Abstract:
In this article, we present a case of apixaban elimination prolonged by 450% in a patient with coronavirus disease 2019 because of multiple conditions, including drug–drug interaction, severe inflammation, and acute kidney injury. Therapeutic drug monitoring was used to explain unusual routine coagulation assays. This grand round highlights the importance of dialog between the clinician and a therapeutic drug monitoring consultant for optimal patient care.
Key Words: apixaban, lopinavir, COVID-19, inflammation, acute kidney failure, drug–drug interaction
CLINICIAN
An 82-year-old man (85 kg, body mass index 30.8 kg/m2, and glomerular filtration rate 62 mL/min/1.73 m2), with a medical history of hypertension and atrial fibrillation (AF), was admitted for fever, cough, fatigue, confusion, vomiting, and diarrhea to our tertiary care hospital on March 9, 2020. His outpatient treatment included apixaban 5 mg twice daily as a preventive treatment for AF, which was continued throughout hospitalization. Coronavirus disease 2019 (COVID-19) was identified after nasopharyngeal swab test using real-time polymerase chain reaction. On day 5, the patient was transferred to the intensive care unit for acute respiratory distress syndrome. Based on the COVID-19 treatment strategy used at that time, lopinavir/ritonavir was introduced on day +5.
Is it possible to monitor lopinavir concentrations?
THERAPEUTIC DRUG MONITORING CONSULTANT
Lopinavir can be monitored using a validated ultrahigh-performance liquid chromatography–mass spectrometry method.
However, apixaban elimination is mediated by metabolism by cytochrome P450 3A4/5 (CYP3A4/5) and renal elimination, with excretion in the gastrointestinal tract by P-glycoprotein (P-gp).1 Ritonavir is a potent inhibitor of CYP3A4 and P-gp,2 resulting in a marked increase in anticoagulant activity.3 Apixaban trough concentrations were +77% to +491% higher than previous concentrations (measured before COVID) in 3 patients with COVID receiving concomitant lopinavir/ritonavir (ranging from 71 to 92 to ng/mL before COVID to 163–420 ng/mL with lopinavir/ritonavir).4 Typically, when lopinavir/ritonavir is introduced, a 50% decrease in the apixaban dose is recommended.5 Switching to parenteral heparin is recommended 12–24 hours after direct oral anticoagulants (DOACs) withdrawal in patients with COVID-19.3
CLINICIAN
Apixaban was discontinued on day +5. After a 24-hour washout period, enoxaparin was introduced on day +6 as a curative treatment (8000 UI/mL twice daily) for AF. On day +7, antifactor Xa activity was >2 UI/mL (<1.2 UI/mL for curative treatment), the activated partial thromboplastin time (aPTT) ratio was increased (1.52), and prothrombin time (PT) was decreased (68%). Accordingly, enoxaparin was withdrawn, with no clinical sign of bleeding. How can we evaluate these unusual results?
THERAPEUTIC DRUG MONITORING CONSULTANT
Apixaban concentrations could be retrospectively assessed using remaining samples stored at +4°C for 7 days because its long-term matrix stability was investigated during method validation according to the International Council for Hamonization M10 Guideline on bioanalytical method validation (European Medicines Agency/Committee for Medicinal Products for Human Use/International Council for Hamonization/172948/2019).
Concentrations were retrospectively investigated from day +2 using the validated ultrahigh-performance liquid chromatography–mass spectrometry method. Before withdrawal, apixaban concentrations were increased by 2-fold when compared with its predicted steady-state exposure in patients with AF (162.8–241.6 versus 104.5 mcg/L using apixaban 5 mg6). The level of C-reactive protein (CRP), a serum biomarker of inflammation, was 72–129 mg/L. However, inflammation, as observed during COVID-19, has recently been identified as a major factor of interindividual and intraindividual variabilities of drug-metabolizing enzymes and transporters,7 such as CYP3A4 and P-gp. The acute phase response can increase circulating proinflammatory cytokines, possibly downregulating both hepatic and intestinal metabolizing cytochrome P450 enzymes, drug transporters, and conjugative enzymes.7 Despite a 24-hour therapeutic window, the high apixaban level persisted (157.7 mcg/L) after enoxaparin initiation. Apixaban is a reversible and selective FXa inhibitor (activated factor X) that inhibits free, clot-bound FXa, and prothrombinase activity.8 The remainder apixaban concentration is responsible for the elevated antifactor Xa activity.
