ABSTRACT
Iron is an essential element for the replication of most bacteria, including Riemerella anatipestifer, a Gram-negative bacterial pathogen of ducks and other birds. R. anatipestifer utilizes hemoglobin-derived hemin as an iron source; however, the mechanism by which this bacterium acquires hemin from hemoglobin is largely unknown. Here, rhuA disruption was shown to impair iron utilization from duck hemoglobin in R. anatipestifer CH-1. Moreover, the putative lipoprotein RhuA was identified as a surface-exposed, outer membrane hemin-binding protein, but it could not extract hemin from duck hemoglobin. Mutagenesis studies showed that recombinant RhuAY144A, RhuAY177A, and RhuAH149A lost hemin-binding ability, suggesting that amino acid sites at tyrosine 144 (Y144), Y177, and histidine 149 (H149) are crucial for hemin binding. Furthermore, rhuR, the gene adjacent to rhuA, encodes a TonB2-dependent hemin transporter. The function of rhuA in duck hemoglobin utilization was abolished in the rhuR mutant strain, and recombinant RhuA was able to bind the cell surface of R. anatipestifer CH-1 ΔrhuA rather than R. anatipestifer CH-1 ΔrhuR ΔrhuA, indicating that RhuA associates with RhuR to function. The sequence of the RhuR-RhuA hemin utilization locus exhibits no similarity to those of characterized hemin transport systems. Thus, this locus is a novel hemin uptake locus with homologues distributed mainly in the Bacteroidetes phylum.
IMPORTANCE In vertebrates, hemin from hemoglobin is an important iron source for infectious bacteria. Many bacteria can obtain hemin from hemoglobin, but the mechanisms of hemin acquisition from hemoglobin differ among bacteria. Moreover, most studies have focused on the mechanism of hemin acquisition from mammalian hemoglobin. In this study, we found that the RhuR-RhuA locus of R. anatipestifer CH-1, a duck pathogen, is involved in hemin acquisition from duck hemoglobin via a unique pathway. RhuA was identified as an exposed outer membrane hemin-binding protein, and RhuR was identified as a TonB2-dependent hemin transporter. Moreover, the function of RhuA in hemoglobin utilization is RhuR dependent and not vice versa. The homologues of RhuR and RhuA are widely distributed in bacteria in marine environments, animals, and plants, representing a novel hemin transportation system of Gram-negative bacteria. This study not only was important for understanding hemin uptake in R. anatipestifer but also enriched the knowledge about the hemin transportation pathway in Gram-negative bacteria.
KEYWORDS: Riemerella anatipestifer, hemin-binding protein
INTRODUCTION
Iron is an essential nutrient for biological activities in most bacteria and is required by most bacteria to cause disease. Hemin, which exists in the host in the form of hemoglobin (1), can be consumed as an important iron source. In Gram-negative bacteria, classical hemin uptake systems consist of outer membrane TonB-dependent hemin receptors, the TonB-ExbB-ExbD complex, periplasmic hemin-binding proteins (HBPs), an ABC transporter, and a hemin-degrading protein/hemin oxygenase (2). TonB-dependent hemin receptors either bind hemin and hemoglobin directly or bind hemin-bound secreted hemophores, which can extract hemin from hemoglobin (3). Hemin is internalized into the periplasm by its receptor in a TonB complex-dependent manner (4). Finally, hemin is transported into the cytoplasm by the ABC transporter. In the cytoplasm, hemin is either incorporated into cytochrome apoproteins or degraded by hemin oxygenase or hemin-degrading proteins to supply iron (5). However, excess hemin or iron is toxic to bacteria. Gram-negative bacteria regulate iron or hemin uptake to maintain iron homeostasis through the ferric uptake regulator (Fur) (6).
Riemerella anatipestifer, a Gram-negative bacterium belonging to the Flavobacteriaceae family, causes the serious disease infectious serositis in ducklings and other birds worldwide (7). R. anatipestifer can utilize hemin from bovine and duck hemoglobin (Hb) (8, 9), but the components of the hemin uptake system and its mechanism of action are largely unknown. In previous studies, it was shown that TonB1 and TonB2, but not TonB3 (TbfA), were involved in hemin uptake (4, 8). However, extensive bioinformatic analyses did not allow us to find homologues of well-characterized hemin-binding proteins, such as hemophores or hemin transporters, in the proteome of R. anatipestifer (10). Although several TonB-dependent receptors have been identified (11–13), only TbdR1 was identified as a putative TonB-dependent hemin transporter (13). Recently, B739_1416-B739_1417 (rhuR-rhuA) of R. anatipestifer CH-1 was found to be significantly upregulated under iron-limited conditions (14), suggesting that this gene locus could be involved in iron or hemin uptake. In this study, RhuA was identified as an outer membrane, surface-exposed hemin-binding protein, and the RhuA amino acids Y144, Y177, and H149 were found to be crucial for its hemin-binding activity. In addition, the adjacent gene rhuR was identified as a TonB2-dependent hemin transporter, and the function of RhuA was RhuR dependent.
RESULTS
RhuA disruption of R. anatipestifer CH-1 significantly decreases iron utilization from duck hemoglobin.
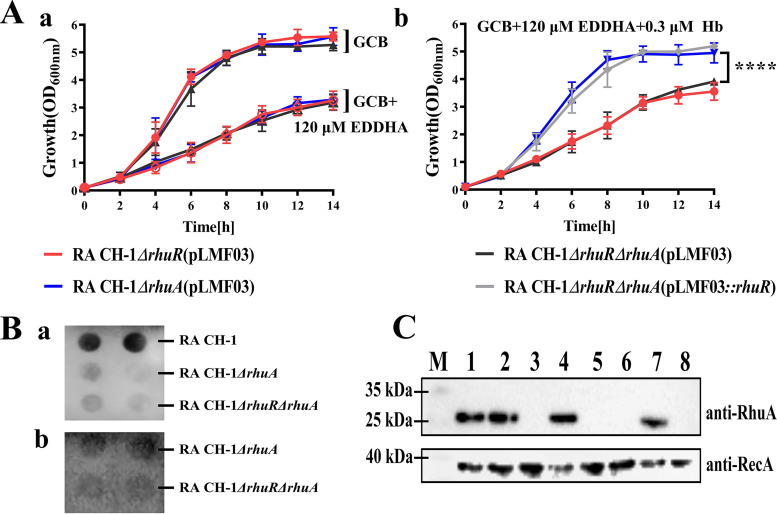
R. anatipestifer can transport hemin from duck hemoglobin (9) and encodes various uncharacterized hemin-binding proteins (15). A previous study showed that B739_1417 was significantly upregulated under iron-limited conditions (14). In the genome of R. anatipestifer CH-1, B739_1417 is annotated as a hypothetical lipoprotein. First, we assessed whether B739_1417 of R. anatipestifer CH-1 is involved in iron utilization from duck hemoglobin. As shown in Fig. 1A, B739_1417 disruption did not affect the growth of R. anatipestifer CH-1 in GC medium base (GCB) (51) or GCB containing 120 μM ethylenediamine-N,N′-bis[(2-hydroxyphenyl)acetic acid] (EDDHA). Moreover, the addition of 120 μM Fe(NO3) to GCB containing 120 μM EDDHA restored the growth of all strains equally (Fig. 1B). However, compared with that of the parent strain, the growth of the B739_1417 mutant was slightly decreased in GCB containing 120 μM EDDHA and 0.3 μM duck hemoglobin. The reintroduction of B739_1417 into the mutant strain via a recombinant plasmid expressing B739_1417 restored the growth ability of the mutant strain under the same conditions (Fig. 1C). This result suggests that the activity of B739_1417 is indeed required for the transport of hemin from duck hemoglobin to obtain an iron source and that residual hemin uptake systems could import iron from duck hemoglobin. Subsequently, we named B739_1417 as RhuA (R. anatipestifer hemin uptake protein A). Furthermore, we assessed whether RhuA is specifically required for duck hemoglobin utilization, with the result that an R. anatipestifer CH-1 rhuA mutant was also disrupted for iron utilization from bovine hemoglobin (see Fig. S1 in the supplemental material).
FIG 1.
Growth curves of R. anatipestifer (RA) CH-1(pLMF03) and its derivatives in different GCB media. The strains were grown in GCB overnight. Next, bacterial cells were inoculated into GCB, GCB containing 120 μM EDDHA, and GCB containing 120 μM EDDHA supplemented with 120 μM Fe(NO3)3 or 0.3 μM duck hemoglobin, separately, at an OD600 of 0.1. The OD600 value was measured at 2-h intervals for 14 h. The experiment was repeated at least three times, and the data were analyzed using Student’s t test. **, P < 0.01.
The RhuA protein is located in the outer membrane with surface exposure.
To better understand the role of RhuA in hemin transport, we determined its subcellular localization. Sequence analysis showed that the predicted N terminus of RhuA contains a prokaryotic membrane lipoprotein lipid attachment site (http://prosite.expasy.org/). In addition, the cysteine in the N terminus (position +17) is followed by a conserved lipoprotein export signal of Gram-negative bacteria (SKDR in RhuA) that is responsible for the flipping of lipoproteins from the inner to the outer membrane in Capnocytophaga canimorsus (16).
To determine the localization of the RhuA protein, R. anatipestifer CH-1 cells were fractionated into the cytoplasmic and membrane fractions; the secreted proteins were also isolated (Fig. S2A). These fractions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis with an anti-recombinant RhuA (rRhuA) antibody. A band corresponding to an ∼25-kDa protein was detected mainly in the membrane fraction but not among the cytoplasmic or secreted proteins (Fig. 2A and Fig. S2B). In contrast, the control cytoplasmic protein RecA was detected only in the cytoplasmic fraction by the antibody specific for recombinant RecA (rRecA). This result indicated that RhuA was a membrane protein.
FIG 2.
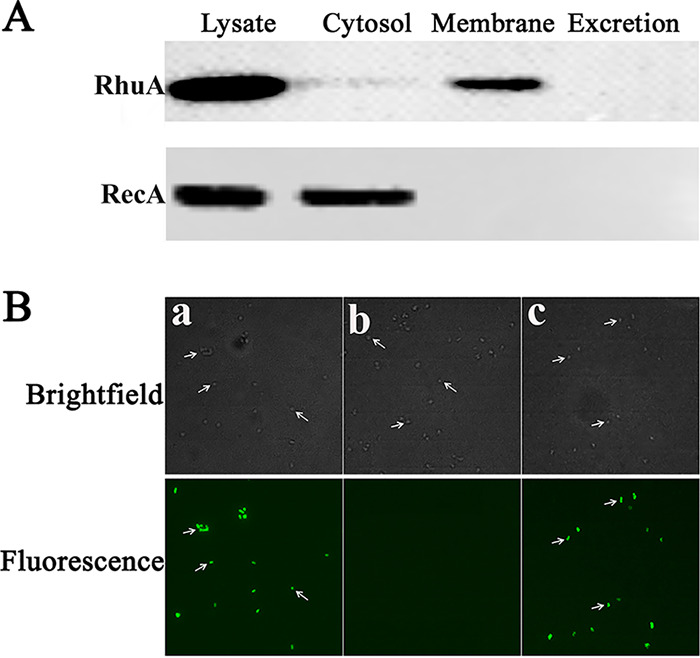
Subcellular localization and surface exposure of RhuA in R. anatipestifer CH-1. (A) Subcellular fractions of R. anatipestifer CH-1 were prepared and subjected to immunoblot analysis using antibodies against rRhuA. The localization of the cytoplasmic protein RecA was used as the control. Lysate, whole-cell lysate of R. anatipestifer CH-1; Cytosol, cytoplasmic protein fraction of R. anatipestifer CH-1; Membrane, membrane protein fraction of R. anatipestifer CH-1; Excretion, secreted proteins isolated from the R. anatipestifer CH-1 culture. (B) Immunofluorescence micrographs of bacteria labeled with anti-rRhuA serum as described in Materials and Methods. Micrographs were acquired with a Nikon Eclipse 80i microscope. (a) Strain R. anatipestifer CH-1(pLMF03); (b) strain R. anatipestifer CH-1 ΔrhuA(pLMF03); (c) strain R. anatipestifer CH-1 ΔrhuA(pLMF03::rhuA).
