Abstract
Background
Older adults commonly face challenges in understanding, obtaining, administering, and monitoring medication regimens after hospitalization. These difficulties can lead to avoidable morbidity, mortality, and hospital readmissions. Pharmacist-led peri-discharge interventions can reduce adverse drug events, but few large randomized trials have examined their effectiveness in reducing readmissions. Demonstrating reductions in 30-day readmissions can make a financial case for implementing pharmacist-led programs across hospitals.
Methods/Design
The PHARMacist Discharge Care, or the PHARM-DC intervention, includes medication reconciliation at admission and discharge, medication review, increased communication with caregivers, providers, and retail pharmacies, and patient education and counseling during and after discharge. The intervention is being implemented in two large hospitals: Cedars-Sinai Medical Center and the Brigham and Women’s Hospital. To evaluate the intervention, we are using a pragmatic, randomized clinical trial design with randomization at the patient level. The primary outcome is utilization within 30 days of hospital discharge, including unforeseen emergency department visits, observation stays, and readmissions. Randomizing 9,776 patients will achieve 80% power to detect an absolute reduction of 2.5% from an estimated baseline rate of 27.5%. Qualitative analysis will use interviews with key stakeholders to study barriers to and facilitators of implementing PHARM-DC. A cost-effectiveness analysis using a time-and-motion study to estimate time spent on the intervention will highlight the potential cost savings per readmission.
Discussion
If this trial demonstrates a business case for the PHARM-DC intervention, with few barriers to implementation, hospitals may be much more likely to adopt pharmacist-led peri-discharge medication management programs.
Trial registration:
ClinicalTrials.gov Identifier: NCT04071951
Keywords: adverse drug events, medication management, geriatrics, readmissions, pharmacist
1. INTRODUCTION
Older adults transitioning from acute hospitals to community care are at high risk of experiencing adverse drug events (ADEs). ADEs account for up to 70% of all adverse events occurring after discharge [1–3]. The oldest, most vulnerable patients are at highest risk for ADEs: they have the most complex and hazardous medication regimens but the fewest social and economic resources and the least physiologic reserve [4]. Post-discharge ADEs occur in 17–19% of older patients [5, 6] and cause 23–38% of readmissions in older adult patients [6–8].
Older adults commonly face challenges in understanding, obtaining, administering, and monitoring new medications prescribed at discharge [9, 10]. These difficulties can lead primarily to avoidable side effects, non-adherence, and suboptimal disease management, and secondarily to outpatient and emergency department visits, hospital readmission, morbidity, and death [5]. Two studies show that most post-discharge ADEs are either preventable (24–27%) or ameliorable (33–38%) [3, 11].
Despite the known evidence about pharmacist-led interventions to reduce ADEs, there has been poor uptake of these interventions across health systems. One argument is that ADE reduction interventions have not focused on 30-day hospital readmissions – outcomes important to payors and health system administrators. In 2012, Medicare began penalizing hospitals for excess readmissions through a value-based care program in order to incentivize better coordination of post-discharge care [12]. One interrupted time series study found that inpatient pharmacy-led interventions can reduce hospital readmissions due to medication reconciliation errors [13–15]. Until now, however, few large randomized controlled trials have examined the effectiveness of pharmacist-led inpatient and post-discharge medication management programs in reducing all-cause 30-day readmissions.
This pragmatic trial, randomized at the patient level and conducted in two large hospitals, aims to fill this important gap. The PHARMacist Discharge Care (PHARM-DC) intervention, described in detail below, includes medication reconciliation at admission and discharge, patient/caregiver counseling, medication review, and at least one post-discharge phone call. Our objectives for this study are as follows: (1) to test the effect of PHARM-DC on post-discharge utilization among patients most at risk for post-discharge ADEs: recently discharged older adults taking ≥10 medications or ≥3 high-risk medications; (2) to study barriers and facilitators of implementing PHARM-DC; and (3) to analyze the costs of PHARM-DC, including the incremental cost per readmission averted and the net incremental cost from the health system perspective.
2. RANDOMIZED CONTROLLED TRIAL STUDY DESIGN
2.1. Trial design
