletes sp. 1 | 2 | 9 | ||
| Knoxisporites sp. cf. K. ruhlandii | 80 | |||
| Seed plants | ||||
| Cordaitales | ||||
| Florinites mediapudens | 1 | |||
| Florinites sp. | 1 | 4 | ||
| Pinidae | ||||
| Potonieisporites species complex | 65 | 25 | 33 | 27 |
| cf. Nuskoisporites | 6 | |||
| “Seed ferns” | ||||
| Paravesicaspora splendens | 8 | 1 | ||
| Schopfipollenites ellipsoides | * | |||
| Vesicaspora sp. cf. V. wilsonii | 17 | 66 | 40 | 45 |
| Vesicaspora sp. ‘rotundus’ | 3 | |||
| Wilsonites sp. | 6 | |||
| Seed plants incertae sedis | ||||
| Colatisporites decorus | 25 | 4 | 136 | 21 |
| Costatascyclus crenatus | 1 | |||
| Illinites unicus | 18 | 3 | 1 | 2 |
| Non-taeniate saccates | ||||
| Alisporites plicatus | 10 | 2 | ||
| Klausipollenites staplinii | 1 | |||
| Platysaccus sp. cf. P. saarensis | 40 | 2 | ||
| Pityosporites communis | 2 | |||
| ISahnisporites | 10 | |||
| Tinnulisporites sp. cf. T. microsaccus | 1 | |||
| Taeniates | ||||
| Hamiapollenites sp. cf. H. bullaeformis | 1 | 2 | ||
| Hamiapollenites perisporites | 1 | 4 | ||
| Stiatoabieites richteri | 6 | 5 | 1 | |
| Vittatina lata | 1 | |||
| Vittatina indet. | 1 | |||
| Incertae sedis (?spore) | ||||
| Leioaletes sp. | 35 | |||
| Total number of specimens | 300 | 300 | 300 | 300 |
| Botryococcus | Present | |||
| Total number of species | 20 | 22 | 16 | 17 |
Raw counts from each sample
Indicates taxa present in sample but not in 300-grain count. Botryococcus was not included in count, but was marked as present
Fig. 14.
Selected palynomorphs from the Kola Switch locality, Archer City Formation, north-central Texas. Specimen names are followed by USNM and slide numbers and England Finder (EF) locator coordinates. a Columinisporites ovalis, USNM40069–1, EF: R56/1. b Calamospora breviradiata, USNM40069–2, EF: J41/3. c Laevigatosporites minor, USNM40069–1, EF: F61. d Cadiospora magna, USNM40069–2, EF: J40/1. e Knoxisporites cf. ruhlandii, USNM40070-BB7, EF: R44/2. f Leioaletes sp., USNM40070-BB7, EF: J45. g Punctatisporites minutus, USNM40070-BB1, EF: W44/4. h Cyclogranisporites minutus, USNM40070-BB1, EF: R51/4. i Dictyotriletes sp., USNM40069–1, EF: R59. j Convolutispora florida, USNM40069–1, EF: Q51. k Illinites unicus, USNM40069–1, EF: M62/4. l Colatisporites decorus, USNM40071–24CP1–2, EF: R45/1. m cf. Nuskoisporites sp., USNM40069–1, EF: K26/2. n Potonieisporites sp., USNM40069–1, EF: W66. o Vesicaspora wilsonii, USNM40069–1, EF: L65/2/4. p Platysaccus sp. cf. P. saarensis, USNM40071–24CP1–2, EF: O34. q Pityosporites communis, USNM40069–1, EF: F52/1. r Alisporites plicatus, USNM40069–1, EF: L66. s Hamiapollenites perisporites, USNM40071–24CP1–2, EF: Y54. t Hamiapollenites sp. cf. H. bullaeformis, USNM40069–2, EF: S41/2. u Striatoabieites richteri, USNM40069–1, EF: P54/3. v Vittatina lata, USNM40070-BB7, EF: T43. Scale bar 20 μm, applies to all images
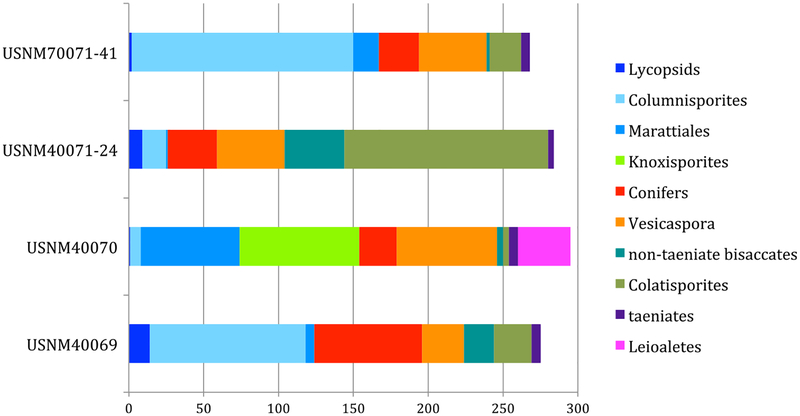
Despite the general similarities in overall composition, there are some striking quantitative differences among the three assemblages (Fig. 15). In the lowest level (USNM 40069), Columinisporites ovalis (36%), Potonieisporites spp., and Vesicaspora are abundant, and Cadiospora magna has a notable presence (5%). The enigmatic taxon Colatispo-rites decorus (?Anguisporites intonsus, Fig. 14l) also is moderately abundant (8%). Except for Columinisporites, spores display low abundance and diversity (Fig. 15).
Fig. 15.
Major group palynomorph composition of Kola Switch samples. Lower bed—USNM 40069. Middle bed—USNM 40070. Upper bed, two separate samples—USNM 40071–24, USNM 40071–41
In contrast, the middle layer (USNM 40070) contains abundant spores (almost 50%), including those produced by Marattiales (Fig. 14g), and an unknown spore we attribute to Knoxisporites sp. cf. K. ruhlandii (Fig. 14e). The latter taxon, of unknown affinity, comprises about a quarter of the total. Columinisporites is also present, at low abundance. Species of Potonieisporites and Vesicaspora make up most of the rest of the assemblage, along with uncommon Colatisporites decorus (Fig. 14). A palynomorph of unknown affinity, Leioaletes sp. (Fig. 14f), either a spore or an algal cyst, also is abundant. The algal genus Botryococcus is present.
The two samples from the upper unit (USNM 40071) differ significantly from one another in terms of relative abundance of common taxa. USNM 40071–24 is dominated by Colatisporites decorus (45%). The non-taeniate bisaccte Platysaccus sp. cf. P. saarensis (Fig. 14p) is also abundant. Potonieisporites and Vesicaspora make up much of the rest of the pollen. Spore abundance and diversity are low and comprise primarily Columinisporites ovalis as well as a spore of unknown affinity (Fig. 14i). In contrast, USNM 40071–41 is dominated by Columinisporites ovalis (nearly 60% of the palynoflora), whereas Colatisporites decorus makes up only 7% of the total. Other spores, except for those produced by Marattiales, are very rare (Fig. 15). Potonieisporites and Vesicaspora remain at roughly the same abundance.
