Abstract
Activation of μ, δ, and κ opioid receptors by endogenous opioid peptides leads to the regulation of many emotional and physiological responses. The three major endogenous opioid peptides, β-endorphin, enkephalins, and dynorphins result from the processing of three main precursors: proopiomelanocortin, proenkephalin, and prodynorphin. Using a knockout approach, we sought to determine whether the absence of endogenous opioid peptides would affect the expression or activity of opioid receptors in mice lacking either proenkephalin, β-endorphin, or both. Since gene knockout can lead to changes in the levels of peptides generated from related precursors by compensatory mechanisms, we directly measured the levels of Leu-enkephalin and dynorphin-derived peptides in the brain of animals lacking proenkephalin, β-endorphin, or both. We find that whereas the levels of dynorphin-derived peptides were relatively unaltered, the levels of Leu-enkephalin were substantially decreased compared to wild-type mice suggesting that preproenkephalin is the major source of Leu-enkephalin. This data also suggests that the lack of β-endorphin and/or proenkephalin does not lead to a compensatory change in prodynorphin processing. Next, we examined the effect of loss of the endogenous peptides on the regulation of opioid receptor levels and activity in specific regions of the brain. We also compared the receptor levels and activity in males and females and show that the lack of β-endorphin and/or proenkephalin leads to differential modulation of the three opioid receptors in a region- and gender-specific manner. These results suggest that endogenous opioid peptides are important modulators of the expression and activity of opioid receptors in the brain.
Electronic supplementary material
The online version of this article (10.1007/s10571-020-01015-w) contains supplementary material, which is available to authorized users.
Keywords: Opioid receptors, Enkephalins, Endorphins, Dynorphins, GPCR
Introduction
The endogenous opioid system, comprising three major sets of endogenous peptide ligands, β-endorphin, enkephalins, and dynorphins activating the three main types of opioid receptors (μOR, δOR, and κOR aka MOPR, DOPR and KOPR), has been implicated in modulation of emotional and physiological responses (Toubia and Khalife 2019; Vaccarino and Kastin 2000). While the effects of exogenous opiates such as morphine and fentanyl are well established and include antinociception, altered mood, and modulation of reward, the roles of individual opioid receptors and their endogenous ligands in modulating these processes were difficult to interpret till the generation of knockout mice due to low receptor selectivity of most endogenous peptides (Gaveriaux-Ruff and Kieffer 2002). The generation of receptor knockout mice has permitted the evaluation of the contribution of individual opioid receptor types towards analgesia (Gaveriaux-Ruff and Kieffer 2002), and demonstrated opposing roles for μ and δ opioid receptors in the regulation of emotional responses (Filliol et al. 2000).
The endogenous opioid peptides, β-endorphin, enkephalins, and dynorphins, are generated by differential post-translational processing of proopiomelanocortin (POMC), proenkephalin, and prodynorphin, respectively, at classical basic amino acid (Lys, Arg) processing sites by prohormone convertases (Day et al. 1998; Zhou et al. 1999; Hoshino and Lindberg 2012). While β-endorphin and dynorphins are solely derived from POMC and prodynorphin, respectively, it was generally thought that Leu-enkephalin is derived from both proenkephalin and prodynorphin (Day et al. 1998; Zhou et al. 1999; Hoshino and Lindberg 2012). However, the relative contribution of each precursor to the endogenous pool of Leu-enkephalin has been a matter of debate. To examine this, in this study, we quantified the levels of Leu-enkephalin in animals lacking proenkephalin and find a significant reduction in levels of this peptide supporting the idea that the majority of Leu-enkephalin arises from proenkephalin.
For a number of years, it was generally thought that β-endorphin is a μ opioid receptor (μOR) agonist, while enkephalins are δ opioid receptor (δOR) agonists and dynorphins are κ opioid receptor ((κOR) agonists (Hollt 1986; Schoffelmeer et al. 1991). However, studies have revealed that endogenous opioid peptides can bind to and signal through the 3 types of opioid receptors (Mansour et al. 1995; Gomes et al. 2020) and this makes it difficult to assign individual roles for these peptides in vivo. Genetic deletion of the peptide precursors has helped elucidate the physiological roles of peptides derived from these precursors; for example, lack of proenkephalin leads to a decreased pain threshold, increased anxiety, and decreased morphine tolerance (Chen et al. 2008; Kung et al. 2010; Nitsche et al. 2002; Ragnauth et al. 2001), while lack of β-endorphin reduces stress-induced analgesia (Rubinstein et al. 1996), and selectively blocks the acute morphine tolerance observed following partial sciatic nerve ligation (Petraschka et al. 2007). Similarly, lack of prodynorphin has been shown to lead to mild hyperalgesia in the late phase of the formalin test (Wang et al. 2001), and a return to basal levels of thermal and mechanical sensitivities in a neuropathic pain model (Wang et al. 2001), implicating a complex role for prodynorphin peptides in nociception (being antinociceptor or pronociceptive depending on the pain model used). The roles of endogenous opioid peptides have been extensively examined using behavioral paradigms in which the levels of the peptides are likely increased (e.g., pain and anxiety); though less is known about the extent to which these peptides regulate opioid receptors in the absence of stress. Mice lacking opioid peptides can be useful tools to infer receptor–ligand interactions in vivo. For example, proenkephalin knockout mice show region-specific upregulation of μOR and δOR binding (Clarke et al. 2003; Brady et al. 1999); however, the role of endogenous opioid peptides in regulating receptor protein levels (Mogil et al. 2000) or signaling activity has not been extensively characterized.
In this study, we examined the roles of enkephalins and/or β-endorphin in the regulation of μOR, δOR, and κOR levels and activity in the brain by using mice lacking proenkephalin and/or β-endorphin. We examined this regulation in brain regions rich in opioid receptors: the midbrain which contains multiple opiate targets (important for descending pain modulation, reward, motivation, cognition, and drug abuse), the cerebral cortex (important in behaviors/mood), and the striatum (important in motivation, reinforcement, and reward). We also compared activity in males and females, since gender-specific effects of opioid agonists on opioid receptors have been reported (Kest et al. 2000; Zubieta et al. 1999; Cicero et al. 2000). We show that endogenous opioid peptides are involved in modulating not only the level of receptor protein but also the activity of the three opioid receptors in the various brain regions examined.
Materials and Methods
Materials
Superdex Peptide HR 10/30 gel exclusion columns were obtained from Amersham Biosciences, Piscataway, NJ, USA. [35S]GTPγS, [3H] DAMGO, [3H]Deltorphin II, and [3H]U69,593 were obtained from Perkin Elmer, Shelton, CT, USA. HEPES, MgCl2, NaCl, GDP, GTPγS, and U69,593 were obtained from Sigma-Aldrich, St. Louis, MO, USA. DAMGO and Deltorphin II were obtained from Tocris Bioscience, Ellisville, MO, USA.
