Abstract
Self-assembled peptides and proteins possess tremendous potential as targeted drug delivery systems and key applications of these well-defined nanostructures reside in anti-cancer therapy. Peptides and proteins can self-assemble into nanostructures of diverse sizes and shapes in response to changing environmental conditions such as pH, temperature, ionic strength, as well as host and guest molecular interactions; their countless benefits include good biocompatibility and high loading capacity for hydrophobic and hydrophilic drugs. These self-assembled nanomaterials can be adorned with functional moieties to specifically target tumor cells. Stimuli-responsive features can also be incorporated with respect to the tumor microenvironment. This review sheds light on the growing interest in self-assembled peptides and proteins and their burgeoning applications in cancer treatment and immunotherapy.
Keywords: Cancer therapy, Drug delivery, Immunotherapy, Self-assembled peptides and proteins, Stimuli-responsive, Virus-like particles
Graphical Abstract
Functionalized, self-assembled peptide and protein nanostructures encapsulate therapeutic agents for targeted cancer therapy.
Introduction
Proteins are building blocks for all known living organisms and comprise different amino acids that are linked together via peptide bonds. By definition, peptides are composed of short sequences of amino acids and these macromolecules may be further connected together to form proteins [1–3]. Peptide and protein self-assembly offers a wide range of applications in the biomedical sectors. During the self-assembly process, well-defined, highly-ordered structures are created permanently from peptides and proteins. Some of the most famous examples of natural peptide and protein self-assembly include the self-assembly of four polypeptide chains, each attached to a heme group, to produce hemoglobin, and the self-assembly of ribosomal RNA molecules and proteins into ribosomes [4,5]. Self-assembly of proteins and peptides is a field of escalating importance, allowing the generation of nanosized particles for carrying, protecting and controlling the release of biotherapeutics for anti-tumor therapy [6].
Cancer is one of the most lethal diseases worldwide, with increasing numbers of new cases every year [7–10]. Nevertheless, cancer fatality has diminished by 29% from 1991 to 2017 [11]. This is attributed to better comprehension of tumor biology, which has enabled the advancement of diagnostic techniques and anti-cancer therapeutics [12–15]. An integral part of anti-cancer treatment is chemotherapy. However, “drug resistance” is a new emerging problem in field of cancer chemotherapy. Frequent application of a chemotherapeutic agent leads to ignorance of cancer cells towards its inhibitory effects. To overcome this issue, high doses of chemotherapeutic agent is applied to suppress cancer progression. However, chemotherapeutic agents have dose-dependent side effects that is a limitation towards using high doses [16,17]. To address this issue, peptide and protein nanovehicles have been used for the preparation of targeted drug delivery systems for anti-cancer treatment. These nanocarriers help to minimize the side effects of anti-cancer therapy by targeting only tumor cells and leaving healthy cells relatively unharmed, thereby resulting in higher therapeutic efficacy [17–20].
Cancer immunotherapy is emerging as an effective approach to fight cancer by activation and potentiation of immune cells using cancer vaccines, checkpoint inhibitors, or adoptive T cell transfer [22]. However, there remain problems for current immunotherapeutic approaches due to presence of immune-related adverse effects, low specificity in tumour cell targeting and rapid elimination and degradation issues. In these regards, nanotechnology offers a wide range of platforms for targeted delivery and controlled release of cancer immunotherapeutics. Peptide and protein-based nanostructures offer several advantages to the field: their small dimensions can promote penetration through the venous endothelium and allow the nanocarriers to reach the antigen-presenting cells (APCs), with consequent improvement of the activation of the immune responses [23]. Moreover, the potential intrinsic immunostimulating effects of peptide and protein-based nanomaterials and biocompatibility, together with the possibility of co-delivery of antigens, adjuvant and targeting molecules, allow nanosystems to play a growing role in the field of cancer immunotherapy [24,25].
Initially, the structural features and functions of peptides and proteins are described in the present review followed by a presentation of the self-assembled nanostructures derived from them in the last 3 years. Targeted delivery and stimuli-responsive self-assembly of peptide and protein nanoplatforms are highlighted, including a discussion on immunotherapy. Finally, the development of vaccine formulations using self-assembling nanomaterials to deliver tumor antigens with the aim of strengthening the immune system and directing the immune response toward cancer cells, is extensively appraised.
Structural features and functions of peptides and proteins
Proteins and peptides are fundamental components of cells that exert and support an enormous range of vital biological functions. Proteins can, for instance, even influence the configuration of cells; another duty of proteins is the transformation of extracellular stimuli into signals. Peptides play an indispensable role in regulating the activities of molecules [25,26]. Structurally, proteins and peptides are rather alike, as one might expect given that both are formed from sequences of amino acid residues held together by peptide bonds. Traditionally, peptides are smaller, containing 2–50 amino acid residues, while proteins encompass more than 50 amino acid residues. Peptides have specific structural characteristics that can also make it possible to form complex confirmations recognized as secondary, tertiary, and quaternary structures. In general, peptides may be divided into two main categories: oligopeptides that include relatively few amino acids (i.e. 2 – 20), or polypeptides that have a long sequence of amino acids. Proteins are, in fact, formed by one or more polypeptides linked together that ultimately give rise to higher order structure and function [27].
Peptides
Peptides, short sequences of amino acid residues connected by peptide (amide) bonds naturally occur in living organisms, responsible for the execution of important biological functions [28]. Both peptides and proteins are formed by translation of the genetic code sequence, deoxyribonucleic acid (DNA). During transcription, a DNA gene sequence is copied into messenger ribonucleic acid (mRNA) that contains information for producing a specific peptide or protein [29]; translation of the mRNA yields a chain of amino acids linked together via peptide bonds. Polymerization of peptides under particular conditions leads to the formation of dipeptides, tripeptides, tetrapeptides, etc. Peptides and proteins are generally differentiated based on their size and structure as a function of their amino acid sequence. Differences between peptides and proteins contribute to their functional diversity [30].
Peptides are fundamental components of cells and tissues, play key roles in nearly all biological activities, and their functionality is determined by 3 parameters: the identity of amino acids, the sequence of amino acids and the shape of the peptide. Peptides such as hormones act as biologic messengers in the blood by carrying information from one tissue to another. To exert their effects, peptides must bind to highly-specific cognate receptors which are present on the membrane of the destined cells (Figure 1). Upon binding, the peptides are dragged into the cell membrane by receptors which consist of two domains: the extracellular domain where the peptides are located, and the intracellular domain through which the peptides exert their functions after penetrating the cell membrane [28,31].
Figure 1.
Schematic representation of the penetration of a peptide into the internal milieu of a cell. (A) The peptide binds to the receptor located on the receptors’ extracellular domain. (B) Receptor activation. (C) Penetration of a peptide through the cell membrane.
Proteins
Protein function is intimately related to its structure. Proteins are biological polymers consisting of a sequence of amino acid residues connected via peptide bonds to form polypeptide chains. There are four orders of protein structure: primary, secondary, tertiary and quaternary structures (Figure 2). Primary structure is the simplest level of protein structure; it is simply the sequence of amino acid residues of a protein [32]. Since the DNA of the gene encoding a protein determines its sequence of amino acid arrangement, any alteration in the DNA sequence of a gene may lead to changes in the amino acid sequence of a protein, potentially impacting the overall structure and function of the protein [33]. Secondary structure contains internal folding of the protein structure that is caused by atomic interaction of its amino acid moieties. The α helix and β pleated sheet are the most common secondary structures, which are formed by hydrogen bonds between different amino acids [34]. Tertiary structure refers to the complex, three-dimensional arrangement of amino acid moieties within a protein and its formation is due to R group interactions of amino acid residues that form the protein. There are different types of R group interactions, such as hydrogen bonding, ionic bonding, dipole-dipole interactions and London dispersion forces [35]. In the protein tertiary structure, hydrophobic amino acid residues cluster together on the inside of the protein, while hydrophilic amino acids on the external surface interact with the adjacent water molecules as part of the free energy minimization assembly process [32]. When a protein is formed from multiple polypeptide chains by similar types of interactions that create the tertiary structure, the protein displays a quaternary structure. Quaternary structure is unnecessary for most proteins; many proteins are formed from a single polypeptide chain representing only three orders of structure [36].
Figure 2.
A schematic depiction of primary, secondary, tertiary and quaternary protein structures.
Because proteins are responsible for the functioning of all living organisms, understanding of proteins’ structures and how they function is critical. Likewise, it is important to clearly understand how biological activities occur at the molecular level. This, in turn, supports the development of precision medicine and novel therapeutics [37,38]. The discovery of new proteins with unknown functions is increasing exponentially because of the rise in the number of sequenced genomes. The identification and characterization of this diverse set of protein functions via manual techniques is highly challenging because of their sheer numbers. Computational automated prediction and identification of protein function is a burgeoning field toward a reliable and convenient approach that saves time, resources, and expense [39–41]. To date, several methods are available to predict protein function. Important basic protein information can be identified with the use of conventional techniques, such as amino acid sequence, genomic contextual data and protein-protein interactions. Advanced computational approaches with higher efficacy and precision are currently employed for prediction of many protein functions. Continuing technological developments in this exciting field are increasing our understanding of the biological processes and functions of proteins [42]. It has long been known that protein 3D structure is directly related to their biochemical functions. Thus, for a protein to maintain its structural and/or functional properties, there are limitations on the amino acid substitutions imposed by different locations of a protein’s 3D structure [43].
Proteins can evolve via a complex process that is realized in the emergence of a novel gene in a sequence of DNA found, for instance, in newly discovered species or in disease states. They are created by processes such as gene duplication, incorporation of mobile elements, domain shuffling, de novo acquisition and gene fission and fusion [44]. The majority of novel genes are created via gene duplication processes [45]. During this process, one copy of a gene resides in a new piece of DNA. The new copy of the duplicated gene, under different environmental circumstances, undergoes certain modifications that create a new adaptive function [46]. The manner in which orphan genes are formed in primate genomes is of increasing interest [47]. Orphan genes are a specific gene type with no recognizable similarities to other species. They are lineage-specific, with a strong tendency to undergo rapid acceleration in their evolution. The majority of orphan genes originate from transposable Alu elements (short stretches of DNA) that move or “jump” from one place on the genome to another [48]. The duplicated genes split into two categories in terms of functionality. The first category of duplicated genes is highly specialized and their functions can only be altered after significant modifications. The second category of duplicated genes are not very specialized and include enzymes such as esterases, cytochrome P450 and glutathione S-transferases and interact with a wide range of different substrates and exhibit better adaptation with a single substrate over time [49]. There is no set constant rate for protein evolution and the process occurs under specific environmental conditions at any given time. Different parameters may be involved in the evolution of proteins, such as functional promiscuity, conformational plasticity and protein fold modularity. For screening the rate of evolution of proteins, the most reliable technique is to perform deep comparison analysis on the changes in amino acid sequence or composition within the related genes [50].
Fundamental Principles and Mechanism of Self-assembly
Self-assembly of peptides and proteins is a powerful phenomenon and its process includes assembly of these macromolecules into an ordered structure. A comprehensive understanding of the hierarchical self-assembly mechanism of peptides and proteins would include knowledge of the kinetic and thermodynamic factors and reaction intermediates of spontaneous self-assembly processes. Insights into the mechanisms of these dynamic processes is essential to ensure control over these interactions [51–53]. The self-assembly process is primarily influenced by thermodynamics, which also helps controls the kinetics, and which is in turn crucial to structural changes and functions [51]. In fact, molecules in this process spontaneously organize upon near-thermodynamic equilibrium conditions and convert into structurally well-defined and stabilized arrangements via noncovalent interactions [54]. Molecules with varied multimeric structures may assemble into well-defined architectures through chemical bonds between their surfaces and form novel shapes with diverse functions (Figure 3). The most well-known self-assembled structures in biological systems are proteins [56], peptides [57], and RNA and DNA complexes [58].
Figure 3.
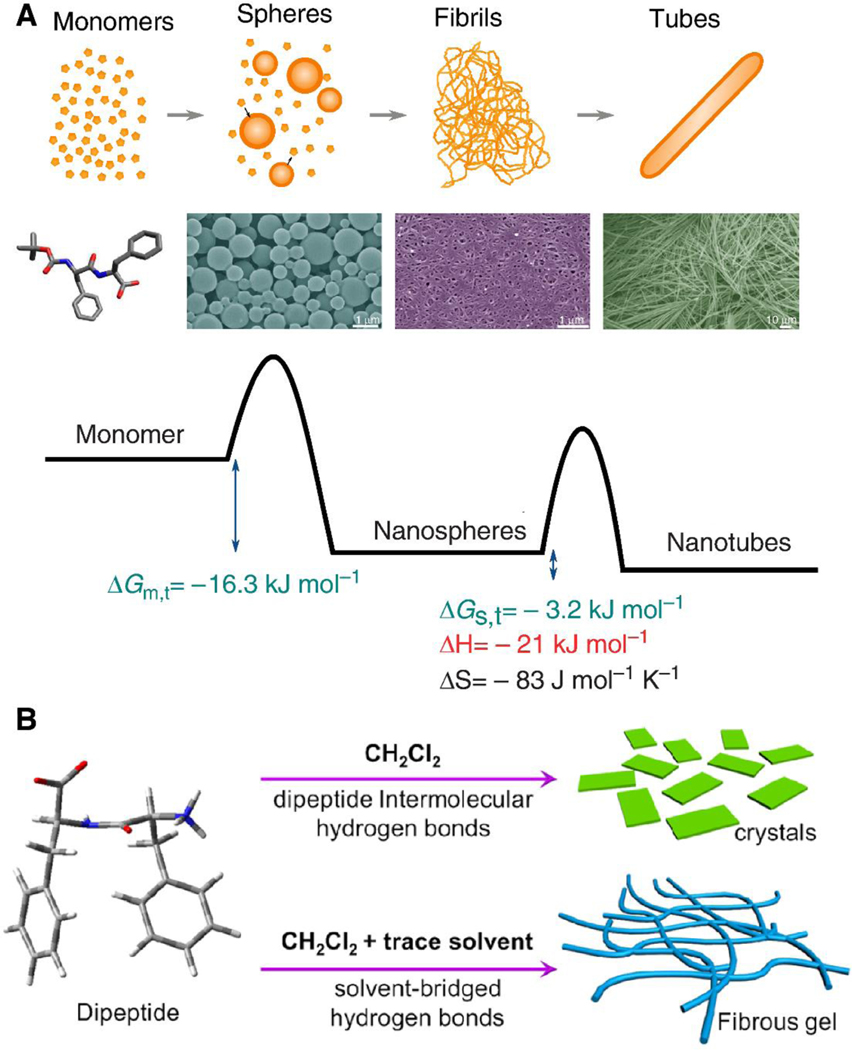
Strategies for thermodynamically favorable protein self-assembly. (A) Scanning electron microscopy (SEM) images and schematic depiction of structural transitions and free energy changes during the phase transition observed in the N-tert-butoxycarbonyl- diphenylalanine (Boc-FF) system. Scale bars represent 1, 1, and 10 μm. Reprinted with permission from [62]. (B) Schematic depiction of a phase transition induced by trace amounts of solvent. Hydrogen-bond-forming solvents, such as ethanol, Dichloromethane (DCM, CH2Cl2), and acetone can induce diphenylalanine (FF) to form fiber structures. Reprinted with permission from [63].
The peptide and protein self-assembly process is influenced by solution conditions (e.g., ionic strength, pH, and assembling rate), as well as a variety of driving forces (e.g., weak non-covalent bonds such as electrostatic interactions (ionic bonds), van der Waals interactions, hydrophobic and hydrophilic interactions, hydrogen bonds, and water-mediated hydrogen bonds) [53,55–59]. While it is true that these forces are weak when they are considered alone, a mixture of different interaction types can form structurally- and chemically-stable structures. Non-covalent interactions have synergistic effects that determine thermodynamic stability and the minimum energy state to control and understand the self-assembly of peptides and proteins [60]. Solution properties (e.g., hydrogen-bonding ability and polarity) precisely affect peptide self-assembly local interactions at the molecular level. As shown in Figure 3B, hydrogen-bond-forming solvents perform a crucial role in forming fibrils in dichloromethane [61].
Nature has exploited the self-assembly of proteins and peptides to produce many molecules with well-defined structures, such as collagen fibrils, keratin, coral, pearl and shell [64]. Common structured shapes formed by the self-assembly of peptides and proteins include nanotube, spherical micelle, lamellar sheet, and vesicle structures (Figure 4) [65].
Figure 4.
Examples of self-assembled structures derived from peptide amphiphiles. Amphiphilic peptides assemble into secondary structures via intramolecular and intermolecular interactions to form larger self-assembled structures. The scheme of sheet, tube, and micelle reprinted with modification from [65].
The process of peptide and protein self-assembly and their resultant sizes are strongly affected by the amino acid sequence and physicochemical parameters such as pH (from acidic to mildly alkaline), temperature (4–95 °C), metal ion concentration (e.g. calcium, potassium, magnesium and sodium) and salt concentration (Figure 5) [66]. Once the environmental parameters are set, self-assembled particles become structurally stable under the same conditions. Scientists have also exploited the self-assembly of proteins and peptides in designing nanoparticles for biomedical applications [67–69]. For example, bovine carbonic anhydrase (BCA) linked with a P114 peptide (BCA-P114) nanoparticles are formed via self-assembly process, and this is by alteration of MgCl2 concentration. Nanoparticles are obtainable using as low as 5 mM MgCl2 and 10 mM Tris-HCl at pH 8.0 (Figure 5B). The maximum size of these nanoparticles is realized when the MgCl2 concentration is 25 mM; particle size decreases when the concentration of MgCl2 exceeds 25 mM (Figure 5C) [66].
