1. Introduction
Manganese (Mn) is an essential trace element that is required to maintain a variety of metabolic and physiological functions in the body (Aschner and Aschner, 2005) since Mn is a cofactor for an array of metabolic and antioxidant enzymes such as glutamine synthetase and Mn superoxide dismutase (MnSOD) (Fridovich, 1974). Although Mn is vital for these biological functions, chronic exposure to excess Mn leads to a neurological condition referred to as manganism, which is characterized by pathological symptoms similar to those of Parkinson’s disease (PD) (Pal et al., 1999). Exposure to high levels of Mn may occur from various sources and means such as inhalation of particles from industrial emissions (Hassani et al., 2012), mining sites (Wang et al., 2018a), fertilizers (i.e., maneb, mancozeb) (de Tollenaer et al., 2006; Harrison Brody et al., 2013), gasoline additives [i.e., methylcyclopentadienyl Mn tricarbonyl (MMT)], as well as exposure to contaminated soil, water, and food sources (Arya and Roy, 2011; Okereafor et al., 2020). Given that these sources of Mn are ubiquitous in the environment, there is an increasing concern regarding the risk of Mn toxicity in the human population. Studies have shown that elevated levels of Mn in the brain of welders and smelters is correlated with higher risk of developing PD-like behavior and motor deficits (Juncos et al., 1968; Sriram et al., 2012). Moreover, Mn overexposure increased the risk of the development of abnormal behavior, cognitive decline and learning deficits in infants and children (Palzes et al., 2019; Rodrigues et al., 2018). In support of these findings, experimental studies have demonstrated that Mn impaired memory and learning ability in a mouse model (Song et al., 2016), along with pathological symptoms of locomotor activities (Alsulimani et al., 2015). Studies have reported that, in addition to basal ganglia, Mn accumulates in several other brain regions such as the frontal cortex, hippocampus and cerebellum upon its exposure (Garcia et al., 2006; Newland et al., 1989), suggesting that Mn may modulate a wide array of brain functions. Mn may be a risk factor for multiple neurological disorders including PD (Harischandra et al., 2019), dementia with Lewy bodies (DLB) (Tong et al., 2009) and Alzheimer’s disease (AD) (Guilarte, 2010).
The underlying molecular mechanisms of Mn-induced neurotoxicity appear to be complex and may involve multiple mechanisms via various neural cell types in the brain, which ultimately deciphers Mn-induced neurotoxicity mechanisms. Studies have shown that mitochondrial impairment, oxidative stress, inflammation and excitotoxicity are involved in Mn-induced neurotoxicity (Brouillet et al., 1993; Gavin et al., 1990; Pajarillo et al., 2020; Popichak et al., 2018). Mitochondrial dysfunction may be an initiating event for Mn toxicity as Mn preferentially accumulates in mitochondria (Lai et al., 1999; Morello et al., 2008), leading to overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS), activation of proinflammatory signaling, and apoptotic cell death (Sarkar et al., 2018). Thus, understanding how Mn dysregulates these processes may provide invaluable information to prevent and treat neurodegenerative disorders associated with Mn-induced neurotoxicity.
This review focuses on Mn-induced neurotoxicity mechanisms (Fig. 1) such as impairment of mitochondrial dynamics and mitophagy, autophagy dysregulation, oxidative and nitrosative stress, proinflammatory signaling and excitotoxicity, all of which eventually leads to Mn-induced neurotoxicity and behavioral consequences. We also discuss several genes involved in these cellular processes.
Fig. 1.
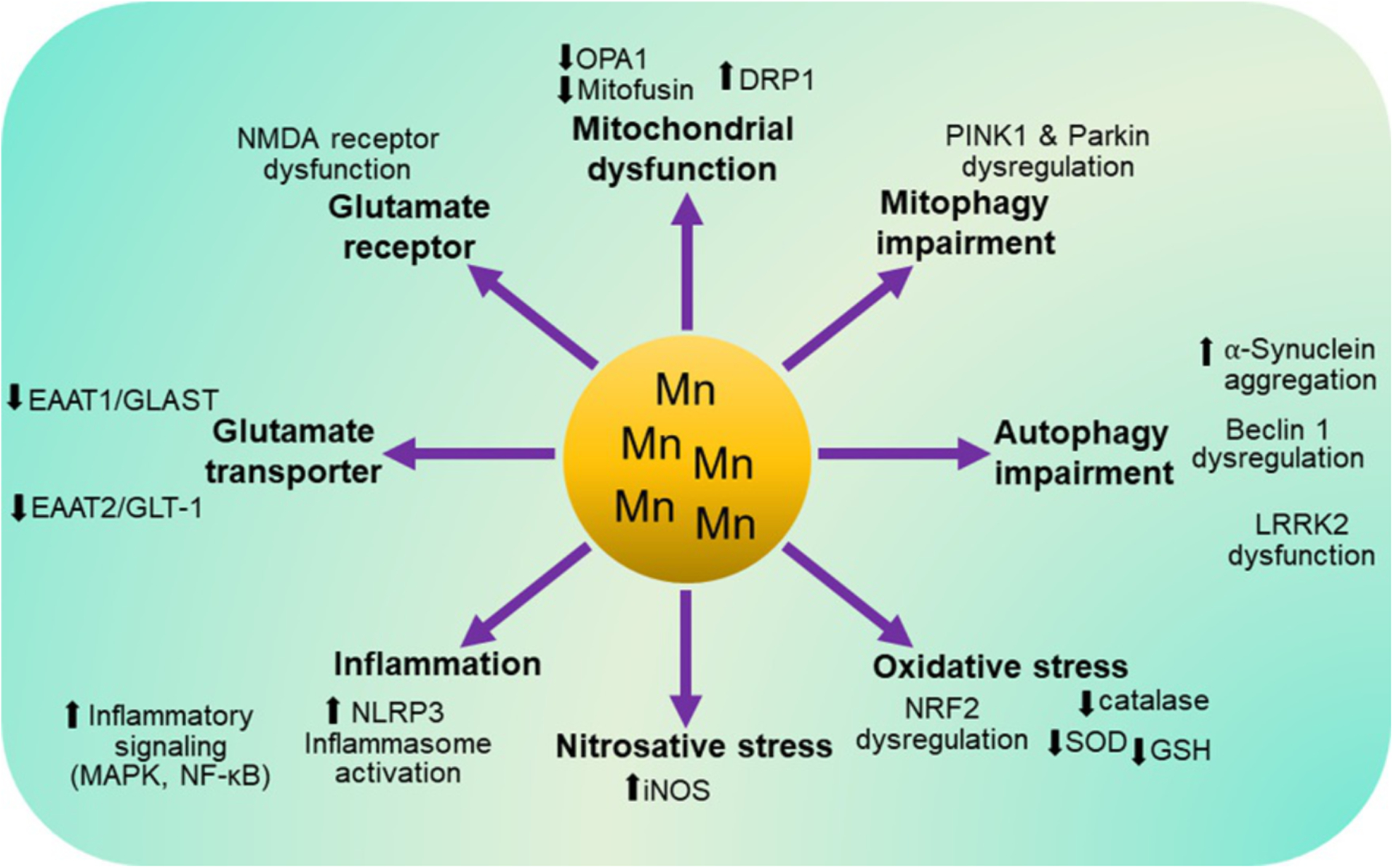
A schematic of the molecular mechanisms involved in Mn-induced toxicity in the central nervous system. Dysfunction of molecular mechanisms such as mitochondrial dynamics, mitophagy, autophagy, antioxidant defense systems, inflammatory signaling (i.e., MAPK, NF-κB), NLRP3 inflammasome activation, glutamate transporters (i.e., GLAST and GLT-1) and NMDA receptors have been implicated in Mn-induced toxicity.
2. Effects of Mn on mitochondrial function
Optimal mitochondrial function is critical for cell survival, and thus, toxic conditions that induce its impairment can lead to cellular injury. The proper functions of the mitochondria are maintained by the mitochondrial quality control (MQC) machinery, which is an integrated network of pathways involving mitochondrial dynamics, biogenesis and mitophagy (Palikaras et al., 2015). It has been shown that Mn accumulates in the mitochondria and impairs MQC machinery in neurons and astrocytes (Lai et al., 1999; Morello et al., 2008). Mn is mainly transported into the mitochondria via the mitochondrial Ca2+ uniporter system (Gunter et al., 1978; Puskin et al., 1976), suggesting that dysfunction of this transporter may lead to Mn accumulation in the mitochondria. Here, we will focus on Mn-induced dysfunction of mitochondrial fusion-fission dynamics and mitophagy degradation pathway.
