Abstract
Lead (Pb2+) is a non-essential metal with numerous industrial applications that have led to ts ubiquity in the environment. Thus, not only occupational-exposed individuals’ health is compromised, but also that of the general population and in particular children. Notably, although the central nervous system is particularly susceptible to Pb2+, other systems are affected as well. The present study focuses on molecular mechanisms that underlie the effects that arise from the presence of Pb2+ in situ in the brain, and the possible toxic effects that follows. As the brain barriers represent the first target of systemic Pb2+, mechanisms of Pb2+ entry into the brain are discussed, followed by a detailed discussion on neurotoxic mechanisms, with special emphasis on theories of ion mimicry, mitochondrial dysfunction, redox imbalance, and neuroinflammation. Most importantly, the confluence and crosstalk between these events is combined into a cogent mechanism of toxicity, by intertwining recent and old evidences from humans, in vitro cell culture and experimental animals. Finally, pharmacological interventions, including chelators, antioxidants substances, anti-inflammatory drugs, or their combination are reviewed as integrated approaches to ameliorate Pb2+ harmful effects in both developing or adult organisms.
Keywords: Metals, Lead, Neurotoxicity, Ion mimicry, Mitochondrial dysfunction, Redox imbalance, Neuroinflammation, Chelators, Antioxidants, Anti-inflammatory drugs
1. INTRODUCTION
Lead is the second most hazardous substance according to the Agency for Toxic Substances and Disease Registry (ATSDR)’s Substance Priority List, a classification that consisted in prioritization of substances based on a combination of their frequency, toxicity, and potential for human exposure at National Priorities List (NPL) sites. Metallic lead (Pb0) is a bluish-gray heavy metal, resistant to corrosion because, when it is exposed to air or water, thin films of lead compounds (oxides and carbonates) are formed and protect this metal from further attacks; additionally, it can combine other metals to form various alloys. This non-essential element (atomic weight 207.2) is primarily found in various mineral forms in the earth’s crust in the divalent form (Pb2+), although Pb4+ is present in organolead. It has been widely used for centuries because it is readily shaped, molded, and resistant to corrosion. It is a ubiquitously distributed environmental toxicant that is naturally present in the earth cortex, but anthropogenic activities are responsible for its widespread in all compartments of the environment. The phasing out of leaded gasoline for vehicles between 1973 and 1995 and the removal of the metal from paint by 1978 have resulted in a substantial lowering of mean blood lead levels (BLL). However, because Pb2+ is a persistent metal, it is still present in all compartments of the environment, water, soil, and dust, making human exposure to occurs via food, water, air, and soil (Gupta, 2016; Patrick, 2006a). After exposure, Pb2+ is absorbed into the bloodstream, binds to circulating erythrocytes with a half-life of approximately 30 days to be distributed throughout the body, transiently accumulating in the brain, liver, and kidney to be eventually stored in the bone for 20–30 years. Nutritional status is a risk factor for Pb2+ intoxication and its effects. Thus, metals like iron (Fe2+), zinc (Zn2+), and calcium (Ca2+) deficiency increase gastrointestinal Pb2+ absorption, and affect the susceptibility to Pb2+ neurotoxicity as will be described later in this chapter. Also, physiological conditions such as pregnancy, menopause, lactation, and aging favor the release of the metal from storage compartments. In the laboratory, several animal models (mostly rodents) are used to simulate Pb2+ exposure on the human body, with the metal mainly administrated via gavage, intraperitoneal injection or drinking water. Following absorption, Pb2+ interferes with many organs such as the liver, kidney, and central nervous system (CNS), the most vulnerable system, particularly during development. Once in the brain, Pb2+ effects can be classified as either morphological or pharmacological (Silbergeld, 1992). Morphological effects are related to structural and pathological changes in the brain cells, including neuronal differentiation, myelination, and synaptogenesis. The pharmacological perspective, on the other side, approaches Pb2+ neurotoxicity from the ion-mimicry mechanism, with special emphasis on Pb2+ competition with Ca2+ and to a lesser extent Zn2+ and Fe2+ for their insertion sites and essential functions. This characteristic is somehow responsible of brain Pb2+ incorporation, synaptic neurotransmission disruption, and impacts on intracellular Ca2+ concentration with consequent mitochondrial dysfunction and redox imbalance. These effects, conjugate with new evidence that ascribe a neuroinflammatory potential to Pb2+, creating a highly disrupted microenvironment in the brain that leads to the neurotoxicant manifestations at both, the neurobehavioral and neurobiological/biochemical level, all pathognomonic of Pb2+ intoxication. Therefore, the present review will focus solely on Pb effects on the nervous system, mostly in cellular models or experimental animals and clinical evidence, with special emphasis on changes associated with the in-situ presence of the metal in the brain. Nevertheless, developmental effects will be also cited in the context of existing effects and potentially enduring consequences of Pb2+ neurotoxicity.
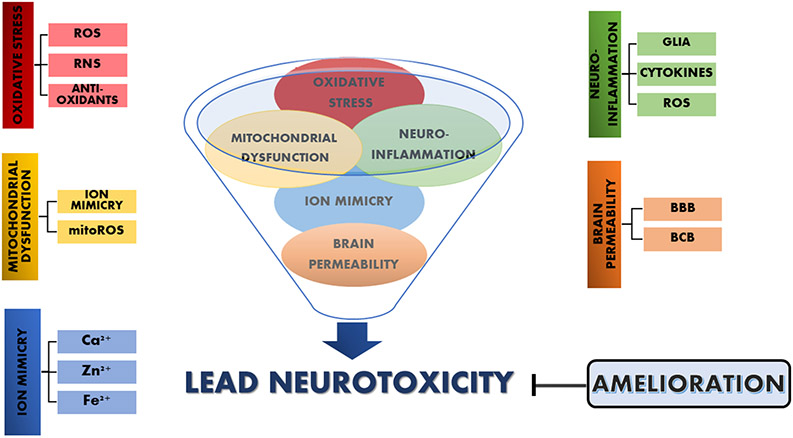
2. CELLULAR AND MOLECULAR MECHANISMS INVOLVED ON LEAD NEUROTOXICITY
2.1. THE ROLE OF THE BRAIN BARRIERS: LEADING Pb2+ INTO THE BRAIN
The brain, an organ with high complexity and functions that are vital for an individual’s life is protected by barriers. They are the blood-brain barrier (BBB) a physical, metabolic and transport-specialized interface shaped by the endothelial cells joined by tight junctions that form the walls of the capillaries accompanied by the astroglia, pericytes, perivascular macrophages, and a basal lamina; the blood-cerebrospinal fluid barrier (BCB) an interface formed by the epithelial cells of the choroid plexus facing the cerebrospinal fluid; and the arachnoid barrier formed by avascular arachnoid epithelium. Among the functions, the BBB provides a stable microenvironment for physiological neural function, ensuring brain homeostasis and thereby protecting a tissue with limited capability to regenerate from chemical insult and damage of endogenous and exogenous toxins. In occasions, although with high restriction, the BBB allows several paths of permeation for incorporation or efflux of substances to and out of the CNS, namely: a) passive diffusion for lipophilic and non-polar molecules; b) efflux transporters including adenosine triphosphate (ATP)-binding cassettes transporters (ABC); c) solute carriers for the incorporation of essential nutrients; d) transcytosis of macromolecules, and e) monocyte-lineage-cell movement in pathological conditions (Abbott et al., 2010). A large number of toxicants including metals, pesticides, mycotoxins, drugs of abuse, and therapeutic drugs are known to disrupt the BBB and/or BCB morphology or function.
In the case of metals, several elements have specialized processes to enters the CNS because they are necessary for optimal CNS function, while other metals that are non-essential can also cross or modify the barriers, including Pb2+. Although severe Pb2+ intoxication in newborn rats and in infants may cause micro vessel damage characterized by brain edema, there is little evidence that blood Pb2+ levels lower than 80 μg/dl were able to damage or disturb transport mechanisms (Bradbury and Deane, 1993). Once inside the brain, Pb2+ accumulates in the choroid plexus, which is not directly affected by the metal toxicity but rather mediated by the inhibition of production and secretion of transthyretin, a protein involved with the transport of thyroid hormones to the developing brain. This selective neurotoxicity may be the consequence of the incomplete tightness of the interepithelial junctions of the BCB or the high expression of numerous organic anion and cation transporters in this interface. In this respect, in vitro studies demonstrated that in contrast to later Pb2+ exposure, the presence of the metal prior to the formation of intercellular tight junctions inhibits the expression of claudin-1, an essential protein for interepithelial junctions (Lewis and Zheng, 2007). A more recent study established that Pb2+-induced a reduction in Cx43 gap junction hemichannel levels by promoting extracellular signal-regulated kinase (ERK) phosphorylation, constituting a novel pathway of cellular uptake through the BCB barrier (Song et al., 2016).
In terms of the BBB, several processes have been described (Martinez-Finley et al., 2012). On one side, Pb2+ may enter the CNS by passive diffusion as free Pb2+ or by the exchange of PbCO3 with an anion, or passively in the form of an inorganic complex, such as PbOH+ with a high dependence on pH. This was suggested after experiments of 203Pb uptake into the brain, with the calcium-ATP-dependent pump playing a pivotal role of Pb2+ transport back into the capillary lumen (Deane and Bradbury, 1990). Other studies performed in cerebellar granule cells from newborn rats indicate that 1,4-dihydropyridine insensitive calcium channels and N-methyl-D-aspartate receptors (NMDAr) are permeable to Pb2+ (Mazzolini et al., 2001). It is also possible that lead traverses the BBB by the Divalent Metal (Ion) Transporter 1 (DMT1), a 12-transmembrane domain protein present in endothelial cells of the BBB in both glia and neurons as an uptake mechanism for Fe2+ and other essential divalent metals (Luo et al., 2012). Furthermore, Fe2+ deficiency enhances Pb2+ transport and DMT1 overexpression promotes an increase in Pb2+ transport (Wang et al., 2011). Related to Ca2+, pioneer experiments performed in bovine adrenal medullary cells, they demonstrate that Pb2+ does not compete for binding sites in Ca2+-dependent channels, but rather permeates through them (Simons and Pocock, 1987; Tomsig and Suszkiw, 1991). Thus, Pb2+ incorporation into the brain seems to be mediated by mechanisms independent of voltage-gated-calcium channels (VGCCs) and of protein transport, although the activation of cation channels by the depletion of intracellular Ca2+ stores may also play a role in the Pb2+ transport through cellular endothelium (Kerper and Hinkle, 1997). More recently, the transient receptor potential (TRPC) canonical channels that mediate Ca2+ influx into the brain and BBB integrity also play a role in Pb2+ incorporation into the brain, provided that these channels can be activated by a variety of signals, including intracellular Ca2+ store depletion (Weber and Muller, 2017). Moreover, Pb2+ is a relatively potent stimulator of the TRPC5 channel and augments Ca2+ entry to the cells (Bouron et al., 2015; Sukumar and Beech, 2010), although TRPC role in Pb2+ incorporation into the brain has not been completely elucidated. It should be noted that modifications in intracellular vs. extracellular Ca2+ balance have detrimental effects in the BBB tight junctions assembly and functionality (Abbott et al., 2010). Most importantly, Pb2+ activates the Ca2+ dependent protein kinase C (PKC) in both BBB and BCB cells (Markovac and Goldstein, 1988; Zhao et al., 1998), an event that determines a loss of epithelial barrier function, an increase in trans endothelial permeability, and the inhibition of astroglia-induced endothelial differentiation (Laterra et al., 1992). Additionally, it has also been reported that Pb2+ induces morphological damage to the BBB accumulating in the brain endothelial cells, opening of the tight junctions and promoting an enhanced pinocytotic activity as evidenced by an extensive cerebral hemorrhage and extravascular distribution of albumin ( Gupta and Gupta, 2019; Zheng et al., 2003). Moreover, Pb2+ exposure activated intracellular non-receptor protein tyrosine kinase Src, leading to a reduction of occludin level, a tight junction protein involved in Pb2+-induced BBB leakage (Song et al., 2014). Besides, Pb2+ may indirectly affect the BBB through its accumulation in the astrocytes (Tiffany-Castiglioni and Qian, 2001), a type of neural cell with a pivotal role in the formation and maintenance of BBB integrity (Bressler and Goldstein, 1991). Although Pb2+ accumulates in both astrocytes and neurons, there is a preferential deposit of the metal in the immature astroglia, providing the basis for the Pb2+ sink hypothesis in which astrocytes act as Pb2+ depot in encephalopathy (Lindahl et al., 1999; Tiffany-Castiglioni et al., 1989), resulting in the accumulation of unfolded protein in the endoplasmic reticulum (ER) and consequent inhibition in the cell-cycle progression and the transcription of certain proteins (Zhang et al., 2008). In this line, Pb2+ alters the astroglial-endothelial interactions, leading to cerebral microvascular dysfunction due to alterations in the expression of human fetal astrocyte genes (Hossain et al., 2000). Finally, oligodendrocytes, the myelin-forming cells in the CNS, are pointed out as the most vulnerable brain cell type for Pb2+ toxicity (Tiffany-Castiglioni, 1993), particularly at the early stages of maturation, affecting both oligodendrocytes and astrocytes progenitor cells with a direct impact in the nerves’ myelin cover (Deng et al., 2001).
In summary, these findings indicate that Pb2+-induced damage to mature and immature brain cellular components may contribute to the neurotoxicant consequences of adult occupational Pb2+ exposure and to the higher vulnerability of children to environmental Pb2+ exposure. Thus, additional research is needed to increase the understanding of how Pb2+ and other neurotoxicants appropriate of the physiological mechanisms aimed to maintain homeostatic control of the CNS microenvironment and the direct or indirect damage to the morphology or functionality of the brain barriers and cellular types (Zheng et al., 2003).
2.2. THE IONIC MIMICRY MECHANISM: THE ROLE OF DIVALENT ESSENTIAL METALS
The ability of Pb2+ to interact with O2 and thiols, both of critical relevance as part of protein metal binding sites, allows its substitution for diverse essential divalent cations such as Ca2+ and Zn2+. It is important to emphasize that the living organisms are provided with many mechanisms of control to maintain physiological intracellular levels of essential metals, whereas the nonessential metals lack of homeostatic regulation. Although there is a consensus now that not a single mechanism can be ascribed to Pb2+ neurotoxicity, its ability to substitute or compete with essential divalent cations, preferentially Ca2+ but also Fe2+ and Zn2+, and thus altering the whole cellular microenvironment, was for some time the prevalent mechanism of Pb2+ toxic action. Pb2+ has an ionic radius 20% larger, broader coordination chemistry, greater electronegativity than Ca2+ and forms complex preferentially with thiols groups and complex ions rather than with O2 ligands. Though all these characteristics lead to a lower affinity of Pb for to Ca2+ binding sites, provided the extensive distribution and physiological importance of Ca2+ effects, including synaptic activity and its actions as an intracellular second messenger, the Pb2+/Ca2+ mimicry mechanism has the most profound impact in living organisms (Garza et al., 2006; Giorgi et al., 2018).
2.2.1. Calcium
The ability of Pb2+ to interchange with Ca2+ is central to its detrimental effects. Unlike Ca2+, which intracellular levels are highly regulated, Pb2+ is an unregulated non-essential heavy metal The 10,000-fold difference between extracellular and cytosolic Ca2+ levels respond to the impermeability of the plasma membrane to Ca2+ and transport mechanisms that remove Ca2+ from the cytoplasm (Florea et al., 2013). Pb2+ binds to the same/near sites than Ca2+ and enters the cell through Ca2+ channels to mimic, substitute, or compete with Ca2+ by a) direct interference with Ca2+ transport systems or storage sites; b) indirect alteration of Ca2+ homeostasis in cells functionality, including the elevation in intracellular cytosolic free Ca2+; or c) competition for Ca2+ binding sites and Ca2+ effector mechanisms (Pounds, 1984; Simons, 1993). Thus, in the first place, Pb2+ gains entrance to the cell in part by permeating Ca2+ channels, contributing to the early stages of toxic metal accumulation being the membrane and its proteins the first components of the cell to be exposed to the metal. Once inside the cell, Pb2+ further competes with Ca2+ to activate or inhibit Ca2+-regulated processes via interactions with Ca2+ binding-proteins (CABPs) such as the ones mentioned below:
2.2.1.1. VGCCs:
presynaptic neuronal depolarization leads to the influx of Ca2+ via the VGCCs, being Pb2+ a potent blocker of all VGCCs types probably through the binding to an external site, as there is no change in the voltage dependence upon Pb2+ binding. The inhibition of presynaptic VGCCs prevents the required rise in intracellular Ca2+ levels ([Ca2+]i), and eventually permits the passage of Pb2+ which is incorporated into the Ca2+ transport systems in the nervous system, all events impacting on synaptic activity with net detrimental effects on neurotransmitter release in a tissue-dependent-fashion ( Atchison, 2003; Audesirk and Audesirk, 1993; Piatt and Biisselberg, 1994; Sadiq et al., 2012). Besides, Pb2+ can induce catecholamine release from its storage vesicles in a Ca2+-independent manner, a mechanism that involves exocytosis stimulation probably due to direct stimulation of calcineurin by Pb2+ (Westerink and Vijverberg, 2002). Notably, several in vivo evidence demonstrate alterations on catecholamine functionality (Cory-Slechta et al., 2008; Verstraeten et al., 2008), revealing that Pb2+ effects on these systems might be the result of either an overall metal-mediated cellular damage or a direct interaction with these enzymes and neurotransmitters.
2.2.1.2. CABPs:
Pb2+ competes with Ca2+ for its binding sites on multiple proteins involved in vesicular mobilization and docking at the presynaptic cleft. This is known to occur by one of the following three mechanisms: 1) occupation of Ca2+ sites and structural inhibition of the protein’s activity; 2) inappropriate activation of the protein with detrimental consequences on downstream events, or 3) allosteric modulation of the protein activity with a binding site different than the one for Ca2+ (Gorkhali et al., 2016). These Ca2+-binding structures are characterized by two main types in terms of their conformational structure: the “EF-hand” motifs, which are characteristic of calmodulin conformation, and the C2 domains, exhibited by protein kinase C (PKC), both having a higher affinity to Pb2+ compared to Ca2+.
2.2.1.2.1. Calmodulin:
small changes in [Ca2+]i modify calmodulin subcellular distribution, its interaction with other proteins, and changes its conformational states (Simons, 1986). Several reports underscore the importance of the cross-reactions between Pb2+ and Ca2+ with calmodulin-dependent systems. In the first place, there are evidence that Pb2+ modifies the expression of calmodulin-related genes (Li et al., 2015). Secondly, pioneer reports indicate that Pb2+ activates calmodulin-dependent phosphodiesterase, calmodulin-inhibitor-sensitive-potassium channels, and calmodulin-independent PKC (Gill et al., 2003; Goldstein, 1993; Habermann et al., 1983). In addition, low Pb2+ concentrations activate calmodulin enzymatic activity by mimicking Ca2+ in the EF-hand binding sites, which leads to an increased calmodulin-mediated syntaxin I that opens VGCCs by phosphorylation. This results in an elevated number of readily releasable synaptic vesicles whereby binding Pb2+ to synaptotagmin I, a synaptic-vesicle-associated protein, whose interaction with Pb2+ induces inhibition of vesicular membrane fusion and therefore a decreased neurotransmitter release. In contrast, at high Pb2+ levels, calmodulin activity is inhibited, preventing the interaction between syntaxin I and synaptotagmin I and suppressing neurotransmitter release (Florea et al., 2013). Besides, calmodulin inhibition could be the result of Pb2+ binding to another region of the protein with resulting allosteric inhibition, a mechanism independent of simply ionic displacement (Kasten-Jolly and Lawrence, 2018). In fact, as a result of binding studies (Kirberger et al., 2013; Kirberger and Yang, 2008) it is demonstrated that Pb2+ alters the conformation of calmodulin in the Ca2+-bound state, particularly at the molecular recognition site, resulting in Pb2+ opportunistic binding to secondary sites of diverse geometric structure and to which Ca2+ does not bind. The authors concluded that: “this allosteric mechanism suggests that the promiscuous nature of lead allows for multiple molecular targets and by extension offers a comprehensive explanation for the resulting systemic pathology of lead toxicity”. Therefore, the presence of Pb2+ in the neurons interferes with a myriad of cellular events resultant of inadequate calmodulin functionality, including phosphorylation and dephosphorylation processes, signaling events, and the interaction with other proteins, all which convert this protein as a recently-described bridging and adaptor molecule (Villalobo et al., 2018), and likely playing a prominent role in Pb2+ neurotoxicity.
