Abstract
One of the key features of addiction is the escalated drug intake. The neural mechanisms involved in the transition to addiction remain to be elucidated. Since abnormal neuronal activity within the subthalamic nucleus (STN) stands as potential general neuromarker common to impulse control spectrum deficits, as observed in obsessive–compulsive disorders, the present study recorded and manipulated STN neuronal activity during the initial transition to addiction (i.e., escalation) and post-abstinence relapse (i.e., re-escalation) in rats with extended drug access. We found that low-frequency (theta and beta bands) neuronal oscillations in the STN increase with escalation of cocaine intake and that either lesion or high-frequency stimulation prevents the escalation of cocaine intake. STN–HFS also reduces re-escalation after prolonged, but not short, protracted abstinence, suggesting that STN–HFS is an effective prevention for relapse when baseline rates of self-administration have been re-established. Thus, STN dysfunctions may represent an underlying mechanism for cocaine addiction and therefore a promising target for the treatment of addiction.
Introduction
The subthalamic nucleus (STN) is a small nucleus within the basal ganglia that has been historically associated with basic motor functions. Abnormal neuronal activity within the STN, mostly increased beta oscillations (12–30 Hz), marks and drives motor symptoms of Parkinson’s disease (PD) [1]. Although their exact origins remain largely unknown, these abnormal beta oscillations may be a secondary consequence of the PD-associated degeneration of midbrain dopamine (DA) neurons. Importantly, neutralizing beta activity by blocking STN activity with high-frequency stimulation (HFS) is a current effective treatment for PD [2, 3].
Over the past 15–20 years, the STN has progressively been shown also to be involved in non-motor functions, such as impulse control, encoding of reward, and motivational regulation [4–11]. As a result, abnormal STN activity has been reinterpreted as a general neuromarker common to impulse control spectrum deficits, as observed in obsessive–compulsive disorders (OCD) [6]. Since addiction is a form of impulse control spectrum disorder, the present study aimed first at identifying these possible abnormal activities within the STN during the transition to addiction. Previous research has shown that extended, as opposed to limited, access to drugs of abuse causes rats to develop several core addiction-like behavioral symptoms, including escalated drug intake and increased drug motivation despite the negative consequences [12, 13].
Since STN inactivation reduces the initial motivation to self-administer cocaine in individuals that have limited access to the drug, while simultaneously increasing the motivation for sweet food [14, 15], targeting STN has been proposed as potential therapeutic strategy for cocaine addiction [16]. Whether and to what extent STN inactivation can affect the transition to more compulsive cocaine self-administration remains to be demonstrated. Here, we thus tested the effects of STN lesions or HFS during both the initial transition to addiction (i.e., escalation of cocaine use) and re-escalation after protracted abstinence.
Materials and methods
Animals
Adult Lister Hooded males (~380 g, Charles River) were housed in groups of two in Plexiglas cages and maintained on an inverted 12 h light/dark cycle (light onset at 7 pm) with food and water available ad libidum, in a temperature-and humidity-controlled environment. Animal care and use conformed to the French regulation (Decree 2010–118) and were approved by local ethic committee and the University of Aix-Marseille under #03129.01.
Surgery
As previously described [14, 15], animals were anesthetized with ketamine (Imalgen, Merial, 100 mg/kg, s.c.) and medetominine (Domitor, Janssen, 30 mg/kg, s.c.) to first implant homemade silicone catheter in the right jugular vein and were then placed in a Kopf stereotaxic apparatus for either lesion or electrode implantation surgery. Animals received a bilateral microinjection of ibotenic acid (9.4 μg/μl; 0.5 μl injected; n = 33) or vehicle solution (PB; 0.1 M, n = 27) in the STN (AP: −3.7 mm; L: ±2.4 mm from bregma; DV: −8.35 mm from skull) [17]. Ninety-seven other animals were implanted with electrodes at these coordinates. Electrodes and four anchoring screws (one on the right frontal lobe designated as the electric reference allowing LFP recordings in some animals) were secured with dental cement.
After surgery, all rats were awakened with an injection of atipamezol (Antisedan, Janssen, 0.15 mg/kg, i.m.). They were allowed to recover for 10 days. Catheters were daily checked for blood reflux and flushed with heparin (Sanofi, 3 g/l) and enroflorilexine (Baytril, Bayer, 8 g/l) in 0.9% saline to prevent blood clots and infection during the recovery period and until the end of experiments. Catheters were also regularly tested with propofol (Propovet, Abbott, 10 mg/ml) to confirm their patency.
Self-administration paradigms
The classical self-administration chambers used are described in Supplementary materials. Each session started with the illumination of the house light and the delivery of a free infusion (250 μg/90 μl) of hydrochloride cocaine (Coopérative pharmaceutique française, dissolved in 0.9% NaCl). A continuous fixed-ratio 1 schedule of reinforcement (FR1) was used: a single nose poke in the active hole (randomized assignment) resulted in a 5-s illumination of the associated cue-light and cocaine delivery, that initiated a time-out period of 20 s during which nose pokes in the active hole were counted as perseverative responses. Alternatively, nose poke in the inactive hole had no programmed consequence. After acquisition of a stable baseline of cocaine consumption over the 2 h-short access (ShA) sessions, they were given daily 6 h-long access (LgA) to cocaine for 15 or 20 days.
One group of animals (n = 13 STN lesion and 11 sham) was tested during this procedure for preventive effect of the lesion on escalation. In parallel, another group (n = 12 ON and 12 OFF) was tested with STN–HFS.
An additional group of animals was only tested with STN–HFS (n = 10 ON and 8 OFF) applied during 15 ShA sessions to test the specificity of STN–HFS effects on escalation process.
In another experiment (n = 16), STN lesion (n = 8) was performed after rats had escalated their cocaine intake. They were then tested for ten LgA sessions after a post-surgical recovery period of 10 days.
In parallel, in a group of animals that had escalated their drug intake during 15 sessions of LgA (n = 16), STN–HFS was applied immediately from the 16th session for 15 consecutive sessions (n = 10) or remained OFF (n = 6).