2.1. Effects of Mn on mitochondrial fusion and fission
Mitochondria are highly dynamic organelles that can build large interconnected intracellular networks (Bereiter-Hahn, 1990). Their shapes change continually through the combined actions of fission, fusion and motility. At the cellular level, mitochondrial fusion-fission dynamics are involved in maintenance of mitochondrial morphology, mitochondrial DNA (mtDNA) stability, respiratory capacity, apoptosis, and response to cellular stress (Chan, 2012). Proteins such as mitochondrial Rho GTPase 1 (MIRO1), mitofusin 1 (MFN1) and 2 (MFN2), dynamin-1-like protein (DLP1/DRP1), and optic atrophy 1 dynamin-like GTPase (OPA1) regulate mitochondrial fusion-fission process (Chang and Blackstone, 2010; Chen et al., 2003; Ishihara et al., 2004; Song et al., 2007, 2009). OPA1 and MFN2 also participate in mitochondrial degradation (Belenguer and Pellegrini, 2013; de Brito and Scorrano, 2008). Therefore, functional defects of these proteins due to exposure to toxicants and genetic mutations have detrimental consequences on bioenergetic supply, oxidative stress and overactivation of proapoptotic signaling, which can contribute to not only Mn-induced neurotoxicity, but also neurodegenerative diseases.
Mitochondrial fusion is a well-coordinated and synchronized process of combining adjacent mitochondrion and its components (Westermann, 2010). In mammals, mitochondrial membrane proteins such as MFN1, MFN2, and OPA1 facilitate mitochondrial fusion, but DRP1 modulates fission, maintaining mitochondrial quality and function (Alexander et al., 2000; Ishihara et al., 2004; Rojo et al., 2002; Song et al., 2009). Studies have shown that Mn reduced levels of OPA1 and MFN2, but increased DRP1 levels in astrocytes, resulting in imbalance of fusion-fission (Alaimo et al., 2013). Mn decreased MFN2 levels parallel with increased inflammation in astrocytes (Sarkar et al., 2018) and neuronal damage in the rat striatum and PC12 cells (Liu et al., 2017). Mn also decreased OPA1, causing mitochondrial depolarization and apoptosis in astrocytes (Alaimo et al., 2014). On the other hand, Mn increased DRP1, resulting in mitochondrial depolarization and dysfunction in astrocytes (Alaimo et al., 2014). These findings suggest that Mn-induced impairment of mitochondrial integrity and the fusion-fission process is the initial step toward the cascade of events leading to mitochondrial dysfunction, oxidative stress and apoptosis by increasing fission and fragmentation.
2.2. Effects of Mn on mitophagy
In addition to the impairment of mitochondrial dynamics by modulating proteins associated with fusion and fission (Sarkar et al., 2018), Mn dysregulates mitophagy, which is the normal process of removing damaged mitochondria and its components by autophagic degradation (Safiulina et al., 2019; Scott and Klionsky, 1998). Mn-induced mitophagy impairment results in the accumulation of damaged mitochondria, producing excess reactive species that ultimately lead to cellular injury. It has been reported that Mn dysregulated mitophagy-regulating proteins PTEN-induced kinase 1 (PINK1) and E3 ubiquitin ligase parkin (PARK2), leading to Mn-induced mitophagy impairment and apoptosis in SH-SY5Y and CATH.a dopaminergic cells (Higashi et al., 2004; Song et al., 2017). Although it is unclear how Mn modulates PINK1 and parkin, transcription factors such as forkhead box O3 (FOXO3) may be involved in Mn-induced dysregulation of PINK1 and parkin, since Mn dysregulated PINK1 and parkin by activating FOXO3 via mitogen-activated protein kinase (MAPK) signaling pathways in SH-SY5Y cells (Exil et al., 2014; Song et al., 2017). Studies have also shown that oxidative stress appears to play a critical role in Mn-induced mitophagy impairment in PC12 cells (Zhou et al., 2018).
3. Effects of Mn on autophagy
Autophagy impairment has emerged as one of the key cellular events in multiple neurodegenerative disorders such as PD, AD and manganism, as it promotes the accumulation of cytokines and damaged mitochondria, excessive build-up of α-synuclein oligomers, β-amyloid plaques and tau aggregates (Ghavami et al., 2014). Autophagy is a highly regulated process which involves several important steps including (1) initiation, (2) elongation and maturation (3) fusion and lysosomal degradation. Mn regulates several steps of autophagy processes by modulating Beclin 1 in initiation step, microtubule-associated protein 1 light chain 3 (LC3-II/I) in maturation step, and SQSTM1/p62 in lysosomal fusion step in the mouse brain, as well as in neurons and astrocytes (Afeseh Ngwa et al., 2011; Song et al., 2017; Zhang et al., 2013, 2019). Growing evidence suggests that autophagy dysregulation is involved in Mn-induced toxicity (Afeseh Ngwa et al., 2011; Zhou et al., 2018). Mn increased autophagy proteins and formation of autophagic vacuoles in mouse striatum (Zhang et al., 2013), while other studies showed that Mn reduced autophagy by decreasing LC3-II/I ratio in astrocytes (Zhang et al., 2019), indicating that Mn modulates autophagy processes differently depending on the experimental conditions. Studies have shown that Mn initially increased autophagic function to degrade Mn-induced damaged proteins and maintain cellular homeostasis by increasing Beclin 1 and LC3-II/LC3-I ratio in the substantia nigra, but longer exposure impaired autophagy by decreasing Beclin 1 and LC3-II/LC3-I ratio (Zhang et al., 2013). Taken together, these findings indicate that Mn dysregulates several autophagy steps to impair autophagy, which may be involved in dopaminergic dysfunction and subsequent movement deficits.
Mn modulates the expression of autophagy genes at multiple levels including transcriptional, translational and posttranslational steps. Mn inhibited the transcription factor EB (TFEB) concomitant with autophagy impairment in primary astrocytes (Zhang et al., 2019). Inhibition of TFEB exacerbated Mn-induced autophagy dysregulation, while TFEB overexpression attenuated these Mn effects by preventing Mn-induced reduction in LC3-II/LC3-I ratio, Atg18, LAMP1 and increased p62 levels (Zhang et al., 2019). TFEB is known to regulate the transcription of genes involved in autophagosome initiation (BECN1, ATG9B) and elongation (ATG5, MAP1LC3B), as well as substrate capture (p62) (Palmieri et al., 2011; Settembre et al., 2011). Mn also altered transcription factor FOXO3 function, resulting in autophagy impairment in SH-SY5Y and primary astrocytes (Exil et al., 2014; Song et al., 2017). FOXO3 regulates autophagy by directly binding to the promoters of genes related to autophagy initiation (e.g., RBCC1 and ULK1), autophagosome formation and elongation (e.g., ATG14, GABARAP, ATG5, ATG10) and p62 (Audesse et al., 2019). Mn-induced activation of the transcription factor activator protein 1 (AP-1) also increases Beclin 1 and LC3-II/LC3-I ratio, while decreasing p62 in mice (Liu et al., 2020).
Mn can also impair autophagy by dysregulating translational and posttranslational steps. Studies have shown that Mn caused autophagy dysfunction in dopaminergic neurons of Caenorhabditis elegans, partly by aberrant translational activities (Vijayan et al., 2019). In addition, Mn appears to induce autophagy dysregulation by impairing Beclin 1 degradation via aberrant posttranslational modifications (Afeseh Ngwa et al., 2011; Ma et al., 2017, 2020). For instance, Mn increased the breakdown of Beclin 1 by enhancing proteolytic activities in N27 dopaminergic cells (Afeseh Ngwa et al., 2011). In addition to abnormal Beclin 1 proteolysis, Mn also promoted aberrant S-nitrosylation to disrupt Beclin 1 and Bcl-2 interaction in SH-SY5Y cells (Ma et al., 2017, 2020). Under normal conditions, antiapoptotic Bcl-2 interacts with Beclin 1 to inhibit autophagy and maintain basal autophagy activity (Maiuri et al., 2007). However, toxicants such as Mn increased nitrosylated Bcl-2, resulting in the excessive release of Beclin 1, leading to autophagy impairment (Ma et al., 2017, 2020). Nitrosative stress also contributes to Mn-induced autophagy impairment, as inhibition of inducible nitric oxide synthase (iNOS) attenuated Mn-induced autophagy impairment in SH-SY5Y cells (Ma et al., 2017). Mn-induced oxidative stress plays a role in Mn-induced autophagy dysregulation in PC12 cells (Zhou et al., 2018). Trehalose, which exerts antioxidant properties, also attenuated Mn-induced abnormal autophagy function, mitochondrial impairment and neuronal injury (Liu et al., 2018).