This is a pragmatic, randomized controlled superiority clinical trial with balanced randomization (1:1) and two parallel groups (intervention and control) conducted in two large, urban, academic hospitals in the United States. See Figure 1 for an overview of the study design.
Figure 1.
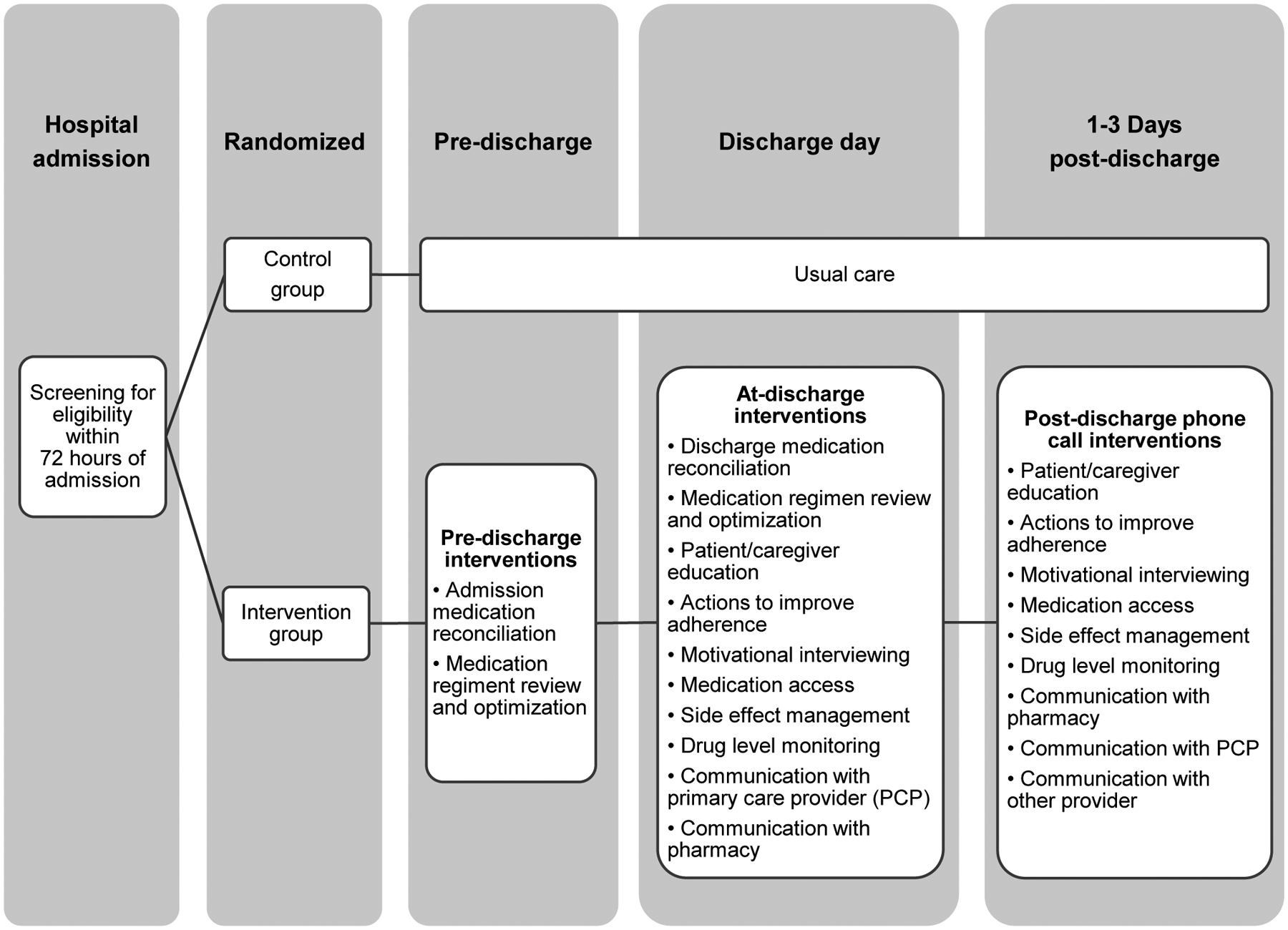
Study design
2.2. Enrollment and eligibility
Consistent with accepted principles of pragmatic trial design [16], we chose inclusion and exclusion criteria that would be readily replicable at other hospitals. At each site, there are already existing pharmacist discharge programs for patients with recent transplants, cardiac catheterization procedures and other programs. Thus, patients eligible for those pharmacy programs are excluded from randomization. Enrollment at both sites began in December 2019 and is planned to last three years.
Inclusion criteria include:
Admitted to a medical ward, AND
- ≥ 55 years old AND
-
≥ 10 chronic prescription medicationsOR
- ≥ 3 high-risk medications (anticoagulants, antiplatelets, insulin, and oral hypoglycemics) at admission
-
Exclusion criteria include:
Expected discharge to another state, acute care facility, psychiatric facility, or locked facility (including locked skilled nursing facility, jail, or prison) OR
Expected leaving hospital against medical advice (AMA) or actual AMA OR
Homeless OR
On hospice OR
Already enrolled into study during prior discharge in previous year OR
Expected to receive pharmacist-led post-discharge medication management regardless of the trial OR
Patients admitted by Primary Medical Doctors who have a specialty that is not Internal Medicine or Family Medicine OR
Expected post-discharge setting not conducive to the studied medication management intervention (e.g. SNF, acute rehabilitation facility). Actual (unexpected) discharge to SNF or rehabilitation is not an exclusion, but if anticipated at the time of patient enrollment, exclusion of these patients minimizes the number of subjects discharged to these facilities.
2.3. Study settings
This study includes two hospitals sharing three critical features: high patient volume, especially of the oldest, sickest patients most likely to benefit from PHARM-DC, allowing us to accumulate the necessary sample size; a Chief Pharmacy Officer committed to using operational resources to test PHARM-DC; and pre-existing use of interventions to ensure high-quality in-hospital medication history-taking, a prerequisite for post-discharge medication management efforts. The two sites are an academic medical center (Brigham and Women’s Hospital in Boston, Massachusetts) and a university-affiliated hospital (Cedars-Sinai Medical Center in Los Angeles, California) that has many practicing community physicians.
We selected these sites because they each have: robust pharmacist programs, including residency programs that can supply some of the labor needed for this study; pharmacist leaders committed to supporting PHARM-DC interventions with institutional resources and to randomly allocating these services to patients to test their benefit; skilled investigators with relevant content knowledge and methodological experience; more than 900 inpatient beds, allowing for timely accumulation of the sample size while minimizing overhead.
Cedars-Sinai Medical Center
Cedars-Sinai Health System is a large health system with two hospitals (Cedars-Sinai Medical Center, [CSMC] 886 beds, and Marina Del Rey Hospital, 133 beds). The system has approximately 55,000 annual admissions, 120,000 annual emergency visits, and 250 primary and specialty care locations throughout Los Angeles County. CSMC has a Level I Trauma Center and is a major teaching hospital [17]. In 2015, the payor mix for the main medical center was 42.9% Medicare, 12.6% Medicaid, 42.3% private insurance, and 2.1% other payers [18].
Brigham and Women’s Hospital
Brigham and Women’s Hospital (BWH) is one of the founding members of Mass General Brigham (formerly Partners HealthCare), a large integrated health care system in Eastern Massachusetts. BWH is a 793-bed teaching hospital affiliated with Harvard Medical School with 46,000 annual admissions 62,000 annual emergency department visits, and 950,000 ambulatory visits annually. In 2019, the payor mix was 40.8% Medicare, 47.4% third-party insurance, and 11.8% Medicaid.
Pharmacist Interventions at Sites Prior to Study
In Table 1, we detail the state of pharmacist-led interventions at the two sites prior to the PHARM-DC study. Medication reconciliation, specifically using trained, dedicated personnel to take “best possible” preadmission medication histories, was implemented at both sites for half (BWH) of medical patients to most (CSMC) to admitted from the emergency department [19]. High-risk patients received post-discharge phone calls at both sites and some patients received bedside delivery of medications to inpatients prior to discharge.
Table 1.
