Abstract
Body mass index (BMI) is a risk factor for Alzheimer’s disease (AD) although the relationship is complex. Obesity in midlife is associated with increased risk for AD, whereas evidence supports both higher and lower BMI increasing risk for AD in late life. This study examined the influence of individual differences in genetic risk for AD to further clarify the relationship between late-life BMI and conversion to AD. Participants included 52 individuals diagnosed as having mild cognitive impairment (MCI) at baseline who converted to AD within 24 months and 52 matched MCI participants from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort. BMI was measured at baseline. Genetic risk for AD was assessed via genome-wide polygenic risk scores. Conditional logistic regression models were run to determine if BMI and polygenic risk predicted conversion to AD. Results showed an interaction between BMI and genetic risk, such that individuals with lower BMI and higher polygenic risk were more likely to convert to AD relative to individuals with higher BMI. These results remained significant after adjusting for cerebrospinal fluid biomarkers of AD. Exploratory sex-stratified analyses revealed this relationship only remained significant in males. These results show that higher genetic risk in the context of lower BMI predicts conversion to AD in the next 24 months, particularly among males. These findings suggest that genetic risk for AD in the context of lower BMI may serve as a prodromal risk factor for future conversion to AD.
Keywords: Genetics, Health factors, Mild cognitive impairment, Neurodegenerative disease, Sex differences
Alzheimer’s disease (AD) is a global health concern that is associated with significant memory loss and impaired functioning in everyday life, causing immense costs and burdens to individuals, their families, and health care systems. Approximately 5.8 million Americans are currently living with AD, and the prevalence of AD is expected to increase to 13.8 million by 2050 as the Baby Boomer generation ages (1). AD is characterized by a long preclinical period, whereby neural and biological changes occur prior to noticeable clinical and cognitive symptoms, including neurodegeneration and buildup of tau and β-amyloid peptide (Aβ) (2,3). Approximately 10%–15% of individuals diagnosed with mild cognitive impairment (MCI) progress to AD each year (4). There are no known cures for AD, making it particularly important to investigate risk and protective factors that could be targeted by intervention techniques to prevent conversion to AD.
Health factors, including body mass index (BMI), are thought to play a role in the development of late-onset AD. The relationship between BMI in midlife and risk for dementia is well characterized, such that BMI in the obese range is associated with heightened risk for AD and other types of dementia (5). Obesity is associated with damage to AD-vulnerable regions, such as the hippocampus (6), as well as AD-related pathology, including Aβ and tau (7). The link between obesity and dementia may be, in part, due to inflammation, insulin resistance, oxidative stress, and metabolic and vascular dysregulation (6,8). Furthermore, genetic variants associated with lower BMI are also associated with higher cognitive abilities (9), which may also contribute to the link between obesity and dementia. However, the relationship between late-life BMI and dementia is less straightforward. Although some work shows that higher BMI is associated with increased risk for progression to AD (10), many studies have demonstrated a protective effect of higher BMI against risk for dementia (11,12). Moreover, lower BMI in late life has been associated with increased AD-related pathology in older adults with cognitive impairment (13). Mechanisms that may explain the relationship between lower BMI and AD risk include AD-related damage to brain regions implicated in eating-related behavior and weight regulation, and serotonergic and noradrenergic system abnormalities (14–17). Further complicating the picture, significant relationships between late-life BMI and dementia are not always observed, as evidenced in a meta-analysis by Danat et al. (18).
To better understand the relationship between late-life BMI and late-onset AD, it is important to consider the influence of genetic risk factors; genetics play an integral role in progression to AD and research suggests late-onset AD is highly heritable (60%–80%) (19). However, the majority of previous research has examined BMI and AD genetic risk independently. The small set of studies that have assessed their combined effects have primarily focused on the role of the apolipoprotein E (APOE) ε4 allele, demonstrating that in late life, lower BMI among APOE ε4 carriers is associated with greater cognitive decline (20) and AD-related pathology (21). APOE is certainly the strongest single genetic predictor of AD, accounting for 13% of the phenotypic variance in late-onset AD (22). Nonetheless, polygenic approaches used to measure genetic propensity for AD integrate APOE and numerous other genetic variants into a single risk score and can explain more phenotypic variance in AD than single candidate genes (22). Polygenic scores for AD have been independently associated with various neurobiological markers of AD, including cortical thickness (23), hippocampal and entorhinal cortex volume, neurofibrillary tangles, and neuritic plaques (24). Our recent work demonstrated that higher BMI and higher polygenic risk were associated with lower volume in medial temporal lobe regions in older adults with normal cognition (25). One limitation of this previous study was that it was cross-sectional, and thus the direct relationships between BMI, genetic risk, and conversion to AD could not be examined.
The primary goal of this study was to examine the interactive effects of polygenic risk for AD and BMI at baseline in predicting conversion to AD within 24 months using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Additionally, given data showing sex differences in the relationship between BMI and dementia (26) and an interaction between BMI and genetics on AD-related pathology (25), we also implemented exploratory analyses to examine the relationship between BMI, polygenic risk for AD, and progression to AD separately in males and females. Investigating the interactive effects of health and genetic factors longitudinally has implications for developing prevention methods to delay or preclude conversion to AD, as well as clinical utility for identifying individuals at heightened risk for future conversion.
Method
Participants
Data were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2004 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Additional information can be found at www.adni-info.org.
