Abstract
Latent-transforming growth factor beta-binding protein 2 (LTBP-2) is a major component of arterial and lung tissue and of the ciliary zonule, the system of extracellular fibers that centers and suspends the lens in the eye. LTBP-2 has been implicated previously in the development of extracellular microfibrils, although its exact role remains unclear. Here, we analyzed the three-dimensional structure of the ciliary zonule in wild type mice and used a knockout model to test the contribution of LTBP-2 to zonule structure and mechanical properties. In wild types, zonular fibers had diameters of 0.5-1.0 micrometers, with an outer layer of fibrillin-1-rich microfibrils and a core of fibrillin-2-rich microfibrils. LTBP-2 was present in both layers. The absence of LTBP-2 did not affect the number of fibers, their diameters, nor their coaxial organization. However, by two months of age, LTBP-2-depleted fibers began to rupture, and by six months, a fully penetrant ectopia lentis phenotype was present, as confirmed by in vivo imaging. To determine whether the seemingly normal fibers of young mice were compromised mechanically, we compared zonule stress/strain relationships of wild type and LTBP-2-deficient mice and developed a quasi-linear viscoelastic engineering model to analyze the resulting data. In the absence of LTBP-2, the ultimate tensile strength of the zonule was reduced by about 50%, and the viscoelastic behavior of the fibers was altered significantly. We developed a harmonic oscillator model to calculate the forces generated during saccadic eye movement. Model simulations suggested that mutant fibers are prone to failure during rapid rotation of the eyeball. Together, these data indicate that LTBP-2 is necessary for the strength and longevity of zonular fibers, but not necessarily their formation.
Keywords: Zonule, ectopia lentis, microspherophakia, saccade, LTBP-2, super-resolution microscopy, quasi-linear viscoelastic model
INTRODUCTION
Latent-transforming growth factor β-binding protein 2 (LTBP-2) is a member of the fibrillin/LTBP superfamily of matrix glycoproteins [1]. LTBP-2 is primarily a microfibril-associated protein [2] and its expression is particularly strong in tissues enriched in microfibrils, such as heart, lung, and skin [1].
Mutations in LTBP2 are associated with a range of inherited conditions, many of which negatively affect the lens or other tissues of the anterior segment of the eye [3]. LTBP2 mutations, for example, cause a recessive form of Weill-Marchesani syndrome (WMS type 3; MIM # 614819), a rare condition characterized by short stature, brachydactyly and ocular symptoms including microspherophakia (an unusually small and spherical lens) and ectopia lentis (a dislocated lens). Mutations in LTBP2 also underlie primary congenital glaucoma (GLC3D; MIM# 613086) [4, 5], a potentially blinding condition characterized by increased intraocular pressure, buphthalmos, corneal edema, and progressive glaucomatous optic atrophy. Although most recessive LTBP2 variants are associated with eye-restricted phenotypes, they can sometimes result in Marfanoid-like phenotypes (i.e., tall stature, and cardiovascular involvement, in addition to ectopia lentis and other ocular symptoms; [6]).
As their name implies, LTBPs bind latent forms of TGFβ, and through interactions with fibrillin and other matrix components, help sequester TGFβ in the extracellular matrix (ECM) and facilitate its release [7, 8]. However, unlike other LTBPs (LTBP-1, -3 and, to a lesser extent, -4), LTBP-2 does not appear to bind TGFβ [9]. Consequently, its physiological role remains obscure, although it may be required for microfibril synthesis [10] or the coalescence of microfibrils into large caliber fibers [11].
One of the challenges in characterizing the physiological roles of ECM proteins is that they are typically embedded in a dense mesh of biopolymers, which are in turn linked mechanically to resident cells in the connective tissue. The complexity associated with the cellular component can be removed through chemical or mechanical decellularization, but these approaches have the potential to alter the native structure of the ECM and its mechanical properties. There are, however, rare exceptions where elaborate ECM structures develop in a region free of cells and can be accessed easily through manual dissection. The ciliary zonule is one such example. The zonule is the system of radial fibers that constitutes the suspensory ligament of the eye lens, as shown in Fig. 1. It centers the lens on the optical axis and, in the human eye, transmits forces that flatten the lens during the process of disaccommodation [12]. The fibers that comprise the zonule are composed entirely of microfibrils [13] and span the relatively wide gap between the ciliary body and the lens equator (about 0.9 mm in the unaccommodated human eye [14] and 0.15 mm in the mouse [15]). Along with its physical accessibility, the zonule offers the further advantage that its composition is relatively well defined [16, 17].
Figure 1.
Arrangement of the lens and ciliary zonule in a one-month-old mouse eye, drawn approximately to scale. A. Organization of the anterior segment, showing the relationship between lens, zonule, and wall of the eye. B. The zonule extends from the posterior portion of the ciliary body (CB, blue) to the lens. Zonular fibers diverge as they approach the lens. The population of zonular fibers is arbitrarily divided into three groupings. Equatorial fibers (eq.) are the shortest fibers and are oriented normal to the equatorial lens surface. The anterior-most fibers (ant.) form an angle, θ1, with the eq. fibers. Likewise, the posterior-most fibers (post.) form an angle, θ2, with the eq. fibers. Total fiber divergence is θ1 + θ2. At the lens surface, the “zonular span” is the linear distance between the anchorage points of the anterior-most and posterior-most fibers (arrowheads).
In the present study, we used Ltbp2-null mice to examine the role of LTBP-2 in the formation of the zonule and its mechanical properties. To visualize zonular fibers in a near-native state, we employed a tissue preparation protocol wherein the eye globe is fixed under physiological intraocular pressure, thereby maintaining the inflation of the globe and tension in the zonular fibers [15]. The organization of zonular fibers and the distribution of key structural proteins (fibrillin-1, fibrillin-2, and LTBP-2) were visualized by immunofluorescence and super-resolution microcopy. These studies were complemented by immuno-electron microscopy, to localize LTBP-2 at the level of individual microfibrils. Finally, we employed a pull-up assay [18] that exerts a uniform stretch across all zonular fibers, to compare the biomechanical properties of the zonule in wild type and LTBP-2-deficient mice. Together, our results show that the absence of LTBP-2 does not affect the development of zonular fibers or their three dimensional architecture, but does diminish their strength and durability.
RESULTS
A. Zonular fiber formation and organization do not require LTBP-2.
To visualize the organization of the zonule in three dimensions, immunofluorescence using specific fibrillin-1 antiserum α-mFbn1-C (Supplemental Data Fig. 1) was performed. Stacks of optical sections from wild type or Ltbp2-null mice were reconstructed (See Fig. 2 for wild type results). Image stacks viewed in XY projection showed the zonule from the perspective of the ciliary body (i.e., as if looking “down” on the lens equator; Fig. 2A), while XZ projections provided the corresponding sagittal view (Fig. 2B). Individual optical sections collected at various distances above the lens surface revealed zonular fibers in transverse section (Fig. 2C, D). Fibers branched repeatedly as they approached the lens. Consequently, optical sections located further from the lens (Fig. 2C) contained fewer cross-sectional fiber profiles than images collected closer to the lens surface (Fig. 2D). The number of cross-sectioned fibers in each optical section was quantified by image analysis and used to calculate the total number of fibers present in the mouse zonule. As a consequence of fiber branching (Fig. 2F and Supplemental Data Movie 1), this value varied from <20,000 fibers near the eye wall to >30,000 near the lens. Close to the lens surface, the presence of meandering, radially-oriented channels cutting through the field of zonular fibers was noted (* in Fig. 2D,E). The channels were approximately 30 μm wide and, as shown in YZ projections (Fig. 2E), about 50 μm high. The channels provided a direct route between the aqueous (anteriorly) and vitreous humors (posteriorly). Approximately 65 such channels were present around the lens circumference in both wild type and Ltbp2-null mice. The location of the channels probably reflected the original disposition of capillaries around the lens surface during embryonic development (see also Fig. 10, below).