Animals
Mice lacking either enkephalins (Enk−/−), β-endorphin (End−/−), or both peptides (Enk−/−/End−/−) were generated as described previously (Hayward et al. 2002, 2004). All three strains were backcrossed to C57BL/6 J mice from Jackson Laboratories for at least 10 consecutive generations. End−/− mice were previously shown to lack β-endorphin but not adrenocorticotropin or α-melanocyte-stimulating hormones (Rubinstein et al. 1996). The Enk−/− mice were previously shown to lack both enkephalin as well as BAM 18 immunoreactivity (Nitsche et al. 2002; Ragnauth et al. 2001; Konig et al. 1996). The four genotypes used in the present study were wild-type (WT), enkephalin-deficient (Enk−/−), β-endorphin-deficient (End−/−), and double knockout mice (Enk−/−/End−/−). Genotyping was performed as previously described (Hayward et al. 2002). All subjects were housed in groups in a 12-h light/dark cycle with food (PicoLab Mouse Diet 20; PMI Feeds, Inc., St Louis, MO; composition: 5% fat, 19% protein, and 5% fiber; 3.4 kcal/g) and water available ad libitum. All procedures involving the animals were approved by the Institutional Animal Care and Use Committee at Vollum Institute, Oregon Health and Science University and at Robert Wood Johnson Medical School, and followed the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Brain Extraction, Gel Filtration, and Radioimmunoassays
Brains from age- and sex-matched Enk−/−, End−/−, Enk−/−/End−/−, or congenic C57BL/6 J (> 10 backcross generations) control (WT) littermates were collected, frozen in liquid nitrogen, and ground into a fine powder. Ten volumes of 0.1 M boiling acetic acid were added and the samples were homogenized and incubated at 100 °C for 15 min. After cooling on ice, the samples were centrifuged for 30 min at 12,000×g at 4 °C. The supernatants were transferred to fresh tubes, dried in a SpeedVac System, and stored at − 80 °C until ready to use.
For gel filtration chromatography, dried samples were rehydrated in 100 mM sodium phosphate buffer (pH 7.4) containing 0.1% Triton X-100. The samples were adjusted to 30% acetonitrile and 0.1% trifluoroacetic acid (TFA), applied to a Superdex Peptide HR 10/30 gel exclusion column (Amersham Bioscience) and fractionated in 30% acetonitrile and 0.1% TFA at a flow rate of 0.5 ml/min. One-min fractions were collected, dried, resuspended, and subjected to radioimmunoassay (RIA).
RIAs for prodynorphin-derived peptides were carried out essentially as previously described (Berman et al. 1994, 1995) using Dyn A8 antisera that is directed against the COOH-terminal portion of Dyn A8 peptide and does not recognize COOH-terminal extensions (Cone and Goldstein 1982; Cone et al. 1983; Xie and Goldstein 1987). The antiserum to measure Leu-enkephalin that does not recognize C- or N-terminal extensions of Leu-enkephalin (Weber et al. 1982) was used to measure immunoreactive Leu-enkephalin as previously described (Devi et al. 1989). Trypsin/CPB treatment to release Leu-enkephalin from enkephalin-containing peptides was carried out as described (Fricker et al. 1996).
Generation and Characterization of Monoclonal Antibodies
Monoclonal antibodies (mAb) to mouse μOR (1A4), δOR (2B1), and κOR (7AG-9) were generated as described previously (Gupta et al. 2007; Gupta and Devi 2006). The μOR mAB (1A4) was generated against the 14SDPLAPASCSPA25 sequence in mouse μOR, the δOR mAb (2B1) to the 3LVPSARAELQSSPLV17 sequence in mouse δOR, and the κOR mAB (7AG-9) to the 46DQQLEPAHISPA57 sequence in rat κOR. These antibodies were characterized for receptor selectivity by ELISA using CHO cells alone or expressing individual opioid receptors as well as membranes from wild-type mice or mice lacking μOR, δOR, or κOR as previously described (Gupta et al. 2007; Gupta and Devi 2006). These antibodies exhibit receptor selectivity since the 1A4 antibody recognizes an epitope only in cells expressing μOR and in wild-type, δOR knockout, κOR knockout but not μOR knockout tissue; the 2B1 antibody recognizes an epitope only in cells expressing δOR and in wild-type, μOR knockout, κOR knockout, but not δOR knockout tissue; the 7AG-9 antibody recognizes an epitope only in cells expressing κOR and in wild-type, μOR knockout, δOR knockout, but not κOR knockout tissue (Supl. Fig. S1).
Enzyme-Linked Immunosorbent Assay (ELISA)
Brain regions from age-matched Enk−/−, End−/−, Enk−/−/End−/−, or control littermates were collected by gross dissection and membranes prepared as described previously (Gomes et al. 2004). For measuring the level of receptor recognition by μOR (1A4), δOR (2B1), or κOR (7AG-9) monoclonal antibodies, ELISA was carried out with CHO cells alone or expressing either μOR, δOR, or κOR (2 × 105 cells /well) or membranes (10 μg) from the cortex, midbrain, or striatum using 1:500 dilution of primary antibodies, and 1:500 dilution of HRP-conjugated anti-mouse IgG (Vector Laboratories) as described previously (Gupta and Devi 2006; Gupta et al. 2010).
In order to correlate antibody recognition with receptor levels (Supl. Fig. S1), ELISA was carried out with a suspension of CHO cells expressing either μOR, δOR, or κOR (0–4 × 106 cells) as described previously (Gupta et al. 2008). Total cell number (4 × 106 cells) was kept constant using CHO cells. Cells were probed with 1:500 dilution of either μOR (1A4), δOR (2B1), or κOR (7AG-9) monoclonal antibodies and 1:500 dilution of HRP-conjugated anti-mouse IgG. Binding assays were carried out in a parallel set of tubes using 10 nM [3H]DAMGO, [3H]Deltorphin II, or [3H]U69,593 in 50 mM Tris–Cl buffer pH 7.4 containing 0.32 M sucrose as described previously (Gomes et al. 2004). Non-specific binding was determined in the presence of 1 μM DAMGO, Deltorphin II, or U69,593 and was less than 10% of the total binding.
[35S]GTPγS-Binding Assay
Brain membranes from age-matched Enk−/−, End−/−, Enk−/−/End−/−, or control littermates were suspended in 50 mM HEPES pH 7.5 containing 5 mM MgCl2, 100 mM NaCl, 10 μM GDP, 0.1 nM [35S]GTPγS, and the agonist 10 μM DAMGO, Deltorphin II, or U69,593 in a final volume of 500 μl. Basal binding was determined in the presence of GDP and absence of agonist, whereas non-specific binding was determined in the presence of 10 μM cold GTPγS. After 1 h at 30 °C, membranes were filtered and washed three times with ice-cold 20 mM HEPES (pH 7.5) using a Brandel cell harvester. Bound radioactivity was determined following overnight incubation in scintillation fluid. Agonist stimulation of [35S]GTPγS binding was expressed as percent of basal values.
Statistical Analysis
Data analyses were carried out using one-way or two-way ANOVAs depending on a given experimental design, followed by appropriate post-hoc pair-wise tests adjusted for multiple comparisons using GraphPad prism version 8. Values p < 0.05 were considered to be significant.