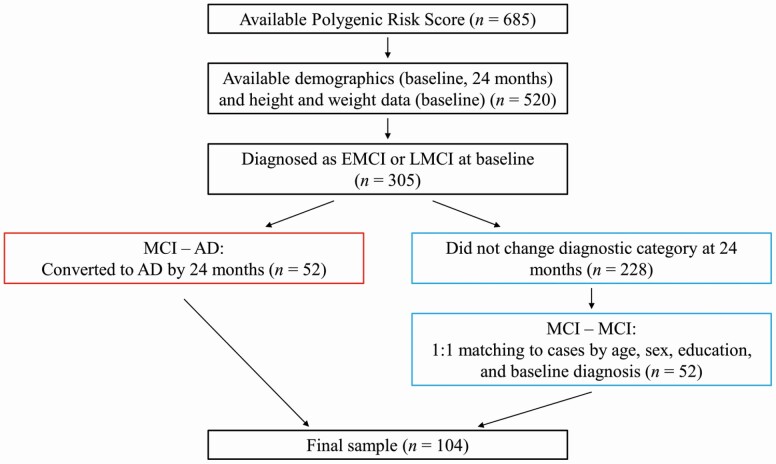
To generate our final sample, we first considered all individuals with available polygenic risk scores (n = 685). Polygenic risk scores were calculated as part of another study for a subset of ADNI participants with available demographic, neuroimaging, and genome-wide genotype data. Moreover, polygenic risk scores were only calculated for participants who identified as White, non-Hispanic/Latino to avoid population stratification effects. Next, we identified participants who also had available demographic data at the baseline and 24-month visits, as well as height and weight data at baseline (n = 520). Next, we identified participants who were diagnosed as early MCI (EMCI) or late MCI (LMCI) at the baseline visit (n = 305). Of these 305 participants, 52 individuals converted to AD within 24 months. The remaining 253 participants were considered for inclusion as matched participants. To generate our matched participants, we first excluded anyone who changed diagnostic categories from baseline to 24 months (n = 25). Next, we used the R function matchControls to select 52 of these 228 individuals to serve as matched participants in the final sample. These participants were specifically matched to individuals who converted to AD based on age, sex, education, and baseline diagnosis. The final sample of 104 older adults aged 55–84 years who were diagnosed with MCI at baseline included 52 individuals who converted to AD within 24 months (referred to as “MCI–AD” for brevity) and 52 matched individuals who did not convert to AD within 24 months (referred to as “MCI–MCI”) (see Figure 1).
Figure 1.
Flow chart detailing generation of the final sample.
Exclusion criteria included any neurological disease other than developing or suspected AD, such as, but not limited to, Parkinson’s disease, multi-infarct dementia, Huntington’s disease, and multiple sclerosis. Moreover, individuals with mental health diagnoses were excluded, including major depression, bipolar disorder, schizophrenia, and alcohol/substance abuse/dependence. Similarly, all participants had to score less than 6 on the Geriatric Depression Scale (Short Form); scores 0–5 are not indicative of depression and scores 6–15 are indicative of depression. Individuals were also excluded if they presented with a history of significant head trauma. To minimize the likelihood of including individuals presenting with dementias other than AD, all participants had to score less than or equal to 4 on the Hachinski Ischemic Scale and show no evidence of infection, infarction, focal lesion, multiple lacunes, or lacunes in memory structures on the baseline MRI scan. Additional details about inclusion/exclusion criteria in ADNI can be found in the ADNI protocol (http://adni.loni.usc.edu/methods/documents/).
EMCI, LMCI, and AD were defined according to ADNI’s diagnostic criteria, which are described below and can be found in greater detail in the ADNI protocol (http://adni.loni.usc.edu/methods/documents/). EMCI was defined as subjective memory concern, abnormal memory functioning on the Logical Memory II subscale (Delayed Paragraph Recall, Paragraph A) of the Wechsler Memory Scale—Revised (ie, 9–11 for 16+ years of education, 5–9 for 8–15 years of education, 3–6 for 0–7 years of education), Mini-Mental State Examination (MMSE) score between 24 and 30, Clinical Dementia Rating of 0.5 with the memory box score being at least 0.5, and did not meet criteria for AD. LMCI had the same criteria as EMCI, except the abnormal memory function on the Logical Memory II subscale had to be more severe than the criteria for EMCI (ie, ≤ 8 for 16+ years of education, ≤ 4 for 8–15 years of education, ≤ 2 for 0–7 years of education). AD was defined as subjective memory concern, abnormal memory function on the Logical Memory II subscale (used the same criteria as LMCI), MMSE between 20 and 26, a Clinical Dementia Rating of 0.5 or 1.0, and met NINCSD/ADRDA criteria for probable AD. Study procedures were approved by site-specific Institutional Review Boards and all participants and/or authorized representatives provided written informed consent.
Body Mass Index
Height (inches or centimeters) and weight (pounds or kilograms) were measured at baseline for all participants. Height measurements were converted to meters and weight measurements were converted to kilograms. BMI was calculated using the formula: [weight (kilograms)/height (meters)2]. There were 25 individuals categorized as normal weight (18.5 ≤ BMI < 25), 56 individuals categorized as overweight (25 ≤ BMI < 30), and 23 individuals categorized as obese (BMI ≥ 30). There were no individuals categorized as underweight (BMI < 18.5).
Polygenic Risk Score Calculation and Genotyping Procedures
We calculated genome-wide polygenic risk scores using beta values to weight all single nucleotide polymorphisms (SNPs) from a genome-wide association study (GWAS), multiplied these weights by the additively coded genotypes (ie, 0, 1, 2), then summed them together to create a single risk score for AD. Our polygenic risk scores integrated all SNPs under a specific p value threshold and were calculated across multiple p value thresholds, as there is no way to predetermine the most predictive threshold. The most predictive score was used for further analyses and was operationalized as the threshold exhibiting the strongest relationship with conversion to AD (described in greater detail below).
