Abstract
Background
Dentinal hypersensitivity is characterized by short, sharp pain from exposed dentine that occurs in response to external stimuli such as cold, heat, osmotic, tactile or chemicals, and cannot be explained by any other form of dental defect or pathology. Laser therapy has become a commonly used intervention and might be effective for dentinal hypersensitivity.
Objectives
To assess the effects of in‐office employed lasers versus placebo laser, placebo agents or no treatment for relieving pain of dentinal hypersensitivity.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 20 October 2020), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2020, Issue 9), MEDLINE Ovid (1946 to 20 October 2020), Embase Ovid (1980 to 20 October 2020), CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 20 October 2020), and LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; from 1982 to 20 October 2020). Conference proceedings were searched via the ISI Web of Science and ZETOC, and OpenGrey was searched for grey literature. The US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
Randomized controlled trials (RCTs) in which in‐office lasers were compared to placebo or no treatment on patients aged above 12 years with tooth hypersensitivity.
Data collection and analysis
Two review authors independently and in duplicate screened the search results, extracted data, and assessed the risk of bias of the included studies. Disagreement was resolved by discussion. For continuous outcomes, we used mean differences (MD) and 95% confidence intervals (CI). We conducted meta‐analyses only with studies of similar comparisons reporting the same outcome measures. We assessed the overall certainty of the evidence using GRADE.
Main results
We included a total of 23 studies with 936 participants and 2296 teeth. We assessed five studies at overall low risk of bias, 13 at unclear, and five at high risk of bias. 17 studies contributed data to the meta‐analyses. We divided the studies into six subgroups based on the type of laser and the primary outcome measure. We assessed the change in intensity of pain using quantitative pain scale (visual analogue scale (VAS) of 0 to 10 (no pain to worst possible pain)) when tested through air blast and tactile stimuli in three categories of short (0 to 24 hours), medium (more than 24 hours to 2 months), and long term (more than 2 months).
Results demonstrated that compared to placebo or no treatment the application of all types of lasers combined may reduce pain intensity when tested through air blast stimuli at short term (MD ‐2.24, 95% CI ‐3.55 to ‐0.93; P = 0.0008; 13 studies, 978 teeth; low‐certainty evidence), medium term (MD ‐2.46, 95% CI ‐3.57 to ‐1.35; P < 0.0001; 11 studies, 1007 teeth; very low‐certainty evidence), and long term (MD ‐2.60, 95% CI ‐4.47 to ‐0.73; P = 0.006; 5 studies, 564 teeth; very low‐certainty evidence). Similarly, compared to placebo or no treatment the application of all types of lasers combined may reduce pain intensity when tested through tactile stimuli at short term (MD ‐0.67, 95% CI ‐1.31 to ‐0.03; P = 0.04; 8 studies, 506 teeth; low‐certainty evidence) and medium term (MD ‐1.73, 95% CI ‐3.17 to ‐0.30; P = 0.02; 9 studies, 591 teeth; very low‐certainty evidence). However, there was insufficient evidence of a difference in pain intensity for all types of lasers when tested through tactile stimuli in the long term (MD ‐3.52, 95% CI ‐10.37 to 3.33; P = 0.31; 2 studies, 184 teeth; very low‐certainty evidence).
Most included studies assessed adverse events and reported that no obvious adverse events were observed during the trials. No studies investigated the impact of laser treatment on participants' quality of life.
Authors' conclusions
Limited and uncertain evidence from meta‐analyses suggests that the application of laser overall may improve pain intensity when tested through air blast or tactile stimuli at short, medium, or long term when compared to placebo/no treatment. Overall, laser therapy appears to be safe. Future studies including well‐designed double‐blinded RCTs are necessary to further investigate the clinical efficacy of lasers as well as their cost‐effectiveness.
Plain language summary
What are the benefits and risks of using lasers to treat tooth hypersensitivity (short, sharp tooth pain)?
Key messages
‐ Lasers may slightly reduce pain after 24 hours. They may reduce pain beyond 24 hours but the evidence is very uncertain. ‐ Lasers do not appear to cause adverse (unwanted) effects. ‐ We need future studies to strengthen the evidence and investigate the impact of laser treatment on quality of life.
What causes tooth hypersensitivity?
Tooth hypersensitivity is short, sharp pain that is not due to a dental disease or problem such as caries (holes in the teeth) and can occur when teeth come into contact with hot or cold food or drinks; cold air; or specific food or drinks such as sugar and fizzy (carbonated) drinks. It can also occur when people brush their teeth or receive professional dental care.
How can we treat tooth hypersensitivity?
An option for treating tooth hypersensitivity is to use laser (light) therapy. Lasers produce a narrow, focused beam of light that is applied to the painful tooth to treat it. Depending on the type of laser used, the treatment either aims to seal off the painful area, or to numb it.
What did we want to find out?
We wanted to find out if lasers work to treat tooth hypersensitivity, and whether they are associated with any unwanted (adverse) effects.
What did we do?
We searched for studies that compared lasers against a placebo (dummy treatment) or no treatment for treating tooth hypersensitivity. We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 23 studies of different durations up to 6 months that involved 936 people (2296 teeth) over 12 years of age with tooth hypersensitivity.
The evidence:
‐ suggests that lasers may slightly reduce pain after 24 hours compared to placebo or no treatment; ‐ is not robust enough to determine if lasers reduce pain beyond 24 hours or not; and ‐ suggests that lasers do not cause unwanted effects.
No studies investigated the impact of laser treatment on people’s quality of life.
What are the limitations of the evidence?
The main limitations of the evidence are that studies:
‐ reported inconsistent results; ‐ were conducted in ways that may have introduced errors into their results; and ‐ produced imprecise results when they were combined together.
Due to these limitations, we have little confidence in the evidence.
How up to date is this evidence?
The evidence is up to date to October 2020.
Summary of findings
Summary of findings 1. Laser compared to placebo/no treatment for patients with dentinal hypersensitivity tested via VAS with the value range from 0 to 10 (no pain to worst possible pain) in response to air blast stimuli.
| Laser compared to placebo/no treatment for patients with dentinal hypersensitivity tested via VAS with the value range from 0 to 10 (no pain to worst possible pain) in response to air blast stimuli | |||||
| Patient or population: patients with dentinal hypersensitivity Setting: dental clinic Intervention: laser Comparison: placebo/no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Number of teeth (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with laser | ||||
| Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) when tested through air blast stimuli ‐ short term (0 to 24 hours) All types of laser | The mean changes in pain intensity ranged from 0.10 to 4.80 | MD 2.24 lower (3.55 lower to 0.93 lower) | 978 (13 RCTs) | ⊕⊕⊝⊝ LOWa | Lasers may reduce pain intensity in the short term when compared to placebo/no treatment. Pain reduction was observed in the lasers group in the short term with a mean VAS score difference of 2.22 suggesting minimal clinical significance. The effective lasers in this category were Er,Cr:YSGG, diode (630 nm to 700 nm), and diode (850 nm to 980 nm) |
| Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) when tested through air blast stimuli ‐ medium term (24 hours to 2 months) All types of laser | The mean changes in pain intensity ranged from 0.10 to 5.18 | MD 2.46 lower (3.57 lower to 1.35 lower) | 1007 (11 RCTs) | ⊕⊝⊝⊝ VERY LOWb,c | Lasers may reduce pain intensity in the medium term when compared to placebo/no treatment but the evidence is very uncertain. Pain reduction was observed in the lasers group in the medium term with a mean VAS score difference of 2.41 suggesting minimal clinical significance. The effective lasers in this category were Er,Cr:YSGG, diode (700 nm to 850 nm), and diode (850 nm to 980 nm) |
| Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) when tested through air blast stimuli ‐ long term (more than 2 months) All types of laser | The mean changes in pain intensity ranged from 0.30 to 4.73 | MD 2.60 lower (4.47 lower to 0.73 lower) | 564 (5 RCTs) | ⊕⊝⊝⊝ VERY LOWc,d | Lasers may reduce pain intensity in the long term when compared to placebo/no treatment but the evidence is very uncertain. Pain reduction was observed in the lasers group in the long term with a mean VAS score difference of 2.60 suggesting minimal clinical significance. The effective lasers in this category were Er,Cr:YSGG and diode (850 nm to 980 nm) |
| Adverse events | No adverse event was noted in the experimental and control groups | 954 (11 RCTs) |
⊕⊕⊝⊝ LOWe | ‐ | |
| Patient‐reported quality of life | Outcome not measured in any of the included studies | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; Er,Cr:YSGG: erbium,chromium:yttrium‐scandium‐gallium‐garnet; MD: mean difference; nm: nanometer; RCT: randomized controlled trial; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded 2 levels for inconsistency: marked heterogeneity is noted among the studies (I2 = 99%). bDowngraded 1 level for risk of bias: blinding of outcome assessors was not performed or unclear in studies that contribute significantly to the participants' pool. Blinding of participants was not performed in 1 of the parallel studies. cDowngraded 2 levels for inconsistency: marked heterogeneity is noted among the studies (I2 = 98%). dDowngraded 1 level for imprecision: wide confidence interval. eDowngraded 1 level each for risk of bias and imprecision.
Summary of findings 2. Laser compared to placebo/no treatment for patients with dentinal hypersensitivity tested via VAS with the value range from 0 to 10 (no pain to worst possible pain) in response to tactile stimuli.
| Laser compared to placebo/no treatment for patients with dentinal hypersensitivity tested via VAS with the value range from 0 to 10 (no pain to worst possible pain) in response to tactile stimuli | |||||
| Patient or population: patients with dentinal hypersensitivity Setting: dental clinic Intervention: laser Comparison: placebo/no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Number of teeth (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with laser | ||||
| Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) when tested through tactile stimuli ‐ short term (0 to 24 hours) All types of laser | The mean changes in pain intensity ranged from 0.10 to 3.17 | MD 0.67 lower (1.31 lower to 0.03 lower) | 506 (8 RCTs) | ⊕⊕⊝⊝ LOWa,b | Lasers may reduce pain intensity in the short term when compared to placebo/no treatment. Pain reduction was observed in the lasers group in the short term with a mean VAS score difference of 0.65 suggesting minimal clinical significance. The effective laser in this category was diode |
| Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) when tested through tactile stimuli ‐ medium term (24 hours to 2 months) All types of laser | The mean changes in pain intensity ranged from 0.30 to 3.60 | MD 1.73 lower (3.17 lower to 0.30 lower) | 591 (9 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c | Lasers may reduce pain intensity in the medium term when compared to placebo/no treatment but the evidence is very uncertain. Pain reduction was observed in the lasers group in the medium term with a mean VAS score difference of 1.14 suggesting minimal clinical significance. The effective lasers in this category were GaAlAs and diode |
| Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) when tested through tactile stimuli ‐ long term (more than 2 months) All types of laser | The mean changes in pain intensity ranged from 0.50 to 2.30 | MD 3.52 lower (10.37 lower to 3.33 higher) | 184 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWd,e,f | There was insufficient evidence of a difference in pain intensity for all types of lasers in the long term |
| Adverse events | No adverse event was noted in the experimental and control groups | 386 (5 RCTs) |
⊕⊕⊝⊝ LOWg | ‐ | |
| Patient‐reported quality of life | Outcome not measured in any of the included studies | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GaAIAs: gallium‐aluminum‐arsenide; MD: mean difference; RCT: randomized controlled trial; VAS: visual analogue scale | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
aDowngraded 1 level for risk of bias: studies with significant contribution to data analysis were rated as at unclear and high risk of bias for randomization and blinding of outcome assessors. bDowngraded 1 level for inconsistency: marked heterogeneity with I2 = 92% and some studies crossing the threshold. cDowngraded 2 levels for inconsistency: marked heterogeneity with I2 = 97% and some studies crossing the threshold. dDowngraded 1 level for risk of bias: the study with significant contribution to data analysis was rated as at unclear risk of bias for selection, detection, and attrition bias. eDowngraded 2 levels for inconsistency: marked heterogeneity with I2 = 99%. fDowngraded 1 level for imprecision: wide confidence interval crossing the threshold. gDowngraded 1 level each for risk of bias and imprecision.
Background
Dentinal hypersensitivity is an independent condition and a common symptom of various dental diseases. Enamel loss and dentine exposure, due to physiological abrasion of enamel or gingival recession, is considered the main cause of dentinal hypersensitivity. Hargreaves and colleagues pointed out that the cervical region of incisors and premolars opposite to the dominant hand tends to be the most affected areas, suggesting that toothbrush abrasion may be an etiologic factor (Hargreaves 2002). Heasman and colleagues aimed to analyze possible risk factors for non‐carious cervical lesions (NCCL) or cervical dentine hypersensitivity in which they concluded that there are no data to support or refute the association between toothbrushing and NCCLs (Heasman 2015). Some iatrogenic factors such as periodontal procedures and tooth whitening techniques have been reported to provoke dentinal hypersensitivity (Addy 2002; Bamise 2008; Litonjua 2003).
The prevalence of dentinal hypersensitivity ranges from 8% to 98% among the adult population, this variety is due to the different diagnostic approaches, time frames, and patient samples used in studies (Addy 2000; Markowitz 2007; Porto 2009; Splieth 2013). Prevalence can be as high as 98% among people with periodontal diseases (Orchardson 2006). The peak prevalence has been variously reported as occurring between the second and fifth decades of life (Al‐Sabbagh 2009).
Description of the condition
Dentinal hypersensitivity (also called dentine hypersensitivity, hyperdentin, hypersensitive dentine, hypersensitive tooth or tooth hypersensitivity) is characterized by short, sharp pain from exposed dentine that occurs in response to external stimuli such as cold, heat, osmotic, tactile, or chemicals (Holland 1997; Shiau 2012; West 2013), and cannot be explained by any other form of dental defect or pathology (Kimura 2000). It is one of the most painful and least predictably treated chronic conditions in dentistry (Kumar 2005), which may cause considerable patient discomfort (Bartold 2006). The sufferers tend to have substantially decreased oral health‐related quality of life (OHRQoL) in comparison with the general population (Bekes 2009). Dentinal hypersensitivity is frequently related with root surface exposure also known as gingival recession (Cortellini 2018).
Description of the intervention
Efficacious management of dentinal hypersensitivity depends upon proper diagnosis in terms of the severity and location of the pain, and elimination of the predisposing factors and causes (CABDH 2003; Porto 2009). To date, numerous clinical interventions have been reported to have a positive effect in reducing dentinal hypersensitivity; these include cavity varnishes, corticosteroids, calcium compounds, oxalates, resins and adhesives, gingival augmentation and laser therapy (Addy 2002; Al‐Sabbagh 2009; Corona 2003; Naghsh 2020; Stabholz 2004). A Cochrane Review on the effects of potassium‐containing toothpastes for the management of dentinal hypersensitivity indicated that there was little reliable evidence to support the effectiveness of these products (Poulsen 2006). Some Chinese authors have reported that the local use of alcoholic extract of propolis might be effective in the treatment of dentinal hypersensitivity (Feng 2010; He 2009), but this evidence should be verified further by more well‐designed clinical trials.
Laser (light amplification by stimulated emission of radiation) is an intense beam of coherent monochromatic light (or other electromagnetic radiation) generated by particular devices. Lasers have a wide range of clinical applications in dentistry and were first used for treatment of dentinal hypersensitivity in 1985 (Matsumoto 1985). To date, several types of lasers have been employed, which could be classified into two categories: low output power lasers (helium‐neon and gallium/aluminium/arsenide (diode)) and middle output power lasers (Nd:YAG (neodymium‐doped:yttrium‐aluminum‐garnet) and carbon dioxide (CO2)) (Kimura 2000). Clinical application of lasers in combination with chemical agents (e.g. fluoride) has also been reported as an effective therapeutic approach for controlling dentinal hypersensitivity (Kumar 2005; Maximiano 2019; Ritter 2006).
How the intervention might work
Several theories have been proposed to explain the mechanism of dentinal hypersensitivity. The most accepted one is the hydrodynamic theory, assuming that the outward fluid movement within the dentinal tubules followed by external stimuli results in neural discharge and subsequently causes a painful sensation (Addy 1990). Scanning electron microscope (SEM) photographs suggest that lasers have the ability to vaporize, fuse, melt, or seal dentinal tubules probably by means of recrystallization of the mineral component of dentine.
The other theory indicates that the Nd:YAG laser could block the depolarization of A delta and C fibres and suppresses generation or transmission or both of the impulse and thus contributes to the direct nerve analgesia (Stabholz 2004). In addition, the combination of Nd:YAG laser and sodium fluoride varnish is reported to have better effectiveness in reducing the number of dentinal tubules (Kumar 2005; Lan 1999).
Most studies apply diode laser at varying wavelengths ranging between 655 nanometers (nm) and 980 nm. In this review, three studies evaluated Er,Cr:YSGG (erbium,chromium:yttrium‐scandium‐gallium‐garnet) laser alone (Lee 2015; Yilmaz 2011b; Yilmaz 2014) or as one experimental group with diode laser as the other (Yilmaz 2011a); one study evaluated Nd:YAG laser (Lier 2002).
Why it is important to do this review
Lasers have a wide range of clinical applications in dentistry and have been used for the treatment of dentinal hypersensitivity since 1985. Several studies have shown that lasers may be more effective compared to other treatments in reducing dentinal hypersensitivity (Kimura 2000; Porto 2009; Sicilia 2009; Yilmaz 2011); however, some researchers argue that they have no significant superiority over placebo (Lier 2002). Moreover, laser therapy has been reported to be less effective in treating severe cases (Al‐Sabbagh 2009). Some adverse effects, such as thermal effects on dental pulp, should not be neglected when lasers are used as a cure for dentinal hypersensitivity (Kimura 2000; Stabholz 2004).
A considerable number of randomized controlled trials (RCTs) have been conducted to explore the effects of different types of lasers in comparison with placebo laser or other treatment methods (Birang 2007; Ipci 2009; Kara 2009; Lier 2002; Sicilia 2009). Several meta‐analysis (He 2010) and systematic reviews (Sgolastra 2011; Sgolastra 2013) have been conducted, but the former was limited to Chinese literature and the latter two were confined to those RCTs only comparing lasers with placebo lasers. Therefore, a Cochrane Review is important to summarize the evidence regarding the effectiveness of lasers in treating dentinal hypersensitivity, and to explore influencing factors such as type of lasers and other associated parameters.
Objectives
To assess the effects of in‐office employed lasers versus placebo laser, placebo agents, or no treatment for relieving pain of dentinal hypersensitivity.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in which in‐office laser therapies were compared to placebo or no treatment. RCTs with split‐mouth design, in which the interventions were randomly assigned to either side of the mouth and the outcomes were blindly assessed, were included.
Types of participants
We considered patients aged above 12 years presenting with self‐reported tooth hypersensitivity confirmed in clinical evaluation.
Types of interventions
The test intervention was any type of in‐office laser having been employed for relieving pain of dentinal hypersensitivity with different radiation parameters such as:
Nd:YAG (neodymium‐doped:yttrium‐aluminum‐garnet);
Er:YAG (erbium‐doped:yttrium‐aluminum‐garnet);
diode of different wavelength (visible red (630 nm to 700 nm), near infrared (700 nm to 850 nm), near infrared 2 (850 nm to 980 nm));
Er,Cr:YSGG (erbium,chromium:yttrium‐scandium‐gallium‐garnet);
CO2 (carbon dioxide) laser.
The control intervention was placebo lasers, placebo agents, or no treatment. We did not consider the at‐home therapeutic approaches due to questionable internal validity and reliability of the study methodology.
Types of outcome measures
Primary outcomes
Changes in intensity of pain using quantitative pain scale (visual analogue scale (VAS)) when tested through air blast stimulus at short (0 to 24 hours), medium (24 hours to 2 months) and long term (more than 2 months).
Changes in intensity of pain using quantitative pain scale (VAS) when tested through tactile stimulus at short (0 to 24 hours), medium (24 hours to 2 months) and long term (more than 2 months).
Secondary outcomes
Adverse outcomes: any unexpected or unpredicted events related to the intervention, especially the serious adverse events leading to discontinuation of treatment.
Patient‐reported quality of life.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials without language or publication status restrictions:
Cochrane Oral Health's Trials Register (to 20 October 2020; see Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9) in the Cochrane Library (searched 20 October 2020; see Appendix 2);
MEDLINE Ovid (1950 to 20 October 2020; see Appendix 3);
Embase Ovid (1980 to 20 October 2020; see Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 20 October 2020; see Appendix 5);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; from 1982 to 20 October 2020; see Appendix 6);
ISI Web of Science (limited to conference proceedings; 1990 to 20 October 2020; see Appendix 7);
ZETOC (limited to conference proceedings; 1993 to 20 October 2020; see Appendix 8);
OpenGrey (1990 to 20 October 2020; see Appendix 9).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid but revised appropriately for each database.
No restrictions were placed on the language or date of publication when searching the electronic databases. Non‐English studies were translated and included in the review.
Searching other resources
The following trial registries were searched for ongoing studies by Cochrane Oral Health's Information Specialist:
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 20 October 2020) (Appendix 10).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 20 October 2020) (Appendix 11);
We also searched the International Association for Dental Research (IADR) website on 20 October 2020 (see Appendix 12).
The reference lists of included studies retrieved by the search were checked to identify additional relevant trials. We contacted researchers in the field to identify unpublished studies.
We checked that none of the included studies in this review were retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
For previous versions of the review, we handsearched the following journals, but we discontinued this search due to poor yield:
Lasers in Medical Science (January 2001 to July 2011)
Photomedicine and Laser Surgery (January 2001 to July 2011)
Periodontology (January 2001 to July 2011)
Journal of Oral Laser Application (January 2001 to July 2011)
West China Journal of Stomatology (January 2001 to July 2011)
Chinese Journal of Stomatology (January 2001 to July 2011)
Journal of Practical Stomatology (January 2001 to July 2011).
Data collection and analysis
Selection of studies
Two review authors (Mina Mahdian (MM) and Soodabeh Behboodi (SB)) assessed the titles and abstracts of studies identified in the searches independently and in duplicate. Full copies of all relevant and potentially relevant trials, those appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, were obtained. The full‐text papers were assessed independently and in duplicate, and any disagreement on the eligibility of trials was resolved through discussion and consensus. All potentially relevant studies that failed to meet the eligibility criteria were excluded and the reasons for their exclusion noted in the 'Characteristics of excluded studies' section of the review. Studies considered suitable for inclusion are described in the 'Characteristics of included studies' section.
Data extraction and management
Two review authors (Yumi Ogata (YO) and SB) extracted data from the included studies independently and in duplicate. The review authors resolved any disagreements by discussion and by consulting a third review author (MM) when necessary. For missing information, review authors contacted corresponding authors of trials to obtain necessary data.
We extracted the following data for each trial: year of publication, study type, duration of trial, duration of follow‐up, study location and setting, inclusion and exclusion criteria, number and demographic characteristics of participants (i.e. sex, age), details of interventions, details of outcomes, sources of funding, adverse events, inclusion of ethics approval and informed consent statements.
Assessment of risk of bias in included studies
Two review authors (YO and Zuhair Natto (ZN)) assessed the risk of bias in the included studies using RoB 1 as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011). Any disagreements between the review authors were discussed and resolved.
RoB 1 is a two‐part tool addressing the following domains.
Sequence generation.
Allocation concealment.
Blinding (of participants, personnel, and outcome assessors).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
Each domain in the tool includes one or more specific entries in a risk of bias table. Within each entry, the first part of the tool describes what was reported to have happened in the study, in sufficient detail to support a judgement about the risk of bias. The second part of the tool assigns a judgement relating to the risk of bias for that entry. This is achieved by assigning a judgement of low risk, high risk, or unclear risk of bias.
After taking into account any additional information provided by the authors of the trials, we grouped studies into the following categories.
Low risk of bias (plausible bias unlikely to seriously alter the results) for all key domains.
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more key domains were assessed as unclear.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more key domains were assessed to be at high risk of bias.
We completed a risk of bias table for each included study and presented the results graphically.
Measures of treatment effect
We compared specific types of lasers employed in dentistry (e.g. Nd:YAG, CO2, He‐Ne (helium–neon) or GaAlAs (gallium‐aluminum‐arsenide) which is a specific type of diode), diode of different wavelengths lasers, with different radiation parameters) for treatment of hypersensitivity with placebo or no treatment. For continuous outcomes (e.g. intensity of pain) we used the mean difference (MD), with respective 95% confidence intervals (CIs).
Unit of analysis issues
If outcomes were reported both at baseline and at follow‐up or at trial endpoints, we extracted both the mean change from baseline and the standard deviation of this mean for each treatment group, as well as the same for endpoint data.
If count data were reported in trials, we extracted the total number of events in each group. We also recorded the total number of participants in each group. If this information was not available, we attempted to extract alternative summary statistics such as rate ratios and confidence intervals, if available. If count data were presented as dichotomous outcomes, we extracted the number of participants in each intervention group and the number of participants in each intervention group who experienced at least one event.
Data from split‐mouth studies were combined with those of parallel studies.
Dealing with missing data
The original trial investigators were contacted to request missing data.
Assessment of heterogeneity
We assessed clinical heterogeneity using the I2 statistic, where values over 50% indicate substantial to considerable heterogeneity (Higgins 2011). However, it was decided not to try to explain the heterogeneity since the main and subgroup analyses were exploratory investigations to generate hypotheses to be tested in future studies. In addition, including different types of laser would cause heterogeneity in the overall analysis. In the subgroup analysis, several factors would consider it such as the distance of stimuli, length of the stimuli, and the time of assessment.
Assessment of reporting biases
We addressed publication bias using a funnel plot if we had at least 10 included studies. However, the number of trials in each meta‐analysis was insufficient to detect any publication bias.
Data synthesis
We pooled data using a fixed‐effect model if there was no significant heterogeneity (I2 ≤ 50%) or if there were less than four studies in an analysis. If there was significant heterogeneity (I2 > 50%) or if there were more than four studies in an analysis then we pooled data using a random‐effects model. Meta‐analyses were done only with studies of similar comparisons reporting the same outcome measures. The analysis of the split‐mouth trials was undertaken using the generic inverse variance method in RevMan (Review Manager 2020), taking into account any clustering. A correlation coefficient of 0.5 was imputed for split‐mouth trials.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was to be performed based on the type of lasers and longevity of effect using the formal test for subgroup differences where there were sufficient studies (at least 10 studies in a meta‐analysis). However, subgroup analyses were not possible due to the small number of studies within each category of comparison. We included these groups as separate analyses instead.
Sensitivity analysis
We planned to conduct sensitivity analyses to investigate the robustness of the results for the primary outcomes by evaluating outcomes in trials with low risk of bias versus those with high risk or unclear risk of bias, or if trials reported dropout rates of 10% or greater, to ascertain differences in outcomes of intention‐to‐treat (ITT) analysis and analysis of completers. However, available data were insufficient to perform these analyses.
Summary of findings and assessment of the certainty of the evidence
We developed summary of findings tables using GRADEpro software (GRADEpro GDT 2015) for the main comparisons and outcomes of this review for all types of lasers. We assessed the certainty of the body of evidence with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias. We categorised the certainty of the body of evidence for each of the outcomes as high, moderate, low, or very low (GRADE 2004).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies tables.
Results of the search
Figure 1 presents the study flow diagram. 1340 records and 4 records were retrieved via electronic searching and other sources, respectively. After removal of duplicates, two review authors independently screened 1307 records; 979 records were discarded after identification of the title or abstract, and full copies of the remaining 328 records were obtained and scrutinized. Any disagreements on the eligibility of trials were resolved through discussions involving at least three review authors. No additional trials in the references of included studies and reviews were considered eligible for this review. The final number of included studies were 23.
1.
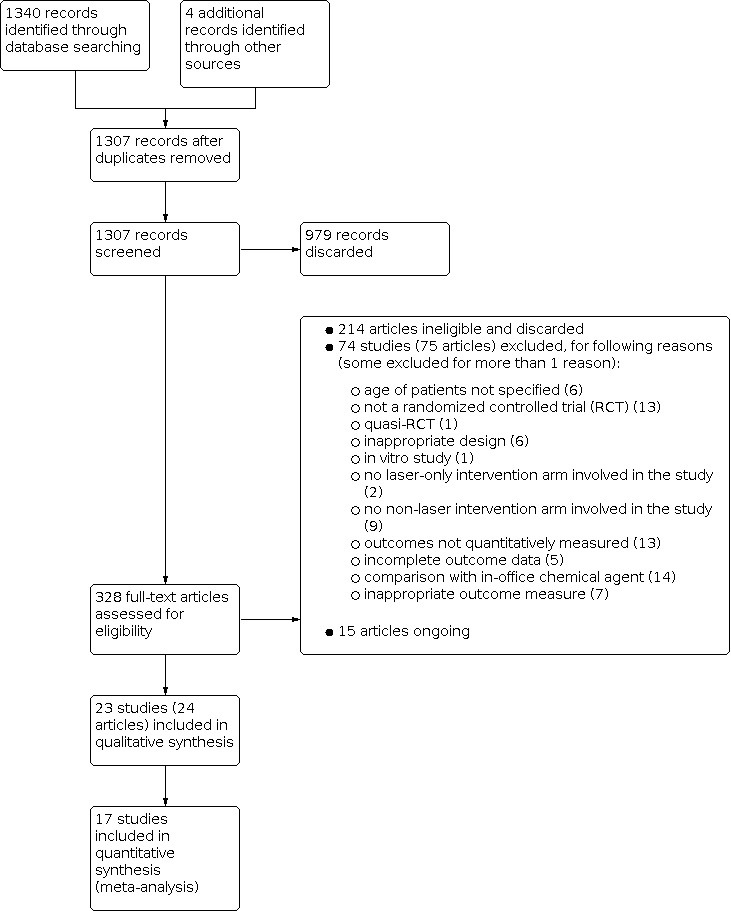
Study flow diagram.
Included studies
Additional Table 3 presents an overview of included studies. The Characteristics of included studies table provides more summarized information.