Results
Characterization of Endogenous Peptide Levels in Knockout Animals
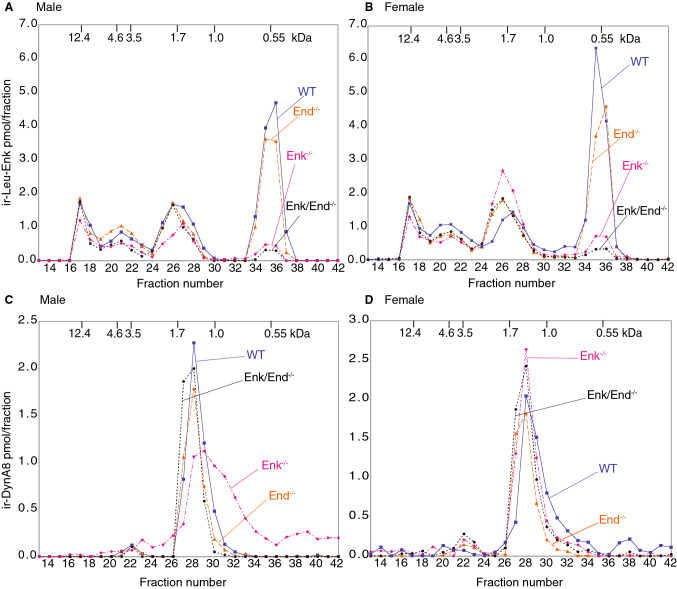
In order to determine whether deletion of enkephalin-encoding sequences from the preproenkephalin gene, or the targeted deletion of β-endorphin from the POMC gene led to compensatory changes in opioid peptide levels, we examined the levels of Leu-enkephalin and of the prodynorphin-derived peptide, Dyn A8, in the whole brain of wild-type, Enk−/−, End−/−, or Enk−/−/End−/− mice of both sexes. For this, we extracted peptides from the brains using a hot acid extraction protocol, separated them by gel filtration, and subjected aliquots to RIA using Leu-enkephalin or Dyn A8 antisera. We find that the levels of free Leu-enkephalin peptide (M.W. 0.55 kDa) are substantially decreased to < 10% in both Enk−/− and Enk−/−/End−/− mice as compared to wild-type controls (Fig. 1 and Supl. Fig. S2). The levels of Leu-enkephalin intermediates were not significantly altered (although a slight decrease was observed in the 1.7 κDa peak in male Enk−/− and a slight increase in female mice). Also, the higher molecular weight forms (12.4–3.5 kDa) representing Dyn-containing intermediates were not significantly altered in any of the groups (Fig. 1a, b). Together, these results support the idea that the major source of fully processed Leu-enkephalin pentapeptide is proenkephalin (and not prodynorphin) and that in the absence of proenkephalin there is no substantial compensatory upregulation of Leu-enkephalin or Leu-enkephalin-containing dynorphin peptides.
Fig. 1.
Analysis of immunoreactive Leu-enkephalin and Dyn A8 in mouse brain. Extracts from the brains of 3 age- and sex-matched wild-type, Enk−/−, End−/−, and End−/−/ Enk−/− mice were pooled, and 100 μl was subjected to gel filtration chromatography on Superdex Peptide 10/30 column as described in Methods. a, b Gel filtration fractions from male (a) and female (b) mice were analyzed for immunoreactive Leu-enkephalin as described in Methods. c, d Gel filtration fractions from male (c) and female (d) mice were analyzed for immunoreactive Dyn A8 as described in Methods. Molecular mass calibration standards are as follows: cytochrome c, 12.4 kDa; ACTH, 4.6 kDa; β-endorphin, 3.5 kDa; α-MSH, 1.7 kDa; Dyn A8, 1.0 kDa, Leu-enkephalin, 0.55 kDa. ir, immunoreactive
Next, we examined the extent of dynorphin processing by measuring the levels of processed dynorphin peptides in gel filtration fractions using an antiserum directed against the C-terminal portion of the Dyn A8 peptide that recognizes the fully processed form. There were no significant changes in Dyn A8 levels in any of the knockout animal groups compared to wild-type animals of either sex (Fig. 1, Supl. Fig. S2). The shift in the peak in male Enk−/− mice is presumably due to a shift during fraction collection and does not represent a change in levels as evident from the area under the curve measurements (Supl. Fig. S2); hence the relative levels of Dyn A8 were unchanged across all study groups. Taken together, these results suggest that the lack of opioid peptides derived from POMC or proenkephalin does not lead to alterations in the levels and/or processing of prodynorphin.
Characterization of μOR Levels and Activity
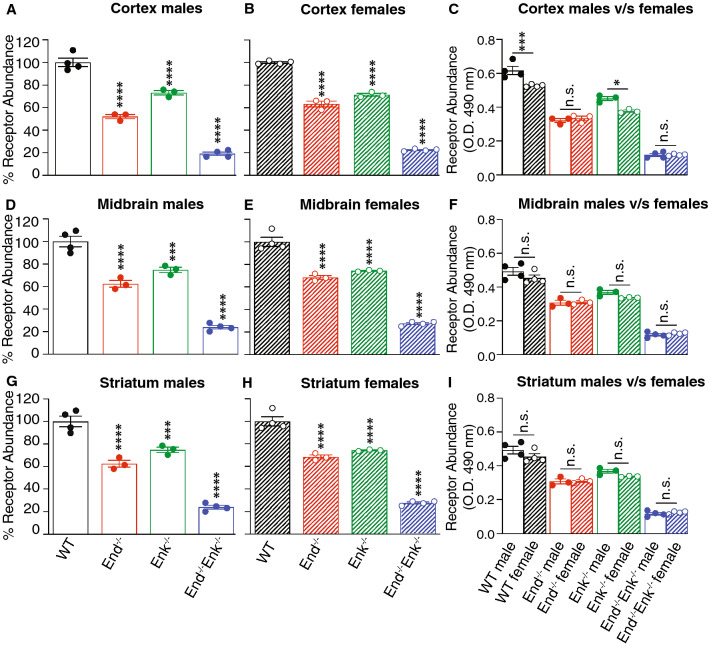
Next we investigated μOR abundance and activity in Enk−/−, End−/−, and End−/−/ Enk−/− mice of both sexes. First, we quantified the receptor abundance using μOR monoclonal antibodies; these antibodies are receptor-selective and suitable for quantitation of receptor abundance (Supl. Fig. S1). The enzyme-based ELISA assay was used since it tends to be simple, straightforward, and allows for a rapid quantitation of receptor levels when the amount of material is limiting. Also, we find that a linear relationship exists between receptor recognition by these antibodies and receptor levels detected by radioligand binding studies (Supl. Fig. S1). Using the ELISA assay to measure receptor abundance, we find that the levels of μOR are significantly lower in the cortex, midbrain, and striatum of all three genotypes as compared to wild-type mice (Fig. 2). Comparison of μOR levels between males and females in wild-type, Enk−/−, End−/−, and End−/−/ Enk−/− mice does not show significant differences between the sexes in the cortex and striatum (Fig. 2c, i), while a significant increase is seen in the midbrain of wild-type and End−/− female mice (Fig. 2f).
Fig. 2.
μOR levels in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/−mice. Membranes (10 μg) from the cortex (a–c), midbrain (d–f), and striatum (g–i) from male (a, d, g) and female (b, e, h) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to ELISA using monoclonal antibodies selective for μOR (1A4). Values obtained with wild-type membranes were taken as 100%. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. (c, f, i) Comparison of μOR levels between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; **p < 0.01; ***p < 0.001, ****p < 0.0001. Data represent Mean ± SE of 3–4 animals in triplicate
Next, we examined μOR signaling in the three brain regions using the [35S]GTPγS-binding assay. First, we focused on basal levels of [35S]GTPγS binding (i.e., in the absence of the agonist). We find that compared to wild-type mice, basal levels are significantly decreased in the cortex (Fig. 3a, b) of Enk−/−, End−/−, and End−/−/ Enk−/− mice of both sexes, and significantly increased in the midbrain (Fig. 3d, e). In the striatum a significant decrease in basal [35S]GTPγS binding is seen only in male End−/− (Fig. 3g) and in female Enk−/− mice, while a significant increase is seen only in female End−/−, and End−/−/ Enk−/− mice (Fig. 3h). Comparison of basal [35S]GTPγS-binding between males and females in wild-type, Enk−/−, End−/−, and End−/−/ Enk−/− mice shows that basal levels are significantly lower in the cortex of female wild-type, Enk−/−, End−/− mice (Fig. 3c), in the midbrain of female End−/− and End−/−/ Enk−/− mice (Fig. 3f), and in the striatum of female Enk−/− mice (Fig. 3i); the basal levels were found to be significantly higher in the cortex and striatum of female End−/−/ Enk−/− mice (Fig. 3c, i), and in the midbrain of female Enk−/− mice (Fig. 3f).