The polygenic risk scores for AD in the present study were calculated based on summary statistics from the largest and most recent GWAS on AD, which was conducted on individuals from the International Genomics of Alzheimer’s Disease Project (IGAP) who identify as White and non-Hispanic (27). The summary results are available for download at https://www.niagads.org/datasets/ng00075. The risk scores in the present study excluded SNPs with Impute2 quality <0.50 and/or minor allele frequency <0.01. Furthermore, to prevent redundancy, the SNP panel was trimmed for linkage disequilibrium using PLINK’s “clumping” procedure prior to imputation (r2 threshold of .2 in a 500 kb window based on linkage disequilibrium patterns in the 1 000 Genomes EUR sample). Polygenic risk scores were computed across 13 p value thresholds: p < 1 × 10–8, p < 1 × 10–7, p < 1 × 10–6, p < 1 × 10–5, p < 1 × 10–4, p < 1 × 10–3, p < .01, p < .05, p < .1, p < .2, p < .3, p < .4, and p < .5.
Genome-wide genotyping was performed using the Illumina HumanOmniExpress BeadChip and processed via GenomeStudio v2011.1 (Illumina). APOE was genotyped using DNA from a blood sample. Additional details regarding the genome-wide and APOE genotyping procedures used in ADNI are available elsewhere (28).
Cerebrospinal Fluid Biomarkers
Cerebrospinal fluid (CSF) Aβ, total tau, and phosphorylated tau (p-tau) were analyzed at the UPenn/ADNI Biomarker laboratory using the fully automated Roche Elecsys immunoassay (29). The Elecsys Aβ CSF immunoassay in use is not a commercially available in vitro diagnostic assay; it is currently under development and for investigational use only. The measuring range of the assay is 200 (lower technical limit) to 1 700 pg/mL (upper technical limit). The performance of the assay beyond the upper technical limit has not been formally established. Therefore, use of values above the upper technical limit, which are provided based on an extrapolation of the calibration curve, is restricted to exploratory research purposes and is excluded for clinical decision making or for the derivation of medical decision points. In our sample, 14 participants had Aβ values greater than the upper technical limit, which were truncated to 1 700. No participants had values below the lower technical limit for Aβ or outside of the technical limits for tau (80–1 300 pg/mL) or p-tau (8–120 pg/mL).
Statistical Approach
Statistical analyses were performed using R version 3.6.1 for Macintosh. MCI–AD and MCI–MCI participants were compared on relevant demographic and outcome variables using Mann–Whitney U tests for continuous variables, as normality was violated for all variables assessed, and Fisher’s exact tests for categorical variables. Baseline BMI and all polygenic risk scores were standardized prior to analyses. To determine the most predictive polygenic risk score threshold to be used in subsequent analyses, conditional logistic regression models were used to examine the effect of genetic risk for AD on conversion to AD within 24 months across all 13 polygenic risk score p value thresholds. We chose the polygenic risk score threshold that showed the strongest relationship (ie, most significant p value) with conversion to AD. The score computed at this threshold was used in all further analyses.
Using hierarchical conditional logistic regression models, our primary analysis was to examine the relationship between BMI and polygenic risk on progression to AD. In the first model, the main effects of BMI and the most significant polygenic score were entered. In the second model, the BMI × polygenic score interaction term was entered. Covariates were not included in the conditional logistic regression models, as MCI–AD and MCI–MCI participants were matched on relevant covariates (age, sex, education, and baseline diagnosis).
Secondarily, we sought to determine if BMI and polygenic risk could explain conversion to AD over and above well-known biomarkers of AD (3). As such, we replicated the BMI × polygenic risk logistic regressions described above including Aβ, tau, and p-tau as covariates. Three participants were missing these CSF biomarkers, which broke MCI–AD/MCI–MCI matching and therefore, unconditional logistic regression models were fit for these analyses. As such, we also included age, sex, education, and baseline diagnosis as covariates. In the first model, age, sex, education, baseline diagnosis, Aβ, tau, and p-tau were entered. In the second model, polygenic risk and BMI were entered. In the third model, the BMI × polygenic score interaction term was entered. We calculated Nagelkerke’s pseudo-R2 and area under the curve (AUC) as measures of goodness of fit and diagnostic accuracy, respectively, for unconditional logistic regression models; AUC of 0.5–0.7 suggests poor discrimination, 0.7–0.8 suggests acceptable discrimination, and 0.8–0.9 suggests excellent discrimination (30).
We also implemented our primary and secondary analyses described above separately in males and females to examine sex differences in the relationship between BMI, polygenic risk, and conversion to AD. Due to small sample sizes when the overall sample is stratified into males (n = 62) and females (n = 42), these analyses are exploratory and findings should be considered provisional.
To ensure our results were not being driven by outliers, we replicated all analyses after excluding 2 bivariate outliers with respect to BMI and polygenic risk (31); results were similar and therefore the final results do not exclude outliers. We also considered the contribution of cerebrovascular risk factors that are commonly associated with BMI and risk for AD, including smoking, hypertension, diabetes, and hypercholesterolemia/hyperlipidemia. These factors were not significant predictors and are therefore not reported in the final results. Additionally, we implemented correlation analyses to examine the relationship between polygenic risk for AD and cognition at baseline and BMI and cognition at baseline to determine if cognitive functioning may be confounding the observed relationships between BMI, polygenic risk, and conversion to AD. Cognitive status at baseline, as measured via the MMSE, Alzheimer’s Disease Assessment Scale 13, and Clinical Dementia Rating sum of boxes, was not associated with polygenic risk for AD or BMI. As such, cognition was not included as a covariate in the final models.
