ABSTRACT
Background:
Selective dorsal rhizotomy (SDR) is one of the surgical alternatives for treating spasticity, especially in children with spastic diplegia secondary to cerebral palsy (CP). It is becoming increasingly used, and the results of this operation need to be further highlighted.
Aim:
The main objective of this article was to present the results of such surgical procedure in a cohort of a specialized center, with a particular focus on a quantitative analysis (goniometry).
Materials and Methods:
Retrospective review of the medical records and gait analyses of a cohort of 34 patients diagnosed with CP submitted to elective SDR at our institution, in a period of 6 years, was carried out. All patients underwent a thorough clinical and neurological assessment, gait analysis at a dedicated laboratory, and magnetic resonance imaging of whole neuro-axis.
Statistical Analysis:
For continuous quantitative variables (goniometric angles and muscle tone), a t-student test was used. A scatterplot regression analysis was used for the comparison of modified Ashworth scale (mAS) scores and goniometry measurements.
Results and Conclusion:
In a mean follow-up of 3.2 years, SDR provides a measurable and consistent improvement in the motor function of spastic patients, as per range of motion and tonus scales, with low complication rates. It also allows for patients to reduce their use of muscle relaxants, even though their global mobility does not change significantly. Therefore, it should be considered for CP patients who suffer with the deleterious effects of spasticity.
KEYWORDS: Cerebral palsy, dorsal rhizotomy, outcome, spastic diple, spasticity
INTRODUCTION
Most patients with cerebral palsy (CP) will develop lower limb spasticity during their lifetime. Although it may not be clinically relevant during the first years, spasticity ultimately damages the musculoskeletal system and leads to a number of problems, such as progressive gait disturbances, pain, decreased functional independence, and hygiene/self-care difficulties.[1] Moreover, it does not disappear spontaneously and tends to worsen with time.[2] Therefore, multiple therapeutic modalities have been used in an attempt to overcome the detrimental effects of spasticity.
Partial section of dorsal lumbar rootlets, called selective dorsal rhizotomy (SDR), has been used for over 30 years in spastic patients.[3,4,5,6,7] The rationale for this procedure is to interrupt the reflex arc, which is hyperactive in CP patients, and it leads to increased muscle tone and excessive tendinous contraction, as part of the upper motor neuron syndrome.[8]
There is a growing body of evidence to support the beneficial effects of SDR in spastic children.[1,8,9,10] The purpose of this study is to describe our own experience with this therapeutic modality and thus contribute with additional data regarding the outcome of CP patients that underwent SDR in a single-tertiary center with a comprehensive rehabilitation program.
MATERIALS AND METHODS
This study consisted of a retrospective review of the medical records and gait analyses of a cohort of 34 patients diagnosed with CP and submitted to elective SDR at our institution, in a period of 6 years (2012–2018). All patients underwent a thorough clinical and neurological assessment, gait analysis at a dedicated laboratory, and magnetic resonance imaging (MRI) of whole neuro-axis. They were all evaluated regularly at our spasticity multidisciplinary clinic, which includes pediatric neurosurgeons, developmental neurologists, pediatricians, psychologists, physical and speech and language therapists, and social workers.
Inclusion criteria consisted of predominant spastic diplegia or diparesis, limited function, absence or minimal concomitant dystonia, absence of other etiologies of spasticity (as ruled out by neuroimaging), and a supportive family with adequate cognitive and motivational ability. Patients with non-CP-related spasticity and severe extrapyramidal pathology were excluded.
Patients’ demographics, such as age at surgery, gender, subtype of CP, ambulation level as per the Gross Motor Function Classification System (GMFCS), use of muscle relaxants (baclofen and/or biperiden), and surgical complications, were noted. Spasticity outcome was assessed by means of changes in muscle tone (modified Ashworth scale [mAS]), range of motion (ROM) of hip abduction, hamstring and foot dorsiflexion muscles, as well as post-operative GMFCS, and the opinion of patients and caregivers about aspects of their quality of life after surgery. All post-operative evaluations reported herein were taken at 1-year follow-up consultations.
