Abstract:
Therapeutic drug monitoring of hydroxychloroquine (HCQ) has been recommended to optimize the treatment of patients with COVID-19. The authors describe an ultrahigh-performance liquid chromatography tandem spectrometry method developed in a context of emergency, to analyze HCQ in both human plasma and blood samples. After adding the labeled internal standard and simple protein precipitation, plasma samples were analyzed using a C18 column. Blood samples required evaporation before analysis. The total chromatographic run time was 4 minutes (including 1.5 minutes of column equilibration). The assay was linear over the calibration range (r2 > 0.99) and up to 1.50 mcg/mL for the plasma samples (5.00 mcg/mL for the blood matrix). The limit of quantification was 0.0150 mcg/mL for plasma samples (0.05 mcg/mL blood matrix) with accuracy and precision ranging from 91.1% to 112% and from 0.750% to 11.1%, respectively. Intraday and interday precision and accuracy values were within 15.0%. No significant matrix effect was observed in the plasma or blood samples. This method was successfully applied to patients treated for COVID-19 infection. A simple and rapid ultrahigh-performance liquid chromatography tandem spectrometry method adapted to HCQ therapeutic drug monitoring in the context of SARS-CoV-2 infection was successfully developed and validated.
Key Words: hydroxychloroquine, tandem mass spectrometry, COVID-19, therapeutic drug monitoring
BACKGROUND
Hydroxychloroquine (HCQ), a widely used antimalarial drug, also exhibits anti-inflammatory and immunomodulatory activity, which justifies its use in autoimmune diseases, such as chronic or acute rheumatoid arthritis and lupus erythematosus.1–3 In the context of the COVID-19 pandemic, several drugs have been repurposed as potential candidates for the treatment of COVID-19 infection. HCQ was among the first to be tested due to an in vitro antiviral activity demonstrated against SARS-CoV-2.4,5 In France, the Ministry of Health authorized the prescription of HCQ in hospitalized patients on March 25, 2020 (Decree-Law no. 2020–31, March 25, 2020). HCQ therapeutic drug monitoring (TDM) was recommended because of its narrow therapeutic index6,7 and an expected high pharmacokinetic interindividual and intraindividual variability given the context: steady-state conditions not achieved, specific population (elderly, intensive care, or dialysis patients), and a systemic inflammation state potentially associated with metabolism downregulation of cytochrome P450 isoenzymes (CYP) (such CYP2D6) that are involved in HCQ metabolism.8–10 Despite no clear association between concentration and response and/or side effects,11,12 a minimal plasma threshold of 0.1 mg/L was proposed, based on in vitro and modeling experiments.4,8 This emergency setting thus requires a simple, rapid, and accurate analytical method to meet the increasing activity and optimize the management of HCQ-based treatment in large and various populations.
Different methods have been developed for the detection and quantification of HCQ from different biological samples (blood, plasma, and tissues). Several of these methods used ultraviolet (UV) or fluorescence detection with high-performance liquid chromatography (HPLC) to analyze HCQ concentrations.13–17 However, most of these methods were not adapted to TDM in patients with COVID-19 because the sample preparation is highly time-consuming (liquid–liquid extraction), it requires a volume of toxic solvents (dimethyl ether), or the method present a high limit of quantification (LOQ) that are not consistent with the expected concentrations.13–16 Similarly, our HPLC-UV analytical method previously developed in our laboratory to perform TDM in autoimmune diseases was not appropriate in the context of the COVID-19 pandemic (high LOQ and liquid–liquid extraction with dimethyl ether). Other methods using ultra-HPLC tandem mass spectrometry (UHPLC-MS/MS) have been developed and present improved sensitivity and a short run time with a simple and safe extraction process.18–21 However, these methods were commonly validated in a whole blood matrix (a matrix recommended in TDM of patients with autoimmune disease), whereas plasma may be more appropriate for TDM in patients with COVID-19.5 HCQ pharmacokinetics is characterized by a protein binding of approximately 45% and an important accumulation in erythrocytes. Consequently, the whole blood concentration does not seem to be an adequate surrogate for concentrations at the site of infection. Moreover, total plasma concentration is preferentially used for the TDM of anti-infective drugs with low or moderate protein binding (as it predicts the concentrations at the site of infection) and pharmacokinetic/pharmacodynamic (PK/PD) relationships are commonly established using total plasma concentration.22,23 An UHPLC-MS/MS method was validated by Fuzery et al19 in plasma using an online extraction system (Thermo Scientific Transcend TLX systems with Turboflow technology). However, an online extraction system was not available in our laboratory, so this method could not be implemented. In the study of Morita et al,24 an LC-MS/MS method was described for the quantification of HCQ in blood and plasma, but a high volume of toxic solvent was used (2 mL of methyl tert-butyl ether).
