1. Introduction
Aluminum (Al) is considered as the most abundant metal in the Earth’s crust, being the third most abundant chemical element after oxygen and silicon. Intensive development of the Al industry (Brough and Jouhara, 2020) due to a wide use of the metal has resulted in a significant increase in environmental Al levels (Crisponi et al., 2012).
The sources of human Al exposure may include diet (Tietz et al., 2019), being responsible for 95% of total body Al (Goullé and Grangeot-Keros, 2020), drinking water (Krupińska, 2020), air (Exley, 2013), as well as cosmetics (Crisponi et al., 2012, 2013) and medicinal drugs, namely antacids (Klotz et al., 2017). Involvement in Al processing industry may also result in occupational Al exposure (Skalny et al., 2018). Earlier studies demonstrated that vaccination could be considered as a source of Al exposure due to the presence of aluminium adjuvants that are currently not widely used thus reducing the risk of vaccine-associated Al exposure (Goullé and Grangeot-Keros, 2020).
Being a non-essential element, Al was shown to be toxic for humans (Exley, 2013), resulting in adverse health effects (Crisponi et al., 2011) including bone pathology (Klein, 2019) and breast cancer (Darbre et al., 2013). Our data also demonstrated the association between obesity (Tinkov et al., 2019), laboratory markers of metabolic syndrome and Al exposure markers (Skalnaya et al., 2018). However, the existing data on adverse effects of Al exposure are limited (Krewski et al., 2007).
Recent studies have demonstrated that the brain may be considered as the target for Al toxicity (Exley, 2014), resulting in neurodegenerative (Exley, 2013; Shaw et al., 2014) and neurodevelopmental disorders (Blaylock, 2012). Recent detailed studies by Exley and the coauthors have highlighted the association between brain Al accumulation and neurological disorders including Alzheimer’s disease, multiple sclerosis and autism spectrum disorder (Exley and Clarkson, 2020; Mirza et al., 2017; Mold et al., 2018). Speciation analysis using hyphenated techniques demonstrated that Al exposure induces a specific bioligand response in Al-exposed neuronal cells (Połeć-Pawlak et al., 2004). However, the particular mechanisms of Al neurotoxicity and their role in Al-associated neurological disorders are still debatable (Morris et al., 2017). This chapter will provide an update on the particular mechanisms of Al neurotoxicity that may be used as targets for development of therapeutic strategies.
Generally, the neurotoxic effect of Al exposure is mediated by its common toxic properties including prooxidant, proinflammatory, proapoptotic activity that are reported for a variety of cell lines and tissues, as well as more specific “neurotropic” effects namely interference with neurotransmitter metabolism and neuronal cytoskeleton.
2. Oxidative stress
Oxidative stress along with mitochondrial dysfunction (see next section) are involved in development of a variety of adverse effects of Al including neurotoxicity (Kumar and Gill, 2014). The observed increase in brain lipid peroxidation under Al exposure (Ghorbel et al., 2016; Nehru and Anand, 2005; Yuan et al., 2012) was shown to be associated with a significant reduction in antioxidant enzyme activity, namely superoxide dismutase (Nehru and Anand, 2005), catalase (Nehru and Anand, 2005), glutathione peroxidase (Sánchez-Iglesias et al., 2009), glutathione reductase (Nehru and Bhalla, 2006), as well as glutathione-S-transferase (Bhalla and Dhawan, 2009). Alteration of glutathione system is also characterized by Al-induced decline in cerebral and cerebellar total, reduced, and oxidized glutathione levels (Anand and Nehru, 2006). In contrast to glutathione, data on involvement of thioredoxin system to Al-induced redox perturbations are insufficient, although recent study revealed a significant decrease in mitochondrial thioredoxin in aluminum chlorohydrate treated SH-SY5Y neuroblastoma cells (Tsialtas et al., 2020).
Al exposure was also shown to reduce mitochondrial Mn-SOD activity thus contributing to mitochondrial dysfunction (Kumar et al., 2009a,b). At the same time, certain studies demonstrated Al-induced increase in SOD activity and gene expression (Ali et al., 2014) that may also contribute to oxidative stress through overproduction of hydrogen peroxide, especially at low catalase and GPx activity. Inhibitory effect of Al on antioxidant system are likely mediated by Al-induced down-regulation of Nrf2/Keap1 signaling pathway (Yu et al., 2019a,b), whereas prevention of this inhibitory effect ameliorated prooxidant activity of the metal (Wang et al., 2017).
In parallel with inhibition of antioxidant system, the role of Al in development of cerebral oxidative stress may be mediated by its impact on prooxidant systems. Particularly, exposure to Al was shown to increase cerebral level of inducible (Bondy et al., 1998) and endothelial NOS (Mokhemer et al., 2020) with a concomitant elevation of brain NO levels (Al-Olayan et al., 2015). The use of the NOS inhibitor N-nitro-l-arginine methyl ester (L-NAME) significantly reduced Al-induced oxidative damage in brain, indicating a critical role of NOS induction in the prooxidant activity of Al (Stevanović et al., 2009). Al-induced alteration of vascular reactivity was shown to be associated with increased superoxide production from activated NADPH-oxidase (Schmidt et al., 2016) due to up-regulation of NAD(P)H oxidase 1 and 2 mRNA expression (Martinez et al., 2017). Stimulatory effect of Al was observed in another enzymatic source of superoxide anion, xanthine oxidase, in liver (Moumen et al., 2001) as well as various brain regions (Sushma et al., 2006).
In addition to enzymatic prooxidants, Al was capable of potentiating prooxidant effect of iron in neuronal cultures (Xie et al., 1996). This effect may also underlie a shift to ferroptosis in PC12 cells exposed to aluminium (Cheng et al., 2020). It is also notable that co-exposure to Al and 6-hydroxydopamine enhanced 6-hydroxydopamine autooxidation-induced oxidative stress in brain mitochondrial preparations (Sánchez-Iglesias et al., 2009). Moreover, Al is directly involved in the formation of highly reactive Al superoxide semi-reduced radical ion (Exley, 2012).
Taken together, increased production of reactive oxygen species due to activation of enzymatic and non-enzymatic prooxidant systems and reduced antioxidant capacity of the cells (Fig. 1) result in Al-induced oxidative stress affecting neuronal protein structure and inducing redox-sensitive pathways.
Fig. 1.
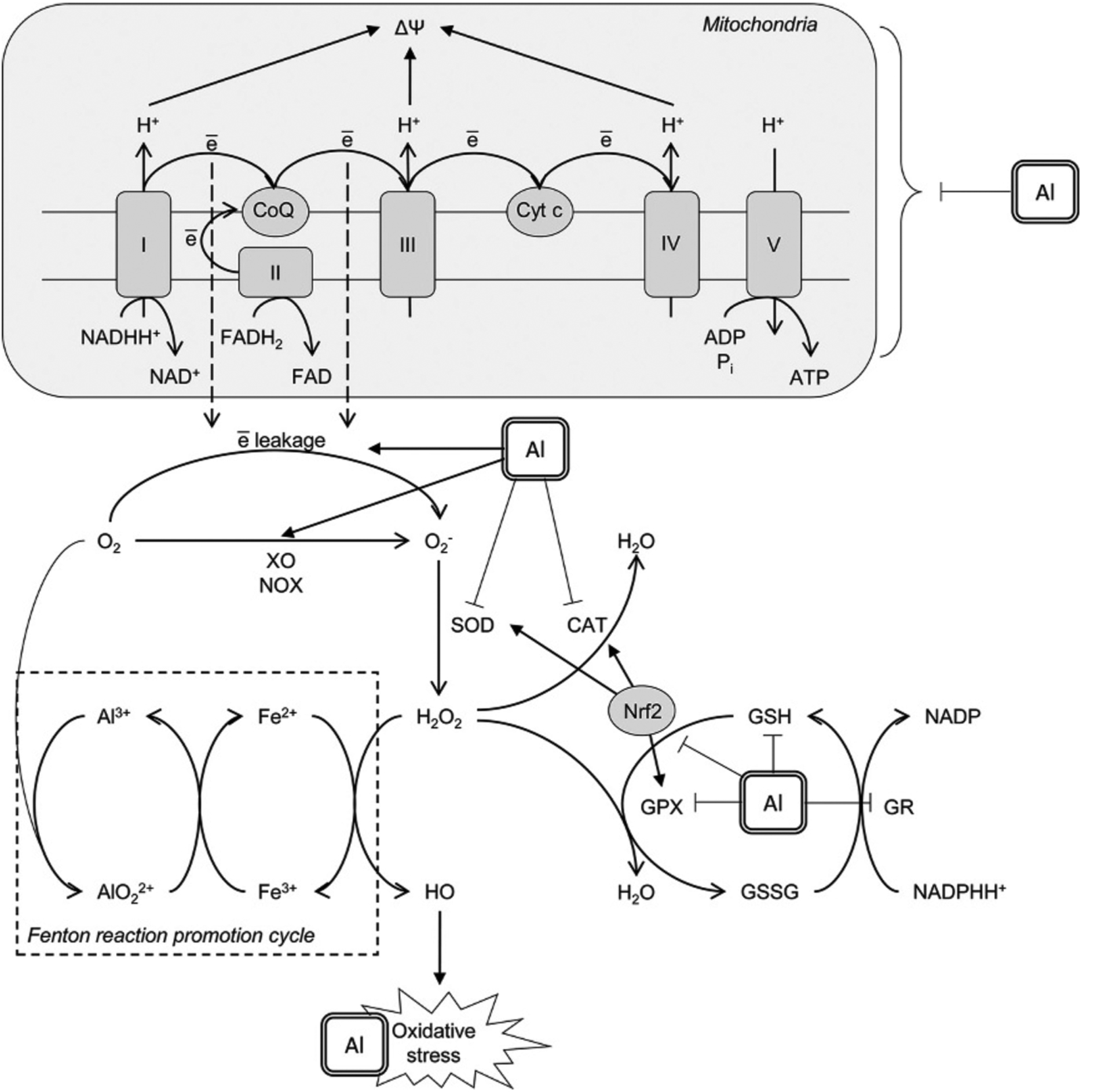
Mechanisms underlying prooxidant effect of aluminium (Al3+). Al3+ was shown to affect mitochondrial electron transport chain thus increasing electron leakage from Complex I and III with subsequent formation of superoxide anion radical . Another mechanism contributing to superoxide production involves Al-dependent increase in xanthine oxidase (XO) and NADPH-oxidase (NOX) activity. Al3+ cation is directly involved in the formation of highly reactive Al superoxide semi-reduced radical ion that was shown to promote prooxidant activity of Fe2+ in Fenton reaction with generation of hydroxyl radical (HO•). Prooxidant activity of Al is also aggravated by its inhibitory effect on enzymatic antioxidants including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR). The latter results in reduced glutathione (GSH) depletion. Moreover, Al was shown to down-regulate nuclear factor erythroid 2–related factor 2 (Nrf2), being the key regulator of the antioxidant system. Taken together, these mechanisms result in development of oxidative stress with increased oxidative modification of lipids, proteins and nucleic acids observed in brain/neuronal cell lines under Al exposure.
3. Mitochondrial dysfunction
Mitochondrial dysfunction is considered as one of the first pathological responses to Al exposure in the cell, resulting in altered energy metabolism, oxidative stress, and apoptosis. Particularly, Al was shown to decrease mitochondrial electron transport chain through inhibition of complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), and complex IV (cytochrome oxidase) in rat cortex and midbrain, as well as cerebellum (Sood et al., 2011). A reduction in cytochrome oxidase activity was also accompanied by significant Al-induced changes in enzyme Arrhenius plot (Dua and Gill, 2004). Complex III activity was found to be significantly inhibited by Al nanoparticles that also induced a sharp decrease in mitochondrial membrane potential and cytochrome c leakage in isolated brain mitochondria (Arab-Nozari et al., 2019). Reduced activity of electron transport chain complexes from Al-treated rats was accompanied by reduction in particular cytochromes cyt a, cyt b, and cyt c, as well as altered mitochondrial ultrastructure in hippocampus and striatum (Kumar et al., 2008). Complex V (ATP-synthase) was also down-regulated along with other fragments of electron transport chain upon Al exposure (Sharma et al., 2013a,b). Correspondingly, analysis of mitochondrial ultrastructure in Al-exposed neurons revealed mitochondrial swelling, cavitation, altered inner mitochondrial membrane and cristae structure (Niu et al., 2005), as well as loss of cristae, chromatin condensation and decreased number of mitochondria (Sharma et al., 2013a,b). The latter is indicative of Al-induced decrease in mitochondrial biogenesis through down-regulation of peroxisome proliferator activated receptor gamma co-activator 1α (PGC-1α) and the downstream genes including Nrf1, Nrf2, and mitochondrial transcription factor A (Tfam) (Sharma et al., 2013a,b). Taken together, these Al-induced changes result in alteration of mitochondrial transmembrane potential and impaired oxidative phosphorylation that may contribute to increased generation of reactive oxygen species (Iglesias-González et al., 2017). However, results from another study indicate that oxidative stress precedes mitochondrial dysfunction in brain of Al-exposed gerbils (Vučetić-Arsić et al., 2013).
4. Apoptosis
Induction of apoptosis in Al-exposed cells represents one of the mechanisms of direct Al-induced cell damage. Specifically, Al was capable of inducing apoptosis in neuronal (SH-SY5Y), glial (U373MG), and retinal epithelial cells (RPE D407) (Toimela and Tähti, 2004). Exposure of co-cultured neurons and astrocytes to Al resulted in significant metal accumulation on both cell lines, whereas Al-induced apoptosis was revealed only in astrocytes (Suárez-Fernández et al., 1999). In agreement, intensified neuronal apoptosis was observed in a number of in vivo studies (Çabuş et al., 2015; Keshava et al., 2019).