Figure 2.
Three-dimensional reconstruction of wild type mouse zonule at 1 month of age, visualized by fibrillin-1 immunofluorescence. A. Maximum intensity orthographic projection of the zonule showing the anterior (ant.), equatorial (eq.), and posterior (post.) fibers as they approach the lens. The posterior fibers connect to the fibrillar girdle (FG) at the lens surface. B. XZ projections show the zonule from the lateral aspect (for orientation, see Fig. 1B). C. An individual optical section from 100 μm above the equatorial lens surface (position arrowed in B). D. An optical section from immediately above the lens surface (z = 0 μm, arrowed in B). Note the presence of channels (*1-3) in the zonular fibers. The channels meander but are generally oriented to the anterior-posterior axis. E. YZ projections show the channels in transverse section. They are approximately 30 μm wide and 50 μm high. F. Volumetric projection of individual zonular fibers showing their branched organization. Scale bars = 50 μm.
Figure 10.
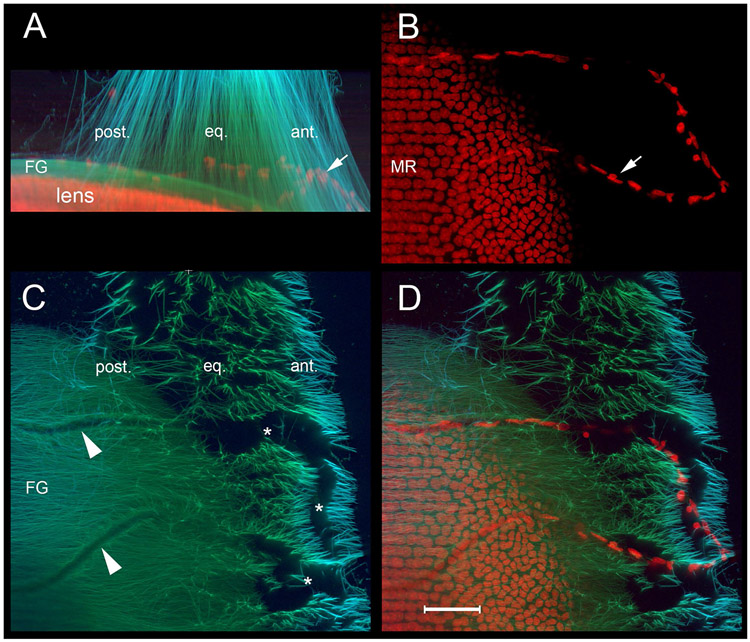
Zonular channels mark the original course of TVL capillaries at the lens surfaces. A. The zonule of a one-month-old wild type mouse is visualized using antibodies against fibrillin-1 (green) and MAGP-1 (light blue). Nuclei (red) are counterstained with a DNA probe. XZ projections show the zonular span and the presence of a capillary remnant (arrow). B. In XY projections, the nuclei of endothelial cells in the capillary (arrow) and equatorial lens cells are visible. The nuclei of differentiating lens fiber cells are aligned in meridional rows (MR). C. In XY projections, channels through the zonule are evident (*). The channels narrow posteriorly (arrowheads), forming ruts in the surface of the FG. Note that the immunofluorescence staining intensities for the two zonule proteins (fibrillin-1 and MAGP-1) are unequal; fibrillin-1 is present throughout the zonule, whereas MAGP-1 is enriched in the anterior and posterior fiber populations. D. Overlay of the fluorescent signals confirm that the capillaries lie entirely within the zonular channels. Scale bar = 50 μm.
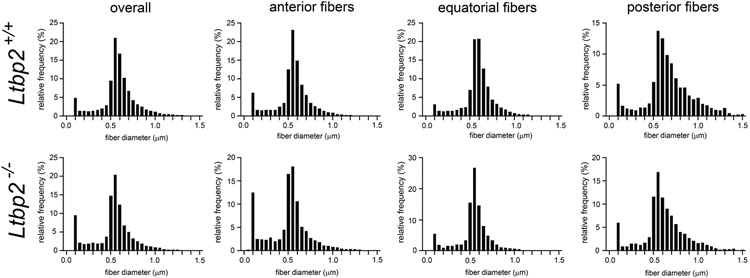
To determine whether the absence of LTBP-2 had a quantitative effect on the number or dimensions of zonular fibers, we analyzed individual optical sections (similar to that shown in Fig. 2C) of the zonule in one-month-old wild type and Ltbp-null eyes. Images were collected 50 μm above the lens surface. The total number of zonular fibers did not differ significantly between genotypes (29,596 ± 4063 (n=12) for one-month-old wild types versus 28,735 ± 3,289 (n=9) for Ltbp2-nulls). The distribution of fiber diameters for the two genotypes is shown in Fig. 3. A range of diameters (0.1 – 1.5 μm) was present in each case. The size distributions were positively skewed, particularly for the posterior fiber population, where although fiber density was reduced, a greater proportion of large diameter fibers was present. The modal value for both genotypes was 0.55-0.60 μm. Thus, the absence of LTBP-2 did not have a discernable effect on the number or thickness of the zonular fibers.
Figure 3.
Distribution of cross-sectional diameters of zonular fibers imaged 50 μm above the equatorial lens surface in 1-month-old wild type (upper panel) or Ltbp2-null mice (lower panel). The distributions are positively skewed, with a modal value in all cases of 0.55-0.60 μm. The posterior fiber distributions are the most heavily skewed, reflecting the presence of a higher proportion of thicker fibers in that region.
Zonular fiber biomechanics and longevity are altered in the absence of LTBP-2.
To test whether the absence of LTBP-2 affected the biomechanical properties of the zonule, we measured the tensile properties of the zonule for the two genotypes using a pull-up assay [18]. The measured force/displacement curves had a characteristic spike and decay pattern that was especially evident at high strain values (Fig. 4). For each displacement step, the measured force decayed (over a period of seconds) to a lower, more stable value. Such time-dependent behavior is typical of viscoelastic materials. Hence, we developed a Quasi-linear viscoelastic (QLV) model to help interpret the empirical measurements. Best fit values for the instantaneous (G0) and equilibrium stiffness (G∞) of the zonular fibers, the decay time constant (τ), and the ultimate tensile strength (σf) were obtained (see Table 1).
Figure 4.
Representative examples of empirical force measurements (black), and best fit of the QLV model for zonular force (red) and fiber failure rates (blue) for the ciliary zonule in one-month-old wild type and Ltbp2-null mice.
Table 1.
Best fit parameters to the QLV model.
| Wild type | Ltbp2−/− | p value from t-tests | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eye # | G0 (Pa) | G∞ (Pa) | τ(s) | σf (Pa) | G0 (Pa) | G∞ (Pa) | τ (s) | σf (Pa) | G0 | G∞ | τ | σf |
| 1 | 1.96E+05 | 1.27E+05 | 4.7 | 9.27E+05 | 1.80E+05 | 5.75E+04 | 12.7 | 3.07E+05 | 0.989117 | 0.020246 | 0.802269 | 0.000152 |
| 2 | 5.06E+05 | 8.84E+04 | 35.1 | 1.05E+06 | 4.94E+05 | 4.25E+04 | 26.9 | 3.64E+05 | ||||
| 3 | 3.15E+04 | 1.02E+05 | 7.6 | 8.52E+05 | 5.91E+04 | 9.23E+04 | 7.9 | 5.33E+05 | ||||
| 4 | 2.13E+05 | 1.24E+05 | 15.9 | 9.39E+05 | 2.06E+05 | 7.25E+04 | 24.4 | 4.17E+05 | ||||
| Mean | 2.37E+05 | 1.10E+05 | 15.8 | 9.43E+05 | 2.35E+05 | 6.62E+04 | 18.0 | 4.05E+05 | ||||
| SD | 1.97E+05 | 1.83E+04 | 13.7 | 8.38E+04 | 1.84E+05 | 2.13E+04 | 9.2 | 9.63E+04 | ||||
| 95%CI | 3.87E+05 | 3.58E+04 | 26.8 | 1.64E+05 | 3.62E+05 | 4.17E+04 | 17.9 | 1.89E+05 | ||||
| +95%CI | 6.24E+05 | 1.46E+05 | 42.7 | 1.11E+06 | 5.96E+05 | 1.08E+05 | 35.9 | 5.94E+05 | ||||
| −95%CI | −1.50E+05 | 7.43E+04 | −11.0 | 7.79E+05 | −1.27E+05 | 2.45E+04 | 0.0 | 2.17E+05 | ||||
The mean values for G∞ and σf differed significantly between genotypes (see Table 1). G∞ was considerably larger in wild types than Ltbp2-nulls (110 kPa vs 66 kPa, respectively) and the ultimate tensile strength (σf) of the wild type zonule was more than twice as large (943 kPa vs 405 kPa). Thus, fibers in the wild type zonule were significantly stronger and stiffer than LTBP-2-depleted fibers.