Results
Demographics of the entire sample and as a function of MCI–AD/MCI–MCI status are shown in Table 1. There were no significant differences between the groups in terms of age, sex, education, or baseline diagnosis, indicating that MCI–AD/MCI–MCI matching was successful. There were also no significant differences between MCI–AD and MCI–MCI participants in terms of BMI. There was a significant difference in APOE ε4 alleles between MCI–AD and MCI–MCI participants (p = .006). Furthermore, MCI–AD participants had significantly lower CSF Aβ (reflecting greater intracranial Aβ burden) (U = 1 813, p < .001), higher tau (U = 637, p < .001), and higher p-tau (U = 599, p < .001). The conditional logistic regression models indicated the strongest main effect of polygenic risk score at the p < 1 × 10–6 threshold (see Supplementary Table 1 for results at each p value threshold). At this threshold (p < 1 × 10–6), MCI–AD participants had significantly higher polygenic risk for AD than MCI–MCI participants (U = 877, p = .002). The genetic architecture of late-onset AD is yet to be fully elucidated; however, recent work suggests p value thresholds that are more stringent may be indicative of an oligogenic architecture, whereas more lenient p value thresholds may be indicative of a polygenic architecture (32). The optimal polygenic risk threshold here is stringent, which supports the interpretation that the genetic risk of AD reflects an oligogenic architecture.
Table 1.
Demographics of the Overall Sample, MCI–AD, and MCI–MCI Participants
| Totala | MCI–ADb | MCI–MCIc | p Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age, y | 71.8 (7.01) | 71.9 (7.42) | 71.7 (6.64) | .687 |
| Sex, N (%) | ||||
| Female | 42 (40.4%) | 21 (40.4%) | 21 (40.4%) | |
| Male | 62 (59.6%) | 31 (59.6%) | 31 (59.6%) | 1 |
| Years of education | 16.0 (2.49) | 15.9 (2.60) | 16.1 (2.39) | .711 |
| Baseline diagnosis, N (%) | ||||
| EMCI | 22 (21.2%) | 11 (21.2%) | 11 (21.2%) | |
| LMCI | 82 (78.8%) | 41 (78.8%) | 41 (78.8%) | 1 |
| Polygenic risk scored | 0.00 (1.00) | 0.30 (0.97) | −0.30 (0.95) | .002* |
| BMIe | 28.0 (5.16) | 27.9 (6.14) | 28.0 (4.01) | .136 |
| APOE ε4 allelesf, N (%) | ||||
| 0 | 46 (44.2%) | 15 (28.8%) | 31 (59.6%) | |
| 1 | 41 (39.4%) | 26 (50.0%) | 15 (28.8%) | |
| 2 | 17 (16.3%) | 11 (21.2%) | 6 (11.5%) | .006* |
| Aβ | 915 (424) | 749 (305) | 1090 (462) | <.001* |
| Tau | 318 (152) | 380 (163) | 252 (107) | <.001* |
| P-tau | 31.2 (17.4) | 38.3 (18.2) | 23.6 (12.7) | <.001* |
Notes: Aβ = β-amyloid peptide; APOE = apolipoprotein E; BMI = body mass index; EMCI = early mild cognitive impairment; LMCI = late mild cognitive impairment; p-tau = phosphorylated tau.
a N = 104. bN = 52. cN = 52. dStandardized value. eUnstandardized value. fCitation: Li et al. (33).
*p < .05.
Overall Sample
Interaction between BMI and polygenic risk score
Hierarchical conditional logistic regression models revealed a significant interaction between BMI and polygenic risk (computed at the p < 1 × 10–6 threshold) in the overall sample (OR = 0.49, 95% CI = 0.25–0.96, p = .036; see Table 2). This interaction can be thought of as indicating that the association between polygenic risk and conversion to AD differed as a function of BMI or conversely that the effect of BMI differed as a function of polygenic risk. To further clarify the interaction between BMI and polygenic risk on conversion to AD, we ran follow-up, unconditional, hierarchical logistic regressions for lower/higher BMI groups (based on median split; unstandardized median = 26.74, standardized median = −0.23). Unconditional logistic regressions were fit since MCI–AD/MCI–MCI matching was broken with the median split. Demographics and statistical comparisons of lower/higher BMI groups are shown in Supplementary Table 2. In the first model, covariates (age, sex, education, and baseline diagnosis) were entered. In the second model, polygenic risk score was entered. Results revealed that among individuals with lower BMI, polygenic risk score significantly predicted conversion to AD (OR = 2.91, 95% CI = 1.51–6.42, p = .003, Nagelkerke’s R2 = .31, AUC = 0.78). Among individuals with higher BMI, polygenic risk did not significantly predict conversion to AD (OR = 1.27, 95% CI = 0.69–2.39, p = .451, Nagelkerke’s R2 = .07, AUC = 0.63).
Table 2.