Although RhuA was predicted to be an outer membrane lipoprotein and was demonstrated to be associated with the membrane of R. anatipestifer CH-1, the orientation of this protein in the membrane is unknown. Bacterial lipoproteins may be located at the cell surface or directed into the periplasmic space (17). To determine whether RhuA is surface exposed, we used immunolabeling to detect the orientation of RhuA on the cell surface of R. anatipestifer CH-1 as described previously (16). As shown in Fig. 2B, in immunolabeling experiments, RhuA was detected on the cell surface of R. anatipestifer CH-1(pLMF03) and the complementation strain R. anatipestifer CH-1 ΔrhuA(pLMF03::rhuA), but it was not detected on the cell surface of R. anatipestifer CH-1 ΔrhuA(pLMF03). This result showed that RhuA is a cell surface protein of R. anatipestifer CH-1.
The recombinant RhuA protein binds free hemin.
Since RhuA is involved in the hemin uptake process of R. anatipestifer CH-1, we performed two hemin-binding experiments to test whether the rRhuA protein can bind hemin in vitro. First, the recombinant protein and a mixture of the recombinant protein and hemin were run on two native gels as described in Materials and Methods. One gel was stained with Coomassie brilliant blue R (Fig. 3Aa). Proteins on the other gel were transferred to a nitrocellulose membrane and then detected using an enhanced chemiluminescence (ECL) method since hemin has intrinsic peroxidase activity (18). As shown in Fig. 3Ab, hemin was detected when it was preincubated with the recombinant hemin-binding protein HasA of Serratia marcescens (19) (positive control) and rRhuA. However, in the lane loaded with only the recombinant protein, hemin was undetectable (Fig. 3Ab).
FIG 3.
Detection of hemin binding to the rRhuA protein. (A) Equal amounts of recombinant protein or mixtures of recombinant protein and hemin were loaded into native polyacrylamide gels. (a) After electrophoresis, one gel was stained with Coomassie brilliant blue. (b) The proteins on the other gel were transferred to a nitrocellulose membrane and detected by ECL. Lane 1, mixture of rHasA and hemin; lane 2, rHasA; lane 3, mixture of rRhuA and hemin; lane 4, rRhuA. (B) Absorption spectra of hemin binding to rRhuA. Increasing concentrations of hemin (1 to 40 μM) were added to 20 μM rRhuA protein. The inset shows the absorbance values of hemin-rRhuA minus those of hemin alone at 397 nm at increasing hemin concentrations. Experiments were performed in triplicate, and the results of a single representative experiment are presented.
To further strengthen the above-described result, the binding of hemin by rRhuA was assessed spectrophotometrically. The spectral properties of hemin change when it binds to a protein, yielding a specific Soret band. In the sample with hemin added to the rRhuA protein, the appearance of a peak at 397 nm indicated the formation of a complex between hemin and rRhuA since the absorbance maximum of free hemin is 378 nm. The complex formed between hemin and rRhuA was also quantified using spectral changes in the visible absorption spectrum of the mixture (Fig. 3B). Plotting the spectral changes observed at 397 nm versus the hemin concentration yielded a saturation curve (Fig. 3B). The binding of hemin to rRhuA occurred in a concentration-dependent manner, and these results showed that rRhuA interacts with hemin in a 1:1 molar ratio (Fig. 3B). Taken together, these results demonstrated that rRhuA can bind hemin in vitro.
The rRhuA protein cannot extract hemin from duck hemoglobin.
Since RhuA is a surface-exposed, outer membrane hemin-binding protein, we sought to determine whether it functions as a hemophore that can extract hemin from hemoglobin. To test this hypothesis, the model Escherichia coli strain C600 ΔhemA(pAM238::hemR) was introduced in this experiment. E. coli C600 ΔhemA cannot grow on Luria-Bertani (LB) plates, as it is deficient in the heme synthesis enzyme HemA (20). This E. coli C600 ΔhemA strain can transport extracellular hemin when the bacterium is engineered to express a functional TonB complex-dependent outer membrane hemin receptor, such as HemR of S. marcescens (21), which can transport both free hemin and hemin from hemoglobin directly (21). As shown in Fig. 4A, the C600 ΔhemA(pAM238::hemR) strain grew around the well containing only hemin; however, it did not grow around the well containing both hemin and recombinant HasA (rHasA), a hemophore of S. marcescens (positive control), which has been shown to be highly dependent on its cognate receptor HasR (19). This result is consistent with the finding that rHasA bound free hemin and prevented HemR from transporting it. Similarly, the C600 ΔhemA(pAM238::hemR) strain did not grow around the well containing both hemin and rRhuA, suggesting that rRhuA binds hemin and that hemin cannot be captured by HemR. As the negative control, the addition of rRecA did not affect the hemin utilization of C600 ΔhemA(pAM238::hemR). In parallel, as shown in Fig. 4B, the C600 ΔhemA(pAM238::hemR) strain grew robustly in the well containing only duck hemoglobin, but it did not grow in the well containing both duck hemoglobin and rHasA (positive control), suggesting that rHasA captures hemin from duck hemoglobin and subsequently prevents hemin from being transported by HemR. However, the addition of rRhuA or rRecA (negative control) to the well did not affect the utilization of duck hemoglobin by C600 ΔhemA(pAM238::hemR). The observation that RhuA is incapable of removing hemin from hemoglobin, similar to the HasA hemophore, suggests that other factors (i.e., proteases) from R. anatipestifer mediate the release of hemin from hemoglobin.
FIG 4.
Detection of hemin extraction by rRhuA from duck hemoglobin. C600 ΔhemA(pAM238::hemR) was mixed with 4 ml of soft LB agar and poured onto LB plates. Holes were cut into the LB plates, and 20 μM hemin, a mixture of 20 μM hemin with 20 μM recombinant protein, 5 μM duck Hb, and a mixture of 5 μM duck Hb with 5 μM recombinant protein were added to the wells. rHasA, recombinant HasA of S. marcescens; rRhuA, recombinant RhuA of R. anatipestifer CH-1; rRecA, recombinant RecA of R. anatipestifer CH-1. The plates were incubated at 37°C overnight. The experiment was repeated three times, and a representative result is presented. The lucid zone represents the zone of C600 ΔhemA(pAM238::hemR) growth.
Affinity of rRhuA for hemin.
To examine the affinity of rRhuA for hemin, we performed localized surface plasmon resonance (LSPR) experiments (22). rRhuA was covalently coupled to a nitrilotriacetic acid (NTA) sensor chip. The signal intensity of rRhuA protein binding increased depending on the hemin concentration (Fig. 5). Kinetic analysis determined a dissociation constant (Kd) of 5 × 10−6 M and an association rate constant (Ka) of 7 × 102 M−1 s−1 for rRhuA protein-hemin binding. The binding affinity of rRhuA for hemin was much lower than that of HasA (Kd = 10−12 M) (23).
FIG 5.

Affinity of rRhuA for hemin. rRhuA was immobilized on an NTA sensor chip (Nicoya). The kinetic parameters and concentrations used for the analysis are indicated. Protein-hemin interactions were monitored over a 5-min time period and reported as signal (picomole [pm]) values.
Identification of the key amino acid site for hemin binding by modeling.
BLAST searches with RhuA of R. anatipestifer CH-1 revealed that RhuA contains the same domain as HmuY of Porphyromonas gingivalis, although the sequence identity is only 15%. The structure of RhuA was generated based on the structure of P. gingivalis HmuY using Phyre2 (24). As RhuA has low sequence identity to HmuY of P. gingivalis, we also used the iterative threading assembly refinement (I-TASSER) server to calculate the structure of RhuA starting from an initial amino acid sequence (25). The Phyre2 model comprises 13 β-strands connected by loops (Fig. 6A). Both the N and C termini are located on the bottom of the protein, and the domain spanning amino acids 141 to 154 (WYTYDMSTHTIMPI) and the domain spanning amino acids 175 to 181 (YYKGAP) form a cavity, which was predicted to bind hemin (Fig. 6A). The I-TASSER model has an organization similar to that of the Phyre2 model but comprises 8 β-strands connected by loops (Fig. 6B). Moreover, the sequence comparison showed that the putative hemin-binding cavity was the most conserved region among RhuA and its homologues (Fig. 6C). Many studies have shown that histidine (H), tyrosine (Y), methionine (M), and cysteine (C) residues are crucial hemin-binding residues for most hemin-binding proteins (23, 26, 27). Inspection of the putative hemin-binding site in RhuA suggested that 142Y, 144Y, 149H, 176Y, and 177Y potentially provide an axial ligand to bind hemin (Fig. 6C). Thus, RhuA142Y-A-144Y-A (RhuA with Y-to-A changes at residues 142 and 144), RhuA177Y-A, RhuA144Y-A, RhuA176Y-A, RhuA144Y-A-176Y-A, and RhuA149H-A mutants were constructed. Compared to rRhuA, purified rRhuA containing the Y176-to-A (176Y-A) substitution did not exhibit significantly altered hemin binding (Fig. 7A). However, rRhuA containing the 142Y-A-144Y-A, 177Y-A, 144Y-A, 144Y-A-176Y-A, or 149H-A mutation displayed a dramatically reduced ability to bind hemin (Fig. 7A). The reduced hemin-binding ability was further demonstrated using dot blotting. At 10−6 M hemin, rRhuA and all the mutants were able to bind hemin, although RhuA142Y-A-144Y-A, RhuA177Y-A, RhuA144Y-A, RhuA144Y-A-176Y-A, and RhuA149H-A had lower hemin-binding abilities than RhuA and RhuA176Y-A. However, when the concentration of hemin was lower than 5 × 10−7 M, only RhuA and RhuA176Y-A retained their hemin-binding ability (Fig. S3). In parallel, hemin binding was also detected using the native PAGE method. As shown in Fig. 7B, hemin was detected only in the mixture of rRhuA and hemin and the mixture of RhuA176Y-A and hemin after native PAGE. Taken together, these results indicated that the Y144, Y177, and H149 residues of RhuA are involved in hemin binding.
FIG 6.