The surgical technique used herein for SDR is the single-level laminotomy, as devised by Park et al.[7] In this technique, the patient is positioned prone and well-padded under areas of bony prominences (shoulders, iliac crests, and knees). The level of the conus medullaris, as seen previously in the MRI scans, is confirmed by fluoroscopy. A 5- 8-cm-long straight lumbar incision is made at this level, and the vertebral laminae (usually around L1) are exposed up to the articular facets laterally. Just prior to dural opening, the patient is put on a slight Trendelenburg position, to avoid excessive cerebrospinal fluid (CSF) drainage, and a direct ultra-sound scan is performed to further assess the position of the conus [Figure 1].
Figure 1.
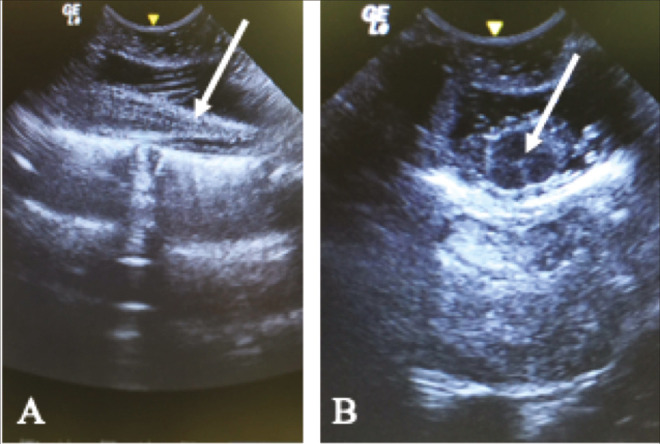
Ultrasound-guided identification of the conus medullaris. (A) Sagittal and (B) Axial incidences. The conus is usually hypoechoic (arrow on A), while the central canal appears as a hyperechogenic point (arrow in B)
The dura-mater is opened longitudinally and tacked up, and the arachnoid membranes are removed. The L1 rootlets are identified by direct visualization of the respective foramina, thus allowing for further identification of the remaining lumbar and sacral rootlets. The dentate ligament and the cleft between ventral and dorsal rootlets are important anatomical landmarks for separation of the motor rootlets, which are isolated using a plastic or cottonoid pattie, along with lower sacral roots. Correct isolation of L1-S1 rootlets is paramount to avoid undesirable post-operative motor and sphincter deficits.
Afterwards, each dorsal root is individually isolated and responses to electrical stimulation with a threshold voltage are recorded from the lower-extremity muscles [Figure 2], using single constant square-wave pulses of 0.1-ms duration at a rate of 0.5 Hz, followed by a gradual increase in intensity until the lower muscular threshold is achieved. At this point, they are further subdivided in three to five fascicles of similar size. Then, a tetanic stimulation with a frequency of 50 Hz is performed in these same subdivisions. The ones that generate the highest degrees of sustained responses, usually in distant and/or contralateral muscular segments, are sectioned. Our policy is to sever 50–75% of a given dorsal rootlet to provide maximum improvement of spasticity while preventing complications related to sensory loss. Motor evoked potentials are also checked after each sectioning to further avoid post-operative deficits.
Figure 2.
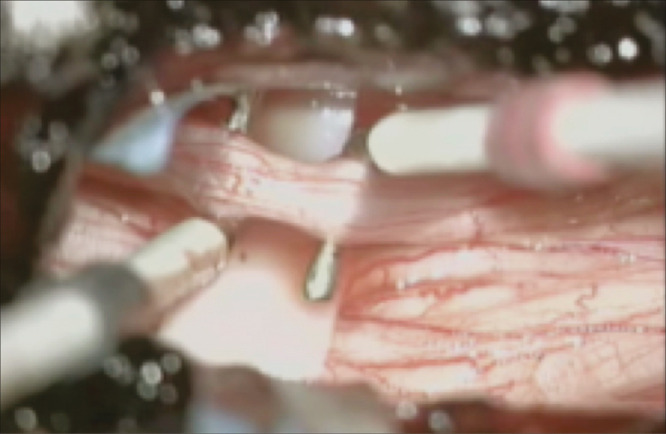
Intraoperative photograph depicting isolation of the L2 sensory nerve root. After threshold stimulation, it is then further subdivided in three to four rootlets which will be sectioned according to their neurophysiological responses
Patients are kept in a semi-intensive care unit for 12–24h, for analgesia and neuro-observation. They can be usually transferred to the pediatric ward on the first day after surgery, where pain control is continued and the bladder catheter is removed. Physical therapy is resumed on the third to fifth post-operative days and children can be discharged 2–3 days later, with a thorough outpatient care plan.
