Abstract

Materials exhibiting high energy/power density are currently needed to meet the growing demand of portable electronics, electric vehicles and large-scale energy storage devices. The highest energy densities are achieved for fuel cells, batteries, and supercapacitors, but conventional dielectric capacitors are receiving increased attention for pulsed power applications due to their high power density and their fast charge–discharge speed. The key to high energy density in dielectric capacitors is a large maximum but small remanent (zero in the case of linear dielectrics) polarization and a high electric breakdown strength. Polymer dielectric capacitors offer high power/energy density for applications at room temperature, but above 100 °C they are unreliable and suffer from dielectric breakdown. For high-temperature applications, therefore, dielectric ceramics are the only feasible alternative. Lead-based ceramics such as La-doped lead zirconate titanate exhibit good energy storage properties, but their toxicity raises concern over their use in consumer applications, where capacitors are exclusively lead free. Lead-free compositions with superior power density are thus required. In this paper, we introduce the fundamental principles of energy storage in dielectrics. We discuss key factors to improve energy storage properties such as the control of local structure, phase assemblage, dielectric layer thickness, microstructure, conductivity, and electrical homogeneity through the choice of base systems, dopants, and alloying additions, followed by a comprehensive review of the state-of-the-art. Finally, we comment on the future requirements for new materials in high power/energy density capacitor applications.
1. Introduction
To limit global warming to <1.50 °C, as set out in the Paris agreement, carbon dioxide emissions need to decrease ∼45% by 2030 and reach net-zero by 2050.1,2 Technologies based on renewable resources such as sun, wind, and tides will play a pivotal role to meet these targets. Although the increasing deployment of renewable energies is encouraging, there still are many barriers to the replacement of power generation from traditionally high CO2-emitting sectors based on coal and gas, which is still a critical and large portion of the energy generation, due to the intermittent nature of renewables. Hence, to simultaneously move away from fossil fuels and to circumvent the unpredictability inherent in clean energy resources, it is necessary to integrate energy-harvesting technologies with energy storage devices.
Energy storage, therefore, is emerging as a key enabler for sustainable renewable technologies, particularly for the electrification of transportation but also in more specialized applications such as heart defibrillators and active armor.3 Technologies already exist to store energy, such as batteries, electrochemical supercapacitors, and electrostatic capacitors.4−16 The latter are electrical energy-storage devices belonging to the category of passive components, which are ubiquitous in electronics. Indeed, every year more than 3 trillion multilayer ceramic capacitors (MLCCs) are manufactured from BaTiO3 (BT), the prototypical ferroelectric (FE) ceramic.17−22
In comparison with Li-ion batteries or fuel cells, the nonpolarized electrostatic or dielectric capacitors possess high power density (∼104–105 W/kg) resulting from their faster charging/discharging characteristics (∼μs), which are advantageous for power electronics in electrical vehicles (EVs) and pulse power applications (Figure 1a).4,23−27 Hence, electrostatic capacitors are emerging as promising candidates for energy storage devices, where high power density in combination with high energy density are important technological requirements, as illustrated by the exponential rise in publications devoted to energy storage involving electrostatic ceramic capacitors, Figure 1b. Apart from high energy density and fast charging–discharging rate, other properties such as temperature/frequency stability, fatigue resistance, lifetime reliability, equivalent series resistance, and manufacturing cost are equally important for dielectric capacitors used in practical applications. New electroceramics are, therefore, required to facilitate near-engine power electronics, exhibit ultrafast charging, and have more durable EV performance at high temperature and voltage. Thus, future electroceramics must (i) deliver high energy density (Wrec > 10 J cm–3) and conversion efficiencies (η > 90%); (ii) endure wider temperature ranges (−50–250 °C) and frequency ranges (1–1000 Hz); (iii) exhibit greater reliability (>105 cycles) and fatigue resistance (<5% change over capacitor lifetime); and (iv) be compatible with cost-effective internal electrodes and be easily integrated with other components.
Figure 1.
(a) Applications for energy storage capacitors. *EMP: electromagnetic pulse. (b) Number of annual publications on lead-based ceramics, lead-free ceramics, ceramic multilayers, and ceramic films for energy storage capacitors from 2010 to 2020. (Collected from Web of Science, search “energy storage/density lead-based ceramic, lead-free ceramic, multilayer ceramic, ceramic capacitor, ceramic films but NOT polymer”). Reproduced with permission from PixaBay, Creative Commons License.
Historically, many different dielectric materials, ranging from paper and plastic to ceramics, have been employed in the fabrication of electrostatic capacitors. Nowadays, capacitors are fabricated from either polymers or ceramics because they offer the best combination of properties in terms of capacitance, dielectric loss, breakdown strength (BDS), and for the latter, thermal stability.
The prospects of employing ceramic capacitors for energy storage can be traced back to the 1960s work by Jaffe28 from the Clevite Corp., USA. One decade later, Burn and Smyth29 from Sprague Electric Company evaluated the energy storage performance in SrTiO3 (ST) and BT with applied electric fields up to 400 kV cm–1. Until that point, quantitative data of energy storage on these materials were limited to fields generally smaller than 150 kV cm–1 due to the relatively low dielectric BDS of the fabricated ceramics. They emphasized that the maximum energy density for a ceramic should be obtained for thinner dielectric layers due to the lower probability for the occurrence of defects (such as pores, voids, or microcracks), which are well-known sources of dielectric breakdown. Later in 1990, Love,30 also from Sprague Electric Company, revisited energy storage in ceramic capacitors and highlighted empirical design principles to achieve enhanced energy storage in capacitors, as shown in Table 1. Commercial C0G-type capacitors are manufactured from low relative permittivity (εr) linear dielectrics but may achieve an energy storage of 1 J cm–3, by virtue of their intrinsically high BDS. The significance of the BDS, to achieve high energy storage becomes apparent in the case of X7R-type capacitors, fabricated from high εr BT. An important correlation between dielectric BDS and the thickness (t) can be extracted from Table 1. Indeed, by halving the t of the dielectric layers, the energy storage appears to increase >3 fold. This effect has been recently captured by Yang and co-workers,31 who compiled BDS data from literature for several dielectric materials of different t and observed decay inversely proportional to (t)a, where a was determined as 0.5. Finally, when comparing the energy storage of Z5U and X7R, it becomes apparent that high εr alone is not a sufficient parameter to achieve high energy storage. Interestingly, Love30 stressed that the capacitor industry was rather conservative in terms of perfecting the BDS of ceramics to reach values near those of single-crystals, which would significantly enhance the energy storage in ceramic capacitors.
Table 1. General Characteristics of Commercial Type Ceramic Materials Relevant for Energy Storage (Adapted from Love30) Using Electronic Industries Alliance (EIA) Classificationsa.
| dielectric type | dielectric BDS (V μm–1) | relative permittivity, εr | t (μm) | energy at 1 kV (J cm–3) |
|---|---|---|---|---|
| C0G (temperature coefficient 0 with tolerance ±30 × 10–6/K) | 65 | 75 | 18.5 | 0.88 |
| Z5U (+10/+85 °C, ΔC/C0 = +22/–56%) | 13.2 | 7500 | 95 | 0.02 |
| X7T (−55/+125 °C, ΔC/C0 = +22/–33%) | 16 | 2800 | 70 | 0.71 |
| X7R (−55/+125 °C, ΔC/C0 = ± 15%) | 30 | 2000 | 38 | 1.40 |
| X7R | 40 | 2000 | 30 | 1.34 |
| X7R | 90 | 1800 | 20 | 4.82 |
Class I ceramic capacitors are accurate, temperature-compensating capacitors, C0G will have 0 drift with a tolerance of ±30 × 10–6/K. Class II ceramic capacitors have a dielectric with a high permittivity. C and C0 are represented capacitance value and capacitance value at 25 °C.
Love30 proposed that maximum energy storage density can be achieved in intermediate rather than high εr materials since they exhibit larger BDS. Fletcher and co-workers32 convincingly postulated that greater energy storage densities can indeed be achieved in FE materials, whose Curie temperature (Tc) is adjusted to ensure that the material is operated in the paraelectric regime, where it shows a relatively small zero-field εr, an approach already mentioned by Jaffe in 1961.28
In 2009, Ogihara and co-workers33 proposed the use of so-called weakly coupled relaxors, such as 0.7BaTiO3–0.3BiScO3 (0.7BT–0.3BS), to fabricate energy storage devices. This new conceptual approach aimed at exploiting the extraordinary temperature stability of εr exhibit by this family of materials. When compared with commercial X7R capacitors, 0.7BT–0.3BS capacitors displayed superior performance, reaching a recoverable energy density (Wrec) of 6.1 J cm–3 at 730 kV cm–1. Again, the large dielectric BDS played a decisive role in this performance. More recently, in 2019 Wang, Reaney and co-workers34 unveiled a novel approach to enhance energy storage characteristics via the fabrication of chemically heterogeneous but electrically homogeneous ceramics, with Wrec reaching 10.5 J cm–3, as detailed later in this review.
Here, we present the principles of energy storage performance in ceramic capacitors, including an introduction to electrostatic capacitors, key parameters for evaluating energy storage properties, microstructural considerations, and critical electrical factors. Second, we will review the current state-of-the-art for lead and lead-free electroceramics for energy storage capacitors with bulk ceramics, ceramic multilayers (MLs), ceramic films and glass ceramics evaluated separately. Third, we will describe strategies for optimizing energy storage in electroceramics. Finally, we will demonstrate, with appropriate examples, a guide to the future development for electroceramics in energy storage capacitors.
2. Principles of Energy Storage in Electroceramics
2.1. Electrostatic Capacitors
The simplest dielectric capacitor consists of two parallel metallic plates separated by an insulator, which becomes polarized under the application of an electric field. This is the defining behavior of a dielectric material. The actual capacitance, C (i.e., ability to store charge), of an ideal capacitor is given by the ratio of the charge, Q, stored on each metallic plate and the applied voltage, V, as shown by eq 1.
| 1 |
Nevertheless, from a practical viewpoint, a more useful equation to compute the C of a real device, as illustrated in Figure 2, encompassing a dielectric material between two parallel plates of area, A, separated by a distance, d, subject to a V, can be obtained through the application of Gauss’s law
| 2 |
where ε is the permittivity of the dielectric, and a measure of its polarizability. Combination of eqs 1 and 2 provides the relationship:
| 3 |
From eq 3, it becomes immediately apparent that the ability of dielectric capacitor to charge and, therefore store energy, is ultimately associated with ε of the dielectric.
Figure 2.

Schematic representation of an electrostatic capacitor, where D, P, and ε0 are electric displacement, polarization, and electric permittivity of free space (electric constant), respectively.
2.2. Key Parameters for Evaluating Energy Storage Properties
During the application of a V, the electrostatic energy stored, W, in the dielectric can be estimated by
| 4 |
where Qmax is the maximum charge achieved at the end of the charging cycle and dq is the incremental charge increase during the charging cycle. The volumetric energy density, Wst (i.e., the energy stored per volume unit, A d), is a common key performance indicator, expressed by
| 5 |
where E is the electric field and Dmax is the electric displacement in the material under the maximum applied field, Emax. The electrical displacement (D) corresponds to the charge density (Q/A) on the metallic plates and is expressed by D = ε0E + P (Figure 2), where P is the polarization (surface charge density).
For high ε materials, D is approximately equal to P, and it follows that D = εE = ε0εrE, where ε0 is the permittivity of free space (= 8.854 × 10–12F m–1) and εr is the relative permittivity, which is the ε/ε0 ratio. This approximation allows stored energy density (Wst) to be defined in terms of P, as follows
| 6 |
where Pmax is the maximum polarization reached at the Emax. From a practical viewpoint, eq 6 is prevalent in the calculation of Wst because several experimental methods exist to determine P under an applied E. In 1961, Jaffe28 pointed out that the recoverable energy (Wrec) corresponds to the area above the discharging curve, whose upper limit is given by the Pmax. Essentially, the mathematical integration of the area above a polarization-electric (P–E) loop provides an estimate of Wrec, as schematically illustrated in Figure 3 for four distinct types of polarization response.
Figure 3.

Four distinctive P–E hysteresis loops and their energy storage behavior: (a) linear, (b) FE, (c) relaxor-ferroelectric (with the schematic of energy storage calculation), and (d) antiferroelectric materials. *Wloss is loss energy density.
For linear dielectrics such as Al2O3, where εr is independent of the applied E. The calculation of Wrec from the P–E response illustrated in Figure. 3a, is given by
| 7 |
which clearly shows that Wrec is dependent on εr and E. Parts b–d of Figure 3 show cases where polarization responses deviate from linearity, and consequently, the computation of Wrec needs to be carried out using eq 6. The response illustrated in Figure 3b is typical of a classical FE material, such as BT, where the hysteresis is linked to polarization switching of macroscopic FE domains, as explained in detail in the review by Damjanovic.35 Already in 1961, Jaffe28 stressed that in FEs, charging energy is mainly absorbed by domain switching and is retained as remanent polarization (Pr). The typically high remanence of classical FEs can be effectively minimized via chemical doping, giving rise to the response shown in Figure 3c, which is characteristic of relaxor-ferroelectrics (RFEs), such as doped-BT and Pb(Mg1/3Nb2/3)O3.36
It is now generally accepted that relaxor behavior originates from the response of polar nanoregions (PNRs) to an alternating E. RFEs remain unsaturated at high applied E, and therefore, any increment of the E will have a contribution to energy storage. Remanence-free materials are therefore, preferable for achieving high Wrec. Linear dielectric materials meet this requirement but due to their low εr, energy storage is limited. Antiferroelectrics (AFEs) display low-remanence under low E but at large E the P–E loop opens due to the stabilization of an FE with respect to AFE phase and they display a saturated polarization, as illustrated in Figure 3d. In principle, therefore, as suggested by Jaffe,28 AFEs should afford advantages for high energy storage, providing that dielectric breakdown issues are eliminated (i.e., the BDS should be high enough to induce the AFE-FE phase transition).
From the above, it becomes evident that nonlinear dielectric materials such as FEs, RFEs, and AFEs exhibit energy dissipation (Wloss); therefore, the Wrec is actually the most important parameter, as schematically illustrated in Figure 3c (red area). Hence, Wrec becomes
| 8 |
Energy conversion efficiency of a capacitor can then be calculated as
| 9 |
where Wloss is the energy loss during discharging, which correlates to the area enclosed by the P–E loop (Figure 3c green area).
Electric-field induced polarization can be determined via the measurement of charge, current, and voltage responses, typically achieved using either the Sawyer–tower, the virtual ground, the shunt or the current step methods. Each presents advantages and disadvantages as listed in Table 2. For details of each method, the reader is referred to Prume and co-workers.37 Prume, Schmitz, and Tiedke proposed that overall the virtual ground method offers the highest precision for the measurement of FEs.
Table 2. Comparison of Different Hysteresis Measurement Methods for FEs37.
| method | measured quantity | reference component | integration necessary | bandwidth requirement | influence of parasitics |
|---|---|---|---|---|---|
| Sawyer–tower | charge Q | capacitor | no | moderate | high |
| virtual ground | current I | no | yes | high | low |
| shunt | current I | resistor | yes | high | high |
| current step | voltage V | no | no | moderate | moderate |
2.3. Key Factors for Optimizing Energy Density
The microstructural features of electroceramics, such as density, grain size, secondary phases and core–shell structures, play an important role in energy storage properties. Simultaneously, the intrinsic electrical response, e.g., band gap, alongside the electrical microstructure, i.e., the distribution of conductive and resistive elements, are equally critical factors for the optimization of energy density. The following section reviews these factors, and gives examples of where and how they may be optimized.
2.3.1. Intrinsic Band Gap
The band gap (Eg) is the forbidden energy between the top of the valence band and bottom of the conduction band. Eg is commonly used to define insulator (Eg > 4.0 eV), semiconductor (0.0 eV < Eg < 4.0 eV), and metal (Eg = 0.0 eV). For semiconductor, the intrinsic BDS can be defined as
| 10 |
where BDS is direct proportional to Eg.38 Thus, semiconductors with wider Eg have higher intrinsic BDS. The electronic structure and band gaps of semiconductor can be studied theoretically using, e.g., linear discriminant analysis, or experimentally, e.g., absorbance spectroscopy and diffuse reflectance spectroscopy.39 A general rule of thumb is that the activation energy (Ea) for conduction is approximately half Eg. Both may be increased by doping or through the formation of solid solutions, often delivering higher BDS and Wrec.40,41
For example, the highest Eg ∼ 3.58 eV among all different kinds of lead-free electroceramics was found in NaNbO3 (NN), as shown in Figure 4a.42 Thus, NN was introduced into Na0.5Bi0.5TiO3 (NBT) and BiFeO3–BaTiO3 (BF–BT) to enhance Eg. The Eg for BF–BT–xNN ceramics increased from 2.5 eV up to 2.95 eV for x ≤ 0.15, as shown in Figure 4b accompanied by significant enhanced Wrec ∼ 8.12 J cm–3 under electric field ∼400 kV cm–1, along with greater thermal stability (±10%, −50 to +250 °C) and ultrafast discharge rate (t0.9 < 100 ns), Figure 4.43
Figure 4.
(a) Comparison of the Eg among dielectric perovskites and a schematic of electronic breakdown. (b) Variation of average grain size and Eg as a function of NN concentration. (c) P–E loops and dP/dE under different E, (d) Wrec and η values, and (e) pulsed overdamped discharging energy density (WD) of the BF–BT–0.10NN ceramic. (a) Reproduced with permission from ref (42). Copyright 2019 John Wiley and Sons. (b-e) Reproduced with permission from ref (43). 2020 John Wiley and Sons.
2.3.2. Electrical Microstructure
The distribution of regions with different conductivity and εr are important aspects of the so-called “electrical microstructure” of electroceramics.44 In many instances, such as the core–shell microstructure or grain boundary response of BT based ceramics, the distributions are markedly heterogeneous and lead to localization of the electrical field strength in lower εr regions or pathways for breakdown through interconnected conducting regions. In 2019, electrical homogeneity was for the first-time proposed by Wang, Reaney and co-workers in the BF–BT system as a key factor to optimize BDS and as a consequence Wrec. Electrical heterogeneity was effectively eliminated by alloying with a third end-member so that it became more difficult to form a conductive pathway at high field, resulting in higher BDS and Wrec.34
A homogeneous electrical microstructure may be obtained in many different ways such as heat-treatment in the appropriate atmosphere (N2, Air, O2) provided the type and magnitude of electrical conductivity is affected by oxygen stoichiometry. Practically, however, in production, a suitable dopant strategy is utilized once the conduction type is known (p vs n type). For example, the conductivity of BF-ST-based compositions is suppressed by doping with 3 mol % Nb on the B-site to compensate for Bi volatilization and the formation of oxygen vacancies (VO..), through variation of the Fe valence (Fe3+ to Fe4+).45
For materials with more than one bulk-like region, e.g., phase mixtures, core–shell microstructures, or surface layers, alternating current (AC) impedance spectroscopy (IS) is able to show multiple responses and the resistance (R) and C can be extracted.46−53 Both the volume fraction and difference in magnitude of R and C for multiple electrical responses are equally important in influencing energy storage performance. Given the importance of the electrical microstructure, a brief outline of the role of IS is described and its advantages with respect to direct current methods are emphasized.
Direct current (DC) electrical measurements are the most commonly employed technique to evaluate the electrical characteristics of materials. However, they merely give the overall response instead of the properties of specific regions (e.g., grains and grain boundaries) unless microprobe techniques are employed.54,55 Such techniques are useful but the sample volume is small, which casts doubt on their ability to represent global behavior and they are difficult to implement experimentally.
An alternative and much more convenient technique is IS. In IS measurements, an AC signal with small voltage over a wide range of frequency, typically 10–2 to 107 Hz, is applied on the sample.44,56 The small voltage prevents any permanent change to the sample as well as yielding a (near) linear relationship between input and output. The wide range of frequencies allows separation of the response of different electro-active regions according to their relaxation times. For energy storage capacitors, impedance is capable of: (i) establishing the contributions to the electrical microstructure (grains, grain boundaries, core–shell structure and electrode–sample interface) and determine their individual conductivity and εr which give an insight into the distribution of electrical components within the sample; (ii) verifying the origin of the dominant electrical behavior (i.e., grains, grain boundary or interfacial layer response);57,58 and (iii) determining the conduction mechanism and charge carrier type which helps further interpret the electrical response of the material.47
Impedance can be defined as a complex number which usually contains both resistive and reactive (capacitive and/or inductive) components:
| 11 |
Different electro-active regions of a material are characterized by a R and a C, usually in parallel. Then the electric relaxation time or time constant, τ, of each region can be expressed as its R and C
| 12 |
at the frequency of maximum loss, ωmax, it holds the relation:
| 13 |
Due to their different R and C values, electro-active regions can be separated in the frequency domain. Once the value of R and C are extracted, they can then be assigned to appropriate regions of the sample.
Normally the impedance measurement needs to be taken over a temperature and/or oxygen partial pressure (pO2) range to gain a better understanding of the conduction mechanism and the charge carrier. The associated activation energy, Ea, can be estimated using Arrhenius equation
| 14 |
where σ is the conductivity, σ0 is pre-exponential factor, k is the Boltzmann constant, and T is temperature. Ea may be related to predominant charge carrier and conduction mechanism. The type of charge carrier may also be determined to some extent by the pO2 dependence of conductivity, i.e., p-type: conductivity increases with increasing pO2; n-type: conductivity decreases with increasing pO2; ionic charge carrier: conductivity is independent with pO2.
2.3.3. Density and Porosity
The density of the ceramic materials plays an essential role on electrical performance, especially BDS. Ceramics with higher density tend to support higher E closer to the intrinsic/theoretical BDS. In contrast, low density ceramics exhibit conductive pathway composed of pores/voids which result in short circuit under modest field strengths. The relationship between the voltage across the pore and the external E based on a “slab” model is shown below
| 15 |
where Vc and Vext are the voltage applied cross the cavity pore and external applied voltage, εc and εd are the permittivity of the cavity and the dielectric, respectively,59,60 and td and tc are the thicknesses of the dielectric and cavity, respectively. Thus, the local E increases markedly for materials with larger pores and pore volumes, resulting in lower BDS.
High density electroceramic materials are commonly obtained by optimization of the sintering conditions, including sintering temperature/time and heating/cooling rate. For ceramics that are difficult to densify using a conventional approach, sintering aids are often added.61−64 Higher density ceramics may be obtained by the addition of ZnO,65 CuO,66 and MgO,62 which enhances BDS and Wrec. For K0.5Na0.5NbO3–Bi(Mg2/3Nb1/3)O3 (KNN–BMN), small amounts of CuO help densify ceramics through the formation of a transient liquid phase, as reported by Qu and co-workers (Figure 5).64 The sintering temperature was also reduced from 1150 to 930 °C, allowing compatibly with Cu or Ag/Pd internal electrode in MLs and giving rise to Wrec ∼ 4.02 J cm–3 at 400 kV cm–1 for 0.9KNN-0.1BMN with 1% mol CuO.66
Figure 5.
Scanning electron microscopy (SEM) images of the 0.9KNN-0.1BMN-x mol % CuO ceramics with (a) x = 0.25; (b) x = 0.5; (c) x = 1.0; (d) x = 1.5, as liquid phase is circled in red. (e) Unipolar P–E hysteresis loops and (f) Calculated W and Wrec under different E of 0.9KNN-0.1BMN-1 mol % CuO ceramics.66 Reproduced with permission from ref (66). Copyright 2017 John Wiley and Sons.
Different sintering technologies, such as spark plasma sintering (SPS), two-step sintering,67 and the formation of coatings using chemical methods,68−81 have also been shown to improve density and give rise to higher BDS and Wrec.
2.3.4. Grain Size
The effect of grain size (G) on energy storage properties has been discussed for several electroceramics because of the relationship between BDS and G, expressed in eq 16
| 16 |
where a is the exponent values being in the range of 0.2–0.4.31,82−84 Waser explained that leakage current in fine-G ceramics is lower than coarse-G ceramics due to the high grain boundary density which act as barriers for charge carriers.85 Thus, dielectric materials with high density and fine-G are required to optimize energy storage. G may be tailored by chemical doping and the formation of solid solution. It may also be modified by the application of an ultrathin coating on the primary particles prior to sintering via chemical coating methods, e.g., SiO2 on BT ceramics.67,77,86−89 The optimization on Emax and Wrec via grain size-engineering for several materials is illustrated in Figure 6.
Figure 6.
Relationship between energy storage properties of ceramics and G: (a) G vs Emax and (b) G vs Wrec. *AN: AgNbO3.
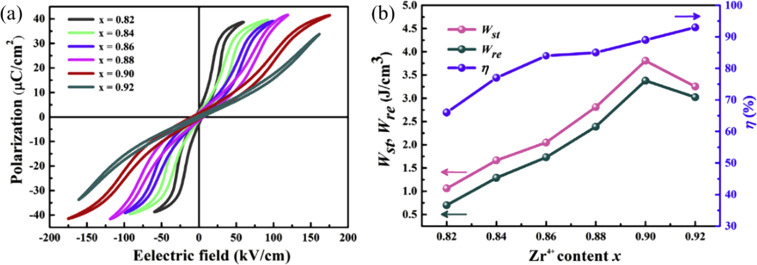
For example, an average G ∼ 10 μm was reported for BF–BT ceramics, which was reduced to <2 μm after A-site Nd doping, as shown in Figure 7. Meanwhile, improved Wrec ∼ 1.8 J cm–3 and η ∼ 88% were obtained for 15 mol % Nd–BF–BT and 40 mol % Nd–BF–BT, respectively.90 Similar optimization behavior has also been found in KNN–BMN and KNN–ST ceramics, resulting in BDS ∼ 400 kV cm–1 and Wrec > 3.5 J cm–3.91,92
Figure 7.
SEM images of x mol % Nd-doped BF–BT with different Nd concentrations: (a) BF–BT, (b) 2.5 mol % Nd–BF–BT, (c) 5 mol % Nd–BF–BT, (d) 7.5 mol % Nd–BF–BT, (e) 10 mol % Nd–BF–BT, (f) 15 mol % Nd–BF–BT, (g) 20 mol % Nd–BF–BT, (h) 30 mol % Nd–BF–BT, and (i) 40 mol % Nd–BF–BT; the G distributions of Nd-doped BF–BT are shown in the insets of the SEM images.90 (j) G, density and (k) energy storage performance at 170 kV cm–1, as a function of x(Nd) mol % in BF–BT ceramics. Reproduced with permission from ref (90). Copyright 2017 Royal Society of Chemistry.
2.3.5. Core–Shell Structure
Core–shell subgrain microstructures are observed in many lead-free ceramics, due to either kinetic limitations of the diffusion process (typical for BT based ceramics) or immiscibility on cooling from high temperature for perovskite end members with dissimilar ion size and bonding (BF based ceramics).22,34,45 The effect of core–shell microstructures on energy storage performance is still unclear. In BT-based ceramics, the cores are often more conducting than the doped shells and core to core conductive pathways lead to breakdown.93−96 For BF based ceramics, the defect chemistry of the cores and shells remains to be elucidated, but initial work suggests that further dopants are needed to create electrical homogeneity and thus eliminate the conducting pathways.34,45 The theoretical modeling has reported a positive influence of core–shell microstructure but none have been unambiguously validated experimentally.97
3. State-of-the-Art in Electroceramics for Energy Storage
3.1. Bulk Ceramics
3.1.1. Lead-Based Ceramics
Lead-based ceramics are used commercially as energy storage materials for high-power pulsed capacitors due to their excellent Wrec and η.98−101 The energy storage properties of RFE and AFE lead-based ceramics are summarized in Table 3.
Table 3. Summary of Energy Storage Properties for Lead-Based Ceramicsa.
| compositions | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| (Pb0.89Ba0.08La0.02)(Zr0.7Sn0.27Ti0.03)O3 | 135 | 22.6 | 2.1 | 76.5 | (150) |
| (Pb1.067La0.02)(Zr0.95Ti0.05)O3 | 90 | 39.5 | 2.12 | 92.98 | (141) |
| 0.90(Pb0.97La0.02)(Zr0.65Sn0.30Ti0.05)O3–0.10Bi(Zn2/3Nb1/3)O3 | 115 | 29 | 2.19 | 95.6 | (162) |
| Pb0.97La0.02(Zr0.58Sn0.35Ti0.07)O3 | 118 | 29.0 | 2.35 | 86.1 | (158) |
| Pb0.91La0.02Ba0.06(Zr0.65Sn0.3Ti0.05)O3 | 150 | 29.5 | 2.4 | 82 | (159) |
| (Pb0.93Ba0.04La0.02)(Zr0.65Sn0.3Ti0.05)O3–0.005Mn2O3 | 308 | 31.5 | 2.64 | 73 | (161) |
| Pb0.97La0.02(Zr0.33Sn0.55Ti0.12)O3@0.05SiO2 | 238 | 34.6 | 2.68 | 83.5 | (87) |
| (Pb0.87Ba0.1La0.02)(Zr0.65Sn0.3Ti0.05)O3–0.75Y | 130 | 46.5 | 2.75 | 71.5 | (149) |
| (Pb0.88La0.08)(Zr0.91Ti0.09)O3 | 170 | 31.5 | 3.04 | 92 | (112) |
| 1.7 mol % Pr3+ doped 0.24Pb (In1/2Nb1/2)O3–0.42Pb(Mg1/3Nb2/3)O3-0.34PbTiO3 | 50 | 20 | 3.1 | 90 | (109) |
| 0.92Pb(Tm0.5Nb0.5)O3–0.08Pb(Mg1/3Nb2/3)O3 | 310 | 17.03 | 3.12 | (136) | |
| (Pb0.87Ba0.1La0.02)(Zr0.68Sn0.24Ti0.08)O3 | 180 | 58.2 | 3.2 | (151) | |
| Pb0.97La0.02(Zr0.50Sn0.46Ti0.04)O3 | 150 | 43 | 3.2 | 86.5 | (124) |
| 0.55(Pb0.97La0.02)(Zr0.93Sn0.05Ti0.02)O3–0.45(Pb0.93Ba0.04La0.02) (Zr0.65Sn0.3Ti0.05)O3 | 180 | 25 | 3.2 | 74.4 | (160) |
| Pb0.97La0.02(Zr0.56Sn0.35Ti0.09)O3 | 175 | 39.4 | 3.3 | 80 | (166) |
| (Pb0.895La0.07)(Zr0.9Ti0.1)O3 | 175 | 42.3 | 3.38 | 86.5 | (115) |
| 0.9PbHfO3–0.1Pb(Mg0.5W0.5)O3 | 155 | 43.5 | 3.7 | 72.5 | (134) |
| Pb0.94La0.04(Lu0.5Nb0.5)O3 | 681 | 3.85 | (137) | ||
| (Pb0.955La0.03)(Zr0.50Sn0.42Ti0.08)O3 | 180 | 41 | 3.99 | 79.2 | (127) |
| Pb0.97La0.02(Zr0.60Sn0.35Ti0.05)O3 | 200 | 34.48 | 4.1 | (121) | |
| (Pb0.97La0.02)(Zr0.5Sn0.44Ti0.06)O3 | 250 | 29.3 | 4.2 | 82 | (117) |
| (Pb0.97La0.02)(Zr0.5Sn0.44Ti0.06)O3 | 250 | 29.3 | 4.2 | 82 | (118) |
| Pb0.955La0.03(Zr0.5Sn0.43Ti0.07)O3 | 200 | 36 | 4.2 | 78 | (126) |
| (Pb0.97La0.02)(Zr0.8Sn0.145Ti0.055)O3 | 225 | 34 | 4.38 | 73 | (124) |
| (Pb0.858Ba0.1La0.02Y0.008)(Zr0.65Sn0.3Ti0.05)O3- (Pb0.97La0.02)(Zr0.9Sn0.05Ti0.05)O3 | 200 | 46.8 | 4.65 | 60 | (152) |
| La0.02Pb0.97[(Yb0.5Nb0.5)0.92Ti0.08]O3 | 240 | 34 | 5.18 | 65 | (135) |
| (Pb0.97La0.02Zr0.85Sn0.12Ti0.03O3)−0.5 wt % Al2O3 | 315 | 35.5 | 5.3 | 88.3 | (72) |
| (Pb0.955Sr0.015La0.02)(Zr0.75Sn0.195Ti0.055)O3 | 350 | 33.5 | 5.56 | 70 | (156) |
| Pb0.97La0.02(Zr0.5Sn0.45Ti0.05)O3 | 400 | 36.2 | 5.6 | 63 | (116) |
| (Pb0.858Ba0.1La0.02Y0.008)(Zr0.65Sn0.3Ti0.05)O3–(Pb0.97La0.02)(Zr0.9Sn0.05Ti0.05)O3 | 306 | 48.5 | 6.4 | 62.4 | (173) |
| Pb[(Lu0.5Nb0.5)–(Mg0.5W0.5)]O3 | 340 | 46 | 6.4 | 71 | (132) |
| Pb0.91La0.06(Zr0.552Sn0.368Ti0.08)O3@1 wt %PbO–B2O3–SiO2–Al2O3–ZnO–MnO2 | 380 | 43 | 7.4 | 91.6 | (77) |
| PbHfO3 | 270 | 44.5 | 7.6 | 80.8 | (133) |
| Pb0.98La0.02(Hf0.45Sn0.55)0.995O3 | 380 | 36 | 7.63 | 94 | (138) |
| (Pb0.91La0.06)(Zr0.96Ti0.04)O3–1.0 mol % MnCO3 | 300 | 43.5 | 7.65 | 87 | (145) |
| (Pb0.98La0.02)(Zr0.55Sn0.45)0.995O3 | 400 | 41.5 | 10.4 | 87 | (131) |
| (Pb0.94La0.02Sr0.04)(Zr0.9Sn0.1)0.995O3 | 400 | 44 | 11.18 | 82.2 | (130) |
t of the bulk ceramics is commonly >0.1 mm.
3.1.1.1. Lead-Based Relaxor-Ferroelectrics
Many lead-based RFEs, including Pb(Mg1/3Nb2/3)O3–PbTiO3 (PMN–PT), Pb(Zn1/3Nb2/3)O3–PbTiO3 (PZN–PT), and (Sr,Pb,Bi)TiO3 (SPBT)-based materials, have been reported as potential candidates for energy storage capacitors.102−110 Zhang and co-workers investigated the relaxation behavior and energy storage properties of (1–x)PMN–xPT ceramic, obtaining Wrec ∼ 0.47 J cm–3 at room temperature.102 Li and co-worker probed the effect of domain structure on Wrec and thermal stability of 0.2PMN–0.8Pb(SnxTi1–x)O3 (PMN–PSxT1–x) ceramics, as illustrated in Figure 8. 0.2PMN–0.8PST ceramics exhibited Wrec ∼ 0.85 J cm–3 with excellent thermal stability which was attributed to the coexistence of ferroelectric domains and PNRs.111
Figure 8.

Wrec of 0.2PMN–0.8PSxT1–x ceramics with different Sn (x) contents at 70 kV cm–1. The insets show the mechanism of enhanced energy storage due to coexistent-phase structure and the Wrec for PMN–PSxT1–x ceramics under different electric fields. Reproduced with permission from ref (111). Copyright 2018 Elsevier.
3.1.1.2. Lead-Based Antiferroelectrics
PbZrO3 (PZ) is the first known AFE and exhibits a double P–E hysteresis loop below TC. However, the high critical switching field required for an AFE–FE phase transition at room temperature limits applications for energy storage. Chemical substitution to reduce switching field is an effective strategy to overcome the problem and three well-known PbZrO3 based compositions are reviewed: (i) (Pb,La)(Zr,Ti)O3 (PLZT);112−115 (ii) (Pb,La)(Zr,Sn,Ti)O3 (PLZST);116−129 and (iii) (Pb,La)(Zr,Sn)O3 (PLZS).130,131 Additionally, some new AFEs have also been identified based on PbHfO3, Pb(Lu0.5Nb0.5)O3, Pb(Yb0.5Nb0.5)O3, and Pb(Tm0.5Nb0.5)O3.132−138
3.1.1.2.1. (Pb,La)(Zr,Ti)O3 (PLZT)
According to the phase diagram (Figure. 9), PLZT exists as homogeneous compositions over a wide range of mol % La in the PbZrO3–PbTiO3 solid solution.139,140
Figure 9.
Phase diagram of PLZT at room temperature.139 Reproduced with permission from ref (139). Copyright 2014 Elsevier.
When Pb ions are replaced by ≤30 mol % La on the A-site in accordance with a lead vacancy (Vpb..) ionic compensation model, an orthorhombic AFE phase similar to PbZrO3 occurs for Zr rich compositions. However, only PLZT with <10 mol % La is typically utilized for energy storage applications141−147 since higher concentrations have lower polarization and therefore lower Wrec. Li and co-workers prepared (Pb0.97La0.02)(Zr0.95Ti0.05)O3 ceramics via a solid-state reaction route, yielding Wrec ∼ 0.83 J cm–3 and η ∼ 70% under an electric field of 55 kV cm–1.114 Jo and co-workers found that AFE and RFE behavior can both be obtained by substitution of La and excess PbO in PLZT, resulting in the enhancement of Wrec by promoting a slim and slanted hysteresis loop. Both high Wrec ∼ 3.04 J cm–3 and η ∼ 92% were obtained along with no performance degradation after 105 cycles.112 Tuning the Zr/Ti ratio has also shown to be an effective way to improve Wrec of PLZT ceramics. Qiao and co-workers reported slimmer P–E loops giving a Wrec ∼ 3.38 J cm–3 in (Pb0.895La0.07)(ZrxTi1–x)O3 ceramics by changing the Zr/Ti ratio (Figure 10), which was attributed to the reduction of tolerance factor and “enhancement of antiferroelectricity”.115 Mn doping is also suggested to improve Wrec of PLZT by “enhancing antiferroelectricity”.143−145
Figure 10.
Effect of Zr/Ti ratio on P–E loops and energy storage properties of PLZT.115 Reproduced with permission from ref (115). Copyright 2019 Elsevier.
3.1.1.2.2. (Pb,La)(Zr,Sn,Ti)O3 (PLZST)
To further optimize the energy storage properties of PLZT ceramics, Sn may be substituted on the B-site of PLZT, which broadens the AFE compositional range,72,77,87,116,122,148−166 in accordance with phase diagram from 1989 (Figure 11).167 (Pb0.97La0.02)(Zr,Sn,Ti)O3 with 2 mol % La has been most commonly studied in which the Zr/Ti/Sn ratio is varied to give a complex phase diagram that contains FE tetragonal (FT), high-temperature nontilted FE rhombohedral (FR(HT)), low temperature FE rhombohedral (FR(LT)) AFE tetragonal (AT), and AFE orthorhombic (AO) phases.
Figure 11.
Phase diagram of (Pb0.97La0.02)(Zr,Sn,Ti)O3, where T, R, and O represent the tetragonal, rhombohedral, and orthorhombic structure, respectively, and HT and LT represent high and low temperature, respectively. Reproduced with permission from ref (167). Copyright 2005 John Wiley and Sons.
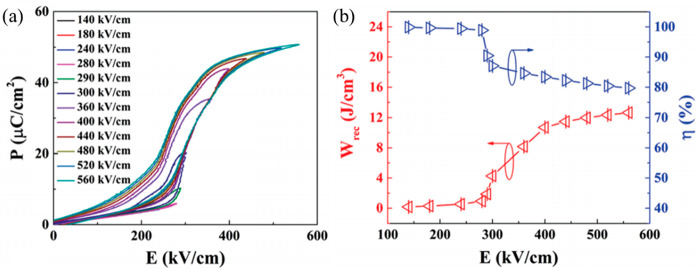
We note that the AT phase in both PLZT and PLZST has been shown to be incommensurate by a number of researchers and might be better described as a AO phase in which there is disorder of antipolar coupling.168−170 PLZST is AFE for concentrations with <15 mol % Ti. For compositions with AO structure, the critical phase switching fields are above BDS but ceramics with the AT phase undergo electric field-induced AFE-FE switching at room temperature, for which the switching field increases with increasing Sn concentration (Figure 12).118 Adjusting the Zr/Sn/Ti ratio leads to optimization of Wrec in PLZST ceramics116,118,148,157 with an increase in Sn concentration leading to a reduction in switching field (forward threshold electric field, EF, and backward threshold electric field, EB) and weakening ferroelectricity.171Wrec ∼ 5.6 J cm–3 and high thermal stability have been obtained in PLZST ceramics with a Zr/Sn/Ti ratio of 0.5:0.45:0.05116 while Wang and co-workers reported, in their study of the (Pb0.97La0.02)(Zr0.5Sn0.5-xTix)O3 solid solution, superior Wrec of 4.2 J cm–3 with η of 82% for (Pb0.97La0.02)(Zr0.5Sn0.44Ti0.06)O3 ceramics with good temperature stability.118 Recently, a ferrielectric (FIE) configuration was reported in PLZST which consists of ferroelectric ordering segments with either magnitude or angular modulation of dipoles.172 The net polarization of field-induced FE order can be tailored by adjusting the Sn/Ti ratio.
Figure 12.
AFE-type P–E hysteresis loops of Pb0.97La0.02(Zr0.5Sn0.5–xTix)O3 with x = (a) 0.10, (b) 0.08, (c) 0.06, and (d) 0.04.118 Reproduced with permission from ref (118). Copyright 2016 Elsevier.