3D model of RhuA and sequence alignment of putative hemin-binding domains. (A) 3D model of RhuA built with Phyre2. The putative hemin-binding domain is shown in red. The potential crucial amino acid sites for hemin binding are indicated. (B) 3D model of RhuA built with I-TASSER. The putative hemin-binding domains are shown in red. The potential crucial amino acid sites for hemin binding are indicated. (C) Sequence alignment of putative hemin-binding domains (HBD) in RhuA and its orthologues. Sequence alignment was carried out using Clustal Omega, and figures were generated using Jalview 2. Universally conserved residues are highlighted in black, while putative conserved hemin-binding sites are highlighted in red. C. kapabacteria, “Candidatus Kapabacteria”; E. sp., Elizabethkingia sp.
FIG 7.
Detection of hemin binding by rRhuA mutants. (A) Absorption spectra of 20 μM hemin binding to rRhuA or its mutants. (B) Mixtures of recombinant mutant proteins and hemin were loaded separately into native polyacrylamide gels. (a) After electrophoresis, one gel was stained with Coomassie brilliant blue. (b) The proteins on the other gel were transferred to a nitrocellulose membrane and detected by ECL (b). Mutant 1, RhuA142Y-A-144Y-A; mutant 2, RhuA177Y-A; mutant 3, RhuA144Y-A; mutant 4, RhuA176Y-A; mutant 5, RhuA144Y-A-176Y-A; mutant 6, RhuA149H-A.
The gene adjacent to rhuA, rhuR, encodes a TonB2-dependent hemin transporter.
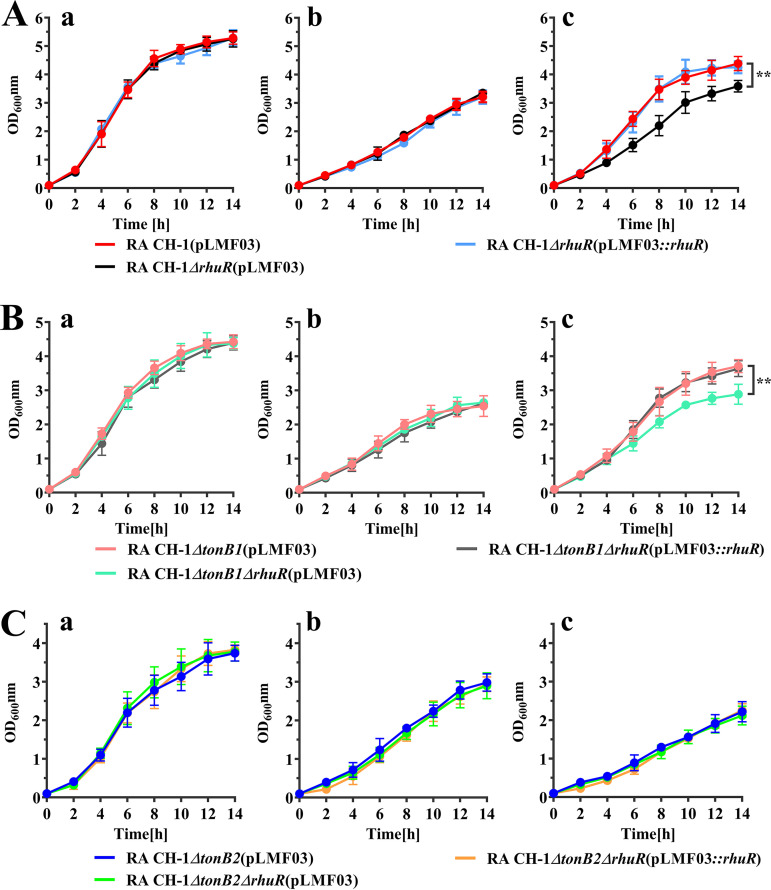
Sequence analysis showed that RhuA is located in the same operon as B739_1416, which encodes a putative TonB-dependent receptor. Thus, we first investigated whether B739_1416 is involved in hemin acquisition from duck hemoglobin as a hemin transporter. As shown in Fig. 8A, compared with the wild type, the B739_1416 mutant did not affect the growth of R. anatipestifer CH-1 in GCB and GCB supplemented with 120 μM EDDHA; however, growth was significantly impaired in iron-limited GCB supplemented with 0.3 μM duck hemoglobin. Moreover, this effect on growth was reversed by the introduction of a recombinant plasmid expressing B739_1416 into the mutant strain. Since B739_1416 is upstream of RhuA, we detected the expression of RhuA in R. anatipestifer CH-1 ΔB739_1416 as a control. Compared with the parent strain, the B739_1416 deletion strain exhibited no effect on the expression of RhuA (data not shown), and we named B739_1416 as RhuR (R. anatipestifer hemin uptake receptor).
FIG 8.
Growth curves of R. anatipestifer CH-1, CH-1 ΔrhuR, and its derivative strains in different GCB media. (A) R. anatipestifer CH-1(pLMF03), CH-1 ΔrhuR(pLMF03), and CH-1 ΔrhuR(pLMF03)::rhuR bacterial cells in the exponential growth phase were inoculated into fresh GCB (a), GCB containing 120 μM EDDHA (b), and GCB containing 120 μM EDDHA supplemented with 0.3 μM duck hemoglobin (c). Bacteria were cultured at 37°C with shaking at 180 rpm, and the OD600 was measured every 2 h for 14 h. (B) R. anatipestifer CH-1 ΔtonB1(pLMF03), CH-1 ΔtonB1 ΔrhuR(pLMF03), and CH-1 ΔtonB1 ΔrhuR(pLMF03::rhuR) bacterial cells in the exponential growth phase were inoculated into fresh GCB (a), GCB containing 120 μM EDDHA (b), and GCB containing 120 μM EDDHA supplemented with 0.3 μM duck hemoglobin (c). Bacteria were cultured at 37°C with shaking at 180 rpm, and the OD600 was measured every 2 h for 14 h. (C) R. anatipestifer CH-1 ΔtonB2(pLMF03), CH-1 ΔtonB2 ΔrhuR(pLMF03), and CH-1 ΔtonB2 ΔrhuR(pLMF03::rhuR) bacterial cells in the exponential growth phase were inoculated into fresh GCB (a), GCB containing 120 μM EDDHA (b), and GCB containing 120 μM EDDHA supplemented with 0.3 μM duck hemoglobin (c). Bacteria were cultured at 37°C with shaking at 180 rpm, and the OD600 was measured every 2 h for 14 h. The error bars indicate the standard deviations from three repeated experiments. The data were analyzed using two-way analysis of variance (ANOVA). A P value of <0.05 indicates a statistically significant difference. **, P < 0.01.
To further identify the dependence of RhuR on TonB for hemin transport, we constructed a tonB1 rhuR double mutant and a tonB2 rhuR double mutant and assessed the effect of these double mutations on hemin utilization from duck hemoglobin. As shown in Fig. 8B, compared with the tonB1 single mutant, the tonB1 rhuR double mutant grew more slowly in iron-limited GCB supplemented with 0.3 μM duck hemoglobin. In contrast, compared with the tonB2 single mutant, the tonB2 rhuR double mutant did not exhibit any difference in growth in iron-limited GCB supplemented with 0.3 μM duck hemoglobin (Fig. 8C). Furthermore, the introduction of a recombinant plasmid expressing RhuR partially restored the growth of the tonB1 rhuR double mutant in iron-limited medium supplemented with 0.3 μM duck hemoglobin. The results suggested that the function of RhuR is dependent on the TonB2 complex.
Attempts to express and purify rRhuR in E. coli were unsuccessful. Therefore, we developed a whole-cell assay to measure RhuR hemin binding as described in a previous study (28). First, we used RhuA as a proof of concept to verify the feasibility and validity of the method. As shown in Fig. 9A, the amount of bound hemin was slightly decreased in R. anatipestifer CH-1 ΔrhuA compared with that in the parent strain. In parallel, the hemin-binding activity observed in R. anatipestifer CH-1 under iron starvation conditions was significantly greater than that observed in R. anatipestifer CH-1 ΔrhuR (Fig. 9B). Moreover, the hemin-binding ability of R. anatipestifer CH-1 ΔrhuR was restored by the introduction of a recombinant plasmid expressing RhuR into the mutant strain (Fig. 9B). Taken together, these results suggested that RhuR is a TonB2-dependent hemin receptor.
FIG 9.
Hemin binding assay of R. anatipestifer CH-1 and its derivative strains. Cells were incubated under iron starvation conditions, and a hemin-binding assay was performed. The mixture of 10 μg of hemin and 1 ml of bacteria (OD600 = 1) was incubated for 2 h and 4 h, respectively. The binding reactions were terminated by centrifugation at 10,000 × g for 5 min. The amount of unbound hemin was determined by measuring the OD of hemin at 405 nm with a NanoDrop 2000 instrument (Thermo Scientific). The amount of bound hemin on the cells was calculated by subtraction. Each value indicates the mean of the results from three experiments, and the vertical bars denote the standard deviations. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
The function of RhuA is associated with RhuR.
Because the gene encoding RhuA is adjacent to the gene encoding RhuR, we reasoned that RhuA might associate with RhuR to transport hemin. To test this hypothesis, we evaluated the effect of rhuA deletion on hemin utilization in R. anatipestifer CH-1 ΔrhuR. As shown in Fig. 10Aa, compared with the rhuR or rhuA single mutant, the rhuR-rhuA double mutant did not exhibit any difference in growth in GCB or GCB containing 120 μM EDDHA. In parallel, as shown in Fig. 10Ab, compared with the rhuA single mutant, the rhuR-rhuA double mutant exhibited a growth deficiency in GCB containing 120 μM EDDHA and 0.3 μM duck hemoglobin. However, compared with the rhuR single mutant, the rhuR-rhuA double mutant did not exhibit any difference in growth in GCB containing 120 μM EDDHA and 0.3 μM duck hemoglobin. These results indicated that RhuA plays a role in the presence of RhuR; however, RhuR was independent of RhuA.
FIG 10.
