Abstract
As a key macronutrient and source of essential macromolecules, dietary protein plays a significant role in health. For many years, protein-rich diets have been recommended as healthy due to the satiety-inducing and muscle-building effects of protein, as well as the ability of protein calories to displace allegedly unhealthy calories from fats and carbohydrates. However, clinical studies find that consumption of dietary protein is associated with an increased risk of multiple diseases, especially diabetes, while studies in rodents have demonstrated that protein restriction can promote metabolic health and even lifespan. Emerging evidence suggests that the effects of dietary protein on health and longevity are not mediated simply by protein quantity but are instead mediated by protein quality – the specific amino acid composition of the diet. Here, we discuss how dietary protein and specific amino acids including methionine, the branched chain amino acids (leucine, isoleucine, and valine), tryptophan and glycine regulate metabolic health, healthspan, and aging, with attention to the specific molecular mechanisms that may participate in these effects. Finally, we discuss the potential applicability of these findings to promoting healthy aging in humans.
Keywords: amino acids, aging, protein restriction, branched-chain amino acids, methionine
Introduction
We live in a rapidly graying society beset with an epidemic of obesity; over 70% of adults in US, and 30% (2.1 billion) of the world population are overweight or obese. Obesity is becoming increasingly prevalent for those over 65, and is a key risk factor for the development of diabetes, which affects 29.1 million Americans, with health care costs of over $176 billion per year in the US alone (1, 2). Obesity and diabetes are risk factors for other serious diseases of aging, including cardiovascular diseases (CVD), cancer, and Alzheimer’s disease, amplifying the impact of obesity considerably (3–6).
While traditional dieting – essentially, reducing calorie intake – can reverse obesity, adherence to a low calorie diet is unsustainable for most. The three major macronutrients, compounds we ingest that are used as fuel, are carbohydrates, fats, and protein. While the prevailing view has long been that calories from one of these sources is equivalent to calories from another, recent studies have demonstrated that a calorie is “not just a calorie” – and that dietary macronutrients have metabolic impacts beyond their simple caloric value (7–9). Diets that alter the level of specific macronutrients, but which do not restrict calories, may therefore be able to promote metabolic health and leanness in a more sustainable way than traditional dieting (10).
Dietary protein is composed of amino acids, twenty of which – those directly encoded by the genome – are considered common. Amino acids are essential building blocks for proteins; but in addition to this function, amino acids also have roles as signaling molecules, and can be catabolized for use as fuel or as building blocks for a range of other macromolecules. Here, we summarize the current knowledge on how the level of protein in the diet, as well as protein quality – the precise amino acid composition of the protein – impacts both healthspan and longevity, with an emphasis on the role of specific dietary amino acids.
Dietary protein regulates the health and longevity of animals
Evidence for the effect of dietary protein on lifespan dates to the 1920’s, when McCay et al. found that trout fed a low protein diet lived longer (11). Following this finding, a series of systematic studies examined the effect of diets containing varying amounts of protein (10%−26% of calories from protein) on growth and lifespan in rats, concluding that lower protein diets reduce growth but extend lifespan (12). In a later study, Sprague Dawley rats fed a 7.8% protein diet were shown to live longer than ones fed on a 20.8% protein diet (13). Despite these intriguing findings, interest in protein restriction (PR) was limited by a number of studies that found dietary PR or supplementation did not affect rat lifespan (14–16). Based on what we know now, the variation in these results were likely due to the highly variable protein sources and quantities used, but the confusion engendered delayed serious consideration of the effects of dietary protein on aging.
Interest in dietary protein as a regulator of longevity re-emerged in this century, stimulated in part by studies in Drosophila which found that the level of dietary protein regulates lifespan (17). Subsequent research using a nutritional geometry approach to study how multiple different diets influence fitness and lifespan found that the ratio of protein to carbohydrate in the diet strongly influenced both the longevity and fecundity of flies. The lifespan of flies was maximized at a very low ratio (1:16) of dietary protein to carbohydrate (18). A similar nutritional geometry approach was undertaken in mice; mice were fed 25 different diets with varying ratios of dietary macronutrients as well as energy density. In agreement with the results found in flies, mice fed a low protein diet, and in particular those animals fed a low protein: high carbohydrate diet, lived longer (19).
Numerous beneficial effects of protein restricted (PR) diets on health in various target organs have now been identified in rodents (Fig. 1). PR has strong effects on the metabolic health of mice and rats, promoting glucose tolerance, insulin sensitivity, and energy expenditure (20–23). A low protein diet prevents age related declines in motor coordination and cognition in female mice (24). In a mouse model of Alzheimer’s disease (AD), periodic protein restriction reduced cognitive deficits as well as phosphorylation of the tau protein (25). PR also decreases the production of reactive oxygen species by mitochondria, and reduced oxidative damage to lipids and endogenous DNA in the livers of rats (26); and in rodent cancer xenograft models, PR inhibits tumor growth (27).
Figure 1: Benefecial effects of protein restricted (PR) diet on health in various target organs.
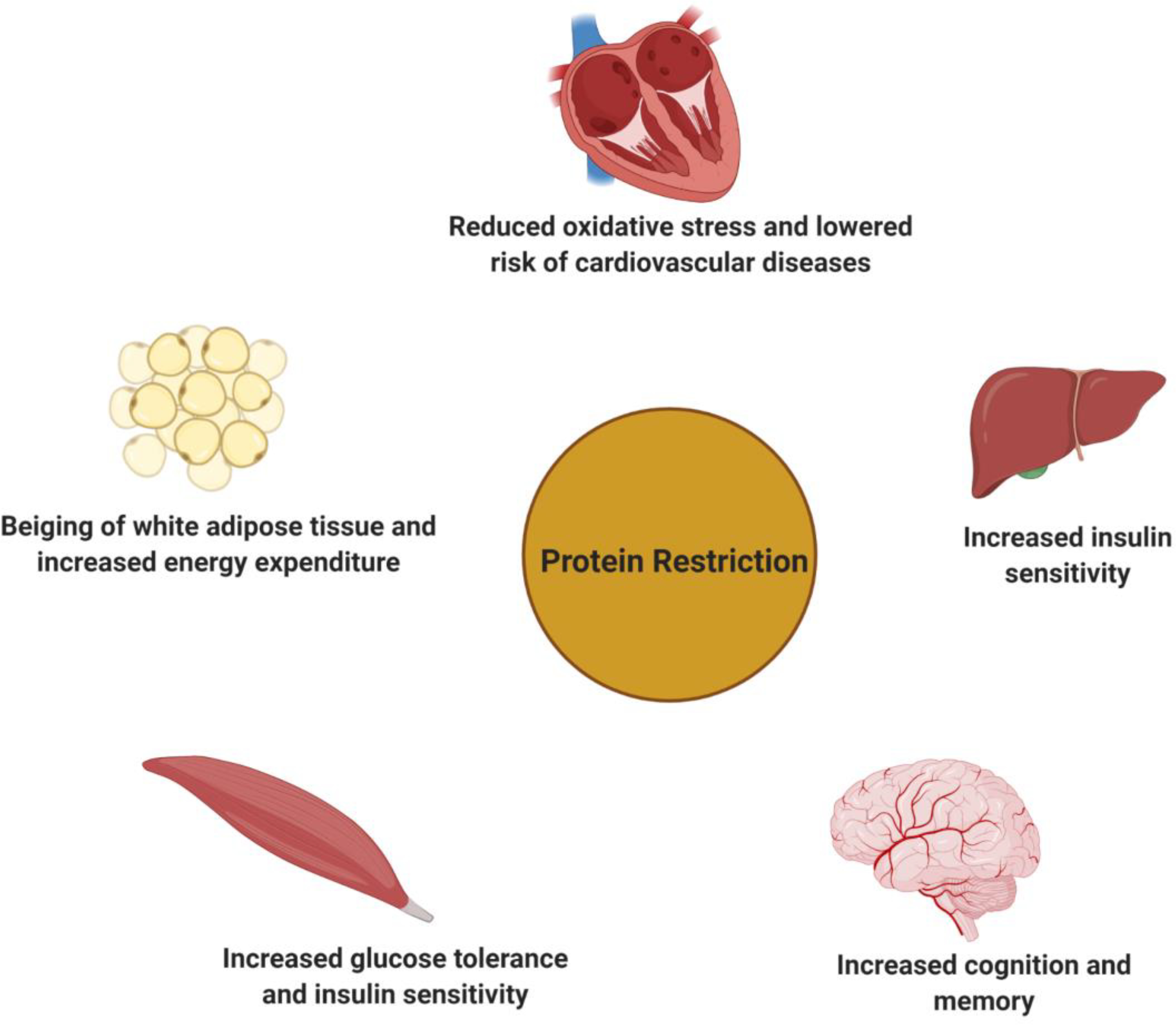
As depicted in this figure, a low protein diet exerts its beneficial effects on metabolic status through actions in multiple tissues, including liver, skeletal muscle and adipose tissue. PR has also been shown to have cardioprotective effects and has positive influence on memory and cognition function. Figure created with Biorender (https://biorender.com).
Dietary protein is negatively associated with metabolic health and lifespan of humans
A number of popular diet plans are based on the widely-held idea that high protein, low carbohydrate diets promote weight loss (28). The efficacy of high protein diets in clinical studies have been mixed, with weight loss observed in some trials (29–31), particularly in highly compliant subjects (32). At least part of the success of high protein diets comes through promoting satiety (33), but one study suggested that high protein diets may also increase thermogenesis, thereby driving weight loss through increased energy expenditure (34). Dietary protein supplementation has also been pursued in the elderly as a means of treating or preventing sarcopenia (35, 36). A limitation to these studies is that they were generally short-term.