3.1. Effects of Mn on the PD-related genes in autophagy
Growing evidence suggest that Mn may be a risk factor to PD pathogenesis by contributing to neuropathology of α-synuclein, a small protein (~140 amino acids) predominantly localized in presynaptic nerve terminals (Bendor et al., 2013; Pajarillo et al., 2019). Mn promotes expression and accumulation of α-synuclein in various experimental models and exerts consequent toxic effects (Cai et al., 2010; Harischandra et al., 2019; Liu et al., 2018; Verina et al., 2013; Wang et al., 2018b; Xu et al., 2014). On the other hand, Mn may also promote the build-up of toxic substances and undegraded cellular components by reducing autophagic activity. Studies have shown that Mn-induced autophagy impairment resulted in increased α-synuclein aggregation in mice (Liu et al., 2018), since inhibition of autophagy exacerbated, but activation of autophagy attenuated Mn-induced α-synuclein accumulation and its toxic effects (Liu et al., 2018; Yan et al., 2019a,b). Moreover, Mn-induced increased α-synuclein itself appears to dysregulate autophagy process by impairing Beclin 1 function at the initiation step in SH-SY5Y cells (Yan et al., 2019b). These findings indicate that autophagy impairment is critically involved in Mn toxicity associated with α-synuclein aggregates. Mn dysregulates the PD-associated protein leucine rich repeat kinase 2 (LRRK2) which is involved in autophagy regulation. Mn impairs autophagy in microglia by modulating Beclin 1 and Atg5 via LRRK2 activation (Chen et al., 2018). Inhibition of LRRK2 attenuated Mn-induced autophagy dysregulation in mice by modulating Beclin 1 and Atg5 levels, resulting in protective effects against Mn-induced neurotoxicity (Chen et al., 2018). Taken together, these indicate that dysregulation of autophagy is critically involved in Mn-induced neurotoxicity.
4. Effects of Mn on oxidative and nitrosative stress
Oxidative stress and nitrosative stress are widely recognized as major contributors to pathological mechanisms of various neurological disorders including manganism (Sayre et al., 2008). Generation of ROS and RNS are normal by-products of aerobic respiration and metabolism of nitric oxide (NO), respectively. However, overproduction of ROS and RNS in response to toxicants such as Mn cause oxidative/nitrosative stress damage in cells and tissues. Thus, elucidating the underlying mechanisms of Mn-induced oxidative and nitrosative stress is critical to understand pathogenesis in Mn′s neurotoxicity.
4.1. Effects of Mn on oxidative stress
Mitochondria appear to be the main intracellular sources of endogenous ROS from oxidative phosphorylation and electron transfer reactions (Cadenas and Davies, 2000). Overproduction of ROS can lead to high levels of lipid peroxidation, increased protein oxidation and DNA damage (Cadenas and Davies, 2000; Prabhakaran et al., 2008; Sayre et al., 2008; Smith et al., 2017). Mn preferentially accumulates in mitochondria and disrupts various mitochondrial functions (Afeseh Ngwa et al., 2011; Alaimo et al., 2013; Brouillet et al., 1993; Gavin et al., 1990; Malthankar et al., 2004; Zhang et al., 2003), leading to a reduction in ATP production (Sarkar et al., 2018) and a leakage of electrons from electron transport chain (ETC), eventually generating excessive ROS (Cadenas and Davies, 2000; Smith et al., 2017; Turrens, 2003). It is well established that Mn-induced oxidative stress is involved in Mn-induced neurotoxicity, as Mn induced excess ROS generation in a plethora of in vitro and in vivo studies (Kim et al., 2019; Milatovic et al., 2007; Pajarillo et al., 2020; Sadeghi et al., 2018; Yang et al., 2018). Several causal sources of Mn-induced oxidative stress may include (1) accumulation of Mn2+ and its conversion into higher oxidative states of Mn which are highly unstable and reactive (Smith et al., 2017), (2) dysregulation of antioxidant systems (e.g., NRF2-ARE) and enzymes (e.g., SOD and catalase) (Bahar et al., 2017; Gawlik et al., 2017; Latronico et al., 2013; Pajarillo et al., 2020), and (3) depletion of oxidative scavengers such as glutathione (GSH) and metallothionein (MT) (Erikson and Aschner, 2002; Erikson et al., 2006; Yang et al., 2018). Mn induced oxidative damage by dysregulating antioxidant enzyme activities of SOD and catalase in the mouse striatum (Gawlik et al., 2017) and the rat brain as well as neuronal cells (Bahar et al., 2017; Pajarillo et al., 2018, 2020). Mn also inhibited antioxidant capacity by reducing GSH synthesis in mouse striatum as well as in neurons and astrocytes (Yang et al., 2018). Mn reduced MT, leading to ROS build-up in astrocytes and mice (Erikson and Aschner, 2002; Erikson et al., 2006). The effect of Mn inducing excess ROS triggers a domino effect that leads to apoptotic cell death by activating caspases, protein kinase C and/or mitogen-activated kinases in different cell types of the brain (Anantharam et al., 2002; Kim et al., 2019; Kitazawa et al., 2005). Antioxidants NAC, GSH, and quercetin attenuated Mn-induced neurotoxicity by scavenging ROS in neuronal cells and mice (Bahar et al., 2017; Stephenson et al., 2013), indicating that oxidative stress is a critical mechanism for Mn neurotoxicity.
4.2. Effects of Mn on nitrosative stress
Mn promotes cellular injury by inducing nitrosative stress by NO overproduction in the brain (Popichak et al., 2018; Prabhakaran et al., 2008). NO functions in vasodilation, neurotransmission and intracellular signaling (Tuteja et al., 2004), and it is generated by nitric oxide synthases (NOS) with three isoforms, namely iNOS, endothelial NOS (eNOS) and neuronal NOS (nNOS) (Forstermann and Sessa, 2012). Mn elevated NO levels, leading to neurotoxicity by increasing iNOS expression and activity in both microglia and astrocytes (Chen et al., 2006; Filipov et al., 2005; Moreno et al., 2011; Popichak et al., 2018) as well as promoting inflammation, and neuronal injury in mice (Popichak et al., 2018; Prabhakaran et al., 2008). Results that deletion of iNOS attenuated Mn-induced movement deficits and dopaminergic cell death in mice indicate the importance of nitrosative stress in Mn-induced dopaminergic dysfunction (Streifel et al., 2012). Although the exact mechanism of Mn-induced NOS expression is not fully understood, studies have reported that Mn-decreased DNA methylation of CpG sites on the iNOS promoter is involved in dysregulation of iNOS expression (Searles Nielsen et al., 2015). Clinically, Mn reduced DNA methylation of the iNOS promoter in Mn-exposed welders who exhibited parkinsonian symptoms with higher inflammation due to increased iNOS expression (Searles Nielsen et al., 2015). Mn also increased iNOS expression by enhancing iNOS promoter activity in microglia (Filipov et al., 2005). In addition to DNA methylation, activation of signaling pathways such as MAPK and NF-κB, may also be involved in Mn-activated NOS expression and nitrosative stress in microglia (Bae et al., 2006; Moreno et al., 2011).