Finally, in a last experiment, STN–HFS was tested on re-escalation in animals that had escalated their drug intake. After the 15 LgA session establishing escalation, a 35 days period of abstinence was observed before the animals were re-exposed to the drug for 10 LgA sessions under STN–HFS (ON, n = 8) or not (OFF, n = 8).
Recording and analysis of LFP activity
Bilateral bipolar platinum electrodes were used for LFP recordings and HFS (see Supplementary methods for details).
Animals (n = 8) were connected to the recording interface and placed in the operant chamber. LFPs were recorded for ten sessions of the ShA and for ten sessions (days 2, 3, 4, 6, 7, 8, 9, 11, 12, and 13) over the LgA. STN electric activity was recorded for 15 min before any access to cocaine and then during the first 30 min of the self-administration session. After the recording, the animals were immediately transferred to another operant chamber to complete their self-administration session.
In a separate experiment (n = 9), LFPs were recorded for 15 min before and after a single 6 h STN–HFS session (equivalent to an LgA session) with no access to cocaine. Additional recordings (15 min) were also performed 2 and 4 days after this HFS session.
Signals were amplified and filtered using a Neuralinx8 amplifier. Data were acquired using Sciworks software (Datawave Tech, USA) with a sampling rate of 1 kHz in the range of 1–475 Hz. Signals were filtered off-line with a Chebyshev low-pass filter (corner 98 Hz, order 10, ripple 0.5) and a notch filter was applied, to remove 50 Hz noise created by surrounding electrical devices, using Spike2 software (CED). Data were then carefully examined to ensure removal of electrical noise and ultimately treated using Matlab (Mathworks) software. As such, the analysis was limited to the following frequency bands: 5–45 Hz and 65–95 Hz. The specific activity of the STN, i.e., the difference of potential between the two wires within the same STN, was then calculated.
Correlational analysis
Relationships between behavioral performances during ShA or LgA (i.e., number of cocaine injections) and STN oscillatory power in the theta, beta, and gamma bands were assessed by plotting for each individual STN LFP powers and the number of cocaine injections taken either the day before each recording session (n = 9 for ShA and LgA) for the baseline recordings (i.e., 15 min before cocaine intake) or during the first 30 min of the self-administration sessions (n = 10 for ShA and LgA) for recordings during cocaine consumption. Power data used in these analyses were derived from all ten recording sessions during ShA and for ten sessions of LgA on days 2, 3, 4, 6, 7, 8, 9, 11, 12, and 13.
High-frequency deep brain stimulation
HFS was delivered to the STN by a digital stimulator (DS8000, WPI) via a stimulus isolator (DLS100, WPI) and a rotating commutator (Plastics One) wired to the implanted electrodes (see supplementary methods). Stimulation parameters were adapted from previous studies [15, 18]. Briefly, stimulation parameters were set with frequency at 130 Hz and 80 μs pulse width. Stimulation intensity (50–150 μA) was individually adjusted below the threshold of induction of hyperkinetic movements. STN–HFS was turned ON just prior the start of each behavioral session and applied during the entire session.
Histology
At the end of the experiment, the rats were killed with pentobarbital (Dolethal; i.p.). Brains were removed and frozen into liquid isopentane (−80 °C) to be further cut in 50-μm-thick frontal slices with a cryostat in a blinded manner. Histological controls, for extend of the lesions and position of the electrodes, were performed after staining with cresyl violet (Supplementary Figure S1). Out of 33 animals tested for STN lesions, ten were discarded from final analysis for missed lesion (no shrinkage and gliosis reaction within the STN). Eleven animals were excluded from the LFP or STN–HFS experiments for mislocation of the electrodes. The final n values after histological verification and exclusion of some animals due to catheter patency issue (n = 18) or head cap loss (n = 3) are indicated in the figure legends.
Statistical analyses
No statistical methods were used to predetermine sample size, but the samples are comparable to those reported in previous studies [14, 15, 19, 20]. However, power analysis (two groups, two tails, and equal variance model) was performed to confirm that our sample sizes were sufficient to detect reliable changes in escalation (power ≥80% at a level of confidence P < 0.05). Group assignment was mostly done in a random fashion, except for re-escalation procedure (Fig. 5), where rats were assigned to treatment group in a counterbalanced manner based on their first escalation level. Data are expressed as mean ± SEM with the sample size indicated for each group. Analyses were performed with Prism (GraphPad) and Matlab (Mathworks) softwares. Only P values <0.05 were considered significant. Most data were analyzed with two-tailed t-test or one- or two-way ANOVA with group (sham or STN lesion; HFS ON or OFF) as a between factor and sessions, and/or blocks of sessions, or time bins (LFP and locomotor activity experiments) as repeated measures, followed by Bonferroni post hoc test or Student’s t test. Correlations between LFPs power and the number of cocaine injections were statistically validated with ANCOVA controlling for inter-individual differences, since rats were treated as a covariate.
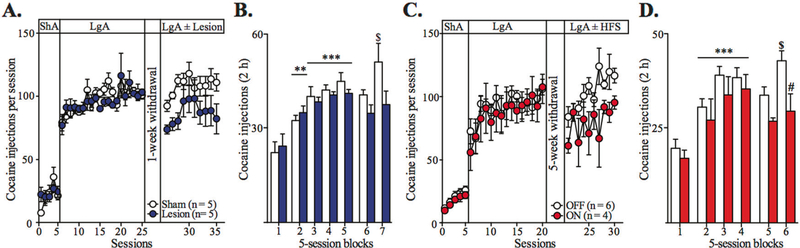
Fig. 5.