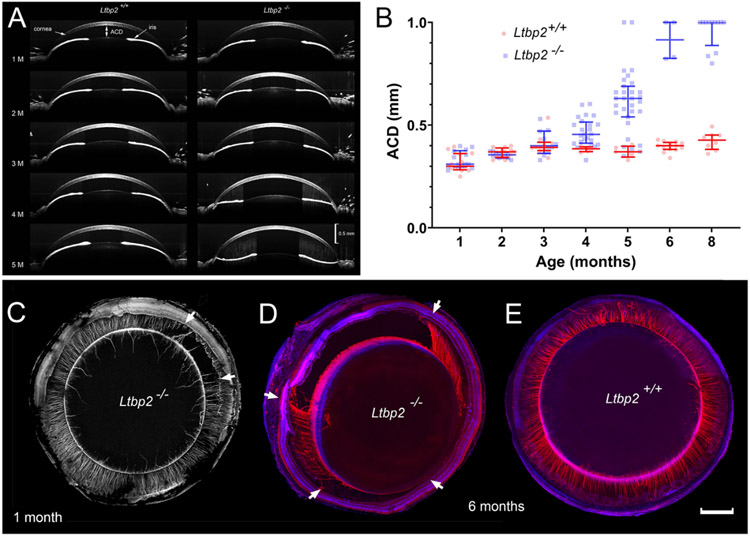
In addition to diminished tensile strength, LTBP-2-depleted fibers were less durable than wild type fibers, as demonstrated by progressive lens luxation in mutant animals. In both wild type and Lbtp2-null mice, the lens was initially centered properly in the eye, with an anterior chamber distance (ACD) of <0.5 mm (Fig. 5A, B). However, from 3 months of age onward, some of the mutant mice showed evidence of lens decentration and, by six months of age, a fully penetrant ectopia lentis phenotype was evident. At that age, ACD values exceeded 0.5 mm for all eyes, indicating that the lens had become displaced into the vitreous cavity. We assessed the integrity of the zonule at intermediate time points using confocal imaging from the posterior aspect [18]. At one month, the zonule was usually intact but in a small proportion of Lbtp2-null mice, broken fibers were present (Fig. 5C). This was some months before frank lens dislocation was apparent by optical coherence tomography (OCT). Initially, the ruptured region involved a small portion of the zonular circumference (<2 clock hours) but, with time, expanded to include most or all of the fibers (Fig. 5D). Close examination suggested that the fibers broke mid-span, rather than at their anchorage points on the surface of the lens or ciliary epithelium. In age-matched wild type mice, the zonular fibers perdured (Fig. 5E).
Figure 5.
Development of ectopia lentis in Ltbp2-null mice. A. OCT images of the anterior segment in age-matched wild type and Ltbp2-null mice. In this example, posterior lens displacement occurs at five months of age in the Ltbp2-null mouse (right hand column). Note that lens dislocation is accompanied by flattening of the iris. B. Variation of anterior chamber depth (ACD; see panel A) with age in eyes from wild type and Ltbp2-null mice as measured by OCT. Ectopia lentis is first apparent at 3 months of age and is fully penetrant by 6 months of age. C. Zonular dehiscence (area between the arrows) is observed as early as one month of age in some Ltbp2-null mice. D. By six months of age, the regions containing broken fibers have expanded significantly. E. In contrast, in six-month-old wild type mice, the zonular fibers are intact. Scale bar C-E = 500 μm.
As with human eyes, mouse eyes constantly undergo rapid involuntary motions called saccades. During a saccadic movement, eyes can accelerate to speeds of >2500°/sec [19]. We developed a harmonic oscillator model to calculate whether wild type and LTBP-2-deficient zonular fibers are likely to withstand the forces generated during such rapid excursions. For this purpose, the mechanical properties of the zonule (G0, G∞, τ, and σf) were obtained from Table 1. The number and size distribution of zonular fibers used in the model was based on the morphometric measurements shown in Figure 3. Rapid horizontal rotation of the eye is predicted to cause a lateral displacement (of the order of 10 to 20 μm) of the lens with respect to the eye wall. This results in the rapid loading and unloading of the zonular fibers, particularly those located in the nasal and temporal quadrants.
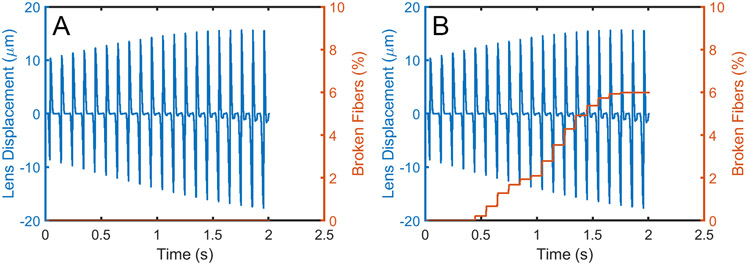
Model simulations suggested that while repetitive eye movements had little effect on zonule integrity in wild type animals (Movie 1), zonular fibers in LTBP-2 deficient mice were prone to failure during repeated cycles (shown in red in Movie 2). In the model, translation was in the horizontal plane. Fibers in the nasal and temporal quadrant were therefore subject to the greatest stress and, as a result, were most at risk of failure. In our model, even a single eye movement sometimes resulted in fiber breakage but usually multiple cycles were required. The likelihood of a fiber breaking depended significantly on the residual tension in the zonule at rest, a value that we have yet to determine experimentally. The proportion of broken fibers is expected to increase in a stepwise fashion (Figure 6) corresponding to the rapid loading and unloading associated with each eye rotation. Once a fiber breaks, the load must be borne by the remaining fibers. This implies that the rate of fiber breakage is likely to increase once the process has begun.
Figure 6.
Displacement of lens relative to the limbus for 20 cycles of simulated saccadic motion (blue) and corresponding fraction of broken zonular fibers (orange). Zonular fibers in the wild type eye (left panel) resist the forces generated by oscillatory eye motion, while fibers in the Ltbp2-null eye (right panel) begin to break.
B. EM and super-resolution light microscopy reveal that LTBP-2 may be more than a bridge between individual microfibrils.
While the precise role of LTBP-2 has yet to be established, it has previously been localized to the base of putative cross-bridges between adjacent fibrillin microfibrils [10]. Such cross-bridging structures are particularly prominent in quick-freeze, deep-etch preparations of the zonule [20]. Given that a zonular fiber is composed entirely of microfibrils, one might expect LTBP-2 to be distributed uniformly throughout the fiber. To investigate this possibility, we employed electron microscopy and super-resolution light microscopy to co-localize fibrillins and LTBP-2 in individual zonular fibers.