The function of RhuA is dependent on RhuR. (Aa) Growth curves of R. anatipestifer CH-1 ΔrhuA(pLMF03), CH-1 ΔrhuR(pLMF03), and CH-1 ΔrhuR ΔrhuA(pLMF03) in GCB and GCB containing 120 μM EDDHA. (b) Growth curves of R. anatipestifer CH-1 ΔrhuA(pLMF03), CH-1 ΔrhuR(pLMF03), CH-1 ΔrhuR ΔrhuA(pLMF03), and CH-1 ΔrhuR ΔrhuA(pLMF03::rhuR) in GCB containing 120 μM EDDHA supplemented with 0.3 μM duck hemoglobin. Bacteria were cultured at 37°C with shaking at 180 rpm, and the OD600 was measured every 2 h for 14 h. (Ba) A total of 108 CFU of whole cells of R. anatipestifer CH-1 and its derivative strains were dropped on the filter as indicated (double spots for each strain). The filter was dried and blocked with a blocking solution. The dot blots were probed with mouse polyclonal anti-RhuA serum at a 1:400 dilution. (b) A total of 108 CFU of whole cells of R. anatipestifer CH-1 ΔrhuA(pLMF03) and CH-1 ΔrhuR ΔrhuA were dropped on the filter as indicated. The filter was dried and blocked with a blocking solution. The filter was incubated with rRhuA at a 4 μM concentration at room temperature for 30 min. After washing three times, the dot blots were probed with mouse polyclonal anti-RhuA serum at a 1:400 dilution. (C) rRhuA binds to the cell surface of R. anatipestifer CH-1 ΔrhuA but not the cell surface of R. anatipestifer CH-1 ΔrhuR. The binding of rRhuA to the cell surface of R. anatipestifer CH-1 ΔrhuA and its derivative strains was assessed as described in Materials and Methods. M, molecular weight; lane 1, mixture of rRhuA and rRecA; lane 2, R. anatipestifer CH-1; lane 3, R. anatipestifer CH-1 ΔrhuA; lane 4, sample of R. anatipestifer CH-1 ΔrhuA incubated with rRhuA and hemin; lane 5, R. anatipestifer CH-1 ΔrhuR ΔrhuA; lane 6, sample of R. anatipestifer CH-1 ΔrhuR ΔrhuA incubated with rRhuA and hemin; lane 7, sample of R. anatipestifer CH-1 ΔrhuA incubated with rRhuA; lane 8, sample of R. anatipestifer CH-1 ΔrhuR ΔrhuA incubated with rRhuA. ****, P < 0.0001.
Given that the function of RhuA was associated with RhuR, we hypothesized that RhuA might interact with the TonB-dependent hemin receptor protein RhuR to transport hemin into the cell. We first determined if RhuA of R. anatipestifer CH-1 was able to be immunodetected by the anti-RhuA polyclonal antibodies through dot blot analysis. As shown in Fig. 10Ba, a clear signal was obtained with approximately 108 CFU of R. anatipestifer CH-1 spotted onto the filters. However, there was no signal observed with the control strain R. anatipestifer CH-1 ΔrhuA and R. anatipestifer CH-1 ΔrhuR ΔrhuA at the same bacterial density (Fig. 10Ba). Next, filters spotted with R. anatipestifer CH-1 ΔrhuA and R. anatipestifer CH-1 ΔrhuR ΔrhuA bacteria were incubated with 4 μM rRhuA and probed with the anti-RhuA polyclonal antibodies. The results showed detectable signals at the sites spotted with R. anatipestifer CH-1 ΔrhuA; however, no signal was detected on the sites spotted with R. anatipestifer CH-1 ΔrhuR ΔrhuA (Fig. 10Bb), suggesting that RhuA has an interaction with RhuR.
Furthermore, we used Western blotting to determine if rRhuA was able to bind to the surface of R. anatipestifer CH-1 ΔrhuA and if hemin loading of rRhuA will strengthen binding, as described in Materials and Methods. As shown in Fig. 10C, RhuA was detectable on R. anatipestifer CH-1 ΔrhuA bacteria incubated with rRhuA but could not be detected on R. anatipestifer CH-1 ΔrhuR ΔrhuA bacteria incubated with rRhuA, whether in the presence or absence of hemin. These data suggested that RhuA and RhuR are involved in direct protein-protein interactions rather than interactions via hemin.
Transcription of the rhuR-rhuA locus is regulated by Fur but not by hemin.
In a previous study, RNA sequencing (RNA-seq) analysis showed that the transcription of rhuR-rhuA was upregulated under iron-limited conditions (14). To further investigate whether this regulation is mediated by Fur, the transcript level of rhuR-rhuA was measured in R. anatipestifer CH-1 Δfur cells in GCB by quantitative real-time PCR. As a control, the transcript level of rhuR-rhuA in R. anatipestifer CH-1 was also measured in GCB and iron-limited GCB. As shown in Fig. 11A, the transcription of rhuR-rhuA in R. anatipestifer CH-1 was upregulated more than 80-fold in iron-limited GCB compared with that in GCB. Similarly, the transcription of rhuR and rhuA in R. anatipestifer CH-1 Δfur was upregulated more than 120-fold relative to rhuR and rhuA of R. anatipestifer CH-1 when the bacteria were grown in GCB (Fig. 11B). Moreover, the increase in transcription was fully reversed to the wild-type level by complementation with fur (Fig. 11B). Analysis of the promoter sequence of the rhuR-rhuA locus showed that it contained the classical R. anatipestifer Fur box sequence (ATTTAAAATTATTCTAAAT) (29). Furthermore, the negative regulatory effect of Fur and iron on rhuR-rhuA locus transcription was further confirmed at the protein level using RhuA production as an indicator. As shown in Fig. 11C, the RhuA expression level was higher in iron-limited GCB than in GCB, and the RhuA expression level was higher in the fur mutant strain than in the wild-type and complementation strains.
FIG 11.
Regulation of the RhuR-RhuA locus in R. anatipestifer CH-1. (A) R. anatipestifer CH-1 bacterial cells were cultured in GCB, GCB containing 120 μM EDDHA, and GCB containing 120 μM EDDHA supplemented with 240 μM Fe(NO3)3. Bacterial cells in the exponential growth phase were harvested, and the relative transcription of rhuR-rhuA was measured as described in Materials and Methods. (B) R. anatipestifer CH-1, CH-1 Δfur, and CH-1 Δfur(pLMF03::fur) bacterial cells were cultured in GCB. In parallel, R. anatipestifer CH-1 Δfur bacterial cells were cultured in GCB containing 120 μM EDDHA. Bacterial cells in the exponential growth phase were harvested, and the relative transcription of rhuR-rhuA was measured as described in Materials and Methods. (C) R. anatipestifer CH-1 bacterial cells were cultured in GCB, GCB containing 120 μM EDDHA, and GCB containing 120 μM EDDHA supplemented with 240 μM Fe(NO3)3. In parallel, R. anatipestifer CH-1 Δfur(pLMF03) and CH-1 Δfur(pLMF03::fur) bacterial cells were cultured in GCB. The bacteria were harvested and subjected to SDS-PAGE, and RhuA and RecA were then detected by Western blotting as described in Materials and Methods. (D) R. anatipestifer CH-1 bacterial cells were cultured in GCB and GCB containing 20 μM hemin. In parallel, R. anatipestifer CH-1 ΔhemA bacterial cells were cultured in GCB containing 20% δ-ala to an OD600 of 1 to 1.5, harvested, and inoculated into GCB and GCB containing 20 μM hemin. ****, P < 0.0001; ns, not significant.
Since hemin can provide a source of both iron and hemin, we sought to determine whether rhuR-rhuA is regulated by hemin. As shown in Fig. 11D, compared with that in the wild-type strain, the transcript level of rhuR-rhuA showed no significant change in R. anatipestifer CH-1 ΔhemA, which cannot synthesize hemin. Similarly, the transcript levels of rhuR-rhuA in R. anatipestifer CH-1 ΔhemA did not significantly differ between bacterial cells grown in GCB containing 20 μM hemin and those grown in GCB. Additionally, the transcript levels of rhuR-rhuA of R. anatipestifer CH-1 did not differ between cells grown in GCB supplemented with 20 μM hemin and cells grown in GCB (Fig. 11D). Furthermore, similar results were obtained by substituting duck hemoglobin for hemin (data not shown). Collectively, these results suggested that in RhuR-RhuA, hemin transport is regulated by iron, mediated by Fur, rather than by hemin.
RhuR-RhuA is not required for virulence of R. anatipestifer CH-1.
Since RhuR-RhuA was shown to be involved in hemin transport from duck hemoglobin, we further tested whether RhuR-RhuA contributes to the virulence of R. anatipestifer CH-1. The R. anatipestifer strains CH-1, CH-1 ΔrhuA, CH-1 ΔrhuR, and CH-1 ΔrhuR ΔrhuA were used to infect 3-day-old ducklings, as described in Materials and Methods. The results showed that the virulence of all the mutants was not significantly attenuated compared to that of the parent strain (Fig. S4). Furthermore, we tested whether the mutants had an effect on colonization in 3-day-old ducklings. The results showed that at 24 h postinfection, all the mutants and the parent strain had colonized the ducklings at similar levels (Fig. S4). Taken together, these results suggest that rhuR-rhuA deletion is not sufficient to reduce the pathogenicity of R. anatipestifer CH-1 toward ducklings.
Distribution of RhuR-RhuA in the Bacteroidetes phylum.
Phylogenetic analysis of the 60 sequenced strains indicates that strains that contain a homologue of RhuA also contain a homologue of RhuR. Genomic analyses of the RhuR-RhuA locus indicated that this system is highly conserved in all sequenced R. anatipestifer strains, with 99% identity, and in other Bacteroidetes such as Riemerella columbina, Elizabethkingia meningoseptica, and Flavobacterium johnsoniae, with 34% to 62% identities. These bacteria are widely distributed in marine environments (39.7%), animals (20.7%), plants (19.0%), soil (10.3%), and freshwater (10.3%) (Fig. S5). These bacteria are proposed to use conserved mechanisms to handle different hemin environments.
DISCUSSION
Iron is an essential element for the replication and infection of most bacteria, including R. anatipestifer (4, 8, 11, 12, 14), as it has key functions in biological catalysis and electron transfer. In vertebrate hosts, most iron is stored intracellularly as hemoglobin (1, 2). Bacteria have evolved diversified hemin uptake systems to obtain hemin as an iron source. A previous study showed that the transcript level of RhuR-RhuA in R. anatipestifer CH-1 was upregulated in iron-limited medium (14); however, whether RhuR and RhuA are involved in the utilization of hemin from hemoglobin and how these proteins function have remained unknown.
In Gram-negative bacteria, hemin is transported from the outer membrane to the periplasm by a specific TonB-dependent hemin receptor (2). In addition to the TonB-dependent hemin receptor, some bacteria also encode outer membrane hemin-binding proteins. Currently, the lipoprotein HpuA of the Neisseriaceae family (22, 30), the lipoprotein HmuY of P. gingivalis (31, 32), and hemin-binding proteins of Bartonella (33, 34) have been characterized as outer membrane proteins and are involved in hemin uptake.
In the HpuAB hemin transporter of Neisseria meningitidis, HpuA and HpuB (a TonB-dependent outer membrane transporter) physically interact to form a functional complex, and both receptor components are required for the use of hemoglobin as a hemin source (35). Initially, direct binding between HpuA of meningococci and hemoglobin was not detected (36). Recently, HpuA proteins of Neisseria gonorrhoeae and Kingella denitrificans were shown to be able to bind human hemoglobin with low affinity (30). Sequence comparison showed that the RhuA protein sequence shares no identity with the HpuA protein sequence of N. gonorrhoeae (less than 8% identity). Moreover, the receptor of RhuA, RhuR, can transport hemin independently of RhuA. Thus, these observations indicate that the hemin uptake mechanism mediated by RhuA is different from that of HpuA of Neisseria.