STN manipulations have therapeutic effects after cocaine escalation. a Summary time graph presenting the effect of STN lesion, performed after escalation, on the total cocaine intake during ten LgA sessions following the surgery recovery period (10-day abstinence). Both groups (sham group: n = 5, white dots; STN lesion group: n = 5, blue dots) display comparable cocaine intake during escalation (session effect F19,152 = 4.084, P < 0.0001, session × group interaction F19,152 = 0.8126, n.s., mixed two-way ANOVA). Although not significant, STN lesion tends to reduce cocaine re-escalation observed in sham control animals after abstinence (session effect: F9,72 = 3.083, P < 0.01; group effect: F1,72 = 4.762, P = 0.06, mixed two-way ANOVA). b Summary bar graph showing the cocaine intake during the first 2 h of each session averaged per blocks of five ShA (block 1), four blocks of five pre-surgical LgA (blocks 2–5) and two blocks of five post-surgical re-escalation LgA (blocks 6–7) sessions. Both groups escalate their cocaine intake before surgery (block effect F4,32 = 24.27, P < 0.0001; group effect F1,32 = 0.07691, n.s., mixed two-way ANOVA). Again, STN lesion tends to reduce cocaine re-escalation after abstinence (block effect: F1,8 = 6.564, P < 0.05, group effect: F1,8 = 3.756, P = 0.08, mixed two-way ANOVA). c Summary time graph showing the effect of STN–HFS on the total cocaine intake during ten LgA sessions following a 35-day period of protracted abstinence (OFF group: n = 6, white dots; ON group: n = 4, red dots). While both groups display comparable intake during escalation (session effect: F14,112 = 2.861, P = 0.01; group effect: F1,112 = 0.1888, n. s., mixed two-way ANOVA), STN–HFS reduces the re-escalation of cocaine escalation (session effect: F9,72 = 2,546, P < 0.05, group effect: F1,72 = 10.01, P < 0.05, mixed two-way ANOVA). d Summary bar graph, averaging the first 2 h of each session per blocks of five ShA (Block 1), three LgA of pre-HFS LgA (Blocks 2–4) and two blocks of five HFS LgA sessions, shows that STN DBS also reduces the cocaine re-escalation (block effect: F1,8 = 7.086, P < 0.05, group effect: F1,8 = 9.186, P < 0.05, mixed two-way ANOVA). All data are means with SEM. **P < 0.01, ***P < 0.001 vs. ShA (block 1); $P < 0.05 vs. first block of re-escalation, #P < 0.05 vs. OFF group (Bonferroni post hoc test)
Results
Oscillations within the STN during the process of escalation
To monitor STN oscillations during the development of cocaine escalation, rats implanted with bipolar HFS electrodes were trained to self-administer cocaine under an FR1 schedule of reinforcement with daily ShA to cocaine for 10 sessions and then for 15 LgA sessions (Fig. 1a). As expected, escalation in the drug intake was observed during LgA sessions (i.e., significant increased number of cocaine injections during the first 30 min spent in the electrophysiological recording chamber) (Fig. 1b).
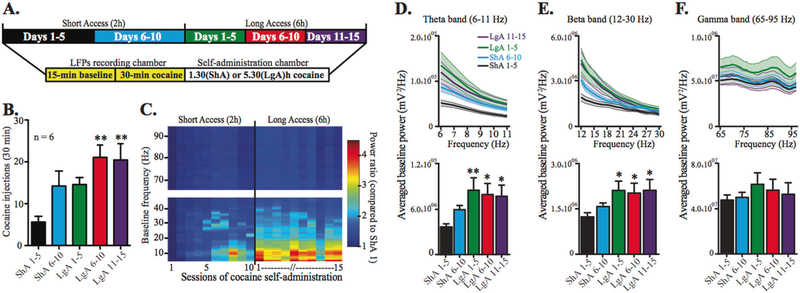
Fig. 1.
Progressive development of low-frequency oscillations within the STN during baseline recording, i.e., in absence of cocaine (15 min before access to cocaine) parallels escalation of cocaine intake. a Schematic of the experiment. b Increase in cocaine intake during the first 30 min of self-administration averaged by blocks of five daily sessions over ShA (black bar: days 1–5, blue bar: days 6–10) and LgA conditions (green bar: days 1–5, red bar: days 6–10, purple bar: days 11–15; block effect: F4,20 = 6.952, P = 0.011, n = 6, repeated measure one-way ANOVA). c Daily time-frequency analysis of the power spectrum, equivalent to a measurement of the signal amplitude. Values indicate the ratio of power spectrum normalized to the first day of ShA. d–f Highlights (upper panels, bold lines indicate average power values, light-colored areas indicate SEM; LgA 6–10 data have been omitted for clarity of presentation) and global quantifications of baseline power (bottom panels) for the theta (d), beta (e), and gamma (f) bands. Cocaine escalation induces an increase in oscillation power during LgA sessions only in the theta and beta bands (d F4,20 = 4.308, P < 0.05; e F4,20 = 4.633, P < 0.01; f F4,20 = 1.917, n.s., repeated measures one-way ANOVA). All bars are mean with SEM. *P < 0.05, **P < 0.01 vs. ShA 1–5 (Bonferroni post hoc test)
Baseline activity (before access to cocaine): STN low-frequency oscillations as possible neuromarkers of reduced control over cocaine use?
LFPs recordings started 15 min before the self-administration sessions, allowing measure of the baseline activity in absence of cocaine (Fig. 1a). There were few variations in STN baseline oscillations recorded before the first ShA sessions, when the animals were naive to cocaine. However, over the course of the experiment, the oscillatory activity significantly increased within the STN during the LgA phase to reach a plateau, in parallel with the increased cocaine self-administration (escalation). Indeed, in absence of cocaine, during the baseline recording, the oscillations in low frequencies increased progressively as a function of the sessions (Supplementary Figure S2), and this increase was even more striking in comparison to the first day of ShA, when the rats had no experience with cocaine (Fig. 1c). Of note, variations in STN oscillatory activities were only observed in low-frequency oscillations. Indeed, specific band analysis revealed a significant progressive power increase in both theta (6–11 Hz) and beta (specifically in its lowest part, 12–20 Hz) bands during the self-administration protocol (Fig. 1d, e), while the gamma band (60–95 Hz) remained stable (Fig. 1f).