Statistical analysis
All statistical analyses were performed with the statistical package GraphPad Prism v. 6.0 (GraphPad Software, Inc., 2014). For continuous quantitative variables (goniometric angles and muscle tone), a t-student test was used. A scatterplot regression analysis was used for the comparison of mAS scores and goniometry measurements.
RESULTS
Thirty-four patients were analyzed in this study (17 males and 17 females), averaging 13.1 years of age (range 5.3–25.4 years). Mean age at surgery was 9.9 years (range 4.9–22.3 years, median 9.05 years), with a mean follow-up of 3.2 years (range 1.1–6.3 years). Their pre-operative spasticity status was: 15 with paraparesis, 11 with triparesis, and 8 with tetraparesis. To evaluate outcome, as mentioned above, we have used the following assessments: GMFCS grade, goniometry, and muscle tone as per the mAs.
Gross Motor Function Classification System
Initial GMFCS of patients was distributed as follows: 2 were GMFCS grade II, 8 grade III, 19 grade IV, and 5 grade V [Table 1]. Six patients (17.6%) experienced improvement in their GMFCS after SDR, while the remaining 28 (82.4%) maintained their pre-operative status; in the follow-up period studied herein, no decline in GMFCS function grades was observed. Improvement in GMFCS was higher in grades II and III, as shown in [Table 1].
Table 1.
Pre- and post-operative GMFCS score of spastic patients submitted to SDR
| GMFCS (pre-operative) | Patients | Improved | % |
|---|---|---|---|
| II | 2 | 1 | 50 |
| III | 8 | 2 | 25 |
| IV | 19 | 3 | 15,7 |
| V | 5 | 0 | 0 |
Patients in the ambulatory groups (II and III) were more prone to improvement in their pre-operative GMFCS than non-ambulatory ones (IV and V)
Goniometry/ROM
To evaluate ROM of the lower limbs, we have measured hip abduction, unilateral popliteal, and foot dorsiflexion angles, using a standard goniometer. Pre-operatively, mean hip abduction angles were 21.61 and 21.47degrees on the right and left, respectively, whereas they became 33.82 and 34.41 after SDR, with an increase of 12.21 (56.4%) and 12.94 (60.7%) (P < 0.01 for both sides; confidence intervals [CIs] of 7.86–16.55 on the right and 9.87–16.01 on the left).
For the unilateral popliteal angle, the following measurements were obtained: before SDR, 63.97degrees on the right limb and 66.28 on the left limb; after SDR, 51.03degrees on the right and 51.47 on the left, resulting in a reduction of 12.94 (20.2%) and 14.71 (22.1%) degrees on the right and left, respectively (P < 0.01 for both sides; CI of 8.98–16.90 on the right and 10.29–19.13 on the left).
Lastly, regarding foot dorsiflexion angles, results were as follows: pre-operatively, 10.14degrees on the right and 8.97 on the left. Post-operatively, 18.82 on right (improvement of 8.68degrees - 86.1%) and 15.88 on the left (improvement of 6.91degrees – 77.5%) (P < 0.01 for the right and P = 0.02 for the left; CI of 6.05–11.3 on the right side and 4.72–9.1 on the left side).
Modified Ashworth scale
Muscle tone of the abductors, hamstring, and triceps surae muscle groups was also evaluated by means of the mAS [Table 2]. Reduction of the average scores was seen in all groups after SDR: 1.53 for the abductors, 1.61 for the hamstring muscles, and 1.79 for the triceps surae (P = 0.08, 0.015, and 0.01, respectively).