2.2.1.2.2. PKC:
Resembling calmodulin, Pb2+ has been reported to interact with PKC isozymes, possibly through different binding sites and at even lower concentrations than those required to activate calmodulin. These enzymes are activated by signals such as increases in the concentration of diacylglycerol (DAG) or in [Ca2+]i. Upon activation, the enzyme is translocated from the cytosol to the membrane and undergo phosphorylation to initiate the activation of transcription factors through signaling cascades (Kasten-Jolly and Lawrence, 2018). In this respect, studies in PC12 cells demonstrate that Pb2+ induces c-jun and egr-1 mRNA, in addition to PKC-dependent c-fos mRNA, as well as the corresponding encoded protein products (Kim et al., 2000). Moreover, PKC activation by picomolar Pb2+ concentrations (Markovac and Goldstein, 1988) triggers a variety of events such as cellular phosphorylation, stimulated basal secretion of the neurotransmitter, spontaneous transmitter release within the nerve terminals (Bressler and Goldstein, 1991; Goldstein, 1993), all of which inhibit Ca2+ entry into cells, and thus inducing an elevation in [Ca2+]i (Simons, 1993). It was later reported that Pb2+ in the micromolar range inhibited the enzyme through a mechanism not related to the competition with Ca2+ (Marchetti, 2003). Overall, Pb2+ modulation of cellular PKC activity remains a controversial subject, although it seems that at low Pb2+ levels, both PLC and PKC activities are enhanced, whereas higher Pb2+ concentrations inhibit these enzymes (Toscano and Guilarte, 2005). In this respect, a recent review indicates that Pb2+ shifts Ca2+ from the two Ca2+ binding loops within the C2 domain of all isoforms of the conventional PKC molecule, with different activation/inactivation outcomes according to the assay system (Kasten-Jolly and Lawrence, 2018).
2.2.1.2.3. AMPA glutamate receptor 2 (GluR2) subunit:
Ionotropic glutamate receptors include NMDA and a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors. The overactivation of glutamate receptors increases cytosolic [Ca2+]i leading to cell death. Different from NMDAr which are Ca2+ permeable, AMPAr containing the GluR2 subunit are impermeable to Ca2+. On the basis of the premise that long-term exposure of rat cortical neurons to Pb2+ acetate decreases GluR2 expression (Ishida et al., 2013), the same authors demonstrated the protective effects of the non-selective AMPA receptor blocker 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) against Pb2+-induced activation of ERK 1/2, mitogen-activated protein kinase 38 (MAPK p38), PKC, and neuronal cell death, suggesting that Ca2+-permeable AMPAr resulting from GluR2 decrease may be involved in Pb2+-induced neurotoxicity (Ishida et al., 2017).
Overall, this evidence indicates that Pb2+ not only affects cell signaling by replacing Ca2+ in protein binding sites, but also alters Ca2+ cellular concentration by modulating the activity of cationic channels.
2.2.2. Zinc
Besides Ca2+, Pb2+ interaction at Zn2+ binding sites is also important. In the CNS, this element is second only to Fe2+ in trace metal abundance and it is considered redox inert. Although mostly protein-bound, it is an essential component of numerous proteins involved in biological defense mechanisms against oxidative stress. There are small pools of the metal in presynaptic vesicles of glutamatergic neurons as well as in intracellular non-vesicular pools.
2.2.2.1. Zn2+ finger proteins:
are transcription factors that contain a Cys2-His2 Zn2+ binding domain, a major structural motif required for sequence-specific DNA-binding. Thus, provided the Pb2+ affinity for thiol sulfur atoms, the Zn2+-binding sites based on thiol groups are those with a higher affinity for Pb2+ (Garza et al., 2006). Most importantly, Pb2+ competes with the Zn2+ binding site of several Zn2+ finger proteins, probably as a result of structural alteration in the Zn2+ coordination site ( Ordemann and Austin, 2016; Zawia et al., 2000). In this regard, a report indicates that Pb2+ inhibits the DNA-binding mechanism of 1) the transcription factor IIIA (TFIIIA), which is related to the internal control region of the gene (Zawia et al., 2000); 2) the specificity protein 1 (Sp1), which is highly important in development (Basha et al., 2003); and, 3) of the early growth response transcription factor 1 (Egr-1), that is implicated in plasticity, learning and memory processes (Reddy and Zawia, 2000).
2.2.2.2. NMDAr channel:
is another putative target of Pb2+ neurotoxicity in the Zn2+ allosteric binding site (Guilarte et al., 1995; Toscano and Guilarte, 2005; Marchetti, 2014), although the exact interaction with Zn2+ binding site is still under debate (Gavazzo et al., 2008). This glutamatergic receptor subtype is implicated in the regulation of neuronal development and cognitive functions, including long term-potentiation (LTP), a form of synaptic plasticity NMDAr-dependent that is believed to form the cellular basis for the cognitive process, including learning and memory. Pioneer studies by Alkondon et al. (1990) concluded: “the elucidation of the actions of Pb2+ on the NMDAr ion channel complex provides important insights into the clinical and toxic effects of this cation” (Alkondon et al., 1990). The extracellular portion of the NMDAr subunits consists of an N-terminal domain that binds Zn2+ allosterically, and a contiguous ligand-binding domain that binds glutamate and glycine, a step required for NMDAr activation. This is accompanied by the removal of the Mg2+ blockade through the depolarization of the neuron’s cellular membrane and the opening of the associated ion channel permeable to Ca2+, Na+ and K+ (Baranowska-Bosiacka et al., 2012). Evidence indicates that Pb2+ is a potent, non-competitive antagonist of the NMDAr, with a high affinity to the NR2A subunit of the receptor by binding at the Zn2+ regulatory site of the NMDAr (Nihei and Guilarte, 1999) in a voltage-independent fashion (Neal and Worley, 2011). Further studies in rats chronically exposed to Pb2+ performed by these authors demonstrated that impairments of spatial learning and hippocampal LTP are associated with changes in gene and protein expression of NMDAr subunits, conforming the mechanistic basis for the well-known deficits in cognitive function resultant of Pb2+ exposure (Nihei et al., 2000; Nihei and Guilarte, 2001). Importantly, the involvement of the glutamatergic system, and in particular NMDA participation in Pb2+-induced learning impairments was already reported in a study that examined the mechanistic relationships between Pb-induced alterations in glutamate neurotransmission and behavioral toxicity (Cory-Slechta et al., 1997). Other studies performed in vitro in cultured hippocampal neurons reported that Pb2+ exposure during synaptogenesis decreases synaptophysin and synaptobrevin proteins, resulting in impaired vesicular release at both the glutamatergic and GABAergic synapses. The authors conclude that these presynaptic deficits resulting from Pb2+ exposure are mediated by disruption of NMDAr-associated cAMP response element-binding (CREB)-dependent transcription of activity-regulated genes such as brain-derived neurotrophic factor (BDNF) that alters the function of its related binding site, the tyrosine receptor kinase B (TrkB) and downstream signaling, being BDNF a trans-synaptic molecule released from both dendrites and axons whose levels are decreased in response to Pb2+ exposure (Neal et al., 2010; Neal and Guilarte, 2010; Stansfield et al., 2012). As a regulator of Ca2+-signaling and homeostasis, BDNF perturbation led to alterations in Ca2+-dependent pathways, which in turn can also be interfered by Pb2+. In further studies, the same authors showed that Pb2+ exposure markedly inhibits presynaptic vesicular release in hippocampal Schaffer collateral-CA1 synapses in young adult rats. This effect was associated with a reduction in vesicle number and a decreased number of presynaptic terminals with multiple mitochondria, while no change in presynaptic Ca2+ influx was reported, effects that contribute to the deficits in synaptic plasticity and intellectual development observed in children (Zhang et al., 2015). More recently, Ding et al. (2018) reported that Pb2+ decreased both excitatory and inhibitory presynaptic transmission due to a disarrange in the distribution and density of presynaptic vesicles, a process dependent on the phosphorylation level of synapsin 1 via cyclin-dependent kinase 5 (CDK5) (Ding et al., 2018).
In addition to the blockage of the NMDAr, Pb2+ inhibits the passage of Na+, Ca2+, and other ions through the postsynaptic membrane, thus reducing excitatory postsynaptic potentials (EPSPs) and the likelihood for generating an action potential (Florea et al., 2013). Moreover, and besides these effects, Pb2+ alters hippocampal NMDAr subunits mRNA expression in rats, modifying the receptor levels or subtypes and altering the development of defined neuronal connections that require its activation (Sanders et al., 2009). However, evidence also exists that the interaction between Pb2+ and Zn2+ occurs via independent allosteric binding sites (Lasley and Gilbert, 1999) that are associated to an elevation in NMDAr density as a result of prenatal Pb2+ exposure in rats (Lasley et al., 2001). Furthermore, developmental Pb2+ exposure reduces the ability of the NMDA antagonist MK-801 to suppress LTP in the rat dentate gyrus (Gilbert and Lasley, 2007) and produces MK-801 sub-sensitivity at the behavioral level associated with postnatal Pb2+ exposure (Cory-Slechta, 1995). Finally, and based on the premise that Pb2+ affinity for NMDAr may not completely explain the mechanisms of Pb2+-induced neurotoxicity, further studies aimed to examine metabotropic glutamate receptors (mGluR) in rat hippocampal neurons. In this regard, both in vitro and in vivo studies revealed that expression of mGluR5 mRNA and protein was decreased, revealing that mGluR5 might be involved in Pb2+-induced neurotoxicity by disturbing mGluR5-induced long-term depression (LTD) and decreasing NMDAr-dependent LTP (Xu et al., 2009; Xu et al., 2009).
2.2.2.3. δ-aminolaevulinic acid dehydratase (δ-ALA-d):
is an enzyme involved in heme synthesis formed by eight identical subunits with eight Zn2+ ions as cofactors. It is also a target of Pb2+ due to mimicry with Zn2+ whereby Pb2+ binds to the sulfhydryl moiety at the active site of the enzyme, resulting in the characteristic anemia observed in organisms exposed to Pb2+ (Bergdahl et al., 1997). In addition, δ-ALA-d inhibition results in δ-ALA accumulation, an unstable compound that can be rapidly converted into free radicals (Ahamed and Siddiqui, 2007) and in part responsible for the redox imbalance associated to Pb2+ exposure, a crucial aspect that will be discussed below.
2.2.3. Iron
Iron is the most abundant trace element in the brain and is considered a redox-active metal that undergoes redox-cycling, including the crucial Fenton reaction. It is an essential cofactor for normal brain development and function as well as for a wide variety of important cellular processes, such as O2 transport, respiration, the tricarboxylic acid (TCA) cycle, lipid metabolism, heme biosynthesis, gene regulation, and DNA and RNA synthesis. It is incorporated in the heme group of hemoglobin, myoglobin, and cytochromes, or is associated with nonheme moieties or Fe-S motifs (Cairo et al., 2006). As a divalent cation, Fe2+ is another important metal with the potential to be a target for Pb2+ displacement, particularly in the DMT-1, which as previously discussed may be involved in the transport and cellular uptake of Pb2+ (Kirberger et al., 2013). Intriguingly, few studies have examined the effect of Pb2+ exposure on cellular Fe2+ homeostasis to provide a hint on possible interactive mechanisms. In this regard, a recent report indicates that Pb2+ exposure increases Fe2+ content in rat brain, which was associated with the decreased expression of ferroportin 1 (FP1), a pivotal Fe2+ efflux protein (Zhu et al., 2013). Further studies from the same group showed that Pb2+ exposure induced an increase in cellular Fe2+ levels which was accompanied by a decrease in FP1 expression, while FP1 overexpression could attenuate Fe2+ accumulation in Pb2+-exposed PC12 cells (Zhou et al., 2014).
2.3. THE MITOCHONDRIAL DYSFUNCTION MECHANISM
Mitochondria, as the major source of cellular energy metabolism, have been studied extensively in xenobiotic-induced neurotoxicity. In particular, metals have been reported to produce some of the toxic effects by targeting mitochondria (Meyer et al., 2013). Although a non-redox-active metal, Pb2+ promotes an imbalance in the mitochondrial homeostasis, in part by decreasing glutathione (GSH) as the result of the inhibition of thiols groups, reducing the antioxidant enzymes activities, substituting Ca2+, Fe2+, or Zn2+ or altering the integrity, permeability and functionality of membranes, favoring lipid peroxidation (Caito and Aschner, 2015). All these events will be discussed in detail in the next section. In comparison to other organelles and on the basis of the high content of unsaturated lipids, the mitochondrial membranes represent a structure with a particular susceptibility to Pb2+. In the same line, mitochondrial enzymes that contain essential sulfhydryl groups are also a target for Pb2+ species. Thus, direct damage to mitochondrial components may disrupt homeostatic mitochondrial functions leading to cell death (Blajszczak and Bonini, 2017). In this regard, pre- and neonatal Pb2+ exposure in rats affects the energy status of cultured primary cerebellar granule neurons from rats through a decrease in ATP concentrations by the inhibition of Na+/K+ ATPase, and the increase in intracellular and mitochondrial reactive oxygen species (ROS) concentration (Baranowska-Bosiacka et al., 2011).
2.3.1. Intramitochondrial ion mimicry
Different from other organelles, the mitochondrion has its own genome, a circular double chain DNA molecule (mtDNA), 22 transference RNAs, and 2 ribosomal RNAs that lack repair mechanisms, although playing a key role in mitochondrial functionality. This unique characteristic is emphasized by Meyer et al, 2013, who consider this organelle as a preferential target of environmental toxicants, with a particular focus on mtDNA (Meyer et al., 2013). In this regard, recent data from participants in the PROGRESS cohort revealed that Pb2+ levels at different time frames during pregnancy were associated with increased mtDNA content in cord blood (Sanchez-Guerra et al., 2019). Notably, mitochondrial dysfunction can affect key regulators of the intracellular signaling such as ROS and Ca2+ that all can be transmitted to the nuclei (mitochondrial to nuclei signaling or retrograde signaling), resulting in changes in the genic expression.
From the ultrastructural view, the mitochondrion is semi-autonomous with a double membrane system, the internal membrane (IMM), and external membrane (EMM) that delimit an intermembrane space (IMS), whereas the IMM brands the matrix, a viscous microenvironment with high enzymatic content. Mitochondrial membranes facilitate the accumulation of lipophilic substances, while the matrix’s negative charge and slightly alkaline pH as a result of the proton pumping associated with oxidative phosphorylation permits the incorporation of cationic metals, including Pb2+ (Castellino and Aloj, 1969). In addition, as mentioned in the previous section, Ca2+-dependent transporters represent another influx/efflux mechanism resultant of molecular mimicry (Lidsky and Schneider, 2003; Meyer et al., 2013; Rocha and Trujillo, 2019; Silbergeld, 1977). In the same line, it is important to highlight that essential metal ions such as manganese (Mn2+), Fe2+, copper (Cu2+), and Zn2+ play pivotal roles in this organelle and their dysregulation can impact on mitochondrial compartments and functions (Nam et al., 2018), which opens up the possibility of Pb2+ competition with these ions, thus affecting mitochondrial homeostasis.
In terms of function, the mitochondria are the energetic center of the cell as a consequence of the electron flux through the mitochondrial respiratory complexes I-IV with resultant oxidative phosphorylation. The complete nutrients oxidation by the tricarboxylic acid cycle produces reduced coenzymes such as reduced nicotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide 2 (FADH2) that act as electron donors. The electron flux through the electron transition chain (ETC) produces an electrochemical gradient that is used by the complex V to generate ATP. Mitochondrial dysfunction is characterized by the inhibition of mitochondrial O2 consumption, altered membrane potential, and reduction in ATP levels by an imbalance in the energy intake and expenditure. Any defect in the ATP-generating capacity causes decreased cellular energy metabolism, cellular dysfunction, and cell death (Fariss et al., 2005). There are some evidence that support the effect of Pb2+ on oxidative mitochondrial processes. First, it was reported that neonatal exposure to low levels of Pb2+ produces changes in phosphorylation activity in rat brain mitochondria (Bull et al., 1979), while in vitro studies in brain mitochondria of young rats (Dumas et al., 1985) and in synaptosomes (Rafałowska et al., 1996) showed that Pb2+ ions alter mitochondrial respiration and Adenosine diphosphate (ADP) phosphorylation. In addition, Pb2+ can also act on cytochrome c and ATP synthase to cause dysfunction of mitochondrial ETC and generation of free radicals (Dressier et al., 1999). Furthermore, it was demonstrated that relatively high Pb2+ concentrations inhibited both the Pb2+-induced respiration and the respiratory response to ADP in cerebral and cerebellar mitochondria from both, immature and adult rats. The authors concluded that matrix NAD-linked dehydrogenase, were more sensitive to inhibition by Pb2+ than were the inner-membrane enzymes (Holtzman et al., 1978).