Meanwhile, the lopinavir trough level was 39.2 mg/L on day +8, whereas the ritonavir level was 0.47 mg/L, and CRP was 251.7 mg/L. High lopinavir trough concentrations have previously been described in patients with coronavirus disease 2019.9 Furthermore, lopinavir elimination is Cytochrome mediated, and its concentration reportedly varies with CRP levels.10
CLINICIAN
Lopinavir dosage was first reduced by 50% on day +8 and then discontinued on day +9. The patient experienced acute kidney injury (glomerular filtration rate = 26 mL/min/1.73 m2 on day +8).
Simultaneously, on days +8 and +9, the antifactor Xa activity was 1.72 and 1.26 UI/mL, respectively, whereas the aPTT ratios were 1.57% and 1.36%, and PT was 68% and 70%, respectively.
THERAPETIC DRUG MONITORING CONSULTANT
Ritonavir-induced reversible acute tubular injury has been previously described.11 However, the disease itself might induce acute kidney injury, as reported in up to 25% of critically ill patients with COVID-19.12 The half-life of lopinavir was 13 hours, indicating a 200% decrease in elimination.
The remaining high antifactor Xa activity was attributed to the extended apixaban elimination. Owing to the drug–drug interaction between a Cytochrome and P-gp inhibitor and apixaban, potentially increased because of inflammation, the direct impact of inflammation on apixaban metabolism, and acute kidney injury, the apixaban half-life was decreased by 450% (half-life was 54 hours versus normal half-life of 12 hours) (Fig. 1).
FIGURE 1.
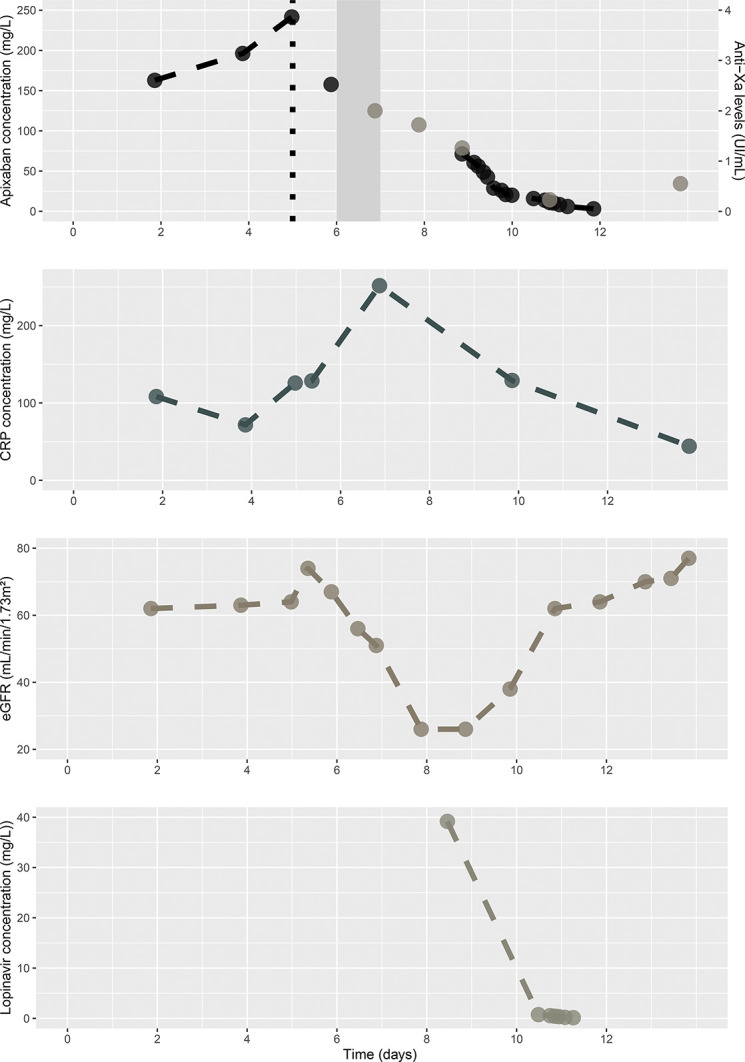
Evolution of apixaban and lopinavir concentrations, CRP, estimated glomerular filtration rate, and antifactor Xa activity over time. The black dotted line represents apixaban withdrawal, and the gray-filled rectangle represents enoxaparin administration.