In stark contrast to these short-term results, long-term retrospective and prospective cohort several studies have found that high protein consumption is associated with increased insulin resistance, diabetes, cancer, and overall mortality (37, 38). A retrospective analysis of data from The National Health and Nutrition Examination Survey (NHANES III), found that dietary protein consumption was also correlated with mortality in individuals under the age of 65 (38). High protein, low carbohydrate diets were associated with cardiovascular mortality as well as overall mortality in a cohort of over 40,000 Swedish women followed for over a decade (39). In line with these results, a recent population-based study from Finland showed that higher protein intake was associated with an increased risk of heart failure in middle aged men (40) and an overall increase in mortality among those with a history of cancer, CVD, or diabetes (41).
Supporting the epidemiological link between dietary protein consumption and the risk of developing diabetes, a recent short-term randomized clinical trial of PR found that reducing dietary protein reduced weight, fat mass, fasting blood glucose levels, and lowered plasma triglycerides in overweight middle-aged males (21, 42). PR also alters biomarkers associated with insulin and leptin signaling in plasma extracellular vesicles (43). However, no long-term clinical trial of PR has yet been undertaken.
Molecular mechanisms by which dietary protein impacts health and longevity
While the precise physiological and molecular mechanisms by which PR promotes metabolic health and longevity are unknown, there are several molecular pathways which likely play important roles in this response (Fig 2). Here we briefly discuss some of the key pathways that likely mediate the beneficial effects of a PR diet, and on metabolic health and lifespan.
Figure 2: Overview of nutrient signaling pathways altered by methionine restriction (MR).
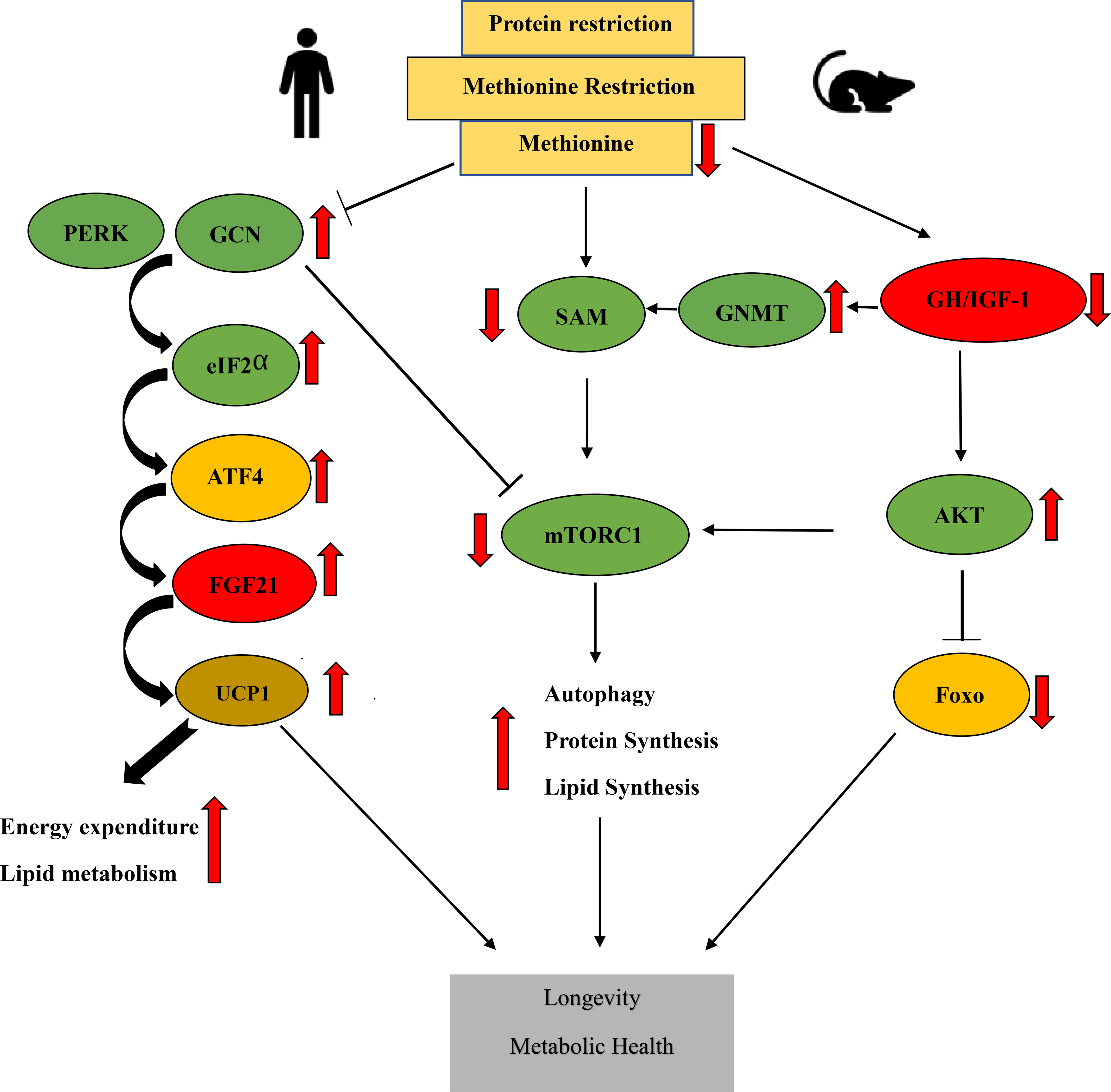
The benefical effects of MR are likely mediated through multiple metabolic pathways and molecular mechanisms. These include the integrated stress response pathway (PERK/GCN2-ATF4-FGF21), decreased GH/IGF-1 signaling, and decreased mTORC1 signaling as a result of decreased IGF-1 signaling, the activation of GNMT and reduced levels of the methionine metabolite SAM. The suppression of mTORC1 activity leads to increased autophagy and decreased protein translation, which act to promote health and longevity. Abbreviations: eIF2α -eukaryotic transcription factor 2α, FGF21 -fibroblast growth factor 21, GH/IGF-1- Growth hormone/insulin-like growth factor 1, mTORC1- mechanistic target of rapamycin complex 1, UCP1 - uncoupling protein 1, PERK- protein kinase R (PKR)-like endoplasmic reticulum kinase, GCN2 -general control nonderepressible 2, GNMT- glycine‐N‐methyl transferase, ATF4- Activating Transcription Factor 4, SAM-S-Adenosyl methionine, AKT- Protein kinase B, Foxo- forkhead box transcription factors.
mTOR
The mechanistic target of Rapamycin (mTOR) is a serine/threonine protein kinase which regulates and coordinates numerous cellular processes by integrating nutrient sensing and growth factor signals. Highly conserved in eukaryotic cells as a major growth regulator, mTOR exerts its function through two different protein complexes mTOR complex 1 (mTORC1) and complex 2 (mTORC2) which are composed of distinct protein subunits and phosphorylate different substrates. The defining components of mTORC1 are mTOR itself, Raptor (regulatory protein associated with mTOR), and mLST8 (mammalian lethal with Sec13 protein 8); while the defining components of mTORC2 are mTOR, Rictor, mLST8, and mSin1; but both complexes have been shown to associate with numerous other proteins in a variety of cell types (reviewed in (44)).
mTORC1 activity is directly regulated by the availability of nutrients, most notably amino acids but also including glucose and cholesterol and requires that growth factor signaling must be permissive for growth. Pharmacological or genetic inhibition of mTORC1 extends life span in numerous diverse species, ranging from yeast to mice (45–52). In contrast to mTORC1, which integrates information about many different environmental and hormonal cues, mTORC2 primarily acts as an effector of insulin/PI3K signaling. While mTORC2 regulates lifespan in worms, flies, and mice (53–59), it does not appear to be key in the response to dietary protein, and thus we will not discuss it in detail.
Over the last decade, major advances have been made in understanding the regulation of mTORC1 by amino acids, which occurs at the lysosomal surface; as this has been thoroughly reviewed elsewhere (60), we will only touch upon it briefly. Amino acid sensing by mTORC1 is controlled by the Rag family of GTPases, which recruits mTORC1 to the lysosomal surface in the presence of amino acids (61). Amino acids regulate the GTP/GDP-bound status of the Rag GTPases through controlling the activity of the Ragulator complex, which has guanine nucleotide exchange factor (GEF) activity for two of the Rag proteins, RagA and RagB; the GATOR1 complex, which has GTPase-activating protein (GAP) activity and is controlled in turn by the GATOR2 complex; and a folliculin (FLCN) and folliculin-interacting proteins 1 and 2 (FNIP1/2) complex that acts as a GAP for Rag C and Rag D heterodimers (61–65). Sensing of specific amino acids occurs via specific sensors, including Sestrin2, CASTOR1, and SAMTOR, which modulate the activity of GATOR 1/2 upon binding to leucine, arginine, and SAM, respectively (66–69). Additional sensing of amino acid availability is mediated by Ragulator via SLC38A9, a lysosomal arginine sensor, as well as a mechanism that requires the vacuolar ATPase (70–72).
After localizing to the lysosome, mTORC1 needs to interact with Rheb-GTP, which binds to mTORC1 allosterically and realigns active-site residues (73). In the absence of insulin/IGF-1 or other growth factor signaling, Rheb is found bound to GDP due to the action of the tuberous sclerosis complex (TSC), which acts as a GAP for Rheb (74). In the presence of insulin/IGF-1 signaling, Akt phosphorylates TSC which leads to its departure from the lysosome, allowing Rheb-GTP to bind to and activate mTORC1 (75).
As amino acids, which function as the building blocks of protein, are known to stimulate mTORC1 activity, it is logical to assume that a low protein diet results in reduced mTORC1 activity. Drosophila fed a high sugar low protein diet have decreased TOR signaling (76). In mice, as the protein: carbohydrate ratio decreased, there was a decrease in hepatic mTOR activation (19). Similarly, tumor-bearing mice fed a PR diet have decreased mTORC1 activity relative to ad libitum fed controls in multiple somatic tissues (77), as well as in mouse models of obesity (78), and mTORC1 is repressed by in a mouse model of ischemia reperfusion injury (79). Given the profound beneficial effects of reduced mTORC1 signaling on healthspan and longevity, decreased mTORC1 activity likely contributes to or mediates the ability of PR to promote health and longevity.