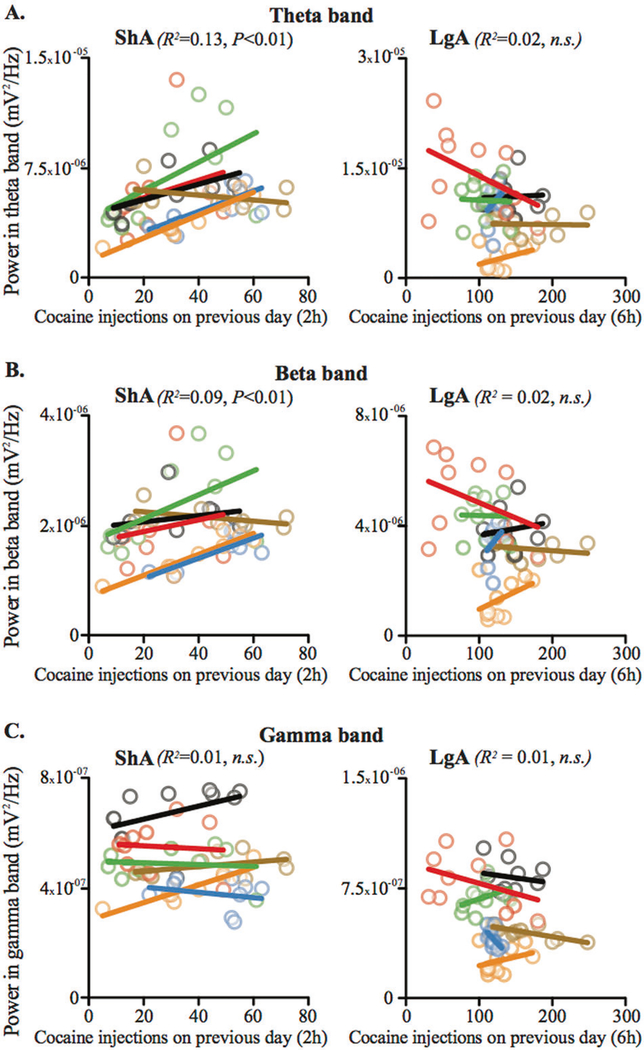
A significant correlation was observed between baseline activity and the number of cocaine injections taken on the previous day during ShA sessions for theta and beta oscillations for most animals. This correlation disappeared during the LgA sessions, when the amount of cocaine injected was very high (Fig. 2a, b). No correlation was found for the gamma oscillations neither in ShA nor in LgA phases (Fig. 2c).
Fig. 2.
During baseline recording, theta and beta oscillations in the STN show a correlation with the number of cocaine injections taken the day before during ShA, but not LgA sessions. Diagrams showing the correlations (bold lines) between STN oscillation power of the theta (a), beta (b), and gamma bands (c) and the amount of cocaine taken on the previous day during the baseline recordings preceding ShA (left panels) and LgA sessions (right panels) for each animal (light shaded-color circles). A significant correlation was found for the theta and beta, but not for the gamma, bands for ShA (a F1,42 = 9.2917, P < 0.01; b F1,42 = 7.55, P < 0.01; c F1,42 = 2.14, n.s.; ANCOVA controlled for inter-individual differences). In contrast, no correlation was found during the baseline sessions preceding LgA sessions (a F1,48 = 2.34, n.s.; b F1,48 = 1.09, n.s.; c F1,48 = 2.78, n.s.)
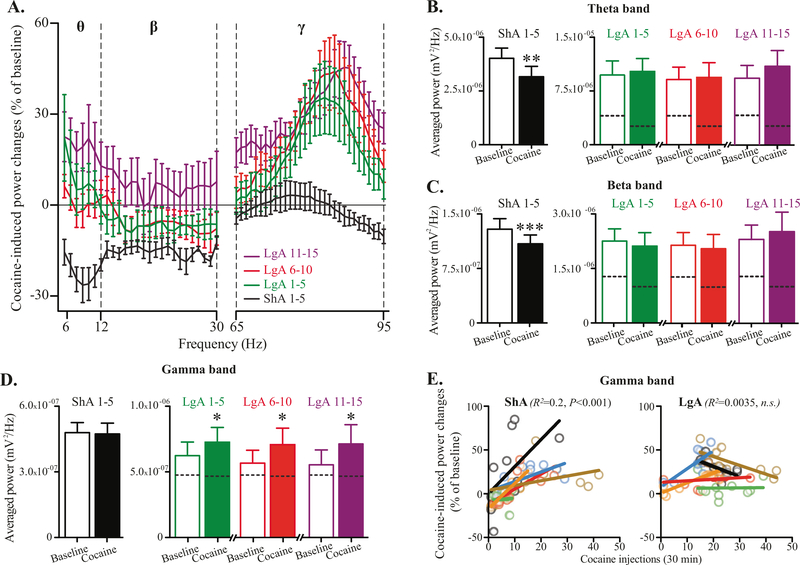
Oscillatory activity during cocaine access: loss of cocaine influence on STN theta and beta activity, but gamma activity as a possible neuromarker of cocaine intake
During the first 30 min of the ShA sessions, cocaine injections reduced the activity in the low-frequency bands (theta and beta) compared to baseline, with no effect on gamma band (Fig. 3a–d). However, during the first 30 min of the LgA sessions, following an increased baseline activity for theta and beta bands, the cocaine injections failed to reduce oscillatory activity (Fig. 3b, c). In contrast, while the baseline activity in the gamma band remained stable between ShA and LgA sessions, the access to cocaine induced a significant increase of the activity (Fig. 3a, d). Here, the increase in oscillatory activity in the gamma band was correlated with the number of cocaine injections taken during these 30 min of recording for most rats in ShA but not in LgA (Fig. 3e). In contrast, no correlation was found for theta and beta activities (Supplementary Figure S3). This result positions gamma oscillatory activity within STN as a discriminative signature of controlled cocaine consumption.
Fig. 3.