1. Overview of included studies.
| Studies | Countries | Study designs | Sample sizes | Age ranges | Experimental groups | Control groups | Outcome assessment | Outcome time pointsa | Notes |
| Alencar 2020 | Brazil | RCT (Patient as unit) | 32 patients with 83 teeth | 18 to 50 years | 1. Diode laser 2. Diode laser + nHAP toothpaste |
Placebo (Placebo laser + nHAP‐free toothpaste) | Tactile + evaporative | ‐ Baseline ‐ After the 1st session (short term) ‐ After the 2nd session (medium term) ‐ 1 month after the 2nd session (medium term) |
The treatments were performed in 2 sessions with a 24‐hour interval |
| Bal 2015 | Turkey | RCT (Split‐mouth design) | 21 patients with 156 teeth | 19 to 60 years (Mean 37 years) | Diode laser (685 nm) | Placebo (Saline) | Air blast | ‐ Baseline ‐ Immediate (short term) ‐ 10 days (medium term) ‐ 30 days (medium term) ‐ 60 days (medium term) ‐ 90 days (long term) |
‐ |
| Doshi 2014 | India | RCT (Split‐mouth design) | 30 patients with 60 teeth | 23 to 56 years | Diode laser (660 nm) | Placebo laser | Air blast | ‐ Baseline ‐ Day 1 (short term) ‐ Day 3 (medium term) ‐ Day 5 (medium term) ‐ Day 7 (medium term) |
‐ |
| Flecha 2013 | Italy | RCT (Split‐mouth design) | 62 patients with 434 teeth | 12 to 60 years | Diode laser (795 nm) | Cyanoacrylate (Toothpaste) | Air blast | ‐ Baseline ‐ 1 day (short term) ‐ 1 month (medium term) ‐ 3 months (long term) ‐ 6 months (long term) |
‐ |
| García 2017 | Spain | RCT (Split‐mouth design) | 30 patients with 120 teeth | 19 to 67 years | Diode laser (660 nm) | Placebo laser | 1. Tactile 2. Thermal (air jet from the syringe, isolating adjacent teeth) |
‐ Baseline ‐ Immediately after treatment (short term) ‐ 2 weeks (medium term) ‐ 1 month (medium term) ‐ 2 months (medium term) |
‐ |
| Gentile 2004 | Brazil | RCT (Patient as unit) | 32 patients with 68 teeth | 20 to 52 years | Diode laser (670 nm) | Placebo laser | 1. Tactile 2. Air blast |
‐ Baseline ‐ 6 to 8 weeks (medium term) |
‐ |
| Gerschman 1994 | Australia | RCT (Patient as unit) | 71 patients | 15 to 69 years (Mean 37.5 years) | 1. Diode laser (830 nm) | Placebo laser | 1. Tactile 2. Air blast |
‐Baseline ‐ 1 week (medium term) ‐ 2 weeks (medium term) ‐ 8 weeks (medium term) |
Participants of experimental intervention group (GaAlAs laser) were re‐assigned into subgroups according to the measurements (tactile and air blast) respectively |
| Lee 2015 | South Korea | RCT (Patient as unit) | 82 patients | 20 to 65 years | Er,Cr:YSGG laser | Strontium chloride (SC) toothpaste | 1. Tactile 2. Air blast |
‐ Baseline ‐ 1 week (medium term) ‐ 2 weeks (medium term) ‐ 1 month (medium term) |
‐ |
| Lier 2002 | Norway | RCT (Split‐mouth design) | 17 patients with 34 teeth | 26 to 66 years | Nd:YAG laser | Placebo laser | Air blast | ‐ Baseline ‐ Immediate (short term) ‐ 1 week (medium term) ‐ 4 weeks (medium term) ‐ 16 weeks (long term) |
‐ |
| Lizarelli 2007 | Brazil | RCT (Split‐mouth design) | 60 patients with144 teeth | Minimum 18 years | 1. Diode laser (780 nm and 660 nm) 2. Diode laser (630nm) |
Placebo | 1. Cold and heat 2. Air blast |
‐ After 1sttreatment ‐ After 2nd treatment ‐ After 3rd treatment ‐ 15 days ‐ 30 days ‐ 60 days |
3 irradiation procedures at 7‐day intervals and 3 follow‐up sessions at 15, 30, and 60 days after the last application were carried out |
| Lund 2013 | Brazil | RCT (Split‐mouth design) | 13 patients with 117 teeth | 19 to 58 years (Mean 35.75 ±15.05 years) | Diode laser (780 nm) | Placebo (Carbomer gel) | Air blast | ‐ Baseline ‐ 5 minutes (short term) ‐ 1 week (medium term) ‐ 2 weeks (medium term) ‐ 1 month (medium term) ‐ 3 months (long term) |
‐ |
| Maximiano 2019 | Brazil | RCT | 70 patients | 18 to 65 years | Nd:YAG laser | 1. Placebo laser 2. CSP paste |
1. Air blast 2. Tactile stimuli |
‐ Baseline ‐ 5 minutes (short term) ‐ 1 week (medium term) ‐ 4 weeks (medium term) |
‐ |
| Naghsh 2020 | Iran | RCT (Patient as unit) | 7 patients with 96 teeth | 25 to 45 years | 1. Diode laser (660 nm) 2. Diode laser (810 nm) |
Placebo laser | Tactile | ‐ Baseline ‐ Immediate (short term) ‐ 1 week (medium term) ‐ 30 days (medium term) ‐ 60 days (long term) |
Treatment was rendered in 4 sessions with a 1‐week interval. Outcome measures following the first treatment session were considered for meta‐analysis |
| Orhan 2011 | Turkey | RCT (Patient as unit) | 16 patients with 64 teeth | 21 to 51 years (Mean 34.31 years) | Diode (655 nm) | 1. Distilled water 2. Placebo laser | Air blast | ‐ Baseline ‐ 24 hours (short term) ‐ 7 days (medium term) |
‐ |
| Ortiz 2019 | Brazil | RCT (Patient as unit) | 24 patients were enrolled. 21 participants with 80 teeth completed all phases of the study | 18 to 50 years (Mean age 30 years) | Photobiomodulation (PBM) 808 nm diode laser | Placebo laser | 1. Tactile 2. Air blast |
‐ Baseline ‐ Immediate ‐ 24 hours (short term) ‐ 48 hours (medium term) ‐ 1 month (medium term) |
3 desensitizing treatment sessions were performed with a 24‐hour interval between each session. Outcome measures following the 1st treatment session were considered for meta‐analysis |
| Pantuzzo 2020 | Brazil | RCT (Patient as unit) | 28 patients | Mean age 48.4 (older than 18 years) | Diode laser (808 nm) | Placebo laser | Tactile + evaporative | ‐ Immediate ‐ 6 hours (short term) ‐ 12 hours (short term) ‐ 24 hours (medium term) |
‐ |
| Suri 2016 | India | RCT (Split‐mouth design) | 30 patients with 120 teeth | 20 to 59 years | Diode laser (980 nm) | Placebo (Distilled water) | 1. Tactile 2. Air blast |
‐ Baseline ‐ 24 hours (short term) ‐ 1 week (medium term) ‐ 1 month (medium term) ‐ 2 months (medium term) |
‐ |
| Tevatia 2017 | India | RCT | 120 patients | 18 to 55 years (mean age 36.5 years) | 1. Diode laser (980 nm) 2. Diode laser (980 nm) + 5% KNO3 |
1. 5% KNO3 2. Placebo | 1. Tactile 2. Air blast 3. Cold water |
‐ Baseline ‐ Immediately after treatment (short term) ‐ 2 weeks (medium term) ‐ 4 weeks (medium term) ‐ 6 weeks (medium term) |
Commercial toothpaste was applied for 60 s as placebo control |
| Vieira 2009 | Brazil | RCT (Split‐mouth design) | 30 patients with 164 teeth | 24 to 68 years | Diode laser (660 nm) | Placebo (laser + gel) | 1. Tactile 2. Air blast |
‐ Baseline ‐ Immediate (short term) ‐ 3 months (long term; 6/30 patients lost to follow‐up) |
The 3‐month dropout rate was 20% |
| Yilmaz 2011a | Turkey | RCT (Split‐mouth design) | 51 patients with 174 teeth | 18 to 60 years (Mean 44 years) | 1. Diode laser (810 nm) 2. Er,Cr:YSGG laser |
No treatment | Air blast | ‐ Baseline ‐ Immediate (short term) ‐ 1 week (medium term) ‐ 1 month (medium term) ‐ 3 months (long term) |
‐ |
| Yilmaz 2011b | Turkey | RCT (Split‐mouth design) | 42 patients with 146 teeth | 18 to 64 years (from author's reply) (Mean 33.8 years) | Er,Cr:YSGG laser | Placebo laser | Air blast | ‐ Baseline ‐ Immediate (short term) ‐ 1 week (medium term) ‐ 1 month (medium term) ‐ 3 months (long term) |
‐ |
| Yilmaz 2011c | Turkey | RCT (Split‐mouth design) | 48 patients with 244 teeth | 18 to 58 years (Mean 41 years) | Diode laser (810 nm) | Placebo laser | Air blast | ‐ Baseline ‐ Immediate (short term) ‐ 1 week (medium term) ‐ 1 month (medium term) ‐ 3 months (long term) ‐ 6 months (long term) |
‐ |
| Yilmaz 2014 | Turkey | RCT (Split‐mouth design) | 20 patients with 60 teeth | 18 to 60 years (Mean 46.3 ± 8.4 years) | 1. Er,Cr:YSGG laser at 0.25 W 2. Er,Cr:YSGG laser at 0.5 W |
Placebo laser | Air blast | ‐ Baseline ‐ Immediately after treatment (short term) |
All teeth were extracted for SEM evaluation after VAS assessment |
aOutcome time points: 'Immediate' is short for 'Immediate after intervention,' '1 week' is short for '1 week after intervention,' '1 month' is short for '1 month after intervention,' and so forth. CSP = calcium sodium phosphosilicate; Er,Cr:YSGG = erbium,chromium:yttrium‐scandium‐gallium‐garnet; Nd:YAG = neodymium‐doped:yttrium‐aluminum‐garnet; nHAP = nano‐hydroxyapatite; nm = nanometers; RCT = randomized controlled trial; s = second; SEM = scanning electron microscopy; VAS = visual analogue scale; W = watt.
We included a total of 23 studies in this review, all but one were single‐center randomized controlled trials (RCTs).
Designs
Among the 23 included studies, 13 studies were RCTs with a split‐mouth design (Bal 2015; Doshi 2014; García 2017; Flecha 2013; Lier 2002; Lizarelli 2007; Lund 2013; Suri 2016; Vieira 2009; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014;). The other 10 included studies were parallel‐group RCTs (Alencar 2020; Gentile 2004; Gerschman 1994; Lee 2015; Maximiano 2019; Naghsh 2020; Orhan 2011; Ortiz 2019; Pantuzzo 2020; Tevatia 2017).
Settings
Nine studies were conducted in Brazil (Alencar 2020; Flecha 2013; Gentile 2004; Lizarelli 2007; Lund 2013; Maximiano 2019; Ortiz 2019; Pantuzzo 2020; Vieira 2009); six in Turkey (Bal 2015; Orhan 2011; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c, Yilmaz 2014); three in India (Doshi 2014; Suri 2016; Tevatia 2017); one in Australia (Gerschman 1994), Norway (Lier 2002), Spain (García 2017), South Korea (Lee 2015), and Iran (Naghsh 2020) each. 21 trials were university based (Alencar 2020; Bal 2015; Doshi 2014; Flecha 2013; Gentile 2004; Gerschman 1994; Lee 2015; Lier 2002; Lizarelli 2007; Lund 2013; Maximiano 2019, Naghsh 2020; Orhan 2011; Ortiz 2019; Pantuzzo 2020; Suri 2016; Vieira 2009; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014). One trial was a multicenter study and conducted collaboratively between university and a private clinic (García 2017) and in one trial, the setting was unclear (Tevatia 2017). One study (Lee 2015) was supported by research foundations. One study (Yilmaz 2011b) was self‐funded by the authors and their institution. One study (Lier 2002) was supported by a company.
Sample sizes
The sample sizes of included studies are presented in Additional Table 3. Eight studies (Alencar 2020; Flecha 2013; Maximiano 2019; Ortiz 2019; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014) conducted sample size calculation. Vieira 2009 reported loss to follow‐up of 6 out of 30 patients and Ortiz 2019 reported loss to follow‐up of 3 out of 24 patients. The 3‐month dropout rate of Vieira 2009 was 20%. No studies carried out intention‐to‐treat analysis.
Participants
A total of 936 patients with dentinal hypersensitivity participated in these 23 studies. One study (Gerschman 1994) failed to report gender ratios among their participants. Of the remaining 22 studies, two studies (Orhan 2011; Suri 2016) had equal number of male and female participants, and one study (Tevatia 2017) had a slightly higher number of male participants. The remaining studies demonstrated higher female preponderance. The age range of participants varied significantly among studies. Flecha 2013 investigated patients aged between 12 and 60 and Gerschman 1994 studied patients ranging between 15 and 69 years. Two studies (Bal 2015; Yilmaz 2011b) reported mean ages (37.0 years and 33.8 years, respectively) and the age range was provided via a follow‐up email to the authors. The participants in the remaining studies were all above 18 years according to their full‐text copies.
Interventions
Experimental interventions
Five laser subgroups were investigated in the included studies. Most studies applied diode laser at varying wavelengths ranging between 655 nanometer (nm) and 980 nm. Three studies evaluated erbium,chromium:yttrium‐scandium‐gallium‐garnet (Er,Cr:YSGG) laser alone (Lee 2015; Yilmaz 2011b; Yilmaz 2014) or as one experimental group with diode laser as the other (Yilmaz 2011a); one study evaluated neodymium‐doped:yttrium‐aluminum‐garnet (Nd:YAG) laser (Lier 2002).
Control interventions
Placebo lasers, placebo agents as well as 'no treatment' were adopted as the control group in the included studies, some of which set up two or three control groups. In five studies, placebo agents such as toothpaste, distilled water or saline were applied as the control intervention (Bal 2015; Flecha 2013; Lee 2015; Lizarelli 2007; Lund 2013). 14 studies employed placebo laser as the only control intervention (Doshi 2014; Gentile 2004; Gerschman 1994; Lier 2002; Maximiano 2019; Naghsh 2020; Ortiz 2019; Pantuzzo 2020; Yilmaz 2011b; Yilmaz 2014) or one of the control interventions or in combination with another chemical therapeutic agent (Alencar 2020; Orhan 2011; Vieira 2009; Yilmaz 2011c). Two studies had multiple experimental groups including one group which investigated the effectiveness of the intervention laser in combination with a chemical agent (Suri 2016; Tevatia 2017). Such groups were also considered as control interventions. 'No treatment' served as the control intervention in two studies (García 2017; Yilmaz 2011a).
Measurement
Pain intensity was measured in response to air blast stimuli alone (Bal 2015; Doshi 2014; Flecha 2013; Lier 2002; Lizarelli 2007; Lund 2013; Orhan 2011; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014) or in combination with tactile stimuli (Alencar 2020; García 2017; Gentile 2004; Gerschman 1994; Lee 2015; Maximiano 2019; Ortiz 2019; Pantuzzo 2020; Suri 2016; Tevatia 2017; Vieira 2009). One study evaluated pain intensity to tactile stimulus only (Naghsh 2020). Included studies assessed dentinal hypersensitivity by visual analogue scale (VAS) with the value range from 0 to 10 (no pain to worst possible pain) (Alencar 2020; Bal 2015; Doshi 2014; Flecha 2013; Gentile 2004; Gerschman 1994; Lier 2002; Lizarelli 2007; Lund 2013; Maximiano 2019; Naghsh 2020; Ortiz 2019; Suri 2016; Tevatia 2017; Vieira 2009; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014) or from 0 to 100 (García 2017; Orhan 2011). One study (Lee 2015) utilized VAS and an air‐sensitivity scale (0 to 3) to assess the severity of dentinal hypersensitivity.
Outcomes
Most included studies reported baseline scores and post‐intervention scores of pain intensities. One study (Gentile 2004) reported baseline and post‐intervention scores as well as change scores, but Gentile 2004 reported its baseline and post‐intervention scores by histograms.
Longevity of effect varied among studies (see Additional Table 3 for details). To date, no research papers have defined 'short‐term', 'medium‐term' or 'long‐term' effectiveness of laser therapy for dentinal hypersensitivity. We have defined 'not greater than 24 hours' as 'short term', 'greater than 24 hours but not greater than 2 months' as 'medium term' and 'greater than 2 months' as 'long term'. For the group 'short term', we chose the time point closest to 0 minutes after treatment. For the group 'medium term', we chose the time point closest to 2 months. For the group 'long term', we chose the time point the least close to 2 months. We realized that defining time points as short, medium, and long term would be clinically meaningful for the following reason: short‐term effects are important to determine the immediate effects (positive and adverse) of laser therapy; medium‐term effects are important to determine the immediate and prolonged effects (positive and adverse) of laser therapy, and long‐term effects are important to determine the longevity and potency of the effects of laser therapy.
Of the 23 included studies, one study reported a temporary, reversible pain sensation occurring in 2 out of 71 patients when lasing but there were no other adverse reactions or instances of oral irritation (Gerschman 1994). Eight studies (Alencar 2020; Doshi 2014; Flecha 2013; Gentile 2004; Lee 2015; Naghsh 2020; Ortiz 2019; Pantuzzo 2020) did not provide any information regarding any adverse events in their experimental groups. The remaining studies reported that no obvious adverse events were observed during the trials.
Excluded studies
See Characteristics of excluded studies table for more summarized information.
We excluded several plausibly eligible studies for the following reasons: age of patients not specified (Aranha 2012; Birang 2007; Dantas 2016; Kripal 2019; Lizarelli 2015; Pandey 2017); controlled clinical trials but not RCTs (Brugnera 1999; Brugnera 2003; Ciaramicoli 2003; Corona 2003; Dilsiz 2009; Dilsiz 2010a; Hu 2004; Kumar 2005; Ladalardo 2002; Oberhofer 2008; Pesevska 2010; Tengrungsun 2008; Wang 1991); quasi‐RCT (Zhao 2008); inappropriate design (Aranha 2012; Birang 2007; Moosavi 2016; Sgolastra 2013; Tabatabaei 2018; Talesara 2014); in vitro trial (Lan 1999); no laser‐only intervention arm involved in the study (Dilsiz 2010b; Moritz 1996); no non‐laser intervention arm involved in the study (Gelskey 1993; Hashim 2014; Hotta 2006; Liu 1994; Pourshahidi 2019; Shintome 2007; Tabibzadeh 2018; Yonaga 1999; Yu 2014); incomplete outcome data (Aranha 2009: Dantas 2016; Lan 1996; Liu 2004; Renton‐Harper 1992); outcomes not quantitatively measured (Chang 1999; He 2004; Kong 2004; Li 2001; Ma 2004; Wang 2004; Wang 2005; Wang 2006; Xiong 2010; Xu 2002; Yamaguchi 1990; Yu 1996; Zhao 2003); used different outcome measures such as numerical rating scale (NRS) (Lima 2017; Tailor 2014), Yeaple probe scores (Ozlem 2018), arbitrary pain scale in four degrees (Schwarz 2002), modified criteria proposed by Uchida et al (Ipci 2009), or verbal rating scale (VRS) (Sicilia 2009; Yadav 2019); therapeutic chemical agent as the only comparison group (Chebel 2018; De Lima Suares 2016; Femiano 2013; Guo 2019; Kara 2009; Lopes 2013; Lopes 2015; Lopes 2017; Moura 2019; Osmari 2018; Praveen 2018; Raichur 2012; Tocarruncho 2018; Wang 2012).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias assessments and the risk of bias graph. We have presented further details in risk of bias tables for each study in the Characteristics of included studies table. We assessed five studies as being at low overall risk of bias, 13 at unclear, and five at high risk of bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Thirteen studies demonstrated adequate methods of random sequence generation and were assessed as being at low risk of bias. The methods used were random number tables/cards (Alencar 2020; García 2017; Gerschman 1994; Ortiz 2019; Suri 2016) or lottery methods (Doshi 2014; Flecha 2013; Orhan 2011; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014). One study performed computerized generated randomization (Maximiano 2019). The other 10 studies did not report a clear method and were assessed as being at unclear risk of bias.
Allocation concealment
Nine studies reported adequate methods of allocation concealment and were assessed as being at low risk of bias. The methods used were sequentially numbered, opaque, sealed envelopes (Alencar 2020; Flecha 2013; Maximiano 2019; Orhan 2011; Ortiz 2019; Pantuzzo 2020; Tevatia 2017; Yilmaz 2011a), or central allocation (Yilmaz 2014). The remaining 14 studies did not clearly mention allocation concealment and were assessed as being at unclear risk of bias.
Blinding
Blinding of participants and personnel (performance bias)
Due to the limitation of the study design, even if placebo lasers were employed in the study, to blind personnel was almost impossible.The influence of blinding of personnel on the outcomes is considered minimal because it affects only during the execution of the test intervention (in‐office lasers) and placebo/no treatment. It is unlikely that blinding of personnel affects other aspects of studies and outcomes. In contrast, blinding of participants is considered very important as it affects the outcomes directly.
16 studies (Alencar 2020; Doshi 2014; García 2017; Gentile 2004; Gerschman 1994; Lier 2002; Lizarelli 2007; Maximiano 2019; Naghsh 2020; Orhan 2011; Ortiz 2019; Pantuzzo 2020; Vieira 2009; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014) blinded participants by using placebo lasers and were assessed as being at low risk of performance bias. One more study (Flecha 2013) was assessed as being at low risk, where they attempted to simulate the application of treatment and because it was a split‐mouth design, they have likely succeeded in simulating the condition. Three studies (Lee 2015; Suri 2016; Tevatia 2017) provided insufficient information on blinding of participants and personnel. The other three studies did not or could not blind participants and were assessed as being at high risk of bias.
Blinding of outcome assessment (detection bias)
11 studies (Alencar 2020; Doshi 2014; Flecha 2013; Lier 2002; Maximiano 2019; Orhan 2011; Ortiz 2019; Vieira 2009; Yilmaz 2011b; Yilmaz 2011c, Yilmaz 2014) blinded outcome assessors and were unlikely to be influenced by performance bias, therefore, they were assessed as being at low risk of detection bias. Four studies were assessed as being at high risk of detection bias. Among them, one study did not perform blinding of outcome assessors (García 2017) and three studies (Bal 2015; Lund 2013; Yilmaz 2011a) lacked blinding of participants, which is likely to lead to detection bias. Eight studies were assessed as being at unclear risk of bias due to insufficient information.
Incomplete outcome data
Of the 23 included studies, seven studies (Alencar 2020; Gentile 2004; Gerschman 1994; Lee 2015; Lizarelli 2007; Lund 2013; Naghsh 2020) did not mention loss to follow‐up and were assessed as being at unclear risk of bias. We assessed Pantuzzo 2020 as at high risk of attrition bias as it failed to provide the exact number of patient dropouts and limited the reporting to 7‐day evaluation being restricted due to limited number of individuals. One study (Vieira 2009) reported that 6 out of 30 patients were lost to follow‐up at the end of the third month. However, since the study was a split‐mouth design, the outcomes would probably not be influenced by the dropouts. Similarly, Ortiz 2019 reported that 3 out of 24 patients were lost to follow‐up and were accounted for in the analysis. One study (Tevatia 2017) reported the authors intended to exclude patients who dropped out of the study from the analysis, however, all recruited patients completed the study. We assessed these studies and the remaining 12 studies which had no dropouts as being at low risk of attrition bias.
Selective reporting
All included studies reported the intended outcomes and were assessed as being at low risk of reporting bias.
Other potential sources of bias
No other potential sources of bias were found.
Effects of interventions
Laser compared to placebo/no treatment
We included 23 studies in our review (936 participants, 2296 teeth) of which 17 (720 participants, 1265 teeth) contributed data to the analyses.
Changes in intensity of pain (visual analogue scale (VAS) with the value range from 0 to 10 (no pain to worst possible pain)) when tested through air blast stimuli
Short term: 0 to 24 hours
There were 13 trials (Alencar 2020; García 2017; Lier 2002; Maximiano 2019; Orhan 2011; Ortiz 2019; Pantuzzo 2020; Tevatia 2017; Vieira 2009; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c; Yilmaz 2014) reporting this outcome measured as changes from the baseline values as the following.
All types of laser
There was evidence of greater reduction of pain intensity (VAS) for all types of laser when compared to placebo/no treatment: mean difference (MD) ‐2.24, 95% confidence interval (CI) ‐3.55 to ‐0.93; P = 0.0008; Analysis 1.1.
1.1. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 1: Short term ‐ all types of laser
Er,Cr:YSGG (erbium,chromium:yttrium‐scandium‐gallium‐garnet)
There was evidence of greater reduction of pain intensity (VAS) for Er,Cr:YSGG when compared to placebo/no treatment: MD ‐4.61, 95% CI ‐4.83 to ‐4.39; P < 0.0001; Analysis 1.2.
1.2. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 2: Short term ‐ Er,Cr:YSGG
Nd:YAG (neodymium‐doped:yttrium‐aluminum‐garnet)
There was insufficient evidence of a difference in pain intensity (VAS) for Nd:YAG when compared to placebo/no treatment: MD 0.22, 95% CI ‐0.55 to 0.99; P = 0.58; Analysis 1.3.
1.3. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 3: Short term ‐ Nd:YAG
Diode: visible red (630 nanometer (nm) to 700 nm)
There was evidence of greater reduction of pain intensity (VAS) for visible red (630 nm to 700 nm) when compared to placebo/no treatment: MD ‐1.95, 95% CI ‐3.14 to ‐0.76; P = 0.001; Analysis 1.4.
1.4. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 4: Short term ‐ diode: visible red (630 nm to 700 nm)
Diode: near infrared (700 nm to 850 nm)
There was insufficient evidence of a reduction of pain intensity (VAS) for near infrared (700 nm to 850 nm) when compared to placebo/no treatment: MD ‐2.62, 95% CI ‐5.61 to 0.38; P = 0.09; Analysis 1.5.
1.5. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 5: Short term ‐ diode: near infrared (700 nm to 850 nm)
Diode: near infrared (850 nm to 980 nm)
There was evidence of greater reduction of pain intensity (VAS) for near infrared (850 nm to 980 nm) when compared to placebo/no treatment: MD ‐1.90, 95% CI ‐2.49 to ‐1.31; P < 0.0001; Analysis 1.6.
1.6. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 6: Short term ‐ diode: near infrared (850 nm to 980 nm)
Medium term: more than 24 hours to 2 months
There were 12 trials (Alencar 2020; García 2017; Gentile 2004; Gerschman 1994; Lier 2002; Maximiano 2019; Orhan 2011; Ortiz 2019; Tevatia 2017; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c) reporting this outcome measured as changes from the baseline values as the following.
All types of laser
There was evidence of greater reduction of pain intensity (VAS) for all types of laser when compared to placebo/no treatment: MD ‐2.46, 95% CI ‐3.57 to ‐1.35; P < 0.0001; Analysis 1.7.
1.7. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 7: Medium term ‐ all types of laser
Er,Cr:YSGG
There was evidence of greater reduction of pain intensity (VAS) for Er,Cr:YSGG when compared to placebo/no treatment: MD ‐4.90, 95% CI ‐5.21 to ‐4.59; P < 0.0001; Analysis 1.8.
1.8. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 8: Medium term ‐ Er,Cr:YSGG
Nd:YAG
There was insufficient evidence of a difference in pain intensity (VAS) for Nd:YAG when compared to placebo/no treatment: MD 0.42, 95% CI ‐0.97 to 1.82; P = 0.55; Analysis 1.9.
1.9. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 9: Medium term ‐ Nd:YAG
Diode: visible red (630 nm to 700 nm)
There was insufficient evidence of a difference in pain intensity (VAS) for visible red (630 nm to 700 nm) when compared to placebo/no treatment: MD ‐1.45, 95% CI ‐4.35 to 1.44; P = 0.32; Analysis 1.10.
1.10. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 10: Medium term ‐ diode: visible red (630 nm to 700 nm)
Diode: near infrared (700 nm to 850 nm)
There was evidence of greater reduction in pain intensity (VAS) for near infrared (700 nm to 850 nm) when compared to placebo/no treatment: MD ‐3.22, 95% CI ‐4.88 to ‐1.56; P = 0.0001; Analysis 1.11.
1.11. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 11: Medium term ‐ diode: near infrared (700 nm to 850 nm)
Diode: near infrared (850 nm to 980 nm)
There was evidence of greater reduction of pain intensity (VAS) for near infrared (850 nm to 980 nm) when compared to placebo/no treatment: MD ‐2.30, 95% CI ‐2.86 to ‐1.74; P < 0.0001; Analysis 1.12.
1.12. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 12: Medium term ‐ diode: near infrared (850 nm to 980 nm)
Long term: more than 2 months
There were five trials (Lier 2002; Vieira 2009; Yilmaz 2011a; Yilmaz 2011b; Yilmaz 2011c) reporting this outcome measured as changes from the baseline values as the following.
All types of laser
There was evidence of greater reduction of pain intensity (VAS) for all types of laser when compared to placebo/no treatment: MD ‐2.60, 95% CI ‐4.47 to ‐0.73; P = 0.006; Analysis 1.13.
1.13. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 13: Long term ‐ all types of laser
Er,Cr:YSGG
There was evidence of greater reduction of pain intensity (VAS) for Er,Cr:YSGG when compared to placebo/no treatment: MD‐5.19, 95% CI ‐5.50 to ‐4.89; P < 0.0001; Analysis 1.14.
1.14. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 14: Long term ‐ Er,Cr:YSGG
Nd:YAG;
There was insufficient evidence of a difference in pain intensity (VAS) for Nd:YAG when compared to placebo/no treatment: MD 0.18, 95% CI ‐0.84 to 1.20; P = 0.73; Analysis 1.15.
1.15. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 15: Long term ‐ Nd:YAG
Diode: visible red (630 nm to 700 nm)
There was insufficient evidence of a difference in pain intensity (VAS) for visible red (630 nm to 700 nm) when compared to placebo/no treatment: MD 0.81, 95% CI 0.78 to 2.40; P = 0.32; Analysis 1.16.
1.16. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 16: Long term ‐ diode: visible red (630 nm to 700 nm)
Diode: near infrared (700 nm to 850 nm)
There was evidence of greater reduction of pain intensity (VAS) for near infrared (700 nm to 850 nm) when compared to placebo/no treatment: MD ‐3.83, 95% CI ‐6.90 to ‐0.77; P = 0.01; Analysis 1.17.
1.17. Analysis.

Comparison 1: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli, Outcome 17: Long term ‐ diode: near infrared (700 nm to 850 nm)
Changes in intensity of pain (VAS with the value range from 0 to 10 (no pain to worst possible pain)) in response to tactile stimuli
Short term: 0 to 24 hours
There were eight trials (Alencar 2020; García 2017; Maximiano 2019; Naghsh 2020; Ortiz 2019; Pantuzzo 2020; Tevatia 2017; Vieira 2009) reporting this outcome measured as changes from the baseline values as the following.
All types of laser
There was evidence of greater reduction of pain intensity (VAS) for all types of laser when compared to placebo/no treatment: MD ‐0.67, 95% CI ‐1.31 to ‐0.03; P = 0.04; Analysis 2.1.
2.1. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 1: Short term ‐ all types of laser
Nd:YAG
There was insufficient evidence of a difference in pain intensity (VAS) for Nd:YAG when compared to placebo/no treatment: MD ‐0.25, 95% CI ‐0.91 to 0.41; P = 0.46; Analysis 2.2).
2.2. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 2: Short term ‐ Nd:YAG
Diode: visible red (630 nm to 700 nm)
There was evidence of greater reduction of pain intensity (VAS) for visible red (630 nm to 700 nm) when compared to placebo/no treatment: MD ‐0.81, 95% CI ‐1.16 to ‐0.45; P < 0.0001; Analysis 2.3.