Discussion
Environmental interpretation of the three floras
The three Kola Switch plant assemblages (Fig. 16) accumulated under different depositional conditions. The two lower beds are separated from the uppermost by nearly a meter of gray mudstone. The lower and middle beds, which are hypoautochthonous and contain the best preserved macrofossils, are highly dominated by a single taxon or taxonomic group. Not surprisingly, the heavily sampled lower bed contains a large number of rarely occurring taxa. In contrast, the allochthonous upper bed has a more evenly distributed abundance, and rarely occurring taxa comprise a smaller proportion of the flora. Because this bed was not heavily sampled, more intensive work doubtlessly would yield a more diverse collection.
Fig. 16.
Macrofloral dominance-diversity curves. a Lower bed. b Middle bed. c Upper bed. Most abundant taxa are noted on the graphs
The lowermost fossiliferous bed accumulated in a channel setting under waning-flow conditions. The underlying sandstone is not a major alluvial sandstone deposit like the informal sandstone members of the formation. Its limited thickness and lateral continuity suggest a flood-stage over-bank deposit or alluvial splay. The fining-upwards characteristics of the fossil-bearing, mudstone-dominated, lower interval are consistent with those of an intermittently active floodplain channel in which plant remains collected in the lower part of the fill. The upper portion of the lowermost plant-bearing deposit is finer grained and flat-bedded to nearly fissile. The flora is well preserved throughout the deposit, and both foliage and leafy shoot fragments are relatively large and abundant. These aspects of plant-fossil preservation suggest limited transport of remains from the site at which the parent plants were growing.
The middle fossiliferous bed lies in conformable but sharp contact with the lower bed. The plant fossils and sedimentary features typical of each bed may occur on opposite sides of single hand specimen without any lithological or biological intergradation. The differences in lithological character and plant-fossil composition between the two suggest that the overlying (middle collecting interval) bed accumulated in standing water conditions within an abandoned floodplain channel. The absence of roots indicates that plant remains are at best parautochthonous, possibly allochthonous, derived from near the environment in which they were deposited and preserved. Given the large size of marattialean tree ferns, their root-mantle supported stems (Ehret and Phillips 1977), and the fact that aerial marattialean macrofossil remains are common in the deposit, roots would be expected to be obvious and abundant had the plant-fossil assemblage accumulated in an autochthonous or even parautochthonous manner (Bateman 1991); roots would have both penetrated the accumulating carbonaceous shale and been part of the aerial litter. In addition, the middle bed contains the green alga Botryococcus, which is characteristic of generally freshwater oligotrophic lakes and ponds (Guy-Ohlson 1992). Thus, the second plant-bearing interval appears to be a record of shed plant parts that accumulated in stagnant water very close to where they grew. The densely matted nature of plant remains within this very fissile deposit further resembles Modern organic-rich muds found below floating mats of vegetation in Louisiana (Zangerl and Richardson 1963).
The occurrence of fossiliferous deposits with the same lithological and fossil-plant characteristics approximately 1.5–5 km (USNM 9998B and TxBEG—Fig. 1) to the east suggests either a deposit of considerable extent or the recurrence of similar depositional environments within the same stratigraphic interval. The latter may seem more likely in an alluvial floodplain setting. We have observed both of these situations, however, in the Wichita and lower Bowie Groups and the Clear Fork Formation and therefore conclude that either interpretation could pertain to the Kola Switch area.
The upper Kola Switch flora is more enigmatic than the lower two. It consists of a mixture of plants with hygromorphic to mesomorphic aspects typical of wetlands or wet soils, and more xeromorphic forms, generally considered characteristic of periodically moisture-limited soils. The fossil preservation quality does not differ noticeably among the taxa, and all the remains are fragmentary and of relatively small size. That, in combination with the remarkably even distribution of relative abundance, which is not typical of most autochthonous or parautochonous assemblages, suggests derivation of the fossil remains from a variety of microhabitats distributed in close proximity. In contrast, the lower and middle beds are quite uniform in the habitat signal provided by their respective floras, the lower bed consisting of forms most typical of periodic moisture stress and the middle bed containing a typical wetland flora. And these beds, in physically sharp contact, lack any evidence of a floristic transition. In fact, the upper bed is compositionally what might have been predicted to lie between the lower and middle beds were sedimentation continuous and floristic change gradual; however, the two lower beds, which are compositionally entirely distinct, lie in sharp contact without a mixed transitional assemblage between them.
Taphonomy and affinities of dispersed pollen and spores
Although the presence of particular pollen and spores in the palynological assemblages is definite proof that certain plant groups were present in the local to regional landscape, several factors complicate the translation of these assemblages into plant communities and the environments they lived in. The composition of the dispersed palynomorph assemblage is influenced by the environment, including physical factors such as seasonality and precipitation and biological factors such as plant phenology, plant stature and canopy structure. Palynological assemblages such as these tend to be time-averaged, in that they may encompass a number of individual depositional events over some unknown interval of time. In addition, assemblages are generally a mix of pollen and spores transported far from their source by wind and water (regional signal) as well as those dispersed from plants growing nearby (local signal). A more detailed discussion of the taphonomy of palynological samples may be found in Looy and Hotton (2014). Further difficulty arises from uncertainty about the parent plant of many dispersed pollen and spore taxa. In some cases, the connection between dispersed palynomorph and parent plant is secure, e.g., between walchian conifers and Potonieisporites, but the affinities of many other dispersed palynomorphs are uncertain. This is particularly true of pollen produced by more xeric taxa, which, in general, are more poorly understood than the Pennsylvanian wetland plants. Affinities of taxa found in the Kola Switch assemblage may be found in Table 3.
Table 3.