Figure 5.
Schematic illustration and data on the preparation of self-assembled protein nanoparticles. (A) Fabrication of protein-peptide nanoparticles (bovine carbonic anhydrase (BCA)-P114 (BCA-P114) by changing pH from 8.0 to 5.6 or adding metal ions (MgCl2). (B) Transmission electron microscopy (TEM) of the prepared peptide nanoparticles in 5 mM MgCl2 at pH 8.0. Scale bar represents 200 nm. (C) Effect of metal ion (MgCl2) concentration on the size of peptide nanoscale particles. Parts B and C reprinted with permission from [66].
Peptides and proteins are naturally functional individually and are prone to self-assembly by hydrogen bonding, hydrophobic interaction, π-π stacking, and other non-covalent bonding forces under specific conditions to perform distinct coding functions [74,75]. Self-assembly of proteins and peptides can improve their stability [70] (i.e. thermal stability, enzyme stability), selectivity [71] and tunable mechanical strength (by tuning both the final ionic strength and the rate of pH change) [72], and impart new functionalities.
Lytic peptides were prepared with the ability to self-assemble into peptide fibrils, which confers improved enzyme stability and selectivity. Lytic peptides in self-assembled peptide fibrils lose their cell lysis activity but become resistant to enzymatic degradation [76]. Self-assembled peptide fibrils also gain a new function: resistance to enzymatic degradation by restricting enzyme access [71]. Self-assembly can also increase the thermal stability of protein fibers [70]. The thermal stability of second-generation protein fibers was greater (TM, 49 °C) than pre-assembled fibers.
The functions of self-assembled peptides/proteins are usually reflected in three aspects: i: functional improvement by enhancing collective behavior. For instance, polyoxometalates can drive the self-assembly of short peptides with hydrophobic and/or p-p interactions, which are sufficiently strong to improve their stability in situ [73]. ii: Gain of new functions that the individual building blocks do not have. For example, self-adjuvanting MUC1 glycopeptide vaccines with Tn glycosylation in the PDTRP domain elicited significant immune response [74]. iii: Gain of even more functions via the incorporation of functional molecules, e.g., incorporation with phototherapeutic agents for photodynamic therapy (PDT) and photothermal therapy (PTT) [75,76].
Nanoparticle architectures of a wide variety of shapes have been fabricated via self-assembly for the delivery of therapeutic agents. For example, tubular nanoparticles have been generated by self-assembly from camptothecin (CPT), a small molecule, hydrophobic anti-cancer drug derived from the bark and stem of the Camptotheca acuminata tree, conjugated to a β-sheet-forming peptide sequence derived from the Tau protein through the reducible disulfylbutyrate (buSS) linker (Figure 6). The nanostructures were well-defined and stable under physiological conditions, with high drug loading capacity. These nanoarchitectures demonstrated excellent killing efficacy against different cancer cell lines in vitro. Apart from protecting CPT from the external deterioration during drug delivery, the nanostructures also control CPT release in a dose-dependent manner [77].
Figure 6.
Drug encapsulation in self-assembled peptides. (A) Schematic illustrating the effect of self-assembly on the susceptibility of drug amphiphiles to degradation, in their capacity as reservoirs for long-term drug release. The species shown in red are hydrophobic drugs, blue are β-sheet peptides, and green are linkers. (B) Low magnification transmission electron microscopy (TEM) image of the nanostructure. (C) High magnification TEM confirms the tubular morphology of the nanostructures. Reprinted with modification from [77].
Self-assembly enables the production of molecular templates and supramolecular structures using nanoengineering techniques [78]. Macromolecules undergo self-assembly to form nanostructures such as nanofibers, nanotubes, vesicles, helical ribbons and fibrous scaffolds [79,80]. An amphiphilic molecule is composed of a nonpolar hydrophobic region(s) and a polar hydrophilic region(s). In aqueous solutions, the polar hydrophilic regions of amphiphilic molecules undergo self-assembly to form unique structures such as micelles, vesicles and tubules. In contrast, the nonpolar hydrophobic regions of the amphiphilic molecules attract each other to avoid interactions with the surrounding water molecules (hydrophobic effect) [81]. An interesting example is the design of the octahedral cage protein O3–33, a 24 subunit, 13 nm diameter complex structure with octahedral symmetry. The architecture of self-assembled O3–33 was predicted by computational techniques. A high-resolution crystal structure of protein O3–33 revealed the backbone root mean square derivation (RMSD) of all 24 subunits. Low-energy protein-protein self-assembly was driven by the designed interface between the building blocks (Figure 7) [82].
Figure 7.
Structure of the octahedral cage protein O3–33. (A) A representative negative-stained TEM micrograph of O3–33. Particles shown in the white boxes (enlarged at right) correspond to nanoparticles along their four-fold, two-fold and three-fold rotational axes, as depicted schematically in (B). (B) The O3–33 design model, represented in ribbon format. Each trimeric building block is shown in a different color. (C) The density map from a 20 Å resolution cryo-EM reconstruction of O3–33 clearly recapitulates the architecture of the design model. (D) The crystal structure of O3–33 (R32 crystal form). Reprinted with permission from [82].
Self-assembled peptides and protein nanostructures appear to be suitable candidates for improving conventional anti-cancer treatments because of their unique features, including capability for precise targeting and selectivity, low toxicity, excellent scalability, and the ease of synthesis [83]. Nanostructures formed by self-assembled peptides and proteins may be used for holding drugs, other bioactive molecules, and imaging agents. They may also be functionalized to increase targeting efficacy (Figure 8) [84,85]. In the following sections, the applications of self-assembled peptides and proteins in anti-cancer therapy are highlighted.
Figure 8.
Schematic of surface-functionalized, self-assembled peptide and protein particles carrying therapeutic agents for targeting tumor and tumor-associated cells.
Peptides and proteins in drug delivery: an overview on the affecting parameters
Many studies have been devoted to exploring the relationship between antitumor effects and the size of nanocarriers [86–91]. Logically, the selection of peptides and proteins (and nanostructures thereof) for cancer therapy could be made based on analysis of the pore size of leaky tumor vasculature [92]. Nevertheless, leaky vasculature is heterogeneous [4], and differences often occur with respect to various tumor models, tumor stage, and site in the body. Notably, pore sizes tend to decrease in tumors that grow in the cranium, e.g., glioma [93]. Therefore, peptide and protein size should be considered owing to the special structure and environment of tumor tissues. There is a general principle, as part of the enhanced permeation and retention (EPR) effect, on the size of nanocarriers that suggests that larger nanoparticles are more likely to be retained in tumor tissue, while smaller nanostructures exhibit improved ability to penetrate in tumor tissues [94–97].
For drug delivery purposes, chemical modifications of proteins and peptides are a highly promising approach for improvement of their enzymatic stability and/or membrane penetration, as well as reducing immunogenicity [98,99]. Advantageously, peptides and proteins include key chemical functional groups and moieties that enable facile modification. There are different potential pathways to exert these modifications. For example, mutations of peptides and proteins using site-directed mutagenesis can significantly alter the physiological properties and, subsequently, increase the efficacy of peptide and protein applications [100]. Another strategy is to increase the hydrophobicity of a peptide or protein via surface modification by lipophilic moieties. This can generate a great advantage specifically for transcellular passive or active absorption by membrane penetration or attachment, respectively. It could also simply increase the stability of the peptides or proteins for a wide range of physicochemical conditions [101,102].
Multifunctional materials may be integrated into a single nanoparticle type to produce targeted drug delivery nanocarriers [103–105]. The most important advantage of self-assembled peptides and proteins in preserving the biologic functions of multifunctional nanostructures is their spontaneous assembly from disordered entities into ordered structures [106]. The key characteristics of these multifunctional nanostructures include their mechanical properties, responsiveness, capacity for biomimicry, and lack of toxicity [107]. The basic requirements for using nanomaterials for drug delivery are high efficacy, biocompatibility, reproducibility, non-cytotoxicity, and biodegradability [108]. The mechanism of drug loading and release by nanocarriers typically involves: (1) the nanocarriers encapsulating the desired biomolecules with high loading capacity; (2) protecting the encapsulated biomolecules from degradation during the delivery journey; and (3) release of the biomolecules in a controlled and prolonged manner [109–111]. However, to improve drug delivery efficacy and decrease toxicity, self-assembling nanomaterials should also contain specific domains for targeting and/or sensing the environment at the ultimate targets [112].
Self-assembled nanovehicles for chemotherapy and gene delivery
This section deals with the application of self-assembled peptides and proteins to encapsulate biotherapeutic agents and their delivery to a targeted site. We first introduce non-responsive self-assembled cargos to deliver drug and gene in targeted fashion. Subsequently, we review stimuli-responsive platforms that respond to internal or external triggers.
Targeted nanoplatforms
Drug delivery
Many studies have been conducted in recent years to design platforms with targeted delivery capability [69,113–117]. Amphiphilic peptides produce promising nanoparticles that enhance the targeting efficacy of the loaded anti-cancer drug [69]. Peptide ligands are highly esteemed in anti-cancer chemotherapeutics because of their low immunogenicity, excellent biodegradability and ease of modification of the nanostructure sizes by changing the peptide composition to cater to different drug loading capacities [118].
Examples of targeting ligands for nanoarchitectures commonly used in chemotherapeutic delivery systems include cyclic arginyl glycyl aspartic acid (RGD) peptide, cell-surface hormone receptor (LHRH), granulocyte colony stimulating factor (G-CSF) and tumor vasculature antigen [85,119–121]. The amphiphilic peptide RGD possesses cell adhesion properties that enable it to mimic extracellular matrix (ECM) proteins [122]. RGD binds to upregulated αvβ3 and αvβ5 integrins (transmembrane glycoproteins) on the surface of vascular endothelial cells and tumor cells during tumor growth and metastasis. This provides rationale for utilizing RGD to activate endocytosis and help penetrate cells [123]. The RGD sequence is handy for the design of peptide amphiphiles for targeted anti-cancer drug delivery of cytotoxic therapeutics to the tumor region [124]. For example, a bioresponsive gel delivery system has been designed by the self-assembly of cisplatin (cis-dichlorodiamine platinum (II)) in a 4% aqueous solution of palmitic acid-GTAGLIGQRGDS at 37 °C [125]. The delivery system assembled into a nanofiber gel by forming complexes of platinum with the carboxylic acid groups of the adjacent nanofibers. The nanofiber gel displayed RGD ligands on its surface, which could be used to target the integrin receptors that are overexpressed on certain cancer cells. The anti-cancer agent could then be released directly into the tumor via cleavage by high local matrix metalloproteinase-2 concentrations which accelerated biodegradation of the gel [126]. In another example, the self-assembling dipeptide LeuΔPhe (containing an α,β-dehydrophenylalanine residue) can spontaneously form a stable hydrogel suitable for the entrapment of anti-cancer drugs. The chemotherapeutic drug mitoxantrone (MT) was incorporated into the gel via hydrophobic interactions during formation. The hydrogel was administered intraperitoneally in tumor-bearing mice, releasing MT to reduce tumor growth. MT entrapped in the hydrogel showed higher efficacy and lower toxicity than MT alone, suggesting suitability of the hydrogel for in vivo applications [127].
A colloidal suspension can be produced using a mixture of two peptides, EAK16-II and EAK16-IV, in the presence of ellipticine, an anti-cancer agent [128–130]. Nanoparticles with protonated ellipticine were efficient in treating both human breast cancer cells (MCF-7) and alveolar basal epithelial adenocarcinoma cells (A549) [131]. Increasing the hydrophobicity of the EAK16-II peptide augmented the hydrophobic interactions between the drug and the peptide. This, in turn, enhanced the formulation’s stability in aqueous environments [129].
Biotherapeutic peptides may under certain conditions form clusters with metal ions or nanoparticles. For example, certain β-catenin/Bcl9 inhibitors, known as anti-cancer peptides, can copolymerize with Au ion and assemble into a nanocomposite (Figure 9); these nanodevices have size- and charge-switching capabilities in acidic microenvironments such as tumor. The small, positively-charged nanoparticles exhibited excellent cellular penetration and can realize drug release in acidic intracellular microenvironments (Figure 9A and B), thereby contributing to tumor growth inhibition (Figure 9C and D) [132].
Figure 9.

Anti-cancer effects of peptide cluster nanoparticles. (A) Depiction of the function of a peptide cluster (pCluster). (B) Schematic illustrating the changes in size and charge of a pCluster. (C) Changes in tumor volume over time after administration of the pCluster. (D) Photographs of tumors collected from mice 12 days after administration of the pCluster. Reprinted with permission from [132].
A tumor-selective cascade activatable self-detained system (TCASS) was designed to accommodate a tumor-specific recognition motif. The peptide molecule (AVPIAQKDEVDKLVFFAEC(Cy)G) contained both self-assembly and recognition motifs. The recognition motif identified the inhibitor of apoptosis protein (XIAP) that undergoes upregulation during cancer progression. Downstream caspase-3/7 was activated during the recognition process, which initiated self-assembly of the peptide into fibrous nanostructures (Figure 10A). Mouse models were employed to monitor peptide accumulation and penetration into tumors, as well as its organ biodistribution. The accumulation of the peptide within tumors was significantly higher than other typical soft/hard nanoparticles such as liposomes and SiO2. The nanostructures exhibited increased accumulation in tumor regions through recognition-induced self-assembly effects (Figure 10B). Additionally, the platform showed comparable clearance pathways to that of small molecules, which are excreted from reticuloendothelial system organs (e.g., liver) and kidneys, whereas they are rather slowly removed from tumor tissues (Figure 10C). Adding an anti-cancer agent such as doxorubicin to TCASS can improve the anti-cancer treatment efficacy without concomitantly increasing toxicity. Ex vivo use of TCASS demonstrated high specificity and sensitivity in screening human bladder cancer (Figure 10E), showing potential clinical translatability [133].
Figure 10.
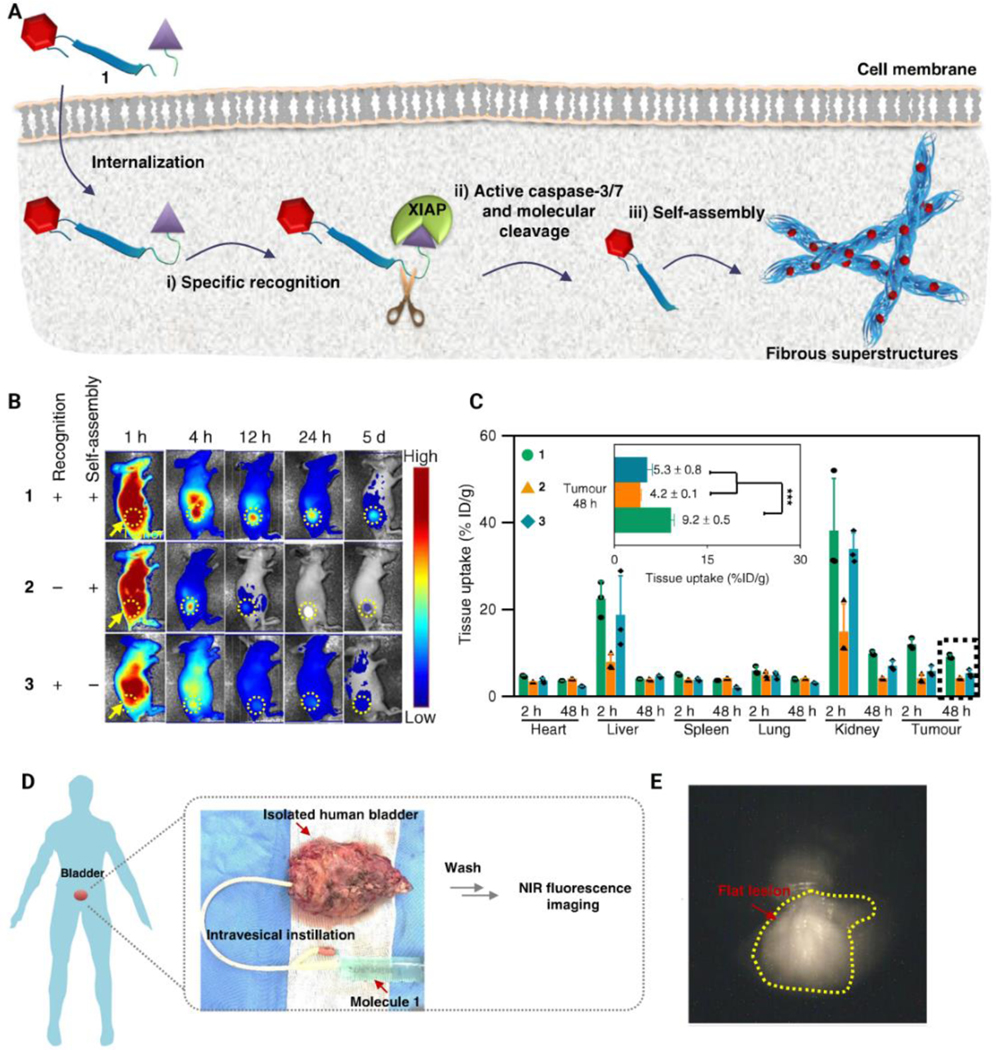
Schematic presentation of a tumor-selective cascade activatable self-detained system (TCASS). (A) The underlying pathways involved in molecular recognition, molecular cleavage, and in-situ self-assembly of molecules. (B) Representative near-infrared (NIR) fluorescence images of molecules 1, 2 and 3 on H460 tumor-bearing nude mice. The sign to show the use of recognition or self-assembly nanoparticles is presented by “+” and without them is by “−”. (C) Molecular uptake of molecules 1, 2 and 3 by the tumor and major organs in H460 tumor-bearing nude mice. (D) Schematic illustrating the procedure of intravesicular instillation of the TCASS using intact human bladder. (E) NIR fluorescence imaging of human bladder tumor tissues of a patient. TCASS: tumor-selective cascade activatable self-detained system. Molecules 1, 2 and 3 are three different synthesized peptides including peptide molecule 1 (AVPIAQKDEVDKLVFFAEC(Cy)G) contained both recognition and self-assembly motifs; peptide molecule 2 (MDEKAQKDEVDKLVFFAEC(Cy)G) contained the self-assembly motif alone; peptide molecule 3 (AVPIAQKDEVDEC(Cy)G) contained the recognition motif alone. Reprinted with permission from [133].