Characteristics and Current Use of PHARM-DC and Associated Interventions at Each Site
| Characteristic: | Cedars-Sinai Medical Center (CSMC) | Brigham and Women’s Hospital (BWH) |
|---|---|---|
| Hospital Type | Academic Medical Center/Community | Academic Medical Center |
| Inpatient med rec interventions at site | Pharmacy technicians (PTs) and pharmacists take medication histories from most patients admitted from ED. Access to Surescripts claims data at hospital admission. Physicians/NPs/PAs use Epic EHR to reconcile medications, pharmacist/PT assistance for some high-risk patients, limited by pharmacist/PT availability. RNs counsel most patients. | Pharmacy students and technicians take medication histories on half of medical patients admitted from ED. Electronic tool consolidates meds from several preadmission sources, including SureScripts claims data. Physicians/PAs use Epic EHR to reconcile meds; RNs counsel most patients; pharmacist assistance for some high-risk patients limited by availability. |
| Current state of PHARM-DC Intervention usage: | ||
| Inpatient pharmacist visit | For some patients | For some patients |
| “Meds-to-Beds” (Delivery of medications to inpatients prior to discharge) | For some patients, based on: patient risk, hospital providers, and patient insurance | On some services |
| Post-discharge pharmacist phone calls | For some patients deemed to be high risk | As part of Transitional Care Management calls by some practices, usually by LPNs |
| Pharmacist communication with PCPs | Only if concerns based on other post-discharge follow-up | Rarely |
| Communication with retail pharmacists, especially regarding discontinued medications | None | None |
| Home pharmacist visits | None | On a limited basis as part of previous studies, not currently in use |
| Electronic pillbox technology | None | As part of a recently completed AHRQ- |
| Electronic (email or other visual) transmission of medication information to recently discharged | Patients using outpatient network can view meds via patient portal | None; patient portal may include soon |
2.4. Leadership and stakeholder engagement
In response to difficulty obtaining funding to implement and disseminate pharmacist-led peri and post-discharge medication management interventions within their own institutions, pharmacist leaders at our study sites conceived this work with the support of their pharmacist colleagues at similar sites. These pharmacist-leaders have assisted us in choosing an outcome, post-discharge utilization, that they believe would affect internal funding for implementation, dissemination, and sustainability of PHARMacist Discharge Care (PHARM-DC) at their own institutions and at others. It is partially with the promise of this future internal operational funding in mind that they have committed to using their operational and political capital to support PHARM-DC at their institutions during the study period.
During the planning phase of the study (November 2018-December 2019), investigators and pharmacy leaders at both sites met periodically to discuss:
Pharmacist-led peri-discharge intervention components
Pharmacist workflow
Electronic health record note templates
Training for pharmacist staff (e.g. Motivational Interviewing training options)
Communication with other hospital staff (e.g. nursing staff, hospitalists, residents, pharmacist technicians)
Harmonization of study with other organizational priorities
During the study implementation period, pharmacist teams meet at least once a month to discuss potential barriers to implementation, challenges, and successes. Pharmacist teams at both sites also meet quarterly over the phone to share challenges and lessons learned.
2.5. Description of the comparators
The study compares usual care (control) to a multi-component medication management intervention. For both intervention patients and control patients, pharmacy staff attempt to obtain a Best Possible Medication History (BPMH) to determine which medications a patient has been prescribed and has been taking prior to admission, and to use this information to make sure physicians are aware of this information when inpatient medication orders are placed[20]. To obtain a BPMH, pharmacists review medications with patients and/or caregivers/family members. Pharmacists may also call the patient’s community pharmacy and/or consult SureScripts pharmacy fill history. To ensure that study pharmacists have enough bandwidth to perform study interventions, pharmacy students and technicians may help obtain BPMHs, which are reviewed by study pharmacists. As part of the BPMH provided to all patients in the study, pharmacists are using a Medication Adherence and Literacy (MedAL) scoring tool to quantify medication adherence and literacy [21, 22], which includes questions in Table 2.
Table 2.
Medication Adherence and Literacy (MedAL) 3.0 Score Components
| 1. Did you forget to take your medications this past week? | Yes, No, Not Assessed |
| 2. Are you taking your medications differently than how your doctor prescribed? | Yes, No, Not Assessed |
Answer “Yes” to questions 1 and 2 and if the patient is taking 10 or more medications denotes a high need for post-discharge follow-up
Intervention Categories
For clarity, we have separated the pharmacist-led peri-discharge medication management interventions into three main categories. Using an accepted conceptual framework of medication management [23, 24], we determined that almost all pharmacist-led interventions reducing post-discharge ADEs do so by addressing (1) medication reconciliation, (2) medication adherence, (3) and medication review. Thus, the intervention in this study includes the following components, outlined in Table 3.
Table 3.
PHARM-DC Intervention Components
| Admission medication reconciliation |
| Medication regimen review/optimization |
| Actions to improve adherence |
| Side effect management |
| Drug level monitoring |
| Discharge Medication Reconciliation |
| Patient/caregiver education: indications, directions, reasons for changes in regimen |
| Motivational interviewing |
| Post discharge follow up phone call |
| Post discharge additional phone call |
| Medication Access (e.g. problems obtaining medications) |
| Communication with PCP |
| Communication with community pharmacy |
Medication Reconciliation
As noted earlier, intervention (and control) patients receive a BPMH on admission or as soon as possible after admission. Intervention patients also receive medication reconciliation at discharge, identifying and correcting any unintentional discrepancies between preadmission and discharge medications. If the patient is discharged before pharmacists can conduct the medication reconciliation, pharmacists attempt to conduct it during the post-discharge phone call (see below). If patients are discharged to a post-acute rehabilitation facility or a skilled nursing facility (SNF), pharmacists aim to communicate with prescribers at the post-acute facility to ensure that the medication administration record (MAR) is correct (i.e., the same as the discharge medication orders, or that any changes are intentional).