Table 2.
Pre- and post-operative muscle tone scores of patients submitted to SDR, as per the modified Ashworth scale
| Mean pre-operative mAS | Mean post-operative mAS | P | |
|---|---|---|---|
| Abductors | 2.53 | 1 | 0.008 |
| Isquio | 2.76 | 1.15 | 0.015 |
| Triceps | 2.76 | 0.97 | 0.01 |
A statistically significant reduction after surgery was found
We have also performed a scatterplot analysis of the linear relationship between goniometry values and mAS scores. This resulted in r values (correlation coefficients) of 0.82, 0.71 and 0.97 between goniometry of hip abduction and abductors tone, the unilateral popliteal angle and hamstrings tone, and foot dorsiflexion and triceps surae tone, respectively.
Use of muscle relaxants
Nineteen patients (55.8%) were using prescribed muscle relaxants regularly prior to SDR (17 baclofen: 89.4%/2 biperiden: 10.6%), which could be weaned off in 11 (57.8%) of them (10 used baclofen and 1 biperiden). This was statistically significant (P = 0.032).
Complications
Surgical complications occurred in two patients (5.8%): one had a CSF leak that required reoperation for closure of a dural dehiscence; another one had a superficial wound infection that was properly treated with systemic antibiotics. We also had a patient who developed post-operative paraplegia secondary to an autoimmune plexopathy, confirmed by electromyography, which was reversed after a course of immunoglobulin; however, we could not find any direct relationship between these symptoms and the surgical procedure itself. All patients recovered well with no further problems. Neuropathic pain was reported by four patients (11.7%) and subsided with gabapentin in all; two patients required continuous prescription and were still using it by the time of their last follow-up.
DISCUSSION
The aim of the present study was to demonstrate that SDR is a safe and effective procedure to reduce spasticity related to CP. In such patients, spastic lower limbs can be a significant drawback for functional and gait rehabilitation as well as the cause of pain and contractures. Our data add to the strong body of evidence published in the literature confirming the benefits of this operation.[8,9,10,11,12,15] Also, we were able to provide quantitative data, using goniometric angular values, which alone confirm loosening of spastic muscles, but which also correlate positively with spasticity improvement as analyzed by muscle tone scales (mAS).
In comparison with the implantation of pumps for intrathecal baclofen therapy (ITB), another frequently used method for managing spasticity of cerebral origin, SDR, offers several advantages: although they both decrease tone and increase ROM, SDR has a larger magnitude effect of reducing the degree of spasticity and improving function.[10] Likewise, there are complications related to the intrathecal device itself, such as infection and the need for further refilling, as well as baclofen dose-related problems (withdrawal or overdose),[11] thus requiring frequent visits to spasticity clinics. On the other hand, intrathecal baclofen is certainly more effective in the control of extrapyramidal symptoms, especially dystonia.[13,14,15] Interestingly, no differences in the occurrence of orthopedic deformities (notably hip dislocations and scoliosis) have been seen after both procedures (SDR and ITB).[16,17] Due to local particularities also, it has been the authors’ policy to manage pure spastic diplegia with SDR and use ITB mainly for dystonic patients.
Regarding motor function, our results have not shown any worsening GMFCS status; patients have either improved or remained in the same group after SDR. Ailon et al.,[18] reported even better results, with 84% of his patients showing GMFCS improvement or stability on early follow-up, whereas Langerak et al.[19] reported improved GMFCS level in 58% of children and no change in 42%. Improvement in motor function as per GMFCS stratification was higher in levels II/III, and modest or no improvement was seen in groups IV/V, which is in keeping with previous reports.[8,18,20,21] It is important to have this in mind while discussing the goals of surgery with the patients and his/her family and/or caregivers, as results may vary according to pre-operative status.