While necrosis and apoptosis are the major forms of regulated cell death, the role of mitochondria in more recently described necroptosis, pyroptosis, ferroptosis, and other cell death processes is still poorly defined. In relation to apoptosis, mitochondrial dysfunction initiated by the opening of the mitochondrial permeability transition pore (MPTP) leads to membrane permeabilization, the release of cytochrome c, followed by activation of caspases and cleavage of downstream effector proteins, resulting in cell death. This is a suicide event triggered by extrinsic, receptor-mediated, or intrinsic, mitochondria-mediated signaling pathways. In vivo evidence indicate that Pb2+ exposure affects the mitochondrial permeability, thereby stimulating the release of mitochondrial proteins, facilitating apoptosis in several brain regions, increasing the Bax/Bcl-2 ratio by down-regulation of Bcl-2 and up-regulation of Bax expression, being their relationship a key indicator of apoptosis susceptibility by the intrinsic pathway (Sharifi et al., 2002) (Pulido and Parrish, 2003). Further studies reported that Pb2+ induced developmental apoptosis in several brain regions, consistent with dying neurons expressing activated caspase-3 early in the degenerative process (Dribben et al., 2011), is the hippocampus the region most vulnerable where caspase 3, Bcl-2, and BDNF increase in developmental Pb2+ exposed rats (Chao et al., 2007). Moreover, in vitro experiments in PC12 cells demonstrated that p53 activation by DNA damage leads to an imbalance in Bax/Bcl-2 and mitochondrial dysfunction that was followed by Pb2+-induced apoptosis (Xu et al., 2006). Likewise, an increase in the production of [Ca2+]i, ROS, and associated oxidative stress in Pb2+-treated PC12 cells accompanied by an upregulation in the expression in both mRNA and protein caspase-3, caspase-9, Bax, p53, and cytochrome-c and downregulation in Bcl-2 (Kumar et al., 2015). In addition, ROS triggers the apoptotic pathway that can be activated by an overload in mitochondrial [Ca2+]i, being the ER-mitochondrial Ca2+ interchange a highly regulated process in the cell in homeostatic conditions (Marchi et al., 2018; Orrenius et al., 2003; Rizzuto et al., 2003). Thus, apoptosis can be caused by loss of control of Ca2+ homeostasis or by changes in Ca2+ distribution within intracellular compartments (Garza-Lombó et al., 2018). In addition, Ca2+ homeostasis is regulated by mitochondrial functions that control the buffering capacity of ER-Ca2+ channels. Ryanodine receptors (RyRs), the major intracellular Ca2+ release channels located in the ER, are crucial for brain function because they mediate Ca2+ release. Interestingly, Pb2+ exposure affects RyRs leading to an increase in [Ca2+]i in cultured cells (Fan et al., 2013; Jia et al., 2018). Thereby, increased [Ca2+]i alter cellular energy balance because high cytoplasmic Ca2+ concentrations increase mitochondrial Ca2+ uptake by the Ca2+ uniporter. In these conditions, the MMP is dissipated, and ATP synthesis is reduced. Additionally, Ca2+ may also impair ATP synthesis by causing oxidative injury to the IMM. Furthermore, a sustained rise in cytoplasmic Ca2+ increases ATP consumption by forcing the Ca2+-ATPases to work to eliminate the excess Ca2+. Importantly, Pb2+-induced elevation of [Ca2+]i resulting from the ER Ca2+ release in resting cells may be involved in the neurotoxicity (including learning and memory impairment) on account of [Ca2+]i which play a major role in the induction and maintenance of LTP (Fan et al., 2013). It should be noted that Ca2+ uptake is driven by a difference in mitochondrial potential product of the ETC force that facilitates positively charged ions to enter the matrix through macromolecular complexes Ca2+ antiporters. In turn, Ca2+ is rapidly extruded into the cytoplasm by different pumps, channels, and auxiliary proteins, in order to restore the basal state. Thus, any alteration of the coordination of these events will disrupt mitochondrial Ca2+ homeostasis (Giorgi et al., 2018). It is the case of Pb2+, a metal that as mentioned affects many Ca2+-dependent events product of ion-mimicry and of its accumulation in the same subcellular compartments than Ca2+ (Bressler and Goldstein, 1991; Dressier et al., 1999; Lidsky and Schneider, 2003; Rocha and Trujillo, 2019). In this respect, Chavez et al. (1987) proposed that Pb2+ stimulates mitochondrial Ca2+ release by promoting a reduction in the NAD(P)H/NAD(P) ratio and a collapse in the transmembrane potential, due to its ability to pass through the Ca2+ uniporter and its affinity to the thiol groups located in the matrix side of the IMM (Chávez et al., 1987). These results confirm previous reports in terms of interference with Ca2+ entrance to the mitochondria (Jurkowitz et al., 1974; Parr and Harris, 1976; Silbergeld and Adler, 1978), and complement others (Kapoor and Van Rossum, 1984), suggesting that suggested that Pb2+ accumulated in the mitochondria displaces Ca2+ from its intramitochondrial binding sites. Thereby, the inhibition of Ca2+uptake into brain mitochondria by Pb2+ would be expected to increase [Ca2+]i in the cytosol, affecting normal neuronal function (Goldstein, 1977).
On the other hand, and as mentioned before, the ETC is also the main source of ROS due to an incomplete O2 reduction in the Complex I and Complex III, affecting proteins, membranes, and promoting caspase activation and the mitochondrial apoptotic pathway. ROS can also facilitate the opening of the MPTP leading to the IMM permeability (Moro, 2020), a mechanism reported in human neuroblastoma SH-SY5Y cells treated with Pb2+ (Ye et al., 2016).
In summary, in addition to its role in energy metabolism, mitochondria regulate cellular redox status, control Ca2+ homeostasis, initiate the intrinsic pathway of apoptosis through activation of MPTP, carry-out anabolic biochemical processes including synthesis of heme and Fe2+-sulfur clusters, regulate the levels of essential intracellular intermediates that activate cellular signal transduction pathways including retrograde signaling, and contribute to additional energy production by other pathways including Krebs cycle and fatty acid oxidation (Meyer et al., 2013). Important homeostatic processes include replication of the mitochondrial genome, mitochondrial biogenesis such fusion and fission processes, and degradation pathways including mitophagy (Murata et al., 2020). The inclusion of the study of mitochondrial targets beyond the ETC, such as mitochondrial metabolism, oxidative stress, mtDNA genetics, and signaling response will broaden the spectrum of environmental agents considered mitotoxicants.
2.3.2. Mitochondrial ROS generation
ROS are chemically reactive chemical species containing O2. At low levels, these species may participate in cell signaling processes, whereas at higher levels they may damage cellular macromolecules, including proteins, lipids, DNA, and RNA. Most ROS are generated in the mitochondria (mtROS), with a reduced proportion is mediated by the activity of enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidases (NOx), enzymes that generate O2•− or H2O2, xanthine oxidase (that contains a Fe2+-sulfur cluster), the heme proteins cyclooxygenases, cytochrome p450 enzymes, lipoxygenases, and myeloperoxidases, as well as the protein folding machinery in the endoplasmic reticulum (Turrens, 2003). ROS generation leads to OH• formation through Fenton/Haber-Weiss reactions, in where H2O2 oxidizes either Fe2+ or Cu2+ respectively, that are reduced back by O2•− initiating free radical chain reactions. Once formed, O2•− undergoes rapid dismutation into H2O2 through the action of superoxide dismutase (SODs), including the Cu/Zn-dependent cytosolic SOD1, extracellular SOD3, and MnSOD (SOD2) which is exclusively localized in the mitochondrial matrix. H2O2 is removed by peroxisomal enzyme catalase (CAT) or by the selenoprotein glutathione peroxidase (GPx) enzymes, the latter one of the most abundant intrinsic antioxidants that help in preventing lipid peroxidation and the resulting cell death by ferroptosis, a process assisted by glutathione reductase (GR) to recycle oxidized glutathione (GSSG). Additionally, other H2O2-removing systems do exist, including peroxidases that use the dithiol-containing protein thioredoxin as a substrate (peroxiredoxins); whereas thioredoxin reductase, which converts oxidized thioredoxin back to the reduced form is also a selenoprotein. The above-mentioned are the main components of the enzymatic antioxidant system that counteracts ROS deleterious effects. The tripeptide GSH constitutes the main non-enzymatic antioxidant, particularly in the mitochondria. Most GSH is used by GPx and peroxiredoxins to catalyze H2O2 reduction. Additional natural antioxidants are ascorbic acid, vitamin E, α-tocopherol, cysteine, Zn2+, Se2+, among others (Halliwell, 2001). The interference with nitric oxide (NO) production might represent another mechanism accounting for Pb neurotoxicity. Reactive nitrogen species (RNS) include the diffusible second messenger NO, considered a free radical because it has an unpaired electron, the nitrogen dioxide radical (NO2), and the peroxynitrite (OONO−), which protonates at relevant pH to form peroxynitrous acid (ONOOH), both strong oxidizing agents (Aschner, 1996). The synthesis of NO is a process initiated by the presynaptic release of glutamate, which binds to its receptors and allows Ca2+ to enter into the cell. The binding of Ca2+/calmodulin to neuronal NO synthase (nNOS) facilitates the oxidation of L-arginine to conclude with the synthesis of L-citrulline and NO. Several shreds of evidences demonstrate that the mimicry between Pb2+ and Ca2+ can alter the activity and expression of Ca2+-dependent nNOS and endothelial NOS synthase (eNOS) in different brain regions, preventing the accessibility of Ca2+ to NOS, thus leading to a decreased activity of nNOS/eNOS and resulting in a reduced NO production in different brain regions (Garcia-Arenas et al., 1999; Nava-Ruiz et al., 2012). In agreement, nNOS expression and activity were reported to be reduced by Pb2+ in the developing rat brain, alterations attributed to changes in [Ca2+]i homeostasis (Chetty et al., 2001). Additionally, provided the importance of the NO signaling in the induction of LTP in the hippocampus, the impairment on the NO transduction system might affect synaptic development and plasticity of brain regions involved in higher cognitive functions that are associated with clinical Pb2+-exposure manifestations (García-Arenas et al., 2004; Selvín-Testa et al., 1997). In addition, Pb2+ competes with Ca2+, Zn2+ is also an important structural element of NOS enzymes, in particular at the NMDAr level, thus interfering with nNOS activation and consequent NO production (Garza-Lombó et al., 2018).
Mitochondrial ROS production depends on the electron flux in the ETC. When reduced, such as by age, pathological conditions, or the presence of xenobiotics, namely, the possibility that electrons move from O2 to form ROS it’s enhanced. Another factor influencing mtROS generation is Ca2+ signaling that involves all the associated processes described above (Meyer et al., 2018). Moreover, the inhibition of the ETC by xenobiotics or by NADH augmentation due to the low ATP demand and consequent low respiration rate will increase the NADH/NAD+ ratio and lead to the O2•− formation. In contrast, in most of the situations in which the mitochondria have a normal functionality and the NADH/NAD+ relationship is relatively low, only small O2•− amounts are produced (Murphy, 2009). Additionally, both the Complex I and the enzyme alpha ketoglutarate dehydrogenase will produce more ROS when the NADH/NAD+ relation is high; then increased ROS generation will compromise the ability of the Complex I to oxidize NADH, originating a vicious circle that may lead to greater mitochondrial dysfunction (Stefanatos and Sanz, 2011).
Additionally, the membrane polyunsaturated fatty acids are very susceptible to the lipidic peroxidation, playing a critical role in the redox state of the cell by the production of toxic aldehydes such as malonaldehyde (MDA) and 4-hydroxynonenal (4-HNE), a process triggered by ROS and catalyzed by both Fe2+ and Cu2+. These aldehydes are highly reactive and able to form adducts with nucleic acids, proteins, amino acids, and polypeptides, leading to enzymatic inactivation, mitochondrial dysfunction, and cell death (Halliwell, 1992). Thus, essential metal ions (Mn2+, Cu2+, Fe2+, and Zn2+) and ROS are capable to alter mitochondrial lipid environments and membrane integrity. In addition, either the overload or depletion of essential metal ions (or their substitutes) interferes with mitochondrial functions, such as the TCA cycle, oxidative phosphorylation, and GSH metabolism (Nam et al., 2018).
In summary, both enzymatic and non-enzymatic antioxidants are involved in the neural protection from oxidative harm resultant of mitochondrial dysfunction. Thus, the orchestrated formation of excessive free radicals, combined with a decrease in cellular enzymatic defenses accompanied by GSH and natural antioxidant depletion might lead to increased sensitivity of neural cells to Pb toxicity. Evidence of these effects will be provided in detail in the next section.
2.4. THE PRO-OXIDANT/ANTI-OXIDANT MECHANISM
Oxidative stress is the redox state resulting from an imbalance between the generation and detoxification of ROS and RNS. Under carefully controlled situations, ROS function as important physiological regulators of intracellular signaling pathways. However, an overloaded amount of ROS promotes a redox imbalance that damage the cells by oxidizing cellular biomolecules, including nucleic acids, proteins, and lipids, all of which lead to and facilitate apoptosis (Finkel, 2011)
The brain is particularly susceptibility to oxidative damage. In the first place, because of elevated O2 consumption, the product of high ATP demand of neurons results in efficient mitochondrial functionality. Secondly, the brain presents low levels of antioxidant defenses when compared to other cells, as well as an abundance of highly polyunsaturated fatty acids. In addition, although mostly stored in ferritin, Fe2+ is present in the brain, which along with Cu2+ in Fenton and Haber-Weiss reactions, respectively are capable to promote H2O2 formation, lipid peroxidation, and autoxidation of neurotransmitters, in particular catecholamines considered highly auto-oxidized molecules. Moreover, the brain generates H2O2 in situ via dopamine metabolism by monoamine oxidases (MAOs), flavoprotein enzymes located in the OMM. In the same line, cytochromes P450 (CYPs), enzymes present in the brain can ‘leak away’ ROS from the catalytic intermediates in the P450 cycle. Finally, brain microglia can produce O2•− and H2O2 upon activation, although neurons and oligodendrocytes seem to be more susceptible to oxidative damage than astrocytes and microglia (Halliwell, 1992; Halliwell, 2001; Wang and Michaelis, 2010).
Even low-environmentally-relevant Pb2+ levels are reported to induce a redox imbalance in the cell by the generation of free radicals resulting in oxidative damage to critical biomolecules, lipids, proteins, and DNA (Ahamed and Siddiqui, 2007; Ercal et al., 2005). Evidence on this regard come from in vitro experiments or studies conducted in animals, most of them at moderate to high Pb2+ doses. In addition, several clinical and epidemiological studies provide associations among workers with high Pb2+ exposure and oxidative stress, while there are fewer reports of environmental Pb2+ exposure in children and adults that assessed this association (Almeida Lopes et al., 2016). This raises the need to develop oxidative stress biomarkers of early Pb2+-adverse effects and potential therapeutically interventions in Pb2+-related negative outcomes. Thus, in the first place, the putative mechanisms of Pb2+ on the pro- and antioxidant balance will be described in detail with a summary of the most relevant results of this topic in experimental and clinical settings, followed by a brief description of state-of-the-art studies aimed to ameliorate these detrimental effects.
2.4.1. Mechanisms for free radical generation
2.4.1.1. Interactions in the heme biosynthetic pathway: pro-oxidative effect of δ-ALA
The δ-ALA undergoes enolization and auto-oxidation at pH 7.0–8.0. The conversion of the δ-ALA keto form into the δ-ALA enol form is shown to be necessary for auto-oxidation reactions, being the latter autoxidized to generate O2•− and reduced ferricytochrome c. In parallel, the reaction between δ-ALA/oxyhemoglobin (oxyHb)-coupled oxidation results in metaHb, δ-ALA radical, and H2O2 generation. The O2•− and H2O2 formed in these reactions can generate HO• radicals. In addition to these mechanisms, some authors have related Pb2+ neurotoxicity to the ability of δ-ALA to inhibit either the K+-stimulated release of γ-aminobutyric acid (GABA) from preloaded rat brain synaptosomes or the binding of GABA to synaptic membranes (Bechara, 1996; Gurer-Orhan et al., 2004).
2.4.1.2. Membrane lipid peroxidation: direct Pb2+ effect
Lipids play a pivotal role in neuronal function as they constitute the plasma membrane, the barrier between intracellular and extracellular spaces. They are prone to the attack of free radicals and undergo lipid peroxidation, leading to a decrease in membrane fluidity and leakiness. This facilitates the entrance of substances usually unable to cross the barrier, except through specific channels (e.g., K+, Na+, Ca2+, etc.). As the neuronal membrane lipids are rich in polyunsaturated fatty acids (PUFA), the side chains are specifically more vulnerable to ROS/RNS, and thus to oxidative stress processes.
Lead is known to have toxic effects on membrane structure and functions, altering membrane fluidity and/or promoting alterations in membrane permeability, which were associated to Pb neurotoxicity (Verstraeten et al., 2008). On cell membrane, the presence of double bonds in the fatty acid weakens the C–H bonds on the carbon atom adjacent to the double bonds and makes H removal easier. Therefore, fatty acids containing zero to two double bonds are more resistant to oxidative stress than is the PUFA with more than two double bonds. Lipid peroxidation results in the formation of a wide variety of oxidation products, the most common of which are aldehydes. Among the most abundantly produced aldehydes are MDA, propanal, hexanal, and 4-HNE. Another mechanism for Pb2+-induced membrane oxidative damage is the effect on changes in the membrane composition. Because fatty acid chain length and unsaturation are associated with membrane susceptibility to peroxidation, Pb2+-induced arachidonic acid elongation might be responsible for the enhanced lipid peroxidation in the membrane. By causing lateral phase separation and/or by increasing lipid peroxidation rates, Pb2+ could affect membrane related processes such as the activity of membrane enzymes, endo- and exocytosis, the transport of solutes across the bilayer, and signal transduction processes. In addition, Pb2+ can also stimulate Fe2+-initiated membrane lipid oxidation by changing membrane physical properties (Adonaylo and Oteiza, 1999; Ahamed and Siddiqui, 2007). Taken together, these data suggest that altered lipid composition of membranes due to Pb2+ exposure may result in altered membrane integrity, permeability, and function, increasing the susceptibility to lipid peroxidation.
2.4.1.3. Effects on antioxidant defense systems of cells
GSH is a tripeptide containing cysteine that has a reactive thiol group with reductive potency against ROS that represents more than 90% of the non-tissue-related thiol pool of the human body. Eventually, GSH can be involved as a cofactor or a coenzyme in the enzymatic detoxification reactions of ROS. In addition, GSH is an important substrate acting in the metabolism of specific drugs and toxins via conjugation in the liver. Pb2+ binds to the –SH group, which decreases GSH levels and its antioxidant activity. An enzymatic component of the antioxidant defense system, GR, reduces GSSG back to GSH and thereby supports the antioxidant defense system indirectly. GR possesses a disulfide at its active site that constitutes a target for Pb2+, resulting in the inhibition of the enzyme, leading to a decreased GSH/GSSG ratio that will render cells more susceptible to oxidative damage (Carocci et al., 2016; Ercal et al., 1996).
The metalloproteins GPx, CAT, and SOD accomplish their antioxidant functions by enzymatically detoxifying the peroxides (–OOH), H2O2, and O2•−, respectively. CAT decomposes H2O2 into H2O and O2 at higher steady- state H2O2 concentration, while GPx requires GSH to decompose H2O2 or other peroxides with the simultaneous oxidation of GSH into GSSG under lower steady-state levels of H2O2. Since these antioxidant enzymes depend on various essential trace elements and prosthetic groups for proper molecular structure and enzymatic activity, they are potential targets for Pb2+ toxicity. SOD dismutates O2•− into H2O2 and requires Cu2+and Zn2+ for its activity. Thus, three types of SODs exist in mammalian cells that use an essential metal as a cofactor. Cu/Zn-dependent SOD1 and SOD3 are localized in the cytosol (SOD1), the extracellular space (SOD3) and to a lesser extent, in the IMM space of the mitochondria (SOD1), while MnSOD (SOD2) is solely localized in the mitochondrial matrix. The transition metal (Cu2+ or Mn2+) in the SOD active site is required for the breakdown of O2•− by catalyzing both the one-electron oxidation-reduction of separate O2•− to give the overall disproportionation reaction that produces O2 and H2O2. Cu2+ ions have a functional role in the reaction by undergoing alternate oxidation and reduction, where Zn2+ ions confer higher thermal stability to the proteins instead of having a role in the catalytic cycle (Garza-Lombó et al., 2018).
2.4.2. Clinical and experimental studies
Provided the numerous reports that relate Pb2+ detrimental effects on redox balance and the possible link to its neurotoxic effects, what follows is a short description of some pivotal studies stressing relevant conclusions on these effects, with a distinction in between clinical evidence and studies performed in vitro and in laboratory animals focused on blood and/or brain biomarkers status.