Medication Adherence
Medication adherence interventions are used to improve patients’ adherence to a prescribed medication regimen, which may include identifying barriers (e.g., cost, side effects, understanding) and addressing them. Medication adherence efforts are individualized based on pharmacists’ assessment of patients’ need. Intervention patients thought to have potential to benefit from this intervention component may receive: motivational interviewing, counseling, and enhanced discharge medication access. For example, if patients and/or caregivers identify side effects that may intervene with adherence, pharmacists may recommend alternative options. Pharmacists may also assess whether medication costs are barriers to adherence and may recommend switching to medications that are on patients’ formularies or facilitating enrollment in drug assistance programs. Intervention patients may also receive bedside medication delivery (“Meds to Beds,”) [25] where the pharmacy team coordinates having the medications brought to the patient prior to discharge. Adherence aids such as pillboxes and medication diaries can also be provided. Study pharmacists may also communicate with community pharmacies regarding prior authorizations.
Medication Review
Many pharmacist-led interventions to reduce inappropriate polypharmacy involve ‘Medication Review,’ wherein pharmacists critically appraise medication regimens and reach out to prescribers to suggest modifications, including addressing under-prescribing and overprescribing [26]. Polypharmacy refers to the fact that many patients, especially older patients with multimorbidity, are overprescribed medications. It is facilitated by multiple factors, including fragmented care and fear of discontinuing old medications. During hospitalization, intervention patients receive tailored medication review and optimization during hospitalization and post-discharge. Tailored medication review and optimization may include: recommendations given to prescribers during hospitalization and to primary care providers, to deprescribe medications, modify doses, manage side effects, substitute medications, or add medications if needed. Recommendations are communicated to providers by phone call, text, or email. Pharmacists will follow up with providers who do not respond to request a response if clinically needed.
To conduct medication review and optimization, pharmacists use past medical history and laboratory results from: the electronic health record, health information exchanges, and Surescripts (a nationwide network that delivers healthcare information to healthcare providers, including prescriptions previously filled, health records, and clinical documents). When less information is available from these sources, pharmacists seek further consultation with prescribers.
On the day of discharge, intervention patients receive a discharge intervention, which may include a brief discussion of medication changes, discussion of potential side effects, and discussion of red flags (worrisome symptoms to watch for) for new medications. Clinical pharmacists may also consult with inpatient clinicians and pharmacists to discuss the medication list and to ensure that e-prescribing is done appropriately.
One to three days after discharge, intervention patients or caregivers receive a post-discharge intervention, which nearly always includes a call with a patient. Issues discussed may include the patient’s medication regimen (for discrepancies and non-adherence), discussion of side effects, red flags for new medications, medication monitoring, a motivational interview, and a discussion of barriers to adherence. Pharmacists may also assist with identifying drug assistance programs for reduced pricing and linkage with community or social service organizations. Other tasks include a phone call with or an email to the patient’s primary care physician as clinically needed, for example, to coordinate a follow-up plan. Some patients, depending on need, receive a second follow-up phone call.
Given the substantial potential benefit of motivational interviewing,[27] all participating study pharmacists completed an 8-hour online Motivational Interviewing training program (comMIt, or comprehensive motivational interviewing training) offered by Purdue University’s College of Pharmacy [28]. The program includes 6 modules focused on topics such as developing rapport with patients, understanding sense-making, reframing, and working with patients who are ambivalent or resistant to change. Pharmacy leaders and pharmacists were also trained on use of standardized medication discharge and post-discharge phone call templates, which include the elements of the intervention below (See Table 3). Periodic meetings with investigators and pharmacists are being used to ensure adherence to the templates and to identify challenges to implementation.
Usual Care
Pharmacy personnel at both sites will attempt to obtain a BPMH and MedAL screening from all study patients. Patients will receive usual care, which may at times include pharmacist consultation and/or post-discharge phone call(s) if deemed clinically necessary and requested or performed by physicians, nurses, or pharmacists in the course of usual clinical care.
2.6. Outcome definitions
Primary outcome
Our primary outcome is 30-day post-discharge utilization, defined as a binary outcome (yes/no) of whether patients had any inpatient readmissions, observation stays, or ED visits within 30 days after hospital discharge. Following other studies, we excluded foreseen readmissions, including: transplants, chemotherapy or radiotherapy, treatment follow-up, rehabilitation care, planned operations [29]. We selected this as our primary outcome because the RE-AIM model points to the importance of institutional-level effectiveness and cost. We recognize the limitations of same-hospital utilization data [30]. As such, the RCT is powered on a primary endpoint of same-state 30-day hospital readmissions and ED visits.
Secondary outcomes
We will also conduct an analysis of post-discharge utilization using same-hospital utilization data, which will be available before statewide data. Additionally, we will examine the rates of 30-day post-discharge utilization stratified by:
Receipt of different intervention components
Diagnosis of congestive heart failure at admission
Having three or more high risk medications (anticoagulants, antiplatelets, insulin, oral hypoglycemics) prior to admission
Having 10 or more medications prior to admission
Study site
Patient medication adherence and literacy
Quintiles of patient socioeconomic status (estimated via median income of home zip code)
Discharge on weekends (compared to weekdays)
We hypothesize that the intervention will work equally at both sites, will work better for those taking more medications, and those from zip codes at lower quintiles of SES status. However, we recognize that these unpowered analyses may be most useful for shaping future hypotheses. To ensure that no results are misinterpreted, we will report that any negative results may be due to inadequate power.
2.7. Data Sources
We will extract age, gender, number of preadmission medications, medication types, comorbidities (including diabetes, congestive heart failure), median income of zip code, first language, and marital status from electronic health records after the study ends at each study site. Post-discharge utilization will be tracked with data from the California’s Office of Statewide Health Planning and Development and the Massachusetts All-Payer Claims Database, both of which include comprehensive encounter-level data for ED, observation, and hospitalizations [31]. We will also conduct a sensitivity analysis using National Death Index data to ensure that any utilization reductions are not due to deaths.
2.8. Stratified Randomization by Site
Using a daily report populated with data from the electronic health record, a study coordinator at each site identifies eligible patients on weekdays (Monday-Friday). Study coordinators also conduct a brief chart review to ensure the patient meets eligibility criteria. Randomization is performed via the Research Electronic Data Capture (REDCap) system, a secure, web-based application supporting data capture for research studies, providing: an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources [32, 33]. A statistician at the primary site (CSMC) created a random number table for each site in R and uploaded the table to REDCap.