2.3. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 3: Short term ‐ diode: visible red (630 nm to 700 nm)
Diode: near infrared (700 nm to 850 nm)
There was insufficient evidence of a difference in pain intensity (VAS) for near infrared (700 nm to 850 nm) when compared to placebo/no treatment: MD 0.27, 95% CI ‐0.60 to 1.14; P = 0.54; Analysis 2.4.
2.4. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 4: Short term ‐ diode: near infrared (700 nm to 850 nm)
Diode: near infrared (850 nm to 980 nm)
There was evidence of greater reduction of pain intensity (VAS) for near infrared (850 nm to 980 nm) when compared to placebo/no treatment: MD ‐2.17, 95% CI ‐2.80 to ‐1.54; P < 0.0001; Analysis 2.5.
2.5. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 5: Short term ‐ diode: near infrared (850 nm to 980 nm)
Medium term: more than 24 hours to 2 months
There were nine trials (Alencar 2020; García 2017; Gentile 2004; Gerschman 1994; Lee 2015; Maximiano 2019; Naghsh 2020; Ortiz 2019; Tevatia 2017) reporting this outcome measured as changes from the baseline values as the following.
All types of laser
There was evidence of greater reduction of pain intensity (VAS) for all types of laser when compared to placebo/no treatment: MD ‐1.73, 95% CI ‐3.17 to ‐0.30; P = 0.02; Analysis 2.6.
2.6. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 6: Medium term ‐ all types of laser
Nd:YAG
There was insufficient evidence of a difference in pain intensity (VAS) for Nd:YAG when compared to placebo/no treatment: MD ‐1.06, 95% CI ‐2.15 to 0.03; P = 0.06; Analysis 2.7.
2.7. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 7: Medium term ‐ Nd:YAG
Diode: visible red (630 nm to 700 nm)
There was evidence of greater reduction of pain intensity (VAS) for visible red (630 nm to 700 nm) when compared to placebo/no treatment: MD ‐2.95, 95% CI ‐5.14 to ‐0.75; P =0.008; Analysis 2.8.
2.8. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 8: Medium term ‐ diode: visible red (630 nm to 700 nm)
Diode: near infrared (700 nm to 850 nm)
There was insufficient evidence of a difference in pain intensity (VAS) for near infrared (700 nm to 850 nm) when compared to placebo/no treatment: MD ‐0.68, 95% CI ‐3.57 to 2.21; P = 0.65; Analysis 2.9.
2.9. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 9: Medium term ‐ diode: near infrared (700 nm to 850 nm)
Diode: near infrared (850 nm to 980 nm)
There was evidence of greater reduction of pain intensity (VAS) for near infrared (850 nm to 980 nm) when compared to placebo/no treatment: MD ‐1.83, 95% CI ‐2.36 to ‐1.30; P < 0.0001; Analysis 2.10.
2.10. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 10: Medium term ‐ diode: near infrared (850 nm to 980 nm)
Er,Cr:YSGG
There was insufficient evidence of a difference in pain intensity (VAS) for Er,Cr:YSGG when compared to placebo/no treatment: MD 0.38, 95% CI ‐0.13 to 0.89; P = 0.14; Analysis 2.11.
2.11. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 11: Medium term ‐ Er,Cr:YSGG
Long term: more than 2 months
There were two trials (Naghsh 2020; Vieira 2009) reporting this outcome measured as changes from the baseline values as the following.
All types of laser
There was insufficient evidence of a difference in pain intensity (VAS) for all types of laser when compared to placebo/no treatment: MD ‐3.52, 95% CI ‐10.37 to 3.33; P = 0.31; Analysis 2.12.
2.12. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 12: Long term ‐ all types of laser
Diode: visible red (630 nm to 700 nm)
There was evidence of greater reduction of pain intensity (VAS) for visible red (630 nm to 700 nm) when compared to placebo/no treatment: MD ‐4.67, 95% CI ‐5.38 to ‐3.96; P < 0.0001; Analysis 2.13.
2.13. Analysis.

Comparison 2: Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli, Outcome 13: Long term ‐ diode: visible red (630 nm to 700 nm)
Adverse events
Fifteen included studies assessed this outcome and reported that no obvious adverse events were observed during the trials.
Patient‐reported quality of life
None of the included studies measured this outcome.
Discussion
Summary of main results
23 randomized controlled trials (RCTs) met our inclusion criteria and were included in this review. 17 studies were included in the meta‐analysis. We summarized the findings for the most important outcomes as the following. The results of the current review have indicated that lasers can reduce pain of dentine hypersensitivity compared to a placebo or no treatment. However, there is very limited evidence available to support the advantages of laser therapy over efficacious conventional in‐office therapy (agents) for the treatment of dentine hypersensitivity. Additionally, there is no evidence of what types of in‐office lasers or laser parameters (e.g. wavelength, mode, power density) are more efficacious than others. Overall, laser therapy appears to be safe. Future studies including well‐designed double‐blinded RCTs are necessary to further investigate the clinical efficacy of lasers as well as their cost‐effectiveness.
Changes in intensity of pain when tested through air blast stimuli in short, medium, and long term
Results from meta‐analyses demonstrated evidence of pain intensity reduction when considering all types of lasers compared with placebo/no treatment in short (0 to 24 hours), medium (24 hours to 2 months), and long term (more than 2 months). The overall certainty of the evidence for all types of lasers was low to very low (Table 1). Separate analyses revealed that diode (visible red (630 nanometer (nm) to 700 nm) and near infrared (850 nm to 980 nm)) and erbium,chromium:yttrium‐scandium‐gallium‐garnet (Er,Cr:YSGG) lasers were more likely to reduce pain intensity in hypersensitivity cases compared with placebo/no treatment in the short term. Furthermore, studies comparing Er,Cr:YSGG laser with placebo/no treatment demonstrated limited evidence of pain intensity reduction at all time points. Statistical analyses (i.e. meta‐analyses) did not reveal sufficient evidence of differences between neodymium‐doped:yttrium‐aluminum‐garnet (Nd:YAG) and placebo/no treatment at short, medium, or long term. We did not find any eligible study to evaluate erbium:yttrium‐aluminum‐garnet (Er:YAG) or carbon dioxide (CO2) laser compared with placebo/no treatment.
Changes in intensity of pain when tested through tactile stimuli in short, medium, and long term
It is interesting to observe that less results from meta‐analyses demonstrated evidence of pain intensity reduction through tactile stimuli compared with air blast stimulus. Low‐ to very low‐certainty evidence showed all types of lasers reduced pain at short and medium term (Table 2). It was also found that diode laser was the only laser showing evidence of pain reduction compared with placebo/no treatment at most terms. However, statistical analyses did not reveal sufficient evidence of differences between Nd:YAG and placebo/no treatment. We did not find any eligible study to evaluate Er:YAG or CO2 laser compared with placebo/no treatment.
Adverse outcomes
No adverse event was noted in the experimental and control groups.
Patient reported quality of life
Patient reported quality of life was not measured in any of the included studies.
Overall completeness and applicability of evidence
The inclusion of 23 trials was insufficient to have a strong and clear conclusion, with only 17 considered for meta‐analysis. In fact, it is very hard to do a systematic review in this topic due to different types of laser with different exposure parameters, types of stimuli, methods of laser application, length and distance of stimuli. Additionally, in order to evaluate the effects of intervention versus no treatment or placebo, we had to eliminate studies that investigated the therapeutic effect of lasers versus other therapeutic agents. This limited the number of participants per subgroup analysis and increased the risk of overestimation of intervention effects (Thorlund 2011). Sensitivity analysis was not conducted due to the small number of included studies in several subgroups. However, the effect of new laser products in dentistry, which will likely increase heterogeneity in the included studies in any future systematic reviews/meta‐analysis should be investigated.
Quality of the evidence
23 studies were included in this review that were divided into six subgroups based on type of stimulus (air blast or tactile) and longevity of effect (short, medium, and long term). For several of the subgroup comparisons, only one or two studies provided data. A summary of the certainty of the evidence, the effectiveness of interventions, and a summary of the available data on the primary outcomes is provided in Table 1 and Table 2.
Changes in intensity of pain when tested through air blast stimuli in the short term: 13 RCTs were included in this category. The overall risk of bias was deemed not serious. Very serious inconsistency was noted among the studies in this category with a wide range of confidence intervals. All included studies directly assessed the objective of this review. The overall certainty of the evidence for this group was low.
Changes in intensity of pain when tested through air blast stimuli in the medium term: 11 RCTs were included in this category. The overall risk of bias was deemed serious. Very serious inconsistency was noted among the studies in this category with a wide range of confidence intervals. All included studies directly assessed the objective of this review. The overall certainty of the evidence for this group was very low.
Changes in intensity of pain when tested through air blast stimuli in the long term: five RCTs were included in this category. The overall risk of bias was deemed not serious. Very serious inconsistency was noted among the studies in this category with a wide range of confidence intervals. All included studies directly assessed the objective of this review. The overall certainty of the evidence for this group was very low.
Changes in intensity of pain when tested through tactile stimuli in the short term: eight RCTs were included in this category. The overall risk of bias was deemed serious. Serious inconsistency was noted among the studies in this category with a wide range of confidence intervals crossing the treatment threshold. All included studies directly assessed the objective of this review. The overall certainty of the evidence for this group was low.
Changes in intensity of pain when tested through tactile stimuli in the medium term: nine RCTs were included in this category. The overall risk of bias was deemed serious. Very serious inconsistency was noted among the studies in this category with a relatively narrow range of confidence intervals resulting in a rating of not serious for imprecision. All included studies directly assessed the objective of this review. The overall certainty of the evidence for this group was very low.
Changes in intensity of pain when tested through tactile stimuli in the long term: two RCTs were included in this category. The overall risk of bias was deemed serious. Very serious inconsistency was noted among the studies in this category with a wide range of confidence intervals. All included studies directly assessed the objective of this review. The overall certainty of the evidence for this group was very low.
We did not downgrade the certainty of the evidence for publication bias, largely due to the small number of studies in most subgroups. We cannot completely discard the possibility of not having identified small studies, especially if they were conducted decades ago. We have, however, minimized the effect of publication bias by undertaking a comprehensive search for all eligible studies.
Potential biases in the review process
We did not identify any potential biases in the review process. However, there are multiple study limitations associated with this review. While there is no evidence to document safety concerns associated with laser treatment in the pediatric and adolescent population, most studies considered participants of 18 years and older. Of the 23 studies in this review, two studies included participants as young as 12 (Flecha 2013) and 15 (Gerschman 1994) years of age. However, the mean age of the study population were 31.4 and 37.5, respectively which reflects that most participants were adults. Additionally, the studies failed to measure other patient‐ and public health‐important outcomes such as patient‐reported quality of life and health economics outcomes. Moreover, as per Holland et al guidelines (Holland 1997) only studies with two stimuli, ideally cold/evaporative and tactile, should be included. In our review of the literature, we encountered many RCTs that only evaluated one stimulus which is considered a potential methodological limitation. Nonetheless, limiting the inclusion criteria to studies with both tactile and air blast stimuli could result in the elimination of potential clinically relevant studies, narrowing the results to very few studies which we believe would not be applicable, practical, or beneficial to our dental community and in daily practice.
Agreements and disagreements with other studies or reviews
Several systematic reviews (He 2011; Hu 2019; Kong 2020; Lin 2013; Rezazadeh 2019; Sgolastra 2011; Sgolastra 2013; Zhou 2021) investigated the effectiveness of laser therapy in the treatment of dental hypersensitivity.
The first systematic reviews were published in 2011 by He 2011 and Sgolastra 2011. Sgolastra 2011 reported that the reduction of dental hypersensitivity following laser treatment was not significant compared with placebo laser treatment. The authors included only three RCTs using strict inclusion and exclusion criteria. For instance, only placebo‐controlled trials were included due to the concerns of the high effectiveness of placebo laser. He 2011 compared the effectiveness of laser therapy with that of topical desensitizing agents including eight studies. In both studies, a meta‐analysis could not be performed due to the high level of heterogeneity among the included studies. In a recent review, Zhou and colleagues (Zhou 2021) compared the effectiveness of laser therapy and topical desensitizing agents for dentine hypersensitivity. They applied the Cochrane risk of bias tool for quality assessment leading to the inclusion of 13 studies comparing Nd:YAG laser and diode laser with topical agents. They reported that given the low quality of the evidence, no conclusion could be made regarding the superiority of lasers or conventional topical agents and due to low cost and ease of use of topical agents, they recommended clinicians to use them as a routine treatment.
Another systematic review and meta‐analysis (Lin 2013) showed that the use of lasers was more effective than placebo laser for the reduction of dental hypersensitivity. However, the review did not analyze the results based on the types of lasers. Therefore, differences in the level of effectiveness among different types of lasers were not considered.
In 2013, Sgolastra and colleagues published another systematic review (Sgolastra 2013) including 13 studies for meta‐analysis. The authors found the differences in favor of Er:YAG, Nd:YAG, and GaAlAs lasers in comparison with placebo. However, significant amount of heterogeneity was found for all these comparisons. There was no significant difference for the comparison between Er,Cr:YSSG laser and placebo. A comparable systematic review and meta‐analysis in 2019 by Hu 2019 further investigated the immediate and long‐term effectiveness of laser therapy versus placebo or no treatment. They limited the outcome measure to air blast stimulus and their search to English and Chinese literature. In their study, long‐term effect ranged between 1 week and 6 months. Within the limitations of their study with 22 eligible RCTs, lasers were reported to render better immediate‐ and long‐term desensitizing effect compared to placebo or no treatment. In 2020, another systematic review (Kong 2020), resembling Hu 2019's study, was published with the difference that long‐term effect was defined up to 1 month. They conducted a network meta‐analysis on 11 included studies with four different types of laser i.e. GaAlAs, Er:YAG, Nd:YAG, Er,Cr:YSGG and concluded that lasers proved to be more effective in the immediate and the long term.
Rezazadeh and colleagues (Rezazadeh 2019) conducted a more inclusive systematic review with four different subgroups: Group 1 investigated laser application as a preventive procedure; Group 2 compared laser with placebo; Group 3 compared laser with desensitizing agents; and Group 4 examined different types of lasers. Due to the significant heterogeneity and inconsistency among the different subgroups, they failed to perform a meta‐analysis and limited their results to descriptive reporting of their search results.
The results of the present study are comparable with Sgolastra 2013 as both studies compared the different types of lasers and placebo or no treatment. Both reviews were similar in regards to heterogeneity among the included studies. In the present study, we categorized the studies based on the type of outcome measure (reduction in pain intensity following air blast stimulus and tactile stimulus), longevity of effect (short, medium, and long term) and type of laser. We also considered adverse events as a significant patient‐relevant outcome. Additionally, we separated the split‐mouth studies from parallel‐group studies. Sgolastra 2013 et al conducted their analysis in the following categories: 1. Er,Cr:YSSG versus placebo, 2. Er:YAG versus placebo, 3. Nd:YAG versus placebo, and 4. GaAlAs versus placebo. The primary outcome in their study was change in pain intensity from baseline to the follow‐up sessions. The secondary outcome was cost‐effectiveness. Our quantitative analysis of all types of lasers combined, revealed a statistically significant reduction in dentine hypersensitivity in short, medium, and long term compared to placebo/no treatment. Additionally, the subgroup analysis suggests that Er,Cr:YSGG and diode laser were superior to placebo/no treatment in reducing dentine hypersensitivity in response to air blast stimulus in short, medium, and long term. Studies that evaluated pain reduction in response to tactile stimulus, revealed that diode was significantly effective in reducing dentine hypersensitivity compared to placebo/no treatment in short, medium, and long term. Sgolastra 2013 et al concluded that Er:YAG, Nd:YAG, and GaAlAs lasers were superior to placebo in reducing pain of dentinal hypersensitivity.
There is a controversial body of evidence in the literature suggesting variable degrees of clinical effectiveness of laser treatment compared to no treatment, placebo, or other therapeutic approaches. This is largely due to lack of complete understanding of the mechanism of laser treatment, variability of treatment protocols and other confounding factors related to the operator's experience, socio‐economic factors i.e. availability and affordability of the treatment, longevity of effect and psychological factors such as placebo effect and the doctor‐patient relationship determining both parties' understanding and expectations of the treatment outcomes (Asnaashari 2013; Kimura 2000; Orchardson 2006; Sgolastra 2011). While the present meta‐analysis reveals statistically significant pain reduction by Er,Cr:YSGG and diode lasers in response to air blast stimulus and statistically significant pain reduction by diode laser in response to tactile stimulus, the mean visual analogue scale (VAS) score difference in the pooled analysis of all laser types was minimal, ranging between 0.91 and 2.61 and the certainty of the evidence was low or very low. This finding is in line with the previous studies suggesting limited clinical importance and magnitude of effect of laser treatment (Asnaashari 2013).
Authors' conclusions
Implications for practice.
Analyses suggested that the application of laser overall may improve pain intensity (visual analogue scale (VAS)) when tested through air blast or tactile stimuli at short, medium, or long term. However, results have to be interpreted with great caution due to the high degree of heterogeneity among trials. Overall, laser therapy appears to be safe with potential of sensitivity pain reduction, with weak magnitude of effect, and low‐ to very low‐certainty of evidence.
Implications for research.
Among 23 randomized controlled trials (RCTs) included in this review, 17 studies were included for meta‐analysis. After dividing these studies into six subgroups based on type of stimulus and pain assessment, only a few RCTs were available to evaluate the long‐term (> 2 months) effectiveness of laser in each subgroup. More information is needed on whether each type of laser can actually reduce or not the pain intensity with a follow‐up of at least 2 months. Additionally, more than half of the included studies were at high or unclear risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel. Overall, high heterogeneity was found among the included studies. Therefore, well‐designed, well‐controlled RCTs or large multicenter trials with long‐term follow‐up are required to further evaluate the effectiveness and safety of different types of lasers for the treatment of dentinal hypersensitivity, the possibility and patients' desire of attending and paying for laser treatment for dentinal hypersensitivity every 2 months, and the effectiveness of studies of lasers against other active interventions.
History
Protocol first published: Issue 11, 2011
Acknowledgements
The review authors would like to thank Anne Littlewood, Information Specialist, and Philip Riley, Deputy Co‐ordinating Editor of Cochrane Oral Health for their generous help and support; Helen Worthington, Thomas Lamont, Jennifer Hilgart, Nicole Pitcher, Professor Nicola West (Periodontology, Bristol Dental School, Lower Maudlin Street, Bristol, UK), and Professor Laurence J Walsh for their helpful feedback; and Luisa Fernandez Mauleffinch, Managing Editor and Copy Editor, Cochrane Oral Health, for her assistance in facilitating this review.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
Cochrane Oral Health's Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see oralhealth.cochrane.org/trials.
#1 ((sensitiv* or hypersensitiv* or hyper‐sensitiv*):AB) AND (INREGISTER) #2 ((sensitiv* or hypersensitiv* or hyper‐sensitiv*):TI) AND (INREGISTER) #3 (#1 or #2) AND (INREGISTER) #4 (laser*:AB) AND (INREGISTER) #5 (laser*:TI) AND (INREGISTER) #6 (#4 or #5) AND (INREGISTER) #7 (#3 and #6) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Dentin sensitivity this term only #2 ((dentin* in All Text near/5 sensitiv* in All Text) or (dentin* in All Text near/5 hypersensitiv* in All Text) or (dentin* in All Text near/5 hyper‐sensitiv* in All Text)) #3 ((tooth in All Text near/5 sensitiv* in All Text) or (tooth in All Text near/5 hypersensitiv* in All Text) or (tooth in All Text near/5 hyper‐sensitiv* in All Text)) #4 ((teeth in All Text near/5 sensitiv* in All Text) or (teeth in All Text near/5 hypersensitiv* in All Text) or (teeth in All Text near/5 hyper‐sensitiv* in All Text)) #5 (#1 or #2 or #3 or #4) #6 MeSH descriptor Lasers explode all trees #7 MeSH descriptor Laser therapy this term only #8 laser* in All Text #9 (#6 or #7 or #8) #10 (#5 and #9)
Appendix 3. MEDLINE Ovid search strategy
1. Dentin sensitivity/ 2. ((dentin$ or tooth or teeth) adj5 (sensitiv$ or hypersensitiv$ or hyper‐sensitiv$)).mp. 3. or/1‐2 4. exp Lasers/ 5. Laser Therapy/ 6. laser$.mp. 7. or/4‐6 8. 3 and 7
Appendix 4. Embase Ovid search strategy
1. dentin sensitivity/ 2. ((dentin$ or tooth or teeth) adj5 (sensitiv$ or hypersensitiv$ or hyper‐sensitiv$)).mp. 3. or/1‐2 4. exp Lasers/ 5. laser Therapy/ 6. laser$.mp. 7. or/4‐6 8. 3 and 7
Appendix 5. CINAHL EBSCO search strategy
S1 MH "Dentin sensitivity" S2 (dentin* N5 sensitiv*) or (dentin* N5 hypersensitiv*) or (dentin* N5 hyper‐sensitiv*) S3 (tooth N5 sensitiv*) or (tooth N5 hypersensitiv*) or (tooth N5 hyper‐sensitiv*) S4 (teeth N5 sensitiv*) or (teeth N5 hypersensitiv*) or (teeth N5 hyper‐sensitiv*) S5 S1 or S2 or S3 or S4 S6 MH "Lasers+" S7 laser* S8 S6 or S7 S9 S5 and S8
Appendix 6. LILACS BIREME search strategy
Mh lasers or laser$ [Words] and Mh Dentin hypersensitivity or sensitiv$ or hypersensitiv$ or hyper‐sensitiv$ [Words]
Appendix 7. ISI Web of Science conference proceedings search strategy
# 3 #1 and #2
# 2 TS=((dentin* or tooth or teeth) and (sensitiv* or hypersensitiv*))
# 1 TS=laser*
Appendix 8. ZETOC search strategy
hypersensitivity and lasers
hypersensitivity and laser
hypersensitive and lasers
hypersensitive and laser
Appendix 9. OpenGrey search strategy
laser* AND tooth or teeth or dentin* AND sensitive or sensitivity or hypersensitive or hypersensitivity or hyper‐sensitive or hyper‐sensitivity
Appendix 10. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
hypersensitive* and laser*
Appendix 11. World Health Organization International Clinical Trials Registry Platform search strategy
hypersensitivity and lasers
hypersensitivity and laser
hypersensitive and lasers
hypersensitive and laser
Appendix 12. IADR website search strategy
laser and hypersensitivity
Data and analyses
Comparison 1. Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through air blast stimuli.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Short term ‐ all types of laser | 13 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.55, ‐0.93] | |
| 1.1.1 Split‐mouth design | 8 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐3.99, ‐1.95] | |
| 1.1.2 Parallel design | 5 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐2.40, 0.15] | |
| 1.2 Short term ‐ Er,Cr:YSGG | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Split‐mouth design | 3 | Mean Difference (IV, Fixed, 95% CI) | ‐4.61 [‐4.83, ‐4.39] | |
| 1.3 Short term ‐ Nd:YAG | 2 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.55, 0.99] | |
| 1.3.1 Split‐mouth design | 1 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.85, 1.25] | |
| 1.3.2 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.90, 1.38] | |
| 1.4 Short term ‐ diode: visible red (630 nm to 700 nm) | 4 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐3.14, ‐0.76] | |
| 1.4.1 Split‐mouth design | 3 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐3.09, ‐0.17] | |
| 1.4.2 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐3.99, ‐1.95] | |
| 1.5 Short term ‐ diode: near infrared (700 nm to 850 nm) | 4 | Mean Difference (IV, Random, 95% CI) | ‐2.62 [‐5.61, 0.38] | |
| 1.5.1 Split‐mouth design | 2 | Mean Difference (IV, Random, 95% CI) | ‐4.69 [‐5.08, ‐4.30] | |
| 1.5.2 Parallel design | 2 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.87, 0.96] | |
| 1.6 Short term ‐ diode: near infrared (850 nm to 980 nm) | 1 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.49, ‐1.31] | |
| 1.6.1 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.49, ‐1.31] | |
| 1.7 Medium term ‐ all types of laser | 11 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐3.57, ‐1.35] | |
| 1.7.1 Split‐mouth design | 6 | Mean Difference (IV, Random, 95% CI) | ‐3.48 [‐4.68, ‐2.29] | |
| 1.7.2 Parallel design | 5 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.37, ‐0.16] | |
| 1.8 Medium term ‐ Er,Cr:YSGG | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Split‐mouth design | 2 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐5.21, ‐4.59] | |
| 1.9 Medium term ‐ Nd:YAG | 2 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.97, 1.82] | |
| 1.9.1 Split‐mouth design | 1 | Mean Difference (IV, Random, 95% CI) | 1.03 [0.02, 2.04] | |
| 1.9.2 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.92, 1.10] | |
| 1.10 Medium term ‐ diode: visible red (630 nm to 700 nm) | 4 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐4.35, 1.44] | |
| 1.10.1 Split‐mouth design | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐6.64, ‐0.57] | |
| 1.10.2 Parallel design | 2 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.80, 2.46] | |
| 1.11 Medium term ‐ diode: near infrared (700 nm to 850 nm) | 4 | Mean Difference (IV, Random, 95% CI) | ‐3.22 [‐4.88, ‐1.56] | |
| 1.11.1 Split‐mouth design | 2 | Mean Difference (IV, Random, 95% CI) | ‐4.67 [‐5.11, ‐4.23] | |
| 1.11.2 Parallel design | 2 | Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐3.82, 0.49] | |
| 1.12 Medium term ‐ diode: near infrared (850 nm to 980 nm) | 1 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐2.86, ‐1.74] | |
| 1.12.1 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐2.86, ‐1.74] | |
| 1.13 Long term ‐ all types of laser | 5 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐4.47, ‐0.73] | |
| 1.13.1 Split‐mouth design | 5 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐4.47, ‐0.73] | |
| 1.14 Long term ‐ Er,Cr:YSGG | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 Split‐mouth design | 2 | Mean Difference (IV, Fixed, 95% CI) | ‐5.19 [‐5.50, ‐4.89] | |
| 1.15 Long term ‐ Nd:YAG | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 Split‐mouth design | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.84, 1.20] | |
| 1.16 Long term ‐ diode: visible red (630 nm to 700 nm) | 1 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.78, 2.40] | |
| 1.16.1 Split‐mouth design | 1 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.78, 2.40] | |
| 1.17 Long term ‐ diode: near infrared (700 nm to 850 nm) | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.83 [‐6.90, ‐0.77] | |
| 1.17.1 Split‐mouth design | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.83 [‐6.90, ‐0.77] |
Comparison 2. Laser versus placebo/no treatment. Outcome: changes in intensity of pain (VAS with value range from 0 to 10) when tested through tactile stimuli.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Short term ‐ all types of laser | 8 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.31, ‐0.03] | |
| 2.1.1 Split‐mouth design | 4 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.68, ‐0.11] | |
| 2.1.2 Parallel design | 4 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.50, 0.62] | |
| 2.2 Short term ‐ Nd:YAG | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Parallel design | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.91, 0.41] | |
| 2.3 Short term ‐ diode: visible red (630 nm to 700 nm) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Split‐mouth design | 4 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐1.16, ‐0.45] | |
| 2.4 Short term ‐ diode: near infrared (700 nm to 850 nm) | 2 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.60, 1.14] | |
| 2.4.1 Parallel design | 2 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.60, 1.14] | |
| 2.5 Short term ‐ diode: near infrared (850 nm to 980 nm) | 1 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐2.80, ‐1.54] | |
| 2.5.1 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐2.80, ‐1.54] | |
| 2.6 Medium term ‐ all types of laser | 9 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.17, ‐0.30] | |
| 2.6.1 Split‐mouth design | 3 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐6.15, ‐1.35] | |
| 2.6.2 Parallel design | 6 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.77, 0.42] | |
| 2.7 Medium term ‐ Nd:YAG | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Parallel design | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐2.15, 0.03] | |
| 2.7.2 Parallel design | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐2.80, ‐1.54] | |
| 2.8 Medium term ‐ diode: visible red (630 nm to 700 nm) | 4 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐5.14, ‐0.75] | |
| 2.8.1 Split‐mouth design | 3 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐6.15, ‐1.35] | |
| 2.8.2 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.98, 1.60] | |
| 2.9 Medium term ‐ diode: near infrared (700 nm to 850 nm) | 2 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐3.57, 2.21] | |
| 2.9.1 Parallel design | 2 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐3.57, 2.21] | |
| 2.10 Medium term ‐ diode: near infrared (850 nm to 980 nm) | 1 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐2.36, ‐1.30] | |
| 2.10.1 Parallel design | 1 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐2.36, ‐1.30] | |
| 2.11 Medium term ‐ Er,Cr:YSGG | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.11.1 Parallel design | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.13, 0.89] | |
| 2.12 Long term ‐ all types of laser | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.52 [‐10.37, 3.33] | |
| 2.12.1 Split‐mouth design | 2 | Mean Difference (IV, Random, 95% CI) | ‐3.52 [‐10.37, 3.33] | |
| 2.13 Long term ‐ diode: visible red (630 nm to 700 nm) | 2 | Mean Difference (IV, Fixed, 95% CI) | ‐4.67 [‐5.38, ‐3.96] | |
| 2.13.1 Split‐mouth design | 2 | Mean Difference (IV, Fixed, 95% CI) | ‐4.67 [‐5.38, ‐3.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alencar 2020.