Summary of Conditional Logistic Regression Analysis for Association With Conversion to AD in the Overall Sample
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variable | Adjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| BMI | 1.06 | 0.70–1.62 | .769 | 1.02 | 0.66–1.57 | .928 |
| PRS | 1.77 | 1.15–2.71 | .009** | 1.78 | 1.11–2.84 | .016* |
| BMI × PRS | 0.49 | 0.25–0.96 | .036* | |||
| Model LRT | 8.21* | 13.31** |
Notes: BMI = body mass index; LRT = likelihood ratio test; PRS = polygenic risk score. BMI and PRS were standardized prior to analyses.
*p < .05. **p < .01.
CSF biomarker analyses
We next conducted hierarchical unconditional logistic regressions with CSF biomarkers to determine if BMI and polygenic risk could predict conversion to AD after adjusting for core biomarkers of AD. Age, sex, education, baseline diagnosis, Aβ, tau, and p-tau significantly predicted conversion to AD with excellent diagnostic accuracy [χ 2 (7, N = 101) = 33.31, p < .001, Nagelkerke’s R2 = .41, AUC = 0.80]. Adding polygenic risk and BMI in model 2 [Δχ 2 (2, N = 101) = 2.37, p = .306, Nagelkerke’s R2 = .43, AUC = 0.81] did not significantly improve the overall model. Adding the BMI × polygenic risk interaction in model 3 significantly improved the overall model [Δχ 2 (1, N = 101) = 5.48, p = .019, Nagelkerke’s R2 = .48, AUC = 0.83].
Sex-Stratified Samples
Interaction between BMI and polygenic risk score
Sex differences in the relationship between BMI and polygenic risk on conversion to AD were explored next. Demographics and statistical comparisons of males and females are shown in Supplementary Table 3. Among males, there was a significant interaction between BMI and polygenic risk on conversion to AD (OR = 0.28, 95% CI = 0.09–0.85, p = .025; see Supplementary Table 4). Among females, there was no significant interaction between BMI and polygenic risk (OR = 1.18, 95% CI = 0.28–4.97, p = .821) or main effect of BMI (OR = 1.17, 95% CI = 0.59–2.30, p = .649); however, there was a main effect of polygenic score on conversion to AD (OR = 2.52, 95% CI = 1.07–5.93, p = .034; see Supplementary Table 5).
To further clarify the interaction between BMI and polygenic risk score on progression to AD in males, we ran follow-up, unconditional, hierarchical logistic regressions for lower/higher BMI groups (based on median split; unstandardized median = 27.57, standardized median = −0.07), as described above for the overall sample. Results revealed that among males with lower BMI, polygenic risk score significantly predicted conversion to AD (OR = 2.99, 95% CI = 1.32–8.31, p = .017, Nagelkerke’s R2 = .37, AUC = 0.81). Among males with higher BMI, polygenic risk did not significantly predict conversion to AD (OR = 0.95, 95% CI = 0.42–2.09, p = .905, Nagelkerke’s R2 = .04, AUC = 0.59).
CSF biomarker analyses
Hierarchical unconditional logistic regressions revealed the sex-stratified results remained significant after adjusting for Aβ, tau, and p-tau. In males, age, education, baseline diagnosis, Aβ, tau, and p-tau predicted conversion to AD with excellent diagnostic accuracy [χ 2 (6, N = 61) = 22.12, p = .001, Nagelkerke’s R2 = .42, AUC = 0.81]. Adding polygenic risk and BMI in model 2 [Δχ 2 (2, N = 61) = 2.74, p = .255, Nagelkerke’s R2 = .46, AUC = 0.83] did not significantly improve the overall model. Adding the BMI × polygenic risk interaction in model 3 significantly improved the overall model [Δχ 2 (1, N = 61) = 10.59, p = .001, Nagelkerke’s R2 = .60, AUC = 0.89]. In females, age, education, baseline diagnosis, Aβ, tau, and p-tau predicted conversion to AD with excellent diagnostic accuracy [χ 2 (6, N = 40) = 16.25, p = .012, Nagelkerke’s R2 = .50, AUC = 0.87]. Adding polygenic risk and BMI in model 2 [Δχ 2 (2, N = 41) = 1.70, p = .427, Nagelkerke’s R2 = .53, AUC = 0.85] and the BMI × polygenic risk interaction in model 3 [Δχ 2 (1, N = 41) = 0.11, p = .740, Nagelkerke’s R2 = .53, AUC = 0.87] did not significantly improve the overall model.
Discussion
This study examined the relationship between BMI, polygenic risk for AD, and conversion to AD within 24 months. There were 2 primary findings. First, the interaction between BMI and polygenic risk predicted conversion to AD, such that lower BMI and higher polygenic risk increased likelihood of conversion, whereas there was no association between polygenic risk and conversion among individuals with higher BMI; this finding remained significant even after adjusting for the effects of Aβ, tau, and p-tau. Second, exploratory analyses revealed that the relationship between BMI, polygenic risk, and conversion to AD was more pronounced in males.