Study participants are randomized 1:1 to the two arms of the study, with stratification by site. Researchers have no access to the random number sequence prior to randomizing a given patient. Arm assignment is not actively masked, but pharmacists are made aware of patients randomized to the intervention arm so that they can conduct the intervention. At one site, pharmacists were also made aware of patients randomized to the control group, so that they could ensure that attempts were made to obtain a BPMH. Patients are not approached prior to randomization (see Waiver of Consent, below).
Once patients are randomized, study coordinators add patients to patient lists created at each site’s electronic health records system. Pharmacists monitor the lists daily to keep track of patients throughout their stay and to help ensure that they receive the intervention components. Each site is also using a separate tracking method to track post-discharge phone calls (Sharepoint at CSMC; SmartData Elements in encounter notes via Epic at BWH).
2.9. Analysis plan and sample size
The descriptive phase of analysis will include an assessment of the distributions and correlations of the aforementioned variables of interest in the two study arms. We will use chi-square tests, Analysis of Variance (ANOVAs), logistic regression, and means/medians for descriptive statistics. We will use a 0.05 cutoff for p-values and 95% confidence intervals for statistical significance. We will also examine the potential interaction of covariates. We will explore collinearity using the condition index and with careful assessment of the correlation matrix.
For the main outcomes (30-day readmissions, 30-day ED visits, 30-day observation stays), we will use a two-sided likelihood ratio test to compare the proportion of patients with utilization within 30 days of discharge between the intervention and usual care groups. We will also use multivariate logistic regression for the main outcomes and will control for age, sex, study site, and common chronic conditions associated with readmissions (e.g. acute myocardial infarction, chronic obstructive pulmonary disease, congestive heart failure, pneumonia, coronary artery bypass graft surgery) [34].
For the secondary outcomes, we will conduct multivariable regression for the following variables and will use interaction terms to look for effect modification: whether the patient has 10 or more medications on admission, whether the patient has 3 or more high-risk (anticoagulants, antiplatelets, insulin, oral hypoglycemics) on admission, the patient’s medication adherence and literacy, and the patient’s socioeconomic status (estimated via median income of home census tract).
Assuming 80% power and a type I error rate of 0.05, a two-sided Z test between two proportions with 4,888 subjects per group would detect a difference of at least 2.49% in the primary outcome of 30-day post-discharge utilization from a baseline of 27.5%. We will also compare the baseline characteristics of those subjects across both arms to ensure there are no major differences. Because the two sites have differences in patient populations, institutional context, and provider characteristics, randomization will be stratified by site. All enrolled patients will be analyzed. To achieve this power, we estimate that we will need to enroll approximately 7 patients per weekday at each site for three years.
We also performed a sensitivity analysis assuming that 7.5% of patients in the intervention arm were unreachable or refused a phone call. At this rate, we estimate that we will have 80% power to detect a difference of 2.54%. Finally, these rates were robust to contamination rates of 2% among patients in the usual care arm. We believe this low rate is a reasonable estimate for contamination, because patients thought to have a clear need for these services will be excluded from the RCT, and because we believe that non-pharmacist clinicians will remain so busy that they will not begin to provide such services to other patients.
We will conduct the following analyses: Intention-to-Treat (ITT) analysis (i.e., all randomized participants); Modified Intention-to-Treat analysis e.g., participants who had the in-hospital intervention but did not respond to post-discharge phone calls); Per-Protocol analysis, which defines a subset of the participants in the full analysis (ITT) set who received the protocol sufficiently to ensure that these data would be likely to represent the effects of study intervention according to the underlying scientific model (e.g. participants who had both the in-hospital intervention and the post-discharge phone call).
2.10. Evaluation Framework
We used the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) Framework to guide our evaluation design [35]. To evaluate interventions’ potential health impact, RE-AIM considers the following dimensions: Reach, i.e., the extent to which the intervention reaches the target population, Effectiveness of the intervention, Adoption by target staff, settings, or institutions, Implementation consistency, fidelity, costs and adaptations made during intervention delivery, Maintenance of intervention effects in individuals and settings over time. Depending on the situation, each dimension may need to be addressed both at the individual level and the institutional level. In the case of PHARM-DC, prior studies examining only individual-level process Efficacy were insufficient to motivate adoption. RE-AIM helped us to see beyond individual-level Efficacy and to include institutional-level Effectiveness, barriers and facilitators to Adoption, and the role of costs.
2.11. Ethical and regulatory approval
Both sites already conduct some components of the PHARM-DC intervention on some patients. Because it is currently used, but allocation is determined by operational convenience (e.g., pharmacists try to provide this service to everyone they think would benefit, but only reach some patients), there is justification for an RCT with a waiver of informed consent. The RCT only changes how the intervention is allocated (from operational convenience to randomization). We requested and received a waiver of informed consent from the single Institutional Review Board (IRB) at Cedars-Sinai Medical Center for the following reason:
Minimal Risk
The risks to the patients are minimal. After the medication history is taken for patients, the patients are randomized into either the treatment or control group. However, if any clinician believes that a patient would benefit from pharmacist services, pharmacists will see the patient for these services regardless of study arm, and regardless of whether they are in the study. Thus, any patient who needs the pharmacist services will receive the appropriate care.
During training, it was emphasized to pharmacists participating in this study that additional, clinically-indicated services may be provided to control group patients to ensure that services that are part of the PHARM-DC intervention would not be withheld from the control group if clinically indicated.
Safety oversight is under the direction of a Data and Safety Monitoring Board (DSMB) composed of individuals with the appropriate expertise. Members of the DSMB are independent from the study conduct and free of conflict of interest. The DSMB, composed of a statistician, a pharmacist scientist, and a geriatrician, meets at least semiannually to review adverse events. Adverse events (deaths, same-hospital readmissions) are collected retrospectively every six months. The DSMB operates under the rules of an approved charter that was written and reviewed at the organizational meeting of the DSMB. Eligibility changes, study updates, and any protocol changes are discussed during biweekly calls with investigators.
3. IDENTIFICATION OF BARRIERS AND FACILITATORS
We will use qualitative methods to investigate several hypotheses developed using the Consolidated Framework for Implementation Research (CFIR) model as a guide [36]. We hypothesize that barriers to staff adoption will include: (1) difficulties associated with coordinating a time for discharge counseling (patients are frequently eager to leave as soon as discharge orders are placed, but the discharge medication regimen is not usually available before this time), and (2) that even when problems are identified, lack of resources may still be an issue (e.g., medications with expensive copayments). We hypothesize that implementation consistency will be higher and that adaptations will be lower when pharmacists are not overwhelmed by other responsibilities.