4.3. Effects of Mn on antioxidative stress responses
Mn generates excessive ROS by reducing antioxidative ability, in addition to direct oxidative stress (Chen and Liao, 2002; Pajarillo et al., 2018, 2020), leading to inflammation and apoptosis. Transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) induces the expression of several antioxidant genes by activating the transcription of its target genes through binding to their antioxidant response elements (ARE). NRF2 is normally found in the cytosol sequestered by the Kelch-like ECH-associated protein 1 (KEAP1) which prevents NRF2’s nuclear translocation and transcriptional activation of target genes. NRF2 is constantly degraded by ubiquitination and proteasomal degradation (Villeneuve et al., 2010). Target genes include heme oxygenase 1 (HO-1), NAD(P)H dehydrogenase, quinone 1 (NQO1), γ-glutamylcysteine synthetase (γ-GCS), glutathione S-transferase (GST) and SOD (Kobayashi et al., 2004).
Accumulating evidence reveals that NRF2 is a key response gene in the adaptive survival against Mn-induced oxidative stress. Mn initially increases NRF2 protein levels to overcome oxidative stress, followed by a gradual decrease of NRF2 levels as Mn′s overproduction of ROS eventually exceeds its capacity (Deng et al., 2015). It has been shown that Mn-containing fungicide maneb significantly reduced NRF2-responsive antioxidant genes such as GPx and HO-1 in NRF2-KO mice accompanied by higher levels of lipid peroxidation and hippocampal neuronal death (Kurzatkowski and Trombetta, 2013), indicating that NRF2 exerts protective effects against Mn-induced neurotoxicity. The protective role of NRF2 against Mn-induced oxidative stress may involve ROS breakdown as well as increasing synthesis of GSH for ROS scavenging (Settivari et al., 2013; Zhang et al., 2017). The molecular mechanisms by which Mn regulates NRF2 function have been extensively studied. Studies have shown that Mn decreased levels of NRF2 and HO-1 by reducing histone acetylation in PC12 cells (Zhang et al., 2017). Mn dysregulated NRF2 and SOD activities by inhibiting acetylcholinesterase activity in the mouse brain (Santos et al., 2012), indicating that increased cholinergic activity may play a role in Mn-induced oxidative stress dysregulation. Other cellular activities by which Mn modulates NRF2 activation and HO-1 expression involves the impairment ofNRF2 degradation through the ubiquitin-proteasome pathway in PC12 cells (Li et al., 2011).
Transcription factor repressor element-1 silencing transcription factor (REST)-induced NRF2 appears to be involved in attenuation of Mn-induced neurotoxicity (Pajarillo et al., 2020). Mn increased ROS generation, accompanied by decreased REST expression, but REST overexpression attenuated these Mn effects in CAD cells (Pajarillo et al., 2020). Moreover, Mn decreased NRF2 levels, concomitant with increasing its ubiquitination, while REST overexpression attenuated Mn-reduced NRF2 as well as Mn-induced NRF2 ubiquitination (Pajarillo et al., 2020). Taken together, these findings indicate that NRF2 play a critical role against Mn-induced oxidative stress, and REST may protect against Mn-induced oxidative stress, at least in part, by attenuating Mn-induced NRF2 dysregulation.
5. Effects of Mn on inflammation
Mn induces activation of inflammatory signaling and molecules that cause irreparable damage in the brain microenvironment. Inflammatory factors such as tumor necrosis factor alpha (TNF-α), interleukins (IL-1, IL-6, IL-8, IL-18), interferons (IFNγ) and chemokines are major contributors to cellular injury in the brain (Akdis et al., 2016). Mn also amplifies inflammatory signaling via ROS and nitric oxide in causing cell death. These Mn effects on inflammation will be discussed in detail in the following sections.
5.1. Glial cells: Microglia and astrocytes as key neural cell types of Mn-induced inflammation
Microglia and astrocytes are key cell sources of inflammation in the brain. Microglia are the main cell type to induce inflammatory responses to external stimuli and environmental toxicants such as Mn. Studies have shown that Mn induced the production of proinflammatory cytokines including TNF-α and ILs in microglia (Chen et al., 2018; Dodd and Filipov, 2011; Kelly et al., 2017; Kim et al., 2019). Microglial-derived cytokines also activate adjacent cells such as astrocytes and neurons, leading to the amplification of inflammatory signaling caused by Mn toxicity (Kirkley et al., 2017; Popichak et al., 2018). Astrocytes may also be an important source of proinflammatory factors as Mn induced astrogliosis in various mouse brain regions including striatum and substantia nigra (Krishna et al., 2014; Tomas-Camardiel et al., 2002). Mn increased inflammatory cytokines such as TNF-α, ILs, and nterferons, and substances such as cyclooxygenase-2 (COX-2) and prostaglandins in astrocytes (Chen and Liao, 2002; Filipov et al., 2005; Liao et al., 2007; Sengupta et al., 2007).
5.2. Effects of Mn on inflammatory signaling
Given that Mn increases proinflammatory cytokines and chemokines in various experimental settings, leading to neuronal cell death, understanding the signaling molecules and pathways (e.g., MAPK, NF-κB) by which Mn promotes the production of proinflammatory factors and inflammation is critical to mitigate the effects of Mn-induced inflammation in the brain.
MAPKs are a family of serine/threonine protein kinases that direct cellular responses to a variety of stimuli, including Mn, that regulate cell survival and apoptotic cell death (Crittenden and Filipov, 2008). Three MAPKs, the extracellular-signal-regulated kinase ERK1/2, p38 and c-Jun N-terminal kinases (JNK), participate in inflammatory signaling (Kaminska et al., 2009; Roux and Blenis, 2004). Mn activated ERK1/2, JNK and p38 in astrocytes and microglia accompanied by increased proinflammatory factors including IL-6, TNF-α and interferons (Chen et al., 2006; Crittenden and Filipov, 2011; Kim et al., 2019). Mn has been shown to activate upstream of ERK1/2, JNK and p38, which are MKK1/2, MKK4 and MKK3/6, respectively, in microglia (Crittenden and Filipov, 2011).
NF-κB is a ubiquitous transcription factor that regulates a variety of biological processes including survival and inflammation (Oeckinghaus and Ghosh, 2009), but the role of NF-κB in Mn-induced neurotoxicity mechanisms, particularly in inflammation, inflammasome activation and impairment of glutamate transporters, will be discussed in this review. NF-κB is activated by inflammatory factors such as cytokines in response to external stimuli such as Mn. Functional NF-κB is a dimer, comprised of various combinations of several subunits including p65, RelB, c-Rel, p50 and p52 (Oeckinghaus and Ghosh, 2009), forming up to 15 different heterodimer and homodimer complexes that can modulate numerous biological processes within the cell. The two most common subunits are p65 (Rela gene) and p50 (Nfkb1 gene) in the brain (Dresselhaus and Meffert, 2019). In general, p65/p50 heterodimers activates transcription, while p50/p50 homodimers represses transcription (Oeckinghaus and Ghosh, 2009; Sheppard et al., 1999), and these NF-κB subunit dimers are sequestered in the cytosol by IκB, an inhibitor of NF-κB, under normal conditions.
Mn activated the canonical NF-κB signaling pathway to induce transcriptional activation of its target proinflammatory cytokines and chemokines in microglia and astrocytes (Kirkley et al., 2017; Popichak et al., 2018). Inhibition of NF-κB attenuated Mn-induced production of proinflammatory cytokines and chemokines such as TNF-α, IL-6, CCL2, and CCL5 (Kirkley et al., 2017), indicating that NF-κB activation is central to Mn-induced inflammation in microglia. Moreover, NF-κB appears to be the target of flavonoid-based compounds rutin or quercetin against Mn-induced inflammation in neuronal cells and rat brain (Bahar et al., 2017; Nkpaa et al., 2019). While glial cells are major sources of cytokines, neurons also produce those factors and induce inflammatory signaling. Several studies have shown that Mn increased inflammation in SK-N-MC and CAD neuronal cells (Bahar et al., 2017; Nkpaa et al., 2019; Pajarillo et al., 2020; Ramesh et al., 2002). These findings indicate that Mn-induced inflammation could be derived from multiple brain cell types.
Neuronal-glial co-culture studies provide better insight into the role of NF-κB and impact of glial-derived proinflammatory factors in neuroinflammation during Mn exposure. Mn targets IKK, an upstream of NF-κB signaling, in astrocytes since the deletion of IKK protected primary neurons from Mn-induced inflammatory injury (Popichak et al., 2018). Although TNF-α is the most well-known proinflammatory gene regulated by NF-κB, other NF-κB-mediated cytokines are also involved in cell-to-cell propagation of inflammation. For example, knockdown of astrocytic CCL2, a chemokine regulated by NF-κB, protected neuronal viability against Mn toxicity in neuron-glia co-culture experiments (Popichak et al., 2018). Taken together, these findings indicate the role of NF-κB in regulating glial-glial interactions and amplification of proinflammatory factors in the brain microenvironment during Mn exposure.