The results presented here show that 24 months prior to an AD diagnosis, lower BMI, in combination with greater genetic risk for AD, is associated with conversion to AD. Genetic risk for AD is associated with alterations and atrophy in various brain regions, including the amygdala, hippocampus, and other medial temporal regions (24,34–36), as well as differential gene expression in regions implicated in AD, such as temporal regions (37). Inflammation and oxidative stress are 2 specific pathways that genetic risk for AD likely acts through to facilitate neurodegeneration (38–40). One interpretation of our results is that lower BMI acts through, and exacerbates, these genetic pathways. It is often proposed that lower BMI in the 10 years preceding an AD diagnosis is a consequence of AD pathology (41); AD pathology may induce damage to brain regions that are implicated in eating-related behavior and weight regulation, such as the amygdala, hypothalamus, cingulate gyrus, hippocampus, and other medial temporal regions (14–17), leading to body weight loss and consequently lower BMI. AD pathology may specifically induce damage to these regions through oxidative stress and inflammation (38,40). This suggests that higher genetic risk for AD and AD-related damage contributing to lower BMI may affect the same neural pathways; therefore, these 2 risk factors in combination may lead to greater neurobiological disruptions and consequently increase the risk of clinical manifestation of AD. Nevertheless, additional work is needed to elucidate the specific, synergistic mechanisms underlying this interaction. One specific factor that warrants consideration and should be further investigated is the role of sarcopenia (ie, losing muscle mass and function). Sarcopenia is associated with lower BMI (42) and may be caused by AD-related pathology, including oxidative stress and inflammation (43), both of which are also genetic pathways of AD (39). Thus, sarcopenia may mediate the relationship between BMI, genetic risk for AD, and conversion to AD. Another factor that warrants consideration is frailty, which is characterized, in part, by weight loss (44) and is associated with increased risk for dementia (45), including AD (46). Furthermore, frailty is associated with inflammation (47) and oxidative stress (48), further demonstrating that it may contribute to the observed relationship between BMI, genetic risk for AD, and conversion to AD. A second interpretation of these findings that warrants consideration is that there is a protective effect of maintaining higher BMI that may help to counteract the negative pathways of genetic risk for AD. Specifically, leptin is a hormone produced by adipose tissue that has neuroprotective properties, including hippocampal synaptic plasticity and corresponding learning and memory outcomes (49). As such, the neuroprotective effects of leptin, specifically in regions vulnerable to AD such as the hippocampus, may offset some of the negative effects of genetic risk for AD in these regions to preclude conversion to AD. Although, to further explore this interpretation, future work should examine how leptin interacts with genetic risk for AD to influence progression to AD.
Findings of this study also showed that the interaction between BMI and polygenic risk for AD remained significant after adjusting for Aβ, tau, and p-tau, which are some of the most well-known biomarkers of AD (3). These results underscore the importance of considering health and genetic factors as well as their synergistic effects when examining risk for late-onset AD, as they have predictive power beyond common AD biomarkers and can help us characterize this multifactorial disease better than any factor can in isolation. Nonetheless, it is important to acknowledge that the effect of BMI and genetic factors is relatively small after accounting for the most predictive biomarkers of AD.
Our exploratory analyses indicated that the relationship between BMI and polygenic risk on conversion to AD was stronger in male participants. This finding was initially surprising, as females are at greater risk for AD (50) and previous research suggests that the relationship between BMI and dementia (26), as well as BMI, genetics, and AD-related pathology (25), may be stronger in females. A potential explanation for the sex differences observed in this study considers the role of testosterone, which decreases throughout the aging process, specifically among males (51,52), and has been associated with lower BMI in older males (53). Low testosterone is consistently identified as a risk factor for AD (53), specifically during the preclinical phase (54), and interacts with APOE ε4 to confer risk for AD (52). Decreased testosterone and genetics may specifically interact and increase risk for late-onset AD via inflammatory pathways (38,51), although additional work is needed to clarify the role of testosterone in the relationship between BMI and genetic risk for AD on AD risk, specifically among males. Furthermore, due to small sample sizes, these findings should be considered provisional and should be replicated with larger sample sizes.
This study has several limitations. First, while it is beneficial that this study was able to examine BMI, genetics, and progression to AD longitudinally, ADNI does not collect data on BMI prior to the baseline assessment. As such, the relationship between lifetime BMI, genetic risk, and late-onset AD could not be examined. Furthermore, it would be useful to examine the relationship between late-life BMI and progression to AD over a longer period of time. However, attrition in ADNI increases at later timepoints; we chose to preserve power and minimize non-random dropout that may bias results, which consequently sacrificed the ability to examine this relationship over a longer timeframe (ie, 4–5 years). Additional work is needed to examine the relationship between BMI at various points across the life span, genetic risk for AD, and AD, including earlier in the disease process as some of the neurobiological changes of AD begin to develop. Similarly, future work should also examine the interplay between BMI and polygenic risk on the progression from cognitively normal to MCI. While we selected the timeframe of this study to maximize power, our sample is still relatively small and is therefore another limitation of the current study. Until this study can be replicated with additional, larger sample sizes, the findings should be considered provisional. Risk for AD is also influenced by various lifestyle factors, such as physical activity, diet, mental stimulation, and social engagement (55). These variables were not assessed in ADNI and therefore we could not determine how these factors may contribute to conversion in the present study, which is another limitation of this study. Similarly, ADNI does not collect data on midlife and therefore the influence of various vascular and lifestyle factors in midlife on conversion to AD in late life could not be examined. Another limitation of this study is that our sample did not include any individuals categorized as underweight. Underweight BMI, genetic risk, and progression to AD should be examined, as there is evidence that late-life, underweight BMI may increase dementia risk (56). Finally, our sample only included older adults who identify as White, non-Hispanic/Latino and additional work is needed to clarify how BMI and genetic risk interact to influence progression to AD in other racial and ethnic groups.
In conclusion, this study showed that polygenic risk for AD had a greater impact on risk in those with lower BMI on the likelihood of conversion to AD within 24 months, specifically among males. No association was observed between polygenic risk and AD in individuals with higher BMI. This interaction remained significant even after adjusting for Aβ, tau, and p-tau, the core biomarkers of AD. These results suggest that genetic risk for AD in the context of lower BMI may serve as a predictor of future progression to AD. Moreover, these results may help clinicians identify individuals at heightened risk of developing late-onset AD who should be monitored more closely for pathological decline and may benefit most from interventions attempting to delay or prevent progression to AD. This study underscores the importance of examining the synergistic effects of health and genetic risk factors to better characterize late-onset AD, which may be used to inform prevention methods.