In the HmuY-HmuR system of P. gingivalis, hmuY encodes a lipoprotein (23 kDa) that is anchored to the outer membrane by a lipid-modified cysteine residue located downstream of the signal peptide cleavage site (31). This protein can be cleaved by an enzyme at a Lys-Asp site, leading to an N-terminally truncated extracellular protein (31). HmuY captures hemin from hemoglobin with the help of arginine (R)-specific gingipain protease A (R-gingipain A [RgpA]) or lysine (K)-specific gingipain protease (K-gingipain [Kgp]) (32). Although the structure of HmuY has been solved (31), hemin transfer from HmuY to its receptor HmuR and the interaction between these proteins have not been demonstrated. Moreover, sequence comparison indicated that the R. anatipestifer genome does not encode homologues of K-gingipain and R-gingipain. Thus, RhuA is unlikely to function in a manner similar to that of HmuY of P. gingivalis.
Although the hemin-binding protein RhuA is annotated as an HmuY-like family protein, the identity between these proteins is low (less than 15%), suggesting that RhuA binds hemin in a unique manner. To determine how RhuA interacts with hemin, we modeled its structure using both Phyre2 and I-TASSER. The model of the putative hemin-binding domain revealed that it is similar to a β-sandwich-type fold. Consistent with the model, this region is conserved in the orthologues of RhuA. To identify the key amino acid sites that contact hemin, we compared the primary sequences of the orthologues and performed targeted mutagenesis. Based on the hemin-binding sites in other hemin-binding proteins, Y142, Y144, H149, Y176, and Y177 are conserved and were predicted as putative hemin-binding sites. Y142, Y144, and H149 are in one of the extended loops, while Y176 and Y177 are located in another extended loop. Mutational replacement of the tyrosine with an alanine at Y144, Y177, and H149 significantly decreased hemin binding, suggesting that the amino acid residues Y144, Y177, and H149 within this domain are important for hemin binding. According to the model, Y176 and Y142 are located at the bottom of the pocket and are unlikely to be involved in hemin binding. A structural study of HmuY-hemin confirmed that H134 and H166 participate in heme ligand binding (31). Thus, the model of RhuA binding to hemin is different from that of HmuY.
Although rhuA encodes an outer membrane hemin-binding protein, it cannot extract hemin from duck hemoglobin, suggesting that it is not the first step in the acquisition of hemin from duck hemoglobin and that it alone cannot transport hemin into the periplasm. Since its adjacent encoding gene, rhuR, was also upregulated under iron-limited conditions and encodes a TonB-dependent receptor, we hypothesized that RhuR is involved in hemin uptake. As expected, the RhuR mutant exhibited a significantly decreased efficiency of hemin utilization from duck hemoglobin under iron-limited conditions. Moreover, the hemin-binding ability of R. anatipestifer CH-1 was decreased significantly when RhuR was knocked out. Finally, the phenotype of decreased hemin uptake efficiency under iron-limited conditions in the strain with an RhuR mutation was abolished in the R. anatipestifer CH-1 ΔtonB2 strain; however, this phenotype was still observed in R. anatipestifer CH-1 ΔtonB1, suggesting that RhuR is TonB2 dependent. Collectively, these results demonstrated that rhuR encodes a TonB-dependent hemin receptor. Sequence comparison showed that the RhuR protein exhibited low homology to the characterized hemin receptor proteins HemR of S. marcescens (20.89% similarity and 10.29% identity), HasR of S. marcescens (23.89% similarity and 10.65% identity), HmuR of P. gingivalis (36.9% similarity and 19.89% identity), ShuA of Shigella dysenteriae (25% similarity and 11.63% identity), HuxC of Haemophilus influenzae (24.52% similarity and 11.36% identity), and ChuA of E. coli O157:H7 (25% similarity and 11.75% identity). However, the RhuR protein sequence was found to contain NPKL/FKAP motifs that share 75% identity with both the NPNL and FRAP motifs in Yersinia enterocolitica HemR, which are conserved in other hemin receptors (37). RhuR also shared low identity (16.72%) with TbdR1 of R. anatipestifer CH3, a putative TonB-dependent receptor that has been shown to be involved in hemin iron acquisition (13). Taken together, these results indicate that RhuR is a newly characterized TonB-dependent hemin receptor.
Furthermore, we provide evidence indicating that RhuA facilitates RhuR-mediated hemin transport. First, the function of RhuA in hemin transport is dependent on the presence of RhuR. Next, rRhuA bound the cell surface of R. anatipestifer CH-1 ΔrhuA but not R. anatipestifer CH-1 ΔrhuR ΔrhuA. We thus suggest that the outer membrane hemin-binding protein RhuA interacts with the outer membrane hemin transporter RhuR to improve hemin uptake. Similarly, it was recently shown that the surface-exposed lipoprotein BtuG interacted with the outer membrane B12 transporter BtuB2 to facilitate B12 transport in Bacteroides thetaiotaomicron (38).
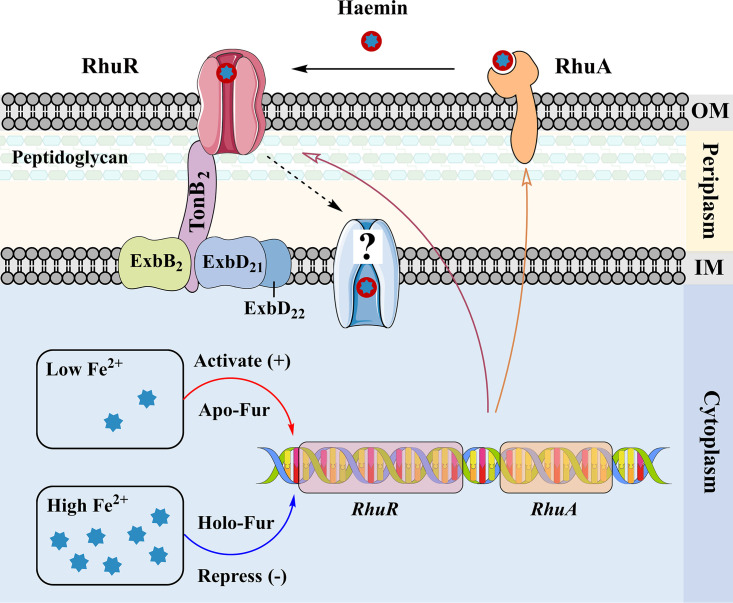
In summary, the model in Fig. 12 shows that on the cell surface, RhuA captures hemin via an unidentified mechanism. Once hemin is transported to RhuR, it is translocated across the outer membrane, in a manner dependent on the TonB2 complex, into the periplasm. Next, hemin is transported into the cytoplasm by an unidentified ABC transporter, where it is used directly or degraded by at least one unidentified oxygenase to release iron for use by R. anatipestifer. Since excessive iron is cytotoxic, the transcription of rhuR-rhuA is negatively regulated by iron and Fur. When the intracellular iron content is insufficient, apo-Fur dissociates from the promoter region of rhuR-rhuA; therefore, the transcription of rhuR-rhuA is upregulated to obtain more iron sources from the extracellular environment. In contrast, when the intracellular iron content is excessive, holo-Fur binds to the promoter region of rhuR-rhuA to repress the transcription of this locus. The function of R. anatipestifer Fur in iron homeostasis has been characterized in a previous study (39). However, the mechanism by which hemin is extracted from duck hemoglobin and transferred to RhuA needs further investigation.
FIG 12.
Model of hemin acquisition from hemoglobin mediated by the RhuR-RhuA locus in R. anatipestifer. RhuA is a membrane-bound, surface-accessible putative lipoprotein. Hemin from hemoglobin is transferred to RhuA via an unidentified pathway. RhuA can transfer hemin to the TonB2-dependent hemin receptor RhuR to facilitate hemin utilization. Alternatively, RhuR can transport hemin from hemoglobin directly. In the cytoplasm, the transcription of rhuR-rhuA is increased when iron is limited, and this event is regulated by Fur. OM, outer membrane; IM, inner membrane.
Although the functions of RhuR and RhuA have been identified in R. anatipestifer, a duck pathogen, homologues of RhuR-RhuA are widely distributed in other environmental (marine, soil, and freshwater) or mammalian-associated bacteria, such as Elizabethkingia species. Consistent with this, R. anatipestifer also survives in soil, freshwater, and other hosts such as pig and guillemot (40). In vertebrates, the majority of iron is predominantly coordinated into a porphyrin ring in the form of hemin (41). In cultured marine phytoplankton, hemin can account for up to 40% of the cellular iron concentration (42). Hemin transport systems of only a few marine bacteria have been studied, although these systems play an important role in the biogeochemical cycling of major nutrient elements (43). Homologues of RhuR-RhuA are found in marine bacteria, and understanding the hemin utilization mechanism of these bacteria will be helpful in the future. In summary, these results provide insight into the potential role of hemin in vertebrate-bacterium and phytoplankton-bacterium interactions and in the broader biogeochemical cycle of iron.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material.
Media and growth conditions.
δ-Aminolevulinic acid (δ-ala) was dissolved in double-distilled water (ddH2O) and filter sterilized through a 0.22-μm Millipore filter (Millipore, China). Hemin (Sigma, USA) was dissolved immediately before use in 0.1 N NaOH and filter sterilized through a 0.22-μm Millipore filter for hemin-binding and hemin utilization experiments. Duck hemoglobin was prepared as described in a previous study (9) and filter sterilized through a 0.45-μm-pore-size Millipore filter for bacterial growth experiments. 2,2′-Dipyridyl (Dip) was obtained from Sigma Chemical (China) and dissolved in ethanol to a final concentration of 100 mM. EDDHA was purchased from Alfa Chemistry, Protheragen Inc. (USA), and dissolved in ddH2O to a final concentration of 20 mM. E. coli strains were grown in Luria-Bertani (LB) liquid medium or on LB agar plates at 37°C. R. anatipestifer was grown on LB agar plates containing 5% defibrinated sheep blood or in GCB at 37°C (44). The final concentrations of antibiotics used for E. coli selection were 100 μg/ml ampicillin (Amp), 50 μg/ml spectinomycin (Spc), and 20 μg/ml gentamicin (Gen). For R. anatipestifer selection, the final antibiotic concentrations were 80 μg/ml Spc and 1 μg/ml cefoxitin (Cfx).
Preparation of cytoplasmic proteins, membrane proteins, and secretory proteins.
Cytoplasmic proteins and membrane proteins were isolated using previously described methods (45). In brief, bacterial pellets were resuspended in 20 mM Tris-HCl (pH 7.4)–10 mM EDTA–1 mM N-α-p-tosyl-l-lysine chloromethyl ketone (TLCK). Bacterial cells were disrupted with a French press cell disrupter (Thermo), with cooling on ice between bursts. Cellular debris was pelleted by centrifugation at 8,000 × g for 30 min at 4°C. The supernatants were transferred to ultracentrifuge tubes and centrifuged at 100,000 × g for 2 h at 4°C. The supernatants were removed and saved for cytosolic protein preparation. The pellets were resuspended in TLCK and analyzed as membrane protein fractions. All samples were stored in 20% glycerol at −80°C.
Secreted proteins were isolated using previously described methods (46). In brief, R. anatipestifer CH-1 was grown overnight at 37°C with shaking in GCB. GCB (20 ml) was inoculated with bacterial cells at an optical density at 600 nm (OD600) of 0.1. Bacterial cells were grown at 37°C to an OD600 of approximately 1. Bacterial cells were harvested by centrifugation for 10 min at 10,000 × g at 4°C, and the supernatants were filtered through a 0.22-μm Millipore filter (Sangon, Shanghai, China). Trichloroacetic acid (TCA) (10%) was added to the filtrate, and the mixture was incubated overnight with stirring. The filtrate was centrifuged for 20 min at 12,000 × g at 4°C. The precipitate was washed with acetone and suspended in protein loading buffer.