Pollen and spore taxa present in the palynological assemblage with citations to an original or emended description (or in a few cases to a description not necessarily conforming to the original description, indicated here by ‘fide’) and inferred affinity
| Taxon | Affinity |
|---|---|
| Cadiospora magna Kosanke 1950: p. 50, pl. 16, fig. 1 | Sigillaria |
| Calamospora breviradiata Kosanke, 1950: p. 41, pl. 9, fig. 4 | Calamitaceae, Sphenophyllales |
| Columinisporites ovalis Peppers 1964: p. 16, pl. 1, figs. 11, 12 | Sphenophyllales |
| Convolutispora florida Hoffmeister, Staplin & Malloy 1955: p. 384, pl. 38, fig. 6 | Polypodiidae |
| Cyclogranisporites aureus (Loose) Potonie & Kremp 1955: p. 61, pl. 13, figs. 184–186 | Marattiales |
| Cyclogranisporites minutus Bhardwaj 1957: p. 83, pl. 22, figs. 22–23, emend. Ravn 1986: p. 31, pl. 3, figs. 3, 4 | Marattiales |
| Cyclogranisporites sp. cf. C. orbicularis (Kosanke) Potonie & Kremp 1955 fide Ravn 1986: | |
| Punctatisporites obliquus Kosanke 1950: p. 16, pl. 2, fig. 5 | Marattiales |
| Dictyotriletes sp. | ?Marattiales |
| Knoxisporites sp. cf. K. ruhlandii Doubinger & Rauscher 1966 fide Playford 1971: p. 35, pl. 12, figs. 1–4 | Probably Polypodiidae |
| Laevigatosporites medius Kosanke 1950: p. 29, pl. 16, fig. 12 | Sphenophyllales |
| Laevigatosporites minor Loose 1934: p. 158, pl. 7, fig. 12 | Sphenophyllales |
| Leioaletes circularis Ravn & Fitzgerald 1982: p. 150, pl. 12, figs. 1–5 | ?Marattiales, ?Sphenopsida |
| Punctatisporites aerarius Butterworth & Williams: p. 360, pl. 1, figs. 10, 11 | Marattiales |
| Punctatisporites minutus Kosanke 1950 fide Smith and Butterworth 1967, p. 126, pl. 1, figs. 15, 16 | Marattiales |
| Punctatosporites minutus Ibrahim 1933: p. 40, pl. 5, fig. 33, fide Smith & Butterworth 1967, pl. 24, figs. 8, 9 | Marattiales |
| Triquitrites cf. T. sculptilis Balme 1952: p. 181, text-fig. 1g (fide Smith & Butterworth 1967, pl. 12, figs. 10–15) | Polypodiidae: Gleicheniaceae |
| Pollen/prepollen | |
| Alisporites cf. A. plicatus Jizba 1962: p. 884, pl. 124, figs. 51–53 | ?Seed fern |
| Colatisporites decorus (Bharadwaj & Venkatachala) Williams, in Neves et al., 1973: p. 41, pl. 2, figs. 11–13 [=Anguisporites intonsus Wilson 1962: p. 12, pl. 1, fig. 3] | ?Lyginopteridales |
| Florinites mediapudens (Loose 1936) Potonie & Kremp, 1956: p. 169, pl. 21, figs. 468–471 | Cordaitales |
| Florinites sp. | Cordaitales |
| Hamiapollenites sp. of Jizba 1962, pl. 123, fig. 38 (as Striatosaccites sp.) | ?Peltaspermales or ?Gnetales |
| Hamiapollenites perisporites (Jizba) Jansonius 1962 (as Striatosaccites perisporites Jizba 1962: p. 882, pl. 123, fig. 39–43 | ?Peltaspermales or ?Gnetales |
| Illinites unicus Kosanke 1950: 51–52, pi. 1, figs. 3, 4 (fide Jizba 1962: p. 879, pl. 121, figs. 1–14, as Complexisporites polymorphus) | ?Voltziales |
| Klausipollenites sp. cf. K. staplinii Jansonius 1962: p. 56, pl. 12, figs. 21–27 | ?Seed fern |
| cf. Nuskoisporites sp. | Voltziales |
| Paravesicaspora splendens (Leschik 1955) Klaus 1963: pl. 18, figs. 90, 91 | ?Peitaspermales |
| Pityosporites communis Tschudy & Kosanke 1966: p. 66, pl. 2, figs. 32, 33 | ?Seed fern |
| Platysaccus sp. cf. P. saarensis (Bhardwaj 1957) Jizba 1962: p. 885, pl. 124, figs. 59–61 | ?Seed fern |
| Potonieisporites sp. cf. P. novicus Bhardwaj 1954 emend. Poort & Veid 1997: fig. 1a-f | Voltziales |
| Potonieisporites sp. cf. P. simplex Wilson 1962: pp. 14–15, pl. 3, figs. 1–3 | Voltziales |
| Schopfipollenites ellipsoides (Ibrahim 1932) Potonie & Kremp 1954: pp. 180, pl. 19, figs. 89–92, pl. 20, fig. 107 | Medullosaceae |
| Striatoabietites richteri (Kiaus 1955) Hart 1964 (fide Jizba 1962: p. 880, pl. 122, figs. 16–21, 25–30, as Striatites richteri (Kiaus) Jizba 1962) | ?Peitaspermaies or ?Voltziales |
| Tinnulisporites sp. cf. T. microsaccus Dempsey 1967: p. 115, pl. 1 figs. F-N | Unknown seed plant |
| Vesicaspora wilsonii Schemei 1951: pp. 749–750, figs. 1, 3 | Callistophytaies or Peltaspermaies (?Autunia) |
| Vesicaspora sp. ‘giobose’ | Callistophytales or ?Peltaspermales or |
| Vittatina lata Wilson 1962, p. 25, pl. 3, fig. 11 | ?Gnetales |
| Wilsonites sp. | ?Peltaspermales |
Proposed affinities follow Looy and Hotton (2014) and literature therein
Comparison of palynofloras and macrofloras
Studies combining macrofloral and palynofloral data often are not possible because one or the other of these preservational modes is not available, but when both are, they yield insights into paleofloristics not accessible through either data set in isolation. For example, combined analyses can clarify patterns of floristic change around major extinction events (Mander et al. 2010) and more accurately characterize regional vegetation (Looy et al. 2014; DiMichele et al. 2016, 2018). For interpretation of vegetation on the broader landscape, palynofloras have an advantage over most macrofloral assemblages in that they include taxa not usually preserved as macrofossils and include taxa drawn from outside the depositional sites where most macrofossils are preserved. Conversely, palynofloras have the disadvantage of lower taxonomic resolution and are not present in oxidized sediments where plant macrofossils may yet be preserved (e.g., many plant-bearing red-bed deposits). Macrofossils and palynofloras together offer a more complete picture of the flora than from either source alone, yet they often record depositional conditions in dramatically different ways. Consequently, disparity between macrofloras and palynofloras is common, and this is especially pronounced at Kola Switch. For example, in the lower bed macroflora, Sphenopteris germanica is overwhelmingly abundant, and conifers are subdominant. In contrast, conifer pollen is a dominant element in the pollen assemblage. Vesicaspora and Paravesicaspora are relatively common but not dominant; the parent plants of these pollen forms are uncertain, but are probably peltasperms, several species of which are present in the macroflora, in the form of callipterids. Sphenophyll macrofossils are absent from the lower bed, although the sphenophyll spore, Columinisporites ovalis, is the single most common element in the palynoflora. Lycopsids also are absent from the macroflora, but Cadiospora (produced by Sigillaria) is relatively common. Medullosan macrofossils are present in the lower bed, but their prepollen, Schopfipollenites, is extremely rare (< 1/300) or absent. This is normal for this taxon, in part because of its large size but also perhaps because of the phenology of the parent plant.