2.4.2.1. Clinical evidence
An excellent review regarding epidemiological data has been published recently (Almeida Lopes et al., 2016). Among the studies that met their criteria, the authors divided the population in environmentally-exposed to Pb2+, mostly composed of children and the general population, or workers occupationally-exposed to the metal. They concluded that Pb2+ exposure induced oxidative stress in both populations, with the most frequent biomarkers that showed association with blood Pb2+ levels (BLL) were the antioxidant enzymes, as well as GSH and MDA.
2.4.2.1.1. Environmental exposures:
many reports compared BLL with biomarkers of effects, in particular δ-ALA-d, SOD and CAT activities, MDA and GSH levels as a biochemical profile of redox toxicity. Ahamed et al., in 2005 and 2006 reported a positive association among BLL, MDA levels, and CAT activity and a negative correlation with GSH levels and δ-ALA-d activity in children (Ahamed et al., 2005) and adolescents living in an urban setting exposed to Pb2+ from environmental sources (Ahamed et al., 2006). The authors propose δ-ALA-d not only a biochemical index of Pb2+ exposure but also an early biomarker of oxidative stress. However, other authors reported no significant variations in δ-ALA-d, CAT, and SOD activities at low BLL in a pediatric population (Martínez et al., 2013). Additionally, Pb2+-exposed adults living in urban centers showed a negative correlation with antioxidant enzymes SOD, CAT, GPx activity, and GSH levels (Jangid et al., 2016). On the other hand, Pb2+-contaminated opium is a frequent cause of Pb2+ toxicity in opium addicts from certain countries. In this subpopulation, high levels of lipid peroxidation and protein carbonylation, and a decrease in total antioxidant capacity and in SOD activity were reported (Shojaeepour et al., 2018).
2.4.2.1.2. Occupational exposures:
A high increase in δ-ALA distribution and accumulation was indicated as a triggering event in Pb2+-induced oxidative stress responses in occupationally-exposed workers (Costa et al., 1997). In accordance with these results, Gurer-Orhan et al, in 2004 evaluated workers of a battery plant exposed to Pb2+, a study that reported decreased blood GSH/GSSG ratios and δ-ALA-d activity accompanied by increased MDA levels and CAT activity, all indicative of oxidative stress (Gurer-Orhan et al., 2004). Two independent studies also performed in battery manufacturing workers that reported an increase in lipid peroxidation levels, while a reduction in SOD and CAT activity was evident (Ghanwat et al., 2016; Patil et al., 2006). In another study, the gene expression levels of both SOD1 and GPx1 and SOD and GPx enzymatic activity were increased, while the expression and activity of CAT were unchanged in red blood cells from Pb2+-exposed workers (Kasperczyk et al., 2012). More recently, Kshirsagar et al. (2020) reported significantly increased serum lipid peroxide levels in painters occupationally exposed to Pb2+, likely due to exaggerated ROS generation caused by SOD and CAT inhibition (Kshirsagar et al., 2020).
Overall, these studies suggest that high BLL seem to be associated to increased lipid peroxidation and reduced δ-ALA-d, while the activity of antioxidant enzymes is less consistent across the studies with divergent results, reductions in the presence of high BLL and increases at lower BLL, probably as a compensatory mechanism.
2.4.2.2. Experimental animals and in vitro studies
2.4.2.2.1. Adult animals:
Pioneer experiments performed by Sandhir et al. (1994) demonstrated that chronic Pb2+ exposure accentuated lipid peroxidation in all regions, preferentially in the hippocampus, the area with higher Pb2+ accumulation. The antioxidant enzymes SOD, CAT, GR, and GPx activity was diminished, with a similar trend in GSH, results that suggest that Pb2+ may exert its neurotoxic effects via peroxidative damage to the membranes (Sandhir et al., 1994). Ercal et al. (1996) demonstrated that chronic adult Pb2+ exposure depletes GSH levels, increases GSSG, and promotes MDA production in both liver and brain samples taken from C57BL/6 mice (Ercal et al., 1996). Under the same experimental conditions, Gurer et al. (1998) reported similar results in blood, as well as increases in CAT and glucose 6-phosphate dehydrogenase (G6PD) activity, accompanied by a reduction in δ-ALA-d activity. Resembling clinical studies, the authors suggested that δ-ALA accumulation and autooxidation might contribute to Pb2+-induced oxidative stress (Gurer et al., 1998). In further studies, the same authors reported enhanced MDA content in Pb2+-treated Chinese hamster ovary (CHO) cells and brain tissue of Fisher 344 rats, accompanied by alterations in their antioxidant defense systems, including decreased GSH levels and increased CAT and G6PD activity (Gurer et al., 1999). Several other studies performed in chronically-Pb2+-exposed rats also demonstrated a reduction in δ-ALA-d and an increase in TBARS as compared to controls (Adonaylo and Oteiza, 1999). Liver GSSG and MDA levels were higher in young chronically-exposed Pb2+ rats than those in the adult group, although both brain and blood MDA levels were higher in the older animals exposed to identical treatment (Aykin-Burns et al., 2003). In agreement, adult rats chronically exposed to high Pb2+ levels had high blood SOD and CAT activity and increased MDA content in blood and brain (Soltaninejad et al., 2003). Moreover, Pb2+ exposure caused an increase in brain TBARs and CAT activity, while brain SOD activity levels were unaffected (Antonio-García and Massó-Gonzalez, 2008a). Interestingly, GPx was increased, while GR was reduced in several brain regions of rats chronically-exposed to Pb2+ (Bokara et al., 2009). On the other hand, there is evidence to support that Pb2+ exposure causes genotoxicity, probably as a result of the production of free radicals, but also by a metal inhibition on DNA repair (García-Lestón et al., 2010). In this respect, an increase in DNA damage in lymphocytes of young and adult female rats chronically exposed to Pb2+ has been reported (Nascimento and Martinez, 2016).
2.4.2.2.2. Developing animals:
Pb2+ concentration in blood and brain regions was associated with enhanced lipid peroxidation in rats continuously exposed to the metal from gestation to adolescence (Villeda-Hernández et al., 2001). However, when SOD, GPx, and GR activity was assessed in several brain regions obtained from pre and postnatally Pb2+-exposed pups, only a decrease in SOD in the hypothalamus was evident (Moreira et al., 2001). Another report indicates that plasma and brain δ-ALA concentrations increased significantly immediately after Pb2+-exposure ended at weaning, along with a decrease in activities of SOD, GPx, and GR enzymes in several brain regions, effects that were not evident in adult rats (Wang et al., 2006). A similar pattern of effects was later published (Bokara et al., 2008) showing an increase in lipid peroxidation and antioxidant enzymes (SOD and CAT) activities in young rats’ brains developmentally exposed to Pb2+, an effect that did not persist into adulthood. The consequences of lactational Pb2+ exposure on the onset of oxidative damage late in life was assessed by measuring the levels of 8-hydroxyl-2’-deoxyguanosine (oxo8dG), one of the major products of DNA oxidation, and the associated activity of the DNA repair enzyme 8-oxoguanine DNA glycosylase (Ogg1) in the brain cerebral tissue of aging rats exposed to Pb2+ during postnatal brain development and/or during old age. The results demonstrated that oxo8dG remained elevated in the frontal cortex of these animals only if Pb2+ exposure started at early postnatal age. However, no changes in GSH, Cu/ZnSOD, or MnSOD levels were affected in either scheme of Pb2+ exposure (Bolin et al., 2006). In addition, both gestational and lactational Pb2+ exposure elevated ROS and MDA levels followed by an increase in the Bax/Bcl-2 ratio, promoting apoptosis, while GSH and SOD were significantly decreased, in the pups’ hippocampus at early postnatal age (Lu et al., 2013). It was also demonstrated that pre-and post-natal low-level Pb2+-exposure in rats increases the GSSG/GSH ratio by decreasing the GSH levels and SOD, CAT, and GPx activity, with inconsistent changes in mRNA and protein expression, an effect that the authors attribute to impairments in the catalytic function of the enzyme protein or a reduction in the availability of cofactors (Baranowska-Bosiacka et al., 2012). Further evidence confirms that developmental Pb2+ exposure induced a redox imbalance evidenced by a significant increase in MDA levels and decrease in antioxidant enzymes (GPx, SOD, and CAT activity) in the pups’ hippocampus, the brain region associated with LTP, and learning and memory impairments frequently reported after early Pb2+ exposure (Soleimani et al., 2016).
Overall, as concluded from a meta-analysis of the available literature regarding the effects of Pb2+ on oxidative stress parameters in rodents, the metal significantly increased the levels of oxidants such as MDA, GSSG, ROS, and H2O2, and reduces the levels of antioxidative substances, such as CAT, GPx, GR, GSH, SOD, and GST (Fan et al., 2020). As mentioned before, the reported decreases in the antioxidant enzymes may be the result of the metal affinity for sulfhydryl groups or Pb2+ competition for metal cofactors such as Cu2+ and Zn2+ in the binding sites of these enzymes.
Finally, and regarding genotoxicity and its impact on redox imbalance, according to the International Agency for Research on Cancer, Pb2+ has been identified as a probable human IIB carcinogen, while its inorganic form Pb2+ is included in class IIA (“Inorganic and organic lead compounds.,” 2006). Experimental evidence suggests a facilitating role for Pb2+ in carcinogenesis, allowing or enhancing carcinogenic events involved in DNA damage, repair, and regulation of tumor suppressor and promoter genes (Silbergeld, 2003). From the mechanistic point of view, it has been reported that the genetic damage induced by the metal could be due to indirect mechanisms provided that can substitute Ca2+ and/or Zn2+ in enzymes involved in DNA processing and repair leading to an inhibition of DNA repair and an enhancement in the genotoxicity when combined with other DNA damaging agents. Besides, oxidative stress produced by the increase in free radical levels induced by Pb2+ exposure may also contribute to the indirect genotoxicity of this metal (García-Lestón et al., 2010). However, its role in human carcinogenesis is still a topic of debate with limited epidemiological evidence (Ahn et al., 2020; Fu and Boffetta, 1995; Ferrante et al., 2017).
2.5. THE INFLAMMATORY COMPONENT
The neurotoxic effects of Pb2+ have been long recognized, although only recently new insights have linked the neuroimmune system to its toxicity (Chibowska et al., 2016; Liu, et al., 2015; Metryka et al., 2018). Neuroinflammation is a complex biological response that involves microglial and astroglial activation and many signaling proteins, receptors, and cell types including the production and release of inflammatory cytokines, augmented ROS generation, diminished antioxidant activity, resulting in neuronal injury or neuronal loss in the CNS. In the first place and regarding cytokines importance for BBB permeability, it is reported that the coadministration of inflammatory cytokines and Pb2+ induces the production of matrix metalloproteinase (MMP)-9, a Zn2+-dependent enzyme that degrades the extracellular matrix and basement membrane in astrocytes, resulting in increased permeability of the BBB (Lahat et al., 2002). There are reports that Pb2+ exposure caused a microgliosis and astrogliosis after continuous or intermittent Pb2+ exposure, with Iba-1, and glial fibrillary acidic protein (GFAP) indicators of gliosis (Shvachiy et al., 2018). Similarly, an increase in the hippocampal number of immunoreactive microglial cells (Iba1+) and (Iba1+/TLR4+) has also been reported. Moreover, the microgliosis and astrogliosis in the hippocampus of young mice were probably mediated by the innate immune reaction of toll-like receptors 4 (TLR4), the signal transfer by the adapter myeloid differentiation primary response gene 88 (MyD88) to the intracellular pathway with resultant nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kβ) activation and inflammatory responses. In the same study, the authors reported enhanced expression levels of interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), p38MAPK, and ERK1/2 in the hippocampus, indicating potential implication of inflammatory response and MAPK signaling activation in Pb2+-induced microgliosis and astrogliosis (Liu et al., 2015). In another study, Pb2+ exposure induced the activation of NF-kβ, activator protein-1 (AP-1), c-jun N-terminal kinase (JNK), MAPK, and caspases in the rats’ brain, which may contribute to its neurotoxic effects by apoptotic mechanisms (Ramesh et al., 2001). In addition, astroglial activation, microglia-induced neuroinflammation, and synaptic degeneration, accompanied by the increased level of glial markers, GFAP and S-100b, as well as the production of proinflammatory cytokines, including IL-1β, TNFα, and IL-6, was reported in immature rats’ brain areas as a result of prolonged Pb2+ exposure (Struzyńska et al., 2007). Moreover, brain gene expression for IL-6 and the transforming growth factor-β1 (TGF-β1) was induced as the result of developmental Pb2+ exposure in mice (Kasten-Jolly et al., 2011). Recent data corroborate these findings, reporting that chronic pre-and neonatal Pb2+exposure induces neuroinflammation in the forebrain cortex, hippocampus, and cerebellum of rat pups evidenced by a significant increase in several cytokines and prostanoids [IL-1, IL-6, TGF, prostaglandin E2 (PGE2) and T-box transcription factor 2 (TXB2)]. The protein and mRNA expression of enzymes involved in the inflammatory process (cyclooxygenases) COX-1 and COX-2 increased in all brain structures, as did NF-kβ expression (Chibowska et al., 2020). Additionally, Kumawat et al. (2014) reported up-regulation of ERK and protein kinase B (Akt) pathways, along with activation of NF-kβ. Moreover, TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1), as well as COX-2 pro-inflammatory enzyme levels were increased in response to Pb2+ exposure. It was proposed that pro-inflammatory cytokines secreted by the microglia in response to Pb2+ can induce apoptotic pathways culminating in the activation of the caspase-3 enzyme. Notably, both NF-kβ and AP-1 are redox-sensitive transcription factors that regulate the induction of antioxidant-specific genes by the “antioxidant responsive element” in the promoter region of the CAT or SOD genes (Kumawat et al., 2014). It has been shown that Pb2+causes the induction of oxidative stress and depletion of cellular GSH content, a situation that triggers the activation of NF-kβ and AP-1 binding (Korashy and El-Kadi, 2008). Finally, microglial and astroglial activation is associated with elevated levels of NADPH oxidase (NOx), the main non-mitochondrial source of O2•− and ROS. Importantly, NOx is ubiquitously expressed in microglia and may be activated by IL-1β, TNF-α, IFN-γ, and other pro-inflammatory cytokines (Kasten-Jolly et al., 2011). Interestingly, NOx is activated by Pb2+, promoting ROS accumulation in the brain (Verstraeten et al., 2008). In addition, Pb2+ stimulates the synthesis and secretion of the proinflammatory cytokine IL-8 in a mechanism dependent on Nrf2, a transcriptional factor responsible for the induction of xenobiotic-metabolizing enzymes (Metryka et al., 2018) and a redox-sensitive counterbalancing component. Notably, the microglia is more sensitive to Pb2+-induced oxidative stress, evidenced as increased production of ROS, down-regulation of GSH, and enhanced Nrf2 protein expression, while no obvious changes in astrocytes were observed (Peng et al., 2019). It is worth to mention that the Nrf2 pathway plays a negative regulatory role in response to oxidative stress, an event that has an important role in primary microglial toxicity caused by Pb2+ exposure and may be associated to the pathological basis of Pb-induced neurological dysfunction. On the other hand, Liu et al, 2012 (Liu et al., 2012) raised the hypothesis that activation of microglia participates in the Pb2+-induced hippocampal LTP impairment and neuronal injury. They supported their idea on the bases that microglia activation can impair learning and memory processes via released IL-1. Thus, Pb2+ can cause microglia activation, which can up-regulate the levels of IL-1b, TNF-α, and inducible NOS synthase (iNOS), proinflammatory factors that either alone or in combination may cause hippocampal neuronal injury and LTP impairment leading to learning and memory deficits. Remarkably, iNOS is a Ca2+-independent enzyme with a similar structure to nNOS and eNOS, which is found primarily in immune cells or glial cells (astrocytes and microglia) and is activated in response to pathogen recognition and cytokine release (Nava-Ruiz et al., 2012). Overall, this evidence reveals the tight relationship between pro-oxidant and inflammatory events that may lead to brain injury and eventually cell death, particularly when homeostatic processes are overwhelmed and the Pb2+ induction of redox imbalance lead to neuroinflammation.
Finally, it is noteworthy that more recent evidences point-out neuroinflammation characterized by astroglial and microglial activation as a key factor in the onset and progression of sporadic Alzheimer’s disease (AD) (Huat et al., 2019). In particular, acute Pb2+ exposure is associated with the formation of amyloid plaques as the result of a significant increase of beta-amyloid (Aß) in brain tissue (Gu et al., 2011). Specifically, Pb2+ produced IL-1-associated amyloid precursor protein (APP) over-expression, as well as β-secretase (BACE) and presenilin (PS) generation, leading to Aß accumulation. Moreover, based on previous reports indicating that ROS contribute to astrocyte degeneration, these authors ascribed the induction of IL-1 in the developing brain to a metal-catalyzed oxidative stress mechanism (Ashok et al., 2015). Interestingly, there are other theories that associate AD occurrence to “fetal programming” as the result of Pb2+-exposure and the potential role of neuroepigenetics in neurodegenerative diseases. In this regard, it has been recently demonstrated in experimental animals that Pb2+ neurotoxicity can be mediated through epigenetic mechanisms, including brain DNA methylation, chromatin remodeling by histone post-translational modifications (both acetylation and methylation), and non-coding RNAs, in particular microRNAs (Khalid and Abdollahi, 2019; Xu et al., 2020). Interestingly, an association between BLLs and aberrant DNA methylation in δ-ALA-d and p16 gene leading to epigenetic silencing was recently reported in an environmentally Pb2+-exposed pediatric population. Given these observations, the authors proposed these epigenetic changes as early biomarkers of susceptibility (Yohannes et al., 2020), while other authors considered them as essential tools in addressing risk assessment and proper decision making in the management of Pb2+ exposure (Mani et al., 2019). Furthermore, sex-dependent epigenetic mechanisms were pointed-out as pivotal in the combined developmental neurotoxicity of Pb2+ and prenatal stress in rats (Sobolewski et al., 2018). For a complete revision of the existing literature on the role of Pb2+ in the “fetal origin of adult disease” hypothesis, in particular related to AD pathogenesis, the reader can refer to (Basha and Rajarami, 2010; Bihaqi, 2019; Wang, et al., 2020).
3. AMELIORATION OF PB-NEUROTOXIC EFFECTS
It is clear that preventive measures are preferred over amelioration because once Pb2+ enters the body and reaches its target organs it is almost impossible to remove it completely or to reverse the harmful effects. However, in an event of a Pb2+ intoxication, various chelators and antioxidants are approved to be used either alone or in combination. Details on these compounds’ benefits and pitfalls are provided below.