Cocaine induces STN theta and beta oscillations decrease when compared to baseline in ShA, but not LgA sessions, while STN gamma oscillations appear as a marker of cocaine intake. a Graph showing the variations in STN oscillation powers induced by cocaine consumption, compared to baseline recordings, during the first 30 min of ShA (days 1–5, black line) and LgA sessions (days 1–5, green line; days 6–10, red line; days 11–15 purple line). When expressed as percentage of the baseline, cocaine induced a decreases in LFPs power spectrum for the low (theta and beta bands) frequencies during ShA that disappeared during LgA sessions, where high-frequency oscillations increased in the gamma band. b–d Bar graphs showing the averaged STN oscillation powers during baseline (open bars) and cocaine recordings (filled bars) for ShA (days 1–5, black bars) and LgA sessions (days 1–5, green bars; days 6–10, red bars; days 11–15 purple bars) in theta (b), beta (c), and gamma (d) bands. (ShA 1–5: b t5 = 5.138, P < 0.01; c t5 = 7.213, P < 0.001; d t5 = 0.396, n.s.; LgA 1–5: b t5 = 0.9415, n.s.; c t5 = 1.416, n.s.; d t5 = 2.873, P < 0.05; LgA 6–10: b t5 = 0.5672, n.s.; c t5 = 0.9004, n.s.; d t5 = 3.607, P < 0.05; LgA 11–15: b t5 = 2.256, P = 0.0738, n.s.; c t5 = 0.9542, P = 0.3838, n.s.; d t5 = 3.847, P = 0.012, paired t-test). Dotted lines in the LgA bars indicate the level reached during ShA sessions. e Diagrams showing the correlations (bold lines) between STN oscillation power of the gamma band and the amount of cocaine taken during the first 30 min of ShA (left panel) and LgA sessions (right panel) for each animal (light shaded-color circles). A significant correlation was found during ShA (F1,48 = 18.39, P < 0.001; ANCOVA controlled for inter-individual differences) that disappeared during the LgA session (F1,46 = 1.96, n.s.). All data are means with SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline, paired t-test
STN inactivation prevents cocaine intake escalation
STN lesions prevent cocaine intake escalation
Sham and STN lesion rats subjected to the escalation procedure took the same amount of cocaine during ShA sessions (Fig. 4a). In contrast, different patterns of intake were revealed when the animals were subjected to LgA sessions. As expected, sham control animals exhibited an escalation in their cocaine intake under extended access, showing a progressive increase in the total number of cocaine injections taken per 6 h session. In contrast, the STN lesion rats exhibited a lower intake than sham rats (Fig. 4a).
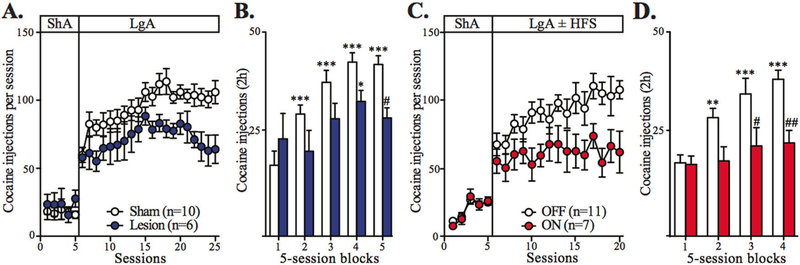
Fig. 4.
STN manipulations have preventive effects on cocaine escalation. a Summary time graph presenting the effect of STN lesion on the total cocaine intake throughout the 5 last ShA and 20 LgA sessions. While STN lesion has no effect on ShA, it reduces the development of cocaine escalation (ShA: group effect: F1,56 = 0.5683, n.s., LgA: session effect: F19,266 = 5.231, P < 0.0001; group effect, F1,266 = 8.939, P < 0.01, mixed two-way ANOVA; sham group, n = 10, white dots; STN lesion group, n = 6, blue dots). b Summary bar graph showing that STN lesion also reduces the cocaine intake during the first 2 h of each session averaged per blocks of five ShA (block 1) and LgA (blocks 2–5) sessions (block × group interaction: F4,56 = 4.329, P < 0.01, mixed two-way ANOVA). c Summary time graph showing the effect of STN–HFS applied during 15 LgA sessions on the total cocaine intake (OFF group: n = 11, white dots; ON group: n = 7, red dots). While both groups exhibit comparable intake during ShA, STN–HFS blocks the development of cocaine escalation (ShA: group effect: F1,64 = 0.01819, n.s.; LgA: session effect: F14,224 = 1.863, P < 0.05; group effect, F1,224 = 13.51, P < 0.01, mixed two-way ANOVA). d Summary bar graph of averaged cocaine intake during the first 2 h of each session per blocks of five ShA (block 1) and LgA (blocks 2–4) also shows that STN–HFS also blocks cocaine escalation development (block × group interaction: F3,48 = 3.081, P < 0.05, mixed two-way ANOVA). All data are means with SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. ShA (block 1); #P < 0.05, ##P < 0.01 vs. sham group or OFF group (Bonferroni post hoc test)
For a better comparison of the cocaine intake during ShA and LgA, the first 2 h of the LgA sessions were analyzed and pooled in blocks of five consecutive sessions and compared to the number of injections taken during the ShA sessions. Consistent with previous studies [12], the sham control animals progressively increased their drug intake during the first 2 h of the LgA sessions and reached a level much higher than that observed during the ShA sessions (Fig. 4b). STN lesion had a preventive effect on escalation since STN lesion rats took less cocaine than the controls during these first 2 h of the LgA sessions with no escalation in their intake (Fig. 4b). It is important to note that this reduced cocaine intake was not observed in animals only subjected to repeated ShA sessions, but was seen in a progressive ratio procedure [14] and was not related to a possible reduced motor activity in animals with STN lesions, since the lesion has no effect on spontaneous or drug-induced locomotor activity (Supplementary Figure S4A–C).
STN–HFS prevents escalation in cocaine intake
To test the effects of STN–HFS on escalation, rats were divided into two groups: the control group remaining OFF stimulation and the group ON stimulation. The two groups were subjected to 15 LgA sessions. As expected, the OFF group exhibited an escalation of cocaine intake under extended access to the drug. Conversely, the ON group did not (Fig. 4c). STN–HFS significantly decreased the amount of cocaine consumed during the entire LgA sessions when compared with the controls (Fig. 4c), as well as during the first 2 h of the LgA sessions (Fig. 4d). This effect of STN–HFS was specific to escalation processes since it was not seen in animals subjected to 15 consecutive 2 h ShA sessions (Supplementary Figure S5).