We thus have developed in a context of emergency a simple, robust, and rapid UHPLC-MS/MS method for the quantification of HCQ in plasma. This method was also adapted to the whole blood matrix to determine the blood-to-plasma ratio in patients with COVID-19 and consolidate HCQ TDM in patients with autoimmune disease.
MATERIALS AND METHODS
Chemicals and Reagents
HCQ was purchased from LGC Standards (Luckenwalde, Germany) and HCQ-d5 was provided by Alsachim (Illkirch, France). All other chemicals were commercially available analytical grade materials: methanol from Biosolve (Dieuze, France), acetonitrile and formic acid from Carlo Erba (Val-de-Reuil, France), and ammonium formate from MP Biomedicals (Illkirch, France). Milli-Ω water (Millipore Milli-Q Gradient A10, Molsheim, France), prepared from demineralized water, was used throughout the study. Ultrapure argon (>99.998% purity) gas was used as the collision gas and was obtained from Air Products (Rognac, France). Nitrogen was used for dissolution and was provided by a nitrogen generator ZEFIRO 36 LC-MS (FDGSi, Evry, France). A plasma blank was used for the development and validation of the procedure and was purchased from French Blood Establishment (EFS, Marseille, France).
Apparatus
Liquid chromatographic–mass spectrometric analysis was performed with an Acquity UPLC H-Class (Waters, Milford, CT) and a triple quadrupole mass spectrometer (Quattro Premier XE; Waters) equipped with an electrospray ionization interface. Data were acquired using the PC-based MassLynx V4.1 software (Waters) and processed using QuanLynx V4.1 quantification software (Waters).
Liquid Chromatography
The UHPLC system was equipped with quaternary solvent manager, sample manager flow-through-needle, and column heater. Chromatography was performed on a Phenomenex Luna Omega Polar C18 column (100 mm × 2.1 mm ID, 1.6 µm PD) (Phenomenex, Torrance, CA). The column temperature and autosampler temperature were maintained at 40°C and 10°C, respectively. Chromatography was performed using a gradient elution method with a binary mobile phase composed of phase A containing 0.01 mol/L ammonium formate and 0.1% formic acid in water/acetonitrile 95/5 (vol/vol), and mobile phase B (acetonitrile). The flow rate was set at 0.4 mL/min, and the injection volume was 5 µL. Gradient conditions were as follows: 0–2.5 minutes linear from 10% to 80% B; 2.5–2.7 minutes linear, 80%–10% B; and 3–4 minutes, isocratic 10% B.
Mass Spectrometry
The mass spectrometry (MS) detector was equipped with an electrospray ionization source operating in multireaction monitoring (MRM) in a positive ion mode. For precursor [M + H]+ and product ion determination, 5 mcg/mL solutions of each compound (prepared in the initial gradient mobile phase) were directly infused into the ion source with a Hamilton syringe pump (250 µL) at a flow rate of 10 µL/min. For LC-optimization of the compound ionization, a T-piece was placed between the outlet of the LC and MS sources. A standard solution (500 ng/mL) of each analyte was then infused using the syringe pump through the T-Piece at a flow rate of 20 µL/min into the eluent stream and then into the spray source to imitate LC-MS/MS conditions.
The fragmentation transitions were m/z = 336.21 → 246.93 for HCQ and m/z = 341.28 → 247.01 for the internal standard (IS) HCQ-d5. The fragmentor voltages for both HCQ and IS were 40 V while their collision energy was 20 and 30 eV, respectively. The MS valve was diverted to waste between 0 and 0.8 minutes and then between 2.5 and 4 minutes.