3.1. AMELIORATION BY ANTIOXIDANTS
Antioxidants are effective in alleviating and treating the oxidative stress-induced toxicity of Pb2+. By interacting with generated ROS, antioxidants prevent radical chain reactions because they inhibit or delay oxidation of a substrate by counteracting free radical-induced damage to macromolecules. The major limitation in the use of antioxidants is related to their poor solubility in aqueous solvents, low absorption, poor bioavailability, and rapid metabolism. To improve their use, nanoparticles, liposomes, micelles, and phospholipid complex have been formulated for better delivery. The most important source of antioxidants is provided by nutritional factors, which can modify Pb2+ absorption, deposition, and toxicity. Next, we will discuss nutrients known to interact with Pb2+ as reviewed by (Ahamed and Siddiqui, 2007; Carocci et al., 2016; Flora et al., 2012; Gurer and Ercal, 2000; Hsu and Guo, 2002):
3.1.1. Metals:
As reviewed before, Pb2+ disrupt Ca2+ homeostasis and interact with its cellular and molecular targets; thereby, a diet with Ca2+ supplementation reduces Pb2+ absorption and toxicity. In this line, there are pieces of evidence that Pb2+ administration during pregnancy and lactation induced significant reductions in antioxidant enzymes, and increased the MDA levels in the cerebellum and hippocampus, effects that were partially reversed by Ca2+ supplementation (Gottipolu and Davuljigari, 2014). In a similar way, the competition between Zn2+ and Pb2+ might decrease the absorption of Pb2+, thus Zn2+ supplementation prevents Pb2+-induced δ-ALA-d inhibition and Pb2+ substitution at various Zn2+-mediated processes and molecular targets, including Cu/ZnSOD. Moreover, combined Zn2+ and Ca2+ supplementation reversed the Pb2+-induced decreases on antioxidant enzymes and an increase in lipid peroxidation and free radical formation, all involved in Pb2+ neurotoxicity in mice (Prasanthi et al., 2010). In the case of Fe2+, its deficiency increases the susceptibility to Pb2+ toxicity and in turn, Pb2+ inhibits δ-ALA-d enzyme and impairs Fe2+ utilization in heme biosynthesis. Finally, selenium influences Pb2+ absorption, being also the cofactor for the antioxidant enzyme GPx, enhancing GSH availability which ultimately leads to ROS neutralization. In this regard, N-acetylcysteine (NAC) supplementation can improve the Pb2+-induced oxidative stress in blood and tissue (including the brain), the burden of Pb2+ on the body, and molecular alterations in DNA (Sharma et al., 2014).
3.1.2. Vitamins and carotenoids:
ascorbic acid (vitamin C) is a low molecular mass antioxidant that binds to and removes Pb2+, increases Pb2+ elimination, and reduces Pb2+-induced lipid peroxidation by quenching ROS together with metal chelation properties. In a study performed in battery manufacturing workers, vitamin C supplementation during one month reduced lipid peroxidation and nitrite formation and enhanced the erythrocytes SOD and CAT activity, although these effects were evident in the absence of a reduction in blood Pb2+ levels (Ghanwat et al., 2016). Vitamin E (α-tocopherol) on the other side, is the generic term used to describe several naturally-occurring compounds that possess the biological activity of α-tocopherol, although is more effective in combination with other antioxidant agents than alone. Vitamin B6 (pyridoxine) has antioxidant effects by promoting GSH generation while its deficiency inhibits GSH biosynthesis by limiting cysteine availability. Finally, β-carotenes prevent lipid peroxidation and increase antioxidant enzymatic activity. In support of these shreds of evidence, experimental studies revealed that alterations in antioxidant enzyme activities and the increase in oxidant production and lipid peroxidation levels in the brain in chronically- Pb2+-intoxicated rats during gestation and lactation were prevented by simultaneous treatment with vitamins A, C, E, B6 and Zn2+ (Antonio-García and Massó-Gonzalez, 2008b). Moreover, combined vitamin E and garlic oil administration act as detoxifying agents and antioxidants by scavenging free radicals, associated to the independent action of garlic oil on the removal of Pb2+ salt as PbS (Sajitha et al., 2010).
3.1.3. Flavonoids and polyphenols:
Flavonoids are naturally polyphenolic compounds present in several foods and beverages that exhibit both antioxidants and chelating properties due to its molecular structure. Alpha-lipoic acid employs a two steps antioxidant activity: first by attacking ROS and preventing the lipid peroxides formation, and second, because it can replenish and regenerate other antioxidants like vitamin C and E. It is frequently used in combination with chelating agents like 2,3-dimercaptosuccinic acid (DMSA). In this regard, a beneficial role of lipoic acid was demonstrated when administered with DMSA/Mi-ADMSA (mono isoamyl meso-2,3-dimercaptosuccinic acid) treatment, due to the effective reversal of altered parameters indicative of oxidative stress compared to monotherapy in acute-Pb2+-intoxicated rats (Pande and Flora, 2002). In the same line, the beneficial effects of lipoic acid on oxidative stress parameters in rats are not related to its ability to remove Pb2+ from target cells as a chelator but rather associated with its thiol antioxidant capacity (Gurer et al., 1999). Quercetin, on the other side, suppressed Pb2+-induced toxicity by reducing oxidative stress, downregulating Hsp-70 and Bak and upregulating Bcl-2 in the rat brain, revealing the involvement of intrinsic pathways in Pb2+-mediated neuronal cells death (Chander et al., 2014). Moreover, quercetin administration decreased Pb content in blood and brain, markedly increased NO production and PKA activity in the brains of Pb-treated mice, and suppressed Pb2+-induced oxidative stress accompanied by an increase in protein kinase B (Akt), calcium/calmodulin kinase II (CaMKII), eNOS, nNOS, and CREB phosphorylation in mice brains, all parameters known to be inhibited by Pb2+ (Liu et al., 2013). Morin is another bioflavonoid that has shown antioxidant and also anti-inflammatory properties. Pb2+ exposure enhanced the Bax/Bcl-2 ratio, facilitated cytochrome c release from the mitochondria resulting in neuronal cell death, all events reversed with morin (Thangarajan et al., 2018). In addition, luteolin is a glycosylated flavonoid compound that is demonstrated to attenuate neuronal damage induced by Pb2+ through the inhibition of the oxidative damage, neuroinflammation, and the cortical cell death in adult rats chronically exposed to Pb2+ (Baty et al., 2020). Moreover, treatment with melatonin can improve motor deficits and oxidative stress protecting the cerebellum against developmental Pb2+-exposure (Bazrgar et al., 2015). In addition, in vivo pharmacological activation of TrkB receptors by small molecules such as 7,8-dihydroxyflavone can reverse long-term effects of chronic Pb2+ exposure on presynaptic terminals, pointing to BDNF-TrkB receptor activation as a promising therapeutic intervention in Pb2+-intoxicated children (Zhang et al., 2018). Tannic acid is another naturally occurring plant polyphenol with antioxidant, anticancer, and anti-inflammatory effects with chelator properties for metals, including Pb2+. In this respect, its administration restored antioxidant status and histopathological alterations in the rat’s brain induced by chronic Pb2+ treatment during adulthood (Ashafaq et al., 2016). Levo-carnitine (L-carnitine) is a water-soluble antioxidant located on the mitochondrial membrane that facilitates the transport of long-chain fatty acids used for the production of energy through the mitochondrial membrane. Its treatment counteracted the effect of Pb2+ in rats, reducing the production of ROS and scavenging free radicals by maintaining and protecting the level of the antioxidant enzymes SOD, CAT, and GPx (El-Sherbini et al., 2017). Ferulic acid, a phenolic acid present in aliments abrogated Pb2+-induced ROS generation, lipid peroxidation, and protein carbonyls in the rat’s cerebellum and hippocampus, while perturbations in the GSH levels and activity of enzymatic antioxidants were also markedly restored (Kumar and Muralidhara, 2014). Ferulic acid treatment significantly, although not completely, protected the cells and could be blocked by Zn2+ protoporphyrin (Zn-PP), and Nrf2 shRNA (Yu et al., 2016). Catechins, on the other side, are polyphenolic compounds contained in green tea, and strong scavengers against O2•−, H2O2, OH•, and NO that resulted in PC12 cells treated by Pb2+ decreased intracellular Ca2+ level and ROS formation as well as improved MMP (Chen et al., 2003). Thymoquinone, which is extracted from Nigella sativa seeds (commonly known as Black seed), possesses therapeutic effects including, antibacterial, anti-inflammatory, and antioxidant properties. Several evidence demonstrate that this compound greatly improves Pb-induced neurotoxicity in early life and provides neuroprotective and antioxidant characteristics in several mouse brain regions (Butt et al., 2018) and improvement of SOD, CAT, and GPx activity and MDA levels in the maternal and fetal brains (Saleh et al., 2019).
In summary, essential elements mainly alter Pb2+ biokinetics both at absorptive and enzymatic sites, whereas vitamins, flavonoids, and polyphenols largely act as antioxidants in preventing toxicity via rebalancing the impaired oxidant/antioxidant balance disrupted by Pb2+ exposure. However, caution should be used as an overdose of some nutrients that may overcome their beneficial effects and produce adverse health effects (Ahamed and Siddiqui, 2007).
3.2. AMELIORATION BY CHELATORS
Numerous evidences indicate that metal toxicity to humans can be treated with human-relevant chelating agents. An ideal chelating agent possesses characteristics like great affinity for the toxic metal, high solubility in water, ability to cross cell membranes, the possibility to oral administration, and low metabolism. A chelating agent forming a stable complex with a toxic metal may shield the metal ion from biological targets, thereby reducing the toxicity, or it may expose the metal to the biological environment, prevent it from being scavenged by biological protective mechanisms and thereby increase the toxicity of the metal (Carocci et al., 2016). In addition to their heavy metal chelating properties, most of these agents have a dithiol group that may act as an O2 radical scavenger and thus inhibit lipid peroxidation (Gurer and Ercal, 2000). Chelating agents are divided into prophylactic chelators, used as a preventive treatment, and therapeutic chelators, used as a treatment for Pb2+ intoxication. The recommended chelator treatment for both acute and chronic Pb2+ intoxication includes: DMSA (succimer), 2,3-dimercaptopropane-1-sulfonate (DMPS), British Anti-Lewisite (BAL) or 2,3-dimercaprol, calcium disodium ethylenediaminetetraacetate (CaNa2EDTA) and D-penicillamine (DPA) (Bjørklund et al., 2017). Penicillamine was the first chelating agent used in the treatment of Pb2+poisoning; BAL on the other side, is rarely used due to the narrow therapeutic window and severe side effects. DMSA is the most efficient and safe chelating agent for Pb2+ exposure while DMPS is less effective than CaNa2EDTA or DMSA. In turn, CaNa2EDTA has side effects because contributes to a greater loss in essential minerals and redistributes Pb2+ to the brain (Kim et al., 2015). The following chelators are under experimentation: ascorbic acid, vitamin B, racemic DMSA, and Mi-ADMS (Blanusa et al., 2012). Besides, some ionophores have been shown to transport Pb2+ across the cell membrane, providing a novel method for reducing the body burden of the metal. Additionally, macrocyclic decadentate or octadentate ligands are suited for the complexation of large metal ions like Pb2+, whereas picolinate ligand is designed to accommodate the Pb2+ ion pair leading to high stability and selectivity (Thuppil and Kaushik, 2012). Finally, herbal compounds are under study, with garlic as a medicinal plant attributed to remarkable therapeutic properties. Its active agent is allicin, which prevents oxidative stress by chelating lead ions and scavenging free radicals (Parsi et al., 2020).
In the first place, the increased ROS, nNOS, and [Ca2+]i along with altered behavioral abnormalities that were supported by changes in neurotransmitter levels as well as a fall in mitochondrial membrane potential, release of cytochrome c, and altered Bcl2/Bax ratio reported in adult rats as a consequence of Pb2+ exposure, were all reverted towards normal levels following combination therapy over monotherapy with CaNa2EDTA or MiADMSA (Flora et al., 2007). In addition, a group of studies performed in developing rats reported an appreciable recovery of Pb2+-induced oxidative stress in several synaptic processes in the brain after ascorbate treatment, suggesting its potential as a therapeutic strategy against early-life Pb2+ exposure (Ahmad et al., 2018; Ahmad et al., 2018; Ahmad et al., 2020). In line with these evidences, concomitant administration of ascorbic acid and Pb2+ reduced Bax protein and increased Bcl-2 in developing rat hippocampus and decreased Pb2+ level in the blood of dams compared with Pb2+-treated only dams, revealing protective effects of ascorbic acid on Pb2+-mediated apoptosis (Han et al., 2007). Further evidence revealed a significant increase in plasma MDA and total oxidant status in Pb-intoxicated rats compared to the control group, while ascorbic acid increased the total antioxidant capacity in these animals and ameliorated the Pb-induced impairment of synaptic plasticity in the hippocampus via its antioxidant activity (Karamian et al., 2015). Furthermore, a group of evidence demonstrated that ascorbic acid treatment during pregnancy can prevent Pb2+-induced impairments in the cerebellar development in rats via the protection of inhibitory neurons and synapses (Nam et al., 2019a, 2019b). On the other hand, Parsi et al. (2020) concluded that garlic is as effective as DPA in significantly reducing blood Pb2+ levels, an amelioration effect that showed fewer side effects and more clinical improvement than DPA (Parsi et al., 2020). Interestingly, Spirulina maxima are more effective than garlic in reducing oxidative stress and lipid peroxidation parameters as well as caspase-3 gene expression in adult rat brain tissue (cerebrum and cerebellum) (Galal et al., 2019).
Although most therapeutic approaches seek to increase Pb2+ excretion by chelation, it should be noted that available chelators can induce adverse health effects and its efficacy in reversing or preventing toxic effects is questionable. Thereby, the search for new substances and therapeutically-safe regimens, including combined chelator administration, is still necessary.
3.3. AMELIORATION BY COMBINATORIAL CHELATING/ANTIOXIDANT THERAPY
When antioxidants are combined with chelating agents, they show a synergism that improved chelating ability. The combination of DMSA with NAC, vitamin E or alpha-lipoic acid was found to have a synergistic effect in preventing oxidative damage. Zinc, at moderate doses, was effective at increasing the chelating ability of CaNa2EDTA. From these assertions, it is evident that antioxidants can be used in standard doses in conjunction with chelating agents with beneficial effects on Pb2+ toxicity (Patrick, 2006b). Multiple studies have demonstrated the effectiveness of coadministration of antioxidant nutrients or essential metal with a chelating agent like CaNa2EDTA or DMSA or the antioxidant alone. Ercal et al, in 1996 demonstrated that pharmacologic interventions with DMSA, which encompass both chelating as well as thiol-mediated antioxidant functions, reduced both, Pb2+ levels in blood and brain as well as indices of oxidative stress (Ercal et al., 1996). Treatment with either NAC (a thiol antioxidant) or succimer (a chelating agent) reversed lead-induced alterations in MDA and GSH content, but only succimer appeared to partially restore δ-ALA-d activity (Gurer et al., 1998). S-adenosyl-L-methionine, the GSH precursor, reduced Pb2+ concentrations in the brain and had beneficial effects in preventing alterations in some biochemical variables, including brain MDA in animals acutely-administered with Pb2+ (Flora and Seth, 1999). The combined administration of NAC and DMSA or MiADMS restored the parameters indicative of Pb2+-induced oxidative stress in rats and decreased the Zn2+ levels that were elevated in response to Pb2+-exposure. The authors concluded that the ideal treatment for Pb2+ intoxication seems to be a combination of a chelator and an antioxidant (Tandon et al., 2002). Nevertheless, in a study performed in Pb2+-poisoned opioid addicts, NAC treatment recovered their antioxidant capacity, although the advantages of NAC in the improvement of DPA efficacy were not evident (Shojaeepour et al., 2018).
With respect to natural herbal compounds, curcumin is the main active constituent of turmeric rhizome (Curcuma longa) with strong anti-inflammatory and antioxidant properties as a radical scavenger and metal chelator against Pb2+ intoxication, reducing the metal levels in rat brain. Although its low aqueous-solubility and rapid metabolism result in poor systemic bioavailability, restricting its oral use, nanocurcumin (curcumin encapsulated in chitosan nanoparticles) has good bioavailability, chelating property, and longer retention time than curcumin (Pal et al., 2015). In corroboration, a recent study performed in adult rats chronically exposed to Pb2+, curcumin attenuates Pb-induced neurotoxicity via inhibition of oxidative stress associated with its chelating activity (Abubakar et al., 2019).
3.4. AMELIORATION BY OTHER COMPOUNDS
Gangliosides are sialic acid-containing glycosphingolipids that are part of cell membranes and abundantly expressed in the CNS. They have referred to as being neuroprotective against Pb2+ induced injuries in the rats’ hippocampal neurons, evidenced as a reduction of cell viability and abnormal autophagic processes (Meng et al., 2016). Olive leaf extract, on the other side, is protected against Pb2+-induced brain damage through apoptosis, oxidative stress, and inflammation inhibition (Seddik et al., 2011). The naturally-occurring cationic polysaccharide, chitosan prevents lipid oxidation in biological systems by scavenging free radical and inhibiting ROS generation. After Pb2+ exposure, GSH and total thiols decreased, while protein oxidation, GSSG, MDA, and ROS increased in blood and tissues compared with the control group. Its administration removed Pb2+ from blood and tissues and reversed partially or totally the alterations in the biochemical variables (Wang et al., 2016). In other studies, tert-butylhydroquinone, an Nrf2 and phase II detoxification enzyme inducer protects against oxidative stress and cell death through the Nrf2/HO-1 (heme oxygenase-1) pathway resultant of Pb2+ toxicity in vivo and in vitro (Ye et al., 2016). In this aspect, it was indicated that hemin administration in SH-SY5Y cells attenuates Pb2+-induced cell death and oxidative stress, providing protection against neurotoxicity by HO-1/carbon monoxide activation (Ye et al., 2018).
4. CONCLUSION
This collective evidence indicates that Pb2+ is a compelling neurotoxicant with not a single well-established molecular mechanism that completely explain its neurotoxicity. Instead, from the vast literature of in vitro, in vivo, and clinical studies available one may conclude that a plethora of effects arise in response to exposure to this metal. Undeniably, Pb2+-associated neurotoxicity is multifactorial in nature and encompasses divalent cation mimicry and oxidative stress as major causes of neuronal cell death. Overall, all the proposed mechanisms discussed above congregate, interrelate, entangle, and lead to neurotoxic manifestations when the compensatory responses are overcome (see Figure 1). As reviewed, Pb2+ exposure results in ROS accumulation and a decrease in antioxidants. This metal is able to bind to GSH sulfhydryl groups and restrict its ability as a ROS scavenger. In addition, increased lipid peroxidation and decreased mitochondrial antioxidant enzymatic activities are serious consequences of Pb2+ toxicity. Thus, the pro-oxidant microenvironment contributes to higher BBB permeability, inducing oxidative damage to cellular molecules, the activation of inflammatory mediators, and damage of tight junctions. Additionally, ROS and RNS overproduction can be secondary to intracellular hypercalcemia, as Ca2+ stimulates enzymes that generate ROS and/or RNS by activating dehydrogenases in the citric acid cycle, a process that accelerates hydrogen output, which in turn increases the electrons flux along with the ETC, an event that increases ROS formation. In turn, Ca2+ activates proteases that convert xanthine dehydrogenase into xanthine oxidase, another important source of ROS. Finally, neurons and endothelial cells constitutively express nNOS and eNOS, respectively, both enzymes activated by Ca2+, a process that further promotes a high reactivity between NO and ROS. Thereby, Pb2+ can perturb Ca2+-dependent functions by direct interference with Ca2+ transport, storage, or Ca2+ binding sites, or by indirectly altering cell functions required for Ca2+ homeostasis. In any case, the Pb2+/Ca2+ but also Zn2+, Fe2+, and eventually Cu2+ interactions lead to important functional impairments in organelles, cells, tissues, and organ systems intoxicated with Pb2+. Eventually, a presumed sequence of events may start with the rise in [Ca2+]i that first triggers intrinsic generation of mitochondrial dysfunction and oxidative damage that altogether promotes apoptotic neuronal death.
Figure 1: Mechanisms implicated in lead neurotoxicity.
The funnel represents the confluent nature of the discussed mechanisms, that alone or in combination may trigger Pb2+ neurotoxicity. See the text for details on amelioration strategies and on key players participating in each mechanism (depicted on both sides of the figure). ROS, reactive oxygen species; RNS, reactive nitrogen species; mitoROS, mitochondrial-generated ROS; Ca2+, calcium; Zn2+, zinc; Fe2+, iron; BBB, blood-brain barrier; BCB, blood-cerebrospinal fluid barrier.