STN–HFS not only prevented escalation, but also reduced the number of perseverative responses (nose pokes in the active hole during the time-out following an injection) in the ON group, compared to the increase in the OFF group (Supplementary Figure S6). These responses can be either interpreted as an index of compulsivity or motivation for cocaine. Similar to what was observed in the lesion experiment, STN–HFS did not affect spontaneous or cocaine-induced locomotor activity (Supplementary Figure S4D–F).
Effects of STN–HFS on STN oscillatory activity
Given that oscillations developed during escalation that is prevented by STN lesions and HFS, we tested whether or not STN–HFS could directly affect oscillatory activity. The results show that STN–HFS applied at 130 Hz immediately drastically diminishes the general oscillatory activity (Supplementary figure S7A) in an indiscriminate manner between bands (Supplementary figure S7B–D) and this effect recovers progressively over time.
Possible therapeutic effect of STN inactivation on cocaine intake after escalation?
To assess whether or not STN lesions could reduce cocaine intake even after a history of escalated intake, they were performed after rats had gone through the escalation procedure. As shown in Fig. 5a, while sham animals re-escalated their overall intake during the 6 h sessions, STN lesion rats did not re-escalate to the same extent. There was a trend toward significant group effect during re-escalation. Similar effects were observed during the first 2 h of the LgA sessions (Fig. 5b).
In the parallel experiment using STN–HFS, no significant effect of STN–HFS was observed when stimulation was activated immediately after escalation (Supplementary Figure S8).
Therapeutic effect of STN–HFS on re-escalation after protracted abstinence
Inactivation of STN immediately after escalation (Supplementary Figure S8) or after a 10-day period of abstinence (Fig. 5a, b) did not result in significant reduction of cocaine intake. We questioned whether or not a longer period of abstinence could allow possible therapeutic effect of STN manipulation. As shown in Fig. 5c, after an abstinence of 35 days, the OFF group exhibited re-escalation, unlike the ON group, indicating that STN–HFS prevented re-escalation of the number of cocaine injections taken overall. Similar effects were observed when analyzing the first 2 h (Fig. 5d).
Discussion
Escalation of drug intake is a hallmark of the transition from use to addiction. In animals, it is associated with decreased reward threshold, increased motivation to work for the drug and resistance to punishment, which are partly mediated by negative reinforcement [12, 21–24]. We show here that STN low-frequency (theta and beta) oscillations, measured before daily cocaine access, increase during the escalation process, while gamma oscillations increase during cocaine consumption. A 130 Hz STN–HFS reduces STN oscillatory activity on the one hand and prevents escalation of cocaine intake on the other hand, suggesting that increased STN oscillatory activity represents an important process in mediating escalation of drug intake. Finally, when applied after a period of protracted abstinence, STN–HFS can then reduce re-escalation of cocaine intake. These results suggest that STN inactivation prevents compulsive-like responding for cocaine possibly through reduction of oscillations in the STN and can restore a controlled consumption even after a history of drug intake escalation.
How can extended access to cocaine lead to oscillatory activity within the STN?
During the controlled consumption phase (ShA), basal low-frequency oscillations (theta and beta) correlate with the amount of cocaine taken the day before. Since the drug intake remained stable when rats were maintained in ShA for 15 days, it is highly probable that the LFPs would not change during this period for these animals according to the correlation observed between STN LFPs and cocaine intake. In contrast, these correlations disappear during the escalation phase (LgA) while low oscillation powers keep increasing, suggesting they are not a direct consequence of the increased drug intake. Consequently, this pathological increase may thus represent a biomarker of loss of control over drug intake.
Beta oscillations in particular have been associated with a DA depletion, as they are found in PD patients and animal models [1, 25, 26]. Interestingly, these oscillations are only present in STN after a chronic DA depletion, but not an acute DA blockade [26], suggesting their development required a long-term plasticity process. This could imply that repeated LgA exposures to cocaine may trigger a long-lasting process mimicking a DA depletion when recorded for baseline after an 18 h period of abstinence, a time-point during which reward efficacy is decreased [27]. In line with previous reports showing that DA replacement therapy reduces PD-abnormal STN oscillations [1], we show here that cocaine consumption reduces STN beta and theta oscillations.
In contrast, access to cocaine increases STN gamma oscillations, in line with their proposed involvement in some aspects of addiction and motor behaviors related to DA activation [28–31]. As for basal low frequencies, gamma band power correlates with the amount of cocaine taken in the ShA phase, but no longer during the LgA phase. This loss of correlation can be either due to a ceiling effect resulting from high amount of cocaine intake or may be interpreted as another biomarker of loss of control over drug intake, independent on the amount of drug consumed.
What are the consequences in the brain and on behavior of STN oscillations?
Although they can be recorded in various sensorimotor regions of the basal ganglia structures, STN LFP oscillations correlate with cholinergic striatal interneurons as well as basal ganglia downstream structures (SNr, GPe, and GPi), but not with medium spiny striatal neurons [31, 32]. Beta activity induced by DA depletion is both higher in the STN than in its surroundings and correlates with increased single neuron or neuronal assembly activity in STN [26, 32, 33]. We thus hypothesize here that the progressive increased beta power observed during escalation process reflects an increased neuronal activity within the STN during baseline, which should result in a general reduction of motor activity. However, our results indicate that animals are not motorically impaired to a level that could prevent them from self-administering the drug since they are able to escalate their intake. In fact, beta oscillations and motor behavior do not always correlate, as acute DA antagonists seriously affect motor behavior without inducing any pathological beta activity in the STN, as in the case of catalepsy [34].
If one considers that STN activity increases in absence of cocaine in the escalated phase, this should lead to increased activity in the NAc, via the direct projections from STN to striatum. In contrast, NAc activity is progressively reduced during the development of escalation and also during re-escalation after protracted abstinence, especially in the shell [19]. This observation further suggests that the reduced NAc activity is probably due to another source of changes during escalation that might be more related to the DA system and confirms that STN oscillatory activity does not correlate with striatal activity [32]. However, STN oscillatory activity has been shown to correlate with motor cortical activity in PD rats [26]. It would thus be interesting to further investigate whether or not the STN biomarkers identified here have a correlate in the motor cortex or are indeed synchronized, as observed in PD, in which case desynchronization either at STN or cortical level might represent an interesting therapeutic strategy to prevent escalated drug intake.