The middle organic palynoflora contains about 22% marattialean fern spores in keeping with the abundant marattialian macofossils. However, the single most common element is a spore of unknown affinities, Knoxisporites sp. cf. K. ruhulandii. Vesicaspora is quite abundant in this bed (22%), and conifer prepollen (cf. Nuskoisporites and Potonieisporites) is relatively common (8%), although neither conifers nor peltasperms were found in the macroflora. Columinisporites is present but not especially abundant in this horizon, in keeping with the presence of a few sphenophylls in the macroflora.
The upper bed assemblages are dominated by either the enigmatic form Colatisporites decorus or Columinisporites. The parent plant of Colatisporites is unknown. Conifer prepollen is relatively abundant in both assemblages from the upper bed, in agreement with the presence of walchian conifers in the macroflora, although the proportion in the macro-flora is smaller than in the palynoflora. Relatively common Vesicaspora agrees with the presence of several forms of callipterids in the macroflora. Calamites are relatively common in the upper bed in the macroflora, but their corresponding spore type, Calamospora, is barely present. This may be due to preservational bias; the upper palynoflora is poorly preserved, and thin-walled spores, subject to crushing and distortion, may not be recognizable. Similar preservational bias might account for the apparent absence of small and morphologically simple marattialean spores in contrast to the diversity and subdominance of marattialean macrofossils. Sphenophylls are the most common macrofossils in the upper bed, as are their spores, a rare example of agreement between macroflora and palynoflora at Kola Switch.
Reasons for the macrofloral-palynofloral disparity are unclear. It is possible that the microfossils and macrofossils record catchment areas of different sizes. All three beds reflect varying degrees of transport. The macrofossil assemblages, for the most part, reflect plants that grew close to the depositional environment, whereas microfossils represent a broader landscape and may even include reworked elements. An additional complicating factor is differential pollen production. Certain plants, such as walchian conifers and, possibly, the parent plants of Vesicaspora and Anguisporites, were copious pollen producers. Thus, these plant groups may be overrepresented in dispersed assemblages relative to their proportions in the parent vegetation, which suppresses the likelihood of capturing other taxa in a 300-grain count.
The difference in abundances in the two samples from the upper bed (USNM 40071) is unexpected. However, this bed is laminated, consisting of alternating layers of darker and lighter mudstone, and it is quite possible that different laminae were collected for each sample. Variation in abundance, alternatively or additionally, could be caused by seasonal variation in precipitation and palynomorph production, resulting in different palynofloras in different portions of the bed that might, at a coarse level of observation, be considered to be lithologically the same. Overall species composition of all four palynological samples is similar and differs primarily in relative abundance from bed to bed, although notable exceptions to this are the presence of Knoxisporites in the middle assemblage and of several rare unknown spore types in the upper bed. Generally speaking, Potonieisporites and Vesicaspora display greater evenness, suggesting that they represent the background vegetation and, thus, are less subject to fortuitous swings in abundance, which might be expected from palynomorphs derived from local vegetation. Overall, the pollen and spore taxa appear to be drawn largely from the same species pool, which is in agreement with the observations in the macrofloral assemblages.
Comparison with other floras
Macrofloral comparisons
Spatially, the most obvious point of comparison to the Kola Switch macroflora is that from the Sanzenbacher Ranch site (DiMichele et al. 2018). These sites are located at the same stratigraphic level in floodplain lithofacies below informal sandstone member 8 and are separated by only about 8.5 km. The Sanzenbacher deposit is a coarsening-upward, mud-stone-dominated deposit. Although lithologically heterogeneous, and internally variable in terms of plant composition, it lacks evidence of organic-rich beds or of a basal channel fill of unique lithological and compositional character. The Sanzenbacher macroflora was sampled three times, at intervals of about 20 years, by three different collecting parties.
All three subcollections of the Sanzenbacher macroflora are dominated by the callipterid Autunia conferta, and both walchian conifers and Sphenopteris germanica are common. These are abundant in the Kola Switch lowermost unit, which we interpret as typical of seasonally moisture-limited soils. Other plants characteristic of microhabitats with some degree of seasonal moisture limitation, occurring in all Sanzenbacher subcollections, include Odontopteris subcrenulata, Rhachiphyllum schenkii, Cordaites spp., and Neurodontopteris auriculata, only some of which occur in the lowermost Kola Switch assemblage.
The Sanzenbacher subcollections also include marattialean fern foliage of several kinds, Sphenophyllum spp. remains, and calamitalean stem and foliage remains, the latter in two of the three subcollections, commonly (15.7%) in one. When compared with the Kola Switch collections, these taxa bring the Sanzenbacher flora closer to the uppermost Kola Switch flora, which is a mixture of taxa from wetland and seasonally dry habitats. The dominants, however, are reversed: at Kola Switch, Sphenophyllum, marattialeans, and calamitaleans—all typical of habitats with relatively high soil moisture—are the dominants, followed by lesser amounts of Sphenopteris germanica, Walchia, and Autunia conferta. Several other elements in the two floras overlap as well.
What the Sanzenbacher flora lacks entirely is an assemblage dominated exclusively by wetland plants. By any measure it, as with Kola Switch, can be described as being drawn from a floristically mixed landscape that included both plants typical of high soil moisture for much of the year (marattialeans, calamitaleans, sphenophylls) and plants typical of soils with moisture deficits for one or more periods of the year (Sphenopteris germanica, walchian conifers, callipterids, cordaitaleans, certain odontopterids, and others). Palynological data from Sanzenbacher underscores the mixed character of the flora. Such a flora suggests a background regional climate with seasonal rainfall, but with persistent areas of high soil moisture, probably immediately surrounding standing water bodies.
Further comparison can be made to Rotliegend floras of Europe. The basic composition of such floras is summed up in the work of Barthel (2009) in addition to several summaries of more site-specific studies (e.g., Barthel 1976, 2016; Lausberg and Kerp 2000; Šimůnek and Martínek 2009; Barthel et al. 2010; Uhl and Jasper 2016). In the following, we focus on only two further summary papers, those of Kerp and Fichter (1985) and Uhl and Lausberg (2008), which examine broader ecological aspects of Lower and Upper Rotliegend floras in the Saar-Nahe Basin. Kerp and Fichter (1985) provide an extensive summary of floras based on presence-absence data, covering the Carboniferous-Permian transition, possibly as young as those of Sanzenbacher and Kola Switch. Most of these floras are compositionally mixed, with varying numbers of wetland and seasonally dry taxa. Strikingly, however, the great majority of wetland taxa, and the most consistently present throughout the entire suite of samples analyzed, are marattialean ferns, calamitaleans, and sphenophylls. Lycopsid remains occur rarely, and only in older deposits. The most commonly encountered medullosan pteridosperm is Odontopteris subcrenulata, generally considered to be mesomorphic. Taxa from seasonally dry habitats occur throughout, including a diversity of callipterids and conifers. At the level of major group composition, these floras are quite similar to both Kola Switch and Sanzenbacher, but with a notable lack of Sphenopteris germanica; this latter species has since been reported from the lowermost Rotliegend (Uhl and Jasper 2016). In a quantitative study of two Lower and two Upper Rotliegend floras, Uhl and Lausberg (2008), who used the same methods of quantification as in the Kola Switch and Sanzenbacher studies, found conifers of various kinds to dominate three of the floras and callipterids to dominate the fourth. Of more typical wetland elements, calamitaleans were present in three of the four collections, and at significant levels in the callipterid-dominated assemblage. Marattialean ferns were rare, but were of the highest diversity and abundance in the callipterid-dominated flora, along with the lycopsids Sigillaria and Asolanus. These comparisons again indicate a general similarity between the Rotliegend floras and those of Kola Switch and Sanzenbacher—a dominance by plants of xeromorphic to mesomorphic morphology, with calamitaleans and marattialeans as the most common representatives of a depauperate wetland component.