As stated earlier, mitochondrial dysfunction causes cytochrome c leakage that binds Apaf-1 with subsequent formation of apoptosome and procaspase 9 activation underlying Al-induced apoptotic neuronal death (Savory et al., 2003), thus indicative of mitochondrial pathway of apoptosis (Fig. 2). Another study demonstrated that prooxidant effect of AlCl3 was associated with a significant increase in cerebral levels of caspase-3 and Bax, whereas antiapoptotic Bcl2 was found to be down-regulated by Al treatment (Mesole et al., 2020). Cortical and hippocampal proapoptotic p53, p21, Bax, caspase 3, and pJNK levels were increased in response to oral Al treatment along with a two- to threefold increase in Fas levels, being indicative of involvement of Fas/FasL-mediated apoptosis (Keshava et al., 2019). The role of Fas/FasL apoptotic pathway has also been confirmed by the observation of Al-induced caspase-8 (Qin et al., 2020) and DAXX up-regulation (Kumar et al., 2009a,b; Lukiw et al., 2005), which is known to be p53-independent (Wasylishen et al., 2018). Taken together with the earlier demonstrated increase in p53-associated proapoptotic signaling under Al exposure (Johnson et al., 2005), these findings indicate that Al-induced apoptosis may involve both p53-dependent and -independent pathways.
Fig. 2.
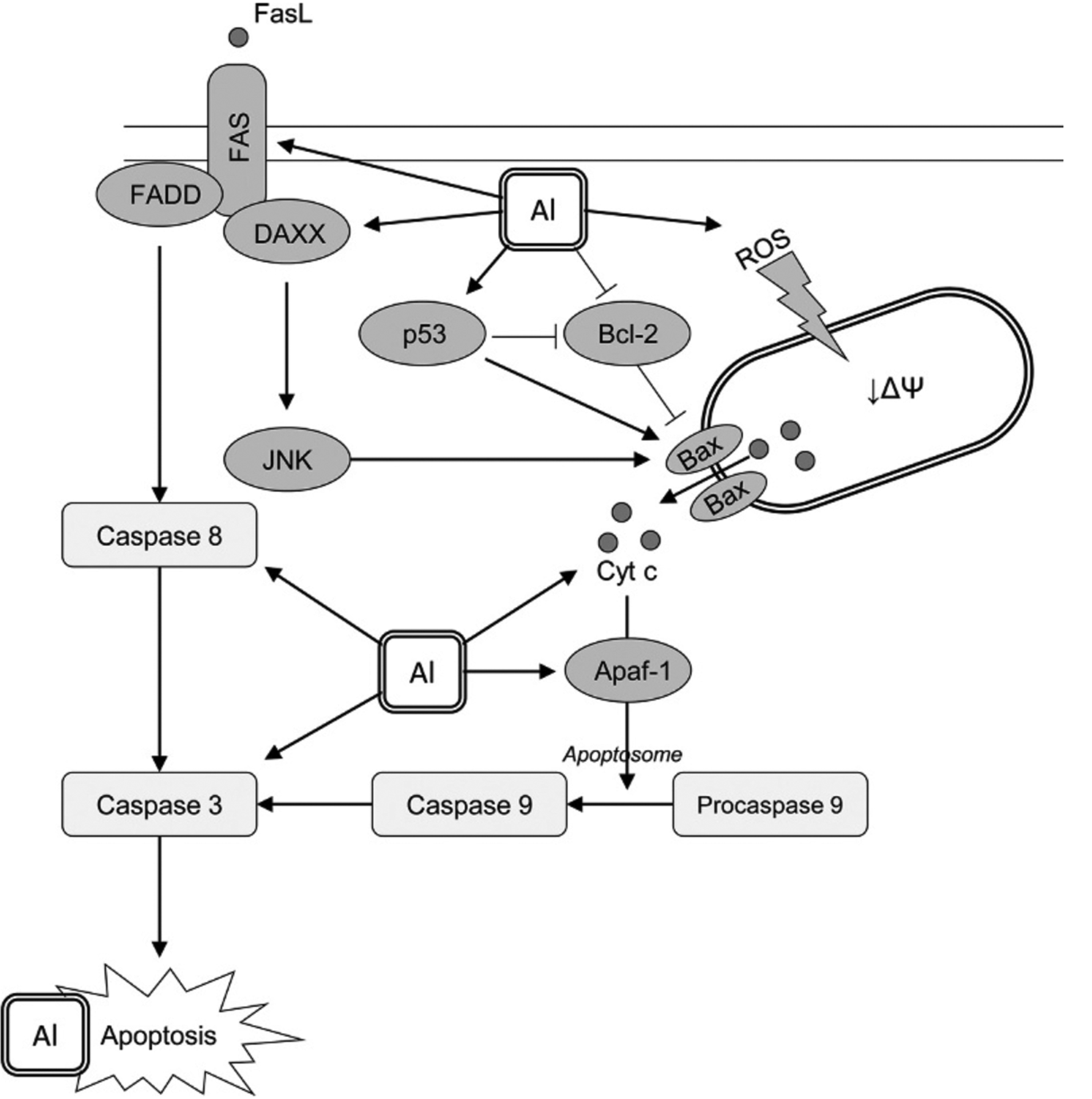
The proposed model of aluminium-induced neuronal apoptosis. Al was shown to induce apoptosis in neuronal cells through distinct pathways. Mitochondrial pathway is directly related to Al-induced mitochondrial dysfunction and subsequent Bax-mediated cytochrome c release. The latter is also stimulated due to positive and negative regulation of p53 and Bcl-2 expression, respectively. Al was shown to increase the rate of cytochrome c binding to Apaf-1 with subsequent formation of apoptosome and caspase 9 activation with subsequent caspase 3 cleavage and activation promoting apoptosis. Another pathway of Al proapoptotic effect involves Fas/FasL signaling with activation of caspase 3 following caspase 8 stimulation. In addition, Al3+-induced up-regulation of DAXX results in JNK signaling that also possesses a stimulatory effect on Bax. Prooxidant activity of Al3+ is also expected to underlie proapoptotic effect of the metal through increased oxidative modification of biomolecules and particularly nucleic acids.
The use of necrostatin 1 and Z-VAD-FMK, a pan-caspase inhibitor, demonstrated that Al-induced neuronal cell death may involve activation of both necrosis and apoptosis (Hao et al., 2019). This observation corresponds to the proposed role of necroptosis in Al neurotoxicity (Zhang, 2018b). The latter was observed at high doses (450mg/kg AlCl3), whereas lower doses more likely induced apoptosis through IL-1β/JNK signaling pathway (Zhang et al., 2020). Another study also demonstrated that Al-induced apoptosis and necrosis differentially involved ERK and p38 MAPK/ERK signaling pathways in SH-SY5Y cells, respectively (Jia et al., 2014).
As Al is capable of inducing apoptosis in brain cells, it clearly modulates cell cycle in brain cells. A recent study by Reichert et al. (2019) demonstrated that Al3+ inhibited proliferation of neural progenitor cells during neural differentiation and induced apoptosis along with modulation of cell cycle by increasing sub-G1 phase and reducing G2/M phase (Reichert et al., 2019). At the same time, in cultured astrocytes Al-induced apoptosis was associated with a shift from S phase to G2/M phase (Guo and Liang, 2001). Al exposure also increases neuronal expression of cyclin-dependent kinase 5 (Cdk5) (Bihaqi et al., 2009), playing an essential role in regulation of neuronal differentiation (Cicero and Herrup, 2005). Several studies demonstrated that a significant increase in cyclin D1 expression in Al-exposed rat brains (Kumar et al., 2009a,b). In contrast, exposure of PC12 cells to Al nanoparticle significantly increased p21 and decreased cyclin D1 expression ultimately resulting in G1 cell cycle arrest (Liu et al., 2020).
5. Neuroinflammation and microglial activation
Existing data demonstrate proinflammatory effects of Al (Lukiw et al., 2005). Specifically, Al exposure was shown to increase expression of pro-inflammatory cytokines IL-1b, IL-6, TNF-a, and MIP-1a whereas expression of brain derived neurotrophic factor (BDNF) was significantly reduced (Cao et al., 2016; Kasbe et al., 2015; Prema et al., 2017). Increased expression of proinflammatory cytokines may be associated with up-regulation of nuclear factor-kappa beta (NF-κB) expression (Sood et al., 2012; Zhao et al., 2020), being the key regulator of inflammation (Shih et al., 2015). Particularly, a significant down-regulation of NF-κB inhibitor gene was observed in brains of mice subcutaneously exposed to Al (Li et al., 2017). It is also notable that co-exposure of Al and mercury (as sulfates) significantly potentiated neurotoxic effect of each of these metals alone via NF-κB induction and subsequent proinflammatory signaling, as noted in human neuronal and glial cells co-culture (Alexandrov et al., 2018). Therefore, NF-κB may be considered as a link between Al-induced neuroinflammation and amyloidogenesis (Alawdi et al., 2017). Together with 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP), a dopaminergic neurotoxicant, Al exposure is capable of inducing hippocampal expression of AP-1 (Li et al., 2007), another redox sensitive transcription factor involved in regulation of inflammatory response.
Proinflammatory effect of Al hydroxide was shown to be associated with inflammasome activation and subsequent IL-1β secretion by microglia (Gustin et al., 2015). The results obtained in another study demonstrated that apurinic/apyrimidinic endonuclease 1 may be also considered as negative regulator of Al neuroinflammation (Zaky et al., 2013). It is also notable that induction of systemic inflammation characterized by elevated proinflammatory cytokine (IL-6, TNF-a) and microRNA (miRNA-9, miRNA-125b and miRNA-146a) levels may also contribute to Al-induced inflammatory neurodegeneration (Pogue et al., 2017).
In addition to overproduction of proinflammatory cytokines, Al exposure was also shown to increase both COX-1 and COX-2 activity in cortex and/or hippocampus (Syed et al., 2015). In agreement, Al-induced COX-downstream signaling involving increased prostaglandin synthase and receptor expression, as well as prostaglandin levels in hippocampus (Wang et al., 2015). It is also notable that the use of synthetic prostaglandin E1 (PGE1) analogue, misoprostol, ameliorated Al-induced memory and learning dysfunction through modulation of PGES-PGE2-EP signaling pathway and decreasing PGE2, mPGES-1, EP2, and EP4 expression (Guo et al., 2016). AlCl3-exposed mice treated with COX inhibitor ibuprofen also demonstrated that Al toxicity is associated with hippocampal overexpression of neuronal pentraxin 1 (NP1) (Jamil et al., 2016) that is considered one of the key factors of Aβ-induced neurite loss and synaptic dysfunction (Abad et al., 2006).
Al -induced (micro)gliosis is considered as the key component of neuroinflammation (Blaylock, 2012). Particularly, exposure to Al was shown to induce oxidative stress-dependent glial activation in rat brain (Akinrinade et al., 2015a,b), being especially observed in striatum and thalamus (Platt et al., 2001). Nanoscaled silica was also shown to induce glial activation in rat brain cortex and hippocampus as assessed by increased number of ED1, GFAP, and nestin-positive cells (Li et al., 2009). Moreover, Campbell et al. (2002) demonstrated that Al sulfate was shown to increase proliferation of glioblastoma cells with the effect comparable to that for LPS (Campbell et al., 2002). It was also demonstrated that glial cells accumulate significantly more Al and iron, although being less sensitive to metal-induced oxidative stress when compared to neurons (Oshiro et al., 2000). These findings also correspond to a recent observation of increased Al deposition in microglia, as well as other inflammatory cells in a patient with cerebral amyloid angiopathy (Mold et al., 2019).
6. Endoplasmic reticulum stress (ERS) and altered Ca2+ homeostasis
It has been demonstrated that Al glycinate exposure down-regulates mRNA expression of molecular chaperone BiP/Grp78, as well as certain Ca-handling proteins (e.g., calnexin, calreticulin, stanniocalcin 2), thus inhibiting adaptive response and predisposing astrocytes to ERS (Aremu et al., 2011). Al-induced ERS characterized by an increase in CHOP and caspase 12 expression was shown to induce p53-independent cell death in SH-SY5Y neuroblastoma cells along with development of oxidative stress and Aβ1-40 accumulation (Rizvi et al., 2014). Similarly, development of ERS characterized by increased GADD153 translocation was accompanied by NF-κB induction and Bcl-2 down-regulation (Ghribi et al., 2001). A later study also demonstrated up-regulation of ERS pathway-associated proteins including PERK, EIF2α, and CHOP as a component of Al-induced neurotoxic effect (Bharathi et al., 2019). It is proposed that Al-dependent induction of PERK-EIF2α signaling with subsequent unfolded protein response may induce inflammation in human neuroblastoma SH-SY5Y cells (Rizvi et al., 2016).
Given the role of ER in Ca2+ handling, altered intracellular Ca levels may be also indicative of ER dysfunction (Rizvi et al., 2014). The role of altered Ca2+ homeostasis as the mechanism of Al neurotoxicity was proposed by Julka and Gill (1996). Chronic Al exposure was shown to increase synaptosomal Ca levels through reduction of Ca2+-ATPase activity (Kaur and Gill, 2005). Taken together with increased Ca2+ release from mitochondria these changes result in increased intracellular Ca2+ levels (Gandolfi et al., 1998). In addition, Al-Aβ conjugate was shown to affect neuronal Ca2+ homeostasis (Drago et al., 2008). Microarray analysis of 35,129 genes in Al-exposed SH-SY5Y cells demonstrated that alteration of Ca2+ homeostasis is the key mechanism mediating neuronal and/or synaptic dysfunction (Gatta et al., 2011). Thus, due to its role in increasing intracellular Ca2+ concentrations, Al can be considered excitotoxic (Exley, 2013).