The synchronization between STN and cortical activities has also been reported for gamma band oscillations [35]. Gamma oscillations have also been associated with arousal to emotional stimuli under DA influence in PD patients [36]. The increased gamma power observed here may therefore possibly affect the sensitivity to DA during cocaine intake and also prevent a proper treatment of emotional context. Indeed, lesion of STN reduces affective responses in rats [37].
STN lesions and HFS effects?
STN lesions and HFS have a preventive effect on escalation that is specific to the LgA phase since they have no effect on animals’ consumption during ShA. One hypothesis to explain the efficacy of STN inactivation on blocking escalation of cocaine self-administration would be that abnormal synchronization within the STN network could be prevented by manipulating STN activity. Indeed, STN–HFS reduces oscillatory activity in all bands which is in line with former work showing that beta oscillations observed in PD patients are blocked by either L-DOPA [38] or STN–HFS [3]. L-DOPA and STN–HFS silence STN neurons in both PD macaques [25] and intact rats [20]. These physiological consequences have been classically associated with the beneficial effect of STN lesion or HFS on motor Parkinsonian symptoms [3, 39]. In a non-Parkinsonian brain, STN inactivation has here no effect on spontaneous or cocaine-induced locomotor activity, but has been shown to increase impulsivity of action that could favor drug self-administration [39, 40]. However, it also slows motor responses in tasks also requiring choice and reduces impulsivity of choice [5, 41, 42], which is often associated with compulsivity, consistent with the STN–HFS beneficial effect on compulsivity in OCD patients [43]. Here, STN–HFS reduces the number of perseverations during the cocaine escalation, an index of either compulsivity or motivation for the drug. Thus, our previous and present data confirm that STN inactivation reduces the animal’s motivation for cocaine [14, 15].
In addition to its local effects, HFS affects STN network activity because stimulation may propagate both orthodromically and antidromically along the axons to disrupt abnormal synchronization of the basal ganglia functional circuits [25, 31] and also to reduce excessive cortical coupling between beta oscillations and broadband activity observed in PD [44]. STN–HFS also reduces c-fos activity in the NAc shell in absence of drug [20] and abolishes the cocaine-induced increased c-fos expression in both NAc core and shell [45]. As such, during escalation, STN–HFS might therefore counteract the changes induced by cocaine in the NAc.
Immediately after cocaine escalation has developed, STN–HFS had no therapeutic-like effect in a procedure during which rats experienced a daily 18 h period of abstinence. Despite a trend, STN lesions also failed to significantly reduce re-escalation of cocaine intake after a 10-day period of abstinence. However, STN–HFS reduces re-escalation after a 35-day period of abstinence. This difference between STN lesions and HFS may be due to the procedure used, since excitotoxic lesions only affect STN neurons, while the HFS mostly inhibits the cells, but also activates the passing fibers. This highlights the issue that to obtain beneficial effects, it is important to reduce STN activity, while acting simultaneously on its network. However, it is more likely that the duration of abstinence is a more critical factor than the procedure. The 10-day period of abstinence imposed by the post-surgery recovery period presumably was too short. This suggests that a prolonged period of abstinence such as 35 days, possibly allowing a return to homeostasis resembling the naive state of the first escalation, seems required to allow STN–HFS effectiveness on cocaine relapse. Future experiments will be required to investigate this issue.
Deep brain stimulation is currently an FDA-approved surgical treatment for some neurodegenerative and psychiatric disorders [46]. Regarding addiction, the best target remains a matter of debate [15, 47, 48, 49]. Since it is important to treat the addiction to a drug without diminishing the desire for alternative, non-drug rewards such as food [14, 15], the STN appears as a solid candidate [16]. Favoring our proposed target, STN–HFS can reduce L-DOPA addiction in PD patients [50]. Accordingly, we identified here an addiction-associated pattern of STN abnormal activity whose reduction may contribute to HFS preventive and therapeutic effects. Furthermore, since addiction evolves through multiple cycles of abstinence/relapse, our data evidence a potential clinical window for STN–HFS application, which may normalize drug intake after abstinence in cocaine abusers.
Supplementary Material
Acknowledgements
The authors thank Drs. P Carrieri, C Bernard, P Belin, F Brocard, and G Masson for critical reading of the manuscript and the technical support from J. Baurberg and animal facilities personal. This research was funded by CNRS, Aix-Marseille Université (AMU), the “Agence Nationale pour la Recherche” (ANR_2010NEUR-005-01 in the framework of the ERA-Net NEURON to CB and supporting to YP), the Fondation pour la Recherche Médicale (FRM DPA20140629789 to CB), National Institutes of Health grants DA (DA029821 to GFK) from the National Institute on Drug Abuse and the Fondation de l’Avenir (ET2-655 to CB).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41380-018-0080-y) contains supplementary material, which is available to authorized users.