We are aware that the study hospitals are currently implementing other interventions to reduce readmission rates. As a result, another continuing challenge of implementing PHARM-DC will be integrating it into existing and evolving programs designed to improve transitions of care more broadly, and understanding any effect of co-interventions implemented during our trial. Our qualitative analysis will shed light on how this was done. Furthermore, conducting the RCT in this environment will increase external validity.
3.1. Recruitment and eligibility
We will meet with leadership for each major stakeholder group (nursing staff, pharmacists, hospitalists) and will ask for a list of potential qualitative study participants. Participants will be eligible if they are: (1) 18 years or older, (2) are directly conducting clinical care for patients enrolled in the PHARM-DC clinical trial or supervising those performing direct clinical care.
3.2. Data collection
During Year 1, we will email eligible study pharmacists and pharmacy leaders to participate in interviews. At least two study investigators will conduct interviews regarding how the intervention is being applied (implementation consistency across patients and sites), barriers/facilitators to adoption and implementation, and adaptations made during delivery. Interviews will take place over video web-conferencing software or by phone. We will use a semi-structured interview guide with open-ended questions and follow-up probes. Interviews will last 60–90 minutes and will be video/audio-taped and transcribed. In Year 2, we will conduct in-person focus groups and/or interviews with physicians and nurses who interacted with study patients. We will obtain lists of potential study participants from physician and nurse leaders and will email all eligible participants. Focus groups and/or interviews will last 60–90 minutes. The guide will focus on the effect of the intervention on these two stakeholder groups, barriers/facilitators to working with study pharmacists during the intervention period, average number of recommendations per patient that are made to providers and patients, acceptance rate of these recommendations, and barriers/facilitators to acceptance of recommendations.
In Year Three, investigators will conduct one-on-one phone interviews with frontline pharmacists and key stakeholders (e.g., pharmacist leaders). Questions will be similar to those of the interviews in Year 1 and will also use a semi-structured guide, but we will place more emphasis on changes during the implementation period, adaptations of the intervention components, whether and how components can be sustained, acceptance rate of components by patients and providers, and barriers/facilitators to patient acceptance of the components. Interviews are expected to last about 25 minutes.
3.3. Data analysis
For the qualitative analysis of interviews and focus groups, we will combine a content-analysis approach with qualitative inquiry allowing us to discover and quantify key stakeholders’ experiences and perceptions. We will use an iterative process to identify “a priori” themes based on CFIR domains and to create “in vivo” themes as they emerge during coding (e.g., specific barriers to PHARM-DC implementation) [37]. Two coders will code each interview independently and then discuss variations until consensus is reached. After coding all interviews, we will use the constant comparative method to combine similar themes with limited data under more general themes [38]. We will use NVivo software which will allow for quantifying the number of key stakeholders who addressed these themes and their density (i.e., number of overall mentions in the interview). Theme density is a valid proxy for significance. In the final step of the analysis, we will prepare NVivo code reports (participant N and density) of salient themes and coding matrices related to significant interview findings. We will perform follow-up interviews as needed to supplement or clarify themes. We will also prepare descriptive statistics of all interview and focus group participants.
We will take several measures to ensure rigorous analysis. All transcripts will be independently analyzed by three investigators who will meet weekly during the qualitative analysis process to discuss each transcript and to resolve disagreements through negotiated consensus [39]. Analysts will also maintain an audit trail throughout this process to document the fit between the raw data and the conclusions drawn. NVivo software will be used to code patterns among sites and individuals, and to code variations within and between key stakeholders. We will later triangulate survey results with interviews, focus groups, and on-site observations to better understand the impact of context on the experiences of key stakeholders.
3.5. Data protection
For the interviews and focus groups, we will provide an information sheet outlining the purpose, risks, and benefits of the study intervention. A member of the study staff will go over the information sheet. Information sheets are IRB-approved and the participant will be asked to read and review the document. The investigator will explain the research study to the participant and answer any questions that may arise. A verbal explanation will be provided in terms suited to the participant’s comprehension of the purposes, procedures, and potential risks of the study and of their rights as research participants. Participants will have the opportunity to carefully review the written information sheet and ask questions prior to participating in the focus group. Participants will be informed that participation is voluntary and that they may withdraw from the study at any time, without prejudice. The decision whether or not to participate in the study and the results will remain anonymous and will never be shared with any hospital personnel.
Interviews and focus groups present no more than minimal risk. Signed consent will not be obtained for interviews and focus group participants. The interviews and/or focus groups will be conducted in areas which the staff frequently occupy as part of their daily tasks and the questions will require no more disclosure of information than would be routinely occur in their conventional work environment. Attendance and participation in the interviews/focus groups will be considered consent to participate. Personal identifiers will be stripped from all transcripts before analysis. Study participants in the qualitative study will be given number identifiers, which will be kept separate from their identifiers/demographic information. Only the study staff will have access to the identifiers.
3. ANALYSIS OF NET COSTS TO THE HEALTH SYSTEMS
Our objective will be to estimate the incremental net cost of the PHARMC-DC intervention, relative to usual care, at each study site over a three-year period. Calculating incremental net costs will encompass both implementation costs and costs associated with return emergency department (ED), observation, and inpatient hospital visits within 30 days. The economic evaluation will use the health system perspective because it encompasses the perspectives of two key stakeholders, hospitals (implementation costs) and healthcare payers (costs associated with return hospital visits). We will not include other types of healthcare utilization, quality of life, time in care, or costs borne by patients.
We will use microcosting techniques to calculate implementation costs. To estimate labor costs associated with start-up and maintenance of the intervention, each site PI will track relevant committee meetings and training sessions, obtain information on the types and numbers of personnel hired or reallocated, and document the hours spent. To estimate the costs associated with changes in workflow by pharmacists during the care of individual patients, we will conduct approximately 300 hours of time and motion analyses at each site. To convert time estimates to costs, we will obtain data on hourly wages and benefits for each hospital region and type of worker, as available from the Bureau of Labor Statistics.