7. Reduced synaptic plasticity and neurotrophin production
Impairment of synaptic plasticity and transmission is known to underlie multiple adverse neurological effects of Al (Zhang, 2018a) affecting both presynaptic and postsynaptic mechanisms (Chen et al., 2002). Correspondingly, laboratory studies demonstrated a significant negative effect of Al on synaptic plasticity (Wang et al., 2002), being strongly dependent on the ontogenetic period with the most prominent effect observed in adulthood (Wang et al., 2002b). Al exposure resulted in a significant decrease of field excitatory postsynaptic potentials after high-frequency stimulation, being indicative of synaptic dysfunction (Qin et al., 2020). Al-induced alteration of synapse ultrastructure are characterized by decreased postsynaptic density thickness, increased synaptic cleft width, high frequency of flat synapses, and reduced number of perforated synapse (Jing et al., 2004). Increased rigidity of synaptosomes under Al exposure due to redox-dependent modulation of membrane fluidity and membrane lipid composition (Ahmed et al., 2020a) may reduce the release of synaptic vesicles into synaptic cleft (Ahmed et al., 2020b). Al was also shown to inhibit synaptic (Na+/K+)ATPase activity and contributing to synaptic dysfunction (Silva and Gonçalves, 2003). Al-maltol exposure resulted in a significant alteration of synaptic structure characterized by decreased number of axon branches and dendritic spines along with neuronal atrophy, being associated with mGluR up-regulation and down-regulation of PKC and the NMDAR subunits (Pan et al., 2020b). Another study proposed Al-induced modulation of the PI3K-Akt-mTOR pathway, could result in alterations in synaptic plasticity (Li et al., 2020a,b).
Adverse effects of Al exposure on synaptic plasticity and neurite growth may be mediated by negative regulation of neurotrophic factor production. Al-maltol induced a significant decrease in nerve growth factor (NGF) and BDNF expression in brain cell cultures (Johnson and Sharma, 2003). The decrease in synaptic long-term potentiation in Al-exposed rats was associated with a significant reduction of BDNF expression through modulation of Histone H3K9 demethylation (H3K9me2) demethylase and plant homeodomain finger protein 8 (PHF8) (Li et al., 2020a,b). Other epigenetic mechanisms involving H3K4me3, H3K27me3 and H3K9me2 may also underlie altered BDNF and early growth response protein 1(EGR1) under occupational Al exposure (Pan et al., 2020a). Correspondingly, hippocampal expression of activity-regulated cytoskeleton-associated protein (ARC) gene, known to play a significant role in synaptic plasticity (Korb and Finkbeiner, 2011), was also found to be reduced following in utero Al exposure (Inohana et al., 2018). The underlying mechanism was shown to involve a decrease in BDNF-induced Arc expression through alteration of ERK signaling (Chen et al., 2011). Other mechanisms involved in altered synaptic plasticity upon Al exposure may include deregulation of SIRT1, TORC1 and pCREB levels (Yan et al., 2017) and cAMP-PKA-CREB pathway (Zhang et al., 2014).
8. Cytoskeletal pathology
Cytoskeleton and microtubules in particular were considered as the potential targets for Al toxicity (Walton, 2014), resulting in altered axonal transport and perikaryal aggregation (Kushkuley et al., 2010). Particularly, Al exposure resulted in a significant aggregation and disruption of cytoskeletal proteins in cerebral cortex, corpus striatum and hippocampus (Kaur et al., 2006). After prolonged exposure Al -rich pyramidal cells are characterized by microtubule depletion with neurite damage and loss of synapse density (Walton, 2009). Particularly, Al is capable of inhibiting neurofilament assembly to axonal cytoskeleton, translocation and degradation of neurofilaments in axonal neurites (Shea et al., 1997). Generally, these observations corroborate earlier data on sharp reduction in neurofilament and tubulin genes in spinal cord motor neurons (Muma et al., 1988) contributing to neuro-fibrillary degeneration (Katsetos et al., 1990). A recent study demonstrated that Al(III) affects microtubule assembly far more effectively than iron (Shevtsov et al., 2016). Al-induced alterations in cytoskeleton may also involve modulatory effects on amyloid β and α-synuclein accumulation.
8.1. Amyloid β
Modulation of amyloid generation and accumulation is considered as the potential pathway of Al-induced neurodegeneration in Alzheimer’s disease (Exley, 2005) (Fig. 3). Generally, this suggestion is based on the observation of cooccurrence between Al and amyloid deposits in neurodegenerative diseases (Mirza et al., 2017; Mold et al., 2019).
Fig. 3.
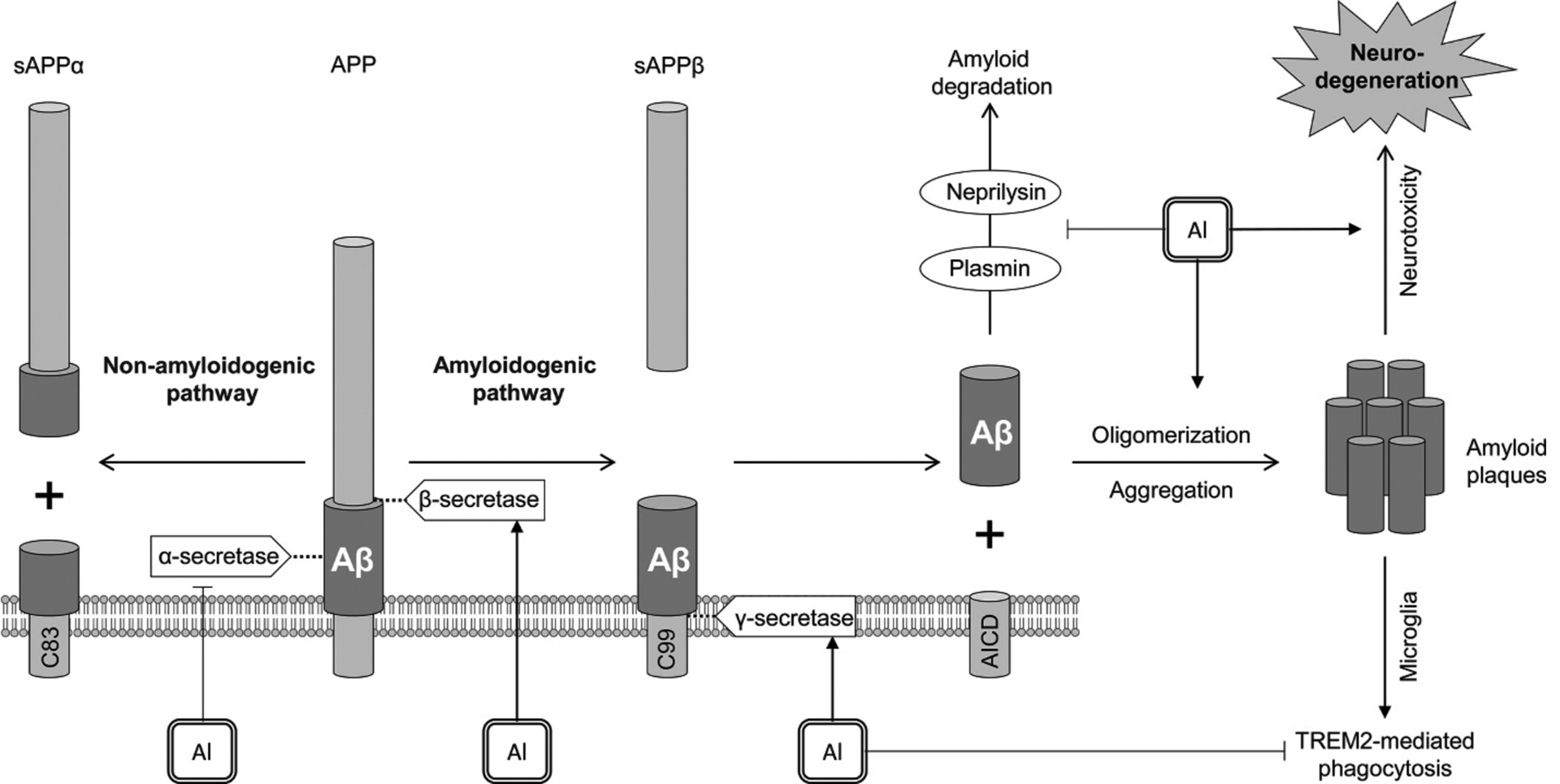
The role of Al in amyloid β generation. Al exposure promotes amyloidogenic pathway through activation of β- (BACE1) and γ-secretase (presenilin-1), as well as down-regulating non-amyloidogenic pathway due to inhibition of α-secretase. Along with promotion of amyloid oligomerization and aggregation, Al3+ also inhibits neprilysin and plasmin that are known to be involved in amyloid degradation. In addition, Al exposure was shown to impair TREM2-mediated phagocytosis of amyloid proteins by microglia. Taken together, these effects of Al exposure result in accumulation of amyloid plaques and Alzheimer disease-like neurodegeneration.
Al-maltolate exposure was shown to increase Aβ1–42 expression in rat brain through up-regulation of APP, β- (BACE1), and γ-secretase (presenilin-1) mRNA transcription and protein expression in rat brain regions (Liang et al., 2013; Thenmozhi et al., 2015). These changes are also associated with a significant decrease of α-secretase proteins (ADAM9, ADAM10, ADAM17) (Wang et al., 2014). In vitro studies in cell lines also demonstrate a significant impact of Al on proteins of amyloidogenic pathway. Al exposure was shown to increase BACE1 (Li et al., 2012) and BACE2 levels (Castorina et al., 2010), although a significant decrease in both enzyme expression could be observed at long-term exposure (Castorina et al., 2010). Certain studies also demonstrate that along with increasing Aβ production, Al exposure may negatively affect its degradation by decreasing neprilysin expression (Luo et al., 2009). Inhibition of plasmin cascade by Al (Korchazhkina et al., 2002) may be also responsible for both APP α-cleavage and Aβ degradation (Zhao and Pei, 2008). Al is also capable of inhibiting microglial phagocytosis of Aβ42 peptides (Zhao et al., 2014) due to TREM2 down-regulation (Alexandrov et al., 2013).
Promotion of amyloid β oligomerization under Al exposure may also play a crucial role in Al-associated Alzheimer’s disease. Pioneer studies by Exley et al. (1993) and Kawahara et al. (1994) demonstrated direct interaction between Al and amyloid β with increasing aggregation of the latter. Later studies have unraveled the particular features of Al-amyloid β interaction and its effects on the protein structure (Narayan et al., 2013; Turner et al., 2019). Particularly, Al(III) is capable of inducing β-sheet formation and subsequent aggregation of Aβ40 (Zhang et al., 2019). Similar effect was observed for Aβ42 with Al(III) and Fe(II)/Fe(III) being more active promoters of Aβ42 aggregation than Cu(II) and Zn(II) (House et al., 2004).
In addition to modulation of Aβ production and aggregation, Al was also shown to be involved in induction of tau hyperphosphorylation (El-Sebae et al., 1993). Correspondingly, d-galactose and Al chloride treatment were shown to increase phosphorylated tau deposition in brain regions (Chiroma et al., 2018). Al oxide nanoparticles directly interact with the hydrophilic residues of tau protein resulting in cytotoxicity in in SH-SY5Y cells (Kermani et al., 2018). Neurotoxic effect of Al and d-galactose were also shown to be dependent on GSK-3β activity (Chiroma et al., 2019), that is known to play a significant role in tau hyperphosphorylation in AD and cerebral ischemia (Culbreth and Aschner, 2018). In turn, reducing GSK-3β activity may be involved in decreasing Aβ production and tau phosphorylation in Al-exposed PC12 cells (Huang et al., 2017).
However, certain negative findings indicate a lack of Al(III) impact on amyloid β and tau accumulation in AβPP and AβPP/tau transgenic mice (Akiyama et al., 2012). Although being somewhat contradictory to other studies, these observations may be indicative of complex mechanisms of Al neurotoxicity involving a wide variety of mechanisms.
8.2. α-Synuclein
The earlier demonstrated association between Al exposure and PD may be at least partially mediated by the interplay between Al(III) and α-synuclein. Particularly, Al treatment was shown to increase α-synuclein fibril formation more effectively than Cu(II), Fe(III), Co(III), and Mn(III) (Uversky et al., 2001). Correspondingly, knockdown of α-synuclein expression in PC12 cells was shown to ameliorate Al-maltolate toxicity, being indicative of the essential role of α-synuclein in Al neurotoxicity (Saberzadeh et al., 2016). In addition, Al(III) was shown to possess synergistic effect on tau oligomer formation with GSK-3β, as well as to promote tau coaggregation with α-synuclein (Nübling et al., 2012).
9. Altered neurotransmitter metabolism
Adverse neurological effects of Al exposure are at least partially mediated by interference of Al with neurotransmitter metabolism and signal transduction. Although the particular mechanisms are still unknown, the existing data clearly demonstrate that Al exposure has a significant impact on glutamatergic, gabaergic, cholinergic, serotoninergic and dopaminergic neurotransmission (Gonçalves and Silva, 2007).
9.1. Glutamatergic-gabaergic systems
The majority of the existing studies were devoted to investigation of the impact of Al on glutamatergic mechanisms. Al exposure was shown to reduce NMDA, AMPA, and glutamate-mediated currents in hippocampal neurons (Platt et al., 1994). Al exposure was shown to reduce hippocampal expression of AMPA receptor subunits GluR-1 and GluR-2 (Song et al., 2013, 2014) that may be at least partially mediated by modulation of Akt/GSK-3β Pathway (Zhang et al., 2016a,b,c). A dose-dependent hippocampal NMDA receptor (NMDAR) expression was observed in orally exposed rats (Jin et al., 2010) with NMDAR 1A and NMDAR 2A/B subunits affected (Yuan et al., 2011). Altered NMDAR expression was also associated with reduced PLC expression (Jin et al., 2010). Al was also shown to aggravate diabetes-associated alteration of neuroblast differentiation and NMDAR signaling (Nam et al., 2019). At the same time, the use of NMDAR antagonist partially reversed adverse effects of Al in primary neural cultures, indicative of excitotoxic component in Al toxicity (Atterwill et al., 1996). It is also proposed that Al exposure may alter ability of astrocytes to protect neurons from excitotoxic effect of high glutamate levels (Sass et al., 1993).