Ultrastructurally, zonular fibers consisted of bundles of 10-12 nm diameter microfibrils (Fig. 7A). In longitudinal sections, faint striations (50 nm periodicity, oriented perpendicular to the fiber long axis) were visible between neighboring microfibrils. Immunogold labeling of zonular fibers with anti-LTBP-2 showed strong but discontinuous labeling of microfibrils throughout the fiber (Fig. 7B). Labeled foci contained 5-10 gold particles, perhaps reflecting the polyclonal nature of the LTBP-2 antibody. In many instances, the gold particles were found in linear arrays along the microfibrils but there were also zones where the labeling was sparse or absent, implying that if LTBP-2 constitutes a bridging structure then not all microfibrils are linked by such bridges. Where individual microfibrils were discernible, labeling foci with a ≈200 nm periodicity were observed (inset Fig. 7B).
Figure 7.
Immunolocalization of fibrillin-1, fibrillin-2, and LTBP-2 in zonular fibers of one-month-old wild type and Ltbp2-null mice. A. TEM image of a wild type fiber in longitudinal section. The fiber is comprised of many microfibrils. Note the presence of faint cross-striations oriented perpendicular to the long axis of the fiber. B. Immuno-gold localization of LTBP-2 in a zonular fiber. LTBP-2 is distributed throughout the fiber. Inset. LTBP-2 labeling of individual microfibrils suggests a periodicity of about 200 nm. C,D. Transverse views of zonular fibers from wild type mice imaged using super-resolution microscopy. C. Fibrillin-2 (red) is present throughout the fiber but the staining is discontinuous and only partially overlaps with LTBP-2 (green) immunofluorescence, which is also discontinuous. D. Fibrillin-1 immunofluorescence (red) is restricted to a ≈200 nm-thick layer at the fiber surface and absent from the core. E,F. Transverse views of zonular fibers from Ltbp2-null mice. E. Fibrillin-2 immunofluorescence (red) is more homogeneous than in the wild type case (C). LTBP-2 immunofluorescence (green) is undetectable. F. As with wild type fibers, fibrillin-1 immunofluorescence (red) is restricted to the outer portion of the fiber. Scale bar inset B = 200 nm, all other panels are shown at the magnification indicated in F.
The observations made from electron micrographs were corroborated with super-resolution light microscopy, which confirmed that LTBP-2 was not uniformly distributed within a fiber (Fig. 7C,D and Supplemental Data Movies 2 and 3). LTBP-2-positive elements were present at the surface of the zonular fiber and within its core, in a variegated pattern. Fibrillin-2 fluorescence was also present throughout the fiber and, like LTBP-2, had a variegated pattern, but there was little spatial overlap between the fibrillin-2 and LTBP-2 signals. Fibrillin-1 immunofluorescence was restricted to a 200 nm-wide layer at the fiber surface. The thickness of this layer was consistent between fibers and did not scale with fiber diameter. In fibers from Ltbp2-null mice, the coaxial organization of the fibers was retained, with fibrillin-2 distributed throughout the core (Fig. 7E) and fibrillin-1 concentrated at the fiber surface (Fig. 7F). A subtle change in fibrillin-2 distribution within the fiber was noted, in that the staining pattern was more uniform in the core of Ltbp2-null fibers than in wild types.
The observed LTBP-2 localization patterns, within and around a fiber, invited questions related to where and when this protein is synthesized, and how it is incorporated into the developing fiber. To visualize Ltbp2 promoter activity, we performed X-gal staining on ocular tissue from Ltbp2-null mice (Fig.8). X-gal staining was undetectable at postnatal day 5 (P5; Fig. 8A,B) but was evident at P7 ( Fig. 8C) and strong by P21 (Fig. 8D). Expression was restricted to the posterior region of the NPCE and the inner wall of Schlemm’s canal. Schlemm’s canal forms part of the outflow pathway of the eye. Increased resistance to outflow and the resulting increase in intraocular pressure (IOP) are hallmarks of glaucoma and, in humans, LTBP2 mutations have been linked to primary congenital glaucoma [4, 5]. We therefore used tonometry to test whether the absence of LTBP-2 affected IOP in the knockout animals. We did not detect a statistically significant difference in IOP between wild type and Ltbp2-null mice (see Supplemental Data Fig. 2). Although null mutations in LTBP2 are associated with the development of microspherophakia in humans, no such growth deficit was noted in the Ltbp2-null mice (Supplemental Data Table 1)
Figure 8.
LacZ expression in the eyes of Ltbp2-null mice. A, B. At postnatal day 5 (P5), LacZ expression is undetectable. C. At P7, weak LacZ expression (blue) is present in the non-pigmented ciliary epithelium (NPCE) and connective tissue of the eye wall. By P21, strong LacZ expression is evident in NPCE cells and the inner wall of Schlemm’s canal (SC). IE, iris epithelium, PCE, pigmented ciliary epithelium. Scale bar A = 250 μm, B-D =25 μm.
At the protein level, LTBP-2 was not detected until near the end of the first postnatal week, when microfibrils at the surface of the NPCE first became positively labeled (Fig. 9). Initial labeling was restricted to circumferentially-oriented fibers and the proximal portions of radial fibers, adjacent to the ciliary epithelial surface (Fig. 9A). By one-month-of-age, however, LTBP-2 immunofluorescence was detected throughout the zonule, strongly labeling the entire length of the fibers, including those in the fibrillar girdle at the lens surface (Fig. 9B).
Figure 9.
Incorporation of LTBP-2 (green) into the nascent zonule. A. At P7, microfibrils (red, visualized with MAGP1/MFAP2 immunofluorescence) are associated with vessels of the tunica vasculosa lentis (TVL; arrowheads) and intervening regions of lens capsule. LTBP-2 is incorporated initially into circumferential fibers at the surface of the ciliary body (CB) and the proximal portions of radial fibers that connect the ciliary body to the lens equator. Nuclei (gray) are stained with DAPI, highlighting the meridional rows (MR) of lens epithelial cells underlying the zonular attachments to the lens capsule. B. By one month of age, LTBP-2 is present throughout the zonule, including the fibrillar girdle at the lens surface (FG). Scale bar = 50 μm.
C. Channels through the zonule matrix mark the original location of embryonic blood vessels.
Three dimensional reconstruction of the zonule in adult mice revealed the presence of radial channels through the zonule matrix (See Fig. 2D,E). During embryological development, the mammalian lens is temporarily nourished by a network of capillaries known as the tunica vasculosa lentis (TVL) [21]. The blood vessels of the mouse TVL regress over the first few weeks of postnatal life [22] but, occasionally, vessel remnants are visible at one month of age (Fig. 10). In such instances, three-dimensional reconstructions confirmed that the persistent blood vessels follow the course of the channels. Together with images of microfibrils at the lens surface at P7 (Fig. 9A), these observations suggest that the channels represent the silhouettes of TVL vessels that usually regress during postnatal development.
DISCUSSION
Coaxial organization of fibers
In conventional transmission electron micrographs, zonular fibers appear as bundles of apparently uniform microfibrils. The only indication that the contents of a fiber may be spatially organized is the presence of faint transverse striations, observed here and in previous studies [23, 24], that suggest a degree of lateral registration between neighboring microfibrils. In the present study, utilizing super-resolution imaging, we found that three major structural components (LTBP-2, fibrillin-1, and fibrillin-2) were compartmentalized within a fiber. Fibrillin-1 was restricted to the outer layer (approximately 200 nm thick) of the zonular fibers. In contrast, LTBP-2 and fibrillin-2 were present throughout the fiber but showed non-overlapping distributions. Of note, fibrillin-2 was the only fibrillin in the fiber core. Thus, zonular fibers have an underlying coaxial organization with regard to fibrillin expression. In skin, ligament, and perichondrium, the two fibrillin isoforms are present within individual microfibrils [25] demonstrating that, at least in those tissues, microfibrils are heteropolymers. This may also be the case in the outer layer of zonular fibers were both fibrillins coexist. However, homopolymers of fibrillin-2 presumably comprise the core of the fibers, as fibrillin-2 is the only fibrillin present in that region.