Fig. 3.
Basal levels of [35S]GTPγS binding in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/− mice. Membranes (10 μg) from the cortex (a–c), midbrain (d–f), and striatum (g–i) from male (a, d, g) and female (b, e, h) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to a [35S]GTPγS-binding assay as described in Methods. Basal values were obtained in the absence of agonist treatment and in the presence of GDP. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. c, f, i Comparison of basal levels of [35S]GTPγS binding between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Next, we examined the agonist (DAMGO)-mediated increase in [35S]GTPγS binding (Fig. 4), and calculated the receptor signaling efficiency by determining the level of G-protein activity/unit of protein (% [35S]GTPγS binding/fmol of μOR). We find that there is a significant increase in μOR signaling efficiency (p < 0.05) for males and a trend towards increase in females in cortical membranes of End−/− mice (Fig. 4d, e). In Enk−/− mice, there is a significant increase (p < 0.001) in μOR signaling efficiency for males and a trend towards increase for females compared to wild-type controls (Fig. 4d, e). In End−/−/ Enk−/− mice, there is an increase in μOR signaling efficiency for both sexes (p < 0.0001 for males and p < 0.05 for females) compared to wild-type controls (Fig. 4d, e). Comparison of DAMGO-mediated increases in [35S]GTPγS binding between males and females shows that there are no significant differences between wild-type, Enk−/−, End−/−, and End−/−/ Enk−/− (Fig. 4c), although the μOR signaling efficiency is significantly decreased in female Enk−/− (p < 0.05), and End−/−/ Enk−/− (p < 0001) mice (Fig. 4f).
Fig. 4.
μOR signaling efficiency in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/− mice. Membranes (10 μg) from the cortex (a–f), midbrain (g–l), and striatum (m–r) from male (a, d, g, j, m, p) and female (b, e, h, k, n, q) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to a [35S]GTPγS-binding assay using a μOR agonist ( DAMGO;10 μM) as described in Methods. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. Signaling efficiency in (d–f; j–l; p–r) is expressed as % [35S]GTPγS binding/fmol of opioid receptor. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. (c, f, i, l, o, r) Comparison of signaling between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.Data represent Mean ± SE of 3 animals in triplicate
In midbrain membranes from End−/− mice, we find a statistically significant decrease (p < 0.01) in μOR signaling efficiency in males and a significant increase (p < 0.05) in females compared to wild-type controls (Fig. 4j, k). In the Enk−/− mice, the OR signaling efficiency in both genders is significantly higher (p < 0.05) than in wild-type mice (Fig. 4j, k). In the End−/−/Enk−/− mice, the μOR signaling efficiency is similar to that of wild-type mice (Fig. 4j, k). Comparison of DAMGO-mediated increases in [35S]GTPγS binding between males and females in the midbrain shows a significant increase (p < 0.01) in female End−/− mice (Fig. 4i) and a significant decrease (p < 0.05) in μOR signaling efficiency in female wild-type and End−/−/ Enk−/− mice (Fig. 4l) compared to males.
In striatal membranes, we find that the μOR signaling efficiency is significantly lower in Enk−/− and End−/−/ Enk−/− mice of both sexes compared to wild-type controls (Fig. 4p, q). Comparison of DAMGO-mediated increases in [35S]GTPγS binding between males and females in the striatum shows significant increases (p < 0.01) in signaling (Fig. 4o) and in OR signaling efficiency (Fig. 4r) only in female Enk−/− mice compared to males. Together these results indicate that endogenous opioid peptides play a role in modulating μOR abundance and signaling.
Characterization of δOR Levels and Activity
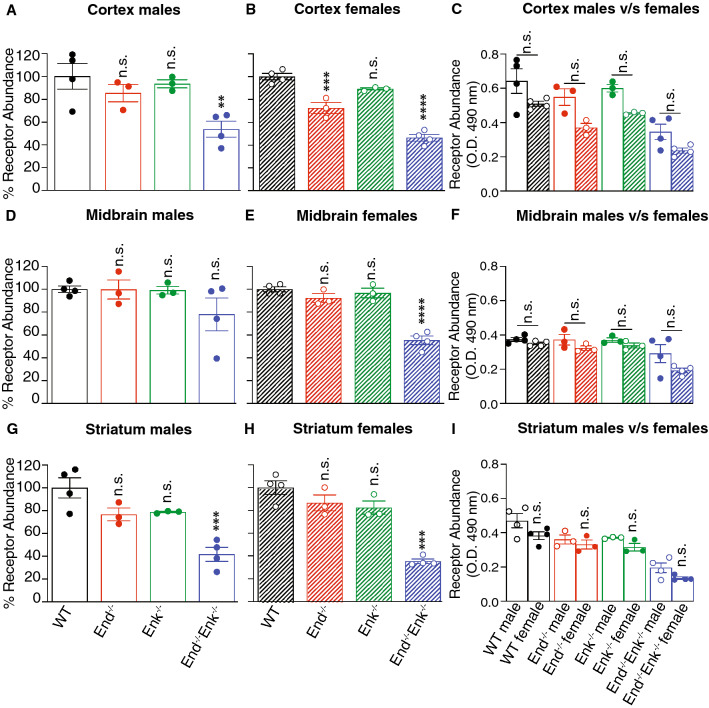
Quantification of δOR abundance by ELISA detects significant decreases in cortical, midbrain, and striatal membranes from End−/−, Enk−/−, and End−/−/ Enk−/− of both sexes compared to wild-type controls (Fig. 5). Comparison of δOR levels between males and females does not show significant differences between the sexes in the midbrain and striatum (Fig. 5f, i), while a significant decrease is seen only in the cortex of female wild-type (p < 0.001) and Enk−/− (p < 0.05) mice (Fig. 5c) compared to males.
Fig. 5.
δOR levels in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/−mice. Membranes (10 μg) from the cortex (a–c), midbrain (d–f), and striatum (g–i) from male (a, d, g) and female (b, e, h) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to ELISA using monoclonal antibodies selective for δOR (2B1). Values obtained with wild-type membranes were taken as 100%. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. (c, f, i) Comparison of δOR levels between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; **p < 0.01; ***p < 0.001, ****p < 0.0001. Data represent Mean ± SE of 3–4 animals in triplicate
Next we examined δOR activity using a δOR selective agonist, Deltorphin II (Fig. 6). In cortical membranes, the δOR signaling efficiency does not change from wild-type mice in End−/− and Enk−/− mice, but is significantly increased (p < 0.001 to p < 0.0001) in End−/−/ Enk−/− mice of both sexes (Fig. 6d, e). Comparison of δOR signaling efficiency between males and females in cortical membranes from wild-type, Enk−/−, End−/−, and End−/−/ Enk−/− mice does not show significant differences between the sexes (Fig. 6f).
Fig. 6.
δOR signaling efficiency in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/− mice. Membranes (10 μg) from the cortex (a–f), midbrain (g–l), and striatum (m–r) from male (a, d, g, j, m, p) and female (b, e, h, k, n, q) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to a [35S]GTPγS-binding assay using a δOR agonist ( Delt II;10 μM) as described in Methods. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. Signaling efficiency in (d–f; j–l; p–r) is expressed as % [35S]GTPγS binding/fmol of opioid receptor. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. (c, f, i, l, o, r) Comparison of signaling between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data represents Mean ± SE of 3 animals in triplicate
In midbrain membranes, we do not detect changes in δOR signaling efficiency in End−/− and Enk−/− of either sex compared to wild-type controls (Fig. 6j, k). Midbrain membranes from male End−/−/ Enk−/− mice exhibit no change in δOR signaling efficiency (Fig. 6j) while female mice show a statistically significant (p < 0.0001) increase (Fig. 6k) compared to wild-type mice. Comparison of δOR signaling and δOR signaling efficiency between males and females in midbrain membranes shows significant differences in signaling and efficiency only in female End−/−/ Enk−/− mice compared to males of the same genotype (Fig. 6i, l).