A delivery peptide hydrogel (VKVKVKVKVDPLPTKVKVKVKV-NH2) for curcumin was produced via gelation in response to an increase in the ionic strength of the medium. Curcumin is a hydrophobic polyphenol drug derived from rhizome of Curcuma longa. It possesses anti-oxidant, anti-inflammatory and anti-tumorigenic properties. The lysine-associated charges in the peptide were first neutralized. Neutralization induced self-assembly of the peptide, both laterally (via intermolecular H-bonds and van der Waals interactions) and facially (i.e., the hydrophobic face of the peptide). The self-assembly process of the peptide resulted in a β-sheet hairpin structure [134]. The peptide hydrogel was used for sustained release of curcumin for over two weeks. The shear-thinning properties of the peptide increased drug stability under physiological conditions while retaining the bioactivity of the loaded curcumin [135].
It is important to point out that tumor cells rely on various molecular pathways and mechanisms to drive their uncontrolled growth and migration. Hence, eradication of tumor cells is generally enhanced using a combination of anti-tumor drugs and genetic tools. Each of these strategies can disrupt a certain oncogene pathway. Further studies should be focused on using self-assembled peptides for co-delivery of anti-tumor drugs and genetic tools. Table 1 summarizes the use of self-assembled peptides and protein nanostructures as drug delivery systems in anti-cancer therapy.
Table 1.
Self-assembled peptide and protein nanostructures as drug delivery systems in anti-cancer therapy.
| Materials composition | Architecture | Size (nm) | Cargo | Remarks | Reference |
|---|---|---|---|---|---|
| Protein-functionalized silica | Spherical nanoparticles | 20 | Doxorubicin | Breast tumor size decreased 3 times compared with free doxorubicin. Targeting protein on the surface of nanosilica enhanced tumor internalization. | [136] |
| C12-PPPPRRRR-NH2 peptide | Nanofibers | - | Doxorubicin and paclitaxel | Increased liposomal uptake because of arginine-rich peptide. Enhanced efficacy of the drugs combined with peptidefunctionalized liposomes. | [137] |
| mPEG-b-P(Glu-co-Phe) poly(ethylene glycol)-b-poly(L-glutamic acid-co-L-phenylalanine) | Micelle-type nanoparticles | 140 | Doxorubicin | Superior drug-loading capacity and pH-triggered drug release. Excellent antitumor activity with negligible side effects compared to free doxorubicin. | [138] |
| VVVVVVKK peptide | Spherical | 100 | Doxorubicin and paclitaxel | Potent toxicity against breast cancer cells when compared to free doxorubicin with negligible side effects. | [139] |
| RADA16 peptide | Nanofibers | 22 | Paclitaxel | High efficiency in inhibiting the growth of breast cancer cell line MDA-MB-435S in vitro. Strong control of drug release. | [140] |
| E3PA (AAAAGGGEEE) peptide | Nanofibers | 12–13 | Camptothecin | Anti-tumor activity against human breast cancer cells in vitro. | [141] |
| cRGDfK peptide conjugated with dextran | Micellar-type nanoparticles | 230 | Paclitaxel | High drug loading efficacy. The conjugate enabled formation of a nanodrug with surface targeting property. | [142] |
| P4 (LDLKLELKLDLKLELK) peptide | Nanofibers | 5 | Ellipticine | Stabilized ellipticine, a hydrophobic anti-cancer agent, in aqueous solution. Reduced the viability of two cancer cell lines, SMMC7721 and EC9706. | [143] |
| GTAGLIGQRGDS peptide | Nanofibers | 8–10 | Cisplatin | Bioresponsive drug release capability for targeted anticancer drug delivery. | [144] |
| H3SSgT peptide | Micelles | ~140 | Anthraquinone | High cellular uptake, providing enhanced permeability and retention, targeted delivery and cytotoxicity against cancer cells. | [145] |
| RGDSEEEEEEEEEEK peptide | Micelles | 255 | Doxorubicin | pH-responsive. Reduced cancer cell proliferation of cancer cells. | [146] |
| Cyclodextrin B | Hydrogel | 100 | Arylazopyrazole | Photodynamic therapy. Reduced viability of cancer cells. | [147] |
| NapFFKY peptide | Micelles | 192 | Doxorubicin | Glutathione responsive, tumor growth inhibition and synergistic chemotherapy. | [148] |
Gene delivery
In addition to drug delivery, peptide-based nanoscale delivery systems can be utilized for gene delivery. The need for gene delivery emanates from a variety of drawbacks associated with anti-tumor compounds including the resistance of cancer cells to chemotherapies [149]. It is thus important to also develop gene delivery nanoparticles for cancer therapy. To date, a number of genetic tools such as small interfering RNA (siRNA) and CRISPR/Cas9 have been developed for cancer therapy [150]. However, these tools have been limited to pre-clinical studies in cancer therapy, with more efficacy in vitro compared to in vivo due to different conditions in animal models. In this section, we provide a mechanistic discussion of the use of self-assembled peptide nanocarriers for gene delivery in cancer therapy.
In pre-clinical cancer therapy, gene delivery has shown higher efficacy in comparison to conventional anti-cancer drugs because of the presence of a certain type of cells in the tumor microenvironment, known as cancer-associated fibroblasts (CAFs) [151]. These fibroblasts are abundant in the tumor microenvironment and can enhance the migration of cancer cells to neighboring and distant sites via induction of angiogenesis [152–154]. There are, however, controversies regarding targeting CAFs in anti-cancer therapy. Recent work indicates that CAF depletion is correlated with enhanced cancer metastasis [155]. Accordingly, inactivation of CAFs is likely a better strategy versus depletion. Although anti-cancer drugs are capable of eliminating CAFs, siRNAs are capable of inactivating CAFs without their depletion, thereby minimizing the risk of metastasis. Oncogenes that are responsible for sending signals to CAFs and increasing cancer progression should be identified in the future to support the development of effective cancer therapies. The chemokine C-X-C Motif Chemokine Ligand 12 (CXCL12) is essential for oncogenic communication of tumor cells with their stroma, which results in increased metastasis [156]. A recently published study revealed the efficacy of a peptide assembly-based nanosystem for the delivery of siRNA-CXCL12. To selectively target CAFs, the self-assembled nanosystem was equipped with fibroblast activation protein-α antibody. After penetrating into CAFs, siRNA-CXCL12 was released to downregulate the CXCL12 gene, resulting in CAF inactivation. Consequently, cytokines and other factors required for angiogenesis were not secreted by the CAFs, giving rise to decreased invasion and migration of the cancer cells [157]. This study demonstrated how self-assembled peptides may be deployed for selective targeting of important cells in the tumor microenvironment for the delivery of certain siRNAs (Figure 11) [157].
Figure 11.
Anti-tumor activity of self-assembled peptide nanoparticles. (A) Preparing the PNP/siRNA/mAb nanoplatform via self-assembly. (B) Suggested pathway of PNP/siCXCL12/mAb-induced metastasis suppression and CPP-mediated transfection of CXCL12 siRNA in CAFs. (C) Tumor progression curves determined by quantification analysis of the in vivo bioluminescence signal. (D) Images of prostate tumors with testicles. Yellow dashed lines represent the locations of the primary tumor. (E) Weight of isolated tumors (without prostate and testicles) in each group. CAF: cancer-associated fibroblasts; CPP: cell-penetrating peptide; CXCL12: C-X-C Motif Chemokine Ligand 12; mAb: monoclonal antibody; PNP: peptide nanoparticle; SiRNA: small interfering RNA. Reprinted with permission from [157].
SiRNA has emerged as a relatively new class of gene therapeutics for the treatment of various diseases including viral infections and cancer [158,159]. SiRNA has been one of the most important gene editing tools over the last couple decades; it degrades and ‘silences’ specific mRNA in a highly sequence-specific manner. It has been employed to down-regulate various oncogenes in anti-cancer therapies [160]. Nevertheless, a number of impediments exist that challenge the efficacy of siRNA for in vivo gene editing. Although siRNA is capable of effectively knocking-down or knocking-out target genes in in vitro experiments, less success has been encountered when siRNA is applied for gene editing in vivo. This is because siRNAs circulating in the blood must evade elimination by kidney filtration, phagocytosis, precipitation by serum proteins, and perhaps most importantly enzymatic degradation before they can reach their targeted sites [161,162]. In addition, siRNAs are generally not easily taken up by cells, due to their anionic and hydrophilic features. These barriers necessitate the use of carriers to successfully deliver siRNAs for gene silencing in living subjects [163,164]. Self-assembled proteins/peptides have been used to deliver siRNAs for cancer treatment [165,166]. For example, siRNA was encapsulated by diphenylalaninamide-based nanoparticles to silence human epidermal growth factor receptor-2 (HER2), an oncogene that plays a role in the development of some breast cancers. Functionalized diphenylalaninamide-based nanoparticles (FFANPs) were prepared using layer-by-layer polyelectrolyte deposition. Because FFANPs degrade at low pH, coating them with poly-L-lysine via cation-dipole interactions promoted their stability, with diphenylalaninamide as a dipole with zero net charge. Coating diphenylalaninamide with poly-L-lysine resulted in a net positive surface charge crucial for the encapsulation of siRNA due to its anionic nature. After formation of the FFANP-poly-L-lysine/siRNA complex, another coating is required to protect the siRNA against degradation by nucleases. The self-assembled peptide-based nanoparticles were capable of effectively delivering and releasing siRNA for silencing HER2 in breast cancer cells [165]. This study provided evidence that the anionic nature of siRNAs prevented them from penetrating into the cell membrane and escaping from endosomes, a problem that was solved using poly-L-lysine. Because siRNA can also affect other genes apart from the targeted gene, the use of self-assembled peptides can help to prevent off-target uptake and undesirable toxicity.
Stimuli-responsive devices
Non-specific distribution of drugs in cells and tissues, and consequent off-target issues of currently-applied genetic tools have driven many scientists to use nanoscale delivery systems in cancer therapy. Importantly, the potential of nanoparticles for drug and gene delivery in cancer can be improved by exploiting distinct features of cancer cells. A key difference between cancerous and normal cells includes the expression of certain surface receptors as well as secreted enzymes. Furthermore, the tumor microenvironment generally displays a mildly acidic pH that can be exploited for developing smart nanostructures. Due to the presence of such differences between diseased and normal tissues, stimuli-responsive nanoparticles have been designed based on two distinct types of stimuli: endogenous and external physical stimuli. Glutathione concentration, levels of enzymes such as matrix metalloproteinases, and pH can be utilized to intelligently design stimuli-responsive devices. For instance, nanoparticles can be synthesized from polymers that degrade at the mild acidic pH of the tumor microenvironment, releasing drug or genes at the tumor site, but are stable at neutral pH such as that found within blood and normal tissues. The second type of stimuli-responsive devices involve those that respond to energy that is externally applied. For example, magnetically-guided delivery can be controlled using ultrasmall iron oxide-based nanoparticles; there also exist a growing variety of thermo-, light- and ultrasound-sensitive nanoarchitectures to treat disease [167,168].
Mesoporous silica nanoparticles (MSN) have been used as carriers for targeted drug delivery in anti-cancer therapy, with high responsiveness toward hyaluronidase. For the formation of a drug delivery system, biotin (or desthiobiotin) binds to the external surface of MSN, followed by decoration of the created nanoparticle by hyaluronic acid for drug release control on cancerous cells. Uptake by cancer cells was mediated via CD44 receptor-mediated endocytosis (Figure 12A and B). The drug delivery system released doxorubicin rapidly in vitro in the presence of biotin and hyaluronidase at pH 6.5 in PBS (Figure 12C). The MSN-hyaluronic acid/doxorubicin nanoparticles displayed good biocompatibility in vitro and in vivo and inhibited tumor growth (Figure 12D). pH-responsive MSN were capable of delivering doxorubicin to improve the efficacy of chemotherapy at a mild acidic pH near the tumor microenvironment [169].
Figure 12.
Self-assembly of biotin on silica nanoparticles for tumor therapy. (A) Preparation of MSN-HA/Dox delivery system. MSN: mesoporous silica; HA: hyaluronic acid; Dox: doxorubicin. (B) Schematic of CD44 receptor-mediated endocytosis and trigger of drug release in tumor cells. (C) Drug release at pH 6.5 in PBS was performed to simulate the condition of the tumor microenvironment. Under different stimuli, biotin (2 mmol/L), hyaluronidase (HAase; 150 U/mL) or both were added to the MSN-HA/Dox solution. MSN-Dox was employed as the control. (D) Representative photos of tumor tissues obtained from tumor-bearing mice treated for 18 days with saline (control), MSN-HA, free Dox or MSN-HA/Dox. Reprinted with permission from [169].
Previous studies have highlighted the importance and potential of pH-sensitive peptide nanocarriers in cancer therapy. Surface functionalization of peptide nanocarriers should further improve their selectivity toward cancer cells. Surface modification of nanoparticles with hyaluronic acid is important for selectively targeting CD44-overexpressing cancer cells. Self-assembly, however, does not occur, due to the hydrophilic nature of hyaluronic acid [170–172]. In light of this, studies were conducted to functionalize hyaluronic acid with hydrophobic segments to produce amphiphilic hyaluronic acid. Such strategies have led to the fabrication of hyaluronic acid-modified nanoparticles for the encapsulation of hydrophobic anti-tumor agents such as doxorubicin. Alternatively, hyaluronic acid-based nanoparticles could be prepared without using hydrophobic groups by electrostatic self-assembly of hyaluronic acid and ε-polylysine, and subsequently, in-situ crosslinking by disulfide bonds. The release of chlorin e6 (Ce6) from nanovehicles was based on alterations in pH and glutathione; in particular, addition of glutathione to pH 7.4 promoted the release of Ce6 from these nanocarriers. Apart from their biocompatibility, the self-assembled nanoparticles demonstrated excellent cellular uptake, with marked improvement in the photodynamic therapeutic efficacy [173].
Smart nanoscale delivery systems based on various endogenous stimuli can be developed for cancer therapy. For example, a photosensitive drug delivery system was designed for photodynamic therapy using dipeptide or amphiphilic amino acid-tuned self-assembly of photosensitizers (Figure 13). Self-assembly was attributed to the presence of opposite charges on the two moieties (i.e. the photosensitizer bearing negative charge and the dipeptide bearing positive charge) (Figure 13A). These nanodrugs displayed valuable characteristics for therapeutic efficacy, such as changeable size, high loading capacity and drug release in response to pH, surfactant and enzyme stimuli (Figure 13B and C). These features significantly increased the effectiveness of the photosensitizers in vitro and in vivo. Tumor eradication was achieved in mice using a single drug dose and a single light exposure (Figure 13D and E) [174].
Figure 13.

Self-assembly of photosensitizers bearing negative charge and a dipeptide bearing positive charge. (A) Fabrication of photosensitive nanoparticles by amphiphilic dipeptide- or amino-acid-tuned self-assembly. (B) Cell internalization of the assembled nanoparticles in vitro. Fmoc-l-Lys/Ce6 nanoparticles (FCNPs) were incubated with MCF7 cells for 24 h at 37 ˚C. Nuclei were stained blue by Hoechst 33342. The cell membrane was stained green by Alexa 488. Red staining is indicative of FCNPs. (C) Cytotoxicity of FCNPs and free Ce6 at different Ce6 concentration after co-culturing for 24 h, with or without irradiation. (D) Whole body fluorescence images of MCF7-tumor-bearing nude mice intravenously injected via the tail vein with FCNPs and free Ce6 (equivalent Ce6 4.0 mgkg−1 body) at different times. Black circles indicate tumor sites. (E) Measured tumor growth for 20 days. Ce6: Chlorin e6, Fmoc-l-Lys: Fluorenylmethoxycarbonyl-l-lysine. PS: photosensitizers. Reprinted with permission from [174].
The efficacy of co-delivered anti-tumor drugs by self-assembled peptides, mentioned in the previous section, has been investigated using stimuli-responsive self-assembled peptide nanocarriers. Small hydrophobic molecules were encapsulated with hydrophobic L-tryptophan-D-leucine repeating units isolated from a truncated sequence of gramicidin A. To endow electrostatic affinity to the nucleotides, the latter were mixed with a hydrophilic moiety of histidine. An artificial amino acid bearing a disulfide functional group (H3SSgT) was used to link the hydrophilic and hydrophobic sequences, and to render the nanoparticles stimuli-responsive and functional. The small size of self-assembled peptide nanoparticles (100–200 nm) made them ideally suited for evading phagocytosis and kidney filtration [175]. In cancer therapy, the self-assembled peptide nanoparticles effectively encapsulated drugs and genes, as well as provided targeted delivery, significantly suppressing the proliferation and invasion of cancer cells [132].