The performance of PLZST ceramics is also influenced by the Pb/La ratio.117,122,126 The AFE phase becomes more stable with a commensurate increase in the AFE–FE switching field as La concentration increases. The energy storage properties of (Pb1–1.5xLax)(Zr0.5Sn0.43Ti0.07)O3 ceramics were optimized (Wrec of 4.2 J cm–1) by Dan and co-workers for compositions with x = 0.03 due to a large switching electric field and high BDS.3,126 Furthermore, doping with Ba and Sr (A-site) improves fatigue behavior and temperature stability, suppresses the stress sensitivity, and enhances energy storage.148,150−153,155,161,173
3.1.1.2.3. (Pb,La)(Zr,Sn)O3 (PLZS)
Wang and co-workers reported a unique E-induced multiphase transition in PLZS for which a conventional AFE–FE phase transition at low E, followed by a second FE-FE phase transition at a higher E, leads to an increase in polarization.131Wrec of 10.4 J cm–3 and η of 87% were achieved at 400 kV cm–1 for (Pb0.98La0.02)(Zr0.55Sn0.45)0.995O3 ceramics, along with superior discharge current density of 1640 A cm–2 and ultrafast discharge speed (75 ns discharge period) (Figure 13a,b).131 Subsequently, a record-high Wrec∼ 11.2 J cm–3 and a high η ∼ 82% were realized in (Pb0.98–xLa0.02Srx)(Zr0.9Sn0.1)0.995O3 ceramics, as illustrated in (Figure 13c,d). The substitution of Pb by Sr gave rise to an increase in BDS and AFE/FE switching fields, leading to further enhancement of energy storage performance.130
Figure 13.
(a) Bipolar P–E hysteresis loops and (b) energy storage properties of (Pb0.98La0.02)(Zr0.55Sn0.45)0.995O3 ceramics under different applied fields.131 (c) Unipolar P–E hysteresis loops of the (Pb0.94La0.02Sr0.04)(Zr0.9Sn0.1)0.995O3 ceramic under different applied fields. (d) Wrec of (Pb0.98-xLa0.02Srx)(Zr0.9Sn0.1)0.995O3 with Sr concentration (x = 0–0.06) as a function of the E.130 (a, b) Reproduced with permission from ref (131). Copyright 2019 John Wiley and Sons; (c, d) Reproduced with permission from ref (130). Copyright 2019 Royal Society of Chemistry.
3.1.2. Lead-Free Ceramics
In the last decades, extensive research has focused on lead-free electroceramics due to concerns over the toxicity of lead/lead oxide-based compounds.174−176 As a result, there has been a steady but continuous improvement in their energy storage performance,31,103,177−180 with a view to replacing existing lead-based materials. Several lead-free ceramic systems are considered as potential candidates to replace PLZT for energy storage applications, including those based on BT, ST, KNN, BF, NBT, AgNbO3 (AN), and NN.
3.1.2.1. BaTiO3-Based Ceramics
BT-based dielectric ceramics have been studied for decades and dominate the commercial market of ceramic capacitors.81,181 Several studies have reported improvements in energy storage performance of BT-based ceramics through (i) substituting oxides to improve BDS, such as Al2O3, La2O3, MgO, SiO2;79,80,182−184 (ii) employing different sintering techniques, such as SPS, citrate precursor, and cold sintering (CS) to increase ceramic density or control grain growth;68,76,185 (iii) adding sintering aids such as ZnNb2O6 and NiNb2O6 to increase density;186,187 (iv) introducing further end-members in the solid solution, Bi(Mg1/2Ti1/2)O3,188,189 BiYbO3,190 BMN,191−193 NBT–Na0.73Bi0.09NbO3,194 Nd(Zn1/2Ti1/2)O3,195 Bi(Zn2/3Nb1/3)O3,196 Bi(Li1/2Nb1/2)O3,197,198 Bi(Zn1/2Zr1/2)O3,199 Bi(Zn1/2Ti1/2)O3,200 YNbO4,201 Bi0.9Na0.1In0.8Zr0.2O3,202 Bi(Li1/2Ta1/2)O3,203 Bi(Mg1/2Zr1/2)O3,204 Bi(Zn1/2Sn1/2)O3,205 K0.5Bi0.5TiO3–KNbO3,206 and K0.5Bi0.5TiO3–NN207 to promote RFE behavior. The energy storage properties of BT-based materials are summarized in Table 4.
Table 4. Energy Storage Properties of BT-Based Materialsa.
| compd | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| 0.9BT–0.1BMN | 143.5 | ∼16 | 1.13 | 95 | (192) |
| Ba0.85Ca0.15Zr0.1Ti0.9O3 | 200 | ∼15 | 1.153 | (182) | |
| 0.9BT–0.1(Bi0.9Na0.1)(In0.8Zr0.2)O3 | 180 | ∼20 | 1.33 | 88 | (202) |
| 0.88(Ba0.8Sr0.2)TiO3–0.12 Bi(Zn2/3Nb1/3)O3 | 225 | 17 | 1.62 | 99.8 | (210) |
| 0.9BT–0.1BMN–0.3 wt % MnCO3 | 205 | 16.28 | 1.7 | 88.5 | (191) |
| 0.92(0.65BT–0.35NBT)–0.08(Na0.73Bi0.09NbO3) | 172 | ∼25 | 1.7 | 82 | (194) |
| 0.92(0.92BT–0.08K0.5Bi0.5TiO3)–0.08NN | 220 | ∼23 | 1.96 | 67.4 | (207) |
| 0.88BT–0.12Bi(Li0.5Nb0.5)O3 | 270 | ∼19 | 2.03 | 88 | (197) |
| 0.9BT–0.1Bi(Li0.5Ta0.5)O3 | 280 | ∼11.9 | 2.2 | 88 | (203) |
| 0.85BT–0.15Bi(Zn0.5Sn0.5)O3 | 280 | ∼23 | 2.41 | 91.6 | (205) |
| 0.9BT–0.1Bi(Zn0.5Zr0.5)O3 | 264 | ∼25 | 2.46 | (199) | |
| 0.85BT–0.15Bi(Mg0.5Zr0.5)O3 | 280 | ∼23 | 2.9 | 86.8 | (204) |
| 0.9Ba0.65Sr0.35TiO3–0.1BMN | 400 | 23 | 3.34 | 85.71 | (211) |
| 0.65(Ba0.98Li0.04)Ti0.98O3–0.35(Sr0.7Bi0.2)TiO3 | 410 | 35 | 3.54 | 77 | (212) |
| 0.6BT–0.4Bi(Mg0.5Ti0.5)O3 | 340 | ∼40 | 4.49 | 93 | (208) |
| BT–0.06Bi2/3(Mg1/3Nb2/3)O3 | 520 | 25 | 4.55 | 91 | (209) |
t of the bulk ceramics is commonly >0.1 mm.
The most effective approach, however, is the introduction of a Bi-based perovskite end member in which the B-site contains multiple cations. Doping with a Bi ion that has a lone pair electronic 6s2 configuration into the Ba-site increases Pmax. Pr is minimized by forming a so-called “weakly coupled relaxor” state in which long-range polar coupling is disrupted by the multiple B-site ions which have different valence and ionic radius to Ti. Using this principle, Hu and co-workers208 reported high Wrec of 4.49 J cm–3 with a η of 93% for 0.6BT–0.4 Bi(Mg0.5Ti0.5)O3 (BT–BMT) ceramics that were temperature stable to 170 °C (variation Wrec < 5% and η < 4%).
Of greater commercial potential, Yang and co-workers reported similar properties with BT–0.06 Bi2/3(Mg1/3Nb2/3)O3 (BT–0.06B2/3MN) but in compositions compatible with Ag/Pd electrodes due to the presence of only 4 mol % Bi on the A-site.209 Similar energy storage properties, Wrec ∼ 4.6 J cm–3 and η ∼ 92% (Figures 14a,b) to BT–BMT were obtained for BT–0.06B2/3MN ceramics which also benefited from the highest BDS, ∼520 kV cm–1, among all reported BT-based compositions.209 BT–0.06B2/3MN is not electrically homogeneous but BDS and Wrec were still optimized by reducing, though not completely eliminating, the difference between the bulk and grain boundary responses with respect to undoped BT, Figures 14c,d.209
Figure 14.

(a) Unipolar P–E loops under Emax and (b) calculated Wrec and η at different electric field for BT–0.06B2/3MN ceramics. (c) Z* plots of (c) BT at 400 °C and (d) BT–0.06B2/3MN at 550 °C.209 Reproduced from ref (209). Copyright 2020 American Chemical Society.
3.1.2.2. SrTiO3-Based Ceramics
ST, which is an incipient ferroelectric, is considered as a promising candidate for energy storage applications due to its relatively high permittivity (εr ∼ 300) and low dielectric loss (<1%) at room temperature. Extensive efforts have been made to improve the energy storage performance of ST-based ceramics, including (i) doping with Ba,213−216 Dy,217 Mg,218 Ce,64 and Bi219−222 on the A-site or Mn223 and Sn224,225 on the B-site; (ii) using sintering aids, such as ZnO,65 MgO,226−228 SiO2,63,229 SrO–B2O3–SiO2,230 ZnO–Li2O,231 Bi2O3–B2O3–SiO2,232 B2O3–SiO2–Bi2O3–CaO–BaO,233 Al2O3,234 BaO–TiO2–SiO2,75 BaCu(B2O5),235 and NiNb2O6;236 (iii) employing different sintering techniques such as microwave sintering73,75 and SPS;70 and (iv) introducing complex end-members, such as NBT,237,238 NBT–Ba(Al0.5Nb0.5)O3,239 NBT–BT,118,240,241 and Bi(Mg0.5Hf0.5)O3.242 The energy storage properties of ST-based materials are summarized in Table 5.
Table 5. Energy Storage Properties of ST-Based Materialsa.
| compounds | E (kV cm–1) | ΔP (μC cm–2) | Wrec(J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| 95 wt % Ba0.4Sr0.6TiO3–5 wt %MgO | 300 | 11.88 | 1.5 | 88.5 | (227) |
| Ba0.4Sr0.6TiO3–8 mol % SiO2 | 400 | 9.11 | 1.6 | 90.9 | (229) |
| 0.6ST–0.4NBT | 210 | ∼20 | 1.7 | 69.93 | (237) |
| 0.45ST–0.2NBT–0.35BT | 170 | ∼25 | 1.78 | 77.06 | (243) |
| (Sr0.99Mg0.01)TiO3 | 362 | ∼12 | 1.86 | 89.3 | (218) |
| 0.5ST–0.5(0.94 Bi0.54Na0.46TiO3–0.06BT) | 180 | ∼30 | 1.88 | 79 | (239) |
| 0.5ST–0.5(0.95NBT–0.05BaAl0.5Nb0.5O3) | 190 | ∼28 | 1.89 | 77 | (240) |
| Ba0.4Sr0.6TiO3–9 wt %(Bi2O3–B2O3–SiO2) | 279 | ∼17 | 1.98 | 90.57 | (232) |
| 0.95(Sr0.5Na0.25Bi0.25TiO3)–5 wt %MgO–0.05KNbO3 | 178.5 | ∼27 | 2 | 76.34 | (244) |
| Ba0.4Sr0.6(Ti0.996Mn0.004)O3–2 wt % MgO | 300 | ∼16 | 2.014 | 88.6 | (228) |
| Ba0.3Sr0.6Ca0.1TiO3–2 wt %MgO | 194.33 | ∼23 | 2.223 | (245) | |
| Sr0.985Ce0.01TiO3–3 wt % SiO2 | 290 | ∼10 | 2.23 | (64) | |
| SrTi0.985(Zn1/3Nb2/3)0.015O3–4.5 wt %ZnNb2O6 | 422 | 9.34 | 2.35 | 77 | (246) |
| 0.9ST–0.1(Bi0.48La0.02Na0.48Li0.02Ti0.98Zr0.02O3) | 323 | ∼20 | 2.59 | 85 | (247) |
| 0.8ST–0.2(NBT– Ba0.94La0.04Zr0.02Ti0.98O3) | 320 | ∼22 | 2.83 | 85 | (248) |
| 0.995(0.6ST−0.4NBT)−0.005ZrO2 | 285 | ∼25 | 2.84 | 71.54 | (74) |
| 0.9(Sr0.7Bi0.2)TiO3–0.1 Bi(Mg0.5Hf0.5)O3 | 360 | ∼22 | 3.1 | 93 | (242) |
| Ba0.3Sr0.7TiO3–1.6 wt % ZnO | 400 | 3.9 | (65) | ||
| 98.5 wt %Ba0.4Sr0.6TiO3–1.254 wt %Al2O3–0.246 wt %SiO2 | 493 | 5.09 | (234) |
The t of the bulk ceramics is commonly >0.1 mm.
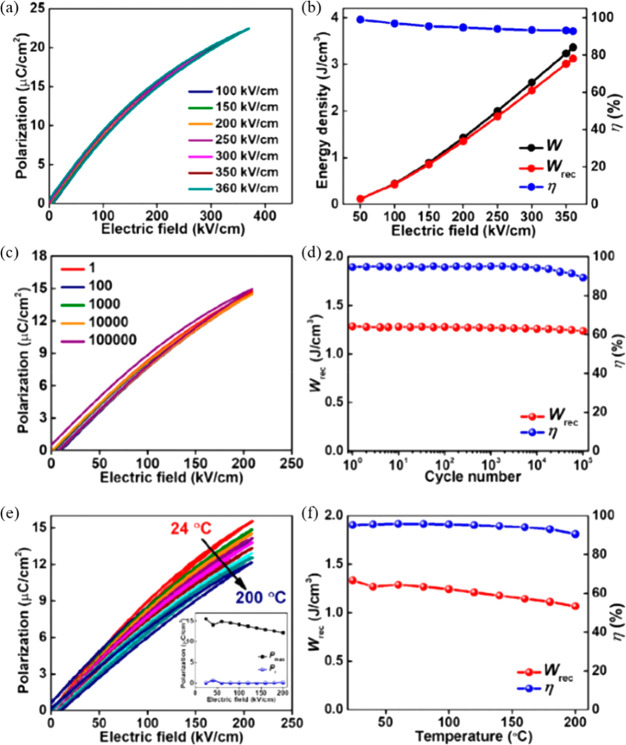
From a review of the literature, doping commonly increases both εr and Pmax but decreases the BDS, sintering aids increase BDS but lower the Pmax. The highest energy densities have therefore been achieved through either dopants and/or alloying with “relaxor end-members” which also act as sintering aids. Adopting these protocols, a Wrec of 3.1 J cm–3 with η ∼ 93% was obtained for 0.9(Sr0.7Bi0.2)TiO3–0.1 Bi(Mg0.5Hf0.5)O3 ceramic at 360 kV cm–1, Figures 15a,b,242 which was also fatigue-resistant up to 105 cycles and temperature stable from 25 to 200 °C, Figures 15c–f.242
Figure 15.
(a) Unipolar P–E loops and (b) W, Wrec, and η of 0.9(Sr0.7Bi0.2)TiO3–0.1Bi(Mg0.5Hf0.5)O3 ceramic as functions of E. (c) Unipolar P–E loops and (d) Wrec and η of 0.9(Sr0.7Bi0.2)TiO3–0.1Bi(Mg0.5Hf0.5)O3 ceramic as functions of cycle numbers up to 105. (e) Unipolar P–E loops, with the inset shows the Pmax and Pr as functions of temperature, and (f) Wrec and η of 0.9(Sr0.7Bi0.2)TiO3–0.1Bi(Mg0.5Hf0.5)O3 ceramics as a function of temperature. Reproduced with permission from ref (242). Copyright 2019 John Wiley and Sons.
3.1.2.3. K0.5Na0.5NbO3-Based Ceramics
In 2016, the energy storage properties of KNN–(Bi,Na)HfO3 solid solutions were first investigated, and Wrec ∼ 0.54 J cm–3 was achieved at 129 kV cm–1.249 ZnO and CuO were introduced as sintering aids improved Wrec in KNN-based ceramics66,250 by decreasing porosity and restricting grain growth. BDS of 400 kV cm–1 was obtained in 0.8KNN-0.2Sr(Sc0.5Nb0.5)O3 ceramic with 0.5 mol % ZnO250 and CuO reduced the sintering temperature and enhanced the density of 0.9KNN–0.1BMN ceramics.66 A third perovskite end-member, e.g., ST, BF, and BMN, has also been shown to optimize energy storage properties.91,92,251Wrec of 4.03 J cm–3 at 400 kV cm–1 was obtained in 0.85KNN–0.15ST bulk ceramics92 with similar energy storage performance realized in 0.90KNN–0.10BMN ceramic with a large Pmax (41 μC cm–2) obtained at 300 kV cm–1, Figure 16.91 The energy storage properties of KNN-based materials are summarized in Table 6.
Figure 16.
(a) Unipolar P–E hysteresis loops and (b) calculated W and Wrec and (c) Wloss and η for 0.90KNN–0.10BMN ceramics under different E. Reproduced with permission from ref (91). Copyright 2017 Royal Society of Chemistry.
Table 6. Energy Storage Properties of KNN-Based Materialsa.
| compounds | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| (K0.48Na0.52)0.88Bi0.04NbO3 | 189 | ∼12.7 | 1.04 | (252) | |
| 0.93KNN–0.07BMN | 150 | ∼22.5 | 1.3 | 58.8 | (253) |
| 0.9KNN–0.1BF | 206 | 23.7 | 2 | 61 | (251) |
| 0.8KNN–0.2Sr(Sc0.5Nb0.5)O3–0.5%ZnO | 400 | ∼11.6 | 2.6 | 73.2 | (250) |
| K0.14Bi0.12Na0.5NbO3–1 mol % CuO | 300 | 29 | 2.89 | 80 | (254) |
| 0.85KNN-0.15 Bi(Zn0.5Zr0.5)O3 | 325 | 30 | 3.5 | 86.8 | (255) |
| 0.9KNN–0.1BMN–1.0 mol % CuO | 400 | ∼21 | 4.02 | 57.3 | (66) |
| 0.85KNN–0.15ST | 400 | ∼26 | 4.03 | ∼52 | (92) |
| 0.9KNN–0.1BMN | 300 | ∼34 | 4.08 | ∼62.7 | (91) |
t of the bulk ceramics is commonly >0.1 mm.
3.1.2.4. BiFeO3-Based Ceramics
BF-based ceramics are best known as multiferroics but have also been explored for high-temperature ferroelectric and piezoelectric applications due to their high TC and large spontaneous polarization.256−259 Compared with other lead-free ceramics, BF-based were not initially considered as good candidates for energy storage applications due to their high leakage current.260p-type electrical conductivity due to the volatilization of Bi and the variation of Fe valence states has been reported frequently in BF system, which limits the BDS and restricts energy density.261−263 However, the introduction of dopants and alloying with end-members suppresses the leakage current, reduces the electrical conductivity, increases intrinsic Eg and induces transitions from a FE to RFE state. The energy storage properties of BF-based materials are summarized in Table 7.
Table 7. Energy Storage Properties of BF-Based Materialsa.
| compounds | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| 3 mol % Nb2O5–0.65BF–0.35BT | 90 | 19 | 0.71 | (265) | |
| 0.61BF–0.33BT–0.06Ba(Mg2/3Nb1/3)O3 | 125 | 32.3 | 1.56 | 75 | (269) |
| 0.61BF–0.33BT–0.06La(Mg0.5Ti0.5)O3 | 130 | 33.3 | 1.66 | 82 | (267) |
| 0.6BF–0.34BT–0.06Sr(Al0.5Nb0.5)O3 | 150 | 35 | 1.75 | 81 | (270) |
| 15%Nd–0.70BF–0.30BT | 170 | 30.7 | 1.82 | 41.3 | (90) |
| 0.65BF-0.30BT–0.05Ba(Zr0.5Zn0.5)O3 | 190 | 32 | 2.04 | 54 | (34) |
| 0.65BF–0.30BT–0.05Bi(Zn2/3Nb1/3)O3 | 180 | 32.8 | 2.06 | 53 | (266) |
| 0.56BF–0.30BT–0.14AN | 195 | 26 | 2.11 | 84 | (268) |
| 0.60BF–0.30BT–0.10Nd(Mg2/3Nb1/3)O3 | 220 | 24 | 2.4 | 77 | (34) |
| 0.7(0.67BF–0.34BT)–0.3(Sr0.7Bi0.2)TiO3 | 180 | 37 | 2.4 | 90.4 | (271) |
| 0.62BF–0.3BT–0.08Nd(Zr0.5Zn0.5)O3 | 240 | 26 | 2.45 | 72 | (34) |
| 0.6BF–0.34BT–0.06Ba(Zn0.5Ta0.5)O3 | 160 | 41 | 2.56 | 71 | (272) |
| 0.67Bi0.9Sm0.1FeO3–0.33BT | 200 | 48 | 2.8 | 55.8 | (273) |
| 0.25Bi0.83Sm0.17Fe0.95Sc0.05O3−0.75[0.85BT-0.15Bi(Mg0.5Zr0.5)O3] | 206 | 36 | 3.2 | 92 | (274) |
| 0.61BF–0.33BST–0.06La(Mg2/3Nb1/3)O3 | 230 | 36.5 | 3.38 | 59 | (275) |
| 0.57BF–0.30BT–0.13 Bi(Li0.5Nb0.5)O3 | 280 | 30 | 3.64 | 74 | (276) |
| 0.57BF–0.33BT–0.10NN | 360 | 51 | 8.12 | 90 | (42) |
| 0.5BF–0.4ST–0.1BMN-0.03Nb | 460 | 45 | 8.2 | 74.1 | (45) |
The t of the bulk ceramics is commonly >0.1 mm.
BF–BT-based materials are purported as potential energy storage compositions due to their excellent BDS and high Pmax.34,45,90 Undoped BF–xBT ceramics exhibit optimized ferroelectric and piezoelectric properties in a mixed-phase region of 0.25≤ x ≤ 0.35.264 The majority of studies have focused on this region and modified compositions either by (i) A and/or B-site chemical doping, including Nd, Nb, Zn0.5Zr0.5, Zn2/3Nb1/3, and Li0.5Nb0.5 or (ii) alloying with a third end-member, such as Nd(Zn0.5Zr0.5)O3, Nd(Mg2/3Nb1/3)O3, La(Mg0.5Ti0.5)O3, Ba(Mg1/3Nb2/3)O3, AN, and NN.34,43,90,265−269
The importance of electrical homogeneity was first postulated in 2019 by Wang, Reaney, and co-workers as a key factor to optimize BDS as well as Wrec.34 Two electrical components with similar capacitance value, C∼ 8 × 10–10 F cm–1 and 1.4 × 10–9 F cm–1, were found in 0.7BF–0.3BT ceramics, as illustrated Figure 17a,b. However, the associated resistances, R1 ∼ 3.8 kΩ cm and R2 ∼ 1.3 MΩ cm, were vastly different. The presence of a large volume fraction of conductive cores (R1) easily led to electrical breakdown at lower electrical field. In contrast, only one single electrical component with C ∼ 1.87 × 10–9 F cm–1 and resistivity of ∼2.3 MΩ cm was observed for 8% Nd(Zr0.5Zn0.5)O3 (NZZ)-doped BF–BT ceramics (Figures 17c,d). The conductive electrical component was effectively eliminated by forming a solid solution with NZZ, inhibiting the formation of conductive pathways at higher electric field (>500 kV cm–1). As a result, optimized Wrec ∼ 2.45 and 10.5 J cm–3 were realized at Emax ∼ 240 and 700 kV cm–1 for 0.08NZZ-BF–BT bulk ceramics and MLs, respectively.
Figure 17.
Combined Z′′ and M′′ spectroscopic plots at 275 °C for (a) BF–BT and (b) BF–BT–0.08NZZ. Unipolar P–E loops of BF–BT–0.08NZZ (c) bulk ceramics and (e) ceramic MLs, with cross-section SEM image as shown in inset figure. Calculated energy storage properties of BF–BT–0.08NZZ (d) bulk ceramic and (e) ceramic MLs. Reproduced from ref (34). Copyright 2019 Royal Society of Chemistry.
Recently, superior energy density through tailored dopant strategies was achieved in BF–ST–xNb–yBMN ceramics, by promoting electrical homogeneity, enhancing Ea and suppressing the p-type conduction, all of which resulted in significantly enhanced BDS. The ceramic without Nb (BF–ST–0.06BMN, x = 0) exhibits a broadened arc in Z* at room temperature (Figure 18a) with at least two electrical components observed in combined Z′′ and M′′ spectroscopic plots (Figure 18b). By donor doping Nb on the B-site, only one ideal semicircle in the Z* plots with a single Debye peak at the same frequency in both Z′′ and M′′ spectroscopic plots was observed for all doped samples (Figures 18c,d), suggesting electrical homogeneity. Nb doping suppresses the formation of Fe4+ associated with the loss of Bi2O3 or/and VO.. during ceramic processing, thus reducing electrical conductivity with respect to x = 0 by several orders of magnitude, coupled with enhanced Ea (Figure 18e). A reduction in Seebeck coefficient from ∼ 600 μV K–1 to zero indicates a commensurate decrease in charge carrier concentration as Nb concentration increases. At x = 0.03, the BDS increases to 360 kV cm–1 (Figures 18g) which is insufficient on its own to optimize Wrec but when combined with an increase in BMN (y) concentration to reduce polar coupling, results in Wrec ∼ 8.2 J cm–3 at Emax ∼ 460 kV cm–1 for BF–ST–0.03Nb–0.1BMN ceramics, Figure 18h.45
Figure 18.
(a) Z* plots and (b) Combined Z′′ and M′′ spectroscopic plots of 0.54BF–0.4ST–0.06BMN–xNb (x = 0); (c) Z* plots and (d) Combined Z′′ and M′′ spectroscopic plots of x = 0.01–0.05; (e) Arrhenius plots, (f) Seebeck coefficients, and (g) unipolar P–E loops under Emax of x = 0.01–0.05. (h) Wrec and η of (0.6–y)BF–0.4ST–0.03Nb–yBMN. Reproduced from ref (45). Copyright 2020 Royal Society of Chemistry.
3.1.2.5. Na0.5Bi0.5TiO3–Based Ceramics
NBT-based ceramics are promising candidates of lead-free dielectrics due to their high Pmax and Tc. However, their large hysteresis and low BDS are not ideal for high energy density capacitor applications.277−284 Attempts to improve their properties generally fall into the following approaches: (i) doping on the A-site (Ba, Sr, K, Li, La, Dy, Nd)222,239,243,285−296 and B-site (commonly Nb)297 and codoping (K,Sr/Nb; K,La/Zr; Li,K,Sr/Ta,Nb; K,Mg/Nb; Ba/Nb; Ba/Sn; Ba/Sn,Zr; Ba/Ta; Ba/Zr; Ba,Ca/Zr; Ba,K,Ca/Nb,Zr; Ba/Mg,Nb; Ba/Mg; Ba,La/Al,Nb; Ba,Sr/Yb,Nb; Ba/Hf; La/Al; La,Ba/Nb; Sr/Sn; Sr/Zr; Sr/Mg; Sr/Mg,Nb);194,298−321 (ii) forming solid solution with other end-members, such as AN, NN, and SBT;297,322−325 (iii) using additives such as MnO, Fe2O3, MgO, SnO2, ZnO, CaO, and ZrO262,74,238,326−334 and (iv) employing different processing methods such as hot-pressing59 and synthesis using sol–gel derived powders.69,71,74,335,336 The energy storage properties of NBT-based materials are summarized in Table 8.
Table 8. Energy Storage Properties of NBT-Based Materialsa.
| compounds | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| 0.95(0.76NBT–0.24ST)-0.05AN | 120 | 43.5 | 2.03 | 61.8 | (324) |
| Bi0.41Na0.35Sr0.21TiO3 | 135 | ∼35.8 | 2.04 | 82.4 | (290) |
| 0.75Na0.25Sr0.5Bi0.25TiO3–0.25MgO | 200 | ∼35 | 2.06 | 84 | (317) |
| 0.8(0.775NBT–0.225BaSnO3)–0.2BaZrO3 | 245 | 20 | 2.08 | 88.8 | (315) |
| 0.85NBT–0.15BaHfO3 | 175 | 29 | 2.1 | 66.1 | (310) |
| 0.775NBT–0.225BaSnO3–5 wt %MgO | 215 | ∼28 | 2.13 | 67.8 | (326) |
| Sn–0.45ST–0.2NBT–0.35BT | 240 | ∼21 | 2.25 | 79.51 | (328) |
| 0.9NBT–0.1Li2TiO3 | 200 | ∼25 | 2.3 | 74.2 | (291) |
| (0.5NBT–0.5ST) −1%SnO2 | 180 | 37.37 | 2.35 | ∼80 | (238) |
| (Na0.5Bi0.5)0.8Ba0.2Ti0.8Sn0.8O3 | 195 | ∼34 | 2.35 | 71.04 | (338) |
| {Bi0.5[(Na0.8K0.2)0.9Li0.1]0.5}0.96Sr0.04(Ti0.975Ta0.025)O3 | 143 | ∼43 | 2.42 | ∼64 | (339) |
| 0.55NBT–0.45(Bi0.2Sr0.7TiO3) | 200 | 25 | 2.5 | 95 | (222) |
| 0.94(BNT–Bi0.2Sr0.7TiO3)–0.06KNN | 180 | 37 | 2.65 | 84.6 | (298) |
| 0.95(0.6ST–0.4NBT)–0.05Zr | 285 | ∼25 | 2.84 | 71.54 | (74) |
| 0.85(0.95NBT–0.05SrZrO3)–0.15NN | 210 | ∼30 | 2.93 | 72 | (325) |
| 0.96(0.65NBT–0.35Sr0.85Bi0.1TiO3)–0.04NN | 220 | 50.46 | 3.08 | 81.4 | (323) |
| 0.6(Bi0.51Na0.47)TiO3–0.4Ba(Zr0.3Ti0.7)O3 | 280 | ∼25 | 3.1 | 91 | (299) |
| 0.9(0.76NBT−0.24NN)−0.1SBT | 200 | 43 | 3.12 | 75 | (340) |
| 0.93NBT–0.07LaAlO3 | 210 | 43 | 3.18 | 60 | (320) |
| (Na0.25Bi0.25Sr0.5)(Ti0.8Sn0.2)O3 | 310 | 26.5 | 3.4 | 90 | (321) |
| 0.85(Na0.5Bi0.5)0.7Sr0.3TiO3−0.15BMN | 250 | 38 | 3.45 | 88.01 | (316) |
| 0.95(0.6 Bi0.5Na0.5TiO3–0.4Sr0.7Bi0.2TiO3)–0.05AN | 246 | 41 | 3.62 | 89 | (322) |
| 0.65(0.84NBT–0.16K0.5Bi0.5TiO3)–0.35(Bi0.2Sr0.7TiO3) | 350 | ∼33.99 | 4.06 | 87.3 | (288) |
| 0.55NBT–0.45SBT | 315 | 19.1 | 4.14 | 92.2 | (295) |
| 0.90(Na0.5Bi0.5)0.7Sr0.3TiO3–0.10 Bi(Ni0.5Sn0.5)O3 | 270 | 47 | 4.18 | 83.64 | (341) |
| 0.75Bi0.58Na0.42TiO3–0.25ST | 535 | 41 | 5.63 | 94 | (342) |
| 0.78NBT–0.22NN | 390 | 45 | 7.02 | 85 | (297) |
| 0.62NBT–0.30SBT–0.08BMN | 470 | 48 | 7.5 | 92 | (337) |
t of the bulk ceramics is commonly >0.1 mm.
Notably, Li and co-workers reported that 0.55NBT–0.45(Sr0.7Bi0.2)TiO3(SBT) achieved Wrec of 2.5 and 9.5 J cm–3 with η > 90% for bulk ceramic and MLs at 200 and 720 kV cm–1 (Figure 19a,b), respectively.222 Superior Wrec ∼ 7.02 J cm–3 and η ∼ 85%, were also reported for 0.78NBT–0.22NN ceramics at Emax ∼ 360 kV cm–1, Figures 19c,d, with <10% variation from 25–250 °C and from 0.1 to 100 Hz.297
Figure 19.
(a) Unipolar P–E loop and the current–E curve for NBT–0.45SBT bulk ceramics. (b) Wrec and η for NBT–0.45SBT ceramic MLs; inset SEM image of the ceramic MLs (c) Bipolar P–E loops and (c) calculated Wrec and η of 0.78NBT–0.22NN ceramic.222,297 (a, b) Reproduced with permission from ref (222). Copyright 2018 John Wiley and Sons; (c, d) Reproduced with permission from ref (297). Copyright 2019 Royal Society of Chemistry.
Recently, Ji and co-workers337 proposed that the key factors for designing an ideal RFE with high energy density were as follows: (i) utilization of a highly polar base system (e.g., NBT); (ii) disruption of long-range polar coupling through forming solid solutions with, e.g., SBT and BMN without sacrificing average ionic polarizability, Figure 20a, and (iii) simultaneously inducing or retaining electrical homogeneity with a highly resistive single component in IS (∼250 kΩ cm at 660 °C), Figure 20b. These factors combined to give Emax ∼ 470 kV cm–1, Wrec ∼ 7.5 J cm–3, and η ∼ 92% for 0.62NBT–0.30SBT–0.08BMN, Figure 20c,d.
Figure 20.
(a) Tolerance factor and average ionic polarizability per unit cell of NBT–SBT–xBMN as a function of BMN concentration. (b) Combined Z′′ and M′′ spectroscopic plots for NBT–SBT–0.08BMN ceramics at 660 °C. (c) P–E loops at the Emax, and (d) Wrec and η for NBT–SBT–xBMN ceramics. Reproduced with permission from ref (337). Copyright 2021 Elsevier.
3.1.2.6. AgNbO3–Based Ceramics
AFEs have long been considered as the prime candidate for energy storage capacitors due to their large Pmax and small Pr. There are only a handful of lead-free AFE systems, with AN showing particular promise because it possesses a large saturation polarization of 52 μC cm–2 under an Emax ∼ 220 kV cm–1.343 Recent research on AN ceramics has focused on stabilizing the AFE phase so that switching field is moved to higher fields while simultaneously optimizing Pmax.344,345
There have been a number of recent studies on AN focusing on (i) substitution of aliovalent B-site oxides such as MnO2 and WO3;346,347 (ii) doping Ba, Sr, Ca, Bi, La, Sm, and Gd on the A-site348−356 often with isovalent Ta doping on the B-site;357−359 and (iii) forming solid solutions with end-members, such as BiMnO3 and Bi(Zn2/3Nb1/3)O3.360,361 Most dopants reduce the G of AN which maximizes BDS but delay the onset of the AFE-FE transition to higher field while simultaneously narrowing hysteresis in the induced FE phase. Wrec of 4.4, 4.5, and 5.2 J cm–3 with η of 70, 63, and 69.2% has been obtained for La, Gd, and Sm A-site doped AN ceramics,348,351,355,359,362 respectively, and B-site Ta-doped AN was reported to exhibit Wrec of 4.2 J cm–3 with η of 69% (Figure 21a,b).357 A-site doping with ions smaller in radius than Ag is suggested to decrease tolerance factor and enhance AFE stability, while donor doping is compensated by A-site vacancies which reduce antipolar and polar coupling of the AFE and field induced FE phases, respectively. Some authors postulate that substituting B-site ions with a lower polarizability than Nb also stabilizes the AFE phase and moves the switching field higher.348,350,359,363 The underlying principles are schematically represented in Figure 21c. The energy storage properties of AN-based materials are summarized in Table 9.
Figure 21.
(a) Bipolar P–E loops of AN and Ag(Nb0.85Ta0.15)O3 ceramics. (b) Energy storage performance of Ag(Nb1–xTax)O3 ceramics prior to their breakdown. (c) Schematic of the underlying principles for enhancing energy storage property in AN-based materials. (a, b) Reproduced with permission from ref (357). Copyright 2017 John Wiley and Sons; (c) Reproduced with permission from ref (359). Copyright 2019 Royal Society of Chemistry.
Table 9. Energy Storage Properties of AN-Based Materialsa.
| compounds | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| AN | 150 | ∼34 | 2.0 | 46 | (345) |
| AN | 175 | ∼33 | 2.1 | 40–50 | (343) |
| Ag0.96Ba0.02NbO3 | ∼180 | ∼34 | 2.3 | 46 | (356) |
| 0.6 mol % BiMnO3–AN | 175 | ∼36 | 2.4 | 54 | (360) |
| 0.1 wt % Mn–AN | 150 | ∼37.2 | 2.5 | 57 | (346) |
| Ag0.91Bi0.03NbO3 | 200 | ∼30 | 2.6 | 86 | (353) |
| Ag0.90Sr0.05NbO3 | 190 | ∼38 | 2.9 | 56 | (354) |
| Ag0.94La0.02NbO3 | 230 | ∼28 | 3.12 | 63 | (352) |
| 0.3 wt % Mn-doped Ag0.97La0.01NbO3 | 142 | 37.8 | 3.2 | 62 | (351) |
| 0.1 wt % W-AN | 200 | ∼42.5 | 3.3 | 50 | (347) |
| AN–0.03NBT | 220 | 33 | 3.4 | 62 | (364) |
| Ag0.90Ca0.05Nb0.95Ta0.05O3 | 210 | 37 | 3.36 | 58 | (363) |
| Ag0.92Ca0.04NbO3 | 220 | ∼37.6 | 3.55 | 63 | (349) |
| Ag(Nb0.8Ta0.2)O3 | 270 | ∼30 | 3.7 | ∼65 | (358) |
| AgNb0.85Ta0.15O3 | 233 | ∼35.1 | 4.2 | 69 | (357) |
| 2 mol % La-doped AN | 273 | ∼30 | 4.4 | 70 | (350) |
| Ag0.88Gd0.04NbO3 | 290 | ∼32.5 | 4.5 | ∼63 | (355) |
| Ag0.94Sm0.02NbO3 | 310 | 31 | 4.5 | 63 | (362) |
| 0.99AN–0.01 Bi(Zn2/3Nb1/3)O3 | 220 | 46.8 | 4.6 | 57.5 | (115) |
| (Sm0.02Ag0.94)(Nb0.9Ta0.1)O3 | 280 | ∼36 | 4.87 | 63.5 | (359) |
| Sm0.03Ag0.91NbO3 | 290 | ∼36 | 5.2 | 69.2 | (348) |
| AgNb0.45Ta0.55O3 | 460 | ∼29 | 6.3 | 90 | (365) |
| Ag0.97Nd0.01Nb0.80Ta0.20O3 | 370 | 38 | 6.5 | 71 | (366) |
| Ag0.76La0.08NbO3 | 476 | 33 | 7.01 | 77 | (367) |
t of the bulk ceramics is typically >0.1 mm.
3.1.2.7. NaNbO3-Based Ceramics
Recently, AFE NN has received attention as a potential candidate for energy storage applications. An AFE double hysteresis loop is difficult to observe in NN because (i) the energy difference between the AFE phase and field-induced FE phase is very small and (ii) E-induced FE phase is metastable. Thus, AFE behavior in NN based materials is commonly stabilized by chemical substitution with end members such as BS and CaHfO3.344,368−370
Emax > 250 kV cm–1 and Wrec > 2.5 J cm–3 have been reported for NN in solid solution with BMN, ST, Bi(Mg2/3Ta1/3)O3, and Bi(Mg0.5Ti0.5)O3 by stabilizing the AFE phase or inducing relaxor behavior,371−374 as summarized in Table 10. Zuo and co-workers42 have also proposed the concept of an “AFE relaxor” to explain the energy storage properties of 0.78NN–0.22NBT ceramics. They argue that the local AFE phase transforms reversibly into an FE monoclinic phase at∼ 400 kV cm–1, giving a large ΔP (Pmax > 50 μC cm–2 and Prem < 5 μC cm–2). Wrec of ∼12.2 J cm–3 was reported with η ∼ 69%, at 680 kV cm–1, Figure 22.42 However, the term “AFE relaxor” has little physical significance since an antipolar phase cannot form short-range polar features characteristic of a relaxor. 0.78NN–0.22NBT may, therefore, be better described as either a relaxor or a short-range AFE phase that undergoes a field induced transition. This intriguing behavior is interesting, but it is the large Emax (680 kVcm–1) that is most likely responsible for the exceptional Wrec rather than the intrinsic crystal chemistry. The underpinning reasons for the large Emax most likely relate to the defect chemistry, band gap and electrical homogeneity, consistent with the key factors proposed by Ji and co-workers.337
Table 10. Energy Storage Properties of NN-Based Materialsa.
| compounds | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|
| NN–0.09Bi(Zn0.5Ti0.5)O3 | 200 | 29 | 2.1 | 76 | (375) |
| 0.9NN–0.10BMN | 300 | ∼23 | 2.8 | 82 | (371) |
| 0.8NN–0.2ST | 323 | 34.5 | 3.02 | 80.7 | (372) |
| 0.78NN–0.22Ba(Mg2/3Nb1/3)O3 | 540 | 18.7 | 3.51 | 87 | (376) |
| Na0.7Bi0.1NbO3 | 250 | ∼30 | 4.03 | 85.4 | (377) |
| NN–MnO2 | 360 | 33 | 4.3 | 90 | (378) |
| Na0.84Bi0.08Nb0.92Zr0.08O3 | 430 | 30 | 4.9 | 88 | (67) |
| 0.9NN–0.1 Bi(Ni0.5Sn0.5)O3 | 550 | 25 | 5 | 68 | (379) |
| 0.78NN–0.22 Bi(Mg2/3Ta1/3)O3 | 620 | 17 | 5.01 | 86.8 | (373) |
| 0.92NN–0.08 Bi(Mg0.5Ti0.5)O3 | 480 | 38 | 5.57 | 71 | (374) |
| (Na0.91La0.09)(Nb0.82Ti0.18)O3 | 550 | 42 | 6.5 | 65.9 | (380) |
| 0.75[0.9NN–0.1 Bi(Mg0.5Ta0.5)O3]–0.25(Bi0.5Na0.5)0.7Sr0.3TiO3 | 800 | 22 | 8 | 90.4 | (381) |
| 0.76NN–0.24NBT | 680 | ∼55 | 12.2 | 69 | (42) |
t of the bulk ceramics is commonly >0.1 mm.
Figure 22.