Preparation of Stock Solutions Calibration Curve and Quality Control
Two independent stock solutions were prepared in Milli-Q water (18.2 MΩ) to yield a final concentration of 0.5 mg/mL for HCQ and 1 mg/mL for the IS. Stock solutions were stored at −20°C. From the stock solution of HCQ, working solutions were then prepared in methanol at a concentration of 150 mcg/mL for HCQ stored at −20°C. The first working solution was used to prepare calibration curve spiking solutions by serial dilutions in methanol, yielding concentrations of 0.150, 0.375, 0.750, 1.50, 3.70, 7.50, 12.0, and 15.0 mcg/mL for plasma samples and 0.5, 1.0, 2.5, 5.0, 10.0, 25.0, and 50.0 mcg/mL for blood samples. A secondary working solution was used to prepare spiking solutions for quality control (QC). Working IS solutions were prepared by diluting the stock solution with water (18.2 MΩ) to obtain a final concentration of 2.5 mcg/mL. The quantification of HCQ was based on the IS method. Seven nonzero samples were prepared by spiking blank plasma or total blood with appropriate amounts of working solutions to obtain 0.015, 0.0375, 0.075, 0.150, 0.375, 0.75, 1.20, or 1.50 mcg/mL of calibration standard concentrations for plasma samples and 0.05, 0.10, 0.25, 0.50, 1.0, 2.5, or 5.0 mcg/mL for blood samples. The lowest standard on the calibration curve was defined as the low LOQ (LLOQ). The QC samples were prepared: low QC (LQC) at 0.060 mcg/mL for plasma and 0.15 mcg/mL for blood, medium QC (MQC) at 0.60 mcg/mL for plasma and 1.50 mcg/mL for blood, and high QC (HQC) at 1.125 mcg/mL for plasma and 4.0 mcg/mL for blood.
Sample Preparation
Two hundred microliter of human plasma samples were processed by precipitation according to the following procedure. A volume of 20 µL of working IS solution was added to the human plasma. Samples were vortex mixed with methanol: 1000 µL for blank, 980 µL for patient samples, and 960 µL for calibration and QC samples. After vortexing for 25–30 seconds, the samples were centrifuged at 19,745g for 10 minutes. The supernatant contained all molecules. Hundred microliter of supernatant was then transferred into an autosampler vial. Finally, 5 µL of solution was injected into the LC-MS/MS system for analysis.
For whole blood HCQ quantification, a volume of 10 µL of working IS solution was added to a 100 µL aliquot of blood. Protein precipitation was effectuated with 1.0 mL of acetonitrile (stored at −20°C before use). The resulting mixture was thoroughly vortexed and then centrifuged at 19,745g for 10 minutes. The supernatant was then transferred into glass tubes for evaporation at 40°C with a gentle nitrogen flow. After approximately 20 minutes, the dry residue was diluted with 400 µL of mobile phase. A sample of the solution (5 µL) was injected into the LC-MS/MS system for analysis.
Validation Procedures
Validation of the assay was performed in accordance with the European European Medical Agency (EMA) Guideline on bioanalytical method validation.25
Full Validation in Plasma
Linearity and Sensitivity
Linearity was estimated by assaying calibration curves in 6 independent runs consisting of a blank plasma sample, a zero sample, and 7 calibrator concentrations: 0.015, 0.0375, 0.075, 0.15, 0.375, 0.750, 1.20, and 1.50 mcg/mL. ISs were used to correct the extraction variability and ion suppression effect produced by the matrix. Calibration curves were prepared for each run based on the peak area ratio of HCQ to that of the IS. Back-calculated concentrations of calibration standards had to be within ±15% of the nominal value, except for the LOQ level that was ±20%. At least 75% of the calibration standards were required to fulfill these criteria.
Accuracy and Precision
Intraday precision (repeatability) was assessed by running 6 replicates at 4 concentrations (LLOQ, LQC, MQC, and HQC) on the same day under the same operating conditions. The interday precision was estimated on 6 different days using the same instrument. The precision is expressed as a relative SD [or coefficient of variation (CV)] and should not exceed 15%, except for the LLOQ, where it should not exceed 20%.
The accuracy was calculated in terms of bias, expressed as the percentage deviation of the mean calculated concentration and the corresponding nominal concentration. The mean value should be within 85%–115% of the nominal concentration, except for LLOQ, which should be 80%–120% of the nominal concentration.
Extraction Recovery
Extraction recovery of the analytes, expressed as a percentage, was determined at 2 different concentrations (LQC and HQC) by comparing peak areas of extracted spiked samples (n = 3) with those found by the direct injection of diluted stock solution at the same concentration (n = 6).