The coaxial distribution of fibrillins may reflect the temporal sequence of gene expression. Fbn2 is strongly expressed early in eye development [24, 26] but expression in the eye wanes in adulthood [18, 26]. Conversely, Fbn1 expression is relatively low in the embryonic eye but increases postnatally, peaking at about one month of age. The coaxial structure of mature zonular fibers implies a laminar growth pattern in which newly synthesized components are added to the surface of extant fibers. In such a model, the core of the fiber is the oldest part. If this model is correct, it suggests that LTBP-2 incorporation occurs after fibrillin polymers are laid down because LTBP-2, which is not synthesized until the end of the first postnatal week, is present in the fiber core.
In situ hybridization experiments have detected Fbn1 mRNA in the ciliary epithelium of adult mice [18] and guinea pigs [27] but there is some uncertainty as to whether microfibril synthesis continues in the adult zonule. In that regard, it will be of interest to determine whether the thickness of the fibrillin-1 rich layer (≈200 nm at one-month-of age) increases with age. In other tissues, fibrillin-2 immuno-reactivity is masked in postnatal tissues but can be revealed by enzymatic treatments that remove fibrillin-1 [28]. If coaxial organization is a general feature of microfibril bundles, it could provide an explanation for the unmasking phenomenon.
Ectopia lentis and microspherophakia phenotypes in Ltbp2-null mice and human WMS3 patients.
In humans, mutations in LTBP2 cause syndromes that affect the eye. Ectopia lentis and microspherophakia are features of WMS3, for example. In human patients, ocular symptoms most often develop postnatally [29]. Ectopia lentis typically manifests in the second decade of life [30]. In this respect, the phenotype of Ltbp2-null mice mirrored the human condition; zonular dehiscence was first detected at one month of age and a fully penetrant ectopia lentis phenotype was present by six months of age. The present findings also confirm an earlier report of ectopia lentis in an independently generated Ltbp2-null strain [10].
Microspherophakia is commonly observed in human WMS patients but was not observed in the Ltbp2-null mice. We note that lens growth is driven by cell division in the germinative zone (GZ) of the equatorial lens epithelium [31] and that the GZ is located immediately beneath the attachment points of the zonule. Further, in vitro studies suggest that lens epithelial cell growth is modulated by forces induced in the lens capsule [32]. In view of this, it is perhaps surprising that lenses from Ltbp-2 null mice did not show a growth deficit. One explanation for this discrepancy may be that during accommodation in humans, the forces applied to the lens surface via the zonule are relatively large, effectively flattening the lens (and thereby bringing distant objects into focus). Forces of this magnitude may be transduced by GZ cells into a proliferative response. Mice, in contrast, do not accommodate and the forces necessary to merely center and stabilize the murine lens may be significantly smaller than those generated during human accommodation.
Effect of LTBP-2 depletion on rheological properties of the zonule
Previous studies have suggested that LTBP-2 is necessary for microfibril production [10] or the tension-induced coalescence of individual microfibrils into larger caliber fibers [11]. Quantitative analyses of the fiber population in one-month-old LTBP-2 knockout mice did not support either contention. We found that the absence of LTBP-2 did not affect significantly the total number of fibers produced nor their size distributions. There was, however, a subtle reorganization of fibrillin-2 within the core of individual fibers. It remains to be determined whether this contributed to the measured loss of tensile strength in LTBP-2 depleted zonular fibers.
Tensile strength
In the absence of LTBP-2, the ultimate tensile strength of the zonule (σf) was reduced by about 50%. At later time points (>2 months of age) the fibers ruptured, leading to ectopia lentis. Lens dislocation was probably a direct consequence of diminished tensile strength of the zonule. Mouse eyes undergo rapid involuntary eye motions that closely resemble the saccadic movements of the human eye [19]. We modeled the forces generated during such movements and conclude that while wild type fibers are likely to withstand such forces, the weakened fibers of the mutant zonule may not. Thus, failure under saccadic motion provides a plausible explanation for the eventual rupture of the mouse (and by implication, human) zonule under conditions where the fibers are weakened. This does not preclude non-mechanical mechanisms, such as fiber proteolysis, however. Fibrillin microfibrils are substrates for matrix metalloproteinases [33] which have been detected in ocular fluids [34] and fibrillin-2 has recently been shown to serve as a substrate for ADAMTS10 (the gene implicated in Weill Marchesani Syndrome Type 1) [35, 36]. If mutant fibers were more susceptible to proteolysis than wild type fibers, this might contribute to eventual mechanical failure. Whatever the initiating event, confocal images of the mutant zonule at 1-2 months of age indicate that failure of fibers occurs in an arc segment (rather than through breakage of dispersed individual fibers) and modeling suggests that once fibers have begun to break the process is expected to accelerate.
Material Stiffness
We found that the force generated in the mouse zonule in response to stepwise displacement of the lens decayed with time. Thus, the zonular fibers did not act as simple springs but rather as viscoelastic elements. Viscoelasticity is a property common to many biopolymers. In the case of zonular fibers, the structural basis of viscoelastic behavior remains to be determined. It is not clear, at present, whether viscoelasticity reflects the deformation of individual microfibrils or the collective behavior of microfibril bundles within the coaxial fiber. Of note, the equilibrium stiffness value, G∞, was significantly reduced in the absence of LTBP-2.
Precisely how LTBP-2 functions to stiffen and strengthen zonular fibers is not known. Recent studies have shown that ectopic expression of LTBP-4 can prevent fragmentation of zonular fibers in Ltbp2−/− mice, suggesting that whatever the mechanism of microfibril stabilization it may not be unique to LTBP-2 [37]. In addition to fibrillins and LTBP-2, the zonule proteome contains a number of putative structural elements, such as MFAP-2, EMILIN-1, ADAMTSL-6, at relatively high abundance [16]. To better understand the collective behavior of the microfibril bundles, it will be important to map these components in relation to the coaxial fiber structure.
Values for the elastic modulus of the human zonule range from 342-248 kPa (for young Vs old eyes [38]), 1500 kPa [39], and 350 kPa [40]. The modulus of the porcine zonule is 200-250 kPa [41]. In the bovine eye, the elastic modulus was found to decay over a period of 20 seconds, from about 250 kPa to about 180 kPa [42], exhibiting similar viscoelastic properties to those reported here for the mouse zonule. Thus, the biomechanical properties of the zonule seem to be broadly similar, regardless of whether or not the species in question is capable of accommodation.
Although the ultimate tensile strength of the zonule was reduced in LTBP-2-deficient mice, this was not attributable to a decrease in the number of zonular fibers, nor a reduction in the fiber diameter (neither parameter differed significantly between Ltbp2-null and wild type animals). A distinction can be drawn, therefore, between the present data and previous reports on the properties of fibrillin-1-depleted zonules [18]. Conditional targeting of the Fbn1 gene in the mouse ciliary epithelium led to loss of fiber tensile strength and ectopia lentis. In that case, however, both the total number of fibers and their diameters were decreased substantially in the absence of fibrillin-1.
Zonular channels
We used pressurized fixation to preserve the three-dimensional arrangement of the mouse zonule. A previously unrecognized anatomical feature revealed through this approach was a series of meandering channels (60 – 70 per eye), which cut through the zonular matrix, providing a direct connection between the aqueous and vitreous humors. The channels marked the original course of blood vessels that envelop the embryonic lens but which regress in the course of postnatal development. We hypothesize that the presence of vessels at the lens surface may physically block zonular fibers from attaching to the underlying lens capsule. If this is the case, it implies that zonule formation is substantially completed during the period in which vessels are present. In mice, the TVL consists of 60-70 vessels until postnatal day 6, after which the vasculature regresses [22]. This suggests that no new fibers are erected across the circumlental space after the first few postnatal weeks. The functional significance, if any, of zonule channels is unknown. The zonule has a relatively open structure and is not considered to represent a substantial barrier to diffusion between the anterior and posterior segments of the eye. Nevertheless, channels through the fibers will constitute a low resistance path that will tend to direct the flow of aqueous humor, the watery fluid produced by the ciliary epithelium that sustains the lens and other tissues of the anterior segment.