(a) Bipolar P–E loops with corresponding current density-field (J–E) curves and (b) Wrec and η values of under different E for the 0.76NN–0.24BNT ceramic at 10 Hz (c) a comparison of Wrec, η, and Emax among the recently reported bulk ceramics; (d) temperature-dependent P–E hysteresis, (e) temperature- and frequency-dependent εr and (f) Wrec and η as a function of temperature for the 0.76NN-0.24NBT ceramic at 450 kV cm–1. Reproduced with permission from ref (42). Copyright 2019 John Wiley and Sons.
3.1.3. Glass Ceramics
Glass-ceramics are composed of one or more crystallized phases (ceramics) dispersed uniformly in amorphous phase (glass). They often exhibit the combined properties of ceramics and glass depending on the induced crystalline phases and their microstructures. Glass-ceramics are prepared by melting the requisite raw materials, cooling to room temperature to form a glass, followed by two step annealing to induce crystal nucleation (approximately at the glass transition temperature, Tg) and growth > TgFigure 23.179,382 The microstructure of a glass ceramic is typically dominated by a largely 2D and 3D defect-free (e.g., no grain boundaries) glass phase and a uniformly distributed (provided the system undergoes homogeneous rather than heterogeneous nucleation) ceramic phase. Wrec and η are both large due to the high BDS associated with the absence of 2D and 3D defects accompanied by a near zero value of Pr. The energy storage properties of glass-based glass ceramics are summarized in Table 11.
Figure 23.
Schematic of the processing step of glass-ceramics.
Table 11. Energy Storage Properties of Glass Ceramicsa.
| compounds | εr (1 kHz, 300 K) | E (kV cm–1) | Wrec (J cm–3) | ref |
|---|---|---|---|---|
| 14.4SrO–17.6BaO–32Nb2O5–36B2O3 | 117 | 1050 | 5.71 | (386) |
| 25.6BaO–6.4Na2O–32Nb2O5–36SiO2 | ∼90 | 1248 | ∼6.2 | (409) |
| 14.3SrO–17.5BaO–31.9Nb2O5–35.8B2O3–0.5ZnO + 0.5La2O3 | 131 | 1127 | 7.1 | (400) |
| 20BaO–20SrO–20Nb2O5-5Al2O3–1.5B2O3–33.5SiO2 + 0.2La2O3 | 92.4 | 1326 | 7.2 | (406) |
| 20SrO–20BaO–10Nb2O5–10TiO2–32SiO2–8Al2O3 | 52.9 | 1817 | 7.73 | (410) |
| 14.3SrO–17.5BaO– 31.9%Nb2O5–35.8%B2O3–0.5ZnO + 0.5Sm2O3 | 143.8 | 1132 | 8.15 | (402) |
| 14.4SrO–17.6BaO–32Nb2O5–36B2O3 + 1%Yb2O3 | 98.3 | 1398 | 8.5 | (403) |
| 15K2CO3–15SrCO3–30Nb2O5–32SiO2–4Al2O3–4B2O3 | 102 (10 kHz) | 1411 | 8.99 | (407) |
| 20BaO–20SrO–20Nb2O5–5Al2O3–1.5B2O3–33.5SiO2 + 0.05MnO2 | 95.8 | 1471 | 9.2 | (411) |
| 42[0.2Na2O–0.8SrO]–28Nb2O5–30SiO2 | 53 (100 kHz) | 2074 | 10.09 | (412) |
| 9.6K2O–22.4BaO–32Nb2O5–36SiO2 | 75 | 1937 | 12.06 | (393) |
| 25.6BaO–6.4K2O–32Nb2O5–36SiO2 + 1Gd2O3 | 83 | 1818 | 12.14 | (405) |
| 20BaO–12K2O–32Nb2O5–36SiO2 | 83 | 1859 | 12.7 | (413) |
| 15.16SrO–6.736BaO–10.104K2O–32Nb2O5–28B2O3–8P2O5 | 85.2 | 1844 | 12.83 | (395) |
| 6.4K2O–25.6SrO–32Nb2O5–36SiO2 + 3CaF2 | 114 | 1623 | 13.5 | (414) |
| 31.2SrO–7.8Na2O–26Nb2O5–35SiO2 | 91 | 1941 | 15.2 | (390) |
| 15 Bi2O3–15Nb2O5–40SiO2–30Al2O3 | 100 | 1861 | 15.3 | (391) |
| 65(48SrO–12Na2O–40Nb2O5)–35SiO2 | 124 | 1669 | 15.3 | (392) |
| 24BaO–6Na2O–30Nb2O5–10Al2O3–30SiO2 | ∼70 | 2322 | 16.6 | (388) |
| 15.4Na2O–15.4PbO–23.1Nb2O5–46.2SiO2 | 175 | 1486 | 17 | (385) |
| 25.6(0.4SrO–0.6BaO)–6.4K2O–32Nb2O5–36SiO2 | 118 | 1828 | 17.45 | (394) |
| 21.25BaO–1PbO–12.75Na2O–34Nb2O5–32SiO2 | 154 | 1638 | 18.29 | (389) |
| 25.6BaO–3.2Na2O–3.2K2O–32Nb2O5–36SiO2 | 22 | 4433 | 19 | (396) |
| 21.6BaO–2.4PbO–6Na2O–30Nb2O5–10Al2O3–30SiO2 | 137 | 1848 | 20.7 | (384) |
| 63SiO2–12BaO–16B2O3–9Al2O3 | 6 | 12,000 | 38.5 | (397) |
t of the bulk ceramics is commonly >0.1 mm.
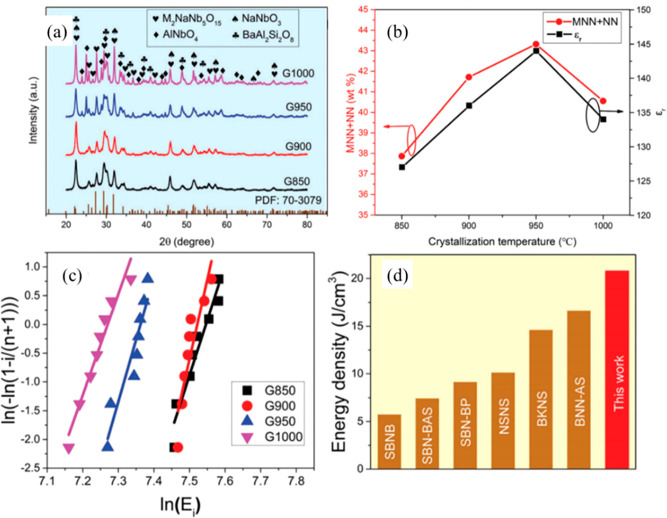
As discussed above, the crystallization of glass ceramics is controlled by the annealing procedure, where the annealing temperature and time are critical for nucleation and growth of the ceramic phase, the microstructure and the properties. Generally, the volume fraction of crystalline phase increases with increasing annealing temperature and time, accompanied by an increase of εr and decrease of BDS. The optimized Wrec is a balance between εr and BDS. Chen and co-workers383 reported that tungsten bronze structured, Ba0.27Sr0.75Nb2O5.78 phase formed from the Na2O–BaO–SrO–Nb2O5–SiO2–B2O3 glass matrix at 800 °C and a secondary phase NaSr1.2Ba0.8Nb5O15 emerged when crystallization temperature exceeded 850 °C. Remarkably high BDS ∼ 1400 kV cm–1 with εr of ∼50 were obtained, leading to a Wrec = 4 J cm–3. Besides, Wang and co-workers reported ultrahigh Wrec of 20.7 J cm–3 in BaO–PbO–Na2O–Nb2O5–SiO2–Al2O3 (BPNN-AS) glass ceramics at the optimized crystallization temperature of 900 °C, as shown in Figure 24.384 With increasing crystallization temperature from 850 to 1000 °C, the BDS decreased from 1890 to 1440 kV cm–1 and the crystallinity increased from 64.5 to 97.3% (Figure 24b). Similar results were also reported in other glass ceramic systems, including Na2O–PbO–Nb2O5–SiO2,385 SrO–BaO–Nb2O5–B2O3,386 K2O–SrO–Nb2O5–SiO2–Al2O3–B2O3,387 BaO–Na2O–Nb2O5–SiO2–Al2O3,388 BaO–PbO–Na2O–Nb2O5–SiO2,389 SrO–Na2O–Nb2O5–SiO2,390 and Bi2O3–Nb2O5–SiO2–Al2O3.391
Figure 24.
(a) X-ray diffraction (XRD) patterns of BPNN-AS glass ceramics annealed from 850 to 1000 °C; (b) εr and M2NaNb5O15 + NN phase proportion with increasing annealing temperature; (c) BDS Weibull distribution plots; (d) Wrec of 900 °C annealed BPNN-AS glass ceramics, compared with other kinds of ferroelectric glass ceramics. Reproduced with permission from ref (384). Copyright 2018 Royal Society of Chemistry.
Each constituent oxide in the glass matrix has an important effect on the crystal phase, microstructure, BDS, and energy storage properties. For example, SiO2 is an important and active studied constituent oxide in glass matrix. With increasing SiO2 content, εr of SrO–Na2O–Nb2O5–SiO2 (SNN-Si) glass ceramics first increased and then decreased as shown in Figure 25a, which was attributed to the change of volume fraction Sr6Nb10O30 (Figure 25b). The optimal εr of 120 and BDS ∼ 1700 kV cm–1 were obtained with 35 mol % SiO2 (Figures 25a,c), resulting in the highest theoretical Wrec of 15.2 J cm–3.392 Wang and co-workers reported that, as K2O concentration increased in K2O–BaO–Nb2O5–SiO2 glass ceramics, grain boundary R and activation energy decreased, indicating the decrease of interfacial polarization, leading to the enhancement of BDS to ∼1900 kV cm–1 and Wrec ∼ 12 J cm–3.393 They also reported that substitution of Sr for Ba in SrO–BaO–K2O–Nb2O5–SiO2 led to the formation of solid phase Sr0.5Ba0.5Nb2O6 and improvement of dielectric properties.394 The highest BDS of ∼1800 kV cm–1 and Wrec of 17.5 J cm–3 were achieved with Sr = 0.4 due to a uniform and dense microstructure and lower interfacial polarization. Li and co-workers reported substitution of K with Ba in SrO–BaO–K2O–Nb2O5–B2O3–P2O5 glass ceramics transformed Ba0.5Sr0.5Nb2O6 to a solid solution of K2xyBa(1-x)ySr5–yNb10O30 and then KSr2Nb5O15 phase, leading to a decrease in εr.395 A maximum theoretical Wrec of 12.8 J cm–3 was obtained under BDS ∼ 1800 kV cm–1, along with dielectric loss <0.3%. Liu and co-workers studied the effect of R2O (R = Li, Na, K) on the phase structure, dielectric properties and BDS in BaO–R2O–Nb2O5–SiO2 glass ceramics, where the highest Wrec ∼ 19 J cm–3 was achieved with εr ∼ 22 and superior BDS of ∼4400 kV cm–1 in composition of BaO–Na2O–K2O–Nb2O5–SiO2.396
Figure 25.
(a) Dielectric properties, (b) XRD patterns, and (c) BDS of SNN-Si glass ceramics as a function of SiO2 concentration (β). Reproduced with permission from ref (392). Copyright 2017 Elsevier.
Compared with alkali based compositions, alkali-free glass compositions were found to deliver lower dielectric loss and fewer defect microstructure. For example, Smith and co-workers reported both ultrahigh Emax ∼ 12000 kV cm–1 and Wrec ∼ 35 J cm–3 in BaO–B2O3–Al2O3–SiO2 glass.397 The effect of Al/Si ratio on the modification of the microstructure and properties of SrO–BaO–Nb2O5–SiO2–Al2O3 glass ceramics was also studied by Xiu and co-workers.398
Additionally, rare-earth oxides, such as La2O3,399−401 Sm2O3,402 Yb2O3,403,404 and Gd2O3,405 are also commonly substituted into glass formulations for energy storage applications. Rare-earth oxides are mainly reported to act as nucleating agents399,402 or crystal growth inhibitors.400,406 Zhang and co-workers revealed that La2O3 leads to a homogeneous microstructure in the BaO–SrO–TiO2–Al2O3–SiO2 glass-ceramics which improved BDS ∼ 1600 kV cm–1 and Wrec ∼ 3.2 J cm–3 (2.5 times of the glass-ceramics without La2O3).399 Zheng and co-workers reported that 0.5 mol % La2O3 in SrO–BaO–Nb2O5–B2O3–ZnO glass-ceramics also optimized εr (∼130) and Wrec ∼ 7.1 J cm–3 through achieving a BDS ∼ 1100 kV cm–1 due to a reduction in crystallite size and precipitation of high εr phase, Sr0.5Ba0.5Nb2O6.400 A similar effect was reported for Sm2O3 by Chen and co-workers in the SrO–BaO–Nb2O5–B2O3–ZnO glass ceramics with Wrec of 8.2 J cm–3 at 1100 kV cm–1.402 Moreover, Yb2O3 is reported to eliminate the impurity phases and form a uniform microstructure in BaO–SrO–TiO2–Al2O3–B2O3–SiO2 glass-ceramics, leading to Wrec of 3.5 J cm–3, ∼ 1.8 times higher than undoped compositions.404
Apart from the conventional annealing, novel methods such as microwave treatment have been reported to improve the energy storage properties of glass ceramics. Zhang and co-workers found that microwave treatment restrained the formation of the dendritic microstructure in BaxSr1-xTiO3−(Ba−B−Al−Si−O) (BST−BBAS) glass-ceramics (Figure 26), leading to the improvement of BDS from 1200 kV cm–1 to 1500 kV cm–1, corresponding to Wrec of 2.8 J cm–3 (950 °C anneal).408 Xiao and co-workers further reported that the precipitation of impurity phases in the K2O–SrO–Nb2O5–SiO2–Al2O3–B2O3 glass-ceramics was limited by controlling the crystallization time using microwave sintering, with optimum εr, BDS of 1400 kV cm–1 and maximum theoretical Wrec (∼9 J cm–3) obtained after 10 min.407
Figure 26.

SEM images of BST-BBAS samples annealed at 950 °C by (a) conventional method and (b) microwave treatment.408 SEM images of BNNS samples annealed at 1000 °C by (c) conventional method and (d) microwave treatment. (e, f) Temperature dependence of dielectric properties of BNNS samples. The BDS plot is inset in (f).409 (a, b) Reproduced with permission from ref (408). Copyright 2014 Elsevier; (c−f) Reproduced with permission from ref (409). Copyright 2017 Elsevier.
3.1.4. Summary of State-of-the-Art in Ceramics
The debate over whether lead-free electroceramics can replace their lead-based counterparts has been ongoing for over two decades. Lead based compositions generally outperform their lead-free counterparts on most metrics. Moreover, lead-free compositions are disparate with a large number of different formulations potentially required to cover the properties achieved with essentially doped PZT. Provided, however, that the performance, reliability and cost of lead-free are competitive with PZT, it is highly likely that lead-free electroceramics will begin to replace their lead-based equivalents and attain large scale production in the coming years as a consequence of environmental legislation.31,103,415
Of all the applications, lead-free high energy density capacitors are the most likely to see large-scale production since (i) the performance of lead-free compositions is approaching that of lead-based; (ii) reduction in intrinsic electrical properties may be compensated by increasing the BDS often through decreasing layer thickness (see section 3.1.2); and (iii) the capacitor industry is dominated by lead-free BT-based MLCCs, and thus, there is an expectation that the related products will not contain lead.34,45,90,209,276,416 This latter statement does not hold for piezoelectric ceramics market which is dominated by PZT and its derivatives.174,417−419
The energy storage performances, Emax, ΔP, Wrec, and η, for lead-based and lead-free ceramics are summarized and plotted in Figure 27 (note: glass ceramics are not included). A comparison of Wrec vs Emax for different lead-based/lead-free bulk ceramics is displayed in Figure 27a. Lead-based bulk ceramics have the advantage of both high Emax (up to ∼400 kV cm–1) and Wrec (up to ∼12 J cm–3) with respect to lead-free candidates. NN-based ceramics currently offer the highest Wrec under high E (>350 kV cm–1) for lead-free compositions, followed by AN-, NBT-, BF-, and KNN-based materials. BT and ST-based ceramics display the lowest Wrec in spite of their high Emax of ∼450 kV cm–1, but they are perhaps the most appealing dielectrics commercially since they are the current basis of MLCC production.
Figure 27.

Comparison of (a) Emax vs Wrec; (b) ΔP vs Wrec; and (c) Wrec vs η for lead-based/lead-free bulk ceramics. *t of the bulk ceramics is commonly >0.1 mm.
ΔP vs Wrec is compared in Figure 27b. Lead-, NBT-, and BF-based materials exhibit extraordinarily high ΔP (up to ∼60 μC cm–2), followed by AFE NN- and AN-based (up to ∼40 μC cm–2) and KNN-based materials (up to ∼35 μC cm–2) with the lowest (up to ∼25 μC cm–2) for BT- and ST-based materials. Bicontaining electroceramics such as NBT and BF, have been heavily studied recently as potential lead-free electroceramic materials due to their large polarization.260,266,281,282,284,420−423 A high ΔP (60 μC cm–1) is obtained in NBT and BF based by reducing the Pr through chemical substitution with other perovskite end members to form a relaxor with an ultraslim P–E loop. To some extent therefore, the advantage of a high intrinsic polarization end member such as BF is weakened. Intermediate ΔP ∼ 40 μC cm–2 values are observed for AFEs such as AN- and NN-based materials but Pmax is often limited as compositions exhibit polarization saturation as a function of applied field.
Figure 27c compares Wrec vs η for a wide range of compositions. ST-based materials display the best η (∼90%) due to their linear-like dielectric behavior. For BT-, NBT-, and lead-based materials, η varies with the material composition since it is a function of many factors. Dielectric loss associated with defects such as VO.. play a role but primarily at high field and high frequency, energy is dissipated during the transition to a field induced long-range ordered state which is manifested by the opening of the P–E loop. If the transition is smeared over for the operational range through alloying or doping to create a so-called “weakly coupled relaxor state”, η > 90% can be achieved.43,209,337
High leakage current and electrical conductivity are considered as major challenges in BF and KNN-based materials but are addressed by appropriate doping, e.g. donor doping to mitigate p-type conductivity in BF-based ceramics.45 In addition, AFE based materials generally suffer from opening of the polarization loop above switching field to form a field induced FE phase which is detrimental to η. Compositional modifications to AN and NN ceramics aim not only to push the AFE-FE transition to higher field and stabilize the AFE phase but also to disrupt the long-range ordering in the field induced FE phase, thereby creating a slimmer portion of the P–E loop (higher η) than that being observed in unmodified materials.366
Apart from the parameters discussed above (Emax, ΔP, Wrec, and η), temperature and frequency stability are also important for practical applications. In the future, high energy density ceramic capacitors will be placed closer to the core engine electronics to optimize the equivalent circuit resistance. Therefore, the temperature requirement for energy storage ceramics is anticipated to increase. According to the white paper “Multilayer Ceramic Capacitors for Electric Vehicles” published by Knowles capacitors in 2017,424 the explosive development of EVs has prompted the appearance of new 200 °C-stable C0G type I dielectric ceramic capacitor on the market. However, these materials still do not fulfill the required high power/voltage, energy density, and temperature requirements (∼250 °C) to facilitate use near-engine. Better frequency stability from 100 Hz to 100 kHz is required to reduce power fluctuations when capacitors are used for DC/DC conversion for battery charging and DC/AC conversion for propulsion.425−429 Enhanced frequency stability also enables the capacitor to be compatible with diodes and thyristors for power switching and control.430
The temperature and frequency stabilities of many high energy density ceramics are evaluated, as shown in Figure 28. Only two compositions to date deliver Wrec > 3.5 J cm–3 up to 250 °C, 0.57BF–0.33BT–0.1NN43 and 0.78NBT–0.22NN.297 Most other compositions either do not sustain or do not have properties reported >200 °C. Typical issues associated with operating at higher temperature include, widening of the P–E loop or early breakdown due to high leakage current and electric field and/or temperature-induced phase transitions. High leakage currents above 200 °C typically arise from oxygen vacancy diffusion.45
Figure 28.

(a) Temperature-42,43,130,131,208,242,297,337,348,352,357,359 and (b) frequency-dependent Wrec for some reported electroceramic materials for high energy density capacitors.276,295,297,337,416
Most compositions have been shown to deliver Wrec at a few hundred Hz but higher frequencies (>kHz) are rarely reported. Wang and co-workers, for example, discussed frequency stability from 10–2 to 102 Hz for 0.57BF-0.3BT–0.13BLN with Wrec ∼ 8 J cm–3 and η ∼ 81% at 400 kV cm–1. All electroceramics for capacitors appear to deliver a charging–discharging speed at or faster than 1 μs. Short times of τ0.9 ∼ 0.15 μs (90% of energy discharge in 0.15 μs) were reported by Li and co-workers in BT based ceramics at 150 kV cm–1197 while Qi et al. described even faster charging–discharging speeds (τ0.9 ∼ 97 ns) in BF-based ceramics at 200 kV cm–1.42 Increasing attention has also been focused on fatigue-resistant behavior as a performance metric in practical applications with 104 cycles (Wrec variation <10%) reported in BF-based ceramic multilayers from 20 to 100 °C, coupled with a low value of electrostrain (<0.03%).276
3.2. Ceramic Multilayers and Films
3.2.1. Ceramic Multilayers
Ceramic MLs are fabricated by a series of processing steps which include slurry preparation, tape-casting, screen printing, lamination, cosintering, and termination, as shown in Figure 29.18,176,422,431,432 This fabrication technology is a powder-based approach that accommodates scale-up from laboratory research to commercial manufacturing. The market of ceramic MLs ∼ $5.3 billion in 2017 but will reach ∼ $7.8 billion by 2024 for electronic applications, including but not limited to mobile phones, laptops and motor vehicles.433 Advanced high energy density ceramic MLs, based on AFEs and RFEs materials, are being developed to facilitate power electronics within hybrid electric vehicles which require higher Wrec and operating temperature. Simultaneously, research into low cost internal electrodes is required so that the highest performant ceramics can be developed. The energy storage properties for different ceramic MLs are summarized in Table 12.
Figure 29.

Ceramic MLs fabrication process (MLs cofire technology). Reproduced with permission from ref (18). Copyright 2010 IEEE.
Table 12. Summary of the Energy Storage Performance of Ceramic MLs.
| materials | t (μm) | electrode | E (kV cm–1) | ΔP (μC cm–2) | Wrec J cm–3 | η (%) | ref |
|---|---|---|---|---|---|---|---|
| [0.94(0.75NBT–0.25NN)–0.06BT]–0.1CaZrO3 | 30 | 70Ag/30Pd | 120 | 0.35 | 77 | (440) | |
| BT + BT@SiO2 *layer structure | 10/20 | 301.4 | 18 | 1.8 | 71.5 | (441) | |
| ST + Li2CO3)/(0.94NBT–0.06BT) *layer structure | 50/12 | 237 | 30 | 2.41 | 68 | (442) | |
| (Pb0.88Ba0.05La0.02Dy0.04) (Zr0.68Sn0.27Ti0.05)O3 | 11 | 95Pd/5Ag | 300 | 28 | 2.7 | 67.4 | (435) |
| 0.6NBT–0.4ST | 27 | 75Ag/25Pd | 270 | 2.83 | 85 | (443) | |
| Pb(Zr0.95Ti0.05)0.98Nb0.02O3 | 32 | 3 | (434) | ||||
| Nb and Mn co-doped 0.9BT–0.1NBT | 30 | 60Ag/40Pd | 480 | 18 | 3.33 | 80 | (444) |
| Ca(Zr0.80Ti0.20)O3 | 10 | Pt | 1500 | 6 | 4 | (445) | |
| BT–0.12 Bi(Li0.5Ta0.5)O3 | 30 | Pt | 466 | 25 | 4.05 | 95.5 | (203) |
| BT–0.12 Bi(Li0.5Nb0.5)O3 | 29 | Pt | 450 | 25 | 4.5 | 91.5 | (198) |
| 0.7BT–0.3BiScO3 | 25 | Pt | 730 | 24 | 6.1 | (33) | |
| 15% Nd doped BF–BT | 33 | Pt | 540 | 39 | 6.74 | 77 | (90) |
| BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3 | 11 | 60Ag/40Pd | 790 | 27 | 7.8 | 88 | (446) |
| BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3 | 11 | 60Ag/40Pd | 750 | 27 | 8.13 | 95 | (437) |
| NBT–0.45(Sr0.7Bi0.2)TiO3 | 30 | Pt | 720 | 30 | 9.5 | 92 | (222) |
| BF–0.3Ba0.8Sr0.2TiO3–0.06La(Mg2/3Nb1/3)O3 | 8 | Pt | 720 | 43 | 10 | 77 | (416) |
| BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3 | 5 | 60Ag/40Pd | 1047 | 30 | 10.12 | 89.4 | (438) |
| BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3 | 9 | 60Ag/40Pd | 1000 | 30 | 10.5 | 93.7 | (447) |
| BF–0.3BT–0.08Nd(Zr0.5Zn0.5)O3 | 17 | Pt | 700 | 34 | 10.5 | 87 | (34) |
| Pb0.98La0.02(Zr0.7Sn0.3)0.995O3 | 20 | Pt | 560 | 50 | 12.6 | 80 | (436) |
| BF–0.3BT–0.13 Bi(Li0.5Nb0.5)O3 | 8 | Pt | 953 | 45 | 13.8 | 81 | (276) |
| BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3 | 4.8 | Pt | 1500 | 36 | 14.1 | 69.7 | (448) |
| 0.50BF–0.40ST–0.10BMN | 8 | Pt | 1000 | 50 | 15.8 | 80 | (45) |
| NBT–0.30(Sr0.7Bi0.2)TiO3–0.08BMN | 8 | Pt | 1000 | 50 | 18 | 93 | (337) |
| BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3@SiO2 | 4.7 | 60Ag/40Pd | 1755 | 35 | 18.24 | 94.5 | (89) |
| ⟨111⟩-textured NBT–0.30SBT | 20 | Pt | 1030 | >65 | 21.5 | 80 | (439) |
Several lead-based AFE ceramic MLs have been reported using different internal electrodes. A giant power density ∼2000 kW cm–3 and Wrec∼ 3 J cm–3 was obtained in Pb(Zr0.95Ti0.05)0.98Nb0.02O3 MLs using Pt as internal conductive electrode.434 Optimized performance of Wrec ∼ 3.8 J cm–3 was also reported by Hao and co-workers for (Pb0.88Ba0.05La0.02Dy0.04)(Zr0.68Sn0.27Ti0.05)O3 MLs using 5%Ag/95%Pd internal electrode. However, this was accompanied by a large electric field-induced strain ∼0.71% at 300 kV cm–1,435 a major drawback for practical applications due to inferior mechanical stability in operation. To date, Hao and co-workers have reported the record-high Wrec ∼ 12.6 J cm–3 and η ∼ 80% under Emax ∼ 560 kV cm–1 for Pb0.98La0.02(Zr0.7Sn0.3)0.995O3 ceramic ML, as shown in Figure 30.436
Figure 30.
(a) P–E loops under electric field up to Emax (b) calculated Wrec and η values for Pb0.98La0.02(Zr0.7Sn0.3)0.995O3 ceramic ML. Reproduced with permission from ref (436). Copyright 2020 Royal Society of Chemistry.
Although lead-free AFE ceramics show great promise, there are no reports of ceramic MLs in the literature. Instead, lead-free RFEs have dominated recent ceramic ML research due to their excellent Wrec and fatigue-resistant behavior accompanied by negligible electric field induced strain. 0.87BT–0.13 Bi(Zn2/3(Nb0.85Ta0.15)1/3)O3 ceramic MLs have been fabricated with a dielectric layer t of 11 μm using 60Ag/40Pd internal electrodes that exhibit excellent Wrec ∼ 8.13 J cm–3 and η ∼ 95% at 750 kV cm–1.437 The energy storage properties of 0.87BT–0.13 Bi(Zn2/3(Nb0.85Ta0.15)1/3)O3 were further enhanced by Zhao and co-authors by decreasing the dielectric layer to ∼5 μm, achieving Wrec ∼ 10.12 J cm–3 at 1012 kV cm–1, Figure 31a,b, as well as demonstrating good temperature stability from 75 to 175 °C.438 NBT–0.45SBT ceramic MLs have also been reported to exhibit Wrec ∼ 9.5 J cm–3 with η ∼ 95% at 720 kV cm–1,222 which were further improved by forming a solid solution with a third perovskite end-member, 10%BMN to give Wrec ∼ 18 J cm–3 with η > 90% at 1000 kV cm–1, by Ji and co-workers, Figures 31c,d.337 The highest among all lead/lead-free ceramic MLs, Wrec ∼ 21.5 J cm–3 at ∼1030 kV cm–1, however, was achieved for textured NBT–0.3SBT ceramic MLs.439 Texturing was achieved through the use of <111>-oriented ST platelets which reduced field-induced strain at high field thus enhancing the BDS greatly. Combination of texturing with alloying with a third end member (BMN) in NBT–SBT may well represent an exciting path to achieve yet higher energy densities.
Figure 31.

(a) Bipolar P–E loops and (b) calculated Wrec and η of 0.87BT−0.13Bi[Zn2/3(Nb0.85Ta0.15)1/3]O3 MLs under different electric fields.438 (c) Unipolar P–E loop, with inset SEM micrograph of ceramic MLs, and (d) calculated Wrec and η of NBT–0.45SBT–0.08BMN ceramic MLs under different E. (a, b) Reproduced with permission from ref (438). Copyright 2019 John Wiley and Sons; (c, d) Reproduced with permission from ref (337). Copyright 2021 Elsevier.
Wang, Reaney, and co-workers have employed a range of different chemical dopants and alloying additions to investigate BF-(B,S)T-based ceramic multilayers.34,45,90,276,416 In 2018, Nd-doped BF-0.3BT was reported to exhibit Wrec ∼ 6.74 J cm–3 (more than 3 times higher than the bulk value) and η ∼ 77% at 540 kV cm–1 with a layer t of 33 μm.90 On the other hand, alloying BF–0.3BT with 8 mol % Nd(Zr0.5Zn0.5)O3 resulted in Wrec ∼ 10.5 J cm–3 with η of 87% at 700 kV cm–1 with a dielectric layer t of ∼17 μm, Figure 17.34 Further studies focused on promoting electrical homogeneity, which was considered to prevent conductive pathways developing in these composition, thereby avoiding the breakdown at high field and facilitating the improvement of Wrec by reducing the dielectric layer thickness. Finally, Wrec ∼ 13.8 J cm–1 at 953 kV cm–1 was achieved for BF–0.3BT–0.13 Bi(Li0.5Nb0.5)O3 (BF–BT–0.13BLN) ceramic MLs at a dielectric layer t of 8 μm, as shown in Figure 32.276
Figure 32.
(a) Backscattering electron (BSE) cross section micrographs of BF–BT–0.13BLN ceramic MLs; (b) energy dispersive X-ray (EDX) mapping of all elemental distribution (c) transmission electron microscopy (TEM) micrograph obtained from an interface between a BF–BT–0.13BLN grain and a Pt grain (electrode); inset shows a high resolution TEM (HRTEM) image (filtered) obtained from the grain at a higher magnification. (d) Unipolar P–E loops and (e) calculated energy storage properties for BF–BT–0.13BLN ceramic MLs. Reproduced from ref (276). Copyright 2020 Royal Society of Chemistry.
3.2.2. Ceramic Films
Higher BDS and Wrec compared to MLs fabricated through powder-based technology have been reported for ceramic films deposited on LaNiO3/Si(100) or Pt/Ti/SiO2/Si substrates by physical vapor or chemical deposition techniques, such as radio frequency magnetron sputtering,449 spin coating,101,450,451 pulsed laser deposition,452 and chemical solution deposition.453 The BDS of ceramic films is significantly improved due to the reduction of t (<1 μm) often attributed to fewer defects (grain boundaries) and/or pore/void concentration. Not only have higher figures of merit been reported for ceramic films, but several researchers have proposed novel underlying mechanisms (beyond reduction in defect density) behind the enhancement, such as the formation of polymorphic nanodomains.452 However, nonpowder based techniques are difficult to scale up into MLCCs, which may constrain these extraordinary results to lab-based fundamental research rather than promising practical output for commercial exploitation. The energy storage properties for ceramic films are summarized in Table 13.
Table 13. Energy Storage Properties for Different Ceramic Films.
| materials | t (μm) | E (kV cm–1) | ΔP (μC cm–2) | Wrec (J cm–3) | η (%) | ref |
|---|---|---|---|---|---|---|
| Pb0.97La0.02(Zr0.97Ti0.03)O3 | 1.7 | 1158 | ∼90 | 20.1 | 64 | (460) |
| 1 mol %Fe–doped 0.72NBT–0.18KBT–0.10ST | 0.9 | 1200 | 58 | 20.34 | 35.17 | (461) |
| Pb0.97Y0.02[(Zr0.6Sn0.4)0.925Ti0.075]O3 | 0.5 | 1300 | 70 | 21 | 91.9 | (462) |
| Pb0.92La0.08(Zr0.52Ti0.48)O3 | 0.4 | 1600 | 35 | 22 | 77 | (463) |
| Pb0.85La0.1ZrO3 | 0.45 | 1500 | 45 | 23.1 | 73 | (464) |
| 0.942(Na0.535K0.480NbO3)−0.058LiNbO3 | 5 | 1400 | ∼52 | 23.4 | 70 | (465) |
| (Sr0.85Bi0.1)Ti0.99Mn0.01O3 | 0.25 | 1982 | 35 | 24.4 | (466) | |
| Pb0.97La0.02(Zr0.98Ti0.02)O3 | 2 | 984 | ∼120 | 25.2 | 52 | (467) |
| Bi(Mg0.5Ti0.5)O3 | 0.11 | 900 | 70 | 26 | 55 | (468) |
| (Pb0.98La0.08)(Zr0.52Ti0.48)O3 | 0.25 | 2200 | 40 | 27.5 | 62.2 | (469) |
| 6 mol % BF-doped (K0.5Na0.5)(Mn0.005Nb0.995)O3 | 1 | 2000 | ∼40 | 28 | 90 | (470) |
| PbZrO3/PbZr0.52Ti0.48O3 | 0.35 | 2615 | 60 | 28.2 | 50 | (471) |
| (Pb0.92La0.08)(Zr0.65Ti0.35)O3 | 0.32 | 3000 | 40 | 29.7 | 50.8 | (472) |
| 1 mol % Mn-doped NBT | 1.2 | 2310 | ∼40 | 30.2 | 48 | (455) |
| (K0.5, Na0.5)(Mn0.005, Nb0.995)O3-6 mol % BF | 1 | 1900 | 45 | 31 | 90.3 | (470) |
| 0.9Pb(Mg1/3Nb2/3)O3–0.1PbTiO3 | 0.375 | 2640 | 35 | 31.3 | 40 | (473) |
| 0.9(0.94NBT–0.06BT)–0.1NN | 0.3 | 3170 | 20 | 32 | 90 | (474) |
| 0.4 Bi(Mg0.5Zr0.5)O3–0.6PbTiO3 | 0.5 | 2000 | 43 | 32.3 | 51.4 | (475) |
| 2 mol % Fe-doped (Na0.85K0.15)0.5Bi0.5TiO3 | 1.15 | 2300 | ∼35 | 33.3 | 51 | (456) |
| 0.95NBT–0.05ST | 1.5 | 1950 | ∼55 | 36.1 | 41 | (476) |
| Ba2Bi4Ti5O18 | 0.41 | 2150 | 38 | 37.1 | 91.5 | (477) |
| Pb0.82La0.12Zr0.85Ti0.15O3 | 1 | 2100 | ∼52 | 38 | 71 | (478) |
| 5.8 mol % SiO2 doped HfO2 | 0.01 | ∼5800 | 40 | 72 | (479) | |
| Pb0.91La0.09 (Zr0.65Ti0.35)0.9775O3 | 1 | 1998 | ∼65 | 40.2 | 62 | (449) |
| 0.89NBT–0.06BT–0.05BF | 0.28 | 1750 | 150 | 42.9 | 65.7 | (480) |
| BaBi4Ti4O15 | 0.45 | 2000 | 40 | 43.3 | 87.1 | (481) |
| Bi3.25La0.75Ti3O12 | 0.5 | 2040 | 50 | 44.7 | 78.4 | (482) |
| Hf0.3Zr0.7O2 | 0.0092 | 4500 | 30 | 46 | 53 | (458) |
| Pb0.97La0.02Zr0.66Sn0.23Ti0.11O3 | 0.65 | 4000 | 55 | 46.3 | 84 | (453) |
| 0.5 Bi(Ni1/2Ti1/2)O3-0.5PT | 0.455 | 2250 | 62 | 46.7 | (483) | |
| Pb0.94La0.04(Zr0.98Ti0.02)O3 | 2 | 3699 | ∼48 | 47.4 | 25 | (454) |
| 0.4ST-0.6 Bi3.25La0.75Ti3O12 | 0.3 | 2470 | 38 | 47.7 | 87.4 | (484) |
| 0.9 Bi0.2Sr0.7TiO3-0.1BF | 0.46 | 4800 | 25 | 48.5 | 47.57 | (485) |
| Si doped Hf0.5Zr0.5O2 | 0.01 | ∼3500 | ∼30 | 50 | 80 | (486) |
| 0.6NBT–0.4 Bi(Ni0.5Zr0.5)O3 | 1 | 2200 | ∼63 | 50.1 | 64 | (457) |
| 0.5 Bi(Ni1/2Ti1/2)O3-0.5PT-excess 20% PbO | 0.45 | 2250 | 75 | 50.2 | (487) | |
| (Pb0.97La0.02)(Zr0.7Sn0.25Ti0.05)O3 | 1.8 | 3750 | ∼75 | 56 | 45 | (488) |
| 0.6BT–0.4 Bi3.25La0.75Ti3O12 | 0.3 | 3200 | 45 | 61.1 | 84.2 | (489) |
| 6 mol % Si-doped HfO2 | 0.01 | 4500 | 61.2 | 65 | (490) | |
| 8 atom % Al doped HfO2 | 0.05 | 4900 | 14 | 63 | 90 | (459) |
| Na0.485Bi0.5(Ti0.96W0.01Ni0.03)O3 | 0.6 | 2500 | 30 | 63.1 | 55 | (491) |
| BF/Bi3.25La0.75Ti3O12 | 0.14 | 2753 | 50 | 65.5 | 74.2 | (492) |
| Bi0.525Na0.5(Ti.96W0.01Ni0.03)O3 | 0.4 | 2500 | 78 | 65.8 | 52.9 | (493) |
| 0.25BF–0.75ST | 0.5 | 4460 | 70 | 70 | (494) | |
| 0.97(0.93NBT–0.07BT)–0.03BF | 0.35 | 2285 | ∼120 | 81.9 | 64.4 | (495) |
| (Ba0.95Sr0.05)(Zr0.2Ti0.8)O3 | 0.1 | 6200 | 102 | 87 | (496) | |
| 0.25BF–0.3BT–0.45ST | 0.45 | 4900 | 90 | 112 | 80 | (452) |
| 0.68PMN–0.32PT | 0.15 | 5800 | ∼100 | 130 | 75 | (497) |
Lead-based ceramic films have been studied heavily in the past decade using different preparation methods, particular for PLZT. (Pb0.94La0.04)(Zr0.98Ti0.02)O3 ceramic film with t of 2 μm was reported to exhibit 47.4 J cm–3 under electric field of 3700 kV cm–1 on a Pt(111)/TiO2/SiO3/Si(100) substrate.454 Further increasing La concentration resulted in superior Wrec ∼ 40.2 J cm–3 and η ∼ 62%, achieved at Emax∼ 1998 kV cm–1 for Pb0.91La0.09(Zr0.65Ti0.35)0.9775O3 on a LaNiO3/F-Mica substrates.449 In addition, for Pb0.97La0.02Zr0.66Sn0.23Ti0.11O3, an improved Wrec ∼ 46.3 J cm–3 was achieved at ∼4000 kV cm–1, accompanied by excellent temperature stability (up to 380 K) and cyclic reliability (up to 105), Figure 33.453
Figure 33.
(a) Unipolar P–E loops, inset image is cross-section SEM image of ceramic film deposited on the substrate, and (b) Wrec and η of Pb0.97La0.02Zr0.66Sn0.23Ti0.11O3 ceramic film under various E. (c) Temperature and (d) cyclic unipolar P–E loops, Wrec, and η of Pb0.97La0.02Zr0.66Sn0.23Ti0.11O3 ceramic film under E∼ 2200 kV cm–1. Reproduced with permission from ref (453). Copyright 2018 Elsevier.
In the past few years, there has been increased focus on lead-free ceramic films due to concerns over toxicity of PbO. NBT and BF based relaxor compositions have dominated research and have competitive or even superior energy storage performance to lead-based films. Mn doped NBT ceramic films on a LaNiO3/Si(100) substrates with t = 1.2 μm were reported to exhibit excellent Wrec ∼ 30.2 J cm–3 under Emax ∼ 2310 kV cm–1.455 Similar properties, Wrec ∼ 33.3 J cm–3 under Emax ∼ 2300 kV cm–1, were also obtained for Fe doped NBT–K0.5Bi0.5TiO3 ceramic film.456 Recently, even higher Wrec ∼ 50.1 J cm–3, η ∼ 63.9% accompanied by fast charge–discharge speed (∼210 ns) were achieved simultaneously at ∼2200 kV cm–1 in relaxor 0.6NBT–0.4 Bi(Ni0.5Zr0.5)O3 films.457 In addition, an ultrahigh Wrec ∼ 112 J cm–3 with η ∼ 80% was reported by Pan and co-workers in BF–BT–ST ceramic films recently (Figure 34). Polymorphic nanodomains with competitive rhombohedral and tetragonal phases with competitive free energy were considered critical for the extraordinary electrical properties. BF was chosen as a main component due to its large spontaneous polarization. BT was introduced to form a solid solution to encourage coexistence of rhombohedral and tetragonal phases and finally ST was incorporated to further disrupt the long-range polar coupling and induce polymorphic nanodomains. By tuning the ratio of BF, BT and ST, a highly disordered composition is produced with rhombohedral and tetragonal nanodomain. The experimental observations were validated by phase-field simulations in the optimized composition, 0.20BF–0.25BT–0.55ST.452 Apart from these perovskite lead-free relaxor candidates, HfO2-based ceramic films have also been explored and demonstrate promising energy storage properties, stabilities/reliabilities, scalability, and integration. Wrec ∼ 46 J cm–3 with excellent temperature stability (up to 175 °C) and cyclic fatigue resistant (up to 109 time) was reported by Park in a 9.2 nm thick Hf0.3Zr0.7O2 film458 and Wrec ∼ 63 J cm–3 with η ∼ 85% were realized in 50 nm thick Al doped HfO2 ceramic films with excellent temperature and frequency stability.459
Figure 34.