Al also induced an increase in hippocampal and cortical glutamine levels through increase in glutamine-synthetase (Struys-Ponsar et al., 2002) and reduced glutaminase activity in astrocytes (Zielke et al., 1993), whereas glutamate accumulation was reduced (Struys-Ponsar et al., 2000). Metabolomic study involving HT-29 cells also revealed a significant Al-induced decrease in intracellular glutamate levels (Yu et al., 2019a,b). In agreement, down-regulation of glutamate-NO-cGMP pathway in neurons under Al treatment was observed both in vitro and in vivo (Canales et al., 2001).
At the same time, i.p. Al administration resulted in a significant increase in glutamate levels in thalamus, hippocampus, and cerebellum along with elevation of glutamate alpha-decarboxylase activity, whereas GABA transaminase was found to be reduced (Nayak and Chatterjee, 2001). Aluminum was shown to have a biphasic effect on GABA-evoked currents by potentiation observed at lower levels (<100μM) and reduction at higher concentration (≥300μM) (Trombley, 1998). Modulatory effect of Al on GABA transport may be mediated through Ca2+/ calmodulin/calcineurin pathway (Cordeiro et al., 2003).
9.2. Cholinergic system
Cholinergic system was shown to be highly vulnerable in response to Al exposure (Yellamma et al., 2010). Al exposure was shown to reduce cerebral acetylcholinesterase (AChE) activity (Sharma et al., 2013a,b) with a biphasic response characterized by an increase in enzyme activity at shorter periods of exposure following by a subsequent depression (Kumar, 1998). Perinatal Al exposure resulted in a significant decrease in cerebellar AChE activity in the offspring (Ghorbel et al., 2016). Expression of choline acetyltransferase and the subsequent acetylcholine synthesis was also found to be reduced in response to Al exposure even despite substrate availability (Farhat et al., 2017). At the same time, certain studies revealed an Al-induced increase in brain AChE activity (Khan et al., 2013). Applying whole-cell patch clamp technique to isolated rat trigeminal ganglion neurons, Hu et al. (2007) observed that Al3+ potentiated nicotine-evoked inward currents in a concentration-dependent manner. The authors concluded that the enhanced function of nAChR induced by Al might underlie the neurological alteration induced by Al (Hu et al., 2007).
Moreover, Al exposure is also known to be associated with reduced binding activity of M1 muscarinic acetylcholine receptors (M1AChR) (Harkany et al., 1996) as well as nicotinic acetylcholine receptor (α7, α4 and β2 nAChR) gene expression (Farhat et al., 2019). Importantly, this reduction in nicotinic receptor gene expression is associated with severen neurodegeneration and impaired hippocampus dependent learning in mice (Farhat et al., 2019).
Cholinergic dysfunction may also occur from Al-dependent oxidative stress-induced increase in choline synaptic uptake (Amador et al., 2001). Correspondingly, the use of NOS inhibitors demonstrated that altered cholinergic neurotransmission under Al exposure may be at least partially mediated by disturbances in NO generation (Stevanović et al., 2010).
9.3. Dopaminergic system
Dopaminergic neurotransmission was found to be significantly inhibited by Al exposure (Laabbar et al., 2019). Correspondingly, cerebral dopamine levels were found to be significantly reduced in Al-exposed animals (Bhalla et al., 2010a,b; Singla and Dhawan, 2017). Dopamine synthesis in striatal synaptosomes was also found to be reduced under Al exposure (Pavandi et al., 2014). In addition, Al reduced dopamine D1-like and D2-like receptor expression predominantly in brain cortex and rostral striatum (Kim et al., 2007). Despite these interactions of Al with the dopaminergic system, results of a 36-year multicenter study suggest that aluminum’s main toxicity is associated with Alzheimer’s disease, Down’s syndrome and dialysis dementia syndrome, but not Parkinson’s disease or other neurological disorders (Lukiw et al., 2019)
9.4. Serotoninergic system
Certain studies demonstrated that Al exposure may differentially affect serotonin levels (Kumar, 2002; Ravi et al., 2000; Said and Abd Rabo, 2017) and 5-HT2C receptor reactivity (Brus et al., 1997) in brain regions. Exposure to Al was also shown to reduce circulating serotonin levels (Zhang et al., 2016a,b,c). Interestingly, however, a recent study concludes that Al-induced depressive-like behavior in rats is due to activation of hippocampal IL-1β/JNK signaling pathway, resulting in neuronal death in this region (Zhang et al., 2020).
10. Treatment strategies
Due to the revealed high potential of Al as neurotoxic agent as well as its role in development of neurodegenerative and neurodevelopmental disorders, multiple studies aimed at investigation of the potential protective strategies. Due to the role of oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and endoplasmic reticulum stress in Al neurotoxicity, the majority of protective substances are expected to mediate their effect through antioxidant and anti-inflammatory activity. However, other more specific mechanisms may also mediate neuroprotective effects. The existing protective agents may be mechanistically divided into the following groups: (i) chelators; (ii) trace elements; (iii) amino acids; (iv) polyphenols; (v) polyphenol-rich phytoextracts; (vi) drugs and other agents.
10.1. Chelation therapy
Chelation therapy in treatment of Al toxicity aims to reduce metal bioavailability and facilitates its excretion from the organism, thus preventing its toxic effects. The majority of prior studies demonstrate effective chelation of Al using iron chelator desferrioxamine or deferoxamine (DFO) (Kumar and Gill, 2014). Particularly, DFO was shown to prevent Al-induced oxidative damage to brain proteins (Sivakumar et al., 2012). Ethylene diamine tetraacetic acid (EDTA) (Fulgenzi et al., 2015) and N(2-hydroxyethyl) ethylenediamine triacetic acid (HEDTA) (Shrivastava, 2012) were also shown to be effective in reducing Al neurotoxicity. At the same time, comparative analysis of efficiency of other existing Al-chelating agents (Crisponi et al., 2012) in treatment of Al neurotoxicity is highly required.
10.2. Essential trace elements
The existing data demonstrate that silicon (Si), selenium (Se), and zinc (Zn) may possess neuroprotective effect against Al toxicity.
Epidemiological studies demonstrated that increased silicon intake may prevent adverse neurological effects of Al (Davenward et al., 2013; Nielsen, 2014) through its direct interaction with Al ion forming aluminosilicate thus reducing Al bioavailability and toxicity (Domingo et al., 2011). Particularly, increased Si intake was shown to reduce brain Al accumulation in mice (Granero et al., 2004).
Being a structural component of selenoproteins including glutathione peroxidase, protective effects of Se against Al neurotoxicity may be mediated by its antioxidant activity. Particularly, Se treatment significantly reversed Al-induced inhibition of catalase and glutathione reductase activity, as well as reduction of GSH levels. These effects were also accompanied by improved brain morphology, muscle strength, locomotion, and acetylcholinesterase activity (Lakshmi et al., 2015). In addition, neuroprotective effects of Se under Al exposure were shown to involve decreased inflammatory response and improvement of NO signaling (Cao et al., 2018).
Zinc was shown to ameliorate adverse effects of Al exposure on brain morphology and redox status, dopamine and serotonin levels, as well as acetylcholinesterase activity (Lu et al., 2013). Particularly, zinc significantly increased total and reduced glutathione levels, as well as improved activity of antioxidant enzymes and metallothionein levels along with reversal of Al-induced neurodegeneration (Singla and Dhawan, 2014). Improved redox status was also associated with lower expression of redox-sensitive transcription factor NF-κB (Singla and Dhawan, 2013). The authors also demonstrated antiapoptotic effect of Zn in Al-exposed animals through reduction of proapoptotic protein expression including Bax, Apaf-1, caspases 3, 6,7, 8, 9 and improvement of cerebral Bcl2 levels (Singla and Dhawan, 2015).
Certain other trace elements were shown to be effective against Al neurotoxicity. Specifically, lithium treatment ameliorated adverse effects of Al on brain ultrastructure (Bhalla et al., 2010a), acetylcholinesterase and monoamine oxidase activity, as well as brain dopamine and serotonin levels (Bhalla et al., 2010b). Another study demonstrated preventive effects of boric acid on neuronal ultrastructure under Al exposure (Colak et al., 2011).
10.3. Polyphenols
Polyphenols represent a wide group of phytochemicals possessing antioxidant and anti-inflammatory effects thus being used as the potential agents in treatment of Al neurotoxicity. Particularly, quercetin was shown to increase cerebral antioxidant enzyme gene expression (Ali et al., 2014) and improve brain mitochondrial biogenesis and function in Al-exposed animals (Sharma et al., 2015). In parallel with improvement of redox status mangiferin (Kasbe et al., 2015) and hesperidin (Jangra et al., 2015) ameliorated Al-induced neuroinflammation, whereas fisetin possessed antiapoptotic effect and reduced Aβ aggregation (Prakash and Sudhandiran, 2015). Naringenin (Haider et al., 2020) and curcumin (Kakkar and Kaur, 2011) were capable of restoration of acetylcholinesterase activity affected by Al.
Significant neuroprotective effect under Al toxicity was observed for phenolic acids. Particularly, chlorogenic acid was shown to improve redox status and antioxidant enzyme activity through up-regulation of Nrf2-ARE signaling pathway in Al-exposed hippocampal neurons (Wang et al., 2018). Adverse effects of Al treatment on cerebral AChE activity were ameliorated by caffeic (Khan et al., 2013) and syringic (Zhao et al., 2020) acids, whereas the latter also reduced neuroinflammation.
Along with antioxidant and anti-inflammatory effect of the phytochemicals, neuroprotective activity may be at least partially attributed to direct interaction with metal ion. Particularly, it has been demonstrated that polyphenols may bind Al(III) thus reducing its bioavailability (Zhang et al., 2016a,b,c). The role of Al chelation in treatment of neurotoxicity was clearly demonstrated for chlorogenic acid where it increased metal excretion and reduced its accumulation in hippocampus (Wang et al., 2018).
10.4. Phytochemical-rich plants and phytoextracts
Due to clearly demonstrated protective effect of particular phytochemicals (polyphenols) against Al neurotoxicity, the potential efficiency of polyphenol-rich plant extracts was also investigated. Specifically, neuroprotective, antioxidant, and anti-inflammatory effects were demonstrated for green (Jelenković et al., 2014) and black tea (Mathiyazahan et al., 2015), Allium cepa (Singh and Goel, 2015), grape (Lakshmi et al., 2014), Ginkgo biloba (Verma et al., 2020), and saffron (Linardaki et al., 2013) to name a few. The growing body of evidence demonstrate the usefulness of ethnobotanical species. However, further studies are required to characterize the particular mechanisms and active agents contributing to neuroprotective effect of phytoextracts under Al exposure.
10.5. Other agents
A variety of agents with different mechanism of action were also shown to be effective against Al neurotoxicity including melatonin (Sadek et al., 2019), anti-inflammatory drugs (e.g., ibuprofen) (Jamil et al., 2016), lipoic acid (Al-Otaibi et al., 2018), N-acetylcysteine (Prakash and Kumar, 2009), etc. Combined therapy may be also considered as a perspective approach due to the use of agents with different mechanism of biological activity (Kumar and Gill, 2014).
11. Conclusion
The growing body of data demonstrate the potential role of Al exposure and its neurotoxicity as contributors to a wide spectrum of neurodegenerative and neurodevelopmental disorders. The underlying mechanisms of Al(III) neurotoxicity were shown to involve oxidative and endoplasmic reticulum stress, mitochondrial dysfunction, inflammation, cell death, interaction with Aβ and α-synuclein, cytoskeletal abnormalities as well as alteration of synaptic plasticity and signal transduction through interference with neurotransmitter systems. Targeting these mechanisms at different stages may be beneficial for treatment of Al neurotoxicity and associated diseases. However, certain contradictions regarding the mechanisms and the overall effects of Al exposure in vivo and in vitro exist. The latter may at least partially arise from the difference in analytical methodology, whereas development of precise and validated methods including speciation analysis and imaging techniques would significantly contribute to understanding of biological effects of Al. Further studies are required to highlight the intimate mechanisms involved in Al neurotoxicity, as well as its particular contribution to neurological disorders for subsequent development of effective therapeutic strategies.
Acknowledgments
The study was performed with the support of the Russian Ministry of Science and Higher Education, Project No. 0856-2020-0008. M.A. was supported in part by grants from the National Institute of Environmental Health Sciences (NIEHS) R01ES07331 and R01ES10563.