In vitro growth rate determination.
The in vitro growth rates of the tested strains were determined by measuring the OD600 with a spectrophotometer as described in a previous study (47). In brief, cultures in the early exponential phase were inoculated in 20 ml of defined medium at an OD600 of 0.1 and incubated at 37°C with shaking at 180 rpm. The OD600 was determined at intervals of 2 h for 14 h.
Hemin utilization assays using the model E. coli strain C600 ΔhemA(pAM238::hemR).
The C600 ΔhemA(pAM238::hemR) strain was mixed with 4 ml of soft agar and poured onto LB plates supplemented with 100 μM Dip. Holes were cut into agar and filled with hemin/hemoglobin or hemin/hemoglobin supplemented with sterile rRhuA protein, mutant rRhuA protein, rHasA protein, and rRecA protein, separately. Growth in the holes was recorded after 48 h of incubation at 37°C.
Site-directed mutagenesis.
Point mutations were introduced into rhuA using overlap PCR as described in a previous study (48). Selected amino acids with potential hemin-binding ability were replaced with alanine, resulting in single or double mutations. In brief, the first fragment was amplified from the genomic DNA of R. anatipestifer CH-1 using the primer RhuA N-His P1, which contains an EcoRI restriction enzyme site, and the primer RhuA up P2, which contains the mutant nucleotides. The second fragment was amplified from the genomic DNA of R. anatipestifer CH-1 using the primer RhuA down P1, which contains the mutant nucleotides, and the primer RhuA down P2, which contains a HindIII restriction enzyme site. The two fragments were ligated by overlap PCR. Next, the amplicon was digested with EcoRI and HindIII and cloned into pBAD24. The inserted mutant gene was confirmed by sequencing. The primers used for the construction of each mutant are shown in Table S2.
Construction of the recombinant plasmids for expression in E. coli.
The rhuA gene lacking the predicted signal peptide sequence was amplified from R. anatipestifer CH-1 using the primer pair RhuA N-his P1/RhuA down P2. The entire coding region of the hasA gene was amplified from S. marcescens using the primer pair HasA P1/P2. The PCR products were purified, digested, and ligated with similarly digested pBAD24 plasmids to yield the pBAD24::rhuA and pBAD24::hasA plasmids. The presence of the correct inserts was confirmed by PCR and sequencing (BGI, Guangzhou, China).
Construction of the suicide plasmids for knockout in R. anatipestifer CH-1.
The ∼800-bp upstream fragment and the ∼800-bp downstream fragment of the gene targeted for deletion, for example, rhuR, rhuA, tonB1, and tonB2, were amplified with the primer pairs shown in Table S2. The upstream and downstream PCR fragments were fused with the primer pair up P1/down P2 by overlap PCR. The resulting PCR products were purified, digested, and ligated into similarly digested pOBS plasmids (48) to generate the corresponding plasmid derivatives pOBSΔrhuR, pOBSΔrhuA, pOBSΔtonB1, and pOBSΔtonB2.
Construction of the plasmids for complementation.
The entire coding regions of rhuR and rhuA were PCR amplified from R. anatipestifer CH-1 chromosomal DNA using the primer pairs RhuR Comp P1/P2 and RhuA Comp P1/P2, respectively (Table S2). The PCR products were purified, digested, and ligated into the similarly digested shuttle plasmid pLMF03 (8) to generate the plasmids pLMF03::rhuR and pLMF03::rhuA. The presence of the correct inserts was confirmed by PCR and sequencing (BGI, Guangzhou, China).
Construction of the markerless deletion and complementation strains of R. anatipestifer CH-1.
For the construction of markerless deletion strains of R. anatipestifer CH-1, pOES derivatives (pOESΔrhuR, pOESΔrhuA, pOESΔtonB1, and pOESΔtonB2) were introduced into the relevant R. anatipestifer CH-1 strains by E. coli S17-1-mediated conjugation as described in a previous study (48). After the first recombination, the transconjugants were selected on GCB or blood agar containing cefoxitin and kanamycin. The positive clones were identified by PCR and cultured in GCB without antibiotics. The second transconjugants were selected on GCB or blood agar plates supplemented with 13 mM p-Cl-Phe. The correct clones were identified by PCR as described in a previous study (48).
For complementation, the pLMF03 derivatives were transformed into the relevant R. anatipestifer CH-1 strains by conjugation, and the correct clones were selected on GCB or blood agar containing cefoxitin and kanamycin and finally identified by PCR as described in a previous study (8).
Real-time PCR.
R. anatipestifer CH-1 and R. anatipestifer CH-1 Δfur were inoculated at an OD600 of 0.1 in 20 ml of GCB at 37°C. The bacterial cells were grown to the mid-exponential growth phase with the same OD value (OD600 = 1 to 1.5). R. anatipestifer CH-1 ΔhemA was inoculated at an OD600 of 0.1 in 20 ml of GCB containing 0.5 μM hemoglobin and cultured to the mid-exponential growth phase (OD600 = 1 to 1.5); the bacteria were then centrifuged, transferred to GCB, and incubated for 2 h. The bacteria were immediately mixed with 2 volumes of RNA Protect bacterial reagent (catalogue number 76506; Qiagen) and centrifuged again at 5,000 × g for 10 min. Total RNA was extracted using an RNeasy Protect bacterial minikit (catalogue number 74524; Qiagen). The purity and concentration of the total RNA were measured with a NanoDrop 2000 instrument (Thermo Scientific). RNA (0.8 μg) was reverse transcribed using HiScript reverse transcriptase and random primers (catalogue number R123-01; Vazyme) according to the manufacturer’s instructions. A control without enzyme was included for all RNA samples to confirm the absence of genomic DNA. Next, quantitative PCR (qPCR) was performed using SYBR green master mix (catalogue number Q111-01; Vazyme) and primers shown in Table S2. Three samples and technical replicates were run for each target and condition. All qPCRs were performed in a CFX Connect real-time system (Bio-Rad) as recommended by the manufacturer, and the following thermal cycling conditions were used: 95°C for 5 min and 40 cycles at 95°C for 10 s and 60.4°C for 30 s. The fold change values were calculated as described previously (49), using the ΔΔCT method and considering the efficiency of the PCR for each target. 16S rRNA was used as the internal reference gene.
Overexpression and purification of the recombinant His-tagged protein.
The plasmids pBAD24::rhuA, pBAD24::rhuA mutant, pBAD24::hasA, and pBAD24::recA were transformed into E. coli host strain JP313 to overexpress rRhuA, the rRhuA mutant, rHasA, and rRecA, respectively. Bacteria were cultured at 37°C in LB medium to an OD600 of 0.5 to 0.8 and induced with 0.05% arabinose for 2 to 4 h. Bacteria were harvested by centrifugation and treated with lysozyme and DNase as described in a previous study (4). The protein was purified with His-Bind Ni-NTA resin according to the manufacturer’s protocol. The purified protein was dialyzed twice against a buffer containing 50 mM Tris-HCl to eliminate any residual imidazole.
Hemin-binding assay using native PAGE.
The purified protein and a mixture of equimolar amounts of the purified protein and free hemin were run on two native gels. One gel was stained with Coomassie brilliant blue. The proteins on the other gel were transferred to a nitrocellulose membrane, which was washed for 10 min with Tris-buffered saline (TBS)–Tween 20 (TBST) (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Tween 20) at room temperature. Hemin was visualized by its intrinsic peroxidase activity using ECL reagents (ECL Plus; GE Healthcare) in a ChemiDoc MP imaging system (Bio-Rad).
Absorption spectroscopy.
The recombinant protein and hemin were diluted to 20 μM and 200 μM, respectively, in 50 mM Tris-HCl (pH 8.0). Aliquots of hemin at increasing concentrations from 2 μM to 40 μM were successively added to tubes containing 100 μl of 20 μM rRhuA protein. Absorbance spectra from 300 nm to 500 nm were recorded every 5 min after each hemin addition in a NanoDrop 2000 spectrophotometer (Thermo). Experiments were performed in triplicate.
Whole-cell hemin-binding assay.
A whole-cell hemin-binding assay was performed as described previously (15, 28). In brief, R. anatipestifer CH-1 and its derivative strains were grown under iron starvation conditions (GCB containing 120 μM EDDHA) at 37°C. Cells were harvested by centrifugation and resuspended in 1 ml of phosphate-buffered saline (PBS) buffer to adjust the OD600 to 1.0. Next, 10 μg of hemin was added, and the tubes were shaken continuously. After incubation for 2 h and 4 h, the bacterial cells were centrifuged at 12,000 × g for 10 min at room temperature. The amount of unbound hemin was determined by measuring the OD of hemin at 405 nm with a NanoDrop 2000 instrument (Thermo Scientific). The amount of bound hemin was calculated by subtraction of the OD value of a bacterium-free control sample. The E. coli strain C600 ΔhemA, which does not encode any outer membrane hemin-binding proteins, was used as a negative control. All experiments were performed in triplicate.
Antibody preparation.
One hundred microliters of purified rRhuA (50 μg) and 100 μl of Freund’s complete adjuvant (Sigma, China) were inoculated by celiac injection into 4-week-old Kunming mice. Two weeks later, the same volumes of purified rRhuA and Freund’s incomplete adjuvant (Sigma, China) were again inoculated into the mice by celiac injection two more times at intervals of half a month. Blood samples were collected every 2 weeks via retro-orbital bleeding. Blood samples were centrifuged at 8,000 × g for 10 min at 4°C to obtain serum, which was stored at −20°C.
Immunoblot analysis.
SDS-PAGE and immunoblotting to detect the expression of the RhuA protein were performed using a previously described protocol (8). In brief, proteins were separated by 12% SDS-PAGE and transferred to a nitrocellulose membrane as recommended by the manufacturer. Nonspecific binding sites were blocked with 5% skim milk in TBS-Tween 20 (0.05%). The immunoblot was probed first with polyclonal mouse sera raised against rRhuA (1:400 dilution) and then with a 1:2,000 dilution of a goat anti-mouse IgG alkaline phosphatase-conjugated secondary antibody (CST). Antibody binding to the RhuA protein was detected with a 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) solution according to the manufacturer’s instructions (Sigma, China). The RecA antiserum was prepared as described in our previous study (8).
Dot blotting.