| Study characteristics | ||
| Methods |
Study type: RCT (double‐blinded). 4 parallel groups Duration of trial: not mentioned in the study Duration of follow‐up: 1 month |
|
| Participants |
Setting: not mentioned in the study. Authors' affiliation is School of Dentistry, Federal University of Para, Belem, PA, Brazil Inclusion criteria: volunteers aged between 18 and 50 years, who were students and employees of the local university, were recruited. The main inclusion criterion was the presence of 2 to 4 hypersensitive teeth with shallow non‐carious cervical lesions up to 1 mm deep, according to Van Landuyt et al, Class I gingival recession according to the Miller classification, or both. The sensitive teeth should present a score > 4 on the VAS for the evaporative stimulus Exclusion criteria: systemic diseases, pulpitis, carious lesions, defective restorations, active periodontal disease, use of analgesic medication, pregnant or lactating women, and the use of professional desensitizing treatment received during the previous 3 months Total number: 32 participants with 83 teeth Age range: 18 to 50 years Sex (M/F): 15/17 |
|
| Interventions | Before the treatments, all the volunteers received professional prophylaxis and a kit containing a soft bristle toothbrush and toothpaste without desensitizing action. Patients were instructed to brush their teeth 3 times a day. The treatments were performed in 2 sessions with a 24‐hour interval After the randomization process (block randomization), the patients were allocated into 4 groups Group 1: (n = 19 teeth): GPlacebo ‐ simulated PBM (without light emission) followed by the application of nHAP‐free toothpaste Group 2: (n = 18 teeth): GLaser ‐ PBM followed by the application of nHAP‐free toothpaste Group 3: (n = 18 teeth): GnHAP ‐ simulated PBM followed by the application of nHAP Group 4: (n = 18 teeth): GLasernHAP ‐ PBM followed by the application of nHAP |
|
| Outcomes | Dentine hypersensitivity was evaluated at 4 evaluation times: baseline (immediately before the 1st session), after the 1st session, after the 2nd session, and 1 month after the 2nd session. The evaluation of pain sensitivity was performed by means of a tactile stimulus followed by an evaporative stimulus associated with a VAS (0 to 10). A 5‐minute interval between tactile and evaporative stimuli was performed | |
| Notes |
Sample size calculation: described in detail Source of funding: the authors declared no conflict of interest Ethics approval: quote: "it was approved by the local university ethics committee (No. 2,402,287)" Informed consent: quote: "All participants in the study signed the informed consent form after being duly informed in accordance with the declaration of Helsinki" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomization was performed, aiming to balance the sample number in the number of existing arms. The number of eight volunteers in each block was predefined. For the formation of each block a stratified randomization was performed considering the sex and age strata. After the formation of the block, a simple numerical draw was carried out among the participants, and subsequently, every two volunteers were allocated to compose one of the four groups. The total number of blocks in the study was four" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation secrecy was maintained throughout the sample compounding process. One of the co‐authors of the study was responsible for the formation of the blocks, numerical drawing within the block and allocation of the sample in the groups. The numerical drawing was performed using numbered and coded papers, for which the volunteers, clinical operator and evaluator did not know to which group the subject was allocated" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The evaluator and the patient were blinded. The volunteers evaluated in the experiment were also not aware of the treatment to which they were submitted. Both the Nano‐P desensitizer and the placebo toothpaste had the same color and visual appearance. The materials were placed in identical containers so that the patients were not identified at the time of application of the product" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluator and the patient were blinded. The study had only one evaluator to assess pain sensitivity, who did not participate in the randomization process" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No descriptions on loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified |
Bal 2015.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth design, 5 groups) Duration of trial: not mentioned in the study Duration of follow‐up: 3 months |
|
| Participants |
Setting: Periodontology Department of Gulhane Military Medical Academy, Ankara, Turkey Inclusion criteria: 19 to 60 years of age, being in good systemic health, having 2 or more teeth showing hypersensitivity to air blast in each of 4 quadrants, good oral hygiene, and agreeing to 3‐month follow‐up Exclusion criteria: benign or malignant pathological oral lesions, chronic disease, chronic medication use, caries in selected or neighbouring teeth, cracked enamel, orthodontic appliances, restorations, congenital enamel and/or dentine defects, history of vital bleaching, periodontal disease, periodontal surgery or hypersensitivity treatment within the past 6 months, non‐surgical treatment of periodontal disease within the past 3 months, use of dentifrice or mouthwash containing a desensitizing agent, being pregnant or lactating, use of antidepressants or analgesics, and allergy to any of the content of the treatment Total number: 21 participants with 156 teeth Age range: 19 to 60 years Sex (M/F): 5/16 |
|
| Interventions | After giving oral hygiene education at the first examination, the 4 mouth quadrants of each patient were randomized to apply 1 of the 4 study treatments. A randomly selected tooth in 1 of the quadrants was defined as the control. In each patient, a randomly selected quadrant was given 1 session of 1 of the following treatments: Group 1: (n = 41 teeth): diode laser (25 mW, 2 J/cm2, 685 nm, 100 s, 1 cm2 area). The laser dosage applied to each tooth was 4 J/cm2 for a pre‐determined time of 2 minutes, according to calibration of the laser device. The laser was applied at a distance of 2 mm from the dental outer surface Group 2: (n = 22 teeth): placebo. Physiological saline solution |
|
| Outcomes | Sensitivity to thermal (air) stimulus was recorded before and immediately post‐treatment, and on days 10, 30, 60, and 90 post‐treatment (VAS score) | |
| Notes |
Sample size calculation: not mentioned in the study Source of funding: none Ethics approval: quote: "Study protocol and consent forms were approved by the Institutional Ethics Committee of Gulhane Military Medical Academy (protocol number: 10‐1539)" Informed consent: quote: "All patients were thoroughly informed about the treatment plan, possibilities of discomfort, and risks. Written informed consent was obtained from all participants" Adverse events: quote: "One‐time application of either a 685 nm low‐level laser, or DP containing 8% arginine‐calcium carbonate, reduced DH both immediately and over the long term was performed without any adverse reactions" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo was used for the control tooth, however, placebo was of different material/content. This is inadequate and the patients are likely be aware of the treatment received in each site due to the differences in perception. The outcome is likely to be influenced by incomplete blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding of participants can lead to high risk for detection bias when reporting pain level |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"All the patients completed the 90‐day study period" |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation was not described in the study |
Doshi 2014.
| Study characteristics | ||
| Methods |
Study type: RCT (double‐blinded, split‐mouth design) Duration of trial: 1 week Duration of follow‐up: 1 week |
|
| Participants |
Setting: Periodontology Department, MA Rangoonwala Dental College and Research Centre, Pune, India Inclusion criteria: having chronic periodontitis with a minimum of 2 sites in each quadrant with probing pocket depth ‡ 5 mm bilaterally, after 1 month following phase 1 therapy Exclusion criteria: smoking, debilitating diseases, being pregnant or lactating, having taken non‐steroidal anti‐inflammatory drugs (NSAIDS) or antibiotics in the past 3 months, having carious lesions, having had desensitizing therapy during the last 6 months, having cervical fillings, or gingival recession Total number: 30 participants with 60 teeth Age range: 23 to 56 years Sex (M/F): 14/16 |
|
| Interventions |
Group 1: (30 patients, n = 30 teeth): GaAlAs laser (25 mW, 11.36 mW/cm2, 660 nm, area: 2.2 cm2). Laser irradiation was performed for the first 3 days following periodontal flap surgery for a duration of 3 minutes Group 2: (30 patients, n = 30 teeth): on the control sites, the laser was used as a placebo. Although it was placed on the marginal gingiva and was used in the similar motion, it was not activated |
|
| Outcomes | Sensitivity to thermal (air) stimulus was recorded before and on days 1, 3, 5, and 7 after the treatment (VAS score and VRS) | |
| Notes |
Baseline characteristics: thorough scaling and root planing were performed as part of phase 1 therapy, and patients were advised to use 0.2% chlorhexidine mouthrinses for 2 weeks. Patients were recalled after 4 weeks. Persistent pockets of ‡ 5 mm at minimum of 2 sites in each quadrant were scheduled for flap surgery. The surgical procedure was performed by an experienced examiner Sample size calculation: not mentioned in the study Source of funding: no competing financial interests reported Ethics approval: not mentioned in the study Informed consent: quote: "The purpose and design of the investigation were explained to patients and an informed consent form was signed" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a coin toss, 30 sites were randomly assigned for laser irradiation (test site) and the other 30 sites served as control sites" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was double blinded such that the subjects and the calibrated examiner performing the measurements were blinded to avoid bias" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was double blinded such that the subjects and the calibrated examiner performing the measurements were blinded to avoid bias" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the patients attended the follow‐up sessions" |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation and source of funding were not mentioned in the study |
Flecha 2013.
| Study characteristics | ||
| Methods |
Study type: RCT (double‐blinded, split‐mouth design) Duration of trial: 12 months Duration of follow‐up: 6 months |
|
| Participants |
Setting: periodontology clinic of the Department of Dentistry of the Federal University of Jequitinhonha and Mucuri Valleys (UFVJM), Diamantina, Minas Gerais, Brazil Inclusion criteria: participants had good general and oral health, complained of pain in teeth located in different hemi‐arches of the mouth, manifested pain or discomfort in response to stimulus caused by the jet of air from a triple syringe, and initially responded to this stimulus with a score > 5 in the numeric rating scale Exclusion criteria: patients who had undergone previous professional desensitizing treatment or had used over‐the‐counter desensitizing products; those with chronic use of anti‐inflammatory, analgesic, or psychotropic drugs; pregnant and breastfeeding females; patients presenting allergies and idiosyncratic responses to product ingredients; eating disorders; systemic conditions that are etiologic factors or predisposing for DH, excessive dietary or environmental exposure to acids; and patients who underwent periodontal surgery or orthodontic treatment in the preceding 3 months. In addition, the following teeth were excluded: teeth or periodontium with pathology or defects likely to cause pain; those that were restored in the preceding 3 months; those that served as abutment for fixed or removable prostheses; those that were crowned or had extensive restorations; and those with restorations extending into the test area (cervical) Total number: 62 participants with 434 teeth Age range: 12 to 60 years Sex (M/F): 15/47 |
|
| Interventions |
Group 1: (62 patients, n = 216 teeth): GaAlAs laser (120 mW, 2.88 J/cm2, 795 nm, area: 0.031 cm2). Laser irradiation was performed for 3 sessions, at intervals of 48 hours Group 2: (62 patients, n = 218 teeth): cyanoacrylate group. 3 applications, at intervals of 48 hours, following the laser protocol |
|
| Outcomes | Sensitivity to thermal (air) stimulus was recorded before and on days 1, 3, 5, and 7 after the treatment (VAS score) | |
| Notes |
Baseline characteristics: thorough scaling and root planing were performed as part of phase 1 therapy, and patients were advised to use 0.2% chlorhexidine mouthrinses for 2 weeks. Patients were recalled after 4 weeks. Persistent pockets of 5 mm at minimum of 2 sites in each quadrant were scheduled for flap surgery. The surgical procedure was performed by an experienced examiner Sample size calculation: detailed mention of the calculation Source of funding: no competing financial interests reported Ethics approval: quote: "The study protocol was approved by both the Research Ethics Committee of the UFVJM (no. 061/06) and Research Ethics Committee of the Federal University of Sao Paulo (no. 0530/08). The study was also conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000" Informed consent: quote: "Participants were informed about the research and signed the statement of free and informed consent" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent researcher (PG), who was masked to the patients and interventions, conducted randomization using opaque envelopes that had been prepared previously and sealed" |
| Allocation concealment (selection bias) | Low risk | Quotes: "An independent researcher (PG), who was masked to the patients and interventions, conducted randomization using opaque envelopes that had been prepared previously and sealed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The researchers who applied the treatments were only informed of the treatment to be performed when it was time to do so [blinding of personnel]. Masking was done by replacing the laser goggles with sleeping masks for each patient and simulating the application of the other treatment [blinding of participants]" Comment: blinding of personnel was inadequate. Blinding of participants was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Interventions were always performed by the same researcher who did not participate in treatment evaluations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A single tooth (treated with laser) presented acute sensitivity, spontaneous pain, had to be covered with glass ionomer cement, and was excluded from statistical analysis" |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified The authors report no conflicts of interest related to this study |
García 2017.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth design) Duration of trial: not mentioned in the study Duration of follow‐up: 2 months |
|
| Participants |
Setting: Periodontal Pathology and Surgery Unit belonging to the Oral Surgery and Implantology Master Degree program of the University of Barcelona and a private clinic of the same area, Spain Inclusion criteria: patients diagnosed with dentine hypersensitivity in at least 2 teeth at different quadrants Exclusion criteria: any desensitizing treatment (current or last month); pregnancy; eating disorders (bulimia, etc.) or diet that cause erosion and/or tooth wear; orthodontic treatment; teeth whitening in the past 3 months; teeth with large fillings or reconstructions affecting the assessment area; teeth with fractures, cracks or untreated caries; non‐vital teeth or pulpal pathology; parafunction Total number: 30 patients (120 teeth) Age range: 19 to 67 years Sex (M/F): 9/21 |
|
| Interventions |
Group 1: (30 patients, n = 60 teeth): control group (placebo). Treatment of 2 teeth was simulated without activating the laser Group 2: (30 patients, n = 60 teeth): laser THOR LX2 (THOR Photomedicine Ltd, Chesham, UK) at a 5 mm distance, with oscillating movements, wavelength 660 nm, power 200 mW, continuous mode, illuminated treatment area 1.15 cm2, irradiance 173 mW/cm2, irradiation time 60 s, energy 12 Joules, fluence 10.4 J/cm2 |
|
| Outcomes | Dentine hypersensitivity for tactile stimulation (touching the tooth neck with a sharp dental probe) and thermal stimulation (with an air jet from the syringe dental chair, isolating adjacent teeth with cotton rolls) was recorded before the laser treatment, immediate post‐treatment (after 2 minutes), 2 weeks, 1 month, and 2 months after treatment (VAS from 0 to 100) | |
| Notes |
Baseline characteristics: patients were treated with scaling and root planing and subsequently referred if diagnosed with dentine hypersensitivity Sample size calculation: not mentioned in the study. Following the baseline examination, each side was randomly allocated either to the treatment or the control side with a series of random numbers Source of funding: the authors state no conflict of interest Ethics approval: quote: "The institutional review board (Ethical Committee of Clinical Investigation, University of Barcelona Dental School) reviewed and approved the study protocol" Informed consent: quote: "All patients were informed of the nature and objectives of the study, and signed consent prior to inclusion in the study" Adverse events: quote: "There were no adverse effects of diode laser treatment and no complications" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following the baseline examination, each side was randomly allocated either to the treatment or the control side with a series of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants is likely adequate due to use of placebo laser |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome measurement is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data from the result table during 2‐month follow‐up period |
| Selective reporting (reporting bias) | Low risk | The study did not report included standard deviations for the estimated marginal means of VAS for tactile and thermal stimulation (Table 1) |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation not mentioned in the study |
Gentile 2004.
| Study characteristics | ||
| Methods |
Study type: RCT (2 parallel groups) Duration of trial: 8 to 11 weeks Duration of follow‐up: 6 to 8 weeks |
|
| Participants |
Setting: Bauru Dental School, Brazil Inclusion criteria: complaint of sensitivity at the cervical part of the anterior and/or posterior teeth to current stimulus such as air, touch, sweet foods or brushing, with or without non‐carious cervical lesions and with gingival recession, independent of their length or wideness; good levels of oral hygiene, with an oral health condition that would allow precise diagnosis of dentine hypersensitivity; absence of serious systemic and psychological diseases; those using any analgesic or anti‐inflammatory medicines were oriented to not make use of it 6 hours before hypersensitivity treatment Exclusion criteria: exclusion of teeth presenting decays, cracks or fissures, large and unsatisfactory restorations, class V restorations, prosthetic elements and abutments of partial prosthesis under abnormal occlusion forces; without periodontal cysts and increased mobility; in cases submitted to periodontal surgery, a minimum period of at least 3 months was respected before treatment of dentine hypersensitivity; teeth should not have been submitted to dentine hypersensitivity treatment in the last 6 months Total number: 32 participants with 68 teeth Age range: 20 to 52 years Sex (M/F): 10/22 |
|
| Interventions |
Group 1: (16 patients, n = 35 teeth): AsGaAl diode (15 mW, 0‐15 J/cm2, 670 nm, tip: 4 mm2). The laser dosage applied to each tooth was 4 J/cm2 for a pre‐determined time of 2 minutes, according to calibration of the laser device. Laser was irradiated at 3 points on each tooth Group 2: (16 patients, n = 33 teeth): the control group were exposed to light cure (as placebo) for 30 seconds in an identical procedure. Treatments in both groups were rendered in 6 sessions with intervals from 48 to 72 hours |
|
| Outcomes | Sensitivity to tactile and thermal (air) stimuli was recorded before and 6 to 8 weeks after the final session of the treatment (VAS score) | |
| Notes |
Baseline characteristics: 1. all patients were submitted to an accurate anamnesis, in order to detect any systemic alteration, use of any medication, or psychological disturbances that could have been omitted at the initial report and that could be relevant for maintenance of the patient in the study. A second approach comprised questioning the patients about their current food habits, oral hygiene and about any periodontal treatments that could act as coadjutant factors triggering dentine hypersensitivity. However, no alteration regarding food habits was introduced, only concerning hygiene methods when the technique was considered traumatic; 2. determination of dentine hypersensitivity patterns: each selected tooth of each patient received 2 stimuli, namely a tactile with a #5 dental probe and an air jet, considered as a thermal‐evaporative stimulus. The first stimulus consisted in investigating all lesion extensions with the probe tip, which can lead to compression of the dentine, thus favouring motion of the dentine fluid, which activates the mechanoreceptors causing the painful sensation. The second stimulus, the air‐jet, was applied to the exposed dentine with an air syringe for 1 second, at room temperature and at a distance of 1 cm from the dentine surface. This stimulus can be considered a combination of thermal and evaporative stimuli, and this way 2 mechanisms operate to cause pain, namely the decrease in temperature at the dentine surface and fluid evaporation from some opened dentinal tubules, activating the hydrodynamic forces at the dentinal tubules and stimulating the painful sensation. An interval was allowed between the stimuli application, which was enough to avoid interferences between them. Measurement of sensitivity was performed after each stimulus by VAS Sample size calculation: not mentioned in the study Source of funding: not mentioned in the study Ethics approval: quote: "All patients participating in the study signed an Informed Consent Term, based on the Guidelines of the Ethics Committee of Bauru Dental School, 1998" Informed consent: quote: "All patients participating in the study signed an Informed Consent Term, based on the Guidelines of the Ethics Committee of Bauru Dental School, 1998" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to the use of placebo laser, the study might be free of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No descriptions on loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation and source of funding were not mentioned in the study |
Gerschman 1994.
| Study characteristics | ||
| Methods |
Study type: RCT (2 parallel groups) Duration of trial: not mentioned in the study Duration of follow‐up: 8 weeks |
|
| Participants |
Setting: Oro‐Facial Pain Clinic, University of Melbourne, Australia Inclusion criteria: age 15 to 69 years; even distribution of males and females; mobility of teeth < 1; VAS ≥ 3 Exclusion criteria: active periodontal disease; periodontal surgery within the last 6 months; teeth with carious lesions; large restorations; pulpitis and cracked enamel; chronic, debilitating disease or chronic disease with daily episodes of pain, for example, arthritis; daily doses of medication, for example, analgesics, anticonvulsants, antihistamines, sedatives, tranquillizers, mood‐altering drugs, or anti‐inflammatory drugs; participation in a desensitizing dentifrice study within the last 3 months Total number: 71 participants enrolled and completed Age range: 15 to 69 years (mean age: 37.5 years) Sex (M/F): not mentioned in the study |
|
| Interventions | Participants were randomly divided into 2 groups
Group 1: placebo group (28 participants enrolled and completed). Details not mentioned in the study Group 2: GaAlAs laser (active group). Details: (1) GaA1As (P‐laser, RHR Marketing Services Ltd, Bentlcigh, Victoria) was used for active treatment. An identical, unpowered placebo laser was also used. The P‐laser system has a power output of 30 mW; and a red aiming beam, with a power output of approximately 0.1 mW. (2) The laser source has a GaAlAs‐diode and a wavelength of 830 nm. (3) The stated power of 30 mW (± 10%) is the precise measure of energy emitted. The diode specification is 50 mW. A linear scale power meter with LCD read‐out designed to measure only light sources at a wavelength of 830 nm was employed. (4) LLLT was applied for 1 minute to both the apex and cervical area of the tooth. Calculation for total irradiance was based on mean power (watts) per time (seconds), for example, 30 mW for 60 s = 1.8 J. The power meter was used to calibrate the laser before each use. LLLT was reapplied at 1 week, 2‐week, and 8‐week intervals and dentinal hypersensitivity was rated on the VAS at the conclusion of each visit Subgroup 2a: tactile sensitivity group (22 participants enrolled and completed) Subgroup 2b: thermal sensitivity group (21 participants enrolled and completed) |
|
| Outcomes | Responses to air blast stimuli and tactile stimuli (VAS) at baseline, and 1 week, 2 weeks, 8 weeks after intervention | |
| Notes |
Baseline characteristics: 1. baseline hypersensitivity assessment included thermal sensitivity (response to a 1‐second air blast from a dental air unit syringe at a temperature of 18 to 21 ℃) and tactile sensitivity (response to passing a dental explorer over the labial cervical area); 2. the subjects rated their response on a continuous 100 mm VAS. Only 1 tooth per subject was tested. No specific tooth type (that is, incisor, premolar, molar) was selected. A VAS >= 3 was required for inclusion in the study; 3. care was taken so that 1 stimulus did not interfere with the succeeding stimuli, that is, tactile first then thermal. Subjects were instructed to brush their teeth with a non‐desensitizing dentifrice twice daily throughout the study Sample size calculation: not mentioned in the study Source of funding: not mentioned in the study Ethics approval: not mentioned in the study Informed consent: quote: "subjects were drawn mainly from a private practice population and on referral by other dentists and were required to sign an informed consent form" Adverse events: 2 patients reported a temporary, reversible pain sensation in response to lasing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects experiencing thermal and tactile sensitivity were assigned randomly to an active or placebo group by means of a computer‐generated randomized code" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to use of placebo laser, the study might be free of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No descriptions on loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation, source of funding and ethics approval were not mentioned in the study |
Lee 2015.
| Study characteristics | ||
| Methods |
Study type: RCT (single‐blinded, 3 parallel groups) Duration of trial: 4 weeks (the first 2‐week treatment period, the strantium chloride (SC) toothpaste, the n‐CAP toothpaste, and the laser treatments were applied to each group. For the subsequent 2‐week maintenance period, all participants performed only toothbrushing with the same fluoride dentifrice) Duration of follow‐up: 4 weeks |
|
| Participants |
Setting: Department of Advanced General Dentistry at Yonsei University Dental Hospital, Seoul, South Korea Inclusion criteria: having at least 2 hypersensitive teeth that were scored > 4 according to the VAS and scored > 1 according to the air blast, and having exposed root surfaces from which a painful response was elicited by a dental probe and air blast Exclusion criteria: chronic disease; carious lesions on the selected or neighbouring teeth; any desensitizing therapy on the selected teeth during the last 2 months; cervical filling, non‐vital teeth, and cracks on the selected teeth; or pregnancy or side effects from a laser Total number: 102 participated in the experiment, however, 20 were excluded because they did not complete the follow‐up sessions. Therefore, a total of 82 participants were evaluated Age range: 20 to 65 years Sex (M/F): 29/53 |
|
| Interventions | Participants were randomly divided into 3 groups Group 1: positive control group, strantium chloride dentifrice (n = 30) Group 2: experimental group 1, 20% n‐CAP dentifrice (n = 30) Group 3: experimental group 2, Er,Cr:YSGG laser (n = 22) Details: the laser irradiation was divided into 3 phases according to the manufacturer's recommended settings. The first and second phases were supplied with water (0.25 W, 20 pulses per sec (pps), 16% water, 16% air), whereas the third phase was conducted without water and the laser treatment was implemented for 30 seconds in each phase |
|
| Outcomes | Responses to tactile stimulus was recorded as VAS and thermal (air blast) stimulus was recorded using the air sensitivity scale by Schiff et al: 0 = tooth/subject does not respond to air stimulus; 1 = tooth/subject responds to air stimulus but subject does not request discontinuation of stimulus; 2 = tooth/subject responds to air stimulus and subject requests discontinuation or moves from stimulus; 3 = tooth/subject responds to air stimulus, subject considers stimulus to be painful, and requests discontinuation of the stimulus; at baseline, 1 week, 2 weeks, and 1 month after intervention |
|
| Notes |
Baseline characteristics: all participants received supra‐ and subgingival scaling prior to entry into the study. All participants received the dentifrice tested in each group and used the same toothbrush with flat and soft bristles (Skydent No 32 SOFT, Skydent, Korea), and they were instructed to brush their teeth twice a day for 1 minute every time with the same toothbrushing method that they usually used Sample size calculation: not calculated; quote: "The small sample size was also another limitation. Therefore, further study is needed to evaluate the combined effects of the n‐CAP dentifrice and the Er,Cr:YSGG laser with sufficient samples based on a sample size estimation" Source of funding: National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology Ethics approval: quote: "The research protocol was initially submitted for approval by the Ethics Committee of the Yonsei Dental Hospital, Seoul, Republic of Korea" Informed consent: quote: "Participants were fully informed about the purpose and procedures of this study, and voluntarily provided written informed consent" Adverse events: not reported in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Single‐blind study" Comment: the patients may be aware of the treatments received due to the differences in perception of each treatment. The outcome is likely to be influenced by incomplete blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Possible incomplete blinding of participants can lead to unclear detection bias when reporting pain level. Neither of the assessors participated in treatment procedure to maintain blindness |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "20 participants dropped out of the study (loss to follow‐up). These 20 were excluded from this experiment because they did not attend follow‐up appointments" Comment: participants who did not complete the follow‐up sessions were excluded from the study. However, reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation was not mentioned in the study |
Lier 2002.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth design, 2 groups) Duration of trial: not mentioned in the study Duration of follow‐up: 16 weeks |
|
| Participants |
Setting: The Institute of Clinical Dentistry, University of Oslo, Norway Inclusion criteria: the subjects had to have 2 teeth that were hypersensitive to thermal/evaporative stimulation with a blast of air Exclusion criteria: being under analgesic anti‐inflammatory, inhalation drugs or tricyclic antidepressive treatment regimens, having eating disorders, pregnancy, being under orthodontic therapy, having cognitive dysfunctions or general communication difficulties; teeth were excluded if they had been subjected to periodontal surgery within the past 3 months, had congenital enamel/dentine defects, were carious, were extensively restored or fractured, were abutments with no exposed tooth surface, were non‐vital or had symptoms of pulp damage, were under other antihypersensitive regimens within the last 30 days Total number: 17 participants enrolled and completed Age range: 26 to 66 years Sex (M/F): 3/14 |
|
| Interventions |
Group 1: (one side) Nd:YAG laser (wavelength: 400 μm; output: 4 W; duration: 2 minutes) Details: a Genius Laser System (Mølsgaard Dental, Denmark) with a 400 micrometers laser tip was used. Laser was first applied without a cooling system for 30 s, then for 90 s, using water as a coolant, in accordance with the manufacturer's instruction. The potency of the laser used was 4 watts, and it was applied on the buccal surface of the tooth in a mesial‐distal direction Group 2: (another side) placebo laser Details: the difference between the test and control procedures was that the laser beam was switched off during the latter, but the coolant was functioning during both |
|
| Outcomes | Responses to air blast stimuli (VAS) at baseline, and immediately after, 1 week, 4 weeks, 16 weeks after intervention | |
| Notes |
Baseline characteristics: 1. hypersensitivity reaction was provoked by means of an air blast 1 cm from the tooth surface at the cervical third of the tooth after removing supragingival plaque with a low speed handpiece with pumice powder and without fluoride. The adjacent teeth were covered by cotton rolls. The stimulus was delivered until reaction, or up to a maximum duration of 10 s. The time before reaction was recorded. All stimuli were given by 1 operator at the same dental chair with the same equipment yielding similar air pressure and temperature each time; 2. after the stimulus, the patient was asked to mark the intensity of pain on a VAS, which was marked on the left end 'no pain' and on the right end 'extreme pain' Sample size calculation: not mentioned in the study Source of funding: DICO MED AS Ethics approval: quote: "The study protocol was approved by the Regional Committee for Medical Research, Oslo, Norway" Informed consent: quote: "After having received oral and written information about the intention and the design of the study, and having signed the informed consent form, the subjects were included in the study" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to use of placebo laser, the study might be free of performance bias It was impossible to blind personnel in this study design, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The laser treatment was performed by one operator and the pain was assessed by another person" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation was not mentioned in the study |
Lizarelli 2007.
| Study characteristics | ||
| Methods |
Study type: RCT (single‐blinded, split‐mouth design) Duration of trial: not mentioned Duration of follow‐up: 3 months |
|
| Participants |
Setting: Sao Carlos Federal University, Brazil Inclusion criteria: male and female patients with cervical dentinal hypersensitivity Exclusion criteria: indication for or already performed endodontic treatment, extensive restorative treatment, decay, and severe periodontal disease Total number: 60 patients; 144 teeth Age range: not mentioned (older than 18) Sex (M/F): not mentioned |
|
| Interventions |
Group 1: treated with light emitting diode laser Details: a twin laser used equipped with 2 handpieces, 1 emitting in infrared (780 nm) and 1 in the red (660 nm) band; the 660 nm handpiece with a power setting of 40 mW was chosen; laser beam spot diameter was 4 mm Group 2: treated with light emitting diode therapy Details: device emits at a spectral band of 630 nm, presenting a constant power of 230 mW. The spot size of 4 mm is equivalent to that of the low‐intensity laser system Both had same delivery tip, in order to focus the light beam from the laser source to the target tissue Group 3: placebo (no irradiation) |
|
| Outcomes | Using VAS at baseline, 15, 30, and 60 days after last application | |
| Notes |
Baseline characteristics: dentinal hypersensitivity was assessed by 2 to 3 s air blast stimulation; after the stimulus, the patient was asked to mark the intensity of pain on a VAS, which was marked on the left end 'no pain' and on the right end 'extreme pain' Sample size calculation: not mentioned in the study Source of funding: not mentioned in the study Ethics approval: not mentioned in the study Informed consent: quote: "Informed consent describing risk and benefits of the respective treatments was obtained from all subjects before participation in the study" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebos might reduce performance bias. It was impossible to blind personnel in this study design, but the outcome is not likely to be influenced by lack of blinding Comment: this study was described as a "single‐blind study" in the abstract. However, the details of blinding (study personnel as outcome assessor or patients) was not described in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided Comment: this study was described as a "single‐blind study" in the abstract. However, the details of blinding (study personnel as outcome assessor or patients) was not described in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions Incomplete outcome data was not mentioned in the study. However, we can appreciate that they had dropouts by comparing the number of teeth between table 1 and 2. It seems that participants who did not complete the follow‐up sessions were excluded from the study |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study. However, tables with number of teeth with improved or worsened hypersensitivity and only bar charts were reported as a result (Fisher's test was used). They cannot be entered in a meta‐analysis Quote: "A 95% confidence interval was used for all analysis" |
| Other bias | Low risk | No other sources of bias were identified Source of funding, ethics approval and sample size calculation were not described in the study |
Lund 2013.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth design) Duration of trial: not mentioned in the study Duration of follow‐up: 3 months |
|
| Participants |
Setting: Dental College at Federal University of Pelotas, Brazil Inclusion criteria: aged between 18 to 60 years, good health conditions, symptoms elicited by cold air, no periodontal surgery in the last 6 months, no use of desensitizing agents in the last 6 months, no use of analgesics and/or anti‐inflammatory medicines, no orthodontic treatment, absence of excessive gingival inflammation and the presence of, at a minimum, 2 teeth with dentine hypersensitivity Exclusion criteria: any other possible causes of sensitivity such as pulpitis, failed restorations, premature contacts, attrition, abrasion, occlusal stress or periodontal disease Total number: 13 participants enrolled and completed Age range: 19 to 58 years Sex (M/F): 5/8 |
|
| Interventions |
Group 1: diode low‐level laser group Details: infrared diode low‐level laser treatment (Biowave Dual – Kondortech, class IV = 780 nm) consisted of 3 sessions of application on the teeth, at intervals of 72 hours. The protocol was followed by 4 punctual applications of 10 s each, 3 at the cervical zone (mesio‐buccal, the bucco‐lingual midpoint and disto‐buccal) and 1 at the root apex with an applied dosage of 5 J/cm which represented the effective optic potency of 20 mW at the entry of the laser equipment Group 2: carbomer 940 gel (placebo) |
|
| Outcomes | Exposure time to air blast (VAS) at baseline, 5 minutes after the execution of the last session of treatment and after 7, 15, 30, and 90 days | |
| Notes |
Baseline characteristics: 1. using an evaporative stimulus (with a dental air syringe) that was applied 2 cm distant from the hypersensitive area for 60 s, with a right angle to the buccal site of the assigned teeth, whilst adjacent teeth were isolated with cotton rolls, until the patient raised his or her hand to indicate the occurrence of sensitivity; 2. soon after pain was detected, the stimulus was interrupted and the Exposure Time to Air Blast (ETAB) was noted; 3. after the stimulus, the patient was asked to mark the intensity of pain on a VAS, which was marked on the left end 'no pain' and on the right end 'extreme pain' Sample size calculation: not mentioned in the study Source of funding: not mentioned in the study Ethics approval: quote: "Ethics Committee of the Dental College at Federal University of Pelotas, RS, Brazil (Reference #046/2003)" Informed consent: quote: "Patients that met all of the inclusion criteria signed consent forms before entering the trial" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided Quote: "the teeth were randomly divided into one of three" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Lack of use of placebos might lead to performance bias Comment: the patients are likely to be aware of the treatments received due to the differences in perception of each treatment. The outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack blinding of participants can lead to high risk for detection bias when reporting pain level |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Low risk | The intended outcome (pain) was reported |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation and source of funding were not mentioned in the study |
Maximiano 2019.