References
- Abad MA, Enguita M, DeGregorio-Rocasolano N, Ferrer I, Trullas R, 2006. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-β and is overexpressed in dystrophic neurites in Alzheimer’s brain. J. Neurosci 26 (49), 12735–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed GA, Khalil SK, Abbas L, Sherif HH, Abdel-Rahman EA, Saber SH, Ali SS, 2020a. ATR-IR and EPR spectroscopy for detecting the alterations in cortical synaptosomes induced by aluminium stress. Spectrochim. Acta A Mol. Biomol. Spectrosc 228, 117535. [DOI] [PubMed] [Google Scholar]
- Ahmed GAR, Khalil SK, El Hotaby W, Abbas L, Farrag ARH, Aal WEA, Hassan MH, 2020b. ATR-IR and EPR spectroscopy for following the membrane restoration of isolated cortical synaptosomes in aluminium-induced Alzheimer’s disease–Like rat model. Chem. Phys. Lipids 231, 104931. [DOI] [PubMed] [Google Scholar]
- Akinrinade ID, Memudu AE, Ogundele OM, 2015a. Fluoride and aluminium disturb neuronal morphology, transport functions, cholinesterase, lysosomal and cell cycle activities. Pathophysiology. 22 (2), 105–115. [DOI] [PubMed] [Google Scholar]
- Akinrinade ID, Memudu AE, Ogundele OM, Ajetunmobi OI, 2015b. Interplay of glia activation and oxidative stress formation in fluoride and aluminium exposure. Pathophysiology. 22 (1), 39–48. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Hosokawa M, Kametani F, Kondo H, Chiba M, Fukushima M, Tabira T, 2012. Long-term oral intake of aluminium or zinc does not accelerate Alzheimer pathology in AβPP and AβPP/tau transgenic mice. Neuropathology. 32 (4), 390–397. [DOI] [PubMed] [Google Scholar]
- Alawdi SH, El-Denshary ES, Safar MM, Eidi H, David MO, Abdel-Wahhab MA, 2017. Neuroprotective effect of nanodiamond in Alzheimer’s disease rat model: a pivotal role for modulating NF-κB and STAT3 signaling. Mol. Neurobiol 54 (3), 1906–1918. [DOI] [PubMed] [Google Scholar]
- Alexandrov PN, Zhao Y, Jones BM, Bhattacharjee S, Lukiw WJ, 2013. Expression of the phagocytosis-essential protein TREM2 is down-regulated by an aluminum-induced miRNA-34a in a murine microglial cell line. J. Inorg. Biochem 128, 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov PN, Pogue AI, Lukiw WJ, 2018. Synergism in aluminum and mercury neurotoxicity. IFNM. 5 (3). 10.15761/IFNM.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HA, Afifi M, Abdelazim AM, Mosleh YY, 2014. Quercetin and omega 3 ameliorate oxidative stress induced by aluminium chloride in the brain. J. Mol. Neurosci 53 (4), 654–660. [DOI] [PubMed] [Google Scholar]
- Al-Olayan EM, El-Khadragy MF, Abdel Moneim AE, 2015. The protective properties of melatonin against aluminium-induced neuronal injury. Int. J. Exp. Pathol 96 (3), 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Otaibi SS, Arafah MM, Sharma B, Alhomida AS, Siddiqi NJ, 2018. Synergistic effect of Quercetin and α-Lipoic acid on aluminium chloride induced neurotoxicity in rats. J. Toxicol 2018, 2817036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador FC, Santos MS, Oliveira CR, 2001. Lipid peroxidation and aluminium effects on the cholinergic system in nerve terminals. Neurotox. Res 3 (3), 223–233. [DOI] [PubMed] [Google Scholar]
- Anand P, Nehru B, 2006. Alterations in glutathione system in adult and pup rat brains following chronic aluminum exposure. Indian J. Occup. Environ. Med 10 (3), 128. [Google Scholar]
- Arab-Nozari M, Zamani E, Latifi A, Shaki F, 2019. Mitochondrial toxicity of aluminium nanoparticles in comparison to its ionic form on isolated rat brain mitochondria. Bratisl. Lek. Listy 120 (7), 516–522. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Ezomo OF, Meshitsuka S, 2011. Gene expression in primary cultured astrocytes affected by aluminum: alteration of chaperons involved in protein folding. Environ. Health Prev. Med 16 (1), 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterwill CK, Johnston HB, Thomas SM, 1996. Reversal of aluminium-induced metabolic changes in primary rat midbrain neural cultures by the NMDA antagonist MK-801. Toxicol. In Vitro 10 (5), 631–635. [DOI] [PubMed] [Google Scholar]
- Bhalla P, Dhawan DK, 2009. Protective role of lithium in ameliorating the aluminium-induced oxidative stress and histological changes in rat brain. Cell. Mol. Neurobiol 29 (4), 513–521. [DOI] [PubMed] [Google Scholar]
- Bhalla P, Singla N, Dhawan DK, 2010a. Potential of lithium to reduce aluminium-induced cytotoxic effects in rat brain. Biometals. 23 (2), 197–206. [DOI] [PubMed] [Google Scholar]
- Bhalla P, Garg ML, Dhawan DK, 2010b. Protective role of lithium during aluminium-induced neurotoxicity. Neurochem. Int 56 (2), 256–262. [DOI] [PubMed] [Google Scholar]
- Bharathi MD, Justin-Thenmozhi A, Manivasagam T, Rather MA, Babu CS, Essa MM, Guillemin GJ, 2019. Amelioration of aluminum maltolate-induced inflammation and endoplasmic reticulum stress-mediated apoptosis by tannoid principles of emblica officinalis in neuronal cellular model. Neurotox. Res 35 (2), 318–330. [DOI] [PubMed] [Google Scholar]
- Bihaqi SW, Sharma M, Singh AP, Tiwari M, 2009. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J. Ethnopharmacol 124 (3), 409–415. [DOI] [PubMed] [Google Scholar]
- Blaylock RL, 2012. Aluminum induced immunoexcitotoxicity in neurodevelopmental and neurodegenerative disorders. Curr. Inorg. Chem 2 (1), 46–53. [Google Scholar]
- Bondy SC, Liu D, Guo-Ross S, 1998. Aluminum treatment induces nitric oxide synthase in the rat brain. Neurochem. Int 33 (1), 51–54. [DOI] [PubMed] [Google Scholar]
- Brough D, Jouhara H, 2020. The aluminium industry: a review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofl, 100007. [Google Scholar]
- Brus R, Szkilnik R, Popieluch I, Kostrzewa RM, Mengel K, 1997. Effect of aluminium exposure on central serotonine and muscarine receptors reactivity in rats. Med. Sci. Monit 3 (5), BR631–BR636. [Google Scholar]
- Çabus N, Oğuz EO, Tufan AÇ, Adıgüzel E, 2015. A histological study of toxic effects of aluminium sulfate on rat hippocampus. Biotech. Histochem 90 (2), 132–139. [DOI] [PubMed] [Google Scholar]
- Campbell A, Yang EY, Tsai-Turton M, Bondy SC, 2002. Pro-inflammatory effects of aluminum in human glioblastoma cells. Brain Res. 933 (1), 60–65. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Corbalán R, Montoliu C, Llansola M, Monfort P, Erceg S, Felipo V, 2001. Aluminium impairs the glutamate-nitric oxide-cGMP pathway in cultured neurons and in rat brain in vivo: molecular mechanisms and implications for neuropathology. J. Inorg. Biochem 87 (1–2), 63–69. [DOI] [PubMed] [Google Scholar]
- Cao Z, Yang X, Zhang H, Wang H, Huang W, Xu F, Li Y, 2016. Aluminum chloride induces neuroinflammation, loss of neuronal dendritic spine and cognition impairment in developing rat. Chemosphere. 151, 289–295. [DOI] [PubMed] [Google Scholar]
- Cao C, Li X, Qin L, Luo J, Zhang M, Ou Z, Wang K, 2018. High Selenium Yeast mitigates aluminum-induced cerebral inflammation by increasing oxidative stress and blocking NO production. Biometals. 31 (5), 835–843. [DOI] [PubMed] [Google Scholar]
- Castorina A, Tiralongo A, Giunta S, Carnazza ML, Scapagnini G, D’Agata V, 2010. Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of ß-amyloid toxicity. Cell Biol. Toxicol 26 (4), 367–377. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang M, Ruan D, She J, 2002. Early chronic aluminium exposure impairs long-term potentiation and depression to the rat dentate gyrus in vivo. Neuroscience. 112 (4), 879–887. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Cheng HM, Wang DC, Hung HS, 2011. Nonlethal aluminum maltolate can reduce brain-derived neurotrophic factor-induced Arc expression through interrupting the ERK signaling in SH-SY5Y neuroblastoma cells. Toxicol. Lett 200 (1–2), 67–76. [DOI] [PubMed] [Google Scholar]
- Cheng L, Liang R, Li Z, Ren J, Yang S, Bai J, Niu Q, Yu H, Zhang H, Xia N, Liu H, 2020. Aluminum maltolate triggers ferroptosis in neurons: mechanism of action. Toxicol. Mech. Methods, 1–10. [DOI] [PubMed] [Google Scholar]
- Chiroma SM, Moklas MAM, Taib CNM, Baharuldin MTH, Amon Z, 2018. D-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed. Pharmacother 103, 1602–1608. [DOI] [PubMed] [Google Scholar]
- Chiroma SM, Baharuldin MTH, Mat Taib CN, Amom Z, Jagadeesan S, Ilham Adenan M, Moklas MAM, 2019. Centella asiatica protects d-galactose/AlCl3 mediated Alzheimer’s disease-like rats via PP2A/GSK-3β signaling pathway in their Hippocampus. Int. J. Mol. Sci 20 (8), 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero S, Herrup K, 2005. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci 25 (42), 9658–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak S, Geyikoğlu F, Keles ON, Türkez H, Topal A, Unal B, 2011. The neuroprotective role of boric acid on aluminum chloride-induced neurotoxicity. Toxicol. Ind. Health 27 (8), 700–710. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Silva VS, Oliveira CR, Goncalves PP, 2003. Aluminium-induced impairment of Ca2+ modulatory action on GABA transport in brain cortex nerve terminals. J. Inorg. Biochem 97 (1), 132–142. [DOI] [PubMed] [Google Scholar]
- Crisponi G, Nurchi VM, Faa G, Remelli M, 2011. Human diseases related to aluminium overload. Monatsh. Chem 142 (4), 331. [Google Scholar]
- Crisponi G, Nurchi VM, Bertolasi V, Remelli M, Faa G, 2012. Chelating agents for human diseases related to aluminium overload. Coord. Chem. Rev 256 (1–2), 89–104. [Google Scholar]
- Crisponi G, Fanni D, Gerosa C, Nemolato S, Nurchi VM, Crespo-Alonso M, Faa G, 2013. The meaning of aluminium exposure on human health and aluminium-related diseases. Biomol. Concepts 4 (1), 77–87. [DOI] [PubMed] [Google Scholar]
- Culbreth M, Aschner M, 2018. GSK-3β, a double-edged sword in Nrf2 regulation: implications for neurological dysfunction and disease. F1000Research 7, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD, Mannello F, Exley C, 2013. Aluminium and breast cancer: sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J. Inorg. Biochem 128, 257–261. [DOI] [PubMed] [Google Scholar]
- Davenward S, Bentham P, Wright J, Crome P, Job D, Polwart A, Exley C, 2013. Silicon-rich mineral water as a non-invasive test of the ‘aluminum hypothesis’ in Alzheimer’s disease. J. Alzheimers Dis 33 (2), 423–430. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Gómez M, Colomina MT, 2011. Oral silicon supplementation: an effective therapy for preventing oral aluminum absorption and retention in mammals. Nutr. Rev 69 (1), 41–51. [DOI] [PubMed] [Google Scholar]
- Drago D, Cavaliere A, Mascetra N, Ciavardelli D, Di Ilio C, Zatta P, Sensi SL, 2008. Aluminum modulates effects of β Amyloid1–42 on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer’s disease. Rejuvenation Res. 11 (5), 861–871. [DOI] [PubMed] [Google Scholar]
- Dua R, Gill KD, 2004. Effect of aluminium phosphide exposure on kinetic properties of cytochrome oxidase and mitochondrial energy metabolism in rat brain. Biochim. Biophys. Acta Gen. Subj 1674 (1), 4–11. [DOI] [PubMed] [Google Scholar]
- El-Sebae AH, Abdel-Ghanv ME, Shalloway D, Zeid MA, Blancato J, Saleh MA, 1993. Aluminum interaction with human brain tau protein phosphorylation by various kinases. J. Environ. Sci. Health B 28 (6), 763–777. [DOI] [PubMed] [Google Scholar]
- Exley C, 2005. The aluminium-amyloid cascade hypothesis and Alzheimer’s disease. In: Alzheimer’s Disease. Springer, Boston, MA, pp. 225–234. [DOI] [PubMed] [Google Scholar]
- Exley C, 2012. The coordination chemistry of aluminium in neurodegenerative disease. Coord. Chem. Rev 256 (19–20), 2142–2146. [Google Scholar]
- Exley C, 2013. Human exposure to aluminium. Environ. Sci. Process Impacts 15 (10), 1807–1816. [DOI] [PubMed] [Google Scholar]
- Exley C, 2014. What is the risk of aluminium as a neurotoxin? Expert Rev. Neurother 14 (6), 589–591. [DOI] [PubMed] [Google Scholar]
- Exley C, Clarkson E, 2020. Aluminium in human brain tissue from donors without neurodegenerative disease: a comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep 10 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley C, Price NC, Kelly SM, Birchall JD, 1993. An interaction of β-amyloid with aluminium in vitro. FEBS Lett. 324 (3), 293–295. [DOI] [PubMed] [Google Scholar]
- Farhat SM, Mahboob A, Iqbal G, Ahmed T, 2017. Aluminum-induced cholinergic deficits in different brain parts and its implications on sociability and cognitive functions in mouse. Biol. Trace Elem. Res 177 (1), 115–121. [DOI] [PubMed] [Google Scholar]