To determine costs associated with 30-day hospital visits, we will obtain data from each site regarding the numbers of ED, observation stays, and readmissions within 30 days of hospital discharge as well as attributable charges. To convert to costs, we will apply hospital-specific cost-to-charge ratios.
4. DISCUSSION
4.1. Strengths
This study has several strengths. The PHARM-DC is a realistic quality improvement project: the resources available to each site are similar to those that could be available to other sites wanting to adopt this intervention outside the context of a research study. Our primary outcome – 30-day utilization – is clinically important, sensitive to change with this sample size, and relatively inexpensive to collect. Qualitative analysis will greatly add to the lessons learned – not just factors contributing to intervention success, but how, when, why, and where the intervention is and is not successful.
4.2. Limitations
We recognize that it may be difficult to conduct some interventions for patients suffering from dementia and delirium, and for non-English speakers. We will address the former issue by engaging caregivers. Our co-investigators with geriatrics training are especially knowledgeable in this area. The latter issue can often be addressed by using pharmacists and other personnel at sites who speak the most common non-English languages of the patients at those hospitals. Otherwise, we will use trained interpreters when needed.
The sites may not be typical of hospitals in general. For example, both study sites have prior experience implementing pharmacist-led peri and post-discharge medication management interventions. We intentionally reduced this aspect of generalizability so as to maximize the study’s chances of success. We appreciate that these hospitals are different from where most patients in the US receive their care. Following this study, next steps should include a larger investigation, based on lessons learned from this study, with an enhanced intervention applied to a broader range of institutions, including more community hospitals. For example, the study could resemble MARQUIS2, a study run by one of our investigators and funded by AHRQ, involving “mentored implementation” of 18 hospitals as they improve their inpatient medication reconciliation processes [40]. MARQUIS2 followed a smaller study (MARQUIS1) at 5 hospitals, which used similar methods as the current study to refine the intervention and provide lessons learned to optimize implementation [41, 42].
It is possible that PHARM-DC will not impact post-discharge utilization. We have hedged against this outcome with an optimized intervention, a highly selected patient population, and a large sample size. Nonetheless, if the trial is negative despite these efforts, this would be an important result because it would show that this intervention will not meaningfully reduce utilization, and that future similar trials would be unnecessary.
4.3. Conclusion
Even though prior studies have suggested that pharmacist-led peri-discharge interventions reduce adverse drug events, they have not been widely implemented. Based on the idea that hospital and health system leaders would need a business case to support these non-billable interventions, we sought to measure their effectiveness in reducing post-discharge utilization. This large pragmatic randomized trial will quantitatively and qualitatively assess the effect of a pharmacist-led peri-and post-discharge medication management intervention on post-discharge utilization.
Highlights:
Prior studies suggest peri- and post-discharge, pharmacist-led medication management interventions may reduce adverse drug events, but few have examined their effectiveness in reducing readmissions – an outcome important to patients, caregivers, and hospital administrators.
The Pharmacist Discharge Care (PHARM-DC) intervention involves intensive, tailored medication reconciliation and review, discharge planning, patient and caregiver counseling and education, and post-discharge medication management.
This large multisite pragmatic randomized trial will study the effect of the PHARM-DC on 30-day readmissions and emergency department visits, examine barriers and facilitators to implementation, and estimate the cost-effectiveness of the intervention.
Funding:
This project was supported by the National Institute on Aging of the National Institutes of Health, United States under award R01AG058911 and NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsor was not involved in the study design, collection, management, analysis, writing, or decision to publish this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsilimingras D, Schnipper J, Duke A, Agens J, Quintero S, Bellamy G, et al. Post-Discharge Adverse Events Among Urban and Rural Patients of an Urban Community Hospital: A Prospective Cohort Study. Journal of general internal medicine. 2015;30(8):1164–71. Epub 2015/03/31. doi: 10.1007/s11606-015-3260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden SK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Annals of internal medicine. 2012;157(1):1–10. doi: 10.7326/0003-4819-157-1-201207030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–7. Epub 2003/02/01. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 4.Burns JM, Sneddon I, Lovell M, McLean A, Martin BJ. Elderly patients and their medication: a post-discharge follow-up study. Age Ageing. 1992;21(3):178–81. Epub 1992/05/01. doi: 10.1093/ageing/21.3.178. [DOI] [PubMed] [Google Scholar]
- 5.Kanaan AO, Donovan JL, Duchin NP, Field TS, Tjia J, Cutrona SL, et al. Adverse drug events after hospital discharge in older adults: types, severity, and involvement of Beers Criteria Medications. J Am Geriatr Soc. 2013;61(11):1894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigenbaum P, Neuwirth E, Trowbridge L, Teplitsky S, Barnes CA, Fireman E, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599–605. Epub 2012/02/23. doi: 10.1097/MLR.0b013e318249ce72. [DOI] [PubMed] [Google Scholar]
- 7.Teymoorian SS, Dutcher D, Woods M. Association between postdischarge adverse drug reactions and 30-day hospital readmission in patients aged 80 and older. J Am Geriatr Soc. 2011;59(5):948–9. Epub 2011/05/17. doi: 10.1111/j.1532-5415.2011.03376.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet-Zamponi D, d’Arailh L, Konrat C, Delpierre S, Lieberherr D, Lemaire A, et al. Drug-related readmissions to medical units of older adults discharged from acute geriatric units: results of the Optimization of Medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2013;61(1):113–21. Epub 2012/12/21. doi: 10.1111/jgs.12037. [DOI] [PubMed] [Google Scholar]
- 9.Conn V, Taylor SG, Stineman A. Medication management by recently hospitalized older adults. Journal of community health nursing. 1992;9(1):1–11. [DOI] [PubMed] [Google Scholar]
- 10.Daliri S, Bekker CL, Buurman BM, Scholte Op Reimer WJM, van den Bemt BJF, Karapinar-Çarkit F. Barriers and facilitators with medication use during the transition from hospital to home: a qualitative study among patients. BMC Health Serv Res. 2019;19(1):204. Epub 2019/03/31. doi: 10.1186/s12913-019-4028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20(4):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James J Medicare hospital readmissions reduction program. Health affairs. 2013;34(2):1–5. [Google Scholar]
- 13.Karapinar-Çarkıt F, Borgsteede SD, Janssen MJA, Mak M, Yildirim N, Siegert CEH, et al. The effect of a pharmaceutical transitional care program on rehospitalisations in internal medicine patients: an interrupted-time-series study. BMC Health Serv Res. 2019;19(1):717. Epub 2019/10/23. doi: 10.1186/s12913-019-4617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karapinar-Carkit F, Borgsteede SD, Zoer J, Siegert C, van Tulder M, Egberts AC, et al. The effect of the COACH program (Continuity Of Appropriate pharmacotherapy, patient Counselling and information transfer in Healthcare) on readmission rates in a multicultural population of internal medicine patients. BMC Health Serv Res. 2010;10:39. Epub 2010/02/17. doi: 10.1186/1472-6963-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karapinar-Çarkıt F, van der Knaap R, Bouhannouch F, Borgsteede SD, Janssen MJA, Siegert CEH, et al. Cost-effectiveness of a transitional pharmaceutical care program for patients discharged from the hospital. PLoS One. 2017;12(4):e0174513. Epub 2017/04/27. doi: 10.1371/journal.pone.0174513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford I, Norrie J. Pragmatic trials. New England journal of medicine. 2016;375(5):454–63. [DOI] [PubMed] [Google Scholar]
- 17.Cedars-Sinai Medical Center. Reports to the Community 2019. [cited 2019 January 8, 2019]. Available from: https://www.cedars-sinai.org/about/facts-and-reports/reports.html.