| Study characteristics | ||
| Methods |
Study type: RCT (double‐blinded, 3 parallel groups) Duration of trial: not mentioned in the study Duration of follow‐up: 1 month |
|
| Participants |
Setting: Dental School of the University of São Paulo, Brazil Inclusion criteria: patients in the age range 18 to 65 years, of both sexes, non‐carious cervical lesions and/or gingival recessions associated with dentine exposure and symptoms of cervical dentine hypersensitivity, and with at least 1 tooth with a minimum pain level of 4 cm on the VAS Exclusion criteria: pregnant patients or those in the breastfeeding stage, those who made continuous use of analgesic or anti‐inflammatory medications, those with active caries lesions or deficient restorations, those undergoing orthodontic treatment, and those with deficient oral hygiene or loss of dental structure that would need restorative treatment were excluded from the study, since these conditions could directly interfere in the results of evaluations Total number: 70 participants enrolled and completed Age range: 18 to 65 years Sex (M/F): not mentioned in the study |
|
| Interventions |
Group 1: placebo laser (n = 23) Details: control/placebo patients received simulations of the 2 treatments. Prophylaxis with Nupro® paste was simulated with a rubber cup and water, taking care that the cup did not touch on the cervical region of the teeth in question, to prevent any possible change in the dentine structure, such as obliteration of tubules. Irradiation with Nd:YAG laser was simulated with the laser switched on, but with the display exhibiting 0 W and with the guide light only Group 2: CSP paste (NovaMin) (n = 23) Details: CSP patients received the treatment with 15% CSP prophylaxis paste (NUPRO Extra Care powered by NovaMin, Dentsply professional, lot 16050201), applied on the vestibular surface of the selected teeth, with a rubber cup at low speed for 60 s in accordance with the manufacturer’s instructions. Afterwards, the surfaces were washed with water and the patient performed a mouthrinse Group 3: Nd:YAG laser irradiation (n = 24) Details: laser patients received irradiation in the vestibular and cervical regions of the selected teeth, with Nd:YAG laser (Power Laser, Lares Research, San Clemente, CA, USA, process FAPESP 07/55497‐0). The equipment worked in a pulsed manner, with a pulse width of 150 μs and a fixed repetition rate of 10 Hz. Its energy system operated by means of a quartz fiber optic of 400 μm. Irradiation was performed with the fibre optic perpendicular to the tooth, in contact mode. 4 (4) irradiations were made with scanning movements: 2 in the mesio‐distal and 2 in the occlusal‐gingival directions. Each irradiation was made for up to 15 s, with an interval of 10 s between each irradiation, time necessary for thermal relaxation of dentine. The parameter used was 1 W of power, repetition rate of 10 Hz, 100 mJ of energy, and 85 J/cm2 of energy density |
|
| Outcomes | Exposure Time to Air Blast and tactile stimuli (VAS) at baseline, 5 minutes, 1 week, and 4 weeks after the treatment | |
| Notes |
Baseline characteristics: an evaporative stimulus, with a jet of air applied in the cervical region of the tooth for 3 s at a distance of 1 mm with a pressure of 40 psi, and under relative isolation, covering the neighboring teeth with cotton wool rolls. Another measurement was made after tactile stimulation, with the exploratory probe in contact on the cervical region, passing over the mesial to the distal region and vice versa under constant pressure Sample size calculation: quote: "The sample calculation was based on comparison of the means, with an expected minimum difference of 2 units between the initial and final values of the VAS and standard deviation of 2. Considering an α of 5% and power of 80%, 26 patients per group would be required" Source of funding: State of São Paulo Research Foundation Ethics approval: quote: "Research Ethics Committee of the Dental School of the University of São Paulo ‐ CEP FOUSP (Report No. 2291636)" Informed consent: quote: "Patients that met all of the inclusion criteria signed consent forms before entering the trial" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a researcher not involved in the study, using the Excel program of the Microsoft Office package. Stratified randomization was performed, considering two strata: moderate (4–6.9 cm) and severe (7–10 cm) pain" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was implemented with sequentially numbered opaque sealed envelopes. Each envelope was only opened by the researcher at the time of performing the treatment. The patients and evaluators did not know what the designated allocation was. The researcher did not know the patients’ level of pain" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded study, the stimuli and pain measurements were made by 2 previously calibrated evaluators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified |
Naghsh 2020.
| Study characteristics | ||
| Methods |
Study type: RCT (double‐blinded, 3 groups) Duration of trial: not mentioned in the study Duration of follow‐up: 2 months |
|
| Participants |
Setting: Faculty of Dentistry, Isfahan University of Medical Sciences, Iran Inclusion criteria: patients who had cervical dentine hypersensitivity in at least 3 teeth in 3 separate quadrants. The selected teeth were free of calculus and plaque and if necessary, the subjects underwent a scaling procedure before the study Exclusion criteria: patients with teeth showing evidence of irreversible pulpitis or necrosis, carious lesions, crown fractures, cracks, caries or restorations, facets of attrition, premature contact, active periodontal disease, use of analgesics during the 72‐hour period before laser application and individuals who had used anti‐sensitivity toothpaste during the previous 3‐month period. Pregnant women and smokers were excluded from the study Total number: 7 patients with 96 teeth enrolled and completed Age range: 25 to 45 years Sex (M/F): 3/4 |
|
| Interventions | Sensitive teeth of 7 patients were divided into 3 groups with a randomized matching method Group 1: 660 nm diode laser beams were applied to the hypersensitive teeth. The power of 30 mW, in contact with and perpendicular to the surface, continuous irradiation for 120 s with a forward and backward (sweeping) movement Group 2: 810 nm diode laser beams were applied. The power of 100 mW, in contact with and perpendicular to the surface, continuous irradiation for 120 s with a forward and backward (sweeping) movement Group 3: not laser‐irradiated and were only exposed to index radiation (control group). Treatment was rendered in 4 sessions with a 1‐week interval in a similar manner in all the 4 sessions |
|
| Outcomes | Responses to applying dry ice sprayed on a small cotton pallet over the tooth surface recorded sing the VAS index (0 to 10). The VAS was evaluated before treatment and immediately after laser irradiation at the first, second, third, and fourth sessions (immediately after it and at 1‐week, 30‐day, and 60‐day postoperative intervals) | |
| Notes |
Baseline characteristics: to register the severity of pain in the affected teeth the VAS was used. An attempt was made to carry out random assignment (randomized allocation) based on the baseline VAS scores of the teeth after they were recorded Sample size calculation: not mentioned in the study Source of funding: none. Quote: "The authors declare no conflict of interest" Ethics approval: quote: "The present randomized clinical trial was approved by the Isfahan University Ethics Committee under the code 396228 and registered on the Iranian Registry of Clinical Trials website (identifier: IRCT2017062022699N4; https://www.irct.ir/)" Informed consent: quote: "All the subjects signed informed consent forms in order to be included in the study" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided Quote: "An attempt was made to carry out random assignment (randomized allocation based on the baseline VAS scores of the teeth after they were recorded" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided Quote: "An attempt was made to carry out random assignment (randomized allocation based on the baseline VAS scores of the teeth after they were recorded" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The teeth in the control group were not laser‐irradiated and for the purpose of blinding, they were only exposed to index radiation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided Comment: although the authors stated that this is a double‐blinded study, the method of blinding the examiner was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified |
Orhan 2011.
| Study characteristics | ||
| Methods |
Study type: RCT (4 parallel groups) Duration of trial: not mentioned in the study Duration of follow‐up: 7 days |
|
| Participants |
Setting: Department of Endodontics, Near East University, Turkey Inclusion criteria: patients were required to present a minimal of 4 teeth with dentinal hypersensitivity; the selected individuals had similar sociocultural characteristics; the patients had more than 1 month dentine hypersensitivity and did not use other hypersensitivity methods such as toothpastes and tubules sealers to decrease the amount of dentine hypersensitivity; the clinical examination of the teeth involved in the study revealed no difference in terms of presence of restorations between test and control teeth Exclusion criteria: participants that presented parafunctional habits, gastric and/or emotional diseases, or frequent ingestion of acidic food as probable etiological factors for dentine hypersensitivity, were ruled out; patients who had dental pathology causing pain similar to cervical dentinal hypersensitivity (such as teeth with caries, the presence of orthodontic appliances and restorations, and/or the presence of a history of periodontal surgery in the area of the tooth during the previous 3 months, postoperative sensitivity), patients who had taken any medication, patients who received professional treatment with desensitizers in the previous 6 months, patients who received any treatment in past 30 days, those patients who were pregnant or lactating and patients who had any systemic diseases and/or the presence of a vital bleaching history; subjects whose test teeth had evidence of pulpitis, carious lesions, cervical fillings, defective restorations, facets of attrition, premature contact, cracked enamel or any other factor that could be responsible for sensitivity complaints, were also excluded Total number: 16 patients with 64 teeth enrolled and completed Age range: 21 to 51 years (mean age: 34.31 years) Sex (M/F): 8/8 |
|
| Interventions | Participants were randomly distributed into 4 groups (n = 4) Group 1: Gluma desensitizer Details: in the first group, 2 layers of desensitizer (Gluma Desensitizer, Heraeus Kulzer, Armonk, NY) containing 2‐hydroxyethylmethacrylate, glutardialdehyde, and purified water were applied to ensure adequate desensitization. Care was taken to ensure that the desensitizer only came into contact with the area to be treated and the smallest possible amount required for the treatment was applied using disposable brushes for avoiding cross‐contamination and left for 60 s. Then the surface was carefully dried applying a stream of compressed air until the fluid film disappeared and the surface was no longer shiny. The surface was then rinsed thoroughly with water Group 2: GaAlAs laser (output 25 mW, wavelength 655 nm) Details: in the second group, a GaAlAs red wavelength low‐intensity diode laser (RJ Lasers, Vienna, Austria) was chosen for LLLT. The system delivers a 25‐mW output that emits a wavelength of 655 nm. The operator and the patient wore laser‐protective eyewear specific to the diode laser's wavelength during the treatments. The laser was applied to the dentine surfaces in continuous mode with contact on the region of exposed dentinal area for 160 s in each session per tooth, in a uniform, sweeping, and scanning motion. The calculated deposited energy density was 4 J/cm2 per dental element. Laser treatments were carried out in 6 sessions, with no intervals between sessions, during a period of 6 consecutive days Group 3: distilled water Details: in the third group, distilled water was used as the placebo group for the desensitizer Group 4: placebo laser Details: in the fourth group, the laser device was positioned but yet not activated |
|
| Outcomes | Responses to air blast stimuli (VAS with the value range between 0 and 100) at baseline, and 24 hours, 7 days after intervention | |
| Notes |
Baseline characteristics: 1. prior to desensitizing treatment, dentine hypersensitivity was assessed by a thermal/evaporative stimuli and patients' response to the examination was considered to be a control. Each patient had 4 incisors, canines, or premolars with exposed cervical dentine on the facial surface that could be affected when air was applied. Sensitivity was assessed by means of evaporative stimuli, a 5‐s cold air blast (temperature range of 19‐20℃) at a distance of 0.5 cm from the site to be tested. All stimuli were given by 1 operator at the same dental chair with the same equipment yielding similar air pressure and temperature each time; 2. all the stimuli were applied on the cervical region of the experimental teeth after removing supragingival plaque with a low‐speed handpiece with pumice powder and without fluoride. The adjacent teeth were isolated with cotton rolls and a suction device. The air stream was not extended longer than necessary to generate a response. The patients were given a VAS upon which they were asked to place a pencil mark at a point on a linear scale marked from 0 to 100 to describe the pain experienced. After each stimulus to the suspected site, the degree of hypersensitivity was determined from 0 to 100 as the baseline VAS score for each individual painful tooth. In order to standardize the sample, the criterion for inclusion in the study was a sensitive dentinal response of a minimum of 40 in the 0 to 100 numeric scale for pain evaluation, characterizing moderate cervical dentine hypersensitivity; 3. after recording the first scores, the subjects were randomly assigned to 1 of the treatment or the placebo groups. The subjects were blinded to the agent being used. The randomization process was conducted before the clinical steps and carried out by using sequentially numbered opaque sealed envelopes prepared with unrestricted (simple) randomization. Each treatment agent and placebo was written and sealed in envelopes before beginning the study. The dental operator who carried out all the treatments opened an envelope for each case at the beginning of the treatment Sample size calculation: not mentioned in the study. Source of funding: not mentioned in the study. Ethics approval: not mentioned in the study. Informed consent: quote: "Informed consent was prepared and obtained according to the Helsinki Declaration Ⅱ. The purpose and design of the investigation were explained and a consent form was signed by all of the participating patients" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process was conducted before the clinical steps and carried out by using sequentially numbered opaque sealed envelopes prepared with unrestricted (simple) randomisation" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation process was conducted before the clinical steps and carried out by using sequentially numbered opaque sealed envelopes prepared with unrestricted (simple) randomisation. Each treatment agent and placebo was written and sealed in envelopes before beginning the study. The dental operator who carried out all the treatments opened an envelope for each case at the beginning of the treatment" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The subjects were blind to the agent being used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatments were performed by the same experienced operator and the pain was assessed by another person to minimize errors and to avoid bias" Comment: adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation, source of funding, and ethics approval were not mentioned in the study |
Ortiz 2019.
| Study characteristics | ||
| Methods |
Study type: RCT (4 parallel groups) Duration of trial: 1 June to 20 October 2018 Duration of follow‐up: 1 month |
|
| Participants |
Setting: Federal University of Para (UFPA), Brazil Inclusion criteria: patients with at least 2 sensitive teeth with a response ≥ 4 on the 10 cm length VAS (0: no pain and 10: unbearable pain) after tactile and evaporative stimulation and/or the presence of a non‐carious lesion up to 2 mm deep, and/or class I gingival recession Exclusion criteria: patients with allergies to milk proteins; systemic diseases; carious lesions and/or pulpitis; presence of restorations on sensitive teeth; periodontal disease; cracks in the enamel; fixed orthodontic treatment; pregnant or nursing women; medication with analgesics or anti‐inflammatories or have received desensitizing treatment during the 3 months prior to the recruitment date Total number: 24 patients were enrolled. 21 participants (87.5%) with 80 teeth completed all phases of the study Age range: 18 to 50 years. The mean age was 30 years (SD = 8.13) Sex (M/F): 6/15 |
|
| Interventions | Participants were allocated into 4 groups Group 1: CPP‐ACPF group Details: CPP‐ACPF group participants received the MI Paste Plus application following the placebo group protocol, the PBM was simulated in the same way Group 2: PBM group Details: the placebo paste was applied as previously described and the laser was applied using an infrared light spectrum with a wavelength of 808 nm, positioning its tip at the previously described points (60 J/cm2 at each point) for 16 s Group 3: CPP‐ACPF + PBM group Details: MI Paste Plus and PBM were applied following the CPP‐ACPF and PBM protocols Group 4: placebo group Details: the placebo group received a water‐based base paste application with a microbrush (Microbrush, 3M ESPE) on the cervical vestibular surfaces of each tooth for 5 minutes that was later rubbed for 20 s with a rubber cup (Prophy Cup, MICRODONT) coupled to a low speed handpiece (Contra angle 500, Kavo). Followed by PBM simulation, that was carried out by positioning the laser tip at 2 points of each tooth, 1 at the center of the cervical region and another 1 in the middle third of the crown without light emission. The sound of the equipment was mimicked by an applicative on the cell‐phone (Beep, FoncannonInc) |
|
| Outcomes | Responses to tactile and air blast stimuli (VAS with the value range between 0 and 10) and dentine hypersensitivity experience questionnaire (DHEQ) Details: dentine hypersensitivity evaluation was performed by requesting the participant to signal a compatible number to their pain sensation on the VAS after 2 stimuli: tactile was performed by sliding an exploratory probe in a cross‐shaped way into the cervical region of the tooth. Evaporative consisted of the application of an air blast from a triple syringe for 3 s at a pressure of 40 psi (20 ± 3˚C), perpendicularly to the buccal surface of the tooth (0.5 cm distance), the adjacent teeth were protected with cotton rolls Dentine hypersensitivity was recorded in 5 moments: 1st registration: before starting the 1st treatment session; 2nd registration: after the 1st treatment session; 3rd registration: after the 2nd treatment session (24 hours after the 1st treatment session); 4th registration: after the 3rd treatment session (24 hours after the 2nd treatment session); and 5th registration: after 1 month of follow‐up The participants’ self‐reported evaluation was performed by giving them a questionnaire (DHEQ) before the start and after 1‐month follow‐up to determine the treatment impact on their HRQL [25,26]. Its summarized version consists of 15 questions that are answered according to a 7‐point Likert scale. Higher scores indicate a poorer HRQL |
|
| Notes |
Baseline characteristics: quote: "All the participants received a toothbrush with soft bristles (Top Plus, Condor) and a dentifrice without a desensitizing action (EVEN Baby, Raymundo da Fonte) with oral hygiene instructions. Three desensitizing treatment sessions were performed with a 24‐hour interval between each session" Sample size calculation: quote: "The data obtained in a pilot study were introduced into the G Power1 program (Heinrich‐Heine‐Universitat Dusseldorf, Germany), with a statistical power of 80%, a probability of error α of 5%, and effect size f estimative of 1.35 a total of 08 teeth per arm would be necessary" Source of funding: quotes: "The study was supported in part by scholarships for postgraduate students of the Organization of American States (OAS), the Coordination for the Improvement of Higher Education Personnel (CAPES) ‐ Finance Code 001. However, the study was not directly funded." "The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article. This study was supported by infrastructural maintenance by the National Council for Scientific and Technological Development (CNPq) and the Federal University of Para" Ethics approval: quote: "This RCT was approved by the health of science institute of ethics research committee of the University Federal of Para (Approval number: 2.858.288 S1 Protocol) and was registered on the clinical trials registry" Informed consent: quote: "After being duly informed about the risks and methods of this study, all the participants signed an informed written consent in accordance with the Declaration of Helsinki" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One of the research collaborators performed a block randomization using a numerical draw which allowed each participant to be allocated into one of the four groups under different arrangements (A4,1; A3,1; A2,1 and A1,1)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation concealment was maintained since the numerical draw was performed using numbered and coded papers. The code for each group was unknown by all: participants, principal investigator, clinical operator, and evaluator" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were unaware of the desensitizing treatment received because both pastes were contained in similar recipients hindering visual identification and the texture, color and odor of the placebo was similar to MI Paste Plus" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluator was not aware of the group to which the participant belonged" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up of 3 out of 24 patients was reported in the study |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pantuzzo 2020.
| Study characteristics | ||
| Methods |
Study type: RCT (single‐blinded, 3 groups) Duration of trial: August 2018 to June 2019 Duration of follow‐up: 7 days |
|
| Participants |
Setting: quote: "The work belongs to the Department of Clinic, Pathology and Dental Surgery, Faculty of Dentistry, Federal University of Minas Gerais, Belo Horizonte, Brazil" Inclusion criteria: individuals diagnosed with a score of dentine hypersensitivity higher than or equal to 2 in the VRS after evaporative and tactile stimulation in at least 1 sound tooth with gingival recession, individuals older than 18 years and those who accepted to participate in the study after signing of the informed consent form Exclusion criteria: individuals submitted to periodontal treatment or the treatment of dentine hypersensitivity within the last 30 days, pregnant women and individuals with decayed or filled teeth Total number: 28 participants Age range: mean age: 48.4 years Sex (M/F): 6/22 |
|
| Interventions | 28 patients were randomly distributed across 3 groups. Treatment was carried out in a single session Group 1: diode laser Details: the GaAlAs infrared semiconductor laser, 808‐nm wavelength was applied over the exposed root region at a central point for 60 s Group 2: fluoride Details: the application of acidulated fluoride phosphate 1.23% was performed under isolation with cotton rolls and after the procedure the tooth was dried with a piece of cotton. Application of the fluoride was performed with a small sterile cotton ball on the exposed root surface for 60 s. After application, the patient was instructed to spit exhaustively for 1 minute Group 3: placebo group Details: the placement of a layer of acrylic resin blocked the photons. The application of the placebo gel was similar to the application of fluoride. However, the cotton used for the application of the placebo on the tooth had no medication for dentine hypersensitivity |
|
| Outcomes | Pain was assessed with VAS. Evaporative stimulus and tactile stimulus were evaluated with the VRS. VAS was applied shortly after, 6 hours after, 12 hours after, and 24 hours after the single‐session treatment for dentine hypersensitivity, whereas VRS was applied shortly after, 15 minutes and 7 days after the treatment. Participants' quality of life was assessed with the validated Brazilian version of the Dentine Hypersensitivity Experience Questionnaire (DHEQ‐15) assessing functional limitations, coping behaviors, emotional, and social impacts caused by dentine hypersensitivity Details: the DHEQ‐15 consisted of 15 items distributed across 5 subscales: “constraints,” “adaptation,” “social impact,” “emotional impact,” and “identity.” The response options for each item are given on a 7‐point Likert scale as follows: 1 = “I strongly disagree;” 2 = “I disagree;” 3 = “I agree a little;” 4 = “I do not agree or disagree;” 5 = “I agree a little;” 6 = “I agree;” and 7 = “I agree a lot.” A higher score indicated a more negative perception of the individual regarding the impact of dentine hypersensitivity on himself/herself |
|
| Notes |
Baseline characteristics: quotes: "There was no difference among groups with respect to participants' age. In addition, the groups were similar with respect to sex, schooling, family income, and gingival recession." "In the baseline, the pain level measured with the VRS scale was very much similar among groups" Sample size calculation: not mentioned in the study Source of funding: quote: "The study was financially supported by PRPq‐UFMG. There are no conflicts of interest" Ethics approval: quote: "Approval of the Ethics Research Committee from the University was obtained (CAAE 87761718.1.0000.5149)" Informed consent: quote: "Participants signed a consent form" Adverse events: not mentioned in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Low risk | Quote: "The distribution of participants among groups was performed randomly with a sealed envelope, in which numbers corresponding to the treatment modalities were placed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "For the placebo, the placement of a layer of acrylic resin blocked the photons. The application of the placebo gel was similar to the application of fluoride. However, the cotton used for the application of the placebo on the tooth had no medication for DH. Participants were unaware to which treatment they would be submitted" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the limited number of participants and losses over the study period, restricting the 7‐day evaluation to a few individuals" |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported |
| Other bias | Low risk | No other sources of bias were identified |
Suri 2016.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth design) Duration of trial: not mentioned in the study Duration of follow‐up: 2 months |
|
| Participants |
Setting: Department of Periodontics, Patil Dental College and Hospital, India Inclusion criteria: patients in good systemic health, good oral hygiene, and clinically demonstrable dentine hypersensitivity teeth, specifically canines and premolars, which were reliable in their response to test measurements Exclusion criteria: patients with any systemic or psychological diseases, constant use of analgesic, antiinflammatory drugs, or allergic responses to dental products, carious lesions, defective restorations, fractures, prosthesis or orthodontics appliances, periodontal pockets, mobility, or evidence of pulpits and those who have used any desensitizing agents or undergone any periodontal surgery in the last 6 months Total number: 30 participants (120 teeth) Age range: 40 to 49 years Sex (M/F): 15/15 |
|
| Interventions |
Group 1: placebo‐controlled group, distilled water was applied by means of a cotton swab for 20 s Group 2: after isolation, 50% NaF varnish was painted on the sensitive surface with a disposable microbrush as per the manufacturer's instructions for 60 s. The cotton roll was removed to allow saliva or moisture to set the varnish Group 3: 980 nm diode laser was applied at 2 W power in a continuous wave mode on the test tooth surface, no contact mode using a fiber of 320 micron diameter held perpendicular to the irradiated surface at a distance of 1 mm. Each area was irradiated twice for 20 s Group 4: treated with both 5% NaF varnish and 980 nm diode laser described in group 2 and 3. NaF varnish was left on the tooth surface for 60 s before the irradiation |
|
| Outcomes | Response to tactile and air blast stimuli was recorded as VAS score at baseline, 24 hours, 1 week, 1 month, and 2 months | |
| Notes |
Baseline characteristics: 1. scaling and polishing was done for all the patients 1 week before the study and subjects were instructed not to use any other desensitizing agents during the study. All patients were taught modified Stillman technique and were instructed to use the same with non‐desensitizing toothpastes and soft bristle tooth brushes; 2. degree of severity was measured in response to tactile stimuli with explorer applied in light pressure on facial surface in mesio‐distal movement on cervical area; 3. air blast stimuli was performed with air syringe (air pressure 25–28 psi) of the dental unit at normal room temperature for 1 s at a distance of 1 cm from the tooth surface; 4. dentine hypersensitivity was assessed by patient's indication of the severity of pain related to each tooth immediately after each stimulus, according to VAS; 5. the sensitivity patterns were recorded at baseline, 24 hours, 1 week, 1 month, and 2 months Sample size calculation: not mentioned in the study Source of funding: none Ethics approval: not mentioned in the study Informed consent: quote: "Participants were informed about the purpose of the investigation and signed an appropriate informed consent form" Adverse events: none. Quote: "Complications such as detrimental pulpal effects or allergic reactions were not observed during this period. All teeth remained vital after treatment, with no reported adverse reactions or clinically detectable complications" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization sequence was generated using a table of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study consists of 4 groups, 1 of which is placebo. Inclusion of a placebo group in the experiment may indicate an attempt to blind participants, however it is considered insufficient as the patients may be aware of the nature of the treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"A total of 120 teeth in thirty patients completed the 2month study period" |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation and ethics approval were not reported |
Tevatia 2017.