- Farhat SM, Mahboob A, Ahmed T, 2019. Oral exposure to aluminum leads to reduced nicotinic acetylcholine receptor gene expression, severe neurodegeneration and impaired hippocampus dependent learning in mice. Drug Chem. Toxicol, 1–9. 10.1080/01480545.2019.1587452. [DOI] [PubMed] [Google Scholar]
- Fulgenzi A, De Giuseppe R, Bamonti F, Vietti D, Ferrero ME, 2015. Efficacy of chelation therapy to remove aluminium intoxication. J. Inorg. Biochem 152, 214–218. [DOI] [PubMed] [Google Scholar]
- Gandolfi L, Stella MP, Zambenedetti P, Zatta P, 1998. Aluminum alters intracellular calcium homeostasis in vitro. Biochim. Biophys. Acta Mol. Basis Dis 1406 (3), 315–320. [DOI] [PubMed] [Google Scholar]
- Gatta V, Drago D, Fincati K, Valenti MT, Dalle Carbonare L, Sensi SL, Zatta P, 2011. Microarray analysis on human neuroblastoma cells exposed to aluminum, β 1–42-amyloid or the β 1–42-amyloid aluminum complex. PloS one. 6 (1), e15965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbel I, Amara IB, Ktari N, Elwej A, Boudawara O, Boudawara T, Zeghal N, 2016. Aluminium and acrylamide disrupt cerebellum redox states, cholinergic function and membrane-bound ATPase in adult rats and their offspring. Biol. Trace Elem. Res 174 (2), 335–346. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Herman MM, DeWitt DA, Forbes MS, Savory J, 2001. Aβ (1–42) and aluminum induce stress in the endoplasmic reticulum in rabbit hippocampus, involving nuclear translocation of gadd 153 and NF-κB. Mol. Brain Res 96 (1–2), 30–38. [DOI] [PubMed] [Google Scholar]
- Gonçalves PP, Silva VS, 2007. Does neurotransmission impairment accompany aluminium neurotoxicity? J. Inorg. Biochem 101 (9), 1291–1338. [DOI] [PubMed] [Google Scholar]
- Goullé JP, Grangeot-Keros L, 2020. Aluminum and vaccines: current state of knowledge. Med. Mal. Infect 50 (1), 16–21. [DOI] [PubMed] [Google Scholar]
- Granero S, Vicente M, Aguilar V, Martínez-Para MC, Domingo JL, 2004. Effects of beer as a source of dietary silicon on aluminum absorption and retention in mice. Trace Elem. Electroly 21 (1), 28–32. [Google Scholar]
- Guo GW, Liang YX, 2001. Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Res. 888 (2), 221–226. [DOI] [PubMed] [Google Scholar]
- Guo Y, Lei W, Wang J, Hu X, Wei Y, Ji C, Yang J, 2016. Misoprostol reverse hippocampal neuron cyclooxygenase-2 downstream signaling imbalance in aluminum-overload rats. Curr. Alzheimer Res 13 (9), 1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, Dostert C, 2015. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PloS one. 10 (6), e0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Liaquat L, Ahmad S, Batool Z, Siddiqui RA, Tabassum S, Naz N, 2020. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. Plos one. 15 (1), e0227631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YX, Li MQ, Zhang JS, Zhang QL, Jiao X, Ji XL, Niu Q, 2019. Aluminum-induced “mixed” cell death in mice cerebral tissue and potential intervention. Neurotox. Res, 1–12. [DOI] [PubMed] [Google Scholar]
- Harkany T, Lengyel Z, Kasa P, Gulya K, 1996. Chronic aluminum treatment results in aluminum deposits and affects Ml muscarinic receptors in rat brain. Neurobiology. 4 (1–2), 35–43. [PubMed] [Google Scholar]
- House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C, 2004. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Aβ 42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J. Alzheimers Dis 6 (3), 291–301. [DOI] [PubMed] [Google Scholar]
- Hu WP, Li XM, Chen JG, Li ZW, 2007. Potentiation of the nicotinic acetylcholine receptor by aluminum in mammalian neurons. Neuroscience. 149 (1), 1–6. [DOI] [PubMed] [Google Scholar]
- Huang W, Cheng P, Yu K, Han Y, Song M, Li Y, 2017. Hyperforin attenuates aluminum-induced Aβ production and Tau phosphorylation via regulating Akt/GSK-3β signaling pathway in PC12 cells. Biomed. Pharmacother 96, 1–6. [DOI] [PubMed] [Google Scholar]
- Iglesias-González J, Sánchez-Iglesias S, Beiras-Iglesias A, Méndez-Álvarez E, Soto-Otero R, 2017. Effects of aluminium on rat brain mitochondria bioenergetics: an in vitro and in vivo study. Mol. Neurobiol 54 (1), 563–570. [DOI] [PubMed] [Google Scholar]
- Inohana M, Eguchi A, Nakamura M, Nagahara R, Onda N, Nakajima K, Shibutani M, 2018. Developmental exposure to aluminum chloride irreversibly affects postnatal hippocampal neurogenesis involving multiple functions in mice. Toxicol. Sci 164 (1), 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil A, Mahboob A, Ahmed T, 2016. Ibuprofen targets neuronal pentraxins expresion and improves cognitive function in mouse model of AlCl3-induced neurotoxicity. Exp. Ther. Med 11 (2), 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra A, Kasbe P, Pandey SN, Dwivedi S, Gurjar SS, Kwatra M, Sarma N, 2015. Hesperidin and silibinin ameliorate aluminum-induced neurotoxicity: modulation of antioxidants and inflammatory cytokines level in mice hippocampus. Biol. Trace Elem. Res 168 (2), 462–471. [DOI] [PubMed] [Google Scholar]
- Jelenković A, Jovanović MD, Stevanović I, Petronijević N, Bokonjić D,Živković J, Igić R, 2014. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother. Res 28 (1), 82–87. [DOI] [PubMed] [Google Scholar]
- Jia X, Zhang Q, Niu Q, 2014. MAPK signaling pathways involved in aluminum-induced apoptosis and necroptosis in SH-SY5Y cells. J. Hygiene Res 43 (6), 917–922. [PubMed] [Google Scholar]
- Jin CH, Wu SW, Zhou P, Liu QF, Lu XB, Shi LD, Cai Y, 2010. Effect of aluminum on Ca2+ concentration and expression of phospholipase C and NMDA receptor α genes in hippocampus of weaning rats as well as their neural behavior through subchronic exposure. Chin. J. Industr. Hygiene Occup. Dis 28 (9), 648–651. [PubMed] [Google Scholar]
- Jing Y, Wang Z, Song Y, 2004. Quantitative study of aluminum-induced changes in synaptic ultrastructure in rats. Synapse. 52 (4), 292–298. [DOI] [PubMed] [Google Scholar]
- Johnson VJ, Sharma RP, 2003. Aluminum disrupts the pro-inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: possible role in neurodegeneration. Neurotoxicology. 24 (2), 261–268. [DOI] [PubMed] [Google Scholar]
- Johnson VJ, Kim SH, Sharma RP, 2005. Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol. Sci 83 (2), 329–339. [DOI] [PubMed] [Google Scholar]
- Julka D, Gill KD, 1996. Altered calcium homeostasis: a possible mechanism of aluminium-induced neurotoxicity. Biochim. Biophys. Acta Mol. Basis Dis 1315 (1), 47–54. [DOI] [PubMed] [Google Scholar]
- Kakkar V, Kaur IP, 2011. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol 49 (11), 2906–2913. [DOI] [PubMed] [Google Scholar]
- Kasbe P, Jangra A, Lahkar M, 2015. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol 31, 107–112. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Savory J, Herman MM, Carpenter RM, Frankfurter A, Hewitt CD, Wills MR, 1990. Neuronal cytoskeletal lesions induced in the CNS by intraventricular and intravenous aluminium maltol in rabbits. Neuropathol. Appl. Neurobiol 16 (6), 511–528. [DOI] [PubMed] [Google Scholar]
- Kaur A, Gill KD, 2005. Disruption of neuronal calcium homeostasis after chronic aluminium toxicity in rats. Basic Clin. Pharmacol. Toxicol 96 (2), 118–122. [DOI] [PubMed] [Google Scholar]
- Kaur A, Joshi K, Minz RW, Gill KD, 2006. Neurofilament phosphorylation and disruption: a possible mechanism of chronic aluminium toxicity in Wistar rats. Toxicology. 219 (1–3), 1–10. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Muramoto K, Kobayashi K, Mori H, Kuroda Y, 1994. Aluminum promotes the aggregation of Alzheimer′ s Amyloid β-Protein in vitro. Biochem. Biophys. Res. Commun 198 (2), 531–535. [DOI] [PubMed] [Google Scholar]
- Kermani ZR, Haghighi SS, Hajihosseinali S, Fashami AZ, Akbaritouch T, Akhtari K, Falahati M, 2018. Aluminium oxide nanoparticles induce structural changes in tau and cytotoxicity of the neuroblastoma cell line. Int. J. Biol. Macromol, 1140–1148. [DOI] [PubMed] [Google Scholar]
- Keshava R, Vazhayil V, Mitra R, Bhagavatula ID, Gope R, 2019. AlCl 3 causes Fas/Fas-L mediated cell death in the cortex and hippocampus of mouse brain. Int. J. Biosci 12 (3), 21–35. [Google Scholar]
- Khan KA, Kumar N, Nayak PG, Nampoothiri M, Shenoy RR, Krishnadas N, Mudgal J, 2013. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J. Pharm. Pharmacol 65 (12), 1745–1752. [DOI] [PubMed] [Google Scholar]
- Kim S, Nam J, Kim K, 2007. Aluminum exposure decreases dopamine D1 and D2 receptor expression in mouse brain. Hum. Exp. Toxicol 26 (9), 741–746. [DOI] [PubMed] [Google Scholar]
- Klein GL, 2019. Aluminum toxicity to bone: a multisystem effect? Osteoporosis Sarcopenia. 5 (1), 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H, 2017. The health effects of aluminum exposure. Dtsch. Arztebl. Int 114 (39), 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S, 2011. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 34 (11), 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchazhkina OV, Ashcroft AE, Kiss T, Exley C, 2002. The degradation of Aβ 25–35 by the serine protease plasmin is inhibited by aluminium. J. Alzheimers Dis 4 (5), 357–367. [DOI] [PubMed] [Google Scholar]
- Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Rondeau V, 2007. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev 10 (S1), 1–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupińska I, 2020. Aluminium drinking water treatment residuals and their toxic impact on human health. Molecules. 25 (3), 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, 1998. Biphasic effect of aluminium on cholinergic enzyme of rat brain. Neurosci. Lett 248 (2), 121–123. [DOI] [PubMed] [Google Scholar]
- Kumar S, 2002. Aluminium-induced changes in the rat brain serotonin system. Food and chemical toxicology 40 (12), 1875–1880. [DOI] [PubMed] [Google Scholar]
- Kumar V, Gill KD, 2014. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 41, 154–166. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bal A, Gill KD, 2008. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res. 1232, 94–103. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bal A, Gill KD, 2009a. Susceptibility of mitochondrial superoxide dismutase to aluminium induced oxidative damage. Toxicology. 255 (3), 117–123. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bal A, Gill KD, 2009b. Aluminium-induced oxidative DNA damage recognition and cell-cycle disruption in different regions of rat brain. Toxicology. 264 (3), 137–144. [DOI] [PubMed] [Google Scholar]
- Kushkuley J, Metkar S, Chan WK, Lee S, Shea TB, 2010. Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res. 1322, 118–123. [DOI] [PubMed] [Google Scholar]
- Laabbar W, Elgot A, Elhiba O, Gamrani H, 2019. Curcumin prevents the midbrain dopaminergic innervations and locomotor performance deficiencies resulting from chronic aluminum exposure in rat. J. Chem. Neuroanat 100, 101654. [DOI] [PubMed] [Google Scholar]
- Lakshmi BVS, Sudhakar M, Anisha M, 2014. Neuroprotective role of hydroalcoholic extract of Vitis vinifera against aluminium-induced oxidative stress in rat brain. Neurotoxicology. 41, 73–79. [DOI] [PubMed] [Google Scholar]
- Lakshmi BVS, Sudhakar M, Prakash KS, 2015. Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: behavioral and biochemical alterations in rats. Biol. Trace Elem. Res 165 (1), 67–74. [DOI] [PubMed] [Google Scholar]
- Li H, Campbell A, Ali SF, Cong P, Bondy SC, 2007. Chronic exposure to low levels of aluminum alters cerebral cell signaling in response to acute MPTP administration. Toxicol. Ind. Health 23 (9), 515–524. [DOI] [PubMed] [Google Scholar]
- Li XB, Zheng H, Zhang ZR, Li M, Huang ZY, Schluesener HJ, Xu SQ, 2009. Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat brains. Nanomedicine. 5 (4), 473–479. [DOI] [PubMed] [Google Scholar]
- Li WQ, Ge CC, Jia ZJ, Liang RF, Niu Q, 2012. Effects of aluminum on the expression of BACE1 proteins and genes in PC12 cells. J. Occup. Environ (4), 3. [Google Scholar]
- Li D, Tomljenovic L, Li Y, Shaw CA, 2017. Subcutaneous injections of aluminum at vaccine adjuvant levels activate innate immune genes in mouse brain that are homologous with biomarkers of autism. J. Inorg. Biochem 177, 39–54. [DOI] [PubMed] [Google Scholar]
- Li H, Xue X, Li Z, Pan B, Hao Y, Niu Q, 2020a. Aluminium-induced synaptic plasticity injury via the PHF8–H3K9me2-BDNF signalling pathway. Chemosphere. 244, 125445. [DOI] [PubMed] [Google Scholar]