References
- 1.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Lazzaro VD. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Broussolle E, Perret JE, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995;345: 91–95. [DOI] [PubMed] [Google Scholar]
- 3.Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Front Integr Neurosci. 2012; 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baunez C, Lardeux S. Frontal cortex-like functions of the subthalamic nucleus. Front Syst Neurosci. 2011;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welter M-L, Burbaud P, Fernandez-Vidal S, Bardinet E, Coste J, Piallat B, et al. Basal ganglia dysfunction in OCD: subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transl Psychiatry. 2011;1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbaky Y, Baunez C, Arecchi P, Legallet E, Apicella P. Reward related neuronal activity in the subthalamic nucleus of the monkey. Neuroreport. 2005;16:1241–4. [DOI] [PubMed] [Google Scholar]
- 8.Lardeux S, Pernaud R, Paleressompoulle D, Baunez C. Beyond the reward pathway: coding reward magnitude and error in the rat subthalamic nucleus. J Neurophysiol. 2009;102:2526–37. [DOI] [PubMed] [Google Scholar]
- 9.Lardeux S, Paleressompoulle D, Pernaud R, Cador M, Baunez C. Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. J Neurophysiol. 2013;110: 1497–510. [DOI] [PubMed] [Google Scholar]
- 10.Breysse E, Pelloux Y, Baunez C. The good and bad differentially encoded within the subthalamic nucleus in rats. eNeuro. 2015;2:e0014–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zénon A, Duclos Y, Carron R, Witjas T, Baunez C, Régis J, et al. The human subthalamic nucleus encodes the subjective value of reward and the cost of effort during decision-making. Brain J Neurol. 2016;139:1830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282: 298–300. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–25. [DOI] [PubMed] [Google Scholar]
- 14.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat Neurosci. 2005;8:484–9. [DOI] [PubMed] [Google Scholar]
- 15.Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci USA. 2010;107:1196–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelloux Y, Baunez C. Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr Opin Neurobiol. 2013;23:713–20. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The rat brain in stereotaxic coordinates.2013. 7th edn. Academic Press, Cambridge. [Google Scholar]
- 18.Darbaky Y, Forni C, Amalric M, Baunez C. High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. Eur J Neurosci. 2003;18:951–6. [DOI] [PubMed] [Google Scholar]
- 19.Guillem K, Ahmed SH, Peoples LL. Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry. 2014;76:31–9. [DOI] [PubMed] [Google Scholar]
- 20.Wade CL, Kallupi M, Hernandez DO, Breysse E, de Guglielmo G, Crawford E, et al. High-frequency stimulation of the subthalamic nucleus blocks compulsive-like re-escalation of heroin taking in rats. Neuropsychopharmacol. 2017;42:1850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78: 199–207. [DOI] [PubMed] [Google Scholar]
- 22.Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantsch JR, Yuferov V, Mathieu-Kia A-M, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. [DOI] [PubMed] [Google Scholar]
- 24.Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. [DOI] [PubMed] [Google Scholar]
- 25.Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, et al. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372–82. [DOI] [PubMed] [Google Scholar]
- 26.Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci 2008;28:4795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–6. [DOI] [PubMed] [Google Scholar]
- 28.Alcaro A, Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev. 2011;35:1805–20. [DOI] [PubMed] [Google Scholar]
- 29.Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–30. [DOI] [PubMed] [Google Scholar]
- 30.Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain J Neurol. 2002;125:1196–209. [DOI] [PubMed] [Google Scholar]
- 31.Hamani C, Florence G, Heinsen H, Plantinga BR, Temel Y, Uludag K, et al. Subthalamic nucleus deep brain stimulation: basic concepts and novel perspectives. eNeuro. 2017;4:140–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deffains M, Iskhakova L, Katabi S, Haber SN, Israel Z, Bergman H. Subthalamic, not striatal, activity correlates with basal ganglia downstream activity in normal and parkinsonian monkeys. eLife. 2016;5:e16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–20. [DOI] [PubMed] [Google Scholar]
- 34.Degos B, Deniau J-M, Thierry A-M, Glowinski J, Pezard L, Maurice N. Neuroleptic-induced catalepsy: electrophysiological mechanisms of functional recovery induced by high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2005;25: 7687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaville C, McCoy AJ, Gerber CM, Cruz AV, Walters JR. Subthalamic nucleus activity in the awake hemiparkinsonian rat: relationships with motor and cognitive networks. J Neurosci. 2015;35:6918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huebl J, Spitzer B, Brücke C, Schönecker T, Kupsch A, Alesch F,et al. Oscillatory subthalamic nucleus activity is modulated by dopamine during emotional processing in Parkinson’s disease. Cortex. 2014;60:69–81. [DOI] [PubMed] [Google Scholar]
- 37.Pelloux Y, Meffre J, Giorla E, Baunez C. The subthalamic nucleus keeps you high on emotion: behavioral consequences of its inactivation. Front Behav Neurosci. 2014;8:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol. 2004;189:369–79. [DOI] [PubMed] [Google Scholar]
- 39.Baunez C, Nieoullon A, Amalric M. In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J Neurosci. 1995;15:6531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baunez C, Robbins TW. Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur J Neurosci. 1997;9:2086–99. [DOI] [PubMed] [Google Scholar]
- 41.Baunez C, Humby T, Eagle DM, Ryan LJ, Dunnett SB, Robbins TW. Effects of STN lesions on simple vs choice reaction time tasks in the rat: preserved motor readiness, but impaired response selection. Eur J Neurosci. 2001;13:1609–16. [DOI] [PubMed] [Google Scholar]
- 42.Adams WK, Vonder Haar C, Tremblay M, Cocker PJ, Silveira MM, Kaur S, et al. Deep-brain stimulation of the subthalamic nucleus selectively decreases risky choice in risk-preferring rats. eNeuro. 2017;4:e94–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallet L, Polosan M, Jaafari N, Baup N, Welter M-L, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive–compulsive disorder. N Engl J Med. 2008;359: 2121–34. [DOI] [PubMed] [Google Scholar]
- 44.de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hachem-Delaunay S, Fournier M-L, Cohen C, Bonneau N, Cador M, Baunez C, et al. Subthalamic nucleus high-frequency stimulation modulates neuronal reactivity to cocaine within the reward circuit. Neurobiol Dis. 2015;80:54–62. [DOI] [PubMed] [Google Scholar]
- 46.Krack Paul, Hariz Marwan I., Baunez Christelle, Guridi Jorge, Obeso Jose A.. Deep brain stimulation: from neurology to psychiatry? Trends in Neurosciences. 2010;33:474–84. [DOI] [PubMed] [Google Scholar]
- 47.Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, et al. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 2012;17:572–83. [DOI] [PubMed] [Google Scholar]
- 48.Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creed M, Pascoli VJ, Lüscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–64. [DOI] [PubMed] [Google Scholar]
- 50.Eusebio A, Witjas T, Cohen J, Fluchère F, Jouve E, Régis J, et al. Subthalamic nucleus stimulation and compulsive use of dopaminergic medication in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84:868–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.