In striatal membranes, all genotypes show statistically significant (p < 0.0001) decreases in δOR signaling efficiency compared to wild-type controls (Fig. 6p, q). Comparison of δOR signaling efficiency between males and females in striatal membranes from wild-type, Enk−/−, End−/−, and End−/−/ Enk−/− mice shows no significant gender differences (Fig. 6o, r). Together these results indicate that endogenous opioid peptides play a role in modulating δOR levels and activity.
Characterization of κOR Levels and Activity
Quantification of κOR abundance by ELISA detects significant decreases in cortical membranes from female End−/− mice (p < 0.001) and in cortical and striatal membranes (p < 0.001) from male and female End−/−/ Enk−/− mice (Fig. 7a, b, g, h) compared to wild-type controls. Statistically significant decreases were also found in midbrain membranes from female End−/−/ Enk−/− mice (p < 0.0001) as compared to controls (Fig. 7e). A comparison of κOR levels between males and females shows no significant genotype-specific differences in any of the regions tested (Fig. 7c, f, i).
Fig. 7.
κOR levels in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/−mice. Membranes (10 μg) from the cortex (a–c), midbrain (d–f), and striatum (g–i) from male (a, d, g) and female (b, e, h) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to ELISA using monoclonal antibodies selective for κOR (7AG-9). Values obtained with wild-type membranes were taken as 100%. One-way ANOVA with Dunnett's multiple comparison tests v/s WT. (c, f, i) Comparison of δOR levels between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; **p < 0.01; ***p < 0.001, ****p < 0.0001. Data represents Mean ± SE of 3–4 animals in triplicate
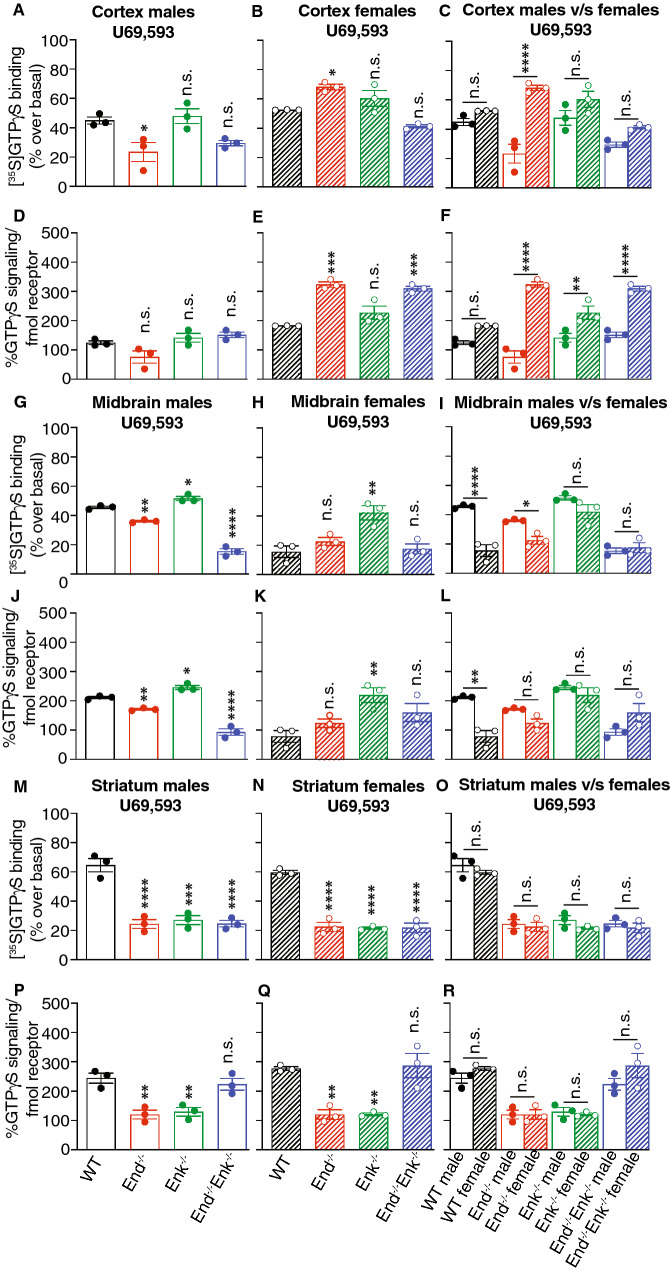
Next we examined κOR activity using a κOR selective agonist, U69,593 (Fig. 8). In cortical membranes, we detect a significant increase in κOR signaling efficiency only in female End−/− and End−/−/ Enk−/− mice (Fig. 8d, e). Comparison of κOR signaling efficiency between males and females shows a statistically significant increase in female mice from the three knockout genotypes (Fig. 8f).
Fig. 8.
κOR signaling efficiency in the cortex, midbrain, and striatum of wild-type, End−/−, Enk−/−, and End−/−/Enk−/− mice. Membranes (10 μg) from the cortex (a–f), midbrain (g–l), and striatum (m–r) from male (a, d, g, j, m, p) and female (b, e, h, k, n, q) wild-type (WT), End−/−, Enk−/−, End−/−/Enk−/− mice were subjected to a [35S]GTPγS-binding assay using a κOR agonist ( U69,593;10 μM) as described in Methods. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. Signaling efficiency in (d–f; j–l; p–r) is expressed as % [35S]GTPγS binding/fmol of opioid receptor. One-way ANOVA with Dunnett’s multiple comparison tests v/s WT. (c, f, i, l, o, r) Comparison of signaling between males and females. Two-way ANOVA with Sidak’s multiple comparison tests; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data represents Mean ± SE of 3 animals in triplicate
In midbrain membranes, we detect significant changes in κOR signaling efficiency showing either a decrease (male End−/− mice, p < 0.01; male End−/−/ Enk−/− mice, p < 0.00001) or an increase (male and female Enk−/− mice) (Fig. 8j, k). Comparison of κOR signaling efficiency between males and females shows a statistically significant decrease only in female wild-type (p < 0.0001) mice (Fig. 8l).
In striatal membranes, we find significant decreases (p < 0.01) in κOR signaling efficiency in End−/−, Enk−/− but not in End−/−/ Enk−/− mice compared to wild-type males and females (Fig. 8p, q). Comparison of κOR signaling efficiency between males and females does not show significant changes among the different genotypes (Fig. 8o, r). Together these results indicate that κOR abundance and activity are modulated by endogenous opioid peptides.
Discussion
Mouse strains lacking genes for the endogenous opioid system have been used to understand the role of the different components of this system in physiological and pathological states. Is this study, we used animals lacking proenkephalin, β-endorphin, or both to determine whether the absence of these endogenous opioid peptides has an effect on μOR, δOR, and kOR levels and activity. It is possible that the lack of these peptides during development could lead to compensatory adaptations. For example, Leu-enkephalin sequences are also present in the preprodynorphin gene in addition to the preproenkephalin gene; therefore, it is possible that in the absence of proenkephalin (in Enk−/− mice), the prodynorphin precursor could be extensively processed to Leu-enkephalin in order to compensate for the lack of enkephalins derived from preproenkephalin. However, we find no evidence for increased processing of prodynorphin in Enk−/− animals supporting the idea that most of the free Leu-enkephalin in wild-type is derived from proenkephalin.