Gcn2-ATF4-FGF21
A second major evolutionarily conserved amino acid sensitive kinase is general control nonderepressible 2 (GCN2), one of four kinases that can activate the integrated stress response pathway (ISR). GCN2 is canonically activated by binding to uncharged transfer ribonucleic acids (tRNAs); following activation, GCN2 phosphorylates eukaryotic initiation factor 2-α (eIF2α), leading to the inhibition of protein translation (80). More recently, it has been recognized that ribosome stalling can also lead to GCN2 activation independently of an increase in uncharged tRNAs; this may be one of the principle mechanism of GCN2 activation under many physiological conditions (81, 82).
Phosphorylation of eIF2α leads to a global decrease in translation, but increases translation of specific stress-responsive transcripts. One of the best characterized of these and a key effector of the ISR is activating transcription factor 4 (ATF4) (83–86). ATF4 is a basic leucine zipper (bZIP) transcription factor that plays a key role in both basal metabolism and stress response, binding to amino acid response elements (AARE), primarily activating as a transcriptional activator, and upregulating transcription of genes involved in amino acid uptake and biosynthesis among other stress response genes (reviewed in (87)). Interestingly, ATF4 is a key factor in coordinating GCN2 and mTORC1 activity; ATF4 induces expression of Sestrin2, and indeed GCN2 and phosphorylation of eIF2α are required to prevent mTORC1 activation by depletion of specific amino acids (88, 89).
Another key gene regulated by ATF4 is fibroblast growth factor 21 (FGF21), a peptide hormone which plays an important role in adaptive responses to starvation (90). FGF21 can be produced by multiple tissues, including the liver, muscle, white adipose tissue, and pancreas (91, 92). FGF21 can be induced by a variety of stresses including PR, which increases levels of FGF21 in both rodents and humans (21, 22). FGF21 is believed to be a critical regulator of many of the effects of a PR diet, as experiments using mice lacking Fgf21 have found that FGF21 is required for PR-mediated changes in food intake, energy expenditure, body weight, and glucose tolerance (21, 22, 93, 94). Loss of Gcn2 does not entirely block the ability of a PR diet to induce Fgf21 transcription, but it does delay it by about 2 weeks (95), highlighting a key role for GCN2 in the response to PR.
In mice, FGF21 induces hepatic insulin sensitivity, in part via inhibition of mTORC1 activity (96). However, the most robust mechanism by which FGF21 promotes metabolic health is by activating UCP1 in white and brown adipose tissue, promoting energy expenditure as well as food intake (93). This physiological response involves signaling through the brain to the adipose tissue, as mice lacking a brain FGF21 receptor are unable to respond to low protein diets (94). Thus, it seems likely that the GCN2-FGF21-UCP1 axis is a key mediator in the response to low protein diets; and as transgenic expression of FGF21 increases the lifespan of mice (97), this hormone may also play a role in the ability of a low protein diet to extend lifespan.
Protein quality impacts health and longevity
For many years, there has been interest in understanding if protein source plays a role in health, with the greatest focus on understanding if there is a difference between the effect of plant protein and animal protein. Several studies have suggested that plant-based protein is healthier. One study found that consumption of a plant-based vegan diet decreased all-cause mortality, coronary heart disease and a decrease in risk of developing obesity in humans (98); a more recent study showed that a plant based diet significantly lowered the incidence of cardiovascular disease (CVD), CVD mortality, and all-cause mortality in a cohort of middle-aged adults (99). Vegan diets have also been implicated in reducing the risk of developing metabolic syndrome, lowering triglycerides, blood pressure, glucose, waist circumference and body mass index (100), and decreasing fat mass and insulin resistance (101).
One possibility that has been advanced to explain the beneficial effects of plant protein is that there is a difference in protein quality – the specific amino acid composition of the protein. Plant-based diets have a reduced level of methionine as compared to animal sources, and humans consuming a vegan diet have reduced plasma levels of methionine compared to humans who eat animal proteins (102, 103). As discussed below, significant data now suggests that the level of methionine – as well as of several other dietary amino acids – has a profound effect on health and longevity, not only in rodents, but also in humans. An overview of recent studies is provided in Table 1 and Table 2).
Table 1:
Summary of recent studies examining the effects of amino acid restriction or supplementation on the lifespan of rodents. Level of intake is the percent of the altered amino acids(s) in the experimental diet relative to the control diet. M -Male, F-Female, BCAAs = branched-chain amino acids (leucine, isoleucine, and valine).
| Species/Strain/Sex | Altered Amino acid | Lifespan | Level of intake relative to control diet | Study |
|---|---|---|---|---|
| Methionine | ||||
| Mice CB6F1 (F) | ↓ Met | Increased | 23–35% | Miller et al., 2005 (104) |
| Mice CB6F1 (M) | ↓ Met | Increased | 7% | Sun et al., 2009 (105) |
| Rats Fisher 344 (M) | ↓ Met | Increased | 20% | Orentreich et al., 1993 (106) |
| Rats Fisher 344 (M) | ↓ Met | Increased | 20% | Richie et al., 1994 (107) |
| Sprague Dawley Brown Norway Wistar |
↓ Met | Increased | 20% | Zimmerman et al., 2003 (108) |
| Tryptophan | ||||
| Swiss Albino Mice (M) | ↓ Trp | Increased | 17% | (De Marte et al., 1986 (109) |
| Long Evans rats (F) | ↓ Trp | Increased | 30% or 40% | Ooka et al., 1988 (110) |
| Branched-chain amino acids | ||||
| Mice C57BL/6J | ↑ BCAAs | Decreased | 200% | Solon-Biet et al., 2019 (111) |
| Mice C57BL/6J | ↓ BCAAs | Increased (M only) | 33% | Richardson et al., 2021 (112) |
| Glycine | ||||
| Rats Fisher 344 (M) | ↑ Gly | Increased | 347% or 522% | Brind et al., 2011 (113) |
| Mice UM‐HET3 | ↑ Gly | Increased | 772% | Miller et al., 2019 (114) |
Table 2: Effect of altered dietary levels of methionine or the branched-chain amino acids on the metabolic health of rodents and humans.
M-Male, DIO – Diet Induced Obesity, T2D-Type 2 Diabetes, BCAAs - branched chain amino acids, FGF21-fibroblast growth factor 21, WAT-white adipose tissue. Table is adapted from Green et al., 2019 (128) with permission.
| Species/Strain/Sex | Altered amino acid | Level of intake relative to control diet | Metabolic health | Length of intervention | Study |
|---|---|---|---|---|---|
| Methionine | |||||
| Mice CB6F1 (F) | Met | 23–35% | Decreased circulating IGF-1, insulin and glucose Increased resistance to liver stress |
Lifespan study | Miller et al., 2005 (104) |
| Mice C57BL/6J (M) | Met | 20% | Increased food intake Reduced body weight Improved hepatic insulin sensitivity Decreased hepatic lipogenic gene expression |
8 weeks | Lees et al., 2014 (115) |
| Mice C57BL/6J (M) | Met | 20% | Improved insulin sensitivity Reduced hepatic glucose production Increased FGF21 |
8 weeks | Stone et al., 2014 (116) |
| Mice C57BL/6J (M) | Met | 15% | Increased food intake Increased energy expenditure Reduced accumulation of body weight and fat mass |
10 weeks | Wanders et al., 2017 (117) |
| Mice C57BL/6 J (M) | Met | 20% | Increased food intake Reduced body and fat mass Improved glycemic control Decreased fasting blood glucose and insulin Increased lipid cycling in WAT Decreased hepatic lipogenic gene expression Elevated FGF21 |
8 weeks | Lees et al., 2017 (118) |
| Mice C57BL/6J (M/F and M/F DIO) | Met | 0% | Increased food intake Reduced weight and adiposity Improved glycemic control Increased energy expenditure Elevated FGF21 (males) |
5 weeks | Yu et al., 2018 (119) |
| Mice C57BL/6J | Met | 20% | Reduced Body Weight and Adiposity Increased food intake Increased expression of thermogenic markers Improved insulin sensitivity Decreased circulating lipid levels |
7 weeks | Forney et al., 2020 (120) |
| Rats Fisher 344 (M) | Met | 20% | Decreased Body weight Reduced visceral fat Decreased serum lipds and IGF-1 |
Lifespan study | Malloy et al., 2006 (121) |
| Humans (O) | Met | 6% | Increased fat oxidation Decreased hepatic lipid metabolism |
16 weeks | Plaisance et al., 2011 (122) |
| Branched-chain amino acids | |||||
| Mice C57BL/6J (M) | Leu or Ile or Val | 0% | Improved insulin sensitivity Improved glucose tolerance Increased hepatic insulin sensitivity (Leu) Decreased fasting blood glucose (Ile or Val) |
1 or 7 days | Xiao et al., 2011 (123) and Xiao et al., 2014 (124) |
| Mice C57BL/6J (M) | Leu | 33% | Increased adiposity | 13 weeks | Fontana et al., 2016 (21) |
| Mice C57BL/6J (M) | BCAAs | 33% | Increased food intake Reduced accumulation of body weight and fat mass Improved glycemic control Decreased fasting blood glucose |
13 weeks | Fontana et al., 2016 (21) |
| Mice C57BL/6 J (M) | Leu | 20% | Increased food intake Decreased body and fat mass Improved glycemic control Decreased fasting insulin Increased lipid cycling in WAT |
8 weeks | Lees et al., 2017 (118) |
| Mice C57BL/6J (M, DIO) | BCAAs | 33% | Rapid weight and fat mass loss Improved glycemic control Increased energy expenditure Transient increase in fasting FGF21 |
14 weeks | Cummings et al., 2018 (20) |
| Mice C57BL/6J | BCAAs | 20%, 50%, or 200% | Increased food intake (200%) Decreased weight and fat mass Decreased leptin Decreased liver triglycerides Decreased fasting insulin (20%) |
Lifespan study | Solon-Biet et al., 2019 (111) |
| Mice C57BL/6J | BCAAs | 33% | Increased food intake Reduced weight and adiposity Improved glycemic control Increased energy expenditure |
Lifespan study | Richardson et al., 2021 (112) |
| Mice C57BL/6J (M) | Ile | 33% | Increased food intake Decreased weight and adiposity Improved glucose tolerance Increased hepatic insulin sensitivity Decreased fasting blood glucose Increased energy expenditure Elevated FGF21 |
12 weeks | Yu and Richardson et al., 2021 (125) |
| Rats Zucker fatty | BCAAs | 55% | Improved skeletal muscle glucose disposal and insulin sensitivity | 15 weeks | White et al., 2016 (126) |
| Humans (T2D) | BCAAs | 40% | Decreased insulin secretion Induced FGF21 production, Improved oral glucose sensitivity Improves white adipose tissue metabolism |
4 weeks | Karusheva et al., 2019 (127) |
Methionine
Orentreich and colleagues first tested the hypothesis that a methionine restricted (MR) diet could extend lifespan in the 1990’s using Fischer 344 rats, finding that MR extended lifespan by about 30% (106). This effect was not strain specific, with a MR diet also able to extend the lifespan of Brown Norway, Sprague Dawley and Wistar rats (108). Subsequent research has demonstrated that restricting dietary methionine can extend the lifespan of many diverse species, including yeast, flies, and mice (104, 129–134).