5.3. Effects of Mn on NLRP3 inflammasome activation
Inflammasomes are multi-component complexes critical for pattern recognition and signaling in response to environmental toxicants such as Mn. They are important for cell-to-cell signaling and propagation of inflammatory signals from immune cells such as macrophages and microglia. The most well-established inflammasomes include the nucleotide-binding oligomerization domain-like receptor (NLR) and pyrin-domain (PYD)-containing (NLRP) protein family. NLRP3 inflammasomes contain the NLRP3 sensor, PYCARD/ASC adapter and caspase 1 (CASP1) protease which can be triggered by multiple factors and events including lysosomal disruption, mitochondrial dysfunction and oxidative stress (Heid et al., 2013; Katsnelson et al., 2016). Mn promotes NLRP3 inflammasome activation in both in vitro and in vivo experimental settings (Fan et al., 2020; Sarkar et al., 2019; Wang et al., 2017a). Mn activated NLRP3-CASP1 inflammasome complex, leading to CASP1 activation and IL-1β maturation in BV2 microglial cells and hippocampus of mice (Wang et al., 2017a). It has been shown that lysosomal fusion is a critical step in Mn-induced inflammasome activation. The dysfunction at this stage may lead to impairment of lysosomal release of cathepsin B which is a critical factor of inflammasome activation and subsequent IL-1β secretion in microglia (Wang et al., 2017a).
Mn-induced inflammasome activation is also affected by dysregulation of mitochondrial dynamics/mitophagy (Zhou et al., 2011). Although current studies lack the mechanistic insight into the role of mitochondrial dysfunction in Mn-induced inflammasome activation, it has been reported that the mitochondrial fusion protein MFN2 may mediate an NLRP3-dependent inflammatory response (Sarkar et al., 2019). In fact, Mn promoted the release of inflammasome-containing exosomes from the microglia to trigger an inflammatory response to other adjacent cells in the brain microenvironment (Sarkar et al., 2019). These findings suggest that Mn-induced inflammasome activation might be critical for propagation of inflammation between cells and Mn-induced inflammatory neurotoxicity.
6. Effects of Mn on excitotoxicity
Growing evidence suggest that excitotoxic neuronal death is implicated in Mn neurotoxicity (Brouillet et al., 1993; Centonze et al., 2001). Excitotoxicity is caused by accumulation of excess glutamate in the extracellular space, resulting in overstimulation of glutamate receptors on postsynaptic neurons, leading to increased Ca2+ influx, mitochondrial impairment, oxidative/nitrosative stress and apoptosis (Ayata et al., 1997; Budd and Nicholls, 1996; Reynolds and Hastings, 1995). Studies have shown that Mn dysregulates astrocytic glutamate transporters as well as the N-methyl-d-aspartate (NMDA) receptor, which will lead to excitotoxic neuronal death.
6.1. Effects of Mn on glutamate transporters GLAST and GLT-1
Mn promotes accumulation of excess glutamate mostly by impairment of astrocytic glutamate transporters (Erikson and Aschner, 2002; Lee et al., 2009, 2012). These transporters are responsible for glutamate reuptake into astrocytes ensuring optimal glutamate levels in the synapse (Rothstein et al., 1996). Mn decreases expression of two main transporters, GLT-1 (EAAT2 in human) and GLAST (EAAT1 in human) (Lehre et al., 1995; Rothstein et al., 1994), which are responsible for the majority of glutamate reuptake from the extracellular space, leading to excitotoxic neuronal death (Rothstein et al., 1996).
Mn decreases expression of GLAST and GLT-1 at the transcriptional level (Karki et al., 2014, 2015; Lee et al., 2009), accompanied by decreased glutamate reuptake by astrocytes (Karki et al., 2014, 2015). Transcription factor Yin Yang 1 (YY1) and epigenetic modifier histone deacetylase (HDAC) play a critical role in Mn-induced reduction of EAAT1 and EAAT2 in astrocytes (Karki et al., 2014, 2015). The promoter regions of EAAT1 and EAAT2 contain multiple cis regulatory elements for YY1 binding to repress EAAT1/2 transcription. YY1 also binds to the promoter regions of GLAST and GLT-1, decreasing their expression in Bergmann glia cells (Rosas et al., 2007), rodent astrocyte cultures and mice (Karki et al., 2014, 2015; Yin et al., 2018). Mn increased YY1 expression at the transcriptional level since mutation of those YY1 binding sites or knockdown of YY1 abrogated Mn-induced reduction in promoter activities of EAAT1 and EAAT2 (Karki et al., 2014, 2015). Mn increased YY1 via NF-κB activation, given that the YY1 promoter contains binding motifs of NF-κB, and subsequently increased YY1 binding to the promoter regions of EAAT1 and EAAT2 (Karki et al., 2014, 2015). It appears that Mn-induced TNF-α enhances YY1 expression and activity via NF-κB, indicating that the TNF-α/NFκB/YY1 pathway is important for Mn-induced repression of EAAT1 and EAAT2 and subsequent glutamate-mediated excitotoxicity.
Epigenetic modifiers HDACs contribute to Mn′s repression of EAAT1 and EAAT2 by interacting with YY1. HDACs which remove acetyl groups from acetylated lysine of histone proteins repress gene expression (Kuo and Allis, 1998). We have previously reported that HDACs, including HDAC1 and 2, repressed EAAT1 and EAAT2 promoter activity, and were critically involved in Mn-induced repression on EAAT1/2. Mn increased interaction of YY1 with HDAC and binding of YY1/HDAC complex to the promoter regions of EAAT1/2 in astrocytes (Karki et al., 2014, 2015). Moreover, inhibition of HDACs protected mice against Mn-induced neurotoxicity by attenuating Mn-induced motor deficits, with concomitant reversal of Mn-induced reduction of GLAST and GLT-1 expression in mice (Johnson et al., 2018), indicating that HDACs, and potentially histone deacetylation, play a significant role in Mn-induced reduction of glutamate transporters and neurotoxicity.
Given the importance of astrocytic glutamate transporters in Mn-induced neurotoxicity, pharmacological agents have been shown to attenuate Mn-induced repression of EAAT1 and EAAT2 (Karki et al., 2014, 2017; Lee et al., 2009; Pajarillo et al., 2018). 17β-estradiol (E2) and selective estrogen receptor modulators (SERM) such as tamoxifen (TX) and raloxifene (RX) increased GLAST and GLT-1 levels in astrocytes (Karki et al., 2014; Lee et al., 2009), by activating their receptors, including ER-α, ER-β, and GPR30, and downstream signaling pathways. Furthermore, E2 and TX exerted neuroprotection against Mn-induced dopaminergic neurotoxicity in mice (Pajarillo et al., 2018), at least in part by attenuating Mn′s reduction of GLAST and GLT-1 in mice (Pajarillo et al., 2018). Studies have also shown that arundic acid, an S100β inhibitor, enhanced EAAT1 expressionand attenuated Mn-induced repression of EAAT1 in astrocytes (Karki et al., 2018).
6.2. Effects of Mn on NMDA receptors
Mn increased excitotoxic neurodegeneration and lesions in rat striatum which may have involved dysregulation of the NMDA receptor (Brouillet et al., 1993), indicating that Mn-induced excitotoxicity may be associated with glutamate receptors and its transporters. It appears that effects of Mn on NMDA receptors are different depending on the experimental conditions. Mn impaired the NMDA receptor in the mouse brain by decreasing its expression (Wang et al., 2017b; Xu et al., 2009) of mRNA and protein levels of its subunits such as NR1, NR2A and NR2B in the hippocampus of rats (Wang et al., 2017b). Mn-induced impairment of the NMDA receptor is accompanied by increased cell death, lactate dehydrogenase release and Ca2+ influx in primary neurons (Xu et al., 2009). Mn-induced impairment of glutamatergic activity leads to dysregulation of dopaminergic neurotransmission as inhibition of the NMDA receptor prevented Mn-inhibited dopamine release in mouse striatum (Cuesta de Di Zio et al., 1995), indicating that Mn can dysregulate dopaminergic neurotransmission by disrupting the crosstalk of dopaminergic and glutamatergic neurons. On the other hand, several studies have shown that reduction of the NMDA receptor expression appears to be a protective mechanism to prevent excitotoxicity as Mn reduced the density of NMDA recognition sites and NMDA receptors in the mouse brain (Cano et al., 1996, 1997). Some studies suggest that Mn impaired glutamatergic neurotransmission by unknown mechanisms instead of overactivation of postsynaptic glutamate receptors (Centonze et al., 2001). Studies of Mn effects on glutamatergic functions, particularly through NMDA receptors, are inconsistent in multiple experimental settings, showing that NMDA receptors are highly dynamic and sensitive in response to Mn exposure.