Although enkephalins and β-endorphin are generally regarded as endogenous agonists for δOR and μOR, respectively, they have been shown to bind and signal through all three opioid receptors (Fricker et al. 2020; Gomes et al. 2020; Mansour et al. 1995). A recent study examining biased signaling by a panel of ~ 20 peptides derived from POMC, proenkephalin, and prodynorphin showed that the opioid peptides derived from these three precursors exhibited binding and signaling at all three opioid receptors (Gomes et al. 2020). Thus, it is not surprising that we find that enkephalins and β-endorphin are required to maintain the normal levels and activity of opioid receptors. The fact that we observe a differential regulation of receptor activity in different brain regions suggests a region-specific modulation of opioid receptor activity by endogenous ligands.
In the present study, using receptor-selective antibodies to measure receptor abundance, we detect decreased μOR and δOR in the cortex, midbrain, and striatum of Enk−/− mice. In contrast, previous studies using quantitative autoradiography reported no changes in binding for opioid receptors in cortical structures and increases in μOR and δOR in non-cortical regions of Enk−/− mice (Brady et al. 1999; Clarke et al. 2003). The differences seen between these studies could be due to the background of the mice (CD1 v/s C57BL/6 J in our study) or the assay used to measure receptor levels (receptor autoradiography v/s ELISA). Moreover, the autoradiographic studies measured receptor binding in discrete sub-regions within the cortex, striatum, and midbrain (with some showing either increase or decrease in binding) while we used total (not discrete sub-regions) cortex, midbrain, or striatal membranes. Another possibility is that autoradiographic studies using radiolabeled agonists detect the functional receptor at agonist configuration, while the antibodies used in this study detect active as well as inactive receptors (Gupta et al. 2007; Heimann et al. 2017).
It is not clear how endogenous opioid peptides modulate receptor levels. Their effects could be at the transcriptional level by regulating opioid receptor synthesis or at the post-translational level by modulating opioid receptor maturation and trafficking. At the transcriptional level, previous studies have identified several transcription factors (activators/repressors) that regulate opioid receptor expression (for review see (Wei and Loh 2011)). It is possible that in wild-type animals, endogenous opioid peptides maintain the tonic state of receptor levels by engaging appropriate transcription factors. This engagement of transcription factors may not occur in the absence of the opioid peptides leading to a decrease in receptor levels as seen in Enk−/−, End−/−, and Enk−/−/ End−/− mice. Another possibility is that the opioid peptide-mediated gene expression induces proteins (such as chaperones) that either stabilize and/or facilitate the maturation of the receptors. It is also possible that the endogenous opioid peptides themselves may function as chaperones and help in the proper folding and packaging of newly synthesized receptors in the endoplasmic reticulum, thus preventing receptor ubiquitination and degradation. Support for the role of an endogenous ligand as a chaperone for its cognate receptor comes from a study showing that dopamine could serve as a pharmacological chaperone and increase the surface expression of D4 dopamine receptors (Van Craenenbroeck et al. 2011). In addition, a study demonstrated colocalization of δOR with met-enkephalin in dense core vesicles in the spinal cord (Cheng et al. 1995) suggesting an exciting possibility that the proenkephalin-derived peptides could serve as δOR chaperones or regulate their cell surface insertion. For example, several studies show that δOR, localized to large peptide storing dense core vesicles, are inserted into the plasma membrane following a variety of stimuli including agonist treatment (Bao et al. 2003; Walwyn et al. 2005; Patwardhan et al. 2005; Zhang et al. 1998; Guan et al. 2005; Cheng et al. 1995; Zhao et al. 2011). It is possible that in wild-type animals enkephalins facilitate the exocytosis of δOR containing dense core vesicles leading to receptor insertion at the cell surface.
A decrease in opioid receptor levels in knockout animals would be expected to be accompanied by a decrease in opioid receptor activity compared to wild-type. However, in some instances we observe either an increase or no change in receptor signaling in mutant mice as compared to wild-type. This could be because the absence of the endogenous peptide leads to a compensatory increase in the activity of the target receptors. A possibility is that absence of the endogenous peptides leads to a decrease in the basal G-protein activity of the target receptor (see Fig. 3a) and reveals an augmented agonist-mediated G protein activity as a compensatory effect (e.g., Fig. 4a, b; Enk−/− mice). Another possibility is that the lack of endogenous opioid peptides could modulate the activity of receptor associated proteins. For example, studies show that RGS9-2, a protein that regulates the duration of G protein signaling by modulating the speed of GTP hydrolysis, negatively modulates μOR activity (Traynor et al. 2009). It is possible that enkephalins promote μOR association with RGS9-2 and set the basal receptor activity levels. The absence of the peptide could lead to a decrease in receptor association with RGS9-2 which would lead to increased receptor signaling efficacy as seen in Enk−/− mice. Alternatively, the absence of enkephalins would increase the association of the opioid receptor with other RGS proteins leading to increases in the functional activity of the receptor. Further studies are needed to evaluate how endogenous opioid peptides modulate the activity of their cognate receptors.
Another finding of this study is gender differences in the signaling efficiency of μOR, δOR, κOR in some brain regions of Enk−/−, End−/−, and Enk−/−/End−/− mice. This suggests that modulation of opioid receptor activity by endogenous ligands in these brain regions may be under the influence of sex hormones. Although a few studies have described region-specific modulation of opioid receptor levels and activity by estrogen and/or progesterone (Huhn et al. 2018) and of opioid peptide levels (Williams et al. 2011), very little is known about how individual opioid peptides contribute to these gender differences in signaling. Thus further studies are needed to elucidate the mechanisms by which sex hormones and endogenous opioid peptides contribute to the signaling efficacy of opioid receptors in specific brain regions.
In summary, our results show a decrease in protein levels of μOR, δOR, and κOR but a paradoxical increase in activity in some brain regions. This suggests that although the absence of enkephalins and/or β-endorphin decreases the expression of opioid receptors, it also leads to compensatory effects at the level of signaling as seen by increased functional activity. Taken together, these studies further extend current knowledge on the regulation of activity of opioid receptors. Although the molecular mechanisms of how endogenous opioid peptides regulate the expression and functional activity of opioid receptors is speculative at this point, results from the present study provide a foundation for future studies exploring the importance of the endogenous opioid system in the regulation of the steady-state activity of their target receptors. This, in turn, would impact the receptor activity under physiological and pathological conditions and may explain differences previously observed in opioid receptor responses to drugs and/or stress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Relationship between antibody recognition and receptor number. CHO cells expressing either µOR (A), δOR (B), or κOR (C) (0-4×106 cells) were subjected to ELISA using μOR (1A4), δOR (2B1) or κOR (7AG-9) monoclonal antibodies as described in Methods. In parallel binding assays were carried out using 10 nM [3H]DAMGO, [3H]Deltorphin II or [3H]U69,593 as described in Methods. Results are Mean±SE of 2 experiments in triplicate. r2 = correlation coefficient. (D-F) ELISA showing selectivity of μOR (D), δOR (E), and κOR (F) monoclonal antibodies in CHO cells alone or expressing either μOR, δOR, or κOR. (G-I) ELISA showing selectivity of μOR (G), δOR (H), and κOR (I) monoclonal antibodies in endogenous tissue. Results are Mean±SE of 3 experiments in quintuplicate. One-way ANOVA; Dunnett’s multiple comparison test v/s wild-type (WT); **p<0.01; ***p<0.001; ****p<0.0001. Supplementary material 1 (492 kb)
Fig. S2 Analysis of immunoreactive Leu-enkephalin and Dyn A8 in mouse brain. Extracts from the brains of 3 age- and sex-matched wild-type, Enk-/-, End-/- and End-/-/ Enk-/- mice were pooled, and 100 μl was subjected to gel filtration chromatography on Superdex Peptide 10/30 column as described in Methods. (A-B) Gel filtration fractions from male (A) and female (B) mice were analyzed for immunoreactive Leu-enkephalin as described in Methods. Area under the curve for ir-Leu-enkephalin with wild-type (WT) animals was taken as 100%. (C-D) Gel filtration fractions from male (C) and female (D) mice were analyzed for immunoreactive Dyn A8 as described in Methods. Area under the curve for ir-Dyn A8 with wild-type (WT) animals was taken as 100%. Molecular mass calibration standards are as follows: cytochrome c, 12.4 kDa; ACTH, 4.6 kDa; β-endorphin, 3.5 kDa; α-MSH, 1.7 kDa; Dyn A8, 1.0 kDa; Leu-enkephalin, 0.55. ir, immunoreactive. One-way ANOVA; Dunnett’s multiple comparison test v/s wild-type (WT); ***p<0.001; ****p<0.0001. Supplementary material 1 (432 kb)
Acknowledgements
This work was supported by the National Institute of Health Grants DA008863 and NS026880 (to LAD), DA08622 (to JP), and DK066604 (to MJL).