(a) Comparative display of Landau energy profiles and P–E loops of an FE with micrometer-size domains, an RFE with nanodomains, and an RFE with polymorphic nanodomains. Phase-field-simulated three-dimensional domain structures of (b) 0.45BF-0.55ST with rhombohedral nanodomains and (c) 0.20BF-0.25BT–0.55ST with coexisting rhombohedral and tetragonal nanodomains. (d) Simulation of the two-dimensional multiple nanodomain structure of 0.20BF-0.25BT–0.55ST in the cubic matrix. Contour plots of the simulated (e) Wrec and (f) η of BF–BT–ST solid solutions. Reproduced with permission from ref (452). Copyright 2019 The American Association for the Advancement of Science.
3.2.3. Summary of State-of-the-Art in Ceramic MLs and Films
The energy storage performances, Wrec, η, and ΔP, between bulk ceramics, ceramic MLs, and ceramic films are shown in Figure 35. The highest Wrec (∼130 J cm–3) and Emax (∼5800 kV cm–1) are obtained in ceramic films, followed by ceramic MLs (∼21 J cm–3 and Emax ∼ 1000 kV cm–1) and bulk ceramics (Wrec (∼12 J cm–3 and Emax ∼ 650 kV cm–1) and scale primarily with t of the dielectric layer (Figure 35d). Wrec in ceramic films is also improved by higher ΔP (up to ∼120 μC cm–2) with respect to ceramic MLs (up to ∼70 μC cm–2) and bulk ceramics (up to ∼60 μC cm–2), as shown in Figure 35b. η for bulk ceramics, ceramic MLs and ceramic films varies significantly with composition and relates to factors such as, energy dissipation through a field induced transition to a long-range polar state, domain switching, polarization rotation, and leakage current relating to the presence of VO.. and associated defect dipoles.
Figure 35.
(a) Emax vs Wrec, (b) ΔP vs Wrec, (c) Wrec vs η, and (d) t vs Wrec and Emax between bulk ceramics, ceramic MLs and ceramic films.
From a commercial perspective, energy storage performance of lead-free ceramic MLs has improved significantly in the past few years with BF and NBT based ceramic MLs now rivalling lead-based ceramic MLs, delivering Wrec > 15 J cm–3 at ∼1000 kV cm–1. However, the selection of inner electrodes for these materials is limited to Pt, currently too costly for mass production. Commercial focus therefore, currently remains mainly on modified BT compositions which are compatible with Ni, Ag and Ag/Pd electrodes depending on composition and pO2 during fabrication. A huge stride forward in the industry would be the development of a low cost equivalent to Ag/Pd electrodes which could permit the fabrication of a wider range of MLs at higher pO2 which would inhibit the formation of VO.. and maintain high BDS.
4. Strategies for Improving Energy Storage Properties
The review of the state-of-the-art of ceramics, MLs, and films presented in section 3 clearly points to a set of criteria that are required to optimize energy storage performance. Though some of these have been alluded to in section 3 as part of the review of the state-of-the-art, it is worth collating these principals for RFE and AFE materials to act as guide for future materials development.
4.1. Optimization through an Induced Relaxor State
The most commonly utilized strategy to optimize energy storage properties is through inducing a relaxor state within a system that contains highly polarizable ionic species. It is typically carried out through strategic doping or alloying to form a pseudoternary solid solution. Levin and co-workers proposed that long-range ferroelectric correlations can be effectively “blocked” by using designed dopants with enhanced local polarizability.498 If this concept is married to dopant strategies to induce or maintain a homogeneous electrical microstructure, ΔP and BDS can be optimized leading to a large Wrec.
These two simple precepts can be applied to most systems; e.g., a frequency dispersion of εr was observed after doping xBi2/3(Mg1/3Nb2/3)O3 (B2/3MN) into BT ceramics, along with reduction on maximum dielectric constant (εm) and associated temperature (Tm).209 After fitting the εr and Tm using modified Curie–Weiss law (as follows)
| 17 |
γ was found to be within the range of 1.61 for BT–0.06B2/3MN ceramics with a Burns temperature ∼154 °C. In addition, XRD revealed a transformation from tetragonal to an average pseudocubic structure as increasing x concentration, coupled with a reduction in Pr, confirming a relaxor state at room temperature, Figure 36a,b.
Figure 36.
(a) XRD patterns with representative peaks and (b) Bipolar P–E loops for xB2/3MN–BT ceramics with x = 0.00–0.10. (c) BSE surface micrographs of Ag–Pd cofired 0.06B2/3MN–BT ceramics. (d) EDX mapping distribution of Ag, Pd, Ba, and Ti elements. Reproduced from ref (209). Copyright 2020 American Chemical Society.
An optimum Wrec ∼ 4.55 J cm–3 at 520 kV cm–1 was recorded for BT–0.06B2/3MN (Figure 36)209 with similar properties reported for Bi(Mg0.5Ti0.5)O3,208 Bi(Li0.5Ta0.5)O3,203 Bi(Zn0.5Zr0.5)O3,199 and K0.73Bi0.09NbO3499 doped BT ceramics. However, the advantage of BT–B2/3MN is in the comparatively low concentration of dopant required to induce a relaxor state. The polar coupling is disrupted through a combination of A-site (Bi and VO..) and B-site (Nband Mg) dopants which provide a range of difference in ionic size, charge and electronegativity. However, only 4 mol % Bi is present on the A-site which minimizes reaction with Ag/Pd (Figure 36c). MLs with Ag–Pd internal electrodes may then be fired in ambient pO2, minimizing VO and reducing leakage current at high fields.
Similar compositional modifications to induce a relaxor state have been adopted in BF-based solid solution. For example, in FE BF–BT compositions, macroscopic herringbone-type domains are observed in (Figure 37a) but doping with 5 mol % Nd, induced a nanodomain state, accompanied by a decrease in Pr and a frequency-dependent permittivity curve, confirming the transformation from FE to RFE. Simultaneously, average G was reduced from ∼10 μm (BF–BT) to ∼2 μm (Nd doped BF–BT). As a result, enhanced Wrec ∼ 1.8 J cm–3 was obtained for 15 mol % Nd doped BF–BT, which was further improved to 6.74 J cm–3 by multilayering (Figure 37b,c)90 with similar optimization reported in BF doped with Bi(Zn2/3Nb1/3)O3 and Bi(Zr0.5Zn0.5)O3.34,266 The same design strategy of inducing an RFE state is also used in thin film BF–BT–ST compositions.452
Figure 37.
(a) TEM images of the domain structure in BF–BT, 5 mol % Nd–BT–BT and 10 mol % Nd–BF–BT. (b) The changes of Wrec as a function of electric field for x mol % Nd–BF–BT ceramics. (d) W, Wrec, and η for 15 mol % Nd–BF–BT MLs, with ceramic MLs microstructure as inset figure. Reproduced with permission from ref (90). Copyright 2018 Royal Society of Chemistry.
In summary, the inferior energy storage performance of all FEs can be improved by forcing a RFE state through strategic doping or alloying, Figure 38.34,66,90,92,199,204,272Pmax is often unsaturated in RFE and increases with E which means ΔP is not only a function of the slimness of the P–E loop but also of the applied E. RFEs are therefore, among the most promising candidates for capacitors in power electronics (Emax > 300 kV cm–1).
Figure 38.
(a) Emax vs Wrec, (b) ΔP vs Wrec and (c) Wrec vs η for FEs and RFEs bulk ceramics to demonstrate the relaxor optimization.
4.2. Optimization of Antiferroelectrics
Many recent publications have focused on optimization of energy storage in lead-free AN-based materials,347,353 although similar strategies date back to early studies of lead-based PLZT and PLZST AFE ceramics.118,126,143−145 The most comprehensive study of AN AFE ceramics was performed by Lu and co-workers in which they used A and B-site substitutions to develop, Ag0.97Nd0.01Nb0.80Ta0.20O3 which yielded Wrec ∼ 6.5 J cm–3 at 370 kV cm–1 with η∼ 71%, Figure 39.366 In their study, Lu and co-workers defined several key points required to optimize AN-based ceramics:
-
i)
Optimization of Pmax through local strain/field coupling around the smaller (with respect to Ag) Nd ion the A-site and its compensating VA, Figure 39a.
-
ii)
Stabilization of the AFE structure through a combination of Nd and Ta doping which leads the induced AFE/FE transition to higher fields. Figure 39(a).
-
iii)
Inducing a slim hysteresis curve in the field induced region of the P–E loop. This was also achieved, through Nd doping that disrupted polar and antipolar coupling which manifested itself as a decrease in domain width from ∼1 to 0.5 μm and streaking of ±1/4(001)c superstructure reflections in electron diffraction patterns for x = 0.03, Figure 40.
Figure 39.
(a) Schematic illustrating how Wrec is optimized through doping in AN. Gray, yellow, green, dark green, and red spheres represent Ag, Nd, Nb, Ta, and O atoms, respectively. The Wrec and η of (b) AN and (c) Nd0.01Ta0.20 codoped AN under the respective electric fields.366
Figure 40.
TEM [210]c (c = cubic) zone axis diffraction patterns and corresponding dark field images obtained using (001) reflections from (a) and (b) AN and (c) and (d) Ag0.91Nd0.03NbO3 (e) [210]c zone axis diffraction pattern of Ag0.97Nd0.01Ta0.20Nb0.80O3. (f) Bright field TEM image of domains in a grain of Ag0.97Nd0.01Ta0.20Nb0.80O3.366
All the above maximize the area of the P–E loop to the left of the curve in the positive quadrant and thus optimize Wrec, Figure 39b,c.
Stabilization of the AFE structure was also confirmed by First-principles calculation and Ginzburg–Landau–Devonshire (GLD) phenomenology, as illustrated in Figure 41.
Figure 41.
Schematic contour diagrams of the free energy difference (ΔG) for (a) AN- and (b) Nd/Ta-codoped AN without electric field. Schematic contour diagrams of GLD phenomenological theory of AFE-to-FIE phase transition for (c) AN- and (d) Nd/Ta-codoped AN under application of electric field.366
Figure 42 summarizes the properties for many AFE systems. Most conventional AFEs exhibit low Wrec (∼1.5–2 J cm–3) and η (∼40%), which can be optimized to Wrec > 3 J cm–3 and η > 50% (enhanced-AFEs) by strategies described in the work of Lu and others.348,350−352,357,359,363,366 Similar values of ΔP (30–40 μC cm–2) are found for AFEs and enhanced-AFEs which reflects an intrinsic limitation of AFE materials, i.e. when antiparallel polar coupling is fully switched to polar under electric field, Pmax reaches saturation and is difficult to enhance unlike for RFEs (section 4.1). η is also difficult to further improve (>80%) due to the hysteresis above the AFE/FE switching field. However, at intermediate electric fields (∼300 kV cm–1), much higher ΔP and Wrec are obtained for AFEs compared with RFEs, indicating that AFEs are more suitable for low/intermediate-voltage energy storage applications.
Figure 42.
(a) Emax vs Wrec and (b) ΔP vs Wrec and (c) Wrec vs η for AFEs and stabilized AFEs bulk ceramics to demonstrate the AFE stabilized optimization.
4.3. Other Strategies
4.3.1. Chemical Coating
Chemical coating is commonly reported as a strategy to optimize energy storage properties in BT ceramics. In general, chemical coatings are synthesized and applied using wet-chemical and sol–gel methods.69,74,79−81 Smaller grain sizes are typically obtained (on the order of a few nm), leading to the enhanced density and BDS. BT ceramics, for example, with a 4 nm thick SiO2 coating, have a good value of Wrec (∼1.43 J cm–3),233 in which SiO2 coating inhibits grain growth, thereby modifying the microstructure and reducing DC current leakage. The effect of SiO2 layer thickness on BT particles has been systematically investigated (Figure 43a-c). The highest Wrec(∼4.8 J cm–3) and η (∼99.1%) were obtained for 20 wt % SiO2 coated BT, as shown in Figure 43d–f.88 Other coating materials such as Al2O3 and La2O3,80,184 are also reported to optimize energy storage properties, as listed in Table 14.
Figure 43.
(a–c) TEM micrographs of BTnanoparticles coated with SiO2: (a) BT@10 wt %SiO2; (b) BT@15 wt %SiO2; (c) BT@20 wt %SiO2. The shell region is defined by red arrows and dash lines. Bipolar P–E loops of BT with (d) 10 wt % (e) 15 wt % (f) 20 wt % SiO2 composite ceramics at the highest applied electric field, measured at 10 Hz and room temperature. Reproduced with permission from ref (88). Copyright 2019 Elsevier.
Table 14. Energy Storage Performance of Chemically Coated Materials.
| materials | coating/thickness | Emax (kV cm–1) | Wrec (J cm–3) | η (%) | G (nm) | ref |
|---|---|---|---|---|---|---|
| BT | Al2O3/2 nm | 108 | 0.51 | 80 | ∼160 | (80) |
| BT | La2O3 SiO2/20 nm | 136 | 0.54 | 85 | ∼250 | (184) |
| BT | BiScO3/ 4 nm | 120 | 0.68 | 81 | ∼100 | (500) |
| BT | SiO2 and Al2O3/6 nm | 190 | 0.725 | 80 | ∼80 | (73) |
| BT | SiO2/10 nm | 200 | 1.2 | 53.8 | ∼200 | (501) |
| BT | SiO2/4 nm | 290 | 1.43 | 53 | ∼120 | (86) |
| Pb0.97La0.02(Zr0.33Sn0.55Ti0.12)O3 | SiO2/2 nm | 238 | 2.68 | 95 | ∼180 | (87) |
| BT | SiO2/21 nm | 370 | 4.799 | 95 | ∼220 | (88) |
| Pb0.91La0.06(Zr0.552Sn0.368Ti0.08)O3 | PbO-B2O3–SiO2–Al2O3–ZnO-MnO2/4 nm | 402 | 7.4 | ∼250 | (77) |
4.3.2. Layered Structure
Layer-structures composed of multiple materials have been reported to optimize energy storage properties and are typically tape cast, followed by lamination. Both BDS and εr are optimized with the final properties related to the type of electroceramic material and the thickness of each layer. The BDS of BT-based ceramics was enhanced to >300 kV cm–1 by laminating layers between BT–x wt % SiO2 layers (t ∼ 20 μm) and BT layer (t ∼ 25 μm).441 εr decreased but this was compensated by an increased BDS with increasing SiO2 concentration in the BT–x wt % SiO2 layers. ST + Li2CO3 (t ∼ 50 μm) and 0.93NBT–0.07Ba0.94La0.04Zr0.02Ti0.98O3 (t ∼ 33 μm) layered structure were also fabricated via tape-casting with improved Wrec ∼ 2.72 J cm–3 at 294 kV cm–1.502 Enhanced Wrec ∼ 2.41 J cm–3 at 237 kV cm–3 was also obtained for layer-structure ceramics with ST + Li2O3 (t ∼ 50 μm) and NBT–0.06BT (t ∼ 50 μm), Figure 44a.b.442 The interface between the ST + Li2O3 and the NBT–0.06BT layer was further investigated using the finite element analysis. BDS was improved by reducing the breakdown paths between the ST + Li2O3 and the NBT–0.06BT layer, Figure 44c–e442 with electrical field redistribution and interface blocking playing essential roles.503 BDS was also influenced by the difference in εr and thickness ratio between the adjacent layers.
Figure 44.
(a) Bipolar P–E loops of the ST+Li2O3, NBT–0.06BT and ST + Li2O3/NBT–0.06BT ceramic MLs. (b) Comparison of Wrec and electric field between the ST + Li2O3/NBT–0.06BT ceramic MLs and some recently reported lead-free ceramics. (c) Distribution of the electric field at 200 kV cm–1, model of electrical tree propagation simulated using the finite element method for the ST + Li2O3/NBT–0.06BT MLs ceramic under (d) 200 kV cm–1 and (e) 250 kV cm–1. Reproduced with permission from ref (442). Copyright 2018 Royal Society of Chemistry.
5. Summary and Perspectives
5.1. Lead-Based Energy Storage Ceramics
Lead-based ceramics have great potential as energy storage materials in modern microelectronics where high voltage and temperature are required, such as in pulsed power and power electronic applications. Lead-based AFE-type ceramics exhibit extremely high energy density but optimizing BDS, η and minimizing electrostrain is problematic. Low BDS (<300 kV cm–1) is often attributed to the volatilisation of lead/lead oxide which leads the formation of lead vacancy (Vpb..) and VO that results in current leakage. Such issues may be partially solved by a combination of improved processing and dopants but achieving the values of BDS observed in lead-free materials has proved elusive. The low η in lead-based AFE-type ceramics (<80%) is mainly a result of opening of the hysteresis loop at high field due to the stabilization of a field induced FE phase. This results in a change in crystal class from tetragonal (AFET) to rhombohedral (FER) giving large strains (>0.3%) which may prevent long-term cycling through mechanical fatigue.
The lack of popularity in researching lead-based compared with lead-free materials in the academic community has meant that exploration of novel systems is rather limited, but there are, for example, interesting mixed Pb- and Bi-based systems with high εr and a spontaneous polarization that would mirror some of the design principles adopted in lead-free ceramics, particularly in solid solutions which combine AFEs and relaxor end members. In addition, further work is required to understand crystal structure and phase transition behavior. Many systems have incommensurate modulations and their influence on AFE/FE switching needs to be explored further using in situ XRD and Raman (temperature/electric field), as well as utilizing advanced aberration corrected TEM to study the local structure.
5.2. Lead-Free Energy Storage Ceramics
Lead-free candidates, including BT, ST, BF, KNN, NBT, AN and NN-based systems, are extensively studied and summarized in this review. Research into lead-free materials far outweighs that in lead-based, due to how the potential environmental legislation surrounding manufacturing and the end use of lead-based products has influenced funding bodies and awards. As a result, the optimization of energy storage properties has progressed rapidly in the last 5 years. Successful strategies to improve properties include, disrupting long-range polar coupling particularly if the average ionic polarizability is increased or unaffected, construction relaxor feature (PNRs) in FEs and AFEs, enhancing Eg and as a consequence Ea, reducing the total electrical conductivity and promoting electrical homogeneity through the use of strategic dopants to modify defect chemistry. If these strategies are married with a reduction in the dielectric layer thickness, high values of Wrec ∼ 20 J cm–3 and η ∼ 90% can be achieved. Recent work on texturing of ceramic MLs has also proved successful in enhancing Wrec but the complexity of this approach may inhibit commercial uptake. However, the two overriding issues with the majority of lead-free compositions, particularly those whose Wrec are >10 J cm–3 are (i) the need to find an effective low cost internal electrode system that permits their commercial exploitation (currently almost all ML data is quoted with Pt internal electrodes) and (ii) pushing their operating window to >200 °C and >100 Hz. Interestingly, electrostrain, a major drawback in lead-based materials, is broadly speaking not an issue in most of the lead-free RFEs and AFEs since the measured values of strain are often significantly lower (<0.2%) even at high fields. As with lead containing ceramics, there are only a few comprehensive investigations of the energy storage mechanisms which require high field in situ studies to be performed. A greater understanding of the role of defect chemistry, doping and alloying is also required, particular on how this influences, Eg, resistivity and electrical homogeneity, and thus the Wrec and η, thermal stability and cyclic reliability. In addition, in commercial MLCCs, the ripple current, equivalent series R, failure mode, voltage rating, the reliability in high humidity need to be evaluated and explored.
5.3. Glass Ceramics
Glass-ceramics have the advantages of facile manufacture, high Wrec, ultrahigh η (low energy dissipation), ultrafast charge–discharge speed, excellent temperature/frequency stability. However, there are still challenges/problems. Generally, increasing the volume fraction of the crystal phase will increase the ε/P but decrease BDS. It is critical to balance the εr and BDS to obtain the highest Wrec. The mechanism of crystallization and control of crystal phase/microstructure is still ambiguous, which should be further investigated using, advanced TEM and in situ XRD/TEM as a function of applied field and temperature. Furthermore, although the theoretical Wrec (>15 J cm–3, due to ultrahigh BDS, >1100 kV cm–1) of glass-ceramics are much higher than other bulk ceramics and even MLs (15–20 J cm–3), the measured/calculated Wrec by P–E loops and discharging processes is low (<2 J cm–3) due to the lower applied electric fields. As a result, we recommend using the same test method (P–E loops and discharging process) to evaluate the practical energy storage performance for glass-ceramics, consistent with other dielectrics.
Acknowledgments
This work was supported by the Engineering and Physical Sciences Research Council (EP/L017563/1 and EP/N010493/1), Henry Royce Institute for Advanced Materials, funded through EPSRC grants EP/R00661X/1, EP/S019367/1, EP/P02470X/1, and EP/P025285/1, and the National Natural Science Foundation of China (51602060 and 51402005). The authors are grateful for support provided by Functional Materials and Devices group from University of Sheffield.
Biographies
Dr. Ge Wang is currently working as a postdoctoral research associate in the Functional Materials and Devices group at the University of Sheffield (UK). He obtained his Ph.D. degree from the Department of Materials at the University of Manchester in 2017, specifically on functional and structural behavior of lead-free ferroelectrics. His research focuses on ferroelectric, piezoelectric, energy density ceramic capacitors, lithium-ion batteries, high entropy oxide materials, and structural analysis using synchrotron X-ray diffraction.
Dr. Zhilun Lu obtained his Ph.D. from the Department of Materials Science and Engineering at the University of Sheffield, in January 2016 with the High-Quality PhD Thesis Prize. He worked as a research scientist in Helmholtz-Zentrum Berlin before joining The Henry Royce Institute and Department of Materials Science and Engineering at the University of Sheffield. His work focuses on functional and energy materials such as thermoelectrics, dielectrics, high permittivity oxides, microwaves, and geometrically frustrated magnets. He has expertise in ceramics synthesis and structural and property characterization. His paper “Superior energy density through tailored dopant strategies in multilayer ceramic capacitors” was selected as a HOT Article in Energy & Environmental Science.
Dr. Yong Li is an Associate Professor at School of Materials and Metallurgy, Inner Mongolia University of Science and Technology. He received his PhD degree in Materials Science and Engineering from Beijing Institute of Technology in 2016. His research focuses on ferroelectric and multiferroic materials for energy storage, photoelectric conversion, and microwave attenuation.
Dr. Linhao Li obtained his Ph.D. in 2017 from the Department of Materials Science and Engineering at the University of Sheffield under the supervision of Professor Derek C. Sinclair. After graduation, he continued his research in Sheffield as a PDRA. Linhao’s expertise is probing the composition–structure–electrical/thermal property relationships of functional materials, particularly using impedance spectroscopy. His current research focuses mainly on developing new materials for energy and electronic applications.
Dr. Hongfen Ji received her Ph.D. degree in 2013 in electronics science and technology from Xi’an Jiaotong University, Shaanxi, China. She has held visiting appointments at Department of Materials Science and Engineering, University of Sheffield, UK (2019–2020). Currently, she works as lecturer in the Laboratory of Thin Film Techniques and Optical Test, and School of Optoelectronic Engineering, Xi’an Technological University, China. Her main research interests are in lead-free piezoelectric and ferroelectric films and ceramics, and functional devices based on piezoelectric and ferroelectric materials.
Prof. Antonio Feteira is currently a Professor of Advanced Functional Materials at Sheffield Hallam University. He received his Ph.D. degree in Materials Science and Engineering from The University of Sheffield, where he holds a visiting position. In the past, he was a Senior Research Fellow at the University of Birmingham, having previously worked in the R&D department of TDK EPC, Austria. He held visiting research positions at Penn State University, Warwick University, Max Planck Institute, and Complutense University of Madrid, UFPeL. His research focuses on advanced ceramics for electronics, including actuators, energy storage devices, temperature sensors and wireless communication resonators. He is an Associate Editor for the Journal of the American Ceramic Society and the Journal of Electronic Materials and Editor for the International Journal of Applied Ceramic Technology.
Prof. Di Zhou is a professor at the School of Electronic and Information Engineering at Xi’an Jiaotong University (China). He received his Ph.D. degree in Electronic Science and Technology from Xi’an Jiaotong University in 2009 under the guidance of Prof. Dr Xi Yao and won the National Excellent Ph.D. Thesis Award nomination. He was a joint Ph.D. student at the Materials Research Institute. Pennsylvania State University from 2008 to 2009 and a research associate from 2015 tp 2018 at the Department of Materials Science and Engineering, University of Sheffield. He is an Associate Editor of the Journal of the American Ceramic Society and International Journal of Applied Ceramic Technology, Editorial Board Member of Materials Research Bulletin and Journal of Advanced Dielectrics, and Editorial Advisory Board member of ACS Applied Materials & Interfaces. His research interests include microwave dielectric materials, low temperature cofired ceramics technology (LTCC), energy storage capacitor materials, microwave absorption materials, and functional composite materials for high frequency communication/energy storage/microwave absorption.
Prof Dawei Wang is a Professor at Shenzhen Institute of Advanced Electronic Materials, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. Previously, he was a Research Associate at the Department of Materials Science and Engineering of the University of Sheffield (2014–2020) and a visiting scholar at the Materials Research Institute of Pennsylvania State University (2016). He received his Ph.D. degree in Materials Processing Engineering from Beijing Institute of Technology in 2012. He is an associate editor for Journal of American Ceramic Society and Frontiers in Materials. His research focuses on the advanced electronic ceramics for energy storage/conversion/harvesting and translation of new materials to prototype devices/components for electronic systems.
Prof Shujun Zhang is a Professor at ISEM/AIIM of UOW, prior to which, he was a Professor at MatSE Department and Senior Scientist at MRI of Pennsylvania State University. He is the Associate EIC of IEEE Transaction UFFC and Associate Editor of Science Bulletin, Journal ACerS, Journal Electronic Materials, and section EIC of Crystals. He is a fellow of IEEE and ACerS, elected AdCom member of IEEE-UFFC. He is now focusing on fabrication microstructure–property-performance relationship of functional materials for piezoelectric sensors, transducers, and energy storage/harvesting applications.
Prof. Ian M. Reaney FIMM FRMS holds the Dyson Chair in Ceramics and leads the Functional Materials & Devices Group in the Department of Materials Science & Engineering, University of Sheffield. He is a Fellow of the Royal Microscopical Society and the Institute of Materials, Minerals and Mining (IOM3). He has won numerous awards including the Verulam Medal (2017) in Ceramics from IOM3 and was recently elected to the World Academy of Ceramics. He is an Adjunct Professor at Pennsylvania State University (PSU) and European site director of the Centre for Dielectrics and Piezoelectrics in partnership with PSU and North Carolina State University.
Author Contributions
‡ G.W., Z.L., and Y. L. contributed equally to this work.
The authors declare no competing financial interest.
References
- Report of the Secretary-General on the 2019 Climate Action Summit and the Way Forward in 2020. Climate Action Summit 2019.
- Ibn-Mohammed T.; Randall C. A.; Mustapha K. B.; Guo J.; Walker J.; Berbano S.; Koh S. C. L.; Wang D.; Sinclair D. C.; Reaney I. M. Decarbonising Ceramic Manufacturing: A Techno-Economic Analysis of Energy Efficient Sintering Technologies in the Functional Materials Sector. J. Eur. Ceram. Soc. 2019, 39, 5213–5235. 10.1016/j.jeurceramsoc.2019.08.011. [DOI] [Google Scholar]
- Yao Z.; Song Z.; Hao H.; Yu Z.; Cao M.; Zhang S.; Lanagan M. T.; Liu H. Homogeneous/Inhomogeneous-Structured Dielectrics and Their Energy-Storage Performances. Adv. Mater. 2017, 29, 1601727. 10.1002/adma.201601727. [DOI] [PubMed] [Google Scholar]
- Sherrill S. A.; Banerjee P.; Rubloff G. W.; Lee S. B. High to Ultra-High Power Electrical Energy Storage. Phys. Chem. Chem. Phys. 2011, 13, 20714–20723. 10.1039/c1cp22659b. [DOI] [PubMed] [Google Scholar]
- Liu C.; Li F.; Ma L.-P.; Cheng H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. 10.1002/adma.200903328. [DOI] [PubMed] [Google Scholar]
- Liu C.; Yu Z.; Neff D.; Zhamu A.; Jang B. Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. 10.1021/nl102661q. [DOI] [PubMed] [Google Scholar]
- Snook G. A.; Kao P.; Best A. S. Conducting-Polymer-Based Supercapacitor Devices and Electrodes. J. Power Sources 2011, 196, 1–12. 10.1016/j.jpowsour.2010.06.084. [DOI] [Google Scholar]
- Zhu Y.; Murali S.; Stoller M. D.; Ganesh K. J.; Cai W.; Ferreira P. J.; Pirkle A.; Wallace R. M.; Cychosz K. A.; Thommes M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. 10.1126/science.1200770. [DOI] [PubMed] [Google Scholar]
- Wang W.; Jiang B.; Xiong W.; Sun H.; Lin Z.; Hu L.; Tu J.; Hou J.; Zhu H.; Jiao S. A New Cathode Material for Super-Valent Battery Based on Aluminium Ion Intercalation and Deintercalation. Sci. Rep. 2013, 3, 3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Wang W.; Yu Z.; Yuan Y.; Wang S.; Jiao S. A New Aluminium-Ion Battery with High Voltage, High Safety and Low Cost. Chem. Commun. 2015, 51, 11892–11895. 10.1039/C5CC00542F. [DOI] [PubMed] [Google Scholar]
- Wang S.; Yu Z.; Tu J.; Wang J.; Tian D.; Liu Y.; Jiao S. A Novel Aluminum-Ion Battery: Al/AlCl3-[Emim]Cl/Ni3S2@Graphene. Adv. Energy Mater. 2016, 6, 1600137. 10.1002/aenm.201600137. [DOI] [Google Scholar]
- Jiao H.; Wang C.; Tu J.; Tian D.; Jiao S. A Rechargeable Al-Ion Battery: Al/Molten AlCl3-Urea/Graphite. Chem. Commun. 2017, 53, 2331–2334. 10.1039/C6CC09825H. [DOI] [PubMed] [Google Scholar]
- Song Y.; Jiao S.; Tu J.; Wang J.; Liu Y.; Jiao H.; Mao X.; Guo Z.; Fray D. J. A Long-Life Rechargeable Al Ion Battery Based on Molten Salts. J. Mater. Chem. A 2017, 5, 1282–1291. 10.1039/C6TA09829K. [DOI] [Google Scholar]
- Wang S.; Jiao S.; Wang J.; Chen H.-S.; Tian D.; Lei H.; Fang D.-N. High-Performance Aluminum-Ion Battery with Cus@C Microsphere Composite Cathode. ACS Nano 2017, 11, 469–477. 10.1021/acsnano.6b06446. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Jiao S.; Li S.; Chen X.; Song W.-L.; Teng T.; Tu J.; Chen H.-S.; Zhang G.; Fang D.-N. Flexible Stable Solid-State Al-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1806799. 10.1002/adfm.201806799. [DOI] [Google Scholar]