- Li H, Xue X, Li L, Li Y, Wang Y, Huang T, Niu Q, 2020b. Aluminum-induced synaptic plasticity impairment via PI3K-Akt-mTOR signaling pathway. Neurotox. Res 37 (4), 996–1008. [DOI] [PubMed] [Google Scholar]
- Liang RF, LI WQ, Hong WANG, Wang JX, Qiao NIU, 2013. Impact of sub-chronic aluminium-maltolate exposure on catabolism of amyloid precursor protein in rats. Biomed. Environ. Sci 26 (6), 445–452. [DOI] [PubMed] [Google Scholar]
- Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M, 2013. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem. Toxicol 52, 163–170. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang W, Fang Y, Yang H, Tian L, Li K, Xi Z, 2020. Neurotoxicity of aluminum oxide nanoparticles and their mechanistic role in dopaminergic neuron injury involving p53-related pathways. J. Hazard. Mater 392, 122312. [DOI] [PubMed] [Google Scholar]
- Lu H, Hu J, Li J, Pang W, Hu Y, Yang H, Jiang Y, 2013. Optimal dose of zinc supplementation for preventing aluminum-induced neurotoxicity in rats. Neural Regen. Res 8 (29), 2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Percy ME, Kruck TP, 2005. Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J. Inorg. Biochem 99 (9), 1895–1898. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Kruck TPA, Percy ME, 2019. Aluminum in neurological disease—a 36 year multicenter study. J. Alzheimers Dis. Parkinsonism 8 (6), 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Niu F, Sun Z, Cao W, Zhang X, Guan D, Xu Y, 2009. Altered expression of Aβ metabolism-associated molecules from d-galactose/AlCl3 induced mouse brain. Mech. Ageing Dev 130 (4), 248–252. [DOI] [PubMed] [Google Scholar]
- Martinez CS, Piagette JT, Escobar AG, Martín Á, Palacios R, Peçanha FM, Salaices M, 2017. Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: a concerted action of NAD (P) H oxidase and COX-2. Toxicology. 390, 10–21. [DOI] [PubMed] [Google Scholar]
- Mathiyazahan DB, Thenmozhi AJ, Manivasagam T, 2015. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: a behavioural, biochemical and molecular approach. J. Funct. Foods 16, 423–435. [Google Scholar]
- Mesole SB, Alfred OO, Yusuf UA, Lukubi L, Ndhlovu D, 2020. Apoptotic inducement of neuronal cells by aluminium chloride and the neuroprotective effect of eugenol in wistar rats. Oxid. Med. Cell Longev 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza A, King A, Troakes C, Exley C, 2017. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol 40, 30–36. [DOI] [PubMed] [Google Scholar]
- Mokhemer SA, El-Tahawy NFG, Rifaai RA, Saber EA, Abd El-Aleem SA, 2020. Comparative study of the effect of chronic aluminium chloride administration on the expression of endothelial nitric oxide synthase in rat brain. Indian J. Public Health 11 (2), 1723–1728. [Google Scholar]
- Mold M, Umar D, King A, Exley C, 2018. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol 46, 76–82. [DOI] [PubMed] [Google Scholar]
- Mold M, Cottle J, King A, Exley C, 2019. Intracellular aluminium in inflammatory and glial cells in cerebral amyloid angiopathy: a case report. Int. J. Environ. Res 16 (8), 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Puri BK, Frye RE, 2017. The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metabol. Brain dis 32 (5), 1335–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moumen R, Ait-Oukhatar N, Bureau F, Fleury C, Bouglé D, Arhan P, Viader F, 2001. Aluminium increases xanthine oxidase activity and disturbs antioxidant status in the rat. J. Trace Elem. Med. Biol 15 (2–3), 89–93. [DOI] [PubMed] [Google Scholar]
- Muma NA, Troncoso JC, Hoffman PN, Koo EH, Price DL, 1988. Aluminum neurotoxicity: altered expression of cytoskeletal genes. Mol. Brain Res 3 (2), 115–121. [DOI] [PubMed] [Google Scholar]
- Nam SM, Yoo DY, Kwon HJ, Kim JW, Jung HY, Kim DW, Yoon YS, 2019. Effects of long-term exposure to aluminum in the hippocampus in the type 2 diabetes model rats. Toxicol. Res 8 (2), 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Krishnarjuna B, Vishwanathan V, Jagadeesh Kumar D, Babu S, Ramanathan KV, Raghothama S, 2013. Does aluminium bind to histidine? An NMR investigation of amyloid β12 and amyloid β16 fragments. Chem. Biol. Drug Des 82 (1), 48–59. [DOI] [PubMed] [Google Scholar]
- Nayak P, Chatterjee AK, 2001. Effects of aluminium exposure on brain glutamate and GABA systems: an experimental study in rats. Food Chem. Toxicol 39 (12), 1285–1289. [DOI] [PubMed] [Google Scholar]
- Nehru B, Anand P, 2005. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J. Trace Elem. Med. Biol 19 (2–3), 203–208. [DOI] [PubMed] [Google Scholar]
- Nehru B, Bhalla P, 2006. Reversal of an aluminium induced alteration in redox status in different regions of rat brain by administration of centrophenoxine. Mol. Cell Biochem 290 (1–2), 185–191. [DOI] [PubMed] [Google Scholar]
- Nielsen FH, 2014. Update on the possible nutritional importance of silicon. J. Trace Elem. Med. Biol 28 (4), 379–382. [DOI] [PubMed] [Google Scholar]
- Niu PY, Niu Q, Zhang QL, Wang LP, He SC, Wu TC, Boscolo P, 2005. Aluminum impairs rat neural cell mitochondria in vitro. Int. J. Immunopathol. Pharmacol 18 (4), 683–689. [DOI] [PubMed] [Google Scholar]
- Nübling G, Bader B, Levin J, Hildebrandt J, Kretzschmar H, Giese A, 2012. Synergistic influence of phosphorylation and metal ions on tau oligomer formation and coaggregation with α-synuclein at the single molecule level. Mol. Neurodegener 7 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro S, Kawahara M, Kuroda Y, Zhang C, Cai Y, Kitajima S, Shirao M, 2000. Glial cells contribute more to iron and aluminum accumulation but are more resistant to oxidative stress than neuronal cells. Biochim. Biophys. Acta Mol. Basis Dis 1502 (3), 405–414. [DOI] [PubMed] [Google Scholar]
- Pan B, Zhou Y, Li H, Li Y, Xue X, Liang L, Niu Q, 2020a. Relationship between occupational aluminium exposure and histone lysine modification through methylation. J. Trace Elem. Med. Biol 61, 126551. [DOI] [PubMed] [Google Scholar]
- Pan B, Li Y, Zhang J, Zhou Y, Li L, Xue X, Niu Q, 2020b. Role of mGluR 1 in synaptic plasticity impairment induced by maltol aluminium in rats. Environ. Toxicol. Pharmacol 78, 103406. [DOI] [PubMed] [Google Scholar]
- Pavandi M, Messripour M, Moshtaghi AA, 2014. Effect of aluminium and copper on dopamine synthesis in striatal synaptosomes of Rat’s brain. Bull. Env. Pharmacol. Life Sci 3, 12–16. [Google Scholar]
- Platt B, Haas H, Büsselberg D, 1994. Aluminium reduces glutamate-activated currents of rat hippocampal neurones. Neuroreport. 5 (17), 2329–2332. [DOI] [PubMed] [Google Scholar]
- Platt B, Fiddler G, Riedel G, Henderson Z, 2001. Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res. Bull 55 (2), 257–267. [DOI] [PubMed] [Google Scholar]
- Pogue AI, Jaber V, Zhao Y, Lukiw WJ, 2017. Systemic inflammation in C57BL/6J mice receiving dietary aluminum sulfate; up-regulation of the pro-inflammatory cytokines IL-6 and TNFα, C-reactive protein (CRP) and miRNA-146a in blood serum. J. Alzheimers Dis. Parkinsonism 7 (6), 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Połeć-Pawlak K, Zambenedetti P, Szpunar J, Łobiński R, Zatta P, 2004. Investigation of the aluminium binding in Al (III)-treated neuroblastoma cells. J. Anal. At. Spectrom 19 (1), 41–45. [Google Scholar]
- Prakash A, Kumar A, 2009. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin. Pharmacol 105 (2), 98–104. [DOI] [PubMed] [Google Scholar]
- Prakash D, Sudhandiran G, 2015. Dietary flavonoid fisetin regulates aluminium chloride-induced neuronal apoptosis in cortex and hippocampus of mice brain. J. Nutr. Biochem 26 (12), 1527–1539. [DOI] [PubMed] [Google Scholar]
- Prema A, Justin Thenmozhi A, Manivasagam T, Mohamed Essa M, Guillemin GJ, 2017. Fenugreek seed powder attenuated aluminum chloride-induced tau pathology, oxidative stress, and inflammation in a rat model of Alzheimer’s disease. J. Alzheimers Dis 60 (s1), S209–S220. [DOI] [PubMed] [Google Scholar]
- Qin X, Li L, Nie X, Niu Q, 2020. Effects of chronic aluminum lactate exposure on neuronal apoptosis and hippocampal synaptic plasticity in rats. Biol. Trace Elem. Res 197 (2), 571–579. [DOI] [PubMed] [Google Scholar]
- Ravi SM, Prabhu BM, Raju TR, Bindu PN, 2000. Long-term effects of postnatal aluminium exposure on acetylcholinesterase activity and biogenic amine neurotransmitters in rat brain. Indian J. Physiol. Pharmacol 44 (4), 473–478. [PubMed] [Google Scholar]
- Reichert KP, Schetinger MRC, Pillat MM, Bottari NB, Palma TV, Gutierres JM, Morsch VM, 2019. Aluminum affects neural phenotype determination of embryonic neural progenitor cells. Arch. Toxicol 93 (9), 2515–2524. [DOI] [PubMed] [Google Scholar]
- Rizvi SHM, Parveen A, Verma AK, Ahmad I, Arshad M, Mahdi AA, 2014. Aluminium induced endoplasmic reticulum stress mediated cell death in SH-SY5Y neuroblastoma cell line is independent of p53. PLoS One. 9 (5), e98409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SHM, Parveen A, Ahmad I, Ahmad I, Verma AK, Arshad M, Mahdi AA, 2016. Aluminum activates PERK-eIF2α signaling and inflammatory proteins in human neuroblastoma SH-SY5Y cells. Biol. Trace Elem. Res 172 (1), 108–119. [DOI] [PubMed] [Google Scholar]
- Saberzadeh J, Arabsolghar R, Takhshid MA, 2016. Alpha synuclein protein is involved in Aluminum-induced cell death and oxidative stress in PC12 cells. Brain Res. 1635, 153–160. [DOI] [PubMed] [Google Scholar]
- Sadek KM, Lebda MA, Abouzed TK, 2019. The possible neuroprotective effects of melatonin in aluminum chloride-induced neurotoxicity via antioxidant pathway and Nrf2 signaling apart from metal chelation. Environ. Sci. Pollut. Res 26 (9), 9174–9183. [DOI] [PubMed] [Google Scholar]
- Said MM, Abd Rabo MM, 2017. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arh. Hig. Rada Toksikol 68 (1), 27–37. [DOI] [PubMed] [Google Scholar]
- Sánchez-Iglesias S, Méndez-Álvarez E, Iglesias-González J, Muñoz-Patiño A, Sánchez-Sellero I, Labandeira-García JL, Soto-Otero R, 2009. Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J. Neurochem 109 (3), 879–888. [DOI] [PubMed] [Google Scholar]
- Sass JB, Ang LC, Juurlink BHJ, 1993. Aluminum pretreatment impairs the ability of astrocytes to protect neurons from glutamate mediated toxicity. Brain Res. 621 (2), 207–214. [DOI] [PubMed] [Google Scholar]
- Savory J, Herman MM, Ghribi O, 2003. Intracellular mechanisms underlying aluminum-induced apoptosis in rabbit brain. J. Inorg. Biochem 97 (1), 151–154. [DOI] [PubMed] [Google Scholar]
- Schmidt PM, Escobar AG, Torres JGD, Martinez CS, Rizzetti DA, Kunz SN, Wiggers GA, 2016. Aluminum exposure for one hour decreases vascular reactivity in conductance and resistance arteries in rats. Toxicol. Appl. Pharmacol 313, 109–118. [DOI] [PubMed] [Google Scholar]
- Sharma DR, Wani WY, Sunkaria A, Kandimalla RJ, Verma D, Cameotra SS, Gill KD, 2013a. Quercetin protects against chronic aluminum-induced oxidative stress and ensuing biochemical, cholinergic, and neurobehavioral impairments in rats. Neurotox. Res 23 (4), 336–357. [DOI] [PubMed] [Google Scholar]
- Sharma DR, Sunkaria A, Wani WY, Sharma RK, Kandimalla RJ, Bal A, Gill KD, 2013b. Aluminium induced oxidative stress results in decreased mitochondrial biogenesis via modulation of PGC-1α expression. Toxicol. Appl. Pharmacol 273 (2), 365–380. [DOI] [PubMed] [Google Scholar]
- Sharma DR, Sunkaria A, Wani WY, Sharma RK, Verma D, Priyanka K, Gill KD, 2015. Quercetin protects against aluminium induced oxidative stress and promotes mitochondrial biogenesis via activation of the PGC-1α signaling pathway. Neurotoxicology. 51, 116–137. [DOI] [PubMed] [Google Scholar]
- Shaw CA, Seneff S, Kette SD, Tomljenovic L, Oller JW, Davidson RM, 2014. Aluminum-induced entropy in biological systems: implications for neurological disease. J. Toxicol 2014, 491316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea TB, Wheeler E, Jung C, 1997. Aluminum inhibits neurofilament assembly, cytoskeletal incorporation, and axonal transport. J. Mol. Neurosci 32 (1–3), 17. [DOI] [PubMed] [Google Scholar]
- Shevtsov PN, Shevtsova EF, Burbaeva GS, 2016. Effect of aluminum, iron, and zinc ions on the assembly of microtubules from brain microtubule proteins. Exp. Biol. Med 161 (4), 451–455. [DOI] [PubMed] [Google Scholar]
- Shih RH, Wang CY, Yang CM, 2015. NF-kappaB signaling pathways in neurological inflammation: a mini review. Frontiers in Molecular Neuroscience 8, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava S, 2012. Combined effect of HEDTA and selenium against aluminum induced oxidative stress in rat brain. J. Trace Elem. Med. Biol 26 (2–3), 210–214. [DOI] [PubMed] [Google Scholar]