It is clear that using stimuli-responsive devices could be a turning point in disease therapy, since far less than 50% of systemically-administered drug typically reaches the tumor site, resulting in poor bioavailability and restricting therapeutic impact. Moreover, genetic therapies can be degraded by enzymes while circulating in blood, and they can potentially affect the expression of other genes when delivered to off-target sites that may cause unexpected impacts. Consequently, smart and stimuli-responsive devices have been developed for gene and drug delivery in cancer therapy and self-assembled peptide nanocarriers may be ideal candidates for drug and gene delivery at the tumor site. Surface modification of self-assembled nanoparticles can promote their selectivity towards cancer cells, and they can be designed to be pH-sensitive to release cargo within tumors. Important barriers to clinical translation of these peptide-based nano-based delivery systems in cancer therapy include complexity of design and difficulties in scaling up production as well as biocompatibility issues.
Self-assembled peptides/proteins nanoplatforms for cancer immunotherapy
Cancer immunotherapy aims to stimulate the body’s immune responses to combat disease. Despite its successes, cancer immunotherapy has encountered many problems including immunotolerance induction, tumor escape, as well as tumor metastasis and recurrence [176,177]. While these issues have been addressed to some degree by developments in checkpoint blockade and adoptive cell therapy, the application of immunotherapy remains limited to certain types of cancer. The use of antibodies to block the interaction of programmed death-ligand 1 (PDL-1) in tumors containing programmed death-1 (PD-1) expressed on T cells is a thus-far successful application of cancer immunotherapy, directed to avoid T cell inhibition of proliferation and activity. However, some tumors do not express PDL-1 or can downregulate its expression under certain conditions. Tumors lacking PD-L1 expression generally show poor clinical outcomes in response to PD-1/PD-L1 checkpoint inhibitors [178]. Mutations in key intermediate components of interferon signaling pathways [179] as well as immunosuppressive factors and cells in the tumor microenvironment or upregulation of alternative inhibitory proteins [180] can contribute to adaptive resistance to immune checkpoint blockade. On the other hand, despite encouraging results in leukemia treatment, the treatment of solid tumors is still limited with respect to the application of chimeric antigen receptor (CAR)-T cell therapy: the loss of antigen expression due to selection pressures that favor tumor escape, off-target toxicity effects, and poor clinical trial outcomes [181–184] suggest that there remain many challenges to overcome for successful CAR-T cell therapies. [185].
Anti-cancer vaccines and platforms for antigen delivery to induce T cell priming and effector functions are promising approaches for cancer treatment [186–188]. Cancer prevention vaccines may be given to healthy animals (or humans, if the vaccine is clinically approved) to prevent development of certain types of cancers. In contrast, cancer treatment vaccines, also called therapeutic vaccines, are a form of immunotherapy. These vaccines work by boosting the body’s natural defenses to combat cancer by recognizing and destroying antigens expressed on cancer cells. Cancer cells contain molecules that are known as cancer-specific antigens, or sometimes neoantigens, on the surface that healthy cells do not display or are relatively under-expressed. When these molecules are given to an animal or human, the molecules act as antigens. They stimulate the immune system to recognize and destroy cancer cells expressing these molecules on their surface. Most cancer vaccines also contain adjuvants, which are substances that help to boost the immune response.
Tumors evade immunosurveillance by playing an active role in the downregulation of antigen presentation, by reducing expression of major histocompatibility complex I (MHC-I) and of costimulatory molecules and by enhancing expression of inhibitory receptors [189]. Moreover, central immunotolerance to tumor self-antigens represents a barrier for anti-tumor immunity. In addition, tumor-induced immunosuppression by regulatory T cells and myeloid-derived suppressor cells within the tumor microenvironment results in damped, inefficient immune responses. As a result, the immunogenicity of a vaccine compromised [190]. For these reasons, highly effective vaccine formulations are required. These vaccines should be capable of inducing a strong and sustained immune response and to overcome immunotolerance [191].
Self-assembling biomaterials represent a promising cancer immunotherapeutic approach. Apart from their use as drug delivery systems, they may be used as anti-cancer vaccines for active immunization. Self-assembly of proteins and peptides to form micrometric and nanometric blocks enable scientists to generate systems that can be drained to lymph nodes, to be captured by immune cells, and to initiate a cascade of signals that leads to the activation of immune responses [192]. Protein and peptide-based nanostructures can exert different effects as a function of their dimensions and of appropriate targeting of immune cells. Nanocarriers of size >100 nm employed in prophylactic and therapeutic immunotherapy are mostly taken up by local antigen presenting cells (APCs) at the site of injection or tend to accumulate in the highly-permeable tumor tissue. Smaller nanostructures (~40–50 nm) instead tend to enter the lymphatic vessels and reach the lymph nodes (LNs); they can be internalized by the LN-resident dendritic cells with higher efficiency, eliciting an effective and long-lasting immune response against cancer cells [193,194].
Several characteristics of self-assembling peptide/protein nanostructures improve their superiority compared to other types of vaccines: they show higher stability than lipid-based delivery systems [195,196] and they are generally considered less toxic than synthetic nanoparticle carriers and polymer-based nanosystems due to their breakdown into purely biological constituents [197]. Their typical size range favors efficient uptake by dendritic cells, while systemically-administered peptide-based cancer vaccines often end up being internalized by non-professional antigen presenting cells, leading to sub-optimal antigen processing and presentation [198]. Moreover, prolonged retention time in tissue can limit the number of boosting doses needed, often with a consequent decrease in toxic effects observed with soluble immunotherapeutic molecule administration [199]. Finally, these nanostructures display unique advantages like self-adjuvanting properties, structural versatility, and the possibility of rapid production of large quantities through existing expression systems [200]. In recent years, self-assembly of peptides and proteins has received increasing attention due to the unique traits, e.g., physical, chemical, biological, and electronic properties, that self-assembled peptides and proteins offer [201–204]. Peptides and proteins are often assembled into ordered structures such as nanofibers, nanowires, nanotubes, films, networks, and other nanostructured materials on surfaces of inorganic, organic, and biological compounds as substrates [203,205,206]. In the peptide and protein self-assembly process, various key physicochemical parameters including pH, temperature, ionic strength, solution, molecular sequence, and molecular concentration exert a great impact on the kinetics and morphology of nanostructure formation. Therefore, the kinetics and morphologies of the created nanomaterials can be controlled by intelligent adjustments of these parameters [207,208]; moreover, it also follows that these nanostructures are affected by physicochemical properties of surface and interface characteristics of substrates, e.g., hydrophobicity, hydrophilicity, and surface charge. It is thus of great importance to have a full understanding of the interactions between peptides/proteins and their substrates [59]. Table 2 summarizes self-assembling peptide- and protein-based structures recently employed for cancer immunotherapy and vaccinations.
Table 2.
Self-assembling proteins and peptides for antigen and/or drug delivery and used for cancer vaccine formulations.
| Protein/peptide system | Antigen | Adjuvant/drug combination | Cancer | Type of study | Reference |
|---|---|---|---|---|---|
| HBcAg | HPV E7 | - | cervical cancer | mouse | [209] |
| HBcAg | IL-33 | - | 4T1 breast cancer | mouse | [210] |
| HBcAg | FGF-2 | - | TC-1 lung cancer | mouse | [211] |
| PVX | - | doxorubicin | B16 melanoma | mouse | [212] |
| PVX | HER2 | - | Her2+ cancer | mouse | [213] |
| CPMV | - | - | B16 melanoma, 4T1 breast cancer, CT26 colon cancer | mouse | [214] |
| Qbeta | Melan-A/MART-1 | CpG, IFA, imiquimod | melanoma | human | [215] |
| Qbeta | TACAs | CFA/IFA; MPLA, cyclophosphamide | TA3H adenocarcinoma | mouse | [216] |
| Fd phage | OVA | galactosylcera amide | B16-OVA melanoma | mouse | [217] |
| MS2 phage | HPV-L2 | alum | cervical cancer | mouse | [218] |
| RHDV | Gp100 | mannosylation | B16.gp33 melanoma | mouse | [219] |
| E2 | NY-ESO, MAGE-A3 | CpG | A375 melanoma MCF-7 breast cancer | mouse | [220] |
| Ferritin | RFP | - | B16-RFP melanoma | mouse | [221] |
| Ferritin | HPV16 E7; MC38 | anti -PD1 antibody | TC1-E7 lung, MC38 colon adenocarcinoma | mouse | [222] |
| HSP65 | STEAP1 | - | RM-1 prostate cancer | mouse | [223] |
| Vault | - | CCL21 chemokine | GL261 glioblastoma | mouse | [224] |
| Q11 | MUC-1 | - | MCF-7 breast cancer | human, mouse | [74] |
| Q11 | OVA | - | - | mouse | [225] |
| KFE8 | OVA | - | - | mouse | [226] |
| Coil29 | EGFRvIII, PADRE, OVA | CFA/IFA | glioblastoma | mouse | [227] |
| Coil29 | MUC1 | - | breast cancer | human | [227] |
| PNC | OFA | - | - | mouse | [228] |
| RADA16 | OVA | DC/anti PD-L1 | EG7-OVA (lymphoma) | mouse | [229] |
| FK | Autologous tumor cells | PD-L1 inhibitor JQ1 | 4T1 breast cancer | mouse | [230] |
Abbreviations: HBcAg, hepatitis B core antigen; FGF-2, fibroblast growth factor; Qbeta, bacteriophage Qbeta; CFA/IFA, Freund’s complete/incomplete adjuvant; TACAs, tumor-associated carbohydrates; OVA, ovalbumin; RFP, red fluorescent protein; STEAP1, six transmembrane epithelial antigen of the prostate 1; PNC, peptide nanoclusters; OFA, oncofetal antigen; DC, dendritic cells; FK, Fmoc-KCRGDK
6.1. Self-assembling proteins
6.1.1. Virus-like particles
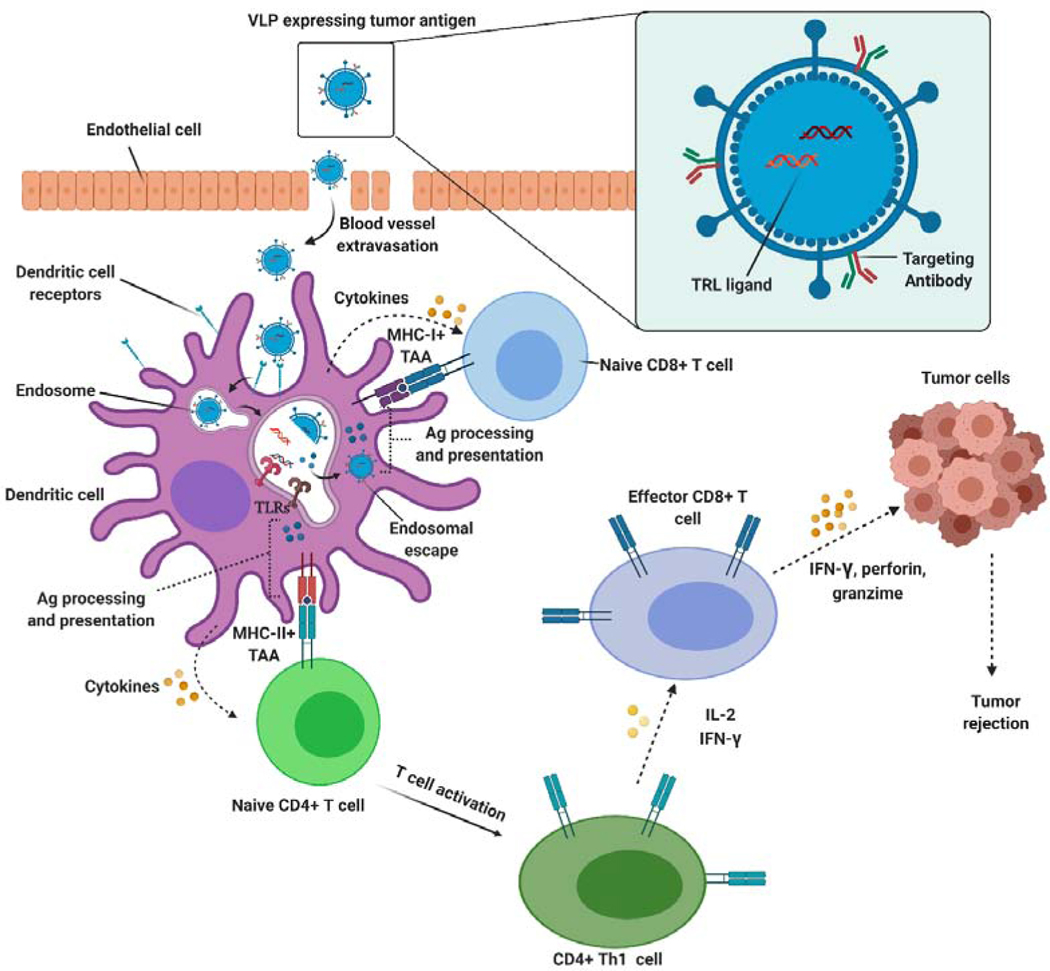
One successful example of self-assembling proteins used for immunotherapeutic approaches is virus-like particles (VLPs). These structural viral proteins self-assemble to form particulate protein scaffolds and resemble viral particles in terms of structure (mainly icosahedral or rod-shaped), size and antigenic composition, but do not carry the viral genome. Hence, VLPs possess a high safety profile and are incapable of replicating within host cells [231].
VLPs may be produced in multiple cell culture systems ranging from bacteria to yeast, plant and mammalian cells. The choice of the expression system is dependent on cost, yield and the need for post-translational modifications [231]. Many VLPs are developed as an alternative to attenuated or inactivated pathogen-based vaccines. These VLPs have the authentic structure of proteinaceous viral capsids but lack the DNA or RNA genome, avoiding any of the risks associated with replication or inactivation by the original virus. Currently, vaccines licensed for human use and based on VLPs are against human papillomavirus (Cervarix®, Gardasil® and Gardasil9®) and against hepatitis B virus, including the 3rd generation Sci-B-Vac™ [232]. The nanometer-scale dimensions, the symmetrical and highly organized structure formed by repeated monomers, and the presentation of antigens in the same conformational structure as native proteins are characteristics that maximize immunogenicity [233,234]. Because of these highly desirable traits, VLPs have been used extensively for vaccination. In addition, VLPs mimic the geometry, size, and shape of the natural pathogens and can be sensed by innate immune cells through recognition of the viral repetitive subunits, producing strong cellular and humoral immune responses [234,235].
Virus-like particles may also be used as platforms for foreign antigen delivery by constructing chimeric particles with surface-exposed heterologous peptides or proteins [236,237]. Using recombinant DNA technology, it is conceivable to engineer genes encoding viral structural proteins. Such techniques produce self-assembling proteins that can fuse with exogenous fragments encoding multiple copies of the designated antigen for inducing immune responses against that antigen. In addition, chemical modifications may be exercised to functionalize the structural proteins of the scaffold, enabling the conjugation of chemical compounds, cell ligands, carbohydrates, antibodies or imaging agents [232,237–239] (Figure 14).
Figure 14.
Schematic representation of multifunctional self-assembling virus-like particles (VLPs). Particulate structure of nanometric size (20–200 nm) to be efficiently taken up by APC; well-defined geometry and highly organized structure composed of repeated subunits; high safety profile due to lack of viral genetic material; delivery of foreign proteins, chemical compounds or peptides on the viral surface; entrapment of drugs or pattern recognition receptors (PRR) ligands (i.e. unmethylated CpG sequences) to activate both innate and adaptive immunity; possibility of specific cell targeting and enhanced internalization by co-delivery of molecules to target cell surface receptors (i.e. antibodies, cell ligands or carbohydrates).
A number of viruses have been used to generate heterologous VLPs, such as adeno-associated virus-2 [240], human papillomavirus (HPV) [241], rabbit hemorrhagic disease virus [242], human hepatitis B virus (HBV) [243,244], and human hepatitis C virus [244]. To maximize safety and reduce production times and costs, plant-derived viruses have also been used, such as cowpea mosaic virus (CPMV) [245], alfalfa mosaic virus [246], tobacco mosaic virus and potato virus X (PVX) [247]. Bacterial viruses have also emerged as promising scaffolds under VLP development, including the bacteriophage Qbeta [248] and the fd filamentous bacteriophage [217].
One of the most widely used VLP platforms is based on the hepatitis B core antigen (HBc) of HBV. This scaffold was used to express heterologous sequences derived from different antigenic proteins, such as HPV E7 [209], HBV PreS1 envelope protein [249] or fibroblast growth factor (FGF)-2 [211]; successful results have been reported in preclinical studies. By using a tandem-core modification, it is possible to covalently assemble two copies of the HBc protein by inserting a flexible peptide linker. This modification enables the optimal folding of viral particles, maximizing the efficacy of assembly and permitting the insertions of larger exogenous proteins on the exposed surface [250]. Large proteins, containing important epitopes requiring three-dimensional structure, can be exposed efficiently by HBc VLPs. For instance, recombinant HBc was covalently fused to the full-length mature form of the cytokine interleukin-33 (IL-33), resulting in VLPs that expose IL-33 on the surface, and have been utilized in vivo to re-modulate the tumor microenvironment and to promote anti-tumor immunity [210].