In addition to these beneficial effects on lifespan, MR has many beneficial effects on metabolic health. Rodents fed a MR diet are leaner, with reduced adiposity; have improvements in glucose homeostasis with lower blood glucose and insulin levels and improved glucose tolerance and insulin sensitivity, and have decreased serum and hepatic triglyceride levels (121, 135–137). These metabolic benefits have led to the idea that MR diets might be effective in the treatment of diabetes and obesity, and indeed MR and methionine depleted dietary regimens promote weight loss and reduced adiposity in obese mice, decreases or reverses liver lipid accumulation, and normalizes glucose homeostasis (119, 138, 139). Beneficial metabolic effects, in particular increased fat oxidation, have also been seen in humans during a clinical trial of MR (122).
MR promotes metabolic health and longevity via multiple pathways and molecular mechanisms (Fig 2). As highlighted in Fig 2., decreased GH/IGF-1 signaling can suppress mTORC1 activity by activating glycine-N-methyl transferase (GNMT) (140, 141), which reduces levels of the key methionine metabolite S-Adenosyl methionine (SAM), which normally activates mTORC1 via binding to SAMTOR (68). In yeast, MR extends chronological lifespan through an autophagy/mitophagy dependent pathway that involves alterations in central carbon metabolism (133, 142). Rodents fed a MR diet have reduced levels of IGF-1, a key effector of the growth hormone signaling pathway that well-characterized as a regulator of lifespan (104, 143). Dietary supplementation with the amino acid selenium, which similarly reduces IGF-1 levels, mimics many of the healthspan benefits of a MR diet, protecting mice against weight gain and fat accumulation, and protecting against diet-induced obesity (144). Many of the metabolic effects of a MR diet are likely mediated by FGF21, which is induced by a MR diet (115). While it was initially believed that MR acted to induce FGF21 via the GCN2-ATF4-FGF21 axis discussed above, it was recently shown that GCN2 is dispensable for the effects of MR (145). Instead, the induction of FGF21 by MR is dependent upon the kinase PKR-like endoplasmic reticulum kinase (PERK), which is activated by MR as a result of oxidative stress, and similarly phosphorylates eIF2α and induces translation of ATF4 (Fig 2.). However, some of the metabolic effects of MR may not require a global increase in FGF21 levels, as female C57BL/6J mice have a robust metabolic response to a methionine depleted diet without an increase in FGF21 levels (119).
The induction of oxidative stress in the liver of MR-treated mice is due to the depletion of the anti-oxidant glutathione, which is generated from methionine via cysteine (145). MR may have many other effects that are mediated indirectly via its metabolites. In addition to the effects of the methionine metabolite SAM on mTORC1 mentioned above, SAM is also a key metabolite for methyltransferases; MR therefore has profound effects on histone methylation and gene expression (146). Finally, the transulfuration pathway has been implicated in longevity and stress resistance, and both PR and MR promote the generation of hydrogen sulfide (H2S), a key longevity regulator, in multiple species (147–150).
Branched-chain amino acids (BCAAs)
The three branched-chain amino acids (BCAAs), leucine, isoleucine, and valine, are so named because they have an aliphatic side-chain with a branched carbon structure. It has long been realized that these amino acids may play an important role in health and metabolism; in 1969, it was observed that the BCAAs are elevated in the blood of obese humans (151). Over the last 15 years, it has become apparent that plasma levels of BCAAs are correlated with obesity and insulin resistance in both humans and rodents (152–154).
Conversely, numerous interventions that reduce obesity and improve metabolic health in humans, including calorie restriction, protein restriction, and gastric bypass surgery, lower plasma levels of BCAAs (21, 155, 156). Branched-chain amino acids are essential, and increased consumption of BCAAs correlates with both plasma BCAAs and the incidence of type 2 diabetes in humans (157, 158). Rodent studies have demonstrated that dietary intake of BCAAs directly regulate metabolic health. Supplementation of BCAAs to a Western diet promotes adiposity and insulin resistance in both mice and rats (20, 153).
Conversely, short-term complete deprivation of any single BCAA promotes hepatic insulin sensitivity (123, 124). As complete deprivation of a BCAA is not physiologically relevant, several groups have recently investigated the results of simply reducing dietary levels of BCAAs. We have shown that reducing dietary levels of BCAAs by 67% improves the metabolic health of lean mice, and rapidly restores metabolic health to diet-induced obese mice, dramatically reducing adiposity and glucose tolerance (20, 21). Similarly, reduced levels of dietary BCAAs slows the accumulation of visceral adipose tissue and preserves the insulin sensitivity of Zucker fatty rats (126).
Overall, these studies demonstrate that BCAAs directly regulate metabolic health, and that reducing dietary levels of the BCAAs may be a strategy to rapidly promote healthy body composition and blood glucose control in overweight or obese humans. A recent randomized controlled study has found that short-term dietary restriction of BCAAs decreases insulin secretion, induces FGF21, improves oral glucose sensitivity index, and improves white adipose tissue metabolism in humans with type 2 diabetes (127). The longer-term effects of reducing dietary BCAAs in humans with and without metabolic syndrome remains to be determined.
The potent metabolic benefits of reduced BCAA consumption strongly suggested that reducing dietary levels of BCAAs might promote mammalian healthspan and even increase lifespan. Such effects could be mediated in part by reducing mTORC1 signaling, as the BCAAs, particularly leucine, are strong agonists of mTORC1 and genetic and pharmacological interventions that inhibit mTORC1 signaling extend mammalian lifespan and healthspan (46, 159–162). In agreement with this hypothesis, circulating levels of the BCAA are associated with hepatic mTOR activity and negatively associated with lifespan (19).
Consistent with a negative effect of BCAAs on longevity, dietary supplementation with extra BCAAs leads not only to impaired metabolic health, but to decreased lifespan (20, 111, 153, 163). Solon-Biet and colleagues did not observe increased or decreased longevity on mice fed a 50% or 80% restricted BCAA diet from 12 weeks of age (111). Similarly, Richardson and colleagues found that 67% restriction of BCAAs improved metabolic health and reduced frailty of male and female mice without increasing lifespan when started at 16 months of age (112). However, lifelong restriction of BCAAs reduced frailty and extended the lifespan of male, but not female mice by over 30%, reducing mTORC1 signaling in multiple tissues specifically in males (112). It is clear that the precise level of restriction, time of diet initiation, and sex may play a role in determining if BCAA restriction will extend lifespan, but both studies support a model in which reduced dietary consumption of BCAAs promote healthspan.
BCAAs have been linked to multiple diseases of aging, including Alzheimer’s disease; and defects in BCAA catabolism induced by diabetes may drive the pathogenesis of Alzheimer’s disease by increasing brain levels of the BCAAs and activating mTORC1 (164). Dietary supplementation of BCAAs has been shown to increase neuropathology and decrease the survival of a mouse model of Alzheimer’s disease (165). BCAAs are also suggested to be critically important in cancer; there is an increased uptake and catabolism of BCAAs by some types of cancer that drives cancer progression (166, 167). Conversely, defective BCAA catabolism leading to an accumulation of BCAAs and mTORC1 hyperactivation have been shown to be important in other cancer types, and dietary BCAA intake has been shown to be correlated with cancer risk (168).
However, not all experiments in model organisms clearly support a model in which reducing dietary BCAAs improves healthspan and extends longevity. In the budding yeast S. cerevisiae, supplementation of leucine actually promotes chronological lifespan during CR (169). In C. elegans, supplementation with BCAAs or impaired expression of a major BCAA catabolic enzyme, branched-chain amino acid transferase 1 (BCAT-1) resulted in extended lifespan and healthy aging without affecting fecundity (170). In contrast, dietary restriction of BCAAs in D. melanogaster improves stress resistance, ameliorates age-related pathologies, and extends lifespan (171); this is likely mediated in part via the regulation of mTORC1 activity by the single fly Sestrin orthologue (172). In contrast to the results described above, consumption of an essential amino acid supplement with extra BCAAs is reported to extend the longevity of male mice (173, 174). However, the BCAA supplement and diet used in these experiments actually alter the levels of many essential and non-essential dietary amino acids, including methionine, making the role of the BCAAs in this response unclear.