- Silva VS, Gonçalves PP, 2003. The inhibitory effect of aluminium on the (Na+/K+) ATPase activity of rat brain cortex synaptosomes. J. Inorg. Biochem 97 (1), 143–150. [DOI] [PubMed] [Google Scholar]
- Singh T, Goel RK, 2015. Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology. 49, 1–7. [DOI] [PubMed] [Google Scholar]
- Singla N, Dhawan DK, 2013. Zinc, a neuroprotective agent against aluminum-induced oxidative DNA injury. Mol. Neurobiol 48 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- Singla N, Dhawan DK, 2014. Zinc modulates aluminium-induced oxidative stress and cellular injury in rat brain. Metallomics. 6 (10), 1941–1950. [DOI] [PubMed] [Google Scholar]
- Singla N, Dhawan DK, 2015. Zinc down regulates Apaf-1-dependent Bax/Bcl-2 mediated caspases activation during aluminium induced neurotoxicity. Biometals. 28 (1), 61–73. [DOI] [PubMed] [Google Scholar]
- Singla N, Dhawan DK, 2017. Zinc improves cognitive and neuronal dysfunction during aluminium-induced neurodegeneration. Mol. Neurobiol 54 (1), 406–422. [DOI] [PubMed] [Google Scholar]
- Sivakumar S, Sivasubramanian J, Raja B, 2012. Aluminium induced structural, metabolic alterations and protective effects of desferrioxamine in the brain tissue of mice: an FTIR study. Spectrochim. Acta A Mol. Biomol. Spectrosc 99, 252–258. [DOI] [PubMed] [Google Scholar]
- Skalnaya MG, Skalny AV, Grabeklis AR, Serebryansky EP, Demidov VA, Tinkov AA, 2018. Hair trace elements in overweight and obese adults in association with metabolic parameters. Biol. Trace Elem. Res 186 (1), 12–20. [DOI] [PubMed] [Google Scholar]
- Skalny AV, Kaminskaya GA, Krekesheva TI, Abikenova SK, Skalnaya MG, Bykov AT, Tinkov AA, 2018. Assessment of hair metal levels in aluminium plant workers using scalp hair ICP-DRC-MS analysis. J. Trace Elem. Med. Biol 50, 658–663. [DOI] [PubMed] [Google Scholar]
- Song J, Liu Y, Wang LP, Niu Q, 2013. Effects of subchronic aluminum exposure on learning and memory and the expression of AMPA receptor in rats. J. Environ. Occup. Med 30, 5–9. [Google Scholar]
- Song J, Ying L, Zhang HF, Zhang QL, Niu Q, 2014. Effects of exposure to aluminum on long-term potentiation and AMPA receptor subunits in rats in vivo. Biomed. Environ. Sci 27 (2), 77–84. [DOI] [PubMed] [Google Scholar]
- Sood PK, Nahar U, Nehru B, 2011. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox. Res 20 (4), 351. [DOI] [PubMed] [Google Scholar]
- Sood PK, Nahar U, Nehru B, 2012. Stress proteins and glial cell functions during chronic aluminium exposures: protective role of curcumin. Neurochem. Res 37 (3), 639–646. [DOI] [PubMed] [Google Scholar]
- Stevanović ID, Jovanović MD, Jelenković A, Ninković M, Đukić M, Stojanović I, Čolić M, 2009. The effect of inhibition of nitric oxide synthase on aluminium-induced toxicity in the rat brain. Gen. Physiol. Biophys 28, 235–242. [PubMed] [Google Scholar]
- Stevanović ID, Jovanović MD, Čolić M, Jelenković A, Bokonjić D, Ninković M, 2010. Nitric oxide synthase inhibitors protect cholinergic neurons against AlCl3 excitotoxicity in the rat brain. Brain Res. Bull 81 (6), 641–646. [DOI] [PubMed] [Google Scholar]
- Struys-Ponsar C, Guillard O, de Aguilar PVDB, 2000. Effects of aluminum exposure on glutamate metabolism: a possible explanation for its toxicity. Exp. Neurol 163 (1), 157–164. [DOI] [PubMed] [Google Scholar]
- Struys-Ponsar C, Guillard O, de Aguilar PVDB, 2002. Effects of Aluminum on Glutamate Metabolism, in: Trace Elements in Man and Animals 10. Springer, Boston, MA, pp. 425–428. [Google Scholar]
- Suárez-Fernández MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernández-Sánchez MT, 1999. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 835 (2), 125–136. [DOI] [PubMed] [Google Scholar]
- Sushma NJ, Sivaiah U, Suraj NJ, Rao KJ, 2006. Aluminium acetate induced oxidative stress in brain of albino mice. J. Pharmacol. Toxicol 1, 579–584. [Google Scholar]
- Syed H, Ikram MF, Yaqinuddin A, Ahmed T, 2015. Cyclooxygenase I and II inhibitors distinctly enhance hippocampal-and cortex-dependent cognitive functions in mice. Mol. Med. Rep 12 (5), 7649–7656. [DOI] [PubMed] [Google Scholar]
- Thenmozhi AJ, Raja TRW, Janakiraman U, Manivasagam T, 2015. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res 40 (4), 767–776. [DOI] [PubMed] [Google Scholar]
- Tietz T, Lenzner A, Kolbaum AE, Zellmer S, Riebeling C, Gürtler R, Merkel S, 2019. Aggregated aluminium exposure: risk assessment for the general population. Arch. Toxicol 93 (12), 3503–3521. [DOI] [PubMed] [Google Scholar]
- Tinkov AA, Skalnaya MG, Aaseth J, Ajsuvakova OP, Aschner M, Skalny AV, 2019. Aluminium levels in hair and urine are associated with overweight and obesity in a non-occupationally exposed population. J. Trace Elem. Med. Biol 56, 139–145. [DOI] [PubMed] [Google Scholar]
- Toimela T, Tähti H, 2004. Mitochondrial viability and apoptosis induced by aluminum, mercuric mercury and methylmercury in cell lines of neural origin. Arch. Toxicol 78 (10), 565–574. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, 1998. Selective modulation of GABAA receptors by aluminumJ. Neurophysiol. 80 (2), 755–761. [DOI] [PubMed] [Google Scholar]
- Tsialtas I, Gorgogietas VA, Michalopoulou M, Komninou A, Liakou E, Georgantopoulos A, Psarra AMG, 2020. Neurotoxic effects of aluminum are associated with its interference with estrogen receptors signaling. Neurotoxicology. 77, 114–126. [DOI] [PubMed] [Google Scholar]
- Turner M, Mutter ST, Kennedy-Britten OD, Platts JA, 2019. Molecular dynamics simulation of aluminium binding to amyloid-β and its effect on peptide structure. PloS one. 14 (6), e0217992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL, 2001. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein a possible molecular link between parkinson′ s disease and heavy metal exposure. J. Biol. Chem 276 (47), 44284–44296. [DOI] [PubMed] [Google Scholar]
- Verma S, Sharma S, Ranawat P, Nehru B, 2020. Modulatory effects of ginkgo biloba against amyloid aggregation through induction of heat shock proteins in aluminium induced neurotoxicity. Neurochem. Res 45 (2), 465–490. [DOI] [PubMed] [Google Scholar]
- Vučetić-Arsić S, Radonjić NV, Jovanović M, Selaković V, Nikolić T, Velimirović M, Petronijević ND, 2013. Oxidative stress precedes mitochondrial dysfunction in gerbil brain after aluminum ingestion. Environ. Toxicol. Pharmacol 36 (3), 1242–1252. [DOI] [PubMed] [Google Scholar]
- Walton JR, 2009. Brain lesions comprised of aluminum-rich cells that lack microtubules may be associated with the cognitive deficit of Alzheimer’s disease. Neurotoxicology. 30 (6), 1059–1069. [DOI] [PubMed] [Google Scholar]
- Walton JR, 2014. Chronic aluminum intake causes Alzheimer’s disease: applying Sir Austin Bradford Hill’s causality criteria. J. Alzheimers Dis 40 (4), 765–838. [DOI] [PubMed] [Google Scholar]
- Wang M, Ruan DY, Chen JT, Xu YZ, 2002. Lack of effects of vitamin E on aluminium-induced deficit of synaptic plasticity in rat dentate gyrus in vivo. Food Chem. Toxicol 40 (4), 471–478. [DOI] [PubMed] [Google Scholar]
- Wang M, Chen JT, Ruan DY, Xu YZ, 2002b. The influence of developmental period of aluminum exposure on synaptic plasticity in the adult rat dentate gyrus in vivo. Neuroscience. 113 (2), 411–419. [DOI] [PubMed] [Google Scholar]
- Wang L, Hu J, Zhao Y, Lu X, Zhang Q, Niu Q, 2014. Effects of aluminium on β-amyloid (1–42) and secretases (APP-cleaving enzymes) in rat brain. Neurochem. Res 39 (7), 1338–1345. [DOI] [PubMed] [Google Scholar]
- Wang H, Ye M, Yu L, Wang J, Guo Y, Lei W, Yang J, 2015. Hippocampal neuronal cyclooxygenase-2 downstream signaling imbalance in a rat model of chronic aluminium gluconate administration. Behav. Brain Funct 11 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fan X, Yuan S, Jiao W, Liu B, Cao J, Jiang W, 2017. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct. 8 (8), 2924–2934. [DOI] [PubMed] [Google Scholar]
- Wang X, Xi Y, Zeng X, Zhao H, Cao J, Jiang W, 2018. Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. J. Funct. Foods 40, 365–376. [Google Scholar]
- Wasylishen AR, Estrella JS, Pant V, Chau GP, Lozano G, 2018. Daxx functions are p53-Independent in vivo. Mol. Cancer Res 16 (10), 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CX, Mattson MP, Lovell MA, Yokel RA, 1996. Intraneuronal aluminum potentiates iron-induced oxidative stress in cultured rat hippocampal neurons. Brain Res. 743 (1–2), 271–277. [DOI] [PubMed] [Google Scholar]
- Yan D, Jin C, Cao Y, Wang L, Lu X, Yang J, Cai Y, 2017. Effects of aluminium on long-term memory in rats and on SIRT 1 mediating the transcription of CREB-dependent gene in hippocampus. Basic Clin. Pharmacol. Toxicol 121 (4), 342–352. [DOI] [PubMed] [Google Scholar]
- Yellamma K, Saraswathamma S, Kumari BN, 2010. Cholinergic system under aluminium toxicity in rat brain. Toxicol. Int 17 (2), 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, Li Y, 2019a. Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotox. Environ. Safe 173, 131–141. [DOI] [PubMed] [Google Scholar]
- Yu L, Wu J, Zhai Q, Tian F, Zhao J, Zhang H, Chen W, 2019b. Metabolomic analysis reveals the mechanism of aluminum cytotoxicity in HT-29 cells. PeerJ. 7, e7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CY, Hsu GSW, Lee YJ, 2011. Aluminum alters NMDA receptor 1A and 2A/B expression on neonatal hippocampal neurons in rats. J. Biomed. Sci 18 (1), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CY, Lee YJ, Hsu GSW, 2012. Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J. Biomed. Sci 19 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaky A, Mohammad B, Moftah M, Kandeel KM, Bassiouny AR, 2013. Apurinic/apyrimidinic endonuclease 1 is a key modulator of aluminum-induced neuroinflammation. BMC Neurosci. 14 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, 2018a. Aluminum-induced electrophysiological variation, synaptic plasticity impairment, and related mechanism. In: Neurotoxicity of Aluminum. Springer, Singapore, pp. 161–172. [DOI] [PubMed] [Google Scholar]
- Zhang Q, 2018b. Aluminum-induced neural cell death. In: Neurotoxicity of Aluminum. Springer, Singapore, pp. 129–160. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jin C, Lu X, Yang J, Wu S, Liu Q, Du Y, 2014. Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology. 323, 95–108. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu R, Gung BW, Tindall S, Gonzalez JM, Halvorson JJ, Hagerman AE, 2016a. Polyphenol–aluminum complex formation: implications for aluminum tolerance in plants. J. Agric. Food Chem 64 (15), 3025–3033. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang X, Qin X, Niu Q, 2016b. Caspase-3 is involved in aluminum-induced impairment of long-term potentiation in rats through the Akt/GSK-3β pathway. Neurotox. Res 29 (4), 484–494. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pi Z, Song F, Liu Z, 2016c. Ginsenosides attenuate d-galactose-and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol 194, 188–195. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang F, Ni Y, Kokot S, 2019. Effects of aluminum on amyloid-beta aggregation in the context of Alzheimer’s disease. Arab. J. Chem 12 (8), 2897–2904. [Google Scholar]
- Zhang H, Wei M, Lu X, Sun Q, Wang C, Zhang J, Fan H, 2020. Aluminum trichloride caused hippocampal neural cells death and subsequent depression-like behavior in rats via the activation of IL-1β/JNK signaling pathway. Sci. Total Environ, 136942. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pei G, 2008. Evoking plasmin for β-amyloid clearance. Cell Res. 18 (8), 803–804. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hill JM, Bhattacharjee S, Percy ME, Pogue AI, Lukiw WJ, 2014. Aluminum-induced amyloidogenesis and impairment in the clearance of amyloid peptides from the central nervous system in Alzheimer’s disease. Front. Neurol 5, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dang M, Zhang W, Lei Y, Ramesh T, Veeraraghavan VP, Hou X, 2020. Neuroprotective effects of Syringic acid against aluminium chloride induced oxidative stress mediated neuroinflammation in rat model of Alzheimer’s disease. J. Funct. Foods 71, 104009. [Google Scholar]
- Zielke HR, Jackson MJ, Tildon JT, Max SR, 1993. A glutamatergic mechanism for aluminum toxicity in astrocytes. Mol. Chem. Neuropathol 19 (3), 219–233. [DOI] [PubMed] [Google Scholar]