| Study characteristics | ||
| Methods |
Study type: parallel, RCT Duration of trial: not mentioned in the study Duration of follow‐up: 6 weeks |
|
| Participants |
Setting: not clearly described in the study. Authors' institution is Department of Periodontology and Oral Implantology, ITS Centre for Dental Studies and Research, Muradnagar, Ghaziabad, Uttar Pradesh, India Inclusion criteria: absence of undergoing desensitizing therapy, absence of gestation or lactation, non‐allergic to the medicament employed in the study, do not have systemic conditions causing or predisposing to dentine hypersensitivity (e.g., chronic acid regurgitation), do not have excessive dietary or environmental exposure to the acids, absence of any teeth or supportive structures with any other painful pathological defects, and have baseline pain of above 5.0 VAS score Exclusion criteria: not mentioned in the study Total number: 120 participants. Quote: "Any drop‐outs were excluded from the study. Each patient included in the study was present at every follow‐up period" Age range: 18 to 55 years (mean 36.5 years) Sex (M/F): 69/51 |
|
| Interventions | Patients were randomly divided into 4 groups (n = 30 per group) Group 1: 5% potassium nitrate (KNO3) Details: 5% KNO was applied for 60 s on the tooth surface. In each visit of a patient, KNO3 was applied using a new disposable brush. Further, patients were restrained to rinse the teeth until 3 minutes Group 2: GaAlA diode laser Details: lased by GaAlA laser Photon Plus, Zolar Co., 980 nm with 62.2 J/cm2 energy in non‐contact mode, power wattage 0.5 W and using a fiber of 320 μ diameter. Each site underwent 3 applications of 60 s Group 3: 5% KNO and diode laser Details: the KNO3 gel was left on the tooth surface for 60 s before laser treatment Group 4: placebo (control) Details: commercial toothpaste was applied (placebo‐control) for 60 s |
|
| Outcomes | Responses to tactile (explorer), air blast and cold water using VAS scores were measured at the baseline, immediate postoperative application, 2, 4, and 6 weeks | |
| Notes |
Baseline characteristics: 1. scaling and root planning were performed before the sensitivity treatment to all the patients. In addition, teeth vitality was assessed. The vitality was checked using an electric Pulp Tester. All selected teeth were vital, which was the inclusion criteria. Oral hygiene instructions were provided to the patients and asked to perform tooth brushing twice daily (toothpaste was standardized after confirming the absence of any sensitivity check agents and handed over to the patients with strict instructions), employing modified bass technique; 2. there was negligible difference in VAS scores between the groups at baseline Sample size calculation: not mentioned in the study Source of funding: none. Quote: "There are no conflicts of interest" Ethics approval: quote: "The research protocol was reviewed and approved by the Ethical Committee of the Institution" Informed consent: quote: "All patients were educated about the study, and then, written consent was acquired before enrolment in the study" Adverse events: none. Quote: "In the recall period of study, no adverse effects for any subjective signs (like an allergic reaction, ulceration, or with objective signs such as redness of mucosa and staining of teeth) were observed" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided |
| Allocation concealment (selection bias) | Low risk | Quote: “Envelopes containing identifications for treatment groups were enclosed, mixed, and then numbered” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided. Possible incomplete blinding of participants can lead to unclear detection bias when reporting pain level Quote: “For all the follow‐ups, the same examiner was assigned every time” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Any drop‐outs were excluded from the study. Each patient included in the study was present at every follow‐up period" Comment: all participants completed the study |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation was not mentioned in the study |
Vieira 2009.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth, 3 groups) Duration of trial: not mentioned in the study Duration of follow‐up: 3 months |
|
| Participants |
Setting: Department of Restorative Dentistry, Federal University of Ceará, Fortaleza, Ceará, Brazil Inclusion criteria: patients who had good oral hygiene and at least 3 hypersensitive teeth Exclusion criteria: patients who had severe systemic and/or psychological diseases, constant use of analgesic and/or anti‐inflammatory drugs, or allergic responses to dental products; patients who used any desensitizing agents and/or submitted to periodontal surgery or scaling in the 6 months before enrolment; teeth which had carious lesions, defective restorations, cracks or fractures, premature contact, prosthesis or orthodontics appliances, periodontal pockets, mobility, or evidence of pulpitis Total number: 30 participants with 164 teeth enrolled, 24 participants with unknown number of teeth completed Age range: 24 to 68 years Sex (M/F): 7/23 |
|
| Interventions |
Group 1: (58 teeth enrolled, unknown number of teeth completed) GaAlAs laser (wavelength: 660 nm; output 30 mW; duration 2 minutes) Details: the treatments were applied under relative isolation by 1 experienced operator other than the examiner. The diode laser device was used on contact mode with the following parameters: continuous emission, 30 mW output power, wavelength of 660 nm, irradiation time of 120 s, and ray diameter of 3 mm resulting in an energy density of 4J/cm2. The laser beam was applied with the laser tip positioned perpendicularly to the tooth surface at 4 points: 1 to the apex (apical point) and 3 to the cervical area (mesio‐buccal, disto‐buccal, and lingual points) of the tooth Group 2: (55 teeth enrolled, unknown number of teeth completed) potassium oxalate gel (3%) Details: potassium oxalate gel was applied according to manufacturer's instructions: passive application, using a brush, for 2 minutes. During this period, the laser device was positioned but not activated Group 3: (51 teeth enrolled, unknown number of teeth completed) placebo gel Details: the placebo gel application followed this same procedure and the laser device was also positioned but not activated. (The treatment was repeated at 7‐day intervals for 4 consecutive weeks. 3 months after the 4th treatment session, patients were recalled for re‐assessment of dentinal hypersensitivity) |
|
| Outcomes | Responses to tactile (probe) and air blast stimuli (VAS) at baseline, and immediately, 3 months after intervention | |
| Notes |
Baseline characteristics: the degree of sensitivity was determined for each tooth in response to tactile (probe) and air blast stimuli. The probe stimulus was applied under slight manual pressure in the mesio‐distal direction on the cervical area of the tooth. The air blast was performed with an air syringe for 1 s at a distance of 1 cm from the tooth surface. Dentinal hypersensitivity was assessed by patient's indication of the amount of pain related to each tooth immediately after each stimulus, according to a VAS. The VAS was 10 cm long and, on the left and right ends, contained an indication of 'no pain' and 'severe pain,' respectively. The participants were instructed to the pain severity of each tooth elicited by the hydrodynamic stimuli. The sensitivity patterns were recorded at baseline and immediately after and 3 months after treatment by an examiner previously calibrated for applying the stimuli. The order in which the teeth were evaluated within each subject was maintained at each visit Sample size calculation: not mentioned in the study Source of funding: not mentioned in the study Ethics approval: quote: "The search protocol was approved by the Committee of Ethics in Research of Federal University of Ceará" Informed consent: quote: "Participants were informed about the purpose and design of the investigation and signed an appropriate informed consent form" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This randomisation was performed by placing all the selected teeth in a list and assigning treatment according to a predefined sequence: 1) laser, 2) potassium oxalate gel, and 3) placebo gel" Comment: insufficient information to permit judgement of low or high risk. Not clearly mentioned how random sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to use of placebos, the study might be free of performance bias Comment: it was impossible to blind personnel in this study design, but the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner and the patients did not know, which type of treatment was applied to which tooth" Comment: adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A loss to follow‐up of 6 out of 30 patients was reported in the study. However, since the study was a split‐mouth design, the outcomes would unlikely be influenced by the dropouts |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Sample size of calculation and source of funding were not mentioned in the study |
Yilmaz 2011a.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth, 3 groups) Duration of trial: not mentioned in the study Duration of follow‐up: 3 months |
|
| Participants |
Setting: Department of Periodontology, Near East University, Turkey Inclusion criteria: 3 or more hypersensitive teeth in different quadrants Exclusion criteria: carious lesions on the selected or neighboring teeth, defective restorations, any professional desensitizing therapy on the selected teeth during the last 6 months, use of desensitizing toothpaste in the last 3 months; being under analgesics/anti‐inflammatory drugs at the time of the study, pregnancy or smoking Total number: 51 participants with 174 teeth enrolled and completed Age range: 18 to 60 years (mean age: 44 years) Sex (M/F): 22/29 |
|
| Interventions |
Group 1: GaAlAs laser (wavelength: 810 nm; output: 8.5 J/cm2; duration: 60 s) Details: in the diode laser group, sensitive teeth were irradiated with the GaAlAs laser (LaserSmile, Biolase Technology, Irvine, CA, USA) using 810 nm continuous waveform at 8.5 J/cm2 energy density Group 2: Er,Cr:YSGG laser (wavelength: 2780 nm; output: 0.25 W; duration: 30 s) Details: in the Er,Cr:YSGG laser group, sensitive teeth were irradiated at 2780 nm with Er,Cr:YSGG laser (Waterlase MD, Biolase Technology, Irvine, CA, USA) in the hard tissue mode with the MZ6 sapphire tip (600 μm diameter, 6 mm length) using non‐contact mode at an energy level of 0.25 W, repetition rate of 20 pulses/s and pulse duration of 140 μs, 0% water and 10% air. Treatment time was 60 s for GaAlAs laser and 30 s for Er,Cr:YSGG laser by scanning the cervical part in an overlapping pattern Group 3: no treatment |
|
| Outcomes | Responses to air blast stimuli (VAS) at baseline, and immediately, 1 week, 1 month, 3 months after intervention | |
| Notes |
Baseline characteristics: the vitality of all experimental teeth was controlled at the beginning and end of the trial by means of an electric pulp tester (Digitest, Parkel, NY, USA). For 4 weeks before treatment, all patients were enrolled in an oral hygiene programme and received oral hygiene instructions on 2 appointments as well as professional tooth cleaning according to individual needs. Prior to the application of lasers in all groups,dentine hypersensitivity was assessed by an evaporative stimulus. A strong air blast from a dental syringe was directed to the exposed cervical area for 3 s at a distance of 1 cm and at right angle to the buccal site of the assigned teeth, whilst adjacent teeth were isolated with cotton rolls. Air stimulus time was controlled by a chronometer and the distance was measured by a UNC‐15 periodontal probe (Hu‐Friedy, Chicago, IL, USA). Patients were asked to record their overall sensitivity by marking a point on a 10 cm VAS, anchored at each end by the phrases 'no pain' and 'unbearable pain'. All stimuli were given by 1 operator in the same dental chair with the same equipment yielding similar air pressure (55 to 60 psi) and air temperature (21 to 22℃) each time Sample size calculation: quote: "A minimum clinically significant difference in VAS scores of 0.6 was determined from the available literature on DH. The power analysis was conducted based on this minimum clinically significant difference in VAS scores, using alpha at level 0.05, at 80% power and a σ of 1.16. On the basis of these data, the number of patients required to be enrolled to conduct this study was calculated as 40" Source of funding: not mentioned in the study Ethics approval: quote: "Study protocol and related consent forms were approved by our Institutional Review Board" Informed consent: quote: "After having received oral and written information about the intention and the design of the study, and having signed the informed consent form, the subjects were included in the study" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In this split‐month study, for each patient, selected teeth were randomly assigned to Er, Cr:YSGG laser group, GaAlAs diode laser group or control group by the lottery method" Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Author's reply: "Sealed, opaque, sequentially numbered, identical envelopes" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Lack of placebos might lead to performance bias Comment: the patients are likely be aware of the treatment received in each site due to the differences in perception (lasers versus no treatment).The outcome is likely to be influenced by incomplete blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding of participants can lead to high risk for detection bias when reporting pain level Quote: "The effectiveness of all treatments was assessed at four examination periods; immediately, at 1 week, 1 and 3 months after treatment by one examiner who was not aware of the type of treatment applied" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Source of funding was not mentioned in the study |
Yilmaz 2011b.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth, 2 groups) Duration of trial: between January and September 2009 Duration of follow‐up: 3 months |
|
| Participants |
Setting: Department of Periodontology, Near East University, Turkey Inclusion criteria: at least 1 or more (maximum 4) contra‐lateral pairs of hypersensitive teeth Exclusion criteria: carious lesions on the selected or neighboring teeth, restorations, active periodontal diseases and more than 3 mm gingival recession on the selected teeth, any professional desensitizing therapy during the last 6 months, use of desensitizing toothpaste in the last 3 months, having any systemic diseases, being under analgesics/anti‐inflammatory drugs at the time of the study, pregnancy and smoking Total number: 42 participants with 146 teeth enrolled and completed Age range: 18 to 64 years (author's reply) (mean age: 33.8 years) Sex (M/F): 18/24 |
|
| Interventions |
Group 1: Er,Cr:YSGG laser (wavelength: 2780 nm; output: 0.25 W; duration: 30 s) Details: for each subject, selected teeth were randomly assigned to the test or the control group by the toss method, and then, half of the sensitive teeth were irradiated with Er,Cr:YSGG laser (Waterlase MD, Biolase, Irvine, CA, USA) on hard tissue mode with a mz6 sapphire tip using non‐contact mode at an energy level of 0.25 W and a repetition rate of 20 kHz, 0% water and 10% air. The treatment time was 30 s per surface by scanning the cervical part of the tooth Group 2: placebo laser Details: in the placebo group, the same Er,Cr:YSGG laser without laser emission was used (If any subjects had more than 1 pair, all teeth on the same side received the same treatment. All active and placebo treatments were performed only at the 1st visit, by the same clinician) |
|
| Outcomes | Responses to air blast stimuli (VAS) at baseline, and immediately, 1 week, 1 month, 3 months after intervention | |
| Notes |
Baseline characteristics: 1. the vitality of all experimental teeth was examined at the beginning and end of the trial by an electric pulp tester (Digitest, Parkel, NY, USA). Each subject received a professional prophylaxis before study and was given oral hygiene instructions at 2 separate appointments during the 4‐week pre‐treatment period; 2. the degree of sensitivity to an evaporative stimulus was determined qualitatively by means of an air blast for 3 s at a distance of approximately 1 cm and at right angle to the buccal site of the assigned teeth, while adjacent teeth were isolated with cotton rolls to prevent false‐positive results. Air stimulus time was controlled by chronometer and the distance was measured by a periodontal probe (UNC‐15, Hu‐Friedy, Chicago, IL, USA). Patients were asked to record their overall sensitivity by marking a point on a 10 cm VAS, which was marked 'no pain' on the left end and 'unbearable pain' on the right end. All stimuli were given by 1 operator at the same dental chair with the same equipment yielding similar air pressure (55 to 60 psi) and temperature (21 to 22℃) each time. A calibration session was performed to determine intra‐examiner consistency. 7 subjects were included for this session and measurements were repeated within 30 minutes. Calibration for plaque index (PI) evaluation was performed with a periodontal probe (UNC‐15, Hu‐Friedy) by repeating measurements at mesial and distal half of the tooth surface adjacent to the gingival margin Sample size calculation: quote: "A minimum clinically significant difference in VAS scores of 0.6 was determined from available literature on DH. The power analysis was conducted based on this minimum clinically significant difference in VAS scores, using alpha at level 0.05, at 80% power and a σ of 1.16. On the basis of these data, the number of patient required to be enrolled to conduct this study has been calculated as 40" Funding source: the study was self‐funded by the authors and their institution Ethics approval: quote: "Study protocol and related consent forms were approved by the Institutional Review Board of Near East University (protocol ID#27.3.3.a)" Informed consent: quote: "Following verbal information about the treatment plan, possible discomforts and potential risks, the subjects who signed the informed consent form were included in the study" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For each subject, selected teeth were randomly assigned to the test or the control group by the toss method" Comment: adequate |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to use of placebo laser, the study might be free of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "DH was assessed with VAS and PI scores were recorded at four examination periods; immediately, at 1 week, 1 and 3 months after treatment by a single calibrated examiner who was not aware of the type of treatment applied" Comment: adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | The study appears to be free of other bias |
Yilmaz 2011c.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth, 4 groups) Duration of trial: not mentioned in the study Duration of follow‐up: 6 months |
|
| Participants |
Setting: Department of Periodontology, Near East University, Turkey Inclusion criteria: patients had to have followed the periodontal maintenance program for > 1 year. They would have been initially diagnosed with chronic periodontitis or gingivitis with gingival recession (maximum 3 mm). The subjects had to have 4 or more hypersensitive teeth at different quadrants Exclusion criteria: criteria for exclusion from the study were carious lesions on the selected or neighboring teeth, defective restorations, any professional desensitizing therapy on the selected teeth during the last 6 months, use of desensitizing toothpaste in the last 3 months, taking analgesics/anti‐inflammatory drugs at the time of the study, pregnancy, and smoking Total number: 48 participants with 244 teeth enrolled and completed Age range: 18 to 58 years (mean age: 41 years) Sex (M/F): 22/26 |
|
| Interventions |
Group 1: GaAlAs laser Details: low‐level laser therapy was performed with GaAlAs diode laser (LaserSmile, Biolase Technology, Irvine, CA) with continuous emission (810 nm) on non‐contact mode (2 mm from the surface). The laser device was used with the following parameters: output power of 500 mW, irradiation time of 60 s, and 3.5 cm2 area of active tip resulting in an energy density of 8.5 J/cm2 Group 2: placebo laser Details: in the placebo laser group, the same GaAlAs laser without laser emission was used. GaAlAs laser was applied by scanning the cervical part in an overlapping pattern Group 3: NaF varnish Details: in the NaF varnish group (Voco, Cuxhaven, Germany), the light yellow varnish was applied with a disposable brush at the cervical region of both the buccal and lingual surfaces strictly, ensuring dry tooth surface by isolation with cotton rolls and an air syringe. The patients were instructed not to eat/drink or brush their teeth for 1 hour following the varnish application, as suggested by the manufacturer Group 4: placebo NaF varnish Details: in the placebo NaF varnish group, the same treatment procedures were performed with a saline solution that was in dark brown bottle like the NaF varnish bottle |
|
| Outcomes | Responses to air blast stimuli (VAS) at baseline, and immediately after, 1 week, 1 month, 3 months, 6 months after intervention | |
| Notes |
Baseline characteristics: the vitality of all experimental teeth was controlled at the beginning and end of the trial by means of an electric pulp tester (Digitest, Parkel, NY). Prior to the application of laser and NaF varnish, dentine hypersensitivity was assessed by an evaporative stimulus. A cold air blast from a dental syringe was directed to the exposed cervical area for 3 s at a distance of 1 cm and at a right angle to the buccal site of the assigned teeth, while the adjacent teeth were isolated with cotton rolls. Patients were asked to record their overall sensitivity by marking a point on a 10 cm VAS, anchored at each end by the phrases ‘no pain’ and ‘unbearable pain.’ After all stimuli, a separate sheet of paper containing the printed VAS was given to the patient so that patients could not be influenced by the previous results. Data from the VAS were recorded by measuring the distance between 0 point and the sign marked by the patient in millimeters on the 10‐cm line. Only patients with baseline VAS scores > 4 were included in the study. All stimuli were given by 1 operator on the same dental chair with the same equipment yielding similar air (55 to 60 ѱ) and temperature (21 to 22℃) each time Sample size calculation: quote: "A minimum clinically significant difference in VAS scores of 0.6 was determined from a previous study on DH. The power analysis was done based on this minimum clinically significant difference in VAS scores, using a at level 0.05, at 80% power, and a σ of 1.12. According to these data, the number of patients required to be involved to conduct this study was calculated as 40" Source of funding: not mentioned in the study Ethics approval: quote: "Study protocol and related consent forms were approved by Institutional Review Board of Near East University" Informed consent: quote: "After having received oral and written information about the intention and design of the study, and having signed the informed consent form, the subjects were included in the study" Adverse events: none. Quote: "No complications such as detrimental pulpal effects or allergic reactions were observed" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In this split‐mouth study for each patient, selected teeth were randomly assigned to the GaAlAs laser group, placebo laser group, NaF varnish group, and the placebo NaF varnish group by lottery method" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebos might reduce performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "DH was assessed with VAS; immediately, at 1 week, and at 1, 3, and 6 months after treatments by a single examiner who was not aware of the type of treatment applied" Comment: adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in the study |
| Other bias | Low risk | No other sources of bias were identified Source of funding was not mentioned in the study |
Yilmaz 2014.
| Study characteristics | ||
| Methods |
Study type: RCT (split‐mouth design, 3 groups) Duration of trial: not mentioned Duration of follow‐up: immediately after treatment |
|
| Participants |
Inclusion criteria: patients with 3 sensitive teeth with VAS score of at least 4, Miller Class III mobility and were indicated to extraction Exclusion criteria: patients with carious lesions in selected or neighboring teeth, defective restoration; patients who had undergone professional desensitizing therapy during the previous 6 months or using desensitizing toothpaste in last 3 months; patients being under analgesics/anti‐inflammatory drugs at time of study, pregnancy or smoking Total number: 20 participants with 60 teeth Age range: 18 to 60 years Sex (M/F): 8/12 |
|
| Interventions |
Group 1: Er,Cr:YSGG laser (025 W, 44 J/cm2) Group 2: Er,Cr:YSGG laser (05 W, 89 J/cm2) Details: 2780 nm wavelength Er,Cr:YSGG laser was applied 30 s to Groups 1 and 2 in the hard tissue mode with the MZ6 tip (600 mm diameter, 6 mm length) using non‐contact mode at repetition rate of 20 pulses s1 and pulse duration of 140 ms, 0% water and 10% air Group 3: same Er,Cr:YSGG laser without laser emission was used |
|
| Outcomes | Responses to evaporative stimuli (air blast) (VAS) was assessed immediately after treatment | |
| Notes |
Baseline characteristics: each patient evaluated the perception of discomfort after the application of an air blast for 3 s at a distance of approximately 1 cm and at right angle to the buccal site of the assigned teeth. The adjacent teeth were isolated with cotton rolls to prevent false‐positive results; all stimuli were given by 1 operator in the same dental chair with the same equipment yielding similar air pressure (55 to 60 psi) and air temperature (21 to 22 °C) each time; after the stimulus, the patient was asked to record their overall sensitivity on a VAS Sample size calculation: the power analysis was conducted based on this minimum clinically significant difference in VAS scores, using alpha at level 005, at 80% power and a r of 07 Source of funding: this research was carried out without funding Ethics approval: quote: "Study protocol and related consent forms were approved by the Institutional Review Board and Ethics committee of Near East University" Informed consent: quote: "Following verbal information about the treatment plan, possible discomforts and potential risks, the subjects who signed the informed consent form were included in the study" Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lottery method was used Quote: "A unique number attended to the each treatment method and these numbers were put in a bowl, mixed" |
| Allocation concealment (selection bias) | Low risk | Quote: "A unique number attended to the each treatment method and these numbers were put in a bowl, mixed and then, without looking, the researcher selected numbers for each tooth" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients did not know what kind of therapy each tooth was receiving" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The effectiveness of all treatments was assessed at immediately after treatment by one calibrated examiner who was not aware of the type of treatment applied" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported in study |
| Other bias | Low risk | No other sources of bias were identified Sample size calculation, source of funding and ethics approval were mentioned in the study |
CPP‐ACPF = casein phosphopeptide amorphous calcium phosphate; CSP = calcium sodium phosphosilicate; DH = dentinal hypersensitivity; Er,Cr:YSGG = erbium,chromium:yttrium‐scandium‐gallium‐garnet; GaAIAs = gallium‐aluminum‐arsenide; J = Joules; LLLT = low‐level laser therapy; M/F = male/female; mW = milliwatt; NaF = sodium fluoride; n‐CAP = nano‐carbonate apatite; Nd:YAG = neodymium‐doped:yttrium‐aluminum‐garnet; nHAP = nano‐hydroxyapatite; nm = nanometer; PBM = photobiomodulation; RCT = randomized controlled trial; s = second; SD = standard deviation; VAS = visual analogue scale; VRS = verbal rating score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aranha 2009 | Incomplete outcome data |
| Aranha 2012 | Inappropriate randomization; no mention of participants' age range |
| Birang 2007 | Inappropriate randomization; no mention of participants' age range |
| Brugnera 1999 | Not an RCT (He:Ne laser versus AsGaAl lasers) |
| Brugnera 2003 | Not an RCT (diode laser) |
| Chang 1999 | Outcomes not quantitatively measured |
| Chebel 2018 | Comparison with in‐office chemical agent |
| Ciaramicoli 2003 | Not an RCT (Nd:YAG laser versus no treatment) |
| Corona 2003 | Not an RCT (GaAlAs laser versus sodium fluoride varnish) |
| Dantas 2016 | Incomplete outcome data; no mention of participants' age range |
| De Lima Suares 2016 | Comparison with in‐office chemical agent |
| Dilsiz 2009 | Not an RCT (Nd:YAG laser versus diode laser) |
| Dilsiz 2010a | Not an RCT (Er:YAG laser, Nd:YAG laser versus GaAlAs laser) |
| Dilsiz 2010b | Desensitizer toothpaste versus diode laser + desensitizer toothpaste |
| Femiano 2013 | Comparison with in‐office chemical agent |
| Gelskey 1993 | He:Ne laser versus He:Ne laser + Nd:YAG laser |
| Guo 2019 | Comparison with in‐office chemical agent |
| Hashim 2014 | No non‐laser intervention arm involved in the study |
| He 2004 | Outcomes not quantitatively measured |
| Hotta 2006 | Laser versus laser + occlusal splint |
| Hu 2004 | Not an RCT (Nd:YAG laser versus sodium fluoride gel) |
| Ipci 2009 | They scored according to the modified criteria proposed by Uchida et al (4 degrees of sensitivity) and not using VAS |
| Kara 2009 | Comparison with in‐office chemical agent |
| Kong 2004 | Outcomes not quantitatively measured |
| Kripal 2019 | No mention of participants' age range |
| Kumar 2005 | Not an RCT (Nd:YAG laser versus sodium fluoride varnish) |
| Ladalardo 2002 | Not an RCT (830 nm GaAlAs laser versus 660 nm GaAlAs laser) |
| Lan 1996 | Incomplete outcome data |
| Lan 1999 | In vitro study (Nd:YAG laser and sodium fluoride varnish) |
| Li 2001 | Outcomes not quantitatively measured |
| Lima 2017 | They used a score ≥ 5 on a numerical rating scale (NRS) not VAS |
| Liu 1994 | Semiconductor laser versus semiconductor laser + sodium fluoride varnish |
| Liu 2004 | Incomplete outcome data |
| Lizarelli 2015 | Age of patients not specified |
| Lopes 2013 | Comparison with in‐office chemical agent |
| Lopes 2015 | Comparison with in‐office chemical agent |
| Lopes 2017 | Comparison with in‐office chemical agent |
| Ma 2004 | Outcomes not quantitatively measured |
| Moosavi 2016 | Hypersensitivity induced by bleaching and not spontaneous |
| Moritz 1996 | Stannous fluoride gel versus CO2 laser + stannous fluoride gel |
| Moura 2019 | Comparison with in‐office chemical agent |
| Oberhofer 2008 | Not an RCT (Er:YAG laser versus sodium fluoride gel) |
| Osmari 2018 | Comparison with in‐office chemical agent |
| Ozlem 2018 | They used Yeaple probe scores ( electronic pressure sensitive probe ) not VAS |
| Pandey 2017 | Only mean age provided. Minimum age not specified |
| Pesevska 2010 | Not an RCT (diode laser versus fluoride) |
| Pourshahidi 2019 | No intervention group. Both study groups are laser |
| Praveen 2018 | Comparison with in‐office chemical agent |
| Raichur 2012 | Comparison with in‐office chemical agent |
| Renton‐Harper 1992 | Not an RCT |
| Schwarz 2002 | Used an arbitrary pain scale in 4 degrees not VAS |
| Sgolastra 2013 | This is a meta‐analysis |
| Shintome 2007 | Nd:YAG laser versus Er:YAG laser |
| Sicilia 2009 | They used verbal rating scale (VRS) not VAS |
| Tabatabaei 2018 | Not an RCT |
| Tabibzadeh 2018 | No intervention group. Both study groups are laser (low versus high level) |
| Tailor 2014 | They used a score ≥ 5 on a numerical rating scale (NRS) not VAS |
| Talesara 2014 | Inappropriate randomization |
| Tengrungsun 2008 | Not an RCT (GaAlAs laser versus dentine bonding agent) |
| Tocarruncho 2018 | Use of in‐office chemical agent as comparison |
| Wang 1991 | Not an RCT (Nd:YAG laser versus NaF) |
| Wang 2004 | Outcomes not quantitatively measured |
| Wang 2005 | Outcomes not quantitatively measured |
| Wang 2006 | Outcomes not quantitatively measured |
| Wang 2012 | Comparison with in‐office chemical agent |
| Xiong 2010 | Outcomes not quantitatively measured |
| Xu 2002 | Outcomes not quantitatively measured |
| Yadav 2019 | Used verbal rating scale (VRS) not VAS |
| Yamaguchi 1990 | Outcomes not quantitatively measured |
| Yonaga 1999 | Various methods using Nd:YAG laser |
| Yu 1996 | Outcomes not quantitatively measured |
| Yu 2014 | No control group |
| Zhao 2003 | Outcomes not quantitatively measured |
| Zhao 2008 | Quasi‐RCT |
AsGaAl = arsenate‐gallium‐aluminum; Er:YAG = erbium:yttrium‐aluminum‐garnet; GaAIAs = gallium‐aluminum‐arsenide; He:Ne = helium:neon laser; Nd:YAG = neodymium‐doped:yttrium‐aluminum‐garnet; nm = nanometer; RCT = randomized controlled trial; VAS = visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐INR‐16010245.
| Study name | Clinical outcomes research of the effect of Er:YAG in treating abrasion caused dentin hypersensitivity: a randomized controlled study |
| Methods | Parallel RCT |
| Participants | Inclusion criteria: 1. patients must have subjective symptom of dentine hypersensitivity caused by abrasion, and the study teeth must be filling free and restoration free; 2. patients must not have used desensitizing toothpaste within the past 3 months, undergone desensitization treatment with desensitizer within the past 1 year, or undergone the desensitization treatment with laser; 3. there must be no caries, crack or other factors which might impact therapy to the study teeth. No other dental therapy except for desensitization to the study teeth; 4. patients must be able to read and understand the consent form and be willing to sign it Exclusion criteria: 1. teeth that have be treated with periodontal surgery within the past 6 months or scaling/root planning within the past 3 months; 2. patients have the contraindications with the laser treatment; 3. patients are not able to independently or accurately describe their self‐subjective feeling; 4. patients suffer from the chronic diseases or pain; 5. patients suffer from serious physical or mental diseases; 6. patients have undergone other clinical trial; 7. patients have history of drug and alcohol addiction; 8. patients are currently being treated with drugs which may influence the sensory nerve or the dental disease |
| Interventions | Group 1: Gluma desensitizer Group 2: Er:YAG |
| Outcomes | VAS, immediately after, 1 week after, 1 month after, 3 months after, 6 months after, and 1 year after air stimulus, slight pressure stimulus and cold stimulus |
| Starting date | ‐ |
| Contact information | cyj1229@fmmu.edu.cn |
| Notes | ‐ |
ChiCTR‐INR‐17013452.
| Study name | Non‐invasive optical therapy for dentin hypersensitivity |
| Methods | Parallel RCT |
| Participants | Sample size: 120 Age: 35 to 65 years |
| Interventions | Group 1: control group Group 2: laser 532 nm Group 3: Gluma desensitizer |
| Outcomes | Dental pulp electrical activity test; cold test; remaining dentine thickness |
| Starting date | 1 December 2017 |
| Contact information | young13doctor@163.com |
| Notes | ‐ |
CTRI/2017/05/008547.
| Study name | Use of diode laser and calcium sodium phosphosilicate for the treatment of dentinal hypersensitivity of tooth ‐ a short‐term randomized clinical trial |
| Methods | Parallel RCT |
| Participants | Total sample size: 45 Age: 25 to 60 years |
| Interventions | Group 1: diode laser Group 2: diode laser + calcium sodium phosphosilicate Group 3: calcium sodium phosphosilicate |
| Outcomes | Numerical Rating Scale (NRS); baseline and 3 days after treatment (short term) |
| Starting date | 11 January 2016 |
| Contact information | Aratrika Mukherjee, draratrika@gmail.com |
| Notes | ‐ |
CTRI/2017/10/010085.
| Study name | Effects of Laser with herbal and chemical toothpastes in the management of dental hypersensitivity |
| Methods | Parallel RCT |
| Participants | Total sample size: 180 Age: 20 to 72 years Inclusion criteria: systemically healthy patient; ability and willingness to comply with all study requirements and written consent; teeth should present dentinal hypersensitivity at least in 2 quadrants Exclusion criteria: smokers, tobacco chewers and alcoholics; pregnant and nursing women; carious exposed teeth, root caries; physical and mental disabilities which will interfere with the maintenance |
| Interventions | Group 1: Nd:YAG laser Group 2: nanocrystalline hydroxyapatite (Aclaim toothpaste) Group 3: herbal toothpaste (HIORA K) |
| Outcomes | Reduction of dentine hypersensitivity after 1 week and 6 months. No scale reported |
| Starting date | 8 August 2016 |
| Contact information | Dr Rakesh Kumar Yadav, rakeshanita10@yahoo.in |
| Notes | ‐ |
CTRI/2018/02/012031.
| Study name | Comparative evaluation of the efficacy of Gluma desensitizer and diode laser in the treatment of dentinal hypersensitivity in chronic periodontitis patients ‐ A single‐blinded split‐mouth study |
| Methods | RCT |
| Participants | Total sample size: 20 Age: 25 to 65 years Inclusion criteria: 1. patients in systemic health; 2. patients with chronic periodontitis; 3. patients with good oral hygiene; 4. patients with clinically demonstrable dentinal hypersensitivity teeth, specifically canines and premolars, which were reliable in their response to test measurements Exclusion criteria: 1. patients who had undergone previous professional desensitizing treatment or had used over‐the‐counter desensitizing products in the past 6 months; 2. those with chronic use of anti‐inflammatory, analgesic, or psychotropic drugs; 3. pregnant and lactating females; 4. patients presenting allergies and idiosyncratic responses to product ingredients; 5. systematic conditions that are etiologic factors or predisposing for dentinal hypersensitivity, excessive dietary or environmental exposure to acids; 6. patients who underwent periodontal surgery in the past 6 months |
| Interventions | Group 1: Gluma desensitizer Group 2: diode laser |
| Outcomes | Numerical Rating Scale (NRS) in response to tactile and air blast stimuli at 30, 60, and 90 days after intervention |
| Starting date | 1 March 2018 |
| Contact information | Amitha Sathish, amithasathish11@gmail.com |
| Notes | ‐ |
IRCT201104104877N5.