Abbreviations
- Dyn A8
Dynorphin A8
- ELISA
Enzyme-linked immunosorbent assay
- PAG
Periaqueductal gray
- POMC
Proopiomelanocortin
- RIA
Radioimmunoassay
- WT
Wild-type
Author Contributions
AG, SG, HP, carried out the research; DLRO helped write the manuscript; MDH, MJL and JEP generated the knockout mice and helped with experimental design, LAD helped with experimental design, data interpretation and manuscript preparation; IG carried out the statistical analysis and wrote the manuscript.
Funding
This work was supported by the National Institutes of Health Grants DA008863 and NS026880 (to LAD), DA08622 (to JP), and DK066604 (to MJL).
Data Availability
All data are included in the publication.
Compliance with Ethical Standards
Conflict of interest
There are no conflict of interests/competing interests for this work.
Ethical Approval
All procedures involving the animals were approved by the Institutional Animal Care and Use Committee at Vollum Institute, Oregon Health and Science University and at Robert Wood Johnson Medical School, and followed the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Consent to Participate
All authors consented to participate in this study.
Consent to Publication
All authors consented to publication of this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Achla Gupta and Srinivas Gullapalli have contributed equally to this work.
Contributor Information
Lakshmi A. Devi, Email: lakshmi.devi@mssm.edu
Ivone Gomes, Email: ivone.gomes@mssm.edu.
References
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hokfelt T, Zhou Z, Zhang X (2003) Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron 37(1):121–133. 10.1016/s0896-6273(02)01103-0 [DOI] [PubMed] [Google Scholar]
- Berman Y, Devi L, Carr KD (1994) Effects of chronic food restriction on prodynorphin-derived peptides in rat brain regions. Brain Res 664(1–2):49–53. 10.1016/0006-8993(94)91952-6 [DOI] [PubMed] [Google Scholar]
- Berman Y, Devi L, Carr KD (1995) Effects of streptozotocin-induced diabetes on prodynorphin-derived peptides in rat brain regions. Brain Res 685(1–2):129–134. 10.1016/0006-8993(95)00419-q [DOI] [PubMed] [Google Scholar]
- Brady LS, Herkenham M, Rothman RB, Partilla JS, Konig M, Zimmer AM, Zimmer A (1999) Region-specific up-regulation of opioid receptor binding in enkephalin knockout mice. Brain Res Mol Brain Res 68(1–2):193–197. 10.1016/s0169-328x(99)00090-x [DOI] [PubMed] [Google Scholar]
- Chen TC, Cheng YY, Sun WZ, Shyu BC (2008) Differential regulation of morphine antinociceptive effects by endogenous enkephalinergic system in the forebrain of mice. Mol Pain 4:41. 10.1186/1744-8069-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, Inturrisi CE, Pickel VM (1995) Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci 15(9):5976–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER (2000) Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav 65(1):91–96. 10.1016/s0091-3057(99)00174-4 [DOI] [PubMed] [Google Scholar]
- Clarke S, Zimmer A, Zimmer AM, Hill RG, Kitchen I (2003) Region selective up-regulation of micro-, delta- and kappa-opioid receptors but not opioid receptor-like 1 receptors in the brains of enkephalin and dynorphin knockout mice. Neuroscience 122(2):479–489. 10.1016/j.neuroscience.2003.07.011 [DOI] [PubMed] [Google Scholar]
- Cone RI, Goldstein A (1982) A dynorphin-like opioid in the central nervous system of an amphibian. Proc Natl Acad Sci USA 79(10):3345–3349. 10.1073/pnas.79.10.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RI, Weber E, Barchas JD, Goldstein A (1983) Regional distribution of dynorphin and neo-endorphin peptides in rat brain, spinal cord, and pituitary. J Neurosci 3(11):2146–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R, Lazure C, Basak A, Boudreault A, Limperis P, Dong W, Lindberg I (1998) Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J Biol Chem 273(2):829–836. 10.1074/jbc.273.2.829 [DOI] [PubMed] [Google Scholar]
- Devi L, Gupta P, Douglass J (1989) Expression and posttranslational processing of preprodynorphin complementary DNA in the mouse anterior pituitary cell line AtT-20. Mol Endocrinol 3(11):1852–1860. 10.1210/mend-3-11-1852 [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL (2000) Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25(2):195–200. 10.1038/76061 [DOI] [PubMed] [Google Scholar]
- Fricker LD, Berman YL, Leiter EH, Devi LA (1996) Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J Biol Chem 271(48):30619–30624. 10.1074/jbc.271.48.30619 [DOI] [PubMed] [Google Scholar]
- Fricker LD, Margolis E, Gomes I, Devi LA (2020) Five decades of research on opioid peptides: Current knowledge and unanswered questions. Mol Pharmacol. 10.1124/mol.120.119388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL (2002) Opioid receptor genes inactivated in mice: the highlights. Neuropeptides 36(2–3):62–71. 10.1054/npep.2002.0900 [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA (2004) A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA 101(14):5135–5139. 10.1073/pnas.0307601101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, Cox BM, Devi LA (2020) Biased signaling by endogenous opioid peptides. Proc Natl Acad Sci USA. 10.1073/pnas.2000712117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X (2005) Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell 122(4):619–631. 10.1016/j.cell.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Gupta A, Decaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, Devi LA (2007) Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem 282(8):5116–5124. 10.1074/jbc.M609254200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Devi LA (2006) The use of receptor-specific antibodies to study G-protein-coupled receptors. Mt Sinai J Med 73(4):673–681 [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA (2010) Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 3(131):54. 10.1126/scisignal.2000807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Rozenfeld R, Gomes I, Raehal KM, Decaillot FM, Bohn LM, Devi LA (2008) Post-activation-mediated changes in opioid receptors detected by N-terminal antibodies. J Biol Chem 283(16):10735–10744. 10.1074/jbc.M709454200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Hansen ST, Pintar JE, Low MJ (2004) Operant self-administration of ethanol in C57BL/6 mice lacking beta-endorphin and enkephalin. Pharmacol Biochem Behav 79(1):171–181. 10.1016/j.pbb.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Hayward MD, Pintar JE, Low MJ (2002) Selective reward deficit in mice lacking beta-endorphin and enkephalin. J Neurosci 22(18):8251–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gupta A, Gomes I, Rayees R, Schlessinger A, Ferro ES, Unterwald EM, Devi LA (2017) Generation of G protein-coupled receptor antibodies differentially sensitive to conformational states. PLoS ONE 12(11):e0187306. 10.1371/journal.pone.0187306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollt V (1986) Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol 26:59–77. 10.1146/annurev.pa.26.040186.000423 [DOI] [PubMed] [Google Scholar]