Certainly, early-life environmental exposures make the brain vulnerable to an adverse priming event. Similarly, late-life vulnerabilities suggest that environmental toxicants may play an important role in the dysfunctions commonly observed in aging and diseases of aging. Accordingly, a better understanding of an organism response to these adverse molecular events may converge to propose corrective approaches for the prevention of Pb-mediated neurotoxicity. The evidence of ameliorative effects of antioxidants, anti-inflammatory compounds, or chelators alone or in combination may emerge as a new perspective for therapeutic interventions against Pb-induced neurotoxicity.
Thus, in spite of the presumed allostatic processes that may take place as a result of synaptic plasticity or eventual ameliorative approaches, it should be recognized that Pb2+ alterations may cause enduring changes in the brain that affect the individual performance at the behavioral, emotional, intellectual, and social level. This is particularly true when exposure occurs during development, making both scenarios not mutually excluding. As Dr. Silbergeld concludes her excellent article: “early-life Pb2+ detrimental effects may reverse, persist, be progressive or remain unmasked until a new event challenges the brain allowing them to reemerge”. Thus, the research community is encouraged to move on and pursue additional studies to better understand the toxic mechanisms associated with Pb exposure during all life-stages, acknowledging that additional information may also provide insight into therapeutic modalities for Pb poisoning.
“TWO DISTINCT FORMS OF LEAD NEUROTOXICITY”.
As Dr. Silbergeld proposed almost 30 years ago, “The complexity of lead neurotoxicity in exposed humans and the interactions of age with dose suggest there may be several mechanisms of toxicity, and at least two distinct forms of lead neurotoxicity, involving specific mechanisms and resulting in specific expressions of altered neuronal function. Both expressions of neurotoxicity may occur at the same time within an organism at a given exposure, but the long-term consequences are likely to differ. Basically, I propose that lead exerts neurotoxic effects in the following distinct ways: first, as a neurodevelopmental toxicant, interfering with the hard wiring and differentiation of the CNS; second, as a neuropharmacological toxicant, interfering with ionic mechanisms of neurotransmission” (Silbergeld, 1992).
This chapter hones on the second proposal, which is to elucidate the molecular mechanisms behind lead (Pb2+) effects in the brain, including those on transmitter release and signal transduction, resulting from the presence of Pb2+ in situ in the synapse. To this end, a special emphasis was directed to theories of ion mimicry, mitochondrial dysfunction, redox imbalance, and neuroinflammation. Most importantly, the crosstalk among all these factors is proposed as the confluent mechanism of toxicity. These alterations were thought to be potentially reversible if the metal was removed from the synapsis microenvironment as the result of the cessation of the exposure or as a consequence of amelioration interventions, including chelators, antioxidants substances, anti-inflammatory drugs, or their combination as integrated approaches to mitigate Pb2+ detrimental effects.
Acknowledgements
Supported in part by grants from the Secretaría de Ciencia y Tecnología (SeCyT-UNC), and Fondo para la Investigación Científica y Tecnológica (FONCyT) from Argentina (MBV), and from the National Institute of Environmental Health Sciences (NIEHS) R01ES07331 and R01ES10563 (MA).
REFERENCES
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, 2010. Structure and function of the blood-brain barrier. Neurobiol. Dis 37, 13–25. [DOI] [PubMed] [Google Scholar]
- Abubakar K, Mailafiya MM, Danmaigoro A, Chiroma SM, Rahim EBA, Zakaria MZAB, 2019. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules 9, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adonaylo VN, Oteiza PI, 1999. Lead intoxication: Antioxidant defenses and oxidative damage in rat brain. Toxicology 135, 77–85. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Siddiqui MKJ, 2007. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 383, 57–64. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Siddiqui MKJ, 2007. Environmental lead toxicity and nutritional factors. Clin. Nutr 26, 400–8. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Verma S, Kumar A, Siddiqui MKJ, 2006. Delta-aminolevulinic acid dehydratase inhibition and oxidative stress in relation to blood lead among urban adolescents. Hum. Exp. Toxicol 25, 547–553. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Verma S, Kumar A, Siddiqui MKJ, 2005. Environmental exposure to lead and its correlation with biochemical indices in children. Sci. Total Environ 346, 48–55. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Haque S, Ravinayagam V, Ahmad A, Kamli MR, Barreto GE, Ghulam Md Ashraf, 2020. Developmental lead (Pb)-induced deficits in redox and bioenergetic status of cerebellar synapses are ameliorated by ascorbate supplementation. Toxicology 440, 152492. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Salahuddin M, Alamoudi W, Acharya S, 2018a. Dysfunction of cortical synapse-specific mitochondria in developing rats exposed to lead and its amelioration by ascorbate supplementation. Neuropsychiatr. Dis. Treat 14, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F, Salahuddin M, Alsamman K, Almulla AA, Salama KF, 2018b. Developmental lead (Pb)-induced deficits in hippocampal protein translation at the synapses are ameliorated by ascorbate supplementation. Neuropsychiatr. Dis. Treat 14, 3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Park MY, Kang MY, Shin IS, An S, Kim HR, 2020. Occupational lead exposure and brain tumors: Systematic review and meta-analysis. Int. J. Environ. Res. Public Health 17, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Costa ACS, Radhakrishnan V, Aronstam RS, Albuquerque EX, 1990. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 261, 124–130. [DOI] [PubMed] [Google Scholar]
- Almeida Lopes ACB, Peixe TS, Mesas AE, Paoliello MMB, 2016. Lead exposure and oxidative stress: A systematic review. In: Reviews of Environmental Contamination and Toxicology 216, 193–238. [DOI] [PubMed] [Google Scholar]
- Antonio-García MT, Massó-Gonzalez EL, 2008a. Toxic effects of perinatal lead exposure on the brain of rats: Involvement of oxidative stress and the beneficial role of antioxidants. Food Chem. Toxicol 46, 2089–2095. [DOI] [PubMed] [Google Scholar]
- Antonio-García MT, Massó-Gonzalez EL, 2008b. Toxic effects of perinatal lead exposure on the brain of rats: involvement of oxidative stress and the beneficial role of antioxidants. Food Chem. Toxicol 46, 2089–2095. [DOI] [PubMed] [Google Scholar]
- Aschner M, 1996. The functional significance of brain metallothioneins. FASEB J. 10, 1129–1136. [DOI] [PubMed] [Google Scholar]
- Ashafaq M, Tabassum H, Vishnoi S, Salman M, Raisuddin S, Parvez S, 2016. Tannic acid alleviates lead acetate-induced neurochemical perturbations in rat brain. Neurosci. Lett 617, 94–100. [DOI] [PubMed] [Google Scholar]
- Ashok A, Rai NK, Tripathi S, Bandyopadhyay S, 2015. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci 143, 64–80. [DOI] [PubMed] [Google Scholar]
- Atchison WD, 2003. Effects of Toxic Environmental Contaminants on Voltage-Gated Calcium Channel Function: From Past to Present. J. Bioenerg. Biomembr 35, 507–532. [DOI] [PubMed] [Google Scholar]
- Audesirk G, Audesirk T, 1993. The effects of inorganic lead on voltage-sensitive calcium channels differ among cell types and among channel subtypes. Neurotoxicology 14, 259–65. [PubMed] [Google Scholar]
- Aykin-Burns N, Laegeler A, Kellogg G, Ercal N, 2003. Oxidative effects of lead in young and adult fisher 344 rats. Arch. Environ. Contam. Toxicol 44, 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska-Bosiacka I, Gutowska I, Marchetti C, Rutkowska M, Marchlewicz M, Kolasa A, Prokopowicz A, Wiernicki I, Piotrowska K, Baśkiewicz M, Safranow K, Wiszniewska B, Chlubek D, 2011. Altered energy status of primary cerebellar granule neuronal cultures from rats exposed to lead in the pre- and neonatal period. Toxicology 280, 24–32. [DOI] [PubMed] [Google Scholar]
- Baranowska-Bosiacka I, Gutowska I, Marchlewicz M, Marchetti C, Kurzawski M, Dziedziejko V, Kolasa A, Olszewska M, Rybicka M, Safranow K, Nowacki P, Wiszniewska B, Chlubek D, 2012a. Disrupted pro- and antioxidative balance as a mechanism of neurotoxicity induced by perinatal exposure to lead. Brain Res. 1435, 56–71. [DOI] [PubMed] [Google Scholar]
- Baranowska-Bosiacka I, Gutowska I, Rybicka M, Nowacki P, Chlubek D, 2012b. Neurotoksyczność ołowiu. hipotetyczny molekularny mechanizm zaburzeń funkcji synaptycznych. Neurol. Neurochir. Pol 46, 569–578. [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH, 2003. Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: In vivo evidence for Pb and Zn competition. Int. J. Dev. Neurosci 21, 1–12. [DOI] [PubMed] [Google Scholar]
- Basha R, Rajarami GR, 2010. Developmental exposure to lead and late life abnormalities of nervous system. Indian J. Exp. Biol 48, 636–641. [PubMed] [Google Scholar]
- Baty RS, Hassan KE, Alsharif KF, El-Hennamy RE, Elmahallawy EK, Hafez MM, Moneim AA, Kassab RB, 2020. Neuroprotective role of luteolin against lead acetate-induced cortical damage in rats. Hum. Exp. Toxicol 39, 1200–1212. [DOI] [PubMed] [Google Scholar]
- Bazrgar M, Goudarzi I, Lashkarbolouki T, Elahdadi Salmani M, 2015. Melatonin ameliorates oxidative damage induced by maternal lead exposure in rat pups. Physiol. Behav 151, 178–188. [DOI] [PubMed] [Google Scholar]
- Bechara EJH, 1996. Oxidative stress in acute intermittent porphyria and lead poisoning may be triggered by 5-aminolevulinic acid. Brazilian J. Med. Biol. Res 29, 841–51. [PubMed] [Google Scholar]
- Bergdahl IA, Grubb A, Schütz A, Desnick RJ, Wetmur JG, Sassa S, Skerfving S, 1997. Lead binding to δ-aminolevulinic acid dehydratase (ALAD) in human erythrocytes. Pharmacol. Toxicol 81, 153–158. [DOI] [PubMed] [Google Scholar]
- Bihaqi SW, 2019. Early life exposure to lead (Pb) and changes in DNA methylation: Relevance to Alzheimer’s disease. Rev. Environ. Health 34, 187–195. [DOI] [PubMed] [Google Scholar]
- Bjørklund G, Mutter J, Aaseth J, 2017. Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch. Toxicol 91, 3787–3797. [DOI] [PubMed] [Google Scholar]
- Blajszczak C, Bonini MG, 2017. Mitochondria targeting by environmental stressors: Implications for redox cellular signaling. Toxicology 391, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanusa M, Varnai VN, Piasek M, Kostial K, 2012. Chelators as Antidotes of Metal Toxicity: Therapeutic and Experimental Aspects. Curr Med Chem. 2005, 12, 2771–2794. [DOI] [PubMed] [Google Scholar]
- Bokara KK, Blaylock I, Denise SB, Bettaiya R, Rajanna S, Yallapragada PR, 2009. Influence of lead acetate on glutathione and its related enzymes in different regions of rat brain. J. Appl. Toxicol 29, 452–458. [DOI] [PubMed] [Google Scholar]
- Bokara KK, Brown E, McCormick R, Yallapragada PR, Rajanna S, Bettaiya R, 2008. Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. BioMetals 21, 9–16. [DOI] [PubMed] [Google Scholar]
- Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, Cardozo-Pelaez F, 2006. Exposure to lead (Pb) and the developmental origin of oxidative DNA damage in the aging brain. FASEB J. 20, 788–790. [DOI] [PubMed] [Google Scholar]
- Bouron A, Kiselyov K, Oberwinkler J, 2015. Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflugers Arch. Eur. J. Physiol 467, 1143–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MWB, Deane R, 1993. Permeability of the blood-brain barrier to lead. Neurotoxicology 14, 131–136. [PubMed] [Google Scholar]
- Bressler JP, Goldstein GW, 1991. Mechanisms of lead neurotoxicity 41, 479–484. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Lutkenhoff SD, McCarty GE, Miller RG, 1979. Delays in the postnatal increase of cerebral cytochrome concentrations in lead-exposed rats. Neuropharmacology 18, 83–92. [DOI] [PubMed] [Google Scholar]
- Butt UJ, Shah SAA, Ahmed T, Zahid S, 2018. Protective effects of Nigella sativa L. seed extract on lead induced neurotoxicity during development and early life in mouse models. Toxicol. Res 7, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo G, Bernuzzi F, Recalcati S, 2006. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 1, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, Aschner M, 2015. Mitochondrial Redox Dysfunction and Environmental Exposures. Antioxidants Redox Signal. 23, 578–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G, 2016. Lead toxicity, antioxidant defense and environment. Reviews of Environmental Contamination and Toxicology 238, 45–67. [DOI] [PubMed] [Google Scholar]
- Castellino N, Aloj S, 1969. Intracellular distribution of lead in the liver and kidney of the rat. Br. J. Ind. Med 26, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander K, Vaibhav K, Ejaz Ahmed M, Javed H, Tabassum R, Khan A, Kumar M, Katyal A, Islam F, Saeed Siddiqui M, 2014. Quercetin mitigates lead acetate-induced behavioral and histological alterations via suppression of oxidative stress, Hsp-70, Bak and upregulation of Bcl-2. Food Chem. Toxicol 68, 297–306. [DOI] [PubMed] [Google Scholar]
- Chao SL, Moss JM, Harry GJ, 2007. Lead-induced alterations of apoptosis and neurotrophic factor mRNA in the developing rat cortex, hippocampus, and cerebellum. J. Biochem. Mol. Toxicol 21, 265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez E, Jay D, Bravo C, 1987. The mechanism of lead-induced mitochondrial Ca2+ efflux. J. Bioenerg. Biomembr 19, 285–295. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang X, Jiao H, Zhao B, 2003. Tea catechins protect against lead-induced ROS formation, mitochondrial dysfunction, and calcium dysregulation in PC12 cells. Chem. Res. Toxicol 16, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D, 2001. Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int. J. Toxicol 20, 113–120. [DOI] [PubMed] [Google Scholar]
- Chibowska K, Baranowska-Bosiacka I, Falkowska A, Gutowska I, Goschorska M, Chlubek D, 2016. Effect of lead (Pb) on inflammatory processes in the brain. Int. J. Mol. Sci 17, 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibowska K, Korbecki J, Gutowska I, Metryka E, Tarnowski M, Goschorska M, Barczak K, Chlubek D, Baranowska-bosiacka I, 2020. Pre- and neonatal exposure to lead (Pb) induces neuroinflammation in the forebrain cortex, hippocampus and cerebellum of rat pups. Int. J. Mol. Sci 21, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, 1995. MK-801 subsensitivity following postweaning lead exposure. Neurotoxicology 16, 83–95. [PubMed] [Google Scholar]
- Cory-Slechta DA, Garcia-Osuna M, Greenamyre JT, 1997. Lead-induced changes in NMDA receptor complex binding: Correlations with learning accuracy and with sensitivity to learning impairments caused by MK-801 and NMDA administration. Behav. Brain Res 85, 161–174. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D, 2008. Lifetime consequences of combined maternal lead and stress. Basic Clin. Pharmacol. Toxicol 102, 218–227. [DOI] [PubMed] [Google Scholar]
- Costa CA, Trivelato GC, Pinto AMP, Bechara EJH, 1997. Correlation between plasma 5-aminolevulinic acid concentrations and indicators of oxidative stress in lead-exposed workers. Clin. Chem 43, 1196–1202. [PubMed] [Google Scholar]
- Deane R, Bradbury MWB, 1990. Transport of Lead-203 at the Blood-Brain Barrier During Short Cerebrovascular Perfusion with Saline in the Rat. J. Neurochem 54, 905–914. [DOI] [PubMed] [Google Scholar]
- Deng W, McKinnon RD, Poretz RD, 2001. Lead exposure delays the differentiation of oligodendroglial progenitors in vitro. Toxicol. Appl. Pharmacol 174, 235–244. [DOI] [PubMed] [Google Scholar]
- Ding JJ, Zou RX, He HM, Lou ZY, Xu Y, Wang HL, 2018. Pb inhibits hippocampal synaptic transmission via cyclin-dependent kinase-5 dependent Synapsin 1 phosphorylation. Toxicol. Lett 296, 125–131. [DOI] [PubMed] [Google Scholar]
- Dressier J, Kim KA, Chakraborti T, Goldstein G, 1999. Molecular mechanisms of lead neurotoxicity. Neurochem. Res 24, 595–600. [DOI] [PubMed] [Google Scholar]
- Dribben WH, Creeley CE, Farber N, 2011. Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol. Teratol 33, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas P, Gueldry D, Loireau A, Chomard P, Buthieau AM, Autissier N, 1985. Effects of lead poisoning on properties of brain mitochondria in young rats. C. R. Seances Soc. Biol. Fil 179, 175–183 [PubMed] [Google Scholar]
- El-Sherbini ES, El-Sayed G, El Shotory R, Gheith N, Abou-Alsoud M, Harakeh SM, Karrouf GI, 2017. Ameliorative effects of L-carnitine on rats raised on a diet supplemented with lead acetate. Saudi J. Biol. Sci 24, 1410–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal N, Treeratphan P, Hammond TC, Matthews RH, Grannemann NH, Spitz DR, 1996. In vivo indices of oxidative stress in lead-exposed C57BL/6 mice are reduced by treatment with meso-2,3-dimercaptosuccinic acid or N- acetylcysteine. Free Radic. Biol. Med 21, 157–161. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N, 2005. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Me-tal induced Oxidative Damage. Curr. Top. Med 1, 529–539. [DOI] [PubMed] [Google Scholar]
- Fan G, Zhou F, Feng C, Wu F, Ye W, Wang C, Lin F, Yan J, Li Y, Chen Y, Bi Y, 2013. Lead-induced ER calcium release and inhibitory effects of methionine choline in cultured rat hippocampal neurons. Toxicol. In Vitro 27, 387–395. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhao X, Yu J, Xie J, Li C, Liu D, Tang C, Wang C, 2020. Lead-induced oxidative damage in rats/mice: A meta-analysis. J. Trace Elem. Med. Biol 58, 126443. [DOI] [PubMed] [Google Scholar]
- Fariss MW, Chan CB, Patel M, Van Houten B, Orrenius S, 2005. Role of mitochondria in toxic oxidative stress. Mol. Interv 5, 94–111. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Ledda C, Conti GO, Fiore M, Rapisarda V, Copat C, Sole G, Terzo N, Travali S, 2017. Lead exposure and plasma mRNA expression in ERBB2 gene. Mol. Med. Rep 15, 3361–3365. [DOI] [PubMed] [Google Scholar]