Compared to vaccines based on mammalian viruses, VLPs built on plant viruses offer advantages in terms of antigen stability, ease of manipulation, safety, enhanced speed of production and high yield [251]. The administration of PVX in conjunction with doxorubicin increased the survival rate of B16 melanoma-bearing mice compared with doxorubicin alone. This was attributed to enhancement of the drug effects by cytokine/chemokine release induced by activation of the innate immune response by the carrier. Using a multiplex assay, the authors demonstrated that PVX administration induces release of proinflammatory cytokines such as interleukin-1® and Macrophage Colony-Stimulating Factor (M-CSF). This release is activated by Pathogen Associated Molecular Patterns (PAMPs) of the PVX and is enhanced by DOX co-administration [212]. Both CPMV and PVX have been used as platforms for HER2-based vaccines for prophylaxis and treatment of HER2+ malignancies, with the capability to rapidly activating antigen-presenting cells (APCs) [213]. Of the two platforms, CPMV demonstrated superior capability for uptake by APCs, which resulted in a stronger HER2-specific antibody response. The CPMV VLPs were used to immunize mice intranasally and reduced B16F10 lung metastatic melanoma in vivo by inducing systemic anti-tumor immunity [214].
Bacterial viruses offer distinctive advantages as antigen delivery systems for vaccine formulations due to their ease of manufacture, safety, inability to infect mammalian cells and low production costs [252]. Because bacteriophages generally resemble PAMPs, these bacterial viruses show intrinsic adjuvant activity, facilitating the activation of innate immune responses [252–254]. A filamentous bacteriophage fd scaffold containing > 2700 copies of the major coat protein pVIII was engineered for the delivery of short tumor-associated antigens (TAAs) in high copy number.
Mouse intratumoral vaccination with fd bacteriophages, delivering a tumor-associated epitope and conjugated to the natural killer T cell-immunostimulating lipid alpha-galactosylceramide, potently boosted adaptive CD8+ T cell responses and delayed tumor progression [217].
Another bacterial virus, the bacteriophage Qbeta, was used as a carrier for tumor-associated carbohydrate antigens (TACAs). The Tn TACAs (αGalNAc-O-Thr and αGalNAc-O-Ser) were chemically conjugated on the Qbeta coat and exposed in around 370 copies per virion in an organized manner on the virion surface. Vaccination of mice with Qbeta-TACAs elicited robust antibody response toward the delivered TACAs [216]. Qbeta scaffold was also employed for the delivery of MART-1 TAA in combination with CpG ODN and imiquimod. The system induced both memory and effector CD8+ T-cell responses in a Phase IIa clinical study [215]. MS2 bacteriophages displaying HPV peptides triggered immune responses toward several HPV strains and demonstrated similar protection against human cervical cancer as commercially available Gardasil-9 [218]. Bacteriophage-based vaccine encapsulation into biodegradable nano/microsystems can further increase their stability and provide improved pharmacokinetics [255].
A further implementation of self-assembling protein-based vaccines consists of chemically attaching a designated antigen to a preformed protein nanocarrier via conjugation methods. Such an approach is particularly useful for the insertion of antigens that interfere with the self-assembly of the nanostructure [256].
6.1.2. Protein nanocages
Apart from virus-derived proteins, non-structural proteins derived from bacteria or from eukaryotic origin that possess self-assembling properties may be used for preparing protein nanocages. The virus-like structure endowed the nanocages with symmetry, highly-organized architecture and a size range that is optimal for them to be trapped by immune cells [236]. The E2 protein of the multi-enzyme complex pyruvate dehydrogenase, derived from Geobacillus stearothermophilus, self-assembles into an icosahedral scaffold that is similar to a VLP. Antigenic determinants can be implemented on the scaffold surface to produce multivalent nanoparticles that resemble virons in size and complexity. This delivery system generated potent antibody response toward the displayed proteins and a Th2-oriented T cell response [257] Engineered E2 scaffolds designed for the simultaneous delivery of two different TAA antigens, in conjunction with a CpG stimulus, induced antigen-specific IFN-γ secretion and cytotoxic T cell responses in transgenic mice that expressed a human HLA-A2 gene [220].
The highly-conserved intracellular protein ferritin is capable of self-assembly into a nanostructure with octahedral symmetry. The nanostructure may be used for the display of heterologous proteins. Ferritin nanoparticles have been developed as delivery agents of tumor-specific antigen. The system was retained in vivo for a prolonged period [221], with the delivered antigen presented by dendritic cells (DC), especially the CD8α+ DC subpopulation [222]. Ferritin-based nanoscaffolds offer several advantages as a delivery vehicle, such as high biocompatibility, biodegradability, remarkable thermal stability, and ease of manipulation. However, some concerns must still be addressed, such as the possible immunogenicity of ferritin derived from non-human origins. Possible strategies to avoid this drawback include the substitution of immunogenic residues to generate partial or total “humanized” ferritin scaffolds, or the development of more efficient and targeted systems that can reduce administered doses [258]. For instance, Archaeoglobus fulgidus ferritin scaffold was recently modified to incorporate the human transferrin receptor 1 on its surface for selective targeting of human acute lymphoid leukemia cells [259].
Among self-assembling proteins, heat shock proteins (HSPs) remain of high interest due to their high binding affinity to certain peptides or proteins, and their capacity to stimulate anti-tumor immune responses either as antigen delivery systems or as natural immunogens. HSPs are intracellular chaperone molecules that get upregulated under stress conditions. They form stable spherical complexes with proteins. The latter bind to the internal central cavity of the chaperone structure [260,261]. Heat shock proteins are overexpressed in several cancer types, including breast [262], lung [263], prostate [264] and gastrointestinal tumors [265]. HSP70 and HSP90 are the most successful and widely used HSPs used in immunotherapy. They have been complexed with tumor antigens extracted from tumors and used for immunization with promising results [266,267].
Increased DC uptake and activation of CD8+T cell responses were attributed to the intrinsic immunomodulatory properties of HSPs [268]. In fact, HSPs alone or in combination with immunogenic peptides have induced dendritic cell maturation, stimulated proinflammatory cytokine release, activated NK functions [269], and promoted antigen cross-presentation of exogenous antigens in the MHC-I pathway for the induction of optimal CD8+ T cell responses [270].
Because HSPs have the capability to form complexes with proteins, they have been used as carriers of antigens or genetic materials. An example of recombinant HSP chaperone vaccine was prepared by complexing large Hsp110 and glucose-regulated protein 170 (Grp170) to tumor protein antigen Gp100 in a baculovirus system. This chaperone-based vaccine efficaciously induced anti-tumor immunity by enhancing DC cross-presentation and IFN-γ release by CD8+ T cells [271]. Recombinant small HSP nanocages fused to tumor peptides were successfully employed to inhibit tumor growth in tumor-bearing mice via activation of a Th1 oriented response, with high levels of secreted IFN-γ and IL-12 proinflammatory cytokines. [223].
One of the key characteristics of protein nanocages is their internal cavity, which governs the number and the size of the molecules that can be incorporated. Protein vaults are natural, self-assembling, multi-ribonucleoproteic structures with an octagonal barrel shape and a hollow cavity that makes them attractive as delivery system for a huge number of molecules [272]. With as-yet unidentified function, they are found in eukaryotic cells. Recombinant vaults can be produced (often using baculovirus-insect cells or yeast expression systems) and effectively exploited to package proteins and antigen for delivery. Several proteins have been genetically fused to the interaction domain of the major vault protein, enabling their spontaneous encapsulation into recombinant vaults [272]. Recombinant vaults have many desirable nanocarrier proprieties, such as nanometric size, safety, biodegradability, a large inner space (with volume of approximately ~5 × 104 nm3) for molecular packaging, and tunable release of the cargo. This latter property can be enhanced through amino acid substitution of the vault interaction domain by histidine. The modification leads to a longer retention of molecular cargoes within the vault hollow cavity, resulting in a prolonged cargo release [273]. The vault structure demonstrated innate immunostimulatory properties, with the ability to induce cellular instead of humoral responses [274] and to induce DC maturation [275]. Packaging of CCL21 chemokine into vaults had been used for chemokine-based immunotherapy in a mouse model of glioblastoma. When injected intratumorally, the CCL21-containing vaults induced recruitment of T cells and dendritic cells, as well as tumor regression [224].
VLPs and protein nano-cages can be internalized by cells of the immune system, such as DCs, and be cross-presented. This enables the delivered antigen to enter the class I pathway for priming CD8+ T lymphocytes. In addition, these nanoparticles can present antigens at high density and repetition, enabling B cell receptor signaling and B cell activation [276].
Inclusion of an appropriate adjuvant or a maturation stimulus in the internal space of these nanoparticles could lead to a successful anti-cancer response. Toll-like receptor (TLR) ligands have high safety profiles and possess the capability to activate antigen presenting cells [277]. The TLR7/8, TLR3 and TLR9 ligands (ssRNA, synthetic dsRNA sequence poly I:C, or unmethylated CpG ODN) have been incorporated into VLPs in association with target antigens; promising results were achieved in preclinical studies [215,278]. The recognition of TLR ligands by pattern recognition receptors (PRRs) initiates signal cascade pathways that lead to upregulation of CD80 and CD86 on the surface of the antigen presenting cells, suggesting activation. Further stimulation of the secretion of proinflammatory cytokines such as IL-6, IFN-γ and TNF-α strongly potentiates the immune responses [234]. The incorporation of TLR ligands into VLP-based vaccines results in early TLR-mediated transcriptional signature activation in DCs, inducing Th1-polarized CD4+ and CD8+ T cell responses [279,280] (Figure 15).
Figure 15.
Schematic of virus-like particle (VLP)-based vaccine’s cellular immune response against cancer. VLPs can cross blood vessels and be captured by antigen presenting cells such as dendritic cells (DCs) via micropinocytosis. Alternatively, VLPs can target DCs and be taken up via receptor-mediated endocytosis. Following uptake, VLPs are degraded and release the antigen and adjuvant. The adjuvant can be recognized by pattern recognition receptors such as toll-like receptors (TLRs). TLRs stimulate the maturation of DCs, upregulate co-stimulatory molecules (CD40, CD80/86) and secrete cytokines. The delivered cancer antigens are processed and presented as major histocompatibility complexes (MHCs) to T cells. The presented antigens enable activation of CD4+ T helper and CD8+ T lymphocytes. Upon activation and recognition of tumor cells, cytotoxic T lymphocytes induce cancer cell death via different pathways, including perforin/granzyme B, Fas-Fas ligand interaction, or the release of pro-inflammatory cytokines.
VLPs and nanocages provide the opportunity to engineer vaccine formulations in both the inner and outer portions of a scaffold. For example, VLPs prepared from rabbit hemorrhagic disease virus (RHDV) delivering tumor antigen gp100(25–33) were functionalized to express an epitope-processing linker. The functionalized VLPs were subsequently mannosylated to enhance uptake by immune cells expressing mannose receptors. The mannosylated VLPs demonstrated anti-tumor response in a murine model and enhanced animal survival.[219] Engineering the surface of VLPs with antibodies (e.g., anti-CD11c, anti-CD40, anti-Fc receptors or anti-C-type lectin receptors) can enable specific cell subpopulations to be targeted for cross-presentation to DCs. Such a process further enhanced vaccine uptake and antigen presentation to T cells [281].
6.2. Self-assembling Peptides
Self-assembling peptides form nanometer-scale supramolecular structures that are suitable for vaccine design. Peptide self-assembly is a naturally occurring process in which peptides spontaneously form ordered aggregates. This process is affected by ionic strength, pH, temperature, and interaction with cargos. Peptides can self-assemble to form nanofibers, nanotubes, nanoribbons and nanovesicles that can be loaded with drugs. Alternatively, these nanostructures may be chemically or covalently bonded to short antigenic peptides derived from TAAs, in conjunction with adjuvant and cell-targeting ligands [282]. Small self-assembling peptides are biocompatible, safe, and offer enhanced stability compared to free peptides. The possibility of guiding the self-assembly process by acting on the biochemical environment to promote formation of structures of various size and morphology represents a further advantage of these nanocarriers [283,284]. The self-assembling property of peptides may be acquired by engineering a peptide sequence to contain β-sheet or α-helix conformations; examples include non-immunogenic fibrillating domain Q11 [(Ac-QQKFQFQFEQQ-Am) [74,225] KFE8 (FKFEFKFE) [226] or Coil29 (QARILEADAEILR-AYARILEAHAEILRAQ)]. [227] These short peptides can be appended with T cell or B cell epitopes to form self-assembled peptide nanofibers which induce humoral, CD4+ and CD8+ T cell response in vivo [225,227,285].
Besides the strategy of increasing peptide antigenicity via conjugation to a particulate nanocarrier, self-assembling structures can also be obtained wherein the building blocks themselves are formed by antigenic peptides. Peptide nanoclusters are subunit peptide vaccine formulations formed by cross-linked peptides. Antigenic peptides may be used to form the nanoclusters, with the advantage of maximizing antigen delivery. This is because the same antigenic determinants are C-terminus modified and cross-linked for the structure assembly. Peptides derived from oncofetal antigen forming nanoclusters of ~ 150 nm in diameter were experimentally used as a peptide subunit vaccine. Peptides were rapidly internalized by DCs and enhanced antigen presentation resulted with improved immunogenicity. The peptide nanocluster showed high in vivo retention at the injection site, suggesting lower clearance, augmented antigen persistence, and increased interactions with resident DCs with subsequent prolonged immune activation [228].
Self-assembled nanogel vaccines offer the advantage of an antigen delivery system with a modulable size that is conjugable to any type of protein. Nanogels may serve as superb platforms for drug delivery thanks to their excellent biocompatibility and stability [286]. Poly(hydroxyethyl methacrylate) (pHEMA) was functionalized with a hydrophobic side chain of pyridine and further complexed with ovalbumin (OVA) to form a nanogel which was rapidly taken up by DCs and induced DC maturation. The activation of PRRs was attributed to the hydrophobic characteristic of the nanogel, enabling activation of OVA-specific T cells [287].
Peptide hydrogels may be used for the encapsulation of antigens together with adjuvants, drugs, antibodies or cells. For example, autologous tumor cells and a programmed death-ligand 1 (PDL1) blocking agent were incorporated into an Fmoc-KCRGDK (FK) peptide hydrogel, together with ICG thus producing a personalized cancer vaccine (PVAX). Activation of the PVAX by near-infrared irradiation enabled release of both antigen and immune checkpoint blockade. This promoted DC maturation and enabled tumor infiltration by cytotoxic T lymphocytes to prevent tumor relapse (Figure 16) [230]. Memory immune responses could be elicited toward a patient’s tumor cells, which can, in turn, prevent metastasis and tumor recurrence.
Figure 16.
Schematic of the fabrication of a personalized cancer vaccine (PVAX) for cancer immunotherapy. (A) Autologous tumor cells, a PD-L1 molecular inhibitor (JQ1) and indocyanine green (ICG) were incorporated into a peptide hydrogel. (B) Simplified mechanism of PVAX-mediated cancer immunotherapy to prevent post-operative tumor recurrence and metastasis. Upon near-infrared laser irradiation, the ICG causes temperature elevation and releases the tumor-specific antigens and JQ1. (C) Therapeutic schedule for PVAX-mediated inhibition of post-surgical tumor metastasis, simulated by injection of circulating tumor cells (CTC). (D) Representative photographs of lung tissue. White arrows indicate pulmonary metastasis. Reprinted with permission from [230].
The RADA16 amphiphilic peptide, consisting of periodically alternating hydrophilic and hydrophobic amino acids, was employed for the self-assembly of a nanofibrous hydrogels. RADA16 peptide scaffold was used for simultaneous delivery of exogenous DCs, OVA antigen and anti-PD-L1 antibody, combining DC vaccination with immune checkpoint therapy (Figure 17) [229]. Upon injection, the peptide hydrogel formed a nodule that promoted DC maturation and CD8+ effector T cells infiltration in the tumor bed. The combination with anti-PD-L1 enhanced the T cell response via inhibition of the PD-1/PD-L1 axis. This resulted in significant enhancement of vaccination effects in vivo, with suppression of tumor growth and survival improvement of the experimental animals [229].
Figure 17.
Self-assembly of RADA 16 peptide and its anti-cancer effect. (A) Assembly and mechanism of action of a DC-based vaccine module engineered in a peptide nanofibrous hydrogel. Tumor antigen was incorporated into a self-assembled peptide nanofibrous hydrogel, together with DCs and anti-PD-1 antibody. The vaccine module was subcutaneously injected into mice. The released antigen recruited and activated host DCs, while immune checkpoint blockade by anti-PD-1 antibody contributed to tumor immunosuppression. (B) Representative tumors observed at day 14 after different treatments. (C) Schematic of the protocol employed for tumor cell inoculation and vaccine injection. (D) Tumor volume changes within 28 days after different treatments. Digital images (E) and mass weight (F) of excised tumors determined with different treatments at day 28. Reprinted with permission from [229]. Copyright © 2018, American Chemical Society
Vaccination strategies focused on self-assembling peptides shed a new light on the use of peptide-based vaccination, usually defined poorly immunogenic. The development of vaccines based on short antigenic peptides is improved by the delivery of antigenic epitopes in high copy number on a particulate structure that needs to be processed by APCs, thus enhancing peptide immunogenicity and strengthening the immune responses [288]. Peptide assembly in supramolecular structures can deliver antigens in a controllable manner. Development of multivalent self-assembling structures grounded on newly identified antigens are likely to improve cancer immunotherapy [289,290].