Matrix Effect
The matrix effect (ME) of plasma components on the HCQ and IS was determined by comparing the postextracted QC samples with the neat samples at equal concentrations. The ME was evaluated by calculating the ratio of peak area in the presence of matrix (a blank matrix spiked after extraction with analyte and IS) to the peak area in the absence of matrix (diluted working solution), at the same concentration. Six lots of matrix were spiked at the LQC and HQC concentrations. IS was added to all samples. The IS-normalized ME was calculated as the ratio of the analyte to that of the IS. The CV of the IS-normalized ME should not be greater than 15%.
Dilution Integrity
Dilution integrity was performed to extend the upper concentration limit with acceptable precision and accuracy. It was considered adequate when the difference in concentration did not exceed 15%.
Carryover
Carryover was assessed by immediately injecting a blank sample after a high concentration sample. The peak area in the blank sample should not be greater than 20% of the LOQ for the compounds and 5% for the IS.
Selectivity
We compared ion chromatograms of 10 plasma samples obtained from different patients with ion chromatograms of corresponding blank plasma samples spiked with HCQ to investigate the interferences from endogenous plasma compounds. Patients were treated for rheumatoid arthritis with chloroquine, for tuberculous with rifampicin and isoniazid, or for other infectious diseases with medications, such as ciprofloxacin, ofloxacin, teicoplanin, doxycycline, and dalbavancin.
Stability
The bench-top stability was then determined after 5 hours and 24 hours at room temperature and compared with freshly spiked plasma QC samples. The freeze/thaw stability was determined after 2 freeze/thaw cycles (from −35°C to room temperature). The long-term stability was evaluated in the validation procedure of our previous HPLC-UV method after storage of QC samples at −35°C for 6 months.
The analyte was considered stable in the matrix when the difference in concentration between the fresh sample and the stability-testing sample did not exceed 15%.
Method Comparison
Patient samples were analyzed successively using HPLC-UV and LC-MS/MS methods. The difference between the 2 values obtained should be within 20% of the mean for at least 67% of the repeats.25 The Bland and Altman approach was used to further assess the difference between both methods by plotting the relative difference between the 2 assays against the determined mean concentration. The mean relative difference (bias) between both methods was also calculated.
Partial Validation in Whole Blood
In the case of matrix modification (ie, plasma to whole blood), the EMA guidelines recommend a partial validation that includes the determination of the intraday precision and accuracy.25 ME and extraction recovery were included to verify the impact of the matrix and sample preparation in the analytical process. Linearity was also determined by analyzing the calibration curves in 3 separate runs. The separate runs included a blank blood sample, a zero sample, and 7 calibrator concentrations of 0.05, 0.10, 0.25, 0.50, 1.0, 2.5, and 5.0 mL in blood. LLOQ was also evaluated.
Clinical Application
The clinical applicability of this method was evaluated by analyzing plasma and whole blood concentrations in patients treated for COVID-19 infection.
Ethics Approval
The data used were anonymous data, and approval was obtained from the Portail d'Accès aux Données de Santé de l'APHM (number PADS20-200).
HCQ is not approved for treatment of SARS-CoV-2 infections.
RESULTS AND DISCUSSION
Mass and Chromatographic Condition Optimization
Chromatographic conditions were optimized through several steps to achieve a high analyte response and reduce the analytical time.
Several columns were tested (such as Waters BEH C18 column 50 × 2.1 mm ID, 1.7 µm PD; Waters Phenyl-Hexyl column 50 × 2.1 mm ID, 1.7 µm PD; Waters HSS T3 column 50 × 2.1 mm ID, 1.7 µm PD; and Phenomenex Luna Omega Polar C18 column 100 × 2.1 mm ID, 1.6 µm PD) to achieve a symmetrical peak shape and optimized response. The Phenomenex Luna Omega Polar C18 column proved to provide the best response and symmetry.
The shape of the peaks and ionization of HCQ and IS were highly affected by the composition of the mobile phase. Various combinations of acetonitrile or methanol as organic modifiers and aqueous buffer were investigated to identify which mobile phase composition provided the highest responses and appropriate peak shape for HCQ and IS. In this study, a mobile phase containing 0.01 mol/L ammonium formate with 0.1% formic acid in water/acetonitrile (95:5, vol/vol) was chosen because it gave a better mass spectrometric response and symmetrical peaks.
Sample preparation is a critical step for an accurate and reliable LC-MS/MS analysis. Therefore, different methods were tested, including precipitation with methanol and acetonitrile and liquid–liquid (LL) extraction.
Finally, a simple precipitation with methanol at a ratio of 1:5 was chosen for the plasma samples. For the blood samples, precipitation/evaporation with ice-cold acetonitrile was preferred. These extractions are not expensive, not time-consuming, and uncomplicated.