- Finkel T, 2011. Signal transduction by reactive oxygen species. J. Cell Biol 194, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Gupta D, Tiwari A, 2012. Toxicity of lead: a review with recent updates. Interdiscip. Toxicol 5, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora GJS, Seth PK, 1999. Beneficial effects of S-adenosyl-L-methionine on aminolevulinic acid dehydratase, glutathione, and lipid peroxidation during acute lead-ethanol administration in mice. Alcohol 18, 103–108. [DOI] [PubMed] [Google Scholar]
- Flora SJS, Saxena G, Mehta A, 2007. Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: Role of reactive oxygen species and intracellular Ca2+. J. Pharmacol. Exp. Ther 322, 108–116. [DOI] [PubMed] [Google Scholar]
- Florea A-M, Taban J, Varghese E, Alost BT, Moreno S, Büsselberg D, 2013. Lead (Pb 2+) neurotoxicity: Ion-mimicry with calcium (Ca 2+) impairs synaptic transmission. A review with animated illustrations of the pre- and post-synaptic effects of lead . J. Local Glob. Heal. Sci 4. [Google Scholar]
- Fu H, Boffetta P, 1995. Cancer and occupational exposure to inorganic lead compounds: A meta-analysis of published data. Occup. Environ. Med 52, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal MK, Elleithy EMM, Abdrabou MI, Yasin NAE, Shaheen YM, 2019. Modulation of caspase-3 gene expression and protective effects of garlic and spirulina against CNS neurotoxicity induced by lead exposure in male rats. Neurotoxicology 72, 15–28. [DOI] [PubMed] [Google Scholar]
- Garcia-Arenas G, Claudio L, Perez-Severiano F, Rios C, 1999. Lead acetate exposure inhibits nitric oxide synthase activity in capillary and synaptosomal fractions of mouse brain. Toxicol. Sci 50, 244–248. [DOI] [PubMed] [Google Scholar]
- García-Arenas G, Ramírez-Amaya V, Balderas I, Sandoval J, Escobar ML, Ríos C, Bermúdez-Rattoni F, 2004. Cognitive deficits in adult rats by lead intoxication are related with regional specific inhibition of cNOS. Behav. Brain Res 149, 49–59. [DOI] [PubMed] [Google Scholar]
- García-Lestón Julia J, Méndez J, Pásaro E, Laffon B, 2010. Genotoxic effects of lead: An updated review. Environ. Int 36, 623–636. [DOI] [PubMed] [Google Scholar]
- Garza-Lombó C, Posadas Y, Quintanar L, Gonsebatt ME, Franco R, 2018. Neurotoxicity Linked to Dysfunctional Metal Ion Homeostasis and Xenobiotic Metal Exposure: Redox Signaling and Oxidative Stress. Antioxidants Redox Signal. 28, 1669–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza A, Vega R, Soto E, 2006. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit 12, 57–65. [PubMed] [Google Scholar]
- Gavazzo P, Zanardi I, Baranowska-Bosiacka I, Marchetti C, 2008. Molecular determinants of Pb2+ interaction with NMDA receptor channels. Neurochem. Int 52, 329–337. [DOI] [PubMed] [Google Scholar]
- Ghanwat G, Patil A, Patil J, Kshirsagar M, Sontakke A, Ayachit RK, 2016. Effect of vitamin C supplementation on blood lead level, oxidative stress and antioxidant status of battery manufacturing workers of western Maharashtra, India. J. Clin. Diagnostic Res 10, BC08–BC011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanwat GH, Patil AJ, Patil JA, Kshirsagar MS, Sontakke A, Ayachit RK, 2016. Biochemical effects of lead exposure on oxidative stress and antioxidant status of battery manufacturing workers of Western Maharashtra, India. J. Basic Clin. Physiol. Pharmacol 27, 141–146. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Lasley SM, 2007. Developmental lead (Pb) exposure reduces the ability of the NMDA antagonist MK-801 to suppress long-term potentiation (LTP) in the rat dentate gyrus, in vivo. Neurotoxicol. Teratol 29, 385–393. [DOI] [PubMed] [Google Scholar]
- Gill KD, Gupta V, Sandhir R, 2003. Ca2+/calmodulin-mediated neurotransmitter release and neurobehavioural deficits following lead exposure. Cell Biochem. Funct 21, 345–353. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Marchi S, Pinton P, 2018. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol 19, 713–730. [DOI] [PubMed] [Google Scholar]
- Goldstein GW, 1977. Lead encephalopathy: the significance of lead inhibition of calcium uptake by brain mitochondria. Brain Res. 136, 185–188. [DOI] [PubMed] [Google Scholar]
- Goldstein GW, 1993. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicology 14, 97–101. [PubMed] [Google Scholar]
- Gorkhali R, Huang K, Kirberger M, Yang JJ, 2016. Defining potential roles of Pb2+ in neurotoxicity from a calciomics approach. Metallomics 8, 563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipolu RR, Davuljigari CB, 2014. Perinatal Exposure to Lead: Reduction in Alterations of Brain Mitochondrial Antioxidant System with Calcium Supplement. Biol. Trace Elem. Res 162, 270–277. [DOI] [PubMed] [Google Scholar]
- Gu H, Wei X, Monnot AD, Fontanilla CV, Behl M, Farlow MR, Zheng W, Du Y, 2011. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci. Lett 490, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Miceli RC, Jett DA, 1995. Biochemical evidence of an interaction of lead at the zinc allosteric sites of the NMDA receptor complex: Effects of neuronal development. Neurotoxicology 16, 63–71. [PubMed] [Google Scholar]
- Gupta PK, 2016. Toxic effects of metals. In: Fundamentals of Toxicology: Essential Concepts and Applications. Academic Press. [Google Scholar]
- Gupta RK, Gupta RC, 2019. Biomarkers in Toxicology. In: Biomarkers of Blood–Brain Barrier Dysfunction,. Elsevier Inc. [Google Scholar]
- Gurer-Orhan H, Sabir HU, Özgüneş H, 2004. Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology 195, 147–154. [DOI] [PubMed] [Google Scholar]
- Gurer H, Ercal N, 2000. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med 29, 927–945. [DOI] [PubMed] [Google Scholar]
- Gurer H, Ozgunes H, Neal R, Spitz DR, Ercal N, 1998. Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128, 181–189. [DOI] [PubMed] [Google Scholar]
- Gurer H, Ozgunes H, Oztezcan S, Ercal N, 1999. Antioxidant role of α-lipoic acid-in lead toxicity. Free Radic. Biol. Med 27, 75–81. [DOI] [PubMed] [Google Scholar]
- Habermann E, Crowell K, Janicki P, 1983. Lead and Other Metals can Substitute for Ca2+ in Calmodulin. Arch Toxicol. 54, 61–70. [DOI] [PubMed] [Google Scholar]
- Halliwell B, 2001. Role of Free Radicals in the Neurodegenerative Diseases Therapeutic Implications for Antioxidant Treatment 18, 685–716. [DOI] [PubMed] [Google Scholar]
- Halliwell B, 1992. Reactive oxygen species and the central nervous system. J Neurochem 59, 1609–1623. [DOI] [PubMed] [Google Scholar]
- Han JM, Chang BJ, Li TZ, Choe NH, Quan FS, Jang BJ, Cho IH, Hong HN, Lee JH, 2007. Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res. 1185, 68–74. [DOI] [PubMed] [Google Scholar]
- Song H, Zheng G, Shen XF, Liu XQ, Luo WJ, Chen JY, 2014. Reduction of Brain Barrier Tight Junctional Proteins by Lead Exposure: Role of Activation of Nonreceptor Tyrosine Kinase Src via Chaperon GRP78. Toxicol. Sci 138, 393–402. [DOI] [PubMed] [Google Scholar]
- Holtzman D, Hsu JS, Mortell P, 1978. In vitro effects of inorganic lead on isolated rat brain mitochondrial respiration. Neurochem. Res 206, 195–206. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Bouton CML, Pevsner J, Laterra J, 2000. Induction of vascular endothelial growth factor in human astrocytes by lead: Involvement of a protein kinase c/activator protein-1 complex-dependent and hypoxia-inducible factor 1-independent signaling pathway. J. Biol. Chem 275, 27874–27882. [DOI] [PubMed] [Google Scholar]
- Hsu PC, Guo YL, 2002. Antioxidant nutrients and lead toxicity. Toxicology 180, 33–44. [DOI] [PubMed] [Google Scholar]
- Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R, 2019. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol 431, 1843–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inorganic and organic lead compounds., 2006. IARC Monogr. Eval. Carcinog. Risks Hum 87, 1–471. [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Kotake Y, Miyara M, Aoki K, Sanoh S, 2013. Involvement of decreased glutamate receptor subunit GluR2 expression in lead-induced neuronal cell death. J. Toxicol Sci 38, 513–521. [DOI] [PubMed] [Google Scholar]
- Ishida K, Kotake Y, Sanoh S, Ohta S, 2017. Lead-Induced ERK Activation Is Mediated by GluR2 Non-containing AMPA Receptor in Cortical Neurons. Biol. Pharm. Bull 40, 303–309. [DOI] [PubMed] [Google Scholar]
- Jangid A, Shekhawat V, Pareek H, Yadav D, Sharma P, John P, 2016. Effect of Lead on Human Blood Antioxidant Enzymes and Glutathione. Int. J. Biochem. Res. Rev 13, 1–9. [Google Scholar]
- Jia Q, Du G, Li Y, Wang Z, Xie J, Gu J, Yin G, Zhang S, Gao Y, Zhou F, Feng C, Fan G, 2018. Pb2+ modulates ryanodine receptors from the endoplasmic reticulum in rat brain. Toxicol. Appl. Pharmacol 338, 103–111. [DOI] [PubMed] [Google Scholar]
- Jurkowitz M, Scott KM, Altschuld RA, Merola AJ, Brierley GP, 1974. Ion transport by heart mitochondria. Arch. Biochem. Biophys 165, 98–113. [DOI] [PubMed] [Google Scholar]
- Kapoor SC, Van Rossum GDV, 1984. Effects of Pb2+ added in vitro on Ca2+ movements in isolated mitochondria and slices of rat kidney cortex. Biochem. Pharmacol 33, 1771–1778. [DOI] [PubMed] [Google Scholar]
- Karamian R, Komaki A, Salehi I, Tahmasebi L, Komaki H, Shahidi S, Sarihi A, 2015. Vitamin C reverses lead-induced deficits in hippocampal synaptic plasticity in rats. Brain Res. Bull 116, 7–15. [DOI] [PubMed] [Google Scholar]
- Kasperczyk A, Machnik G, Dobrakowski M, Sypniewski D, Birkner E, Kasperczyk S, 2012. Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology 301, 79–84. [DOI] [PubMed] [Google Scholar]
- Kasten-Jolly J, Heo Y, Lawrence DA, 2011. Central nervous system cytokine gene expression: Modulation by lead. J. Biochem. Mol. Toxicol 25, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten-Jolly J, Lawrence DA, 2018. The cationic (calcium and lead) and enzyme conundrum. J. Toxicol. Environ. Heal. - Part B Crit. Rev 21, 400–413. [DOI] [PubMed] [Google Scholar]
- Kerper LE, Hinkle PM, 1997. Lead uptake in brain capillary endothelial cells: Activation by calcium store depletion. Toxicol. Appl. Pharmacol 146, 127–133. [DOI] [PubMed] [Google Scholar]
- Khalid M, Abdollahi M, 2019. Epigenetic modifications associated with pathophysiological effects of lead exposure. J. Environ. Sci. Heal. - Part C Environ. Carcinog. Ecotoxicol. Rev 37, 235–287. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Ye BJ, Kim BG, Jeon MJ, Kim SY, Hong YS, 2015. Evaluation and management of lead exposure. Ann. Occup. Environ. Med 27, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Chakraborti T, Goldstein GW, Bressler JP, 2000. Immediate Early Gene Expression in PC12 Cells Exposed to Lead : Requirement for Protein Kinase C. J. Neurochem 74, 1140–1146. [DOI] [PubMed] [Google Scholar]
- Kirberger M, Wong HC, Jiang J, Yang JJ, 2013. Metal toxicity and opportunistic binding of Pb2+ in proteins. J. Inorg. Biochem 125, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirberger M, Yang JJ, 2008. Structural differences between Pb2+- and Ca2+-binding sites in proteins: Implications with respect to toxicity. J. Inorg. Biochem 102, 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AOS, 2008. The role of redox-sensitive transcription factors NF-κB and AP-1 in the modulation of the Cyp1a1 gene by mercury, lead, and copper. Free Radic. Biol. Med 44, 795–806. [DOI] [PubMed] [Google Scholar]
- Kshirsagar MS, Patil JA, Patil A, 2020. Increased blood lead level induces oxidative stress and alters the antioxidant status of spray painters. J. Basic Clin. Physiol. Pharmacol 31, 1–7. [DOI] [PubMed] [Google Scholar]
- Kumar V, Tripathi VK, Jahan S, Agrawal M, Pandey A, Khanna VK, Pant AB, 2015. Lead Intoxication Synergies of the Ethanol-Induced Toxic Responses in Neuronal Cells—PC12. Mol. Neurobiol 52, 1504–1520. [DOI] [PubMed] [Google Scholar]
- Kumawat KL, Kaushik DK, Goswami P, Basu A, 2014. Acute exposure to lead acetate activates microglia and induces subsequent bystander neuronal death via caspase-3 activation. Neurotoxicology 41, 143–153. [DOI] [PubMed] [Google Scholar]
- Lahat N, Shapiro S, Froom P, Kristal-Boneh E, Inspector M, Miller A, 2002. Inorganic lead enhances cytokine-induced elevation of matrix metalloproteinase MMP-9 expression in glial cells. J. Neuroimmunol 132, 123–128. [DOI] [PubMed] [Google Scholar]
- Lalith Kumar V, Muralidhara, 2014. Ameliorative Effects of Ferulic Acid Against Lead Acetate-Induced Oxidative Stress, Mitochondrial Dysfunctions and Toxicity in Prepubertal Rat Brain. Neurochem. Res 39, 2501–2515. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME, 1999. Lead inhibits the rat N-methyl-D-aspartate receptor channel by binding to a site distinct from the zinc allosteric site. Toxicol. Appl. Pharmacol 159, 224–233. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Green MC, Gilbert ME, 2001. Rat hippocampal NMDA receptor binding as a function of chronic lead exposure level. Neurotoxicol. Teratol 23, 185–189. [DOI] [PubMed] [Google Scholar]
- Laterra J, Dressler JP, Inourti RR, Belloni-Olivi L, Goldstein GW, 1992. Inhibition of astroglia-induced endothelial differentiation by inorganic lead: A role for protein kinase C. Proc. Natl. Acad. Sci. U. S. A 89, 10748–10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ZS, Zheng W, 2007. Early lead exposure increases the leakage of the blood-cerebrospinal fluid barrier, in vitro. Hum. Exp. Toxicol 26, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu XL, Zhou XL, Jiang SJ, Yuan H, 2015. Expression of calmodulin-related genes in lead-exposed mice. Interdiscip. Toxicol 8, 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS, 2003. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 126, 5–19. [DOI] [PubMed] [Google Scholar]
- Lindahl LS, Bird L, Legare ME, Mikeska G, Bratton GR, Tiffany-Castiglioni E, 1999. Differential ability of astroglia and neuronal cells to accumulate lead: Dependence on cell type and on degree of differentiation. Toxicol. Sci 50, 236–243. [DOI] [PubMed] [Google Scholar]
- Liu C, Zheng G, Cheng C, Sun J, 2013. Quercetin Protects Mouse Brain against Lead-induced Neurotoxicity Quercetin Protects Mouse Brain against Lead-induced Neurotoxicity. J. Agric. Food Chem 61, 7630–7635. [DOI] [PubMed] [Google Scholar]
- Liu JT, Chen BY, Zhang JQ, Kuang F, Chen LW, 2015a. Lead exposure induced microgliosis and astrogliosis in hippocampus of young mice potentially by triggering TLR4-MyD88-NFκB signaling cascades. Toxicol. Lett 239, 97–107. [DOI] [PubMed] [Google Scholar]
- Liu JT, Dong MH, Zhang JQ, Bai Y, Kuang F, Chen LW, 2015b. Microglia and astroglia: The role of neuroinflammation in lead toxicity and neuronal injury in the brain. Neuroimmunol. Neuroinflammation 2, 131–137. [Google Scholar]
- Liu MC, Liu XQ, Wang W, Shen XF, Che HL, Guo YY, Zhao MG, Chen JY, Luo WJ, 2012. Involvement of Microglia Activation in the Lead Induced Long-Term Potentiation Impairment. PLoS One 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Jin C, Yang J, Liu Q, Wu S, Li D, Guan Y, Cai Y, 2013. Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats’ hippocampus. Biol. Trace Elem. Res 151, 75–84. [DOI] [PubMed] [Google Scholar]
- Luo W, Ruan D, Yan C, Yin S, Chen J, 2012. Effects of chronic lead exposure on functions of nervous system in Chinese children and developmental rats. Neurotoxicology 33, 862–871. [DOI] [PubMed] [Google Scholar]
- Mani MS, Kabekkodu SP, Joshi MB, Dsouza HS, 2019. Ecogenetics of lead toxicity and its influence on risk assessment. Hum. Exp. Toxicol 38, 1031–1059. [DOI] [PubMed] [Google Scholar]
- Marchetti C, 2003. Molecular targets of lead in brain neurotoxicity. Neurotox. Res 5, 221–235. [DOI] [PubMed] [Google Scholar]
- Marchetti C, 2014. Interaction of metal ions with neurotransmitter receptors and potential role in neurodiseases. [DOI] [PubMed] [Google Scholar]
- Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P, 2018. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69, 62–72. [DOI] [PubMed] [Google Scholar]
- Markovac J, Goldstein GW, 1988. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 334, 71–37. [DOI] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Chakraborty S, Fretham SJB, Aschner M, 2012. Cellular transport and homeostasis of essential and nonessential metals. Metallomics 4, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez SA, Simonella L, Hansen C, Rivolta S, Cancela LM, Virgolini MB, 2013. Blood lead levels and enzymatic biomarkers of environmental lead exposure in children in Córdoba, Argentina, after the ban of leaded gasoline. Hum. Exp. Toxicol 32, 449–63. [DOI] [PubMed] [Google Scholar]
- Mazzolini M, Traverso S, Marchetti C, 2001. Multiple pathways of Pb(2+) permeation in rat cerebellar granule neurones J. Neurochem 79, 407–416. [DOI] [PubMed] [Google Scholar]
- Meng H, Wang L, He J, Wang Z, 2016. The protective effect of gangliosides on lead (Pb)-induced neurotoxicity is mediated by autophagic pathways. Int. J. Environ. Res. Public Health 13, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, Chlubek D, Baranowska-Bosiacka I, 2018. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int. J. Mol. Sci 19, 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Hartman JH, Mello DF, 2018. Mitochondrial Toxicity. Toxicol. Sci 162, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Leung MCK, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS, 2013. Mitochondria as a target of environmental toxicants. Toxicol. Sci 134, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira EG, Rosa G.J. de M., Barros SBM, Vassilieff VS, Vassillieff I, 2001. Antioxidant defense in rat brain regions after developmental lead exposure. Toxicology 169, 145–151. [DOI] [PubMed] [Google Scholar]
- Moro L, 2020. Mitochondria at the Crossroads of Physiology and Pathology. J. Clin. Med 9, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata D, Arai K, Iijima M, Sesaki H, 2020. Mitochondrial division, fusion and degradation. J. Biochem 167, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, 2009. How mitochondria produce reactive oxygen species 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam E, Han J, Suh JM, Yi Y, Lim MH, 2018. Link of impaired metal ion homeostasis to mitochondrial dysfunction in neurons. Curr. Opin. Chem. Biol 43, 8–14. [DOI] [PubMed] [Google Scholar]
- Nam SM, Cho IS, Seo JS, Go TH, Kim JH, Nahm SS, Chang BJ, Lee JH, 2019a. Ascorbic Acid Attenuates Lead-Induced Alterations in the Synapses in the Developing Rat Cerebellum. Biol. Trace Elem. Res 187, 142–150. [DOI] [PubMed] [Google Scholar]