Dot blotting was performed as described in a previous study (50). In brief, bacteria were grown under iron starvation conditions (GCB containing 120 μM EDDHA) at 37°C and harvested when they reached an OD600 of ∼1 to 2. Cell pellets were suspended in TBS buffer to an OD600 of 0.5. Aliquots of 20 μl (approximately 108 CFU) were dropped on the filters. The filters were dried at room temperature and blocked at 4°C overnight in the TBST containing 5% skim milk. Next, one filter (filter 1) was incubated for 1 h at 37°C with polyclonal anti-RhuA antibodies diluted 1:400 in blocking buffer. After three washes in blocking buffer, filter 1 was incubated with the horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody diluted 1:5,000 in TBST containing 2.5% skim milk. After three washes in TBS, antibody binding was visualized using ECL reagents (ECL Plus; GE Healthcare) in the ChemiDoc MP imaging system (Bio-Rad). Another filter (filter 2) was incubated with TBST containing 4 μM rRhuA for 30 min at room temperature. After washing three times, filter 2 was incubated for 1 h at 37°C with polyclonal anti-RhuA antibodies diluted 1:400 in blocking buffer. After three washes in blocking buffer, filter 2 was incubated with HRP-conjugated goat anti-mouse IgG diluted 1:5,000 in TBST containing 2.5% skim milk. After three washes in TBS, antibody binding was visualized using ECL reagents (ECL Plus; GE Healthcare) in the ChemiDoc MP imaging system (Bio-Rad).
Confirmation of rRhuA binding to the R. anatipestifer CH-1 cell surface.
The R. anatipestifer strains CH-1, CH-1 ΔrhuA, and CH-1 ΔrhuR ΔrhuA were cultured separately to the exponential growth phase in GCB containing 120 μM EDDHA. After centrifugation at 8,000 × g for 10 min, the bacterial cells were resuspended in PBS to an OD600 of 1. In parallel, 5 μM rRhuA was mixed with 5 μM hemin for 30 min at 37°C. Next, the mixture of rRhuA and hemin or rRhuA alone was added to the bacterial suspension and further incubated for 1 h at 37°C. Next, the samples were washed three times with TBST for 10 min each time. Finally, the samples were resuspended in SDS loading buffer and subjected to SDS-PAGE and immunoblotting. The immunoblot was probed first with polyclonal mouse sera raised against rRhuA (1:400 dilution) and then with a 1:5,000 dilution of a goat anti-mouse IgG HRP-conjugated secondary antibody. The secondary antibody was visualized using ECL reagents (ECL Plus; GE Healthcare) in the ChemiDoc MP imaging system (Bio-Rad) according to the manufacturer’s instructions.
Indirect immunofluorescence analysis.
Immunofluorescence (IF) was used to detect the surface exposure of RhuA as described in a previous study (27). In brief, bacterial cells were grown to exponential phase in GCB at 37°C with shaking. The OD600 of the bacterial suspensions was measured, and approximately 107 bacteria were collected for each strain. The bacterial cells were resuspended in 200 μl of PBS containing 1% (wt/vol) bovine serum albumin (BSA) and incubated for 30 min at room temperature. The bacterial cells were then centrifuged at 8,000 × g for 5 min, resuspended in 200 μl of a 1:400 dilution of mouse anti-rRhuA antiserum, and incubated for 30 min at room temperature. After centrifugation, the bacterial cells were washed three times with PBS, resuspended in 200 μl of a 1:2,000 dilution of fluorescein-conjugated goat anti-mouse IgG (ZSGB-Bio, China), and incubated for 30 min at room temperature in the dark. The bacterial cells were washed three times with PBS, resuspended in 200 μl of 4% (wt/vol) paraformaldehyde (PFA), and incubated for 15 min at room temperature in the dark. Finally, the bacterial cells were washed and resuspended in 700 μl of PBS. The labeled bacterial cells were added on top of poly-l-lysine-coated coverslips and allowed to adhere for 30 min at room temperature in the dark. After the removal of the bacterial suspension, the coverslips were washed three times, mounted inverted on glass slides, and allowed to dry overnight at room temperature in the dark. Micrographs were acquired with a Nikon Eclipse 80i microscope.
LSPR.
All LSPR experiments were performed using an OpenSPR system (Nicoya). LSPR measurements were carried out in HBS-ET running buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% Tween 20 [pH 7.4]). To detect the affinity of rRhuA for hemin, an NTA sensor chip (Nicoya) was used. rRhuA was immobilized onto a flow cell at a flow rate of 20 μl/min for 5 min to a ligand density of ∼50 picomoles. After ligand capture, hemin at different concentrations (1, 6, 10, 20, and 40 μM) was injected into the flow cell at 20 μl/min for 2 min to observe association, and dissociation was then allowed for 5 min. With TraceDrawer evaluation software, a 1:1 binding model was used to fit the binding response curves, and the dissociation constant (Kd) was calculated.
Amino acid sequence alignment and 3D modeling prediction.
Sequence alignment and database searches were carried out using the BLAST tool on the BLAST server at the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/). The phylogenetic tree was constructed with the MEGA 6.0 program. The three-dimensional (3D) models of RhuA were generated using Phyre2 (24) and I-TASSER (25).
Ethics statement.
Animals were handled in strict accordance with good animal practices as defined by local animal welfare bodies. Animal work performed at Sichuan Agricultural University was reviewed and approved by the Sichuan Agriculture University ethics committee in September 2015.
Duckling infection experiments.
Duckling infection experiments were performed according to previously described methods (47). The virulence of the mutants was assessed using two infection models. For mortality, 3-day-old ducklings (10/group) were inoculated in the leg with 5 × 108 CFU of each strain and monitored for near mortality for 7 days. For colonization, 3-day-old ducklings (5/group) were injected with 5 × 108 CFU of each strain in the leg. At 24 h postinoculation, ducklings were euthanized by forced inhalation of CO2, and the tissues were collected, weighed, ground, and diluted in PBS. Finally, dilutions were plated on blood agar plates to determine the CFU of bacteria per milliliter or gram of tissues.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 6 software for Windows. The statistical significance of the data was ascertained with Student’s t test. A P value of <0.05 was considered significant.
Data availability.
The nucleotide sequences of R. anatipestifer CH-1 were deposited in GenBank under accession number CP003787. The nucleotide sequences of RhuR were deposited in GenBank under accession number AFR36014.1. The GenBank accession numbers of RhuA and its homologues are as follows: AFR36015.1 for RhuA of R. anatipestifer CH-1, AWW00704.1 for the homologue in Arcticibacterium luteifluviistationis, QLH54181.1 for the homologue in “Candidatus Kapabacteria,” QCX53080.1 for the homologue in Elizabethkingia sp., NAW52280.1 for the homologue in Elizabethkingia argenteiflava, OPC28768.1 for the homologue in Elizabethkingia meningoseptica, OPC06910.1 for the homologue in Elizabethkingia ursingii, AJW63993.1 for the homologue in Elizabethkingia miricola, WP_035589631.1 for the homologue in Elizabethkingia anophelis, WP_026350584.1 for the homologue in Dyadobacter beijingensis, WP_143829056.1 for the homologue in Dyadobacter fermentans, and WP_018675664.1 for the homologue in Riemerella columbina. The protein ribbon structure of RhuA was predicted using I-TASSER (https://zhanglab.ccmb.med.umich.edu/I-TASSER/) and Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/index.cgi) and visualized using PyMOL molecular visualization software. The data sets generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant number 31772772) (http://www.nsfc.gov.cn/), the Sichuan Science and Technology Program (2020YJ0344), the China Agricultural Research System (CARS-42-17), and the Sichuan Veterinary Medicine and Drug Innovation Group of the China Agricultural Research System (SCCXTD-2020-18).
We thank Francis Biville (Institute Pasteur) and Philippe Delepelaire (Institut de Biologie Physico-Chimique, CNRS Université Paris Diderot) for valuable discussions.
Conceived and designed the experiments, M.L. and A.C. Performed the experiments, S.L., M.H., Y. Wang, Mengying Wang, L.L., X.T., Mingshu Wang, D.Z., R.J., S.C., X.Z., and Y.L.Y. Analyzed the data, Z.Y., Q.Y., Y. Wu, S.Z., J.H., X.O., S.M., and Q.G. Contributed reagents/materials/analysis tools, D.S., M.L., and A.C. Wrote the paper, M.L. and A.C. All authors have reviewed the manuscript.
We declare no competing financial interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Mafeng Liu, Email: liumafengra@163.com.
Anchun Cheng, Email: chenganchun@vip.163.com.