MATERIALS AND METHODS
Animals
ES cells containing a targeted Ltbp2 allele (Ltbp2tm1e(KOMP)Wtsi) were obtained from the KOMP repository, injected into blastocysts and implanted into pseudopregnant females (C57BL/6J) using standard procedures. Age-matched wild type mice on the same genetic background were used for comparative analysis. The animal studies committee at Washington University School of Medicine approved all procedures.
Optical Coherence Tomography (OCT)
The position of the lens was assessed in anesthetized animals by OCT imaging (Biotogen Envisu R2210), as described [18], using a 10 mm tele-centric bore lens. Anterior chamber depth (ACD; distance from the corneal endothelium to the anterior surface of the lens) was measured using software supplied with the instrument.
Intraocular Pressure Measurements
Intraocular pressure (IOP) was measured with a rebound tonometer (iCare Tonolab tonometer, Vantaa, Finland). The tonometer was fixed to a stage and anesthetized mice were positioned such that the center of the probe was aligned with the center of the cornea. Three or more measurements were performed on each eye.
Eye and lens size and shape
The axial and equatorial dimensions of freshly dissected globes were measured from digital photographs taken using a dissecting microscope. Lenses were dissected from the eye and placed in warm tissue culture medium in a spectrophotometer cuvette. A profile view of the lens was obtained by photographing its reflection in a 45° glass prism. Images were taken with a vertical beam path, to avoid parallax errors. Preliminary measurements indicated that lenses from male mice were generally larger than from age-matched females, so all size measurements were made on animals of a single gender (male), 1-6 months of age.
Confocal imaging
The three-dimensional arrangement of the zonule in one-month-old wild type or LTBP-2-deficient animals was visualized and quantified, as described [15, 18]. Briefly, mice were killed by CO2 inhalation. Isolated globes were fixed overnight in 4% paraformaldehyde/PBS at an intraocular pressure of 15 mmHg. Fixation under pressure preserved the circumlental space (gap between the lens equator and the ciliary processes), ensuring that zonular fibers were imaged under tension. The globe was infiltrated with molten 5% low-melting-point agarose/PBS. After allowing the gel to set, 270 μm-thick sagittal slices were prepared using a tissue sectioner (Leica VT1000S). Tissue slices were incubated with appropriate primary and secondary antibodies, counterstained with a DNA probe (NucBlue, ThermoFisher), mounted in anti-fade medium (Vectashield, Vector Laboratories) and imaged by confocal microscopy (FV-1000, Olympus). The number of zonular fibers and their size distribution was determined from individual optical sections collected 50 μm above the equatorial lens surface. Local area thresholding (Bernsen algorithm) was used to segment images, which were then analyzed using the particle analysis routine in Image J [43].
To visualize the substructure of individual fibers, agarose-embedded tissue was incubated with appropriate antibodies and imaged using a Zeiss 800 LSM confocal microscope (63x/1.40 NA PlanApocromat Obj.) equipped with an Airyscan detector.
Antibodies
Polyclonal chicken antibodies were raised against recombinant mouse LTBP-2 [10]. Goat anti-mouse MAGP-1/MFAP2 was obtained commercially (R & D systems; Cat # AF4977). Rabbit antibodies were raised against the C-termini of recombinant mouse fibrillin-1 and fibrillin-2, as follows. Recombinant mouse fibrillin-1 (mrFbn1-C) and -2 (mrFbn2-C) C-terminal halves (amino acid position 1281-2727) were purified from HEK293 cell conditioned media and used to raise the polyclonal antisera α-mFbn1-C and α-mFbn2-C in rabbits (Supplemental Data Fig. 1). ELISA showed high titers of the antisera. Indirect immunofluorescence using mouse NIH3T3 fibroblasts, and immunohistochemistry analyses using aortae from 6-month-old mice confirmed the specificity of the antisera and excluded cross-reactivity of the antisera between fibrillin-1 and -2. However, ELISA analyses with other relevant proteins demonstrated that α-mFbn1-C (but not α-mFbn2C) cross-reacted with fibronectin (Supplemental Data Fig. 1F). Therefore, α-mFbn1-C was passed over a fibronectin-coupled sepharose column (10mg of fibronectin/mL of sepharose), which resulted in complete loss of cross-reactivity against fibronectin (Supplemental Data Fig. 1G). This adsorbed α-mFbn1-C antiserum was used for all experiments. The specificities of LTBP-2, fibrillin-1, and fibrillin-2 antibodies were also verified on ocular tissue from appropriate knockout animals.
Electron microscopy (EM) and Immuno-EM.
Samples were fixed in 4% paraformaldehyde/PBS, using a pressurized fixation method [15] and non-specific antibody binding minimized by preincubation in 10% BSA/PBS. For immuno-EM, hemisected globes were incubated overnight in 1:100 dilution of LTBP-2 antibody. After washing, tissue was incubated in colloidal gold (6 nm) donkey anti-chicken antibody (1:30 dilution; Jackson Immunoresearch Labs) for 3 hours and then fixed for 30 minutes with 2.5% glutaraldehyde. Samples were infiltrated with molten 5% agarose and sectioned using a tissue slicer. Agarose sections were post-fixed in 1% osmium tetroxide and stained en bloc with 1% aqueous uranyl acetate. Tissue was then dehydrated through graded alcohols and embedded in resin (Eponate 12, Ted Pella, Inc.). Sections (90 nm thick) were stained with uranyl acetate and lead citrate and viewed on a transmission electron microscope (JEOL 1200EX) equipped with an 8-megapixel digital camera.
LacZ histochemistry
X-Gal staining (Genlantis, San Diego, CA) was used to visualize LacZ expression in eyes from Ltbp2-null and age-matched wild type mice.
Biomechanical measurements
The tensile properties of the fixed zonule were measured with a pull-up device, as described [18]. Briefly, the corneal surface of an isolated globe was attached to the base of a chamber positioned on the weighing pan of a sensitive balance. The back of the eye was removed, exposing the posterior lens surface. A probe was fixed to the lens surface and the chamber was filled with PBS. The probe was raised in 50 μm steps and the accompanying weight reduction recorded continuously. Typically, at each displacement value, there was an instantaneous change in measured force, followed by slow and partial relaxation of the zonule towards a new, equilibrium force value. Four independent biological replicates were performed for each genotype.
Analysis of the pull-up assay
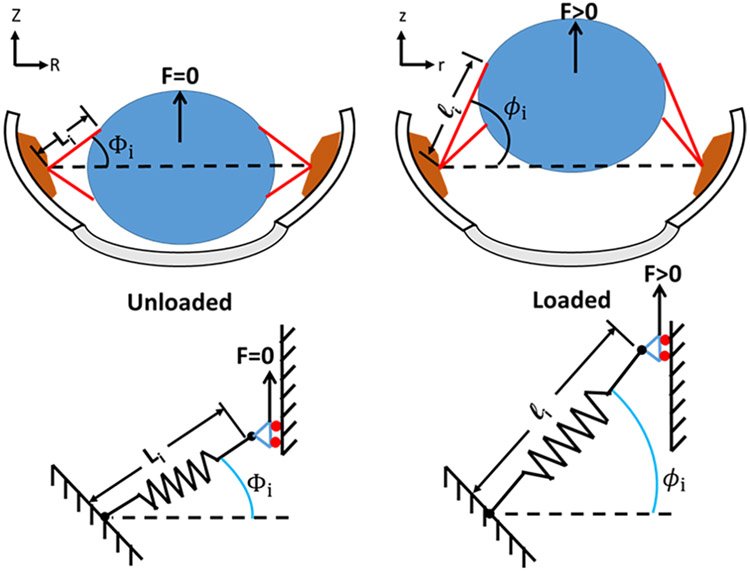
The kinematic relationship between the experimentally observed parameters (time, displacement, and force) and the loading on a given zonular fiber depends on the anatomy and geometry of the test (Fig. 11). The unloaded geometry was specified on the basis of population-based averages for span, maximum anterior/posterior angles, equatorial radius, and total number of zonular fibers; empirical distributions of fiber cross-sectional area and number of fibers in the anterior/equatorial/posterior groupings; and the initial angular orientation of fibers within a given grouping were assumed to be uniformly distributed.