In contrast to the generally deleterious effects of life-long consumption of high levels of the BCAAs, short-term supplementation with BCAAs has shown promise in preclinical and clinical studies in the aged. BCAA supplementation along with low resistance exercise increased muscle strength and physical function in sarcopenic older adults (175). A clinical prospective study on blood metabolites revealed an inverse relation between BCAA levels, dementia and AD. Reduced levels of BCAAs were associated with higher risk of AD (176). BCAA supplementation enhanced cognitive recovery in patients with severe traumatic brain injury (177).Supplementation of BCAAs has also been shown to extend the survival time, reduce complications, and reduce the recurrence rate of patients treated for hepatocellular carcinoma (178, 179)
While one potential explanation for the conflicting results of different studies may be age or disease status, another explanation for the conflicting results of different studies may be the fact that the BCAAs have usually been considered as a group due to their structural similarity and shared catabolic pathways. However, there is emerging evidence that the individual BCAAs have unique effects on signaling and metabolism. Leucine, for example, is a strong activator of mTORC1 in most cell types, binding to Sestrin2 and activating mTORC1 by promoting the recruitment of mTORC1 to the lysosomal surface via a Rag-GTPase dependent pathway (67, 69, 180–182). This crucial signaling pathway leads to the activation of many downstream pathways that regulate metabolism and aging (44). In contrast, isoleucine and valine do not strongly interact with Sestrin2 and may be less potent activators of mTORC1.
Distinct roles for the individual BCAAs have also been observed in vivo. We have observed thicker dermal white adipose tissue and heavier epididymal fat pads in mice fed a diet in which the levels of leucine were specifically reduced (21). An intermediate valine catabolite, 3-hydroxy-isobutyrate (3-HIB), is secreted from muscle cells and can activate trans-endothelial fatty acid transport, thereby causing lipid accumulation and insulin resistance (183). Finally, in a recent study we demonstrated that each of the BCAAs has distinct metabolic effects in mice, with restriction of dietary isoleucine being necessary and sufficient for the metabolic benefits of a low protein diet. As highlighted in Fig. 3, these effects were independent of hepatic mTORC1 and GCN2 signaling, and mediated in part through FGF21, which was strongly induced by isoleucine restriction and not by restriction of leucine or valine alone (125). In humans, we found that dietary levels of isoleucine are positively associated with BMI (125); and another recent study found that blood levels of isoleucine are correlated with increased mortality in humans (184). In contrast, blood levels of leucine and valine were associated with decreased human mortality (184).
Figure 3: Overview of major signaling pathways altered by restriction or supplementation of amino acids.
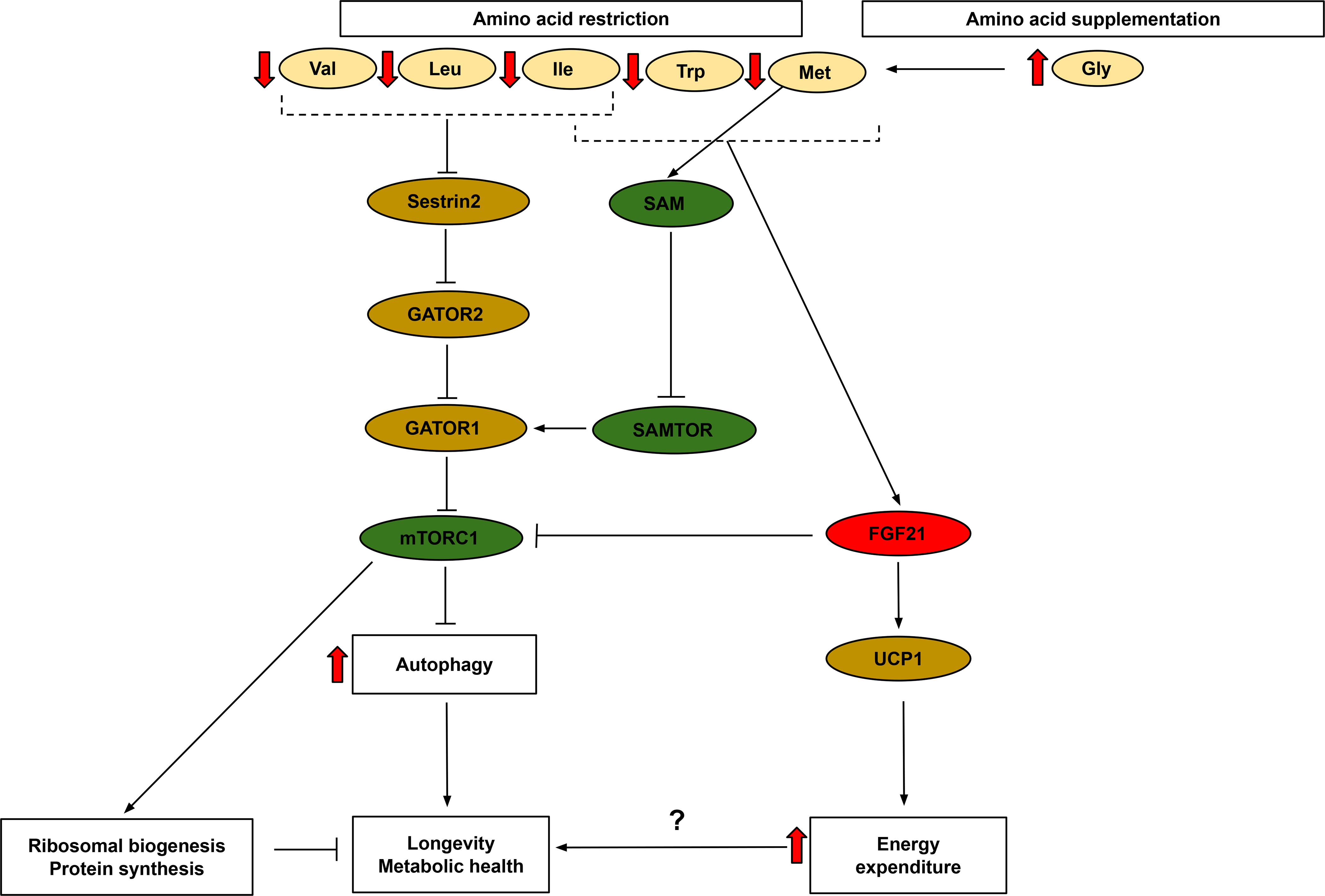
Restriction of methionine, tryptophan or the BCAAs extends lifespan and improves metabolic health. Restriction of methionine, tryptophan, or isoleucine, but not restriction of leucine or valine, induces FGF21 and increases energy expenditure through the FGF21-UCP1 axis. All three BCAAs, Trp, and methionine (via SAM) stimulate mTORC1 activity, and restriction reduces mTORC1 activity. Glycine supplementation promotes longevity through its regulation of methionine and SAM levels. Abbreviations:Trp-tryptophan, Ile-Isoleucine, Val-valine, Leu-leucine, Met-methionine, Gly-glycine, FGF21 -fibroblast growth factor 21, mTORC1- mechanistic target of rapamycin complex 1, UCP1 - uncoupling protein 1, SAM-S-Adenosyl methionine
Tryptophan
The essential amino acid tryptophan and its metabolites have been widely studied as key regulators of metabolic health. Segall and Timiras were the first to report that a tryptophan deficient diet (185) could delay aging in rats, finding that a chronic deficiency of tryptophan delayed pathological signs of aging, including the onset of tumors, and increased lifespan (186). Although this small study was not carried to completion, follow up work demonstrated that tryptophan restriction reduced age-related pathologies and extended the lifespan of both rats (110) and mice (109). Similar beneficial responses have been shown in yeast, worms, and flies, where ibuprofen has been shown to extend longevity by destabilizing tryptophan permeases and reducing tryptophan uptake (187).
The physiological and molecular mechanisms by which tryptophan restriction promotes health and longevity are still under investigation. It was recently shown that tryptophan restriction in the context of a casein-based diet induces FGF21, promoting metabolic health (Fig. 3) (188). The major catabolic pathway of tryptophan is the kynurenine pathway, which produces metabolites like kynurenic acid and nicotinamide adenine dinucleotide (NAD+) (189). This pathway is in fact the sole de novo biosynthetic pathway for NAD+, which has been implicated in many metabolic processes, including mitochondrial function, and NAD+ supplementation has been shown to be beneficial in many diseases of aging (reviewed in (185)). Numerous studies have also investigated links between tryptophan metabolism, immune cell function, and inflammation (190–193). Finally, a recent study found that the serum level of tryptophan was associated with onset of diabetes (194).
As with protein and the BCAAs, while some studies suggest that tryptophan restriction is beneficial, several studies have found that instead dietary supplementation with tryptophan may be beneficial for aging and specific age-related diseases. Tryptophan supplementation extends the lifespan of C. elegans (195), while low serum tryptophan levels and alterations in tryptophan catabolism are associated with reduced life expectancy in people with coronary artery disease (196, 197). Dietary supplementation of tryptophan suppresses blood glucose level and thereby delays diabetes progression in diabetic rats (198).
Tryptophan is a well-known precursor of serotonin, which has been implicated in many neurodegenerative disorders. Tryptophan-rich diets can prevent age-associated cognitive decline in rats (199), and in humans there is a negative correlation between tryptophan levels and cognitive functioning during aging (200). Humans with Alzheimer’s disease have been shown to have increased tryptophan catabolism and altered kynurenine levels, which may be linked to impaired cognitive function (200). However, tryptophan does not simply have beneficial effects on the aging brain; a study in young adults recently showed that consumption of a tryptophan-rich diet has a positive effect on anxiety and depression (201).
Glycine
Multiple studies have demonstrated that dietary supplementation of glycine can increase longevity and promote metabolic health. Dietary glycine increases lifespan in C. elegans, suppressing many genes involved in aging processes (202). This effect requires methionine synthase and S-adenosyl methionine synthetase, suggesting that glycine promotes longevity through alterations in methionine metabolism (Fig. 3). Dietary glycine regulates the action of GNMT, an enzyme that removes the methyl group from methionine (203, 204).