7. Concluding remarks
It is well established that Mn causes neurological disorders, but how Mn causes this neurotoxicity at the molecular level is not well understood. In the current review, we have discussed several aspects of Mn-induced toxicity mechanisms, highlighting the molecular mechanisms involved in Mn-induced neurotoxicity including mitochondrial dysfunction, autophagy, oxidative stress, inflammation and excitotoxicity. Given that a comprehensive understanding of Mn-induced neurotoxicity mechanisms will identify the molecular targets, studying further on those findings discussed in this review is critical to develop optimal neurotherapeutics to treat Mn toxicity.
Acknowledgment
This research was funded by National Institute of Environmental Health Sciences: R01 ES024756 (EL), R01 ES031282 (EL), R01 ES10563 (MA), and R01 ES07331.
References
- Afeseh Ngwa H, et al. , 2011. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol 256 (3), 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis M, et al. , 2016. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J. Allergy Clin. Immunol 138 (4), 984–1010. [DOI] [PubMed] [Google Scholar]
- Alaimo A, et al. , 2013. Manganese induces mitochondrial dynamics impairment and apoptotic cell death: a study in human Gli36 cells. Neurosci. Lett 554, 76–81. [DOI] [PubMed] [Google Scholar]
- Alaimo A, et al. , 2014. Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis. PLoS One 9 (3), e91848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, et al. , 2000. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet 26 (2), 211–215. [DOI] [PubMed] [Google Scholar]
- Alsulimani HH, Ye Q, Kim J, 2015. Effect of Hfe deficiency on memory capacity and motor coordination after manganese exposure by drinking water in mice. Toxicol. Res 31 (4), 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam V, et al. , 2002. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J. Neurosci 22 (5), 1738–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya SK, Roy BK, 2011. Manganese induced changes in growth, chlorophyll content and antioxidants activity in seedlings of broad bean (Vicia faba L.). J. Environ. Biol 32 (6), 707–711. [PubMed] [Google Scholar]
- Aschner JL, Aschner M, 2005. Nutritional aspects of manganese homeostasis. Mol. Aspects Med 26 (4–5), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audesse AJ, et al. , 2019. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. 15 (4), e1008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C, et al. , 1997. Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knock-out mice. J. Neurosci 17 (18), 6908–6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, et al. , 2006. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci. Lett 398 (1–2), 151–154. [DOI] [PubMed] [Google Scholar]
- Bahar E, Kim JY, Yoon H, 2017. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-kappaB and HO-1/Nrf2 pathways. Int. J. Mol. Sci 18 (9), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P, Pellegrini L, 2013. The dynamin GTPase OPA1: more than mitochondria? Biochim. Biophys. Acta 1833 (1), 176–183. [DOI] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH, 2013. The function of alpha-synuclein. Neuron 79 (6), 1044–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J, 1990. Behavior of mitochondria in the living cell. Int. Rev. Cytol 122, 1–63. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, et al. , 1993. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol 120 (1), 89–94. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG, 1996. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurochem 67 (6), 2282–2291. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ, 2000. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med 29 (3–4), 222–230. [DOI] [PubMed] [Google Scholar]
- Cai T, et al. , 2010. Manganese induces the overexpression of alpha-synuclein in PC12 cells via ERK activation. Brain Res. 1359, 201–207. [DOI] [PubMed] [Google Scholar]
- Cano G, Suarez-Roca H, Bonilla E, 1996. Manganese poisoning reduces strychnine-insensitive glycine binding sites in the globus pallidus of the mouse brain. Invest. Clin 37 (4), 209–219. [PubMed] [Google Scholar]
- Cano G, Suarez-Roca H, Bonilla E, 1997. Alterations of excitatory amino acid receptors in the brain of manganese-treated mice. Mol. Chem. Neuropathol 30 (1–2), 41–52. [DOI] [PubMed] [Google Scholar]
- Centonze D, et al. , 2001. Impaired excitatory transmission in the striatum of rats chronically intoxicated with manganese. Exp. Neurol 172 (2), 469–476. [DOI] [PubMed] [Google Scholar]
- Chan DC, 2012. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet 46, 265–287. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C, 2010. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci 1201, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Liao SL, 2002. Oxidative stress involves in astrocytic alterations induced by manganese. Exp. Neurol 175 (1), 216–225. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. , 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol 160 (2), 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, et al. , 2006. Manganese modulates pro-inflammatory gene expression in activated glia. Neurochem. Int 49 (1), 62–71. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. , 2018. Role of LRRK2 in manganese-induced neuroinflammation and microglial autophagy. Biochem. Biophys. Res. Commun 498 (1), 171–177. [DOI] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM, 2008. Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK. Toxicol. In Vitro 22 (1), 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM, 2011. Manganese modulation of MAPK pathways: effects on upstream mitogen activated protein kinase kinases and mitogen activated kinase phosphatase-1 in microglial cells. J. Appl. Toxicol 31 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta de Di Zio MC, et al. , 1995. Autoreceptor presynaptic control of dopamine release from striatum is lost at early stages of manganese poisoning. Life Sci. 56 (22), 1857–1864. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L, 2008. Mitofusin 2: a mitochondria-shaping protein with signaling roles beyond fusion. Antioxid. Redox Signal 10 (3), 621–633. [DOI] [PubMed] [Google Scholar]
- de Tollenaer SM, et al. , 2006. Life threatening central nervous system manifestations and hypothermia due to maneb intoxication in a child: a case report. Ther. Drug Monit 28 (6), 813–815. [DOI] [PubMed] [Google Scholar]
- Deng Y, et al. , 2015. Melatonin antagonizes Mn-induced oxidative injury through the activation of keap1-Nrf2-ARE signaling pathway in the striatum of mice. Neurotox. Res 27 (2), 156–171. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Filipov NM, 2011. Manganese potentiates LPS-induced heme-oxygenase 1 in microglia but not dopaminergic cells: role in controlling microglial hydrogen peroxide and inflammatory cytokine output. Neurotoxicology 32 (6), 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus EC, Meffert MK, 2019. Cellular specificity of NF-kappaB function in the nervous system. Front. Immunol 10, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson K, Aschner M, 2002. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology 23 (4–5), 595–602. [DOI] [PubMed] [Google Scholar]
- Erikson KM, et al. , 2006. Alterations of oxidative stress biomarkers due to in utero and neonatal exposures of airborne manganese. Biol. Trace Elem. Res 111 (1–3), 199–215. [DOI] [PubMed] [Google Scholar]
- Exil V, et al. , 2014. Activation of MAPK and FoxO by manganese (Mn) in rat neonatal primary astrocyte cultures. PLoS One 9 (5), e94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XM, et al. , 2020. Chronic manganese administration with longer intervals between injections produced neurotoxicity and hepatotoxicity in rats. Neurochem. Res 45 (8), 1941–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA, 2005. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol. Sci 84 (1), 139–148. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC, 2012. Nitric oxide synthases: regulation and function. Eur. Heart J 33 (7), 829–837. 837a–d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I, 1974. Superoxide dismutases. Adv. Enzymol. Relat. Areas Mol. Biol 41, 35–97. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, et al. , 2006. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol. Sci 92 (2), 516–525. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE, 1990. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem. J 266 (2), 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik M, et al. , 2017. Manganese neurotoxicity and protective effects of resveratrol and quercetin in preclinical research. Pharmacol. Rep 69 (2), 322–330. [DOI] [PubMed] [Google Scholar]
- Ghavami S, et al. , 2014. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol 112, 24–49. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, 2010. APLP1, Alzheimer’s-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology 31 (5), 572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, et al. , 1978. Uptake of calcium and manganese by rat liver submitochondrial particles. Ann. N. Y. Acad. Sci 307, 246–247. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, et al. , 2019. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci. Signal 12 (572), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Brody A, et al. , 2013. Mancozeb-induced behavioral deficits precede structural neural degeneration. Neurotoxicology 34, 74–81. [DOI] [PubMed] [Google Scholar]