- 18.Vizient I JD Healthcare I. Effect of the Affiliation of Torrance Health Association, Inc. with Cedars-Sinai Health System on the Availability and Accessibility of Healthcare Services Sacramento, Calif.: Office of the California Attorney General, 2017. October 31, 2017. Report No. [Google Scholar]
- 19.Chhibbar S, Ingram S, Fernandes O, Watt A. Best possible medication history interview guide. Toronto (ON): Institute for Safe Medication Practices Canada. 2008. [Google Scholar]
- 20.Remtulla S, Brown G, Frighetto L. Best possible medication history by a pharmacy technician at a tertiary care hospital. The Canadian journal of hospital pharmacy. 2009;62(5):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shane R, Amer K, Noh L, Luong D, Simons S. Necessity for a pathway for “high-alert” patients. American Journal of Health-System Pharmacy. 2018;75(13):993–7. doi: 10.2146/ajhp170397. [DOI] [PubMed] [Google Scholar]
- 22.Rosen OZ, Fridman R, Rosen BT, Shane R, Pevnick JM. Medication adherence as a predictor of 30-day hospital readmissions. Patient preference and adherence. 2017;11:801–10. doi: 10.2147/PPA.S125672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKibbon KA, Lokker C, Handler SM, Dolovich LR, Holbrook AM, O’Reilly D, et al. Enabling medication management through health information technology (Health IT). Evid Rep Technol Assess (Full Rep). 2011;(201):1–951. [PMC free article] [PubMed] [Google Scholar]
- 24.Bell DS, Cretin S, Marken RS, Landman AB. A conceptual framework for evaluating outpatient electronic prescribing systems based on their functional capabilities. J Am Med Inform Assoc. 2004;11(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lash DB, Mack A, Jolliff J, Plunkett J, Joson JL. Meds-to-Beds: The impact of a bedside medication delivery program on 30-day readmissions. Journal of the American College of Clinical Pharmacy. 2019;2(6):674–80. [Google Scholar]
- 26.Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–54. Epub 2011/04/22. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 27.Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an In-Hospital Multifaceted Clinical Pharmacist Intervention on the Risk of Readmission: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(3):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.College of Pharmacy PU. MI Introduction West Lafayette, IN: Purdue Pharmacy Continuing Education; 2020. [cited 2020 March 16, 2020]. Available from: https://ce.pharmacy.purdue.edu/mi/introduction. [Google Scholar]
- 29.Halfon P, Eggli Y, van Melle G, Chevalier J, Wasserfallen JB, Burnand B. Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–87. Epub 2002/06/14. doi: 10.1016/s0895-4356(01)00521-2. [DOI] [PubMed] [Google Scholar]
- 30.Nasir K, Lin Z, Bueno H, Normand S-LT, Drye EE, Keenan PS, et al. Is same-hospital readmission rate a good surrogate for all-hospital readmission rate? Medical care. 2010;48(5):477–81. [DOI] [PubMed] [Google Scholar]
- 31.Center for Health Information and Analysis. CHIA Data Boston, MA: Commonwealth of Massachusetts; 2020. Available from: http://www.chiamass.gov/chia-data/. [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. Journal of biomedical informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Medicare & Medicaid Services. Hospital Readmissions Reduction Program (HRRP) 2020. [updated 1/6/2020; cited 2020 August 7, 2020]. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.
- 35.Glasgow RF, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health. 1999;89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science. 2009;4(1):1–15. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strauss AL. Qualitative analysis for social scientists: Cambridge university press; 1987. [Google Scholar]
- 38.Corbin J, Strauss AC. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: Sage Publications, Inc.; 2008. 2008//. [Google Scholar]
- 39.Hill CE, Knox S, Thompson BJ, Williams EN, Hess SA, Ladany N. Consensual qualitative research: An update. Journal of counseling psychology. 2005;52(2):196. [Google Scholar]
- 40.Mixon AS, Smith GR, Mallouk M, Nieva HR, Kripalani S, Rennke S, et al. Design of MARQUIS2: study protocol for a mentored implementation study of an evidence-based toolkit to improve patient safety through medication reconciliation. BMC health services research. 2019;19(1):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salanitro AH, Kripalani S, Resnic J, Mueller SK, Wetterneck TB, Haynes KT, et al. Rationale and design of the multicenter medication reconciliation quality improvement study (MARQUIS). BMC health services research. 2013;13(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnipper JL, Mixon A, Stein J, Wetterneck TB, Kaboli PJ, Mueller S, et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: final results of the MARQUIS study. BMJ Quality & Safety. 2018;27(12):954–64. [DOI] [PubMed] [Google Scholar]