Supplementary Material
Acknowledgments
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
Funding
This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) (grant numbers R21AG056921 to S.M.H. and R01AG058822 to J.P.H.) and The Ohio State University Discovery Themes Chronic Brain Injury Initiative (to S.M.H. and J.P.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the NIA, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Inc., Biogen, Bristol-Myers Squibb Company, CereSpir, Inc., Cogstate, Eisai Inc., Elan Pharmaceuticals, Inc., Eli Lilly and Company, EuroImmun, F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc., Fujirebio, GE Healthcare, IXICO Ltd., Janssen Alzheimer Immunotherapy Research & Development, LLC., Johnson & Johnson Pharmaceutical Research & Development LLC., Lumosity, Lundbeck, Merck & Co., Inc., Meso Scale Diagnostics, LLC., NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc., Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflict of Interest
None declared.
Author Contributions
J.N.M. designed and conceptualized the study, analyzed and interpreted the data, and drafted the manuscript. K.E.V. analyzed and interpreted the data and critically revised the manuscript for intellectual content. A.N.H. analyzed the data and critically revised the manuscript for intellectual content. S.P. interpreted the data and critically revised the manuscript for intellectual content. M.W.L. analyzed and interpreted the data and critically revised the manuscript for intellectual content. S.M.H. conceptualized the study and critically revised the manuscript for intellectual content. J.P.H. designed and conceptualized the study, interpreted the data, and critically revised the manuscript for intellectual content. All authors approve of the final manuscript and agree to be accountable for all aspects of the work.
References
- 1. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 3. Jack CR Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst). 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16(6):465–477. doi: 10.1016/S1474-4422(17)30084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 8. Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obes Rev. 2018;19(2):269–280. doi: 10.1111/obr.12629 [DOI] [PubMed] [Google Scholar]
- 9. Marioni RE, Yang J, Dykiert D, et al. Assessing the genetic overlap between BMI and cognitive function. Mol Psychiatry. 2016;21(10): 1477–1482. doi: 10.1038/mp.2015.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524 [DOI] [PubMed] [Google Scholar]
- 11. Bell SP, Liu D, Samuels LR, et al. Late-life body mass index, rapid weight loss, apolipoprotein E ε4 and the risk of cognitive decline and incident dementia. J Nutr Health Aging. 2017;21(10):1259–1267. doi: 10.1007/s12603-017-0906-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cova I, Clerici F, Maggiore L, et al. Body mass index predicts progression of mild cognitive impairment to dementia. Dement Geriatr Cogn Disord. 2016;41(3–4):172–180. doi: 10.1159/000444216 [DOI] [PubMed] [Google Scholar]
- 13. Mathys J, Gholamrezaee M, Henry H, von Gunten A, Popp J. Decreasing body mass index is associated with cerebrospinal fluid markers of Alzheimer’s pathology in MCI and mild dementia. Exp Gerontol. 2017;100:45–53. doi: 10.1016/j.exger.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 14. Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90 [DOI] [PubMed] [Google Scholar]
- 15. Cross AJ. Serotonin in Alzheimer-type dementia and other dementing illnesses. Ann N Y Acad Sci. 1990;600(1):405–415; discussion 415. doi: 10.1111/j.1749-6632.1990.tb16897.x [DOI] [PubMed] [Google Scholar]
- 16. Grundman M, Corey-Bloom J, Jernigan T, Archibald S, Thal LJ. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46(6):1585–1591. doi: 10.1212/wnl.46.6.1585 [DOI] [PubMed] [Google Scholar]
- 17. Morris CH, Hope RA, Fairburn CG. Eating habits in dementia. A descriptive study. Br J Psychiatry. 1989;154(6):801–806. doi: 10.1192/bjp.154.6.801 [DOI] [PubMed] [Google Scholar]
- 18. Danat IM, Clifford A, Partridge M, et al. Impacts of overweight and obesity in older age on the risk of dementia: a systematic literature review and a meta-analysis. J Alzheimers Dis. 2019;70(suppl. 1):S87–S99. doi: 10.3233/JAD-180763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- 20. Rajan KB, Skarupski KA, Rasmussen HE, Evans DA. Gene–environment interaction of body mass index and apolipoprotein E ε4 allele on cognitive decline. Alzheimer Dis Assoc Disord. 2014;28(2):134–140. doi: 10.1097/WAD.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blautzik J, Kotz S, Brendel M, et al. Relationship between body mass index, ApoE4 status, and PET-based amyloid and neurodegeneration markers in amyloid-positive subjects with normal cognition or mild cognitive impairment. J Alzheimers Dis. 2018;65(3):781–791. doi: 10.3233/JAD-170064 [DOI] [PubMed] [Google Scholar]