Conclusions and outlook
Self-assembling peptides and proteins have immense potential to be leveraged in drug delivery. Some of these nanomaterials are capable of combining several functions into a single nano-formulation [103,291,292] to facilitate precision targeted drug delivery[293]. Efficient tumor therapy requires potent interaction of drugs/bioactive molecules at the cellular level, so that the released drug may disseminate to all areas within a tumor. The elimination of only the outer cells of a tumor will leave the overall tumor machinery intact. Partial peripheral treatment results in tumors that are resistant to the medication [288,294,295].
Self-assembled peptides and proteins with tunable size are promising vehicles for encapsulation of therapeutic agents to preserve them against degradation in delivery transit. The surface of the nanoparticles can be adorned with functional proteins and other bioactive molecules for extracellular delivery with higher targeting efficacy [296]. Some self-assembled peptides and proteins have already been clinically applied in different targeted anti-cancer drug delivery systems. Examples include cyclic arginylglycylaspartic acid (RGD) peptides [120,121,297,298], mixtures of two peptides, EAK16-II and EAK16-IV [128,129] and M-ΔF peptide [299].
Immunity against carriers, which limits the effectiveness of vaccination, is a limiting factor in the development of VLP-based vaccines. This limitation may be circumvented by choosing viral vectors of non-human origin such as bacterial- or plant-derived viruses. The other limiting factor is the high manufacturing cost of VLP. Eukaryotic cells such as yeast, mammalian or insect cells often must be used for VLP manufacturing because of the need for post-translational modifications, a requirement that cheaper bacterial systems cannot fulfill.
Self-assembling peptide-based materials typically offer the advantage of biocompatibility and biodegradability. However, their in vivo application can be limited by the complex chemical modifications usually required which may adversely alter biocompatibility by inducing inflammation or other side effects. Peptide assembly can develop unwanted immune responses as a result of the change in size, valence and deposition of the nanomaterial. Undesirable immunogenicity may be attenuated by deleting residues recognized by T cells or by altering key amino acids in self-assembling domains [226].
A second issue pertains to stability of self-assembled platforms. Potential structural disassembly in physiological environments can reduce the efficacy of drug delivery. For self-assembling nanomaterials to be clinically acceptable, it is necessary to further improve their safety and efficacy profiles. Moreover, efforts should be devoted to the development of controllable, reproducible and scalable production of self-assembling platforms. The efficacy of self-assembled vaccines is highly dependent on the selection of tumor antigens. Selection of highly immunogenic scaffolds, conjugation or co-administration of antigens with adjuvants or other immunostimulatory molecules is likely to lead to more efficacious therapeutics. The establishment of the factors that maximize immunogenicity, such as optimal antigen number, spacing and orientation, together with understanding the factors that facilitate the extravasation, targeting and penetration of vaccines into the tumor microenvironment, will contribute to success in clinical translation. It is thus highly likely that self-assembling materials will be used as prophylactic and therapeutic regimes against cancer in the near future [300].
Highlights.
Self-assembled peptide and protein nanostructures deliver therapeutics to cancer cells
Stimuli-responsive nanoarchitectures of peptide/protein respond to tumor microenvironment
Self-assembled peptide and protein nanomaterials are employed for cancer therapy
Virus-like nanoparticles are used for immunotherapeutic approaches
Acknowledgments
M.D. was supported by PON CCI 2014IT16M2OP005 from the Italian Ministry of University and Research. B.R.S. is supported by the American Associate for Cancer Research and Breast Cancer Research Foundation (7-20-26-SMIT), American Heart Association (18TPA34230113), and National Cancer Institute of the US NIH (R01CA244491).
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Di Natale C, Celetti G, Scognamiglio PL, Cosenza C, Battista E, Causa F, Netti PA, Chem. Commun. 2018, 54, 10088. [DOI] [PubMed] [Google Scholar]
- [2].Di Natale C, La Manna S, Avitabile C, Florio D, Morelli G, Netti PA, Marasco D, Bioorg. Chem. 2020, 96, 103594. [DOI] [PubMed] [Google Scholar]
- [3].La Manna S, Scognamiglio PL, Di Natale C, Leone M, Mercurio FA, Malfitano AM, Cianfarani F, Madonna S, Caravella S, Albanesi C, Biochimie 2017, 138, 106. [DOI] [PubMed] [Google Scholar]
- [4].Zhu X, Vo C, Taylora M, Smith BR, Mater. Horizons 2019, 6, 1094. [Google Scholar]
- [5].Magna G, Monti D, Di Natale C, Paolesse R, Stefanelli M, Molecules 2019, 24, 4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Steiert E, Ewald J, Wagner A, Hellmich UA, Frey H, Wich PR, Polym. Chem. 2020, 11, 551. [Google Scholar]
- [7].Ashrafizadeh M, Zarrabi A, Orouei S, Zarrin V, Rahmani Moghadam E, Zabolian A, Mohammadi S, Hushmandi K, Gharehaghajlou Y, Makvandi P, Najafi M, Mohammadinejad R, Biology (Basel). 2020, 9, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL, CA. Cancer J. Clin. 2019, 69, 438. [DOI] [PubMed] [Google Scholar]
- [9].Mohammadinejad R, Dehshahri A, Sagar Madamsetty V, Zahmatkeshan M, Tavakol S, Makvandi P, Khorsandi D, Pardakhty A, Ashrafizadeh M, Ghasemipour Afshar E, Zarrabi A, J. Control. Release 2020, 325, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ashrafizadeh M, Zarrabi A, Orouei S, Hushmandi K, Hakimi A, Zabolian A, Daneshi S, Samarghandian S, Baradaran B, Najafi M, Eur. J. Pharmacol. 2020, 173660. [DOI] [PubMed] [Google Scholar]
- [11].Siegel RL, Miller KD, Jemal A, CA. Cancer J. Clin. 2017, 67, 7. [DOI] [PubMed] [Google Scholar]
- [12].Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A, CA. Cancer J. Clin. 2020. [DOI] [PubMed] [Google Scholar]
- [13].Ashrafizadeh M, Najafi M, Makvandi P, Zarrabi A, Farkhondeh T, Samarghandian S, J. Cell. Physiol. 2020, n/a. [DOI] [PubMed] [Google Scholar]
- [14].Ashrafizadeh M, Zarrabi A, Hushmandi K, Hashemi F, Hashemi F, Samarghandian S, Najafi M, Life Sci. 2020, 117973. [DOI] [PubMed] [Google Scholar]
- [15].Ashrafizadeh M, Najafi M, Ang HL, Moghadam ER, Mahabady MK, Zabolian A, Jafaripour L, Bejandi AK, Hushmandi K, Saleki H, Biomedicines 2020, 8, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pucci C, Martinelli C, Ciofani G, Ecancermedicalscience 2019, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ashrafizadeh M, Zarrabi A, Hushmandi K, Hashemi F, Rahmani Moghadam E, Raei M, Kalantari M, Tavakol S, Mohammadinejad R, Najafi M, ACS Comb. Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dorsam RT, Gutkind JS, Nat. Rev. cancer 2007, 7, 79. [DOI] [PubMed] [Google Scholar]
- [19].Meng L, Yang L, Zhao X, Zhang L, Zhu H, Liu C, Tan W, PLoS One 2012, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang XX, Eden HS, Chen X, Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates. J. Control. Release 2012, 159, 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.[] Barbari C, Fontaine T, Parajuli P, Lamichhane N, Jakubski S, Lamichhane P, Deshmukh RR, Immunotherapies and combination strategies for immuno-oncology. Int. J. Mol. Sci. 2020, 21, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou Q, Zhang L, Wu H, Nanomaterials for cancer therapies. Nanotechnol. Rev. 2017, 6, 473–496. [Google Scholar]
- [23].Huang Z, Song W, Chen X, Supramolecular Self-Assembled Nanostructures for Cancer Immunotherapy. Front. Chem. 2020, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iscaro A, Howard NF, Muthana M, Curr. Pharm. Des. 2019, 25, 1962. [DOI] [PubMed] [Google Scholar]
- [25].V Gorshkov A, Rozdina IG, Pridatchenko ML, V Evreinov V, Polym. Sci. Ser. A 2020, 62, 437. [Google Scholar]
- [26].Degrado WF, In Advances in protein chemistry; Elsevier, 1988; Vol. 39, pp. 51–124. [DOI] [PubMed] [Google Scholar]
- [27].SCHERMANN J-P, In Spectroscopy and Modeling of Biomolecular Building Blocks; Elsevier, 2008; Vol. 4, pp. 251–296. [Google Scholar]
- [28].de Araujo CB, Heimann AS, Remer RA, Russo LC, Colquhoun A, Forti FL, Ferro ES, Biomolecules 2019, 9, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Groß A, Hashimoto C, Sticht H, Eichler J, Front. Bioeng. Biotechnol. 2016, 3, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ucar B, Acar T, Pelit Arayici P, Sen M, Derman S, Mustafaeva Z, In Peptide Synthesis; IntechOpen, 2019. [Google Scholar]
- [31].Hirano Y, Mooney DJ, Adv. Mater. 2004, 16, 17. [Google Scholar]
- [32].Greene LH, Brief. Funct. Genomics 2012, 11, 469. [DOI] [PubMed] [Google Scholar]
- [33].Tlusty T, The self-referring DNA and protein: A remark on physical and geometrical aspects. arXiv 2018, 374, 20150070. [DOI] [PubMed] [Google Scholar]
- [34].Banach M, Konieczny L, Roterman I, In Protein Supersecondary Structures; Springer, 2019; pp. 347–378. [DOI] [PubMed] [Google Scholar]
- [35].Schneider M, Belsom A, Rappsilber J, Trends Biochem. Sci. 2018, 43, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tung CH, Chen CW, Guo RC, Ng HF, Chu YW, Biomed Res. Int. 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rehman HU, Zafar U, Benso A, Islam N, In BIOINFORMATICS; 2016; pp. 237–244.27306641 [Google Scholar]
- [38].Benso A, Di Carlo S, ur Rehman H, Politano G, Savino A, Suravajhala P, Proteome Sci. 2013, 11, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mitrofanova A, Pavlovic V, Mishra B, IEEE/ACM Trans. Comput. Biol. Bioinforma 2010, 8, 775. [DOI] [PubMed] [Google Scholar]
- [40].Rehman HU, Benso A, Di Carlo S, Politane G, Savino A, Suravajhala P, In 2012 IEEE Internationa: l Conference on Bioinformatics and Biomedicine; IEEE, 2012; pp. 1–4. [Google Scholar]
- [41].Zhang H, Chen Z, Liu Z, Zhu Y, Wu C, PLoS One 2016, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rehman HU, Azam N, Yao J, Benso A, PLoS One 2017, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hicks M, Bartha I, di Iulio J, Abagyan R, Craig Venter J, Telenti A, Functional characterization of 3D-protein structures informed by human genetic diversity. bioRxiv 2017, 116, 8960–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Klasberg S, Bitard-Feildel T, Mallet L, Bioinform. Biol. Insights 2016, 10, BBI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Innan H, Kondrashov F, Nat. Rev. Genet. 2010, 11, 97. [DOI] [PubMed] [Google Scholar]
- [46].Reams AB, Roth JR, Cold Spring Harb. Perspect. Biol. 2015, 7, a016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rogers J, Gibbs RA, Nat. Rev. Genet. 2014, 15, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Toll-Riera M, Castelo R, Bellora N, Albà MM, In Biochemical Society Transactions; Portland Press Ltd., 2009; Vol. 37, pp. 778–782. [DOI] [PubMed] [Google Scholar]
- [49].Laskowski RA, Thornton JM, Sternberg MJE, Biochem. Soc. Trans. 2009, 37, 723. [DOI] [PubMed] [Google Scholar]
- [50].Agozzino L, Dill KA, Proc. Natl. Acad. Sci. 2018, 115, 9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Braun GA, Ary BE, Dear AJ, Rohn MCH, Payson AM, Lee DSM, Parry RC, Friedman C, Knowles TPJ, Linse S, Åkerfeldt KS, Biomacromolecules 2020. [DOI] [PubMed] [Google Scholar]
- [52].Pandit G, Roy K, Agarwal U, Chatterjee S, ACS Omega 2018, 3, 3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yuan C, Li S, Zou Q, Ren Y, Yan X, Multiscale simulations for understanding the evolution and mechanism of hierarchical peptide self-assembly. Phys. Chem. Chem. Phys. 2017, 19, 23614–23631. [DOI] [PubMed] [Google Scholar]
- [54].Okesola BO, Mata A, Chem. Soc. Rev. 2018, 47, 3721. [DOI] [PubMed] [Google Scholar]
- [55].Mandal D, Shirazi AN, Parang K, Org. Biomol. Chem. 2014, 12, 3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang L, Liu A, Cao S, Putri RM, Jonkheijm P, Cornelissen J, Chem.-Eur. J 2016, 22, 15570. [DOI] [PubMed] [Google Scholar]
- [57].James S, Quinn MK, McManus JJ, Phys. Chem. Chem. Phys. 2015, 17, 5413. [DOI] [PubMed] [Google Scholar]
- [58].Liao HS, Lin J, Liu Y, Huang P, Jin A, Chen X, Nanoscale 2016, 8, 14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yang B, Adams DJ, Marlow M, Zelzer M, Langmuir 2018, 34, 15109. [DOI] [PubMed] [Google Scholar]
- [60].Wang J, Liu K, Xing R, Yan X, Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [DOI] [PubMed] [Google Scholar]
- [61].Mason TO, Chirgadze DY, Levin A, Adler-Abramovich L, Gazit E, Knowles TPJ, Buell AK, ACS Nano 2014, 8, 1243. [DOI] [PubMed] [Google Scholar]
- [62].Levin A, Mason TO, Adler-Abramovich L, Buell AK, Meisl G, Galvagnion C, Bram Y, Stratford SA, Dobson CM, Knowles TPJ, Nat. Commun. 2014, 5, 1. [DOI] [PubMed] [Google Scholar]
- [63].Wang J, Liu K, Yan L, Wang A, Bai S, Yan X, ACS Nano 2016, 10, 2138. [DOI] [PubMed] [Google Scholar]
- [64].Solomonov A, Shimanovich U, Self-Assembly in Protein-Based Bionanomaterials. Isr. J. Chem. 2019, 1152–1170. [Google Scholar]
- [65].Yang S, Yan Y, Huang J, V Petukhov A, Kroon-Batenburg LMJ, Drechsler M, Zhou C, Tu M, Granick S, Jiang L, Nat. Commun. 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Shanbhag BK, Liu C, Haritos VS, He L, ACS Nano 2018, 12, 6956. [DOI] [PubMed] [Google Scholar]
- [67].Sun L, Zheng C, Webster TJ, Int. J. Nanomedicine 2017, 12, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yadav S, Sharma AK, Kumar P, Front. Bioeng. Biotechnol. 2020, 8, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Habibi N, Kamaly N, Memic A, Shafiee H, Nano Today 2016, 11, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Smith AM, Banwell EF, Edwards WR, Pandya MJ, Woolfson DN, Adv. Funct. Mater. 2006, 16, 1022. [Google Scholar]
- [71].Wang H, Feng Z, Xu B, Adv. Drug Deliv. Rev. 2017, 110–111, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pins GD, Christiansen DL, Patel R, Silver FH, Biophys. J. 1997, 73, 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li J, Chen Z, Zhou M, Jing J, Li W, Wang Y, Wu L, Wang L, Wang Y, Lee M, Angew. Chemie - Int. Ed 2016, 55, 2592. [DOI] [PubMed] [Google Scholar]
- [74].Huang ZH, Shi L, Ma JW, Sun ZY, Cai H, Chen YX, Zhao YF, Li YM, J. Am. Chem. Soc. 2012, 134, 8730. [DOI] [PubMed] [Google Scholar]
- [75].Rong P, Huang P, Liu Z, Lin J, Jin A, Ma Y, Niu G, Yu L, Zeng W, Wang W, Chen X, Nanoscale 2015, 7, 16330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tang CY, Liao YH, Tan GS, Wang XM, Lu GH, Yang YH, RSC Adv. 2015, 5, 50572. [Google Scholar]
- [77].Cheetham AG, Zhang P, Lin Y, Lock LL, Cui H, J. Am. Chem. Soc. 2013, 135, 2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wong CK, Qiang X, Müller AHE, Gröschel AH, Prog. Polym. Sci. 2020, 101211. [Google Scholar]
- [79].Dong Y, Chen Y, Zhu D, Shi K, Ma C, Zhang W, Rocchi P, Jiang L, Liu X, J. Control. Release 2020. [DOI] [PubMed] [Google Scholar]
- [80].Hu X, Liao M, Gong H, Zhang L, Cox H, Waigh TA, Lu JR, Curr. Opin. Colloid Interface Sci. 2020, 45, 1. [Google Scholar]