For the analysis of ROM, we have used goniometry, which is a standard quantitative method for the measurement of angles formed by any given joints. In order to evaluate both proximal and distal motion, hip abduction, unilateral popliteal, and foot dorsiflexion angles were selected. Substantial improvement was seen in all of them, reaching strong statistical significance. This is very much in accordance with the current medical literature, which shows similar results.[1,9,20] Gul et al. have found the following degrees of hip abduction ROM (pre-operative, 1-year, and 5-year follow-up, respectively): 20.4, 39.9, and 31.7.[22] These authors state that there has been significant increase at 1 and 5 years compared to baseline, and that there was some regression between 1 and 5 years, though not to the baseline level.
Similarly, Nordmark et al.,[23] studying 35 children consecutively operated, have found an increased average ROM during a 5-year follow-up for hip abduction, popliteal angle, and ankle dorsiflexion (P > 0.001). The greatest changes were detected at 6 months after SDR. Similar results were reported by Dudley et al., Tedroff et al., and Ailon et al.,[18,20,21] even though there remains some controversy regarding the long-term effects of SDR; while all these groups demonstrated satisfactory results in the quality of life after more than 10 years post-operatively, two of them[18,21] found that ROM angles were close to pre-operative baseline measurements after more than 10 and 17 years of follow-up.
Increased tonus of lower limb muscles is also another factor for impaired quality of life in spastic patients, making their mobilization and hygiene more difficult. The goal of SDR, in this aspect, is to reduce muscle tone and facilitate passive motion of the legs. To evaluate this matter, we have used the mAS, which is the most universally accepted tone rating scale. Our results have shown significant reduction in the mean mAS both proximally and distally, as seen by tone scores of abductors, hamstrings, and foot dorsiflexion muscles. Furthermore, these findings were parallel to the changes in the ROM, which was confirmed statistically by a positive linear correlation. Most patients were also able to withdraw or diminish the use of muscle relaxants.
Ailon et al.[18] have shown that lower limb spasticity decreased significantly from pre-operative to early post-operative evaluation, with a mean drop of 1.5 in the mAS score. Interestingly, in their series, there was further decline at late follow-up too, for a total decrease in mean mAS of 2.3. Tedroff et al.[21] also found significantly lower mAS scores in the long-term follow-up (10 years). The group of Engsberg et al.,[24] studying 60 patients, both disabled and non-disabled, found that SDR offers a reduction of spasticity (thus providing functional gains) at 8 and 20 months after surgery. Agrawal et al.[11] performed a systematic review of the role of SDR in the management of post-traumatic spasticity, whose qualitative analysis included 10 studies and 57 patients; they have found that almost all reports demonstrate favorable outcomes, with follow-up mAS scores of 1 to 2 in the majority of patients, who usually attain improved ambulatory status. They have also concluded that slightly better outcomes have been reported for SDR following spinal cord injury in comparison with traumatic brain injury cases.
Lastly, it is currently well established that SDR has a low rate of complications. Overall, we had a 5.8% complication rate which was directly linked to the surgical procedure. This rate is analogous to the ones published in other series: Park et al.,[7] studying his large case series of over 3800 patients, reported on only seven cases of CSF leak and three cases of wound infections, which were resolved straightforwardly. None of his patients developed urinary incontinence or muscle weakness directly related to rhizotomy, with an overall major complication rate of 0.3%. Post-operative neuropathic pain and dysesthesia can be expected, and usually resolve either spontaneously or with the use of anti-neuropathic medication. We had only 11.7% of patients with such symptom, and they were all resolved within 2–3 months. Jeffery et al.