| Study name | Comparative efficacy of gel and laser for treatment of dentine hypersensitivity |
| Methods | Unclear, probably RCT |
| Participants | Inclusion criteria: having dentine hypersensitivity for more than 1 month; not using other hypersensitivity methods such as toothpastes or tubules sealers Exclusion criteria: pregnancy; non‐vital teeth; cracked teeth; deep restorations, crown and abutments |
| Interventions | Group 1: use of Sensikin gel, 3 times a day for 1 week Group 2: use of Nd:YAG laser (1 W, 10 Hz, 60 s, twice) Group 3: with laser device while it was off as placebo |
| Outcomes | Unclear |
| Starting date | Unclear |
| Contact information | Unclear |
| Notes | ‐ |
IRCT201105316664N1.
| Study name | Comparative of effect of sodium fluoride varnish and Nd: YAG laser and their combative use on dentine hypersensitivity treatment |
| Methods | Unclear, probably RCT |
| Participants | Inclusion criteria: each patient had at least 4 hypersensitive teeth; having dentine hypersensitivity more than 6 months; not using other hypersensitivity methods Exclusion criteria: non‐vital teeth; gingivitis; having calculus; decayed teeth |
| Interventions | Group 1: control group: did not receive any treatment Group 2: treated with 5% sodium fluoride varnish (a Durashield company product) Group 3: received Nd:YAG laser (1 W, 20 Hz, 120 s) Group 4: Nd:YAG laser (1 W, 20 Hz, 120 s) + 5% sodium fluoride varnish |
| Outcomes | Unclear |
| Starting date | Unclear |
| Contact information | Unclear |
| Notes | ‐ |
IRCT2015102624715N1.
| Study name | Efficacy of 940 nm diode laser on the treatment of dentin hypersensitivity in periodontal maintenance patients |
| Methods | RCT, split‐mouth design Sample size: 45 teeth Study duration: 30 days |
| Participants | Age: 22 to 57 years Inclusion criteria: individuals who have passed 1st phase of periodontal treatments and have dentine hypersensitivity in teeth with no caries, fracture; any clear tooth crack, fillings and periapical lesion, which will respond to both air and tactile stimuli application tests Exclusion criteria: individuals with systemic diseases; allergy to materials used in the study; smokers; individuals with active periodontal diseases; pregnant and lactating women; patients who have received treatments related to increased tooth sensitivity; teeth with occlusal overload and recent occlusal re‐adjustment therapy |
| Interventions | Group 1: control group: placebo Group 2: potassium fluoride gel 2% Group 3: laser diode of 940 wavelength Group 4: laser + potassium fluoride gel 2% |
| Outcomes | Sensitivity to air and tactile stimulation using VAS score |
| Starting date | 11 January 2016 |
| Contact information | Tahere Pourseyedian, th.1362@yahoo.com |
| Notes | ‐ |
IRCT2016111230858N1.
| Study name | Clinical trial of comparison between Nd:YAG laser and propolis in treatment of dental sensitivity in patients with dentin hypersensitivity |
| Methods | Double‐blinded, parallel RCT |
| Participants | Sample size: 25 Inclusion criteria: having at least 2 hypersensitive teeth in 2 sides of mouth (1 right and 1 left); age of ≥ 18 years; vitality of examined teeth; no carious lesions; fractures or cracks and restorations in examined teeth Exclusion criteria: patient absent in follow‐up appointments; systemic diseases; pregnancy; periodontal surgery within last 3 months; orthodontic treatment in that same course; desensitizing therapy within last 1 month; existing of pulpitis and crown in examined teeth |
| Interventions | Group 1: laser radiation in cervical area of sensitive teeth in 3 sessions with 1 week intervals. Nd:YAG laser with 1064 nm wavelength, 1 W power, 10 Hz frequency, short pulse mode, 300 μm fiber tip was used for a time of 60 s on each tooth Group 2: 40% propolis gel applying in cervical area of sensitive teeth in 3 sessions with 1 week interval |
| Outcomes | VAS score before treatment and immediately after, 1 week after, 2 weeks after, 1 month after, and finally 3 months after treatment |
| Starting date | ‐ |
| Contact information | marasti68@yahoo.com; dr.rasti68@gmail.com |
| Notes | ‐ |
IRCT201707269014N176.
| Study name | Comparison of the effect of Nd:YAG laser, diode laser and CPP/ACP on treatment of patient with dentin hypersensitivity: a randomized clinical trial |
| Methods | RCT |
| Participants | 7 patients with 8 teeth each Age: 18 to 65 years Inclusion criteria: age of 18 to 65 years; dentine hypersensitivity; suitable plaque index with no pocket Exclusion criteria: pregnancy or breastfeeding; systemic disease; periodontal surgery during the last 6 months; whitening treatment of teeth in the last 6 months; using antihypersensitivity and anti‐inflammatory mouthwash or toothpaste during the last 72 hours; using antidepressive drugs; using orthodontic devices; the presence of fractures, cracks, decay and deep repair in the teeth and adjacent teeth |
| Interventions | Group 1: Nd:YAG laser Group 2: diode laser Group 3: casein phosphopeptide phosphate calcium amorphic gel Group 4: no treatment |
| Outcomes | VAS after 1 hour |
| Starting date | 31 October 2016 |
| Contact information | Jalal Poorolajal, poorolajal@umsha.ac.ir |
| Notes | No blinding of participants or examiners |
NCT02931734.
| Study name | Assessment of different protocols for cervical dentin hypersensitivity treatment (CDH) |
| Methods | Double‐blinded, parallel RCT |
| Participants | Sample size: not mentioned Inclusion criteria: presence of dentine exposure lesions with clinical diagnosis of moderate or severe tooth sensitivity; good oral hygiene Exclusion criteria: cavities; presence of periodontal disease and/or parafunctional habits; cracks or enamel fractures; extensive or unsatisfactory restorations; recent restorations involving the labial surface; dentures; orthodontics |
| Interventions | ‐ Group 1: active comparator: resin modified glass ionomer (RMGI) Resin modified glass ionomer; an application every 48 hours; 4 sessions ‐ Group 2: active comparator: potassium nitrate 2% (KF) Potassium nitrate and sodium fluoride 2%; an application every 48 hours; 4 sessions ‐ Group 3: active comparator: low‐level laser therapy ‐ GaAlAs (LLLT) Low level laser therapy ‐ GaAlAs; an application every 48 hours; 4 sessions ‐ Group 4: active comparator: RMGI and KF Resin modified glass ionomer and potassium nitrate and sodium fluoride 2%; an application of the 2 associated products, every 48 hours; 4 sessions ‐ Group 5: active comparator: RMGI and LLLT Resin modified glass ionomer and low‐level laser therapy ‐ GaAlAs; an application of the 2 associated products, every 48 hours; 4 sessions ‐ Group 6: active comparator: KF and LLLT Potassium nitrate and sodium fluoride 2% and low‐level laser therapy ‐ GaAlAs; an application of the 2 associated products, every 48 hours; 4 sessions ‐ Group 7: active Comparator: RMGI, KF, LLLT Resin modified glass ionomer, potassium nitrate and sodium fluoride 2%, and low‐level laser therapy ‐ GaAlAs; an application of the 3 associated products, every 48 hours; 4 sessions |
| Outcomes | Level of cervical dentine hypersensitivity, measured by VAS (time frame: 24 weeks) |
| Starting date | ‐ |
| Contact information | ‐ |
| Notes | ‐ |
NCT03237793.
| Study name | Treatment of hypersensitivity using diode laser and desensitising agent on fluorosed and non‐fluorosed teeth |
| Methods | Parallel RCT |
| Participants | Sample size: 90 patients Age: 18 to 60 years Inclusion criteria: patients with a positive response for hypersensitivity testing (i.e. cold water testing using ice cold water, air blow test, electric tactile stimulation test); patients presenting with non‐carious cervical lesions in the enamel; fluorosed teeth wherein fluorotic enamel staining confirmed by clinical examination, healthy non‐fluorosed teeth, which was confirmed by clinical examination Exclusion criteria: patients undergoing any form of restorative endodontic, orthodontic treatment or crown restorations; local defects including caries and fractures; presence of any systemic diseases; acute pain conditions (like apical periodontitis, periapical abscess); presence of periodontal disease or a history of periodontal treatment in last 6 months; usage of desensitizing toothpaste or mouthrinse in the last 4 weeks; patients allergic to ingredients used in the study product; teeth with intrinsic stains caused by other reasons |
| Interventions | Group 1: desensitizing agent (potassium nitrate) Group 2: diode laser Group 3: desensitizing agent + diode laser |
| Outcomes | Response to air blast stimulus, ice cold water and electrical tactile sensitivity after 30 minutes |
| Starting date | July 2014 |
| Contact information | Rashmi Paramashivaiah, Krishnadevaraya College of Dental Sciences & Hospital, India |
| Notes | ‐ |
NCT03553290.
| Study name | Therapeutic evaluation of lower‐level laser for treating dentinal hypersensitivity |
| Methods | RCT (double‐blinded, split‐mouth design) |
| Participants | 80 adults Age range not mentioned |
| Interventions | Group 1: control group: only debridement Group 2: low level laser therapy |
| Outcomes | VAS, pocket depth, clinical attachment level, gingival recession |
| Starting date | 10 August 2017 |
| Contact information | changpc@ntu.edu.tw |
| Notes | ‐ |
NCT03750851.
| Study name | CPP‐ACPF and low level laser therapy effect on symptomatology and quality of life of people with dentin hypersensitivity: a clinical, randomized, double‐blind study |
| Methods | Placebo‐controlled RCT |
| Participants | 45 participants Age range: 18 to 50 years |
| Interventions | Group 1: placebo Group 2: CPP/ACP Group 3: AsGaAl laser (808 nm) Group 4: laser + CPP/ACP |
| Outcomes | VAS; quality of life change (questionnaire) |
| Starting date | 11 June 2018 |
| Contact information | Cecy M Silva (contact details not mentioned) |
| Notes | ‐ |
RBR‐235jts.
| Study name | Dental sensitivity: prevalence and treatment |
| Methods | Parallel, prospective, double‐blinded, 4‐arm, RCT This project is subdivided into 4 studies: the Orto1 and Perio1 studies are observational studies; the Orto2 and Perio2 studies are clinical researches. All interventions consist of topical application of substances for treatment of cervical dentine hypersensitivity |
| Participants | Inclusion criteria: ‐ Orto2 study: patients older than 18 years; completed corrective orthodontic treatment; permanent dentition erupted prior to orthodontic treatment; agree to participate; good general health; clinically diagnosed dentine hypersensitivity ‐ Perio2 study: patients older than 18 years; completed non‐surgical periodontal treatment; agree to participate; good general health; clinically diagnosed dentine hypersensitivity Exclusion criteria: ‐ Orto2 study: in orthodontic retreatment; use of anti‐inflammatory, analgesic and/or psychiatric medications (chronic or up to 72 hours prior to clinical evaluations); pregnant or lactating women; carious lesions; endodontic treatment; pulp and/or periapical lesion; in treatment for dentine hypersensitivity; who underwent periodontal surgery in the last 3 months; restored in the last 3 months; fixed or removable prosthetic abutments; with prosthetic crowns; extensively restored; restorations covering the cervical region and/or regions that interfere with the assessment of dentine hypersensitivity; allergic to the components of the formulations that will be used ‐ Perio2 study: use of anti‐inflammatory, analgesic and/or psychiatric medications (chronic or up to 72 hours prior to clinical evaluations); pregnant or lactating women; carious lesions; endodontic treatment; pulp and/or periapical lesion; in treatment for dentine hypersensitivity; who underwent periodontal surgery in the last 3 months; restored in the last 3 months; fixed or removable prosthetic abutments; with prosthetic crowns; extensively restored; restorations covering the cervical region and/or regions that interfere with the assessment of dentine hypersensitivity; allergic to the components of the formulations that will be used |
| Interventions | The groups of interventions of Orto2 and Perio2 studies are: Group 1: placebo group (PL): 26 individuals in each trial; application of nitrocellulose varnish and LED light in the buccal cervical region in 3 points (1 mesial point, 1 midpoint, and 1 distal point) perpendicular to the long axis of the tooth, each application will last 30 s Group 2: potassium oxalate (OX) group: 26 individuals in each trial; application of potassium oxalate and LED light in the buccal cervical region in 3 points (1 mesial point, 1 midpoint, and 1 distal point) perpendicular to the long axis of the tooth, each application will last 30 s Group 3: laser group (LA): 26 individuals in each trial; application of nitrocellulose varnish and low‐power laser semiconductor diode GaAIAs and InGaAIP in the buccal cervical region in 3 points (1 mesial point, 1 midpoint, and 1 distal point) perpendicular to the long axis of the tooth, each application will last 30 s Group 4: bioglass group (BV): 26 individuals in each trial; application of experimental bioglass containing K and Sr in vehicle of nitrocellulose varnish and LED light in the buccal cervical region in 3 points (1 mesial point, 1 midpoint, and 1 distal point) perpendicular to the long axis of the tooth, each application will last 30 s The treatments will be applied in 3 moments, with intervals of 72 hours between them, that is, 3 treatment applications will be performed for each patient, within their respective group |
| Outcomes | Primary output for Orto2 and Perio2 studies: HSDC values measured by the evaporative (air blast) and tactile (clinical probe) stimuli method using the NRS pain scale from 0 to 10 (where 0 corresponds to 'no pain' and 10 corresponds to 'worst pain imaginable'), so that the experimental bioglass group might have similarity with the HSDC values measured for the groups that used laser and the groups that used potassium oxalate |
| Starting date | 05 January 2019 |
| Contact information | Ana Cláudia Dalmolin, anaclaudiadalmolin@gmail.com |
| Notes | ‐ |
CPP‐ACP = casein phosphopeptide amorphous calcium phosphate; Er:YAG = erbium:yttrium‐aluminum‐garnet; GaAIAs = gallium‐aluminum‐arsenide; Hz = hertzs; InGaAIP = indium‐gallium‐aluminum phosphide; LLLT = low‐level laser therapy; Nd:YAG = neodymium‐doped:yttrium‐aluminum‐garnet; nm = nanometers; NRS = numerical rating scale; RCT = randomized controlled trial; s = second; VAS = visual analogue scale; W = watt.
Differences between protocol and review
Types of studies: in the protocol we stated that randomized cross‐over studies with adequate wash‐out period would be included. However, throughout the review process, it was deemed that cross‐over studies were not applicable in these types of laser studies, and therefore this statement was removed.
Types of participants: we considered patients aged above 12 years rather than 18 years as stated in the protocol. In reviewing the literature, there was no mention of any adverse events of laser treatment for the paediatric and adolescent population. The participants' age varied significantly, however, the mean age for all studies was in the adult range. The two studies including younger participants also had mean ages in the 30s, so we decided to include them.
Objectives and types of interventions: we had initially intended to include in‐office therapeutic chemical agents as a separate comparison group. However, upon review of the eligible studies, it was noted that there is significant variation between the effects of different chemical agents. In consultation with Cochrane Oral Health, we decided to exclude studies evaluating lasers in comparison with therapeutic chemical agents as it is not possible to study the effects of all the various chemical agents in a meta‐analysis and data cannot be pooled.
Types of outcome measures: due to significant heterogeneity in the type of stimulus and follow‐up periods among the eligible studies, this section was reorganized to include patient‐important outcomes in a meaningful manner. In a preliminary review of the manuscript by Cochrane Oral Health, it was deemed that it would be more appropriate to analyze the data for studies that used tactile stimulus and air blast stimulus separately. Furthermore, to simplify data analysis and presentation, we incorporated the longevity of effect, formerly noted as a separate outcome in the protocol, into the primary outcomes.
We did not search the China National Knowledge Infrastructure (CNKI) database as stated in the protocol due to poor yield.
Contributions of authors
All authors: drafting the protocol and the review.
Mina Mahdian (MM) and Zuhair Natto (ZN): co‐leading the review (designing and co‐ordinating the review).
MM and Soodabeh Behboodi (SB): screening the search results against the inclusion criteria.
ZN and Yumi Ogata (YO): assessing the quality of included studies.
SB and MM: organizing the papers and writing to trial authors for additional information.
MM, ZN, SB and YO: extracting data and entering into Review Manager (Review Manager 2020).
MM and SB: obtaining and screening data on unpublished studies.
ZN: interpretation and analysis of data.
MM and ZN: updating the review.
Sources of support
Internal sources
Shahid Beheshti University of Medical Sciences, Iran
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the NHS, or the Department of Health and Social Care.
-
Cochrane Oral Health Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships-alliances). Contributors in the last 2 years have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Swiss Society of Endodontology, Switzerland.
Declarations of interest
Mina Mahdian: none known. Soodabeh Behboodi: none known. Yumi Ogata: none known. Zuhair S Natto: none known.
New
References
References to studies included in this review
Alencar 2020 {published data only}
- Alencar CD, Ortiz MI, Silva FA, Alves EB, Araujo JL, Silva CM. Effect of nanohydroxyapatite associated with photobiomodulation in the control of dentin hypersensitivity: a randomized, double-blind, placebo-controlled clinical trial. American Journal of Dentistry 2020;33(3):138-44. [PubMed] [Google Scholar]
Bal 2015 {published and unpublished data}
- Bal MV, Keskiner I, Sezer U, Acikel C, Saygun I. Comparison of low level laser and arginin-calcium carbonate alone or combination in the treatment of dentinal hypersensitivity: a randomized split-mouth clinical study. Photomedicine and Laser Surgery 2015;33(4):200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doshi 2014 {published and unpublished data}
- Doshi S, Jain S, Hegde R. Effect of low-level laser therapy in reducing dentinal hypersensitivity and pain following periodontal flap surgery. Photomedicine and Laser Surgery 2014;32(12):700-6. [DOI] [PubMed] [Google Scholar]
Flecha 2013 {published and unpublished data}
- Flecha OD, Azevedo CG, Matos FR, Vieira-Barbosa NM, Ramos-Jorge ML, Gonçalves PF, et al. Cyanoacrylate versus laser in the treatment of dentin hypersensitivity: a controlled, randomized, double-masked and non-inferiority clinical trial. Journal of Periodontology 2013;84(3):287-94. [DOI] [PubMed] [Google Scholar]
García 2017 {published and unpublished data}
- García-Delaney C, Abad-Sánchez D, Arnabat-Domínguez J, Valmaseda-Castellón E, Gay-Escoda C. Evaluation of the effectiveness of the photobiomodulation in the treatment of dentin hypersensitivity after basic therapy. A randomized clinical trial. Journal of Clinical and Experimental Dentistry 2017;9(5):e694-e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentile 2004 {published and unpublished data}
- Gentile LC, Greghi SL. Clinical evaluation of dentin hypersensitivity treatment with the low intensity Gallium-Aluminum-Arsenide laser - AsGaAl. Journal of Applied Oral Science 2004;12(4):267-72. [DOI] [PubMed] [Google Scholar]
Gerschman 1994 {published and unpublished data}
- Gerschman JA, Ruben J, Gebart-Eaglemont J. Low level laser therapy for dentinal tooth hypersensitivity. Australian Dental Journal 1994;39(6):353-7. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published and unpublished data}
- Lee SY, Jung HI, Jung BY, Cho YS, Kwon HK, Kim BI. Desensitizing efficacy of nano-carbonate apatite dentifrice and Er,Cr:YSGG laser: a randomized clinical trial. Photomedicine and Laser Surgery 2015;33(1):9-14. [DOI] [PubMed] [Google Scholar]
Lier 2002 {published and unpublished data}
- Lier BB, Rösing CK, Aass AM, Gjermo P. Treatment of dentin hypersensitivity by Nd:YAG laser. Journal of Clinical Periodontology 2002;29(6):501-6. [DOI] [PubMed] [Google Scholar]
Lizarelli 2007 {published and unpublished data}
- Lizarelli RFZ, Miguel FAC, Villa GEP, Filho EC, Pelino JEP, Bagnato VS. Clinical effects of low-intensity laser vs light-emitting diode therapy on dentine hypersensitivity. Journal of Oral Laser Applications 2007;7(2):129-36. [Google Scholar]
Lund 2013 {published and unpublished data}
- Lund RG, Silva AF, Piva E, Da Rosa WL, Heckmann SS, Demarco FF. Clinical evaluation of two desensitizing treatments in southern brazil: a 3-month follow-up. Acta Odontologica Scandinavica 2013;71(6):1469-74. [DOI] [PubMed] [Google Scholar]
Maximiano 2019 {published and unpublished data}
- Maximiano V, Machado AC, Yoshida ML, Pannuti CM, Scaramucci T, Aranha ACC. Nd:YAG laser and calcium sodium phosphosilicate prophylaxis paste in the treatment of dentin hypersensitivity: a double-blind randomized clinical study. Clinical Oral Investigations 2019;23(8):3331-8. [DOI] [PubMed] [Google Scholar]
Naghsh 2020 {published and unpublished data}
- IRCT2017062022699N4. Evaluation of the effect of two low level diode lasers 660 nm and 810 nm in the treatment of dentin hypersensitivity. en.irct.ir/trial/19526 (first received 31 July 2017).
- Naghsh N, Kachuie M, Kachuie M, Birang R. Evaluation of the effects of 660-nm and 810-nm low-level diode lasers on the treatment of dentin hypersensitivity. Journal of Lasers in Medical Sciences 2020;11(2):126-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orhan 2011 {published and unpublished data}
- Orhan K, Aksoy U, Can-Karabulut DC, Kalender A. Low-level laser therapy of dentin hypersensitivity: a short-term clinical trial. Lasers in Medical Science 2011;26(5):591-8. [DOI] [PubMed] [Google Scholar]
Ortiz 2019 {published and unpublished data}
- Guanipa Ortiz MI, Alencar CM, Freitas De Paula BL, Alves EB, Nogueira Araújo JL, Silva CM. Effect of the casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and photobiomodulation (PBM) on dental hypersensitivity: a randomized controlled clinical trial. PLOS One 2019;14(12):e0225501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pantuzzo 2020 {published and unpublished data}
- Pantuzzo ÉS, Cunha FA, Abreu LG, Esteves Lima RP. Effectiveness of diode laser and fluoride on dentin hypersensitivity treatment: a randomized single-blinded clinical trial. Journal of Indian Society of Periodontology 2020;24(3):259-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suri 2016 {published and unpublished data}
- Suri I, Singh P, Shakir QJ, Shetty A, Bapat R, Thakur R. A comparative evaluation to assess the efficacy of 5% sodium fluoride varnish and diode laser and their combined application in the treatment of dentin hypersensitivity. Journal of Indian Society of Periodontology 2016;20(3):307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tevatia 2017 {published and unpublished data}
- Tevatia S, Khatri V, Sharma N, Dodwad V. Comparative clinical evaluation of gallium-aluminum-arsenide diode laser and potassium nitrate in treating dentinal hypersensitivity. Journal of Indian Society of Periodontology 2017;21:391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieira 2009 {published and unpublished data}
- Vieira AH, Passos VF, Assis JS, Mendonça JS, Santiago SL. Clinical evaluation of a 3% potassium oxalate gel and a GaAlAs laser for the treatment of dentinal hypersensitivity. Photomedicine and Laser Surgery 2009;27(5):807-12. [DOI] [PubMed] [Google Scholar]
Yilmaz 2011a {published and unpublished data}
- Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y. Clinical evaluation of Er,Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. Journal of Dentistry 2011;39(3):249-54. [DOI] [PubMed] [Google Scholar]
Yilmaz 2011b {published and unpublished data}
- Yilmaz HG, Cengiz E, Kurtulmus-Yilmaz S, Leblebicioglu B. Effectiveness of Er,Cr:YSGG laser on dentine hypersensitivity: a controlled clinical trial. Journal of Clinical Periodontology 2011;38(4):341-6. [DOI] [PubMed] [Google Scholar]
Yilmaz 2011c {published and unpublished data}
- Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E. Long-term effect of diode laser irradiation compared to sodium fluoride varnish in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized controlled clinical study. Photomedicine and Laser Surgery 2011;29(11):721-5. [DOI] [PubMed] [Google Scholar]
Yilmaz 2014 {published and unpublished data}
- Yilmaz HG, Bayindir H. Clinical and scanning electron microscopy evaluation of the Er,Cr:YSGG laser therapy for treating dentine hypersensitivity: short-term, randomised, controlled study. Journal of Oral Rehabilitation 2014;41(5):392-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aranha 2009 {published data only}
- Aranha AC, Pimenta LA, Marchi GM. Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Brazilian Oral Research 2009;23(3):333-9. [DOI] [PubMed] [Google Scholar]
Aranha 2012 {published data only}
- Aranha AC, Eduardo Cde P. Effects of Er:YAG and Er,Cr:YSGG lasers on dentine hypersensitivity. Short-term clinical evaluation. Lasers in Medical Science 2012;27(4):813-8. [DOI] [PubMed] [Google Scholar]
Birang 2007 {published data only}
- Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M. Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers in Medical Science 2007;22(1):21-4. [DOI] [PubMed] [Google Scholar]
Brugnera 1999 {published data only}
- Brugnera A, Cruz FM, Zanin FAA, Pécora JD. Clinical results evaluation of dentinal hypersensitivity patients treated with laser therapy. In: Featherstone JDB, Rechmann P, Fried D , editors(s). Lasers in Dentistry V. BIOS'99 International Biomedical Optics Symposium; 1999 Jan 23-29; San Jose (CA). Vol. 3593. San Jose (CA): SPIE, International Society for Optics and Photonics, 1999:66-8. [DOI: 10.1117/12.348328] [DOI]
Brugnera 2003 {published data only}
- Brugnera A, Garrini AEC, Pinheiro ALB, Campos DHS, Donamaria E, Magalhaes F, et al. LLLT in treating dentinal hypersensitivity: a histologic study and clinical application. In: Rechmann P, Fried D, Hennig T , editors(s). Lasers in Dentistry IX. SPIE Biomedical Optics Symposium; 2003 Jan 25-31; San Jose (CA). Vol. 4950. San Jose (CA): SPIE, International Society for Optics and Photonics, 2003:46-53.
Chang 1999 {published data only}
- Chang Q, Li XX. Clinical evaluation of GaAlAs laser for treating dentinal hypersensitivity [用砷化镓半导体激光治疗牙本质过敏症的临床对照]. Journal of Shanghai Medical University 1999;26(6):459-61. [Google Scholar]
Chebel 2018 {published and unpublished data}
- Chebel FB, Zogheib CM, Baba NZ, Corbani KA. Clinical comparative evaluation of Nd:YAG laser and a new varnish containing casein phosphopeptides-amorphous calcium phosphate for the treatment of dentin hypersensitivity: a prospective study. Journal of Prosthodontics 2018;27:860-7. [DOI] [PubMed] [Google Scholar]
Ciaramicoli 2003 {published data only}
- Ciaramicoli MT, Carvalho RC, Eduardo CP. Treatment of cervical dentin hypersensitivity using neodymium:yttrium-aluminum-garnet laser. Clinical evaluation. Lasers in Surgery and Medicine 2003;33(5):358-62. [DOI] [PubMed] [Google Scholar]
Corona 2003 {published data only}
- Corona SA, Nascimento TN, Catirse AB, Lizarelli RF, Dinelli W, Palma-Dibb RG. Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. Journal of Oral Rehabilitation 2003;30(12):1183-9. [DOI] [PubMed] [Google Scholar]
Dantas 2016 {published data only}
- Dantas EM, Amorim FK, Nóbrega FJ, Dantas PM, Vasconcelos RG, Queiroz LM. Clinical efficacy of fluoride varnish and low-level laser radiation in treating dentin hypersensitivity. Brazilian Dental Journal 2016;27(1):79-82. [DOI] [PubMed] [Google Scholar]
De Lima Suares 2016 {published and unpublished data}
- De Lima Soares M, Bandeira Porciúncula G, Ilka Holanda Medeiros De Lucena M, Alcino Monteiro Gueiros L, Carneiro Leão J, De Albuquerque Tavares Carvalho A. Efficacy of Nd:YAG and GaAlAs lasers in comparison to 2% fluoride gel for the treatment of dentinal hypersensitivity. General Dentistry 2016;64(6):66-71. [PubMed] [Google Scholar]
Dilsiz 2009 {published data only}
- Dilsiz A, Canakci V, Ozdemir A, Kaya Y. Clinical evaluation of Nd:YAG and 685-nm diode laser therapy for desensitization of teeth with gingival recession. Photomedicine and Laser Surgery 2009;27(6):843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dilsiz 2010a {published data only}
- Dilsiz A, Aydin T, Canakci V, Gungormus M. Clinical evaluation of Er:YAG, Nd:YAG, and diode laser therapy for desensitization of teeth with gingival recession. Photomedicine and Laser Surgery 2010;28(Suppl 2):S11-7. [DOI] [PubMed] [Google Scholar]
Dilsiz 2010b {published data only}
- Dilsiz A, Aydin T, Emrem G. Effects of the combined desensitizing dentifrice and diode laser therapy in the treatment of desensitization of teeth with gingival recession. Photomedicine and Laser Surgery 2010;28(Suppl 2):S69-74. [DOI] [PubMed] [Google Scholar]
Femiano 2013 {published and unpublished data}
- Femiano F, Femiano R, Lanza A, Festa MV, Rullo R, Perillo L. Efficacy of diode laser in association to sodium fluoride vs Gluma desensitizer on treatment of cervical dentin hypersensitivity. A double blind controlled trial. American Journal of Dentistry 2013;26(4):214-8. [PubMed] [Google Scholar]
Gelskey 1993 {published data only}
- Gelskey SC, White JM, Pruthi VK. The effectiveness of the Nd:YAG laser in the treatment of dental hypersensitivity. Journal of Canadian Dental Association 1993;59(4):377-8. [PubMed] [Google Scholar]
Guo 2019 {published and unpublished data}
- Guo L, Kayastha PK, Chen L, Shakya M, Chen X. Clinical evaluation of Nd:YAG laser with and without dentin bonding agent for the treatment of occlusal hypersensitivity. Operative Dentistry 2019;44(3):227-34. [DOI] [PubMed] [Google Scholar]
Hashim 2014 {published data only}
- Hashim NT, Gasmalla B, Sabahalkheir A, Awooda A. Effect of clinical application of diode laser (810 nm) in treatment of dentine hypersensitivity. BMC Research Notes 2014;7:31-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2004 {published data only}
- He HB, Ou JG, Yu B, Song HW. Effects of combined sodium fluoride and pulsed Nd:YAG laser irradiation on treatment of hypersensitive dental necks. Academic Journal of Kunming Medical College 2004;25(3):40-3. [Google Scholar]
Hotta 2006 {published data only}
- Hotta TH, Marchesan JT, Santos TM, Silva MA, Silva RS, Pécora JD. Use of laser and occlusal splint for dentin hypersensitivity of bruxers [Uso de laser e placa oclusal na sensibilidade dentinária de bruxômanos]. RGO (Porto Alegre) 2006;54(2):195-8. [Google Scholar]
Hu 2004 {published data only}
- Hu C. Dentine hypersensitivity treated with Nd: YAG laser: experience with 262 cases [掺钕钇铝石榴石激光治疗牙本质过敏症262例]. Journal of First Military Medical University 2004;24(3):319-20. [PubMed] [Google Scholar]
Ipci 2009 {published and unpublished data}
- Ipci SD, Cakar G, Kuru B, Yilmaz S. Clinical evaluation of lasers and sodium fluoride gel in the treatment of dentine hypersensitivity. Photomedicine and Laser Surgery 2009;27(1):85-91. [DOI] [PubMed] [Google Scholar]
Kara 2009 {published and unpublished data}
- Kara C, Orbak R. Comparative evaluation of Nd:YAG laser and fluoride varnish for the treatment of dentinal hypersensitivity. Journal of Endodontics 2009;35(7):971-4. [DOI] [PubMed] [Google Scholar]
Kong 2004 {published data only}
- Kong LH, Lu HX. Clinical evaluation of Nd:YAG laser for treating dentinal hypersensitivity [Nd:YAG激光照射治疗牙本质过敏症]. Journal of Naval General Hospital 2004;17(3):177-8. [Google Scholar]
Kripal 2019 {published data only}
- CTRI/2018/06/014395. The comparison of the efficacy of 3 materials propolis (a natural bee product), laser and their combination for the reduction of tooth sensitivity - A clinical study [To compare and evaluate the efficacy of 10% propolis varnish, diode laser, and their combined effects in the treatment of dentinal hypersensitivity: a randomized clinical trial]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=24046&EncHid=&userName=CTRI/2018/06/014395 (first received 4 June 2018). [16709544]
- Kripal K, Dileep A, Rajan S, Fathima T, Manjunath SM. A three way method to mitigate dentinal hypersensitivity - A randomized controlled trial. International Journal of Innovative Science and Research Technology 2019;4(8):711-6. [Google Scholar]
Kumar 2005 {published data only}
- Kumar NG, Mehta DS. Short-term assessment of the Nd:YAG laser with and without sodium fluoride varnish in the treatment of dentin hypersensitivity - a clinical and scanning electron microscopy study. Journal of Periodontology 2005;76(7):1140-7. [DOI] [PubMed] [Google Scholar]
Ladalardo 2002 {published data only}
- Ladalardo GP, Albernaz PLM, Brugnera AB, Zanin F, Siqueira JTT. Comparative clinical evaluation of the immediate and late analgesic effect of GaAIAs diode lasers of 830 nm and 660 nm in treatment of dentine pain: preliminary results. In: Rechmann P, Fried D, Hennig T , editors(s). Lasers in Dentistry VIII. International Symposium on Biomedical Optics; 2002 Jan 19-25; San Jose (CA). Vol. 4610. San Jose (CA): SPIE, International Society for Optics and Photonics, 2002:178-82.