Glycine supplementation also increases the lifespan of rodents, including a small study conducted in Fisher 344 rats (113), and a large study conducted in UM-HET3 mice (114) by the NIA Interventions Testing Program. Although the overall effect of glycine supplementation on longevity was small, numerous rodent and human studies have found benefits from glycine supplementation. Glycine supplementation in aged mice enhanced activation of T cells as well as mitochondrial biogenesis (205), and has anti-inflammatory properties via inhibition of pro-inflammatory cytokines (206). Glycine intake also promotes metabolic health, reducing the accumulation of abdominal fat, plasma triglyceride levels and blood pressure induced by a high sucrose diet in rats (207).
In humans, glycine supplementation has been shown to be protective against chronic inflammation, oxidative stress and immune responses caused by type 2 diabetes (208). Cell culture studies suggest that glycine rescues age-related mitochondrial defects in human cells (209). Glycine is essential for the proliferation of muscle progenitor cells in cell culture and conditionally essential in vivo in mice (210), and humans with a genetic variant that raises blood levels of glycine have a reduced risk of cardiovascular disease (211). In humans, blood levels of glycine are positively associated with insulin sensitivity (212), and higher blood levels of glycine are associated with a reduced risk of diabetes (213).
Conclusions
Promoting healthy aging is becoming of increasing importance around the world as the population grays. Many people, particularly the elderly, are also threatened by the increasing prevalence of obesity and diabetes, which are both deleterious in themselves and increase the risk of developing many other diseases of aging. Here, we have discussed how instead of cutting calories, changing the composition of the diet – in particular, altering the levels of dietary protein or of specific dietary amino acids – may be a translatable and sustainable method to promote healthy aging.
An emerging consensus of animal and human data suggests that, contrary to long-held popular beliefs, lower protein consumption is more beneficial for health and longevity than high protein consumption. Recent human studies have found that lower protein intake is correlated with improved metabolic health as well as increased longevity, while a high protein intake correlates with an increased risk of diabetes and cardiovascular disease. This is not to say that there may not be risks of reducing protein consumption; in older people, lower protein intake has been associated with frailty and sarcopenia, and increased protein intake has been suggested as an intervention to preserve muscle mass in this population (214–216). Long-term clinical trials of PR will be critical to determining if PR can promote healthy aging and longevity in humans, as well as the time periods when PR may be beneficial and identifying any portions of the life where PR may be detrimental.
It is now becoming clear that dietary protein quality – the specific amino acid composition of the dietary protein – has a profound effect on metabolic health and longevity in mammals. In this review, we have discussed current knowledge on how dietary amino acids can affect metabolic health and longevity. These include studies demonstrating that restriction of methionine, BCAAs or tryptophan can improve healthspan and lifespan in rodents. While only a few human studies on these types of diets have been conducted, preliminary evidence suggests that restriction of methionine or BCAAs may also have metabolic benefits in humans. Data from rodents already suggests that the age of initiation of methionine or BCAA restriction, as well as the degree of restriction, will influence the ultimate effect of these diets (111, 112, 217). As with PR, long-term clinical trials will be critical to determining if restriction of specific dietary amino acids – and which ones, and when – can promote healthy aging in humans.
As discussed here, multiple molecular mechanism – including mTORC1, eIF2α, ATF4, and FGF21 – engaged by PR or restriction of specific amino acids may contribute to the beneficial effects of protein and amino acids restricted diets. While long-term adherence to amino acid restricted diets in humans is likely to be low, an expanding range of chemicals to modulate signaling through these pathways is being developed (218–223). It therefore seems likely that as we achieve a deeper understanding of the shared and distinctive molecular mechanisms engaged by restriction of different amino acids, pharmaceuticals can be developed that mimic the benefits of these diets to promote health and longevity.
ACKNOWLEDGEMENTS
We would like to thank all members of the Lamming lab for their valuable insights and comments. The Lamming lab is supported in part by the NIH/National Institute on Aging (AG056771, AG062328, and AG061635 to D.W.L.), startup funds from the University of Wisconsin-Madison School of Medicine and Public Health and Department of Medicine to D.W.L, and funds from the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. The Lamming lab is also supported in part by the U.S. Department of Veterans Affairs (I01-BX004031), and this work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
CONFLICT OF INTEREST STATEMENT
D.W.L has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. The University of Wisconsin-Madison has applied for a patent for the use of diets with reduced levels of specific amino acids to promote metabolic health, for which DWL is an inventor.
References
- 1.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 3.Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res 2016;118(11):1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005;26 Suppl 1:11–6. [DOI] [PubMed] [Google Scholar]
- 5.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res 2007;4(2):111–6. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab 2019;30(1):67–77 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab 2015;22(3):427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 2016;104(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weickert MO. Nutritional modulation of insulin resistance. Scientifica. 2012;2012:424780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCay CM, Bing FC, Dilley WE. Factor H in the Nutrition of Trout. Science. 1928;67(1731):249–50. [DOI] [PubMed] [Google Scholar]
- 12.Sloanaker JR. The effect of difference per cents of protein in the diet. American Journal of Physiology. 1931;98(2):266–75. [Google Scholar]
- 13.Ross MH. Length of life and nutrition in the rat. The Journal of nutrition. 1961;75:197–210. [DOI] [PubMed] [Google Scholar]
- 14.Davis TA, Bales CW, Beauchene RE. Differential effects of dietary caloric and protein restriction in the aging rat. Exp Gerontol 1983;18(6):427–35. [DOI] [PubMed] [Google Scholar]
- 15.Carlson AJ, Hoelzel F. Prolongation of the life span of rats by bulk-formers in the diet. The Journal of nutrition. 1948;36(1):27–40. [DOI] [PubMed] [Google Scholar]
- 16.Kao H-C, Conner RT, Sherman HC. Influence of Protein Intake upon Growth, Reproduction, and Longevity Studied at Different Calcium Levels. The Journal of nutrition. 1941;22(3):327–31. [Google Scholar]
- 17.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 2005;3(7):e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105(7):2498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014;19(3):418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. The Journal of physiology. 2018;596(4):623–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell reports. 2016;16(2):520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014;124(9):3913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Metabolism. 1983;32(3):257–61. [DOI] [PubMed] [Google Scholar]
- 24.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol 1987;42(1):78–81. [DOI] [PubMed] [Google Scholar]
- 25.Parrella E, Maxim T, Maialetti F, Zhang L, Wan J, Wei M, et al. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell. 2013;12(2):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayala V, Naudi A, Sanz A, Caro P, Portero-Otin M, Barja G, et al. Dietary protein restriction decreases oxidative protein damage, peroxidizability index, and mitochondrial complex I content in rat liver. J Gerontol A Biol Sci Med Sci 2007;62(4):352–60. [DOI] [PubMed] [Google Scholar]
- 27.Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, et al. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 2013;4(12):2451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik VS, Hu FB. Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med 2007;4(1):34–41. [DOI] [PubMed] [Google Scholar]
- 29.Due A, Toubro S, Skov AR, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord 2004;28(10):1283–90. [DOI] [PubMed] [Google Scholar]
- 30.Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999;23(5):528–36. [DOI] [PubMed] [Google Scholar]
- 31.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 2005;82(1):41–8. [DOI] [PubMed] [Google Scholar]
- 32.Campos-Nonato I, Hernandez L, Barquera S. Effect of a High-Protein Diet versus Standard-Protein Diet on Weight Loss and Biomarkers of Metabolic Syndrome: A Randomized Clinical Trial. Obes Facts 2017;10(3):238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015;101(6):1320S–9S. [DOI] [PubMed] [Google Scholar]
- 34.Cuenca-Sanchez M, Navas-Carrillo D, Orenes-Pinero E. Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Advances in nutrition. 2015;6(3):260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract 2013;28(6):684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. The Journal of physiology. 2006;575(Pt 1):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care. 2010;33(1):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014;19(3):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, et al. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med 2007;261(4):366–74. [DOI] [PubMed] [Google Scholar]
- 40.Virtanen HEK, Voutilainen S, Koskinen TT, Mursu J, Tuomainen TP, Virtanen JK. Intake of Different Dietary Proteins and Risk of Heart Failure in Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Circ Heart Fail. 2018;11(6):e004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virtanen HEK, Voutilainen S, Koskinen TT, Mursu J, Kokko P, Ylilauri MPT, et al. Dietary proteins and protein sources and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2019;109(5):1462–71. [DOI] [PubMed] [Google Scholar]
- 42.Trevino-Villarreal JH, Reynolds JS, Bartelt A, Langston PK, MacArthur MR, Arduini A, et al. Dietary protein restriction reduces circulating VLDL triglyceride levels via CREBH-APOA5-dependent and -independent mechanisms. JCI Insight 2018;3(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eitan E, Tosti V, Suire CN, Cava E, Berkowitz S, Bertozzi B, et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell. 2017;16(6):1430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23(6):990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–6. [DOI] [PubMed] [Google Scholar]
- 46.Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice. J Gerontol A Biol Sci Med Sci 2016;71(7):876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–6. [DOI] [PubMed] [Google Scholar]
- 50.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 2004;14(10):885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 2006;20(2):174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. [DOI] [PubMed] [Google Scholar]