- Nam SM, Seo JS, Nahm SS, Chang BJ, 2019b. Effects of ascorbic acid treatment on developmental alterations in calcium-binding proteins and gamma-aminobutyric acid transporter 1 in the cerebellum of lead-exposed rats during pregnancy and lactation. J. Toxicol. Sci 44, 799–809. [DOI] [PubMed] [Google Scholar]
- Nascimento CRB, Martinez C.B. dos R., 2016. Daily intake of lead in Wistar rats at different ages: Biochemical, genotoxic and physiological effects. Environ. Toxicol. Pharmacol 41, 132–141. [DOI] [PubMed] [Google Scholar]
- Nava-Ruiz C, Méndez-Armenta M, Ríos C, 2012. Lead neurotoxicity: Effects on brain nitric oxide synthase. J. Mol. Histol 43, 553–563. [DOI] [PubMed] [Google Scholar]
- Neal AP, Guilarte TR, 2010. Molecular neurobiology of lead (Pb2+): Effects on synaptic function. Mol. Neurobiol 42, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR, 2010. Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: Potential role of NMDA receptor-dependent BDNF signaling. Toxicol. Sci 116, 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AP, Worley PF, 2011. NeuroToxicology Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition 32, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR, 2000. N-methyl-D-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience 99, 233–242. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Guilarte TR, 1999. NNMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Mol. Brain Res 66, 42–49. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Guilarte TR, 2001. Molecular changes in glutamatergic synapses induced by Pb2+: Association with deficits of LTP and spatial learning. Neurotoxicology 22, 635–643. [DOI] [PubMed] [Google Scholar]
- Ordemann JM, Austin RN, 2016. Lead neurotoxicity: Exploring the potential impact of lead substitution in zinc-finger proteins on mental health. Metallomics 8, 579–588. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P, 2003. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol 4, 552–565. [DOI] [PubMed] [Google Scholar]
- Pal M, Sachdeva M, Gupta N, Mishra P, Yadav M, Tiwari A, 2015. Lead Exposure in Different Organs of Mammals and Prevention by Curcumin–Nanocurcumin: a Review. Biol. Trace Elem. Res 168, 380–391. [DOI] [PubMed] [Google Scholar]
- Pande M, Flora SJS, 2002. Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology 177, 187–96. [DOI] [PubMed] [Google Scholar]
- Parr DR, Harris EJ, 1976. The effect of lead on the calcium handling capacity of rat heart mitochondria. Biochem. J 158, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsi A, Ghorbani A, Hesam S, Hosseini M, 2020. Comparison of Garlic therapeutic effects and standard therapy with De Penicillamine in patients with Lead poisoning. J. Adv. Pharm. Edu. Res 10, 84–89. [Google Scholar]
- Patil AJ, Bhagwat VR, Patil JA, Dongre NN, Ambekar JG, Jailkhani R, Das KK, 2006. Effect of lead (Pb) exposure on the activity of superoxide dismutase and catalase in battery manufacturing workers (BMW) of Western Maharashtra (India) with reference to heme biosynthesis. Int. J. Environ. Res. Public Health 3, 329–337. [DOI] [PubMed] [Google Scholar]
- Patrick L, 2006a. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern. Med. Rev 11, 2–22. [PubMed] [Google Scholar]
- Patrick L, 2006b. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev 11, 114–127. [PubMed] [Google Scholar]
- Peng J, Zhou F, Wang Y, Xu Y, Zhang H, Zou F, Meng X, 2019. Differential response to lead toxicity in rat primary microglia and astrocytes. Toxicol. Appl. Pharmacol 363, 64–71. [DOI] [PubMed] [Google Scholar]
- Piatt B, Biisselberg D, 1994. Combined Actions of Pb2+ Zn2+, and A13+ on Voltage-Activated Calcium Channel Currents. Cell. Mol. Neurobiol 14, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounds JG, 1984. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: A review. Neurotoxicology 5, 295–331. [PubMed] [Google Scholar]
- Prasanthi RPJ, Devi CB, Basha DC, Reddy NS, Reddy GR, 2010. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int. J. Dev. Neurosci 28, 161–167. [DOI] [PubMed] [Google Scholar]
- Pulido MD, Parrish AR, 2003. Metal-induced apoptosis : mechanisms Mutat. Res 533, 227–241. [DOI] [PubMed] [Google Scholar]
- Rafałowska U, Struzyńska L, Dabrowska-Bouta B, Lenkiewicz A, 1996. Is lead toxicosis a reflection of altered energy metabolism in brain synaptosomes? Acta Neurobiol. Exp. (Wars) 56, 611–617. [DOI] [PubMed] [Google Scholar]
- Ramesh GT, Manna SK, Aggarwal BB, Jadhav AL, 2001. Lead exposure activates nuclear factor kappa B, activator protein-1, c-Jun N-terminal kinase and caspases in the rat brain. Toxicol. Lett 123, 195–207. [DOI] [PubMed] [Google Scholar]
- Reddy GR, Zawia NH, 2000. Lead exposure alters Egr-1 DNA-binding in the neonatal rat brain. Int. J. Dev. Neurosci 18, 791–795. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhães PJ, Di Virgilio F, Pozzan T, 2003. Calcium and apoptosis: Facts and hypotheses. Oncogene 22, 8619–8627. [DOI] [PubMed] [Google Scholar]
- Rocha A, Trujillo KA, 2019. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 73, 58–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq S, Ghazala Z, Chowdhury A, Büsselberg D, 2012. Metal toxicity at the synapse: Presynaptic, postsynaptic, and long-term effects. J. Toxicol 2012, 132671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajitha GR, Jose R, Andrews A, Ajantha KG, Augustine P, Augusti KT, 2010. Garlic oil and vitamin e prevent the adverse effects of lead acetate and ethanol separately as well as in combination in the drinking water of rats. Indian J. Clin. Biochem 25, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh HA, Abd El-Aziz GS, Mustafa HN, El-Fark M, Mal A, Aburas M, Deifalla AH, 2019. Thymoquinone ameliorates oxidative damage and histopathological changes of developing brain neurotoxicity. J. Histotechnol 42, 116–127. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guerra M, Peng C, Trevisi L, Cardenas A, Wilson A, Osorio-Yáñez C, Niedzwiecki MM, Zhong J, Svensson K, Acevedo MT, Solano-Gonzalez M, Amarasiriwardena CJ, Estrada-Gutierrez G, Brennan KJM, Schnaas L, Just AC, Laue HE, Wright RJ, Téllez-Rojo MM, Wright RO, Baccarelli AA, 2019. Altered cord blood mitochondrial DNA content and pregnancy lead exposure in the PROGRESS cohort. Environ. Int 125, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, Tchounwou PB, 2009. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review 24, 15–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Julka D, Dip Gill K, 1994. Lipoperoxidative damage on lead exposure in rat brain and its implications on membrane bound enzymes. Pharmacol. Toxicol 74, 66–71. [DOI] [PubMed] [Google Scholar]
- Seddik L, Bah TM, Aoues A, Slimani M, Benderdour M, 2011. Elucidation of mechanisms underlying the protective effects of olive leaf extract against lead-induced neurotoxicity in Wistar rats. J. Toxicol. Sci 36, 797–809. [DOI] [PubMed] [Google Scholar]
- Selvín-Testa A, Capani F, Fabián Loidl C, Pecci-Saavedra J, 1997. The nitric oxide synthase expression of rat cortical and hippocampal neurons changes after early lead exposure. Neurosci. Lett 236, 75–78. [DOI] [PubMed] [Google Scholar]
- Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M, 2002. Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo Neurosci Lett. 329, 45–48. [DOI] [PubMed] [Google Scholar]
- Sharma S, Raghuvanshi BPS, Shukla S, 2014. Toxic effects of lead exposure in rats: Involvement of oxidative stress, genotoxic effect, and the beneficial role of N-acetylcysteine supplemented with selenium. J. Environ. Pathol. Toxicol. Oncol 33, 19–32. [DOI] [PubMed] [Google Scholar]
- Shojaeepour S, Fazeli M, Oghabian Z, Pourgholi L, Mandegary A, 2018. Oxidative stress in opium users after using lead-adulterated opium: The role of genetic polymorphism. Food Chem. Toxicol 120, 571–577. [DOI] [PubMed] [Google Scholar]
- Shvachiy L, Geraldes V, Amaro-Leal Â, Rocha I, 2018. Intermittent low-level lead exposure provokes anxiety, hypertension, autonomic dysfunction and neuroinflammation. Neurotoxicology 69, 307–319. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, 1977. Interactions of lead and calcium on the synaptosomal uptake of dopamine and choline. Life Sci. 20, 309–318. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, 1992. Mechanisms of lead neurotoxicity, or looking beyond the lamppost. FASEB J. 13, 3201–3206. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, 2003. Facilitative mechanisms of lead as a carcinogen. Mutat. Res 533, 121–133. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Adler HS, 1978. Subcellular mechanisms of lead neurotoxicity. Brain Res. 148, 451–467. [DOI] [PubMed] [Google Scholar]
- Simons TJB, 1986. The role of anion transport in the passive movement of lead across the human red cell membrane. J. Physiol 378, 287–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons TJB, 1993. Lead-calcium interactions in cellular lead toxicity. Neurotoxicology 14, 77–85. [PubMed] [Google Scholar]
- Simons TJB, Pocock G, 1987. Lead Enters Bovine Adrenal Medullary Cells Through Calcium Channels. J. Neurochem 48, 383–389. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Varma G, Adams B, Anderson DW, Schneider JS, Cory-Slechta DA, 2018. Developmental lead exposure and prenatal stress result in sex-specific reprograming of adult stress physiology and epigenetic profiles in brain. Toxicol. Sci 163, 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani E, Goudarzi I, Abrari K, Lashkarbolouki T, 2016. The combined effects of developmental lead and ethanol exposure on hippocampus dependent spatial learning and memory in rats: Role of oxidative stress. Food Chem. Toxicol 96, 263–272. [DOI] [PubMed] [Google Scholar]
- Soltaninejad K, Kebriaeezadeh A, Minaiee B, Ostad SN, Hosseini R, Azizi E, Abdollahi M, 2003. Biochemical and ultrastructural evidences for toxicity of lead through free radicals in rat brain. Hum. Exp. Toxicol 22, 417–423. [DOI] [PubMed] [Google Scholar]
- Song H, Zheng G, Liu Y, Shen XF, Zhao ZH, Aschner M, Luo WJ, Chen JY, 2016. Cellular uptake of lead in the blood-cerebrospinal fluid barrier: Novel roles of Connexin 43 hemichannel and its down-regulations via Erk phosphorylation. Toxicol. Appl. Pharmacol 297, 1–11. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Richard Pilsner J, Lu Q, Wright RO, Guilarte TR, 2012. Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb 2+: Implications for an environmental basis of neurodevelopmental disorders. Toxicol. Sci. 127, 277–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanatos R, Sanz A, 2011. Mitochondrial complex I: A central regulator of the aging process. Cell Cycle 10, 1528–1532. [DOI] [PubMed] [Google Scholar]
- Struzyńska L, Da̧browska-Bouta B, Koza K, Sulkowski G, 2007. Inflammation-like glial response in lead-exposed immature rat brain. Toxicol. Sci 95, 156–162. [DOI] [PubMed] [Google Scholar]
- Sukumar P, Beech DJ, 2010. Stimulation of TRPC5 cationic channels by low micromolar concentrations of lead ions (Pb2+). Biochem. Biophys. Res. Commun 393, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon SK, Singh S, Prasad S, Srivastava S, Siddiqui MKJ, 2002. Reversal of lead-induced oxidative stress by chelating agent, antioxidant, or their combination in the rat. Environ. Res 90, 61–66. [DOI] [PubMed] [Google Scholar]
- Thangarajan S, Vedagiri A, Somasundaram S, Sakthimanogaran R, Murugesan M, 2018. Neuroprotective effect of morin on lead acetate- induced apoptosis by preventing cytochrome c translocation via regulation of Bax/Bcl-2 ratio. Neurotoxicol. Teratol 66, 35–45. [DOI] [PubMed] [Google Scholar]
- Thuppil V, Kaushik VS, 2012. Future of lead chelation - Distribution and treatment. J. Krishna Inst. Med. Sci. Univ 1, 6–23. [Google Scholar]
- Tiffany-Castiglioni E, 1993. Cell culture models for lead toxicity in neuronal and glial cells. Neurotoxicology. [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Qian Y, 2001. Astroglia as metal depots: Molecular mechanisms for metal accumulation, storage and release. Neurotoxicology 22, 577–592. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Sierra EM, Wu JN, Rowles TK, 1989. Lead toxicity in neuroglia. In: NeuroToxicology 10, 417–443. [PubMed] [Google Scholar]
- Tomsig JL, Suszkiw JB, 1991. Permeation of Pb2+ through calcium channels: fura-2 measurements of voltage- and dihydropyridine-sensitive Pb2+ entry in isolated bovine chromaffin cells. BBA - Biomembr. 1069, 197–200. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR, 2005. Lead neurotoxicity: From exposure to molecular effects. Brain Res. Rev 49, 529–554. [DOI] [PubMed] [Google Scholar]
- Turrens JF, 2003. Mitochondrial formation of reactive oxygen species. J. Physiol 552, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten SV, Aimo L, Oteiza PI, 2008. Aluminium and lead: Molecular mechanisms of brain toxicity. Arch. Toxicol 82, 789–802. [DOI] [PubMed] [Google Scholar]
- Villalobo A, Ishida H, Vogel HJ, Berchtold MW, 2018. Calmodulin as a protein linker and a regulator of adaptor/scaffold proteins. Biochim. Biophys. Acta - Mol. Cell Res 1865, 507–521. [DOI] [PubMed] [Google Scholar]
- Villeda-Hernández J, Barroso-Moguel R, Méndez-Armenta M, Nava-Ruíz C, Huerta-Romero R, Ríos C, 2001. Enhanced brain regional lipid peroxidation in developing rats exposed to low level lead acetate. Brain Res. Bull 55, 247–251. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu J, Zhang Z, 2006. Oxidative stress in mouse brain exposed to lead. Ann. Occup. Hyg 50, 405–409. [DOI] [PubMed] [Google Scholar]
- Wang Q, Luo W, Zhang W, Liu M, Song H, Chen J, 2011. Involvement of DMT1 +IRE in the transport of lead in an in vitro BBB model. Toxicol. Vitr 25, 991–998. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang J, Xu Y, 2020. Epigenetic basis of lead-induced neurological disorders. Int. J. Environ. Res. Public Health 17, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK, 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci 2, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yan Y, Yu X, Li W, Li B, Qin C, 2016. Protective effects of chitosan and its water-soluble derivatives against lead-induced oxidative stress in mice. Int. J. Biol. Macromol 83, 442–449. [DOI] [PubMed] [Google Scholar]
- Weber EW, Muller WA, 2017. Roles of transient receptor potential channels in regulation of vascular and epithelial barriers. Tissue Barriers 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink RHS, Vijverberg HPM, 2002. Ca2+-independent vesicular catecholamine release in PC12 cells by nanomolar concentrations of Pb2+. J. Neurochem 80, 861–873. [DOI] [PubMed] [Google Scholar]
- Xu J, Ji LD, Xu LH, 2006. Lead-induced apoptosis in PC 12 cells: Involvement of p53, Bcl-2 family and caspase-3. Toxicol. Lett 166, 160–167. [DOI] [PubMed] [Google Scholar]
- Xu J, Yan C. huai, Yang B, Xie H. fang, Zou X. yu, Zhong L, Gao Y, Tian Y, Shen X. ming, 2009a. The role of metabotropic glutamate receptor 5 in developmental lead neurotoxicity. Toxicol. Lett 191, 223–230. [DOI] [PubMed] [Google Scholar]
- Xu J, Yan HC, Yang B, Tong LS, Zou YX, Tian Y, 2009b. Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. J. Negat. Results Biomed 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang T, Zhang J, 2020. Epigenetics and Lead Neurotoxicity. In: Lead Chemistry [Working Title]. [Google Scholar]
- Ye F, Li X, Li F, Li J, Chang W, Yuan J, Chen J, 2016a. Cyclosporin A protects against Lead neurotoxicity through inhibiting mitochondrial permeability transition pore opening in nerve cells. Neurotoxicology 57, 203–213. [DOI] [PubMed] [Google Scholar]
- Ye F, Li X, Li L, Yuan J, Chen J, 2016b. t-BHQ Provides Protection against Lead Neurotoxicity via Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev 2016, 2075915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Li X, Liu Y, Chang W, Liu W, Yuan J, Chen J, 2018. Hemin provides protection against lead neurotoxicity through heme oxygenase 1/carbon monoxide activation. J. Appl. Toxicol 38, 1353–1364. [DOI] [PubMed] [Google Scholar]
- Yohannes YB, Nakayama SM, Yabe J, Nakata H, Toyomaki H, Kataba A, Muzandu K, Ikenaka Y, Choongo K, Ishizuka M, 2020. Blood lead levels and aberrant DNA methylation of the ALAD and p16 gene promoters in children exposed to environmental-lead. Environ. Res 188, 109759. [DOI] [PubMed] [Google Scholar]
- Yu CL, Zhao XM, Niu YC, 2016. Ferulic Acid Protects Against Lead Acetate-Induced Inhibition of Neurite Outgrowth by Upregulating HO-1 in PC12 Cells: Involvement of ERK1/2-Nrf2 Pathway. Mol. Neurobiol 53, 6489–6500. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Crumpton T, Brydie M, Reddy GR, Razmiafshari M, 2000. Disruption of the zinc finger domain: A common target that underlies many of the effects of lead. NeuroToxicology 21, 1069–1080. [PubMed] [Google Scholar]
- Zhang XL, Guariglia SR, McGlothan JL, Stansfield KH, Stanton PK, Guilarte TR, 2015. Presynaptic mechanisms of lead neurotoxicity: Effects on vesicular release, vesicle clustering and mitochondria number. PLoS One 10, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, McGlothan JL, Miry O, Stansfield KH, Loth MK, Stanton PK, Guilarte TR, 2018. 7,8-dihydroxyflavone rescues lead-induced impairment of vesicular release: A novel therapeutic approach for lead intoxicated children. Toxicol. Sci 161, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun LG, Ye LP, Wang B, Li Y, 2008. Lead-induced stress response in endoplasmic reticulum of astrocytes in CNS. Toxicol. Mech. Methods 18, 751–757. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Slavkovich V, Zheng W, 1998. Lead exposure promotes translocation of protein kinase C activities in rat choroid plexus in vitro, but not in vivo. Toxicol. Appl. Pharmacol 149, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF, 2003. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol 192, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Chen Y, Fan G, Feng C, Du G, Zhu G, Li Y, Jiao H, Guan L, Wang Z, 2014. Lead-induced iron overload and attenuated effects of ferroportin 1 overexpression in PC12 cells. Toxicol. Vitr 28, 1339–1348. [DOI] [PubMed] [Google Scholar]
- Zhu G, Fan G, Feng C, Li Y, Chen Y, Zhou F, Du G, Jiao H, Liu Z, Xiao X, Lin F, Yan J, 2013. The effect of lead exposure on brain iron homeostasis and the expression of DMT1/FP1 in the brain in developing and aged rats. Toxicol. Lett 216, 108–123. [DOI] [PubMed] [Google Scholar]