Figure 11.
Schematic representation of the pull-up assay, showing geometric relationships between lens (blue), ciliary body (brown) and zonular fibers (red) in the unloaded (left) and loaded (right) states. In this assay, the eye is positioned “cornea down” and a lifting force (F) is applied to the back of the lens (blue). Li and li = zonular fiber length; Z,z = position on z-axis; R,r = radius.
The axial stretch ratio of a given zonular fiber λi can be computed from the unloaded geometry (φi,0, Li,0) and applied axial displacement u as
| (1) |
The axial Cauchy stress within a given fiber σi under a state of uniaxial tension and assuming incompressibility, is given by
| (2) |
where Fi is the tensile force applied to the ith fiber having an unloaded radius ai,0. Rearranging and summing over N fibers gives the total upward Fz,total force applied to the lens as
| (3) |
Thus, the total vertical force could be estimated on the basis of the unloaded geometry, the test parameters (time, displacement), and the stress functional σi[λi(t)]. The latter can have an arbitrary form depending on the selection of an appropriate constitutive model.
A quasi-linear viscoelastic (QLV) model was used to derive the ultimate tensile strength (σf), initial stiffness (G0), equilibrium stiffness (G∞), and relaxation time constant (τ) for each zonular fiber from force displacement data obtained from the pull-up assay [44, 45]. The hyperelastic contribution was assumed to be accurately described by an incompressible, isotropic neo-Hookean (NH) model, while the stress history component was assumed to follow a one-term Prony series. This model was modified to account for a lack of non-tensile stiffness in the zonule by setting the instantaneous elastic stresses equal to zero when λi ≤ 1. A tensile failure mode was also considered, by setting the total stress of each fiber to zero for all time points after its total stress exceeded the failure stress σf. Thus, the modified QLV stress function σi[λi(t)] was given by
| (4) |
where tf is the earliest time at which σi(t)≥σf. Note that evaluating σi[λi(t)] at any time point requires numerical integration of the stress history.
Parameter Estimation Algorithm
A typical one-month-old mouse lens is attached to the wall of the eye by 25,000-30,000 zonular fibers. Solving the full series of equations for each fiber at each time point for the duration of the pull-up test required about one hour of processing time on a standard workstation. Since estimation of material properties required thousands of iterations, and owing to the fact that the calculations were highly redundant (due to the presence of many fibers with similar geometries), we elected to bin the fibers based on their unloaded angle relative to horizontal ϕ0 and cross-sectional area A0.
Computation time increases linearly with the number of bins selected for ϕ0, but an insufficient number will render the modeling predictions inaccurate, since all zonular fibers within a given bin break simultaneously. These considerations were balanced by binning zonular fibers into 100 equally spaced bins, based on their respective ϕ0 values. Each of these 100 bins were further subdivided into 20 bins corresponding to equal increments in unloaded cross-sectional area A0 of the zonular fibers. Thus, the stress history was computed for each time step for each ϕ0 bin (Eqn. 4), then those results were used to compute the total lifting force given by Eqn. 3.
An initial parameter vector p0 = [G, G∞, τ, σf] was selected arbitrarily. The Nelder-Mead simplex method [46] was then used to estimate the optimal parameter values by minimizing the relative error metric er, defined in terms of the model-predicted force history Fmodel(t) and measured force Fdata(t):
| (5) |
Failure of zonular fibers under saccadic eye movement
A harmonic oscillator model of the eye-lens-zonule complex was developed as shown in Fig. 12. The limbus and lens were considered rigid and the lens free-floating, other than for its interaction with the zonular fibers. The initial geometry and constitutive model for each zonular fiber was specified as the neo-Hookean QLV model described above. Genotype-averaged material properties were assigned (Table 1).
Figure 12.
Schematic of the harmonic oscillator model of rapid eye movement.
Electrooculographic data from a C57BL/6 mouse rapid eye movement (REM) were extracted from Fig. 3A of Sakatani and Isa [19]. While a unidirectional motion was reported, it was assumed that the return motion was symmetric, yielding the limbus velocity history shown (Fig. 13). This was numerically integrated to produce the displacement waveform applied to the limbus u(t).
Figure 13.

Linear velocity of the limbus used to simulate a single rapid eye movement event. Angular velocity data were taken from Sakatani and Isa [19]. Eye sizes were measured elsewhere in this study (see Supplemental Data Table 1).
A numerical scheme was implemented to solve for the displacement of the lens at each time step such that Newton’s second law was satisfied. Briefly, a bounded minimization algorithm was used to estimate the instantaneous displacement ulens. A numerical approach was used to calculate the resulting acceleration of the lens alens in the x direction. The objective function f(ulens) to be minimized was then the squared error in satisfying Newton’s second law, or
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by NIH R01 EY024607 (SB), P30 EY002687 (SB), HL53325 and HL74138 (RPM), Natural Sciences and Engineering Research Council of Canada (RGPIN-2016-06278; DPR), Australian NHMRC project grant 519211 (MAG, RPM), the Marfan Foundation, the Ines Mandl Research Foundation, and an unrestricted grant to the Department of Ophthalmology & Visual Sciences at Washington University from Research to Prevent Blindness. The authors gratefully acknowledge Dr. Tomo Nakamura for kindly providing LTBP-2 antibodies and Nguyen Hong Phuc Pham for important contributions in generating the anti-mouse fibrillin antisera.