For a broader comparison with other western Pangean early Permian floras of approximately the same age as Kola Switch, the reader is referred to the description of the Sanzenbacher flora (DiMichele et al. 2018, section 6.3.2). These comparisons show a consistency in certain patterns in western Pangea, which have come to be generalized to the plant groups involved. One is the widespread occurrence of marattialean ferns and calamitalean sphenopsids in mixed floras, dominating some, but occurring rarely in many others, including those otherwise heavily dominated by xeromorphic taxa presumed to favor seasonally dry habitats. This pattern may reflect differential abilities, less common in the other lineages typical of high-moisture soils, to establish populations in small areas of high water table and, via their propagules, to locate those areas, even if widely separated by tracts of unfavorable physical conditions. Also seen are consistent associations of certain xeromorphic forms (conifers, taeniopterids, neoggerathialeans, supaioids, comioids, gigantopterids, and to a lesser degree, callipterids) that, by their patterns of cooccurrence, have come to be considered as indicators of soils with periodic moisture stress.
There are some notable exceptions to these patterns. In Euramerica, there are reported occurrences of callipterids in organic-rich, dark shales, presumed to have accumulated in somewhat swampier settings (although not necessarily under humid to perhumid climates—an analogue might be the Taxodium swamps of eastern North America [e.g., see papers in Ewel and Odum 1984]). There are several examples of this in strata of early Permian age. One of the best described is the occurrence of unique ecomorph of the callipterid Autunia conferta, or a species closely related to it (with different cuticular characteristics), from a peat-forming paleoenvironment in central Europe (the Rotliegend Crock locality in Thuringia—see description in Kerp 1988, 1996). Another is the occurrence of Lodevia oxydata in an organic-rich siltstone from the Dunkard Group at the Brown’s Bridge locality in West Virginia (DiMichele et al. 2013), possibly a stagnant lake.
One of the most important exceptional floras is that from the early Permian Wuda coal field of Inner Mongolia (Wang et al. 2012). In this case, a peat-swamp flora, dominated by marattialean tree ferns, and containing other typical Pennsylvanian-Permian wetland taxa, such as Sigillaria, was found also to contain noeggerathialeans, large cordaitaleans, and the suspected cycadophytes, Taeniopteris and Pterophyllum. The noeggerathialeans and cycadophytes, in particular, and the cordaitaleans, in part, belong to lineages generally considered to be centered ecologically in periodically dry habitats. The occurrence of these plants in a peat swamp is unequivocal because they are buried beneath and within an ash parting separating two coal layers (Pfefferkorn and Wang 2007). As noted by Wang et al. (2012), the elements of the Wuda flora with evolutionary ties to drought-tolerant line-ages differ from Euramerican forms at the specific, and often generic, levels. This may be an indication of greater evolutionary dynamism in seasonally dry species pools, mainly because they were richer in seed plants than the wetland species pool, and it was among seed plants that changes in ecological tolerances were most likely to occur. Seeds remove the absolute requirement for environmental water to effect fertilization, thus functionally uniting the gametophyte and sporophyte phases of the plant life cycle. As a result, the dominant lineages centered in seasonally dry habitats may have been able to produce taxa capable of living in wetlands, particularly where opportunities for colonization of those habitats were made possible by reduced competition during the time of environmental change and coincident ecological disruption (Kerp 1988; DiMichele and Aronson 1992).
Palynofloral comparisons
The Kola Switch palynoflora is notably impoverished when compared with temporally and geographically close assemblages. The recently published Sanzenbacher flora is a case in point (DiMichele et al. 2018). Three rich palynological assemblages were described from Sanzenbacher, with a total diversity of 57 taxa from 300 grain counts, in contrast to 42 for the Kola Switch assemblage. The difference in diversity is due almost entirely to the presence of taxa in the Sanzenbacher assemblages, albeit in low abundance, typical of Late Pennsylvanian Euramerican wetland floras (Fig. 17). These include lycopsids (e.g., Endosporites globosus and Crassispora kosankei), diverse calamitaleans (Calamospora spp.), Marattiales (Punctatisporites, Punctatosporites, Cyclogranisporites, small Laevigatosporites), herbaceous fern taxa (such as Triquitrites spp., Verrucosisporites sp., Convolutispora spp.), and cordaitalean prepollen (Florinites). The dominants in the Sanzenbacher palynoflora consist of Vesicaspora spp., the single most common taxon in all three assemblages, as well as Wilsonites sp. and Colatisporites decorus. Marattiales are moderately abundant, and Potonieisporites spp. are relatively uncommon (3–6.5% of the Sanzenbacher flora). Typical Permian taeniate forms, including Vittatina spp. and Striatoabieites richteri, are present at similarly low abundance as in the Kola Switch palynofloras (Fig. 17).
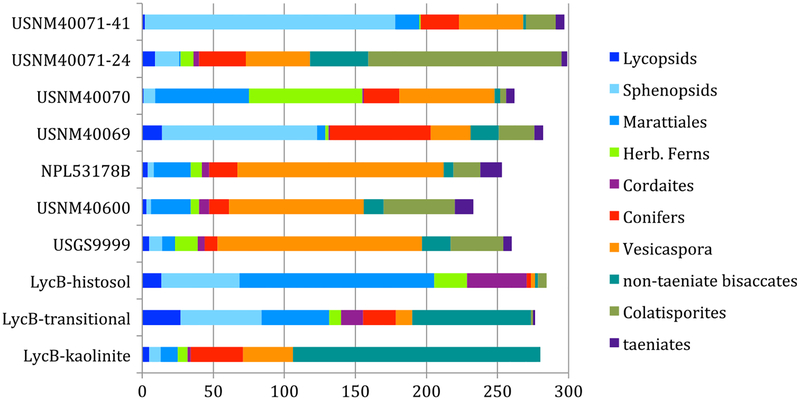
Fig. 17.