- Hassani H, et al. , 2012. Occupational exposure to manganese-containing welding fumes and pulmonary function indices among natural gas transmission pipeline welders. J. Occup. Health 54 (4), 316–322. [DOI] [PubMed] [Google Scholar]
- Heid ME, et al. , 2013. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol 191 (10), 5230–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, et al. , 2004. Parkin attenuates manganese-induced dopaminergic cell death. J. Neurochem 89 (6), 1490–1497. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K, 2004. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci 117 (Pt. 26), 6535–6546. [DOI] [PubMed] [Google Scholar]
- Johnson J Jr., et al. , 2018. Valproic acid attenuates manganese-induced reduction in expression of GLT-1 and GLAST with concomitant changes in murine dopaminergic neurotoxicity. Neurotoxicology 67, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncos RA, Abdala J, Celis DO, 1968. Parkinsonism due to manganese intoxication. Rev. Fac. Cienc. Med. Cordoba 26 (1), 57–63. [PubMed] [Google Scholar]
- Kaminska B, et al. , 2009. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. (Hoboken) 292 (12), 1902–1913. [DOI] [PubMed] [Google Scholar]
- Karki P, et al. , 2014. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol. Cell. Biol 34 (7), 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, et al. , 2015. Transcriptional regulation of the astrocytic excitatory amino acid transporter 1 (EAAT1) via NF-kappaB and Yin Yang 1 (YY1). J. Biol. Chem 290 (39), 23725–23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, et al. , 2017. Transcriptional regulation of human transforming growth factor-alpha in astrocytes. Mol. Neurobiol 54 (2), 964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, et al. , 2018. Arundic acid increases expression and function of astrocytic glutamate transporter EAAT1 via the ERK, Akt, and NF-kappaB pathways. Mol. Neurobiol 55 (6), 5031–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson MA, et al. , 2016. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx. Am. J. Physiol. Cell Physiol 311 (1), C83–C100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CM, et al. , 2017. A manganese pre-catalyst: mild reduction of amides, ketones, aldehydes, and esters. Angew. Chem. Int. Ed. Engl 56 (50), 15901–15904. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. , 2019. LRRK2 kinase plays a critical role in manganese-induced inflammation and apoptosis in microglia. PLoS One 14 (1), e0210248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley KS, et al. , 2017. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflammation 14 (1), 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, et al. , 2005. Activation of protein kinase C delta by proteolytic cleavage contributes to manganese-induced apoptosis in dopaminergic cells: protective role of Bcl-2. Biochem. Pharmacol 69 (1), 133–146. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, et al. , 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol 24 (16), 7130–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, et al. , 2014. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch. Toxicol 88 (1), 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MH, Allis CD, 1998. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20 (8), 615–626. [DOI] [PubMed] [Google Scholar]
- Kurzatkowski DM, Trombetta LD, 2013. Maneb causes pro-oxidant effects in the hippocampus of Nrf2 knockout mice. Environ. Toxicol. Pharmacol 36 (2), 427–436. [DOI] [PubMed] [Google Scholar]
- Lai JC, et al. , 1999. Manganese mineral interactions in brain. Neurotoxicology 20 (2–3), 433–444. [PubMed] [Google Scholar]
- Latronico T, et al. , 2013. Impact of manganese neurotoxicity on MMP-9 production and superoxide dismutase activity in rat primary astrocytes. Effect of resveratrol and therapeutical implications for the treatment of CNS diseases. Toxicol. Sci 135 (1), 218–228. [DOI] [PubMed] [Google Scholar]
- Lee ES, et al. , 2009. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J. Neurochem 110 (2), 530–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, et al. , 2012. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia 60 (7), 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, et al. , 1995. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J. Neurosci 15 (3 Pt. 1), 1835–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. , 2011. Nrf2/HO-1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin-proteasome pathway, not MAPKs signaling. J. Appl. Toxicol 31 (7), 690–697. [DOI] [PubMed] [Google Scholar]
- Liao SL, et al. , 2007. Induction of cyclooxygenase-2 expression by manganese in cultured astrocytes. Neurochem. Int 50 (7–8), 905–915. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. , 2017. Downregulation of Mfn2 participates in manganese-induced neuronal apoptosis in rat striatum and PC12 cells. Neurochem. Int 108, 40–51. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. , 2018. Effect of the cross-talk between autophagy and endoplasmic reticulum stress on Mn-induced alpha-synuclein oligomerization. Environ. Toxicol 33 (3), 315–324. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. , 2020. IRE1 signaling pathway mediates protective autophagic response against manganese-induced neuronal apoptosis in vivo and in vitro. Sci. Total Environ 712, 136480. [DOI] [PubMed] [Google Scholar]
- Ma Z, et al. , 2017. The role S-nitrosylation in manganese-induced autophagy dysregulation in SH-SY5Y cells. Environ. Toxicol 32 (12), 2428–2439. [DOI] [PubMed] [Google Scholar]
- Ma Z, et al. , 2020. Manganese induces autophagy dysregulation: the role of S-nitrosylation in regulating autophagy related proteins in vivo and in vitro. Sci. Total Environ 698, 134294. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, et al. , 2007. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy 3 (4), 374–376. [DOI] [PubMed] [Google Scholar]
- Malthankar GV, et al. , 2004. Differential lowering by manganese treatment of activities of glycolytic and tricarboxylic acid (TCA) cycle enzymes investigated in neuroblastoma and astrocytoma cells is associated with manganese-induced cell death. Neurochem. Res 29 (4), 709–717. [DOI] [PubMed] [Google Scholar]
- Milatovic D, et al. , 2007. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Sci 98 (1), 198–205. [DOI] [PubMed] [Google Scholar]
- Morello M, et al. , 2008. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats an electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology 29 (1), 60–72. [DOI] [PubMed] [Google Scholar]
- Moreno JA, et al. , 2011. Manganese-induced NF-kappaB activation and nitrosative stress is decreased by estrogen in juvenile mice. Toxicol. Sci 122 (1), 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, et al. , 1989. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp. Neurol 106 (3), 251–258. [DOI] [PubMed] [Google Scholar]
- Nkpaa KW, Onyeso GI, Kponee KZ, 2019. Rutin abrogates manganese-induced striatal and hippocampal toxicity via inhibition of iron depletion, oxidative stress, inflammation and suppressing the NF-kappaB signaling pathway. J. Trace Elem. Med. Biol 53, 8–15. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S, 2009. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol 1 (4), a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereafor U, et al. , 2020. Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health 17 (7), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E, et al. , 2018. 17beta-estradiol and tamoxifen protect mice from manganese-induced dopaminergic neurotoxicity. Neurotoxicology 65, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E, et al. , 2019. The role of posttranslational modifications of alpha-synuclein and LRRK2 in Parkinson’s disease: potential contributions of environmental factors. Biochim. Biophys. Acta Mol. Basis Dis 1865 (8), 1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E, et al. , 2020. The transcription factor REST up-regulates tyrosine hydroxylase and antiapoptotic genes and protects dopaminergic neurons against manganese toxicity. J. Biol. Chem 295 (10), 3040–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB, 1999. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20 (2–3), 227–238. [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, Tavernarakis N, 2015. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 22 (9), 1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri M, et al. , 2011. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet 20 (19), 3852–3866. [DOI] [PubMed] [Google Scholar]
- Palzes VA, et al. , 2019. Manganese exposure and working memory-related brain activity in smallholder farmworkers in Costa Rica: results from a pilot study. Environ. Res 173, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popichak KA, et al. , 2018. Glial-neuronal signaling mechanisms underlying the neuro-inflammatory effects of manganese. J. Neuroinflammation 15 (1), 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K, et al. , 2008. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res. Bull 76 (4), 361–367. [DOI] [PubMed] [Google Scholar]