- [81].Lombardo D, Kiselev MA, Magazù S, Calandra P, Adv. Condens. Matter Phys 2015, 2015. [Google Scholar]
- [82].King NP, Sheffler W, Sawaya MR, Vollmar BS, Sumida JP, André I, Gonen T, Yeates TO, Baker D, Science (80-. ). 2012, 336, 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Abbas M, Zou Q, Li S, Yan X, Adv. Mater. 2017, 29. [DOI] [PubMed] [Google Scholar]
- [84].Tyagi A, Tuknait A, Anand P, Gupta S, Sharma M, Mathur D, Joshi A, Singh S, Gautam A, Raghava GPS, Nucleic Acids Res. 2015, 43, D837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Craig EA, Gambill BD, Nelson RJ, Heat shock proteins: Molecular chaperones of protein biogenesis. Microbiol. Rev. 1993, 57, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pang L, Pei Y, Uzunalli G, Hyun H, Lyle LT, Yeo Y, Pharm. Res. 2019, 36, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li J, Wu H, Hong J, Xu X, Yang H, Wu B, Wang Y, Zhu J, Lai R, Jiang X, Lin D, Prescott MC, Rees HH, PLoS One 2008, 3, e2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nguyen DH, Lee JS, Choi JH, Park KM, Lee Y, Park KD, Acta Biomater. 2016, 35, 109. [DOI] [PubMed] [Google Scholar]
- [89].Bansal K, Aqdas M, Kumar M, Bala R, Singh S, Agrewala JN, Katare OP, Sharma RK, Wangoo N, Bioconjug. Chem. 2018, 29, 1102. [DOI] [PubMed] [Google Scholar]
- [90].Verma D, Gulati N, Kaul S, Mukherjee S, Nagaich U, J. Pharm. 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jao D, Xue Y, Medina J, Hu X , Protein-based drug-delivery materials. Materials (Basel). 2017, 10, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM, Am. J. Pathol. 2000, 156, 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Brettle A, Current status and future prospects. Health Info. Libr. J. 2008, 25, 32–34. [DOI] [PubMed] [Google Scholar]
- [94].Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW, Nano Lett. 2009, 9, 1909. [DOI] [PubMed] [Google Scholar]
- [95].Sykes EA, Chen J, Zheng G, Chan WCW, ACS Nano 2014, 8, 5696. [DOI] [PubMed] [Google Scholar]
- [96].She W, Luo K, Zhang C, Wang G, Geng Y, Li L, He B, Gu Z, Biomaterials 2013, 34, 1613. [DOI] [PubMed] [Google Scholar]
- [97].Liu X, Chen Y, Li H, Huang N, Jin Q, Ren K, Ji J, ACS Nano 2013, 7, 6244. [DOI] [PubMed] [Google Scholar]
- [98].Kapri S, Majee R, Bhattacharyya S, ACS Sustain. Chem. Eng. 2018, 6, 8503. [Google Scholar]
- [99].Gholami A, Mousavi SM, Hashemi SA, Ghasemi Y, Chiang WH, Parvin N, Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [DOI] [PubMed] [Google Scholar]
- [100].Mahato RI, Narang AS, Thoma L, Miller DD, Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev. Ther. Drug Carrier Syst. 2003, 20, 153–214. [DOI] [PubMed] [Google Scholar]
- [101].Schade M, Berti D, Huster D, Herrmann A, Arbuzova A, Lipophilic nucleic acids - A flexible construction kit for organization and functionalization of surfaces. Adv. Colloid Interface Sci. 2014, 208, 235–251. [DOI] [PubMed] [Google Scholar]
- [102].González-Toro DC, Ryu JH, Chacko RT, Zhuang J, Thayumanavan S, J. Am. Chem. Soc. 2012, 134, 6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chen G, Roy I, Yang C, Prasad PN, Chem. Rev. 2016, 116, 2826. [DOI] [PubMed] [Google Scholar]
- [104].Zhou J, Yu G, Huang F, Chem. Soc. Rev. 2017, 46, 7021. [DOI] [PubMed] [Google Scholar]
- [105].Fan W, Yung B, Huang P, Chen X, Chem. Rev. 2017, 117, 13566. [DOI] [PubMed] [Google Scholar]
- [106].Ulbrich K, Holá K, Šubr V, Bakandritsos A, Tuček J, Zbořil R, Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [DOI] [PubMed] [Google Scholar]
- [107].Webber MJ, Appel EA, Meijer EW, Langer R, Nat. Mater. 2016, 15, 13. [DOI] [PubMed] [Google Scholar]
- [108].Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C, J. Funct. Biomater. 2019, 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Xu X, Li Y, Li H, Liu R, Sheng M, He B, Gu Z, Small 2014, 10, 1133. [DOI] [PubMed] [Google Scholar]
- [110].Santana H, Avila CL, Cabrera I, Páez R, Falcón V, Pessoa A, Ventosa N, Veciana J, Itri R, Barbosa LRS, Soft Matter 2014, 10, 9260. [DOI] [PubMed] [Google Scholar]
- [111].Gajendran B, Varier KM, Liu W, Yao Y, Ben-David Y, Li Y, Chinnasamy A, Biol. Synth. Nanoparticles Their Appl. 2020, 159. [Google Scholar]
- [112].Boekhoven J, Zha RH, Tantakitti F, Zhuang E, Zandi R, Newcomb CJ, Stupp SI, RSC Adv. 2015, 5, 8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhou W, Qiao Z, Nazarzadeh Zare E, Huang J, Zheng X, Sun X, Shao M, Wang H, Wang X, Chen D, Zheng J, Fang S, Li YM, Zhang X, Yang L, Makvandi P, Wu A, J. Med. Chem. 2020, 63, 8003. [DOI] [PubMed] [Google Scholar]
- [114].Moeini A, Pedram P, Makvandi P, Malinconico M, D’Ayala GG, Carbohydr. Polym. 2020, 223, 115839. [DOI] [PubMed] [Google Scholar]
- [115].Zare EN, Makvandi P, Tay FR, Carbohydr. Polym. 2019, 212, 450. [DOI] [PubMed] [Google Scholar]
- [116].Wang P, Kim T, Harada M, Contag C, Huang X, Smith BR, Nanoscale Horizons 2020. [DOI] [PubMed] [Google Scholar]
- [117].Kanthi Y, de la Zerda A, Smith BR, ACS Nano 2020, 14, 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tarvirdipour S, Schoenenberger C-A, Benenson Y, Palivan CG, Soft Matter 2020, 16, 1678. [DOI] [PubMed] [Google Scholar]
- [119].Stephenson R, Varamini P, Butcher N, Minchin R, Toth I, Nanomedicine Nanotechnology, Biol. Med 2014, 10, 1799. [DOI] [PubMed] [Google Scholar]
- [120].Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS, Nano Lett. 2008, 8, 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Smith BR, Cheng Z, De A, Rosenberg J, Gambhir SS, Small 2010, 6, 2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ishida A, Oshikawa M, Oshikawa M, Ajioka I, Ajioka I, Muraoka T, Muraoka T, ACS Appl. Bio Mater. 2020, 3, 3605. [DOI] [PubMed] [Google Scholar]
- [123].Javali NM, Raj A, Saraf P, Li X, Jasti B, Pharm. Res. 2012, 29, 3347. [DOI] [PubMed] [Google Scholar]
- [124].Smith BR, Zavaleta C, Rosenberg J, Tong R, Ramunas J, Liu Z, Dai H, Gambhir SS, Nano Today 2013, 8, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Spoerke ED, Anthony SG, Stupp SI, Adv. Mater. 2009, 21, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Duan X, He C, Kron SJ, Lin W, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2016, 8, 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Thota CK, Yadav N, Chauhan VS, Sci. Rep. 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Fung SY, Yang H, Chen P, Colloids Surfaces B Biointerfaces 2007, 55, 200. [DOI] [PubMed] [Google Scholar]
- [129].Calvanese L, Brun P, Messina GML, Russo T, Zamuner A, Falcigno L, D’Auria G, Gloria A, Vitagliano L, Marletta G, Dettin M, ACS Biomater. Sci. Eng. 2020, 6, 1154. [DOI] [PubMed] [Google Scholar]
- [130].Stiborová M, Poljaková J, Martínková E, Bořek-Dohalská L, Eckschlager T, Kizek R, Frei E, Interdiscip. Toxicol. 2011, 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wu F, Fu D, J. Comput. Theor. Nanosci. 2016, 13, 2334. [Google Scholar]
- [132].He W, Wang S, Yan J, Qu Y, Jin L, Sui F, Li Y, You W, Yang G, Yang Q, Ji M, Shao Y, Ma PX, Lu W, Hou P, Adv. Funct. Mater. 2019, 29, 1807736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].An HW, Li LL, Wang Y, Wang Z, Hou D, Lin YX, Qiao SL, Di Wang M, Yang C, Cong Y, Ma Y, Zhao XX, Cai Q, Chen WT, Lu CQ, Xu W, Wang H, Zhao Y, Nat. Commun. 2019, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP, Proc. Natl. Acad. Sci. 2007, 104, 7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Altunbas A, Lee SJ, Rajasekaran SA, Schneider JP, Pochan DJ, Biomaterials 2011, 32, 5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Li B, Feng Z, He L, Li W, Wang Q, Liu J, Huang J, Zheng Y, Ma Y, Yang X, ChemMedChem 2018, 13, 2037. [DOI] [PubMed] [Google Scholar]
- [137].Sardan M, Kilinc M, Genc R, Tekinay AB, Guler MO, Faraday Discuss. 2013, 166, 269. [DOI] [PubMed] [Google Scholar]
- [138].Lv S, Li M, Tang Z, Song W, Sun H, Liu H, Chen X, Acta Biomater. 2013, 9, 9330. [DOI] [PubMed] [Google Scholar]
- [139].Jabbari E, Yang X, Moeinzadeh S, He X, Eur. J. Pharm. Biopharm. 2013, 84, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Liu J, Int. J. Nanomedicine 2011, 6, 2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Soukasene S, Toft DJ, Moyer TJ, Lu H, Lee H-K, Standley SM, Cryns VL, Stupp SI, ACS Nano 2011, 5, 9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wang Z, Lee TY, Ho PC, Nanomedicine Nanotechnology, Biol. Med 2012, 8, 194. [DOI] [PubMed] [Google Scholar]
- [143].Wu M, Ye Z, Liu Y, Liu B, Zhao X, Mol. Biosyst. 2011, 7, 2040. [DOI] [PubMed] [Google Scholar]
- [144].Kim J-K, Anderson J, Jun H-W, Repka MA, Jo S, Mol. Pharm. 2009, 6, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Richard PU, Craciun I, Gaitzsch J, Weiner L, Palivan CG, Helv. Chim. Acta 2018, 101, e1800064. [Google Scholar]
- [146].Chang C, Liang P, Chen L, Liu J, Chen S, Zheng G, Quan C, J. Biomater. Sci. Polym. Ed. 2017, 28, 1338. [DOI] [PubMed] [Google Scholar]
- [147].Nowak BP, Ravoo BJ, Faraday Discuss. 2019, 219, 220. [DOI] [PubMed] [Google Scholar]
- [148].Guo WW, Zhang ZT, Wei Q, Zhou Y, Lin MT, Chen JJ, Wang TT, Guo NN, Zhong XC, Lu YY, Yang QY, Han M, Gao J, Biomacromolecules 2020, 21, 444. [DOI] [PubMed] [Google Scholar]
- [149].Vasan N, Baselga J, Hyman DM, Nature 2019, 575, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Rahimi H, Salehiabar M, Charmi J, Barsbay M, Ghaffarlou M, Razlighi MR, Davaran S, Khalilov R, Sugiyama M, Nosrati H, Nano Today 2020, 34, 100895. [Google Scholar]
- [151].Chen X, Song E, Nat. Rev. Drug Discov. 2019, 18, 99. [DOI] [PubMed] [Google Scholar]
- [152].Najafi M, Goradel NH, Farhood B, Salehi E, Solhjoo S, Toolee H, Kharazinejad E, Mortezaee K, J. Cell. Physiol. 2019, 234, 5700. [DOI] [PubMed] [Google Scholar]
- [153].Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, Huang D, Zhao J, Yang L, Liao D, Su F, Li M, Liu Q, Song E, Cell 2018, 172, 841. [DOI] [PubMed] [Google Scholar]
- [154].Liu T, Zhou L, Li D, Andl T, Zhang Y, Front. cell Dev. Biol. 2019, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, V Novitskiy S, DeJesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R, Cancer Cell 2014, 25, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mortezaee K, Life Sci. 2020, 117534. [Google Scholar]
- [157].Lang J, Zhao X, Qi Y, Zhang Y, Han X, Ding Y, Guan J, Ji T, Zhao Y, Nie G, ACS Nano 2019, 13, 12357. [DOI] [PubMed] [Google Scholar]
- [158].Tai W, Molecules 2019, 24, 2211. [Google Scholar]
- [159].Diao L, Tao J, Wang Y, Hu Y, He W, Int. J. Nanomedicine 2019, 14, 8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].jun Wan W, xi Qu C, juan Zhou Y, Zhang L, tian Chen M, Liu Y, gang You B, Li F, dan Wang D, nong Zhang X, Int. J. Pharm. 2019, 566, 731. [DOI] [PubMed] [Google Scholar]
- [161].Raja MAG, Katas H, Amjad MW, Design, mechanism, delivery and therapeutics of canonical and Dicer-substrate siRNA. Asian J. Pharm. Sci. 2019, 14, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kim B, Park J, Sailor MJ, Adv. Mater. 2019, 31, 1903637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yao Y, Wang T, Liu Y, Zhang N, Artif. cells, nanomedicine, Biotechnol. 2019, 47, 1374. [DOI] [PubMed] [Google Scholar]
- [164].Ye C, Pan B, Xu H, Zhao Z, Shen J, Lu J, Yu R, Liu H, J. Mol. Med. 2019, 97, 1575. [DOI] [PubMed] [Google Scholar]
- [165].Bozdoğan B, Akbal Ö, Çelik E, Türk M, Denkbaş EB, RSC Adv. 2017, 7, 47592. [Google Scholar]
- [166].Guan X, Chang Y, Sun J, Song J, Xie Y, Macromol. Biosci. 2018, 18, 1800013. [DOI] [PubMed] [Google Scholar]
- [167].Gupta MN, Mukherjee J, In Nanomedicine for Drug Delivery and Therapeutics; Nature Publishing Group, 2013; Vol. 12, pp. 411–436. [Google Scholar]
- [168].Yerushalmi R, Scherz A, Van Der Boom ME, Kraatz H-B, J. Mater. Chem. 2005, 15, 4480. [Google Scholar]
- [169].Zhang M, Xu C, Wen L, Han MK, Xiao B, Zhou J, Zhang Y, Zhang Z, Viennois E, Merlin D, Cancer Res. 2016, 76, 7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lin CJ, Kuan CH, Wang LW, Wu HC, Chen Y, Chang CW, Huang RY, Wang TW, Biomaterials 2016, 90, 12. [DOI] [PubMed] [Google Scholar]
- [171].Lim HJ, Cho EC, Lee JA, Kim J, Colloids Surfaces A Physicochem. Eng. Asp. 2012, 402, 80. [Google Scholar]
- [172].Lee CS, Na K, Biomacromolecules 2014, 15, 4228. [DOI] [PubMed] [Google Scholar]
- [173].Sun H, Li S, Qi W, Xing R, Zou Q, Yan X, Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 795. [Google Scholar]
- [174].Liu K, Xing R, Zou Q, Ma G, Möhwald H, Yan X, Angew. Chemie - Int. Ed 2016, 55, 3036. [DOI] [PubMed] [Google Scholar]
- [175].Sigg SJ, Postupalenko V, Duskey JT, Palivan CG, Meier W, Biomacromolecules 2016, 17, 935. [DOI] [PubMed] [Google Scholar]
- [176].Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A, Cell 2017, 168, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Ashrafizadeh M, Zarrabi A, Hushmandi K, Zarrin V, Moghadam ER, Zabolian A, Tavakol S, Samarghandian S, Najafi M, Life Sci. 2020, 117899. [DOI] [PubMed] [Google Scholar]
- [178].Muller M, Schouten RD, De Gooijer CJ, Baas P, Expert Rev. Anticancer Ther. 2017, 17, 399. [DOI] [PubMed] [Google Scholar]
- [179].Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TNM, Lo RS, Ribas A, N. Engl. J. Med 2016, 375, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS, Nat. Commun. 2016, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, Isaacs R, Mohan S, Plesa G, Lacey SF, Navenot JM, Zheng Z, Levine BL, Okada H, June CH, Brogdon JL, Maus MV, Sci. Transl. Med. 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW, Sharma SK, Chester KA, Westwood NB, Halford SER, Nabarro S, Wan S, Austin E, Hawkins RE, Cancer Immunol. Immunother. 2017, 66, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Lamers CHJ, Sleijfer S, Van Steenbergen S, Van Elzakker P, Van Krimpen B, Groot C, Vulto A, Den Bakker M, Oosterwijk E, Debets R, Mol. Ther. 2013, 21, 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Beatty GL, Haas AR, V Maus M, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Cancer Immunol. Res. 2014, 2, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Han D, Xu Z, Zhuang Y, Ye Z, Qian Q, Current progress in CAR-T cell therapy for hematological malignancies. J. Cancer 2021, 12, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Patel A, Kaufman HL, Disis ML, Chinese Clin. Oncol. 2017, 6, 19. [DOI] [PubMed] [Google Scholar]
- [187].Hollingsworth RE, Jansen K, NPJ vaccines 2019, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Maeng HM, Berzofsky JA, F1000Research 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Nurieva R, Wang J, Sahoo A, Immunotherapy 2013, 5, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Facciabene A, Motz GT, Coukos G, Cancer Res. 2012, 72, 2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Sau S, Alsaab HO, Bhise K, Alzhrani R, Nabil G, Iyer AK, J. Control. release 2018, 274, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Kelly HG, Kent SJ, Wheatley AK, Expert Rev. Vaccines 2019, 18, 269. [DOI] [PubMed] [Google Scholar]
- [193].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Eur. J. Immunol. 2008, 38, 1404. [DOI] [PubMed] [Google Scholar]