[25] had a rate of post-operative neuropathic pain of 5.3% and sensory symptoms of 8.7%, with persistent neuropathic symptoms reported in 6.5% at 24 months. Earlier studies by Abbott et al.[26] and Steinbok and Schrag[27] report higher complication rates, of 17.5% and 43.6%, respectively; however, these data might reflect further refinements of the surgical technique, since these patients were all operated on before 2003. We have not experienced any other complications such as urinary retention, orthostatic headache, nausea / vomiting, and urine or chest infections, but these might rarely occur.[25]
As for limitations, this was a retrospective case-note review of a single center (with well-known drawbacks), in which follow-up symptoms were reported by parents and caregivers, and this may underestimate the rate of post-operative undesired events, as well as overestimate positive results. Also, our results were based on a short-term follow-up, and, as mentioned above, some patients might have a different spasticity status on the long-term. Nevertheless, it should be noted that currently several reports confirm the beneficial effects of SDR after 10–20 years of follow-up, as mentioned above,[18,20,21,28] from functional, motor and quality of life standpoints. Therefore, there seems to remain no doubt that SDR is a strong tool that will certainly continue to be part of the pediatric neurosurgeons’ armamentarium for the management of spastic children in the foreseeable future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Park TS, Dobbs MB, Cho J. Evidence supporting selective dorsal rhizotomy for treatment of spastic cerebral palsy. Cureus. 2018;10:e3466. doi: 10.7759/cureus.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth MD, Peacock WJ. The surgical treatment of spasticity. Muscle Nerve. 2000;23:153–63. doi: 10.1002/(sici)1097-4598(200002)23:2<153::aid-mus3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Peacock WJ, Staudt LA. Spasticity in cerebral palsy and the selective posterior rhizotomy procedure. J Child Neurol. 1990;5:179–85. doi: 10.1177/088307389000500303. [DOI] [PubMed] [Google Scholar]
- 4.Peacock WJ, Arens LJ. Selective posterior rhizotomy for the relief of spasticity in cerebral palsy. S Afr Med J. 1982;62:119–24. [PubMed] [Google Scholar]
- 5.Georgoulis G, Brînzeu A, Sindou M. Dorsal rhizotomy for children with spastic diplegia of cerebral palsy origin: usefulness of intraoperative monitoring. J Neurosurg Pediatr. 2018;22:89–101. doi: 10.3171/2018.1.PEDS17577. [DOI] [PubMed] [Google Scholar]
- 6.Sindou M, Georgoulis G. Keyhole interlaminar dorsal rhizotomy for spastic diplegia in cerebral palsy. Acta Neurochir (Wien) 2015;157:1187–96. doi: 10.1007/s00701-015-2453-1. [DOI] [PubMed] [Google Scholar]
- 7.Park TS, Johnston JM. Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note. Neurosurg Focus. 2006;21:e7. [PubMed] [Google Scholar]
- 8.Bolster EA, van Schie PE, Becher JG, van Ouwerkerk WJ, Strijers RL, Vermeulen RJ. Long-term effect of selective dorsal rhizotomy on gross motor function in ambulant children with spastic bilateral cerebral palsy, compared with reference centiles. Dev Med Child Neurol. 2013;55:610–6. doi: 10.1111/dmcn.12148. [DOI] [PubMed] [Google Scholar]
- 9.Grunt S, Becher JG, Vermeulen RJ. Long-term outcome and adverse effects of selective dorsal rhizotomy in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2011;53:490–8. doi: 10.1111/j.1469-8749.2011.03912.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharan D. Recent advances in management of cerebral palsy. Indian J Pediatr. 2005;72:969–73. doi: 10.1007/BF02731674. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal M, Samala R, Doddamani R, Agrawal D, Chandra SP. The role of selective dorsal rhizotomy in the management of post-traumatic spasticity: systematic review. Neurosurg Rev. 2020 doi: 10.1007/s10143-020-01255-w. doi: 10.1007/s10143-020-01255-w. [DOI] [PubMed] [Google Scholar]
- 12.Behari M. Spasticity. Neurol India. 2002;50:235–7. [PubMed] [Google Scholar]