- Zhang X.; Jiao S.; Tu J.; Song W.-L.; Xiao X.; Li S.; Wang M.; Lei H.; Tian D.; Chen H.; et al. Rechargeable Ultrahigh-Capacity Tellurium-Aluminum Batteries. Energy Environ. Sci. 2019, 12, 1918–1927. 10.1039/C9EE00862D. [DOI] [Google Scholar]
- Kishi H.; Mizuno Y.; Chazono H. Base-Metal Electrode-Multilayer Ceramic Capacitors: Past, Present and Future Perspectives. Jpn. J. Appl. Phys. 2003, 42, 1–15. 10.1143/JJAP.42.1. [DOI] [Google Scholar]
- Pan M.; Randall C. A. A Brief Introduction to Ceramic Capacitors. IEEE Electrical Insulation Magazine 2010, 26, 44–50. 10.1109/MEI.2010.5482787. [DOI] [Google Scholar]
- Wang D.; Zhou D.; Song K.; Feteira A.; Randall C. A.; Reaney I. M. Cold-Sintered C0G Multilayer Ceramic Capacitors. Adv. Electron. Mater. 2019, 5, 1900025. 10.1002/aelm.201900025. [DOI] [Google Scholar]
- Sakabe Y. Multilayer Ceramic Capacitors. Curr. Opin. Solid State Mater. Sci. 1997, 2, 584–587. 10.1016/S1359-0286(97)80049-6. [DOI] [Google Scholar]
- Yamamatsu J.; Kawano N.; Arashi T.; Sato A.; Nakano Y.; Nomura T. Reliability of Multilayer Ceramic Capacitors with Nickel Electrodes. J. Power Sources 1996, 60, 199–203. 10.1016/S0378-7753(96)80011-5. [DOI] [Google Scholar]
- Saito H.; Chazono H.; Kishi H.; Yamaoka N. X7R Multilayer Ceramic Capacitors with Nickel Electrodes. Jpn. J. Appl. Phys. 1991, 30, 2307–2310. 10.1143/JJAP.30.2307. [DOI] [Google Scholar]
- Christen T.; Carlen M. W. Theory of Ragone Plots. J. Power Sources 2000, 91, 210–216. 10.1016/S0378-7753(00)00474-2. [DOI] [Google Scholar]
- Yano M.; Uchida R. History of Power Electronics in Japan. IEEJ. Transactions on Fundamentals and Materials 2001, 121, 2–10. 10.1541/ieejfms1990.121.1_2. [DOI] [Google Scholar]
- Whittingham M. S. Materials Challenges Facing Electrical Energy Storage. MRS Bull. 2008, 33, 411–419. 10.1557/mrs2008.82. [DOI] [Google Scholar]
- Li Q.; Liu F.; Yang T.; Gadinski M. R.; Zhang G.; Chen L.-Q.; Wang Q. Sandwich-Structured Polymer Nanocomposites with High Energy Density and Great Charge-Discharge Efficiency at Elevated Temperatures. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 9995. 10.1073/pnas.1603792113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Physical Laboratory . New Capacitors to Improve Electric Vehicles. Phys. Org. 2013. [Google Scholar]
- Jaffe B. Antiferroelectric Ceramics with Field-Enforced Transitions: A New Nonlinear Circuit Element. Proc. IRE 1961, 49, 1264–1267. 10.1109/JRPROC.1961.287917. [DOI] [Google Scholar]
- Burn I.; Smyth D. M. Energy Storage in Ceramic Dielectrics. J. Mater. Sci. 1972, 7, 339–343. 10.1007/BF00555636. [DOI] [Google Scholar]
- Love G. R. Energy Storage in Ceramic Dielectrics. J. Am. Ceram. Soc. 1990, 73, 323–328. 10.1111/j.1151-2916.1990.tb06513.x. [DOI] [Google Scholar]
- Yang L.; Kong X.; Li F.; Hao H.; Cheng Z.; Liu H.; Li J.-F.; Zhang S. Perovskite Lead-Free Dielectrics for Energy Storage Applications. Prog. Mater. Sci. 2019, 102, 72–108. 10.1016/j.pmatsci.2018.12.005. [DOI] [Google Scholar]
- Fletcher N. H.; Hilton A. D.; Ricketts B. W. Optimization of Energy Storage Density in Ceramic Capacitors. J. Phys. D: Appl. Phys. 1996, 29, 253–258. 10.1088/0022-3727/29/1/037. [DOI] [Google Scholar]
- Ogihara H.; Randall C. A.; Trolier-McKinstry S. High-Energy Density Capacitors Utilizing 0.7batio3-0.3bisco3 Ceramics. J. Am. Ceram. Soc. 2009, 92, 1719–1724. 10.1111/j.1551-2916.2009.03104.x. [DOI] [Google Scholar]
- Wang G.; Li J.; Zhang X.; Fan Z.; Yang F.; Feteira A.; Zhou D.; Sinclair D. C.; Ma T.; Tan X.; et al. Ultrahigh Energy Storage Density Lead-Free Multilayers by Controlled Electrical Homogeneity. Energy Environ. Sci. 2019, 12, 582–588. 10.1039/C8EE03287D. [DOI] [Google Scholar]
- Damjanovic D. Ferroelectric, Dielectric and Piezoelectric Properties of Ferroelectric Thin Films and Ceramics. Rep. Prog. Phys. 1998, 61, 1267–1324. 10.1088/0034-4885/61/9/002. [DOI] [Google Scholar]
- Bokov A. A.; Ye Z. G. Recent Progress in Relaxor Ferroelectrics with Perovskite Structure. J. Mater. Sci. 2006, 41, 31–52. 10.1007/s10853-005-5915-7. [DOI] [Google Scholar]
- Prume K.; Schmitz T.; Tiedke S. In Polar Oxides: Properties, Characterization, and Imaging; Waser R., Böttger U., Tiedke S., Eds.; Wiley, 2004. [Google Scholar]
- Wang L.-M. Relationship between Intrinsic Breakdown Field and Bandgap of Materials. 2006 25th International Conference on Microelectronics 2006, 576–579. 10.1109/ICMEL.2006.1651032. [DOI] [Google Scholar]
- Wang D.; Wang G.; Lu Z.; Al-Jlaihawi Z.; Feteira A. Crystal Structure, Phase Transitions and Photoferroelectric Properties of KNbO3-Based Lead-Free Ferroelectric Ceramics: A Brief Review. Front. Mater. 2020, 7, 91. 10.3389/fmats.2020.00091. [DOI] [Google Scholar]
- Neudeck P. G.; Okojie R. S.; Chen L.-Y. High-Temperature Electronics - a Role for Wide Bandgap Semiconductors?. Proceedings of the IEEE 2002, 90, 1065–1076. 10.1109/JPROC.2002.1021571. [DOI] [Google Scholar]
- Dyalsingh H. M.; Kakalios J. Thermopower and Conductivity Activation Energies in Hydrogenated Amorphous Silicon. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54, 7630–7633. 10.1103/PhysRevB.54.7630. [DOI] [PubMed] [Google Scholar]
- Qi H.; Zuo R.; Xie A.; Tian A.; Fu J.; Zhang Y.; Zhang S. Ultrahigh Energy-Storage Density in NaNbO3-Based Lead-Free Relaxor Antiferroelectric Ceramics with Nanoscale Domains. Adv. Funct. Mater. 2019, 29, 1903877. 10.1002/adfm.201903877. [DOI] [Google Scholar]
- Qi H.; Xie A.; Tian A.; Zuo R. Superior Energy-Storage Capacitors with Simultaneously Giant Energy Density and Efficiency Using Nanodomain Engineered BiFeO3-BaTiO3-NaNbO3 Lead-Free Bulk Ferroelectrics. Adv. Energy Mater. 2020, 10, 1903338. 10.1002/aenm.201903338. [DOI] [Google Scholar]
- Irvine J. T. S.; Sinclair D. C.; West A. R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. 10.1002/adma.19900020304. [DOI] [Google Scholar]
- Lu Z.; Wang G.; Bao W.; Li J.; Li L.; Mostaed A.; Yang H.; Ji H.; Li D.; Feteira A.; et al. Superior Energy Density through Tailored Dopant Strategies in Multilayer Ceramic Capacitors. Energy Environ. Sci. 2020, 13, 2938–2948. 10.1039/D0EE02104K. [DOI] [Google Scholar]
- Morrison F. D.; Sinclair D. C.; West A. R. Doping Mechanisms and Electrical Properties of La-Doped BaTiO3 Ceramics. Int. J. Inorg. Mater. 2001, 3, 1205–1210. 10.1016/S1466-6049(01)00128-3. [DOI] [Google Scholar]
- Li M.; Pietrowski M. J.; De Souza R. A.; Zhang H.; Reaney I. M.; Cook S. N.; Kilner J. A.; Sinclair D. C. A Family of Oxide Ion Conductors Based on the Ferroelectric Perovskite Na0.5Bi0.5TiO3. Nat. Mater. 2014, 13, 31–35. 10.1038/nmat3782. [DOI] [PubMed] [Google Scholar]
- Sinclair D. C.; West A. R. Impedance and Modulus Spectroscopy of Semiconducting BaTiO3 Showing Positive Temperature Coefficient of Resistance. J. Appl. Phys. 1989, 66, 3850–3856. 10.1063/1.344049. [DOI] [Google Scholar]
- Adams T. B.; Sinclair D. C.; West A. R. Influence of Processing Conditions on the Electrical Properties of CaCu3Ti4O12 Ceramics. J. Am. Ceram. Soc. 2006, 89, 3129–3135. 10.1111/j.1551-2916.2006.01184.x. [DOI] [Google Scholar]
- Yang F.; Li M.; Li L.; Wu P.; Pradal-Velázquez E.; Sinclair D. C. Defect Chemistry and Electrical Properties of Sodium Bismuth Titanate Perovskite. J. Mater. Chem. A 2018, 6, 5243–5254. 10.1039/C7TA09245H. [DOI] [Google Scholar]
- Li M.; Li L.; Zang J.; Sinclair D. C. Donor-Doping and Reduced Leakage Current in Nb-Doped Na0.5Bi0.5TiO3. Appl. Phys. Lett. 2015, 106, 102904. 10.1063/1.4914509. [DOI] [Google Scholar]
- Smyth D. M. The Defect Chemistry of Donor-Doped BaTiO3: A Rebuttal. J. Electroceram. 2002, 9, 179–186. 10.1023/A:1023213208904. [DOI] [Google Scholar]
- Chan N. H.; Sharma R. K.; Smyth D. M. Nonstoichiometry in Undoped BaTiO3. J. Am. Ceram. Soc. 1981, 64, 556–562. 10.1111/j.1151-2916.1981.tb10325.x. [DOI] [Google Scholar]
- Sumino H.; Sakurai O.; Shinozaki K.; Kato M.; Mizutani N. A New Instrument to Measure the Electrical Properties in Very Narrow Regions in Ceramics. J. Mater. Sci. Lett. 1991, 10, 1026–1028. 10.1007/BF00721835. [DOI] [Google Scholar]
- Dimos D.; Chaudhari P.; Mannhart J.; LeGoues F. K. Orientation Dependence of Grain-Boundary Critical Currents in Yba2 cu3o7-Δ Bicrystals. Phys. Rev. Lett. 1988, 61, 219–222. 10.1103/PhysRevLett.61.219. [DOI] [PubMed] [Google Scholar]
- Sinclair D. C. Characterization of Electro-Materials Using Ac Impedance Spectroscopy. Bol. Soc. Esp. Ceram. Vidrio 1995, 34, 55–65. [Google Scholar]
- Schmidt R.; Sinclair D. C. Anomalous Increase of Dielectric Permittivity in Sr-Doped Ccto Ceramics Ca1-X Srx Cu3 Ti4O12 (0 ≤ X ≤ 0.2). Chem. Mater. 2010, 22, 6–8. 10.1021/cm903220z. [DOI] [Google Scholar]
- Li M.; Feteira A.; Sinclair D. C.; West A. R. Influence of Mn Doping on the Semiconducting Properties of CaCu3Ti4O12 Ceramics. Appl. Phys. Lett. 2006, 88, 232903. 10.1063/1.2200732. [DOI] [Google Scholar]
- Ray S.An Introduction to High Voltage Engineering; Prentice-Hall of India: New Delhi, 2004. [Google Scholar]
- H F. On the Theory of Dielectric Breakdown in Solids. Proc. R Soc. Lond A 1947, 188, 521–532. 10.1098/rspa.1947.0023. [DOI] [Google Scholar]
- Wang J.; Chen X.; Wang J.; Wang G.; Nie H.; Cao F.; Dong X. Improved Dielectric Breakdown Strength of Pb0.99(Zr0.95Ti0.05)0.98Nb0.02O3 Ferroelectric Ceramics with the Addition of Cuo. J. Mater. Sci.: Mater. Electron 2015, 26, 8207–8211. 10.1007/s10854-015-3482-5. [DOI] [Google Scholar]
- Pu Y.; Yao M.; Zhang L.; Chen M. Enhanced Energy Storage Density of 0.55Bi0.5Na0.5TiO3-0.45Ba0.85Ca0.15Ti0.85Zr0.1Sn0.05O3 with MgO Addition. J. Alloys Compd. 2017, 702, 171–177. 10.1016/j.jallcom.2017.01.249. [DOI] [Google Scholar]
- Diao C.; Liu H.; Hao H.; Cao M.; Yao Z. Effect of SiO2 Additive on Dielectric Response and Energy Storage Performance of Ba0.4Sr0.6TiO3 Ceramics. Ceram. Int. 2016, 42, 12639–12643. 10.1016/j.ceramint.2016.04.169. [DOI] [Google Scholar]
- Qi J.; Cao M.; Heath J. P.; Dean J. S.; Hao H.; Yao Z.; Yu Z.; Liu H. Improved Breakdown Strength and Energy Storage Density of a Ce Doped Strontium Titanate Core by Silica Shell Coating. J. Mater. Chem. C 2018, 6, 9130–9139. 10.1039/C8TC03181A. [DOI] [Google Scholar]
- Dong G.; Ma S.; Du J.; Cui J. Dielectric Properties and Energy Storage Density in ZnO-Doped Ba0.3Sr0.7TiO3 Ceramics. Ceram. Int. 2009, 35, 2069–2075. 10.1016/j.ceramint.2008.11.003. [DOI] [Google Scholar]
- Qu B.; Hongliang D.; Yang Z.; Liu Q. Large Recoverable Energy Storage Density and Low Sintering Temperature in Potassium-Sodium Niobate-Based Ceramics for Multilayer Pulsed Power Capacitors. J. Am. Ceram. Soc. 2017, 100, 1517–1526. 10.1111/jace.14728. [DOI] [Google Scholar]
- Yang L.; Kong X.; Cheng Z.; Zhang S. Enhanced Energy Storage Performance of Sodium Niobate-Based Relaxor Dielectrics by a Ramp-to-Spike Sintering Profile. ACS Appl. Mater. Interfaces 2020, 12, 32834–32841. 10.1021/acsami.0c08737. [DOI] [PubMed] [Google Scholar]
- Zhan D.; Xu Q.; Huang D.-P.; Liu H.-X.; Chen W.; Zhang F. Contributions of Intrinsic and Extrinsic Polarization Species to Energy Storage Properties of Ba0.95Ca0.05Zr0.2Ti0.8O3 Ceramics. J. Phys. Chem. Solids 2018, 114, 220–227. 10.1016/j.jpcs.2017.10.038. [DOI] [Google Scholar]
- Xu Q.; Xie J.; He Z.; Zhang L.; Cao M.; Huang X.; Lanagan M. T.; Hao H.; Yao Z.; Liu H. Energy-Storage Properties of Bi0.5Na0.5TiO3-BaTiO3-KNbO3 Ceramics Fabricated by Wet-Chemical Method. J. Eur. Ceram. Soc. 2017, 37, 99–106. 10.1016/j.jeurceramsoc.2016.07.011. [DOI] [Google Scholar]
- Huang Y. H.; Wu Y. J.; Li J.; Liu B.; Chen X. M. Enhanced Energy Storage Properties of Barium Strontium Titanate Ceramics Prepared by Sol-Gel Method and Spark Plasma Sintering. J. Alloys Compd. 2017, 701, 439–446. 10.1016/j.jallcom.2017.01.150. [DOI] [Google Scholar]
- Luo H.; Ke H.; Zhang H.; Zhang L.; Li F.; Cao L.; Jia D.; Zhou Y. Enhanced Ferroelectric and Energy-Storage Properties of Nb-Doped 0.94Na0.5Bi0.5TiO3-0.06BaTiO3 Ceramics Prepared by a Multi-Ionic Sol-Gel Method. Phys. B 2019, 567, 17–24. 10.1016/j.physb.2019.05.008. [DOI] [Google Scholar]
- Li C.; Yao M.; Gao W.; Yao X. High Breakdown Strength and Energy Density in Antiferroelectric PLZST Ceramics with Al2O3 Buffer. Ceram. Int. 2020, 46, 722–730. 10.1016/j.ceramint.2019.09.025. [DOI] [Google Scholar]
- Song Z.; Zhang S.; Liu H.; Hao H.; Cao M.; Li Q.; Wang Q.; Yao Z.; Wang Z.; Lanagan M. T. Improved Energy Storage Properties Accompanied by Enhanced Interface Polarization in Annealed Microwave-Sintered BST. J. Am. Ceram. Soc. 2015, 98, 3212–3222. 10.1111/jace.13741. [DOI] [Google Scholar]
- Cui C.; Pu Y. Improvement of Energy Storage Density with Trace Amounts of ZrO2 Additives Fabricated by Wet-Chemical Method. J. Alloys Compd. 2018, 747, 495–504. 10.1016/j.jallcom.2018.03.058. [DOI] [Google Scholar]
- Jin Q.; Pu Y.; Wang C.; Gao Z.; Wang Y.; Zheng H.; Yao M. Microstructure, Dielectric Properties and Energy Storage Performance of Ba0.4Sr0.6TiO3 Ceramics Prepared by Hydrothermal Method and Microwave Sintering. Mater. Lett. 2017, 188, 159–161. 10.1016/j.matlet.2016.11.032. [DOI] [Google Scholar]
- Ma J.-P.; Chen X.-M.; Ouyang W.-Q.; Wang J.; Li H.; Fang J.-L. Microstructure, Dielectric, and Energy Storage Properties of BaTiO3 Ceramics Prepared Via Cold Sintering. Ceram. Int. 2018, 44, 4436–4441. 10.1016/j.ceramint.2017.12.044. [DOI] [Google Scholar]
- Xie J.; Yao M.; Gao W.; Su Z.; Yao X. Ultrahigh Breakdown Strength and Energy Density in PLZST@ Pbsazm Antiferroelectric Ceramics Based on Core-Shell Structure. J. Eur. Ceram. Soc. 2019, 39, 1050–1056. 10.1016/j.jeurceramsoc.2018.12.044. [DOI] [Google Scholar]
- Zhou H. Y.; Liu X. Q.; Zhu X. L.; Chen X. M. Catio3 Linear Dielectric Ceramics with Greatly Enhanced Dielectric Strength and Energy Storage Density. J. Am. Ceram. Soc. 2018, 101, 1999–2008. 10.1111/jace.15371. [DOI] [Google Scholar]
- Liu B.; Wang X.; Zhao Q.; Li L. Improved Energy Storage Properties of Fine-Crystalline BaTiO3 Ceramics by Coating Powders with Al2O3 and SiO2. J. Am. Ceram. Soc. 2015, 98, 2641–2646. 10.1111/jace.13614. [DOI] [Google Scholar]
- Zhao Q.; Wang X.; Gong H.; Liu B.; Luo B.; Li L. The Properties of Al2O3 Coated Fine-Grain Temperature Stable BaTiO3-Based Ceramics Sintered in Reducing Atmosphere. J. Am. Ceram. Soc. 2018, 101, 1245–1254. 10.1111/jace.15287. [DOI] [Google Scholar]
- Lai X.; Hao H.; Liu Z.; Li S.; Liu Y.; Emmanuel M.; Yao Z.; Cao M.; Wang D.; Liu H. Structure and Dielectric Properties of MgO-Coated BaTiO3 Ceramics. J. Mater. Sci.: Mater. Electron. 2020, 31, 8963–8970. 10.1007/s10854-020-03430-7. [DOI] [Google Scholar]
- Tunkasiri T.; Rujijanagul G. Dielectric Strength of Fine Grained Barium Titanate Ceramics. J. Mater. Sci. Lett. 1996, 15, 1767–1769. 10.1007/BF00275336. [DOI] [Google Scholar]
- Lee H. Y.; Cho K. H.; Nam H.-D. Grain Size and Temperature Dependence of Electrical Breakdown in BaTiO3 Ceramic. Ferroelectrics 2006, 334, 165–169. 10.1080/00150190600694415. [DOI] [Google Scholar]
- Liu B.; Wang X.; Zhang R.; Li L. Grain Size Effect and Microstructure Influence on the Energy Storage Properties of Fine-Grained BaTiO3-Based Ceramics. J. Am. Ceram. Soc. 2017, 100, 3599–3607. 10.1111/jace.14802. [DOI] [Google Scholar]
- Waser R. Tri4: The Role of Grain Boundaries in Conduction and Breakdown of Perovskite-Type Titanates. Ferroelectrics 1992, 133, 109–114. 10.1080/00150199208217984. [DOI] [Google Scholar]
- Lu X.; Zhang L.; Talebinezhad H.; Tong Y.; Cheng Z. Y. Effects of Cuo Additive on the Dielectric Property and Energy-Storage Performance of BaTiO3-SiO2 Ceramic-Glass Composite. Ceram. Int. 2018, 44, 16977–16983. 10.1016/j.ceramint.2018.06.139. [DOI] [Google Scholar]
- Bian F.; Yan S.; Xu C.; Liu Z.; Chen X.; Mao C.; Cao F.; Bian J.; Wang G.; Dong X. Enhanced Breakdown Strength and Energy Density of Antiferroelectric Pb,La(Zr,Sn,Ti)O3 Ceramic by Forming Core-Shell Structure. J. Eur. Ceram. Soc. 2018, 38, 3170–3176. 10.1016/j.jeurceramsoc.2018.03.028. [DOI] [Google Scholar]
- Xu C.; Su R.; Wang Z.; Wang Y.; Zhang D.; Wang J.; Bian J.; Wu C.; Lou X.; Yang Y. Tuning the Microstructure of BaTiO3@SiO2 Core-Shell Nanoparticles for High Energy Storage Composite Ceramics. J. Alloys Compd. 2019, 784, 173–181. 10.1016/j.jallcom.2019.01.009. [DOI] [Google Scholar]
- Zhao P.; Cai Z.; Chen L.; Wu L.; Huan Y.; Guo L.; Li L.; Wang H.; Wang X. Ultra-High Energy Storage Performance in Lead-Free Multilayer Ceramic Capacitors Via a Multiscale Optimization Strategy. Energy Environ. Sci. 2020, 13, 4882–4890. 10.1039/D0EE03094E. [DOI] [Google Scholar]
- Wang D.; Fan Z.; Zhou D.; Khesro A.; Murakami S.; Feteira A.; Zhao Q.; Tan X.; Reaney Ian M. Bismuth Ferrite-Based Lead-Free Ceramics and Multilayers with High Recoverable Energy Density. J. Mater. Chem. A 2018, 6, 4133–4144. 10.1039/C7TA09857J. [DOI] [Google Scholar]
- Shao T.; Du H.; Ma H.; Qu S.; Wang J.; Wang J.; Wei X.; Xu Z. Potassium-Sodium Niobate Based Lead-Free Ceramics: Novel Electrical Energy Storage Materials. J. Mater. Chem. A 2017, 5, 554–563. 10.1039/C6TA07803F. [DOI] [Google Scholar]
- Yang Z.; Du H.; Qu S.; Hou Y.; Ma H.; Wang J.; Wang J.; Wei X.; Xu Z. Significantly Enhanced Recoverable Energy Storage Density in Potassium-Sodium Niobate-Based Lead Free Ceramics. J. Mater. Chem. A 2016, 4, 13778–13785. 10.1039/C6TA04107H. [DOI] [Google Scholar]
- Dale G.; Strawhorne M.; Sinclair D. C.; Dean J. S. Finite Element Modeling on the Effect of Intra-Granular Porosity on the Dielectric Properties of BaTiO3 Mlccs. J. Am. Ceram. Soc. 2018, 101, 1211–1220. 10.1111/jace.15261. [DOI] [Google Scholar]
- Heath J. P.; Dean J. S.; Harding J. H.; Sinclair D. C. Simulation of Impedance Spectra for Core-Shell Grain Structures Using Finite element Modeling. J. Am. Ceram. Soc. 2015, 98, 1925–1931. 10.1111/jace.13533. [DOI] [Google Scholar]
- Heath J. P.; Harding J. H.; Sinclair D. C.; Dean J. S. The Analysis of Impedance Spectra for Core-Shell Microstructures: Why a Multiformalism Approach Is Essential. Adv. Funct. Mater. 2019, 29, 1904036. 10.1002/adfm.201904036. [DOI] [Google Scholar]
- Morshead F. H.; Foeller P. Y.; Freeman C. L.; Zhang H.; Reaney I. M.; Sinclair D. C.; Dean J. S. How to Extract Reliable Core-Volume Fractions from Core-Shell Polycrystalline Microstructures Using Cross Sectional Tem Micrographs. J. Eur. Ceram. Soc. 2017, 37, 2795–2801. 10.1016/j.jeurceramsoc.2017.03.006. [DOI] [Google Scholar]
- Wu L.; Wang X.; Li L. Enhanced Energy Density in Core-Shell Ferroelectric Ceramics: Modeling and Practical Conclusions. J. Am. Ceram. Soc. 2016, 99, 930–937. 10.1111/jace.14063. [DOI] [Google Scholar]
- Yuan J.; Wang D.-W.; Lin H.-B.; Zhao Q.-L.; Zhang D.-Q.; Cao M.-S. Effect of ZnO Whisker Content on Sinterability and Fracture Behaviour of PZT Peizoelectric Composites. J. Alloys Compd. 2010, 504, 123–128. 10.1016/j.jallcom.2010.05.067. [DOI] [Google Scholar]
- Wang D.-W.; Cao M.-S.; Yuan J.; Zhao Q.-L.; Li H.-B.; Zhang D.-Q.; Agathopoulos S. Enhanced Piezoelectric and Ferroelectric Properties of Nb2O5 Modified Lead Zirconate Titanate-Based Composites. J. Am. Ceram. Soc. 2011, 94, 647–650. 10.1111/j.1551-2916.2010.04309.x. [DOI] [Google Scholar]
- Wang D.; Cao M.; Zhang S. Piezoelectric Ceramics in the PbSnO3-Pb(Mg1/3Nb2/3)O3-PbTiO3 Ternary System. J. Am. Ceram. Soc. 2011, 94, 3690–3693. 10.1111/j.1551-2916.2011.04857.x. [DOI] [Google Scholar]
- Zhao Q. L.; Cao M. S.; Yuan J.; Lu R.; Wang D. W.; Zhang D. Q. Thickness Effect on Electrical Properties of Pb(Zr0.52Ti0.48)O3 Thick Films Embedded with ZnO Nanowhiskers Prepared by a Hybrid Sol-Gel Route. Mater. Lett. 2010, 64, 632–635. 10.1016/j.matlet.2009.12.028. [DOI] [Google Scholar]
- Zhang T. F.; Tang X. G.; Liu Q. X.; Jiang Y. P.; Huang X. X.; Zhou Q. F. Energy-Storage Properties and High-Temperature Dielectric Relaxation Behaviors of Relaxor Ferroelectric Pb(Mg1/3Nb2/3)O3-PbTiO3 Ceramics. J. Phys. D: Appl. Phys. 2016, 49, 095302. 10.1088/0022-3727/49/9/095302. [DOI] [Google Scholar]
- Liu Z.; Lu T.; Ye J.; Wang G.; Dong X.; Withers R.; Liu Y. Antiferroelectrics for Energy Storage Applications: A Review. Advanced Materials Technologies 2018, 3, 1800111. 10.1002/admt.201800111. [DOI] [Google Scholar]
- Li Y.; Sun N.; Du J.; Li X.; Hao X. Stable Energy Density of a PMN-PST Ceramic from Room Temperature to Its Curie Point Based on the Synergistic Effect of Diversified Energy. J. Mater. Chem. C 2019, 7, 7692–7699. 10.1039/C9TC02172H. [DOI] [Google Scholar]
- Perumal R. N.; Athikesavan V. Investigations on Electrical and Energy Storage Behaviour of PZN-PT, PMN-PT, PZN-PMN-PT Piezoelectric Solid Solutions. J. Mater. Sci.: Mater. Electron. 2019, 30, 902–913. 10.1007/s10854-018-0361-x. [DOI] [Google Scholar]
- Zhang T.-F.; Tang X.-G.; Huang X.-X.; Liu Q.-X.; Jiang Y.-P.; Zhou Q.-F. High-Temperature Dielectric Relaxation Behaviors of Relaxer-Like PbZrO3-SrTiO3 Ceramics for Energy-Storage Applications. Energy Technology 2016, 4, 633–640. 10.1002/ente.201500436. [DOI] [Google Scholar]
- Bikyashev E. A.; Ryush I. O.; Reshetnikova E. A. Structures of Pb1-X Lax[Zr0.9 Mg(0.1+X)/3 Nb(0.2-X)/3]O3 Solid Solutions, Electrostriction and Energy Storage Characteristics of a New Antiferroelectric Phase with Disturbed Translational Symmetry. Ceram. Int. 2017, 43, 1429–1436. 10.1016/j.ceramint.2016.10.109. [DOI] [Google Scholar]
- Chao M.; Liu J.; Zeng M.; Wang D.; Yu H.; Yuan Y.; Zhang S. High Discharge Efficiency of (Sr,Pb,Bi)TiO3 Relaxor Ceramics for Energy-Storage Application. Appl. Phys. Lett. 2018, 112, 203903. 10.1063/1.5009949. [DOI] [Google Scholar]
- Qi X.; Zhao Y.; Sun E.; Du J.; Li K.; Sun Y.; Yang B.; Zhang R.; Cao W. Large Electrostrictive Effect and High Energy Storage Performance of Pr3+-Doped Pin-PMN-Pt Multifunctional Ceramics in the Ergodic Relaxor Phase. J. Eur. Ceram. Soc. 2019, 39, 4060–4069. 10.1016/j.jeurceramsoc.2019.05.045. [DOI] [Google Scholar]
- Shkuratov S. I.; Baird J.; Antipov V. G.; Hackenberger W.; Luo J.; Zhang S.; Lynch C. S.; Chase J. B.; Jo H. R.; Roberts C. C. Complete Stress-Induced Depolarization of Relaxor Ferroelectric Crystals without Transition through a Non-Polar Phase. Appl. Phys. Lett. 2018, 112, 122903. 10.1063/1.5019593. [DOI] [Google Scholar]
- Li Y.; Sun N.; Li X.; Du J.; Chen L.; Gao H.; Hao X.; Cao M. Multiple Electrical Response and Enhanced Energy Storage Induced by Unusual Coexistent-Phase Structure in Relaxor Ferroelectric Ceramics. Acta Mater. 2018, 146, 202–210. 10.1016/j.actamat.2017.12.048. [DOI] [Google Scholar]
- Jo H. R.; Lynch C. S. A High Energy Density Relaxor Antiferroelectric Pulsed Capacitor Dielectric. J. Appl. Phys. 2016, 119, 024104. 10.1063/1.4939617. [DOI] [Google Scholar]
- Gao J.; Liu Y.; Wang Y.; Wang D.; Zhong L.; Ren X. High Temperature-Stability of (Pb0.9 La0.1)(Zr0.65 Ti0.35)O3 Ceramic for Energy-Storage Applications at Finite Electric Field Strength. Scr. Mater. 2017, 137, 114–118. 10.1016/j.scriptamat.2017.05.011. [DOI] [Google Scholar]
- Li B.; Liu Q.; Tang X.; Zhang T.; Jiang Y.; Li W.; Luo J. High Energy Storage Density and Impedance Response of PLZT2/95/5 Antiferroelectric Ceramics. Materials 2017, 10, 143. 10.3390/ma10020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao P.; Zhang Y.; Chen X.; Zhou M.; Yan S.; Dong X.; Wang G. Enhanced Energy Storage Properties and Stability in (Pb0.895 La0.07)(Zrx Ti1-X)O3 Antiferroelectric Ceramics. Ceram. Int. 2019, 45, 15898–15905. 10.1016/j.ceramint.2019.05.096. [DOI] [Google Scholar]
- Chen S.; Wang X.; Yang T.; Wang J. Composition-Dependent Dielectric Properties and Energy Storage Performance of (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics. J. Electroceram. 2014, 32, 307–310. 10.1007/s10832-014-9900-x. [DOI] [Google Scholar]
- Zhuo F.; Qiang L.; Li Y.; Gao J.; Yan Q.; Zhang Y.; Chu X.; Cao W. Effect of a-Site La3+ Modified on Dielectric and Energy Storage Properties in Lead Zironate Stannate Titanate Ceramics. Mater. Res. Express 2014, 1, 045501. 10.1088/2053-1591/1/4/045501. [DOI] [Google Scholar]
- Wang X.; Shen J.; Yang T.; Dong Y.; Liu Y. High Energy-Storage Performance and Dielectric Properties of Antiferroelectric (Pb0.97 La0.02) (Zr0.5 Sn0.5-X Tix)O3 Ceramic. J. Alloys Compd. 2016, 655, 309–313. 10.1016/j.jallcom.2015.09.167. [DOI] [Google Scholar]
- Xu R.; Xu Z.; Feng Y.; Wei X.; Tian J.; Huang D. Evaluation of Discharge Energy Density of Antiferroelectric Ceramics for Pulse Capacitors. Appl. Phys. Lett. 2016, 109, 032903. 10.1063/1.4959139. [DOI] [Google Scholar]
- Liu Z.; Dong X.; Liu Y.; Cao F.; Wang G. Electric Field Tunable Thermal Stability of Energy Storage Properties of PLZST Antiferroelectric Ceramics. J. Am. Ceram. Soc. 2017, 100, 1–5. 10.1111/jace.14867. [DOI] [Google Scholar]
- Shen J.; Wang X.; Yang T.; Wang H.; Wei J. High Discharge Energy Density and Fast Release Speed of (Pb, La)(Zr, Sn, Ti)O3 Antiferroelectric Ceramics for Pulsed Capacitors. J. Alloys Compd. 2017, 721, 191–198. 10.1016/j.jallcom.2017.05.325. [DOI] [Google Scholar]
- xu R.; Tian J.; Zhu Q.; Zhao T.; Feng Y.; Wei X.; Xu Z. Effects of La-Induced Phase Transition on Energy Storage and Discharge Properties of PLZST Ferroelectric/Antiferroelectric Ceramics. Ceram. Int. 2017, 43, 13918–13923. 10.1016/j.ceramint.2017.07.120. [DOI] [Google Scholar]
- Zhang Q.; Dan Y.; Chen J.; Lu Y.; Yang T.; Yao X.; He Y. Effects of Composition and Temperature on Energy Storage Properties of (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics. Ceram. Int. 2017, 43, 11428–11432. 10.1016/j.ceramint.2017.06.005. [DOI] [Google Scholar]
- Dan Y.; Xu H.; Zou K.; Zhang Q.; Lu Y.; Chang G.; Huang H.; He Y. Energy Storage Characteristics of (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics with High Sn Content. Appl. Phys. Lett. 2018, 113, 063902. 10.1063/1.5044712. [DOI] [Google Scholar]
- Dan Y.; Xu H.; Zhang Y.; Zou K.; Zhang Q.; Lu Y.; Chang G.; Zhang Q.; He Y. High-Energy Density of Pb0.97La0.02(Zr0.50Sn0.45Ti0.05)O3 Antiferroelectric Ceramics Prepared by Sol-Gel Method with Low-Cost Dibutyltin Oxide. J. Am. Ceram. Soc. 2019, 102, 1776–1783. 10.1111/jace.16041. [DOI] [Google Scholar]
- Dan Y.; Zou K.; Chen G.; Yu Y.; Zhang Y.; Zhang Q.; Lu Y.; Zhang Q.; He Y. Superior Energy-Storage Properties in (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics with Appropriate La Content. Ceram. Int. 2019, 45, 11375–11381. 10.1016/j.ceramint.2019.03.001. [DOI] [Google Scholar]
- Yu Y.; Zhang Y.; Zou K.; Chen G.; Zhang Y.; Li H.; Lu Y.; Zhang Q.; He Y. High Energy Density and Efficiency in (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics with High La3+ Content and Optimized Sn4+ Content. Ceram. Int. 2019, 45, 24419–24424. 10.1016/j.ceramint.2019.08.164. [DOI] [Google Scholar]
- Zhao Q.; Lei H.; He G.; Di J.; Wang D.; Tan P.; Jin H.; Cao M. Effects of Thickness on Energy Storage of (Pb, La)(Zr, Sn, Ti) O3 Antiferroelectric Films Deposited on Lanio3 Electrodes. Ceram. Int. 2016, 42, 1314–1317. 10.1016/j.ceramint.2015.09.067. [DOI] [Google Scholar]
- Zhao Q.; Lei H.; He G.; Di J.; Wang D.; Tan P.; Jin H.; Cao M. Effects of Thickness on Energy Storage of (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Films Deposited on Lanio3 Electrodes. Ceram. Int. 2016, 42, 1314–1317. 10.1016/j.ceramint.2015.09.067. [DOI] [Google Scholar]
- Liu X.; Li Y.; Hao X. Ultra-High Energy-Storage Density and Fast Discharge Speed of (Pb0.98-X La0.02 Srx)(Zr0.9 Sn0.1)0.995O3 Antiferroelectric Ceramics Prepared Via the Tape-Casting Method. J. Mater. Chem. A 2019, 7, 11858–11866. 10.1039/C9TA02149C. [DOI] [Google Scholar]
- Wang H.; Liu Y.; Yang T.; Zhang S. Ultrahigh Energy-Storage Density in Antiferroelectric Ceramics with Field-Induced Multiphase Transitions. Adv. Funct. Mater. 2019, 29, 1807321. 10.1002/adfm.201807321. [DOI] [Google Scholar]
- Yang X.; Zhuo F.; Wang C.; Liu Y.; Wang Z.; Tailor H.; He C.; Long X. High Energy Storage Density and Ultrafast Discharge in Lead Lutetium Niobate Based Ceramics. J. Mater. Chem. A 2019, 7, 8414–8422. 10.1039/C9TA00463G. [DOI] [Google Scholar]
- Wei J.; Yang T.; Wang H. Excellent Energy Storage and Charge-Discharge Performances in PbHfO3 Antiferroelectric Ceramics. J. Eur. Ceram. Soc. 2019, 39, 624–630. 10.1016/j.jeurceramsoc.2018.09.039. [DOI] [Google Scholar]
- Gao P.; Liu Z.; Zhang N.; Wu H.; Bokov A. A.; Ren W.; Ye Z.-G. New Antiferroelectric Perovskite System with Ultrahigh Energy-Storage Performance at Low Electric Field. Chem. Mater. 2019, 31, 979–990. 10.1021/acs.chemmater.8b04470. [DOI] [Google Scholar]
- Bao Y.; Zhou M.; Yan S.; Cao F.; Dong X.; Wang G. Novel Complex B-Site Lead Oxide Antiferroelectric System Developed by Compositional Design for Dielectric Energy Storage. J. Eur. Ceram. Soc. 2019, 39, 4785–4793. 10.1016/j.jeurceramsoc.2019.07.037. [DOI] [Google Scholar]
- Xu L.; He C.; Yang X.; Wang Z.; Li X.; Tailor H. N.; Long X. Composition Dependent Structure, Dielectric and Energy Storage Properties of Pb(Tm1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3 Antiferroelectric Ceramics. J. Eur. Ceram. Soc. 2017, 37, 3329–3334. 10.1016/j.jeurceramsoc.2017.04.005. [DOI] [Google Scholar]
- Yang X.; Liu Y.; He C.; Tailor H.; Long X. La-Modified Pb(Lu1/2Nb1/2)O3 Antiferroelectric Ceramics with High Energy Storage Density. J. Eur. Ceram. Soc. 2015, 35, 4173–4180. 10.1016/j.jeurceramsoc.2015.07.027. [DOI] [Google Scholar]
- Chao W.; Yang T.; Li Y. Achieving High Energy Efficiency and Energy Density in PbHfO3-Based Antiferroelectric Ceramics. J. Mater. Chem. C 2020, 8, 17016–17024. 10.1039/D0TC04617E. [DOI] [Google Scholar]
- Hao X.; Zhai J.; Kong L. B.; Xu Z. A Comprehensive Review on the Progress of Lead Zirconate-Based Antiferroelectric Materials. Prog. Mater. Sci. 2014, 63, 1–57. 10.1016/j.pmatsci.2014.01.002. [DOI] [Google Scholar]
- Haertling G. H.; Land C. E. Hot-Pressed (Pb,La)(Zr,Ti)O3 Ferroelectric Ceramics for Electrooptic Applications. J. Am. Ceram. Soc. 1971, 54, 1–11. 10.1111/j.1151-2916.1970.tb12105.x-i1. [DOI] [Google Scholar]
- Li B.; Liu Q.-X.; Tang X.-G.; Zhang T.-F.; Jiang Y.-P.; Li W.-H.; Luo J. Antiferroelectric to Relaxor Ferroelectric Phase Transition in PbO Modified (Pb0.97 La0.02)(Zr0.95 Ti0.05)O3 Ceramics with a Large Energy-Density for Dielectric Energy Storage. RSC Adv. 2017, 7, 43327–43333. 10.1039/C7RA08621K. [DOI] [Google Scholar]
- Niu Z.-H.; Jiang Y.-P.; Tang X.-G.; Liu Q.-X.; Li W.-H. B-Site Non-Stoichiometric (Pb0.97 La0.02)(Zr0.95 Ti0.05)O3 Antiferroelectric Ceramics for Energy Storage. Journal of Asian Ceramic Societies 2018, 6, 240–246. 10.1080/21870764.2018.1501126. [DOI] [Google Scholar]
- Samanta S.; Sankaranarayanan V.; Sethupathi K. Effect of Nb and Fe Co-Doping on Microstructure, Dielectric Response, Ferroelectricity and Energy Storage Density of PLZT. J. Mater. Sci.: Mater. Electron. 2018, 29, 20383–20394. 10.1007/s10854-018-0173-z. [DOI] [Google Scholar]
- Kumar A.; Yoon J.; Thakre A.; Peddigari M.; Jeong D.-Y.; Kong Y.-M.; Ryu J. Dielectric, Ferroelectric, Energy Storage, and Pyroelectric Properties of Mn-Doped (Pb0.93 La0.07)(Zr0.82 Ti0.18)O3 Anti-Ferroelectric Ceramics. Han'guk Seramik Hakhoechi 2019, 56, 412–420. 10.4191/kcers.2019.56.4.10. [DOI] [Google Scholar]