Comparative major-group palynological composition of samples from three sites. LycB sites from the Markley Formation, Late Pennsylvanian (Looy and Hotton 2014). USGS 9999, USNM 40600, and NPL 53178B from Sanzenbacher from the early Permian Archer City Formation (DiMichele et al. 2018). USNM 40069, 40070, and 40071 from Kola Switch
Kola Switch is similar to many fossiliferous sites in the Markley Formation of North-Central Texas (DiMichele et al. 2005; Tabor et al. 2013) in terms of its lithological heterogeneity and the succession of beds on the outcrop. Most of the Markley sites were not palynologically productive. Only one, Lycopod B, yielded palynomorphs from all three main lithotypes (Looy and Hotton 2014): a thick basal kaolinitic bed, overlain by thin coals and carbonaceous shales about 3 m thick, in turn overlain by mudrocks interpreted as floodplain deposits. The basal kaolinite is dominated by seed plant (pre)pollen, especially non-taeniate bisaccates (Alisporites, Falcisporites, and Pityosporites), Potonieisporites spp., and, to a lesser degree, Vesicaspora (Fig. 17). Most spore taxa, with the exception of Cyclogranisporites and Punctatisporites (Marattiales), are rare or absent. This assemblage bears some resemblance to those of the lower and upper layers at Kola Switch. However, one striking difference is that the dominant in the Lycopod B kaolinite is the bisaccate complex Alisporites/Falcisporites, which is apparently replaced by Vesicaspora at Kola Switch (Fig. 14). The kaolinite bed is overlain by a thin layer with a transitional flora that contains many elements of the kaolinite, including taeniate pollen, as well as those of the organic rich beds above (Fig. 17). In contrast to the carbonaceous layer at Kola Switch, the interbedded coals and carbonaceous shales at Lycopod B include many elements of the typical Late Pennsylvanian, wetland Euramerican palynoflora, similar to the Sanzenbacher assemblage, but with even greater species richness. Almost all of these Pennsylvanian taxa are absent from the organic-rich horizon at Kola Switch, the exceptions being Florinites and Illinites unicus. Total diversity for the organic-rich horizon at Lycopod B is 44 compared with 22 for the organic-rich bed at Kola Switch. Even taking into account that the tally for Lycopod B includes nine separate samples, this is a significant drop in diversity.
Detrended Correspondence Analysis of the palynofloral assemblages at Lycopod B (Looy and Hotton 2014) shows that most taxa are spread along an axis interpreted as water availability based on lithological correlation, with wetland Late Pennsylvanian taxa clustered near one end and seed plants, including Potonieisporites, Vesicaspora, non-taeniate bisaccates, Colatisporites, and Illinites unicus, on the other end. The few spore taxa that cluster with the presumed seasonally dry taxa include Cadiospora magna, Columinisporites ovalis, and Calamospora spp. These are the same spore taxa present at Kola Switch, along with the addition of marattialian spores, and a few taxa of uncertain affinity. Declining species diversity from Lycopod B to Sanzenbacher and thence to Kola Switch is therefore a consequence of the loss of wetland taxa. These observations constitute strong evidence of decreasing water availability from Late Pennsylvanian to early Permian, whether through a drop in water table, as would occur under seasonally dry conditions, increase in evapotranspiration, or simple lithofacies change. Of particular note is the presence of Hamiapollenites in the Kola Switch assemblage. This stratigraphically important group, which becomes more prominent in the younger Permian flora in Texas and elsewhere, is not present in either the Lycopod B or Sanzenbacher assemblages. Whether Hamiapollenites serves as a chronostratigraphic indicator, an environmental indicator, or both requires additional study.
Conclusions
The three stratigraphically distinct macrofloras at Kola Switch represent two very different assemblages, and one that provides overlap between the two end points. The lower flora is composed almost entirely of plants of xeromorphic to mesomorphic foliar form, considered typical of habitats with seasonal moisture distribution. The middle collecting level, an organic-rich shale, is dominated by hygromorphic marattialean ferns, with smaller numbers of calamitealeans and sphenophylls, but entirely lacking plants characteristic of better drained soils. Both of these assemblages appear to be hypoautochthonous, with only limited transport of plant remains from the source vegetation. Furthermore, the contact between the lower and middle beds is abrupt, and a transitional floral assemblage is lacking. In contrast, the uppermost flora includes elements found in both the lower and middle assemblages. Based on the smaller size of the remains, the lower quality of preservation, and the relatively even biomass distribution among taxa, this flora appears to be allochthonous and thus more likely to be a mixture of plants drawn from different microhabitats.
In striking contrast to these macrofloral differences, the palynoflora, particularly if examined at the presence-absence level of resolution, suggests a consistent regional species pool. Quantitative variability among samples may reflect such factors as proximity of source plants to the depositional site, different transport modes, differences in population sizes, or all of the above. The differences between the two assemblages from the upper deposit even suggest that seasonality of release of pollen and spores may be a variable to consider. Such factors would account for the differences in the proportional variation of different plant groups in the macro- and microfloras. Taking the middle bed as an example, marattialeans almost completely dominate the macroflora but make up less than half of the palynofloras. The remainder of the palynological assemblage indicates the presence of a background of plants typically associated with seasonal rainfall patterns, such as conifers and callipterids.
The combination of macrofloral, palynological, and sedimentological data from Kola Switch, in conjunction with similar data from the nearby and coeval deposit of the Sanzenbacher Ranch (DiMichele et al. 2018), indicates that the Wolfcampian-aged alluvial floodplains of North-Central Texas hosted a considerable variety of plant communities. In addition to edaphic end members based upon complementary paleobotanical and sedimentological inferences, there are floral and depositional intermediates. Similar conclusions have been drawn previously at a regional scale (DiMichele et al. 2006). At Kola Switch, however, this variability is recorded in a rock interval of < 1.5 m in thickness.
The Kola Switch floras, when compared with those of the nearby and age equivalent Sanzenbacher locality, and further afield to those of the Central European Rotliegend, are typical of the early Permian. Callipterids and conifers are important to dominant elements, along with small numbers of odontopterid medullosans, which record largely xeromorphic-to-mesomorphic plant communities. However, certain groups of plants with strong ties to relatively wet soils, including calamitaleans, sphenophylls, and marattialean ferns, remain conspicuous within these landscapes. Floristic diversity in these assemblages is low, which is in keeping with findings from other studies.
Acknowledgements
This paper is dedicated to our colleague, mentor, and friend, Hans Kerp. We thank the late Sergius Mamay (US Geological Survey), the late Nicholas Hotton, III (Smithsonian Institution), Kenneth Craddock (Denton, Texas), and the late Louis Todd (Denton, Texas) for assistance with property access and collecting. Tucker Hentz (Texas Bureau of Economic Geology) and Rudolf Serbet (University of Kansas) assisted materially in identifying the location and composition of the collection made by Theordore Delevoryas. Hermann Pfefferkorn, an anonymous reviewer, and associate editor Benjamin Bomfleur are gratefully acknowledged for providing comments that materially improved the paper. The research of Carol Hotton was supported in part by the Intramural Research Program of the National Institutes of Health, National Library of Medicine. The fieldwork of Hook and DiMichele was supported by a Scholarly Studies grant from the Smithsonian Institution.