- Huhn AS, Berry MS, Dunn KE (2018) Systematic review of sex-based differences in opioid-based effects. Int Rev Psychiatry 30(5):107–116. 10.1080/09540261.2018.1514295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Palmese C, Hopkins E (2000) A comparison of morphine analgesic tolerance in male and female mice. Brain Res 879(1–2):17–22. 10.1016/s0006-8993(00)02685-8 [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A (1996) Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 383(6600):535–538. 10.1038/383535a0 [DOI] [PubMed] [Google Scholar]
- Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC (2010) Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci 17:29. 10.1186/1423-0127-17-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H (1995) The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res 700(1–2):89–98. 10.1016/0006-8993(95)00928-j [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Hayward MD, Bales JR, Rubinstein M, Belknap JK, Low MJ (2000) Disparate spinal and supraspinal opioid antinociceptive responses in beta-endorphin-deficient mutant mice. Neuroscience 101(3):709–717. 10.1016/s0306-4522(00)00422-x [DOI] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE (2002) Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci 22(24):10906–10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM (2005) Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25(39):8825–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraschka M, Li S, Gilbert TL, Westenbroek RE, Bruchas MR, Schreiber S, Lowe J, Low MJ, Pintar JE, Chavkin C (2007) The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience 146(4):1795–1807. 10.1016/j.neuroscience.2007.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW (2001) Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci USA 98(4):1958–1963. 10.1073/pnas.041598498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ (1996) Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci USA 93(9):3995–4000. 10.1073/pnas.93.9.3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Warden G, Hogenboom F, Mulder AH (1991) Beta-endorphin: a highly selective endogenous opioid agonist for presynaptic mu opioid receptors. J Pharmacol Exp Ther 258(1):237–242 [PubMed] [Google Scholar]
- Toubia T, Khalife T (2019) The Endogenous Opioid System: Role and Dysfunction Caused by Opioid Therapy. Clin Obstet Gynecol 62(1):3–10. 10.1097/GRF.0000000000000409 [DOI] [PubMed] [Google Scholar]
- Traynor JR, Terzi D, Caldarone BJ, Zachariou V (2009) RGS9-2: probing an intracellular modulator of behavior as a drug target. Trends Pharmacol Sci 30(3):105–111. 10.1016/j.tips.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino AL, Kastin AJ (2000) Endogenous opiates: 1999. Peptides 21(12):1975–2034. 10.1016/s0196-9781(00)00345-4 [DOI] [PubMed] [Google Scholar]
- Van Craenenbroeck K, Borroto-Escuela DO, Romero-Fernandez W, Skieterska K, Rondou P, Lintermans B, Vanhoenacker P, Fuxe K, Ciruela F, Haegeman G (2011) Dopamine D4 receptor oligomerization–contribution to receptor biogenesis. FEBS J 278(8):1333–1344. 10.1111/j.1742-4658.2011.08052.x [DOI] [PubMed] [Google Scholar]
- Walwyn W, Maidment NT, Sanders M, Evans CJ, Kieffer BL, Hales TG (2005) Induction of delta opioid receptor function by up-regulation of membrane receptors in mouse primary afferent neurons. Mol Pharmacol 68(6):1688–1698. 10.1124/mol.105.014829 [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP Jr, Lai J, Porreca F (2001) Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 21(5):1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Evans CJ, Chang JK, Barchas JD (1982) Brain distributions of alpha-neo-endorphin and beta-neo-endorphin: evidence for regional processing differences. Biochem Biophys Res Commun 108(1):81–88. 10.1016/0006-291x(82)91834-4 [DOI] [PubMed] [Google Scholar]
- Wei LN, Loh HH (2011) Transcriptional and epigenetic regulation of opioid receptor genes: present and future. Annu Rev Pharmacol Toxicol 51:75–97. 10.1146/annurev-pharmtox-010510-100605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Mitterling KL, Thompson LI, Torres-Reveron A, Waters EM, McEwen BS, Gore AC, Milner TA (2011) Age- and hormone-regulation of opioid peptides and synaptic proteins in the rat dorsal hippocampal formation. Brain Res 1379:71–85. 10.1016/j.brainres.2010.08.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie GX, Goldstein A (1987) Characterization of big dynorphins from rat brain and spinal cord. J Neurosci 7(7):2049–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T (1998) Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience 82(4):1225–1242 [DOI] [PubMed] [Google Scholar]
- Zhao B, Wang HB, Lu YJ, Hu JW, Bao L, Zhang X (2011) Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res 21(5):741–753. 10.1038/cr.2011.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Webb G, Zhu X, Steiner DF (1999) Proteolytic processing in the secretory pathway. J Biol Chem 274(30):20745–20748. 10.1074/jbc.274.30.20745 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Dannals RF, Frost JJ (1999) Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry 156(6):842–848. 10.1176/ajp.156.6.842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between antibody recognition and receptor number. CHO cells expressing either µOR (A), δOR (B), or κOR (C) (0-4×106 cells) were subjected to ELISA using μOR (1A4), δOR (2B1) or κOR (7AG-9) monoclonal antibodies as described in Methods. In parallel binding assays were carried out using 10 nM [3H]DAMGO, [3H]Deltorphin II or [3H]U69,593 as described in Methods. Results are Mean±SE of 2 experiments in triplicate. r2 = correlation coefficient. (D-F) ELISA showing selectivity of μOR (D), δOR (E), and κOR (F) monoclonal antibodies in CHO cells alone or expressing either μOR, δOR, or κOR. (G-I) ELISA showing selectivity of μOR (G), δOR (H), and κOR (I) monoclonal antibodies in endogenous tissue. Results are Mean±SE of 3 experiments in quintuplicate. One-way ANOVA; Dunnett’s multiple comparison test v/s wild-type (WT); **p<0.01; ***p<0.001; ****p<0.0001. Supplementary material 1 (492 kb)
Fig. S2 Analysis of immunoreactive Leu-enkephalin and Dyn A8 in mouse brain. Extracts from the brains of 3 age- and sex-matched wild-type, Enk-/-, End-/- and End-/-/ Enk-/- mice were pooled, and 100 μl was subjected to gel filtration chromatography on Superdex Peptide 10/30 column as described in Methods. (A-B) Gel filtration fractions from male (A) and female (B) mice were analyzed for immunoreactive Leu-enkephalin as described in Methods. Area under the curve for ir-Leu-enkephalin with wild-type (WT) animals was taken as 100%. (C-D) Gel filtration fractions from male (C) and female (D) mice were analyzed for immunoreactive Dyn A8 as described in Methods. Area under the curve for ir-Dyn A8 with wild-type (WT) animals was taken as 100%. Molecular mass calibration standards are as follows: cytochrome c, 12.4 kDa; ACTH, 4.6 kDa; β-endorphin, 3.5 kDa; α-MSH, 1.7 kDa; Dyn A8, 1.0 kDa; Leu-enkephalin, 0.55. ir, immunoreactive. One-way ANOVA; Dunnett’s multiple comparison test v/s wild-type (WT); ***p<0.001; ****p<0.0001. Supplementary material 1 (432 kb)
Data Availability Statement
All data are included in the publication.