HCQ was optimized before its quantification by UHPLC-MS/MS. The analyte was monitored using 2 transitions. The first MRM transition was used for quantification and the second for confirmation. Optimized MRM transitions, cone voltages, and collision energies were selected for the maximum response of the product ion. MRM transitions were (collision energy) m/z = 336.21 → 246.93 (20.0 eV) and m/z = 336.21 → 157.9 (24.0 eV) for HCQ and m/z = 341.28 → 247.01 (30.0 eV) and m/z = 341.28 → 162.9 (30.0 eV) for HCQ-d5. The acquisition settings were set as follows: source temperature, 120°C; dissolution temperature, 350°C; dissolution gas flow, 900 L/h; cone gas flow, 25 L/h; cone voltage, 40.0 V; and collision gas pressure (argon), 4 × 10−3 mbar. Using the diverter valve integrated in the MS system, the first 0.8 minutes and the last 1.5 minutes of chromatographic eluents were diverted to waste.
HCQ was detected and quantified over a total run time of 4 minutes, including 1.5 minutes for column equilibration. The Luna Omega Polar C 18 (100 × 2.1 mm ID, 1.6 µm PD) analytical column was maintained at 40°C. The retention times were 1.59 minutes for HCQ and 1.57 minutes for HCQ-d5. Extracted ion chromatograms of blank plasma samples, spiked plasma samples at LLOQ concentrations, and plasma samples obtained from patients are shown in Figure 1.
FIGURE 1.
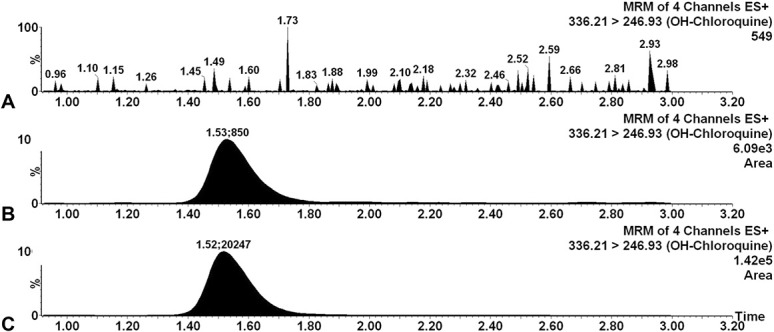
Extracted individual ion chromatograms of HCQ: Blank sample (A), blank plasma spiked with the LLOQ level (0.015 mcg/mL, B), and the plasma sample from patients treated with HCQ (C).
Optimization of analytical conditions has allowed the development of a rapid, simple, sensitive, and specific quantification of HCQ, adapted to assist in the SARS-CoV-2 pandemic.
METHOD VALIDATION
Linearity and Sensitivity
A linear correlation was identified for HCQ using a weighting factor of 1/X. The blank and zero samples were not taken into consideration when calculating the curve parameters. The coefficient of determination (r2) was higher than 0.99. Back-calculated concentrations over 6 runs for the plasma and over 3 runs in the whole blood samples did not deviate more than 12.0% from nominal concentrations for the nonzero calibration standards in plasma and 11.6% in whole blood. The LLOQ was established at 0.015 mcg/mL in plasma with a precision and accuracy of 4.28% and 104%, respectively. The LLOQ was established at 0.05 mcg/mL in blood with a precision and accuracy of 4.11% and 91.1%, respectively. The assay was linear over the concentration range of 0.015–1.5 mcg/mL (0.050–5.0 mcg/mL for whole blood), in coherence with the HCQ expected concentrations in patients with SARS-CoV-2 infection.26
Accuracy and Precision
Intraday and interday accuracy and precision are reported in Table 1. In plasma samples, the intraday accuracy and precision were 91.8%–113.69% and 0.51%–8.95%, respectively. The interday accuracy and precision were 92.28%–112.3% and 1.40%–11.1%, respectively. In the whole blood matrix, the interday accuracy was 95.31%–102.83%, whereas the intraday accuracy was 98.09%–104.67%. Both intraday and interday precision values were 0.75%–3.95% and 1.38%–6.26%, respectively.
TABLE 1.