References
- Barthel M 1976. Die Rotliegendflora Sachsens. Abhandlungen des Staatlichen Museums für Mineralogie und Geologie Dresden 24: 1–190. [Google Scholar]
- Barthel M 2009. Die Rotliegendflora des Thüringer Waldes, 2003–2008. Veröffentlichungen Naturhistorisches Museum Schleusingen: Special Publication, Combined Printing of Chapters from previous issues. [Google Scholar]
- Barthel M 2016. Die Rotliegendflora der Döhlen-Formation. Geologica Saxonica 61: 105–228. [Google Scholar]
- Barthel M, Eichler B, and Reichel W. 2010. Die Rotliegendflora des Weißig-Beckens. Geologica Saxonica 56: 159–192. [Google Scholar]
- Bashforth AR, and Nelson WJ. 2015. A Middle Pennsylvanian macrofloral assemblage from below the Rock Island (no. 1) coal member, Illinois: resolving the Bolsovian-Asturian boundary in the Illinois Basin. Review of Palaeobotany and Palynology 222: 67–83. [Google Scholar]
- Bashforth AR, and Zodrow EL. 2007. Partial reconstruction and palaeoecology of Sphenophyllum costae (Middle Pennsylvanian, Nova Scotia, Canada). Bulletin of Geosciences 82: 365–382. [Google Scholar]
- Bateman RM 1991. Palaeoecology. In Plant Fossils in Geological Investigation. The Palaeozoic, ed. Cleal CJ, 34–116. New York, N.Y: Ellis Harwood. [Google Scholar]
- Batenburg LH 1981. Vegetative anatomy and ecology of Sphenophyllum zwickaviense, S. emarginatum, and other “compression species” of Sphenophyllum. Review of Palaeobotany and Palynology 32: 275–313. [Google Scholar]
- Brown LF Jr., and Goodson JL. 1972. Abilene Sheet. Geological Atlas of Texas, scale 1:250,000, with explanatory pamphlet. Austin, Texas: The University of Texas and Bureau of Economic Geology. [Google Scholar]
- DiMichele WA, and Aronson RB. 1992. The Pennsylvanian-Permian vegetational transition: a terrestrial analogue to the onshoreoffshore hypothesis. Evolution 46: 807–824. [DOI] [PubMed] [Google Scholar]
- DiMichele WA, Tabor NJ, and Chaney DS. 2005. Outcrop-scale environmental heterogeneity and vegetational complexity in the Permo-Carboniferous Markley Formation of North Central Texas. New Mexico Museum of Natural History and Science Bulletin 30: 60–66. [Google Scholar]
- DiMichele WA, Tabor NJ, Chaney DS, and Nelson WJ. 2006. From wetlands to wet spots: environmental tracking and the fate of Carboniferous elements in Early Permian tropical floras. Geological Society of America Special Papers 399: 223–248. [Google Scholar]
- DiMichele WA, Kerp H, Sirmons R, Fedorko N, Skema V, Blake BM Jr., and Cecil CB. 2013. Callipterid peltasperms of the Dunkard Group, central Appalachian Basin. International Journal of Coal Geology 119: 56–78. [Google Scholar]
- DiMichele WA, Schneider JW, Lucas SG, Eble CF, Falcon-Lang HJ, Looy CV, Nelson WJ, Elrick SD, and Chaney DS. 2016. Megaflora and palynoflora associated with a Late Pennsylvanian coal bed (Bursum Formation, Carrizo Arroyo, New Mexico, U.S.A.) and paleoenvironmental significance. New Mexico Geological Society Field Conference Guidebook 67: 351–368. [Google Scholar]
- DiMichele WA, Hook RW, Kerp H, Hotton CL, Looy CV, and Chaney DS. 2018. Lower Permian flora of the Sanzenbacher Ranch, Clay County, Texas. In Transformative Paleobotany, eds. Krings M, Harper CJ, Cuneo NR, and Rothwell GW, 95–126. London: Academic Press. [Google Scholar]
- Ehret DL, and Phillips TL. 1977. Psaronius root systems-morphology and development. Palaeontographica (B: Paläophytologie) 161: 147–164. [Google Scholar]
- Ewel KC, and Odum HT (eds.). 1984. Cypress Swamps. Gainesville: University of Florida Press. [Google Scholar]
- Guy-Ohlson D 1992. Botryococcus as an aid in the interpretation of palaeoenvironment and depositional processes. Review of Palaeo-botany and Palynology 71: 1–15. [Google Scholar]
- Hentz TF 1988. Lithostratigraphy and paleoenvironments of upper Paleozoic continental red beds, North-Central Texas: Bowie (new) and Wichita (revised) Groups. The University of Texas at Austin Bureau of Economic Geology Report of Investigations 170: 1–55. [Google Scholar]
- Hentz TF 1989. Permo-Carboniferous lithostratigraphy of the vertebrate-bearing Bowie and Wichita Groups, North-Central Texas. In Permo-Carboniferous Vertebrate Paleontology, Lithostratigraphy, and Depositional Environments of North-Central Texas, ed. Hook RW, 1–21. Austin, Texas: Society of Vertebrate Paleontology. (=Field Trip Guidebook 2). [Google Scholar]
- Hentz TF, and Brown LF Jr. 1987. Wichita Falls–Lawton Sheet. Geological Atlas of Texas, scale 1:250,000, with explanatory pamphlet. Austin, Texas: The University of Texas and Bureau of Economic Geology. [Google Scholar]
- Kerp JHF 1982. Aspects of Permian palaeobotany and palynology. II. On the presence of the ovuliferous organ Autunia milleryensis (Renault) Krasser (Peltaspermaceae) in the Lower Permian of the Nahe area (FGR) and its relationship to Callipteris conferta (Sternberg) Brongniart. Acta Botanica Neerlandica 31: 417–427. [Google Scholar]
- Kerp JHF 1984. Aspects of Permian palaeobotany and palynology. III. A new reconstruction of Lilpopia raciborskii (Lilpop) Conert et Schaarschmidt (Sphenopsida). Review of Palaeobotany and Palynology 40: 237–261. [Google Scholar]
- Kerp JHF 1988. Aspects of Permian palaeobotany and palynology. X. The West- and Central european species of the genus Autuia Krasser emend. Kerp (Peltaspermaceae) and he form-genus Rhachiphyllum Kerp (callipterid foliage). Review of Palaeobotany and Palynology 54: 249–360. [Google Scholar]
- Kerp H 1996. Post-Variscan late Palaeozoic Northern Hemisphere gymnosperms: the onset to the Mesozoic. Review of Palaeobotany and Palynology 90: 263–285. [Google Scholar]
- Kerp H, and Fichter J. 1985. Die Makrofloren des saarpfälzischen Rotliegenden (? Ober-Karbon–Unter-Perm; SW-Deutschland). Mainzer Geowissenschaftliche Mitteilungen 14: 159–286. [Google Scholar]
- Kerp H, and Haubold H. 1988. Towards a reclassification of the West-European and Central-European species of the form-genus Callipteris Brongniart 1849. Zeitschrift für Geologische Wissenschaften 16: 865–876. [Google Scholar]