- Qiao P.; Zhang Y.; Chen X.; Zhou M.; Wang G.; Dong X. Effect of Mn-Doping on Dielectric and Energy Storage Properties of (Pb0.91 La0.06)(Zr0.96Ti0.04)O3 Antiferroelectric Ceramics. J. Alloys Compd. 2019, 780, 581–587. 10.1016/j.jallcom.2018.11.371. [DOI] [Google Scholar]
- Ciuchi I. V.; Mitoseriu L.; Galassi C. Antiferroelectric to Ferroelectric Crossover and Energy Storage Properties of (Pb1-X Lax)(Zr0.90 Ti0.10)1-X/4O3 (0.02 ≤ X ≤ 0.04) Ceramics. J. Am. Ceram. Soc. 2016, 99, 2382–2387. 10.1111/jace.14246. [DOI] [Google Scholar]
- Kumar A.; Kim S.; Peddigari M.; Jeong D.-H.; Hwang G.; Ryu J. High Energy Storage Properties and Electrical Field Stability of Energy Efficiency of (Pb0.89 La0.11)(Zr0.70 Ti0.30)0.9725O3 Relaxor Ferroelectric Ceramics. Electron. Mater. Lett. 2019, 15, 323–330. 10.1007/s13391-019-00124-z. [DOI] [Google Scholar]
- Jiang S.; Zhang L.; Zhang G.; Liu S.; Yi J.; Xiong X.; Yu Y.; He J.; Zeng Y. Effect of Zr:Sn Ratio in the Lead Lanthanum Zirconate Stannate Titanate Anti-Ferroelectric Ceramics on Energy Storage Properties. Ceram. Int. 2013, 39, 5571–5575. 10.1016/j.ceramint.2012.12.071. [DOI] [Google Scholar]
- Zhang L.; Jiang S.; Zeng Y.; Fu M.; Han K.; Li Q.; Wang Q.; Zhang G. Y Doping and Grain Size Co-Effects on the Electrical Energy Storage Performance of (Pb0.87 Ba0.1 La0.02) (Zr0.65 Sn0.3 Ti0.05)O3 Anti-Ferroelectric Ceramics. Ceram. Int. 2014, 40, 5455–5460. 10.1016/j.ceramint.2013.10.131. [DOI] [Google Scholar]
- Wang X.; Shen J.; Yang T.; Xiao Z.; Dong Y. Phase Transition and Energy Storage Performance in Ba-Doped PLZST Antiferroelectric Ceramics. J. Mater. Sci.: Mater. Electron. 2015, 26, 9200–9204. 10.1007/s10854-015-3612-0. [DOI] [Google Scholar]
- Zhang G.; Zhu D.; Zhang X.; Zhang L.; Yi J.; Xie B.; Zeng Y.; Li Q.; Wang Q.; Jiang S. High-Energy Storage Performance of (Pb0.87 Ba0.1 La0.02)(Zr0.68 Sn0.24 Ti0.08)O3 Antiferroelectric Ceramics Fabricated by the Hot-Press Sintering Method. J. Am. Ceram. Soc. 2015, 98, 1175–1181. 10.1111/jace.13412. [DOI] [Google Scholar]
- Zhang L.; Jiang S.; Fan B.; Zhang G. High Energy Storage Performance in (Pb0.858 Ba0.1 La0.02Y0.008)(Zr0.65 Sn0.3 Ti0.05)O3-(Pb0.97 La0.02)(Zr0.9 Sn0.05 Ti0.05)O3 Anti-Ferroelectric Composite Ceramics. Ceram. Int. 2015, 41, 1139–1144. 10.1016/j.ceramint.2014.09.041. [DOI] [Google Scholar]
- Zhang Q.; Liu X.; Zhang Y.; Song X.; Zhu J.; Baturin I.; Chen J. Effect of Barium Content on Dielectric and Energy Storage Properties of (Pb,La,Ba)(Zr,Sn,Ti)O3 Ceramics. Ceram. Int. 2015, 41, 3030–3035. 10.1016/j.ceramint.2014.10.139. [DOI] [Google Scholar]
- Xu R.; Xu Z.; Feng Y.; Tian J.; Huang D. Energy Storage and Release Properties of Sr-Doped (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics. Ceram. Int. 2016, 42, 12875–12879. 10.1016/j.ceramint.2016.05.053. [DOI] [Google Scholar]
- Zhang Q.; Chen J.; Lu Y.; Yang T. Q.; Yao X.; He Y. (Pb,Sm)(Zr,Sn,Ti)O3 Multifunctional Ceramics with Large Electric-Field-Induced Strain and High-Energy Storage Density. J. Am. Ceram. Soc. 2016, 99, 3853–3856. 10.1111/jace.14592. [DOI] [Google Scholar]
- Zhang Q.; Tong H.; Chen J.; Lu Y.; Yang T.; Yao X.; He Y. High Recoverable Energy Density over a Wide Temperature Range in Sr Modified (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Ceramics with an Orthorhombic Phase. Appl. Phys. Lett. 2016, 109, 262901. 10.1063/1.4973425. [DOI] [Google Scholar]
- Guo B.; Liu P.; Song Y.; Liu D. Effect of Ti Content on Energy Storage Properties of (Pb0.87 Ba0.10 La0.02)(Zr0.60 Sn0.40-X Tix)O3 Bulk Ceramics. Ferroelectrics 2017, 510, 152–160. 10.1080/00150193.2017.1328256. [DOI] [Google Scholar]
- Liu Z.; Bai Y.; Chen X.; Dong X.; Nie H.; Cao F.; Wang G. Linear Composition-Dependent Phase Transition Behavior and Energy Storage Performance of Tetragonal PLZST Antiferroelectric Ceramics. J. Alloys Compd. 2017, 691, 721–725. 10.1016/j.jallcom.2016.08.328. [DOI] [Google Scholar]
- Zhang G.; Liu P.; Fan B.; Liu H.; Zeng Y.; Qiu S.; Jiang S.; Li Q.; Wang Q.; Liu J. Large Energy Density in Ba Doped Pb0.97La0.02(Zr0.65Sn0.3Ti0.05)O3 Antiferroelectric Ceramics with Improved Temperature Stability. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 744–748. 10.1109/TDEI.2017.006161. [DOI] [Google Scholar]
- Liu P.; Li M.-Y.; Zhang Q.; Li W.; Zhang Y.; Shen M.; Qiu S.; Zhang G.; Jiang S. High Thermal Stability in PLZST Anti-Ferroelectric Energy Storage Ceramics with the Coexistence of Tetragonal and Orthorhombic Phase. J. Eur. Ceram. Soc. 2018, 38, 5396–5401. 10.1016/j.jeurceramsoc.2018.08.003. [DOI] [Google Scholar]
- Liu P.; Zhang Y.; Zhu Y.; Fan B.; Li W.; Zhang H.; Jiang S. Structure Variation and Energy Storage Properties of Acceptor-Modified Pblzst Antiferroelectric Ceramics. J. Am. Ceram. Soc. 2018, 102, 1912–1920. 10.1111/jace.16094. [DOI] [Google Scholar]
- Mohapatra P.; Fan Z.; Cui J.; Tan X. Relaxor Antiferroelectric Ceramics with Ultrahigh Efficiency for Energy Storage Applications. J. Eur. Ceram. Soc. 2019, 39, 4735–4742. 10.1016/j.jeurceramsoc.2019.07.050. [DOI] [Google Scholar]
- Wei M.; Tang Z.; Wang W.; Yu H.; Chen H.; Zhang J.; Zhang W. Enhanced Energy Storage Properties of (1-X)PLZST-Xbiyo3 Ceramics. J. Electron. Mater. 2019, 48, 2162–2167. 10.1007/s11664-019-07004-0. [DOI] [Google Scholar]
- Yu H.; Zhang J.; Wei M.; Huang J.; Chen H.; Yang C. Enhanced Energy Storage Density Performance in (Pb0.97 La0.02)(Zr0.5 Sn0.44 Ti0.06)-BiYO3 Anti-Ferroelectric Composite Ceramics. J. Mater. Sci.: Mater. Electron 2016, 28, 832–838. 10.1007/s10854-016-5597-8. [DOI] [Google Scholar]
- Xu R.; Zhu Q.; Tian J.; Feng Y.; Xu Z. Effect of Ba-Dopant on Dielectric and Energy Storage Properties of PLZST Antiferroelectric Ceramics. Ceram. Int. 2017, 43, 2481–2485. 10.1016/j.ceramint.2016.11.043. [DOI] [Google Scholar]
- Chen S.; Yang T.; Wang J.; Yao X. Effects of Glass Additions on the Dielectric Properties and Energy Storage Performance of Pb0.97La0.02(Zr0.56Sn0.35Ti0.09)O3 Antiferroelectric Ceramics. J. Mater. Sci.: Mater. Electron 2013, 24, 4764–4768. 10.1007/s10854-013-1471-0. [DOI] [Google Scholar]
- Pan W.; Zhang Q.; Bhalla A.; Cross L. E. Field-Forced Antiferroelectric-to-Ferroelectric Switching in Modified Lead Zirconate Titanate Stannate Ceramics. J. Am. Ceram. Soc. 1989, 72, 571–578. 10.1111/j.1151-2916.1989.tb06177.x. [DOI] [Google Scholar]
- Reaney I. M.; Glazounov A.; Chu F.; Bell A.; Setter N. Tem of Antiferroelectric-Ferroelectric Phase Boundary in (Pb1-X Bax)(Zr1-X Tix)O3 Solid Solution. Br. Ceram. Trans. 1997, 96, 217–224. [Google Scholar]
- MacLaren I.; Villaurrutia R.; Peláiz-Barranco A. Domain Structures and Nanostructures in Incommensurate Antiferroelectric Pbx La1-X(Zr0.9 Ti0.1)O3. J. Appl. Phys. 2010, 108, 034109. 10.1063/1.3460106. [DOI] [Google Scholar]
- Villaurrutia R.; MacLaren I.; Peláiz-Barranco A. Study of Incommensurate Phases in Lanthanum-Doped Zirconium-Rich Lead Zirconate Titanate Ceramics. Journal of Physics: Conference Series 2010, 241, 012038. 10.1088/1742-6596/241/1/012038. [DOI] [Google Scholar]
- Bersuker I. B. Pseudo Jahn-Teller Origin of Perovskite Multiferroics, Magnetic-Ferroelectric Crossover, and Magnetoelectric Effects: The D0-D10 Problem. Phys. Rev. Lett. 2012, 108, 137202. 10.1103/PhysRevLett.108.137202. [DOI] [PubMed] [Google Scholar]
- Fu Z.; Chen X.; Li Z.; Hu T.; Zhang L.; Lu P.; Zhang S.; Wang G.; Dong X.; Xu F. Unveiling the Ferrielectric Nature of PbZrO3-Based Antiferroelectric Materials. Nat. Commun. 2020, 11, 3809. 10.1038/s41467-020-17664-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Jiang S.; Fan B.; Zhang G. Enhanced Energy Storage Performance in (Pb0.858 Ba0.1 La0.02Y0.008)(Zr0.65 Sn0.3 Ti0.05)O3-(Pb0.97 La0.02)(Zr0.9 Sn0.05 Ti0.05)O3 Anti-Ferroelectric Composite Ceramics by Spark Plasma Sintering. J. Alloys Compd. 2015, 622, 162–165. 10.1016/j.jallcom.2014.09.171. [DOI] [Google Scholar]
- Ibn-Mohammed T.; Koh S. C. L.; Reaney I. M.; Sinclair D. C.; Mustapha K. B.; Acquaye A.; Wang D. Are Lead-Free Piezoelectrics More Environmentally Friendly?. MRS Commun. 2017, 7, 1–7. 10.1557/mrc.2017.10. [DOI] [Google Scholar]
- Ibn-Mohammed T.; Koh S. C. L.; Reaney I. M.; Acquaye A.; Wang D.; Taylor S.; Genovese A. Integrated Hybrid Life Cycle Assessment and Supply Chain Environmental Profile Evaluations of Lead-Based (Lead Zirconate Titanate) Versus Lead-Free (Potassium Sodium Niobate) Piezoelectric Ceramics. Energy Environ. Sci. 2016, 9, 3495–3520. 10.1039/C6EE02429G. [DOI] [Google Scholar]
- Wang D.; Wang G.; Murakami S.; Fan Z.; Feteira A.; Zhou D.; Sun S.; Zhao Q.; Reaney I. M. BiFeO3-BaTiO3: A New Generation of Lead-Free Electroceramics. J. Adv. Dielectr. 2018, 08, 1830004. 10.1142/S2010135X18300049. [DOI] [Google Scholar]
- Peddigari M.; Palneedi H.; Hwang G.-T.; Ryu J. Linear and Nonlinear Dielectric Ceramics for High-Power Energy Storage Capacitor Applications. Han'guk Seramik Hakhoechi 2019, 56, 1–23. 10.4191/kcers.2019.56.1.02. [DOI] [Google Scholar]
- Sun Z.; Wang Z.; Tian Y.; Wang G.; Wang W.; Yang M.; Wang X.; Zhang F.; Pu Y. Progress, Outlook, and Challenges in Lead-Free Energy-Storage Ferroelectrics. Adv. Electron. Mater. 2020, 6, 1900698. 10.1002/aelm.201900698. [DOI] [Google Scholar]
- Hao X. A Review on the Dielectric Materials for High Energy-Storage Application. J. Adv. Dielectr. 2013, 03, 1330001. 10.1142/S2010135X13300016. [DOI] [Google Scholar]
- Du H.-L.; Yang Z.-T.; Gao F.; Jin L.; Cheng H.-L.; Qu S.-B. Lead-Free Nonlinear Dielectric Ceramics for Energy Storage Applications: Current Status and Challenges. Journal of Inorganic Materials 2018, 33, 1046–1058. 10.15541/jim20170594. [DOI] [Google Scholar]
- Han D.; Wang C.; Lu D.; Hussain F.; Wang D.; Meng F. A Temperature Stable (Ba1-X Cex)(Ti1-X/2 Mgx/2)O3 Lead-Free Ceramic for X4D Capacitors. J. Alloys Compd. 2020, 821, 153480. 10.1016/j.jallcom.2019.153480. [DOI] [Google Scholar]
- Yang H.; Yan F.; Zhang G.; Lin Y.; Wang F. Dielectric Behavior and Impedance Spectroscopy of Lead-Free Ba0.85Ca0.15Zr0.1Ti0.9O3 Ceramics with B2O3-Al2O3-SiO2 Glass-Ceramics Addition for Enhanced Energy Storage. J. Alloys Compd. 2017, 720, 116–125. 10.1016/j.jallcom.2017.05.158. [DOI] [Google Scholar]
- Ren P.; Wang Q.; Li S.; Zhao G. Energy Storage Density and Tunable Dielectric Properties of BaTi0.85Sn0.15O3/MgO Composite Ceramics Prepared by Sps. J. Eur. Ceram. Soc. 2017, 37, 1501–1507. 10.1016/j.jeurceramsoc.2016.12.016. [DOI] [Google Scholar]
- Ma R.; Cui B.; Shangguan M.; Wang S.; Wang Y.; Chang Z.; Wang Y. A Novel Double-Coating Approach to Prepare Fine-Grained BaTiO3@La2O3@SiO2 Dielectric Ceramics for Energy Storage Application. J. Alloys Compd. 2017, 690, 438–445. 10.1016/j.jallcom.2016.08.062. [DOI] [Google Scholar]
- Liu B.; Wu Y.; Huang Y. H.; Song K. X.; Wu Y. J. Enhanced Dielectric Strength and Energy Storage Density in BaTi0.7Zr0.3O3 Ceramics Via Spark Plasma Sintering. J. Mater. Sci. 2019, 54, 4511–4517. 10.1007/s10853-018-3170-y. [DOI] [Google Scholar]
- Wang T.; Wei X.; Hu Q.; Jin L.; Xu Z.; Feng Y. Effects of Znnb2o6 Addition on BaTiO3 Ceramics for Energy Storage. Mater. Sci. Eng., B 2013, 178, 1081–1086. 10.1016/j.mseb.2013.07.003. [DOI] [Google Scholar]
- Zeng M.; Liu J.; Yu H.; Chao M.; Tang B.; Zhang S. NiNb2O6-BaTiO3 Ceramics for Energy-Storage Capacitors. Energy Technology 2018, 6, 899–905. 10.1002/ente.201700461. [DOI] [Google Scholar]
- Hu Q.; Jin L.; Wang T.; Li C.; Xing Z.; Wei X. Dielectric and Temperature Stable Energy Storage Properties of 0.88batio3-0.12bi(Mg1/2 ti1/2)O3 Bulk Ceramics. J. Alloys Compd. 2015, 640, 416–420. 10.1016/j.jallcom.2015.02.225. [DOI] [Google Scholar]
- Zhang Q.; Li Z. Weakly Coupled Relaxor Behavior of BaTiO3-Bi(Mg1/2 Ti1/2)O3 Lead-Free Ceramics. J. Adv. Dielectr. 2013, 03, 1320001. 10.1142/S2010135X13200014. [DOI] [Google Scholar]
- Shen Z.; Wang X.; Luo B.; Li L. BaTiO3-BiYbO3 Perovskite Materials for Energy Storage Applications. J. Mater. Chem. A 2015, 3, 18146–18153. 10.1039/C5TA03614C. [DOI] [Google Scholar]
- Li W.-B.; Zhou D.; Pang L.-X. Enhanced Energy Storage Density by Inducing Defect Dipoles in Lead Free Relaxor Ferroelectric BaTiO3-Based Ceramics. Appl. Phys. Lett. 2017, 110, 132902. 10.1063/1.4979467. [DOI] [Google Scholar]
- Wang T.; Jin L.; Li C.; Hu Q.; Wei X. Relaxor Ferroelectric BaTiO3-Bi(Mg2/3Nb1/3)O3 Ceramics for Energy Storage Application. J. Am. Ceram. Soc. 2015, 98, 559–566. 10.1111/jace.13325. [DOI] [Google Scholar]
- Liu G.; Li Y.; Shi M.; Yu L.; Chen P.; Yu K.; Yan Y.; Jin L.; Wang D.; Gao J. An Investigation of the Dielectric Energy Storage Performance of Bi(Mg2/3Nb1/3)O3-Modifed BaTiO3 Pb-Free Bulk Ceramics with Improved Temperature/Frequency Stability. Ceram. Int. 2019, 45, 19189–19196. 10.1016/j.ceramint.2019.06.166. [DOI] [Google Scholar]
- Yang H.; Yan F.; Lin Y.; Wang T.; Wang F.; Wang Y.; Guo L.; Tai W.; Wei H. Lead-Free BaTiO3-Bi0.5Na0.5TiO3-Na0.73Bi0.09NbO3 Relaxor Ferroelectric Ceramics for High Energy Storage. J. Eur. Ceram. Soc. 2017, 37, 3303–3311. 10.1016/j.jeurceramsoc.2017.03.071. [DOI] [Google Scholar]
- Li W.-B.; Zhou D.; He B.; Li F.; Pang L.-X.; Lu S.-G. Structure and Dielectric Properties of Nd(Zn1/2Ti1/2)O3-BaTiO3 Ceramics for Energy Storage Applications. J. Alloys Compd. 2016, 685, 418–422. 10.1016/j.jallcom.2016.05.311. [DOI] [Google Scholar]
- Wu L.; Wang X.; Li L. Lead-Free BaTiO3-Bi(Zn2/3Nb1/3)O3 Weakly Coupled Relaxor Ferroelectric Materials for Energy Storage. RSC Adv. 2016, 6, 14273–14282. 10.1039/C5RA21261H. [DOI] [Google Scholar]
- Li W.-B.; Zhou D.; Pang L.-X.; Xu R.; Guo H.-H. Novel Barium Titanate Based Capacitors with High Energy Density and Fast Discharge Performance. J. Mater. Chem. A 2017, 5, 19607–19612. 10.1039/C7TA05392D. [DOI] [Google Scholar]
- Li W.-B.; Zhou D.; Xu R.; Wang D.-W.; Su J.-Z.; Pang L.-X.; Liu W.-F.; Chen G.-H. BaTiO3-Based Multilayers with Outstanding Energy Storage Performance for High Temperature Capacitor Applications. ACS Appl. Energy Mater. 2019, 2, 5499–5506. 10.1021/acsaem.9b00664. [DOI] [Google Scholar]
- Yuan Q.; Yao F.; Wang Y.; Ma R.; Wang H. Relaxor Ferroelectric 0.9BaTiO3-0.1Bi(Zn0.5 Zr0.5)O3 Ceramic Capacitors with High Energy Density and Temperature Stable Energy Storage Properties. J. Mater. Chem. C 2017, 5, 9552–9558. 10.1039/C7TC02478A. [DOI] [Google Scholar]
- Zhao X.; Zhou Z.; Liang R.; Liu F.; Dong X. High-Energy Storage Performance in Lead-Free (1-X)BaTiO3-XBi(Zn0.5 Ti0.5)O3 Relaxor Ceramics for Temperature Stability Applications. Ceram. Int. 2017, 43, 9060–9066. 10.1016/j.ceramint.2017.04.051. [DOI] [Google Scholar]
- Dong X.; Chen H.; Wei M.; Wu K.; Zhang J. Structure, Dielectric and Energy Storage Properties of BaTiO3 Ceramics Doped with Ynbo4. J. Alloys Compd. 2018, 744, 721–727. 10.1016/j.jallcom.2018.01.276. [DOI] [Google Scholar]
- Li F.; Zhou M.; Zhai J.; Shen B.; Zeng H. Novel Barium Titanate Based Ferroelectric Relaxor Ceramics with Superior Charge-Discharge Performance. J. Eur. Ceram. Soc. 2018, 38, 4646–4652. 10.1016/j.jeurceramsoc.2018.06.038. [DOI] [Google Scholar]
- Li W.-B.; Zhou D.; Xu R.; Pang L.-X.; Reaney I. M. BaTiO3-Bi(Li0.5 Ta0.5)O3, Lead-Free Ceramics, and Multilayers with High Energy Storage Density and Efficiency. ACS Appl. Energy Mater. 2018, 1, 5016–5023. 10.1021/acsaem.8b01001. [DOI] [Google Scholar]
- Yuan Q.; Li G.; Yao F.-Z.; Cheng S.-D.; Wang Y.; Ma R.; Mi S.-B.; Gu M.; Wang K.; Li J.-F.; et al. Simultaneously Achieved Temperature-Insensitive High Energy Density and Efficiency in Domain Engineered BaTiO3-Bi(Mg0.5 Zr0.5)O3 Lead-Free Relaxor Ferroelectrics. Nano Energy 2018, 52, 203–210. 10.1016/j.nanoen.2018.07.055. [DOI] [Google Scholar]
- Zhou M.; Liang R.; Zhou Z.; Dong X. Novel BaTiO3-Based Lead-Free Ceramic Capacitors Featuring High Energy Storage Density, High Power Density, and Excellent Stability. J. Mater. Chem. C 2018, 6, 8528–8537. 10.1039/C8TC03003K. [DOI] [Google Scholar]
- Wan J.; Pu Y.; Hui C.; Cui C.; Guo Y. Effect of KNbO3 on Microstructure and Electrical Properties of Lead-Free 0.92BaTiO3-0.08K0.5Bi0.5TiO3 Ceramic. J. Mater. Sci.: Mater. Electron 2018, 29, 6556–6563. 10.1007/s10854-018-8639-6. [DOI] [Google Scholar]
- Wan J.; Pu Y.; Hui C.; Cui C.; Guo Y. Synthesis and Characterizations of NaNbO3 Modified 0.92BaTiO3-0.08K0.5Bi0.5TiO3 Ceramics for Energy Storage Applications. J. Mater. Sci.: Mater. Electron 2018, 29, 5158–5162. [Google Scholar]
- Hu Q.; Tian Y.; Zhu Q.; Bian J.; Jin L.; Du H.; Alikin D. O.; Shur V. Y.; Feng Y.; Xu Z.; et al. Achieve Ultrahigh Energy Storage Performance in BaTiO3-Bi(Mg1/2 Ti1/2)O3 Relaxor Ferroelectric Ceramics Via Nano-Scale Polarization Mismatch and Reconstruction. Nano Energy 2020, 67, 104264. 10.1016/j.nanoen.2019.104264. [DOI] [Google Scholar]
- Yang H.; Lu Z.; Li L.; Bao W.; Ji H.; Li J.; Feteira A.; Xu F.; Zhang Y.; Sun H.; et al. Novel BaTiO3-Based, Ag/Pd-Compatible Lead-Free Relaxors with Superior Energy Storage Performance. ACS Appl. Mater. Interfaces 2020, 12, 43942–43949. 10.1021/acsami.0c13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Pang L.-X.; Li W.-B.; Zhou D. Extreme High Energy Storage Efficiency in Perovskite Structured (1-X)(Ba0.8 sr0.2)TiO3-Xbi(Zn2/3Nb1/3)O3 (0.04 ≤ X ≤ 0.16) Ceramics. J. Eur. Ceram. Soc. 2020, 40, 3343–3347. 10.1016/j.jeurceramsoc.2020.03.015. [DOI] [Google Scholar]
- Dai Z.; Xie J.; Liu W.; Wang X.; Zhang L.; Zhou Z.; Li J.; Ren X. Effective Strategy to Achieve Excellent Energy Storage Properties in Lead-Free BaTiO3-Based Bulk Ceramics. ACS Appl. Mater. Interfaces 2020, 12, 30289–30296. 10.1021/acsami.0c02832. [DOI] [PubMed] [Google Scholar]
- Liu G.; Li Y.; Guo B.; Tang M.; Li Q.; Dong J.; Yu L.; Yu K.; Yan Y.; Wang D.; et al. Ultrahigh Dielectric Breakdown Strength and Excellent Energy Storage Performance in Lead-Free Barium Titanate-Based Relaxor Ferroelectric Ceramics Via a Combined Strategy of Composition Modification, Viscous Polymer Processing, and Liquid-Phase Sintering. Chem. Eng. J. 2020, 398, 125625. 10.1016/j.cej.2020.125625. [DOI] [Google Scholar]
- Wu T.; Pu Y.; Gao P.; Liu D. Influence of Sr/Ba Ratio on the Energy Storage Properties and Dielectric Relaxation Behaviors of Strontium Barium Titanate Ceramics. J. Mater. Sci.: Mater. Electron. 2013, 24, 4105–4112. 10.1007/s10854-013-1368-y. [DOI] [Google Scholar]
- Jin Q.; Pu Y.-P.; Wang C.; Gao Z.-Y.; Zheng H.-Y. Enhanced Energy Storage Performance of Ba0.4Sr0.6TiO3 Ceramics: Influence of Sintering Atmosphere. Ceram. Int. 2017, 43, S232–S238. 10.1016/j.ceramint.2017.05.229. [DOI] [Google Scholar]
- Song Z.; Liu H.; Zhang S.; Wang Z.; Shi Y.; Hao H.; Cao M.; Yao Z.; Yu Z. Effect of Grain Size on the Energy Storage Properties of (Ba0.4Sr0.6)TiO3 Paraelectric Ceramics. J. Eur. Ceram. Soc. 2014, 34, 1209–1217. 10.1016/j.jeurceramsoc.2013.11.039. [DOI] [Google Scholar]
- Song Z.; Liu H.; Hao H.; Zhang S.; Zhang S.; Cao M.; Yao Z.; Wang Z.; Hu W.; Shi Y.; et al. The Effect of Grain Boundary on the Energy Storage Properties of (Ba0.4 Sr0.6M)TiO3 Paraelectric Ceramics by Varying Grain Sizes. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 2015, 62, 609–616. 10.1109/TUFFC.2014.006927. [DOI] [PubMed] [Google Scholar]
- Hu Q.-G.; Shen Z.-Y.; Li Y.-M.; Wang Z.-M.; Luo W.-Q.; Xie Z.-X. Enhanced Energy Storage Properties of Dysprosium Doped Strontium Titanate Ceramics. Ceram. Int. 2014, 40, 2529–2534. 10.1016/j.ceramint.2013.07.126. [DOI] [Google Scholar]
- Yao Z.; Luo Q.; Zhang G.; Hao H.; Cao M.; Liu H. Improved Energy-Storage Performance and Breakdown Enhancement Mechanism of Mg-Doped SrTiO3 Bulk Ceramics for High Energy Density Capacitor Applications. J. Mater. Sci.: Mater. Electron. 2017, 28, 11491–11499. 10.1007/s10854-017-6945-z. [DOI] [Google Scholar]
- Ang C.; Yu Z. High Remnant Polarization in (Sr0.7Bi0.2)TiO3-(Na0.5 Bi0.5)TiO3 Solid Solutions. Appl. Phys. Lett. 2009, 95, 232908. 10.1063/1.3271180. [DOI] [Google Scholar]
- Ang C.; Yu Z. Dielectric and Ferroelectric Properties in (Sr,Ni,Na)TiO3 Solid Solutions. J. Appl. Phys. 2010, 107, 114106. 10.1063/1.3429234. [DOI] [Google Scholar]
- Ang C.; Yu Z. Phonon-Coupled Impurity Dielectric Modes in Sr1–1.5x BixTiO3. Phys. Rev. B: Condens. Matter Mater. Phys. 2000, 61, 11363–11366. 10.1103/PhysRevB.61.11363. [DOI] [Google Scholar]
- Li J.; Li F.; Xu Z.; Zhang S. Multilayer Lead-Free Ceramic Capacitors with Ultrahigh Energy Density and Efficiency. Adv. Mater. 2018, 30, 1802155. 10.1002/adma.201802155. [DOI] [PubMed] [Google Scholar]
- Ye X. Y.; Li Y. M.; Bian J. J. Dielectric and Energy Storage Properties of Mn-Doped Ba0.3 Sr0.475 La0.12 Ce0.03 TiO3 Dielectric Ceramics. J. Eur. Ceram. Soc. 2017, 37, 107–114. 10.1016/j.jeurceramsoc.2016.08.002. [DOI] [Google Scholar]
- Xie J.; Hao H.; Liu H.; Yao Z.; Song Z.; Zhang L.; Xu Q.; Dai J.; Cao M. Dielectric Relaxation Behavior and Energy Storage Properties of Sn Modified SrTiO3 Based Ceramics. Ceram. Int. 2016, 42, 12796–12801. 10.1016/j.ceramint.2016.05.042. [DOI] [Google Scholar]
- Luo Q.; Li X.; Yao Z.; Zhang L.; Xie J.; Hao H.; Cao M.; Manan A.; Liu H. The Role of Dielectric Permittivity in the Energy Storage Performances of Ultrahigh-Permittivity (Srx Ba1-X)(Ti0.85Sn0.15)O3 Ceramics. Ceram. Int. 2018, 44, 5304–5310. 10.1016/j.ceramint.2017.12.146. [DOI] [Google Scholar]
- Zhang Q.; Wang L.; Luo J.; Tang Q.; Du J. Ba0.4Sr0.6TiO3/MgO Composites with Enhanced Energy Storage Density and Low Dielectric Loss for Solid-State Pulse-Forming Line. Int. J. Appl. Ceram. Technol. 2010, 7, E124–E128. 10.1111/j.1744-7402.2009.02456.x. [DOI] [Google Scholar]
- Huang Y. H.; Wu Y. J.; Qiu W. J.; Li J.; Chen X. M. Enhanced Energy Storage Density of Ba0.4Sr0.6TiO3-MgO Composite Prepared by Spark Plasma Sintering. J. Eur. Ceram. Soc. 2015, 35, 1469–1476. 10.1016/j.jeurceramsoc.2014.11.022. [DOI] [Google Scholar]
- Li W.-B.; Zhou D.; Pang L.-X. Structure and Energy Storage Properties of Mn-Doped (Ba,Sr)TiO3-MgO Composite Ceramics. J. Mater. Sci.: Mater. Electron. 2017, 28, 8749–8754. 10.1007/s10854-017-6600-8. [DOI] [Google Scholar]
- Huang Y. H.; Wu Y. J.; Liu B.; Yang T. N.; Wang J. J.; Li J.; Chen L.-Q.; Chen X. M. From Core-Shell Ba0.4Sr0.6TiO3@SiO2 Particles to Dense Ceramics with High Energy Storage Performance by Spark Plasma Sintering. J. Mater. Chem. A 2018, 6, 4477–4484. 10.1039/C7TA10821D. [DOI] [Google Scholar]
- Chen K.; Pu Y.; Xu N.; Luo X. Effects of SrO-B2O3-SiO2 Glass Additive on Densification and Energy Storage Properties of Ba0.4Sr0.6TiO3 Ceramics. J. Mater. Sci.: Mater. Electron 2012, 23, 1599–1603. 10.1007/s10854-012-0635-7. [DOI] [Google Scholar]
- Liu B.; Wang X.; Zhang R.; Li L. Energy Storage Properties of Ultra Fine-Grained Ba0.4Sr0.6TiO3-Based Ceramics Sintered at Low Temperature. J. Alloys Compd. 2017, 691, 619–623. 10.1016/j.jallcom.2016.08.317. [DOI] [Google Scholar]
- Yang H.; Yan F.; Lin Y.; Wang T. Enhanced Energy Storage Properties of Ba0.4Sr0.6TiO3 Lead-Free Ceramics with Bi2o3-B2O3-SiO2 Glass Addition. J. Eur. Ceram. Soc. 2018, 38, 1367–1373. 10.1016/j.jeurceramsoc.2017.11.058. [DOI] [Google Scholar]
- Shen Z.-Y.; Yu Y.-Y.; Wang Y.; Zhang L.; Luo W.-Q.; Wang Z.-M.; Li Y.-M. Reduced High Temperature Dielectric Loss in Bsb Glass Modified Ba0.3Sr0.7TiO3 Ceramics for Energy Storage. J. Mater. Sci.: Mater. Electron 2018, 29, 1093–1097. 10.1007/s10854-017-8010-3. [DOI] [Google Scholar]
- Wang J.; Xu C.; Shen B.; Zhai J. Enhancing Energy Storage Density of (Ba, Sr)TiO3 Ceramic Particles by Coating with Al2O3 and SiO2. J. Mater. Sci.: Mater. Electron 2013, 24, 3309–3314. 10.1007/s10854-013-1248-5. [DOI] [Google Scholar]
- Li L.; Yu X.; Cai H.; Liao Q.; Han Y.; Gao Z. Preparation and Dielectric Properties of BaCu(B2O5)-Doped SrTiO3-Based Ceramics for Energy Storage. Mater. Sci. Eng., B 2013, 178, 1509–1514. 10.1016/j.mseb.2013.08.016. [DOI] [Google Scholar]
- Li Z. C.; Chen G. H.; Yuan C. L.; Zhou C. R.; Yang T.; Yang Y. Effects of NiNb2O6 Doping on Dielectric Property, Microstructure and Energy Storage Behavior of Sr0.97La0.02TiO3 Ceramics. J. Mater. Sci.: Mater. Electron 2017, 28, 1151–1158. 10.1007/s10854-016-5640-9. [DOI] [Google Scholar]
- Cui C.; Pu Y.; Gao Z.; Wan J.; Guo Y.; Hui C.; Wang Y.; Cui Y. Structure, Dielectric and Relaxor Properties in Lead-Free ST-NBT Ceramics for High Energy Storage Applications. J. Alloys Compd. 2017, 711, 319–326. 10.1016/j.jallcom.2017.04.023. [DOI] [Google Scholar]
- Zhang L.; Wang Z.; Li Y.; Chen P.; Cai J.; Yan Y.; Zhou Y.; Wang D.; Liu G. Enhanced Energy Storage Performance in Sn Doped Sr0.6(Na0.5 Bi0.5)0.4 TiO3 Lead-Free Relaxor Ferroelectric Ceramics. J. Eur. Ceram. Soc. 2019, 39, 3057–3063. 10.1016/j.jeurceramsoc.2019.02.004. [DOI] [Google Scholar]
- Wang C.; Yan F.; Yang H.; Lin Y.; Wang T. Dielectric and Ferroelectric Properties of SrTiO3-Bi0.54Na0.46TiO3-BaTiO3 Lead-Free Ceramics for High Energy Storage Applications. J. Alloys Compd. 2018, 749, 605–611. 10.1016/j.jallcom.2018.03.195. [DOI] [Google Scholar]
- Yan F.; Yang H.; Lin Y.; Wang T. Dielectric and Ferroelectric Properties of SrTiO3-Bi0.5Na0.5TiO3-BaAl0.5Nb0.5O3 Lead-Free Ceramics for High-Energy-Storage Applications. Inorg. Chem. 2017, 56, 13510–13516. 10.1021/acs.inorgchem.7b02181. [DOI] [PubMed] [Google Scholar]
- Yang H.; Yan F.; Lin Y.; Wang T.; He L.; Wang F. A Lead Free Relaxation and High Energy Storage Efficiency Ceramics for Energy Storage Applications. J. Alloys Compd. 2017, 710, 436–445. 10.1016/j.jallcom.2017.03.261. [DOI] [Google Scholar]
- Kong X.; Yang L.; Cheng Z.; Zhang S. Bi-Modified SrTiO3-Based Ceramics for High-Temperature Energy Storage Applications. J. Am. Ceram. Soc. 2020, 103, 1722–1731. 10.1111/jace.16844. [DOI] [Google Scholar]
- Cui C.; Pu Y.; Shi R. High-Energy Storage Performance in Lead-Free (0.8-X)SrTiO3-0.2Na0.5Bi0.5TiO3-xBaTiO3 Relaxor Ferroelectric Ceramics. J. Alloys Compd. 2018, 740, 1180–1187. 10.1016/j.jallcom.2018.01.106. [DOI] [Google Scholar]
- Cui C.; Pu Y.; Li X.; Cui Y.; Liu G. High Energy Storage Density of Temperature-Stable X9R Ceramics. Mater. Res. Bull. 2018, 105, 114–120. 10.1016/j.materresbull.2018.04.043. [DOI] [Google Scholar]
- Li Y.; Dong G.; Liu Q.; Wu D. Preparation and Investigation on Properties of BST-Base Ceramic with High-Energy Storage Density. J. Adv. Dielectr. 2013, 03, 1350005. 10.1142/S2010135X13500057. [DOI] [Google Scholar]
- Pan W.; Cao M.; Jan A.; Hao H.; Yao Z.; Liu H. High Breakdown Strength and Energy Storage Performance in (Nb, Zn) Modified SrTiO3 Ceramics Via Synergy Manipulation. J. Mater. Chem. C 2020, 8, 2019–2027. 10.1039/C9TC06256D. [DOI] [Google Scholar]
- Yang H.; Yan F.; Lin Y.; Wang T. Enhanced Recoverable Energy Storage Density and High Efficiency of SrTiO3-Based Lead-Free Ceramics. Appl. Phys. Lett. 2017, 111, 253903. 10.1063/1.5000980. [DOI] [Google Scholar]
- Yang H.; Yan F.; Lin Y.; Wang T. Novel Strontium Titanate-Based Lead-Free Ceramics for High-Energy Storage Applications. ACS Sustainable Chem. Eng. 2017, 5, 10215–10222. 10.1021/acssuschemeng.7b02203. [DOI] [Google Scholar]
- Tao H.; Wu W.; Wu J. Electrical Properties of Holmium Doped (K,Na)(Nb,Sb)O3-(Bi,Na)HfO3 Ceramics with Wide Sintering and Poling Temperature Range. J. Alloys Compd. 2016, 689, 759–766. 10.1016/j.jallcom.2016.08.037. [DOI] [Google Scholar]
- Qu B.; Du H.; Yang Z.; Liu Q.; Liu T. Enhanced Dielectric Breakdown Strength and Energy Storage Density in Lead-Free Relaxor Ferroelectric Ceramics Prepared Using Transition Liquid Phase Sintering. RSC Adv. 2016, 6, 34381–34389. 10.1039/C6RA01919F. [DOI] [Google Scholar]
- Yang Z.; Gao F.; Du H.; Jin L.; Yan L.; Hu Q.; Yu Y.; Qu S.; Wei X.; Xu Z.; et al. Grain Size Engineered Lead-Free Ceramics with Both Large Energy Storage Density and Ultrahigh Mechanical Properties. Nano Energy 2019, 58, 768–777. 10.1016/j.nanoen.2019.02.003. [DOI] [Google Scholar]
- Tao H.; Wu J. Optimization of Energy Storage Density in Relaxor (K, Na, Bi)NbO3 Ceramics. J. Mater. Sci.: Mater. Electron. 2017, 28, 16199–16204. 10.1007/s10854-017-7521-2. [DOI] [Google Scholar]
- Qiao X.; Zhang X.; Wu D.; Chao X.; Yang Z. Influence of Bi Nonstoichiometry on the Energy Storage Properties of 0.93knn-0.07BixMN Relaxor Ferroelectrics. J. Adv. Dielectr. 2018, 08, 1830006. 10.1142/S2010135X18300062. [DOI] [Google Scholar]
- Li Y.; Zhen Y.; Wang W.; Fang Z.; Jia Z.; Zhang J.; Zhong H.; Wu J.; Yan Y.; Xue Q.; et al. Enhanced Energy Storage Density and Discharge Efficiency in Potassium Sodium Niobite-Based Ceramics Prepared Using a New Scheme. J. Eur. Ceram. Soc. 2020, 40, 2357–2365. 10.1016/j.jeurceramsoc.2020.01.050. [DOI] [Google Scholar]
- Zhang M.; Yang H.; Li D.; Ma L.; Lin Y. Giant Energy Storage Efficiency and High Recoverable Energy Storage Density Achieved in K0.5Na0.5NbO3-Bi(Zn0.5 Zr0.5)O3 Ceramics. J. Mater. Chem. C 2020, 8, 8777–8785. 10.1039/D0TC01711F. [DOI] [Google Scholar]
- Li Y.; Cao M.-S.; Wang D.-W.; Yuan J. High-Efficiency and Dynamic Stable Electromagnetic Wave Attenuation for La Doped Bismuth Ferrite at Elevated Temperature and Gigahertz Frequency. RSC Adv. 2015, 5, 77184–77191. 10.1039/C5RA15458H. [DOI] [Google Scholar]
- Li Y.; Cao W.-Q.; Yuan J.; Wang D.-W.; Cao M.-S. Nd Doping of Bismuth Ferrite to Tune Electromagnetic Properties and Increase Microwave Absorption by Magnetic-Dielectric Synergy. J. Mater. Chem. C 2015, 3, 9276–9282. 10.1039/C5TC01684C. [DOI] [Google Scholar]
- Wang D.; Wang M.; Liu F.; Cui Y.; Zhao Q.; Sun H.; Jin H.; Cao M. Sol-Gel Synthesis of Nd-Doped BiFeO3 Multiferroic and Its Characterization. Ceram. Int. 2015, 41, 8768–8772. 10.1016/j.ceramint.2015.03.100. [DOI] [Google Scholar]
- Li Z.-J.; Hou Z.-L.; Song W.-L.; Liu X.-D.; Wang D.-W.; Tang J.; Shao X.-H. Mg-Substitution for Promoting Magnetic and Ferroelectric Properties of BiFeO3 Multiferroic Nanoparticles. Mater. Lett. 2016, 175, 207–211. 10.1016/j.matlet.2016.04.016. [DOI] [Google Scholar]
- Wang G.; Fan Z.; Murakami S.; Lu Z.; Hall D. A.; Sinclair D. C.; Feteira A.; Tan X.; Jones J. L.; Kleppe A. K.; et al. Origin of the Large Electrostrain in BiFeO3-BaTiO3 Based Lead-Free Ceramics. J. Mater. Chem. A 2019, 7, 21254–21263. 10.1039/C9TA07904A. [DOI] [Google Scholar]
- Rojac T.; Bencan A.; Malic B.; Tutuncu G.; Jones J. L.; Daniels J. E.; Damjanovic D. BiFeO3 Ceramics: Processing, Electrical, and Electromechanical Properties. J. Am. Ceram. Soc. 2014, 97, 1993–2011. 10.1111/jace.12982. [DOI] [Google Scholar]
- Masó N.; West A. R. Electrical Properties of Ca-Doped BiFeO3 Ceramics: From P-Type Semiconduction to Oxide-Ion Conduction. Chem. Mater. 2012, 24, 2127–2132. 10.1021/cm300683e. [DOI] [Google Scholar]
- Yuan G. L.; Baba-Kishi K. Z.; Liu J. M.; Wing Or S.; Wang Y. P.; Liu Z. G. Multiferroic Properties of Single-Phase Bi0.85 la0.15 feo3 Lead-Free Ceramics. J. Am. Ceram. Soc. 2006, 89, 3136–3139. 10.1111/j.1551-2916.2006.01186.x. [DOI] [Google Scholar]
- Leontsev S.; Eitel R. Topical Review: Progress in Engineering High Strain Lead-Free Piezoelectric Ceramics. Sci. Technol. Adv. Mater. 2010, 11, 044302. 10.1088/1468-6996/11/4/044302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Jin L.; Tian Y.; Shu L.; Hu Q.; Wei X. Microstructure and Ferroelectric Properties of Nb2O5-Modified BiFeO3-BaTiO3 Lead-Free Ceramics for Energy Storage. Mater. Lett. 2014, 137, 79–81. 10.1016/j.matlet.2014.08.133. [DOI] [Google Scholar]