- 22. Ridge PG, Hoyt KB, Boehme K, et al. Assessment of the genetic variance of late-onset Alzheimer’s disease. Neurobiol Aging. 2016;41:200.e13–200.e20. doi: 10.1016/j.neurobiolaging.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabuncu MR, Buckner RL, Smoller JW, Lee PH, Fischl B, Sperling RA. The association between a polygenic Alzheimer score and cortical thickness in clinically normal subjects. Cereb Cortex. 2012;22(11):2653–2661. doi: 10.1093/cercor/bhr348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14(3):e1002258. doi: 10.1371/journal.pmed.1002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes JP, Moody JN, Guzmán Roca J, Hayes SM. Body mass index is associated with smaller medial temporal lobe volume in those at risk for Alzheimer’s disease. NeuroImage Clin. 2020;25:102156. doi: 10.1016/j.nicl.2019.102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joo SH, Yun SH, Kang DW, Hahn CT, Lim HK, Lee CU. Body mass index in mild cognitive impairment according to age, sex, cognitive intervention, and hypertension and risk of progression to Alzheimer’s disease. Front Psychiatry. 2018;9:142. doi: 10.3389/fpsyt.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saykin AJ, Shen L, Yao X, et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: progress, opportunities, and plans. Alzheimers Dement. 2015;11(7):792–814. doi: 10.1016/j.jalz.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bittner T, Zetterberg H, Teunissen CE, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517–526. doi: 10.1016/j.jalz.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 30. Hosmer DW, Lemeshow S, Sturdivant RX.. Applied Logistic Regression. 2nd ed.John Wiley & Sons; 2013. [Google Scholar]
- 31. Rousseeuw PJ, Ruts I, Tukey JW. The bagplot: a bivariate boxplot. Am Stat. 1999;53(4):382–387. doi: 10.1080/00031305.1999.10474494 [DOI] [Google Scholar]
- 32. Zhang Q, Sidorenko J, Couvy-Duchesne B, et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat Commun. 2020;11(1):4799. doi: 10.1038/s41467-020-18534-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Loewenstein DA, Duara R, Cabrerizo M, Barker W, Adjouadi M. The relationship of brain amyloid load and APOE status to regional cortical thinning and cognition in the ADNI cohort. J Alzheimers Dis. 2017;59(4):1269–1282. doi: 10.3233/JAD-170286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stein JL, Hua X, Morra JH, et al. Genome-wide analysis reveals novel genes influencing temporal lobe structure with relevance to neurodegeneration in Alzheimer’s disease. Neuroimage. 2010;51(2):542–554. doi: 10.1016/j.neuroimage.2010.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wachinger C, Nho K, Saykin AJ, Reuter M, Rieckmann A. A longitudinal imaging genetics study of neuroanatomical asymmetry in Alzheimer’s disease. Biol Psychiatry. 2018;84(7):522–530. doi: 10.1016/j.biopsych.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walhovd KB, Fjell AM, Sørensen Ø, et al. Genetic risk for Alzheimer disease predicts hippocampal volume through the human lifespan. Neurol Genet. 2020;6(5):e506. doi: 10.1212/NXG.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang M, Roussos P, McKenzie A, et al. Integrative network analysis of nineteen brain regions identifies molecular signatures and networks underlying selective regional vulnerability to Alzheimer’s disease. Genome Med. 2016;8(1):104. doi: 10.1186/s13073-016-0355-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naj AC, Schellenberg GD. Genomic variants, genes, and pathways of Alzheimer’s disease: an overview. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):5–26. doi: 10.1002/ajmg.b.32499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta. 2000;1502(1):139–144. doi: 10.1016/s0925-4439(00)00040-5 [DOI] [PubMed] [Google Scholar]
- 41. Kivimäki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. 2018;14(5):601–609. doi: 10.1016/j.jalz.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Senior HE, Henwood TR, Beller EM, Mitchell GK, Keogh JW. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82(4):418–423. doi: 10.1016/j.maturitas.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 43. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11(4):1509–1526. doi: 10.3390/ijms11041509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 45. Petermann-Rocha F, Lyall DM, Gray SR, et al. Associations between physical frailty and dementia incidence: a prospective study from UK Biobank. Lancet Healthy Longev. 2020;1(2):e58–e68. doi: 10.1016/S2666-7568(20)30007-6 [DOI] [PubMed] [Google Scholar]
- 46. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18(2):177–184. doi: 10.1016/S1474-4422(18)30371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soysal P, Isik AT, Carvalho AF, et al. Oxidative stress and frailty: a systematic review and synthesis of the best evidence. Maturitas. 2017;99:66–72. doi: 10.1016/j.maturitas.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 49. Mejido DCP, Peny JA, Vieira MNN, Ferreira ST, De Felice FG. Insulin and leptin as potential cognitive enhancers in metabolic disorders and Alzheimer’s disease. Neuropharmacology. 2020;171:108115. doi: 10.1016/j.neuropharm.2020.108115 [DOI] [PubMed] [Google Scholar]
- 50. Laws KR, Irvine K, Gale TM. Sex differences in Alzheimer’s disease. Curr Opin Psychiatry. 2018;31(2):133–139. doi: 10.1097/YCO.0000000000000401 [DOI] [PubMed] [Google Scholar]
- 51. Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140–1152. doi: 10.1093/gerona/gls068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moser VA, Pike CJ. Obesity and sex interact in the regulation of Alzheimer’s disease. Neurosci Biobehav Rev. 2016;67:102–118. doi: 10.1016/j.neubiorev.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39(11):1633–1639. doi: 10.1016/j.exger.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 54. Verdile G, Laws SM, Henley D, et al. Associations between gonadotropins, testosterone and β amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry. 2014;19(1):69–75. doi: 10.1038/mp.2012.147 [DOI] [PubMed] [Google Scholar]
- 55. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–666. doi: 10.1038/s41582-018-0070-3 [DOI] [PubMed] [Google Scholar]
- 56. Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(6):431–436. doi: 10.1016/S2213-8587(15)00033-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.