Footnotes
Declarations of interest: None
REFERENCES
- [1].Shipley JM, Mecham RP, Maus E, Bonadio J, Rosenbloom J, McCarthy RT, Baumann ML, Frankfater C, Segade F, Shapiro SD, Developmental expression of latent transforming growth factor beta binding protein 2 and its requirement early in mouse development, Mol Cell Biol 20(13) (2000) 4879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gibson MA, Hatzinikolas G, Davis EC, Baker E, Sutherland GR, Mecham RP, Bovine latent transforming growth factor beta 1-binding protein 2: molecular cloning, identification of tissue isoforms, and immunolocalization to elastin-associated microfibrils, Mol Cell Biol 15(12) (1995) 6932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rifkin DB, Rifkin WJ, Zilberberg L, LTBPs in biology and medicine: LTBP diseases, Matrix Biol 71-72 (2018) 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF, Null mutations in LTBP2 cause primary congenital glaucoma, Am J Hum Genet 84(5) (2009) 664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Narooie-Nejad M, Paylakhi SH, Shojaee S, Fazlali Z, Rezaei Kanavi M, Nilforushan N, Yazdani S, Babrzadeh F, Suri F, Ronaghi M, Elahi E, Paisan-Ruiz C, Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma, Hum Mol Genet 18(20) (2009) 3969–77. [DOI] [PubMed] [Google Scholar]
- [6].Morlino S, Alesi V, Cali F, Lepri FR, Secinaro A, Grammatico P, Novelli A, Drago F, Castori M, Baban A, LTBP2-related "Marfan-like" phenotype in two Roma/Gypsy subjects with the LTBP2 homozygous p.R299X variant, Am J Med Genet A 179(1) (2019) 104–112. [DOI] [PubMed] [Google Scholar]
- [7].Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB, Latent TGF-beta-binding proteins, Matrix Biol 47 (2015) 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yanagisawa H, Wagenseil J, Elastic fibers and biomechanics of the aorta: Insights from mouse studies, Matrix Biol 85-86 (2020) 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saharinen J, Keski-Oja J, Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta, Mol Biol Cell 11(8) (2000) 2691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Inoue T, Ohbayashi T, Fujikawa Y, Yoshida H, Akama TO, Noda K, Horiguchi M, Kameyama K, Hata Y, Takahashi K, Kusumoto K, Nakamura T, Latent TGF-beta binding protein-2 is essential for the development of ciliary zonule microfibrils, Hum Mol Genet 23(21) (2014) 5672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsuruga E, Oka K, Hatakeyama Y, Isokawa K, Sawa Y, Latent transforming growth factor-beta binding protein 2 negatively regulates coalescence of oxytalan fibers induced by stretching stress, Connect Tissue Res 53(6) (2012) 521–7. [DOI] [PubMed] [Google Scholar]
- [12].Bassnett S, Zinn's zonule, Prog Retin Eye Res (2020) 100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Godwin ARF, Singh M, Lockhart-Cairns MP, Alanazi YF, Cain SA, Baldock C, The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly, Matrix Biol 84 (2019) 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khan A, Pope JM, Verkicharla PK, Suheimat M, Atchison DA, Change in human lens dimensions, lens refractive index distribution and ciliary body ring diameter with accommodation, Biomed Opt Express 9(3) (2018) 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bassnett S, A method for preserving and visualizing the three-dimensional structure of the mouse zonule, Exp Eye Res 185 (2019) 107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Maria A, Wilmarth PA, David LL, Bassnett S, Proteomic Analysis of the Bovine and Human Ciliary Zonule, Invest Ophthalmol Vis Sci 58(1) (2017) 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eckersley A, Mellody KT, Pilkington S, Griffiths CEM, Watson REB, O'Cualain R, Baldock C, Knight D, Sherratt MJ, Structural and compositional diversity of fibrillin microfibrils in human tissues, J Biol Chem 293(14) (2018) 5117–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jones W, Rodriguez J, Bassnett S, Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome, Dis Model Mech 12(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sakatani T, Isa T, Quantitative analysis of spontaneous saccade-like rapid eye movements in C57BL/6 mice, Neurosci Res 58(3) (2007) 324–31. [DOI] [PubMed] [Google Scholar]
- [20].Davis EC, Roth RA, Heuser JE, Mecham RP, Ultrastructural properties of ciliary zonule microfibrils, J Struct Biol 139(2) (2002) 65–75. [DOI] [PubMed] [Google Scholar]
- [21].Lutty GA, McLeod DS, Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye, Prog Retin Eye Res 62 (2018) 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ito M, Yoshioka M, Regression of the hyaloid vessels and pupillary membrane of the mouse, Anat Embryol (Berl) 200(4) (1999) 403–11. [DOI] [PubMed] [Google Scholar]
- [23].Raviola G, The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils, Invest Ophthalmol 10(11) (1971) 851–69. [PubMed] [Google Scholar]
- [24].Beene LC, Wang LW, Hubmacher D, Keene DR, Reinhardt DP, Annis DS, Mosher DF, Mecham RP, Traboulsi EI, Apte SS, Nonselective assembly of fibrillin 1 and fibrillin 2 in the rodent ocular zonule and in cultured cells: implications for Marfan syndrome, Invest Ophthalmol Vis Sci 54(13) (2013) 8337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Charbonneau NL, Dzamba BJ, Ono RN, Keene DR, Corson GM, Reinhardt DP, Sakai LY, Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences, J Biol Chem 278(4) (2003) 2740–9. [DOI] [PubMed] [Google Scholar]
- [26].Shi Y, Tu Y, De Maria A, Mecham RP, Bassnett S, Development, composition, and structural arrangements of the ciliary zonule of the mouse, Invest Ophthalmol Vis Sci 54(4) (2013) 2504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hanssen E, Franc S, Garrone R, Synthesis and structural organization of zonular fibers during development and aging, Matrix Biol 20(2) (2001) 77–85. [DOI] [PubMed] [Google Scholar]
- [28].Charbonneau NL, Jordan CD, Keene DR, Lee-Arteaga S, Dietz HC, Rifkin DB, Ramirez F, Sakai LY, Microfibril structure masks fibrillin-2 in postnatal tissues, J Biol Chem 285(26) (2010) 20242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McGavic JS, Weill-Marchesani syndrome. Brachymorphism and ectopia lentis, Am J Ophthalmol 62(5) (1966) 820–3. [DOI] [PubMed] [Google Scholar]
- [30].Chu BS, Weill-Marchesani syndrome and secondary glaucoma associated with ectopia lentis, Clin Exp Optom 89(2) (2006) 95–9. [DOI] [PubMed] [Google Scholar]
- [31].Bassnett S, Sikic H, The lens growth process, Prog Retin Eye Res 60 (2017) 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kumar B, Chandler HL, Plageman T, Reilly MA, Lens Stretching Modulates Lens Epithelial Cell Proliferation via YAP Regulation, Invest Ophthalmol Vis Sci 60(12) (2019) 3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM, Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling, Biochem J 340 (Pt 1) (1999) 171–81. [PMC free article] [PubMed] [Google Scholar]
- [34].Brown D, Hamdi H, Bahri S, Kenney MC, Characterization of an endogenous metalloproteinase in human vitreous, Curr Eye Res 13(9) (1994) 639–47. [DOI] [PubMed] [Google Scholar]
- [35].Wang LW, Kutz WE, Mead TJ, Beene LC, Singh S, Jenkins MW, Reinhardt DP, Apte SS, Adamts10 inactivation in mice leads to persistence of ocular microfibrils subsequent to reduced fibrillin-2 cleavage, Matrix Biol 77 (2019) 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mularczyk EJ, Singh M, Godwin ARF, Galli F, Humphreys N, Adamson AD, Mironov A, Cain SA, Sengle G, Boot-Handford RP, Cossu G, Kielty CM, Baldock C, ADAMTS10-mediated tissue disruption in Weill-Marchesani syndrome, Hum Mol Genet 27(21) (2018) 3675–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fujikawa Y, Yoshida H, Inoue T, Ohbayashi T, Noda K, von Melchner H, Iwasaka T, Shiojima I, Akama TO, Nakamura T, Latent TGF-beta binding protein 2 and 4 have essential overlapping functions in microfibril development, Sci Rep 7 (2017) 43714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Michael R, Mikielewicz M, Gordillo C, Montenegro GA, Pinilla Cortes L, Barraquer RI, Elastic properties of human lens zonules as a function of age in presbyopes, Invest Ophthalmol Vis Sci 53(10) (2012) 6109–14. [DOI] [PubMed] [Google Scholar]
- [39].van Alphen GW, Graebel WP, Elasticity of tissues involved in accommodation, Vision Res 31(7-8) (1991) 1417–38. [DOI] [PubMed] [Google Scholar]
- [40].Fisher RF, The ciliary body in accommodation, Trans Ophthalmol Soc U K 105 (Pt 2) (1986) 208–19. [PubMed] [Google Scholar]
- [41].Bocskai ZI, Sandor GL, Kiss Z, Bojtar I, Nagy ZZ, Evaluation of the mechanical behaviour and estimation of the elastic properties of porcine zonular fibres, J Biomech 47(13) (2014) 3264–71. [DOI] [PubMed] [Google Scholar]
- [42].Wright DM, Duance VC, Wess TJ, Kielty CM, Purslow PP, The supramolecular organisation of fibrillin-rich microfibrils determines the mechanical properties of bovine zonular filaments, J Exp Biol 202(Pt 21) (1999) 3011–20. [DOI] [PubMed] [Google Scholar]
- [43].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Fiji: an open-source platform for biological-image analysis, Nat Methods 9(7) (2012) 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fung YC, Elasticity of soft tissues in simple elongation, Am J Physiol 213(6) (1967) 1532–44. [DOI] [PubMed] [Google Scholar]
- [45].De Pascalis R, Abrahams ID, Parnell WJ, On nonlinear viscoelastic deformations: a reappraisal of Fung's quasi-linear viscoelastic model, Proc Math Phys Eng Sci 470(2166) (2014) 20140058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lagarias JC, Reeds JA, Wright MH, Wright PE, Convergence properties of the Nelder-Mead simplex method in low dimensions, Siam J Optimiz 9(1) (1998) 112–147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.