- Wang D.; Fan Z.; Li W.; Zhou D.; Feteira A.; Wang G.; Murakami S.; Sun S.; Zhao Q.; Tan X.; et al. High Energy Storage Density and Large Strain in Bi(Zn2/3Nb1/3)O3-Doped BiFeO3-BaTiO3 Ceramics. ACS Appl. Energy Mater. 2018, 1, 4403–4412. 10.1021/acsaem.8b01099. [DOI] [Google Scholar]
- Zheng D.; Zuo R. Enhanced Energy Storage Properties in La(Mg1/2 ti1/2)O3-Modified BiFeO3-BaTiO3 Lead-Free Relaxor Ferroelectric Ceramics within a Wide Temperature Range. J. Eur. Ceram. Soc. 2017, 37, 413–418. 10.1016/j.jeurceramsoc.2016.08.021. [DOI] [Google Scholar]
- Sun H.; Wang X.; Sun Q.; Zhang X.; Ma Z.; Guo M.; Sun B.; Zhu X.; Liu Q.; Lou X. Large Energy Storage Density in BiFeO3-BaTiO3-AgNbO3 Lead-Free Relaxor Ceramics. J. Eur. Ceram. Soc. 2020, 40, 2929–2935. 10.1016/j.jeurceramsoc.2020.03.012. [DOI] [Google Scholar]
- Zheng D.; Zuo R.; Zhang D.; Li Y. Novel BiFeO3-BaTiO3-Ba(Mg1/3Nb2/3)O3 Lead-Free Relaxor Ferroelectric Ceramics for Energy-Storage Capacitors. J. Am. Ceram. Soc. 2015, 98, 2692–2695. 10.1111/jace.13737. [DOI] [Google Scholar]
- Liu N.; Liang R.; Zhao X.; Xu C.; Zhou Z.; Dong X. Novel Bismuth Ferrite-Based Lead-Free Ceramics with High Energy and Power Density. J. Am. Ceram. Soc. 2018, 101, 3259–3265. 10.1111/jace.15546. [DOI] [Google Scholar]
- Chen Z.; Bu X.; Ruan B.; Du J.; Zheng P.; Li L.; Wen F.; Bai W.; Wu W.; Zheng L.; et al. Simultaneously Achieving High Energy Storage Density and Efficiency under Low Electric Field in BiFeO3-Based Lead-Free Relaxor Ferroelectric Ceramics. J. Eur. Ceram. Soc. 2020, 40, 5450–5457. 10.1016/j.jeurceramsoc.2020.06.073. [DOI] [Google Scholar]
- Liu N.; Liang R.; Zhou Z.; Dong X. Designing Lead-Free Bismuth Ferrite-Based Ceramics Learning from Relaxor Ferroelectric Behavior for Simultaneous High Energy Density and Efficiency under Low Electric Field. J. Mater. Chem. C 2018, 6, 10211–10217. 10.1039/C8TC03855D. [DOI] [Google Scholar]
- Chen Z.; Bai X.; Wang H.; Du J.; Bai W.; Li L.; Wen F.; Zheng P.; Wu W.; Zheng L.; et al. Achieving High-Energy Storage Performance in 0.67Bi1-xSmxFeO3-0.33BaTiO3 Lead-Free Relaxor Ferroelectric Ceramics. Ceram. Int. 2020, 46, 11549–11555. 10.1016/j.ceramint.2020.01.181. [DOI] [Google Scholar]
- Li Q.; Ji S.; Wang D.; Zhu J.; Li L.; Wang W.; Zeng M.; Hou Z.; Gao X.; Lu X.; et al. Simultaneously Enhanced Energy Storage Density and Efficiency in Novel BiFeO3-Based Lead-Free Ceramic Capacitors. J. Eur. Ceram. Soc. 2021, 41, 387–393. 10.1016/j.jeurceramsoc.2020.08.032. [DOI] [Google Scholar]
- Yang H.; Qi H.; Zuo R. Enhanced Breakdown Strength and Energy Storage Density in a New BiFeO3-Based Ternary Lead-Free Relaxor Ferroelectric Ceramic. J. Eur. Ceram. Soc. 2019, 39, 2673–2679. 10.1016/j.jeurceramsoc.2019.03.001. [DOI] [Google Scholar]
- Wang G.; Lu Z.; Yang H.; Ji H.; Mostaed A.; Li L.; Wei Y.; Feteira A.; Sun S.; Sinclair D. C.; et al. Fatigue Resistant Lead-Free Multilayer Ceramic Capacitors with Ultrahigh Energy Density. J. Mater. Chem. A 2020, 8, 11414–11423. 10.1039/D0TA00216J. [DOI] [Google Scholar]
- Li B.; Cao M.-S.; Liu J.; Wang D.-W. Domain Structure and Enhanced Electrical Properties in Sodium Bismuth Titanate Ceramics Sintered from Crystals with Different Morphologies. J. Am. Ceram. Soc. 2016, 99, 2316–2326. 10.1111/jace.14211. [DOI] [Google Scholar]
- Zhang Y.; Liu X.; Wang G.; Li Y.; Zhang S.; Wang D.; Sun H. Enhanced Mechanical Energy Harvesting Capability in Sodium Bismuth Titanate Based Lead-Free Piezoelectric. J. Alloys Compd. 2020, 825, 154020. 10.1016/j.jallcom.2020.154020. [DOI] [Google Scholar]
- Lu R.; Yuan J.; Shi H.; Li B.; Wang W.; Wang D.; Cao M. Morphology-Controlled Synthesis and Growth Mechanism of Lead-Free Bismuth Sodium Titanate Nanostructures Via the Hydrothermal Route. CrystEngComm 2013, 15, 3984–3991. 10.1039/c3ce40139a. [DOI] [Google Scholar]
- McLaughlin K.; Pascual-Gonzalez C.; Wang D.; Feteira A. Site Occupancy and Electric-Field Induced Strain Response of Er-Doped (Bi0.4 Na0.4 Sr0.2)TiO3 Ceramics. J. Alloys Compd. 2019, 779, 7–14. 10.1016/j.jallcom.2018.11.121. [DOI] [Google Scholar]
- Wang G.; Hall D. A.; Comyn T. P.; Daniel L.; Kleppe A. K. Structure and Ferroelectric Behaviour of Na0.5Bi0.5TiO3-KNbO3 Ceramics. Adv. Appl. Ceram. 2016, 115, 89–95. 10.1080/17436753.2015.1104053. [DOI] [Google Scholar]
- Wang G.; Hall D. A.; Li Y.; Murray C. A.; Tang C. C. Structural Characterization of the Electric Field-Induced Ferroelectric Phase in Na0.5Bi0.5TiO3-KNbO3 Ceramics. J. Eur. Ceram. Soc. 2016, 36, 4015–4021. 10.1016/j.jeurceramsoc.2016.06.022. [DOI] [Google Scholar]
- Wang G.; Li Y.; Murray C. A.; Tang C. C.; Hall D. A. Thermally-Induced Phase Transformations in Na0.5Bi0.5TiO3-KNbO3 Ceramics. J. Am. Ceram. Soc. 2017, 100, 3293–3304. 10.1111/jace.14856. [DOI] [Google Scholar]
- Wang G.; Lu Z.; Zhang Z.; Feteira A.; Tang C. C.; Hall D. A. Electric Field-Induced Irreversible Relaxor to Ferroelectric Phase Transformations in Na0.5Bi0.5TiO3-NaNbO3 Ceramics. J. Am. Ceram. Soc. 2019, 102, 7746–7754. 10.1111/jace.16676. [DOI] [Google Scholar]
- Chen P.; Zhang L.; Cai J.; Wang Z.; Shi W.; Jing J.; Wei F.; Liu G.; Yan Y.; Liu H.; et al. Effect of Dy2O3 Content on the Dielectric, Ferroelectric, and Energy Storage Properties of Lead-Free 0.5Na0.5Bi0.5TiO3-0.5SrTiO3 Bulk Ceramics. J. Mater. Sci.: Mater. Electron 2019, 30, 13556–13566. [Google Scholar]
- Kumar A.; Kumar R.; Singh K.; Singh S. Enhanced Electrocaloric Effect and Energy Storage Density in Lead-Free 0.8Na0.5Bi0.5TiO3-0.2SrTiO3 Ceramics. Phys. Status Solidi A 2019, 216, 1800786. 10.1002/pssa.201800786. [DOI] [Google Scholar]
- Kandula K. R.; Banerjee K.; Raavi S. S. K.; Asthana S. Enhanced Electrocaloric Effect and Energy Storage Density of Nd-Substituted 0.92NBT-0.08BT Lead Free Ceramic. Phys. Status Solidi A 2018, 215, 1700915. 10.1002/pssa.201700915. [DOI] [Google Scholar]
- Hu D.; Pan Z.; Zhang X.; Ye H.; He Z.; Wang M.; Xing S.; Zhai J.; Fu Q.; Liu J. Greatly Enhanced Discharge Energy Density and Efficiency of Novel Relaxation Ferroelectric BNT-BKT-Based Ceramics. J. Mater. Chem. C 2020, 8, 591–601. 10.1039/C9TC05528B. [DOI] [Google Scholar]
- Cao W.; Li W.; Zhang T.; Sheng J.; Hou Y.; Feng Y.; Yu Y.; Fei W. High-Energy Storage Density and Efficiency of (1-X)[0.94NBT-0.06BT]-xST Lead-Free Ceramics. Energy Technol. 2015, 3, 1198–1204. 10.1002/ente.201500173. [DOI] [Google Scholar]
- Liu Z.; Zhang A.; Xu S.; Lu J.; Xie B.; Guo K.; Mao Y. Mediating the Confliction of Polarizability and Breakdown Electric-Field Strength in Bnst Relaxor Ferroelectric for Energy Storage Applications. J. Alloys Compd. 2020, 823, 153772. 10.1016/j.jallcom.2020.153772. [DOI] [Google Scholar]
- Zhang L.; Pu Y.; Chen M.; Wei T.; Peng X. Novel Na0.5Bi0.5TiO3 Based, Lead-Free Energy Storage Ceramics with High Power and Energy Density and Excellent High-Temperature Stability. Chem. Eng. J. 2020, 383, 123154. 10.1016/j.cej.2019.123154. [DOI] [Google Scholar]
- Wu J.; Mahajan A.; Riekehr L.; Zhang H.; Yang B.; Meng N.; Zhang Z.; Yan H. Perovskite Srx(Bi1-X Na0.97-X Li0.03)0.5 TiO3 Ceramics with Polar Nano Regions for High Power Energy Storage. Nano Energy 2018, 50, 723–732. 10.1016/j.nanoen.2018.06.016. [DOI] [Google Scholar]
- Borkar H.; Singh V. N.; Singh B. P.; Tomar M.; Gupta V.; Kumar A. Room Temperature Lead-Free Relaxor-Antiferroelectric Electroceramics for Energy Storage Applications. RSC Adv. 2014, 4, 22840–22847. 10.1039/C4RA00094C. [DOI] [Google Scholar]
- Luo W.-Q.; Shen Z.-Y.; Yu Y.-Y.; Song F.-S.; Wang Z.-M.; Li Y.-M. Structure and Dielectric Properties of NBT-xBT-ST Lead-Free Ceramics for Energy Storage. J. Adv. Dielectr. 2018, 08, 1820004. 10.1142/S2010135X18200047. [DOI] [Google Scholar]
- Qiao X.; Zhang F.; Wu D.; Chen B.; Zhao X.; Peng Z.; Ren X.; Liang P.; Chao X.; Yang Z. Superior Comprehensive Energy Storage Properties in Bi0.5Na0.5TiO3-Based Relaxor Ferroelectric Ceramics. Chem. Eng. J. 2020, 388, 124158. 10.1016/j.cej.2020.124158. [DOI] [Google Scholar]
- Li F.; Yang K.; Liu X.; Zou J.; Zhai J.; Shen B.; Li P.; Shen J.; Liu B.; Chen P.; et al. Temperature Induced High Charge-Discharge Performances in Lead-Free Bi0.5Na0.5TiO3-Based Ergodic Relaxor Ferroelectric Ceramics. Scr. Mater. 2017, 141, 15–19. 10.1016/j.scriptamat.2017.07.010. [DOI] [Google Scholar]
- Qi H.; Zuo R. Linear-Like Lead-Free Relaxor Antiferroelectric (Bi0.5Na0.5)TiO3-NaNbO3 with Giant Energy-Storage Density/Efficiency and Super Stability against Temperature and Frequency. J. Mater. Chem. A 2019, 7, 3971–3978. 10.1039/C8TA12232F. [DOI] [Google Scholar]
- Pan Z.; Hu D.; Zhang Y.; Liu J.; Shen B.; Zhai J. Achieving High Discharge Energy Density and Efficiency with NBT-Based Ceramics for Application in Capacitors. J. Mater. Chem. C 2019, 7, 4072–4078. 10.1039/C9TC00087A. [DOI] [Google Scholar]
- Huang Y.; Li F.; Hao H.; Xia F.; Liu H.; Zhang S. (Bi0.51 Na0.47)TiO3 Based Lead Free Ceramics with High Energy Density and Efficiency. J. Materiomics 2019, 5, 385–393. 10.1016/j.jmat.2019.03.006. [DOI] [Google Scholar]
- Qiu Y.; Lin Y.; Liu X.; Yang H. Bi(Mg2/3Nb1/3)O3 Addition Inducing High Recoverable Energy Storage Density in Lead-Free 0.65BaTiO3-0.35Bi0.5Na0.5TiO3 Bulk Ceramics. J. Alloys Compd. 2019, 797, 348–355. 10.1016/j.jallcom.2019.05.092. [DOI] [Google Scholar]
- Ren P.; Liu Z.; Wang X.; Duan Z.; Wan Y.; Yan F.; Zhao G. Dielectric and Energy Storage Properties of SrTiO3 and Srzro3 Modified Bi0.5Na0.5TiO3-Sr0.8Bi0.1□0.1 tio3 Based Ceramics. J. Alloys Compd. 2018, 742, 683–689. 10.1016/j.jallcom.2018.01.254. [DOI] [Google Scholar]
- Yao M.; Pu Y.; Zhang L.; Chen M. Enhanced Energy Storage Properties of (1-X)Bi0.5Na0.5TiO3-XBa0.85 Ca0.15 Ti0.9 Zr0.1O3 Ceramics. Mater. Lett. 2016, 174, 110–113. 10.1016/j.matlet.2016.03.089. [DOI] [Google Scholar]
- Yin J.; Lv X.; Wu J. Enhanced Energy Storage Properties of {Bi0.5[(Na0.8K0.2)1-Z Liz]0.5}0.96 Sr0.04(Ti1-X-Y Tax Nby)O3 Lead-Free Ceramics. Ceram. Int. 2017, 43, 13541–13546. 10.1016/j.ceramint.2017.07.060. [DOI] [Google Scholar]
- Liu X.; Yang H.; Yan F.; Qin Y.; Lin Y.; Wang T. Enhanced Energy Storage Properties of BaTiO3-Bi0.5Na0.5TiO3 Lead-Free Ceramics Modified by SrY0.5Nb0.5O3. J. Alloys Compd. 2019, 778, 97–104. 10.1016/j.jallcom.2018.11.106. [DOI] [Google Scholar]
- Hu B.; Fan H.; Ning L.; Gao S.; Yao Z.; Li Q. Enhanced Energy-Storage Performance and Dielectric Temperature Stability of (1-X)(0.65bi0.5 na0.5 tio3-0.35bi0.1 sr0.85 tio3)-Xknbo3 Ceramics. Ceram. Int. 2018, 44, 10968–10974. 10.1016/j.ceramint.2018.03.176. [DOI] [Google Scholar]
- Zhang L.; Pu Y.; Chen M.; Li R.; Guo X.; Cui Y. Enhanced Energy-Storage Properties of (1-X)Na0.5Bi0.5TiO3-XBaSnO3 Ceramics. Ceram. Int. 2018, 44, S207–S210. 10.1016/j.ceramint.2018.08.113. [DOI] [Google Scholar]
- Xu Y.; Hou Y.; Zhao H.; Zheng M.; Zhu M. Enhanced High-Temperature Energy Storage Properties in Fine-Grained Lead-Free Ceramic with High Insulation Resistivity. Mater. Lett. 2019, 249, 21–24. 10.1016/j.matlet.2019.04.057. [DOI] [Google Scholar]
- Yang Y.; Wang H.; Bi L.; Zheng Q.; Fan G.; Jie W.; Lin D. High Energy Storage Density and Discharging Efficiency in La3+/Nb5+-Co-Substituted (Bi0.5Na0.5)0.94 Ba0.06 TiO3 Ceramics. J. Eur. Ceram. Soc. 2019, 39, 3051–3056. 10.1016/j.jeurceramsoc.2019.04.031. [DOI] [Google Scholar]
- Hu B.; Fan H.; Ning L.; Wen Y.; Wang C. High Energy Storage Performance of [(Bi0.5Na0.5)0.94 Ba0.06]0.97 La0.03 Ti1-X(Al0.5 Nb0.5)XO3 Ceramics with Enhanced Dielectric Breakdown Strength. Ceram. Int. 2018, 44, 15160–15166. 10.1016/j.ceramint.2018.05.154. [DOI] [Google Scholar]
- Zhang L.; Pu Y.; Chen M.; Wei T.; Keipper W.; Shi R.; Guo X.; Li R.; Peng X. High Energy-Storage Density under Low Electric Fields and Improved Optical Transparency in Novel Sodium Bismuth Titanate-Based Lead-Free Ceramics. J. Eur. Ceram. Soc. 2020, 40, 71–77. 10.1016/j.jeurceramsoc.2019.09.001. [DOI] [Google Scholar]
- Sui J.; Fan H.; Hu B.; Ning L. High Temperature Stable Dielectric Properties and Enhanced Energy-Storage Performance of (1- X)(0.85na0.5 bi0.5 tio3 - 0.15ba0.8 ca0.2 ti0.8 zr0.2o3)-xK0.5Na0.5NbO3 Lead-Free Ceramics. Ceram. Int. 2018, 44, 18054–18059. 10.1016/j.ceramint.2018.07.008. [DOI] [Google Scholar]
- Butnoi P.; Manotham S.; Jaita P.; Randorn C.; Rujijanagul G. High Thermal Stability of Energy Storage Density and Large Strain Improvement of Lead-Free Bi0.5(Na0.40K0.10)TiO3 Piezoelectric Ceramics Doped with La and Zr. J. Eur. Ceram. Soc. 2018, 38, 3822–3832. 10.1016/j.jeurceramsoc.2018.04.024. [DOI] [Google Scholar]
- Chen P.; Chu B. Improvement of Dielectric and Energy Storage Properties in Bi(Mg1/2 Ti1/2)O3-Modified (Na1/2 Bi1/2)0.92 Ba0.08 TiO3 Ceramics. J. Eur. Ceram. Soc. 2016, 36, 81–88. 10.1016/j.jeurceramsoc.2015.09.029. [DOI] [Google Scholar]
- Zhang L.; Pu X.; Chen M.; Bai S.; Pu Y. Influence of BaSnO3 Additive on the Energy Storage Properties of Na0.5Bi0.5TiO3-Based Relaxor Ferroelectrics. J. Eur. Ceram. Soc. 2018, 38, 2304–2311. 10.1016/j.jeurceramsoc.2017.11.053. [DOI] [Google Scholar]
- Zhang L.; Pu Y.; Chen M. Influence of Bazro3 Additive on the Energy-Storage Properties of 0.775Na0.5 Bi0.5 TiO3-0.225BaSnO3 Relaxor Ferroelectrics. J. Alloys Compd. 2019, 775, 342–347. 10.1016/j.jallcom.2018.10.025. [DOI] [Google Scholar]
- Lin Y.; Li D.; Zhang M.; Yang H. (Na0.5 Bi0.5)0.7 Sr0.3 TiO3 Modified by Bi(Mg2/3Nb1/3)O3 Ceramics with High Energy-Storage Properties and an Ultrafast Discharge Rate. J. Mater. Chem. C 2020, 8, 2258–2264. 10.1039/C9TC06218A. [DOI] [Google Scholar]
- Liu G.; Dong J.; Zhang L.; Yu L.; Wei F.; Li Y.; Gao J.; Hu J.; Yan Y.; Li Q.; et al. Na0.25 sr0.5 bi0.25 tio3 Relaxor Ferroelectric Ceramic with Greatly Enhanced Electric Storage Property by a B-Site Ion Doping. Ceram. Int. 2020, 46, 11680–11688. 10.1016/j.ceramint.2020.01.199. [DOI] [Google Scholar]
- Yang Z.; Yuan Y.; Cao L.; Li E.; Zhang S. Relaxor Ferroelectric (Na0.5 Bi0.5)0.4 Sr0.6 TiO3-Based Ceramics for Energy Storage Application. Ceram. Int. 2020, 46, 11282–11289. 10.1016/j.ceramint.2020.01.154. [DOI] [Google Scholar]
- Xu Q.; Liu H.; Zhang L.; Xie J.; Hao H.; Cao M.; Yao Z.; Lanagan M. T. Structure and Electrical Properties of Lead-Free Bi0.5Na0.5TiO3-Based Ceramics for Energy-Storage Applications. RSC Adv. 2016, 6, 59280–59291. 10.1039/C6RA11744A. [DOI] [Google Scholar]
- Zhang L.; Pu Y.; Chen M. Ultra-High Energy Storage Performance under Low Electric Fields in Na0.5Bi0.5TiO3-Based Relaxor Ferroelectrics for Pulse Capacitor Applications. Ceram. Int. 2020, 46, 98–105. 10.1016/j.ceramint.2019.08.238. [DOI] [Google Scholar]
- Yang L.; Kong X.; Cheng Z.; Zhang S. Ultra-High Energy Storage Performance with Mitigated Polarization Saturation in Lead-Free Relaxors. J. Mater. Chem. A 2019, 7, 8573–8580. 10.1039/C9TA01165J. [DOI] [Google Scholar]
- Qiao X.; Wu D.; Zhang F.; Chen B.; Ren X.; Liang P.; Du H.; Chao X.; Yang Z. Bi0.5Na0.5TiO3-Based Relaxor Ferroelectric Ceramic with Large Energy Density and High Efficiency under a Moderate Electric Field. J. Mater. Chem. C 2019, 7, 10514–10520. 10.1039/C9TC03597D. [DOI] [Google Scholar]
- Wu Y.; Fan Y.; Liu N.; Peng P.; Zhou M.; Yan S.; Cao F.; Dong X.; Wang G. Enhanced Energy Storage Properties in Sodium Bismuth Titanate-Based Ceramics for Dielectric Capacitor Applications. J. Mater. Chem. C 2019, 7, 6222–6230. 10.1039/C9TC01239G. [DOI] [Google Scholar]
- Ma W.; Zhu Y.; Marwat M. A.; Fan P.; Xie B.; Salamon D.; Ye Z.-G.; Zhang H. Enhanced Energy-Storage Performance with Excellent Stability under Low Electric Fields in BNT-St Relaxor Ferroelectric Ceramics. J. Mater. Chem. C 2019, 7, 281–288. 10.1039/C8TC04447C. [DOI] [Google Scholar]
- Zhu C.; Cai Z.; Luo B.; Guo L.; Li L.; Wang X. High Temperature Lead-Free BNT-Based Ceramics with Stable Energy Storage and Dielectric Properties. J. Mater. Chem. A 2020, 8, 683–692. 10.1039/C9TA10347C. [DOI] [Google Scholar]
- Zhang L.; Pu Y.; Chen M.; Liu G. Antiferroelectric-Like Properties in MgO-Modified 0.775Na0.5Bi0.5TiO3-0.225BaSnO3 Ceramics for High Power Energy Storage. J. Eur. Ceram. Soc. 2018, 38, 5388–5395. 10.1016/j.jeurceramsoc.2018.08.010. [DOI] [Google Scholar]
- Tao C.-W.; Geng X.-Y.; Zhang J.; Wang R.-X.; Gu Z.-B.; Zhang S.-T. Bi0.5Na0.5TiO3-BaTiO3-K0.5Na0.5NbO3:ZnO Relaxor Ferroelectric Composites with High Breakdown Electric Field and Large Energy Storage Properties. J. Eur. Ceram. Soc. 2018, 38, 4946–4952. 10.1016/j.jeurceramsoc.2018.07.006. [DOI] [Google Scholar]
- Cui C.; Pu Y. Effect of Sn Substitution on the Energy Storage Properties of 0.45SrTiO3-0.2Na0.5Bi0.5TiO3-0.35BaTiO3 Ceramics. J. Mater. Sci. 2018, 53, 9830–9841. 10.1007/s10853-018-2282-8. [DOI] [Google Scholar]
- Yao Y.; Li Y.; Sun N.; Du J.; Li X.; Zhang L.; Zhang Q.; Hao X. Enhanced Dielectric and Energy-Storage Properties in ZnO-Doped 0.9(0.94Na0.5Bi0.5TiO3-0.06BaTiO3)-0.1NaNbO3 Ceramics. Ceram. Int. 2018, 44, 5961–5966. 10.1016/j.ceramint.2017.12.174. [DOI] [Google Scholar]
- Shi W.; Zhang L.; Chen P.; Li Y.; Wang Y.; Liu G.; Yu K.; Yan Y. The Ferroelectric, Dielectric and Energy Storage Properties of Pb-Free 0.6Na0.5Bi0.5TiO3-0.4SrTiO3 Bulk Ceramics Modified by Fe2O3. Mater. Res. Express 2019, 6, 086329. 10.1088/2053-1591/ab25c7. [DOI] [Google Scholar]
- Chen P.; Zhang L.; Wang Z.; Wang Y.; Song C.; Hu J.; Liu G.; Yan Y. Improved Dielectric Energy Storage Performance of Pb-Free 0.5Na0.5Bi0.5TiO3-0.5SrTiO3 Ceramics Modified with Cao. J. Adv. Dielectr. 2018, 08, 1850042. 10.1142/S2010135X1850042X. [DOI] [Google Scholar]
- Cao W. P.; Sheng J.; Qiao Y. L.; Jing L.; Liu Z.; Wang J.; Li W. L. Optimized Strain with Small Hysteresis and High Energy-Storage Density in Mn-Doped NBT-ST System. J. Eur. Ceram. Soc. 2019, 39, 4046–4052. 10.1016/j.jeurceramsoc.2019.05.064. [DOI] [Google Scholar]
- Li F.; Zhai J.; Shen B.; Liu X.; Zeng H. Simultaneously High-Energy Storage Density and Responsivity in Quasi-Hysteresis-Free Mn-Doped Bi0.5Na0.5TiO3-BaTiO3-(Sr0.7 Bi0.2□0.1)TiO3 Ergodic Relaxor Ceramics. Mater. Res. Lett. 2018, 6, 345–352. 10.1080/21663831.2018.1457095. [DOI] [Google Scholar]
- Li F.; Hou X.; Wang J.; Zeng H.; Shen B.; Zhai J. Structure-Design Strategy of 0–3 Type (Bi0.32 sr0.42 na0.20)TiO3/MgO Composite to Boost Energy Storage Density, Efficiency and Charge-Discharge Performance. J. Eur. Ceram. Soc. 2019, 39, 2889–2898. 10.1016/j.jeurceramsoc.2019.03.047. [DOI] [Google Scholar]
- Verma A.; Yadav A. K.; Kumar S.; Srihari V.; Jangir R.; Poswal H. K.; Biring S.; Sen S. Enhanced Energy Storage Properties in a-Site Substituted Na0.5Bi0.5TiO3 Ceramics. J. Alloys Compd. 2019, 792, 95–107. 10.1016/j.jallcom.2019.03.304. [DOI] [Google Scholar]
- Verma A.; Yadav A. K.; Kumar S.; Srihari V.; Jangir R.; Poswal H. K.; Biring S.; Sen S. Structural, Thermally Stable Dielectric, and Energy Storage Properties of Lead-Free (1-X)(Na0.50Bi0.50)TiO3-xKSbO3 Ceramics. J. Mater. Sci.: Mater. Electron 2019, 30, 15005–15017. 10.1007/s10854-019-01873-1. [DOI] [Google Scholar]
- Ji H.; Bao W.; Lu Z.; Wang G.; Li J.; Yang H.; Mostaed A.; Li L.; Feteira A.; Xu F.; et al. Ultrahigh Energy Density in Short-Range Tilted Lead-Free Multilayer Ceramic Capacitors by Nanodomain Percolation. Energy Storage Mater. 2021, 38, 113–120. 10.1016/j.ensm.2021.01.023. [DOI] [Google Scholar]
- Pu Y.; Zhang L.; Cui Y.; Chen M. High Energy Storage Density and Optical Transparency of Microwave Sintered Homogeneous (Na0.5Bi0.5)(1-x)BaxTi(1-y)SnyO3 Ceramics. ACS Sustainable Chem. Eng. 2018, 6, 6102–6109. 10.1021/acssuschemeng.7b04754. [DOI] [Google Scholar]
- Yin J.; Zhang Y.; Lv X.; Wu J. Ultrahigh Energy-Storage Potential under Low Electric Field in Bismuth Sodium Titanate-Based Perovskite Ferroelectrics. J. Mater. Chem. A 2018, 6, 9823–9832. 10.1039/C8TA00474A. [DOI] [Google Scholar]
- Zhao X.; Bai W.; Ding Y.; Wang L.; Wu S.; Zheng P.; Li P.; Zhai J. Tailoring High Energy Density with Superior Stability under Low Electric Field in Novel (Bi0.5Na0.5)TiO3-Based Relaxor Ferroelectric Ceramics. J. Eur. Ceram. Soc. 2020, 40, 4475–4486. 10.1016/j.jeurceramsoc.2020.05.078. [DOI] [Google Scholar]
- Li D.; Lin Y.; Yuan Q.; Zhang M.; Ma L.; Yang H. A Novel Lead-Free Na0.5Bi0.5TiO3-Based Ceramic with Superior Comprehensive Energy Storage and Discharge Properties for Dielectric Capacitor Applications. J. Materiomics 2020, 6, 743–750. 10.1016/j.jmat.2020.06.005. [DOI] [Google Scholar]
- Yan F.; Huang K.; Jiang T.; Zhou X.; Shi Y.; Ge G.; Shen B.; Zhai J. Significantly Enhanced Energy Storage Density and Efficiency of BNT-Based Perovskite Ceramics Via a-Site Defect Engineering. Energy Storage Mater. 2020, 30, 392–400. 10.1016/j.ensm.2020.05.026. [DOI] [Google Scholar]
- Tian Y.; Jin L.; Zhang H.; Xu Z.; Wei X.; Politova E. D.; Stefanovich S. Y.; Tarakina N. V.; Abrahams I.; Yan H. High Energy Density in Silver Niobate Ceramics. J. Mater. Chem. A 2016, 4, 17279–17287. 10.1039/C6TA06353E. [DOI] [Google Scholar]
- Yang D.; Gao J.; Shu L.; Liu Y.-X.; Yu J.; Zhang Y.; Wang X.; Zhang B.-P.; Li J.-F. Lead-Free Antiferroelectric Niobates AgNbO3 and NaNbO3 for Energy Storage Applications. J. Mater. Chem. A 2020, 8, 23724–23737. 10.1039/D0TA08345C. [DOI] [Google Scholar]
- Gao J.; Zhao L.; Liu Q.; Wang X.; Zhang S.; Li J.-F. Antiferroelectric-Ferroelectric Phase Transition in Lead-Free AgNbO3 Ceramics for Energy Storage Applications. J. Am. Ceram. Soc. 2018, 101, 5443–5450. 10.1111/jace.15780. [DOI] [Google Scholar]
- Zhao L.; Liu Q.; Zhang S.; Li J.-F. Lead-Free AgNbO3 Anti-Ferroelectric Ceramics with an Enhanced Energy Storage Performance Using Mno2 Modification. J. Mater. Chem. C 2016, 4, 8380–8384. 10.1039/C6TC03289C. [DOI] [Google Scholar]
- Zhao L.; Gao J.; Liu Q.; Zhang S.; Li J.-F. Silver Niobate Lead-Free Antiferroelectric Ceramics: Enhancing Energy Storage Density by B-Site Doping. ACS Appl. Mater. Interfaces 2018, 10, 819–826. 10.1021/acsami.7b17382. [DOI] [PubMed] [Google Scholar]
- Luo N.; Han K.; Zhuo F.; Xu C.; Zhang G.; Liu L.; Chen X.; Hu C.; Zhou H.; Wei Y. Aliovalent a-Site Engineered AgNbO3 Lead-Free Antiferroelectric Ceramics toward Superior Energy Storage Density. J. Mater. Chem. A 2019, 7, 14118–14128. 10.1039/C9TA02053E. [DOI] [Google Scholar]
- Luo N.; Han K.; Zhuo F.; Liu L.; Chen X.; Peng B.; Wang X.; Feng Q.; Wei Y. Design for High Energy Storage Density and Temperature-Insensitive Lead-Free Antiferroelectric Ceramics. J. Mater. Chem. C 2019, 7, 4999–5008. 10.1039/C8TC06549G. [DOI] [Google Scholar]
- Gao J.; Zhang Y.; Zhao L.; Lee K.-Y.; Liu Q.; Studer A.; Hinterstein M.; Zhang S.; Li J. Enhanced Antiferroelectric Phase Stability in La-Doped AgNbO3: Perspectives from the Microstructure to Energy Storage Properties. J. Mater. Chem. A 2019, 7, 2225–2232. 10.1039/C8TA09353A. [DOI] [Google Scholar]
- Xu C.; Fu Z.; Liu Z.; Wang L.; Yan S.; Chen X.; Cao F.; Dong X.; Wang G. La/Mn Codoped AgNbO3 Lead-Free Antiferroelectric Ceramics with Large Energy Density and Power Density. ACS Sustainable Chem. Eng. 2018, 6, 16151–16159. 10.1021/acssuschemeng.8b02821. [DOI] [Google Scholar]
- Luo N.; Han K.; Liu L.; Peng B.; Wang X.; Hu C.; Zhou H.; Feng Q.; Chen X.; Wei Y. Lead-Free Ag1–3x LaxNbO3 Antiferroelectric Ceramics with High-Energy Storage Density and Efficiency. J. Am. Ceram. Soc. 2019, 102, 4640–4647. 10.1111/jace.16309. [DOI] [Google Scholar]
- Tian Y.; Jin L.; Zhang H.; Xu Z.; Wei X.; Viola G.; Abrahams I.; Yan H. Phase Transitions in Bismuth-Modified Silver Niobate Ceramics for High Power Energy Storage. J. Mater. Chem. A 2017, 5, 17525–17531. 10.1039/C7TA03821F. [DOI] [Google Scholar]
- Han K.; Luo N.; Mao S.; Zhuo F.; Chen X.; Liu L.; Hu C.; Zhou H.; Wang X.; Wei Y. Realizing High Low-Electric-Field Energy Storage Performance in AgNbO3 Ceramics by Introducing Relaxor Behaviour. J. Materiomics 2019, 5, 597–605. 10.1016/j.jmat.2019.07.006. [DOI] [Google Scholar]
- Li S.; Nie H.; Wang G.; Xu C.; Liu N.; Zhou M.; Cao F.; Dong X. Significantly Enhanced Energy Storage Performance of Rare-Earth-Modified Silver Niobate Lead-Free Antiferroelectric Ceramics Via Local Chemical Pressure Tailoring. J. Mater. Chem. C 2019, 7, 1551–1560. 10.1039/C8TC05458D. [DOI] [Google Scholar]
- Han K.; Luo N.; Jing Y.; Wang X.; Peng B.; Liu L.; Hu C.; Zhou H.; Wei Y.; Chen X.; et al. Structure and Energy Storage Performance of Ba-Modified AgNbO3 Lead-Free Antiferroelectric Ceramics. Ceram. Int. 2019, 45, 5559–5565. 10.1016/j.ceramint.2018.12.014. [DOI] [Google Scholar]
- Zhao L.; Liu Q.; Gao J.; Zhang S.; Li J.-F. Lead-Free Antiferroelectric Silver Niobate Tantalate with High Energy Storage Performance. Adv. Mater. 2017, 29, 1701824. 10.1002/adma.201701824. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Jin L.; Hu Q.; Yu K.; Zhuang Y.; Viola G.; Abrahams I.; Xu Z.; Wei X.; Yan H. Phase Transitions in Tantalum-Modified Silver Niobate Ceramics for High Power Energy Storage. J. Mater. Chem. A 2019, 7, 834–842. 10.1039/C8TA10075F. [DOI] [Google Scholar]
- Han K.; Luo N.; Mao S.; Zhuo F.; Liu L.; Peng B.; Chen X.; Hu C.; Zhou H.; Wei Y. Ultrahigh Energy-Storage Density in a-/B-Site Co-Doped AgNbO3 Lead-Free Antiferroelectric Ceramics: Insight into the Origin of Antiferroelectricity. J. Mater. Chem. A 2019, 7, 26293–26301. 10.1039/C9TA06457E. [DOI] [Google Scholar]
- Song A.; Song J.; Lv Y.; Liang L.; Wang J.; Zhao L. Energy Storage Performance in Bimno3-Modified AgNbO3 Anti-Ferroelectric Ceramics. Mater. Lett. 2019, 237, 278–281. 10.1016/j.matlet.2018.11.105. [DOI] [Google Scholar]
- Yan Z.; Zhang D.; Zhou X.; Qi H.; Luo H.; Zhou K.; Abrahams I.; Yan H. Silver Niobate Based Lead-Free Ceramics with High Energy Storage Density. J. Mater. Chem. A 2019, 7, 10702–10711. 10.1039/C9TA00995G. [DOI] [Google Scholar]
- Gao J.; Liu Q.; Dong J.; Wang X.; Zhang S.; Li J.-F. Local Structure Heterogeneity in Sm-Doped AgNbO3 for Improved Energy-Storage Performance. ACS Appl. Mater. Interfaces 2020, 12, 6097–6104. 10.1021/acsami.9b20803. [DOI] [PubMed] [Google Scholar]
- Chao W.; Yang T.; Li Y.; Liu Z. Enhanced Energy Storage Density in Ca and Ta Co-Doped AgNbO3 Antiferroelectric Ceramics. J. Am. Ceram. Soc. 2020, 103, 7283–7290. 10.1111/jace.17415. [DOI] [Google Scholar]
- Xu Y.; Guo Y.; Liu Q.; Wang G.; Bai J.; Tian J.; Lin L.; Tian Y. High Energy Storage Properties of Lead-Free Mn-Doped (1-X)AgNbO3-Xbi0.5 Na0.5TiO3 Antiferroelectric Ceramics. J. Eur. Ceram. Soc. 2020, 40, 56–62. 10.1016/j.jeurceramsoc.2019.09.022. [DOI] [Google Scholar]
- Luo N.; Han K.; Cabral M. J.; Liao X.; Zhang S.; Liao C.; Zhang G.; Chen X.; Feng Q.; Li J.-F.; et al. Constructing Phase Boundary in AgNbO3 Antiferroelectrics: Pathway Simultaneously Achieving High Energy Density and Efficiency. Nat. Commun. 2020, 11, 4824. 10.1038/s41467-020-18665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Bao W.; Wang G.; Sun S.-K.; Li L.; Li J.; Yang H.; Ji H.; Feteira A.; Li D.; et al. Mechanism of Enhanced Energy Storage Density in AgNbO3-Based Lead-Free Antiferroelectrics. Nano Energy 2021, 79, 105423. 10.1016/j.nanoen.2020.105423. [DOI] [Google Scholar]
- Li S.; Hu T.; Nie H.; Fu Z.; Xu C.; Xu F.; Wang G.; Dong X. Giant Energy Density and High Efficiency Achieved in Silver Niobate-Based Lead-Free Antiferroelectric Ceramic Capacitors Via Domain Engineering. Energy Storage Mater. 2021, 34, 417–426. 10.1016/j.ensm.2020.09.021. [DOI] [Google Scholar]
- Gao L.; Guo H.; Zhang S.; Randall C. A. Stabilized Antiferroelectricity in xBiScO3-(1-X)NaNbO3 Lead-Free Ceramics with Established Double Hysteresis Loops. Appl. Phys. Lett. 2018, 112, 092905. 10.1063/1.5017697. [DOI] [Google Scholar]
- Gao L.; Guo H.; Zhang S.; Randall C. A. A Perovskite Lead-Free Antiferroelectric xCaHfO3-(1-X) NaNbO3 with Induced Double Hysteresis Loops at Room Temperature. J. Appl. Phys. 2016, 120, 204102. 10.1063/1.4968790. [DOI] [Google Scholar]
- Guo H.; Shimizu H.; Randall C. A. Direct Evidence of an Incommensurate Phase in NaNbO3 and Its Implication in NaNbO3-Based Lead-Free Antiferroelectrics. Appl. Phys. Lett. 2015, 107, 112904. 10.1063/1.4930067. [DOI] [Google Scholar]
- Ye J.; Wang G.; Zhou M.; Liu N.; Chen X.; Li S.; Cao F.; Dong X. Excellent Comprehensive Energy Storage Properties of Novel Lead-Free NaNbO3-Based Ceramics for Dielectric Capacitor Applications. J. Mater. Chem. C 2019, 7, 5639–5645. 10.1039/C9TC01414D. [DOI] [Google Scholar]
- Zhou M.; Liang R.; Zhou Z.; Yan S.; Dong X. Novel Sodium Niobate-Based Lead-Free Ceramics as New Environment-Friendly Energy Storage Materials with High Energy Density, High Power Density, and Excellent Stability. ACS Sustainable Chem. Eng. 2018, 6, 12755–12765. 10.1021/acssuschemeng.8b01926. [DOI] [Google Scholar]
- Shi J.; Chen X.; Li X.; Sun J.; Sun C.; Pang F.; Zhou H. Realizing Ultrahigh Recoverable Energy Density and Superior Charge-Discharge Performance in NaNbO3-Based Lead-Free Ceramics Via a Local Random Field Strategy. J. Mater. Chem. C 2020, 8, 3784–3794. 10.1039/C9TC06711F. [DOI] [Google Scholar]
- Tian A.; Zuo R.; Qi H.; Shi M. Large Energy-Storage Density in Transition-Metal Oxide Modified NaNbO3-Bi(Mg0.5 Ti0.5)O3 Lead-Free Ceramics through Regulating the Antiferroelectric Phase Structure. J. Mater. Chem. A 2020, 8, 8352–8359. 10.1039/D0TA02285C. [DOI] [Google Scholar]
- Shi R.; Pu Y.; Wang W.; Guo X.; Li J.; Yang M.; Zhou S. A Novel Lead-Free NaNbO3-Bi(Zn0.5 Ti0.5)O3 Ceramics System for Energy Storage Application with Excellent Stability. J. Alloys Compd. 2020, 815, 152356. 10.1016/j.jallcom.2019.152356. [DOI] [Google Scholar]
- Sun C.; Chen X.; Shi J.; Pang F.; Dong X.; Chen H. Y.; Wang K.; Zhou X.; Zhou H. Simultaneously with Large Energy Density and High Efficiency Achieved in NaNbO3-Based Relaxor Ferroelectric Ceramics. J. Eur. Ceram. Soc. 2021, 41, 1891–1903. 10.1016/j.jeurceramsoc.2020.10.049. [DOI] [Google Scholar]
- Zhou M.; Liang R.; Zhou Z.; Dong X. Superior Energy Storage Properties and Excellent Stability of Novel NaNbO3-Based Lead-Free Ceramics with a-Site Vacancy Obtained Via a Bi2o3 Substitution Strategy. J. Mater. Chem. A 2018, 6, 17896–17904. 10.1039/C8TA07303A. [DOI] [Google Scholar]
- Yang L.; Kong X.; Cheng Z.; Zhang S. Enhanced Energy Density and Electric Cycling Reliability Via Mno2 Modification in Sodium Niobate-Based Relaxor Dielectric Capacitors. J. Mater. Res. 2021, 1–9. 10.1557/s43578-020-00085-2. [DOI] [Google Scholar]
- Dong X.; Li X.; Chen X.; Chen H.; Sun C.; Shi J.; Pang F.; Zhou H. High Energy Storage Density and Power Density Achieved Simultaneously in NaNbO3-Based Lead-Free Ceramics Via Antiferroelectricity Enhancement. J. Materiomics 2021, 7, 629–639. 10.1016/j.jmat.2020.11.016. [DOI] [Google Scholar]
- Chen J.; Qi H.; Zuo R. Realizing Stable Relaxor Antiferroelectric and Superior Energy Storage Properties in (Na1-X/2 Lax/2)(Nb1-X Tix)O3 Lead-Free Ceramics through a/B-Site Complex Substitution. ACS Appl. Mater. Interfaces 2020, 12, 32871–32879. 10.1021/acsami.0c09876. [DOI] [PubMed] [Google Scholar]
- Chen H.; Shi J.; Chen X.; Sun C.; Pang F.; Dong X.; Zhang H.; Zhou H. Excellent Energy Storage Properties and Stability of NaNbO3-Bi(Mg0.5Ta0.5)O3 Ceramics by Introducing (Bi0.5Na0.5)-0.7Sr0.3TiO3. J. Mater. Chem. A 2021, 9, 4789. 10.1039/D0TA11022A. [DOI] [Google Scholar]