CLINICIAN
Activated charcoal was administered on day +10, and acute kidney injury resolved on day +11 (GFR = 62 mL/min/1.73 m2). Enoxaparin was reintroduced on day +11, with the antifactor Xa activity at 0.23 UI/mL, aPTT ratio at 1.26, and PT at 83%.
THERAPETIC DRUG MONITORING CONSULTANT
Apixaban may undergo some degree of recycling between the systemic circulation and gastrointestinal tract. The presence of activated charcoal in the gastrointestinal tract interrupted this recycling, resulting in enhanced elimination of apixaban through the feces13; the apixaban concentration was 20 mcg/L, and its half-life was 18 hours after day +10 because of the activated charcoal and improved kidney function. However, it was still detectable 7 days after withdrawal.
CLINICIAN
Finally, the patient was discharged from the intensive care unit after 16 days.
CONCLUSION
COVID-19 can be associated with severe inflammation and acute kidney injury, inducing considerable pharmacological interference. Consequently, repurposed drugs should be carefully monitored using therapeutic drug monitoring to avoid critical drug–drug interactions and subsequent toxicity. Other CYP3A4 and/or P-gp inhibitors (such as antifungal agents or macrolides), as well as CYP3A4 and/or P-gp substrates (such as midazolam, cyclosporine, and tacrolimus), remain widely used in patients with COVID-19, and the situation observed in the current patient could recur.
ACKNOWLEDGMENTS
The authors thank Dr Anne-Camille Faure for her support with analytical specifications of the antifactor Xa activity assay.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Anne-Laure Demartin, Email: anne-laure.demartin@orange.fr.
Sophie Perinel Ragey, Email: sophie.perinel-ragey@chu-st-etienne.fr.
Patrick Mismetti, Email: patrick.mismetti@chu-st-etienne.fr.
Elisabeth Botelho-Nevers, Email: elisabeth.botelho-nevers@chu-st-etienne.fr.
Xavier Delavenne, Email: xavier.delavenne@chu-st-etienne.fr.
REFERENCES
- 1.Kubisz P, Stanciakova L, Dobrotova M, et al. Apixaban—metabolism, pharmacologic properties and drug interactions. Curr Drug Metab. 2017;18:609–621. [DOI] [PubMed] [Google Scholar]
- 2.Bierman WF, Scheffer GL, Schoonderwoerd A, et al. Protease inhibitors atazanavir, lopinavir and ritonavir are potent blockers, but poor substrates, of ABC transporters in a broad panel of ABC transporter-overexpressing cell lines. J Antimicrob Chemother. 2010;65:1672–1680. [DOI] [PubMed] [Google Scholar]
- 3.Testa S, Paoletti O, Giorgi-Pierfranceschi M, et al. Switch from oral anticoagulants to parenteral heparin in SARS-CoV-2 hospitalized patients. Intern Emerg Med. 2020;15:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels' striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18:1320–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeitouni M, Giczewska A, Lopes RD, et al. Clinical and pharmacological effects of apixaban dose adjustment in the ARISTOTLE Trial. J Am Coll Cardiol. 2020;75:1145–1155. [DOI] [PubMed] [Google Scholar]
- 7.Stanke-Labesque F, Gautier-Veyret E, Chhun S, et al. ; French Society of Pharmacology and Therapeutics. Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215:107627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieland E, Shipkova M. Pharmacokinetic and pharmacodynamic drug monitoring of direct-acting oral anticoagulants: where do we stand? Ther Drug Monit. 2019;41:180–191. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire M, Le Turnier P, Gaborit BJ, et al. Lopinavir pharmacokinetics in COVID-19 patients. J Antimicrob Chemother. 2020;75:2702–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzolini C, Stader F, Stoeckle M, et al. Effect of systemic inflammatory response to SARS-CoV-2 on lopinavir and hydroxychloroquine plasma concentrations. Antimicrob Agents Chemother. 2020;64:e01177–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafi T, Choi MJ, Racusen LC, et al. Ritonavir-induced acute kidney injury: kidney biopsy findings and review of literature. Clin Nephrol. 2011;75(suppl 1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Mondal S, Wang J, et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs. 2014;14:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]