Charles M. Dozois, INRS—Institut Armand-Frappier
REFERENCES
- 1.Palmer LD, Skaar EP. 2016. Transition metals and virulence in bacteria. Annu Rev Genet 50:67–91. 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choby JE, Skaar EP. 2016. Heme synthesis and acquisition in bacterial pathogens. J Mol Biol 428:3408–3428. 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandersman C, Delepelaire P. 2012. Haemophore functions revisited. Mol Microbiol 85:618–631. 10.1111/j.1365-2958.2012.08136.x. [DOI] [PubMed] [Google Scholar]
- 4.Liao H, Cheng X, Zhu D, Wang M, Jia R, Chen S, Chen X, Biville F, Liu M, Cheng A. 2015. TonB energy transduction systems of Riemerella anatipestifer are required for iron and hemin utilization. PLoS One 10:e0127506. 10.1371/journal.pone.0127506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giardina BJ, Shahzad S, Huang W, Wilks A. 2019. Heme uptake and utilization by hypervirulent Acinetobacter baumannii LAC-4 is dependent on a canonical heme oxygenase (abHemO). Arch Biochem Biophys 672:108066. 10.1016/j.abb.2019.108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinochet-Barros A, Helmann JD. 2018. Redox sensing by Fe(2+) in bacterial Fur family metalloregulators. Antioxid Redox Signal 29:1858–1871. 10.1089/ars.2017.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramaniam S, Chua KL, Tan HM, Loh H, Kuhnert P, Frey J. 1997. Phylogenetic position of Riemerella anatipestifer based on 16S rRNA gene sequences. Int J Syst Bacteriol 47:562–565. 10.1099/00207713-47-2-562. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Wang M, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Chen X, Biville F, Cheng A. 2016. Investigation of TbfA in Riemerella anatipestifer using plasmid-based methods for gene over-expression and knockdown. Sci Rep 6:37159. 10.1038/srep37159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Huang M, Huang L, Biville F, Zhu D, Wang M, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Huang J, Tian B, Chen X, Liu Y, Zhang L, Yu Y, Pan L, Ur Rehman M, Cheng A. 2019. New perspectives on Galleria mellonella larvae as a host model using Riemerella anatipestifer as a proof of concept. Infect Immun 87:e00072-19. 10.1128/IAI.00072-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Liu W, Zhu D, Yang L, Liu M, Yin S, Wang M, Jia R, Chen S, Sun K, Cheng A, Chen X. 2014. Comparative genomics of Riemerella anatipestifer reveals genetic diversity. BMC Genomics 15:479. 10.1186/1471-2164-15-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Huang M, Shui Y, Biville F, Zhu D, Wang M, Jia R, Chen S, Sun K, Zhao X, Yang Q, Wu Y, Chen X, Cheng A. 2018. Roles of B739_1343 in iron acquisition and pathogenesis in Riemerella anatipestifer CH-1 and evaluation of the RA-CH-1DeltaB739_1343 mutant as an attenuated vaccine. PLoS One 13:e0197310. 10.1371/journal.pone.0197310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Zhang P, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Chen X, Biville F, Cheng A, Liu M. 2017. Identification of the ferric iron utilization gene B739_1208 and its role in the virulence of R. anatipestifer CH-1. Vet Microbiol 201:162–169. 10.1016/j.vetmic.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Lu F, Miao S, Tu J, Ni X, Xing L, Yu H, Pan L, Hu Q. 2013. The role of TonB-dependent receptor TbdR1 in Riemerella anatipestifer in iron acquisition and virulence. Vet Microbiol 167:713–718. 10.1016/j.vetmic.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Huang M, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Wu Y, Biville F, Cheng A. 2017. Identifying the genes responsible for iron-limited condition in Riemerella anatipestifer CH-1 through RNA-Seq-based analysis. Biomed Res Int 2017:8682057. 10.1155/2017/8682057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao H, Liu M, Cheng X, Zhu D, Wang M, Jia R, Chen S, Sun K, Yang Q, Biville F, Cheng A. 2016. The detection of hemin-binding proteins in Riemerella anatipestifer CH-1. Curr Microbiol 72:152–158. 10.1007/s00284-015-0932-5. [DOI] [PubMed] [Google Scholar]
- 16.Lauber F, Cornelis GR, Renzi F. 2016. Identification of a new lipoprotein export signal in Gram-negative bacteria. mBio 7:e01232-16. 10.1128/mBio.01232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson MM, Bernstein HD. 2016. Surface-exposed lipoproteins: an emerging secretion phenomenon in Gram-negative bacteria. Trends Microbiol 24:198–208. 10.1016/j.tim.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier C, Smith A, Delepelaire P. 2011. Haem release from haemopexin by HxuA allows Haemophilus influenzae to escape host nutritional immunity. Mol Microbiol 80:133–148. 10.1111/j.1365-2958.2011.07562.x. [DOI] [PubMed] [Google Scholar]
- 19.Arnoux P, Haser R, Izadi N, Lecroisey A, Delepierre M, Wandersman C, Czjzek M. 1999. The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat Struct Biol 6:516–520. 10.1038/9281. [DOI] [PubMed] [Google Scholar]
- 20.Ghigo JM, Letoffe S, Wandersman C. 1997. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J Bacteriol 179:3572–3579. 10.1128/jb.179.11.3572-3579.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benevides-Matos N, Wandersman C, Biville F. 2008. HasB, the Serratia marcescens TonB paralog, is specific to HasR. J Bacteriol 190:21–27. 10.1128/JB.01389-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis LA, Gray E, Wang YP, Roe BA, Dyer DW. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol 23:737–749. 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 23.Gao JL, Kwan AH, Yammine A, Zhou X, Trewhella J, Hugrass BM, Collins DAT, Horne J, Ye P, Harty D, Nguyen KA, Gell DA, Hunter N. 2018. Structural properties of a haemophore facilitate targeted elimination of the pathogen Porphyromonas gingivalis. Nat Commun 9:4097. 10.1038/s41467-018-06470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 Web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. 2015. The I-TASSER suite: protein structure and function prediction. Nat Methods 12:7–8. 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knippel RJ, Wexler AG, Miller JM, Beavers WN, Weiss A, de Crecy-Lagard V, Edmonds KA, Giedroc DP, Skaar EP. 2020. Clostridioides difficile senses and hijacks host heme for incorporation into an oxidative stress defense system. Cell Host Microbe 28:411–421.e6. 10.1016/j.chom.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent AT, Wilks A. 2020. Contributions of the heme coordinating ligands of the Pseudomonas aeruginosa outer membrane receptor HasR to extracellular heme sensing and transport. J Biol Chem 295:10456–10467. 10.1074/jbc.RA120.014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai SS, Wang TR, Lee CJ. 1997. Characterization of hemin binding activity of Streptococcus pneumoniae. Infect Immun 65:1083–1087. 10.1128/IAI.65.3.1083-1087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Hu D, Guo J, Li X, Guo J, Wang X, Xiao Y, Jin H, Liu M, Li Z, Bi D, Zhou Z. 2017. The role of the regulator Fur in gene regulation and virulence of Riemerella anatipestifer assessed using an unmarked gene deletion system. Front Cell Infect Microbiol 7:382. 10.3389/fcimb.2017.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong CT, Xu Y, Gupta A, Garnett JA, Matthews SJ, Hare SA. 2015. Structural analysis of haemoglobin binding by HpuA from the Neisseriaceae family. Nat Commun 6:10172. 10.1038/ncomms10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojtowicz H, Guevara T, Tallant C, Olczak M, Sroka A, Potempa J, Sola M, Olczak T, Gomis-Ruth FX. 2009. Unique structure and stability of HmuY, a novel heme-binding protein of Porphyromonas gingivalis. PLoS Pathog 5:e1000419. 10.1371/journal.ppat.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smalley JW, Byrne DP, Birss AJ, Wojtowicz H, Sroka A, Potempa J, Olczak T. 2011. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PLoS One 6:e17182. 10.1371/journal.pone.0017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll JA, Coleman SA, Smitherman LS, Minnick MF. 2000. Hemin-binding surface protein from Bartonella quintana. Infect Immun 68:6750–6757. 10.1128/IAI.68.12.6750-6757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battisti JM, Smitherman LS, Sappington KN, Parrow NL, Raghavan R, Minnick MF. 2007. Transcriptional regulation of the heme binding protein gene family of Bartonella quintana is accomplished by a novel promoter element and iron response regulator. Infect Immun 75:4373–4385. 10.1128/IAI.00497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohde KH, Gillaspy AF, Hatfield MD, Lewis LA, Dyer DW. 2002. Interactions of haemoglobin with the Neisseria meningitidis receptor HpuAB: the role of TonB and an intact proton motive force. Mol Microbiol 43:335–354. 10.1046/j.1365-2958.2002.02745.x. [DOI] [PubMed] [Google Scholar]
- 36.Rohde KH, Dyer DW. 2004. Analysis of haptoglobin and hemoglobin-haptoglobin interactions with the Neisseria meningitidis TonB-dependent receptor HpuAB by flow cytometry. Infect Immun 72:2494–2506. 10.1128/iai.72.5.2494-2506.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bracken CS, Baer MT, Abdur-Rashid A, Helms W, Stojiljkovic I. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J Bacteriol 181:6063–6072. 10.1128/JB.181.19.6063-6072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wexler AG, Schofield WB, Degnan PH, Folta-Stogniew E, Barry NA, Goodman AL. 2018. Human gut Bacteroides capture vitamin B(12) via cell surface-exposed lipoproteins. Elife 7:e37138. 10.7554/eLife.37138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, Liu M, Liu J, Zhu D, Tang Q, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Huang J, Ou X, Mao S, Gao Q, Sun D, Wang M, Cheng A. 2021. Functional characterization of Fur in iron metabolism, oxidative stress resistance and virulence of Riemerella anatipestifer. Vet Res 52:48. 10.1186/s13567-021-00919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz KH, Ryll M, Kohler B, Glunder G. 1998. Phenotypic characteristics of Riemerella anatipestifer and similar micro-organisms from various hosts. Avian Pathol 27:33–42. 10.1080/03079459808419272. [DOI] [PubMed] [Google Scholar]
- 41.Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6:e1000949. 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strzepek RF, Harrison PJ. 2004. Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431:689–692. 10.1038/nature02954. [DOI] [PubMed] [Google Scholar]
- 43.Durham BP, Sharma S, Luo HW, Smith CB, Amin SA, Bender SJ, Dearth SP, Van Mooy BAS, Campagna SR, Kujawinski EB, Armbrust EV, Moran MA. 2015. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc Natl Acad Sci U S A 112:453–457. 10.1073/pnas.1413137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Wang MS, Liu MF, Zhu DK, Biville F, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Zhao XX, Chen XY, Cheng AC. 2017. Contribution of RaeB, a putative RND-type transporter to aminoglycoside and detergent resistance in Riemerella anatipestifer. Front Microbiol 8:2435. 10.3389/fmicb.2017.02435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boonjakuakul JK, Gerns HL, Chen YT, Hicks LD, Minnick MF, Dixon SE, Hall SC, Koehler JE. 2007. Proteomic and immunoblot analyses of Bartonella quintana total membrane proteins identify antigens recognized by sera from infected patients. Infect Immun 75:2548–2561. 10.1128/IAI.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cwerman H, Wandersman C, Biville F. 2006. Heme and a five-amino-acid hemophore region form the bipartite stimulus triggering the has signaling cascade. J Bacteriol 188:3357–3364. 10.1128/JB.188.9.3357-3364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian X, Huang L, Wang M, Biville F, Zhu D, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Huang J, Zhang L, Yu Y, Cheng A, Liu M. 2020. The functional identification of Dps in oxidative stress resistance and virulence of Riemerella anatipestifer CH-1 using a new unmarked gene deletion strategy. Vet Microbiol 247:108730. 10.1016/j.vetmic.2020.108730. [DOI] [PubMed] [Google Scholar]
- 48.Liu M, Huang Y, Liu J, Biville F, Zhu D, Wang M, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Chen X, Liu Y, Zhang L, You Y, Yu Y, Cheng A. 2018. Multiple genetic tools for editing the genome of Riemerella anatipestifer using a counterselectable marker. Appl Microbiol Biotechnol 102:7475–7488. 10.1007/s00253-018-9181-4. [DOI] [PubMed] [Google Scholar]
- 49.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Letoffe S, Nato F, Goldberg ME, Wandersman C. 1999. Interactions of HasA, a bacterial haemophore, with haemoglobin and with its outer membrane receptor HasR. Mol Microbiol 33:546–555. 10.1046/j.1365-2958.1999.01499.x. [DOI] [PubMed] [Google Scholar]
- 51.Yi K, Rasmussen AW, Gudlavalleti SK, Stephens DS, Stojiljkovic I. 2004. Biofilm formation by Neisseria meningitidis. Infect Immun 72:6132–6138. 10.1128/iai.72.10.6132-6138.2004. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2, Figures S1 to S5. Download AEM.00367-21-s0001.pdf, PDF file, 1.02 MB (1MB, pdf)
Data Availability Statement
The nucleotide sequences of R. anatipestifer CH-1 were deposited in GenBank under accession number CP003787. The nucleotide sequences of RhuR were deposited in GenBank under accession number AFR36014.1. The GenBank accession numbers of RhuA and its homologues are as follows: AFR36015.1 for RhuA of R. anatipestifer CH-1, AWW00704.1 for the homologue in Arcticibacterium luteifluviistationis, QLH54181.1 for the homologue in “Candidatus Kapabacteria,” QCX53080.1 for the homologue in Elizabethkingia sp., NAW52280.1 for the homologue in Elizabethkingia argenteiflava, OPC28768.1 for the homologue in Elizabethkingia meningoseptica, OPC06910.1 for the homologue in Elizabethkingia ursingii, AJW63993.1 for the homologue in Elizabethkingia miricola, WP_035589631.1 for the homologue in Elizabethkingia anophelis, WP_026350584.1 for the homologue in Dyadobacter beijingensis, WP_143829056.1 for the homologue in Dyadobacter fermentans, and WP_018675664.1 for the homologue in Riemerella columbina. The protein ribbon structure of RhuA was predicted using I-TASSER (https://zhanglab.ccmb.med.umich.edu/I-TASSER/) and Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/index.cgi) and visualized using PyMOL molecular visualization software. The data sets generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.