- Puskin JS, et al. , 1976. Evidence for more than one Ca2+ transport mechanism in mitochondria. Biochemistry 15 (17), 3834–3842. [DOI] [PubMed] [Google Scholar]
- Ramesh GT, Ghosh D, Gunasekar PG, 2002. Activation of early signaling transcription factor, NF-kappaB following low-level manganese exposure. Toxicol. Lett 136 (2), 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG, 1995. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci 15 (5 Pt. 1), 3318–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JLG, et al. , 2018. Airborne manganese exposure and neurobehavior in school-aged children living near a ferro-manganese alloy plant. Environ. Res 167, 66–77. [DOI] [PubMed] [Google Scholar]
- Rojo M, et al. , 2002. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci 115 (Pt. 8), 1663–1674. [DOI] [PubMed] [Google Scholar]
- Rosas S, et al. , 2007. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: a role for YY1. J. Neurochem 101 (4), 1134–1144. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. , 1994. Localization of neuronal and glial glutamate transporters. Neuron 13 (3), 713–725. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. , 1996. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16 (3), 675–686. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J, 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev 68 (2), 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi L, Babadi VY, Tanwir F, 2018. Manganese dioxide nanoparticle induces Parkinson like neurobehavioral abnormalities in rats. Bratisl. Lek. Listy 119 (6), 379–384. [DOI] [PubMed] [Google Scholar]
- Safiulina D, et al. , 2019. Miro proteins prime mitochondria for Parkin translocation and mitophagy. EMBO J. 38 (2), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, et al. , 2012. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 292 (2–3), 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, et al. , 2018. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 64, 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, et al. , 2019. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal 12 (563), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA, 2008. Oxidative stress and neurotoxicity. Chem. Res. Toxicol 21 (1), 172–188. [DOI] [PubMed] [Google Scholar]
- Scott SV, Klionsky DJ, 1998. Delivery of proteins and organelles to the vacuole from the cytoplasm. Curr. Opin. Cell Biol 10 (4), 523–529. [DOI] [PubMed] [Google Scholar]
- Searles Nielsen S, et al. , 2015. Inducible nitric oxide synthase gene methylation and parkinsonism in manganese-exposed welders. Parkinsonism Relat. Disord 21 (4), 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, et al. , 2007. Gene expression profiling of human primary astrocytes exposed to manganese chloride indicates selective effects on several functions of the cells. Neurotoxicology 28 (3), 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, et al. , 2011. TFEB links autophagy to lysosomal biogenesis. Science 332 (6036), 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settivari R, et al. , 2013. The Nrf2/SKN-1-dependent glutathione S-transferase pi homologue GST-1 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of manganism. Neurotoxicology 38, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard KA, et al. , 1999. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol. Cell. Biol 19 (9), 6367–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, et al. , 2017. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun 482 (3), 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, et al. , 2007. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol 178 (5), 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, et al. , 2009. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 20 (15), 3525–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, et al. , 2016. Manganese-disrupted interaction of dopamine D1 and NMDAR in the striatum to injury learning and memory ability of mice. Mol. Neurobiol 53 (10), 6745–6758. [DOI] [PubMed] [Google Scholar]
- Song D, et al. , 2017. FOXO3 promoted mitophagy via nuclear retention induced by manganese chloride in SH-SY5Y cells. Metallomics 9 (9), 1251–1259. [DOI] [PubMed] [Google Scholar]
- Sriram K, et al. , 2012. Manganese accumulation in nail clippings as a biomarker of welding fume exposure and neurotoxicity. Toxicology 291 (1–3), 73–82. [DOI] [PubMed] [Google Scholar]
- Stephenson AP, et al. , 2013. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol. Lett 218 (3), 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streifel KM, et al. , 2012. Gene deletion of nos2 protects against manganese-induced neurological dysfunction in juvenile mice. Toxicol. Sci 126 (1), 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Camardiel M, et al. , 2002. Differential regulation of glutamic acid decarboxylase mRNA and tyrosine hydroxylase mRNA expression in the aged manganese-treated rats. Brain Res. Mol. Brain Res 103 (1–2), 116–129. [DOI] [PubMed] [Google Scholar]
- Tong M, Dong M, de la Monte SM, 2009. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. J. Alzheimers Dis 16 (3), 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, 2003. Mitochondrial formation of reactive oxygen species. J. Physiol 552 (Pt. 2), 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, et al. , 2004. Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. J. Biomed. Biotechnol 2004 (4), 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verina T, Schneider JS, Guilarte TR, 2013. Manganese exposure induces alpha-synuclein aggregation in the frontal cortex of non-human primates. Toxicol. Lett 217 (3), 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan B, et al. , 2019. Spermine protects alpha-synuclein expressing dopaminergic neurons from manganese-induced degeneration. Cell Biol. Toxicol 35 (2), 147–159. [DOI] [PubMed] [Google Scholar]
- Villeneuve NF, Lau A, Zhang DD, 2010. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal 13 (11), 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, et al. , 2017a. The role of NLRP3-CASP1 in inflammasome-mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal-dependent impairment of learning and memory ability. Autophagy 13 (5), 914–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. , 2017b. The effect of postnatal manganese exposure on the NMDA receptor signaling pathway in rat hippocampus. J. Biochem. Mol. Toxicol 31 (12), 1–6. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. , 2018a. Plant species diversity for vegetation restoration in manganese tailing wasteland. Environ. Sci. Pollut. Res. Int 25 (24), 24101–24110. [DOI] [PubMed] [Google Scholar]
- Wang TY, et al. , 2018b. Manganese-induced alpha-synuclein overexpression impairs synaptic vesicle fusion by disrupting the Rab3 cycle in primary cultured neurons. Toxicol. Lett 285, 34–42. [DOI] [PubMed] [Google Scholar]
- Westermann B, 2010. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol 11 (12), 872–884. [DOI] [PubMed] [Google Scholar]
- Xu B, Xu ZF, Deng Y, 2009. Effect of manganese exposure on intracellular Ca2+ homeostasis and expression of NMDA receptor subunits in primary cultured neurons. Neurotoxicology 30 (6), 941–949. [DOI] [PubMed] [Google Scholar]
- Xu B, et al. , 2014. Alpha-synuclein is involved in manganese-induced ER stress via PERK signal pathway in organotypic brain slice cultures. Mol. Neurobiol 49 (1), 399–412. [DOI] [PubMed] [Google Scholar]
- Yan DY, et al. , 2019a. Mn-induced neurocytes injury and autophagy dysfunction in alpha-synuclein wild-type and knock-out mice: highlighting the role of alpha-synuclein. Neurotox. Res 36 (1), 66–80. [DOI] [PubMed] [Google Scholar]
- Yan D, et al. , 2019b. Corynoxine B ameliorates HMGB1-dependent autophagy dysfunction during manganese exposure in SH-SY5Y human neuroblastoma cells. Food Chem. Toxicol 124, 336–348. [DOI] [PubMed] [Google Scholar]
- Yang X, et al. , 2018. Mn inhibits GSH synthesis via downregulation of neuronal EAAC1 and astrocytic xCT to cause oxidative damage in the striatum of mice. Oxid. Med. Cell. Longev 2018, 4235695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, et al. , 2018. Astrocyte elevated gene-1 is a novel regulator of astrogliosis and excitatory amino acid transporter-2 via interplaying with nuclear factor-kappaB signaling in astrocytes from amyotrophic lateral sclerosis mouse model with hSOD1(G93A) mutation. Mol. Cell. Neurosci 90, 1–11. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. , 2003. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J. Neurochem 84 (2), 336–346. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. , 2013. The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotox. Res 24 (4), 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. , 2017. Role of histone acetylation in activation of nuclear factor erythroid 2-related factor 2/heme oxygenase 1 pathway by manganese chloride. Toxicol. Appl. Pharmacol 336, 94–100. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. , 2019. Dysregulation of TFEB contributes to manganese-induced autophagic failure and mitochondrial dysfunction in astrocytes. Autophagy 16, 1506–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, et al. , 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469 (7329), 221–225. [DOI] [PubMed] [Google Scholar]
- Zhou Q, et al. , 2018. Autophagy plays a protective role in Mn-induced toxicity in PC12 cells. Toxicology 394, 45–53. [DOI] [PubMed] [Google Scholar]