Nominal and Mean Calculated Concentrations With Accuracy and Precision of HCQ in Plasma and Blood
| Analyte | Nominal Concentration (mcg/mL) | Intraday (n = 6) | Interday (n = 6) | ||||
| Measured Mean Concentration (mcg/mL) | Accuracy (%) | Precision (%) | Measured Mean Concentration (mcg/mL) | Accuracy (%) | Precision (%) | ||
| HCQ plasma | 0.0150 | 0.0157 | 104 | 5.3 | 0.0169 | 112 | 8.0 |
| 0.0600 | 0.0682 | 114 | 0.5 | 0.0554 | 92.3 | 1.4 | |
| 0.600 | 0.551 | 91.8 | 1.8 | 0.575 | 95.7 | 9.9 | |
| 1.125 | 1.19 | 105 | 7.5 | 1.20 | 107 | 7.6 | |
| HCQ blood | 0.050 | 0.0456 | 91.1 | 4.1 | 0.0513 | 103 | 6.7 |
| 0.150 | 0.157 | 104 | 3.9 | 0.151 | 100 | 1.4 | |
| 1.50 | 1.47 | 98.1 | 0.8 | 1.43 | 95.3 | 3.8 | |
| 4.00 | 4.02 | 100 | 3.1 | 4.11 | 103 | 2.5 | |
Our LC-MS/MS method provides adequate accuracy and precision for HCQ determination in human plasma and whole blood samples.
Extraction Recovery
The results show an efficient mean extraction recovery ranging from 94.08% to 103.95% with a precision below 4.1% at the 2 QC concentrations.
Carryover
Carryover was evaluated by injecting 3 blank plasma samples after the higher concentration of HCQ (5 mcg/mL). The blank plasma chromatogram did not signal higher than 20% of the LLOQ at the HCQ retention time or higher than 5% at the HCQ-d5 retention time. Therefore, the carryover effect was considered negligible.
Matrix Effect
ME was evaluated by comparing the obtained area from a blank plasma extract spiked with HCQ concentrations versus a direct injection of diluted working solution at the same concentrations. Regarding HCQ, the ME value was 1.04 for the LQC concentration (0.97 for HQC) in plasma samples and at 1.03 for the LQC (0.96 for HQC) in whole blood. Regarding IS, the ME values were at 1.10 in plasma samples and at 1.02 in whole blood. The IS-normalized ME values were 0.95 for LQC (0.96 for HQC) in plasma samples and 1.01 for LQC (0.99 for HQC) in whole blood. The CV was 6.11% (9.86% for HQC) for plasma samples and at 2.01% (1.57% for HQC) for whole blood. No ME was observed at 0.1125 mcg/mL or 1.125 mcg/mL for plasma and neither at 0.15 mcg/mL nor at 4.0 mcg/mL for whole blood.
Dilution Integrity
Dilution integrity was proved by spiking 5 blank plasmas with concentrations above the highest calibrator (1.50 mcg/mL). These were diluted with blank plasma to achieve a 1/2 dilution. The concentration difference between the undiluted and diluted samples was 13.3% with a CV of 5.70%.
Selectivity
No significant interferences at the retention time of HCQ were observed after the injection of plasma samples from different patients treated for autoimmune or infectious diseases.
Stability
In the bench‐top stability analyses, the mean percentage changes of the peak area when maintained at room temperature ranged from 1.02% to 8.78% after 5 hours and from −9.59% to 8.00% at 24 hours (n = 3 for each QC). This result allowed us to conclude that HCQ was stable for 5 hours and 24 hours at room temperature. Freeze–thaw stability experiments showed that the QCs (LQC and HQC) were stable for 3 freeze–thaw cycles (deviations were from 0.52% to 13.9%). After 6 months of storage at −35°C, the QC samples showed no significant deviation from the nominal concentration, 0.00%–12.2% (data are from the validation step of our previous HPLC-UV method).
METHOD COMPARISON
The mean bias between the results of patient samples (n = 31) previously analyzed by HPLC-UV and subsequently by the UHPLC-MS/MS method was ±7.51% (0.1%–25.0%), and the Bland–Altman plot is shown in Figure 2. The difference between the 2 values was within 20% of the mean for 29 of the 31 samples. These results demonstrate that the data generated by these 2 LC methods are reproducible despite differing modes of detection.
FIGURE 2.