Lan 1996 {published data only}
- Lan WH, Liu HC. Treatment of dentin hypersensitivity by Nd:YAG laser. Journal of Clinical Laser Medicine and Surgery 1996;14(2):89-92. [DOI] [PubMed] [Google Scholar]
Lan 1999 {published data only}
- Lan WH, Liu HC, Lin CP. The combined occluding effect of sodium fluoride varnish and Nd:YAG laser irradiation on human dentinal tubules. Journal of Endodontics 1999;25(6):424-6. [DOI] [PubMed] [Google Scholar]
Li 2001 {published data only}
- Li LN. The effect of YAG laser irradiation on tooth hypersensitivity [YAG激光治疗牙本质敏感症的疗效观察]. Chinese Journal of Dental Materials and Devices 2001;10(4):187-8. [Google Scholar]
Lima 2017 {published and unpublished data}
- Lima TC, Vieira-Barbosa NM, Grasielle de Sá Azevedo C, Matos FR, Douglas de Oliveira DW, Oliveira ES, et al. Oral health-related quality of life before and after treatment of dentin hypersensitivity with cyanoacrylate and laser. Journal of Periodontology 2017;88(2):166-72. [DOI] [PubMed] [Google Scholar]
Liu 1994 {published data only}
- Liu HC, Lan WH. The combined effectiveness of the semiconductor laser with Duraphat in the treatment of dentin hypersensitivity. Journal of Clinical Laser Medicine and Surgery 1994;12(6):315-9. [Google Scholar]
Liu 2004 {published data only}
- Liu HC, Lan WH. Treatment of cervical dentin abrasion hypersensitivity by diode laser and glass ionomer cement. In: The Second Asian and Pacific Rim Symposium on Biophotonics, APBP; 2004 Dec 14-17; Taipei. Taipei: IEEE (Institute of Electrical and Electronics Engineers), 2004:117.
Lizarelli 2015 {published data only}
- Lizarelli RFZ, Pizzo RCA, Florez FLE, Grecco C, Speciali JG, Bagnato VS. Comparative clinical study using laser and LED-therapy for orofacial pain relief: dentin hypersensitivity and cervicogenic headache. In: Kurachi C, Svanberg K, Tromberg BJ, Bagnato VS , editors(s). SPIE Biophotonics South America; 2015 May 23-25; Rio de Janeiro. Vol. 9531. Rio de Janeiro: SPIE, International Society for Optics and Photonics, 2015:95311Q.
Lopes 2013 {published and unpublished data}
- Lopes AO, Aranha AC. Comparative evaluation of the effects of Nd:YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomedicine and Laser Surgery 2013;31(3):132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopes 2015 {published and unpublished data}
- Lopes AO, Eduardo C de P, Aranha AC. Clinical evaluation of low power laser and a desentizing agent on dentine hypersensitivity. Lasers in Medical Science 2015;30(2):823-9. [DOI] [PubMed] [Google Scholar]
Lopes 2017 {published and unpublished data}
- Lopes AO, Eduardo CP, Aranha ACC. Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial T2. Lasers in Medical Science 2017;32(5):1023-30. [DOI] [PubMed] [Google Scholar]
Ma 2004 {published data only}
- Ma JJ, Wang GL. Clinical evaluation of pulsed Nd:YAG laser for treating dentinal hypersensitivity [脉冲Nd:YAG 激光治疗牙本质过敏症的临床观察]. Modern Practical Medicine 2004;16(7):398-403. [Google Scholar]
Moosavi 2016 {published data only}
- Moosavi H, Arjmand N, Ahrari F, Zakeri M, Maleknejad F. Effect of low-level laser therapy on tooth sensitivity induced by in-office bleaching. Lasers in Medical Science 2016;31(4):713-9. [DOI] [PubMed] [Google Scholar]
Moritz 1996 {published data only}
- Moritz A, Gutknecht N, Schoop U, Goharkhay K, Ebrahim D, Wernisch J, et al. The advantage of CO2-treated dental necks, in comparison with a standard method: results of an in vivo study. Journal of Clinical Laser Medicine and Surgery 1996;14(1):27-32. [DOI] [PubMed] [Google Scholar]
Moura 2019 {published and unpublished data}
- Moura GF, Zeola LF, Silva MB, Sousa SC, Guedes FR, Soares PV. Four-session protocol effectiveness in reducing cervical dentin hypersensitivity: a 24-week randomized clinical trial. Photobiomodulation, Photomedicine, and Laser Surgery 2019;37(2):117-23. [DOI] [PubMed] [Google Scholar]
Oberhofer 2008 {published data only}
- Oberhofer O, Sculean A. Er:YAG laser and desensitizing effects on dentin and dental cervices. Journal of Oral Laser Applications 2008;8(3):189-94. [Google Scholar]
Osmari 2018 {published and unpublished data}
- Osmari D, Fraga S, Ferreira ACO, Eduardo CP, Marquezan M, Silveira BLD. In-office treatments for dentin hypersensitivity: a randomized split-mouth clinical trial. Oral Health and Preventive Dentistry 2018;16(2):125-30. [DOI] [PubMed] [Google Scholar]
Ozlem 2018 {published and unpublished data}
- Ozlem K, Esad GM, Ayse A, Aslihan U. Efficiency of lasers and a desensitizer agent on dentin hypersensitivity treatment: a clinical study. Nigerian Journal of Clinical Practice 2018;21:225-30. [DOI] [PubMed] [Google Scholar]
Pandey 2017 {published and unpublished data}
- Pandey R, Koppolu P, Kalakonda B, Lakshmi BV, Mishra A, Reddy PK, et al. Treatment of dentinal hypersensitivity using low-level laser therapy and 5% potassium nitrate: a randomized, controlled, three-arm parallel clinical study. International Journal of Applied and Basic Medical Research 2017;7(1):63-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pesevska 2010 {published data only}
- Pesevska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, et al. Dentinal hypersensitivity following scaling and root planing: comparison of low-level laser and topical fluoride treatment. Lasers in Medical Science 2010;25(5):647-50. [DOI] [PubMed] [Google Scholar]
Pourshahidi 2019 {published data only}
- Pourshahidi S, Ebrahimi H, Mansourian A, Mousavi Y, Kharazifard M. Comparison of Er,Cr:YSGG and diode laser effects on dentin hypersensitivity: a split-mouth randomized clinical trial. Clinical Oral Investigations 2019;23(11):4051-8. [DOI] [PubMed] [Google Scholar]
Praveen 2018 {published and unpublished data}
- Praveen R, Thakur S, Kirthiga M, Narmatha M. Comparative evaluation of a low-level laser and topical desensitizing agent for treating dentinal hypersensitivity: a randomized controlled trial. Journal of Conservative Dentistry 2018;21(5):495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raichur 2012 {published and unpublished data}
- Raichur PS, Setty S, Thakur SL. Comparative evaluation of diode laser, stannous fluoride gel, and potassium nitrate gel in the treatment of dentinal hypersensitivity. General Dentistry 2013;61(3):66-71. [PubMed] [Google Scholar]
Renton‐Harper 1992 {published data only}
- Renton-Harper P, Midda M. Nd:YAG laser treatment of dentinal hypersensitivity. British Dental Journal 1992;172(1):13-6. [DOI] [PubMed] [Google Scholar]
Schwarz 2002 {published and unpublished data}
- Schwarz F, Arweiler N, Georg T, Reich E. Desensitizing effects of an Er:YAG laser on hypersensitive dentine. Journal of Clinical Periodontology 2002;29(3):211-5. [DOI] [PubMed] [Google Scholar]
Sgolastra 2013 {published data only}
- Sgolastra F, Petrucci M, Severino RG, Monaca A. Lasers for the treatment of dentin hypersensitivity: a meta-analysis. Journal of Dental Research 2013;92(6):492-9. [DOI] [PubMed] [Google Scholar]
Shintome 2007 {published data only}
- Shintome LK, Umetsubo LS, Nagayassu MP, Jorge ALC, Goncalves SEP, Torres CRG. Clinical evaluation of laser therapy on dentin hypersensitivity treatment [Avaliação clínica da laserterapia no tratamento da hipersensibilidade dentinária]. Ciência Odontológica Brasileira [Brazilian Dental Science] 2007;10(1):26-33. [Google Scholar]
Sicilia 2009 {published and unpublished data}
- Sicilia A, Cuesta-Frechoso S, Suárez A, Angulo J, Pordomingo A, De Juan P. Immediate efficacy of diode laser application in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized clinical trial. Journal of Clinical Periodontology 2009;36(8):650-60. [DOI] [PubMed] [Google Scholar]
Tabatabaei 2018 {published data only}
- Tabatabaei MH, Chiniforush N, Hashemi G, Valizadeh S. Efficacy comparison of Nd:YAG laser, diode laser and dentine bonding agent in dentine hypersensitivity reduction: a clinical trial. Laser Therapy 2018;27(4):265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabibzadeh 2018 {published data only}
- Tabibzadeh Z, Fekrazad R, Esmaeelnejad A, Shadkar MM, Khalili Sadrabad Z, Ghojazadeh M. Effect of combined application of high- and low-intensity lasers on dentin hypersensitivity: a randomized clinical trial. Journal of Dental Research, Dental Clinics, Dental Prospects 2018;12(1):49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tailor 2014 {published and unpublished data}
- Tailor A, Shenoy N, Thomas B. To compare and evaluate the efficacy of bifluoride 12, diode laser and their combined effect in treatment of dentinal hypersensitivity: a clinical study. Nitte University Journal of Health Science 2014;4(2):54-8. [Google Scholar]
Talesara 2014 {published data only}
- Talesara K, Kulloli A, Shetty S, Kathariya R. Evaluation of potassium binoxalate gel and Nd:YAG laser in management of dentinal hypersensitivity: a split-mouth clinical and ESEM study. Lasers in Medical Science 2014;29:61-8. [DOI] [PubMed] [Google Scholar]
Tengrungsun 2008 {published data only}
- Tengrungsun T, Sangkla W. Comparative study in desensitizing efficacy using the GaAlAs laser and dentin bonding agent. Journal of Dentistry 2008;36(6):392-5. [DOI] [PubMed] [Google Scholar]
Tocarruncho 2018 {unpublished data only}
- Tocarruncho O, Espitia-Roballo XA, Ibañez-Pinilla E, Rios-Osorio NR. Comparison between 940 nm laser diode and topical 5% potassium nitrate for tooth desensitization. Randomized controlled clinical trial [Comparación entre el láser diodo de 940 nm y el nitrato de potasio tópico al 5 % para la desensibilización dental. Estudio clínico controlado aleatorizado]. Universitas Odontologica 2018;37(79):1-22. [Google Scholar]
Wang 1991 {published data only}
- Wang JY, Yang L, Chen XJ. Use of Nd:YAG laser in the treatment of dentin hypersensitivity. In: Li J , editors(s). SPIE International Conference on Photodynamic Therapy and Laser Medicine; 1991 Oct 15-17; Beijing. Vol. 1616. Beijing: SPIE, International Society for Optics and Photonics, 1993:482-6.
Wang 2004 {published data only}
- Wang HQ, Zhong WJ, Qu XF, Gao WH. The clinical evaluation of Nd:YAG laser in the treatment of dentine hypersensitivity [Nd:YAG激光治疗牙本质过敏症的临床疗效观察]. Journal of Practical Stomatology 2004;20(2):218-9. [Google Scholar]
Wang 2005 {published data only}
- Wang Y, Li QX, Liu GS. Clinical evaluation of Nd:YAG laser and sodium fluoride gel for treating dentin hypersensitivity [脉冲Nd:YAG激光与氟化钠甘油治疗牙本质过敏症的临床应用]. Hebei Medical Journal 2005;27(10):757-8. [Google Scholar]
Wang 2006 {published data only}
- Wang L. Clinical evaluation of Nd:YAG laser and sodium fluoride gel for treating dentinal hypersensitivity [Nd:YAG激光和氟化钠治疗牙本质过敏症的临床疗效观察]. Journal of Practical Stomatology 2006;22(2):274-5. [Google Scholar]
Wang 2012 {published and unpublished data}
- Wang MH, Zhao Y. The effectiveness of an Er:YAG laser on dentine hypersensitivity: an eight-week clinical study [Er:YAG激光治疗牙本质敏感的临床疗效观察]. Laser Journal 2012;33(1):61-2. [Google Scholar]
Xiong 2010 {published data only}
- Xiong JP, Yang X, Si JB, Cui L. Clinical evaluation of three different therapies for treating dentinal hypersensitivity: experience with 153 cases. China Practical Medicine 2010;5(14):66-8. [Google Scholar]
Xu 2002 {published data only}
- Xu Y. Clinical evaluation of Nd:YAG laser and sodium fluoride gel for treating dentine hypersensitivity [Nd:YAG 激光与氟化钠分组治疗牙本质过敏的疗效分析]. Chinese Journal of Laser Medicine and Surgery 2002;11(2):105. [Google Scholar]
Yadav 2019 {published and unpublished data}
- Yadav RK, Verma UP, Tiwari R. Comparative evaluation of neodymium-doped yttrium aluminum garnet laser with nanocrystalline hydroxyapatite dentifrices and herbal dentifrices in the treatment of dentinal hypersensitivity. National Journal of Maxillofacial Surgery 2019;10(1):78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaguchi 1990 {published data only}
- Yamaguchi M, Ito M, Miwata T, Horiba N, Matsumoto T, Nakamura H, et al. Clinical study on the treatment of hypersensitive dentin by GaAlAs laser diode using the double blind test. Aichi Gakuin Daigaku Shigakkai Shi 1990;28(2):703-7. [PubMed] [Google Scholar]
Yonaga 1999 {published data only}
- Yonaga K, Kimura Y, Matsumoto K. Treatment of cervical dentin hypersensitivity by various methods using pulsed Nd:YAG laser. Journal of Clinical Laser Medicine and Surgery 1999;17(5):205-10. [DOI] [PubMed] [Google Scholar]
Yu 1996 {published data only}
- Yu Q, Wu BL, Chen XM, Xiao MZ. Treatment of hypersensitive dentine by Nd:YAG laser [Nd:YAG 激光治疗牙本质过敏症]. Chinese Journal of Conservative Dentistry 1996;6(1):13-4. [Google Scholar]
Yu 2014 {published data only}
- Yu CH, Chang YC. Clinical efficiacy of the Er:YAG treatment on hypersensitve dentin. Journal of Formosan Medical Association 2014;113:388-91. [DOI] [PubMed] [Google Scholar]
Zhao 2003 {published data only}
- Zhao M, Chen Z. Clinical evaluation of semiconductor diode laser for treating dentinal hypersensitivity [半导体激光治疗牙本质敏感症的临床疗效观察]. Chinese Journal of Physical Medicine and Rehabilitation 2003;25(12):766-7. [Google Scholar]
Zhao 2008 {published data only}
- Zhao G. Clinical evaluation of different therapies for treating dentinal hypersensitivity [不同方法治疗牙颈部牙本质敏感症的疗效观察]. China Modern Doctor 2008;46(8):36-7. [Google Scholar]
References to ongoing studies
ChiCTR‐INR‐16010245 {published data only}
- ChiCTR-INR-16010245. Clinical evaluation of the effect of Er:YAG in treating abrasion caused dentin hypersensitivity [Clinical outcomes research of the effect of Er:YAG in treating abrasion caused dentin hypersensitivity: a randomized controlled study]. www.chictr.org.cn/showproj.aspx?proj=17089 (first received 24 December 2016).
ChiCTR‐INR‐17013452 {published data only}
- ChiCTR-INR-17013452. Non-invasive optical therapy for dentin hypersensitivity. www.chictr.org.cn/com/25/showprojen.aspx?proj=22771 (first received 20 November 2017).
CTRI/2017/05/008547 {published data only}
- CTRI/2017/05/008547. Diode lasers: a virtuous desensitizer [Use of diode laser and calcium sodium phosphosilicate for the treatment of dentinal hypersensitivity of tooth - a short-term randomized clinical trial]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=18200&EncHid=&modid=&compid=%27,%2718200det%27 (first received 12 May 2017).
CTRI/2017/10/010085 {published data only}
- CTRI/2017/10/010085. Effects of laser with herbal and chemical toothpastes in the management of dental hypersensitivity [Comparative evaluation of Nd:YAG laser with nanocrystalline hydroxyapattite dentifrices and herbal dentifrices in the treatment of dentinal hypersensitivity]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=18043&EncHid=&modid=&compid=%27,%2718043det%27 (first received 13 October 2017).
CTRI/2018/02/012031 {published data only}
- CTRI/2018/02/012031. A study comparing the ability of a gel and laser light in the treatment of tooth sensitivity [Comparative evaluation of the efficacy of gluma desensitizer and diode laser in the treatment of dentinal hypersensitivity in chronic periodontitis patients - A single-blinded split-mouth study]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=22500&EncHid=&modid=&compid=%27,%2722500det%27 (first received 21 February 2018).
IRCT201104104877N5 {published data only}
- IRCT201104104877N5. Comparative efficacy of gel and laser for treatment of dentine hypersensitivity [Comparative efficacy of desensitizing gel (sensikin) and Nd:Yag laser in comparison with placebo for treatment of dentine hypersensitivity]. en.irct.ir/trial/5218 (first received 28 April 2011).
IRCT201105316664N1 {published data only}
- IRCT201105316664N1. Comparative evaluation of effect of 5% sodium fluoride varnish and Nd:YAG laser and their combative use on dentine hypersensitivity treatment. en.irct.ir/trial/7089/pdf/download (first received 14 June 2011).
IRCT2015102624715N1 {published data only}
- IRCT2015102624715N1. Efficacy of 940 nm diode laser on the treatment of dentin hypersensitivity in periodontal maintenance patients. en.irct.ir/trial/20805 (first received 18 May 2016).
IRCT2016111230858N1 {published data only}
- IRCT2016111230858N1. Effect of laser and propolis in treatment of dentin hypersensitivity [Clinical trial of comparison between Nd:YAG laser and propolis in treatment of dental sensitivity in patients with dentin hypersensitivity]. en.irct.ir/trial/24400/pdf/download (first received 23 March 2017).
IRCT201707269014N176 {published data only}
- IRCT201707269014N176. Comparison of the effect of Nd:YAG laser, diode laser and CPP/ACP on treatment of patient with dentin hypersensitivity: a randomized clinical trial. en.irct.ir/trial/9614/pdf/download (first received 31 July 2017).
NCT02931734 {published data only}
- NCT02931734. Assessment of different protocols for cervical dentin hypersensitivity treatment (CDH) [Analysis of the association of products for the treatment of cervical dentin hypersensitivity]. clinicaltrials.gov/ct2/show/NCT02931734 (first received 13 October 2016).
NCT03237793 {published data only}
- NCT03237793. Treatment of hypersensitivity using diode laser and desensitising agent on fluorosed and non-fluorosed teeth [Treatment of hypersensitivity using diode laser and potassium nitrate desensitising agent - a comparison of treatment effects on fluorosed and non-fluorosed teeth - an in vivo randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT03237793 (first received 3 August 2017).
NCT03553290 {published data only}
- NCT03553290. Therapeutic evaluation of lower-level laser for treating dentinal hypersensitivity [Lower-level laser for treating dentinal hypersensitivity]. clinicaltrials.gov/ct2/show/NCT03553290 (first received 12 June 2018).
NCT03750851 {published data only}
- NCT03750851. CPP-ACPF and laser's effect on dentin hypersensitivity [CPP-ACPF and low level laser therapy effect on symptomatology and quality of life of people with dentin hypersensitivity: a clinical, randomized, double-blind study]. clinicaltrials.gov/ct2/show/NCT03750851 (first received 23 November 2018).
RBR‐235jts {published data only}
- RBR-235jts. Dental sensitivity: prevalence and treatment [Hipersensibilidade dentinária cervical: prevalência e tratamento]. ensaiosclinicos.gov.br/rg/RBR-235jts (first received 29 October 2019).
Additional references
Addy 1990
- Addy M. Etiology and clinical implications of dentine hypersensitivity. Dental Clinics of North America 1990;34(3):503-14. [PubMed] [Google Scholar]
Addy 2000
- Addy M. Dentine hypersensitivity: definition, prevalence, distribution and aetiology. In: Addy M, Embery G, Edgar WM, Orchardson R, editors(s). Tooth Wear and Sensitivity: Clinical Advances in Restorative Dentistry. Taylor & Francis, 2000:239-48. [Google Scholar]
Addy 2002
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. International Dental Journal 2002;52(5 Suppl 1):367-75. [DOI] [PubMed] [Google Scholar]
Al‐Sabbagh 2009
- Al-Sabbagh M, Brown A, Thomas MV. In-office treatment of dentinal hypersensitivity. Dental Clinics of North America 2009;53(1):47-60. [DOI] [PubMed] [Google Scholar]
Asnaashari 2013
- Asnaashari M, Moeini M. Effectiveness of lasers in the treatment of dentin hypersensitivity. Journal of Lasers in Medical Sciences 2013;4(1):1-7. [PMC free article] [PubMed] [Google Scholar]
Bamise 2008
- Bamise CT, Olusile AO, Oginni AO. An analysis of the etiological and predisposing factors related to dentin hypersensitivity. Journal of Contemporary Dental Practice 2008;9(5):52-9. [PubMed] [Google Scholar]
Bartold 2006
- Bartold PM. Dentinal hypersensitivity: a review. Australian Dental Journal 2006;51(3):212-8. [PubMed] [Google Scholar]
Bekes 2009
- Bekes K, John MT, Schaller H-G, Hirsch C. Oral health-related quality of life in patients seeking care for dentin hypersensitivity. Journal of Oral Rehabilitation 2009;36(1):45-51. [DOI] [PubMed] [Google Scholar]
CABDH 2003
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Journal of the Canadian Dental Association 2003;69(4):221-6. [PubMed] [Google Scholar]
Cortellini 2018
- Cortellini P, Bissada NF. Mucogingival conditions in the natural dentition: narrative review, case definitions, and diagnostic considerations. Journal of Periodontology 2018;89 Suppl 1:S204-S213. [DOI] [PubMed] [Google Scholar]
Feng 2010
- Feng C. Progress in research of treatment of anaphylaxis [牙本质过敏症治疗的研究进展]. Chinese Journal of Dental Materials and Devices 2010;19(2):100-3. [Google Scholar]
GRADE 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Version accessed 1 February 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hargreaves 2002
- Hargreaves KM, Seltzer S. Pharmacologic control of dental pain. In: Hargreaves KM, Goodis HE, editors(s). Seltzer and Bender's Dental Pulp. Quintessence Publishing Co, Inc, 2002:205-25. [Google Scholar]
He 2009
- He SL, Hu DY. Development of study on treatment for dentin hypersensitivity [牙本质敏感治疗研究进展]. Chinese Journal of Practical Stomatology 2009;2(7):434-6. [Google Scholar]
He 2010
- He SL, Hu DY. Meta-analysis of the effectiveness of laser therapy in the treatment of dentin hypersensitivity in China [我国激光治疗牙本质敏感疗效的循证医学分析]. Journal of Practical Stomatology 2010;26(4):541-4. [Google Scholar]
He 2011
- He S, Wang Y, Li X, Hu D. Effectiveness of laser therapy and topical desensitising agents in treating dentine hypersensitivity: a systematic review. Journal of Oral Rehabilitation 2011;38(5):348-58. [DOI] [PubMed] [Google Scholar]
Heasman 2015
- Heasman PA, Holliday R, Bryant A, Preshaw PM. Evidence for the occurrence of gingival recession and non-carious cervical lesions as a consequence of traumatic toothbrushing. Journal of Clinical Periodontology 2015;42:237-55. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 1997
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. Journal of Clinical Periodontology 1997;24(11):808-13. [DOI] [PubMed] [Google Scholar]
Hu 2019
- Hu M, Zheng G, Han J, Yang M, Zhang Y, Lin H. Effect of lasers on dentine hypersensitivity: evidence from a meta-analysis. Journal of Evidence-based Dental Practice 2019;19(2):115-30. [DOI] [PubMed] [Google Scholar]
Kaiyan 2021
- Zhou K, Liu Q, Yu X, Zeng X. Laser therapy versus topical desensitising agents in the management of dentine hypersensitivity: a meta-analysis. Oral Diseases 2021;27(3):422-30. [DOI] [PubMed] [Google Scholar]
Kimura 2000
- Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K. Treatment of dentine hypersensitivity by lasers: a review. Journal of Clinical Periodontology 2000;27(10):715-21. [DOI] [PubMed] [Google Scholar]
Kong 2020
- Kong Y, Lei Y, Zhang Y, Han J, Hu M. Network meta-analysis of the desensitizing effects of lasers in patients with dentine hypersensitivity. Clinical Oral Investigations 2020;24(6):1917-28. [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin PY, Cheng YW, Chu CY, Chien KL, Lin CP, Tu YK. In-office treatment for dentin hypersensitivity: a systematic review and network meta-analysis. Journal of Clinical Periodontology 2013;40(1):53-64. [DOI] [PubMed] [Google Scholar]
Litonjua 2003
- Litonjua LA, Andreana S, Bush PJ, Cohen RE. Toothbrushing and gingival recession. International Dental Journal 2003;53(2):67-72. [DOI] [PubMed] [Google Scholar]
Markowitz 2007
- Markowitz K, Pashley DH. Personal reflections on a sensitive subject. Journal of Dental Research 2007;86(4):292-5. [DOI] [PubMed] [Google Scholar]
Matsumoto 1985
- Matsumoto K, Funai H, Shirasuka T, Wakabayashi H. Effects of Nd:YAG laser in treatment of cervical hypersensitive dentine. Japan Journal of Conservative Dentistry 1985;28:760-5. [Google Scholar]
Orchardson 2006
- Orchardson R, Gillam DG. Managing dentin hypersensitivity. Journal of the American Dental Association 2006;137(7):990-8. [DOI] [PubMed] [Google Scholar]
Porto 2009
- Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. Journal of Oral Science 2009;51(3):323-32. [DOI] [PubMed] [Google Scholar]
Poulsen 2006
- Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD001476. [DOI: 10.1002/14651858.CD001476.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rezazadeh 2019
- Rezazadeh F, Dehghanian P, Jafarpour D. Laser effects on the prevention and treatment of dentinal hypersensitivity: a systematic review. Journal of Lasers in Medical Sciences 2019;10(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ritter 2006
- Ritter AV, L Dias W, Miguez P, Caplan DJ, Swift EJ Jr. Treating cervical dentin hypersensitivity with fluoride varnish: a randomized clinical study. Journal of the American Dental Association 2006;137(7):1013-20. [DOI] [PubMed] [Google Scholar]
Sgolastra 2011
- Sgolastra F, Petrucci A, Gatto R, Monaco A. Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. Journal of Endodontics 2011;37(7):297-303. [DOI] [PubMed] [Google Scholar]
Shiau 2012
- Shiau HJ. Dentin hypersensitivity. Journal of Evidence-based Dental Practice 2012;12 (3 Suppl):220-8. [DOI] [PubMed] [Google Scholar]
Splieth 2013
- Splieth CH, Tachou A. Epidemiology of dentin hypersensitivity. Clinical Oral Investigations 2013;17:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stabholz 2004
- Stabholz A, Sahar-Helft S, Moshonov J. Lasers in endodontics. Dental Clinics of North America 2004;48(4):809-32. [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis - a simulation study. PLOS One 2011;6(11):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2013
- West NX, Lussi A, Seong J, Hellwig E. Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clinical Oral Investigations 2013;17 Suppl 1:S9-S19. [DOI] [PubMed] [Google Scholar]
Yilmaz 2011
- Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y. Clinical evaluation of Er,Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. Journal of Dentistry 2011;39(3):249-54. [DOI] [PubMed] [Google Scholar]
Zhou 2021
- Zhou K, Liu Q, Yu X, Zeng X. Laser therapy versus topical desensitising agents in the management of dentine hypersensitivity: a meta‐analysis. Oral Diseases 2021;27(3):422-30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Deng 2011
- Deng Y, Mahdian M, George AT, Blake B, Huang D, Shi Z. Laser therapy for dentinal hypersensitivity. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD009434. [DOI: 10.1002/14651858.CD009434] [DOI] [PMC free article] [PubMed] [Google Scholar]