- Liu S.; Shen B.; Hao H.; Zhai J. Glass-Ceramic Dielectric Materials with High Energy Density and Ultra-Fast Discharge Speed for High Power Energy Storage Applications. J. Mater. Chem. C 2019, 7, 15118–15135. 10.1039/C9TC05253D. [DOI] [Google Scholar]
- Chen G.-H.; Zhang W.-J.; Liu X.-Y.; Zhou C.-R. Preparation and Properties of Strontium Barium Niobate Based Glass-Ceramics for Energy Storage Capacitors. J. Electroceram. 2011, 27, 78–82. 10.1007/s10832-011-9652-9. [DOI] [Google Scholar]
- Wang S.; Tian J.; Liu J.; Yang K.; Shen B.; Zhai J. Ultrahigh Energy Storage Density and Instantaneous Discharge Power Density in BaO-PbO-Na2O-Nb2O5-SiO2-Al2O3 Glass-Ceramics. J. Mater. Chem. C 2018, 6, 12608–12614. 10.1039/C8TC04096F. [DOI] [Google Scholar]
- Luo J.; Du J.; Tang Q.; Mao C. Lead Sodium Niobate Glass-Ceramic Dielectrics and Internal Electrode Structure for High Energy Storage Density Capacitors. IEEE Trans. Electron Devices 2008, 55, 3549–3554. 10.1109/TED.2008.2006541. [DOI] [Google Scholar]
- Yang Y.; Song J.; Chen G.; Yuan C.; Li X.; Zhou C. Effect of Crystallization Temperature on the Dielectric Property and Energy Density of SrO-BaO-Nb2O6-B2O3 Glass-Ceramics. J. Non-Cryst. Solids 2015, 410, 96–99. 10.1016/j.jnoncrysol.2014.12.007. [DOI] [Google Scholar]
- Xiao S.; Xiu S.; Xue S.; Shen B.; Zhai J. Crystallization Behavior and Dielectric Properties of K2O-SrO-Nb2O5-B2O3-Al2O3-SiO2 Glass-Ceramic for Energy Storage. J. Alloys Compd. 2015, 648, 745–750. 10.1016/j.jallcom.2015.07.047. [DOI] [Google Scholar]
- Liu J.; Yang K.; Zhai J.; Shen B. Effects of Crystallization Temperature on Phase Evolution and Energy Storage Properties of BaO-Na2O-Nb2O5-SiO2-Al2O3 Glass-Ceramics. J. Eur. Ceram. Soc. 2018, 38, 2312–2317. 10.1016/j.jeurceramsoc.2018.01.003. [DOI] [Google Scholar]
- Jiang T.; Chen K.; Shen B.; Zhai J. Excellent Energy Storage and Charge-Discharge Performances in Sodium-Barium-Niobium Based Glass Ceramics. Ceram. Int. 2019, 45, 19429–19434. 10.1016/j.ceramint.2019.06.197. [DOI] [Google Scholar]
- Wang H.; Liu J.; Zhai J.; Shen B.; Pan Z.; Liu J. R.; Yang K. Effect of Crystallization Temperature on Dielectric and Energy-Storage Properties in SrO-Na2O-Nb2O5-SiO2 Glass-Ceramics. Ceram. Int. 2017, 43, 8898–8904. 10.1016/j.ceramint.2017.04.026. [DOI] [Google Scholar]
- Tian J.; Wang S.; Jiang T.; Chen K.; Zhai J.; Shen B. Dielectric Characterization of a Novel Bi2o3-Nb2O5-SiO2-Al2O3 Glass-Ceramic with Excellent Charge-Discharge Properties. J. Eur. Ceram. Soc. 2019, 39, 1164–1169. 10.1016/j.jeurceramsoc.2018.11.040. [DOI] [Google Scholar]
- Liu J.; Wang H.; Shen B.; Zhai J.; Pan Z.; Yang K.; Liu J. Crystallization Kinetics, Breakdown Strength, and Energy-Storage Properties in Niobate-Based Glass-Ceramics. J. Alloys Compd. 2017, 722, 212–218. 10.1016/j.jallcom.2017.06.066. [DOI] [Google Scholar]
- Wang H.; Liu J.; Zhai J.; Shen B.; Pan Z.; Yang K.; Liu J. Effect of K2O Content on Breakdown Strength and Energy-Storage Density in K2O-BaO-Nb2O5-SiO2 Glass-Ceramics. Ceram. Int. 2017, 43, 4183–4187. 10.1016/j.ceramint.2016.12.042. [DOI] [Google Scholar]
- Wang H.; Liu J.; Zhai J.; Pan Z.; Shen B. Effects of Sr Substitution for Ba on Dielectric and Energy-Storage Properties of SrO-BaO-K2O-Nb2O5-SiO2 Glass-Ceramics. J. Eur. Ceram. Soc. 2017, 37, 3917–3925. 10.1016/j.jeurceramsoc.2017.04.049. [DOI] [Google Scholar]
- Li B.; Wang D.; Chen G.; Liu X.; Yuan C. Effect of K:Ba Ratio on Energy Storage Properties of Strontium Barium Potassium Niobate-Glass Ceramics. J. Mater. Sci.: Mater. Electron. 2019, 30, 19262–19269. 10.1007/s10854-019-02285-x. [DOI] [Google Scholar]
- Liu J.; Yang K.; Zhai J.; Shen B.; Wang H.; Li F. High Energy Storage Density and Rapid Discharge Speed of Niobosilicate Glasses. Mater. Chem. Phys. 2018, 206, 29–34. 10.1016/j.matchemphys.2017.12.003. [DOI] [Google Scholar]
- Smith N. J.; Rangarajan B.; Lanagan M. T.; Pantano C. G. Alkali-Free Glass as a High Energy Density Dielectric Material. Mater. Lett. 2009, 63, 1245–1248. 10.1016/j.matlet.2009.02.047. [DOI] [Google Scholar]
- Xiu S.; Xiao S.; Xue S.; Shen B.; Zhai J. Effect of Different Al/Si Ratios on the Structure and Energy Storage Properties of Strontium Barium Niobate-Based Glass-Ceramics. J. Electron. Mater. 2016, 45, 1017–1022. 10.1007/s11664-015-4264-9. [DOI] [Google Scholar]
- Zhang W.; Wang J.; Xue S.; Liu S.; Shen B.; Zhai J. Effect of La2O3 Additive on the Dielectric Properties of Barium Strontium Titanate Glass-Ceramics. J. Mater. Sci.: Mater. Electron. 2014, 25, 4145–4149. 10.1007/s10854-014-2141-6. [DOI] [Google Scholar]
- Zheng J.; Chen G. H.; Yuan C. L.; Zhou C. R.; Chen X.; Feng Q.; Li M. Dielectric Characterization and Energy-Storage Performance of Lead-Free Niobate Glass-Ceramics Added with La2O3. Ceram. Int. 2016, 42, 1827–1832. 10.1016/j.ceramint.2015.09.146. [DOI] [Google Scholar]
- Zhou Y.; Zhang Q.; Luo J.; Tang Q.; Du J. Structural Optimization and Improved Discharged Energy Density for Niobate Glass-Ceramics by La2O3 Addition. J. Am. Ceram. Soc. 2013, 96, 372–375. 10.1111/jace.12159. [DOI] [Google Scholar]
- Chen G. H.; Zheng J.; Li Z. C.; Yuan C. L.; Zhou C. R. Optimized Microstructure and Energy-Storage Density of Sm2O3-Added Lead-Free Borate Glass-Ceramic Composites. J. Mater. Sci.: Mater. Electron. 2016, 27, 8499–8503. 10.1007/s10854-016-4865-y. [DOI] [Google Scholar]
- Li B.; Wang D.; Chen G.; Liu X. Effect of Yb2o3 Content on Dielectric and Energy-Storage Properties of Lead-Free Niobate Glass-Ceramics. J. Mater. Sci.: Mater. Electron. 2018, 29, 19238–19244. 10.1007/s10854-018-0050-9. [DOI] [Google Scholar]
- Xiu S.; Xiao S.; Zhang W.; Xue S.; Shen B.; Zhai J. Effect of Rare-Earth Additions on the Structure and Dielectric Energy Storage Properties of Bax sr1-X tio3-Based Barium Boronaluminosilicate Glass-Ceramics. J. Alloys Compd. 2016, 670, 217–221. 10.1016/j.jallcom.2016.02.022. [DOI] [Google Scholar]
- Yang K.; Liu J.; Shen B.; Zhai J.; Wang H. Large Improvement on Energy Storage and Charge-Discharge Properties of Gd2O3-Doped BaO-K2O-Nb2O5-SiO2 Glass-Ceramic Dielectrics. Mater. Sci. Eng., B 2017, 223, 178–184. 10.1016/j.mseb.2017.06.011. [DOI] [Google Scholar]
- Xiu S.; Xiao S.; Shen B.; Zhai J. The Structure, Dielectric and Energy Storage Properties of Strontium Barium Niobate-Based Glass-Ceramics Doped with La2O3. J. Electron. Mater. 2017, 46, 4557–4561. 10.1007/s11664-017-5422-z. [DOI] [Google Scholar]
- Xiao S.; Xiu S.; Shen B.; Zhai J. Microstructure Evolution and Energy Storage Properties of Potassium Strontium Niobate Boroaluminosilicate Glass-Ceramics by Microwave Crystallization. J. Eur. Ceram. Soc. 2016, 36, 4071–4076. 10.1016/j.jeurceramsoc.2016.06.044. [DOI] [Google Scholar]
- Zhang W.; Xue S.; Liu S.; Wang J.; Shen B.; Zhai J. Structure and Dielectric Properties of BaxSr1-xTiO3-Based Glass Ceramics for Energy Storage. J. Alloys Compd. 2014, 617, 740–745. 10.1016/j.jallcom.2014.08.077. [DOI] [Google Scholar]
- Xue S.; Zhai J.; Xiao S.; Xiu S.; Shen B. Improved Dielectric Breakdown Strength and Suppressed Energy Loss in Niobate Glass-Ceramics Crystallized by Microwave Process. Mater. Lett. 2017, 190, 154–156. 10.1016/j.matlet.2017.01.002. [DOI] [Google Scholar]
- Liu J.; Wang H.; Shen B.; Zhai J.; Li P.; Pan Z. Significantly Enhanced Energy-Storage Density in the Strontium Barium Niobate-Based/Titanate-Based Glass-Ceramics. J. Am. Ceram. Soc. 2017, 100, 506–510. 10.1111/jace.14627. [DOI] [Google Scholar]
- Xiu S.; Shen B.; Zhai J. The Effects of Mno2 Addition on the Structure and Dielectric Properties of the Strontium Barium Niobate Glass-Ceramics. Mater. Res. Bull. 2017, 95, 349–353. 10.1016/j.materresbull.2017.08.008. [DOI] [Google Scholar]
- Wang H.; Liu J.; Zhai J.; Shen B.; Xiu S.; Xiao S.; Pan Z. Enhanced Energy Storage Density and Discharge Efficiency in the Strontium Sodium Niobate-Based Glass-Ceramics. J. Alloys Compd. 2016, 687, 280–285. 10.1016/j.jallcom.2016.06.024. [DOI] [Google Scholar]
- Tian J.; Wang S.; Yang K.; Liu J.; Zhai J.; Shen B. Enhanced Energy Storage Properties of BaO-K2O-Nb2O5-SiO2 Glass Ceramics Obtained through Microwave Crystallization. Ceram. Int. 2018, 44, 15490–15494. 10.1016/j.ceramint.2018.05.208. [DOI] [Google Scholar]
- Wang S.; Tian J.; Yang K.; Liu J.; Zhai J.; Shen B. Crystallization Kinetics Behavior and Dielectric Energy Storage Properties of Strontium Potassium Niobate Glass-Ceramics with Different Nucleating Agents. Ceram. Int. 2018, 44, 8528–8533. 10.1016/j.ceramint.2018.02.054. [DOI] [Google Scholar]
- Hong K.; Lee T. H.; Suh J. M.; Yoon S.-H.; Jang H. W. Perspectives and Challenges in Multilayer Ceramic Capacitors for Next Generation Electronics. J. Mater. Chem. C 2019, 7, 9782–9802. 10.1039/C9TC02921D. [DOI] [Google Scholar]
- Wang G.; Lu Z.; Li J.; Ji H.; Yang H.; Li L.; Sun S.; Feteira A.; Yang H.; Zuo R.; et al. Lead-Free (Ba,Sr)TiO3-BiFeO3 Based Multilayer Ceramic Capacitors with High Energy Density. J. Eur. Ceram. Soc. 2020, 40, 1779–1783. 10.1016/j.jeurceramsoc.2019.12.009. [DOI] [Google Scholar]
- Shrout T. R.; Zhang S. J. Lead-Free Piezoelectric Ceramics: Alternatives for PZT?. J. Electroceram. 2007, 19, 113–126. 10.1007/s10832-007-9047-0. [DOI] [Google Scholar]
- Rödel J.; Jo W.; Seifert K. T. P.; Anton E.-M.; Granzow T.; Damjanovic D. Perspective on the Development of Lead-Free Piezoceramics. J. Am. Ceram. Soc. 2009, 92, 1153–1177. 10.1111/j.1551-2916.2009.03061.x. [DOI] [Google Scholar]
- Rödel J.; Webber K. G.; Dittmer R.; Jo W.; Kimura M.; Damjanovic D. Transferring Lead-Free Piezoelectric Ceramics into Application. J. Eur. Ceram. Soc. 2015, 35, 1659–1681. 10.1016/j.jeurceramsoc.2014.12.013. [DOI] [Google Scholar]
- Wang D.; Khesro A.; Murakami S.; Feteira A.; Zhao Q.; Reaney I. M. Temperature Dependent, Large Electromechanical Strain in Nd-Doped BiFeO3-BaTiO3 Lead-Free Ceramics. J. Eur. Ceram. Soc. 2017, 37, 1857–1860. 10.1016/j.jeurceramsoc.2016.10.027. [DOI] [Google Scholar]
- Murakami S.; Ahmed N. T. A. F.; Wang D.; Feteira A.; Sinclair D. C.; Reaney I. M. Optimising Dopants and Properties in Bimeo3 (Me = Al, Ga, Sc, Y, Mg2/3Nb1/3, Zn2/3Nb1/3, Zn1/2Ti1/2) Lead-Free BaTiO3-BiFeO3 Based Ceramics for Actuator Applications. J. Eur. Ceram. Soc. 2018, 38, 4220–4231. 10.1016/j.jeurceramsoc.2018.05.019. [DOI] [Google Scholar]
- Murakami S.; Wang D.; Mostaed A.; Khesro A.; Feteira A.; Sinclair D. C.; Fan Z.; Tan X.; Reaney I. M. High Strain (0.4%) Bi(Mg2/3Nb1/3)O3-BaTiO3-BiFeO3 Lead-Free Piezoelectric Ceramics and Multilayers. J. Am. Ceram. Soc. 2018, 101, 5428–5442. 10.1111/jace.15749. [DOI] [Google Scholar]
- Wang G.; Goetzee-Barral A.; Lu Z.; Keeble D. S.; Hall D. A. Thermally-Induced Local Structural Transformations in Na0.5Bi0.5TiO3-KNbO3 Ceramics. J. Eur. Ceram. Soc. 2021, 41, 3832–3837. 10.1016/j.jeurceramsoc.2021.01.040. [DOI] [Google Scholar]
- Knowles Capacitors . Multilayer Ceramic Capacitors in Automotive. Power Electronics Europe 2017, 36.
- Sharma S. K.; Shyam A. Design and Testing of 45 Kv, 50 Khz Pulse Power Supply for Dielectric Barrier Discharges. Rev. Sci. Instrum. 2016, 87, 105115. 10.1063/1.4964507. [DOI] [PubMed] [Google Scholar]
- Elsied M.; Salem A.; Oukaour A.; Gualous H.; Chaoui H.; Youssef F. T.; Belie D.; Melkebeek J.; Mohammed O. 2015 IEEE Vehicle Power and Propulsion Conference (VPPC) 2015, 1–6. 10.1109/VPPC.2015.7352941. [DOI] [Google Scholar]
- Obaid Z. A.; Cipcigan L. M.; Abrahim L.; Muhssin M. T. Frequency Control of Future Power Systems: Reviewing and Evaluating Challenges and New Control Methods. J. Mod. Power Syst. Clean Energy 2019, 7, 9–25. 10.1007/s40565-018-0441-1. [DOI] [Google Scholar]
- Siami S.; Joubert C.; Glaize C. High Frequency Model for Power Electronics Capacitors. IEEE Trans. Power Electron. 2001, 16, 157–166. 10.1109/63.911139. [DOI] [Google Scholar]
- Yano M.; Abe S.; Ohno E. In IEEE Conference on the History of Electronics; IEEE, 2004.
- Rashid M. H.Power Electronics Handbook [Electronic Resource]: Devices, Circuits, and Applications; 3rd ed.; Elsevier/BH: Amsterdam, 2011. [Google Scholar]
- Hussain F.; Khesro A.; Lu Z.; Wang G.; Wang D. Lead Free Multilayer Piezoelectric Actuators by Economically New Approach. Front. Mater. 2020, 7, 87. 10.3389/fmats.2020.00087. [DOI] [Google Scholar]
- Khesro A.; Wang D.; Hussain F.; Sinclair D. C.; Feteira A.; Reaney I. M. Temperature Stable and Fatigue Resistant Lead-Free Ceramics for Actuators. Appl. Phys. Lett. 2016, 109, 142907. 10.1063/1.4964411. [DOI] [Google Scholar]
- Multilayer Ceramic Capacitor Market, Global Opportunity Analysis and Industry Forecast, 2018–2024. Allied Market Research 2018, SE_184655.
- Shkuratov S. I.; Baird J.; Antipov V. G.; Zhang S.; Chase J. B. Multilayer PZT 95/5 Antiferroelectric Film Energy Storage Devices with Giant Power Density. Adv. Mater. 2019, 31, 1904819. 10.1002/adma.201904819. [DOI] [PubMed] [Google Scholar]
- Chen L.; Sun N.; Li Y.; Zhang Q.; Zhang L.; Hao X. Multifunctional Antiferroelectric Mlcc with High-Energy-Storage Properties and Large Field-Induced Strain. J. Am. Ceram. Soc. 2018, 101, 2313–2320. 10.1111/jace.15380. [DOI] [Google Scholar]
- Liu X.; Li Y.; Sun N.; Hao X. High Energy-Storage Performance of Plzs Antiferroelectric Multilayer Ceramic Capacitors. Inorg. Chem. Front. 2020, 7, 756–764. 10.1039/C9QI01416K. [DOI] [Google Scholar]
- Cai Z.; Zhu C.; Wang H.; Zhao P.; Chen L.; Li L.; Wang X. High-Temperature Lead-Free Multilayer Ceramic Capacitors with Ultrahigh Energy Density and Efficiency Fabricated Via Two-Step Sintering. J. Mater. Chem. A 2019, 7, 14575–14582. 10.1039/C9TA04317A. [DOI] [Google Scholar]
- Zhao P.; Wang H.; Wu L.; Chen L.; Cai Z.; Li L.; Wang X. High-Performance Relaxor Ferroelectric Materials for Energy Storage Applications. Adv. Energy Mater. 2019, 9, 1803048. 10.1002/aenm.201803048. [DOI] [Google Scholar]
- Li J.; Shen Z.; Chen X.; Yang S.; Zhou W.; Wang M.; Wang L.; Kou Q.; Liu Y.; Li Q.; et al. Grain-Orientation-Engineered Multilayer Ceramic Capacitors for Energy Storage Applications. Nat. Mater. 2020, 19, 999–1005. 10.1038/s41563-020-0704-x. [DOI] [PubMed] [Google Scholar]
- Jia W.; Hou Y.; Zheng M.; Xu Y.; Yu X.; Zhu M.; Yang K.; Cheng H.; Sun S.; Xing J. Superior Temperature-Stable Dielectrics for Mlccs Based on Bi0.5Na0.5TiO3-NaNbO3 System Modified by CaZrO3. J. Am. Ceram. Soc. 2018, 101, 3468–3479. 10.1111/jace.15519. [DOI] [Google Scholar]
- Yuan Q.; Cui J.; Wang Y.; Ma R.; Wang H. Significant Enhancement in Breakdown Strength and Energy Density of the BaTiO3/BaTiO3@SiO2 Layered Ceramics with Strong Interface Blocking Effect. J. Eur. Ceram. Soc. 2017, 37, 4645–4652. 10.1016/j.jeurceramsoc.2017.06.028. [DOI] [Google Scholar]
- Yan F.; Yang H.; Ying L.; Wang T. Enhanced Energy Storage Properties of a Novel Lead-Free Ceramic with a Multilayer Structure. J. Mater. Chem. C 2018, 6, 7905–7912. 10.1039/C8TC02368A. [DOI] [Google Scholar]
- Tong X.-Y.; Yang Y.-T.; Song M.-W.; Zhou J.-J.; Wang K.; Guan C.-L.; Liu H.; Fang J.-Z. Energy-Storage Properties of Low-Temperature Co-Fired BNT-St/Agpd Multilayer Lead-Free Ceramic Capacitors. J. Alloys Compd. 2020, 827, 154260. 10.1016/j.jallcom.2020.154260. [DOI] [Google Scholar]
- Chen L.; Wang H.; Zhao P.; Zhu C.; Cai Z.; Cen Z.; Li L.; Wang X. Multifunctional BaTiO3-(Bi0.5Na0.5)TiO3-Based Mlcc with High-Energy Storage Properties and Temperature Stability. J. Am. Ceram. Soc. 2019, 102, 4178–4187. 10.1111/jace.16292. [DOI] [Google Scholar]
- Lee H.; Kim J. R.; Lanagan M. J.; Trolier-McKinstry S.; Randall C. A. High-Energy Density Dielectrics and Capacitors for Elevated Temperatures: Ca(Zr,Ti)O3. J. Am. Ceram. Soc. 2013, 96, 1209–1213. 10.1111/jace.12184. [DOI] [Google Scholar]
- Cai Z.; Wang H.; Zhao P.; Chen L.; Zhu C.; Hui K.; Li L.; Wang X. Significantly Enhanced Dielectric Breakdown Strength and Energy Density of Multilayer Ceramic Capacitors with High Efficiency by Electrodes Structure Design. Appl. Phys. Lett. 2019, 115, 023901. 10.1063/1.5110527. [DOI] [Google Scholar]
- Wang H.; Zhao P.; Chen L.; Wang X. Effects of Dielectric Thickness on Energy Storage Properties of 0.87batio3-0.13bi(Zn2/3(Nb0.85 ta0.15)1/3)O3 Multilayer Ceramic Capacitors. J. Eur. Ceram. Soc. 2020, 40, 1902–1908. 10.1016/j.jeurceramsoc.2020.01.032. [DOI] [Google Scholar]
- Wang H.; Zhao P.; Chen L.; Li L.; Wang X. Energy Storage Properties of 0.87BaTiO3-0.13Bi(Zn2/3(Nb0.85 Ta0.15)1/3)O3 Multilayer Ceramic Capacitors with Thin Dielectric Layers. J. Adv. Ceram. 2020, 9, 292–302. 10.1007/s40145-020-0367-8. [DOI] [Google Scholar]
- Shen B.-Z.; Li Y.; Hao X. Multifunctional All-Inorganic Flexible Capacitor for Energy Storage and Electrocaloric Refrigeration over a Broad Temperature Range Based on PLZT 9/65/35 Thick Films. ACS Appl. Mater. Interfaces 2019, 11, 34117–34127. 10.1021/acsami.9b12353. [DOI] [PubMed] [Google Scholar]
- Zhao Q. L.; Cao M. S.; Yuan J.; Song W. L.; Lu R.; Wang D. W.; Zhang D. Q. Preparation and Electrical Properties of Pb(Zr0.52Ti0.48)O3 Thick Films Embedded with ZnO Nanowhiskers by a Hybrid Sol-Gel Route. J. Alloys Compd. 2010, 492, 264–268. 10.1016/j.jallcom.2009.11.062. [DOI] [Google Scholar]
- Zhao Q.; Su D.; Cao M.; He G.; Di J.; Yuan J.; Wang D. Thickness-Dependent Electrical Properties of Sol-Gel Derived Pb(Zr0.52Ti0.48)O3 Thick Films Using PbTiO3 Buffer Layers. J. Mater. Sci.: Mater. Electron 2013, 24, 3521–3525. 10.1007/s10854-013-1279-y. [DOI] [Google Scholar]
- Pan H.; Li F.; Liu Y.; Zhang Q.; Wang M.; Lan S.; Zheng Y.; Ma J.; Gu L.; Shen Y.; et al. Ultrahigh-Energy Density Lead-Free Dielectric Films Via Polymorphic Nanodomain Design. Science 2019, 365, 578. 10.1126/science.aaw8109. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Chen Y.; Liu Z.; Wang G.; Rémiens D.; Dong X. Large Energy Storage Density, Low Energy Loss and Highly Stable (Pb0.97 La0.02)(Zr0.66 Sn0.23 Ti0.11)O3 Antiferroelectric Thin-Film Capacitors. J. Eur. Ceram. Soc. 2018, 38, 3177–3181. 10.1016/j.jeurceramsoc.2018.03.004. [DOI] [Google Scholar]
- Sun N.; Wang Y.; Zhang L.; Zhang X.; Hao X.; Li M. Effects of Thickness on the Microstructure and Energy-Storage Performance of PLZT Antiferroelectric Thick Films. J. Adv. Dielectr. 2013, 03, 1350021. 10.1142/S2010135X13500215. [DOI] [Google Scholar]
- Wang J.; Sun N.; Li Y.; Zhang Q.; Hao X.; Chou X. Effects of Mn Doping on Dielectric Properties and Energy-Storage Performance of Na0.5Bi0.5TiO3 Thick Films. Ceram. Int. 2017, 43, 7804–7809. 10.1016/j.ceramint.2017.03.094. [DOI] [Google Scholar]
- Wang J.; Li Y.; Sun N.; Zhang Q.; Zhang L.; Hao X.; Chou X. Effects of Fe3+ Doping on Electrical Properties and Energy-Storage Performances of the (Na0.85K0.15)0.5 Bi0.5TiO3 Thick Films Prepared by Sol-Gel Method. J. Alloys Compd. 2017, 727, 596–602. 10.1016/j.jallcom.2017.08.169. [DOI] [Google Scholar]
- Sun N.; Li Y.; Zhang Q.; Hao X. Giant Energy-Storage Density and High Efficiency Achieved in (Bi0.5Na0.5)TiO3-Bi(Ni0.5 Zr0.5)O3 Thick Films with Polar Nanoregions. J. Mater. Chem. C 2018, 6, 10693–10703. 10.1039/C8TC03481H. [DOI] [Google Scholar]
- Park M. H.; Kim H. J.; Kim Y. J.; Moon T.; Kim K. D.; Hwang C. S. Thin Hfx zr1-Xo2 Films: A New Lead-Free System for Electrostatic Supercapacitors with Large Energy Storage Density and Robust Thermal Stability. Adv. Energy Mater. 2014, 4, 1400610. 10.1002/aenm.201400610. [DOI] [Google Scholar]
- Payne A.; Brewer O.; Leff A.; Strnad N. A.; Jones J. L.; Hanrahan B. Dielectric, Energy Storage, and Loss Study of Antiferroelectric-Like Al-Doped Hfo2 Thin Films. Appl. Phys. Lett. 2020, 117, 221104. 10.1063/5.0029706. [DOI] [Google Scholar]
- Liu Y.; Wang Y.; Hao X.; Xu J. Preparation and Energy-Storage Performance of PLZT Antiferroelectric Thick Films Via Sol-Gel Method. Ceram. Int. 2013, 39, S513–S516. 10.1016/j.ceramint.2012.10.124. [DOI] [Google Scholar]
- Wu S.; Chen P.; Zhai J.; Shen B.; Li P.; Li F. Enhanced Piezoelectricity and Energy Storage Performances of Fe-Doped BNT-BKT-St Thin Films. Ceram. Int. 2018, 44, 21289–21294. 10.1016/j.ceramint.2018.08.179. [DOI] [Google Scholar]
- Ahn C. W.; Amarsanaa G.; Won S. S.; Chae S. A.; Lee D. S.; Kim I. W. Antiferroelectric Thin-Film Capacitors with High Energy-Storage Densities, Low Energy Losses, and Fast Discharge Times. ACS Appl. Mater. Interfaces 2015, 7, 26381–26386. 10.1021/acsami.5b08786. [DOI] [PubMed] [Google Scholar]
- Tong S.; Ma B.; Narayanan M.; Liu S.; Koritala R.; Balachandran U.; Shi D. Lead Lanthanum Zirconate Titanate Ceramic Thin Films for Energy Storage. ACS Appl. Mater. Interfaces 2013, 5, 1474–1480. 10.1021/am302985u. [DOI] [PubMed] [Google Scholar]
- Cai H.; Yan S.; Zhou M.; Liu N.; Ye J.; Li S.; Cao F.; Dong X.; Wang G. Significantly Improved Energy Storage Properties and Cycling Stability in La-Doped PbZrO3 Antiferroelectric Thin Films by Chemical Pressure Tailoring. J. Eur. Ceram. Soc. 2019, 39, 4761–4769. 10.1016/j.jeurceramsoc.2019.07.024. [DOI] [Google Scholar]
- Peddigari M.; Palneedi H.; Hwang G.-T.; Lim K. W.; Kim G.-Y.; Jeong D.-Y.; Ryu J. Boosting the Recoverable Energy Density of Lead-Free Ferroelectric Ceramic Thick Films through Artificially Induced Quasi-Relaxor Behavior. ACS Appl. Mater. Interfaces 2018, 10, 20720–20727. 10.1021/acsami.8b05347. [DOI] [PubMed] [Google Scholar]
- Yang X.; Li W.; Qiao Y.; Zhang Y.; He J.; Fei W. High Energy-Storage Density of Lead-Free (Sr1–1.5x Bix)Ti0.99 Mn0.01O3 Thin Films Induced by Bi3+-Vsr Dipolar Defects. Phys. Chem. Chem. Phys. 2019, 21, 16359–16366. 10.1039/C9CP01368G. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Hao X.; Xu J. Effects of PbO Insert Layer on the Microstructure and Energy Storage Performance of (042)-Preferred PLZT Antiferroelectric Thick Films. J. Mater. Res. 2012, 27, 1770–1775. 10.1557/jmr.2012.124. [DOI] [Google Scholar]
- Xie J.; Yao Z.; Hao H.; Xie Y.; Li Z.; Liu H.; Cao M. A Novel Lead-Free Bismuth Magnesium Titanate Thin Films for Energy Storage Applications. J. Am. Ceram. Soc. 2019, 102, 3819–3822. 10.1111/jace.16288. [DOI] [Google Scholar]
- Dang H. T.; Trinh T. T.; Nguyen C. T. Q.; Do T. V.; Nguyen M. D.; Vu H. N. Enhancement of Relaxor Behavior by La Doping and Its Influence on the Energy Storage Performance and Electric Breakdown Strength of Ferroelectric Pb(Zr0.52Ti0.48)O3 Thin Films. Mater. Chem. Phys. 2019, 234, 210–216. 10.1016/j.matchemphys.2019.06.005. [DOI] [Google Scholar]
- Won S. S.; Kawahara M.; Kuhn L.; Venugopal V.; Kwak J.; Kim I. W.; Kingon A. I.; Kim S.-H. BiFeO3-Doped (K0.5,Na0.5)(Mn0.005,Nb0.995)O3 Ferroelectric Thin Film Capacitors for High Energy Density Storage Applications. Appl. Phys. Lett. 2017, 110, 152901. 10.1063/1.4980113. [DOI] [Google Scholar]
- Zhang T.; Li W.; Hou Y.; Yu Y.; Song R.; Cao W.; Fei W. High-Energy Storage Density and Excellent Temperature Stability in Antiferroelectric/Ferroelectric Bilayer Thin Films. J. Am. Ceram. Soc. 2017, 100, 3080–3087. 10.1111/jace.14876. [DOI] [Google Scholar]
- Sun B.; Guo M.; Wu M.; Ma Z.; Gao W.; Sun H.; Lou X. Large Enhancement of Energy Storage Density in (Pb0.92 la0.08)(Zr0.65 ti0.35)O3/PbZrO3 Multilayer Thin Film. Ceram. Int. 2019, 45, 20046–20050. 10.1016/j.ceramint.2019.06.266. [DOI] [Google Scholar]
- Wang X.; Zhang L.; Hao X.; An S. High Energy-Storage Performance of 0.9Pb(Mg1/3Nb2/3)O3-0.1PbTiO3 Relaxor Ferroelectric Thin Films Prepared by Rf Magnetron Sputtering. Mater. Res. Bull. 2015, 65, 73–79. 10.1016/j.materresbull.2015.01.038. [DOI] [Google Scholar]
- Yao Y.; Li Y.; Sun N.; Du J.; Li X.; Zhang L.; Zhang Q.; Hao X. High Energy-Storage Performance of BNT-BT-Nn Ferroelectric Thin Films Prepared by Rf Magnetron Sputtering. J. Alloys Compd. 2018, 750, 228–234. 10.1016/j.jallcom.2018.03.383. [DOI] [Google Scholar]
- Wang C.; Sun N.; Hao X. Dielectric Property and Energy-Storage Performance Of (1-X)PbTiO3-Xbi(Mg0.5 Zr0.5)O3 Relaxor Ferroelectric Thin Films. J. Mater. Sci.: Mater. Electron 2020, 31, 2063–2072. 10.2139/ssrn.3694124. [DOI] [Google Scholar]
- Xu Z.; Hao X.; An S. Dielectric Properties and Energy-Storage Performance of (Na0.5 Bi0.5)TiO3-SrTiO3 Thick Films Derived from Polyvinylpyrrolidone-Modified Chemical Solution. J. Alloys Compd. 2015, 639, 387–392. 10.1016/j.jallcom.2015.03.133. [DOI] [Google Scholar]
- Yang B.; Guo M.; Tang X.; Wei R.; Hu L.; Yang J.; Song W.; Dai J.; Lou X.; Zhu X.; et al. Lead-Free A2 Bi4Ti15O18 Thin Film Capacitors (a = Ba and Sr) with Large Energy Storage Density, High Efficiency, and Excellent Thermal Stability. J. Mater. Chem. C 2019, 7, 1888–1895. 10.1039/C8TC05558K. [DOI] [Google Scholar]
- Zhao Y.; Hao X.; Zhang Q. Energy-Storage Properties and Electrocaloric Effect of Pb(1–3x/2)Lax Zr0.85Ti0.15O3 Antiferroelectric Thick Films. ACS Appl. Mater. Interfaces 2014, 6, 11633–11639. 10.1021/am502415z. [DOI] [PubMed] [Google Scholar]
- Kühnel K.; Czernohorsky M.; Mart C.; Weinreich W. High-Density Energy Storage in Si-Doped Hafnium Oxide Thin Films on Area-Enhanced Substrates. J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom. 2019, 37, 021401. 10.1116/1.5060738. [DOI] [Google Scholar]
- Tang Z.; Ge J.; Ni H.; Lu B.; Tang X.-G.; Lu S.-G.; Tang M.; Gao J. High Energy-Storage Density of Lead-Free BiFeO3 Doped Na0.5Bi0.5TiO3-BaTiO3 Thin Film Capacitor with Good Temperature Stability. J. Alloys Compd. 2018, 757, 169–176. 10.1016/j.jallcom.2018.05.072. [DOI] [Google Scholar]
- Song D. P.; Yang J.; Yang B. B.; Wang Y.; Chen L. Y.; Wang F.; Zhu X. B. Energy Storage in BaBi4Ti4O15 Thin Films with High Efficiency. J. Appl. Phys. 2019, 125, 134101. 10.1063/1.5086515. [DOI] [Google Scholar]
- Yang B. B.; Guo M. Y.; Song D. P.; Tang X. W.; Wei R. H.; Hu L.; Yang J.; Song W. H.; Dai J. M.; Lou X. J.; et al. Bi3.25La0.75Ti3O12 Thin Film Capacitors for Energy Storage Applications. Appl. Phys. Lett. 2017, 111, 183903. 10.1063/1.4997351. [DOI] [Google Scholar]
- Xie Z.; Peng B.; Zhang J.; Zhang X.; Yue Z.; Li L. Effects of Thermal Anneal Temperature on Electrical Properties and Energy-Storage Density of Bi(Ni1/2 Ti1/2)O3-PbTiO3 Thin Films. Ceram. Int. 2015, 41, S206–S212. 10.1016/j.ceramint.2015.03.297. [DOI] [Google Scholar]
- Zhang L.; Yang B.; Yu J.; Deng Y.; Huang K.; Zhang M.; Zhu X. Energy Storage Properties in SrTiO3-Bi3.25La0.75Ti3O12 Thin Films. J. Alloys Compd. 2019, 799, 66–70. 10.1016/j.jallcom.2019.05.333. [DOI] [Google Scholar]
- Song B.; Wu S.; Li F.; Chen P.; Shen B.; Zhai J. Excellent Energy Storage Density and Charge-Discharge Performance of a Novel Bi0.2Sr0.7TiO3-BiFeO3 Thin Film. J. Mater. Chem. C 2019, 7, 10891–10900. 10.1039/C9TC03032H. [DOI] [Google Scholar]
- Lomenzo P. D.; Chung C.-C.; Zhou C.; Jones J. L.; Nishida T. Doped Hf0.5Zr0.5O2 for High Efficiency Integrated Supercapacitors. Appl. Phys. Lett. 2017, 110, 232904. 10.1063/1.4985297. [DOI] [Google Scholar]
- Xie Z.; Yue Z.; Peng B.; Li L. Effect of PbO Excess on the Microstructure, Dielectric and Piezoelectric Properties, and Energy-Storage Performance of Bi(Ni1/2 Ti1/2)O3-PbTiO3 Thin Films. Jpn. J. Appl. Phys. 2014, 53, 08NA02. 10.7567/JJAP.53.08NA02. [DOI] [Google Scholar]
- Hao X.; Wang Y.; Zhang L.; Zhang L.; An S. Composition-Dependent Dielectric and Energy-Storage Properties of (Pb,La)(Zr,Sn,Ti)O3 Antiferroelectric Thick Films. Appl. Phys. Lett. 2013, 102, 163903. 10.1063/1.4802794. [DOI] [Google Scholar]
- Yang B. B.; Guo M. Y.; Song D. P.; Tang X. W.; Wei R. H.; Hu L.; Yang J.; Song W. H.; Dai J. M.; Lou X. J.; et al. Energy Storage Properties in BaTiO3-Bi3.25La0.75Ti3O12 Thin Films. Appl. Phys. Lett. 2018, 113, 183902. 10.1063/1.5053446. [DOI] [Google Scholar]
- Ali F.; Liu X.; Zhou D.; Yang X.; Xu J.; Schenk T.; Müller J.; Schroeder U.; Cao F.; Dong X. Silicon-Doped Hafnium Oxide Anti-Ferroelectric Thin Films for Energy Storage. J. Appl. Phys. 2017, 122, 144105. 10.1063/1.4989908. [DOI] [Google Scholar]
- Yang C. H.; Yao Q.; Qian J.; Han Y. J.; Chen J. Growth, Microstructure, Energy-Storage and Dielectric Performances of Chemical-Solution Nbt-Based Thin Films: Effect of Sodium Nonstoichimometry. Ceram. Int. 2018, 44, 9152–9158. 10.1016/j.ceramint.2018.02.123. [DOI] [Google Scholar]
- Yang B. B.; Guo M. Y.; Jin L. H.; Tang X. W.; Wei R. H.; Hu L.; Yang J.; Song W. H.; Dai J. M.; Lou X. J.; et al. Ultrahigh Energy Storage in Lead-Free BiFeO3/Bi3.25La0.75Ti3O12 Thin Film Capacitors by Solution Processing. Appl. Phys. Lett. 2018, 112, 033904. 10.1063/1.5002143. [DOI] [Google Scholar]
- Yang C.; Yao Q.; Qian J.; Han Y.; Chen J. Comparative Study on Energy Storage Performance of Na0.5 bi0.5(Ti,W,Ni)O3 Thin Films with Different Bismuth Contents. Ceram. Int. 2018, 44, 9643–9648. 10.1016/j.ceramint.2018.02.191. [DOI] [Google Scholar]
- Pan H.; Ma J.; Ma J.; Zhang Q.; Liu X.; Guan B.; Gu L.; Zhang X.; Zhang Y.-J.; Li L.; et al. Giant Energy Density and High Efficiency Achieved in Bismuth Ferrite-Based Film Capacitors Via Domain Engineering. Nat. Commun. 2018, 9, 1813. 10.1038/s41467-018-04189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; Lv P.; Qian J.; Han Y.; Ouyang J.; Lin X.; Huang S.; Cheng Z. Fatigue-Free and Bending-Endurable Flexible Mn-Doped Na0.5Bi0.5TiO3-BaTiO3-BiFeO3 Film Capacitor with an Ultrahigh Energy Storage Performance. Adv. Energy Mater. 2019, 9, 1803949. 10.1002/aenm.201803949. [DOI] [Google Scholar]
- Wang K.; Ouyang J.; Wuttig M.; Zhao Y.-Y.; Cheng H.; Zhang Y.; Su R.; Yan J.; Zhong X.; Zeng F. Superparaelectric (Ba0.95,Sr0.05)(Zr0.2,Ti0.8)O3 Ultracapacitors. Adv. Energy Mater. 2020, 10, 2001778. 10.1002/aenm.202001778. [DOI] [Google Scholar]
- Kim J.; Saremi S.; Acharya M.; Velarde G.; Parsonnet E.; Donahue P.; Qualls A.; Garcia D.; Martin L. W. Ultrahigh Capacitive Energy Density in Ion-Bombarded Relaxor Ferroelectric Films. Science 2020, 369, 81–84. 10.1126/science.abb0631. [DOI] [PubMed] [Google Scholar]
- Levin I.; Laws W. J.; Wang D.; Reaney I. M. Designing Pseudocubic Perovskites with Enhanced Nanoscale Polarization. Appl. Phys. Lett. 2017, 111, 212902. 10.1063/1.5007700. [DOI] [Google Scholar]
- Lin Y.; Li D.; Zhang M.; Zhan S.; Yang Y.; Yang H.; Yuan Q. Excellent Energy-Storage Properties Achieved in BaTiO3-Based Lead-Free Relaxor Ferroelectric Ceramics Via Domain Engineering on the Nanoscale. ACS Appl. Mater. Interfaces 2019, 11, 36824–36830. 10.1021/acsami.9b10819. [DOI] [PubMed] [Google Scholar]
- Wu L.; Wang X.; Li L. Core-Shell BaTiO3@BiScO3 Particles for Local Graded Dielectric Ceramics with Enhanced Temperature Stability and Energy Storage Capability. J. Alloys Compd. 2016, 688, 113–121. 10.1016/j.jallcom.2016.07.057. [DOI] [Google Scholar]
- Zhang Y.; Cao M.; Yao Z.; Wang Z.; Song Z.; Ullah A.; Hao H.; Liu H. Effects of Silica Coating on the Microstructures and Energy Storage Properties of BaTiO3 Ceramics. Mater. Res. Bull. 2015, 67, 70–76. 10.1016/j.materresbull.2015.01.056. [DOI] [Google Scholar]
- Yang H.; Liu P.; Yan F.; Lin Y.; Wang T. A Novel Lead-Free Ceramic with Layered Structure for High Energy Storage Applications. J. Alloys Compd. 2019, 773, 244–249. 10.1016/j.jallcom.2018.09.252. [DOI] [Google Scholar]
- Heath J. P.; Harding J. H.; Sinclair D. C.; Dean J. S. Electric Field Enhancement in Ceramic Capacitors Due to Interface Amplitude Roughness. J. Eur. Ceram. Soc. 2019, 39, 1170–1177. 10.1016/j.jeurceramsoc.2018.10.033. [DOI] [Google Scholar]