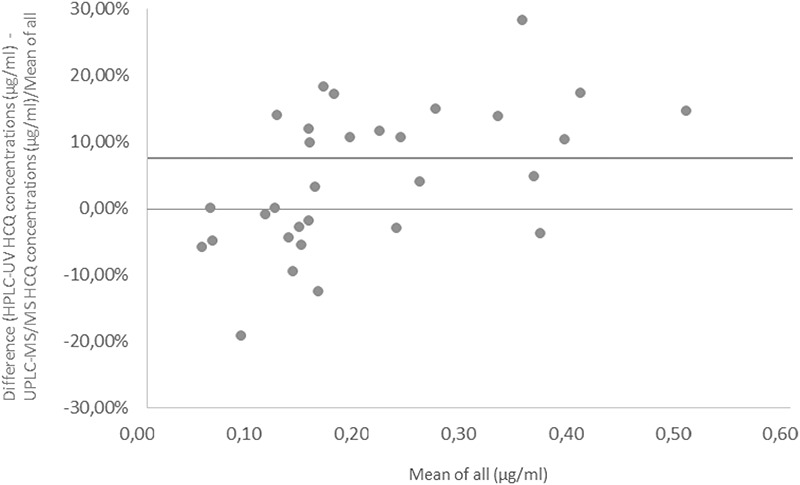
Plot of the percentage difference between the HCQ concentrations measured using HPLC-UV and the UHPLC-MS/MS. The mean difference (bias) is represented using a solid line.
Clinical Application
The validated method was successfully applied to the TDM of HCQ in patients with SARS-CoV-2 infection. Plasma concentrations ranged from 0.028 to 0.692 mcg/mL (n = 90, median concentration: 0.167 mcg/mL, CV = 66.6%). These results confirmed the large variability of HCQ concentrations observed in patients with COVID-19 and the lower exposure compared with that reported in autoimmune disease populations.26 The lower exposure reported in patients with COVID-19 could be explained by the nonachievement of the pharmacokinetic steady state or by the administration of the drug through enteral feeding tubes in intensive care patients.
In 6 patients, whole blood concentrations were also determined and ranged from 0.305 to 1.82 mcg/mL (n = 6, median concentration: 0.522 mcg/mL). Important between-subject variability was also observed in the blood-to-plasma ratio ranging from 1.15 to 8.78 (median: 3.89). This result is probably explained by differences in comorbidities and equilibrium distribution of the drug. These results are in accordance with previously described data in autoimmune disease and healthy volunteers.24,27 It confirmed that the HCQ blood-to-plasma ratio could not be extrapolated from one patient to another.
CONCLUSION
In the emergency context of the COVID-19 pandemic, a sensitive, accurate, and precise analytical method based on UHPLC-MS/MS has been developed and validated for the determination of HCQ in both plasma and whole blood samples. This method was successfully implemented for HCQ TDM in the context of the COVID-19 pandemic with an adjusted LLOQ, a simple and rapid sample preparation, and a short runtime (4 minutes). More than 100 plasma samples can be assayed daily, including sample preparation, data acquisition, and processing. Another advantage is the consolidation of HCQ determination in both plasma and whole blood matrixes. This method may be applied for HCQ quantification in the COVID-19 and autoimmune disease populations.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Natalia Doudka, Email: natalia.doudka@ap-hm.fr.
Madeleine Giocanti, Email: madeleine.giocanti@ap-hm.fr.
Manon Basso, Email: manon.basso@ap-hm.fr.
Renée Ugdonne, Email: renee.ugdonne@ap-hm.fr.
Karine Barthelemy, Email: karine.barthelemy@univ-amu.fr.
Bruno Lacarelle, Email: bruno.lacarelle@ap-hm.fr.
Olivier Blin, Email: olivier.blin@ap-hm.fr.
Caroline Solas, Email: caroline.solas@ap-hm.fr.