- [194].Buabeid MA, Arafa E-SA, Murtaza G, J. Immunol. Res. 2020, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Doll TAPF, Raman S, Dey R, Burkhard P, Soc JR. Interface 2013, 10, 20120740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Hashemzadeh H, Javadi H, Darvishi MH, Sci. Rep. 2020, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ, J. Adv. Res. 2019, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Bijker MS, van den Eeden SJF, Franken KL, Melief CJM, van der Burg SH, Offringa R, Eur. J. Immunol. 2008, 38, 1033. [DOI] [PubMed] [Google Scholar]
- [199].Davar D, Ding F, Saul M, Sander C, Tarhini AA, Kirkwood JM, Tawbi HA, J. Immunother. cancer 2017, 5, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Yan D, Wei YQ, Guo HC, Sun SQ, The application of virus-like particles as vaccines and biological vehicles. Appl. Microbiol. Biotechnol. 2015, 99, 10415–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Wang H, Wang Y, Han A, Cai Y, Xiao N, Wang L, Ding D, Yang Z, ACS Appl. Mater. Interfaces 2014, 6, 9815. [DOI] [PubMed] [Google Scholar]
- [202].Liu X, Gao W, ACS Appl. Mater. Interfaces 2017, 9, 2023. [DOI] [PubMed] [Google Scholar]
- [203].Wei G, Su Z, Reynolds NP, Arosio P, Hamley IW, Gazit E, Mezzenga R, Chem. Soc. Rev. 2017, 46, 4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Zhang W, Yu X, Li Y, Su Z, Jandt KD, Wei G, Prog. Polym. Sci. 2018, 80, 94. [Google Scholar]
- [205].Tsutsumi H, Sawada T, Mihara H, KOBUNSHI RONBUNSHU 2017, 74, 162. [Google Scholar]
- [206].Hwang W, Kim B-H, Dandu R, Cappello J, Ghandehari H, Seog J, Langmuir 2009, 25, 12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Liu Y, Xu XD, Chen JX, Cheng H, Zhang XZ, Zhuo RX, Colloids Surfaces B Biointerfaces 2011, 87, 192. [DOI] [PubMed] [Google Scholar]
- [208].Gurevich L, Poulsen TW, Andersen OZ, Kildeby NL, Fojan P, J. Nanosci. Nanotechnol. 2010, 10, 7946. [DOI] [PubMed] [Google Scholar]
- [209].Chu X, Li Y, Long Q, Xia Y, Yao Y, Sun W, Huang W, Yang X, Liu C, Ma Y, Int. J. Nanomedicine 2016, 11, 2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Feng X, Liu H, Chu X, Sun P, Huang W, Liu C, Yang X, Sun W, Bai H, Ma Y, Acta Biomater. 2019, 100, 316. [DOI] [PubMed] [Google Scholar]
- [211].Shu C, Sun P, Xie H, Huang W, Qi J, Ma Y, Int. J. Nanomedicine 2020, 15, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Lee KL, Murray AA, Le DHT, Sheen MR, Shukla S, Commandeur U, Fiering S, Steinmetz NF, Nano Lett. 2017, 17, 4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, Huang AY, Levine AD, Steinmetz NF, Biomaterials 2017, 121, 15. [DOI] [PubMed] [Google Scholar]
- [214].Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S, Nat. Nanotechnol. 2016, 11, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, Willers J, Geldhof C, Prior JO, Kündig TM, Michielin O, Bachmann MF, Speiser DE, Eur. J. Immunol. 2012, 42, 3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sungsuwan S, Wu X, Huang X, In Methods in enzymology; Elsevier, 2017; Vol. 597, pp. 359–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Sartorius R, D’Apice L, Barba P, Cipria D, Grauso L, Cutignano A, De Berardinis P, Front. Immunol. 2018, 9, 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Zhai L, Peabody J, Pang Y-YS, Schiller J, Chackerian B, Tumban E, Antiviral Res. 2017, 147, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Kramer K, Al-Barwani F, Baird MA, Young VL, Larsen DS, Ward VK, Young SL, J. Immunol. Res. 2019, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Neek M, Tucker JA, Il Kim T, Molino NM, Nelson EL, Wang S-W, Biomaterials 2018, 156, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Lee B-R, Ko HK, Ryu JH, Ahn KY, Lee Y-H, Oh SJ, Na JH, Kim TW, Byun Y, Kwon IC, Sci. Rep. 2016, 6, 35182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Wang W, Liu Z, Zhou X, Guo Z, Zhang J, Zhu P, Yao S, Zhu M, Nanomedicine Nanotechnology, Biol. Med 2019, 16, 69. [DOI] [PubMed] [Google Scholar]
- [223].Chen X, Wang R, Chen A, Wang Y, Wang Y, Zhou J, Cao R, Biomed. Pharmacother. 2019, 111, 1124. [DOI] [PubMed] [Google Scholar]
- [224].Voth BL, Pelargos PE, Barnette NE, Bhatt NS, Chen CHJ, Lagman C, Chung LK, Nguyen T, Sheppard JP, Romiyo P, J. Neurooncol. 2020. [DOI] [PubMed] [Google Scholar]
- [225].Si Y, Wen Y, Kelly SH, Chong AS, Collier JH, Control J. Release 2018, 282, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, Chong AS, Collier JH, ACS Nano 2012, 6, 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Wu Y, Norberg PK, Reap EA, Congdon KL, Fries CN, Kelly SH, Sampson JH, Conticello VP, Collier JH, ACS Biomater. Sci. Eng. 2017, 3, 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Tsoras AN, Champion JA, Bioconjug. Chem. 2018, 29, 776. [DOI] [PubMed] [Google Scholar]
- [229].Yang P, Song H, Qin Y, Huang P, Zhang C, Kong D, Wang W, Nano Lett. 2018, 18, 4377. [DOI] [PubMed] [Google Scholar]
- [230].Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, Zhou L, Jiao S, Li Y, Nat. Commun. 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Fuenmayor J, Gòdia F, Cervera L, N. Biotechnol. 2017, 39, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF, In Seminars in immunology; Elsevier, 2017; Vol. 34, pp. 123–132. [DOI] [PubMed] [Google Scholar]
- [233].Bachmann MF, Jennings GT, Nat. Rev. Immunol. 2010, 10, 787. [DOI] [PubMed] [Google Scholar]
- [234].Mohsen MO, Gomes AC, Vogel M, Bachmann MF, Vaccines 2018, 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].D Hill B, Zak A, Khera E, Wen F, Curr. Protein Pept. Sci. 2018, 19, 112. [DOI] [PubMed] [Google Scholar]
- [236].López-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ, Comput. Struct. Biotechnol. J. 2016, 14, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Brune KD, Howarth M, Front. Immunol. 2018, 9, 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Schwarz B, Douglas T, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2015, 7, 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Al-Barwani F, Young SL, Baird MA, Larsen DS, Ward VK, PLoS One 2014, 9, e104523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Singer J, Manzano-Szalai K, Fazekas J, Thell K, Bentley-Lukschal A, Stremnitzer C, Roth-Walter F, Weghofer M, Ritter M, Pino Tossi K, Oncoimmunology 2016, 5, e1171446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Pouyanfard S, Müller M, Biol. Chem. 2017, 398, 871. [DOI] [PubMed] [Google Scholar]
- [242].Donaldson B, Al-Barwani F, Pelham SJ, Young K, Ward VK, Young SL, J. Immunother. cancer 2017, 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Aston-Deaville S, Carlsson E, Saleem M, Thistlethwaite A, Chan H, Maharjan S, Facchetti A, Feavers IM, Siebert CA, Collins RF, Vaccine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Masavuli MG, Wijesundara DK, Torresi J, Gowans EJ, Grubor-Bauk B, Front. Microbiol. 2017, 8, 2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Wang C, Beiss V, Steinmetz NF, J. Virol. 2019, 93, e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, Lee CK, Ockenhouse CF, Morin MJ, Streatfield SJ, Vaccine 2018, 36, 5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Evtushenko EA, Ryabchevskaya EM, Nikitin NA, Atabekov JG, V Karpova O, Sci. Rep. 2020, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Neek M, Il Kim T, Wang SW, Protein-based nanoparticles in cancer vaccine development. Nanomedicine Nanotechnology, Biol. Med 2019, 15, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Whitacre DC, Peters CJ, Sureau C, Nio K, Li F, Su L, Jones JE, Isogawa M, Sallberg M, Frelin L, Hum. Vaccin. Immunother. 2020, 16, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Stephen SL, Beales L, Peyret H, Roe A, Stonehouse NJ, Rowlands DJ, In Virus-Derived Nanoparticles for Advanced Technologies; Springer, 2018; pp. 97–123. [Google Scholar]
- [251].Chen Q, Lai H, Hum. Vaccin. Immunother. 2013, 9, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Tao P, Zhu J, Mahalingam M, Batra H, Rao VB, Adv. Drug Deliv. Rev. 2019, 145, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Sartorius R, D’Apice L, Prisco A, De Berardinis P, Pharmaceutics 2019, 11, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Carroll-Portillo A, Lin HC, Microorganisms 2019, 7, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Jamaledin R, Sartorius R, Di Natale C, Vecchione R, De Berardinis P, Netti PA, Microorganisms 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Frietze KM, Peabody DS, Chackerian B, Curr. Opin. Virol. 2016, 18, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Trovato M, Maurano F, D’Apice L, Costa V, Sartorius R, Cuccaro F, McBurney SP, Krebs SJ, Prisco A, Ciccodicola A, BMC Microbiol. 2016, 16, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Khoshnejad M, Parhiz H, V Shuvaev V, Dmochowski IJ, Muzykantov VR, Control J. Release 2018, 282, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Macone A, Masciarelli S, Palombarini F, Quaglio D, Boffi A, Trabuco MC, Baiocco P, Fazi F, Bonamore A, Sci. Rep. 2019, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Zangabad PS, Karimi M, Mehdizadeh F, Malekzad H, Ghasemi A, Bahrami S, Zare H, Moghoofei M, Hekmatmanesh A, Hamblin MR, Nanoscale 2017, 9, 1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Craig EA, Gambill BD, Nelson RJ, Microbiol. Mol. Biol. Rev. 1993, 57, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Klimczak M, Biecek P, Zylicz A, Zylicz M, Sci. Rep. 2019, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Zhang SAI, Hu Y, Huang Y, Xu H, Wu G, Dai H, Oncol. Lett. 2015, 9, 2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Voll EA, Ogden IM, Pavese JM, Huang X, Xu L, Jovanovic BD, Bergan RC, Oncotarget 2014, 5, 2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Soleimani A, Zahiri E, Ehtiati S, Norouzi M, Rahmani F, Fiuji H, Avan A, Ferns GA, Khazaei M, Hashemy SI, Hassanian SM, Biochem. Cell Biol. 2019, 97, 85. [DOI] [PubMed] [Google Scholar]
- [266].Zong J, Wang C, Liu B, Liu M, Cao Y, Sun X, Yao Y, Sun G, Oncol. Rep. 2013, 30, 407. [DOI] [PubMed] [Google Scholar]
- [267].Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, Butowski N, Chang SM, Clarke J, Berger MS, Clin. cancer Res. 2013, 19, 205. [DOI] [PubMed] [Google Scholar]
- [268].Kelly M, McNeel D, Fisch P, Malkovsky M, Immunol. Lett. 2018, 193, 1. [DOI] [PubMed] [Google Scholar]
- [269].Specht HM, Ahrens N, Blankenstein C, Duell T, Fietkau R, Gaipl US, Günther C, Gunther S, Habl G, Hautmann H, Hautmann M, Huber RM, Molls M, Offner R, Rödel C, Rödel F, Schuetz M, Combs SE, Multhoff G, Heat shock protein 70 (Hsp70) peptide activated Natural Killer (NK) cells for the treatment of patients with non-small cell lung cancer (NSCLC) after radiochemotherapy (RCTx) - From preclinical studies to a clinical phase II trial. Front. Immunol. 2015, 6, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Oura J, Tamura Y, Kamiguchi K, Kutomi G, Sahara H, Torigoe T, Himi T, Sato N, Int. Immunol. 2011, 23, 223. [DOI] [PubMed] [Google Scholar]
- [271].Guo C, Subjeck JR, Wang XY, In Methods in Molecular Biology; Springer, 2018; Vol. 1709, pp. 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Muñoz-Juan A, Carreño A, Mendoza R, Corchero JL, Pharmaceutics 2019, 11, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Yu K, Yau YH, Sinha A, Tan T, Kickhoefer VA, Rome LH, Lee H, Shochat SG, Lim S, Sci. Rep. 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Kar UK, Jiang J, Champion CI, Salehi S, Srivastava M, Sharma S, Rabizadeh S, Niazi K, Kickhoefer V, Rome LH, PLoS One 2012, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Nagasawa DT, Yang J, Romiyo P, Lagman C, Chung LK, Voth BL, Duong C, Kickhoefer VA, Rome LH, Yang I, J. Neurooncol. 2020. [DOI] [PubMed] [Google Scholar]
- [276].Zabel F, Mohanan D, Bessa J, Link A, Fettelschoss A, Saudan P, Kündig TM, Bachmann MF, J. Immunol. 2014, 192, 5499. [DOI] [PubMed] [Google Scholar]
- [277].Anwar MA, Shah M, Kim J, Choi S, Recent clinical trends in Toll-like receptor targeting therapeutics. Med. Res. Rev. 2019, 39, 1053–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Speiser DE, Schwarz K, Baumgaertner P, Manolova V, Devevre E, Sterry W, Walden P, Zippelius A, Conzett KB, Senti G, Voelter V, Cerottini JP, Guggisberg D, Willers J, Geldhof C, Romero P, Kündig T, Knuth A, Dummer R, Trefzer U, Bachmann MF, J. Immunother. 2010, 33, 848. [DOI] [PubMed] [Google Scholar]
- [279].Pitoiset F, Vazquez T, Levacher B, Nehar-Belaid D, Dérian N, Vigneron J, Klatzmann D, Bellier B, J. Virol. 2017, 91, e01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Gomes AC, Mohsen MO, Mueller JE, Leoratti FMS, Cabral-Miranda G, Bachmann MF, Front. Immunol. 2019, 10, 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Hossain MK, Wall KA, Cancers (Basel). 2019, 11, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Eskandari S, Guerin T, Toth I, Stephenson RJ, Adv. Drug Deliv. Rev. 2017, 110, 169. [DOI] [PubMed] [Google Scholar]
- [283].Aggeli A, Bell M, Carrick LM, Fishwick CWG, Harding R, Mawer PJ, Radford SE, Strong AE, Boden N, J. Am. Chem. Soc. 2003, 125, 9619. [DOI] [PubMed] [Google Scholar]
- [284].Kaur H, Sharma P, Patel N, Pal VK, Roy S, Langmuir 2020, 36, 12107. [DOI] [PubMed] [Google Scholar]
- [285].Wu Y, Kelly SH, Sanchez-Perez L, Sampson JH, Collier JH, Biomater. Sci. 2020, 8, 3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, Tahriri M, Tayebi L, Hamblin MR, Acta Biomater. 2019, 92, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Purwada A, Tian YF, Huang W, Rohrbach KM, Deol S, August A, Singh A, Adv. Healthc. Mater. 2016, 5, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Jamaledin R, Di Natale C, Onesto V, Taraghdari ZB, Zare EN, Makvandi P, Vecchione R, Netti PA, J. Clin. Med. 2020, 9, 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Hirayama M, Nishimura Y, Int. Immunol. 2016, 28, 319. [DOI] [PubMed] [Google Scholar]
- [290].Desrichard A, Snyder A, Chan TA, Clin. Cancer Res. 2016, 22, 807. [DOI] [PubMed] [Google Scholar]
- [291].Liu J, Bu W, Shi J, Chem. Rev. 2017, 117, 6160. [DOI] [PubMed] [Google Scholar]
- [292].Smith BR, Gambhir SS, Chem. Rev. 2017, 117, 901. [DOI] [PubMed] [Google Scholar]
- [293].Chen Q, Li C, Yang X, Huang J, Liu S, Liu W, Liu J, Wang K, J. Mater. Chem. B 2017, 5, 7529. [DOI] [PubMed] [Google Scholar]
- [294].V Katti K, Khoobchandani M, Thipe VC, Al-Yasiri AY, Katti KK, Loyalka SK, Sakr TM, Lugão AB, J. Radioanal. Nucl. Chem. 2018, 318, 1737. [Google Scholar]
- [295].Heydari Sheikh Hossein H, Jabbari I, Zarepour A, Zarrabi A, Ashrafizadeh M, Taherian A, Makvandi P, Molecules 2020, 25, 4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Herrera Estrada LP, Champion JA, Biomater. Sci. 2015, 3, 787. [DOI] [PubMed] [Google Scholar]
- [297].Temming K, Schiffelers RM, Molema G, Kok RJ, Drug Resist. Updat. 2005, 8, 381. [DOI] [PubMed] [Google Scholar]
- [298].Smith BR, Ghosn EEB, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS, Nat. Nanotechnol. 2014, 9, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Tomeh MA, Hadianamrei R, Zhao X, Int. J. Mol. Sci. 2019, 20, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Shi J, Kantoff PW, Wooster R, Farokhzad OC, Nat. Rev. Cancer 2017, 17, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]