- 53.Chellappa K, Brinkman JA, Mukherjee S, Morrison M, Alotaibi MI, Carbajal KA, et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell. 2019;18(5):e13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu D, Tomasiewicz JL, Yang SE, Miller BR, Wakai MH, Sherman DS, et al. Calorie-Restriction-Induced Insulin Sensitivity Is Mediated by Adipose mTORC2 and Not Required for Lifespan Extension. Cell reports. 2019;29(1):236–48 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arriola Apelo SI, Lin A, Brinkman JA, Meyer E, Morrison M, Tomasiewicz JL, et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. eLife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamming DW, Mihaylova MM, Katajisto P, Baar EL, Yilmaz OH, Hutchins A, et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014;13(5):911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 2012;15(5):713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev 2009;23(4):496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang K, Kang P, Liu Y, Huang K, Miao T, Sagona AP, et al. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy. 2019:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamming DW, Bar-Peled L. Lysosome: The metabolic signaling hub. Traffic. 2019;20(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52(4):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150(6):1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340(6136):1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, et al. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell. 2016;165(1):153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell reports. 2014;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu X, Orozco JM, Saxton RA, Condon KJ, Liu GY, Krawczyk PA, et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science. 2017;358(6364):813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347(6218):188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334(6056):678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung J, Genau HM, Behrends C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol Cell Biol 2015;35(14):2479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang H, Jiang X, Li B, Yang HJ, Miller M, Yang A, et al. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature. 2017;552(7685):368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 2003;17(15):1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156(4):771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galikova M, Klepsatel P. Obesity and Aging in the Drosophila Model. Int J Mol Sci 2018;19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamming DW, Cummings NE, Rastelli AL, Gao F, Cava E, Bertozzi B, et al. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget 2015;6(31):31233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maida A, Chan JSK, Sjoberg KA, Zota A, Schmoll D, Kiens B, et al. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Molecular metabolism. 2017;6(8):873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robertson LT, Trevino-Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, et al. Protein and Calorie Restriction Contribute Additively to Protection from Renal Ischemia Reperfusion Injury Partly via Leptin Reduction in Male Mice. The Journal of nutrition. 2015;145(8):1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68(3):585–96. [DOI] [PubMed] [Google Scholar]
- 81.Harding HP, Ordonez A, Allen F, Parts L, Inglis AJ, Williams RL, et al. The ribosomal P-stalk couples amino acid starvation to GCN2 activation in mammalian cells. eLife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inglis AJ, Masson GR, Shao S, Perisic O, McLaughlin SH, Hegde RS, et al. Activation of GCN2 by the ribosomal P-stalk. Proc Natl Acad Sci U S A. 2019;116(11):4946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108. [DOI] [PubMed] [Google Scholar]
- 84.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101(31):11269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 2006;34(Pt 1):7–11. [DOI] [PubMed] [Google Scholar]
- 86.Ishimura R, Nagy G, Dotu I, Chuang JH, Ackerman SL. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. eLife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep 2016;17(10):1374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 2015;29(22):2331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikonorova IA, Mirek ET, Signore CC, Goudie MP, Wek RC, Anthony TG. Time-resolved analysis of amino acid stress identifies eIF2 phosphorylation as necessary to inhibit mTORC1 activity in liver. J Biol Chem 2018;293(14):5005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106(26):10853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000;1492(1):203–6. [DOI] [PubMed] [Google Scholar]
- 92.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 2010;24(10):2050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud HR, Munzberg H, et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Scientific reports. 2017;7(1):8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell reports. 2019;27(10):2934–47 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, et al. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell reports. 2016;16(3):707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H, et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64(2):425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife. 2012;1:e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr 2009;89(5):1607S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-Based Diets Are Associated With a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J Am Heart Assoc 2019;8(16):e012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rizzo NS, Sabate J, Jaceldo-Siegl K, Fraser GE. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the adventist health study 2. Diabetes Care. 2011;34(5):1225–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahleova H, Fleeman R, Hlozkova A, Holubkov R, Barnard ND. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: metabolic benefits of plant protein. Nutr Diabetes. 2018;8(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, et al. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr 2016;70(3):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCarty MF, Barroso-Aranda J, Contreras F. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Medical hypotheses. 2009;72(2):125–8. [DOI] [PubMed] [Google Scholar]
- 104.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4(3):119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 2009;64(7):711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. The Journal of nutrition. 1993;123(2):269–74. [DOI] [PubMed] [Google Scholar]
- 107.Richie JP, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. The FASEB Journal. 1994;8(15):1302–7. [DOI] [PubMed] [Google Scholar]
- 108.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol 2003;38(1–2):47–52. [DOI] [PubMed] [Google Scholar]
- 109.De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev 1986;36(2):161–71. [DOI] [PubMed] [Google Scholar]
- 110.Ooka H, Segall PE, Timiras PS. Histology and survival in age-delayed low-tryptophan-fed rats. Mech Ageing Dev 1988;43(1):79–98. [DOI] [PubMed] [Google Scholar]
- 111.Solon-Biet SM, Cogger VC, Pulpitel T, Wahl D, Clark X, Bagley E, et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab 2019;1(5):532–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nature Aging. 2021;1(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brind J, Malloy V, Augie I, Caliendo N, Vogelman JH, Zimmerman JA, et al. Dietary glycine supplementation mimics lifespan extension by dietary methionine restriction in Fisher 344 rats. The FASEB Journal. 2011;25(S1):528.2–.2. [Google Scholar]
- 114.Miller RA, Harrison DE, Astle CM, Bogue MA, Brind J, Fernandez E, et al. Glycine supplementation extends lifespan of male and female mice. Aging Cell. 2019;18(3):e12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, et al. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13(5):817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63(11):3721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW. FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but Not Its Effects on Hepatic Lipid Metabolism. Diabetes. 2017;66(4):858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lees EK, Banks R, Cook C, Hill S, Morrice N, Grant L, et al. Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Scientific reports. 2017;7(1):9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu D, Yang SE, Miller BR, Wisinski JA, Sherman DS, Brinkman JA, et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J. 2018;32(6):3471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Forney LA, Fang H, Sims LC, Stone KP, Vincik LY, Vick AM, et al. Dietary Methionine Restriction Signals to the Brain Through Fibroblast Growth Factor 21 to Regulate Energy Balance and Remodeling of Adipose Tissue. Obesity (Silver Spring). 2020;28(10):1912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–14. [DOI] [PubMed] [Google Scholar]
- 122.Plaisance EP, Greenway FL, Boudreau A, Hill KL, Johnson WD, Krajcik RA, et al. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):E836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60(3):746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 2014;63(6):841–50. [DOI] [PubMed] [Google Scholar]
- 125.Yu D, Richardson NE, Green CL, Spicer AB, Murphy ME, Flores V, et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab 2021;33(5):905–22 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Molecular metabolism. 2016;5(7):538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karusheva Y, Koessler T, Strassburger K, Markgraf D, Mastrototaro L, Jelenik T, et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr 2019;110(5):1098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green CL, Lamming DW. Regulation of metabolic health by essential dietary amino acids. Mech Ageing Dev 2019;177:186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462(7276):1061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, et al. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nature communications. 2014;5:3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, et al. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Dordr). 2007;29(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu Z, Song L, Liu SQ, Huang D. Independent and additive effects of glutamic acid and methionine on yeast longevity. PLoS One. 2013;8(11):e79319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet 2014;10(5):e1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnson JE, Johnson FB. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS One. 2014;9(5):e97729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hasek BE, Boudreau A, Shin J, Feng D, Hulver M, Van NT, et al. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes. 2013;62(10):3362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol 2010;299(3):R728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Orgeron ML, Stone KP, Wanders D, Cortez CC, Van NT, Gettys TW. The impact of dietary methionine restriction on biomarkers of metabolic health. Prog Mol Biol Transl Sci 2014;121:351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malloy VL, Perrone CE, Mattocks DA, Ables GP, Caliendo NS, Orentreich DS, et al. Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice. Metabolism 2013;62(11):1651–61. [DOI] [PubMed] [Google Scholar]
- 139.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One. 2012;7(12):e51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aida K, Tawata M, Negishi M, Onaya T. Mouse glycine N-methyltransferase is sexually dimorphic and regulated by growth hormone. Horm Metab Res 1997;29(12):646–9. [DOI] [PubMed] [Google Scholar]
- 141.Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev 2005;126(3):389–98. [DOI] [PubMed] [Google Scholar]
- 142.Plummer JD, Johnson JE. Extension of Cellular Lifespan by Methionine Restriction Involves Alterations in Central Carbon Metabolism and Is Mitophagy-Dependent. Front Cell Dev Biol 2019;7:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miura Y, Kato H, Noguchi T. Effect of dietary proteins on insulin-like growth factor-1 (IGF-1) messenger ribonucleic acid content in rat liver. Br J Nutr 1992;67(2):257–65. [DOI] [PubMed] [Google Scholar]
- 144.Plummer JD, Postnikoff SD, Tyler JK, Johnson JE. Selenium supplementation inhibits IGF-1 signaling and confers methionine restriction-like healthspan benefits to mice. eLife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wanders D, Stone KP, Forney LA, Cortez CC, Dille KN, Simon J, et al. Role of GCN2-Independent Signaling Through a Noncanonical PERK/NRF2 Pathway in the Physiological Responses to Dietary Methionine Restriction. Diabetes. 2016;65(6):1499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haws SA, Yu D, Ye C, Wille CK, Nguyen LC, Krautkramer KA, et al. Methyl-Metabolite Depletion Elicits Adaptive Responses to Support Heterochromatin Stability and Epigenetic Persistence. Mol Cell. 2020;78(2):210–23 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1–2):132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Trevino-Villarreal JH, et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell. 2018;173(1):117–29 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, et al. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell. 2018;173(1):74–89 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104(51):20618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281(15):811–6. [DOI] [PubMed] [Google Scholar]