- Kerp JHF, Poort RJ, Swinkels HAJM, and Verwer R. 1990. Aspects of Permian palaeobotany and palynology. IX. Conifer-dominated Rotliegend floras from the Saar-Nahe Basin (? Late Carboniferous-Early Permian; SW-Germany) with special reference to the reproductive biology of early conifers. Review of Palaeobotany and Palynology 62: 205–248. [Google Scholar]
- Kerp H, Noll R, and Uhl D. 2007. Übersicht über Pflanzengruppen und Vegetationsentwicklung im Permokarbon des Saar–Nahe-Beckens. In Kohlesümpfe, Seen und Halbwüsten, eds. Schindler T and Heidtke U-HJ. Pollichia, Sonderveröffentlichung 10: 42–74. [Google Scholar]
- Lausberg S, and Kerp H. 2000. Conifer-dominated flora from the Lower Rotliegend near Alsenz, Saar–Nahe-Basin (Germany). Feddes Repertorium 111: 399–426. [Google Scholar]
- Looy CV, and Hotton CL. 2014. Spatiotemporal relationships among Late Pennsylvanian plant assemblages: Palynological evidence from the Markley Formation, West Texas, U.S.A. Review of Palaeobotany and Palynology 211: 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looy CV, Stevenson RA, van Hoof TB, and Mander L. 2014. Evidence for coal forest refugia in the seasonally dry Pennsylvanian tropical lowlands of the Illinois Basin, USA. PeerJ 2: e630. 10.7717/peerj.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SG 2006. Global Permian tetrapod biostratigraphy and bio-chronology. Geological Society of London, Special Publications 265: 65–93. [Google Scholar]
- Mamay SH 1967. Lower Permian plants from the Arroyo Formation in Baylor County, north-central Texas. US Geological Survey Professional Paper 575-C: C120–C126. [Google Scholar]
- Mamay SH 1968. Russellites, new genus, a problematical plant from the Lower Permian of Texas. US Geological Survey Professional Paper 593-I: I1–I15. [Google Scholar]
- Mamay SH 1989. Evolsonia, a new genus of Gigantopteridaceae from the Lower Permian Vale Formation, north-central Texas. American Journal of Botany 76: 1299–1311. [Google Scholar]
- Mamay SH 1990. Charliea manzanitana, n. gen., n. sp., and other enigmatic parallel-veined foliar forms from the Upper Pennsylvanian of New Mexico and Texas. American Journal of Botany 77: 858–866. [Google Scholar]
- Mamay SH, and Bateman RM. 1991. Archaeocalamites lazarii, sp. nov.: the range of Archaeocalamitaceae extended from the lower-most Pennsylvanian to the mid-Lower Permian. American Journal of Botany 78: 489–496. [Google Scholar]
- Mamay SH, Chaney DS, and DiMichele WA. 2009. Comia, a seed plant possibly of peltaspermous affinity: A brief review of the genus and description of two new species from the early Permian (Artinskian) of Texas, C. greggii sp. nov. and C. craddockii sp. nov. International Journal of Plant Sciences 170: 267–282. [Google Scholar]
- Mander L, Kürschner WM, and McElwain JC. 2010. An explanation for conflicting records of Triassic-Jurassic plant diversity. Proceedings of the National Academy of Sciences 107: 15351–15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowen JH, Hentz TF, Owen DE, Pieper MK, Shelby CA, and Barnes VE. 1991. Sherman Sheet. Geological Atlas of Texas, scale 1:250,000, with explanatory pamphlet. Austin, Texas: The University of Texas and Bureau of Economic Geology. [Google Scholar]
- Pfefferkorn HW, and Wang J. 2007. Early Permian coal-forming floras preserved as compressions from the Wuda District (Inner Mongolia, China). International Journal of Coal Geology 69: 90–102. [Google Scholar]
- Pfefferkorn HW, Mustafa H, and Hass H. 1975. Quantitative Charakterisierung oberkarboner Abdruckfloren. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 150: 253–269. [Google Scholar]
- Read CB 1947. Pennsylvanian floral zones and floral provinces. Journal of Geology 55: 271–279. [Google Scholar]
- Read CB, and Mamay SH. 1964. Upper Paleozoic floral zones and floral provinces of the United States. US Geological Survey Professional Paper 454-K: K1–K19, K31–K35. [Google Scholar]
- Šimůnek Z, and Martínek K. 2009. A study of Late Carboniferous and Early Permian plant assemblages from the Boskovice Basin, Czech Republic. Review of Palaeobotany and Palynology 155: 275–307. [Google Scholar]
- Stull GW, Labandeira CC, DiMichele WA, and Chaney DS. 2013. The “seeds” on Padgettia readi are insect galls: reassignment of the plant to Odontopteris, the gall to Ovofoligallites n. gen., and the evolutionary implications thereof. Journal of Paleontology 87: 217–231. [Google Scholar]
- Tabor NJ, and Montañez IP. 2004. Morphology and distribution of fossil soils in the Permo-Pennsylvanian Wichita and Bowie Groups, north-central Texas, USA: implications for western equatorial Pangean palaeoclimate during icehouse–greenhouse transition. Sedimentology 51: 851–884. [Google Scholar]
- Tabor NJ, Romanchock CM, Looy CV, Hotton CL, DiMichele WA, and Chaney DS. 2013. Conservatism of Late Pennsylvanian vegetational patterns during short-term cyclic and long-term directional environmental change, western equatorial Pangea. Geological Society of London, Special Publications 376: 201–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl D, and Jasper A. 2016. New data on the macroflora of the basal Rotliegend Group (Remigiusberg Formation; Gzhelian) in the Saar-Nahe Basin (SW-Germany). Fossil Imprint 72: 239–250. [Google Scholar]
- Uhl D, and Lausberg S. 2008. Land plant diversity in selected latest Pennsylvanian?–Permian deposits from Saar-Nahe Basin (SW-Germany) and German Zechstein Basin. Studia Geologica Polonica 129: 81–106. [Google Scholar]
- Wang J, Pfefferkorn HW, Zhang Y, and Feng Z. 2012. Permian vegetational Pompeii from Inner Mongolia and its implications for landscape paleoecology and paleobiogeography of Cathaysia. Proceedings of the National Academy of Sciences 109: 4927–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D 1912. The characters of the fossil plant Gigantopteris Schenk and its occurrence in North America. Proceedings of the United States National Museum 41: 493–516. [Google Scholar]
- Zangerl R, and Richardson ES Jr. 1963. The paleoecological history of two Pennsylvanian black shales. Fieldiana: Geology Memoirs 4: 1–352. [Google Scholar]