- 13.Kan P, Gooch J, Amini A, Ploeger D, Grams B, Oberg W, et al. Surgical treatment of spasticity in children: comparison of selective dorsal rhizotomy and intrathecal baclofen pump implantation. Childs Nerv Syst. 2008;24:239–43. doi: 10.1007/s00381-007-0457-8. [DOI] [PubMed] [Google Scholar]
- 14.Davidson B, Schoen N, Sedighim S, Haldenby R, Dalziel B, Breitbart S, et al. Intrathecal baclofen versus selective dorsal rhizotomy for children with cerebral palsy who are nonambulant: a systematic review. J Neurosurg Pediatr. 2019;18:1–9. doi: 10.3171/2019.8.PEDS19282. [DOI] [PubMed] [Google Scholar]
- 15.Gulati S, Sondhi V. Cerebral palsy: an overview. Indian J Pediatr. 2018;85:1006–16. doi: 10.1007/s12098-017-2475-1. [DOI] [PubMed] [Google Scholar]
- 16.Silva S, Nowicki P, Caird MS, Hurvitz EA, Ayyangar RN, Farley FA, et al. A comparison of hip dislocation rates and hip containment procedures after selective dorsal rhizotomy versus intrathecal baclofen pump insertion in nonambulatory cerebral palsy patients. J Pediatr Orthop. 2012;32:853–6. doi: 10.1097/BPO.0b013e31826ba7b2. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida K, Kajiura I, Suzuki T, Kawabata H. Natural history of scoliosis in cerebral palsy and risk factors for progression of scoliosis. J Orthop Sci. 2018;23:649–52. doi: 10.1016/j.jos.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Ailon T, Beauchamp R, Miller S, Mortenson P, Kerr JM, Hengel AR, et al. Long-term outcome after selective dorsal rhizotomy in children with spastic cerebral palsy. Childs Nerv Syst. 2015;31:415–23. doi: 10.1007/s00381-015-2614-9. [DOI] [PubMed] [Google Scholar]
- 19.Langerak NG, Vaughan CL, Peter JC, Fieggen AG, Peacock WJ. Long-term outcomes of dorsal rhizotomy. J Neurosurg Pediatr. 2013;12:664–5. doi: 10.3171/2013.7.PEDS13353. [DOI] [PubMed] [Google Scholar]
- 20.Dudley RW, Parolin M, Gagnon B, Saluja R, Yap R, Montpetit K, et al. Long-term functional benefits of selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg Pediatr. 2013;12:142–50. doi: 10.3171/2013.4.PEDS12539. [DOI] [PubMed] [Google Scholar]
- 21.Tedroff K, Löwing K, Åström E. A prospective cohort study investigating gross motor function, pain, and health-related quality of life 17 years after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol. 2015;57:484–90. doi: 10.1111/dmcn.12665. [DOI] [PubMed] [Google Scholar]
- 22.Gul SM, Steinbok P, McLeod K. Long-term outcome after selective posterior rhizotomy in children with spastic cerebral palsy. Pediatr Neurosurg. 1999;31:84–95. doi: 10.1159/000028839. [DOI] [PubMed] [Google Scholar]
- 23.Nordmark E, Josenby AL, Lagergren J, Andersson G, Strömblad LG, Westbom L. Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr. 2008;8:54. doi: 10.1186/1471-2431-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engsberg JR, Ross SA, Collins DR, Park TS. Effect of selective dorsal rhizotomy in the treatment of children with cerebral palsy. J Neurosurg. 2006;105:8–15. doi: 10.3171/ped.2006.105.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffery SMT, Markia B, Pople IK, Aquilina K, Smith J, Mohamed AZ, et al. Surgical outcomes of single-level bilateral selective dorsal rhizotomy for spastic diplegia in 150 consecutive patients. World Neurosurg. 2019;125:e60–6. doi: 10.1016/j.wneu.2018.12.187. [DOI] [PubMed] [Google Scholar]
- 26.Abbott R, Johann-Murphy M, Shiminski-Maher T, Quartermain D, Forem SL, Gold JT, et al. Selective dorsal rhizotomy: outcome and complications in treating spastic cerebral palsy. Neurosurgery. 1993;33:851–7; discussion 857. doi: 10.1227/00006123-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Steinbok P, Schrag C. Complications after selective posterior rhizotomy for spasticity in children with cerebral palsy. Pediatr Neurosurg. 1998;28:300–13. doi: 10.1159/000028668. [DOI] [PubMed] [Google Scholar]
- 28.Munger ME, Aldahondo N, Krach LE, Novacheck TF, Schwartz MH. Long-term outcomes after selective dorsal rhizotomy: a retrospective matched cohort study. Dev Med Child Neurol. 2017;59:1196–203. doi: 10.1111/dmcn.13500. [DOI] [PubMed] [Google Scholar]