- 152.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature reviews Endocrinology. 2014;10(12):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9(4):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013;62(7):961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes. 2013;62(8):2757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr 2016;103(2):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol 2016;45(5):1482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ribeiro RV, Solon-Biet SM, Pulpitel T, Senior AM, Cogger VC, Clark X, et al. Of Older Mice and Men: Branched-Chain Amino Acids and Body Composition. Nutrients. 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dumas SN, Lamming DW. Next Generation Strategies for Geroprotection via mTORC1 Inhibition. J Gerontol A Biol Sci Med Sci 2020;75(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, McDonald CE, et al. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest 2015;125(8):2952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mu WC, VanHoosier E, Elks CM, Grant RW. Long-Term Effects of Dietary Protein and Branched-Chain Amino Acids on Metabolism and Inflammation in Mice. Nutrients. 2018;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li H, Ye D, Xie W, Hua F, Yang Y, Wu J, et al. Defect of branched-chain amino acid metabolism promotes the development of Alzheimer’s disease by targeting the mTOR signaling. Biosci Rep 2018;38(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tournissac M, Vandal M, Tremblay C, Bourassa P, Vancassel S, Emond V, et al. Dietary intake of branched-chain amino acids in a mouse model of Alzheimer’s disease: Effects on survival, behavior, and neuropathology. Alzheimers Dement (N Y). 2018;4:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017;545(7655):500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li JT, Yin M, Wang D, Wang J, Lei MZ, Zhang Y, et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat Cell Biol 2020;22(2):167–74. [DOI] [PubMed] [Google Scholar]
- 168.Ericksen RE, Lim SL, McDonnell E, Shuen WH, Vadiveloo M, White PJ, et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab 2019;29(5):1151–65 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aris JP, Alvers AL, Ferraiuolo RA, Fishwick LK, Hanvivatpong A, Hu D, et al. Autophagy and leucine promote chronological longevity and respiration proficiency during calorie restriction in yeast. Exp Gerontol 2013;48(10):1107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mansfeld J, Urban N, Priebe S, Groth M, Frahm C, Hartmann N, et al. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nature communications. 2015;6:10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Juricic P, Gronke S, Partridge L. Branched-Chain Amino Acids Have Equivalent Effects to Other Essential Amino Acids on Lifespan and Aging-Related Traits in Drosophila. J Gerontol A Biol Sci Med Sci 2020;75(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu J, Temp U, Müller-Hartmann A, Esser J, Grönke S, Partridge L. Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nature Aging. 2021;1(1):60–72. [DOI] [PubMed] [Google Scholar]
- 173.D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 2010;12(4):362–72. [DOI] [PubMed] [Google Scholar]
- 174.Ruocco C, Ragni M, Rossi F, Carullo P, Ghini V, Piscitelli F, et al. Manipulation of Dietary Amino Acids Prevents and Reverses Obesity in Mice Through Multiple Mechanisms That Modulate Energy Homeostasis. Diabetes. 2020;69(11):2324–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takeuchi I, Yoshimura Y, Shimazu S, Jeong S, Yamaga M, Koga H. Effects of branched-chain amino acids and vitamin D supplementation on physical function, muscle mass and strength, and nutritional status in sarcopenic older adults undergoing hospital-based rehabilitation: A multicenter randomized controlled trial. Geriatr Gerontol Int 2019;19(1):12–7. [DOI] [PubMed] [Google Scholar]
- 176.Tynkkynen J, Chouraki V, van der Lee SJ, Hernesniemi J, Yang Q, Li S, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2018;14(6):723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aquilani R, Iadarola P, Contardi A, Boselli M, Verri M, Pastoris O, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil 2005;86(9):1729–35. [DOI] [PubMed] [Google Scholar]
- 178.Shiozawa S, Usui T, Kuhara K, Tsuchiya A, Miyauchi T, Kono T, et al. Impact of Branched-Chain Amino Acid-Enriched Nutrient on liver Cirrhosis with Hepatocellular Carcinoma Undergoing Transcatheter Arterial Chemoembolization in Barcelona Clinic Liver Cancer Stage B: A Prospective Study. J Nippon Med Sch. 2016;83(6):248–56. [DOI] [PubMed] [Google Scholar]
- 179.Nojiri S, Fujiwara K, Shinkai N, Iio E, Joh T. Effects of branched-chain amino acid supplementation after radiofrequency ablation for hepatocellular carcinoma: A randomized trial. Nutrition. 2017;33:20–7. [DOI] [PubMed] [Google Scholar]
- 180.Kim JS, Ro SH, Kim M, Park HW, Semple IA, Park H, et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific reports. 2015;5:9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saxton RA, Knockenhauer KE, Schwartz TU, Sabatini DM. The apo-structure of the leucine sensor Sestrin2 is still elusive. Sci Signal. 2016;9(446):ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351(6268):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 2016;22(4):421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deelen J, Kettunen J, Fischer K, van der Spek A, Trompet S, Kastenmuller G, et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nature communications. 2019;10(1):3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Castro-Portuguez R, Sutphin GL. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol 2020;132:110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Segall PE, Timiras PS. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Ageing Dev 1976;5(2):109–24. [DOI] [PubMed] [Google Scholar]
- 187.He C, Tsuchiyama SK, Nguyen QT, Plyusnina EN, Terrill SR, Sahibzada S, et al. Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import. PLoS Genet 2014;10(12):e1004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yap YW, Rusu PM, Chan AY, Fam BC, Jungmann A, Solon-Biet SM, et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nature communications. 2020;11(1):2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Badawy AA. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res 2017;10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev 2012;249(1):135–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108(13):4118–25. [DOI] [PubMed] [Google Scholar]
- 192.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002;196(4):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–42. [DOI] [PubMed] [Google Scholar]
- 194.Chen T, Zheng X, Ma X, Bao Y, Ni Y, Hu C, et al. Tryptophan Predicts the Risk for Future Type 2 Diabetes. PLoS One. 2016;11(9):e0162192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, et al. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet 2015;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mangge H, Stelzer I, Reininghaus EZ, Weghuber D, Postolache TT, Fuchs D. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med Chem 2014;21(17):1931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murr C, Grammer TB, Kleber ME, Meinitzer A, Marz W, Fuchs D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur J Clin Invest 2015;45(3):247–54. [DOI] [PubMed] [Google Scholar]
- 198.Inubushi T, Kamemura N, Oda M, Sakurai J, Nakaya Y, Harada N, et al. L-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J Nutr Sci Vitaminol (Tokyo). 2012;58(6):415–22. [DOI] [PubMed] [Google Scholar]
- 199.Esteban S, Nicolaus C, Garmundi A, Rial RV, Rodriguez AB, Ortega E, et al. Effect of orally administered L-tryptophan on serotonin, melatonin, and the innate immune response in the rat. Mol Cell Biochem. 2004;267(1–2):39–46. [DOI] [PubMed] [Google Scholar]
- 200.Ramos-Chavez LA, Roldan-Roldan G, Garcia-Juarez B, Gonzalez-Esquivel D, Perez de la Cruz G, Pineda B, et al. Low Serum Tryptophan Levels as an Indicator of Global Cognitive Performance in Nondemented Women over 50 Years of Age. Oxid Med Cell Longev 2018;2018:8604718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lindseth G, Helland B, Caspers J. The effects of dietary tryptophan on affective disorders. Arch Psychiatr Nurs 2015;29(2):102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu YJ, Janssens GE, McIntyre RL, Molenaars M, Kamble R, Gao AW, et al. Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet 2019;15(3):e1007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem 2009;284(34):22507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rowling MJ, McMullen MH, Chipman DC, Schalinske KL. Hepatic glycine N-methyltransferase is up-regulated by excess dietary methionine in rats. The Journal of nutrition. 2002;132(9):2545–50. [DOI] [PubMed] [Google Scholar]
- 205.Ron-Harel N, Notarangelo G, Ghergurovich JM, Paulo JA, Sage PT, Santos D, et al. Defective respiration and one-carbon metabolism contribute to impaired naive T cell activation in aged mice. Proc Natl Acad Sci U S A. 2018;115(52):13347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R, Roman R, et al. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharmacol 2008;599(1–3):152–8. [DOI] [PubMed] [Google Scholar]
- 207.El Hafidi M, Perez I, Zamora J, Soto V, Carvajal-Sandoval G, Banos G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol 2004;287(6):R1387–93. [DOI] [PubMed] [Google Scholar]
- 208.Cruz M, Maldonado-Bernal C, Mondragon-Gonzalez R, Sanchez-Barrera R, Wacher NH, Carvajal-Sandoval G, et al. Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes. Journal of endocrinological investigation. 2008;31(8):694–9. [DOI] [PubMed] [Google Scholar]
- 209.Hashizume O, Ohnishi S, Mito T, Shimizu A, Ishikawa K, Nakada K, et al. Epigenetic regulation of the nuclear-coded GCAT and SHMT2 genes confers human age-associated mitochondrial respiration defects. Scientific reports. 2015;5:10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gheller BJ, Blum JE, Lim EW, Handzlik MK, Hannah Fong EH, Ko AC, et al. Extracellular serine and glycine are required for mouse and human skeletal muscle stem and progenitor cell function. Molecular metabolism. 2021;43:101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hartiala JA, Tang WH, Wang Z, Crow AL, Stewart AF, Roberts R, et al. Genome-wide association study and targeted metabolomics identifies sex-specific association of CPS1 with coronary artery disease. Nature communications. 2016;7:10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Thalacker-Mercer AE, Ingram KH, Guo F, Ilkayeva O, Newgard CB, Garvey WT. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes. 2014;63(2):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J, et al. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2016;39(5):833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Coelho-Junior HJ, Rodrigues B, Uchida M, Marzetti E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Coelho-Junior HJ, Calvani R, Picca A, Goncalves IO, Landi F, Bernabei R, et al. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients. 2020;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015;14(4):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Forney LA, Stone KP, Gibson AN, Vick AM, Sims LC, Fang H, et al. Sexually Dimorphic Effects of Dietary Methionine Restriction are Dependent on Age when the Diet is Introduced. Obesity (Silver Spring). 2020;28(3):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, et al. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol 2011;7(9):610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mahoney SJ, Narayan S, Molz L, Berstler LA, Kang SA, Vlasuk GP, et al. A small molecule inhibitor of Rheb selectively targets mTORC1 signaling. Nature communications. 2018;9(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA, Velarde MC, Syed FA, et al. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nature communications. 2019;10(1):3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife. 2013;2:e00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim D, Lee J, Bae I, Kim M, Huh Y, Choi J, et al. Preparation, characterization, and pharmacological study of a novel long-acting FGF21 with a potential therapeutic effect in obesity. Biologicals. 2021;69:49–58. [DOI] [PubMed] [Google Scholar]
- 223.Sengupta S, Giaime E, Narayan S, Hahm S, Howell J, O’Neill D, et al. Discovery of NV-5138, the first selective Brain mTORC1 activator. Scientific reports. 2019;9(1):4107. [DOI] [PMC free article] [PubMed] [Google Scholar]