REFERENCES
- 1.Ben-Zvi I, Kivity S, Langevitz P, et al. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcon GS, McGwin G, Bertoli AL, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis. 2007;66:1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petri M. Use of hydroxychloroquine to prevent thrombosis in system lupus erythematosus and in antiphospholipid antibody-positive patients. Curr Rheumatol Rep. 2011;13:77–80. [DOI] [PubMed] [Google Scholar]
- 4.Yao X, Ye F, Zhang M, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury MS, Rathod J, Gernsheimer, et al. A rapid systematic review of clinical trials utilizing chloroquine and hydroxychloroquine as a treatment for COVID-19. Acad Emerg Med. 2020;27:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdulaziz N, Shah AR, McCune WJ. Hydroxychloroquine: balancing the need to maintain therapeutic levels with ocular safety: an update. Curr Opin Rheumatol. 2018;30:249–255. [DOI] [PubMed] [Google Scholar]
- 7.Chi Chiu M. Therapeutic monitoring of the immuno-modulating drugs in systemic lupus erythematosus. Expert Rev Clin Immunol. 2017;13:35–41. [DOI] [PubMed] [Google Scholar]
- 8.Recommendations for Therapeutic Drug Monitoring of Lopinavir/r and Hydroxychloroquine in Patients Treated for SARS-CoV-2 (COVID-19) Infection of ANTS-AC43 Clinical Pharmacology Committee and SFTP Therapeutic Drug Monitoring and Treatment Personalization Committees. Available at: https://sfpt-fr.org/recommandations-et-publications, French. [Google Scholar]
- 9.Roustit M, Guilhaumou R, Molimard M, et al. French Society of Pharmacology and Therapeutics (SFTP). Chloroquine and hydroxychloroquine in the management of COVID-19: much kerfuffle but little evidence. Therapie. 2020;S0040-5957:30100–30101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanke-Labesque F, Gautier-Veyret E, Chhun S, et al. French Society of Pharmacology and Therapeutics (SFPT). Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215:107627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenfant T, Salah S, Leroux G, et al. Risk factors for hydroxychloroquine retinopathy in systemic lupus erythematosus: a case-control study with hydroxychloroquine blood-level analysis. Rheumatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha C, Alexander S, Ashby D, et al. Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome? Nephrol Dial Transpl. 2018;33:1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volin P. Simple and specific reversed-phase liquid chromatographic method with diode-array detection for simultaneous determination of serum hydroxychloroquine, chloroquine and soma corticosteroids. J Chromatogr B Biomed Appl. 1995;666:347–353. [DOI] [PubMed] [Google Scholar]
- 14.Qu Y, Noe G, Breaud AR, et al. Development and validation of a clinical HPLC method for the quantification of hydroxychloroquine and its metabolites in whole blood. Future Sci OA. 2015;1:FSO26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh A, Roopkishora, Singh CL, et al. Development and validayion of reversed-phase high performance liquid chromatographic method for hydroxychloroquine sulphate. Indian J Pharm Sci. 2015;77:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong N, Richez M, Raoult D, et al. Simultaneous UPLC-UV analysis of hydroxychloroquine, minocycline and doxycycline from serum samples for the therapeutic drug monitoring of Q fever and Whipple's disease. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:166–172. [DOI] [PubMed] [Google Scholar]
- 17.Noé G, Amoura Z, Combarel D, et al. Development and validation of a fast Ultra-High Performance Liquid Chromatography-Fluorescent Method for quantitation of hydroxychloroquine and its metabolites in patients with lupus. Ther Drug Monit. 2019;41:476–482. [DOI] [PubMed] [Google Scholar]
- 18.Wang LZ, Ong RYL, Chin TM, et al. Method development and validation for rapid quantification of hydroxychloroquine in human blood using liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2012;61:86–92. [DOI] [PubMed] [Google Scholar]
- 19.Fuzery AK, Breaud AR, Emezienna N, et al. A rapid and reliable method for the quantitation of hydroxychloroquine in serum using turbulent flow liquid chromatography-tandem mass spectrometry. Clinica Chim Acta. 2013;421:79–84. [DOI] [PubMed] [Google Scholar]
- 20.Chhonker YS, Sleightholm RL, Li J, et al. Simultaneous quantification of hydroxychloroquine and its metabolites in mouse blood and tissues using LC-ESI-MS/MS: an application for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1072:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soichot M, Mégarbane B, Houzé P, et al. Development, validation and clinical application of a LC-MS/MS method for the simultaneous quantification of hydroxychloroquine and its active metabolites in human whole blood. J Pharm Biomed Anal. 2014;100:131–137. [DOI] [PubMed] [Google Scholar]
- 22.Rizk ML, Hang Y, Luo W-L, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. 2012;56:3101–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46:1127–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita S, Takahashi T, Yoshida Y, et al. Population pharmacokinetics of hydroxychloroquine in Japanese patients with cutaneous or systemic lupus erythematosus. Ther Drug Monit. 2016;38:259–267. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. Guideline on Bioanalytical Method Validation 2011, EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf.
- 26.Martin-Blondel G, Ruiz S, Murris M, et al. Hydroxychloroquine in COVID-19 patients: what still needs to Be known about the kinetics. Clin Infect Dis. 2020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tett SE, Cutler DJ, Day RO, et al. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988;26:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]


